Abstract
OBJECTIVE
Down syndrome (DS) is the most common form of chromosomal trisomy. Genetic factors in DS may increase the risk for diabetes. This study aimed to determine whether DS is associated with an increased incidence of diabetes and the relationship with obesity across the life span compared with control patients.
RESEARCH DESIGN AND METHODS
This matched population–based cohort study analyzed UK Clinical Practice Research Datalink data from 1990 to 2020.
RESULTS
A total of 9,917 patients with DS and 38,266 control patients were analyzed. Diabetes rates were higher in patients with DS (incidence rate ratio 3.67; 95% CI 2.43–5.55; P < 0.0001) and peaked at a younger age (median age at diagnosis 38 [interquartile range 28–49] years vs. 53 [43–61] years in control patients). Incidence rates (per 1,000 person-years) for type 1 diabetes mellitus were 0.44 (95% CI 0.31–0.61) in patients with DS vs. 0.13 (0.09–0.17) in control patients. Type 2 diabetes mellitus (T2DM) rates were higher in patients with DS versus control patients in age-groups from 5 years up to 34 years. In patients with DS, peak mean BMI was higher and at a younger age (males 31.2 kg/m2 at age 31 years; females 32.1 kg/m2 at 43 years) versus control patients (males 29.5 kg/m2 at 54 years; females 29.2 kg/m2 at 51 years). Obesity was associated with an increased incidence of T2DM.
CONCLUSIONS
At younger ages, the incidence of diabetes in patients with DS is up to four times that of control patients. Peak mean BMI is higher and established earlier in DS, contributing to T2DM risk. Further investigation into the relationship between obesity and diabetes in DS is required to inform treatment and prevention measures.
Introduction
Down syndrome (DS) is the most common form of trisomy, birth anomaly, and genetic cause of intellectual disability (1). People with DS have a constellation of well-recognized facial features as well as other medical conditions, including congenital heart defects, gastrointestinal abnormalities, hematological abnormalities, and immune dysregulation caused by an extra copy of chromosome 21 (2). The incidence of DS is estimated at 1 in 1,000 births worldwide and in the U.K. (3).
People with DS are at increased risk of type 1 diabetes mellitus (T1DM) compared with the general population. The increased incidence of T1DM is thought to be due to trisomy of genes on chromosome 21 and increased defects of the immune system in DS (4,5). The gene for a transcriptional regulator, autoimmune regulatory protein (AIRE), is located on chromosome 21, and it is established that AIRE expression is reduced in patients with DS. It is thought that this may promote autoimmunity in DS and, thus, the development of T1DM (6). Exact mechanisms are not clearly established, but it is suggested that mitochondrial dysfunction and increased oxidative stress are features of DS cells that may increase susceptibility to diabetes (7).
Obesity and type 2 diabetes mellitus (T2DM) rates have increased in the general population, but it is not known whether this similarly affects people with DS (8–10). Nonetheless, studies have shown that there is a higher incidence of obesity in DS that predisposes to insulin resistance (11). Furthermore, patients with DS show an increased rate of nonalcoholic fatty liver disease, which is closely associated with insulin resistance (12).
As well as obesity, a number of genes located on the DS critical region of chromosome 21, including dual-specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A) and regulator of calcineurin 1 (RCAN1), are being investigated and are thought to contribute to diabetes in DS (13,14). Loss of β-cell mass is a key feature in both T1DM and T2DM (13,15). Studies have shown that inhibition of DYRK1A induces β-cell proliferation (16,17). Wang et al. (17) further explored the role of DYRK1A as a regulator of proliferation in human β-cells and found that overexpression of DYRK1A attenuated β-cell proliferation in human islet cells, and conversely, reducing endogenous DYRK1A led to an increase in human β-cell proliferation. Similarly, rodent studies have found that overexpression of RCAN1 causes diabetes, age-associated hyperglycemia, impaired glucose tolerance, hypoinsulinemia, loss of β-cells, and the downregulation of key β-cell genes (14).
To determine the natural history in a representative sample of people with DS across age ranges and trend over the decades of diabetes and obesity in DS, we performed a large retrospective cohort study using linked electronic health records. We examined diabetes risk, age of onset, and obesity in DS compared with the general population over the past three decades using a nationally representative primary care database. Specifically, with use of this data set, we analyzed the association of DS with diabetes and the relationship with obesity across the life span compared with a control population.
Research Design and Methods
Data Source
We performed a matched population–based cohort study using the U.K. Clinical Practice Research Datalink (CPRD) GOLD database. We used data collected over three decades from 1990 to 2020. The CPRD GOLD database is one of the world’s largest of primary care electronic health records, with participation of ∼7% of U.K. family practices and with ongoing collection of anonymized data from ∼9 million patients (18). The high quality of CPRD GOLD data has been confirmed in many studies (19). The protocol was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol 20-048R).
All patients with DS were identified from the July 2020 release of CPRD GOLD, using 10 Read codes for DS or trisomy 21. Control patients were sampled from the list of all registered patients in the database without DS. Control patients were exactly matched on family practice, sex, year of birth, and calendar date of start of record to within 1.5 years. Up to four control patients were randomly sampled for each patient with DS using the sample function in R statistical software (20).
Main Measures
Patients were classified as having diabetes if a diabetes diagnosis was recorded in CPRD GOLD clinical or referral files (diagnostic code list in Supplementary Table 1), if they were prescribed oral hypoglycemic drugs or insulin (medication code list in Supplementary Table 2), or if they had an HbA1c ≥48 mmol/mol recorded on two or more occasions. The date of the first event of any type that was >365 days after the start of the patient’s registration was considered as the diabetes incidence date. In supplementary analyses, patients were classified as having T1DM if they were first prescribed insulin within 91 days of the diabetes diagnosis and were aged <35 years (21); otherwise, they were classified as having T2DM. Records for BMI were analyzed after calculating BMI from height and weight where appropriate. BMI records for adults aged ≥18 years were classified according to the World Health Organization categories of underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥30 kg/m2). Children and young people aged 2–17 years were grouped into the same categories by international BMI standards (1,22) using the zanthro package (23) in Stata statistical software (24). The mean of BMI values recorded in each year of age were used for each patient. Overall mean BMI values were plotted by single year of age, only including mean values where there were five or more patients with observations, with no imputation. Since BMI was not recorded in every year, BMI categories were imputed using the method of last observation carried forward, or backward, allowing patients to remain in the same category for up to 5 years following a measurement.
Analysis
To analyze changes in diabetes rates over time, incidence per 1,000 person-years was used. Person time was analyzed between 1990 and 2020. The start of each patient’s record was the latest of the patient registration date, the practice up-to-standard date, or 1 January 1990, while the end of record was the earliest of the end of registration, patient death date, last data collection date at the practice, or 1 July 2020. Records of patients who developed diabetes were censored at the index date. New diagnoses of diabetes were compared for patients with DS and control patients by aggregating over age-group, sex, and period. Age was divided into the following categories: 0–4 years, 5–14 years, and 10-year ranges thereafter up to 65–75 years. Periods were divided into 1990–1999, 2000–2009, and 2010–2020. Incidence rates were estimated per 1,000 person-years, and 95% CIs were derived from Poisson distribution. A Poisson regression model was fitted to calculate an adjusted incidence rate ratio (IRR). Age was fitted as a continuous predictor in the regression model, with a quadratic term to allow for nonlinearity; similarly, calendar year and year squared were fitted. Sex and DS status were fitted as factors. An interaction term between DS and age was included. Predicted rates were plotted.
Results
There were 10,247 patients who were diagnosed with DS and registered in CPRD GOLD at any time between 1990 and 2020. Forty-three patients recorded as having DS who reached ages >75 years were excluded because they were outliers in terms of typical DS life expectancy (2). This left 10,204 (5,372 female) patients with DS and 39,814 (20,681 female) control patients as comparators. There were 287 cases of preexisting diabetes in patients with DS and 433 in control patients at cohort entry. Because the study focused on incidence (new-onset) rates from birth to 75 years of age, these patients with preexisting diabetes were excluded, leaving 9,917 patients with DS eligible for the analysis of diabetes incidence. In the control group, prevalent cases of diabetes were removed, as well as 1,115 patients who did not have comparator patients with DS. After these exclusions, the final sample of control patients eligible for analysis was 38,266.
There were 287 new diagnoses of diabetes in patients with DS and 1,254 in control patients (Supplementary Table 4). There were 83,160.5 person-years of follow-up for patients with DS and 389,136.1 person-years for control patients (Table 1). Of the 287 new cases in DS, 37 (12.9%) were T1DM and 250 (87.9%) were T2DM. In the control population, of 1,254 new cases, 49 (3.9%) were T1DM and 1,205 (96.1%) were T2DM. There were similar proportions of male and female patients in both groups (Supplementary Table 4).
Table 1.
Incidence of diabetes by sex, age-group, period, and BMI category for patients with DS and control patients
| DS | Control | |||||
|---|---|---|---|---|---|---|
| Diabetes diagnoses | Person-years at risk | Incidence rate per 1,000 person-years (95% CI) | Diabetes diagnoses | Person-years at risk | Incidence rate per 1,000 person-years (95% CI) | |
| Total | 287 | 83,160.5 | 3.45 (3.06–3.87) | 1,254 | 389,136.1 | 3.22 (3.05–3.41) |
| Period | ||||||
| 1990–1999 | 15 | 11,602.1 | 1.29 (0.72–2.13) | 71 | 60,947.0 | 1.16 (0.91–1.47) |
| 2000–2009 | 122 | 37,480.8 | 3.26 (2.70–3.89) | 550 | 180,986.6 | 3.04 (2.79–3.30) |
| 2010–2020 | 150 | 34,077.6 | 4.40 (3.73–5.17) | 633 | 147,202.5 | 4.30 (3.97–4.65) |
| Sex | ||||||
| Male | 137 | 38,770.7 | 3.53 (2.97–4.18) | 617 | 190,023.8 | 3.25 (3.00–3.51) |
| Female | 150 | 44,389.8 | 3.38 (2.86–3.97) | 637 | 199,112.3 | 3.20 (2.96–3.46) |
| Age-group, years | ||||||
| 0–4 | 1 | 6,104.5 | 0.16 (0.00–0.91) | 4 | 33,986.7 | 0.12 (0.03–0.30) |
| 5–14 | 20 | 12,916.8 | 1.55 (0.95–2.39) | 25 | 65,148.9 | 0.38 (0.25–0.57) |
| 15–24 | 34 | 12,379.2 | 2.75 (1.90–3.84) | 38 | 56,126.1 | 0.68 (0.48–0.93) |
| 25–34 | 57 | 14,750.9 | 3.86 (2.93–5.01) | 87 | 52,598.1 | 1.65 (1.32–2.04) |
| 35–44 | 77 | 16,366.0 | 4.70 (3.71–5.88) | 186 | 63,677.5 | 2.92 (2.52–3.37) |
| 45–54 | 61 | 13,704.9 | 4.45 (3.40–5.72) | 356 | 64,138.6 | 5.55 (4.99–6.16) |
| 55–64 | 32 | 6,006.0 | 5.33 (3.64–7.52) | 384 | 39,821.9 | 9.64 (8.70–10.66) |
| 65–75 | 5 | 932.1 | 5.36 (1.74–12.52) | 174 | 13,638.2 | 12.76 (10.93–14.80) |
| BMI category | ||||||
| Underweight | 4 | 895.5 | 4.47 (1.22–11.44) | 2 | 4,450.8 | 0.45 (0.05–1.62) |
| Normal weight | 31 | 12,408.2 | 2.50 (1.70–3.55) | 133 | 53,086.0 | 2.51 (2.10–2.97) |
| Overweight | 51 | 12,053.3 | 4.23 (3.15–5.56) | 306 | 41,988.1 | 7.29 (6.49–8.15) |
| Obese | 148 | 18,334.7 | 8.07 (6.82–9.48) | 678 | 38,601.2 | 17.12 (15.86–18.46) |
| Not recorded | 53 | 39,468.8 | 1.34 (1.01–1.76) | 135 | 250,010.0 | 0.54 (0.45–0.64) |
Changes Through the Decades
Cases of diabetes increased more than three times over the past three decades in both patients with DS and control patients (Table 1). In the period 1990–1999, the incidence rate of diabetes per 1,000 person-years was 1.29 and 1.16 in patients with DS and control patients, respectively. This increased to 3.26 and 3.04, respectively, in the period 2000–2009 and increased further to 4.40 and 4.30, respectively, in the period 2010–2020.
Changes With Age
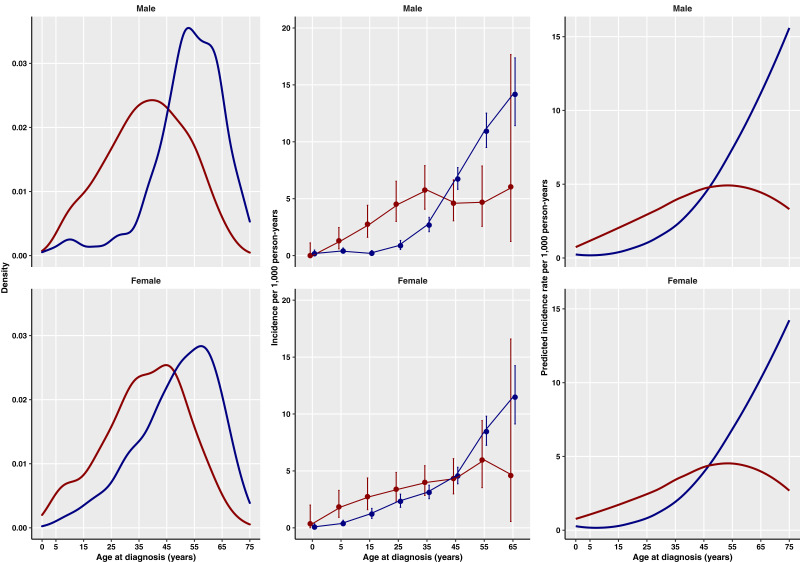
The median age at diagnosis of diabetes was 38 (interquartile range 28–49) years in patients with DS vs. 53 (43–61) years in control patients (Supplementary Table 4). Figure 1 shows the distribution of age at diagnosis of diabetes by sex for patients with DS and control patients, with increased cases of diabetes seen in both men and women with DS compared with control patients up to age ∼45 years. In children and young adults, diabetes was four times more common in patients with DS than in control patients (Table 1); the incidence rate per 1,000 person-years was 1.55 (95% CI 0.95–2.39) in patients with DS vs. 0.38 (0.25–0.57) in control patients at ages 5–14 years and 2.75 (1.90–3.84) vs. 0.68 (0.48–0.93), respectively, at ages 15–24 years. The incidence of diabetes was approximately double in patients with DS aged 25–44 years; the incidence rate per 1,000 person-years was 3.86 (2.93–5.01) in patients with DS vs. 1.65 (1.32–2.04) in control patients at ages 25–34 years and 4.70 (3.71–5.88) vs. 2.92 (2.52–3.37), respectively, at ages 35–44 years. At ages 45–54 years, the incidence rate was comparable in both groups, and in older age-groups, diabetes was approximately twice as common in control patients compared with patients with DS.
Figure 1.
Distribution of age at diagnosis of diabetes by sex for patients with DS (red) and control patients (blue). Left: Density plot of incident cases. Middle: Age-specific incidence rates (95% CIs). Right: Predicted incidence by age from regression model.
IRR for Diabetes
After accounting for age, age-DS interaction, sex, calendar year, and BMI (Table 2), the incidence of diabetes was nearly four times higher in patients with DS than control patients (IRR 3.67 [95% CI 2.43–5.55]; P < 0.0001). However, the effect declined with age (0.96 [0.96–0.97]; P < 0.0001), and at older ages, patients with DS had lower IRRs of diabetes (Fig. 1).
Table 2.
Results of the Poisson regression model
| Variable and category | IRR (95% CI) | P |
|---|---|---|
| DS | 3.67 (2.43–5.55) | 0.000 |
| Calendar year (per year) | 1.09 (1.04–1.13) | 0.000 |
| Calendar year squared | 1.00 (1.00–1.00) | 0.001 |
| Age (per year) | 1.06 (1.04–1.08) | 0.000 |
| Age squared | 1.00 (1.00–1.00) | 0.031 |
| DS-age interaction | 0.96 (0.96–0.97) | 0.000 |
| BMI category | ||
| Not recorded | 0.32 (0.26–0.40) | 0.000 |
| Underweight | 0.60 (0.26–1.35) | 0.22 |
| Normal weight | Reference | |
| Overweight | 1.92 (1.59–2.31) | 0.000 |
| Obese | 4.43 (3.74–5.25) | 0.000 |
| Sex | ||
| Male | Reference | |
| Female | 0.76 (0.69–0.84) | 0.000 |
Diabetes IRRs were adjusted for each of the variables shown.
T1DM and T2DM
The overall incidence rate of T1DM per 1,000 person-years was more than three times higher in patients with DS than control patients (0.44 [95% CI 0.31–0.61] vs. 0.13 [0.09–0.17], respectively) (Supplementary Table 5). Patients with DS aged 15–24 years had the highest incidence rate of T1DM per 1,000 person-years (1.13 [0.62–1.90] vs. 0.18 [0.09 to 0.33] in control patients). In all other age-groups (up to 34 years of age), there was no clear difference in the incidence of T1DM between groups (Supplementary Table 5 and Supplementary Fig. 1).
Overall, the incidence rate of T2DM per 1,000 person-years was similar between patients with DS (3.01 [95% CI 2.65–3.40]) and control patients (3.10 [2.92–3.28]) (Supplementary Table 6). However, when broken down by age-group, there was a much higher incidence of T2DM in patients with DS than control patients from age 5 to 34 years (Supplementary Table 6 and Supplementary Fig. 2). At ages 5–14 years, the incidence rate of T2DM per 1,000 person-years was 10 times higher in patients with DS than control patients (0.62 [0.27–1.22] vs. 0.06 [0.02–0.16], respectively) (Supplementary Table 6 and Supplementary Fig. 2). In both groups, incidence increased by age, with a higher incidence in patients with DS in age-groups up to 45 years. Rates were comparable between groups at age 45–54 years. There was approximately double the incidence of T2DM in control patients compared with patients with DS in age-groups >54 years.
Impact of Obesity
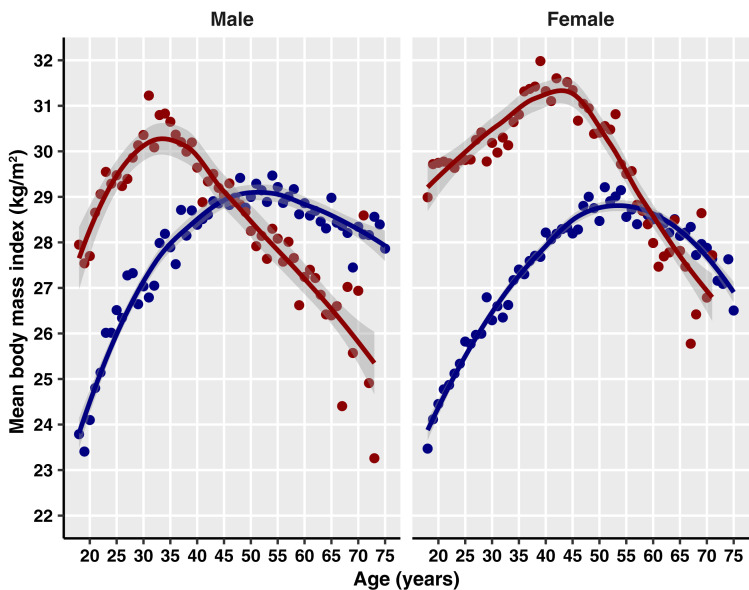
There was an overall increased risk of diabetes in overweight and obese patients compared with those with normal weight (IRR 1.92 [95% CI 1.59–2.31] and 4.43 [3.74–5.25], respectively) (Table 2). Mean BMI was 34.3 (SD 8.7) kg/m2 in patients with DS and 33.2 (7.1) kg/m2 in control patients (Supplementary Table 4). Figure 2 shows increased mean BMI at up to ∼45 years of age in men and 60 years of age in women with DS compared with control patients. Younger patients with DS had higher peaks in mean BMI than control patients as did women with DS compared with their male counterparts and control patients (Fig. 2). In DS, the greatest mean BMI was reached at age 31 years in men (31.2 kg/m2) and 43 years in women (32.1 kg/m2) vs. 54 years in male control patients (29.5 kg/m2) and 51 years in female control patients (29.2 kg/m2). In both groups, raised BMI was associated with increased incidence of diabetes. In patients with DS, the incidence rate of diabetes per 1,000 person-years was 8.07 (95% CI 6.82–9.48) in those with obesity vs. 4.23 (3.15–5.56) in those who were overweight and 2.50 (1.70–3.55) in those with normal weight (Table 1). In control patients, the incidence rate of diabetes per 1,000 person-years was markedly higher with increasing BMI (normal weight 2.51 [2.10–2.97]; overweight 7.29 [6.49–8.15]; obese 17.12 [15.86–18.46]) (Table 1).
Figure 2.
Mean BMI by single year of age and sex for patients with DS (red) and control patients (blue).
There was no difference in incidence of T1DM by BMI category for either group (Supplementary Table 5). There was an increased incidence of T2DM with increased BMI in both groups (Supplementary Table 6). The incidence rate of T2DM per 1,000 person-years in patients with DS rose from 1.93 (95% CI 1.24–2.88) in those with normal weight to 3.90 (2.87–5.19) and 7.58 (6.37–8.95) in those who were overweight and obese, respectively. In control patients, the incidence rate of T2DM per 1,000 person-years rose with increased weight gain from 2.24 (1.86–2.68) in patients with normal weight to 7.12 (6.34–7.98) and 16.89 (15.63–18.22) in patients who were overweight and obese, respectively.
Management
Patients with DS were less likely to be recorded as receiving oral hyperglycemic medication within the first 5 years from diagnosis than control patients (113 [39%] of 287 and 722 [58%] of 1,254, respectively). Fifty-eight (20%) patients with DS and 138 (11%) control patients were receiving insulin therapy. Median levels of HbA1c (53 mmol/mol) were similar between groups.
Conclusions
In this U.K.-based cohort study using a nationally representative primary care database, we show evidence of an increased incidence of diabetes over the past three decades from 1990 to 2020 in both patients with DS and control patients. This is in keeping with global and U.K.-based studies that have shown increasing incidence of T2DM across populations up until approximately the mid-2000s, with stable or falling rates in subsequent years (8).
National surveys in the U.K. have shown a clear increase in the proportion of overweight or obese adults between 1993 and 2001, with small changes seen since then (9). Similarly, surveys of children have shown an increase in obesity between 1995 and 2004, with a subsequent, broadly steady incidence (10). Obesity is a recognized risk factor in other diseases, with clear associations between obesity and T2DM and coronary heart disease (25,26). Studies have shown an increased incidence of overweight and obesity in both adults and children with DS (27,28).
Our study shows an adjusted increased IRR of 3.67 (95% CI 2.43–5.55) of diabetes in DS. There is a difference in the age of onset of diabetes between the DS and control populations of our study, with incidence in DS increased in age-groups <44 years. With regard to age-group–specific incidence, our study shows that patients with DS are at significantly increased risk of diabetes at a younger age than the general population, with more than four times the risk in children and young adults (aged 5–24 years) and more than double the risk in patients aged 25–44 years. We show that there is an increased risk for T1DM in children and young patients with DS compared with general population, with almost three times the risk in children aged 5–14 years and seven times the risk in patients aged 15–24 years. The incidence for T2DM is >10 times higher in patients aged 5–14 years with DS compared with control patients. There remains an increased incidence for T2DM (although less profound a difference) in age-groups up to 45 years. In older age-groups >55 years, there is an increased incidence of T2DM in control patients compared with patients with DS.
Our study also reiterates that raised BMI is associated with an increased incidence of diabetes and additionally shows that in patients with DS, obesity affects younger age-groups. We show that being overweight confers an increased risk of T2DM in both groups and that this additional risk is higher in control patients than in patients with DS.
The underlying mechanisms for this increased susceptibility for diabetes in DS still need further investigation. A combination of factors, including genetic susceptibility, predisposition to autoimmunity, mitochondrial dysfunction, increased oxidative stress, and cellular dysfunction, are thought to contribute to this risk. Candidate genes include AIRE, DYRK1A, and RCAN1 (6,14,16,17). Some of the increased risk of diabetes at a younger age in people with DS may be explained by a predisposition to autoimmune conditions, more specifically of T1DM. DS may be associated with a reduction in β-cell mass and impaired insulin secretion in DS (13–17).
In support of these various mechanisms, we found evidence for an increase in both T1DM and T2DM in patients with DS. Furthermore, we show that patients with DS have a similar increase in diabetes incidence over time and thus, are also subject to the environmental factors related to this increase, such as increased rates of obesity and dietary risk factors. However, the extent to which obesity predisposes to diabetes in people with DS is somewhat reduced compared with the general population, suggesting that additional biological mechanisms associated with DS may be related to an increased incidence of diabetes. Our study supports the theory for an underlying genetic predisposition to diabetes in patients with DS that may be in addition to their risk of obesity.
HbA1c is a marker for glycemic control and has been shown in some studies to correlate with risk of diabetic complications (29). Reassuringly, our analysis found similar median levels of HbA1c between patients with DS and control patients.
Limitations
In the U.K., primary care is the usual first point of contact for adult patients with T2DM, and repeat prescriptions for both adults and children with diabetes of any kind are issued by primary care. In this way, the CPRD database is likely to better represent the burden of disease compared with secondary care records. However, T1DM in adults and diabetes of any type in children is managed in secondary care, though with input from primary care; nevertheless, the CPRD database might reveal an underestimate of the true incidence of T1DM. Furthermore, in contrast to adults with DS, annual health reviews in children with DS are usually performed by community pediatricians (in secondary care) with differing access to primary care health records. This is likely to be reflected in our study with missing BMI data for children with DS.
Our classification of T1DM (patients who were first prescribed insulin within 91 days of the diabetes diagnosis and were aged <35 years) may include patients with very poorly controlled diabetes of other types who may require insulin therapy earlier on, therefore leading to misclassifying these patients as having T1DM. Other published works using similar large clinical data sets also use nonstandard definitions of T1DM and T2DM to reduce the number of missing cases due to incomplete coding (30–33). Since we have been consistent in our definitions between our control group and DS group, we believe that the analyses nevertheless show a valuable difference between population groups.
In this study, we classified diabetes in DS as T1DM and T2DM. We recognize that there are some classification systems, such as the International Society for Pediatric and Adolescent Diabetes, that would classify diabetes in DS under the alternative heading of other genetic syndromes sometimes associated with diabetes (34). However this is not the case for classification systems such as the International Statistical Classification of Diseases and Related Health Problems, where diabetes in DS would fall under T1DM and T2DM categories (35). Furthermore, in practice, clinicians caring for patients with DS with diabetes would generally classify diabetes as T1DM and T2DM on the basis of clinical and biochemical phenotype, which has direct implications in understanding mechanisms as well as clinical management and prevention. Importantly, within the DS field, it is accepted to classify diabetes as T1DM and T2DM, as evidenced in previous publications, including those by the Down Syndrome Medical Interest Group (4,36–38). We acknowledge that the differentiation between T1DM and T2DM may require additional biochemical investigations that may not always be possible in the primary care setting. Finally, we report on overall incidence of diabetes in patients with DS compared with control patients, which includes both T1DM and T2DM classifications as well as other types of diabetes (Supplementary Table 1). In this way, our data are likely to better capture incidence of diabetes and reduce classification errors.
In conclusion, between 1990 and 2020, the median age at diabetes diagnosis was 15 years earlier in patients with DS and more than four times more common in children and young adults with DS compared with control patients. There was also an increased incidence of obesity in children and young adults with DS, with increasing rates over time. Our study suggests the need for close monitoring and early identification of diabetes (and obesity) in this susceptible population. Health promotion is vital in the prompt detection and treatment of T1DM, and annual health checks for children with DS should ensure that those with parental responsibility are given adequate information of signs and symptoms of T1DM (namely polyuria, polydipsia, and weight loss). HbA1c is not routinely measured in children and adolescents with DS. Our study suggests that they have an additional risk of T2DM and are also susceptible to the environmental factors driving increased rates of obesity and T2DM. We would therefore recommend proactive monitoring of HbA1c at annual health checkups in adolescents with DS. Furthermore, exploration of the genetic predisposition to diabetes and obesity in DS is necessary for development of effective therapies and to inform future preventive measures.
Article Information
Acknowledgment. The Gene Overdosage and Comorbidities During the Early Lifetime in Down Syndrome (GO-DS21) Consortium contributed to the discussion of the data and final version of the manuscript.
Funding. The GO-DS21 project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement 848077. A.A.A. is supported by a William Harvey Research Limited clinical research fellowship. L.F.C. received funding from Medical Research Council UK/Academy of Medical Sciences fellowship grant G0802796 and Wellcome Trust grant 217543/Z/19/Z. S.E.P. is supported by Alzheimer’s Society fellowship grant AS-CP-18-0020. A.S. received funding from Medical Research Council grants MR/S011277/1, MR/S005145/1, and MR/R024901/1. For the purpose of open access, the author has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission.
The funders of the study had no role in study design, data collection, data analysis, interpretation of data, or writing of the report.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.A.A., R.A.B., S.E.P., A.S., M.C.G., and L.F.C. were involved in the conception, design, and conduct of the study. A.A.A., R.A.B., M.C.G., and L.F.C. wrote the manuscript. All authors reviewed and edited the manuscript. R.A.B. and M.C.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20810833.
A.A.A. and R.A.B. contributed equally.
A list of principal investigators for the GO-DS21 Consortium can be found in the supplementary material online.
Contributor Information
GO-DS21 Consortium:
Yann Herault, Andre Strydom, Li Chan, Marie-Claude Potier, Johannes Beckers, Pietro Liò, and Mara Dierssen
References
- 1. Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA, Saal HM. Retrospective study of obesity in children with down syndrome. J Pediatr 2016;173:143–148 [DOI] [PubMed] [Google Scholar]
- 2. Antonarakis SE, Skotko BG, Rafii MS, et al. Down syndrome. Nat Rev Dis Primers 2020;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Public Health England . Research and analysis, NCARDRS statistics 2017 summary report, 2020. Accessed 8 January 2022. Available from https://www.gov.uk/government/publications/ncardrs-congenital-anomaly-annual-data/ncardrs-statistics-2017-summary-report
- 4. Anwar AJ, Walker JD, Frier BM. Type 1 diabetes mellitus and Down’s syndrome: prevalence, management and diabetic complications. Diabet Med 1998;15:160–163 [DOI] [PubMed] [Google Scholar]
- 5. Cruz NV, Mahmoud SA, Chen H, Lowery-Nordberg M, Berlin K, Bahna SL. Follow-up study of immune defects in patients with dysmorphic disorders. Ann Allergy Asthma Immunol 2009;102:426–431 [DOI] [PubMed] [Google Scholar]
- 6. Skogberg G, Lundberg V, Lindgren S, et al. Altered expression of autoimmune regulator in infant down syndrome thymus, a possible contributor to an autoimmune phenotype. J Immunol 2014;193:2187–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helguera P, Seiglie J, Rodriguez J, Hanna M, Helguera G, Busciglio J. Adaptive downregulation of mitochondrial function in down syndrome. Cell Metab 2013;17:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab 2017;19:1537–1545 [DOI] [PubMed] [Google Scholar]
- 9. Barker C. Obesity Statistics, 2021. Accessed 8 January 2022. Available from https://commonslibrary.parliament.uk/research-briefings/sn03336/
- 10. Moody A. Overweight and obesity in adults and children, 2020. Accessed 8 January 2022. Available from https://files.digital.nhs.uk/9D/4195D5/HSE19-Overweight-obesity-rep.pdf
- 11. Fonseca CT, Amaral DM, Ribeiro MG, Beserra ICR, Guimarães MM. Insulin resistance in adolescents with Down syndrome: a cross-sectional study. BMC Endocr Disord 2005;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreau M, Benhaddou S, Dard R, et al. Metabolic diseases and Down syndrome: how are they linked together? Biomedicines 2021;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peiris H, Raghupathi R, Jessup CF, et al. Increased expression of the glucose-responsive gene, RCAN1, causes hypoinsulinemia, beta-cell dysfunction, and diabetes. Endocrinology 2012;153:5212–5221 [DOI] [PubMed] [Google Scholar]
- 15. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 16. Shen W, Taylor B, Jin Q, et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat Commun 2015;6:8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R Project for Statistical Computing . R: a language and environment for statistical computing, 2019. Accessed 8 January 2022. Available from https://www.R-project.org/
- 21. Imkampe AK, Gulliford MC. Trends in type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991–2008. Diabet Med 2011;28:811–814 [DOI] [PubMed] [Google Scholar]
- 22. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidmar SCT, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: update. Stata J 2013;13:366–378 [Google Scholar]
- 24. StataCorp . Stata Statistical Software: Release 14. College Station, TX, StataCorp LP, 2015 [Google Scholar]
- 25. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–969 [DOI] [PubMed] [Google Scholar]
- 26. Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 27. Melville CA, Cooper SA, McGrother CW, Thorp CF, Collacott R. Obesity in adults with Down syndrome: a case-control study. J Intellect Disabil Res 2005;49:125–133 [DOI] [PubMed] [Google Scholar]
- 28. Bertapelli F, Pitetti K, Agiovlasitis S, Guerra-Junior G. Overweight and obesity in children and adolescents with Down syndrome-prevalence, determinants, consequences, and interventions: a literature review. Res Dev Disabil 2016;57:181–192 [DOI] [PubMed] [Google Scholar]
- 29. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc 2017;1:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. le Roux CW, Chubb B, Nortoft E, Borglykke A. Obesity and healthcare resource utilization: results from Clinical Practice Research Database (CPRD). Obes Sci Pract 2018;4:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farmer RE, Beard I, Raza SI, et al. Prescribing in type 2 diabetes patients with and without cardiovascular disease history: a descriptive analysis in the UK CPRD. Clin Ther 2021;43:320–335 [DOI] [PubMed] [Google Scholar]
- 33. Rodgers LR, Weedon MN, Henley WE, Hattersley AT, Shields BM. Cohort profile for the MASTERMIND study: using the Clinical Practice Research Datalink (CPRD) to investigate stratification of response to treatment in patients with type 2 diabetes. BMJ Open 2017;7:e017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes 2009;10(Suppl. 12):3–12 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), 2016 [Google Scholar]
- 36. Down’s Syndrome Association . Health series: diabetes, 2018. Accessed 8 January 2022. Available from https://www.downs-syndrome.org.uk/wp-content/uploads/2020/06/Diabetes-1st-May-KP-27th-June-SM_DSMIG.pdf
- 37. Tsou AY, Bulova P, Capone G, et al. Medical care of adults with Down syndrome: a clinical guideline. JAMA 2020;324:1543–1556 [DOI] [PubMed] [Google Scholar]
- 38. Gillespie KM, Dix RJ, Williams AJ, et al. Islet autoimmunity in children with Down’s syndrome. Diabetes 2006;55:3185–3188 [DOI] [PubMed] [Google Scholar]