Abstract
Our understanding of how peripheral damage-sensing neurons (nociceptors) respond to noxious stimuli is fundamental to the development of effective analgesics. To date, numerous studies have presented diverging hypotheses on how nociceptors encode modality-specific stimuli, including labelled-line, intensity dependence or pattern theory. In this short review, we appraise data from electrophysiological, behavioural, imaging and molecular expression studies from the last 60 years, in order to obtain a coherent view of modality-specific sensing in peripheral sensory neurons. We propose a mechanistic explanation for the broad range of values obtained for the incidence of polymodal nociceptors that reconciles apparently contradictory data.
Introduction
A fundamental characteristic of the somatosensory system is the ability to detect and distinguish innocuous and noxious stimuli in order to initiate behavioural responses to evade or minimise harm. Theories of sensation have invoked modality-specific labelled lines, intensity coding or patterned input to explain the link between sensory neuron activity and sensation, and for more than 50 years, countless studies have investigated the mechanisms by which damage-sensing neurons (nociceptors) are activated, with the aim of developing effective analgesics [1]. An intriguing observation from these studies, is that a significant proportion of nociceptors are sensitive to multiple types of noxious stimulation (such as noxious heat and noxious mechanical stimulation), thus making them polymodal. However, this observation is difficult to reconcile with the modality-specific sensations associated with pain, and the evidence for modality-specific pain pathways obtained from behavioural studies after the ablation of subsets of sensory neurons. Indeed, experiments performed by Magnus Blix and Alfred Goldscheider, conducted almost 150 years ago, provided evidence for modality-specific spots on human skin (see Ref. [2] for review). The debate about polymodality has important practical consequences for drug development: if all nociceptors are polymodal, then all pain may be considered a single pathology; a view at odds with much experimental data. Here, we summarise our understanding of modality sensing in sensory neurons, considering electrophysiological, behavioural, imaging and molecular expression data. Taken together, these studies show that plasticity in somatosensation, particularly in response to inflammatory mediators, can reconcile the apparently disparate data in the literature to give a coherent view of modality sensing in peripheral somatosensory neurons.
Electrophysiological analysis of sensory neuron modality
Electrophysiological studies of nociceptor function have been performed on a variety of animal species. This work began in earnest in the late 1960s, with the work by Burgess and Perl (1967) and later Bessou and Perl (1969). These seminal papers characterised a subset of peripheral sensory neurons that were exclusively activated by noxious stimuli, demonstrating the existence of specialised nociceptors [3,4]. A key observation from the work of Bessou and Perl in 1969 was that some unmyelinated neurons responded to more than one noxious modality (e.g. heat, mechanical and chemical), giving rise to the term ‘polymodal nociceptor’ [4]. To date, the majority of sensory physiology studies use the term polymodal to define a neuron that is responsive to both mechanical and thermal stimulation, mainly driven by the fact that chemical sensitivity is infrequently tested. Here, the term polymodal will refer to sensory neurons that respond to more than one noxious modality (e.g. mechanical and heat). Using this more testable definition, numerous studies have characterised the modality responses of nociceptors from different species, yielding variable results. While the reported incidence of C-fibre polymodality is high in some studies (67−100%) [5–10], it is much lower in others (11−56%) [4,11–14]. Beyond the variability in reported levels of polymodality, there are also significant inconsistencies in the response profiles of polymodal nociceptors. For example, several studies investigating the polymodality of C-fibres found that none of the fibres that responded to noxious heat and noxious mechanical stimulation responded additionally to noxious cold [5,14,15]. In contrast, a study by Baumann et al. in 1991 showed that 78% of C-fibre nociceptors responded to noxious mechanical, heat and cold stimuli [10].
How can such substantial differences between studies be reconciled? While factors such as species, site of stimulation, as well as the mode and strength of a given stimulus, are all likely causes of variation between studies, the degree of variation may also reflect the plasticity of the nociceptive system. It is well documented that nerve injury and/or inflammation induces functional changes in nociceptors [see Ref. [16] for review]. Of note, Simone and Kajandar (1997) showed that while ~20% of Aδ-nociceptors are excited at 2°C, this proportion increases to almost 100% at the tissue damaging temperature of −18°C [17]. Similar variations in modality sensitivity have also been reported for heat and mechanical stimuli following the actions of neurotrophic factors [18–21], nerve injury and/or inflammation [22], and between in vivo and in vitro experimental preparations [23]. In addition, a significant population (up to a quarter) of nociceptive afferents are unresponsive to noxious stimulation under basal conditions in both rodents [21,23,24] and humans [25]. These afferents become awakened following injury and/or inflammation, and typically exhibit sensitivity to noxious mechanical stimulation, with some also responding to noxious heat stimulation. Understanding the physiology of silent nociceptors is fundamental to our understanding of the nociceptive system, as they offer clear insight into the plasticity of the system. Relatively little is known about silent nociceptors, perhaps caused by their variable incidence, which, as outlined above, is likely to be affected by local inflammatory mediators.
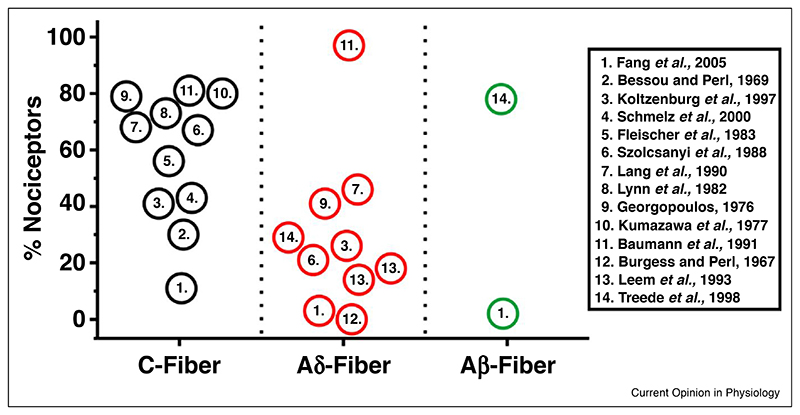
To date, the majority of polymodal classification studies have been restricted to C-fibres. However, a significant proportion of Aδ and Aβ fibres are also activated by noxious stimulation, and are therefore considered nociceptors [26]. Unfortunately, far fewer modality studies have been performed on A nociceptors compared to C-fibres, and even fewer on the largely overlooked Aβ population. One of the first studies to investigate the noxious sensitivity of myelinated fibres was performed by Burgess and Perl in 1967. From the 513 fibres recorded, 74, mainly Aδ fibres, responded exclusively to noxious mechanical stimulation [3]. Intriguingly, none of these fibres responded to noxious heat, noxious cold, acid application or bradykinin injection. Subsequent electrophysiological assessments from rats, cats and monkeys have reported the incidence of polymodality to vary between 3−97% in Aδ fibres [3,5,6,8,10–12,27,28]. Within the Aβ nociceptor population, one study on rats showed that only 3% were polymodal, with the vast majority (86%) responding to noxious mechanical stimulation [11]. In contrast, a study in monkeys showed that 78% of Aβ nociceptors were responsive to both noxious mechanical and heat stimulation [28]. The observed incidence of polymodal nociceptors between, C, Aδ and Aβ fibres is summarised in Figure 1.
Figure 1. Reported incidence of polymodality from nociceptors.
Summary of the reported incidence of mechano-heat polymodal nociceptors as a proportion of total C-fibre, Aδ-fibre and Aβ-fibre nociceptors, obtained from electrophysiological studies.
From the large number of electrophysiology studies undertaken, it is clear that polymodal neurons exist; however, their relative incidence within the nociceptor population, as well as their response profiles, remains to be fully understood, and due to the reasons outlined above, these issues are unlikely to be resolved through electrophysiological studies alone. Therefore, alternative approaches that interrogate neuronal physiology need to be considered.
Behavioural studies identify modality-specific subsets of neurons
Our ability to distinguish between discrete environmental stimuli suggests that there are specific receptors within sensory neurons that are adapted for their detection. Numerous molecular transducers (e.g. Trpv1, Trpa1, Trpm8, Piezo 2) have been identified that respond to a range of noxious and innocuous stimuli. Knockout studies on mice have shown that individual thermal or mechanical transducers are largely dispensable for pain perception, demonstrating redundancy in nociceptive mechanisms. In contrast, ablation of specific neuronal populations has provided strong evidence for labelled modality specific lines of sensation [29–33,34••,35]. Ablation of Nav1.8 expressing neurons (about 85% of the peripherin-positive C-fibre associated neurons) was found to result in a complete loss of noxious mechanosensation with no effect on acute noxious heat responses [29], likely due to a surviving subset of sensory neurons expressing one of the three molecular transducers necessary for heat transduction [36•]. Additional studies by Cavanaugh et al. investigated the behavioural effect of ablating non-overlapping subsets of sensory neurons, marked by either Trpv1, or Mrgprd [30]. The authors found that the ablation of the Mrgprd population of neurons led to significant deficits in noxious mechanical sensation, without affecting noxious heat sensitivity [30]. In contrast, ablation of the Trpv1-expressing population had no effect on noxious mechanical sensitivity, but sensitivity to noxious heat was completely absent. Additional studies investigating the role of the Trpm8-expressing population of sensory neurons, a population that is distinct from Mrgprd and Trvp1, have also been undertaken. Ablation of the Trpm8-expressing population causes a significant loss of noxious cold sensitivity (at temperatures >0°C), while having no effect on noxious mechanical or noxious heat sensitivity [32,33]. A summary of behavioural data obtained following the ablation of specific neuronal populations is shown in Table 1.
Table 1. The effect of ablating specific sub-populations of peripheral sensory neurons on nocifensive behaviours in mice.
The targeted neuronal population is shown along with the method of ablation (capsaicin treatment − experimenter controlled; diphtheria toxin fragment A (DTA) − genetically controlled; diphtheria toxin receptor activation (DTR) − experimenter controlled). Changes in nocifensive phenotype to noxious heat, mechanical (Mech.) and cold are shown (deficit: red; no change/normal: green; not studied: grey)
| Neuronal population | Ablation type | Nocifensive behaviour | Reference | ||
|---|---|---|---|---|---|
| Heat | Mech. | Cold | |||
| Trpv1 | Capsaicin | Cavanaugh et al., 2009 | |||
| Trpv1 | DTA | Mishra et al., 2011 | |||
| Trpv1 | DTR | Pogorzala et al., 2013 | |||
| Mrgprd | DTR | Pogorzala et al., 2013 | |||
| Mrgprd | DTR | Cavanaugh et al., 2009 | |||
| Trpm8 | DTR | Pogorzala et al., 2013 | |||
| Trpm8 | DTR | Knowlton et al., 2013 | |||
| Trpa1 | DTR | Yarmolinsky et at., 2016 | |||
| CGRP | DTR | McCoy et al., 2013 | |||
| NaV1.8 | DTA | Abrahamsen et al., 2008 | |||
 Deficit;
Deficit;  Normal;
Normal;  Not studied.
Not studied.
The observation that distinct populations of neurons are responsible for transducing modality-specific behaviours is compelling, offering strong support for the specificity theory. However, how can discrete modalities be carried by neurons that respond to multiple modalities? A possible answer to this conundrum is that the pattern/combinatorial behaviour of incoming afferent activity allows for modality discrimination at the level of the spinal cord and beyond. However, the ablation of peripheral Trpv1-expressing afferents causes a near complete loss of noxious heat-induced activity from both superficial and deep dorsal horn neurons, without affecting the coded response to noxious mechanical stimulation, or cooling [37]. This suggests that while Trpv1-expressing afferents are essential for detecting noxious heat, Trpv1-negative afferents alone are not sufficient to activate dorsal horn neurons following noxious heat stimulation. This result is also consistent with data from combined electrophysiological and immunohistochemical studies showing that Trpv1 expression is restricted to DRG neurons that are responsive to noxious heat, but not noxious mechanical stimulation [22,38]. These data strongly suggest that, under basal conditions, Trpv1-expressing neurons represent a modality-specific population responsible for noxious heat sensing, however, given the broad expression of Trpv1 among C-fibre nociceptors [39,40,41••], it is difficult to reconcile these data with the high incidences of polymodality reported for C-fibres.
Beyond ablation studies, there is also evidence of analgesics targeting specific subsets of sensory neurons to affect distinct modalities. In 2009, Scherrer et al. showed that μ-opioid and δ-opioid receptors are expressed by different subsets of sensory primary afferents in mice, and moreover, the use of specific μ- (DAMGO) or δ- (SNC80) agonists specifically reduced acute heat or mechanical pain, respectively [42]. More recently, it has been shown that local administration of DAMGO significantly attenuates post-operative mechanical and heat pain, supporting the hypothesis that injury induces changes in the modality sensitivities of sensory neurons [43].
Resolving anomalies: the role of in vivo imaging
Recent studies have exploited genetically encoded calcium indicators (e.g. GCaMP) to monitor neuronal activity in vivo in sensory neurons [34••,44–51,52•]. An obvious limitation of these studies is that, by definition, monitoring changes in intracellular Ca2+ provides an indirect means to monitoring neuronal electrical activity (i.e. action potential generation); however, numerous studies have championed the fidelity of this technique, showing that low frequency electrical stimulation (equivalent to a single action potential) reliably coincides with a reproducible change in GCaMP fluorescence [46•,48,49•]. A major advantage of in vivo imaging is that any associated damage caused by culturing neurons is avoided. Importantly, a number of studies have used in vivo GCaMP imaging to investigate the modality specificity of DRG neurons in mice. One of these studies used single photon confocal microscopy to study modality responses of individual DRG neurons to mechanical, heat and cold stimulation, before and after PGE2-induced inflammation. This study also reported on the number of polymodal neurons observed within each preparation, which was observed to be ~15% of the mechanically sensitive population [46•]. Importantly, following the injection of the pro-inflammatory mediator PGE2, there was a substantial increase in the number of neurons responding to noxious heat stimulus, as well as those that were deemed polymodal, supporting the hypothesis than endogenous inflammatory mediators can regulate the modality responses of sensory afferents. Beyond heat sensitivity, a more recent study has shown that noxious cold sensing can be attributed to two discrete neuronal populations based on whether the stimulus is likely to be damaging [52•].
In vivo imaging studies using two-photon microscopy have been performed to investigate the incidence of polymodality within DRG neurons. Wang et al. observed that in response to plantar stimulation, the vast majority (~70%) of pinch sensing neurons did not respond to noxious heat stimulation [49•]. Despite these low levels of observed polymodality, the authors of this study conclude that >50% of sensory neurons are polymodal. In the study by Emery et al., (2016), neurons that responded to brush stimulation were excluded in order to reduce the artefactual inclusion of low-threshold mechanically sensitive neurons. However, in the study by Wang et al., (2018), the authors deemed neurons that responded to both brush and pinch stimuli as polymodal, despite the fact that some of these neurons did not respond to thermal stimulation. Thus, while the data are similar between these studies, the interpretation differs.
Beyond the DRG, in vivo imaging studies have also been performed on trigeminal ganglia. Although restricted to temperature sensing, Yarmolinksy et al. recently showed that heat and cold sensing neurons form non-overlapping populations, consistent with specific peripheral pathways for thermal discrimination [34••]. Moreover, they observed that injury, caused by the application of a 55μC stimulus for 15 s, profoundly altered neuronal responses to discrete thermal stimuli, highlighting the dynamic plasticity of the peripheral nociceptive system [34••]. Similarly, imaging studies from spinal cord neurons have shown that there is an almost complete absence of heat-induced neuronal activity following diphtheria-mediated ablation of Trpv1-expressing DRG neurons [50•], consistent with electrophysiological recordings from the dorsal horn following capsaicin-mediated afferent ablation [37].
Conclusions
Data from electrophysiological, behavioural, molecular and imaging studies all support the existence of modality-specific nociceptive neurons, yet the prevailing view within the pain field is that the vast majority of nociceptors are polymodal. As discussed above, polymodality within peripheral sensory neurons is a commonly observed phenomenon; however, its incidence is clearly dependent upon environmental context. If a specific stimulus is intense enough to cause tissue damage, or if such damage is caused by experimental preparation, the ensuing inflammatory response is likely to increase the number of responsive sensory neurons, as well as regulate their modality sensitivity, increasing the overall incidence of polymodality. These effects reconcile the variability in the reported incidence of polymodality between different studies, and more importantly, highlight the remarkable plasticity of peripheral sensory neurons in the detection of noxious stimuli in acute and chronic pain states.
Acknowledgements
We thank the Wellcome Trust and Versus Arthritis for invaluable support, and James Cox, Donald MacDonald and Ana Luiz for critical comments and help with experiments.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
•
of outstanding interest
- 1.Perl ER. Pain mechanisms: a commentary on concepts and issues. Prog Neurobiol. 2011;94:20–38. doi: 10.1016/j.pneurobio.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norrsell U, Finger S, Lajonchere C. Cutaneous sensory spots and the “law of specific nerve energies”: history and development of ideas. Brain Res Bull. 1999;48:457–465. doi: 10.1016/s0361-9230(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 3.Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 5.Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- 6.Lang E, Novak A, Reeh PW, Handwerker HO. Chemosensitivity of fineafferentsfromratskininvitro. JNeurophysiol. 1990;63:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- 7.Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- 8.Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976;39:71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated(C)afferentfibers. JNeurophysiol. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- 10.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 11.Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 13.Schmelz M, Schmid R, Handwerker HO, Torebjőrk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer E, Handwerker HO, Joukhadar S. Unmyelinated nociceptive units in two skin areas of the rat. Brain Res. 1983;267:81–92. doi: 10.1016/0006-8993(83)91041-7. [DOI] [PubMed] [Google Scholar]
- 15.Croze S, Duclaux R, Kenshalo DR. The thermal sensitivity of the polymodalnociceptorsinthemonkey. JPhysiol. 1976;263:539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- 18.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elitt CM, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci. 2007;26:1801–1812. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol. 1994;71:941–949. doi: 10.1152/jn.1994.71.3.941. [DOI] [PubMed] [Google Scholar]
- 22.Jankowski MP, et al. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 24.Wetzel C, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt R, et al. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 28.Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knowlton WM, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarmolinsky DA, et al. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92:1079–1092. doi: 10.1016/j.neuron.2016.10.021. [•• This article uses in vivoimaging to investigate the responses of trigeminal neurons to thermal stimuli. They show that cold and heat responsive neurons form non-overlapping populations, and that injury causes a reorganisation of thermosensitive afferents] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy ES, et al. Peptidergic CGRPa primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78:138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandewauw I, et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555:662–666. doi: 10.1038/nature26137. [• This article presents evidence for the essential role of three Trp channels involved in the transduction of noxious heat] [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J Physiol. 2013;591:1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 40.Usoskin D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 41.Zeisel A, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.:e1022. doi: 10.1016/j.cell.2018.06.021. [•• This article provides a comprehensive and searchable database of single cell transcriptomic data for the mouse nervous system] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherrer G, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mecklenburg J, Patil MJ, Koek W, Akopian AN. Effects of local and spinal administrations of mu-opioids on postoperative pain in aged versus adult mice. Pain Rep. 2017;2:e584. doi: 10.1097/PR9.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YS, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YS, et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron. 2016;91:1085–1096. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emery EC, et al. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600990. [• This article uses in vivo imaging to investigate different noxious modalities of peripheral sensory neurons. It also shows that the endogenous inflammatory mediator PGE2 causes a substantial increase in the number of polymodal neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith-Edwards KM, De Berry JJ, Saloman JL, Davis BM, Woodbury CJ. Profound alteration in cutaneous primary afferent activity produced by inflammatory mediators. eLife. 2016;5 doi: 10.7554/eLife.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chisholm KI, Khovanov N, Lopes DM, La Russa F, McMahon SB. Large scale in vivo recording of sensory neuron activity with GCaMP6. eNeuro. 2018;5 doi: 10.1523/ENEURO.0417-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F, et al. Sensory afferents use different coding strategies for heat and cold. Cell Rep. 2018;23:2001–2013. doi: 10.1016/j.celrep.2018.04.065. [• This article uses in vivoimaging to investigate neuronal modality sensing in peripheral sensory neurons, and proposes distinct strategies by which heat and cold stimuli are encoded] [DOI] [PubMed] [Google Scholar]
- 50.Ran C, Hoon MA, Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci. 2016;19:1201–1209. doi: 10.1038/nn.4350. [• This article uses in vivoimaging to investigate the activation of spinal cord neurons in response to discrete thermal stimuli, and how specific Trp channels are involved in their transduction] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawes JM, et al. Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron. 2018;97:806–822.:e810. doi: 10.1016/j.neuron.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luiz AP, et al. Cold sensing by Nav1.8-positive and Nav1.8-negative sensory neurons. Proc Natl Acad Sci U S A. 2019:1–6. doi: 10.1073/pnas.1814545116. [• This article uses in vivo imaging to investigate the role of Nav1.8-positive/negative sensory neurons in cold sensing. The study finds that the detection of acute noxious cold and prolonged noxious cold involves different populations of sensory neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]