The molecular mechanisms underlying the distinct sensations of warmth and heat have remained unclear until recently. In 2016 we showed that the TRPM2 ion channel mediates the sensation of warmth in vivo1, while in 2018 Vandewauw et al2 found that three channels, TRPV1, TRPM3 and TRPA1, jointly mediate the sensation of painful heat. In one important respect, though, these papers disagreed: we found that TRPM2 contributed to the thermal response of isolated somatosensory neurons1,3, while Vandewauw et al proposed that TRPA1, and not TRPM2, was the only thermally-activated ion channel in somatosensory neurons apart from TRPV1 and TRPM32. In the work presented here we sought to resolve the roles of TRPM2 and TRPA1 in the thermal responses of sensory neurons.
In our original paper1 we used calcium imaging to show that around 10% of somatosensory neurons from dorsal root ganglia (DRG) were activated by heat but did not express functional TRPV1 or TRPM3, and we proposed that the thermal responses in these neurons was driven by TRPM2. In Fig 1A (protocol of solution application shown in Fig. 2A) we used a similar method to test whether expression of TRPA1 could instead be the cause of thermal responses in these neurons. Fig 1A shows that responses to the selective TRPA1 agonist allyl isothiocyanate (AITC) are observed in around 25% of DRG neurons, almost all of which (24%) also express TRPV1 and/or TRPM3, in agreement with previous work4,5. A similar result was obtained in facial neurons from the trigeminal ganglion (TG), in which 41.5% in total express TRPA1, of which only 2.5% express TRPA1 in the absence of both TRPV1 and TRPM3 (Extended data Fig. 1A). Around 10% of DRG neurons, and 8% of TG neurons, were activated by heat but did not express functional TRPA1, TRPV1 or TRPM3, similar to the proportion of TRPM2-expressing neurons identified in our original study1.
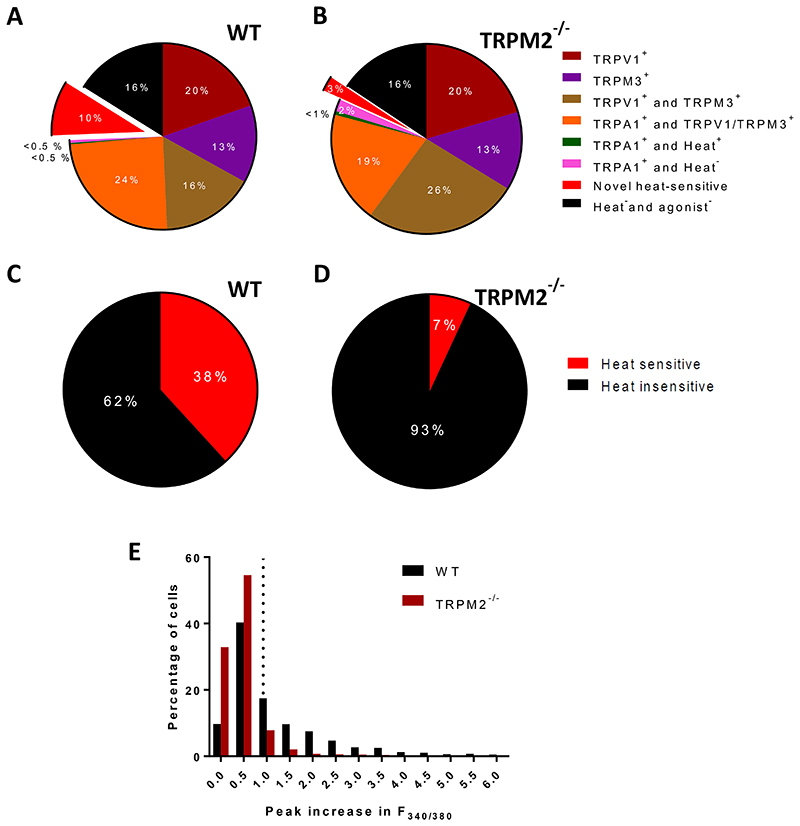
Fig. 1. Novel heat-sensitive neurons do not express TRPA1.
A. Proportions of DRG neurons expressing TRPV1, TRPM3 and TRPA1, and of novel heat sensitive neurons expressing none of these TRP channels, from increases in F340/F380 in response to application of selective agonists (see Methods for details). Experimental protocol shown in Fig. 2A (except that TRPM2 blocker 2-APB was not applied). Pie chart shows data from 3 experiments as follows: neurons expressing only TRPV1 (19.6 ± 2.0%, mean ± SEM); only TRPM3 (13.5 ± 0.2%); both TRPV1 and TRPM3 but not TRPA1 (16.2 ± 3.5%); TRPA1 together with either TRPV1 or TRPM3 (24.4 ± 1.6%); TRPA1 alone, and responding to heat (0.2 ± 0.1%); TRPA1 alone and not responding to heat (0.49 ± 0.2%); novel heat-sensitive neurons responding to heat but not to agonists for any of TRPV1, TRPM3 or TRPA1 (9.5 ± 0.2%); and neurons not responding to heat nor to any of the TRP agonists but identified as viable from response to final pulse of KCl (16.1 ± 2.6%). See criteria in Methods for identifying positive heat and agonist responses. Data from 3 separate experiments on a total of 1563 neurons (13 cover slips from 3 male wild-type mice).
B. Similar experiment performed on DRG neurons from TRPM2-/- mice. There is no significant difference in the proportions of any of the agonist-sensitive neurons from WT (p>0.05, multiple t test with Bonferroni correction). However, the proportion of novel heat-sensitive neurons is significantly reduced by deletion of TRPM2, from 9.5 ± 0.2% in WT to 2.5 ± 0.8% in TRPM2-/-(p = 0.009, multiple t test with Bonferroni correction). Data from 3 separate experiments on a total of 660 neurons (14 cover slips from 3 male TRPM2-/- mice).
C. Proportion of DRG neurons responding to heat in the presence of inhibitor cocktail blocking TRPV1, TRPM3 and TRPA1. See Extended data Fig. 1C, top trace, for protocol. In 4 separate experiments percentage of heat-responsive neurons = 38.2 ± 4.4%, and Δ F340/F380 = 1.90 ± 0.06 (mean ± SEM). Results from 1315 WT neurons.
D. Similar experiment to that shown in C, on DRG neurons from TRPM2-/- mouse. Mean percentage of “unidentified” heat-responsive neurons in n = 4 separate experiments was 6.9 ± 2.2% (mean ± SEM, significant difference from WT, p = 0.0007, unpaired t-test) and ΔF340/F380 = 1.59 ± 0.16 (mean ± SEM, significant difference from WT, p = 0.0237, unpaired t-test). Results from 1794 neurons.
E. Amplitudes of ΔF340/F380 in response to heat ramp to 48°C from the experiments shown in C (WT neurons) and D (TRPM2-/- neurons). Threshold for accepting a heat response shown by vertical dotted line giving intrinsic heat-sensitivity of the fura2 dye obtained from heat ramp applied in Ca-free solution (see details in Methods). Mean ΔF340/F380 in response to heat (48°C) in all WT neurons (i.e. both heat-sensitive and heat-insensitive) was 1.24 ± 0.04 (n = 1315) and in all TRPM2-/- neurons was 0.46 ± 0.01 (n = 1794). Difference significant, p <0.0001, unpaired t-test.
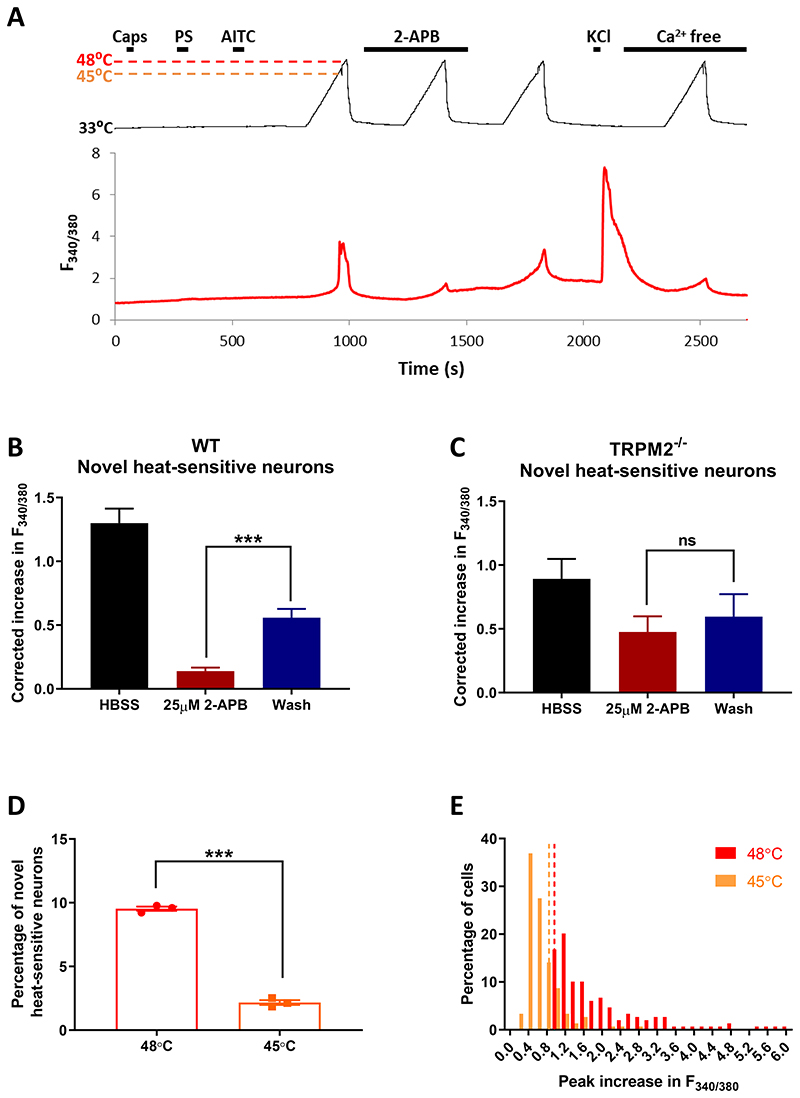
Fig. 2. Pharmacological block of TRPM2 inhibits the heat response of novel heat-sensitive neurons.
A. Effect of TRPM2 blocker 2-APB (25μM) on identified somatosensory neurons. Neurons expressing TRPV1, TRPM3 and/or TRPA1 identified by brief application of the specific agonists capsaicin (1μM), pregnenolone sulphate (PS, 100μM) and allyl isothiocyanate (AITC, 50 μM), respectively, followed by heat ramp to 48°C to activate all heat-sensitive neurons. Effect of TRPM2 blocker 2-aminoethoxydiphenyl borate (2-APB, 25μM) on heat-activated neurons is tested, followed by a pulse of KCl (50mM) to test neuronal viability. At end, heat pulse repeated in Ca-free solution, to identify changes in F340/F380 fluorescence ratio (ΔF340/F380) arising from sensitivity of the fura2 dye to temperature. Response to heat in a novel heat-sensitive neuron not expressing any of TRPV1, TRPM3 or TRPA1 is strongly suppressed by the TRPM2 blocker 2-APB (25μM).
B. Increase in calcium-dependent fluorescence ratio (ΔF340/F380) in response to heat in novel heat-sensitive neurons (n = 149 neurons from 3 wild-type mice) is strongly suppressed by 2-APB (25μM). Increase in ΔF340/F380 corrected for each neuron by subtracting the increase in ΔF340/F380 measured from heat ramp applied in Ca-free solution.
C. Similar experiment on neurons from 3 TRPM2-/- mice. The few novel-heat-sensitive neurons observed (see Fig. 1B) show no significant effect of 2-APB on response amplitude.
D. Effect of terminating temperature ramp at 48°C1 and at an earlier time point when temperature had reached 45°C2. Reducing final temperature had a dramatic effect on proportion of novel heat-sensitive neurons, from 9.5 ± 0.2% in response to heat to 48°C to 2.2 ± 0.2% in response to heat to 45°C (p<0.0001, unpaired t-test). n = 1563 neurons from 3 WT mice.
E. Amplitudes of ΔF340/F380 in response to heat ramp reaching 48°C and 45°C from the experiment shown in B. Threshold for accepting a heat response shown by vertical dotted lines showing intrinsic heat-sensitivity of the fura2 dye obtained from heat ramp applied in 0Ca solution (see details in Methods). Mean ΔF340/F380 in response to heat to 48°C was 2.04 ± 0.11 and to 45°C was 0.67± 0.03 (n = 149 neurons, p<0.0001, unpaired t-test).
Fig. 1B shows that genetic deletion of TRPM2 reduced from 10% to 3% the proportion of neurons in which thermal responses are not attributable to any of TRPV1, TRPM3 or TRPA1, without significantly changing the proportions of neurons in other classes. Apart from this small proportion expressing TRPM2 in isolation, thermal responses attributable to TRPM2 are also co-expressed with TRPV1 and TRPM31. Fig. 1C confirms this by showing that 38% of neurons are thermally activated when responses mediated by TRPV1, TRPM3 and TRPA1 are blocked by selective antagonists. Genetic deletion of TRPM2 reduces the proportion of neurons responding to heat when TRPV1, TRPM3 and TRPA1 are blocked from c. 38% to c. 7% (the latter are “unidentified” heat responses not attributable to any of TRPV1, TRPM3, TRPA1 or TRPM2, Fig. 1D). The mean response amplitude in WT neurons in the presence of inhibitors was ΔF340/F380 = 1.90 ± 0.06, while unidentified heat responses had mean response amplitude of ΔF340/F380 = 1.59 ± 0.16. The unidentified heat response in a small number of TRPM2-/- neurons in the presence of blockers is likely to be due to expression of an additional heat sensitive mechanism, perhaps Anoctamin 16, although the possibility that it may be due to a non-specific response to heat cannot be excluded.
An alternative approach to determining the role of TRPM2 is to use a selective pharmacological blocker. We chose 2-aminoethoxydiphenyl borate (2-APB), which largely blocks TRPM2 at a concentration of 25μM7, although higher concentrations activate members of the TRPV family8-10. Fig. 2B shows that the response amplitude in thermally activated neurons whose responses are not attributable to TRPV1, TRPM3 or TRPA1 (“novel heat-sensitive neurons”) is strongly inhibited by 2-APB. When neurons from TRPM2-/- mice were tested in the same way (Fig. 2C) there was no significant effect of 2-APB on the amplitude of the “unidentified” thermal responses.
In neurons responding to only one of the agonists for TRPV1, TRPM3 or TRPA1 (Extended Data Fig 2A), 2-APB caused a small reduction in thermal response amplitude (not significant in the case of the very small number of neurons expressing only TRPA1 in isolation). Because many neurons co-express TRPM2 with other TRP channels (Fig. 1C, D) it is likely that this reduction in response is due to an inhibitory effect of 2-APB on co-expressed TRPM2. Extended Data Fig. 2B supports this possibility, because in neurons from TRPM2-/- mice there was either no change of amplitude on application of 2-APB, or an increase in the case of TRPV1, which is known to be activated by 2-APB8–10.
These experiments show that the responses of the TRPM2-expressing neurons identified in our original study1 are not due to expression of TRPA1, as proposed by Vandewauw et al2. Thermal responses that are not attributable to TRPV1, TRPM3 or TRPA1 are almost completely eliminated by either genetic deletion or pharmacological block of TRPM2, confirming our original proposal that these neurons express TRPM21.
We sought to identify the cause of the discrepancy between the above results and the work of Vandewauw et al2. Changing the source of neurons (DRG or TG), order of application of agonists and heat stimulus, culture conditions, rise time of heat application and starting temperature did not explain the discrepancy (Extended data Fig. 1). However, terminating the temperature ramp at a lower level of 45°C, as used by Vandewauw et al, rather than the temperature of 48°C used in our experiments, sharply reduced the proportion of novel heat-sensitive neurons (Fig. 2D, E), suggesting that the reason why Vandewauw et al2 did not identify thermally activated neurons expressing TRPM2 was that their heat stimulus did not reach a high enough temperature to activate TRPM2 in these isolated neurons.
As noted previously, TRPM2 deletion alters the behavioural sensation of warmth in vivo1, while deletion of TRPM2 does not affect in vivo thermal sensation in the range of noxious heat, at 42°C and above1,3,11. In addition, noxious heat sensation is abolished when TRPV1, TRPM3 and TRPA1, but not TRPM2, have been deleted2. These observations suggest that TRPM2 is activated in the range 30-40°C in vivo. However, following neuronal isolation, the temperature threshold becomes more elevated, as is also found in expression systems12. A possible explanation is that a factor regulating the thermal sensitivity of TRPM2 may be lost following neuronal isolation.
Finally, we tested whether somatosensory neurons expressing TRPA1 can respond to thermal stimuli, as proposed by Vandewauw et al2. We tested the heat sensitivity of identified TRPA1-expressing neurons from TRPM2-/- mice, with TRPV1 and TRPM3 pharmacologically blocked (Extended data Fig. 3). In these experiments we found that c. 30% of neurons were activated by a TRPA1 agonist, similar to the proportion found in Fig 1. In 34.7% of these TRPA1+ neurons we observed a response to heat that was inhibited by TRPA1 block. In a further 5.5% of neurons the response to heat was not due to any of TRPM2, TRPV1, TRPM3 or TRPA1 (unidentified heat-responders, see also Fig. 1D). We conclude that TRPA1, often assumed to be activated only by cold13, does contribute significantly to heat responses in somatosensory neurons, as reported by Vandewauw et al2. In summary, there is clear evidence that TRPV1, TRPM3, TRPM2 and TRPA1 contribute to the thermal responses of somatosensory neurons, and that a further unidentified heat sensor is present in a small minority of neurons.
Methods
Animals
Male mice on a C57BL/6J background, older than 9 weeks old, were used for all experiments. Trpm2-/- mice were gifts from Y. Mori. Mice were maintained on a 12 h day/12 h night cycle.
Primary neuron cultures
After mice had been euthanized by cervical dislocation, DRG or TG ganglia were excised then incubated in papain (2 mg ml-1 in Ca2+-free and Mg2+-free HBSS) for 30 min at 37 °C, followed by incubation in collagenase (2.5 mg ml-1 in Ca2+-free and Mg2+-free HBSS) for 30 min at 37°C. Ganglia were re-suspended and mechanically dissociated in Neurobasal-A/B27 growing medium, which was prepared with Neurobasal-A Medium supplemented with 0.25% (v/v) l-glutamine 200 mM (Invitrogen), 2% (v/v) B-27 supplement (Invitrogen), 1% (v/v) penicillin-streptomycin (Invitrogen), and nerve growth factor (NGF) (Sigma-Aldrich) at 50 ng ml-1 unless otherwise specified in the figure legend. Dissociated neurons were centrifuged and plated onto coverslips pre-coated with poly-l-lysine (10 μg ml-1) and laminin (40 μg ml-1). Neurons were kept in a 37 °C incubator with a 95% air / 5% CO2 atmosphere for at least 12 h before use, and all neurons were used within 24 h.
Extracellular solutions and perfusion system
Unless otherwise specified, all experiments were carried out with an extracellular solution containing 140 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 5 mM glucose; pH was adjusted to 7.4 with NaOH and osmolarity was between 295–310 mOsm. Calcium-free extracellular solution was prepared with the formulation above except for omission of calcium chloride. An 8-line manifold gravity-driven system controlled by an automated solution changer with a common outlet was used to apply solution to the cells. Solution was heated with a Peltier device regulated by a proportional gain feedback controller designed by Dr V. Vellani (CV Scientific). In separate control experiments, the temperature in each experimental protocol was recorded by a miniature thermocouple at the cell location. All compounds applied were prepared as stock solutions first and then diluted to the concentration needed before experiments. Drugs used were 1 μM capsaicin (5 mM stock in ethanol), 100 μM pregnenolone sulphate (500mM stock in DMSO), 25 μM 2-APB (500 mM stock in DMSO), 50 μM AITC (200 mM stock in DMSO), 100 μM HC030031 (100mM stock in DMSO), 10 μM naringenin (100mM stock in DMSO) and 5 μM AMG9810 (50 mM stock in DMSO). The inhibitor cocktail was composed of the TRPA1 antagonist HC030031, the TRPV1 antagonist AMG9810, and the TRPM3 antagonist naringenin.
Calcium imaging
Cells were loaded with 5 μM fura-2 AM (Invitrogen) with 0.02% (v/v) pluronic acid (Invitrogen) for 30 min. After loading, coverslips were put in an imaging chamber and transferred to a Nikon Eclipse Ti-E inverted microscope. Cells were continuously perfused with extracellular solution and were illuminated with a monochromator alternating between 340 and 380 nm (OptoScan; Cairn Research), controlled by WinFluor 3.2 software (J. Dempster, University of Strathclyde, UK). Emission was collected at 510 nm and the resulting pairs of images were acquired every two seconds with a 100 ms exposure time using an iXon 897 EM-CCD camera (Andor Technology, Belfast, UK). Image time series were converted to TIFF files and processed with ImageJ software. Images of the background fluorescence intensity were obtained for both wavelengths and subtracted from the respective image stack before calculating the F340/380 ratio images. A minority of neurons exhibited an unstable F340/380 baseline in the absence of any applied stimulus, usually caused by poor dye loading but in some cases apparently due to low-frequency repetitive firing even in the absence of any treatment, and were removed from analysis. A positive response to all agonists was therefore defined from the rate of increase of [Ca]i following agonist application, as an increase of F340/380 ratio, between two consecutive time points following application of agonist, which exceeds the mean + 3.09 s.d. (cumulative probability value of 99.9%) of all such differences in the absence of any agonist. A heat-sensitive neuron is defined as a neuron with a peak increase in F340/380 during a heat stimulus larger than the mean + 3.09 s.d. of the peak increase in F340/380 during a heat stimulus in Ca-free solution in the same experiment (see Fig. 2A).
Statistical analysis and data availability
All data are expressed as means ± s.e.m. Statistical analyses were performed with GraphPad Prism version 7.04 and data were tested for approximation to normality. All statistical tests were two-sided. All measurements were taken from distinct samples. The statistical test used is stated in each figure legend. All data not provided within the paper are available on request from the authors.
Ethical approval
Ethical approval was given by the Animal Welfare Ethical Review Board, King’s College London and by the Home Office, UK Government, or in the case of the experiment shown in Extended Data Fig. 1A, by the Institutional Animal Care and Use Committee, Kaohsiung Medical University.
Extended Data
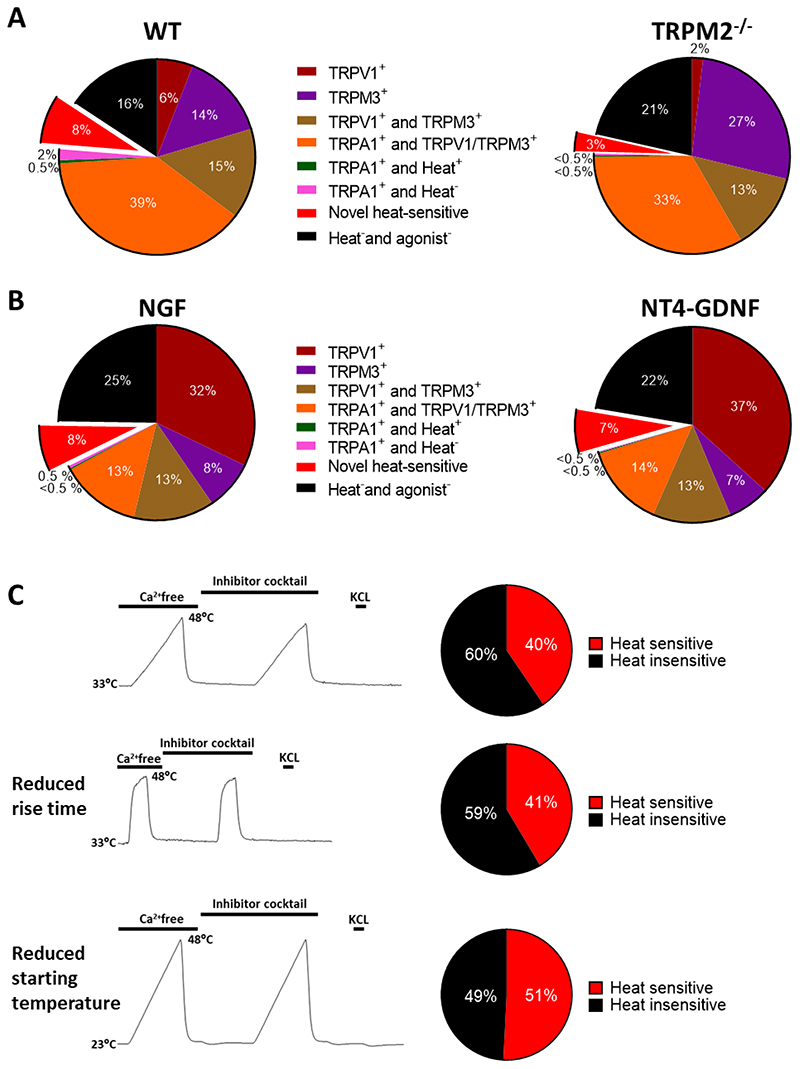
Extended data Fig. 1. Changing the source of neurons, the order of application of agonists and the heat stimulus, the culture conditions, the rise time of heat application and the starting temperature do not significantly affect the proportions of novel heat-sensitive neurons identified in heat activated somatosensory neurons.
A. Neurons from trigeminal ganglion (TG) tested for heat and agonist sensitivity using protocol shown in Fig. 2A, but without application of 2-APB.
Left: Percentage distributions of heat and agonist-sensitive neurons. TRPA1 is strongly co-expressed with TRPV1 or TRPM3 (39%), with few neurons expressing TRPA1 alone (2.5%). Novel heat-sensitive neurons that respond to heat but do not express any of TRPV1, TRPM3 or TRPA1 can be clearly identified (8%). n = 388 neurons from 1 WT mouse.
Right: Similar experiment on n = 382 TG neurons from TRPM2-/- mouse. Proportion of novel heat sensitive neurons significantly reduced (from 8% to 3%, p = 0.0077, Fisher’s exact test). Mean response amplitude of novel heat-sensitive neurons not reduced (ΔF340/F380 = 1.13 ± 0.05, n=31, to 0.96 ± 0.08, n=13). Difference not significant, p = 0.0539, unpaired t-test.
B. Neurons from DRG tested for heat and agonist sensitivity using protocol shown in Fig. 2A, but without application of 2-APB and with agonists presented after heat.
Left: Neurons cultured for 12h in NGF1. See Methods for details. Pie chart shows data from 3 experiments on a total of 1312 neurons from 3 WT male mice on 12 coverslips as follows: neurons expressing only TRPV1 (32.2 ± 4.2%, mean ± SEM); only TRPM3 (8.2 ± 1.2%); both TRPV1 and TRPM3 but not TRPA1 (13.3 ± 0.6%); TRPA1 together with either TRPV1 or TRPM3 (13.3 ± 0.9%); TRPA1 alone, and responding to heat (0.2 ± 0.2%); TRPA1 alone and not responding to heat (0.4 ± 0.2%); novel heat-sensitive neurons responding to heat but not to agonists for any of TRPV1, TRPM3 or TRPA1 (7.6 ± 2.0%); and neurons not responding to heat nor to any of the TRP agonists but identified as viable from response to final pulse of KCl (24.8 ± 1.8%). The application of agonists before (Fig. 1) or after heat pulse (this Fig) does not significantly affect the proportion of novel heat-sensitive neurons detected (9.5 ± 0.2% when agonists applied before, see Fig. 1A, and 7.6 ± 2.0% when after (p = 0.3740, unpaired t-test)). Proportions of neurons in other categories also not significantly affected, apart from a small reduction in the proportion of neurons expressing TRPA1 in combination with TRPV1 and/or TRPM3 (compare Fig. 1A).
Right: Neurons cultured for 12h in NT4-GDNF2. n = 1521 neurons from 3 WT male mice on 12 coverslips. These culture conditions had no significant effect on the proportions of heat and agonist-sensitive neurons. p>0.05, multiple t test with Bonferroni correction.
C. Protocol for determining total proportion of novel heat-sensitive neurons from DRG, including those co-expressing TRPV1, TRPM3 and/or TRPA1. Heat ramp is first applied in Ca-free solution, to identify changes in F340/F380 fluorescence ratio (ΔF340/F380) arising from sensitivity of the fura2 dye to temperature, followed by an identical ramp in the presence of a cocktail of inhibitors of TRPV1 (AMG9810, 5μM), TRPM3 (naringenin, 10μM), and TRPA1 (HC-030031, 100μM) (see details in Methods). Final pulse of KCl confirms neuronal viability.
Top: Heat application rising from 33°C to 48°C in 180 sec1. n = 710 neurons from 2 WT mice on 3 coverslips. Middle: Heat application rising from 33°C to 48°C in 25 sec2. n= 99 neurons from 1 WT mouse on 2 coverslips. Bottom: Heat application rising from 23°C to 48°C in 180 sec2. n= 657 neurons from 1 WT mouse on 2 coverslips.
Reducing rise time or starting temperature of heat application does not reduce the total proportion of novel heat-sensitive neurons. Difference not significant, p = 0.9197 (middle) and 0.3339 (bottom) respectively, unpaired t-test compared to top panel.
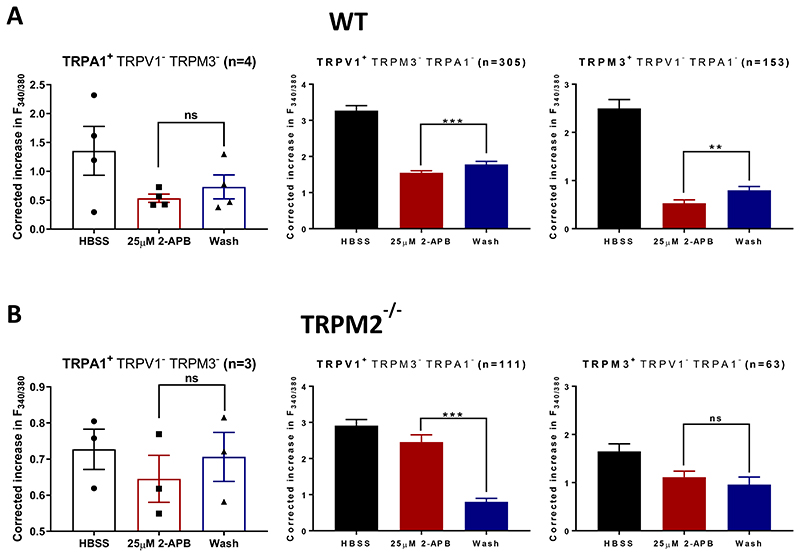
Extended data Fig. 2. Effect of TRPM2 blocker 2-APB on heat responses in neurons expressing only TRPA1, TRPV1 or TPRPM3.
Neurons were identified as expressing only one of TRPA1 (left), TRPV1 (middle) or TRPM3 (right) and the effect of 2-APB (25μM) was tested as shown in Fig. 2A. A, WT neurons. Note that many of these neurons will co-express TRPM2, which is extensively expressed in somatosensory neurons (Fig. 1C).
B, Similar experiments on TRPM2-/- neurons. Significance level during 2-APB application compared to that after wash is shown as: *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., p > 0.05. One-way ANOVA followed by Bonferroni’s post-hoc correction.
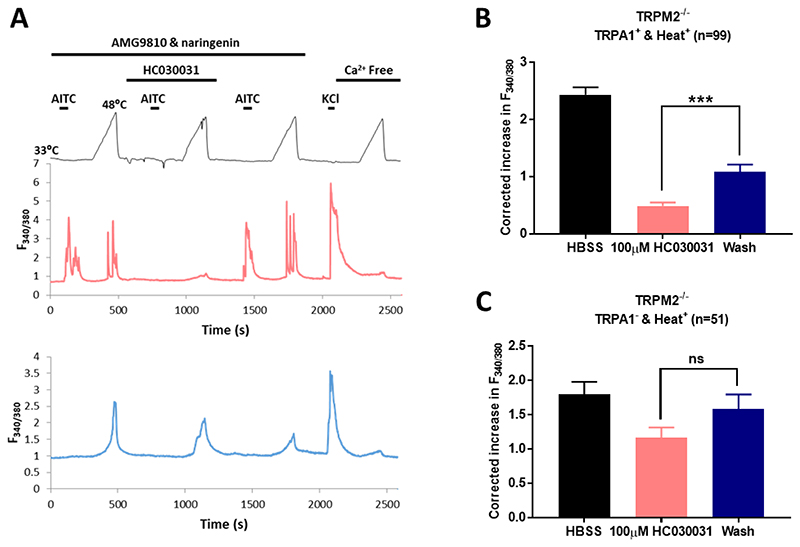
Extended data Fig. 3. A fraction of TRPA1+ neurons from TRPM2-/- mice respond to heat when TRPV1 and TRPM3 are blocked.
A. Upper: Protocol of solution changes used to identify neurons expressing TRPA1 and to test their activation by heat. TRPV1 and TRPM3 were blocked by AMG9810 (5μM) and naringenin (10μM) as shown. The calcium increase elicited by a heat ramp from 33°C to 48°C was tested in the absence (heat ramps 1 and 3) and presence (ramp 2) of the TRPA1 blocker HC030031 (100μM). Responses to the TRPA1-selective agonist AITC were tested as shown. Response to KCl (50mM) and heat ramp in absence of external Ca tested at end. Middle trace: increases in F340/380 in a neuron expressing TRPA1 in which the heat-activated Ca increase was suppressed by the TRPA1 blocker HC030031. TRPA1 was expressed in 31.2 ± 4.2% of the total population, but only a fraction of TRPA1+ neurons (34.7% ± 9.81%) gave a significant Ca increase in response to 48°C heat. Lower trace: a different neuron in same preparation is activated by heat but does not express TRPA1 (unidentified heat-responders). Data from 3 separate experiments on a total of 964 neurons.
B. Heat response in TRPA1-expressing neurons is suppressed by TRPA1 blocker HC030031 (protocol and example trace shown in A. ***, p<0.001, one-way ANOVA followed by Bonferroni’s post-hoc correction). Heat response in neurons not expressing TRPA1 is not significantly reduced by the TRPA1 blocker HC030031 (100μM). Proportion of unidentified heat-responders (5.5% ± 1.4%) is not significantly different from that in Fig. 1D.
References
- 1.Tan CH, McNaughton PA. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 2016;536:460–463. doi: 10.1038/nature19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandewauw I, et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555:662–666. doi: 10.1038/nature26137. [DOI] [PubMed] [Google Scholar]
- 3.Tan CH, McNaughton PA. TRPM2 and warmth sensation. Pflugers Archiv : European journal of physiology. 2018;470:787–798. doi: 10.1007/s00424-018-2139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15:1015–1021. doi: 10.1038/nn.3111. doi:nn.3111 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A. Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci U S A. 2009;106:1626–1631. doi: 10.1073/pnas.0812209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamatova KN, Kang TM. Activation of rat transient receptor potential cation channel subfamily V member 1 channels by 2-aminoethoxydiphenyl borate. Integrative medicine research. 2013;2:112–123. doi: 10.1016/j.imr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraguchi K, et al. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci. 2012;32:3931–3941. doi: 10.1523/JNEUROSCI.4703-11.2012. doi:32/11/3931 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashio M, et al. Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci U S A. 2012;109:6745–6750. doi: 10.1073/pnas.1114193109. doi:1114193109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]