Abstract
Quantitative Phase Imaging (QPI) has gained popularity in bioimaging because it can avoid the need for cell staining, which in some cases is difficult or impossible. However, as a result, QPI does not provide labelling of various specific intracellular structures. Here we show a novel computational segmentation method based on statistical inference that makes it possible for QPI techniques to identify the cell nucleus. We demonstrate the approach with refractive index tomograms of stain-free cells reconstructed through the tomographic phase microscopy in flow cytometry mode. In particular, by means of numerical simulations and two cancer cell lines, we demonstrate that the nucleus can be accurately distinguished within the stain-free tomograms. We show that our experimental results are consistent with confocal fluorescence microscopy (FM) data and microfluidic cytofluorimeter outputs. This is a significant step towards extracting specific three-dimensional intracellular structures directly from the phase-contrast data in a typical flow cytometry configuration.
Introduction
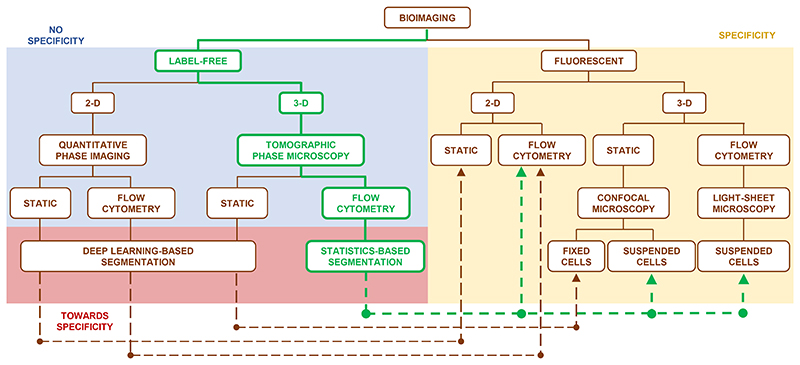
Traditional tools of histopathology will evolve soon, and the future of precision medicine will pass through the accurate screening of single cells. A key challenge that will allow the next jump forward is achieving a more informative label-free microscopy. Nowadays, the gold standard imaging tool in cell biology is FM, in which stains or fluorescent tags are used to make the biological sample visible on a selective basis1,2. Although FM has allowed to achieve significant progress in cellular biology, thus greatly advancing the scientific knowledge, it has some limitations. In fact, FM is intrinsically an indirect imaging method mediated by chemical agents. As a result, drawbacks exist that limit its applications, such as photo-bleaching and photo-toxicity2. Avoiding staining will permit one to access non-destructive, rapid, and chemistry-free analysis in biology and medicine. On the other hand, QPI is emerging as a very useful tool in label-free microscopy, and recently many significant results have been achieved in this field2–11. Starting from QPI, Tomographic Phase Microscopy (TPM) can be performed to map the refractive index (RI) of a biological specimen in 3D, thus providing a full quantitative measurement of 3D morphologies and RI volumetric distributions at the single-cell level12–20. However, the advantages of QPI are counterbalanced by the lack of direct intra-cellular specificity. Among all intra-cellular structures, the nucleus is the principal one in the eukaryotic cell since it contains most of the genetic material and it is responsible for the cellular lifecycle. Identifying the nucleus with label-free 3D imaging is a challenging task since the nuclear size and RI can vary among different cell lines, within the same cell line, and even within the same cell, depending on the lifecycle’s phases. In addition, different subcellular structures show similar RI values21, thus making any threshold-based detection method ineffective. Recently, significant progresses have been reported to introduce specificity in QPI by Artificial Intelligence (AI). Generative networks for cross-modality imaging and virtual staining are good examples in this sense. Networks for improving data analysis have been reported and commercialized as well, as in the case of the Nikon NIS.AI software suite that virtually stains and can segment the nuclei from label-free images of cells in adhesion. In NIS.AI, conventional segmentation of 2D labelled images is used to pre-train the network, which can then emulate the process when requested by the users. Deep learning was employed to virtually stain unlabelled tissues22–24 as well as single cells25,26 in quantitative phase maps (QPMs). The concept of virtual staining has then been extended to 3D RI tomograms of adherent samples, thus showing an AI-based RI to fluorescence mapping for the identification of the stain-free nucleus27 and other endogenous subcellular components28. In particular, fluorescent signals are inferred from 3D RI distributions with remarkable generalization power, which is one of the suggestable pathways towards label-free specificity. To illustrate the state of the art for intra-cellular specificity, the summary diagram shown in Fig. 1 compares various label-free and fluorescent techniques.
Fig. 1. Comparison between label-free and fluorescent bioimaging in microscopy.
Unlike the label-free bioimaging (blue box), the fluorescent bioimaging (yellow box) has sub-cellular specificity because nucleus is marked, but it is qualitative and limited by the staining itself. The methods in the red box allow to fill the specificity gap between the label-free and fluorescent techniques (dashed lines). In particular, virtual staining of unlabelled tissues22–24 and single cells25,26 in 2D imaging case has been demonstrated with the aid of deep learning. In the 3D imaging case, nuclei of unlabelled and adhered cells have been identified using a deep convolutional neural network27,28 to introduce specificity in TPM reconstructions, thus making 3D label-free TPM equivalent to the well-established 3D confocal microscopy, but only for static analysis of fixed cells at rest on a surface. The proposed technology (green pathway) fills a blank in the bioimaging realm because, in terms of specificity, the statistics-based segmentation (CSSI method) makes the TPM in flow cytometry consistent with both the 2D FM flow-cytometry and the 3D FM confocal microscopy of suspended cells. Moreover, it can also emulate the light-sheet FM, which has been recently integrated to microfluidic circuits thus creating a 3D FM flow cytometer33,34.
In this paper, we propose a new 3D shape retrieval method, named here as Computational Segmentation based on Statistical Inference (CSSI), to identify the nucleus in 3D TPM reconstructions in flow cytometry. To this aim, we employ the Learning Tomography (LT) reconstruction algorithm29 on holographic images recorded through a TPM system in flow cytometry condition30,31. Our method is completely different from the others followed so far22–28, since it avoids the learning step and exploits a robust ad hoc clustering algorithm, i.e., it recognizes statistical similarities among groups of nucleus voxels. The output of the CSSI algorithm is the convex hull that overlaps to the cell nucleus, i.e., the smallest convex set that, on average, contains it. For this reason, hereafter we will refer to the segmented region as nuclear organelle convex hull (OCH). However, since the nucleus has mostly a convex shape, a potential misestimation of the nuclear shape is negligible. To the best of our knowledge, for the first time we are posing the problem of delineating the stain-free nucleus in 3D within single suspended cells flowing in a microfluidic cytometer. Beyond the numerical assessment through virtual 3D cell phantoms, we show that the CSSI algorithm can fill the specificity gap with 2D FM cyto-fluorimetry and with 3D FM confocal microscopy. In addition, CSSI allows for direct measurements at the stain-free nuclear level of intrinsic 3D parameters (morphology, RI, and their derivatives, like dry-mass) correlated to cell physiology and health state11, thus providing a whole label-free quantitative characterization exploitable for analyzing large numbers of flowing single-cells32.
Results
Description and assessment of the CSSI method
To reconstruct the 3D RI distribution at the single-cell level in-flow, the TPM system in flow cytometry condition sketched in Fig. 2a has been employed, which is described in the Methods section along with the numerical processing summarized in Fig. 2b. Since the sub-cellular structures (i.e. organelles) often cannot be detected within the tomogram by conventional RI-based thresholding methods, the CSSI algorithm is proposed to identify the nuclear OCH.
Fig. 2. TPM in flow cytometry technique.
a Sketch of the in-flow TPM setup based on a digital holographic microscope in off-axis configuration. BSF, Beam Splitter Fiber; MP, Microfluidic Pump; MC, Microfluidic Channel; OB, Object Beam; MO, Microscope Objective; BE, Beam Expander; RB, Reference Beam; BC, Beam Combiner; PC, Personal Computer. According to the reference system, cells flow along the y-axis, rotate around the x-axis, and are illuminated along the z-axis. b Block diagram of the holographic processing pipeline to reconstruct the stain-free 3D RI tomograms of flowing and rotating single-cells. Multiple digital holograms are recorded around the cell, from which the corresponding QPMs are numerically retrieved. The pose of each flowing cell is calculated by the 3D holographic tracking method and the rolling angles recovery approach. Finally, LT algorithm is exploited to enhance the initial tomographic reconstruction obtained by the inverse Radon transform.
To validate the CSSI method, we preliminarily tested and assessed it on a 3D numerical cell phantom simulation, modelled with the cell membrane, nucleus, cytoplasm, and mitochondria, as shown in Fig. 3a (see the Methods section). As reported by the histogram in Fig. 3b, a RI distribution has been assigned to each of the four sub-cellular structures. This 3D numerical cell phantom has been used to assess our CSSI algorithm for nucleus segmentation. However, nucleus segmentation is only one case of a more general technique which in principle can segment any kind of subcellular structure with a suitable spatial resolution, because it only exploits the hypothesis of knowing the location of a group of voxels belonging to the organelle to be segmented, considered as the initial reference set. In fact, the CSSI method is based on the Wilcoxon–Mann–Whitney (WMW) test35,36, that is a statistical test we use to reject or not the hypothesis for which a test set has been drawn from the same distribution as the designed reference set. In particular, the steps depicted in the scheme in Fig. 3c are performed as follows
Rough clustering of the organelle voxels, exploiting the WMW test to infer the statistical similarity between different voxel clouds (i.e. the test sets) and a certain voxel cloud (i.e. the initial reference set) which contains the voxels supposed to belong to the organelle of interest.
Filtering of the outlier organelle voxels when they are too far away from the centroid of the rough organelle cluster in terms of both geometric and statistical distances.
Refinement of the filtered organelle cluster to improve its external shape by adding/removing smaller voxel clouds.
Filling of the holes and smoothing of the corners of the refined organelle cluster by common morphological operators.
Fig. 3. Numerical assessment of the CSSI algorithm applied to segment the 3D nuclear OCH from a 3D numerical cell phantom (Supplementary Video 1).
a Isolevels representation of the 3D cell model, simulated with four sub-cellular components, i.e., cell membrane, cytoplasm, nucleus, and 18 mitochondria. b Histogram of the RI values assigned to each simulated sub-cellular structure in (a). The red arrow at the top highlights the RI values assigned to the transition region between the nucleus and cytoplasm. c Block diagram of the CSSI method to segment the nuclear OCH from a stain-free 3D RI tomogram. d Visual comparison between the simulated 3D nucleus and the 3D nuclear OCH segmented from the simulated RI tomogram in (a). The simulated nucleus and the segmented nucleus are marked in red within the blue cell shell. The clustering performances obtained in this simulation are reported below (see their definitions in Table S3).
Notice that, whenever the WMW test is used, the reference set is randomly selected from the last estimation of the organelle cluster until that moment, to match its dimensionality with that of the test set, thus preserving the fairness of the statistical test. Due to this random selection, by repeating several times the described steps, at each iteration j = 1,2, …, K we can obtain a slightly different estimation of the OCH. The output of each iteration is a binary valued 3D volume whose non-null values correspond to the voxels associated with the organelle. Therefore, the sum of all the K outputs provides a tomogram of occurrences, from which the probability that a voxel belongs to the organelle can be inferred through a normalization operation. Finally, the OCH is identified by a suitable probability threshold. A detailed description of the CSSI algorithm is reported in Section S1, Figs. S1,S2, and Table S1 in the Supplementary Information. In this paper we focus on the problem of stain-free nucleus segmentation, therefore we associate the initial reference set to the central voxels of the cell. Indeed, for many kinds of suspended cells, the central voxels belong to the nucleus. This property occurs especially in the case of cancer cells. This is confirmed by the 2D images of the neuroblastoma cells recorded through a FM cyto-fluorimeter (see Fig. S3), by the 3D morphological parameters reported in the literature for MCF-7 cells imaged through a confocal microscope37, and more generally by the increase of the nucleus-cytoplasm ratio demonstrated in cancer cells38–42.
On the left in Fig. 3d, the sole simulated nucleus is reported in red within the blue cell shell, while on the right we show the nucleus segmented from the 3D numerical cell phantom through the CSSI algorithm. The visual comparison in Fig. 3d suggests that the proposed CSSI method allows segmenting a nucleus region very close to the original one, as also confirmed by the great quantitative performances reported below the tomograms. Moreover, to numerically assess the proposed 3D CSSI algorithm, it has been applied to reconstruct the nuclei of 1000 numerical cell phantoms simulated by randomly drawing their morphological and RI parameters from the distributions described in the Methods section and in Table S2.
The overall CSSI performances are summarized in the first column of Table S3 by means of 9 metrics, which corresponding histograms are displayed in blue in Fig. S4. It is worth underlying that, in order to simplify the description of the CSSI algorithm and highlight its results about the stain-free nucleus identification, so far we neglected the presence of the nucleolus within the 3D numerical cell phantoms. The extended analysis with the simulation of the nucleolus reported in Section S2 and Figs. S4,S5 in the Supplementary Information highlights that the presence of nucleoli inside the nucleus does not substantially deteriorate the performances of the CSSI nucleus segmentation algorithm (see the second column of Table S3 and the corresponding orange histograms in Fig. S4). For example, the accuracy passes from an average value of ACC = 96.28 % in the case without the nucleolus to an average value of ACC = 95.86 % in the case with nucleolus.
Experimental consistency with 2D FM cyto-fluorimetry
The proposed CSSI method has been used to retrieve the 3D nuclear OCHs from five stain-free human neuroblastoma cancer cells (SK-N-SH cell line), reconstructed by TPM in flow cytometry. The isolevels representation of an SK-N-SH cell is shown in Fig. 4a, highlighting in red the 3D segmented nuclear OCH within the blue cell shell. Moreover, its central slice is displayed in Fig. 4b, in which the segmented nucleus is marked by the red line, while in Fig. 4c we report in green the corresponding 3D RI histogram, separating in red and in blue the contributions of the 3D nuclear OCH and the 3D non-nucleus region, respectively. To experimentally assess the 3D segmentation technique, we digitally projected the segmented 3D TPM reconstruction back to 2D where the experimental 2D FM images are available for comparison. In particular, the segmented RI tomogram is digitally rotated from 0° to 150° with 30° angular step around x-, y-, and z-axes, and then its silhouettes along the z-, x-, and y-axes, respectively, are considered to create 2D TPM segmented projections, as sketched in Fig. 4a. According to the ray optics approximation, the phase measured by digital holography (DH) is directly proportional to the integral of the RI values along the direction perpendicular to the plane of the camera. In this way, 18 unlabelled QPMs were obtained. As shown in a re-projected QPM on the left in Fig. 4d, it is cumbersome to recognize a sub-cellular structuring since no label is employed. However, thanks to the proposed 3D CSSI algorithm, the region occupied by the nucleus can be also marked (red line) in the 2D TPM projection within the outer cell (blue line). We exploit this process to further assess the proposed segmentation algorithm, by comparing the 3D results obtained through the in-flow TPM technique with a conventional 2D FM cyto-fluorimeter (i.e. ImageStreamX, see the Methods section). The latter has been used to record 11549 2D FM images of flowing SK-N-SH single cells, in which the nuclei have been stained through fluorescent dyes. On the right in Fig. 4d, the bright-field image of an SK-N-SH cell has been combined with the corresponding fluorescent image of the marked nucleus, therefore the false-color visualization makes the nucleus easily distinguishable (red line) with respect to the outer cell (blue line). ImagesStreamX can record a single random 2D image for each cell since it goes through the field of view (FOV) once. Instead, TPM allows the 3D tomographic reconstruction of a single cell. Through the reprojection process, we simulate the transition of the reconstructed cell within the ImageStreamX FOV at different 18 3D orientations with respect to the optical axis. In this way, we digitally replicate the ImageStreamX recording process and we also increase the dataset of 2D TPM images avoiding a high correlation between the reprojections of the same cell, thanks to the choice of a big angular step (i.e., 30°). Hence, in the 3D scatter plot in Fig. 4e, we quantitively compare some 2D morphological parameters representative of nucleus size, nucleus shape, and nucleus position, i.e. nucleus-cell area ratio (NCAR), nucleus aspect ratio (NAR), and normalized nucleus-cell centroid distance (NNCCD), respectively, measured from both 90 TPM images (red dots) and 11549 FM images (blue dots). In particular, we computed the NAR as the ratio between the minor axis and the major axis of the best-fitted ellipse to the nucleus surface, while the nucleus-cell centroid distance refers to 2D centroids and has been normalized to the radius of a circle having the same area of the cell, thus obtaining NNCCD. The 3D scatter plot highlights the very good agreement between TPM and FM 2D nuclear features since the TPM red dots are completely contained within the FM blue cloud. In addition, by using the one-sample multivariate Hotelling's T2 test43 between TPM and FM measurements about NCAR, NAR, and NNCCD, we also obtained a high p-value, i.e. 0.962, according to which it is not rejected with high confidence level the hypothesis that TPM and FM 2D nuclear features have been drawn from the same distributions. This quantitative comparison is summarized in Table S4. Moreover, to better visualize the 3D scatter plot in Fig. 4e, we split it into three different 2D scatter plots, shown in Figs. 4f-h.
Fig. 4. Experimental assessment of the CSSI algorithm applied to segment the 3D nuclear OCHs from unlabelled in-flow TPM reconstructions of five SK-N-SH cells, by comparison with the morphological parameters measured through a 2D FM cyto-fluorimeter (Supplementary Video 2).
a 3D segmented nucleus (red) within the 3D cell shell (blue) of an SK-N-SH cell reconstructed by TPM. The segmented tomogram is rotated around the x-, y-, and z-axes (orange arrows) and then reprojected along the z-, x-, and y-axes (white arrows), thus obtaining 2D TPM segmented projections in xy-, yz-, and xz-planes, respectively. b Central slice of the isolevels representation in (a), with nucleus marked by the red line. c RI histogram of the SK-N-SH cell in (a,b) reconstructed by 3D in-flow TPM (green), along with the RI distributions of its 3D nuclear OCH (red) and non-nucleus region (blue) segmented by CSSI algorithm. d 2D segmented projection with nucleus (red line) and non-nucleus (blue line) regions, obtained (on the left) by reprojecting 3D unlabelled TPM RI reconstruction in (a,b) and (on the right) by recording 2D labelled FM images. The scale bar is 5 μm. e 3D scatter plot of nucleus size vs. nucleus shape vs. nucleus position measured in 11549 FM (blue dots) and 90 TPM (red dots) 2D projections. Nucleus size is NCAR, nucleus shape is NAR, and nucleus position is NNCCD. f-h 2D scatter plots of nucleus size vs. nucleus shape, nucleus size vs. nucleus position, and nucleus shape vs. nucleus position, respectively, containing the same points in (e).
Experimental consistency with 3D FM confocal microscope
For the second experimental assessment, three stain-free human breast cancer cells (MCF-7 cells) have been reconstructed by TPM in flow cytometry and then segmented by the CSSI method, as shown in the example in Figs. 5a,b. In particular, the nucleus shell is marked in red within the blue cell shell in the isolevels representation of Fig. 5a, which segmented central slice is displayed in Fig. 5b. Moreover, in Fig. 5c, we display in green its 3D RI histogram, also separating the RI distribution of the 3D nuclear OCH (red) and the 3D non-nucleus region (blue). In this case, the experimental assessment is based on a quantitative comparison with the 3D morphological parameters measured in ref. 37, in which a confocal microscope has been employed to find differences between viable and apoptotic MCF-7 cells through 3D morphological features extraction. In this study, 206 suspended cells were stained with three fluorescent dyes to measure average values and standard deviations of 3D morphological parameters about the overall cell and its nucleus and mitochondria. A synthetic description of 3D nucleus size, shape, and position is given by nucleus-cell volume ratio (NCVR), nucleus surface-volume ratio (NSVR), and normalized nucleus-cell centroid distance (NNCCD), respectively. In particular, in this case, the nucleus-cell centroid distance refers to 3D centroids and has been normalized with respect to the radius of a sphere having the same cell volume, thus obtaining NNCCD. Moreover, it is worth underlining that NCVR and NSVR are direct measurements reported in ref. 37, while NNCCD is an indirect measurement since it has been computed by using the direct ones in ref. 37. In the 2D scatter plots in Figs. 5d-f regarding nucleus size, shape, and position, the three TPM measurements (red dots) are reported along with three blue rectangles, which are the intervals μ ± 1 σ, μ ± 2 σ, and μ ± 3 σ, with μ the average value and σ the standard deviation of the same parameters measured by FM confocal microscope. These scatter plots highlight a very good agreement between the 3D nucleus identified in labelled static MCF-7 cells by confocal microscope and the 3D nucleus segmented in unlabelled flowing MCF-7 cells by the proposed CSSI algorithm. In fact, all the TPM values are located in the 1σ-interval around the FM average values, except for shape measurement, that is anyway located in the 2σ-interval around the FM average value (Figs. 5d,f). The values shown in Figs. 5d-f are summarized in Table S5.
Fig. 5. Experimental assessment of the CSSI algorithm applied to segment the 3D nuclear OCHs from unlabelled in-flow TPM reconstructions of three MCF-7 cells, by comparison with the morphological parameters measured through a 3D FM confocal microscope (Supplementary Video 2).
a 3D segmented nuclear OCH (red) within unlabelled 3D cell shell (blue) reconstructed through in-flow TPM. b Central slice of the isolevels representation in (a), with nucleus marked by the red line. c RI histogram of the MCF-7 cell in (a,b) reconstructed by 3D in-flow TPM (green), along with the RI distributions of its 3D nuclear OCH (red) and non-nucleus region (blue) segmented by CSSI algorithm. d-f Scatter plots of nucleus size vs. nucleus shape, nucleus size vs. nucleus position, and nucleus shape vs. nucleus position, respectively, measured in three segmented TPM MCF-7 nuclei (red dots) along with the corresponding FM intervals (blue rectangles) around the average values, with half-width 1σ, 2σ, and 3σ (σ is the standard deviation of the measurements). Nucleus size is NCVR, nucleus shape is NSVR, and nucleus position is NNCCD.
Discussion
In this paper, we introduce and discuss an entirely new strategy for bridging the gap between FM and TPM in terms of subcellular specificity. In particular, we demonstrate for the first time the capability to identify the cell nucleus from 3D phase-contrast tomograms in unstained cells analysed in a flow cytometry modality. Exploiting the learning-based tomographic reconstruction algorithm, we developed the CSSI method to identify a stain-free organelle region. The numerical simulations yielded great performances in accomplishing the task of nuclei extraction and measurement by our new proposed approach. In addition, we performed the CSSI experimental assessment by retrieving the stain-free nuclei for two distinct cancer cell lines starting from the 3D RI reconstructions of the TPM system in flow cytometry, and we discussed the consistency with the classical FM microscopic methods (see green dashed lines in Fig. 1).
To provide a general overview of the differences, advantages, and limitations of the main methods that aim at introducing nucleus specificity, in Table 1 we compare the most significant efforts towards this goal by just underlying the field of application of each listed technique in respect of what was claimed in the related work. Therefore, each cell of Table 1 is filled with flags or crosses depending on whether the corresponding method possesses or lacks a certain attribute, each of them being highly pursued in the bioimaging field. PhaseStain22, PICS25, and HoloStain26 are virtual staining-based methods which use AI (Generative Adversarial Networks) to emulate fluorescence in a stain-free manner, which is certainly one of the most interesting approaches shown lately to overcome the main limitation of QPI towards the organelle specificity. In the 3D case, the most promising approach to add specificity to TPM is based on a Convolutional Neural Network27,28, which so far has been validated by identifying cells’ nuclei and other subcellular structures in label-free modality for adherent samples. Again, the approach relies on AI to segment the nucleus. In this sense, we believe the CSSI method is very promising to promote label-free TPM with nucleus specificity since it addresses all the required attributes shown in Table 1. In particular, the proposed CSSI algorithm allows in-flow TPM to reach the same results of 2D FM cyto-fluorimeter, but without using dyes and preserving its high-throughput property. Furthermore, the TPM reprojections are much more informative than the FM images (Fig. 4d). Indeed, the phase values contain a quantitative measurement about both the 3D sub-cellular morphology and RI distribution, which can be associated to the cell biology, instead of the 2D FM images, from which the sole 2D morphological parameters can be inferred. Similarly, besides the 3D morphological analysis of the confocal microscopy, in our technology a complete 3D label-free quantitative characterization of the RI-based fingerprint at the sub-cellular single-cell level is possible, as reported in the histograms in Fig. 4c and Fig. 5c. Furthermore, the confocal microscope can only image static samples. Instead, in the range of fluorescent based methods, recently a light-sheet FM strategy has been implemented to retrieve volumetric imaging of single cells while they are flowing in microfluidic circuits33,34. Although promising compared with confocal microscopy, light-sheet FM combined to flow-cytometry is still qualitative and limited by the staining drawbacks as the a priori-knowledge of the target proteins, the phototoxicity and photobleaching.
Table 1. Properties of the methods for the nucleus identification.
As demonstrated in the present paper, the CSSI approach is an ad hoc clustering algorithm based on the computation of statistical similarities among groups of voxels inside the same cell. CSSI strength lies in completely avoiding training neural networks through FM images. This feature comes with two main associated advantages. i) Any network that learns from examples of fluorescence emissions is inherently biased by the labelling process itself (e.g., photobleaching and photodamaging) and thus cannot perform better than the ground-truth. ii) In the case of flowing biological cells in suspension, a voxel-level registration between 3D RI and 3D fluorescence is not obtainable, so that deep neural networks (DNNs) cannot learn from examples of tomograms pairs. In the absence of ground-truth, we rely on the statistical correlation between the tomogram voxels to calculate their associations to the cell regions. At the same time, our approach comes with two main drawbacks. i) The computational times are significantly higher than DNN inference times. ii) The accuracy of the estimated associations depends on the statistical significance of the tests the algorithm performs. Thus, a poor tomographic resolution can limit the accuracy in identifying small subcellular structures. However, we expect that a combined approach between deep learning and statistical inference could be successfully attempted in the future. For instance, CSSI could be used to generate a dataset of tomograms pairs to train a DNN to emulate the CSSI process and obtain real-time inference in nucleus identification in flowing cells. It is worth pointing out that, to date, the CSSI is the sole method able to retrieve the 3D nuclear specificity in stain-free suspended single-cells in flow cytometry mode, thus providing quantitative measurements at the sub-cellular level with statistical significance on a large number of cells by potentially exploiting the high-throughput property. Finally, the CSSI algorithm could be prospectively transferred to other scenarios. In fact, the sole property requested by the CSSI method consists in having different statistical distributions of the reconstructed quantity among the several intracellular organelles, as occurs for example in the RI case32. Therefore, the same method can be also applied to other flow cytometric tomographic phase imaging techniques44. In particular, the WMW statistical test is a non-parametric hypothesis test capable to disclose differences between the medians of two statistical distributions. Therefore, the proposed CSSI method is, in principle, able to segment a desired organelle whether the difference between its RI median value and the RI median values of all the other intracellular structures is greater than the RI resolution of the employed tomographic system. Thus, the CSSI is expected to work well when the signal-to-noise ratio is high enough to make perceptible the differences between the RI statistical distributions of the several cell organelles. In fact, in the considered simulations, the RI values were rounded at the 3rd decimal place to investigate the CSSI robustness against the noise in the worst resolution case. Of course, a higher signal-to-noise ratio (i.e., a better RI resolution) would improve the CSSI performances.
Furthermore, the steps of the CSSI algorithm described in Section S1 have been developed around the discussed statistical working principle at the aim of identifying a single compact organelle having a distinctive RI distribution inside the cell, and it has been demonstrated for segmenting the nucleus. However, in view of the statistical strategy adopted, the CSSI algorithm could be in principle adapted to investigate other cell types and single compact organelles. To prove this, we demonstrated that, by only changing the starting reference cube CR, the same pipeline is able to provide also the stain-free nucleolus segmentation in case of cells having a single nucleolus (see Section S3, Figs. S6,S7, and the third column of Table S3 in the Supplementary Information). Actually, the main factor that could limit the success of the CSSI algorithm is a low imaging spatial resolution with respect to the size of the analyzed organelle, which means that the stain-free organelle must be represented by a suitable number of voxels (see the analysis in Section S3 and Fig. S8 in the Supplementary Information). Future experiments will be dedicated to the application of the CSSI algorithm for identifying other single stain-free intracellular organelles and to its extension to multiple stain-free intracellular organelles through the introduction of slight changes of the CSSI algorithm to avoid the constraints herein used to gather all the candidate cubes at the aim of segmenting a single compact region, i.e. the nucleus.
In conclusion, we believe that, thanks to the above-mentioned properties, the CSSI algorithm combined with the TPM in flow cytometry condition could open a new route for label-free microscopy as biomedical tool. By starting from this conceptually ground breaking strategy, such a tool could revolutionize cancer diagnosis45–47, e.g. through the search for circulating tumour cells in the liquid biopsy paradigm48, or more in general affirm TPM as a viable method for intracellular quantitative characterization at the single-cell level, which can be further exploited for therapeutic purposes in personalized medicine49.
Methods
Sample preparation
The human breast cancer cells (MCF-7 cell line) and the human neuroblastoma cells (SK-N-SH cell line) were selected for tomographic experiments. The MCF-7 and the SK-N-SH cells were cultured in RPMI 1640 and in Minimum Essential Medium Eagle (MEM) from Sigma Aldrich, respectively. Both cell culture media were supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 μg ml−1 streptomycin, and 100 U ml−1 penicillin. Then, cells were collected from the Petri dish by incubation for 5 min with a 0.05% trypsin–EDTA solution (Sigma, St. Louis, MO). Finally, the MCF-7 and the SK-N-SH cells were centrifuged for 5 min at 125 x g, resuspended in complete medium, and injected into the microfluidic channel for the TPM experiment in flow cytometry condition. The SK-N-SH cells were also analysed through a FM cyto-fluorimeter. To this aim, 7 million SK-N-SH cells were resuspended in Phosphate Buffered Saline (PBS), 1X (Sigma) and exposed to 25 μM DRAQ5 Fluorescent Probe for 5 min at room temperature under agitation (#62254, Thermo Scientific™).
Tomographic phase microscopy setup in flow cytometry condition
In a QPM, phase-contrast is due to the optical path length difference between the unlabelled biological specimen and its background because of the combination of its thickness and its RI2. These two quantities can be decoupled by recording multiple 2D QPMs at different viewing angles around the sample, thus performing the 3D TPM. However, unlike conventional TPM methods, in our setup the DH microscope acquires multiple digital holograms of flowing and rotating cells within a microfluidic channel, exploiting the hydrodynamic forces produced by a laminar flow30,31. In fact, the light beam generated by the laser (Laser Quantum – Torus, emitting at a wavelength λ = 532 nm) is coupled into an optical fibre, which splits it into an object beam and a reference beam in order to constitute a Mach−Zehnder interferometer in off-axis configuration. The object beam exits from the fibre and is collimated to probe the biological sample that flows at 7 nL/s along a commercial microfluidic channel with cross section 200 μm × 200 μm (Microfluidic Chip-Shop). The flux velocity is controlled by a pumping system (CETONI – neMESYS) that ensures temporal stability of the parabolic velocity profile into the microchannel. The wavefield passing throughout the sample is collected by the Microscope Objective (Zeiss 40× – oil immersion – 1.3 numerical aperture) and directed to the 2048 × 2048 CMOS camera (USB 3.0 U-eye, from IDS) by means of a Beam-Splitter that allows the interference with the reference beam. The interference patterns of the single cells rotating into a 170 μm × 170 μm FOV are recorded at 35 fps. The proposed TPM system in flow cytometry has the advantage to work in label-free modality, so the sample preparation time related to the nucleus staining is completely skipped. Instead, the experiment to acquire 1000 cells can take 30-60 minutes, since tens of images per cell must be recorded to retrieve their 3D tomographic reconstruction. The described opto-fluidic setting is able to provide potentially a throughput of about 100 cells/minute. It is important to underline that the maximum throughput achievable by a tomographic system of rotating cells in continuous flow is theoretically much lower than the conventional imaging flow cytometer. In fact, in order to retrieve the tomographic data, one needs to collect from tens to hundreds images per cell instead of a single snapshot. This automatically reduces the throughput of the in-flow TPM system by one or two orders of magnitude with respect to the conventional imaging flow cytometer. On the other hand, our system does not need the cells to be in microfluidic focusing, thus multiple cells can be simultaneous imaged within the same FOV (e.g. by using a larger camera sensor and/or by increasing the cell concentration) in order to increase the throughput. Moreover, also the flow rate can be increased over 7 nL/sec but with the constraint to avoid cell deformations and to guarantee that a full cell rotation occurs within the imaged FOV. Finally, it is possible to use multiple parallel microfluidic channels on the same chip, proportionally increasing the throughput. Actually, some of these opto-fluidics solutions to increase the throughput have been exploited in our very recent work50.
According to the reference system sketched in Fig. 2a, cells flow along the y-axis and continuously rotate around the x-axis thanks to the microfluidic properties51, while their holograms are recorded along the z-axis. Then, as summarized in Fig. 2b, numerical operations are performed to reconstruct the stain-free 3D RI tomograms of the recorded flowing single-cells. The off-axis configuration allows demodulating each hologram of the recorded sequence by filtering the real diffraction order52. Then, the 3D positions of the flowing cells within the microfluidic channel are computed through a holographic tracking algorithm53. Each demodulated hologram is numerically propagated along the z-axis through the Angular Spectrum formula52 in order to minimize a contrast-based metric, i.e. the Tamura Coefficient53 (TC), to recover the z-position of the cell and refocus it. The QPMs are then obtained by implementing the phase unwrapping algorithm52 on the corresponding refocused complex wavefronts. In each QPM, the weighted centroids provide the xy-positions of the cell, which are used to center it in its cropped region of interest (ROI), as shown in Fig. 4d, thus avoiding motion artefacts in the final 3D tomogram. Moreover, the microfluidic properties and the high frame rate allow to linearize the relationship between the angular and the translational speeds51. Therefore, the K unknown rolling angles ϑK are estimated by using the computed y-positions as follows
| (1) |
where K = 1, …, K is the frame index. The f180 value is the index of the frame at which the cell has rotated of 180° with respect to the first frame of the sequence. It is computed by minimizing the Tamura Similarity Index (TSI), that is a phase image similarity metric based on the evaluation of the local contrast calculated on all the QPMs of the rolling cell though the TC. The tomographic reconstruction is firstly obtained by the inverse Radon transform, and it is then enhanced though the LT algorithm.
Learning Tomography algorithm
LT is an iterative reconstruction algorithm based on a nonlinear forward model, beam propagation method (BPM), to capture high orders of scattering29. Using the BPM, we propagate an incident light illumination on an initial guess acquired by the inverse Radon transform and compare the resulting field with the experimentally recorded field. The error between the two fields is backpropagated to calculate the gradient54. At each iteration of LT, the gradient calculation is repeated for 8 randomly selected rotation angles, and the corresponding gradients are rotated and summed to update the current solution. As an intermediate step, the total variation regularization was employed. The total iteration number is 200 with a step size of 0.00025 and a regularization parameter of 0.005. In order to run LT, we need two electric fields, incident and total electric fields. The amplitude of the incident field was estimated from the amplitude of the total electric field by low-pass filtering in the Fourier domain with a circular aperture whose radius is 0.176 k0, where given a wavelength λ = 532 nm in a vacuum. In other words, we assume that the high-frequency information in the amplitude of the total electric field was only attributed to the light interference caused by a sample when illuminated by an illumination with slowly varying amplitude.
FM cyto-fluorimeter
In order to record thousands of 2D FM images, a commercial multispectral flow cyto-fluorimeter has been employed, i.e., Amnis ImageStreamX®. Cells are hydrodynamically focused within a micro-channel, and then they are probed both by a transversal brightfield light source and by orthogonal lasers. The fluorescence emissions and the light scattered and transmitted from the cells are collected by an objective lens. After passing through a spectral decomposition element, the collected light is divided into multiple beams at different angles according to their spectral bands. The separated light beams propagate up to 6 different physical locations of one of the two CCD cameras (256 rows of pixels), which operates in time operation. Therefore, the image of each single flowing cell is decomposed into 6 separate sub-images on each of the two CCD cameras, based on their spectral band, thus allowing the simultaneous acquisition of up to 12 images of the same cell, including brightfield, scatter, and multiple fluorescent images. Hence, Amnis ImageStreamX® combines the single-cell analysis of the standard FM microscopy with the statistical significance due to large number of samples provided by standard flow-cytometry.
ImageStreamX Mark II Flow Cytometer (Luminex Corporation) was used to acquire 11549 single cells images at 60× magnification. For each single cell, we recorded two simultaneous images, i.e., a brightfield image of the flowing cell and its corresponding FM image. To segment nucleus, we analysed the single cells using the IDEAS software (version 6.2.64.0), which combines fluorescence intensity (DRAQ5) and morphometric measures (darkfield), generating a global threshold of the FM signal corresponding to the nucleus size. The automatic calibration provided by the instrument, corresponding to 0.33 μm/pixel, was employed to measure 2D morphological parameters related to the cell and the nucleus, in order to realize a quantitative comparison with the tomographic reconstructions. Three of the recorded brightfield and fluorescent images are shown at the top and at the bottom of Fig. S3, respectively, with overlapped in red the contour of the segmented nuclei. In the FM cyto-fluorimeter experiments, the sample preparation step takes ~ 45 minutes, consisting of adding a specific stain (DRAQ5) to label nuclei, while the experiment to collect 1000 images (one per cell) takes at least 5 minutes.
3D numerical cell phantom
In ref. 37, a confocal microscope has been employed to find differences between viable and apoptotic MCF-7 cells through 3D morphological features extraction. In particular, 206 cells were stained with three fluorescent dyes in order to measure the average value and standard deviation of 3D morphological parameters about the overall cell and its nucleus and mitochondria. We exploit these measurements to simulate a 3D numerical cell phantom, by setting 1 px = 0.12 μm. It is made of four sub-cellular structures, i.e., cell membrane, cytoplasm, nucleus, and mitochondria. We shape cell, nucleus, and mitochondria as ellipsoids, then we make irregular the cell external surface, and finally we obtain cytoplasm through a morphological erosion of the cell shape. Moreover, in each simulation, the number of mitochondria is drawn from the uniform distribution U1{a1, b1}. A 3D numerical cell phantom is displayed in Fig. 3a, in which 18 mitochondria have been simulated. To each simulated 3D sub-cellular component, we assign a RI distribution, as shown by the RI histogram in Fig. 3b. Measuring accurate RI values at sub-cellular level is still a deeply debated topic55,56. Hence, we cannot replicate realistic RIs since they are not yet well known, therefore we simulate the unfavourable case for our testing purpose segmenting the nucleus from cytoplasm, i.e., we model overlapped subcellular distributions of the RI values. In particular, for each cell membrane voxel, we draw its RI from distribution N1(μ1, σ2). Instead, without knowing if the nucleus RIs are greater than the cytoplasm ones or vice versa, in each simulation we randomly assign cytoplasm and nucleus to distributions N2(μ2, σ2) or N3(μ3, σ2). It is worth remarking that, to strengthen the numerical assessment, we increase the randomness of the RI assignments among the different simulations, because each voxel belonging to cell membrane, nucleus, and cytoplasm is drawn from gaussian distributions N1, N2, and N3 (or N1, N3, and N2), respectively, which average values μ1, μ2, and μ3 are in turn drawn from other gaussian distributions for each voxel extraction, i.e. , and , respectively. Instead, as regards mitochondria, each of them has a RI gaussian distribution N4(μ4, σ2) which average value μ4 is drawn from the gaussian distribution Nμ4(μμ4, ) for each mitochondrion and not for each voxel. Moreover, we create a RI transition zone straddling the nucleus to cytoplasm, thus avoiding any discontinuity that could somehow facilitate the segmentation. In particular, after drawing all nucleus and cytoplasm values, RIs that are in the middle of their average values are assigned to the voxels of the transition zone, as highlighted by the red arrow at the top of Fig. 3b. This transition zone is obtained through morphological erosion and dilation of the nucleus ellipsoid, by using a spherical structuring element, which radius is drawn from the uniform distribution U2{a2, b2} px for each simulation, thus resulting in an internal nucleus volume that is about 85-95 % of the total nucleus volume. In the example in Figs. 3a,b, a 3 px radius has been selected. All the described parameters are reported in Table S2.
Supplementary Material
Acknowledgements
This work was funded by a PRIN 2017 project (Morphological Biomarkers for early diagnosis in Oncology – MORFEO, Prot. 2017N7R2CJ) received by P.F., and by a project from Swiss National Science Foundation (SNF 2200021_188419) and by the Innosuisse project (34247.1 IP-ENG) received by D.Ps.
Footnotes
Author contributions
L.M. and F.M. designed and conducted the holographic experiments; M.M., F.C. and A.M. took care of cell culture; D.Pi., P.M., J.L., V.B. designed and performed the numerical analysis. M.C. and A.I. contributed to conceive the experiments from biomedical point of view and validated the results of the experiments; F.V. and F.C. conducted the cytofluorimeter measurements and provided data; D.Ps. and P.F. coordinated and guided the research.
D.Pi., P.M., L.M., V.B., J.L., P.F., D.Ps. prepared the manuscript.
All authors supported data analysis and interpretation.
All authors reviewed the manuscript.
Competing interests
D.Pi., J.L., L.M., V.B., P.M., D.Ps., P.F. have filed a patent pending (application number IT102021000019490) about some key aspects described in the paper.
The authors declare no other competing interests.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The codes are available from the corresponding authors upon reasonable request.
References
- 1.Lichtman J, Conchello JA. Fluorescence Microscopy. Nat Methods. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 2.Park Y, Depeursinge C, Popescu G. Quantitative phase imaging in biomedicine. Nat Photonics. 2018;12:578–589. [Google Scholar]
- 3.Merola F, et al. Diagnostic tools for lab-on-chip applications based on coherent imaging microscopy. Proc IEEE. 2015;103(2):192–204. [Google Scholar]
- 4.Cotte Y, et al. Marker-free phase nanoscopy. Nat Photonics. 2013;7:113–117. [Google Scholar]
- 5.Yoffe GD, Mirsky SK, Barnea I, Shaked NT. High-resolution 4-D acquisition of freely swimming human sperm cells without staining. Sci Adv. 2020;6(15):eaay7619. doi: 10.1126/sciadv.aay7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemper B, et al. Label-free quantitative cell division monitoring of endothelial cells by digital holographic microscopy. J Biomed Opt. 2010;15(3):036009. doi: 10.1117/1.3431712. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Motility-based label-free detection of parasites in bodily fluids using holographic speckle analysis and deep learning. Light Sci Appl. 2018;7(1):1–18. doi: 10.1038/s41377-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo Y, et al. Quantitative Phase Imaging and Artificial Intelligence: A Review. IEEE J Sel Top Quantum Electron. 2019;25(1):1–14. [Google Scholar]
- 9.Zhang JK, He YR, Sobh N, Popescu G. Label-free colorectal cancer screening using deep learning and spatial light interference microscopy (SLIM) APL Photonics. 2020;5(4):040805. doi: 10.1063/5.0004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim G, Jo Y, Cho H, Min HS, Park YK. Learning-based screening of hematologic disorders using quantitative phase imaging of individual red blood cells. Biosens Bioelectron. 2019;123:69–76. doi: 10.1016/j.bios.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 11.Zangle TA, Teitell MA. Live-cell mass profiling: an emerging approach in quantitative biophysics. Nat Methods. 2014;11(12):1221–1228. doi: 10.1038/nmeth.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin D, Zhou R, Yaqoob Z, So P. Tomographic phase microscopy: Principles and applications in bioimaging. J Opt Soc Am B. 2017;34(5):B64–B77. doi: 10.1364/josab.34.000b64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y, et al. Profiling individual human red blood cells using common-path diffraction optical tomography. Sci Rep. 2014;4:6659. doi: 10.1038/srep06659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuś A, Dudek M, Kemper B, Kujawińska M, Vollmer A. Tomographic phase microscopy of living three-dimensional cell cultures. J Biomed Opt. 2014;19:046009. doi: 10.1117/1.JBO.19.4.046009. [DOI] [PubMed] [Google Scholar]
- 15.Sung Y, Choi W, Lue N, Dasari RR, Yaqoob Z. Stain-Free Quantification of Chromosomes in Live Cells Using Regularized Tomographic Phase Microscopy. PLoS ONE. 2012;7(11):e49502. doi: 10.1371/journal.pone.0049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon J, et al. Label-free characterization of white blood cells by measuring 3D refractive index maps. Biomed Opt Express. 2015;6:3865–3875. doi: 10.1364/BOE.6.003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, et al. Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes. Sci Rep. 2016;6:36815. doi: 10.1038/srep36815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, et al. Optical diffraction tomography techniques for the study of cell pathophysiology. J Biomed Photonics Eng. 2016;2:020201 [Google Scholar]
- 19.Pirone D, et al. Three-Dimensional Quantitative Intracellular Visualization of Graphene Oxide Nanoparticles by Tomographic Flow Cytometry. Nano Lett. 2021;21:5958–5966. doi: 10.1021/acs.nanolett.1c00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, et al. Dehydration of plant cells shoves nuclei rotation allowing for 3D phase-contrast tomography. Light: Sci Appl. 2021;10:187. doi: 10.1038/s41377-021-00626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, et al. Advanced Imaging and Bio Techniques for Convergence Science. Springer; Singapore: 2021. Holotomography: refractive index as an intrinsic imaging contrast for 3-D label-free live cell imaging; pp. 211–238. [DOI] [PubMed] [Google Scholar]
- 22.Rivenson Y, et al. PhaseStain: the digital staining of label-free quantitative phase microscopy images using deep learning. Light: Sci Appl. 2019;8:23. doi: 10.1038/s41377-019-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borhani N, Bower AJ, Boppart SA, Psaltis D. Digital staining through the application of deep neural networks to multi-modal multi-photon microscopy. Biomed Opt Express. 2019;10:1339–1350. doi: 10.1364/BOE.10.001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivenson Y, et al. Virtual histological staining of unlabelled tissue-autofluorescence images via deep learning. Nat Biomed Eng. 2019;3(6):466–477. doi: 10.1038/s41551-019-0362-y. [DOI] [PubMed] [Google Scholar]
- 25.Kandel ME, et al. Phase imaging with computational specificity (PICS) for measuring dry mass changes in sub-cellular compartments. Nat Commun. 2020;11:6256. doi: 10.1038/s41467-020-20062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nygate YN, et al. Holographic virtual staining of individual biological cells. Proc Natl Acad Sci USA. 2020;117:9223–9231. doi: 10.1073/pnas.1919569117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, et al. Deep-Learning-Based Label-Free Segmentation of Cell Nuclei in Time-Lapse Refractive Index Tomograms. IEEE Access. 2019;7:83449–83460. [Google Scholar]
- 28.Jo Y, et al. Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning. Nat Cell Biol. 2021;23:1329–1337. doi: 10.1038/s41556-021-00802-x. [DOI] [PubMed] [Google Scholar]
- 29.Merola F, et al. Tomographic flow cytometry by digital holography. Light Sci Appl. 2017;6:e16241. doi: 10.1038/lsa.2016.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villone MM, et al. Full-angle tomographic phase microscopy of flowing quasi-spherical cells. Lab Chip. 2018;18(1):126–131. doi: 10.1039/c7lc00943g. [DOI] [PubMed] [Google Scholar]
- 31.Lim J, Goy A, Shoreh MH, Unser M, Psaltis D. Learning tomography assessed using Mie theory. Phys Rev Appl. 2018;9(3):034027 [Google Scholar]
- 32.Liu Y, et al. Cell Refractive Index for Cell Biology and Disease Diagnosis: Past, Present and Future. Lab Chip. 2016;16:634–644. doi: 10.1039/c5lc01445j. [DOI] [PubMed] [Google Scholar]
- 33.Gualda EJ, Pereira H, Martins GG, Gardner R, Moreno N. Three-dimensional imaging flow cytometry through light-sheet fluorescence microscopy. Cytometry A. 2017;91(2):144–151. doi: 10.1002/cyto.a.23046. [DOI] [PubMed] [Google Scholar]
- 34.Han Y, et al. Cameraless high-throughput three-dimensional imaging flow cytometry. Optica. 2019;6(10):1297–1304. [Google Scholar]
- 35.Mann HB, Whitney DR. On a test of whether one of 2 random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 36.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 37.Wen Y, et al. Quantitative analysis and comparison of 3D morphology between viable and apoptotic MCF-7 breast cancer cells and characterization of nuclear fragmentation. PLoS ONE. 2017;12(9):e0184726. doi: 10.1371/journal.pone.0184726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntire PJ, et al. Digital image analysis supports a nuclear-tocytoplasmic ratio cutoff value below 0.7 for positive for high-grade urothelial carcinoma and suspicious for high-grade urothelial carcinoma in urine cytology specimens. Cancer Cytopathol. 2019;127(2):120–124. doi: 10.1002/cncy.22061. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, et al. Machine learning of diffraction image patterns for accurate classification of cells modeled with different nuclear sizes. J Biophotonics. 2020;13(9):e202000036. doi: 10.1002/jbio.202000036. [DOI] [PubMed] [Google Scholar]
- 40.Moore MJ, Sebastian JA, Kolios MC. Determination of cell nucleus-to-cytoplasmic ratio using imaging flow cytometry and a combined ultrasound and photoacoustic technique: a comparison study. J Biomed Opt. 2019;24:106502. doi: 10.1117/1.JBO.24.10.106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 42.Takaki T, et al. Actomyosin drives cancer cell nuclear dysmorphia and threatens genome stability”. Nat Commun. 2017;8:16013. doi: 10.1038/ncomms16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems G, Pison G, Rousseeuw PJ, Van Aelst S. A robust Hotelling test. Metrika. 2002;55(1):125–138. [Google Scholar]
- 44.Sung Y, et al. Three-dimensional holographic refractive-index measurement of continuously flowing cells in a microfluidic channel. Phys Rev Appl. 2014;1(1):014002. doi: 10.1103/PhysRevApplied.1.014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Backman V, et al. Detection of preinvasive cancer cells. Nature. 2000;406:35–36. doi: 10.1038/35017638. [DOI] [PubMed] [Google Scholar]
- 46.Uttam S, et al. Early Prediction of Cancer Progression by Depth-Resolved Nanoscale Mapping of Nuclear Architecture from Unstained Tissue Specimens. Cancer Res. 2015;75:4718–4727. doi: 10.1158/0008-5472.CAN-15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Bista R, Bhargava R, Brand RE, Liu Y. Spatial-domain low-coherence quantitative phase microscopy for cancer diagnosis. Opt Lett. 2010;35(17):2840–2842. doi: 10.1364/OL.35.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miccio L, et al. Perspectives on liquid biopsy for label-free detection of “circulating tumor cells” through intelligent lab-on-chips. View. 2020;1(3):20200034 [Google Scholar]
- 49.Sung W, et al. Computational modeling and clonogenic assay for radioenhancement of gold nanoparticles using 3D live cell images. Radiat Res. 2018;190(5):558–564. doi: 10.1667/RR15134.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirone D, et al. Speeding up reconstruction of 3D tomograms in holographic flow cytometry via deep learning. Lab Chip. 2022;22:793–804. doi: 10.1039/d1lc01087e. [DOI] [PubMed] [Google Scholar]
- 51.Pirone D, et al. Rolling angles recovery of flowing cells in holographic tomography exploiting the phase similarity. Appl Opt. 2021;60(4):A277–A284. doi: 10.1364/AO.404376. [DOI] [PubMed] [Google Scholar]
- 52.Kim MK. Principles and techniques of digital holographic microscopy. SPIE Rev. 2010;1:018005-018048 [Google Scholar]
- 53.Memmolo P, et al. Recent advances in holographic 3d particle tracking. Adv Opt Photon. 2015;7:713–755. [Google Scholar]
- 54.Kamilov US, et al. Optical Tomographic Image Reconstruction Based on Beam Propagation and Sparse Regularization. IEEE Trans Comput Imaging. 2016;2(1):59–70. [Google Scholar]
- 55.Schürmann M, Scholze J, Müller P, Guck J, Chan CJ. Cell nuclei have lower refractive index and mass density than cytoplasm. J Biophotonics. 2016;9(10):1068–1076. doi: 10.1002/jbio.201500273. [DOI] [PubMed] [Google Scholar]
- 56.Yurkin MA. How a phase image of a cell with nucleus refractive index smaller than that of the cytoplasm should look like? J Biophotonics. 2018;11:e201800033. doi: 10.1002/jbio.201800033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
The codes are available from the corresponding authors upon reasonable request.