Abstract
Schistosomiasis, the most important helminthic disease of humanity, is caused by infection with parasitic flatworms of the genus Schistosoma. The disease is driven by parasite eggs becoming trapped in host tissues, followed by inflammation and granuloma formation. Despite abundant transcriptome data for most developmental stages of the three main human-infective schistosome species—Schistosoma mansoni, S. japonicum and S. haematobium—the transcriptomic profiles of developing eggs remain under unexplored. In this study, we performed RNAseq of S. mansoni eggs laid in vitro during early and late embryogenesis, days 1-3 and 3-6 post-oviposition, respectively. Analysis of the transcriptomes identified hundreds of up-regulated genes during the later stage, including venom allergen-like (VAL) proteins, well-established host immunomodulators, and genes involved in organogenesis of the miracidium larva. In addition, the transcriptomes of the in vitro laid eggs were compared with existing publicly available RNA-seq datasets from S. mansoni eggs collected from the livers of rodent hosts. Analysis of enriched GO terms and pathway annotations revealed cell division and protein synthesis processes associated with early embryogenesis, whereas cellular metabolic processes, microtubule-based movement, and microtubule cytoskeleton organization were enriched in the later developmental time point. This is the first transcriptomic analysis of S. mansoni embryonic development, and will facilitate our understanding of infection pathogenesis, miracidial development and life cycle progression of schistosomes.
Keywords: Schistosoma mansoni, eggs, RNAseq, early embryogenesis, late embryogenesis, neglected tropical disease (NTD)
Introduction
Schistosomes are parasitic flatworms that infect more than 250 million people worldwide, mainly in Low and Middle-Income Countries (1). There is only a single effective drug (praziquantel), and an ongoing threat of drug resistance emerging (2). While adult worms can dwell within the blood vessels of humans for years, it is their eggs rather than the worms themselves that drive pathology (3). It is estimated that one pair of Schistosoma mansoni adult worms can lay >300 eggs per day (4). Once the eggs are laid by the worms in the mammalian bloodstream, about half migrate, over the course of six days, through the endothelium of blood vessels, across the epithelium of the gut and are released into the intestinal lumen (5–7). Eggs that reach water hatch into miracidium larvae that must infect a freshwater snail to continue the life cycle. The remaining eggs are swept around the body by the bloodstream and become trapped in host tissues, mainly in the liver, intestines and spleen, where they induce immune responses, severe inflammation and granuloma formation (8). The granuloma surrounding the trapped egg consists of an immune cellular complex that includes macrophages, lymphocytes and eosinophils (3). In addition, fibroblasts in the granuloma produce collagen that leads to periportal fibrosis and induction of collateral circulation, including varices, which increase the risk of life-threatening haemorrhage in the digestive tract (9).
The study of the schistosome egg and its interaction with the host is critical to understand not only the pathogenesis associated with the infection, but also parasite strategies to exit the mammalian host to perpetuate the life cycle (6,7). Thus, numerous reports have focused on soluble egg antigens (SEA) and several excreted-secreted products, including proteins and glycans that interact with the host tissues, inducing immune responses and facilitating the egress of the egg to the external environment (10–14). Notably, the immune modulatory roles of egg-specific antigens, such as Omega, Kappa and IPSE have been validated by functional approaches such as shRNA-mediated knock-down (15) and CRISPR-Cas-based genome editing (16). More recently, the molecular and cellular mechanisms involved in granuloma formation have been dissected using a zebrafish model of macrophage dependent granuloma induction (17). This novel infection model demonstrated that host and parasite molecules play key roles in shaping the granulomatous response and that the response is dependent on the level of egg maturity (17,18).
Notwithstanding this progress, few studies have focused on the development of the miracidium within the egg capsule (19). Jurberg et al. (20) provided a detailed morphological description of embryogenesis of the miracidium inside the egg capsule while migrating through the host tissue. In addition, these investigators provided a revised staging scheme for the miracidial development that comprises eight discrete stages (20). No transcriptome analysis underlying this developmental progression has yet been performed, with only a single RNA-seq report of S. mansoni eggs isolated from the liver of experimentally-infected hamsters (21). Aiming to address this information deficit, we performed comparative transcriptomics (RNA-seq) on in vitro laid eggs of S. mansoni at two time points - early and late embryogenesis. More than 1,300 genes were differentially expressed, including up-regulated in the late development stage of genes associated with organogenesis of the miracidium. The investigation revealed transcriptional signatures in developing in vitro laid eggs that will facilitate our understanding of the pathogenesis associated with the infection, miracidial development and life cycle progression of schistosomes.
Material and Methods
Ethics Statement
The complete life cycle of the NMRI (Puerto Rican) strain of S. mansoni is maintained at the Wellcome Sanger Institute (WSI) by breeding and infecting susceptible Biomphalaria glabrata snails and mice. The mouse experimental infections and other regulated procedures were conducted under the Home Office Project Licence No. P77E8A062 held by GR. All protocols were revised and approved by the Animal Welfare and Ethical Review Body (AWERB) of the WSI. The AWERB is constituted as required by the UK Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012.
In Vitro Laid Eggs
Schistosome eggs laid in vitro by cultured adult worms (in vitro laid eggs or IVLE) were collected as described (22). Briefly, mixed-sex adult worms were recovered from mice by portal perfusion 6 weeks after infection, washed in 1X PBS supplemented with 200 U/ml penicillin, 200 mg/ml streptomycin and 500 ng/ml amphotericin B (ThermoFisher Scientific), transferred to 6-well plates, and maintained in culture in complete Basch media at 37°C in 5% CO2 (23). All media components were purchased from ThermoFisher Scientific. The eggs laid by the worms in culture during the first 72 h post-perfusion were recovered. Fifty percent of the eggs were collected at this time for the early embryogenesis samples (D3 eggs), concentrated by gravity, resuspended in 750 μl Trizol reagent, snap frozen and stored at -80°C. The remaining IVLE were cultured for a further 3 days to allow further development before collection for the late embryogenesis samples (D6 eggs) (24). A total number of eggs ranging from 500 to 1000 IVLE were collected at each time point. We performed a separate collection of IVLE, as above, for each of three independent perfusions of adult schistosomes from experimentally infected mice.
RNA Extraction From In Vitro Laid Eggs
IVLE frozen in Trizol reagent were subjected to 3 freeze-thaw cycles by manually transferring tubes between a water bath at 95°C and dry ice. This procedure enhanced the total RNA yield from the samples. Thereafter, the eggs were transferred to MagNA Lyser tubes (Roche) containing ceramic beads, homogenized in FastPrep (FastPrep-24, MP Biomedicals) at setting 6 with two 20-second pulses and incubated for 5 minutes at room temperature. To each sample, 150μl chloroform was added, shaken vigorously for 10 seconds, incubated for 3 minutes at room temperature and centrifuged at 15,000g for 15 minutes at room temperature. The aqueous phase was carefully removed to a clean centrifuge tube, and the RNA was precipitated by adding one volume of 100% ethanol and incubating at -80°C overnight. The samples were centrifuged at maximum speed for 30 minutes at 4°C, the RNA pellet washed in 70% ethanol, air dried and resuspended in nuclease free water. The RNA quality was assessed and quantified using the Bioanalyzer (2100 Bioanalyzer Instrument, Agilent Technologies). Although the yield of RNA was modest, <30 ng total, high-quality RNA was recovered from each replicate sample (Supplementary Figure S1A).
Library Preparation and Sequencing
Given the minimal amount of RNA obtained from IVLE, the Smart-Seq2 protocol was adapted for low-input RNA library preparation (25). Two different amounts of input RNA, 12 ng and 3 ng, for each sample and its replicates were prepared. Polyadenylated mRNA was enriched using 5 mg/ml Dynabead Oligo(dT)20 in 1X PBS (pH 7.4) from mRNA DIRECT kit (Thermo Fisher). Beads were washed with 10mM Tris-HCl pH 7.5, 150 mM LiCl, 1mM EDTA pH 8.0, 0.1% w/v LiDS in nuclease free water and RNA eluted using 10 mM Tris-HCl pH 8.0 at 75°C for 2 minutes. The poly A-enriched RNA was reverse transcribed by Smart-Seq2 as described (25) with 10 reverse transcriptase cycles, and cDNA further amplified using IsoSeq PCR (ISPCR) primers with 10 or 11 PCR cycles. Dual indexed sequencing libraries were made out of 5 ng cDNA from the above preparations using Illumina Nextera library preparation kit according to manufacturer’s instructions (Illumina). Quality checked and equimolar pooled libraries were sequenced in a HiSeq 4000 Illumina system and 75bp paired- non-stranded- reads generated. Sequence data were deposited in the European Nucleotide Archive (ENA) with the study number ERP128933; NCBI BioProject ID PRJEB44842 (accession numbers for each sample are shown in Supplementary Table S1).
Mapping of RNA-Seq Reads and Gene Counting
Sequence reads from eggs isolated from the liver of experimentally-infected hamsters 6 to 8 weeks after infection (‘liver eggs’) were obtained from published data (21). The reads were mapped to S. mansoni v7 genome (WormBase Parasite WBPS14) using Hisat 2.1.0 (26) due to unequal read lengths generated on Roche 454. Sequence reads for IVLE were mapped using STAR 2.5.0a (27) with the option –alignIntronMin 10. Counts per gene were summarised with FeatureCounts v1.4.5-p1 (28) based on the exon feature, using the annotation from WormBase Parasite WBPS14 (https://parasite.wormbase.org/) (29).
Differential Gene Expression Analysis
Raw read counts from both liver eggs (21) and IVLE samples (this study) were combined and used as input for DESeq2 v1.26.0 (30). The Pearsons’s correlations between replicates were examined. For differential expression analysis, we set cooksCutoff=TRUE to remove extreme outlier genes. Genes with adjusted p-value (Padj) < 0.01 and fold-difference > 2 were regarded as significantly differentially expressed. Log-transformed count data were used for principle component analysis (PCA) and calculating the Euclidean distance between samples.
Gene Ontology Enrichment Analysis of Differentially Expressed Genes
Gene Ontology (GO) annotation for S. mansoni genes were obtained by running InterProScan v5.25 (31). Enrichment analysis of differentially expressed genes (DEGs) was performed using topGO v2.38.1 (32), with 5 nodes and the weight 01 method. GO terms with FDR < 0.05 were considered as significantly enriched. Gene product descriptions were obtained using the Biomart tool at WormBase Parasite (https://parasite.wormbase.org/).
Enrichment of Pfam Family and InterPro Domains
The annotations of Pfam family and InterPro domain in S. mansoni gene products were obtained from InterProScan v5.25. Analysis of functional enrichment in DEGs was conducted via Fisher’s Exact test followed by a p-value correction using the Benjamini-Hochberg procedure. Terms with FDR < 0.05 were considered as significant.
KEGG Pathway Mapping
Mapping of S. mansoni gene products to the KEGG pathway database was performed on the KAAS server (https://www.genome.jp/kegg/kaas/) using the GHOSTX program and BBH method. The significance of DEGs enrichment in pathways was assessed using Fisher’s Exact test and resulting p-values were adjusted using the Benjamini-Hochberg procedure for each of KEGG categories 1-5 (https://www.genome.jp/kegg/pathway.html; excluding pathways for prokaryotes, yeast, and plant). Pathways with FDR < 0.05 were considered as significant. The scripts used for functional enrichment (GO, Pfam, InterPro, KEGG) analysis can be accessed at https://github.com/zglu/Gene-function-enrichment.
Results
Transcriptional Signatures Underlie the In Vitro Development of Schistosoma mansoni Eggs
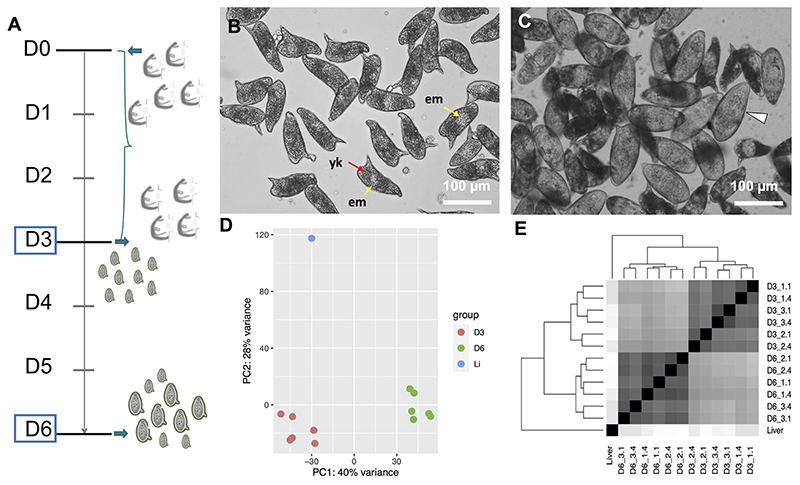
To study the transcriptional profiles associated with in vitro development of eggs, we performed RNA-seq on samples corresponding to early embryogenesis, 1–3 days post-oviposition (D3), and late embryogenesis, 4–6 days post-oviposition (D6) (Figure 1A). According to previously described criteria, most D3 eggs belonged to stages I (nonvisible embryo under the light microscope) and II (visible embryo as a clear central disk that occupies one third of the egg) (19, 20) (Figure 1B and Supplementary Figure S2A). By D6, the eggs were further developed and had increased in size by one third, as previously reported (20). In addition, 40–45% of the D6 eggs had progressed to stages III (enlarged embryo that occupies two thirds of the egg length), IV (embryo occupying almost the entire egg), or V (fully mature miracidium inside the eggshell before hatching, some motile miracidia) (19, 20) (Figure 1C and Supplementary Figure S2B and Supplementary Video S1).
Figure 1.
(A) Timeline depicting the experimental design. On day 0 (D0) adult worms perfused from infected mice were washed and placed in culture for three days. The worms laid eggs, i.e. in vitro laid eggs (IVLE), for three days (bracket). On day 3 (D3) the worms were removed from the culture, and half of the IVLE were collected for RNAseq - D3, early embryogenesis sample. The remaining IVLE were cultured for three more days, and on day 6 (D6) they were collected for RNAseq - D6, late embryogenesis sample. (B, C) Representative micrographs of 3 days old- (B) and 6 days old- (C) in vitro laid eggs (IVLE). Scale bar: 100 μm. em, embryo (yellow arrow); yk, yolk (red arrow); white arrowhead, fully developed egg containing the mature miracidium. (D) Clustering of egg samples using Principal Component Analysis. D3, D3 IVLE; D6, D6 IVLE; Li, liver eggs (E) Clustering of egg samples using Sample Distance Matrix. Names of samples are described in Supplementary Table S1.
The quality of the total RNA extracted from IVLE was much higher than that of the highly-degraded RNA usually obtained from eggs collected from the liver of experimentally-infected mice (Supplementary Figure S1B). On the other hand, the total RNA yield isolated from ~500–1000 IVLE was <30 ng. Therefore, we adapted a Smart-Seq2 protocol originally designed for single-cell RNA-seq (25) to produce high-quality RNA-seq libraries from 3 or 12 ng of input RNA. We obtained 0.2–3.3 million RNA-seq raw reads per library, with three-quarters of them having at least two-fold more reads than the published liver egg sample (21) (0.36 million reads; Supplementary Table S1). We note that the latter sample was sequenced using Roche 454 sequencing technology and 1000 ng of polyA+ RNA. All replicates of IVLEs showed good correlations (Pearson’s r > 0.83; Supplementary Table S2) and similar numbers of detected genes (counts per million, CPM > 10; Supplementary Table S1). From both Principal Component Analysis (PCA) and Sample Distance Matrix analyses, the samples clustered according to their developmental stage (Figures 1D, E).
In Vitro Developed Eggs Are Transcriptionally Distinct From Eggs Collected From the Host
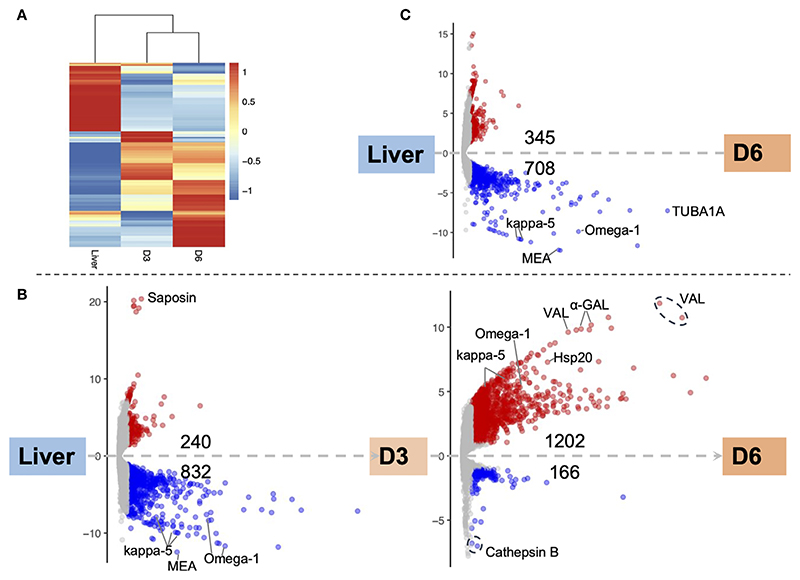
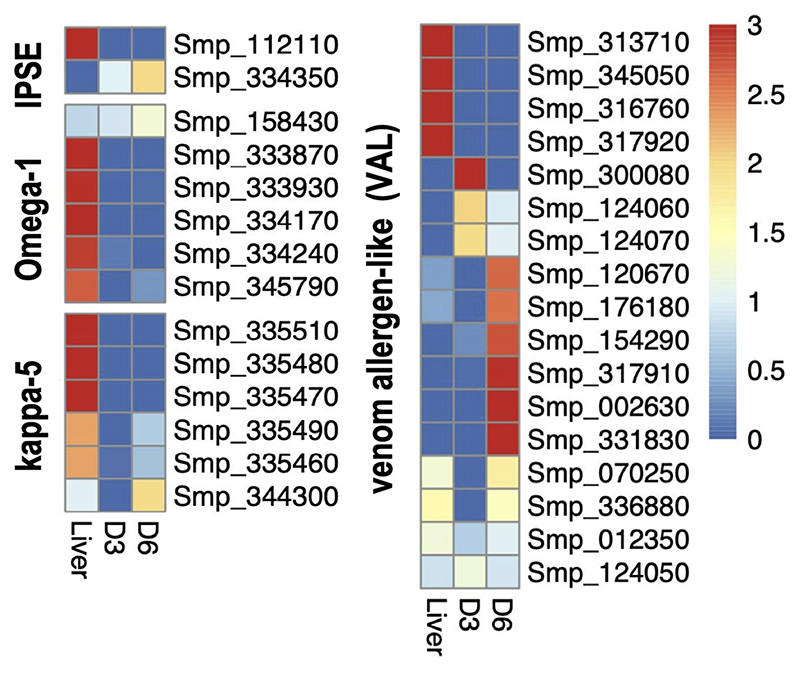
Major transcriptomic differences were evident among the three egg samples: the two IVLE samples—early embryogenesis (D3), late embryogenesis (D6)—and the liver-collected eggs. However, the gene expression profiles of D3 and D6 IVLE partially overlapped suggesting progressive transcriptome changes during egg development (Figure 2A). Conversely, the transcriptome of liver eggs was distinct to that of IVLE; 240 and 832 genes, respectively, were significantly up- and down-regulated in D3 IVLE compared to liver eggs; 345 and 708 genes, respectively, were significantly up- and down-regulated in D6 IVLE compared to liver eggs (adjusted p < 0.01 & fold-difference >2; Figure 2 and Supplementary Figure S3, Supplementary Tables S3, S4). Strikingly, the expression of well-described immunomodulatory egg-specific genes, including omega-1 (Smp_345790 & Smp_334170) and kappa-5 (Smp_335470, Smp_335480 & Smp_335490) (15, 16) was significantly higher in liver eggs compared to IVLE (Figures 2B, C and 3, Supplementary Table S4). Meanwhile, one omega-1 (Smp_345790) and two kappa-5 (Smp_335490 & Smp_344300) genes showed more than a 30-fold increase in expression at D6 compared to D3 (Figure 2). In addition, expression of four the putative major egg antigens (Smp_302350, Smp_302340, Smp_247170 and Smp_302280), including one extensively studied as a key component in the T-cell-mediated response during the granuloma formation (33), significantly increased during in vitro egg development (Supplementary Table S4). In addition to these well-characterised immunomodulatory egg-specific genes, micro-exon genes (MEG) were also identified in D3 or D6 IVLE; however, only MEG 2 (Smp_159830) showed significant upregulation in D6 compared to D3 IVLEs (Supplementary Table S4).
Figure 2. Differential gene expression among egg samples.
(A) Hierarchical clustering showing divergent transcriptomic signatures among the three samples. Liver, liver eggs; D3, D3 IVLE; D6, D6 IVLE. The colour scale indicates the Z-score values. (B) Volcano plots showing differentially expressed genes (DEGs) in D3 IVLE compared to liver eggs (left) and in D6 IVLE compared to D3 IVLE (right). Highlighted genes: Saposin (Smp_105420), kappa-5 (left: Smp_335470, Smp_335480 & Smp_335490; right: Smp_335490 & Smp_344300); Omega-1 (left: Smp_334170 & Smp_345790; right: Smp_345790), Hsp20 (Smp_302270), VAL (venom-allergen like protein - Smp_070250, Smp_176180 & Smp_120670), a-GAL (Alpha-N-acetylgalactosaminidase - Smp_247750 & Smp_247760), Cathepsin B (Smp_067060 & Smp_103610). (C) Volcano plot showing DEGs in D6 IVLE compared to liver eggs. Highlighted genes: kappa-5 (Smp_335470 & Smp_335480), MEA (major egg antigen - Smp_302350), Omega-1 (Smp_334170), TUBA1A (Tubulin alpha-1A chain - Smp_090120). In the volcano plots the x-axes represent -log10Padj values and the y-axes represent log2FoldChange. The volcano plots are available as interactive charts at http://schisto.xyz/IVLE/.
Figure 3.
Heatmaps showing the relative gene expression of the egg antigens IPSE, Omega-1, kappa-5 and VALs in liver, D3 and D6 IVLE, as indicated. The colour scale shows relative values to the mean of each row using normalised counts from DESeq2.
Miracidium-Enriched Genes Up-Regulated in Late Egg Development
Further pairwise analysis between both IVLE transcriptomes, showed 1202 up- and 166 down-regulated genes in D6 compared to D3 eggs (Figure 2B and Supplementary Table S4), indicating an overall upregulation of gene expression as embryogenesis proceeded. Of the 1202 upregulated genes in D6, 524 are among marker genes for different cell types previously defined by single cell RNA-seq in schistosomula, the first intra-mammalian stage (34). Of these, 53.8% (282/524) belong to neurons, 18.7% (98/524) to muscles, 18.9% (99/524) to the parenchyma tissue, and 8.6% (45/524) to germinal cells (Supplementary Table S5).
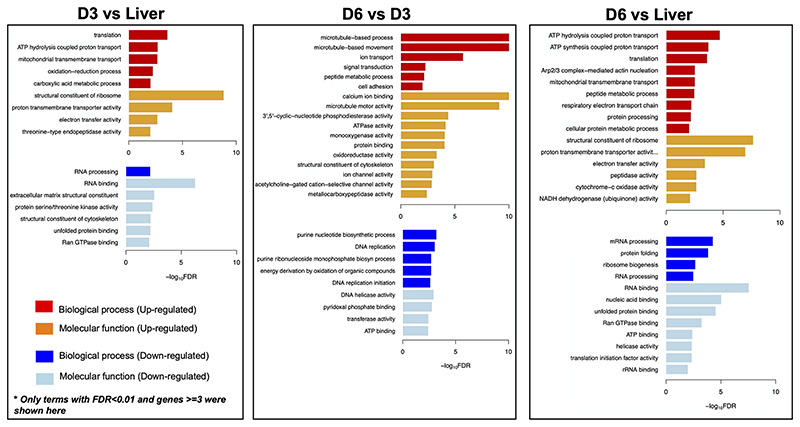
Genes involved in the interaction between the miracidium and the snail were among the 10 with highest fold differences in expression between D6 and D3 eggs (Supplementary Table S4). For example, genes encoding venom allergen-like (VAL) proteins were upregulated in D6 eggs (Figure 3), including VAL 9 (Smp_176180), VAL 5 (Smp_120670), and VAL 15 (Smp_070250) (Figure 2B and Supplementary Table S4). Functional analysis based on Gene Ontology (GO) and KEGG Pathways revealed biological processes and molecular functions associated with differentially-expressed genes among the three egg samples (Figure 4 and Supplementary Table S6). The transcriptome of D3 compared to D6 eggs or liver eggs, showed an enrichment for DNA replication, cell cycle, ribosome biogenesis and RNA translation (Figure 4). This is consistent with the cell division and protein synthesis which are critical processes in the early developing embryo (20). In contrast, up-regulated genes in D6 eggs were associated with processes associated with movement and signalling, e.g. microtubule-based movement, signal transduction, and GPCR signalling pathways (FDR<0.05; Figure 4 and Supplementary Table S6). The microtubule motor activity associated with the later developmental time point may be related to the development of the ciliary plates of the miracidium, which enable swimming.
Figure 4.
Gene Ontology (GO) enrichment in differentially expressed genes. For each comparison - D3 vs Liver; D6 vs D3; D6 vs Liver, only GO terms with FDR<0.01 and at least three genes are visualised. D3, D3 IVLE; D6, D^ IVLE; Liver, liver eggs.
Several common features between the mature egg and the miracidium were identified, and indeed the presence of fully-developed miracidia within eggshells was evident in the D6 eggs (Figure 1B and Supplementary Figure S2B). Therefore, we asked whether a transcriptional footprint was found during the development of D6 eggs towards miracidium. To this end, we examined the top 100 genes that were previously shown to be enriched in miracidium-sporocyst (35) among all developmental stages, and found that around a third were significantly up-regulated in D6 eggs (Supplementary Table S7). The rest of the genes showed either no significant differential expression, or no expression in IVLEs (Supplementary Table S7). When considering the top 200 miracidium-enriched genes, only 4.4% were more abundant in D3 eggs, but 21.9% displayed higher expression in D6 eggs (Supplementary Tables S4,S7), including SmVAL2 (Smp_002630) and SmVAL15 (Smp_070250), which were previously shown to be highly expressed in the miracidium (36). In addition, in D6 eggs we found an evident upregulation of tektin (Smp_162540) and tubulin (Smp_079960) genes, which are essential for microtubule assembly and physiology, key components of the ciliary machinery.
Discussion
The schistosome egg is the main driver of the chronic pathology associated with schistosomiasis (3). In addition, it is a developmental stage that ensures the propagation of the parasite from the definitive host to the intermediate host via the external environment. It has been recently shown that the timing of granuloma formation is actively manipulated by developing eggs, to avoid immune destruction or premature extrusion from the host (17). Thus, it is critical to understand the transcriptome landscape driving the egg development. Other than a handful of descriptive reports on embryogenesis (20) and egg secretions (37), the number of studies focused on gene expression changes in schistosome eggs seems surprisingly scarce. There is only one public RNAseq transcriptome dataset for S. mansoni eggs and one for S. haematobium eggs (21, 38). In addition, a few previous studies have employed expressed-sequence tags (ESTs) or microarrays to described changes in gene expression across developmental stages, including eggs of S. mansoni (39, 40) and S. japonicum (41, 42). Mass spectrometrically-determined proteomes of the soluble egg and secreted proteins of egg, recovered from liver of S. haematobium-infected mice, also have been reported (43). In all these studies, the eggs were isolated from livers of experimentally-infected rodents and the egg transcriptomes/proteomes were compared to those of male and female adult worms aiming at identifying genes involved in host-parasite interaction. The studies did not explore changes in gene expression during embryogenesis.
Investigation of the transcriptome of the schistosome egg has been impeded by intrinsic difficulties of this stage, including the presence of the eggshell and abundance of egg-derived RNases that degrade the transcripts during recovery of RNA. Here, we optimised a protocol to isolate high-quality RNA from eggs by consecutive rounds of freeze-thawing cycles. The quality of total RNA isolated from in vitro laid eggs (IVLE), even after 3 cycles of freeze-thawing was significantly superior to that of RNA isolated from liver eggs. Similarly, it has been reported that for biospecimens stored in biobanks, such as tumour sections, the RNA quality is optimal for downstream analyses after 3 freeze-thawing cycles, but it is dramatically negatively affected after five freeze-thaw cycles (44). In addition, we successfully employed the SmartSeq2 protocol (25) to produce bulk RNA-seq data starting with few nanograms of input RNA, as has been recently shown for transcriptomic studies in Trichuris muris larvae (45). Repurposing single-cell RNAseq protocols to generate high-quality bulk transcriptomic data from pico- to nanograms of total RNA or from few cells (46) becomes a promising approach when the amount of RNA is limited.
We have previously optimised the collection of schistosome IVLE, followed and quantified their daily development (24, 47). We have now produced RNA-seq data from developing eggs of S. mansoni. Although less total RNA was used for library preparation, we observed similar transcriptome coverage in IVLE compared to the only published data from liver eggs. Normalisation using the DESeq2 median-of-ratios method has also accounted for the differences in various library sizes, resulting in robust differential expression analysis (30). We observed drastic changes in the transcriptome profile of developing eggs and inferred functional roles of differentially expressed genes associated with embryo development (20). Our findings are consistent with descriptions of the egg development in vitro and the characterisation of excreted-secreted proteins by mature eggs (37). The authors describe highly abundant protein synthesis in 3-day cultured eggs compared to freshly isolated eggs. Our findings show that the up-regulated genes in D3 vs liver eggs and D6 vs D3 eggs are consistently associated with protein synthesis-associated processes such as translation, translational elongation, and regulation of phosphorylation. We found that genes with established immunomodulatory roles, e.g. omega-1 (13), kappa-5 (12) and the putative major egg antigen Sm-p40 (33) show a higher expression in mature eggs compared to immature eggs. However, the overall expression of these genes was much higher in eggs isolated from the host liver compared to IVLE suggesting that host factors may be required for driving their expression and/or stimulating the genesis of the subshell membrane where some of these proteins seem to be produced (12, 33). The eggs collected from the liver of experimentally-infected rodents likely comprised eggs ranging in developmental stage from newly laid to mature and likely also included eggs already dead due to immune responses and other factors. Thus, any comparison between the liver eggs and IVLE need to be taken cautiously. The in vitro culture conditions do not completely mimic the in vivo development of the schistosome and hence, the production of fully viable eggs is limited. This is consistent with the low percentage (ranging from 10% to 15%) of IVLE that hatch fully viable and infectious miracidia (24). Recent improvements in culture conditions offer novel and informative approaches to sustain and study in vitro and ex vivo parasite development, including sexual differentiation and fecundity (48–50).
Notwithstanding the limitations of our culture system, we identified a transcriptional footprint consistent with the developmental transition from D3 to D6 egg samples and from D6 egg samples towards the miracidium. In the D6 samples, we identified tissue-specific markers for muscle cells, nerve system, parenchymal and germ cells (34). We speculate that these genes may be involved in the specification and differentiation of diverse somatic and germinal tissues in the developing miracidia (51). Products of several genes upregulated in D6 eggs had previously been annotated as “larval transformation proteins” during in vitro miracidium-to-sporocyst transformation (52), including venom allergen-like proteins (SmVALs) (36). The role of SmVALs is not yet confirmed, but in some nematodes, VALs are involved in mechanisms of infection establishment and host immunomodulation (53). More recently, using a combination of RNAi and in situ hybridisation, Perally and colleagues demonstrated the critical role of SmVAL6 in the maintenance of the tegumental barrier in adult worms (54). These findings suggest that the SmVALs may be tentative targets for drug development. Whether SmVALs, already upregulated in the mature egg, display similar functions during infection of the snail remains to be addressed. Proteomic approaches in S. japonicum identified proteins in the mature egg that are associated with miracidium motility (55). Similarly, we identified an upregulation of tektin (Smp_162540) and tubulin (Smp_079960) genes, both essential proteins for microtubule assembly and cilium physiology. These findings were consistent with previous microarray-based findings in which genes overexpressed in liver eggs were associated with microtubule motor activity, microtubule-based movement/process and cytoskeleton organisation and biogenesis (40). Remarkably, the authors also showed that the transcriptome profiles of the egg and daughter sporocyst are highly divergent, in contrast with those of egg and miracidium/mother sporocyst described in the current study. Here, a transcriptional landscape transition from D3–D6 egg samples to miracidium was revealed, with upregulation of previously described genes expressed in the mature egg such as the micro-exon gene 2 (MEG 2) (56). MEG may represent a novel mechanism for immune evasion by schistosomes (57).
In this study, we have reported the first transcriptome analysis of developing eggs from S. mansoni, central drivers of this major neglected tropical disease. Their transcriptomes have clear signatures of the parasite gearing up for life cycle progression, including key proteins required for the structure and motility of the miracidium and for the subsequent infection of snails. Along with highlighting proteins already known to drive egg-induced pathology, the transcriptome analysis has revealed dozens of other genes with similar profiles, not previously associated with pathogenesis but now warranting deeper investigation. Follow up studies on key genes involved in life cycle progression would reveal tentative targets for novel control strategies for this Neglected Tropical Disease.
Supplementary Material
(A) Bioanalyzer electropherogram of RNA preparations isolated from D3 and D6 IVLE and processed for sequencing using the Smart-Seq2 protocol. Samples and concentrations are indicated in the bottom panel. (B) Representative bioanalyzer traces of RNA preparations isolated from IVLE and liver eggs (LE), as indicated.
Representative micrographs of D3- (A) and D6 (B) IVLE. Scale bar: 300 μm.
Left. Venn diagram indicating the number of shared/unshared upregulated genes amongst the three comparisons: D6 vs D3 IVLE (1202 genes); D3 IVLE vs liver eggs (240 genes); D6 IVLE vs liver eggs (345 genes). Right. Venn diagram indicating the number of shared/unshared downregulated genes amongst the three comparisons: D6 vs D3 IVLE (166 genes); D3 IVLE vs liver eggs (832 genes); D6 IVLE vs liver eggs (708 genes). The gene names and identifiers are provided in Supplementary Table S3.
Pie chart indicating the differential expression of the top 200 miracidium-sporocyst enriched genes in D3 and D6 IVLE. Filtered: genes that were filtered out for differential expression analysis in DESeq2, as were detected as outlier genes; DEG: differentially expressed genes.
Representative video showing D6 IVLE. Fully mature, motile miracidia can be seen within their eggshells. Scale bar: 50 μm.
RNA-Seq mapping statistics and accession numbers for all samples. Sample names refer to the day of the egg collection (D3 or D6), the number of the biological replicate (e.g. D3_1, D3_2, D3_3), and to the number of technical replicates employing 3 ng (1 μl - 1) or 12 ng (4 μl - 4), respectively.
Pearson’s correlations among the biological replicates of the in vitro laid eggs.
Gene IDs and normalised counts for the genes numbered in the Venn diagrams in Supplementary Figure S3.
Lists of differentially expressed genes among liver eggs, D3, and D6 IVLEs.
Lists of up-regulated genes in D6 (vs D3) IVLE that were enriched in different schistosomula cell clusters identified in (34).
Enriched functions in identified differentially expressed genes (DEGs), includingGeneOntology, KEGGPathway, and Pfam/InterPro domains.
List of differentially expressed genes in D3 and D6 IVLEs enriched in miracidium-sporocyst.
Acknowledgments
We are very grateful to our colleagues at the Wellcome Sanger Institute: Simon Clare, Cordelia Brandt, Catherine McCarthy, Katherine Harcourt and Lisa Seymour for assistance and technical support with animal infections and maintenance of the Schistosoma mansoni life cycle; Arthur Talman and Virginia Howick for providing expertise and support with the Smart-Seq2 library preparation; Mandy Sanders for project administration. We would also like to thank Alan Wilson and Karl Hoffmann for fruitful discussions.
Funding
This work was funded by Wellcome Trust (grant number 206194).
Footnotes
Ethics Statement
The mouse experimental infections and other regulated procedures were conducted under the Home Office Project Licence No. P77E8A062 held by GR. All protocols were revised and approved by the Animal Welfare and Ethical Review Body (AWERB) of the WSI. The AWERB is constituted as required by the UK Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012.
Author Contributions
GR conceived the project, designed the experiments, collected and cultured the in vitro laid eggs, interpreted the results and directed the study. ZL analysed the data, generated and interpreted the results. GS performed RNA isolation and RNA-seq. library preparation. VO performed the original bioinformatic analysis. KR interpreted and discussed the results, and performed the search of S. mansoni orthologs of genes involved embryogenesis. PB interpreted the results. MB provided resources and interpreted the results. ZL and GR wrote the original draft and all the authors edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.
References
- 1.Toor J, Alsallaq R, Truscott JE, Turner HC, Werkman M, Gurarie D, et al. Are We on Our Way to Achieving the 2020 Goals for Schistosomiasis Morbidity Control Using Current World Health Organization Guidelines? Clin Infect Dis. 2018;66:S245–52. doi: 10.1093/cid/ciy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crellen T, Allan F, David S, Durrant C, Huckvale T, Holroyd N, et al. Whole Genome Resequencing of the Human Parasite Schistosoma Mansoni Reveals Population History and Effects of Selection. Sci Rep. 2016;6:20954. doi: 10.1038/srep20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce EJ, MacDonald AS. The Immunobiology of Schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 4.Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of Egg Production and Egg Excretion by Schistosoma Mansoni and S. Japonicum in Mice Infected With a Single Pair of Worms. Am J Trop Med Hyg. 1994;50:281–95. doi: 10.4269/ajtmh.1994.50.281. [DOI] [PubMed] [Google Scholar]
- 5.Jourdane J, Theron A. In: The Biology of Schistosomes: From Genes to Latrines. Rollinson D, Simpson AJG, editors. Academic Press; New York, NY: 1987. Larval Development: Eggs to Cercariae. [Google Scholar]
- 6.Schwartz C, Fallon PG. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front Immunol. 2018;9:2492. doi: 10.3389/fimmu.2018.02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costain AH, MacDonald AS, Smits HH. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front Immunol. 2018;9:3042. doi: 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross AGP, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 9.Gryseels B, Polman K, Clerinx J, Kestens L. Human Schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 10.Asahi H, Stadecker MJ. Analysis of Egg Antigens Inducing Hepatic Lesions in Schistosome Infection. Parasitol Int. 2003;52:361–7. doi: 10.1016/s1383-5769(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 11.Schramm G, Falcone FH, Gronow A, Haisch K, Mamat U, Doenhoff MJ, et al. Molecular Characterization of an Interleukin-4-Inducing Factor From Schistosoma Mansoni Eggs. J Biol Chem. 2003;278:18384–92. doi: 10.1074/jbc.M300497200. [DOI] [PubMed] [Google Scholar]
- 12.Schramm G, Hamilton JV, Balog CIA, Wuhrer M, Gronow A, Beckmann S, et al. Molecular Characterisation of Kappa-5, A Major Antigenic Glycoprotein From Schistosoma Mansoni Eggs. Mol Biochem Parasitol. 2009;166:4–14. doi: 10.1016/j.molbiopara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Fitzsimmons CM, Schramm G, Jones FM, Chalmers IW, Hoffmann KF, Grevelding CG, et al. Molecular Characterization of Omega-1: A Hepatotoxic Ribonuclease From Schistosoma Mansoni Eggs. Mol Biochem Parasitol. 2005;144:123–7. doi: 10.1016/j.molbiopara.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Meevissen MHJ, Yazdanbakhsh M, Hokke CH. Schistosoma Mansoni Egg Glycoproteins and C-Type Lectins of Host Immune Cells: Molecular Partners That Shape Immune Responses. Exp Parasitol. 2012;132:14–21. doi: 10.1016/j.exppara.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Hagen J, Young ND, Every AL, Pagel CN, Schnoeller C, Scheerlinck J-PY, et al. Omega-1 Knockdown in Schistosoma Mansoni Eggs by Lentivirus Transduction Reduces Granuloma Size In Vivo. Nat Commun. 2014;5:5375. doi: 10.1038/ncomms6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ittiprasert W, Mann VH, Karinshak SE, Coghlan A, Rinaldi G, Sankaranarayanan G, et al. Programmed Genome Editing of the Omega-1 Ribonuclease of the Blood Fluke, Schistosoma Mansoni. Elife. 2019;8:e41337. doi: 10.7554/eLife.41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaki KK, Rinaldi G, Berriman M, Pagán AJ, Ramakrishnan L. Schistosoma Mansoni Eggs Modulate the Timing of Granuloma Formation to Promote Transmission. Cell Host Microbe. 2021a;29:58–67.:e5. doi: 10.1016/j.chom.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaki KK, Roca FJ, Schramm G, Wilbers RHP, Ittiprasert W, Brindley PJ, et al. Tumor Necrosis Factor and Schistosoma Mansoni Egg Antigen Omega-1 Shape Distinct Aspects of the Early Egg-Induced Granulomatous Response. PLoS Negl Trop Dis. 2021b;15:e0008814. doi: 10.1371/journal.pntd.0008814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaels RM, Prata A. Evolution and Characteristics of Schistosoma Mansoni Eggs Laid In Vitro. J Parasitol. 1968;54:921–30. doi: 10.2307/3277120. [DOI] [PubMed] [Google Scholar]
- 20.Jurberg AD, Gonçalves T, Costa TA, de Mattos ACA, Pascarelli BM, de Manso PPA, et al. The Embryonic Development of Schistosoma Mansoni Eggs: Proposal for a New Staging System. Dev Genes Evol. 2009;219:219–34. doi: 10.1007/s00427-009-0285-9. [DOI] [PubMed] [Google Scholar]
- 21.Anderson L, Amaral MS, Beckedorff F, Silva LF, Dazzani B, Oliveira KC, et al. Schistosoma Mansoni Egg, Adult Male and Female Comparative Gene Expression Analysis and Identification of Novel Genes by RNA-Seq. PloS Negl Trop Dis. 2015;9:e0004334. doi: 10.1371/journal.pntd.0004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldi G, Eckert SE, Tsai IJ, Suttiprapa S, Kines KJ, Tort JF, et al. Germline Transgenesis and Insertional Mutagenesis in Schistosoma Mansoni Mediated by Murine Leukemia Virus. PloS Pathog. 2012;8:e1002820. doi: 10.1371/journal.ppat.1002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann VH, Morales ME, Rinaldi G, Brindley PJ. Culture for Genetic Manipulation of Developmental Stages of Schistosoma Mansoni. Parasitology. 2010;137:451–62. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann VH, Suttiprapa S, Rinaldi G, Brindley PJ. Establishing Transgenic Schistosomes. PloS Negl Trop Dis. 2011;5:e1230. doi: 10.1371/journal.pntd.0001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picelli S, Faridani OR, Björklund AK, Winberg G, Sagasser S, Sandberg R. Full-Length RNA-Seq From Single Cells Using Smart-Seq2. Nat Protoc. 2014;9:171–81. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Langmead B, Salzberg SL. HISAT: A Fast Spliced Aligner With Low Memory Requirements. Nat Methods. 2015;12:357–60. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Smyth GK, Shi W. Featurecounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics. 2014;30:923–30. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 29.Howe KL, Bolt BJ, Shafie M, Kersey P, Berriman M. WormBase ParaSite - a Comprehensive Resource for Helminth Genomics. Mol Biochem Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love MI, Huber W, Anders S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data With Deseq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, et al. InterPro in 2019: Improving Coverage, Classification and Access to Protein Sequence Annotations. Nucleic Acids Res. 2019;47:D351–60. doi: 10.1093/nar/gky1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexa A, Rahnenfuhrer J. topGO: Enrichment Analysis for Gene Ontology. R Packag version 2.26.0. R Packag. version 2.26.0. 2020 [Google Scholar]
- 33.Stadecker MJ, Hernandez HJ. The Immune Response and Immunopathology in Infection With Schistosoma Mansoni: A Key Role of Major Egg Antigen Sm-P40. Parasite Immunol. 1998;20:217–21. doi: 10.1046/j.1365-3024.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 34.Diaz Soria CL, Lee J, Chong T, Coghlan A, Tracey A, Young MD, et al. Single-Cell Atlas of the First Intra-Mammalian Developmental Stage of the Human Parasite Schistosoma Mansoni. Nat Commun. 2020;11:6411. doi: 10.1038/s41467-020-20092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z, Berriman M. Meta-Analysis of RNA-Seq Studies Reveals Genes Responsible for Life Stage-Dominant Functions in Schistosoma Mansoni. Cold Spring Harbor Lab. 2018:308189. doi: 10.1101/308189. [DOI] [Google Scholar]
- 36.Farias LP, Chalmers IW, Perally S, Rofatto HK, Jackson CJ, Brown M, et al. Schistosoma Mansoni Venom Allergen-Like Proteins: Phylogenetic Relationships, Stage-Specific Transcription and Tissue Localization as Predictors of Immunological Cross-Reactivity. Int J Parasitol. 2019;49:593–9. doi: 10.1016/j.ijpara.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashton PD, Harrop R, Shah B, Wilson RA. The Schistosome Egg: Development and Secretions. Parasitology. 2001;122:329–38. doi: 10.1017/s0031182001007351. [DOI] [PubMed] [Google Scholar]
- 38.Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, et al. Whole-Genome Sequence of Schistosoma Haematobium. Nat Genet. 2012;44:221–5. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 39.Verjovski-Almeida S, DeMarco R, Martins EA, Guimarães PE, Ojopi EP, Paquola AC, et al. Transcriptome Analysis of the Acoelomate Human Parasite Schistosoma Mansoni. Nat Genet. 2003;35(2):148–57. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick JM, Peak E, Perally S, Chalmers IW, Barrett J, Yoshino TP, et al. Anti-Schistosomal Intervention Targets Identified by Lifecycle Transcriptomic Analyses. PloS Negl Trop Dis. 2009;3(11):e543. doi: 10.1371/journal.pntd.0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gobert GN, Moertel L, Brindley PJ, McManus DP. Developmental Gene Expression Profiles of the Human Pathogen Schistosoma Japonicum. BMC Genomics. 2009;10:128. doi: 10.1186/1471-2164-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai P, Liu S, Piao X, Hou N, You H, McManus DP, et al. A Next-Generation Microarray Further Reveals Stage-Enriched Gene Expression Pattern in the Blood Fluke Schistosoma Japonicum. Parasit Vectors. 2017;10(1):19. doi: 10.1186/s13071-016-1947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sotillo J, Pearson MS, Becker L, Mekonnen GG, Amoah AS, van Dam G, et al. In-Depth Proteomic Characterization of Schistosoma Haematobium: Towards the Development of New Tools for Elimination. PloS Negl Trop Dis. 2019;13(5):e0007362. doi: 10.1371/journal.pntd.0007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu K, Xing J, Zhang J, Zhao R, Zhang Y, Zhao L. Effect of Multiple Cycles of Freeze-Thawing on the RNA Quality of Lung Cancer Tissues. Cell Tissue Bank. 2017;18:433–40. doi: 10.1007/s10561-016-9600-7. [DOI] [PubMed] [Google Scholar]
- 45.Duque-Correa MA, Goulding D, Rodgers FH, Cormie C, Rawlinson K, Andrew Gillis J, et al. Defining the Early Stages of Intestinal Colonisation by Whipworms. 2020 doi: 10.1101/2020.08.21.261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid AJ, Talman AM, Bennett HM, Gomes AR, Sanders MJ, Illingworth CJR, et al. Single-Cell RNA-Seq Reveals Hidden Transcriptional Variation in Malaria Parasites. Elife. 2018;7:e33105. doi: 10.7554/eLife.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan H-B, Smout MJ, Ju C, Folley AE, Skinner DE, Mann VH, et al. Developmental Sensitivity in Schistosoma Mansoni to Puromycin To Establish Drug Selection of Transgenic Schistosomes. Antimicrob Agents Chemother. 2018;62(8):e02568–17. doi: 10.1128/AAC.02568-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Chen R, Collins JJ., 3rd Systematically Improved In Vitro Culture Conditions Reveal New Insights Into the Reproductive Biology of the Human Parasite Schistosoma Mansoni. PloS Biol. 2019;17:e3000254. doi: 10.1371/journal.pbio.3000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anisuzzaman A, Frahm S, Prodjinotho UF, Bhattacharjee S, Verschoor A, et al. Host-Specific Serum Factors Control the Development and Survival of Schistosoma Mansoni. Front Immunol. 2021;12:635622. doi: 10.3389/fimmu.2021.635622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchter V, Schneeberger PHH, Keiser J. Validation of a Human-Serum-Based In Vitro Growth Method for Drug Screening on Juvenile Development Stages of Schistosoma Mansoni. PloS Negl Trop Dis. 2021;15:e0009313. doi: 10.1371/journal.pntd.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawlinson KA. Embryonic and Post-Embryonic Development of the Polyclad Flatworm Maritigrella Crozieri Implications for the Evolution of Spiralian Life History Traits. Front Zool. 2010;7:12. doi: 10.1186/1742-9994-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu XJ, Sabat G, Brown JF, Zhang M, Taft A, Peterson N, et al. Proteomic Analysis of Schistosoma Mansoni Proteins Released During In Vitro Miracidium-to-Sporocyst Transformation. Mol Biochem Parasitol. 2009;164(1):32–44. doi: 10.1016/j.molbiopara.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilbers RHP, Schneiter R, Holterman MHM, Drurey C, Smant G, Asojo OA, et al. Secreted Venom Allergen-Like Proteins of Helminths: Conserved Modulators of Host Responses in Animals and Plants. PLoS Pathog. 2018;14(10):e1007300. doi: 10.1371/journal.ppat.1007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perally S, Geyer KK, Farani PSG, Chalmers IW, Fernandez-Fuentes N, Maskell DR, et al. Schistosoma Mansoni Venom Allergen-Like Protein 6 (SmVAL6) Maintains Tegumental Barrier Function. Int J Parasitol. 2021;51(4):251–61. doi: 10.1016/j.ijpara.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Marco Verissimo C, Potriquet J, You H, McManus DP, Mulvenna J, Jones MK. Qualitative and Quantitative Proteomic Analyses of Schistosoma Japonicum Eggs and Egg-Derived Secretory-Excretory Proteins. Parasitol Vectors. 2019;12:173. doi: 10.1186/s13071-019-3403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Marco R, Mathieson W, Manuel SJ, Dillon GP, Curwen RS, Ashton PD, et al. Protein Variation in Blood-Dwelling Schistosome Worms Generated by Differential Splicing of Micro-Exon Gene Transcripts. Genome Res. 2010;20(8):1112–21. doi: 10.1101/gr.100099.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson RA. Virulence Factors of Schistosomes. Microbes Infect. 2012;14(15):1442–50. doi: 10.1016/j.micinf.2012.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Bioanalyzer electropherogram of RNA preparations isolated from D3 and D6 IVLE and processed for sequencing using the Smart-Seq2 protocol. Samples and concentrations are indicated in the bottom panel. (B) Representative bioanalyzer traces of RNA preparations isolated from IVLE and liver eggs (LE), as indicated.
Representative micrographs of D3- (A) and D6 (B) IVLE. Scale bar: 300 μm.
Left. Venn diagram indicating the number of shared/unshared upregulated genes amongst the three comparisons: D6 vs D3 IVLE (1202 genes); D3 IVLE vs liver eggs (240 genes); D6 IVLE vs liver eggs (345 genes). Right. Venn diagram indicating the number of shared/unshared downregulated genes amongst the three comparisons: D6 vs D3 IVLE (166 genes); D3 IVLE vs liver eggs (832 genes); D6 IVLE vs liver eggs (708 genes). The gene names and identifiers are provided in Supplementary Table S3.
Pie chart indicating the differential expression of the top 200 miracidium-sporocyst enriched genes in D3 and D6 IVLE. Filtered: genes that were filtered out for differential expression analysis in DESeq2, as were detected as outlier genes; DEG: differentially expressed genes.
Representative video showing D6 IVLE. Fully mature, motile miracidia can be seen within their eggshells. Scale bar: 50 μm.
RNA-Seq mapping statistics and accession numbers for all samples. Sample names refer to the day of the egg collection (D3 or D6), the number of the biological replicate (e.g. D3_1, D3_2, D3_3), and to the number of technical replicates employing 3 ng (1 μl - 1) or 12 ng (4 μl - 4), respectively.
Pearson’s correlations among the biological replicates of the in vitro laid eggs.
Gene IDs and normalised counts for the genes numbered in the Venn diagrams in Supplementary Figure S3.
Lists of differentially expressed genes among liver eggs, D3, and D6 IVLEs.
Lists of up-regulated genes in D6 (vs D3) IVLE that were enriched in different schistosomula cell clusters identified in (34).
Enriched functions in identified differentially expressed genes (DEGs), includingGeneOntology, KEGGPathway, and Pfam/InterPro domains.
List of differentially expressed genes in D3 and D6 IVLEs enriched in miracidium-sporocyst.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.