Abstract
The preservation of cognitive function into old age is a public health priority. Cerebral hypoperfusion is a hallmark of dementia but its impact on maintaining cognitive ability across the lifespan is less clear. We investigated the relationship between baseline cerebral blood flow (CBF) and blood oxygenation level-dependent (BOLD) response during a fluid reasoning task in a population-based adult lifespan cohort. As age differences in CBF could lead to non-neuronal contributions to the BOLD signal, we introduced commonality analysis to neuroimaging to dissociate performance-related CBF effects from the physiological confounding effects of CBF on the BOLD response. Accounting for CBF, we confirmed that performance- and age-related differences in BOLD responses in the multiple-demand network were implicated in fluid reasoning. Age differences in CBF explained not only performance-related BOLD responses, but also performance-independent BOLD responses. Our results suggest that CBF is important for maintaining cognitive function, while its non-neuronal contributions to BOLD signals reflect an age-related confound. Maintaining perfusion into old age may serve to support brain function and preserve cognitive performance.
Keywords: ageing, functional magnetic resonance imaging (fMRI), cerebral blood flow, multiple demand network, commonality analysis
1. Introduction
The world’s population is ageing, with every sixth person expected to be over 65 by 2050 (United Nations, 2020).Cognitive decline has emerged as a major health threat in old age, including but not limited to dementia (Piguet et al., 2009; Yarchoan et al., 2012). To combat this threat, there is increasing demand to identify factors that facilitate the maintenance of cognitive function across the lifespan. Ageing causes changes to our brains in vascular, structural and functional domains (Cabeza et al., 2018a; Kennedy and Raz, 2015). However, these effects are normally reported separately, and only through their integration one can better understand how these domains influence cognitive decline in old age (Kamen A Tsvetanov et al., 2020).
Cerebral blood flow (CBF) decreases with age (Leenders et al., 1990; Nagata et al., 2016; Tsvetanov et al., 2021) and in early dementia (Toth et al., 2017; Kalaria and Hase, 2019), leading to neuronal dysfunction that is independent of amyloid-β-dependent contributions (Iadecola, 2004; Kisler et al., 2017; Sweeney et al., 2019, 2018; Zlokovic, 2011). Regional CBF can be measured non-invasively using arterial spin labelling (ASL) methods of magnetic resonance imaging (Belliveau et al., 1991; Detre et al., 2009).Mechanisms that account for age-related decreases in CBF are not fully understood but are likely multifactorial, including changes in cerebral metabolic rate, hemodynamics, cerebrovascular reactivity and neurovascular coupling (Claassen et al., 2021). Maintaining high levels of resting CBF sustains good cognitive health in individuals with high risk of dementia, indicating the potential protective effect of preserved resting CBF in delaying cognitive decline (Mutsaerts et al., 2019). In healthy ageing, previous reports have linked the effects of age on baseline CBF to behavioural performance measured outside of the scanner (Bangen et al., 2014; de Vis et al., 2018; Hays et al., 2017; Leeuwis et al., 2018; Wolters et al., 2017; Xekardaki et al., 2014). However, brain perfusion measurements are highly dependent on other physiological factors such as autoregulation modulators (Lemkuil et al., 2013), medication, time of day, levels of wakefulness (Patricia et al., 2014), physical exercise, caffeine or smoking before the scan (Addicott et al., 2009; Domino et al., 2004; Merola et al., 2017). The time course, the level of contribution and the interaction between each factor varies across brain regions, individuals and ageing, making it difficult to control experimentally across all factors in large-scale studies. Therefore, such factors may introduce an age-related bias in the estimation of cerebral perfusion (Grade et al., 2015). Moreover, it remains unclear whether the observed CBF dysregulation in ageing reflects a general link between somatic differences in vascular health and global cognition (King et al., 2022), or whether CBF modifies regional brain activations underlying specific cognitive processes. Thus, to understand the role of baseline CBF in cognitive ageing, one must also test whether baseline CBF is associated with performance-related brain activity during cognitive tasks.
The field of neurocognitive ageing research has often used functional magnetic resonance imaging (fMRI) to study age differences in brain activity during cognitive tasks. FMRI data are usually interpreted in terms of neuronal activity, but the blood oxygenation level-dependent (BOLD) signal measured by fMRI includes differences in cerebrovasculature and neurovascular coupling (Mishra et al., 2021), which also change with age (Kamen A Tsvetanov et al., 2020). Though the processes contributing to coupling between baseline CBF and neural activity are multifaceted, they likely reflect alteration in the properties of cerebrovasculature, rather than the reduction in cardiac output (Xing et al., 2017). Such alterations lessen the ability of small arteries and arterioles to dilate or constrict under the influence of innate neurogenic, myogenic and chemogenic pathways to blood flow regulation (Claassen et al., 2021; Iadecola, 2017; Willie et al., 2014). Alterations in the vasomotor tone and regulation of baseline CBF can reflect and even lead to alterations in neurovascular coupling (Acharya et al., 2022; Payne, 2006; Rosengarten et al., 2001; Spronck et al., 2012), which in turn can affect the sign and magnitude of the evoked BOLD signal with or without affecting underlying neural activity (Brown et al., 2003a; Eric R Cohen et al., 2002; Stefanovic et al., 2006a). Furthermore, global CBF capacity (Fabiani et al., 2014; Tarumi and Zhang, 2018) and systemic vascular health (Jennings et al., 2021; Ogoh et al., 2008; Thayer et al., 2021; Zhang et al., 2002) limit the changes in CBF in response to increasing demand levels. This is important because the relationship between blood flow and oxygen metabolism responses (Stefanovic et al., 2004) is disrupted at high levels of cognitive demand in ageing (Turner et al., 2022), which in turn can bias the relationship between BOLD signal and cognitive performance (Zhao et al., 2021). Thus, failure to account for vascular health alterations leads to misinterpretation of fMRI BOLD signals (Hutchison et al., 2013a; Liu et al., 2013; Tsvetanov et al., 2015a; Zhao et al., 2021) and their cognitive relevance (Geerligs et al., 2017; Geerligs and Tsvetanov, 2016; Tsvetanov et al., 2016).
Several approaches exist to separate vascular from neural contributions to the BOLD signals, including the use of baseline CBF to normalise for age differences in cerebrovascular function (Krishnamurthy et al., 2020; Kamen A Tsvetanov et al., 2020). Normalisation with baseline CBF would improve detection of “true” neuronal changes by accounting for age-related differences in non-neuronal physiology and behaviourally irrelevant confounding effects. But such normalisation approach would also control behaviourally relevant neurobiological effects, where cerebral hypoperfusion relates to neuronal function and loss. An integrative approach would not simply control for baseline CBF differences in task-based BOLD studies, but allow us to dissociate confounding from performance-related effects of CBF on age-related differences in the BOLD fMRI responses.
To distinguish confounding from performance effects of CBF, it is important to understand the neuronal substrates of multiple cognitive demands with ageing (Kaufman and Horn, 1996a; Kievit et al., 2014; Salthouse, 2012a). Demanding, complex or executive functions depend on a distributed network of brain regions known as the multiple-demand network (MDN), which is readily activated across a wide range of cognitive tasks (Tschentscher et al., 2017; Woolgar et al., 2018a). MDN regions can be divided into two subnetworks, frontoparietal and cingulo-opercular networks (Crittenden et al., 2016), and provide the flexibility to parse complex tasks into simple, more solvable subcomponents or sub-goals (Camilleri et al., 2018a; Duncan, 2013a). MDN activity has been linked to general cognitive abilities, such as fluid intelligence (Assem et al., 2020a; Woolgar et al., 2018b), which is an index of people’s ability to solve novel problems. Fluid intelligence is one of the cognitive abilities most sensitive to ageing (Kievit et al., 2016; Salthouse, 2012b). One possible reason for this may be the substantial spatial overlap between MDN and the brain regions with impaired baseline CBF in ageing (Kamen A Tsvetanov et al., 2020; Tsvetanov et al., 2021) and brain regions with high metabolic demands (Vaishnavi et al., 2010) that are sensitive to cerebrovascular impairments (Hosp et al., 2021; Tsvetanov et al., 2022).Therefore, some of the age differences in MDN and domain-general cognition (Kievit et al., 2016; Samu et al., 2017; Tsvetanov et al., 2016; Zhao et al., 2021) may reflect confounding and/or performance-related effects of CBF dysregulation.
To characterise neurocognitive ageing, we propose the use of commonality analysis to dissociate confounding from performance-related effects of CBF on age-related differences in brain functional measures. Commonality analysis, unlike the normalisation approach, allows for adjustment of multiple variables simultaneously by identifying the variance in a dependent variable associated with each predictor uniquely, as well as the variance in common to two or more predictors (Kraha et al., 2012a; Nimon et al., 2008). Here, we identify unique and common effects of age, performance, and baseline CBF on fMRI BOLD responses during a fluid reasoning task in a population-based adult lifespan cohort (age 19-87, N = 223, www.camcan.org). Reasoning was measured by the common Cattell task of fluid intelligence, which requires solving a number of problems, and is known to decline dramatically with age (Kievit et al., 2014).
We predicted that the integration of baseline CBF with task-based fMRI BOLD with the proposed analytical framework would improve detection of confounding and performance-related effects of CBF associated with reasoning. Performance-related effects of CBF would be indicated by variance in the BOLD response that is common to age, task performance and CBF, whereas confounding effects of CBF would be indicated by variance that is common to age and CBF, but not shared with performance.
2. Methods
2.1. Participants
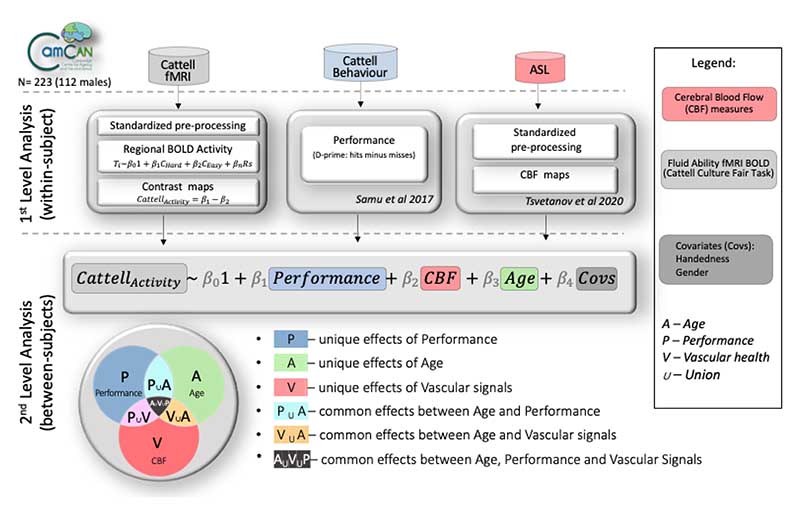
Figure 1 illustrates the study design, data processing and analysis pipeline. The data were acquired from Phase 3 of the Cambridge Centre for Aging and Neuroscience (Cam-CAN), a large population-based study of the healthy adult life span (Shafto et al., 2014; Taylor et al., 2017). The ethical approval for the study was approved by the Cambridge 2 Research Ethics Committee and written informed consent was provided by all participants. Exclusion criteria included poor hearing (a sensitive threshold of 35 dB at 1000 Hz in both ears) and poor vision (below 20/50 on the Snellen test; Snellen, 1862), low Mini-Mental Status Examination (score of 24 or less; Folstein et al., 1975), self-reported substance abuse as assessed by the Drug Abuse Screening Test (Skinner, 1982), significant psychiatric disorders (e.g., schizophrenia, bipolar disorder, personality disorder), or neurological diseases (e.g. a history of stroke, epilepsy, traumatic brain injury). Demographic characteristics of the sample are described in Table 1.
Figure 1.
Schematic represenation of various modality datasets in the study, their processing pipelines on a within-subject level, and analytical strategy on between-subject level to test for uniuque and common effects of performance, CBF and age on Cattell task activity. P, performance; A, age; V, vascular; CBF – cerebral blood flow; Covs – covariates of no interest; U – union.
Table 1.
Participants’ demographic information
| N=223 | Decile | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age range [years] | 19-27 | 28-37 | 38-47 | 48-57 | 58-67 | 68-77 | 78-87 |
| Numbers | 21 | 39 | 37 | 35 | 35 | 30 | 26 |
| Gender | |||||||
| Male | 9 | 19 | 18 | 17 | 18 | 16 | 14 |
| Female | 12 | 20 | 19 | 18 | 17 | 14 | 12 |
| Handednessa | |||||||
| Mean/SD | 79/44 | 89/25 | 81/27 | 94/11 | 77/50 | 95/10 | 87/33 |
| Range[min/max] | -100/100 | -56/100 | -56/100 | 58/100 | -78/100 | 53/100 | -56/100 |
| Educationb | |||||||
| None | 0 | 0 | 0 | 0 | 0 | 5 | 1 |
| GCSE | 2 | 2 | 6 | 3 | 3 | 3 | 3 |
| A-level | 4 | 1 | 3 | 11 | 9 | 8 | 9 |
| University | 15 | 36 | 28 | 21 | 23 | 14 | 13 |
Higher scores indicate greater right-hand preference, as assessed by Edinburgh Handedness Inventory (Oldfield, 1971).
Educational attainment categorized according to the British education system: “None” = no education over the age of 16 yrs; “GCSE” = General Certificate of Secondary Education; “A Levels” = General Certificate of Education Advanced Level; “University” = undergraduate or graduate degree.
2.2. Stimuli, task and procedure
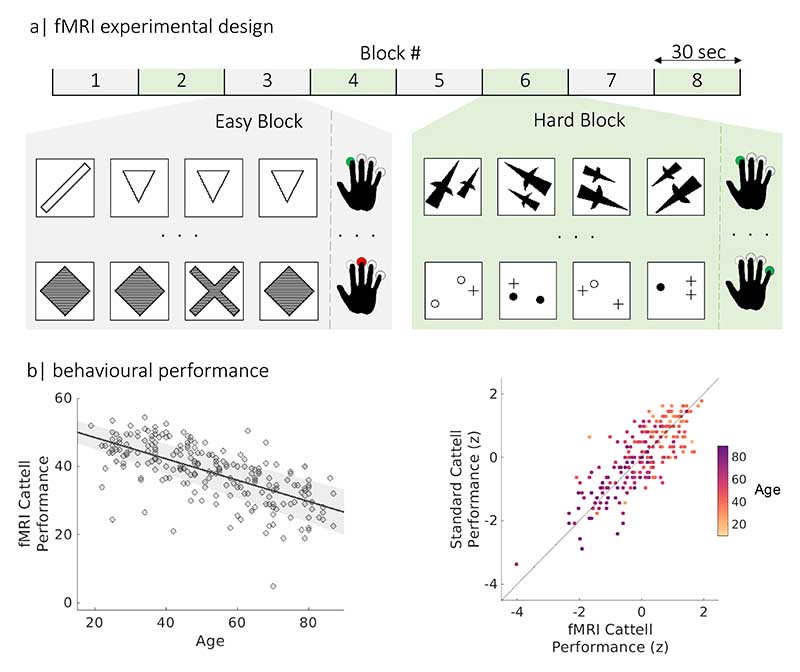
Participants undertook a Fluid Intelligence task which draws on critical cognitive process of fluid reasoning, which underlies many complex cognitive operations (Duncan, 2013b), and which declines with age (Duncan, 2010; Horn and Cattell, 1967; Kaufman and Horn, 1996b; Kievit et al., 2014; Salthouse, 2012c; Salthouse et al., 2003). We used an adapted version of the Cattell Culture Fair test (Cattell, 1971), modified to be used in the scanner (Samu et al., 2017; Woolgar et al., 2013) at Phase III of Cam-CAN (Shafto et al., 2014), see Figure 2. On each trial, participants were presented with a display of four patterns and had to select the “odd one out”. The task employed a block design, with 30-seconds blocks of trials alternating between two conditions with different difficulty level (“easy” and “hard” puzzles). There was a total of four blocks per condition. Because there was a fixed time to perform as many trials as possible, behavioural performance was measured by subtracting the number of incorrect trials from the number of correct trials (averaged over hard and easy bocks, following Samu et al., (Samu et al., 2017). This ensured that someone responding quickly but randomly did not score highly. The suitability of this performance score was confirmed by its strong correlation (Pearson’s r[95% CI]: r(223) = 0.70 [0.63, 0.76], P < 0.001, Figure 2) with scores obtained from the standard Cattell test, administered outside the scanner at Phase II of Cam-CAN (Shafto et al., 2014). We also excluded n = 28 participants who had disproportionately poor performance with 10 or more incorrect trials (17 females, with age range 31-88); leaving N = 223 remaining (111 females, age range 19 - 87 years).
Figure 2.
Experimental design and performance on the fMRI Cattell task. (a) The task consisted of eight 30-second blocks with two difficulties (Easy and Hard). In each trial (each row in grey and green panels), participants selected the odd-one-out of four patterns by pressing a corresponding button with one of their fingers (green and red button presses for correct and incorrect responses, respectively). (b) scatter plots of the relationship between performance on fMRI Cattell task and age (left panel) and performance on the standard Cattell task, where participants age is colour coded using yellow-purple gradient palette (right panel).
2.3. MRI Acquisition and Preprocessing
Imaging data were acquired using a 3T Siemens TIM Trio System with a 32-channel head-coil at the MRC Cognition and Brain sciences Unit (CBU; www.mrc-cbu.cam.ac.uk). Of the initial cohort, 256 participants had valid T1, T2, arterial spin labelling (ASL) data, and task-induced BOLD data from a fluid intelligence task.
A 3D-structural MRI was acquired on each participant using T1-weighted sequence (Generalized Auto-calibrating Partially Parallel Acquisition (GRAPPA) with the following parameters: repetition time (TR) = 2,250 ms; echo time (TE) = 2.99 ms; inversion time (TI) = 900 ms; flip angle α= 9°; field of view (FOV) = 256 × 240 × 192 mm3; resolution = 1 mm isotropic; accelerated factor = 2; acquisition time, 4 min and 32 s. A T2-weighted spatially-selective single-slab 3D turbo-spin-echo (SPACE) image was acquired with the following parameters: TR = 2800ms, TE = 408ms, TI = 900ms; FOV=256x256x192mm; 1mm isotropic; GRAPPA=2; and total acquisition time of 4minsand 30s.
We used Release003 of the CamCAN Automatic Analysis pipelines for Phase III data Taylor et al.,(2017), which called functions from SPM12 (Wellcome Department of Imaging Neuroscience, London, UK). The T1 image from Phase II was rigid-body coregistered to the MNI template, and the T2 image from Phase II was then rigid-body coregistered to the T1 image. The coregistered T1 and T2 images were used in a multimodal segmentation to extract probabilistic maps of six tissue classes: gray matter (GM), white matter (WM), CSF, bone, soft tissue, and residual noise. The native space GM and WM images were submitted to diffeomorphic registration to create group template images. Each template was normalized to the MNI template using a 12-parameter affine transformation.
2.4. EPI image acquisition and processing
For the Cattell-based fMRI in Phase III of CamCAN, Gradient-Echo Echo-Planar Imaging (EPI) of 150 volumes captured 32 axial slices (sequential descending order) of thickness of 3.7 mm with a slice gap of 20% for whole-brain coverage with the following parameters: TR = 1970 ms; TE = 30 ms; flip angle α = 78°; FOV = 192 × 192 mm2; resolution = 3 × 3 × 4.44 mm3, with a total duration of 5 min.
EPI data preprocessing included the following steps: (1) spatial realignment to adjust for linear head motion, (2) temporal realignment of slices to the middle slice, (3) coregistration to the T1 anatomical image from Phase II above, (4) application of the normalization parameters from the T1 stream above to warp the functional images into MNI space, and (5) smoothing by an 8mm Gaussian kernel.
For the participant-level modelling, every voxel’s time-course was regressed in a multiple linear regression on the task’s design matrix which consisted of time-courses for hard and easy conditions convolved with a canonical haemodynamic response function (HRF). Regressors of no interest included WM, CSF, 6 standard realignment parameters (accounting for in-scanner head motions), and harmonic regressors that capture low-frequency changes (1/128 Hz) in the signal typically associated with scanner drift and physiological noise. WM and CSF signals were estimated for each volume from the mean value of WM and CSF masks derived by thresholding SPM’s tissue probability maps at 0.75. The contrast of parameter estimates for hard minus easy conditions for each voxel and participant was then calculated, termed here Cattell activation.
2.5. Arterial spin labelling (ASL) image acquisition and processing
Perfusion-weighted images of cerebral blood flow used pulsed arterial spin labelling (PASL, PICORE-Q2T-PASL with background suppression). The sequence is used with the following parameters: repetition time (TR) = 2500 ms, echo time (TE) = 13 ms, field of view (FOV) = 256 × 256 × 100 mm3, 10 slices, 8 mm slice thickness, flip angle = 90°, inversion time 1 (TI1) = 700 ms, TI2 = 1800 ms, Saturation stop time = 1600 ms, tag width = 100 mm and gap = 20.9 mm, 90 repetitions giving 45 control-tag pairs, voxel-size = 4 mm × 4 mm × 8 mm, 25% interslice gap, acquisition time of 3 minutes and 52 seconds. CBF images with artefacts (n = 5) based on visual inspection were excluded from analysis.In addition, a single-shot EPI (M0) equilibrium magnetization scan was acquired. Pulsed arterial spin labelling time series were converted to maps of CBF using Explore ASL toolbox (https://github.com/ExploreASL/ExploreASL; Mutsaerts et al., 2018). Note that we used ‘relative CBF’ maps, given that CBF quantification of single post-label delay data may be susceptible to age-related biases (Alsop et al., 2015). Importantly, absolute CBF quantification (i.e. absolute CBF values) is not required for the proposed regression-based approach, which aims to determine relative relationships between variables across participants. Following rigid-body alignment, the images were coregistered with the T1 from Phase II above, normalised with normalization parameters from the T1 stream above to warp ASL images into MNI space. Given the ASL data was based on a sequence with lower resolution (e.g. slice thickness of 8 mm), we smoothed the data with a kernel size 1.5 times larger than the slice thickness (12 mm FWHM Gaussian kernel, Tsvetanov et al., 2021), consistent with the efficacy of ASL data with heavier smoothing kernels (Wang et al., 2005).Nonetheless, sensitivity analysis demonstrated that the results were robust to different smoothing strategies (e.g. 6mm FWHM Gaussian kernel, see Figure S1).
2.6. Analytical approach
To model random effects across participants, we performed voxel-wise analysis using multiple linear regression (MLR) with age as the main independent variable of interest, and sex and handedness as covariates of no interest. This MLR was applied to maps of both Cattell activation (BOLD) and baseline CBF.
To evaluate the confounding and performance-related effects of resting CBF on BOLD activation, we conducted commonality analysis (Kraha et al., 2012b; Nimon et al., 2008). Commonality analysis partitions the variance explained by all predictors in MLR into variance unique to each predictor and variance shared between each combination of predictors. Therefore, unique effects indicate the (orthogonal) variance explained by one predictor over and above that explained by other predictors in the model, while common effects indicate the variance shared between correlated predictors. More specifically, coefficients of common effects can indicate how much variance is explained in the dependent variable jointly by two or more correlated predictors. Notably, the sum of variances, also known as commonality coefficients, equals the total R2 for the regression model. Commonality analysis can also provide evidence for suppressor effects, as indicated by negative commonality coefficients, whereby partialling out irrelevant variance of other predictor(s) increases contributions to the overall model R2 (Nimon and Oswald, 2013; Zientek and Thompson, 2006a).
We adapted a commonality analysis algorithm (Nimon et al., 2008) for neuroimaging analysis to facilitate voxel-wise nonparametric testing in Matlab (Mathworks, https://uk.mathworks.com/). The commonality analysis was applied to the Cattell activation in each voxel separately (see Figure 1). The independent variables in the model were baseline CBF for the corresponding voxel, age and fMRI Cattell performance (henceforth termed performance). The model can therefore identify unique variance explained by each of the predictors (UCBF, UAge and UP for CBF, Age and Performance, respectively). Common effects of interest were the confounding effects, defined by the shared variance between CBF and age (CCBF,Age), and performance-related effects, defined by the common variance between CBF, Age and Performance (CCBF,Age,P). We report significant clusters related to effects of interest as identified with nonparametric testing using 1000 permutations and threshold-free cluster enhancement with significance level of 0.05 (Smith and Nichols, 2009), unless otherwise specified. This Matlab version of commonality analysis for neuroimaging with TFCE implementation is available at https://github.com/kamentsvetanov/CommonalityAnalysis/.
Given the level of education or anti-hypertensive medication may play a role on the relationship between age, baseline CBF, BOLD activation and performance, we have considered each of these factors in addition to other covariates of no interest (such as sex and handedness) in a set of additional models. More specifically, we performed the following models on significant clusters identified by the TFCE-analysis.
Model 1 reports the commonality coefficients for fMRI Cattell performance, CBF and age on Cattell activity with sex and handedness as covariates of no interest.
Model 2 extended Model 1 to include anti-hypertensive medication status, which was based on whether an individual was taking a prescribed medication for high blood pressure.
Model 3 extended Model 1 to include educational attainment, based on highest education qualification according to the British education system.
2.7. Data and code availability
The dataset analysed in this study is part of the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) research project (www.cam-can.com). Raw and minimally pre-processed MRI (Taylor et al., 2017) and behavioural data from Phase II are available by submitting a data request to Cam-CAN (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/). Phase III data are available on request from the authors.
As part of this study, MATLAB-based commonality analysis for neuroimaging with TFCE implementation was developed and made available at https://github.com/kamentsvetanov/CommonalityAnalysis/. Task-based fMRI data was post-processed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm; Friston et al., 2007). Arterial spin labelling data were post-processed using ExploreASL toolbox (Mutsaerts et al., 2018). Visualisation of all neuroimaging results was generated using MRIcroGL (https://github.com/rordenlab/MRIcroGL; Rorden and Brett, 2000). Gradient palettes used Matplotlib library (Hunter, 2007) via PyColormap4Matlab (https://github.com/f-k-s/PyColormap4Matlab).
3. Results
3.1. Main effect and effect of age on BOLD in Cattell task
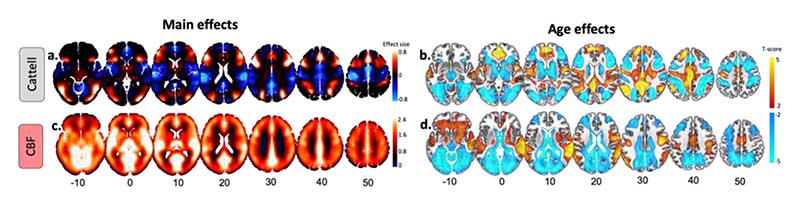
Group-level analysis confirmed activations for the hard vs easy condition in the lateral prefrontal cortex, anterior insula, dorsal anterior cingulate cortex, frontal eye fields, pre-supplementary motor area, and areas along both the intraparietal sulcus and lateral temporal lobe, recapitulating the multiple demand network (Camilleri et al., 2018b; MDN, Duncan, 2013b), as well as lateral occipital cortex and calcarine cortex (Figure 3a). Additionally, we observed deactivations in the ventral medial prefrontal cortex (vmPFC), posterior cingulate cortex (PCC) and inferior parietal lobe (IPL), recapitulating the default network (Buckner et al., 2008; Buckner and DiNicola, 2019a; Raichle, 2015). With respect to ageing, there were weaker activations in regions of the MDN, and weaker deactivations in regions of the DMN, associated with increasing age, see Figure 3b, consistent with previous studies (Samu et al., 2017).
Figure 3.
Main and age effects on task-based activity and cerebral blood flow (CBF) maps. (a) Main effects of BOLD activity in response to Hard vs Easy blocks with over- and underactivations shown in warm and cold colours, respectively. (b) Age-related decreases (cold colours) and increases (warm colours) in Cattell task. (c) Main effect of baseline CBF across all participants. Note the use of ‘relative CBF’, i.e. ‘unquantified CBF’ in our study. (d) Age-related decreases (cold colours) and increases (warm colours) in baseline CBF. Slices are numbered by z level in Montreal Neurological Institute (MNI) space.
3.2. Main effect and effect of age on baseline CBF
Group-level results revealed a pattern of relatively high cerebral blood flow in cortical and subcortical brain areas associated with high perfusion and high metabolism (Henriksen et al., 2018; Figure 3c), such as caudal middle-frontal, posterior cingulate, pericalcarine, superior temporal and thalamic regions. Moderate to low CBF values in the superior-parietal and inferior-frontal areas of the cortex (Figure 3c) may reflect the axial positioning of the partial brain coverage sequence used in the study.
We observed age-related declines in CBF in the bilateral dorsolateral prefrontal cortex, lateral parietal cortex, anterior and posterior cingulate, pericalcarine, and cerebellum (Figure 3d), in agreement with previous reports (Chen et al., 2011; Lu et al., 2011; Zhang et al., 2018). Also, we observed age-related CBF increase in regions susceptible to individual and group differences in arterial transit time that can bias accuracy of CBF estimation, including middle temporal gyrus and middle cingulate cortex (Mutsaerts et al., 2017).
3.3. Commonality analysis of BOLD Cattell activation
3.3.1. Unique effects
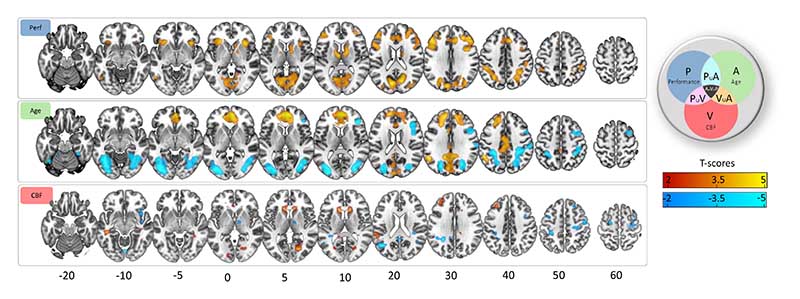
Unique effects of individual differences in performance levels on Cattell activation (BOLD) were found in regions similar to those activated by the main effect of the Cattell task (e.g., MDN), with the exception of the lateral occipital cortex and inclusion of inferior temporal gyrus, primary visual cortex, caudate and thalamus (cf. Figure 2a, Figure 3 top panel, Table 2 and Table S1). Unlike the case for main effects, task-negative regions (e.g., DMN) showed small to no significant associations with performance.
Table 2.
Table of unique and shared effects for three models corresponding to statistically significant clusters of interest at TFCE-level. Each TFCE-cluster is represented by its name according to AAL atlas and coordinates in MNI space. Unique effects included effects of i) Age, ii) CBF and iii) Performance. Common effects included shared signals between i) age and CBF (Age,CBF) , ii) age and performance (Age,Performance), iii) CBF and performance (CBF,Performance), and iv) age, CBF and performance (Age,CBF,Perfomance). In Model 1, Cattell activity was the dependent variable, while age, CBF and performance were dependent variables, and education and sex were covariates of no interest. Model 2 is the same as Model 1, but with anti-hypertensive medications added as covariate of no interest. Model 3 is the same as Model 1, but with educational attainment added as covariate of no interest. Significance was determined based on a null distribution of 5.000 permutations. Effects at p-value < 0.05 shown in bold font.
| Cluster Name | Contrast Name | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| T-score | p-value | T-score | p-value | T-scare | p-value | ||
| R. Thalamus [6, 12,12] | Age | -0.37 | 0.356 | -0.25 | 0.400 | -0.31 | 0.379 |
| CBF | 1.49 | 0.069 | 1.49 | 0.069 | 1.57 | 0.059 | |
| Performance | 3.32 | <0.001 | 3.31 | <0.001 | 3.03 | <0.001 | |
| Age,CBF | 0.86 | 0.197 | 0.73 | 0.232 | 0.86 | 0.196 | |
| Age,Performance | 3.13 | <0.001 | 2.57 | 0.005 | 2.56 | 0.006 | |
| CBF,Performance | 0.72 | 0.237 | 0.72 | 0.236 | 0.91 | 0.183 | |
| Age,CBF,Performance | 3.11 | <0.001 | 2.48 | 0.007 | 2.94 | 0.002 | |
| L. Precuneus [-3, -66,30] | Age | 3.50 | <0.001 | 3.19 | <0.001 | 3.49 | <0.001 |
| CBF | -1.65 | 0.051 | -1.64 | 0.051 | -1.64 | 0.052 | |
| Performance | -1.81 | 0.036 | -1.80 | 0.036 | -1.76 | 0.040 | |
| Age,CBF | 1.98 | 0.025 | 1.83 | 0.035 | 1.99 | 0.024 | |
| Age,Performance | 5.38 | <0.001 | 4.35 | <0.001 | 4.92 | <0.001 | |
| CBF,Performance | 0.41 | 0.343 | 0.41 | 0.342 | 0.52 | 0.302 | |
| Age,CBF,Performance | 3.24 | <0.001 | 2.52 | 0.006 | 3.15 | <0.001 | |
| L. Superior Parietal Lobule [-21, -63,42] | Age | -4.64 | 0.000 | -4.53 | <0.001 | -4.66 | <0.001 |
| CBF | 0.70 | 0.243 | 0.67 | 0.251 | 0.69 | 0.245 | |
| Performance | 3.07 | <0.001 | 3.08 | 0.001 | 3.19 | <0.001 | |
| Age,CBF | 1.31 | 0.096 | 1.27 | 0.103 | 1.31 | 0.097 | |
| Age,Performance | 8.24 | <0.001 | 6.80 | <0.001 | 7.85 | <0.001 | |
| CBF,Performance | 0.77 | 0.222 | 0.76 | 0.225 | 0.77 | 0.220 | |
| Age,CBF,Performance | 3.72 | <0.001 | 3.01 | <0.001 | 3.48 | <0.001 | |
| L. Middle Occipital Gyrus [-21, -87,0] | Age | -4.31 | <0.001 | -4.14 | <0.001 | -4.32 | <0.001 |
| CBF | 2.24 | 0.013 | 2.19 | 0.015 | 2.26 | 0.013 | |
| Performance | 0.77 | 0.221 | 0.77 | 0.220 | 0.91 | 0.183 | |
| Age,CBF | 2.95 | 0.002 | 2.85 | 0.002 | 2.95 | 0.002 | |
| Age,Performance | 4.93 | <0.001 | 4.05 | <0.001 | 4.82 | <0.001 | |
| CBF,Performance | -0.37 | 0.355 | -0.37 | 0.357 | -0.44 | 0.331 | |
| Age,CBF,Performance | 3.25 | <0.001 | 2.58 | 0.005 | 2.99 | 0.002 | |
| L. Middle Cingulate Cortex [-12, -45, 30] | Age | 3.67 | <0.001 | 3.31 | 0.001 | 3.64 | <0.001 |
| CBF | 1.94 | 0.027 | -1.94 | 0.027 | 1.96 | 0.026 | |
| Performance | -1.89 | 0.030 | -1.89 | 0.030 | -1.77 | 0.039 | |
| Age,CBF | 2.09 | 0.019 | 1.96 | 0.026 | 2.11 | 0.018 | |
| Age,Performance | 5.86 | <0.001 | 4.66 | <0.001 | 5.22 | <0.001 | |
| CBF,Performance | -0.31 | 0.378 | -0.30 | 0.381 | 0.23 | 0.409 | |
| Age,CBF,Performance | 2.96 | 0.002 | 2.34 | 0.010 | 2.93 | 0.002 | |
Unique effects of age were similar but weaker to the effect of age in the model without other predictors (cf. Figure 3b, Figure 4 middle panel, Table 2 and Table S1). Unique positive associations between age and Cattell activation was observed in middle frontal gyrus and cuneus regions. Negative associations were observed in insular regions, posterior cingulate cortex, bilateral angular gyrus, precentral gyrus and superior frontal gyrus.
Figure 4.
Unique effects in commonality analysis. (top panel) Age-related decreases (cold colours) and increases (warm colours) using TFCE-correction. (middle panel) Performance-related decreases (cold colours) and increases (warm colours) using TFCE-correction. (bottom panel) CBF-related decreases (cold colours) and increases (warm colours) in Cattell task are shown at uncorrected p-values of 0.05 for more complete description of the spatial representation. Slices are numbered by z level in Montreal Neurological Institute (MNI) space.
Unique effects of CBF were not significant using TFCE-correction. At uncorrected level, CBF showed positive associations with activation in the middle frontal gyrus, the putamen and the cuneus. Additionally, CBF was associated negatively with activation in task negative regions, namely the angular gyrus and precentral gyrus.
3.3.2. Common effects
There were many common effects between age and performance, with a positive commonality coefficient (CAge,P, Figure 5, cyan colour, Table 2 and Table S2) indicating that a portion of the age effects on Cattell activation was related to performance effects on Cattell activation. These effects were observed in task positive (e.g., MDN) and task negative regions (e.g., DMN), in addition to thalamus, caudate, primary motor cortex.
Figure 5.
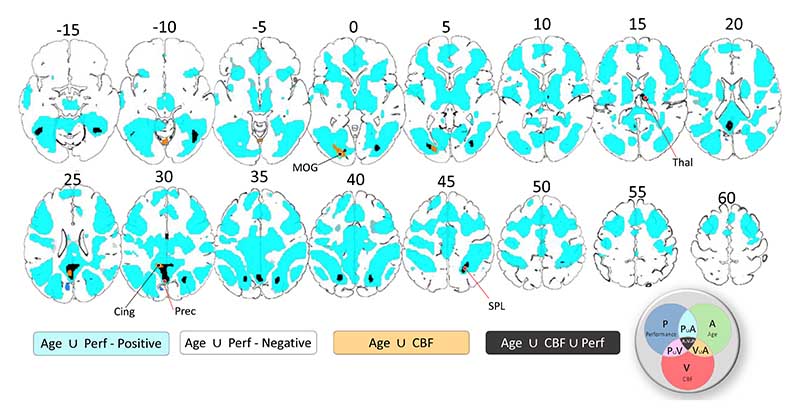
Common Effects in commonality analysis. Positive and negative common effects between age and performance are shown in cyan and dark blue colours, respectively. Common effects between age and baseline CBF are shown in orange colour. Common effects between age, performance and CBF are shown in black colour. P – performance, A – age, V – vascular, CBF. Slices are numbered by z level in Montreal Neurological Institute (MNI) space.
Negative commonality coefficients between performance and age were observed in the cuneus, bilateral middle frontal gyrus, anterior and middle cingulate gyrus, and bilateral superior temporal gyrus (dark blue colour in Figure 5, Table 2 and Table S2). Negative values of commonality coefficients indicate a suppressor relationship between predictors (Zientek and Thompson, 2006b), indicating that the effects of age and/or performance are stronger with their joint consideration in the model.
Confounding effects of baseline CBF on Cattell activation were characterised by the common effect between Age and CBF (CCBF,Age) (separate from that also shared with performance, i.e. CCBF,Agge,P. Significant confounding effects were localised within posterior cingulate cortex, fusiform gyrus and inferior occipital gyrus (orange colour in Figure 5, Table 2 and Table S2).
Performance-related effects of baseline CBF on Cattell activation were characterised by the common effect between Age, CBF and Performance (CCBF,Age,P, black colour in Figure 5 and Table S2). Regions included intraparietal sulcus, posterior cingulate cortex, precuneus, thalamus and fusiform gyrus. Furthermore, consistent with previous findings (Tsvetanov et al., 2018), behaviourally-relevant effects were seen in inferior temporal and adjacent occipital regions, presumably due to attentional enhancement of visual representations in the more difficult conditions (Fedorenko et al., 2013a). There was no evidence for common effects between performance and CBF (CCVF,P) on Cattell activation on TFCE-cluster level.
3.3.3. Effects of educational attainment and anti-hypertensive medications
Finally, we tested whether anti-hypertensive medication and educational attainment can explain the observed unique and common effects of predictors on Cattell activity, within the clusters showing significant unique and common effects of age or performance and common effects with CBF (Figure 5). To this end, we performed three statistical models (see Method section) for clusters showing significant unique and common effects of age or performance and common effects with CBF (see regions of interest in Figure 5). The unique and common effects for the model including anti-hypertensive medication (Model 2) and educational attainment (Model 3) were highly consistent with the initial model, having predictors and other covariates of not interest (Model 1), see Table 2.
4. Discussion
The study confirmed the prediction that age-related decline in regional cerebral blood flow (CBF) can explain performance-related components of the fMRI BOLD signal in parts of the multiple-demand network (MDN) associated with more complex reasoning during a common test of fluid intelligence (Cattell task). The age-dependent differences in baseline CBF also explained variance in fMRI BOLD signal in some regions that was not related to task-performance. We propose that modelling the effects of age on baseline CBF, and in general cerebrovascular and neurovascular health (Kamen A Tsvetanov et al., 2020), improves the interpretation of fMRI studies, with implications for understanding brain health with ageing and disease, and that maintaining brain perfusion as we get older may have a protective effect on brain function and cognition.
4.1. Age differences in baseline cerebral blood flow are related to behaviour-relevant Cattell BOLD activity
Our results showed that age-related decreases in baseline cerebral blood flow (CBF), assessed with a non-invasive MR-perfusion technique, related to behaviourally-relevant BOLD activity evoked by demanding problem-solving. This is consistent with previous studies relating baseline CBF to performance on tasks performed outside the scanner (Bangen et al., 2014; de Vis et al., 2018; Hays et al., 2017; Leeuwis et al., 2018; Wolters et al., 2017; Xekardaki et al., 2014). We extend these lines of work by showing that baseline CBF is linked to BOLD activity, with behavioural correlation across individuals. Age-related decreases in CBF and decline in performance related to a lower range of activation in task-positive regions and less deactivation of task-negative regions.
Of all task-positive regions, the bilateral intra-parietal sulcus, thalamus, and fusiform gyrus showed significant common effects between age, CBF and performance. The intraparietal sulcus and thalamus also showed a unique association between performance and BOLD activity, which is consistent with neurophysiological studies (Tschentscher and Hauk, 2016; Tschentscher and Sauseng, 2022) and may suggest a neural origin of the effects in these regions. The processes contributing to coupling between baseline CBF and neural activity are multifaceted, probably comprising neurogenic vasodilation, cardiac output and arterial remodelling (Gaballa et al., 1998; Li et al., 2015; Ohanian et al., 2014), all of which change with age and regulate baseline and stimulus-evoked CBF (Willie et al., 2014). While our findings cannot speak directly to studies modulating the neural activity baseline (i.e. the spiking frequency of neuronal ensembles) without manipulation of cognitive demands, we speculate that baseline CBF is associated with the ability to upregulate CBF in response to increasing task demands, in line with the metabolic insufficiency hypothesis (Shetty et al., 2011; Turner et al., 2022; Zhao et al., 2021). This is consistent with age-related decline in global CBF capacity (Fabiani et al., 2014; Tarumi and Zhang, 2018) and systemic vascular health limiting the changes in CBF in response to increasing demand levels (Jennings et al., 2021; Ogoh et al., 2008; Thayer et al., 2021; Zhang et al., 2002). This in turn can contribute to age-related disruptions in demand-dependent neurovascular coupling (Turner et al., 2022; Zhao et al., 2021), which is otherwise preserved in young adults with good cardiovascular health (Stefanovic et al., 2004). It is important to note that the task-positive regions observed in our study overlap with the distribution of regions with high metabolic demands (Vaishnavi et al., 2010) and sensitivity to cerebrovascular impairments (Hosp et al., 2021; Tsvetanov et al., 2022, 2021, 2015b). This suggests that maintaining CBF regulation in old age may be especially important for MDN-dependent cognitive abilities (Zhao et al., 2021), such as fluid intelligence and speed processing, which are among the most affected abilities in ageing (Kievit et al., 2016; Salthouse, 2012b).Establishing the relative contribution of regional baseline to modulating CBF in response to increasing cognitive demands, and the importance of these processes to behavioural consequences to other cognitive functions, warrants future research.
Of all task-negative regions, only the posterior cingulate cortex showed common effects between age, CBF and performance in predicting BOLD activation in the Cattell task. In this region, age-related reduction in CBF and performance correlated with less deactivation in the posterior cingulate cortex. The posterior cingulate cortex did not show unique effects between performance and BOLD activity, suggesting a mechanism different from the one observed in task-positive regions, likely reflecting a non-neuronal origin of the effects (see also “Unique effects of performance, age and CBF in Cattell task”). While the deactivation of the default network in young adults is thought to reflect suppression of neuronal activity (Fox et al., 2018a), in the present study, some of the poor performing older adults showed an over-activation, not less deactivation. This again suggests a different involvement of the posterior cingulate cortex in older adults compared to young adults, for instance, signals of non-neuronal origin caused by physiological artifacts (Birn et al., 2006) or ‘vascular steal’ (Shmuel et al., 2002). Taken together, these findings may reflect compromised vasodilatory reserve, resulting in an inefficient redirection of resources from task-positive regions to task-negative regions in the attempt to meet higher energy demands in task-positive regions, perhaps reflecting blood flow-dependent glycolysis and oxidative metabolism. The breadth of these associations is consistent with theories of vasoactive and cardiovascular regulation of cerebral blood flow (Digernes et al., 2017a; Sobczyk et al., 2014a).
4.2. Vascular Confounding effects of CBF on task-related activity
Only a portion of the age differences in performance-independent BOLD activation were associated with CBF decreases. Furthermore, the effects were observed in non-classical demand network task-positive and task-negative regions not showing unique associations between performance and BOLD activity, namely the fusiform gyrus and the posterior cingulate cortex (Figure 4, orange regions). This is consistent with the view that differences in baseline CBF can affect the sign and the magnitude of the evoked BOLD signal, without affecting changes in the underlying neural activity (Brown et al., 2003b; Eric R. Cohen et al., 2002; Stefanovic et al., 2006b). We extend prior findings by showing that only a portion of the CBF effects can introduce such a behaviourally irrelevant bias; other parts of the CBF variance might be related to behaviour-relevant signal, highlighting that differences in CBF could be important in their own right. Unlike the normalisation approach described in Introduction to control for CBF differences, the current commonality framework allows partition of CBF effects into effects of interest and effects of no interest. We propose that modelling the effects of age on baseline CBF, and in general cerebrovascular and neurovascular health (Kamen A Tsvetanov et al., 2020), has implications for the interpretation of fMRI studies of ageing, whereby it can improve brain-behaviour relationships and provide a viable mechanistic account of maintaining and improving cognitive function in old age.
4.3. Unique effects of performance, age and CBF on task-related activity
Ageing was associated with weaker activation of the multiple demand network and less efficient suppression of the default network. These effects were over and above performance and CBF, suggesting the involvement of additional factors leading to age-related difference in BOLD activity. Some factors include genetics (Shan et al., 2016), cardiovascular and neurovascular signals not captured by baseline CBF (Abdelkarim et al., 2019; Kamen A Tsvetanov et al., 2020) or effects of functional connectivity captured by regional activity (Tsvetanov et al., 2018). Age differences in the shape of the haemodynamic response function (West et al., 2019) are less likely to introduce bias in the current study given its block-related fMRI design (Liu et al., 2001). The nature of these age effects should be elucidated through further investigation. The commonality analysis framework provides a useful tool to disentangle the multifactorial nature of age-related BOLD differences.
After accounting for age, baseline CBF and other covariates of not interest, the level of activity in the multiple-demand regions remained positively associated with performance during the Cattell task in the scanner. Our findings are in line with previous studies during diverse demanding tasks, including manipulations of working memory, target detection, response inhibition (Assem et al., 2020b, 2020c; Fedorenko et al., 2013b; Tschentscher et al., 2017). Given that both age and cerebrovascular reactivity could introduce a very strong effect on the activity-behaviour associations (even with narrow age range and healthy populations), our approach to control for these factors, in combination with the population-based, large-sample, provide the strongest evidence to date that individual differences variance in executive abilities is selectively and robustly associated with the level of activity in the multiple demand network.
Our study adds evidence to the nature of suppression of the default network during an externally directed task (Buckner and DiNicola, 2019b). The task-induced default network deactivations were consistent with previous findings in the Cattell task (Samu et al., 2017) and in general with the extent to which task conditions are cognitively demanding (Anticevic et al., 2012; Sripada et al., 2020). The effects in the default network were related to age or baseline CBF, but not uniquely related to performance, suggesting that the level of BOLD deactivations during Cattell task do not reflect individual variability in cognitive performance. The nature of default network suppression remains to be fully defined (Fox et al., 2018b), but future findings about the default network cannot be interpreted independent of age and baseline CBF, at least when aiming to understand the relevance of DMN suppression in health and disease.
After accounting for age and performance, higher baseline cerebral blood flow did not show strong effects with the level of BOLD activity in cortical regions modulated by demanding problem-solving processes. Uncorrected results suggested a weak effect between higher baseline CBF and higher range of activation in task positive regions under more demanding processing, including the middle frontal gyrus, the putamen, and the cuneus. The effects were spatially adjacent or overlapping with behaviour-relevant region suggesting that higher baseline CBF may provide the conditions to upregulate activity in these regions, possibly through functional hyperaemia. Additionally, higher CBF provided higher range of deactivation in task negative regions, namely the angular gyrus and precentral gyrus. These effects were spatially adjacent or overlapping with regions showing inefficient deactivation with ageing and suggest that higher baseline CBF may facilitate suppression of activity in task-negative regions. This may reflect the effect of having an intact vasodilatory reserve (Digernes et al., 2017b; Sobczyk et al., 2014b). Our findings have direct implications for task-based BOLD imaging whereby higher baseline CBF levels contribute to stronger changes in BOLD signal amplitude in response to demanding cognitive conditions. The myogenic response and cardiac output are two major modulators of resting CBF (Hill et al., 2006; Meng et al., 2015), which require future consideration to establish the mechanism underlying our findings.
Finally, accounting for antihypertensive treatment did not change the results, suggesting that the link between baseline perfusion and task-based BOLD activations cannot be explained by antihypertensive medications. These findings are consistent with previous studies (Alosco et al., 2014; Claassen et al., 2021; Foster-Dingley et al., 2015), suggesting a protective role of antihypertensive drugs against hypertension-associated vascular dysfunction in the context of baseline cerebral blood flow. In similar fashion, our findings could not be explained by the level of educational attainment, contemplating the possibility that baseline perfusion may support cognitive function in old age in the context of brain maintenance theory (Cabeza et al., 2018b; Nyberg et al., 2012) over the cognitive reserve theory (Meng and D’Arcy, 2012; Stern, 2012, 2002).
5. Issues and future directions
There are issues to the study. Our findings are based on a population-based cross-sectional cohort, which cannot directly speak to individual’s progression over time (i.e, the ageing process). Longitudinal studies are warranted to clarify the conditions and order of events determining the findings in our study. We only assessed the brain activations/co-activations, but do not quantify brain connectivity (Bethlehem et al., 2020; Geerligs et al., 2017; Samu et al., 2017; Kamen A. Tsvetanov et al., 2020; Tsvetanov et al., 2016), even though both may change differentially in cognitive ageing (Tsvetanov et al., 2018). The relationship between baseline CBF and functional connectivity decouples with ageing (Galiano et al., 2019), but the behavioural relevance of such decoupling is only starting to be explored (Liu et al., 2022), albeit motivated by prior work controlling for vascular effects from fMRI BOLD data (Geerligs et al., 2017; Tsvetanov et al., 2016). Future work should also i) evaluate the effects of baseline CBF under different cognitive states (Campbell et al., 2015; Geerligs and Tsvetanov, 2016) and baseline conditions (Hutchison et al., 2013b; Pasley et al., 2007), ii) consider nonlinearities between CBF and BOLD signal within individuals (Chen, 2019a) and across the lifespan (Tibon et al., 2021; Tsvetanov et al., 2016), and iii) explore the relevance of baseline CBF to stimulus-evoked CBF (Jennings et al., 2005) and neurodegenerative diseases (Chen, 2019b).
Our findings should be extended using approaches with improved accuracy of ASL imaging in older populations, including individual-based arterial transit times and higher resolution ASL sequences complemented with partial volume correction (Chappell et al., 2021; Dai et al., 2017). Cerebrovascular ageing is multifactorial and there are other means to assess cerebrovascular function in ageing (Kamen A Tsvetanov et al., 2020), including cerebrovascular reactivity (Chappell et al., 2021; Kannurpatti and Biswal, 2008) based on CO2-inhalation-induced hypercapnia (Liu et al., 2019), breath-hold-induced hypercapnia (Handwerker et al., 2007; Mayhew et al., 2010; Riecker et al., 2003; Thomason et al., 2007, 2005), hyperventilation-induced hypocapnia (Bright et al., 2009; Krainik et al., 2005), and venous oxygenation (Liau and Liu, 2009; Lu et al., 2010; Restom et al., 2007). It is possible these measures to explain additional effects of age on BOLD where baseline CBF does not.For example, age-related alterations in cerebrovascular reactivity are more prevalent than those of baseline CBF (Lu et al., 2011) and only partly explained by baseline CBF (Tsvetanov et al., 2021).Importantly, the proposed modelling approach enables future studies to integrate more of these factors in the same model in order to dissociate their unique and shared contributions to confounding and behaviourally-relevant effects of the BOLD signal response. Future studies should explore the utility of additional estimates from resting ASL-based CBF data to complement CBF quantification. For instance, little is known about whether resting CBF variability, which is statistically similar to resting state fluctuation amplitudes (Kannurpatti and Biswal, 2008), is sensitive to cerebrovascular reactivity and other vascular origins (Robertson et al., 2017). Furthermore, while the proposed approach is based on plausible neurophysiology that can be used to evaluate its contribution to cognitive function, future studies could improve absolute quantification of neural function together with its integration with deoxyhemoglobin-dilution-based modeling (Davis et al., 1998; Hoge et al., 1999a, 1999b), hemodynamic response function modeling (West et al., 2019), generative modeling (Friston et al., 2003; Jafarian et al., 2020; Tsvetanov et al., 2016) and model-free decomposition (Bethlehem et al., 2020; Campbell et al., 2015; Samu et al., 2017; Tsvetanov et al., 2018) of fMRI BOLD data.
6. Conclusion
We introduce a novel approach to neuroimaging that can dissociate between shared and unique signals across multiple neuroimaging modalities. Using this method, we show the effects of age on cerebral blood flow, task-related BOLD responses and performance. The results demonstrate that aspects of cerebrovascular health (i.e., baseline cerebral blood flow) explain confounding but also performance-related BOLD responses in fluid ability across the lifespan. They highlight the importance of using resting CBF data to model, rather than simply normalise for, differences in vascular health in task-based fMRI BOLD data (Kamen A Tsvetanov et al., 2020). Unlike the normalisation approach, our approach allows simultaneous modelling of multiple measures with independent contributions to cerebrovascular health. Here, we provide empirical evidence highlighting the important role of baseline CBF in neurocognitive function across the adult lifespan. The insights from our results may facilitate the development of new strategies to maintain cognitive ability across the lifespan in health and disease.
Supplementary Material
Highlight.
The impact of CBF on maintaining cognitive ability across the lifespan is unclear.
Age differences in CBF explain behaviour-relevant activity during an fMRI task.
CBF explaining non-neuronal contributions to fMRI signals reflects an age confound.
Maintaining CBF into old age may support brain function with behavioural advantage.
Acknowledgements
This work is supported by the Guarantors of Brain (G101149), SCNU Study Abroad Program for Elite Postgraduate Students, the Medical Research Council (MC_UU_00005/12/SUAG004/051/RG91365; SUAG04/51 R101400) and the Cambridge NIHR Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) research was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1). We thank the Cam-CAN respondents and their primary care teams in Cambridge for their participation in this study. Further information about the Cam-CAN corporate authorship membership can be found at https://www.cam-can.org/index.php?content=corpauth#13.
Footnotes
Competing Interests statement
J.B.R. serves as an associate editor to Brain and is a non- remunerated trustee of the Guarantors of Brain, Darwin College Cambridge, and the PSP Association (UK). He has provided consultancy to Asceneuron, Biogen, UCB and has research grants from AZ-Medimmune, Janssen, Lilly and WAVE as industry partners in the Dementias Platform UK. The other authors have no disclosures.
References
- Abdelkarim D, Zhao Y, Turner MP, Sivakolundu DK, Lu H, Rypma B. A neural-vascular complex of age-related changes in the human brain: Anatomy, physiology, and implications for neurocognitive aging. Neuroscience and Biobehavioral Reviews. 2019;107:927–944. doi: 10.1016/j.neubiorev.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Acharya D, Ruesch A, Schmitt S, Yang J, Smith MA, Kainerstorfer JM. Changes in neurovascular coupling with cerebral perfusion pressure indicate a link to cerebral autoregulation. 2022 doi: 10.1177/0271678X221076566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addicott MA, Yang LL, Peiffer AM, Burnett LR, Burdette JH, Chen MY, Hayasaka S, Kraft RA, Maldjian JA, Laurienti PJ. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Human Brain Mapping. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, Terrero G, Schwarz NF, Walsh EG, Poppas A, Cohen RA, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. Journal of the American Society of Hypertension. 2014;8:561–570. doi: 10.1016/J.JASH.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJP, et al. Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Blank IA, Mineroff Z, Ademoğlu A, Fedorenko E. Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex. 2020a;131:1–16. doi: 10.1016/j.cortex.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Blank IA, Mineroff Z, Ademoğlu A, Fedorenko E. Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex. 2020b;131:1–16. doi: 10.1016/j.cortex.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Glasser MF, Van Essen DC, Duncan J. A Domain-General Cognitive Core Defined in Multimodally Parcellated Human Cortex. Cerebral Cortex. 2020c;30:4361–4380. doi: 10.1093/cercor/bhaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Nation DA, Clark LR, Harmell AL, Wierenga CE, Dev SI, Delano-Wood L, Zlatar ZZ, Salmon DP, Liu TT, Bondi MW. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by magnetic resonance imaging. Science (1979) 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, Paquola C, Seidlitz J, Ronan L, Bernhardt B, Consortium C-C, Tsvetanov KA. Dispersion of functional gradients across the adult lifespan. Neuroimage. 2020:117299. doi: 10.1016/j.neuroimage.2020.117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith Ma, Bandettini Pa. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bright MG, Bulte DP, Jezzard P, Duyn JH. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. Neuroimage. 2009;48:166–75. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, EylerZorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003a;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Brown GG, EylerZorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003b;23:829–37. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience. 2019a doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience. 2019b doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience. 2018a;19:701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience. 2018b;19:701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri JA, Müller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, Eickhoff SB. Definition and characterization of an extended multiple-demand network. Neuroimage. 2018a;165:138–147. doi: 10.1016/j.neuroimage.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri JA, Müller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, Eickhoff SB. Definition and characterization of an extended multiple-demand network. Neuroimage. 2018b;165:138–147. doi: 10.1016/j.neuroimage.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Shafto MA, Wright P, Tsvetanov KA, Geerligs L, Cusack R, Tyler LKLK, Brayne C, Bullmore EE, Calder A, Dalgleish T, et al. Idiosyncratic responding during movie-watching predicted by age differences in attentional control. Neurobiology of Aging. 2015;36:3045–3055. doi: 10.1016/j.neurobiolaging.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. Abilities: Their structure growth and action. Boston, MA: Houghton Mifflin; 1971. [Google Scholar]
- Chappell MA, McConnell FAK, Golay X, Günther M, Hernandez-Tamames JA, van Osch MJ, Asllani I. Partial volume correction in arterial spin labeling perfusion MRI: A method to disentangle anatomy from physiology or an analysis step too far? Neuroimage. 2021;238:118236. doi: 10.1016/J.NEUROIMAGE.2021.118236. [DOI] [PubMed] [Google Scholar]
- Chen JJ. Functional MRI of brain physiology in aging and neurodegenerative diseases. Neuroimage. 2019a;187:209–225. doi: 10.1016/j.neuroimage.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Chen JJ. Functional MRI of brain physiology in aging and neurodegenerative diseases. Neuroimage. 2019b;187:209–225. doi: 10.1016/j.neuroimage.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–78. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/PHYSREV.00022.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Eric R, Ugurbil K, Kim S-G. Effect of Basal Conditions on the Magnitude and Dynamics of the Blood Oxygenation Level-Dependent fMRI Response. Journal of Cerebral Blood Flow & Metabolism. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen Eric R, Ugurbil K, Kim S-G. Effect of Basal Conditions on the Magnitude and Dynamics of the Blood Oxygenation Level-Dependent fMRI Response. Journal of Cerebral Blood Flow & Metabolism. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J. Task encoding across the multiple demand cortex is consistent with a frontoparietal and cingulo-opercular dual networks distinction. Journal of Neuroscience. 2016;36:6147–6155. doi: 10.1523/JNEUROSCI.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Fong T, Jones RN, Marcantonio E, Schmitt E, Inouye SK, Alsop DC. Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. Journal of Magnetic Resonance Imaging. 2017;45:472–481. doi: 10.1002/jmri.25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vis JB, Peng SL, Chen X, Li Y, Liu P, Sur S, Rodrigue KM, Park DC, Lu H. Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study. J MagnReson Imaging. 2018;48:449–458. doi: 10.1002/JMRI.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre Ja, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. CurrOpinNeurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Digernes I, Bjørnerud A, Vatnehol SAS, Løvland G, Courivaud F, Vik-Mo E, Meling TR, Emblem KE. A theoretical framework for determining cerebral vascular function and heterogeneity from dynamic susceptibility contrast MRI. Journal of Cerebral Blood Flow & Metabolism. 2017a;37:2237–2248. doi: 10.1177/0271678X17694187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digernes I, Bjørnerud A, Vatnehol SAS, Løvland G, Courivaud F, Vik-Mo E, Meling TR, Emblem KE. A theoretical framework for determining cerebral vascular function and heterogeneity from dynamic susceptibility contrast MRI. Journal of Cerebral Blood Flow & Metabolism. 2017b;37:2237–2248. doi: 10.1177/0271678X17694187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013a;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013b;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Tan CH, Zimmerman B, Fletcher MA, Schneider-Garces N, Maclin EL, Chiarelli AM, Sutton BP, Gratton G. Taking the pulse of aging: mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology. 2014;51:1072–1088. doi: 10.1111/PSYP.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013a;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013b;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. Journal of Psychiatric Research. 1975 doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster-Dingley JC, Moonen JEF, de Craen AJM, de Ruijter W, van der Mast RC, van der Grond J. Blood pressure is not associated with cerebral blood flow in older persons. Hypertension. 2015;66:954–960. doi: 10.1161/HYPERTENSIONAHA.115.05799. [DOI] [PubMed] [Google Scholar]
- Fox K, Foster B, Kucyi A, Daitch A, Parvizi J. Intracranial Electrophysiology of the Human Default Network. Trends Cogn Sci. 2018a;22:307–324. doi: 10.1016/J.TICS.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Foster B, Kucyi A, Daitch A, Parvizi J. Intracranial Electrophysiology of the Human Default Network. Trends Cogn Sci. 2018b;22:307–324. doi: 10.1016/J.TICS.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, Penny WD. Statistical parametric mapping: the analysis of funtional brain images. Elsevier Academic Press; 2007. [DOI] [Google Scholar]
- Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large Artery Remodeling During Aging. Hypertension. 1998;32:437–443. doi: 10.1161/01.HYP.32.3.437. [DOI] [PubMed] [Google Scholar]
- Galiano A, Mengual E, García de Eulate R, Galdeano I, Vidorreta M, Recio M, Riverol M, Zubieta JL, Fernández-Seara MA. Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging and Behavior. 2019:1–15. doi: 10.1007/s11682-019-00157-w. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Tsvetanov KA. The use of resting state data in an integrative approach to studying neurocognitive ageing – Commentary on Campbell and Schacter (2016) Language, Cognition and Neuroscience. 2016;32 doi: 10.1080/23273798.2016.1251600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Tsvetanov KA, Cam-CAN , Henson RN. Challenges in measuring individual differences in functional connectivity using fMRI: The case of healthy aging. Human Brain Mapping. 2017;38:4125–4156. doi: 10.1002/hbm.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015 doi: 10.1007/s00234-015-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker Da, Gazzaley A, Inglis Ba, D’Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp. 2007;28:846–59. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, Campbell L, Meloy MJ, Wierenga CE. Temporal gradient during famous face naming is associated with lower cerebral blood flow and gray matter volume in aging. Neuropsychologia. 2017;107:76–83. doi: 10.1016/j.neuropsychologia.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen OM, Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, Lisbjerg K, Christensen SJ, Rasmussen P, Olsen NV, Forman JL, Larsson HBW, Law I. Interindividual and regional relationship between cerebral blood flow and glucose metabolism in the resting brain. 2018;125:1080–1089. doi: 10.1152/japplphysiol.00276.2018. [DOI] [PubMed] [Google Scholar]
- Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clinical Hemorheology and Microcirculation. 2006;34:67–79. [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-X. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, Thurow J, Wagner D, Waller C, Niesen WD, Frings L, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. doi: 10.1093/BRAIN/AWAB009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JD. Matplotlib: A 2D graphics environment. Computing in Science and Engineering. 2007;9:90–95. doi: 10.1109/MCSE.2007.55. [DOI] [Google Scholar]
- Hutchison JL, Lu H, Rypma B. Neural Mechanisms of Age-Related Slowing: The ΔCBF/ΔCMRO2 Ratio Mediates Age-Differences in BOLD Signal and Human Performance. Cerebral cortex. 2013a;23:2337–2346. doi: 10.1093/cercor/bhs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JL, Lu H, Rypma B. Neural Mechanisms of Age-Related Slowing: The ΔCBF/ΔCMRO2 Ratio Mediates Age-Differences in BOLD Signal and Human Performance. Cerebral cortex. 2013b;23:2337–2346. doi: 10.1093/cercor/bhs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Reviews Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Allen B, Ginty AT, Gianaros PJ. Cerebrovascular function in hypertension: Does high blood pressure make you old? Psychophysiology. 2021;58:e13654. doi: 10.1111/PSYP.13654. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Hase Y. In: Biochemistry and Cell Biology of Ageing: Part II Clinical Science. Subcellular Biochemistry. Harris J, Korolchuk V, editors. Springer; Singapore: 2019. Neurovascular Ageing and Age-Related Diseases; pp. 477–499. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Horn JL. Age changes on tests of fluid and crystallized ability for women and men on the Kaufman Adolescent and Adult Intelligence Test (KAIT) at ages 17-94 years. Archives of Clinical Neuropsychology. 1996a;11:97–121. doi: 10.1016/0887-6177(95)00003-8. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Horn JL. Age changes on tests of fluid and crystallized ability for women and men on the Kaufman Adolescent and Adult Intelligence Test (KAIT) at ages 17-94 years. Archives of Clinical Neuropsychology. 1996b;11:97–121. doi: 10.1016/0887-6177(95)00003-8. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. An Encyclopedic Reference. Elsevier Inc; 2015. Normal Aging of the Brain, Brain Mapping. [DOI] [Google Scholar]
- Kievit RA, Davis SW, Griffiths J, Correia MM, Cam-CAN. Henson RN. A watershed model of individual differences in fluid intelligence. Neuropsychologia. 2016;91:186–198. doi: 10.1016/J.NEUROPSYCHOLOGIA.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit RA, Davis SW, Mitchell DJ, Taylor JR, Duncan J, Tyler LK, Brayne C, Bullmore E, Calder A, Cusack R, Dalgleish T, et al. Distinct aspects of frontal lobe structure mediate age-related differences in fluid intelligence and multitasking. Nature Communications. 2014;5:5658. doi: 10.1038/ncomms6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DL, Henson RNA, Kievit R, Wolpe N, Tyler LK, Brayne C, Rowe JB, Cam-CAN , Tsvetanov KA. Distinct components of cardiovascular health associate with age-related differences in cognitive abilities. medRxiv. 2022 doi: 10.1038/s41598-022-27252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nature Reviews Neuroscience. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraha A, Turner H, Nimon K, Zientek LR, Henson RK. Tools to Support Interpreting Multiple Regression in the Face of Multicollinearity. Frontiers in Psychology. 2012a;3:44. doi: 10.3389/fpsyg.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraha A, Turner H, Nimon K, Zientek LR, Henson RK. Tools to Support Interpreting Multiple Regression in the Face of Multicollinearity. Frontiers in Psychology. 2012b;3:44. doi: 10.3389/fpsyg.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional Impairment of Cerebrovascular Reactivity and BOLD Signal in Adults After Stroke. Stroke. 2005;36:1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy V, Krishnamurthy LC, Drucker JH, Kundu S, Ji B, Hortman K, Roberts SR, Mammino K, Tran SM, Gopinath K, McGregor KM, et al. Correcting Task fMRI Signals for Variability in Baseline CBF Improves BOLD-Behavior Relationships: A Feasibility Study in an Aging Model. Frontiers in Neuroscience. 2020;14:336. doi: 10.3389/fnins.2020.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma aa, Heather JD, Buckingham P, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Leeuwis AE, Smith LA, Melbourne A, Hughes AD, Richards M, Prins ND, Sokolska M, Atkinson D, Tillin T, Jäger HR, Chaturvedi N, et al. Cerebral Blood Flow and Cognitive Functioning in a Community-Based, Multi-Ethnic Cohort: The SABRE Study. Frontiers in Aging Neuroscience. 2018;10:279. doi: 10.3389/fnagi.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkuil BP, Drummond JC, Patel PM. Central Nervous System Physiology: Cerebrovascular. Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application. 2013:123–136. doi: 10.1016/B978-1-4377-1679-5.00008-9. [DOI] [Google Scholar]
- Li Y, Shen Q, Huang S, Li W, Muir E, Long J, TQ D. Cerebral angiography, blood flow and vascular reactivity in progressive hypertension. Neuroimage. 2015;111:329–337. doi: 10.1016/J.NEUROIMAGE.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau J, Liu TT. Inter-subject variability in hypercapnic normalization of the BOLD fMRI response. Neuroimage. 2009;45:420–30. doi: 10.1016/j.neuroimage.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage. 2019;187:104–115. doi: 10.1016/J.NEUROIMAGE.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Section J, Park DC, Lu H. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage. 2013;78:415–25. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection Power, Estimation Efficiency, and Predictability in Event-Related fMRI. Neuroimage. 2001;13:759–773. doi: 10.1006/NIMG.2000.0728. [DOI] [PubMed] [Google Scholar]
- Liu X, Tyler LK, Cam-Can |, James |, Rowe B, Tsvetanov KA. Multimodal fusion analysis of functional, cerebrovascular and structural neuroimaging in healthy aging subjects. Human Brain Mapping. 2022 doi: 10.1002/HBM.26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yezhuvath US, Xiao G. Improving fMRI sensitivity by normalization of basal physiologic state. Hum Brain Mapp. 2010;31:80–7. doi: 10.1002/hbm.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew SD, Li S, Storrar JK, Tsvetanov KA, Kourtzi Z. Learning shapes the representation of visual categories in the aging human brain. Journal of Cognitive Neuroscience. 2010;22 doi: 10.1162/jocn.2010.21415. [DOI] [PubMed] [Google Scholar]
- Meng L, Hou W, Chui J, Han R, Gelb A. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015;123:1198–1208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- Meng X, D’Arcy C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. PLoS ONE. 2012;7 doi: 10.1371/JOURNAL.PONE.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola A, Germuska MA, Warnert EA, Richmond L, Helme D, Khot S, Murphy K, Rogers PJ, Hall JE, Wise RG. Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. Neuroimage. 2017;155:331–343. doi: 10.1016/J.NEUROIMAGE.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hall CN, Howarth C, Freeman RD. Key relationships between non-invasive functional neuroimaging and the underlying neuronal activity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2021;376:20190622. doi: 10.1098/rstb.2019.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts HJMM, Mirza SS, Petr J, Thomas DL, Cash DM, Bocchetta M, de Vita E, Metcalfe AWS, Shirzadi Z, Robertson AD, Tartaglia MC, et al. Cerebral perfusion changes in presymptomatic genetic frontotemporal dementia: a GENFI study. Brain. 2019;142:1108–1120. doi: 10.1093/brain/awz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts HJMM, Petr J, Thomas DL, de Vita E, Cash DM, van Osch MJP, Golay X, Groot PFC, Ourselin S, van Swieten J, Laforce R, et al. Comparison of arterial spin labeling registration strategies in the multi-centerGENetic frontotemporal dementia initiative (GENFI) Journal of Magnetic Resonance Imaging. 2018;47:131–140. doi: 10.1002/jmri.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts HJMM, Petr J, Václavů L, van Dalen JW, Robertson AD, Caan MW, Masellis M, Nederveen AJ, Richard E, MacIntosh BJ. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. Journal of Cerebral Blood Flow and Metabolism. 2017;37:3184–3192. doi: 10.1177/0271678X16683690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Research Reviews. 2016;30:49–60. doi: 10.1016/J.ARR.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Nimon K, Lewis M, Kane R, Haynes RM. An R package to compute commonality coefficients in the multiple regression case: An introduction to the package and a practical example. Behavior Research Methods. 2008;40:457–466. doi: 10.3758/BRM.40.2.457. [DOI] [PubMed] [Google Scholar]
- Nimon KF, Oswald FL. Understanding the Results of Multiple Linear Regression: Beyond Standardized Regression Coefficients. Organizational Research Methods. 2013;16:650–674. doi: 10.1177/1094428113493929. [DOI] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- Ohanian J, Liao A, Forman SP, Ohanian V. Age-related remodeling of small arteries is accompanied by increased sphingomyelinase activity and accumulation of long-chain ceramides. Physiological Reports. 2014;2 doi: 10.14814/PHY2.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/J.NEUROIMAGE.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricia C, Henk-Jan M, Eidrees G, Marion S, Marjan A, Egill R, Francesca Benedetta P, Jorge J, Mervi K, Ritva V, António B-L, et al. Review of confounding effects on perfusion measurements. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/conf.fnhum.2014.214.00073. [DOI] [Google Scholar]
- Payne SJ. A model of the interaction between autoregulation and neural activation in the brain. Math Biosci. 2006;204:260–281. doi: 10.1016/J.MBS.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Shelley BP, Kipps CM, Hodges JR. Sensitivity of current criteria for the diagnosis of behavioral variant frontotemporal dementia. Neurology. 2009;72:732–737. doi: 10.1212/01.wnl.0000343004.98599.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Brain’s Default Mode Network. Annu Rev Neurosci. 2015:413–427. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–9. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Grodd W, Klose U, Schulz JB, Gröschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–73. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- Robertson AD, Matta G, Basile VS, Black SE, Macgowan CK, Detre JA, MacIntosh BJ. Temporal and spatial variances in arterial spin-labeling are inversely related to large-artery blood velocity. American Journal of Neuroradiology. 2017;38:1555–1561. doi: 10.3174/ajnr.A5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Huwendiek O, Kaps M. Neurovascular coupling and cerebral autoregulation can be described in terms of a control system. Ultrasound in Medicine & Biology. 2001;27:189–193. doi: 10.1016/S0301-5629(00)00332-X. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Consequences of age-related cognitive declines. Annu Rev Psychol. 2012a;63:201–26. doi: 10.1146/annurev-psych-120710-100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. Consequences of age-related cognitive declines. Annu Rev Psychol. 2012b;63:201–226. doi: 10.1146/annurev-psych-120710-100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. Consequences of age-related cognitive declines. Annu Rev Psychol. 2012c;63:201–26. doi: 10.1146/annurev-psych-120710-100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen. 2003;132:566–94. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Samu D, Campbell KL, Tsvetanov KA, Shafto MA, Consortium C-C. Brayne C, Bullmore ET, Calder AC, Cusack R, Dalgleish T, Duncan J, et al. Preserved cognitive functions with age are determined by domain-dependent shifts in network responsivity. Nature Communications. 2017;8:ncomms14743. doi: 10.1038/ncomms14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK, Dixon M, Taylor JR, Rowe JB, Cusack R, Calder AJ, Marslen-Wilson WD, Duncan J, Dalgleish T, Henson RN, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan ZY, Vinkhuyzen AAE, Thompson PM, McMahon KL, Blokland GAM, de Zubicaray GI, Calhoun V, Martin NG, Visscher PM, Wright MJ, Reutens DC. Genes influence the amplitude and timing of brain hemodynamic responses. Neuroimage. 2016;124:663–671. doi: 10.1016/j.neuroimage.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty PK, Galeffi F, Turner DA. Age-Induced Alterations in Hippocampal Function and Metabolism. Aging and Disease. 2011;2:196. [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002 doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive behaviors. 1982 doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Snellen H. Probebuchstabenzurbestimmung der sehscharfe. Van de Weijer; Utrecht: 1862. [Google Scholar]
- Sobczyk O, Battisti-Charbonney a, Fierstra J, Mandell DM, Poublanc J, Crawley aP, Mikulis DJ, Duffin J, Fisher Ja. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage. 2014a;92C:56–68. doi: 10.1016/j.neuroimage.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Sobczyk O, Battisti-Charbonney a, Fierstra J, Mandell DM, Poublanc J, Crawley a P, Mikulis DJ, Duffin J, Fisher Ja. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage. 2014b;92C:56–68. doi: 10.1016/j.neuroimage.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Spronck B, Martens EGHJ, Gommer ED, van de Vosse FN. A lumped parameter model of cerebral blood flow control combining cerebral autoregulation and neurovascular coupling. Am J Physiol Heart CircPhysiol. 2012;303 doi: 10.1152/AJPHEART.00303.2012. [DOI] [PubMed] [Google Scholar]
- Sripada C, Angstadt M, Rutherford S, Taxali A, Shedden K. Toward a “treadmill test” for cognition: Improved prediction of general cognitive ability from the task activated brain. Human Brain Mapping. 2020;41:3186–3197. doi: 10.1002/hbm.25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–778. doi: 10.1016/J.NEUROIMAGE.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006a;30:726–734. doi: 10.1016/j.neuroimage.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006b;30:726–734. doi: 10.1016/j.neuroimage.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nature Neuroscience. 2018 doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: From physiology to disease and back. Physiological Reviews. 2019 doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. Journal of Neurochemistry. 2018;144:595–608. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Williams N, Cusack R, Auer T, Shafto MA, Dixon M, Tyler LK, Cam-CAN. Henson RN. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Mather M, Koenig J. Stress and aging: A neurovisceral integration perspective. Psychophysiology. 2021;58:e13804. doi: 10.1111/PSYP.13804. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JDE, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage. 2005;25:824–37. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum Brain Mapp. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibon R, Tsvetanov KA, Price D, Nesbitt D, CAN C, Henson R. Transient neural network dynamics in cognitive ageing. Neurobiology of Aging. 2021;105:217–228. doi: 10.1016/J.NEUROBIOLAGING.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American Journal of Physiology-Heart and Circulatory Physiology. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschentscher N, Hauk O. Frontal and Parietal Cortices Show Different Spatiotemporal Dynamics across Problem-solving Stages. Journal of Cognitive Neuroscience. 2016;28:1098–1110. doi: 10.1162/JOCN_A_00960. [DOI] [PubMed] [Google Scholar]
- Tschentscher N, Mitchell D, Duncan J. Fluid intelligence predicts novel rule implementation in a distributed frontoparietal control network. Journal of Neuroscience. 2017;37:4841–4847. doi: 10.1523/JNEUROSCI.2478-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschentscher N, Sauseng P. Spatio-Temporal Brain Dynamic Differences in Fluid Intelligence. Frontiers in Human Neuroscience. 2022;16 doi: 10.3389/FNHUM.2022.820780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov Kamen A, Gazzina S, Jones PS, Swieten J, Borroni B, Sanchez-Valle R, Moreno F, Laforce R, Graff C, Synofzik M, Galimberti D, et al. Brain functional network integrity sustains cognitive function despite atrophy in presymptomatic genetic frontotemporal dementia. Alzheimer’s & Dementia alz. 2020:12209. doi: 10.1002/alz.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RNA, Jones PS, Mutsaerts H, Fuhrmann D, Tyler LK, Rowe JB. Psychophysiology. Blackwell Publishing Inc; 2021. The effects of age on resting-state BOLD signal variability is explained by cardiovascular and cerebrovascular factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov Kamen A, Henson RNA, Rowe JB. Separating vascular and neuronal effects of age on fMRI BOLD signals. Philosophical Transactions of the Royal Society B: Biological Sciences. 2020 doi: 10.1098/rstb.2019.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RNA, Tyler LK, Davis SW, Shafto MA, Taylor JR, Williams N, Rowe JB. The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human Brain Mapping. 2015a;36:2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RNA, Tyler LK, Davis SW, Shafto MA, Taylor JR, Williams N, Rowe JB. The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human Brain Mapping. 2015b;36:2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RNA, Tyler LK, Razi A, Geerligs L, Ham TE, Rowe JB. Extrinsic and intrinsic brain network connectivity maintains cognition across the lifespan despite accelerated decay of regional brain activation. Journal of Neuroscience. 2016;36:3115–26. doi: 10.1523/JNEUROSCI.2733-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Spindler LRB, Stamatakis EA, Newcombe VFJ, Lupson VC, Chatfield DA, Manktelow AE, Outtrim JG, Elmer A, Kingston N, Bradley JR, et al. Hospitalisation for COVID-19 predicts long lasting cerebrovascular impairment: A prospective observational cohort study. medRxiv. 2022 doi: 10.1016/j.nicl.2022.103253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Ye Z, Hughes L, Samu D, Treder MS, Wolpe N, Tyler LK, Rowe JB, for Cambridge Centre for Ageing and Neuroscience Activity and connectivity differences underlying inhibitory control across the adult lifespan. J Neurosci. 2018;38:7887–7900. doi: 10.1523/JNEUROSCI.2919-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MP, Zhao Y, Abdelkarim D, Liu P, Spence JS, Hutchison JL, Sivakolundu DK, Thomas BP, Hubbard NA, Xu C, Taneja K, Lu H, et al. Altered linear coupling between stimulus-evoked blood flow and oxygen metabolism in the aging human brain. Cerebral Cortex. 2022 doi: 10.1093/CERCOR/BHAC057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, D of E and SAPD. World Population Ageing 2019. 2020 [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/PNAS.1010459107/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Z, Aguirre GK, Detre JA. To smooth or not to smooth? ROC analysis of perfusion fMRI data. Magnetic Resonance Imaging. 2005;23:75–81. doi: 10.1016/J.MRI.2004.11.009. [DOI] [PubMed] [Google Scholar]
- West KL, Zuppichini MD, Turner MP, Sivakolundu DK, Zhao Y, Abdelkarim D, Spence JS, Rypma B. BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage. 2019;188:198–207. doi: 10.1016/j.neuroimage.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Tzeng Y-C, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA. Cerebral perfusion and the risk of dementia: A population-based study. Circulation. 2017;136:719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- Woolgar A, Bor D, Duncan J. Global increase in task-related fronto-parietal activity after focal frontal lobe lesion. J CognNeurosci. 2013;25:1542–52. doi: 10.1162/jocn_a_00432. [DOI] [PubMed] [Google Scholar]
- Woolgar A, Duncan J, Manes F, Fedorenko E. Fluid intelligence is supported by the multiple-demand system not the language system. Nature Human Behaviour. 2018a;2:200–204. doi: 10.1038/s41562-017-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar A, Duncan J, Manes F, Fedorenko E. Fluid intelligence is supported by the multiple-demand system not the language system. Nature Human Behaviour. 2018b;2:200–204. doi: 10.1038/s41562-017-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekardaki A, Rodriguez C, Montandon ML, Toma S, Tombeur E, Herrmann FR, Zekry D, Lovblad KO, Barkhof F, Giannakopoulos P, Haller S. Arterial Spin Labeling May Contribute to the Prediction of Cognitive Deterioration in Healthy Elderly Individuals. 2014;274:490–499. doi: 10.1148/RADIOL.14140680. [DOI] [PubMed] [Google Scholar]
- Xing C-Y, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan L-J, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. Journal of Cerebral Blood Flow & Metabolism. 2017;37:2848–2856. doi: 10.1177/0271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VMY, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, Ma Y, Chi B, Gomar JJ, Peng S, Kingsley PB, Eidelberg D, Goldberg TE. The age-related perfusion pattern measured with arterial spin labeling MRI in healthy subjects. Frontiers in Aging Neuroscience. 2018;10:1–11. doi: 10.3389/fnagi.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.CIR.0000031798.07790.FE. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu P, Turner MP, Abdelkarim D, Lu H, Rypma B. The neural–vascular basis of age-related processing speed decline. Psychophysiology. 2021;58:e13845. doi: 10.1111/PSYP.13845. [DOI] [PubMed] [Google Scholar]
- Zientek LR, Thompson B. Commonality analysis: Partitioning variance to facilitate better understanding of data. Journal of Early Intervention. 2006a;28:299–307. doi: 10.1177/105381510602800405. [DOI] [Google Scholar]
- Zientek LR, Thompson B. Commonality analysis: Partitioning variance to facilitate better understanding of data. Journal of Early Intervention. 2006b;28:299–307. doi: 10.1177/105381510602800405. [DOI] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed in this study is part of the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) research project (www.cam-can.com). Raw and minimally pre-processed MRI (Taylor et al., 2017) and behavioural data from Phase II are available by submitting a data request to Cam-CAN (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/). Phase III data are available on request from the authors.
As part of this study, MATLAB-based commonality analysis for neuroimaging with TFCE implementation was developed and made available at https://github.com/kamentsvetanov/CommonalityAnalysis/. Task-based fMRI data was post-processed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm; Friston et al., 2007). Arterial spin labelling data were post-processed using ExploreASL toolbox (Mutsaerts et al., 2018). Visualisation of all neuroimaging results was generated using MRIcroGL (https://github.com/rordenlab/MRIcroGL; Rorden and Brett, 2000). Gradient palettes used Matplotlib library (Hunter, 2007) via PyColormap4Matlab (https://github.com/f-k-s/PyColormap4Matlab).