Abstract
Individual differences in working memory capacity (WMC) strongly predict variations in real-world cognitive functioning. However, little is known about how WMC is influenced by the ubiquitously present affective information in our everyday environments. Here, we present a series of 3 experiments investigating a novel WMC paradigm performed in affective (versus neutral) contexts. The paradigm requires simultaneous performance of a visuospatial search and a verbal storage task. These tasks are performed in the presence of either neutral or negative emotional distractor images. Experiments 1 & 2 confirmed our prediction that WMC would be reduced in the context of emotional compared to neutral distractors in student and community samples. Experiment 3 extended these findings to a clinical sample. WMC in motor vehicle accident survivors with a history of PTSD was selectively reduced in the presence of trauma-related emotional distraction compared to survivors without a history of PTSD. Implications of these findings for affective cognitive science are discussed.
Keywords: working memory capacity, emotion, posttraumatic stress disorder, complex span, trauma
Introduction
Working memory capacity (WMC) describes our ability to process and store goal-relevant information while inhibiting the processing of distracting material (Engle, 2002). WMC has proved a powerful explanatory construct in cognitive psychology. Individual differences in WMC strongly predict variations in higher cognitive functioning and performance on real-world cognitive tasks (e.g., reading and language comprehension, reasoning, emotion processing; Barrett, Tugade, & Engle, 2004; Kane & Engle, 2003). In daily life, WMC is recruited when negotiating cognitively dynamic environments with changing goal-demands, motivational states, and affective contexts. In such real-world scenarios, much of the demand on WMC is exerted by information or processing contexts with salient personal and/or emotional characteristics. Despite this, our present corpus of laboratory research has generally eschewed examining WMC in such situations and so the question of how the emotional nature of the material being processed impacts upon WMC remains a neglected one (Baddeley, 2003, 2013).
There is good reason to suppose that the involvement of emotional information as memoranda or as distractors in situations demanding of WMC will have a significant impact because we know that key cognitive processes (incl., executive attention and response inhibition) that underpin WMC are affected by such information. Specifically, attention which is critical to the central executive’s function (Baddeley, 1998; Engle, 2002; Engle, Kane, & Tuholski, 1999) is altered in the presence of emotional information (Vuilleumier, 2005). That is, it is harder to inhibit attention and responses to emotional relative to neutral distractors and such information appears to receive prioritized neural processing, arguably due to its survival relevance (LeDoux, 2012; Vuilleumier & Huang, 2009). The perceptual and potentially higher-order processing of affective information arguably recruits resources from capacity-limited WM – the central executive, especially – and its neural substrates in the fronto-parietal control network (Nee et al., In Press). For a recent review on the neural network involved in the top-down control of emotional distraction specifically see Iordan and colleagues (2013). If the affective information is presented as distracting stimuli this would consequently lead to impaired WM function, alternatively if the affect is inherent to the to-be-remembered stimuli the increased elaboration of emotional vs. neutral material would lead to improved WM performance. Similarly, short-term retention seems to be enhanced if the memoranda are emotional (e.g. Kousta, Vincent & Vigliocco, 2009). There is also a small body of research, with equivocal findings, that has studied the influence of emotional contexts on WM (e.g., Kensinger & Corkin, 2003; Perlstein, Elbert, & Stenger, 2002). However, these studies have mostly used simple operationalizations of WM such as the delayed-match-to-sample task that requires the retention of a small set of stimuli over a short time interval during which emotional distractors are presented (Anticevic, Repovs, Corlett, & Barch, 2011; Dolcos & McCarthy, 2006; Mather et al., 2006). These kinds of tasks tap solely into the storage component of WM (Baddeley, 2003), which is not a reliable predictor of WMC and higher cognitive functions (Conway et al., 2005; Kane et al., 2004).
The primary aim of the present series of experiments was therefore to investigate the influence of affective material on WMC. WMC is prototypically operationalized as the number of items that can be recalled on so-called complex span tasks (Conway et al., 2005). These tasks require parallel performance on a cognitively-demanding target memory storage task and a distractor task. The challenges posed are therefore analogous to the multiple goal-demands of everyday life (Conway et al., 2005). We reasoned that WMC, as assessed by complex span, would be differentially affected by the presence of emotional (relative to affectively neutral) information. In particular, we hypothesized that the presence of an emotionally salient context (i.e., task-irrelevant background images) would impair WMC, relative to an affectively neutral context. We expected a complex span task to be particularly sensitive to the effects of emotional distractors – to a greater extent than simple span tasks and simple retention-distraction WM paradigms (e.g., delayed-match-to-sample task) – as it relies more heavily on executive attention (Conway et al., 2005). To investigate this we designed a complex span task that could be presented against different contextual backgrounds - the Affective Picture Span Paradigm (APSP) - to measure ‘affective WMC’. The APSP constitutes a verbal word span component (the target task) and a visuospatial search component (the operation task). The target task required sequential storage of words, whereas the operation task was designed to be cognitively engaging but with sufficiently low processing demands so as to minimally engage WM. Critically, the target and operation tasks are performed on a computer in the presence of either neutral or emotional full-screen background images.
The hypothesized impairing effect of negative context (i.e., task-irrelevant distractor images) on WMC was investigated in a series of three experiments, which included different samples and a potential clinical application of the task. First, we tested the effect of emotional context in a student sample (Experiment 1). We next investigated whether these findings were replicable in a larger, more socioeconomically and educationally diverse community sample (Experiment 2). Finally, we investigated the clinical application of the task using trauma-relevant background images in a sample of motor vehicle accident (MVA) survivors (Experiment 3). For Experiment 3, we hypothesized that trauma-survivors with a history of posttraumatic stress disorder (PTSD) would be more impaired by the negative trauma-relevant background images than trauma-survivors who never experienced PTSD.
Experiment 1
Experiment 1 tested the hypothesis that APSP performance in negatively-valenced visual contexts would be poorer than in neutrally-valenced contexts in an unselected student sample.
Methods
Participants
Thirty-one participants (M=23 years; SD=2.6; 19 women) were recruited from the University of Cambridge graduate and undergraduate populations via newsletters. Participants met study inclusion for age (>18 years), language (English fluency), vision (normal or corrected normal), and neurological health (no history of neurological disorders or head injuries).
Measures and Materials
APSP
The APSP comprised two cognitively-engaging components – a target (storage) task and an operation task – which were performed simultaneously in the presence of either neutral (APSP-Neu) or negatively emotional (APSP-Neg) background images. The storage component required participants to retain a set of 4-7 words, presented one word per trial, in 4-7-trial blocks against a background image (see Supplementary Methods for stimuli details). For the operation task, in each trial, participants counted shapes that popped-up over the same background image before and after the word was presented. There were two types of shapes (turquoise circles and pink squares) in each trial and the target shape for counting (circle or square) varied across blocks. Valence of the task was manipulated by presenting a background image that was either emotionally neutral or negatively aversive in content.
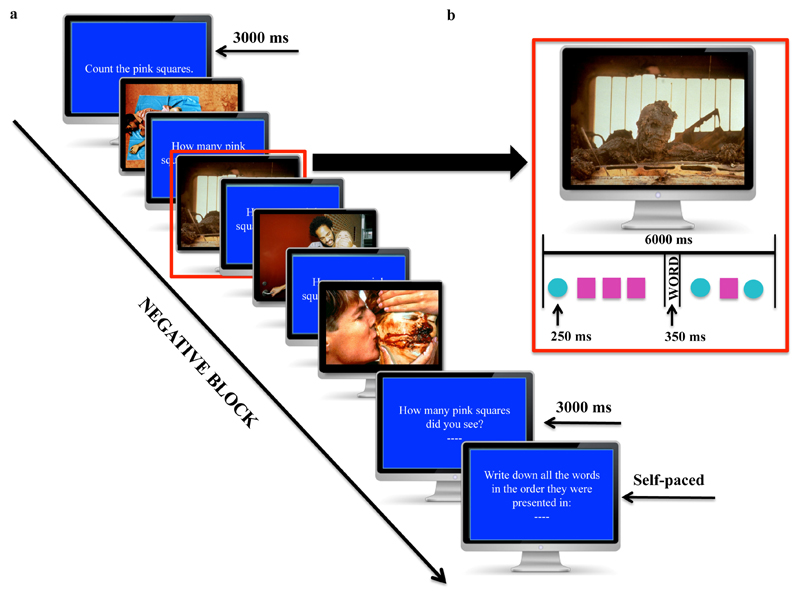
For each trial 7 shapes appeared over the course of 6 seconds against the background image that was continually present during this time (Figure 1). Within the first 3 seconds of the trial, four shapes popped-up over the background image for 250 milliseconds each1. Next the to-be-remembered-word for that trial appeared for 350 milliseconds over the image. Finally, the last three shapes (duration: 250ms/shape) were presented. The screen, including the image, then cleared and participants were prompted to enter the number of target shapes they had counted. There were four to six target shapes per trial for participants to count, with an average of 5 target shapes per trial, within each trial block. At the end of a block, participants were asked to recall as many presented words as possible, and it was stressed that they should recall them in the correct presentation order. This recall screen was displayed until participants pressed a key to continue. As the screen presented seven boxes to enter the recalled words into, there was no cue as to how many trials participants had seen in the current block. Each block length (4-7) was presented twice in each valence condition, giving a total of 88 trials over 16 blocks.
Figure 1. Sample trial sequence for the Affective Picture Span Paradigm (APSP).
(a) A negatively valenced trial block comprising 4 trials. In this example, the target shapes are the pink squares. That is, participants have to count the pink squares that appear during the trial, while ignoring the turquoise circles. (b) The time course of a single trial in which four target shapes are presented.
To compute complex span scores for the negative (APSP-Neg) and neutral (APSP-Neu) conditions we calculated the proportion of all words that were recalled in the correct position (Conway et al., 2005).
Procedure
Participants provided written consent, and the study was approved by the Cambridge Psychology Research Ethics Committee. Participants then provided demographic information on a structured questionnaire. We next administered the National Adult Reading Test2 before participants completed the APSP. The presentation order of the APSP’s two conditions was counterbalanced. Testing was conducted in a soundproofed cubicle on a desktop computer with an 18 inch screen. At the end of the testing session participants were compensated for their time (£6/hour; consistent across all 3 studies).
Results and Discussion
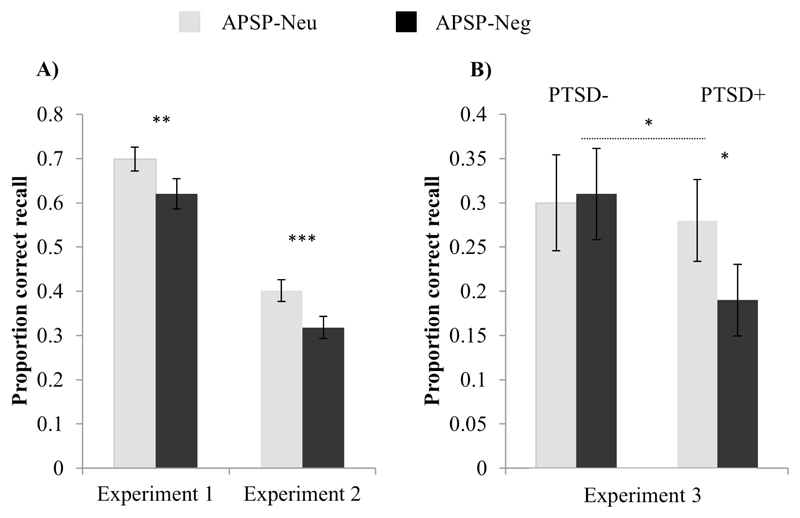
In line with our hypothesis, participants’ APSP-performance was significantly impaired by negative (APSP-Neg) compared to neutral (APSP-Neu) background images, t(30)=2.91, p=.007, d=.52, 95% CI[.02, .13] (Figure 2A). That is, the emotional context appeared to recruit attentional control away from memory storage to a greater degree, relative to neutral contexts, even though the emotional context was irrelevant to the WMC task. This is consistent with the affective neuroscience literature reporting that emotional distractors are processed even if they are irrelevant to the current task, and require attentional control to inhibit distraction (Vuilleumier & Huang, 2009).
Figure 2. WMC across APSP conditions.
Error bars=1 SEM. * P<.05;** P<.01; *** P<.001. PTSD+= trauma-exposed individuals with a lifetime history of PTSD, PTSD-= trauma-exposed individuals with no lifetime history of PTSD.
Experiment 2
Given the risk of Type II error when investigating novel paradigms with relatively small sample sizes and the premium placed on replication (Ioannidis, 2012), Experiment 2 sought to directly replicate our first experiment, this time in an unselected community sample.
Methods
Participants
Fifty-nine participants (M=45 years; SEM=1.68; 45 women) were recruited by community advertisement. Inclusion criteria and all materials and procedures were identical to Experiment 1. It should be noted that a nonparametric binomial test of the distribution, revealed a trend effect toward a greater proportion of women in the sample of Experiment 2. Gender has previously been shown to affect emotion processing both at the behavioural and neural level of analysis (McClure, 2000; Wager, Phan, Liberzon, & Taylor, 2003), the analysis was therefore repeated with gender as a covariate.
Results and Discussion
Directly replicating the results from Experiment 1, Experiment 2 provided further support for the detrimental effect of emotional context on WMC. Performance on APSP-Neg was significantly reduced compared to APSP-Neu, t(58)=4.22, p<.001, d=.55, 95% CI[-.07, -.03] (Figure 2A). The effect size was almost identical in the two studies, although overall performance was, not unexpectedly, lower in the community sample compared to the student sample.
As noted above we repeated the analysis including gender as a covariate. This showed no significant effect of gender on valence; that is, there was no significant interaction (p = .850) and the main effect of valence remained significant, p = .009. Moreover, the large proportion of women in a sample with a mean age of 45 years raised the possibility of effects of menopause on WMC performance. To address the issue we repeated the analyses excluding women between 51 years (average age of menopause in a representative sample; Bruin et al., 2001) and 60 years (conservative upper age limit for menopause). The paired sample t-test comparison remained significant after excluding these women (reduced n = 39), t (38) = 3.44, p = .001.
Experiment 3
Research in cognitive neuroscience and the clinical sciences reliably shows that the capacity to direct attention and responses away from negative distractors is relatively impaired in individuals with emotional disorders (Etkin & Wager, 2007; Joormann, Levens, & Gotlib, 2011). However, to our knowledge only one study has compared WMC using affective material in emotional disorders on a complex span task – in this case a reading span task (Schweizer & Dalgleish, 2011). The task required trauma-exposed individuals with and without a lifetime history of PTSD to make a semantic decision about neutral or trauma-relevant sentences (operation task) and remember an unrelated word at the end of these sentences for recall at the end of each block (storage task). Compared to the group who had never suffered PTSD, WMC in individuals with a lifetime history of PTSD was relatively more impaired in the context of trauma-related sentences.
Experiment 3 sought to extend upon this earlier research by examining the effects of an affective context (as opposed to an affective operation task) on WMC using the APSP. We therefore examined APSP-performance in trauma-exposed individuals (survivors of motor vehicle accidents [MVAs]) who had developed PTSD (PTSD+). In the light of previous research showing greater cognitive interference from disorder-specific as opposed to unselected negative stimuli (Moradi, Taghavi, Neshat Doost, Yule, & Dalgleish, 1999) we adapted the APSP by using trauma-related material as the negative emotional context (mildly evocative images of MVAs) and compared this to relevant neutral material (images of traffic scenes). As the comparison sample, we selected a trauma-exposed group of MVA survivors who had never suffered from PTSD (PTSD-). This ensured that any case-control differences were not a function of differential familiarity with the emotional material. That is, the inclusion of trauma-survivors without PTSD as a control group (as opposed to non-trauma-exposed individuals) allowed us to infer with more confidence that differential group effects were due to the effects of PTSD symptomatology rather than trauma-exposure. We hypothesized that MVA-survivors with a history of PTSD would show reduced WMC in the context of MVA pictures (APSP-Neg) compared to traffic pictures (APSP-Neu), relative to MVA survivors who had never suffered from PTSD.
Methods
Participants
Twenty-seven survivors of life-threatening or fatal MVAs (M=46 years; SEM=1.92; 16 women) were recruited through newspaper advertisements and from local clinics. We assessed whether participants had developed PTSD at any point as a result of the MVA using the Structured Clinical Interview for the DSM (SCID; First, Spitzer, Gibbon, & Williams, 1995).
The PTSD+ (n=12; 10 female, 5 currently suffering from PTSD, 7 with remitted PTSD) and PTSD- (n=15, 6 female) groups did not significantly differ on age, gender ratio, or verbal IQ as assessed by the National Adult Reading Test (Nelson, 1982). As expected there were significant differences in depressive symptomatology on the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and a trend for differences trait anxiety on the Spielberger State and Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970). However, there were no differences in current levels of state anxiety (Table S1).
APSP
The APSP-Neu was populated with emotionally neutral images of traffic scenes and the APSP-Neg with images of MVAs. The two sets of images were rated on valence, arousal, image complexity and picture quality by seven independent raters. We selected raters who had never been involved in a MVA to minimize the influence of personal circumstances on ratings. The two sets of images differed in valence and arousal, but were matched for image complexity and picture quality (Table S2). It should be noted, however, that MVA images, although somewhat affectively negative, were only rated as mildly evocative with an emotional impact that was significantly lower than that of the emotional images used in Experiments 1 and 2 (difference in mean distress ratings: F(1,114)=20.05, p<.001, ηp2=.88). We therefore did not anticipate that picture content would markedly affect WMC for PTSD- participants, despite their involvement in an MVA.
Participants completed the SCID, questionnaire measures and the NART, before completing the APSP.
Results and Discussion
A group (PTSD+, PTSD-) by condition (APSP-Neg, APSP-Neu) mixed model ANOVA revealed the predicted condition by group interaction, F(1, 26)=5.74, p=.025, ηp2=.19 (Figure 2B), with the PTSD+ group being significantly more impaired by MVA versus traffic distractors, t(11)=2.01, p=.03, d=.61, 95% CI[00, .18], compared to PTSD- participants who showed no significant effect of background context, t<1. This interaction qualified a weak trend for a main effect of APSP condition F(1,25)=2.93, p=.099, ηp2 =.11, with performance being more impaired by MVA scenes relative to traffic scenes.
These findings reveal a selective deficit in WMC as a function of a trauma-relevant visual context in trauma survivors who have a history of PTSD. This is unlikely to be due to differential familiarity with the trauma-related information as the effect was not found in trauma survivors who had never suffered PTSD. These results add to the corpus of studies revealing profound effects of PTSD on multiple domains of cognition (see Dalgleish, 2004) and extend the findings of Schweizer and Dalgleish (2011) detailing the effects of trauma-related operation task material on WMC. Moreover, the lack of impact of negative distractor material in trauma-survivors without PTSD is noteworthy. These findings are likely due to the reduced emotional impact of the negative MVA images compared to the IAPS images used in Experiments 1 and 2. Alternatively, however, this finding could indicate a general resilience factor of good cognitive control over emotional material in trauma-survivors who have not succumbed to PTSD. Future research should investigate whether this population shows a generalized ability to successfully ignore task-irrelevant affective information.
Finally, it should be noted that the overall WMC performance was lower and the variability greater (as expressed by the greater error variance in the standard error of the mean) in Experiment 3 compared to Experiments 1 and 2. This profile is common in clinical and trauma-exposed groups (e.g., DePrince, Weinzierl, & Combs, 2009).
General Discussion
Here we show for the first time to our knowledge that emotionally highly negative contexts can impair WMC in groups of student and unselected community participants in a replicable manner, relative to performance in affectively neutral contexts (Experiments 1 & 2). This is consistent with other work on the automatic attention grabbing properties of negative information that, while adaptive in surroundings with survival threats, appears to impair cognitive performance in everyday life (Vuilleumier & Huang, 2009). In addition or alternatively to these putative effects of negative distractors on lower level attentional processes (Öhman, Flykt, & Esteves, 2001; Yamasaki, LaBar, & McCarthy, 2002), negative background images may engender differential cognitive processing (e.g., depth of processing, rumination) compared to neutral background information (Baddeley, In Press; Wisco, 2009) and thereby place greater demands on executive functions underlying WMC such as inhibition and its neural substrates in the fronto-parietal control network (Goeleven, De Raedt, Baert, & Koster, 2006; Joormann et al., 2011; Nee et al., In Press). Interestingly, individuals with greater WMC show better emotion regulation capacity (Schmeichel, Volokhov, & Demaree, 2008) and the neural network involved in successful WM performance has also been implicated in emotion regulation (Buhle et al., In Press; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). The WMC impairment in emotional contexts may thus partially be accounted for by the recruitment of the lateral prefrontal cortex and superior parietal lobe for the regulation of emotions elicited by the task-irrelevant distractor images, making these neural regions and their cognitive correlates less available for the performance of the WM task.
These findings are complemented by those of Experiment 3 which reveal that trauma-relevant contexts that are of insufficient negative impact to impair WMC in trauma survivors who have never suffered PTSD, nevertheless significantly reduce WMC, relative to neutral contexts, in individuals who have suffered PTSD in the aftermath of trauma. These findings are in line with both Morey (2009) and Zhang (2013) and colleagues who have shown impaired WM performance on simple delayed-match-to-sample tasks in the presence of trauma-specific and generally negative distractor materials, respectively. These deficits in individuals with PTSD compared to healthy controls were associated with increased activation in the amygdala and reduced activation of the fronto-parietal control network, while performing the WM task with negative relative to neutral distractors. Improving individuals’ WMC in affective contexts may therefore particularly benefit those suffering from affective psychopathology such as PTSD. We recently showed that training WM for affective material in healthy controls can improve both lower-level attentional control over emotional information (Schweizer, Hampshire, & Dalgleish, 2011) and higher-order emotion regulation (Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, 2013). Future research should investigate whether these benefits extend to clinical populations, such as patients with PTSD.
A number of additional interesting questions arise from the present study which could be addressed in future research. Namely, is the impairing effect of background images on WMC specific to negative distractors or would positive images evoke the same impairment due to preferential attentional processing. Pessoa’s (2008) neural account of emotion-cognition interactions suggests that only high arousal positive images would have a detrimental effect on WMC whereas low arousal positive material would not affect WMC. Moreover, the effects (typically impairing) of negative stimuli in a wide-range of cognitive tasks (e.g., the emotional Stroop paradigm) have commonly been found to be exacerbated in those suffering from emotional disorders (Williams, Mathews, & MacLeod, 1996). It would therefore be interesting to see whether the effects found in Experiment 3 can be replicated in other clinical groups (e.g., depression) and whether they are dependent on the background images being disorder-specific. It could further be that individuals with PTSD, compared to other types of psychopathology, are more sensitive to pictorial distractors given the frequently perceptual nature of triggers of intrusive re-experiencing (Ehlers, Hackmann, & Michael, 2004). Finally, an issue that remains unexplored by the present study is whether the effects might be enhanced if the presentation-modality of the distractors was congruent with that of the memoranda. That is, if instead of a series of words a series of images had to be retained over the course of each block or alternatively if the visuospatial operation task was replaced by a verbal operation task in the presence of either neutral or negative verbal distractors. There is some evidence from the affective WM literature, which suggests that modality-congruent distractors may indeed potentiate the impairing effect of emotional information on WM performance (King & Schaefer, 2011). Specifically, the authors showed that performing an n-back task for faces in the presence of pictorial distractors impaired performance, whereas performance on an n-back task for words remained unaffected by distracting images.
In addition to these questions that remain unexplored by the current study, there are a number of study limitations that merit consideration when evaluating the current results. A limitation was that participants did not provide their individual ratings of the distractor images, which would have allowed us to investigate whether the effects of valence were parametrically related to either the images’ impact (Ewbank, Barnard, Croucher, Ramponi, & Calder, 2009) or to the subjective self-reported arousal that they elicited. Research into the effects of emotional distraction has shown that the interaction between the effects of valence on perceptual processing and attention (Lavie, 2005) can be dependent on the perceived emotionality of the stimuli (Shafer et al., 2012; i.e., the effects are only present for stimuli subjectively rated as highly emotional). Moreover, individuals’ ratings of emotional distractors during a WM task have been shown to correlate with amygdala activation (Dolcos, Diaz-Granados, Wang, & McCarthy, 2008), the down-regulation of which in turn may reduce executive capacity available for the WM task at hand. Another potential issue with the current study is that, in Experiment 3, we did not control for the possible effects of sleep problems, which are both common in PTSD (Germain, Buysse, Shear, Fayyad, & Austin, 2004) and impact WMC (Backhaus et al., 2007; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Walker, 2009). There is however preliminary evidence that the effects of valence may be independent of sleep, with WM performance deteriorating following sleep-deprivation irrespective of distractor valence, that is, there was no significant interaction of condition (sleep-deprived vs. rested) and valence (negative vs. neutral distractors; Chuah et al., 2010). Finally, it should be noted that the current study did not include positive distractors, consequently no inferences can be drawn about the effects of valence versus arousal, which have been shown to affect long-term memory for emotional events through differential cognitive and neural pathways (Kensinger & Corkin, 2004). As noted in the suggestions for future research above, the effects of positive distractors on WMC should be explored, while systematically varying the arousal of the differentially valenced material.
In conclusion, the present study shows that WMC as measured by a novel complex span task is impaired in the presence of negative (relative to neutral) distractor images. These effects appear to be especially prominent in individuals with PTSD for trauma relevant material. Future research should investigate the predictive utility of individual differences in APSP performance in terms of mental health and how people negotiate emotionally-charged cognitively-demanding situations in everyday life.
Supplementary Material
Footnotes
Accurate target detection rate was above the 75% cutoff in all three experiments see Supplementary Results for further details.
Extreme scores on the National Adult Reading Test were an exclusion criterion (fewer than 10 words read correctly). No participants in any of the experiments met the threshold for exclusion.
Contributor Information
Susanne Schweizer, Medical Research Council Cognition and Brain Sciences Unit, Cambridge, UK.
Tim Dalgleish, Medical Research Council Cognition and Brain Sciences Unit, Cambridge, UK And Cambridgeshire and Peterborough NHS Foundation Trust.
References
- Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and nonemotional interference with visual working memory in schizophrenia. Biological Psychiatry. 2011;70(12):1159–1168. doi: 10.1016/j.biopsych.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learning & Memory. 2007;14(5):336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. Working memory and emotion: Rumintions on a theory of depression. Review of General Psychology. In Press. [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255(5044):556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The central executive: A concept and some misconceptions. Journal of the International Neuropsychological Society. 1998;4(05):523–526. doi: 10.1017/s135561779800513x. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–39. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory and emotion: Ruminations on a theory of depression. Review of General Psychology. 17(1):20. [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130(4):553–73. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Cooperation; 1996. [Google Scholar]
- de Bruin JP, Bovenhuis H, van Noord PAH, Pearson PL, van Arendonk JAM, te Velde ER, et al. Dorland M. The role of genetic factors in age at natural menopause. Human Reproduction. 2001;16(9):2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. doi: 10.1093/cercor/bht154. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah LYM, Dolcos F, Chen AK, Zheng H, Parimal S, Chee MWL. Sleep deprivation and interference by emotional distracters. Sleep. 2010;33(10):1305–1313. doi: 10.1093/sleep/33.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–86. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Croucher CJ, Calder AJ, Ramponi C, Barnard PJ, Murphy FC. Disgust enhances the recollection of negative emotional images. PloS One. 2011;6:e26571. doi: 10.1371/journal.pone.0026571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child Abuse & Neglect. 2009;33(6):353–361. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Diaz-Granados P, Wang L, McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46(1):326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory. 2004;12(4):403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11(1):19–23. [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. In: Models of working memory: Mechanisms of active maintenance and executive control. Miyake A, Shah P, editors. New York, N.Y.: Cambridge University Press; 1999. Individual differences in working memory capacity and what they tell us about controlled attentions, general fluid intelligence, and functions of the prefrontal cortex; pp. 102–134. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager T. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive and Affective Neuroscience. 2009;4(2):127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Shear MK, Fayyad R, Austin C. Clinical correlates of poor sleep quality in posttraumatic stress disorder. Journal of Traumatic Stress. 2004;17(6):477–484. doi: 10.1007/s10960-004-5796-6. [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Baert S, Koster EHW. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93(1-3):149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Iordan AD, Dolcos S, Dolcos F. Neural signatures of the response to emotional distraction: A review of evidence from brain imaging investigations. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Levens SM, Gotlib IH. Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychological Science. 2011;22(8):979–983. doi: 10.1177/0956797611415539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133(2):189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3(4):378–393. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R, Schaefer A. The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology. 2011;48(2):269–272. doi: 10.1111/j.1469-8986.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. 2008
- Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Greene EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18(4):614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126(3):424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: Use of the modified Stroop paradigm. Journal of Traumatic Stress. 1993;6(1):33–41. [Google Scholar]
- Moradi AR, Taghavi MR, Neshat Doost HT, Yule W, Dalgleish T. Performance of children and adolescents with PTSD on the Stroop colour-naming task. Psychological Medicine. 1999;29(2):415–419. doi: 10.1017/s0033291798008009. [DOI] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, LaBar KS, McCarthy G. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatry Research. 2009;43(8):809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cerebral Cortex. doi: 10.1093/cercor/bhs007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Sciences. 2002;99(3):1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95(6):1526. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Dalgleish T. Emotional working memory capacity in posttraumatic stress disorder (PTSD) Behaviour Research and Therapy. 2011;49(8):498–504. doi: 10.1016/j.brat.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the emotional brain: Improving affective control through emotional working memory training. Journal of Neuroscience. 2013;33(12):5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Hampshire A, Dalgleish T. Extending brain-training to the affective domain: Increasing cognitive and affective executive control through emotional working memory training. PLoS ONE. 2011;6(9):e24372. doi: 10.1371/journal.pone.0024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AT, Matveychuk D, Penney T, O’Hare AJ, Stokes J, Dolcos F. Processing of emotional distraction is both automatic and modulated by attention: evidence from an event-related fMRI investigation. Journal of Cognitive Neuroscience. 2012;24(5):1233–1252. doi: 10.1162/jocn_a_00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for State Trait Inventory. Palo Alto, CA: Counseling Psychologists Press; 1970. [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin. 2007;133(6):1038–66. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Huang Y-M. Emotional attention: Uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science. 2009;18(3):148–152. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/S1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156(1):168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Wisco BE. Depressive cognition: Self-reference and depth of processing. Clinical Psychology Review. 2009;29(4):382–392. doi: 10.1016/j.cpr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-N, Xiong K-L, Qiu M-G, Zhang Y, Xie B, Wang J, et al. Zhang J-J. Negative emotional distaction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Research. 2013;1531:94–101. doi: 10.1016/j.brainres.2013.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.