Abstract
The MS2-MCP imaging system is widely used to study mRNA spatial distribution in living cells. Here, we report that the MS2-MCP system destabilizes some tagged mRNAs by targeting them to the nonsense-mediated mRNA decay pathway. We introduce an improved version, which counteracts this effect by increasing the efficiency of translation termination of the tagged mRNAs. Improved versions were developed for both yeast and mammalian systems.
The mRNA spatial distribution is important in establishing the localization of their protein products1. The MS2-MCP system is widely used to study mRNA localization in living cells2. In this approach, an array of MS2 binding sites (MBS) is inserted downstream of an open reading frame such that the MBS array is transcribed as part of the 3’ untranslated region (UTR) (“MBS tagging”). The MBS array becomes bound by ectopically expressed fluorescently labeled MS2-coat proteins (MCP), allowing the detection of mRNAs as diffraction-limited spots by fluorescence microscopy3. A potential drawback of the system is that the MBS array may affect mRNA-associated processes. For instance, the MBS was reengineered to prevent the accumulation of decay-resistant RNA fragments3. Here, we report that the MS2-MCP system may destabilize the tagged mRNA. We improved the system and eliminated this perturbation of mRNA stability.
Nonsense-mediated mRNA decay (NMD) is a conserved quality control and gene regulatory pathway4. One class of NMD substrates are the mRNAs that experience inefficient translation termination5–8. For normal translation termination, the poly(A) binding protein, PAB1 in yeast and PABPC1 in mammals, recruits the polypeptide chain release factor, SUP35 in yeast and eRF3 in mammals, to the stop codon. When the poly(A) tail is distant from the stop codon, the recruitment of SUP35/eRF3 by PAB1/PABPC1 is inefficient, triggering mRNA decay via NMD5–7,9–11. As 24xMBS array (1,660 nt3) is 16-fold longer than an average 3’ UTR (104 nt12) in Saccharomyces cerevisiae, MBS tagging increases the distance from the stop codon to the poly(A) tail and could potentially activate NMD. To explore this possibility, we generated eight yeast strains, each of which had an MBS array integrated into the 3’ UTR of a different gene. We examined the abundance of these eight mRNAs via reverse transcription followed by quantitative polymerase chain reaction (RT-qPCR). MBS tagging did not affect the mRNA levels of ACT1, MDN1, CLB2, ATP3, or ATP4 but reduced the mRNA levels of ATP2, HAC1, and PMA1 to about 30%, 50%, and 75%, respectively (Figure 1A, Extended Data Figure 1A-C). Since ATP2, which encodes the ß subunit of F1-ATP synthase13, had the largest mRNA reduction, we used it as an experimental substrate. The ATP2 mRNA reduction was confirmed by single-molecule RNA fluorescent in situ hybridization (smFISH) (Figure 1B-C). When we co-expressed MCP-GFP or reduced the number of MBS repeats from 24 to 12, which is the minimum required for single-molecule imaging, the mRNA reduction remained the same (Figure 1D). To examine the effect of tagging site, we compared mRNAs with MBS arrays placed at 1 or 162 nt downstream from the stop codon. The mRNA levels were comparable, suggesting that the mRNA reduction was not caused by the tagging site specifically (Extended Data Figure 2A-B). To examine whether the mRNA reduction resulted from mRNA destabilization, we blocked de novo transcription with phenanthroline and monitored the mRNA levels over time using RT-qPCR. When compared with the corresponding untagged mRNAs, the MBS-tagged ATP2, HAC1, and PMA1 mRNAs degraded more rapidly, whereas the MBS-tagged ACT1 mRNAs degraded at a similar rate (Figure 1E, Extended Data Figure 3A-C). Hence, MBS tagging decreased the stability and subsequently the abundance of some mRNAs in yeast.
Figure 1. MBS tagging may destabilize mRNAs through the NMD pathway in yeast.
(A) RT-qPCR of MBS-tagged mRNAs in wildtype (W) or Δupf1 (Δ) strain background. When measuring HAC1 mRNA abundance, cells were treated with 1 μg/ml tunicamycin for 2 hours. The mRNA levels were normalized to their corresponding wildtype (WT) mRNAs. P values (left to right) are 0.39, <0.0001, 0.93, 0.69, 0.0099, 0.60, 0.35, 0.0092, 0.12, 0.35, 0.13, 0.22. n = 3 biologically independent experiments. (B) Representative images of ATP2 mRNA (green) and mitochondria (magenta). ATP2 mRNAs were detected by smFISH. Mitochondria were labeled using mitochondria-targeted GFP. Images were max-Z projected. Scale bars are 2 μm. (C) Number of ATP2 mRNAs per cell as quantified from the smFISH images. n = 291 and 457 cells. P value is <0.0001. (D) RT-qPCR of ATP2 mRNAs tagged with 24x or 12xMBS array in the presence or absence of MCP-GFP. P values are <0.0001, 0.0002, <0.0001. n = 3 biologically independent experiments. (E) mRNA stability assay of the ATP2 mRNA in indicated strains. Cells were treated with phenanthroline to inhibit transcription. mRNA levels were measured by RT-qPCR. The fraction of remaining mRNAs was calculated by normalizing the ATP2 mRNA level to the level at time point 0 minute. A two-sided Student’s t-test was performed to compare WT and ATP2-24xMBS strains. P values are 0.012, 0.015, 0.0067. n = 3 biologically independent experiments. (F) Bar graph showing the normalized levels of Atp2 protein as quantified from Western blots. Pgk1 was used as a loading control. P value is 0.0002. n = 3 biologically independent experiments. (G, H) Serial dilutions of yeast cells were spotted on agar plates with indicated carbon source (G) or the ER protein folding stress inducer tunicamycin (H). In Figure 1, all error bars indicate mean ± SD. In Figure 1, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, ns, not significant (all analysis are two-sided Student’s t-test).
To examine the impact of MBS tagging at the protein level, we performed Western blotting for Atp2 C-terminally fused with a myc epitope. The presence of MBS array decreased the Atp2 protein level to about 30% (Figure 1F, Extended Data Figure 4), comparable to the degree of ATP2 mRNA reduction, suggesting that MBS tagging mainly affected mRNA abundance but not translation efficiency. To study the impact of protein reduction on mitochondrial function, yeast cells were grown on a plate containing glycerol, a non-fermentable carbon source. Cells harboring MBS-tagged ATP2 grew slower, indicating that the Atp2 protein reduction caused inefficient oxidative phosphorylation (Figure 1G). Similarly, the MBS-induced reduction of HAC1 mRNA, which encodes the transcription factor of the endoplasmic reticulum (ER) unfolded protein response14, resulted in slower growth under ER stress conditions (Figure 1H). Hence, the MBS-induced mRNA destabilization reduced protein abundance and compromised cellular function.
To examine whether the tagged mRNAs were destabilized via NMD, we deleted a core mediator of the pathway, UPF115. As a result, the abundance and stability of MBS-tagged ATP2, HAC1, and PMA1 mRNA were fully restored (Figure 1A&E, Extended Data Figure 3A-B). The Δupf1 cells harboring MBS-tagged ATP2 or HAC1 had wildtype-like growth rates under non-fermentable or ER stress conditions, respectively (Figure 1G-H). Thus, MBS tagging destabilized mRNAs via NMD.
To make the MS2-MCP system a generally useful imaging tool, it was imperative to counteract the mRNA destabilization. Previous studies demonstrated that tethering SUP35/eRF3 or PAB1/PABPC1 proteins to the vicinity of the stop codon increased the efficiency of translation termination and protected the mRNA from NMD in yeast5,6 and mammalian cells9,16–19 (Supplementary Table 1). Inspired by these studies, we generated yeast strains expressing the fusion protein MCP-GFP-SUP35 or MCP-GFP-PAB1. The fusion proteins should bind to the MBS array and fluorescently label the mRNA through MCP-GFP and correct the mRNA stability through SUP35- or PAB1-promoted translation termination (Figure 2A). In cells expressing MCP-GFP-PAB1, the MBS-tagged mRNAs were not detected using fluorescent microscopy (Extended Data Figure 5A). It was likely that the MCP-GFP-PAB1 bound to the poly(A) tails of other mRNAs through the PAB1 RNA-binding domain, thereby diluting the specific signal of the MBS-tagged mRNAs below detection levels. To test this idea, we used a mutant, PAB1(F170V,F366V) (“PAB1*”) that harbored two point mutations, which were located at conserved residues of PAB1’s RNA-binding domain (Extended Data Figure 5B-C) and decreased PAB1’s RNA-binding affinity by nearly 1,000-fold20. As a result, MCP-GFP-PAB1* should lose its affinity to poly(A) tails and only bind to the MBS array via the MCP’s RNA-binding domain. Indeed, MBS-tagged mRNAs were readily detected by fluorescent microscopy in cells expressing MCP-GFP-PAB1* (Extended Data Figure 5A). Notably, the expression of MCP-GFP-SUP35 or MCP-GFP-PAB1* restored the mRNA abundance and stability of the MBS-tagged ATP2, HAC1, and PMA1 (Figure 2B-C, Extended Data Figure 6A-B), the protein abundance of Atp2 (Figure 2D, Extended Data Figure 4), and the yeast growth phenotypes associated with MBS-tagged ATP2 or HAC1 (Figure 2E-F). ACT1 represents the group of mRNAs that were not destabilized by MBS tagging. None of the MCP fusion proteins affected the level or stability of the MBS-tagged ACT1 mRNA (Extended Data Figure 6C-D). Therefore, the MBS-induced mRNA destabilization was corrected by MCP-GFP-SUP35 and MCP-GFP-PAB1*.
Figure 2. The improved MS2-MCP system minimizes mRNA destabilization in yeast and mammalian cells.
(A) Illustration of the improved MS2-MCP system. (B) RT-qPCR of indicated mRNAs. P values (left to right) are <0.0001, <0.0001, 0.001, 0.002, 0.013, 0.003, 0.006, 0.019, 0.001. n = 3 biologically independent experiments. (C) mRNA stability assay of the ATP2 mRNA. A two-sided Student’s t-test was performed to compare ATP2-24xMBS and ATP2-24xMBS+MCP-GFP-SUP35 strains. P values are 0.012, 0.020, 0.029. n = 3 biologically independent experiments. (D) Bar graph showing the normalized levels of Atp2 protein as quantified from Western blots. Pgk1 was used as a loading control. P values are 0.0002, 0.0001, 0.0058, 0.016. n = 3 biologically independent experiments. (E, F) Cell growth assay with the same conditions as in Figure 1G-H. (G) Representative images of ATP2 mRNA (green) and mitochondria (magenta). The experimental setup and scale bars are the same as in Figure 1B. (H) Fraction of mitochondria-localized ATP2 mRNAs as quantified from the smFISH images. n = 167, 120, 75, 123 cells. ns, not significant. (I) RT-qPCR of MBS-tagged P21 mRNA in U2OS cells and ß-actin mRNA in MEF cells. The mRNA levels were normalized to their corresponding WT mRNAs. P values are 0.0002, 0.0015, 0.0005, 0.037, 0.19. n = 3 biologically independent experiments. (J) mRNA stability assay of the P21 mRNA. Cells were treated with actinomycin D to inhibit transcription. A two-sided Student’s t-test was performed to compare P21-24xMBS and P21-24xMBS+MCP-GFP-eRF3 cell lines. n = 3 biologically independent experiments. P values are 0.19, 0.10, 0.048. (K) Number of P21 mRNAs per cell as quantified from smFISH images. n = 48, 39, 42, 51, 46 cells. (L) Bar graph showing the normalized levels of p21 protein as quantified from Western blots. ß-actin protein was used as a loading control. P values are < 0.0001, < 0.0001, 0.0006. n = 3 biologically independent experiments. In Figure 2, all error bars indicate mean ± SD. In Figure 2, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, ns, not significant (all analysis are two-sided Student’s t-test).
The improved MS2-MCP system expressing SUP35- or PAB1*-fusion proteins was tested for its ability to accurately image mRNAs. Studies showed that ATP2 mRNA is translated at the mitochondrial periphery, thereby promoting the import of nascent Atp2 proteins21. To examine the ATP2 mRNA localization with respect to mitochondria in fixed cells, we imaged ATP2 mRNAs and mitochondria using smFISH and a mitochondria-targeted fluorescent protein. About half of the ATP2 mRNAs co-localized with mitochondria in untagged cells. This spatial distribution remained the same irrespective of the tagged mRNA being destabilized or restored (Figure 2G-H), indicating that the improved MS2-MCP system did not perturb mRNA localization. Live-cell imaging using the improved system revealed that ATP2 mRNAs moved in trajectories along the mitochondrial network (Extended Data Figure 7, Supplementary Movie 1-2). Hence, the improved MS2-MCP system faithfully reported ATP2 mRNA distribution and mobility in living cells.
To extend this study to mammalian cells, P21, which encodes an inhibitor of cyclin-dependent kinase22, and ß-actin mRNA, which encodes an actin isoform23, were examined in U2OS and mouse embryonic fibroblast (MEF) cells, respectively. Both genes had been previously tagged with the MBS array at their endogenous loci23,24. Using RT-qPCR and smFISH, we found that MBS tagging did not affect the level of ß-actin mRNA but reduced the abundance and stability of P21 mRNA (Figure 2I-K, Extended Data Figure 8). Thus, similar to yeast, the MBS-induced mRNA reduction was gene-dependent in mammalian cells. When UPF1 mRNA levels were reduced using shRNA (Extended Data Figure 9), the level of the MBS-tagged P21 mRNA was restored (Figure 2I), suggesting that the mRNA reduction in mammalian cells, as in yeast, occurred through NMD. To minimize the mRNA destabilization, the fusion proteins MCP-GFP-eRF3 or MCP-GFP-PABPC1(F142V,F337V) were generated. PABPC1(F142V,F337V) (“PABPC1*”) is the mammalian homolog of the yeast PAB1* (Extended Data Figure 5B-C). Cells expressing the fusion proteins were isolated by fluorescence-activated cell sorting. The abundance and stability of MBS-tagged P21 mRNA were fully restored by MCP-GFP-eRF3 and partially restored by MCP-GFP-PABPC1* (Figure 2I-K). Of note, higher levels of MCP-GFP-PABPC1* better restored the P21 mRNA abundance (Extended Data Figure 10A). To examine the impact of MBS tagging at the protein level, we performed Western blotting on p21 and showed that the MBS tagging reduced the protein abundance to about 20% (Figure 2L) and slightly increased p21’s molecular weight (Extended Data Figure 10B), the latter of which might have been caused by changes in p21’s posttranslational modification22. Expression of MCP-GFP-eRF3 or MCP-GFP-PABPC1* restored the p21 protein’s abundance and molecular weight (Figure 2L, Extended Data Figure 10B). Using this improved MS2-MCP system, cytoplasmic P21 mRNAs were detected by fluorescent microscopy in living cells and displayed rapid movements (Supplementary Movie 3-5). Thus, the improved approach with NMD-corrective fusion proteins can be used to faithfully image P21 mRNAs in mammalian cells.
In summary, this work reports that MBS tagging may decrease mRNA stability via the NMD pathway. We introduce an improved MS2-MCP system, in which MCP-GFP-SUP35/eRF3 or MCP-GFP-PAB1*/PABPC1* protects MBS-tagged mRNAs from NMD in yeast and mammalian cells. Orthogonal RNA imaging methods, including the PP7-PCP system or aptamer-based RNA imaging methods, achieve single-molecule resolution using a similar strategy of repeated tags25. These tags increase 3’ UTR length and may analogously destabilize mRNAs. The improved MS2-MCP system informs an approach to antagonize NMD and correct mRNA stability. We noticed a correlation between p21 protein abundance and molecular weight in mammalian cells (Extended Data Figure 10B). While the underlying mechanism is unclear, we speculate that reduced p21 protein might affect cell cycle progression or the expression of the receptor tyrosine kinase HER2/neu, and hence alter posttranslational modifications on p2126,27. In this study, MBS-induced NMD varied from gene to gene even though the MBS-tagged mRNAs had similar 3’ UTR lengths (Figure 1A, Extended Data Figure 1A). This resembles previous observations that NMD differentially regulates endogenous mRNAs that have comparable 3’ UTR sizes10,28. The differential regulation by NMD may be determined by additional factors, including mRNA cis-elements, RNA-binding proteins, and translation readthrough rate29–32. It is recommended to assess the effect of MBS tagging on an mRNA of choice before choosing the MCP construct. If MBS tagging does not change the mRNA stability33–35, the original MCP-GFP can be used. Otherwise, the MCP-GFP-SUP35/eRF3 (version 8) or MCP-GFP-PAB1*/PABPC1* (version 9) should be used. Altogether, this improved system can faithfully report mRNA stability and spatial distributions. It will likely see a wide application in future studies.
Methods
Yeast strains and plasmids
Strains used in this study were derived from the S. cerevisiae background W303 (MATa; ura3-1; trp1Δ2; leu2-3,112; his3-11,15; ADE2; can1-100) and are listed in Supplementary Table 2. All the yeast MBS arrays used in this study were MBSv63. Cells carrying MBS-tagged ATP2, PMA1, ACT1, MDN1, CLB2, ATP3,or ATP4 were generated by integrating the MBS array at the endogenous locus of the corresponding gene through homologous recombination and uracil selection. The MBS array was inserted 1 nt downstream of the stop codon unless specified otherwise. WT and MBS-tagged HAC1 were integrated at the HO locus in Δhac1 strain background (backbone plasmid: HO-Poly-KanMX4-HO). The MBS array was inserted 283 nt downstream from the HAC1 stop codon, the same location as the U1A array was inserted in a previous study36. To image mitochondria, we used a plasmid integrated at the HO locus expressing GFP (for imaging in fixed cells) or mKate2 (for imaging in live cells) fused to Su9 mitochondrial targeting sequence. Cells were grown exponentially in yeast synthetic medium with auxotrophy complementing amino acids and 2% glucose under continuous shaking at 26°C.
Expressing MCP-GFP-SUP35/eRF3 or MCP-GFP-PAB1*/PABPC1* in yeast and mammalian cells
The plasmids expressing MCP fusion proteins used in this study is summarized in Supplementary Table 3. In yeast, the MCP constructs were stably integrated at the leu2 locus (backbone plasmid: pRS305) and expressed under the cyc1 promoter. The plasmids were linearized by the ClaI enzyme before transformation. Positive cells were selected on -LEU plates and verified by RT-qPCR analysis and imaging. Compared to expressing MCP constructs on CEN/ARS plasmids, genomic integration has the advantage of less cell-to-cell variability in MCP expression level, and MCP-positive cells do not need to be continuously selected using selective medium. It should be noted that the pRS305 plasmids cannot be integrated at the leu2 locus in yeast strain backgrounds that has a complete deletion of leu2, like BY4741. In this case, a non-integrative plasmid backbone, like the YCplac111 used in a previous study3, should be employed.
In mammalian cells, the MCP-GFP-eRF3 and MCP-GFP-PABPC1* were expressed under UBC promoter. The fusion protein has an SV40 nuclear localization signal and an HA epitope tag at its N-terminus. The following mammalian cell lines were used in this study: WT human U2OS cells (ATCC, HTB-96)37, U2OS cells with one allele of P21 being MBS tagged24, WT MEF cells (ATCC, CRL-2991), and MEF cells expressing MBS-tagged β-actin23. Cells were continuously cultured in DMEM (4.5 g/L, Corning) supplemented with Pen/Strep (Gibco) and 10% FBS (Atlanta Biologicals). To generate stable U2OS cell lines, we infected the cells with MCP-GFP-eRF3 or MCP-GFP-PABPC1* lentivirus and selected positive cells with low, medium, or high expression by fluorescence-activated cell sorting (FACS). Cells with low levels of MCP-GFP-eRF3 or high levels of MCP-GFP-PABPC1* were used unless specified otherwise. To make stable U2OS cell lines expressing the shRNA targeting UPF1, we used the Addgene plasmid #13603738. Cells were infected with the lentivirus and selected using puromycin at 3 μg/ml media. The plasmids expressing the MCP fusion proteins will be available through Addgene.
Single-molecule fluorescence in situ Hybridization (smFISH)
The sequences of smFISH probes (LGC Biosearch Technologies) are listed in Supplementary Table 4. Yeast strains were grown overnight at 26°C in synthetic medium (2% glucose) and auxotrophy complementing amino acids. Cells were diluted to OD600 0.1 in the morning and incubated at 26°C. After the culture reached OD600 0.3-0.4, cells were fixed by gently shaking at room temperature for 45 minutes in 4% paraformaldehyde (32% solution, EM grade; Electron Microscopy Science #15714). Cells were washed with ice-cold buffer B (1.2 M sorbitol and 100 mM potassium phosphate buffer pH=7.5), resuspended, and incubated for about 10 min at 30°C in 500 μL of spheroplast buffer (1.2 M sorbitol, 100 mM potassium phosphate buffer pH=7.5, 20 mM Ribonucleoside–vanadyl complex (NEB #S1402S), and 25 U of Lyticase enzyme (Sigma #L2524) per OD of cells). Digested cells were washed and resuspended in 1 ml of buffer B and seeded onto 18 mm poly-lysine (Sigma) treated coverslips (Fisher Scientific). The coverslips were incubated at 4°C for 30 minutes to allow cells to attach, washed with buffer B, and stored in 70% ethanol at -20°C for at least 3 hours. The coverslips were washed with 2xSSC at room temperature twice. A pre-hybridization mix (10% formamide (ACROS organics #205821000) in 2xSSC) was added to the coverslips. During the 30-minute room temperature incubations, the smFISH probes were prepared. For each coverslip, a speed vac was used to dry a mixture of 0.125 μL of 25 μM smFISH probe, 2.5 μL of 10 mg/μL E. coli tRNA, and 2.5 μl of 10 mg/μl E. coli ssDNA. The dried pellet was resuspended in 25 μL of hybridization mix (10% formamide, 2×SSC, 1 mg/ml BSA, 10 mM ribonucleoside-vanadyl complex (NEB), and 5 mM NaHPO4 pH 7.5) and boiled at 95°C for 2 minutes. The resuspended smFISH probes were applied to the coverslips for hybridization and incubated in the dark for 3 hours at 37°C. Next, coverslips were washed twice with pre-hybridization mix at 37°C for 15 minutes, followed by another three 10-minute washes at room temperature using 2xSSC with 0.1% Triton X-100, 1xSSC, and 1xPBS, respectively. Coverslips were mounted on microscope slides (Thermo Fisher Scientific) using ProLong Gold antifade with DAPI (Thermo Fisher Scientific).
To image P21 mRNA by smFISH, U2OS cells were washed with PBS (Corning), and cultured in fresh DMEM with 10% FBS and 10 μM of Nutlin-3 (Sigma-Aldrich). After 2 hours, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 20 min on ice. Then, paraformaldehyde was quenched by keeping the sample in PBS with 0.1 M glycine solution for 10 min at room temperature. The cells were washed with PBS+MC buffer (PBS with 1mM MgCl2 and 0.1mM CaCl2) three times at room temperature for 5 min each. To permeabilize cells, the sample was incubated with PBS-MC + 0.1% triton-x (PBS with 1mM MgCl2, 0.1mM CaCl2, 0.1% triton-x) on ice for 15 min. The cells were washed with PBS+MC buffer three times at room temperature. The sample was incubated with pre-hybridization buffer (10% Formamide, 2X SSC) for 30 min at 37°C. Next, the smFISH probes were hybridized to the sample in the hybridization buffer (10% deionized formamide, 1 mg/ml E. coli tRNA, 10% dextran sulfate, 0.2 mg/ml BSA, 2X SSC, 2 mM ribonucleoside–vanadyl complex (NEB), 10 U/ml Superase In (Thermo Fisher Scientific), 125 nM probe) overnight in a humidified chamber at 37°C. The sample was washed with pre-hybridization buffer twice for 20 min at 37°C, and then with PBS+MC buffer twice at room temperature. Coverslips were mounted on microscope slides (Thermo Fisher Scientific) using ProLong Gold antifade with DAPI (Thermo Fisher Scientific).
smFISH and mitochondria image acquisition and analysis
smFISH images were acquired on an Olympus BX63 wide-field epi-fluorescence microscope using a 100X/1.35NA UPlanApo objective, an X-cite 120 PC lamp (EXFO), and the ORCA-R2 Digital CCD camera (Hamamatsu). The Metamorph software (Molecular Devices) was used to control the microscope. Images were acquired at multiple stage positions with Z-sections at 200 nm intervals over an optical range of 8 μm. The smFISH images were quantified using FISH-quant39. The mitochondrial images were deconvolved with Huygens Professional version 19.04 (Scientific Volume Imaging, The Netherlands, http://svi.nl) and quantitatively analyzed using the MitoGraph software40. mRNA positions were determined using FISH-quant39. An in-house Matlab script was employed to determine the distance between each mRNA and the mitochondrial network. The Matlab script is available at the GitHub repository (https://github.com/WeihanLi-biology/An-improved-MS2-MCP-imaging-system-with-minimal-perturbation-of-mRNA-stability).
Live-cell fluorescence imaging and image analysis
Yeast cells were cultured in synthetic complete medium at 26°C until reaching an OD600 of 0.2-0.3. 200 μl of cell culture was pipetted onto glass-bottom MatTek dishes pre-coated with 1mg/ml Concanavalin A (Cayman chemical company). For live cell imaging of the P21 mRNA, the U2OS cells were washed with PBS, and treated with 10 μM of Nutlin-3 (Sigma-Aldrich) in Leibovitz’s L-15 Medium (Gibco) containing 10% FBS for 4 hours at 37°C. Live-cell imaging was performed using a microscope that can simultaneously acquire images at 488 nm- and 560 nm- channels. Specifically, the microscope was built around an IX71 stand (Olympus) controlled by the Metamorph software. A 491 nm laser (CalypsoTM, Cobolt) and a 561 nm laser (JiveTM, Cobolt) were used for excitation. The lasers were controlled by an acoustic-optic tunable filter (AOTF, AOTFnC-400.650-TN, AA Opto-electronic), combined and coupled into a single-mode optical fiber (Qioptiq). The fiber was collimated and delivered through the back port of the microscope. It was then reflected into an oil immersion objective lens (Olympus 150x 1.45 N.A) with a dichroic mirror (zt405/488/561rpc, 2mm substrate, Chroma). The 180 mm focal length tube lens was removed from the microscope to be placed outside the right port. Scattered light was filtered by a triple band notch emission filter (zet405/488/561m), and a dichroic mirror (T560LPXR, 3mm substrate, Chroma) enabled splitting of the fluorescence onto two aligned alignment stage mounted (x, y, z, θ- and φ- angle) EMCCD cameras (Andor iXon3, Model DU897). In front of the green and red channel cameras, Semrock emission filters were placed (FF03-525/50-25 and FF01-607/70-25). A DAQ board (Measurement Computing) generated a TTL pulse to trigger the two cameras for exposure. Additionally, a piezo stage (ASI) and Delta-T incubation system (Biopteck) were added to the microscope to allow fast z-stack and live-cell imaging, respectively. Images were streamed at 50 ms on a single Z-plane. Live-cell single-molecule tracking analysis was performed in Diatrack after pre-processing the images by subtracting the background and applying a Gaussian blur filter in ImageJ.
Yeast RNA extraction to analyze mRNA steady-state levels
The protocol of RNA extraction from yeast cells has been described in detail41. Specifically, yeast cells were cultured overnight in yeast extract peptone dextrose (YPD) medium at 26°C, and diluted to OD600 0.05 the next morning. The cells grew to OD600 0.4-0.5 and were harvested. For the RT-qPCR of HAC1 mRNAs, cells were treated with 1 μg/ml tunicamycin for 2 hours before being harvested. The samples were then resuspended in 200 μl AE buffer (50 mM sodium acetate, 10 mM EDTA), 10 μl 20% SDS and 250 μl Acid Phenol pH 4.5 (Ambion) and incubated at 65°C for 10 minutes in a thermal shaker at maximum speed. The samples were then centrifuged for 5 min at 21,000 g at room temperature. The upper aqueous phase was combined with 250 μl chloroform and added to phase-lock tubes (Thermo Fisher Scientific) for centrifugation at 11,000 g for 3 min at room temperature. The upper aqueous phase was combined with 15 μl 3 M Sodium Acetate and 375 μl 100% ethanol and precipitated at -80 degrees overnight. The RNA was pelleted by 30 min centrifugation at 4°C at 21,000 g, washed with ethanol, and dried on ice before being resuspended in water. RNA integrity was examined on agarose gels stained with SYBR Safe (Thermo Fisher Scientific).
Mammalian cell RNA extraction to analyze mRNA steady-state levels
RNA extraction was performed as described in the TRIzol Reagent User Guide (Ambion). In brief, TRIzol was added to the cells. After a 5 min incubation at room temperature, chloroform was added. The samples were centrifuged, and the aqueous phase isolated. Isopropanol was added to precipitate the RNA at 4°C. After centrifugation, the RNA pellet was washed with 75% ethanol before the dried pellet was resuspended in water. DNase treatment of the RNA was performed by adding 1 μl buffer and 1 μl DNase to the RNA sample and incubating at 37°C for 30 minutes. EDTA was thereafter added, and the samples were incubated at 65°C for 10 minutes. The RNA concentration was measured using nanodrop and diluted in water to 500 or 1000 ng/μl. RNA integrity was examined on agarose gels stained with SYBR Safe (Thermo Fisher Scientific).
cDNA synthesis and RT-qPCR analysis
cDNA was synthesized from 1 μg RNA using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific) and random hexamer primers (Thermo Fisher Scientific). For RT-qPCR, 1 μl diluted cDNA (1:10 in water) and SYBR green qPCR kit (Thermo Fisher Scientific) was used. The RT-qPCR was performed in triplicates on a ViiA 7 Real-Time PCR system (Thermo Fisher Scientific). mRNA levels were normalized to GAPDH mRNAs in yeast, ß-actin mRNA in U2OS, and GAPDH in MEFs. The RT-qPCR results analyzed using GraphPad Prism. Error bars of standard deviations were calculated from experimental triplicates. The RT-qPCR primers used in this study are listed in Supplementary Table 5.
RNA stability assay
Yeast cells at exponential phase OD600 ~0.4-1 were treated with 100 μg/ml phenanthroline for the indicated duration before being collected and analyzed by RT-qPCR. For HAC1 mRNA stability assay, yeast cells were treated with 1 μg/ml tunicamycin for 2 hours before adding 100 μg/ml phenanthroline. For P21 mRNA stability assay, U2OS cells were treated with 5 ug/ml actinomycin D. Samples at the indicated time points were collected and analyzed by RT-qPCR.
Protein extraction and western blot
Yeast cells were grown overnight at 26°C in YPD medium with 2% glucose. Cells were diluted to OD600 0.1 in the morning and grew to exponential phase OD600~0.4-1. Cells were lysed by adding 1 ml H2O with 150 μl lysing buffer (1.85 M NaOH,7.5% 2-mercaptoethanol) to the cell pellet consisting of ~3x107 cells. Proteins were then precipitated using 50% TCA. Cells were pelleted and resuspended in 100 ul 1x NuPAGE LDS sample buffer (Invitrogen).
U2OS cells were treated with 10 μM Nutlin-3 for 24 hours to induce P21 expression before lysing the cells with RIPA buffer (Thermo Fisher) with supplemented cOmplete ULTRA protease inhibitor cocktail (Roche). Cells were pelleted, and the supernatant containing the whole cell lysates was used for the western blot assay.
Electrophoresis was performed using NuPAGE Bis Tris 4-12% gradient gels (Invitrogen). Yeast cell protein lysates were examined by Western blot using mouse anti-myc (Sigma) (1:2000 dilution) and mouse anti-Pgk1antibodies (Thermo Fisher Scientific) (1:10000 dilution). Mammalian cell protein lysates were examined using rabbit anti-p21 (Thermo Fisher Scientific) (1:1000 dilution) and mouse anti-β-actin (Thermo Fisher Scientific) (1:1000 dilution) antibodies. For quantitative analyses, fluorescence secondary anti-mouse (IRDye 680RD) (1:2000 dilution) and anti-rabbit (IRDye 800CW) (1:2000 dilution) antibodies were used and visualized on a LYCOR scanner. Quantification of the signal was done in the LITE Software.
Yeast growth assays
Serial dilutions of S. cerevisiae cells were spotted onto YPD plates (glucose carbon source), YPG plates (glycerol carbon source), or YPD plates with 0.2 μg/ml tunicamycin. Plates were photographed after incubating at 26°C for 3-5 days.
Extended Data
Extended Data Fig. 1. The abundance of yeast MDN1, CLB2, ATP3, and ATP4 mRNAs are not reduced by MBS tagging.
(A) RT-qPCR of WT and MBS-tagged mRNAs. The mRNA levels were normalized to their corresponding WT mRNAs. n = 3 biologically independent experiments. Error bars indicate mean ± SD. ns, not significant (two-sided Student’s t-test). (B) Representative smFISH images of CLB2 mRNA (green). DNA was stained with DAPI (blue). Scale bars are 2 μm. (C) The number of CLB2 mRNAs per cell as quantified from the smFISH images. Each dot corresponds to one individual cell. The number of cells being analyzed are 658 (WT) and 1060 (CLB2-24xMBS). Three replicate experiments were performed. Error bars indicate mean ± SD.
Extended Data Fig. 2. Altering the location of the MBS array does not restore the ATP2 mRNA abundance.
(A) Illustration of the MBS-tagging positions on ATP2 mRNA. (B) RT-qPCR of the ATP2 mRNA in the indicated strains. The mRNA levels were normalized to the WT ATP2 mRNA level. Data were analyzed from three replicate experiments. The P values are <0.0001, 0.0001. Error bars indicate mean ± SD. ***P ≤ 0.001, ****P ≤ 0.0001 (two-sided Student’s t-test).
Extended Data Fig. 3. MBS tagging decreases the mRNA stability of HAC1 and PMA1.
(A, B, C) mRNA stability assay of HAC1 (A), PMA1 (B), and ACT1 (C). Cells were treated with phenanthroline to inhibit transcription. In the experiments measuring HAC1 mRNA stability, cells were treated with 1 μg/ml tunicamycin for 2 hours before adding phenanthroline. mRNA levels at the indicated time points were measured by RT-qPCR. A two-sided Student’s t-test was performed to compare WT and 24xMBS strains at the indicated time points. In (A), the P values from left to right are 0.0066, 0.0031, 0.0048. In (B), the P values from left to right are 0.031, 0.091, 0.59. *P ≤ 0.05, **P ≤ 0.01; ns, not significant. n = 3 biologically independent experiments. Error bars indicate mean ± SD.
Extended Data Fig. 4. The abundance of yeast Atp2 protein is reduced by MBS tagging and restored by expressing MCP-GFP-SUP35 or MCP-GFP-PAB1*.
Western blot analysis of Atp2 proteins in the indicated strains. Atp2 protein was c-terminally tagged with myc epitope. Pgk1 protein was used as a loading control. One representative image is shown from three replicate experiments.
Extended Data Fig. 5. MCP-GFP-PAB1* can be used to image MBS-tagged ATP2 mRNAs.
(A) Live-cell imaging of yeast cells expressing MCP-GFP-PAB1 (left) or MCP-GFP-PAB1* (right). Images were max-Z projected. Scale bars are 2 μm. The cell outline is marked with a white dashed line. (B, C) Sequence alignment of PAB1 homologs flanking the two conserved phenylalanines, which are F170 and F366 in yeast PAB1 (F142 and F337 in human PABPC1). These two phenylalanines were mutated to valines in PAB1*/PABPC1*.
Extended Data Fig. 6. Expression of MCP-GFP-SUP35 or MCP-GFP-PAB1* restores the stability of HAC1 and PMA1 mRNA.
(A, B, C) mRNA stability assay of the HAC1 (A), PMA1 (B), and ACT1 (C) mRNA. Experimental conditions were the same as Extended Data Figure 3. n = 3 biologically independent experiments. A two-sided Student’s t-test was performed to compare 24xMBS and 24xMBS + MCP-GFP-SUP35 strains at the indicated time points. *P ≤ 0.05, **P ≤ 0.01; ns, not significant (Student’s t-test). In (A), the P values from left to right are 0.0074, 0.0020, 0.0039. In (B), the P values from left to right are 0.014, 0.045, 0.15. Error bars indicate mean ± SD. (D) RT-qPCR of ACT1 mRNAs. The mRNA levels were normalized to the WT mRNA level. n = 3 biologically independent experiments. Error bars indicate mean ± SD. ns, not significant (two-sided Student’s t-test).
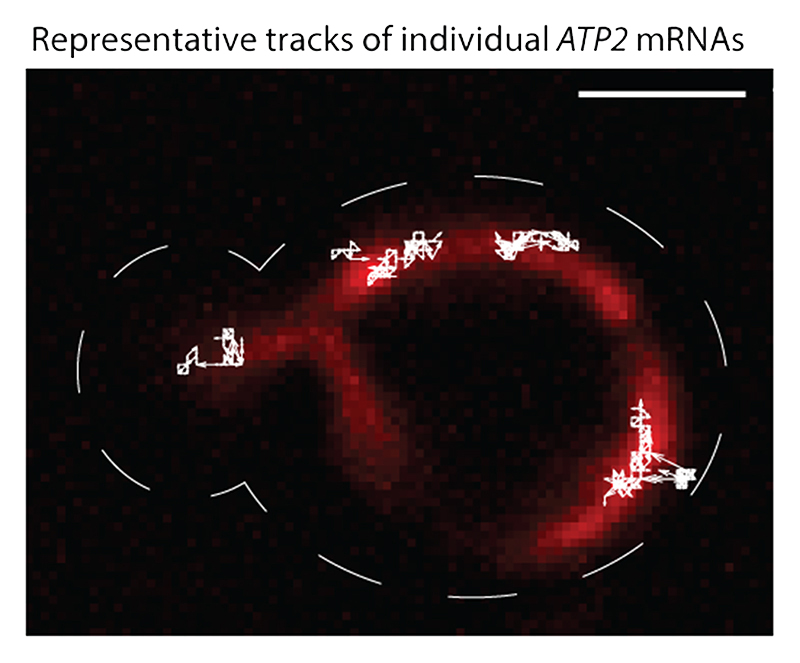
Extended Data Fig. 7. Tracking single-molecule ATP2 mRNAs using the improved MS2-MCP system.
The original movie is shown in Supplementary Movie 1. Mitochondria were labeled using mitochondria-targeted mKate2 (red). MBS-tagged ATP2 mRNAs were imaged using MCP-GFP-SUP35. The movie was acquired at 20 frames per second in one z plane. The figure shows the molecular trajectories of the mRNAs that were tracked for at least 40 consecutive frames (white arrowed lines). The cell outline is marked with a white dashed line. Scale bar is 2 μm.
Extended Data Fig. 8. smFISH images of P21 mRNAs in U2OS cells.
Representative images of P21 mRNA (green). DNA was stained with DAPI (blue). To induce the expression of P21, U2OS cells were treated with 10 μM Nutlin-3 for 2 hours. Scale bars are 4 μm. Two replicate experiments were performed.
Extended Data Fig. 9. Knock-down of UPF1 mRNA in U2OS cells.
RT-qPCR of UPF1 mRNA in the presence or absence of the UPF1 shRNA. n = 3 biologically independent experiments. Error bars indicate mean ± SD. ***P ≤ 0.001 (two-sided Student’s t-test). The P value is 0.0007.
Extended Data Fig. 10. Expression of MCP-GFP-eRF3 or MCP-GFP-PABPC1* restores the mRNA and protein abundance of P21 in U2OS cells.
(A) Increasing levels of MCP-GFP-PABPC1* better restored the P21 mRNA abundance. Cells expressing different levels of MCP-GFP-eRF3 or MCP-GFP-PABPC1* were sorted by FACS (L: low; M: medium; H: high). The level of the P21 mRNA was examined by RT-qPCR. n = 3 biologically independent experiments. Error bars indicate mean ± SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant (two-sided Student’s t-test). The P values from left to right are 0.0037, 0.0006, 0.010, <0.0001, 0.0080, 0.0018, 0.015. (B) Western blot of p21 protein. To induce the expression of P21, U2OS cells were treated with 10 μM Nutlin-3 for 24 hours. A monoclonal antibody against p21 was used. β-actin was used as a loading control. One representative image is shown from three replicate experiments.
Supplementary Material
Acknowledgments
We thank Allan Jacobson, U. Thomas Meier, Robert A. Coleman, and members of the Singer lab for their insightful discussions. We thank Xiuhua Meng for her help with cloning. This work was supported by American Heart Association Postdoctoral Fellowship #903024 (W.L), 1R35 GM136296-01 (R.H.S), and the European Research Council ERCStG-714739 IlluMitoDNA (C.O).
Footnotes
Author Contributions Statement
W.L and A.M designed and performed the experiments and analyzed the data. H.S provided guidance in generating mammalian cell lines expressing MCP-GFP-eRF3/PABPC1*. C.O provided the analysis pipeline to determine the distance between mitochondria and mRNA. W.L, A.M, and R.H.S conceived ideas and wrote the manuscript with input from H.S and C.O.
Competing Interests Statement
The authors declare no competing interests.
Editor’s summary
An improved version of the MS2-MCP system for imaging RNA dynamics involves tethering translation termination factors to tagged mRNAs to bypass destabilization caused by NMD machinery.
Data Availability
All the data and reagents used in this study are available upon request. The key plasmids of the MS2-MCP systems V8 and V9 will be available through Addgene.
Code availability statement
The MATLAB scripts used to measure the distance between mRNAs and mitochondria are available at the GitHub repository (https://github.com/WeihanLi-biology/An-improved-MS2-MCP-imaging-system-with-minimal-perturbation-of-mRNA-stability).
References
- 1.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tutucci E, Livingston NM, Singer RH, Wu B. Imaging mRNA In Vivo, from Birth to Death. Annu Rev Biophys. 2018;47:85–106. doi: 10.1146/annurev-biophys-070317-033037. [DOI] [PubMed] [Google Scholar]
- 3.Tutucci E, et al. An improved MS2 system for accurate reporting of the mRNA life cycle. Nat Methods. 2018;15:81–89. doi: 10.1038/nmeth.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosaki T, Popp MW, Maquat LE. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat Rev Mol Cell Biol. 2019;20:406–420. doi: 10.1038/s41580-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amrani N, et al. A faux 3’-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- 6.Kervestin S, Li C, Buckingham R, Jacobson A. Testing the faux-UTR model for NMD: analysis of Upf1p and Pab1p competition for binding to eRF3/Sup35p. Biochimie. 2012;94:1560–1571. doi: 10.1016/j.biochi.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peixeiro I, et al. Interaction of PABPC1 with the translation initiation complex is critical to the NMD resistance of AUG-proximal nonsense mutations. Nucleic Acids Res. 2012;40:1160–1173. doi: 10.1093/nar/gkr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C, Roy B, He F, Yan K, Jacobson A. Poly(A)-Binding Protein Regulates the Efficiency of Translation Termination. Cell Rep. 2020;33:108399. doi: 10.1016/j.celrep.2020.108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberle AB, Stalder L, Mathys H, Orozco RZ, Muhlemann O. Posttranscriptional gene regulation by spatial rearrangement of the 3’ untranslated region. PLoS Biol. 2008;6:e92. doi: 10.1371/journal.pbio.0060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebaara BW, Atkin AL. Long 3’-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:2771–2778. doi: 10.1093/nar/gkp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlrad D, Parker R. Aberrant mRNAs with extended 3’ UTRs are substrates for rapid degradation by mRNA surveillance. RNA. 1999;5:1299–1307. doi: 10.1017/s1355838299990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagalakshmi U, et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda M, Vassarotti A, Douglas MG. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Primary sequence analysis of ATP2 encoding the F1-ATPase beta-subunit precursor. J Biol Chem. 1985;260:15458–15465. [PubMed] [Google Scholar]
- 14.Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Maquat LE. UPFront and center in RNA decay: UPF1 in nonsense-mediated mRNA decay and beyond. RNA. 2019;25:407–422. doi: 10.1261/rna.070136.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatscher T, Gehring NH. Harnessing short poly(A)-binding protein-interacting peptides for the suppression of nonsense-mediated mRNA decay. Sci Rep. 2016;6:37311. doi: 10.1038/srep37311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatscher T, Boehm V, Weiche B, Gehring NH. The interaction of cytoplasmic poly(A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay. RNA. 2014;20:1579–1592. doi: 10.1261/rna.044933.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh G, Rebbapragada I, Lykke-Andersen J. A competition between stimulators and antagonists of Upf complex recruitment governs human nonsense-mediated mRNA decay. PLoS Biol. 2008;6:e111. doi: 10.1371/journal.pbio.0060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva AL, Ribeiro P, Inacio A, Liebhaber SA, Romao L. Proximity of the poly(A)-binding protein to a premature termination codon inhibits mammalian nonsense-mediated mRNA decay. RNA. 2008;14:563–576. doi: 10.1261/rna.815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deardorff JA, Sachs AB. Differential effects of aromatic and charged residue substitutions in the RNA binding domains of the yeast poly(A)-binding protein. J Mol Biol. 1997;269:67–81. doi: 10.1006/jmbi.1997.1013. [DOI] [PubMed] [Google Scholar]
- 21.Margeot A, et al. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J. 2002;21:6893–6904. doi: 10.1093/emboj/cdf690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lionnet T, et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods. 2011;8:165–170. doi: 10.1038/nmeth.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvajal LA, et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aao3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braselmann E, Rathbun C, Richards EM, Palmer AE. Illuminating RNA Biology: Tools for Imaging RNA in Live Mammalian Cells. Cell Chem Biol. 2020;27:891–903. doi: 10.1016/j.chembiol.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash BC, El-Deiry WS. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell Biol. 2005;25:3364–3387. doi: 10.1128/MCB.25.8.3364-3387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia W, et al. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res. 2004;10:3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- 28.Karousis ED, Gypas F, Zavolan M, Muhlemann O. Nanopore sequencing reveals endogenous NMD-targeted isoforms in human cells. Genome Biol. 2021;22:223. doi: 10.1186/s13059-021-02439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Echevarria MJ, Peltz SW. The RNA binding protein Pub1 modulates the stability of transcripts containing upstream open reading frames. Cell. 2000;101:741–751. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 30.Ge Z, Quek BL, Beemon KL, Hogg JR. Polypyrimidine tract binding protein 1 protects mRNAs from recognition by the nonsense-mediated mRNA decay pathway. Elife. 2016;5 doi: 10.7554/eLife.11155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annibaldis G, et al. Readthrough of stop codons under limiting ABCE1 concentration involves frameshifting and inhibits nonsense-mediated mRNA decay. Nucleic Acids Res. 2020;48:10259–10279. doi: 10.1093/nar/gkaa758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurosaki T, Maquat LE. Rules that govern UPF1 binding to mRNA 3’ UTRs. Proc Natl Acad Sci U S A. 2013;110:3357–3362. doi: 10.1073/pnas.1219908110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Vieira M, Kim HJ, Kesawat MS, Park HY. MS2 Labeling of Endogenous Beta-Actin mRNA Does Not Result in Stabilization of Degradation Intermediates. Mol Cells. 2019;42:356–362. doi: 10.14348/molcells.2019.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das S, Moon HC, Singer RH, Park HY. A transgenic mouse for imaging activity-dependent dynamics of endogenous Arc mRNA in live neurons. Sci Adv. 2018;4:eaar3448. doi: 10.1126/sciadv.aar3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Puerta S, et al. Live imaging of the co-translational recruitment of XBP1 mRNA to the ER and its processing by diffuse, non-polarized IRE1alpha. Elife. 2022;11 doi: 10.7554/eLife.75580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aragon T, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457:736–740. doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato H, Singer RH. Cellular variability of nonsense-mediated mRNA decay. Nat Commun. 2021;12:7203. doi: 10.1038/s41467-021-27423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer JW, Busa VF, Shao Y, Leung AKL. Structure-Mediated RNA Decay by UPF1 and G3BP1. Mol Cell. 2020;78:70–84.:e76. doi: 10.1016/j.molcel.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller F, et al. FISH-quant: automatic counting of transcripts in 3D FISH images. Nat Methods. 2013;10:277–278. doi: 10.1038/nmeth.2406. [DOI] [PubMed] [Google Scholar]
- 40.Viana MP, Lim S, Rafelski SM. Quantifying mitochondrial content in living cells. Methods Cell Biol. 2015;125:77–93. doi: 10.1016/bs.mcb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Singer RH. Detecting the Non-conventional mRNA Splicing and Translational Activation of HAC1 in Budding Yeast. Methods Mol Biol. 2022;2378:113–120. doi: 10.1007/978-1-0716-1732-8_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data and reagents used in this study are available upon request. The key plasmids of the MS2-MCP systems V8 and V9 will be available through Addgene.
The MATLAB scripts used to measure the distance between mRNAs and mitochondria are available at the GitHub repository (https://github.com/WeihanLi-biology/An-improved-MS2-MCP-imaging-system-with-minimal-perturbation-of-mRNA-stability).