Abstract
Crescentic glomerulonephritis is characterized by vascular necrosis and the formation of parietal epithelial cell (PEC) hyperplasia, but little is known about the molecular mechanisms driving this process. Inducing crescentic glomerulonephritis in several Pax2Cre reporter mouse lines revealed that crescents derive from clonal expansion of single immature PECs. Preemptive and delayed histone deacetylase inhibition with panobinostat, a drug used for the treatment of hematopoietic stem cell disorders, attenuated crescentic glomerulonephritis, and recovered kidney function. 3D confocal microscopy and STED super-resolution imaging of glomeruli showed that, in addition to exerting an anti-inflammatory and immunosuppressive effect, panobinostat turned the uncontrolled hyperplasia of a specific immature PEC subset into a controlled differentiation into podocytes thereby restoring the injured glomerular filtration barrier. Single cell RNA sequencing of human renal progenitor cultures identified an immature stratifin+ subset and expansion of a stratifin-expressing progenitor in human crescentic glomerulonephritis was associated with a poor outcome. Treatment of human PECs with panobinostat attenuated stratifin expression in podocyte progenitors, reduced their proliferation but promoted their differentiation into podocytes. These results offer mechanistic insights into the formation of glomerular crescents and demonstrate that selective targeting of progenitors can attenuate crescent formation and the deterioration of kidney function in crescentic glomerulonephritis.
Keywords: glomerulonephritis, proteinuria, stem cell, vasculitis
Introduction
Crescentic glomerulonephritis encompasses a group of diverse disorders characterized by the presence of massive hyperplasia of parietal epithelial cells (PEC) as the main histopathological lesion at kidney biopsy (1, 2). It is frequently associated with a rapid decline in kidney function referred to altogether as rapidly progressive glomerulonephritis (1, 2). Anti-neutrophil cytoplasmic antibody-associated small-vessel vasculitis is the most frequent underlying diagnosis (3), but crescents form also in cryoglobulinemic vasculitis, anti-glomerular basement membrane (GBM) disease (4), lupus nephritis (LN), and other forms of immune complex glomerulonephritis. Crescents involving more than 50% of glomeruli associate with poor prognosis in terms of kidney function and patient survival.
Crescent formation is the consequence of diverse upstream pathomechanisms that fuel into a shared response involving the specific activation of PECs. PECs normally reside along Bowman capsule and represent in part renal progenitor cells (RPC), kept in quiescence via a humoral feedback loop by the differentiated podocytes of the intact glomerular filtration barrier (1, 2, 5, 6). Podocyte injury disrupts this feedback loop and activates PECs via surface glycoproteins, such as CD44 (7) and CD9 (8), that promote their activation, migration, and proliferation (9, 10). RPCs have a high survival capacity (11) and proliferative potential (11) and RPC markers are observed in crescents from patients with different types of glomerulonephritis (12).
Based on similarities between stem cell niches of bone marrow and kidney (13), we hypothesized that crescents result from monoclonal expansion of a single RPC clone conceptually similar to monoclonal diseases originating from hematopoietic stem cells. According to this analogy, we further hypothesized that drugs known to cure monoclonal diseases of the hematopoietic stem cells by enforcing their terminal differentiation could also attenuate crescentic glomerulonephritis (6, 14, 15).
Results
Crescents derive from Pax2+ progenitors of the Bowman capsule
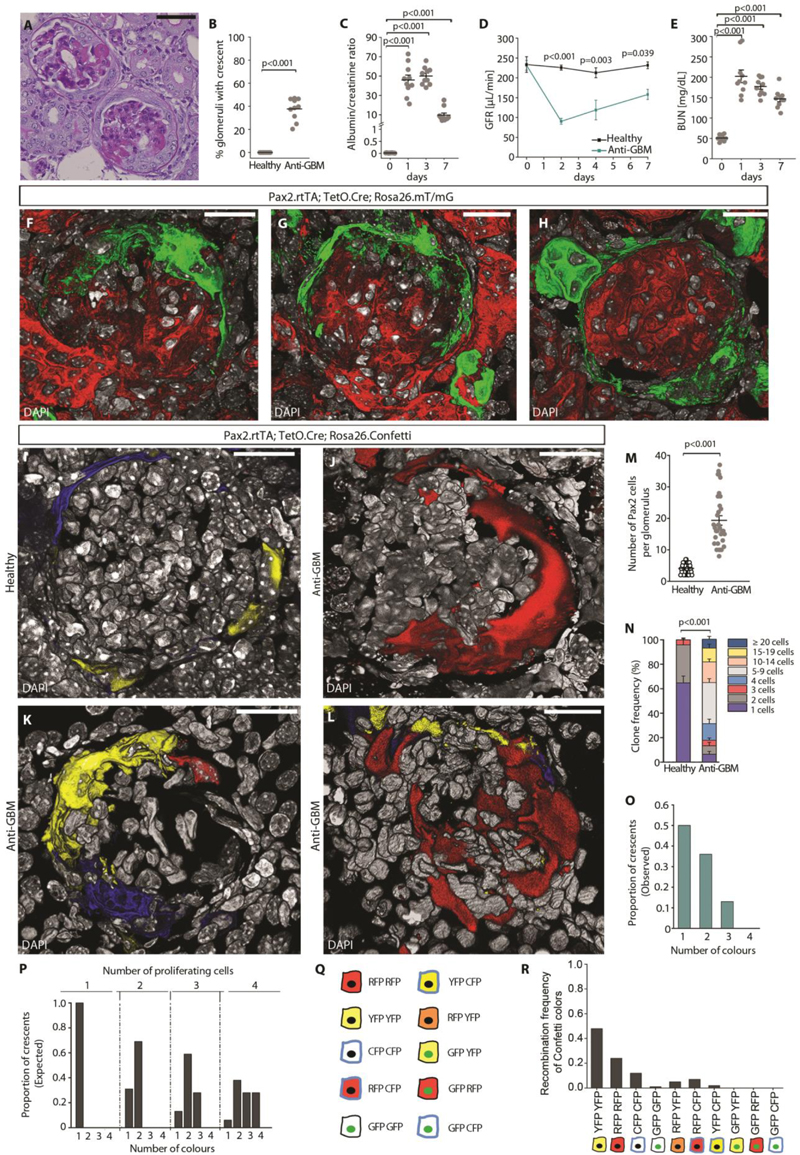
To evaluate the contribution of renal progenitors among the PECs to the generation of crescents, we employed Pax2.rtTA;TetO.Cre;mT/mG conditional transgenic reporter mice that allow to genetically label progenitors with green fluorescent proteins (GFP) and to perform lineage tracing (6). Following transgene induction by doxycycline administration, all Pax2+ progenitors among the PECs were marked in green (Fig. S1A). Following a washout period (16), we induced crescentic glomerulonephritis by intravenous injection of sheep anti-GBM serum (Fig. S1B). On day 7 we observed sheep IgG deposits in the glomeruli (Fig. S1C). PAS staining revealed focal segmental capillary loop necrosis and crescent-like proliferation of PECs in the Bowman space (Fig. 1A) in 37.7 ± 3.0 % of the glomeruli (Fig. 1B). Glomerular damage was associated with a rapid increase in albuminuria at day 1, that declined in the following days remaining higher than in healthy mice at day 7 (Fig. 1C). Proteinuria was accompanied by a loss of kidney function, shown by drop of glomerular filtration rate (GFR) (Fig. 1D) and by an increase in blood urea nitrogen (BUN) (Fig. 1E), i.e., altogether corresponding to severe glomerulonephritis. Lineage tracing experiments and 3D confocal analysis showed that the Pax2+ subset of PECs expanded circumferentially among the Bowman capsule and largely constituted the glomerular crescents (Fig. 1F-H). Taken altogether, crescents originate from immature Pax2+ cells among the PECs that expand massively.
Fig. 1. Crescents are generated by clonal proliferation of single RPCs.
(A) Representative image of a PAS-stained kidney section showing crescents and glomerulosclerosis in anti-GBM treated mice. Bars=50 μm. (B) Quantification of glomeruli with crescent at day 7 on PAS-stained sections (n=10). (C) Assessment of urine albumin/creatinine ratio in anti-GBM treated mice (n=10). (D) GFR monitored over time in healthy mice and in mice following anti-GBM serum injection. Data are mean ± SEM (healthy n=5 and anti-GBM n=5). (E) BUN monitored over time in anti-GBM mice (n=10). (F-H) 3D reconstruction of glomeruli showing Pax2-tracked hyperplastic lesions (green) in Pax2.rtTA;TetO.Cre;Rosa26.mT/mG at day 7. DAPI (white) counterstains nuclei. Bars=20 μm. (I-L) 3D reconstruction of representative glomeruli of healthy Pax2.rtTA;TetO.Cre;Rosa26.Confetti (I) and of anti-GBM mice (J-L). DAPI (white) counterstains nuclei. Bars=20 μm. (M) Dot plot showing the number of Pax2+ cells per glomerulus in healthy mice (n=9) and in anti-GBM mice (n=6) at 7 days observed in 3D analysis. (N) Clone frequency analysis of Pax2+ cells in glomeruli of healthy (n=9) and anti-GBM mice (n=6) at day 7. Data are mean ± SEM. (O) Bar chart showing the number of colours observed per crescent in anti-GBM mice (n=6). (P) Bar chart showing the theoretical distribution of colours per crescents resulting from proliferation of 1, 2, 3 or 4 Pax2+ cells labelled at the recombination frequency observed in induced animals. (Q) Colour combinations generated by recombination of the four possible fluorescent reporter proteins (nuclear GFP, membrane associated CFP, cytoplasmic RFP, cytoplasmic YFP) in homozygous mice for the R26R-Confetti allele, upon doxycycline induction (64). (R) Bar chart showing the proportion of each Confetti colour in 3D reconstructed glomeruli of healthy mice following induction period (n=9 mice).
Signals for fluorescent mT/mG proteins are GFP, green, and tdTomato, red. Signals for fluorescent Confetti proteins are GFP, green, CFP, cyan, RFP, red and YFP, yellow.
In dot plots (B, C, E and M) bars indicate mean values. Individual scores are shown. Statistical significance was calculated by Mann-Whitney test (B, C, E, M and N) or two-way ANOVA with Bonferroni post hoc test (D); numbers on graph represent p-values.
GFR, glomerular filtration rate; BUN, blood urea nitrogen; GBM, glomerular basement membrane;
DAPI, 4',6-diamidino-2-phenylindole.
Crescents originate from clonal expansion of single Pax2+ progenitors
The origin of crescents from proliferation of Pax2+ progenitors suggested possible similarity to disorders induced by uncontrolled clonal proliferation and/or differentiation arrest of subpopulation of hematopoietic stem cells such as myelodysplastic syndrome, leukemia or myelofibrosis (14, 15). In order to evaluate the possibility that also crescents could be clonal lesions, we employed the Pax2.rtTA; TetO.Cre; Rosa26.Confetti conditional transgenic mouse model that allowed clonal lineage tracing by genetical labelling of individual Pax2+ cells with one of the four fluorescent proteins (Fig. S1D) (6). To detect the entire crescentic lesion, we performed Z-stack analysis and 3D reconstructions of glomeruli in 30 μm-thick sections (5) (Fig. 1I-L). Quantification of 3D analysis showed that in healthy mice, the number of Pax2+ cells per glomerulus was 4.6 ± 0.2, while in mice with crescentic glomerulonephritis Pax2+ cells expanded to 18.9 ± 1.8 per glomerulus (Fig. 1M). Clone frequency analysis revealed that over 60% of clones were longer than 5 cells with a maximum clone size of 25 cells (Fig. 1N). In particular, 50% of crescents showed a single color, 36% two colors, and only 13% three colors (Fig. 1O). The observed color frequency matched the theoretically expected color pattern generated if no more than 1-2 Pax2+ cells proliferate to generate a crescent (Fig. 1P), calculated on the basis of the recombination frequency of each color observed in glomeruli of healthy mice (Fig. 1Q, R). Taken together, these results show that crescents derive from clonal proliferation of single Pax2+ PECs conceptually like in stem cell-related proliferative disorders.
Panobinostat attenuates crescentic glomerulonephritis
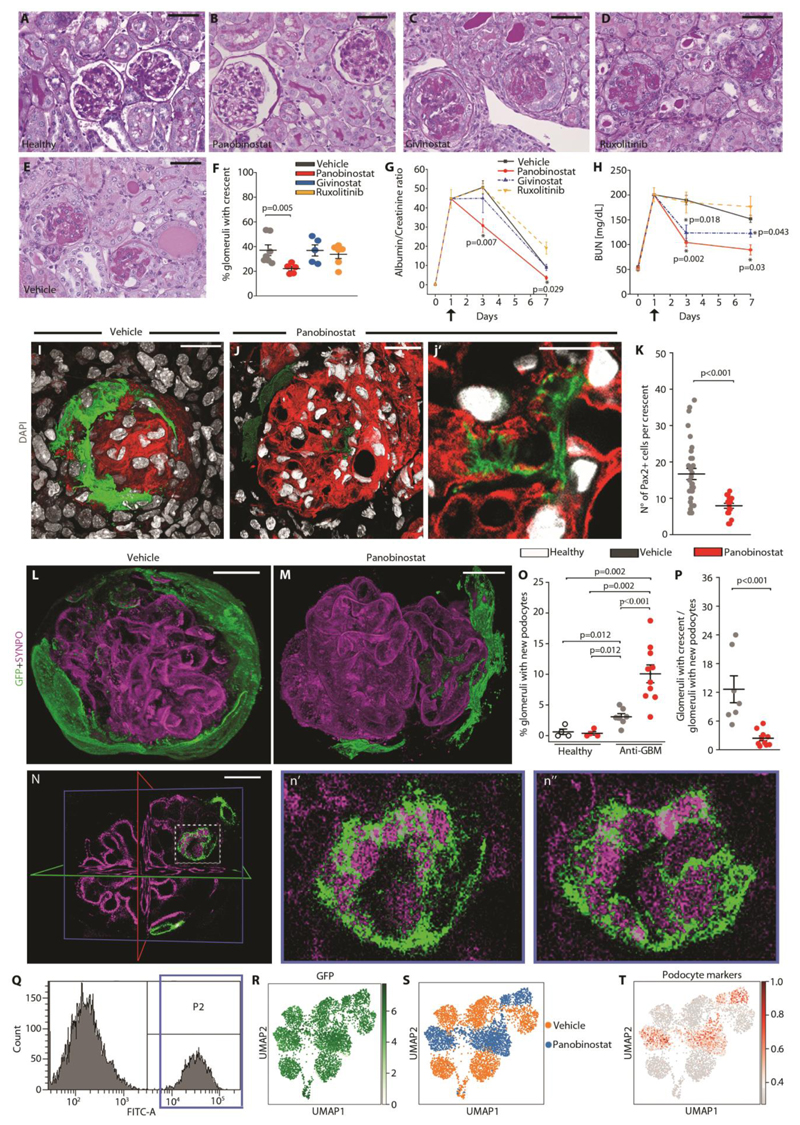
The origin of crescents from clonal proliferation of single progenitors suggested a possible pathogenic similarity to proliferative hematopoietic stem cell disorders (17). Therefore, we examined the effect of three drugs successfully used for the treatment of acute myeloid leukemia and primary myelofibrosis by inhibiting the clonal expansion of the pathogenic hematopoietic stem cell clone, the two histone deacetylase inhibitors panobinostat and givinostat and the Jak1/2 inhibitor ruxolitinib (17-19). We treated Pax2.rtTA;TetO.Cre;mT/mG and Pax2.rtTA;TetO.Cre;Rosa26.Confetti mice with crescentic glomerulonephritis with the three compounds for 6 days starting 18 hours after anti-GBM serum injection. Animals were sacrificed at day 7 (Fig. S1E). Panobinostat treatment decreased the number of crescents in comparison to the vehicle treated group at day 7 after treatment (Fig. 2A, B, E, F), while no effects were observed in animals receiving givinostat and ruxolitinib (Fig. 2C-F). Consistently, the albumin/creatinine ratio was improved in animals treated with panobinostat in comparison to the vehicle treated group, at both day 3 and day 7 (Fig. 2G). Likewise, panobinostat ameliorated excretory kidney function, and a small effect was observed with givinostat, as shown by improvement of BUN (Fig. 2H). By contrast, no effect was observed with ruxolitinib (Fig. 2H). The doses of givinostat and ruxolitinib were sufficient to block their targets as demonstrated respectively by increase in lysine 9-acetylated histone H3 (Fig. S1F) and reduction in phosphorylated STAT3 (Fig. S1G) in comparison to vehicle treated mice. These results show that panobinostat can attenuate crescent formation and ameliorate proteinuria and excretory kidney function.
Fig. 2. Panobinostat ameliorates crescentic glomerulonephritis and enhances generation of new podocytes.
(A-E) Representative images of PAS-stained kidney sections at 7 days in the four experimental groups of mice and in healthy mice. Bars= 50 μm. (F) Percentage of glomeruli presenting crescents at day 7 in the four experimental groups of mice (vehicle n=7, panobinostat n=5, givinostat n=5, ruxolitinib n=6). (G) Time course of urine albumin/creatinine ratio and (H) BUN determined in the four experimental groups of mice (vehicle n=7, panobinostat n=5, givinostat n=5, ruxolitinib n=6). Data are mean ± SEM. Black arrows indicate the starting point for drug treatment. (I, J, j’) 3D reconstruction of representative glomeruli of Pax2.rtTA;TetO.Cre;R26.mT/mG mouse. (I) Representative glomerulus of crescentic glomerulonephritis showing a Pax2+ positive hyperproliferative lesion in vehicle group. (J) Representative image of a glomerulus showing Pax2+ derived cells inside the glomerular tuft in panobinostat treated Pax2.rtTA;TetO.Cre;R26.mT/mG mouse. Bars = 20 μm (j’) Higher magnification showing foot processes of the Pax2+ derived cell in (J) showing formation. DAPI counterstains nuclei (white). Bar = 10 μm. (K) Dot plot showing the number of Pax2+ cells within the crescent in vehicle (n=6) and panobinostat (n=4) treated mice in 3D analysis. (L-M) 3D reconstructions of whole glomeruli using optical tissue clearing in Pax2.rtTA;TetO.Cre;R26.mT/mG. The panel shows main patterns of glomerular lesion based on GFP and synaptopodin (SYNPO) expression (magenta) in anti-GBM injected mice treated with vehicle (L) or panobinostat (M). (N) A z-plane within the glomerulus in M showing a de novo formed green podocyte. The position of x (green), y (red) and z (blue) axis are reported. Bar = 20 μm. (n’ and n”) Higher magnifications of the boxed area in (N). Different planes along Z axis show foot processes of the Pax2+ derived podocyte (green) interdigitating with those of preexisting podocytes (magenta).
(O) Percentage of glomeruli with new podocyte(s) over total number of glomeruli in vehicle (n=4) and panobinostat (n=4) treated healthy mice and in vehicle (n=7) and panobinostat (n=10) treated anti-GBM mice. New podocytes were identified as GFP+ cells within the tuft presenting feature of podocyte cells. (P) Dot plot showing ratio of glomeruli with crescent to glomeruli with new podocytes in vehicle (n=7) and panobinostat (n=10) treated mice. (Q) Gating strategy to isolate Pax2+ cells from renal cells of the Pax2.rtTA;TetO.Cre;R26.mT/mG mouse (n=2). The far right peak (P2) containing GFP+ cells was isolated by cell sorting. (R) UMAP visualization of GFP expression in Pax2+ isolated cells. (S) UMAP projection of control and panobinostat treated Pax2+ cell scRNAseq dataset splitted by treatment. (T) UMAP visualization of podocyte marker expression.
Signals for fluorescent mT/mG proteins are GFP, green, and tdTomato, red. In dot plots (F, K, O and P) bars indicate mean values. Individual scores are shown. Statistical significance was calculated by Mann-Whitney test; numbers on graph represent p-values. BUN, blood urea nitrogen; DAPI, 4’,6-diamidino-2-phenylindole; GFP, green fluorescent protein; SYNPO, synaptopodin; UMAP, uniform manifold approximation and projection.
Panobinostat promotes the generation of new podocyte
To establish how panobinostat could improve proteinuria and BUN, we performed single cell RNA sequencing (RNAseq) on kidney samples obtained from mice with crescentic glomerulonephritis treated with panobinostat and compared it with those treated with givinostat and ruxolitinib (Fig. S2A-E). We first performed an unbiased evaluation in order to identify genes more differentially modulated among immune system components by panobinostat in comparison to controls, givinostat, and ruxolitinib. We did not observe any effect that was specific of panobinostat (Fig. S2F). Likewise, we observed no panobinostat specific effect on the expression of molecules reported in the context of crescentic glomerulonephritis in the literature (Fig. S3A) (1, 20-28). Since macrophages have important roles in the pathogenesis of crescentic glomerulonephritis, we further analyzed the effect of panobinostat, givinostat or ruxolitinib on the expression of major histocompatibility complex class II (MHCII), CD86, CD80, CD40, and interleukin 6 (IL6) as well as on the phagocytic capacity in murine bone marrow-derived macrophages (Fig. S3B-G). We did not observe any effect specific to panobinostat suggesting that all the three drugs elicit similar anti-inflammatory and immunosuppressive properties. However, only panobinostat strongly reduces proteinuria and recovers excretory kidney function in vivo, so the consistent anti-inflammatory effects of the three compounds cannot serve as an explanation and other unique properties of panobinostat must apply.
We then further evaluated the effect of panobinostat on resident kidney cells. Similar to what we had observed for the immune system, there was no apparent panobinostat specific effect on tubular, endothelial and glomerular cells either by an unbiased evaluation (Fig. S4A) or when checking the expression of molecules with a reported role in crescentic glomerulonephritis in the literature (Fig. S4B) (1, 20-28). To further evaluate the effect of panobinostat on the tubule, we used Pax8.rtTA;TetO.Cre;FUCCI2aR mice (Fig. S5A), which allows to track all tubular cells and their cell cycle phases via the Fluorescent Ubiquitin-based Cell cycle Indicator (FUCCI2aR) reporter (11). We observed no changes in the total number of tubular cells after treatment with panobinostat, suggesting no effect of panobinostat on tubular cell viability or proliferation (Fig. S5B).
Finally, we further explored the effects of panobinostat on resident glomerular cells. In the glomerulus, panobinostat reduced fibrin deposition at day 7, whereas no effect was observed at day 3 (Fig. S6A, B). Consistently, quantification of glomerular injury in PAS-stained sections demonstrated that panobinostat treatment reduced the percentage of injured glomeruli at day 7 in comparison to vehicle treated mice (Fig. S6C, D). By performing 3D analysis on kidneys of Pax2.rtTA;TetO.Cre;R26.mT/mG mice, we observed smaller crescents in those treated for 7 days with panobinostat in comparison to vehicle (Fig. 2I-K). This associated with a reduction of proliferating Pax2-labelled renal progenitors within crescents (Fig. S7A, B) as well as with the appearance of Pax2-derived GFP+ cells inside the glomerular tuft showing morphological features of podocytes, such as primary and secondary foot processes (Fig. 2J, j’). In a time-course analysis, Pax2-derived GFP+ cells appeared first as mostly localized in the Bowman space moving toward the tuft and starting to express synaptopodin at day 3 and later engrafting into the tuft at day 7 (Fig. S7C-F). Upon tissue clearing and staining with synaptopodin and anti-GFP, we confirmed the reduction in crescent dimensions (Fig. 2L-N) and observed a specular increase of glomeruli containing Pax2-derived podocytes clearly engrafted within the tuft after treatment with panobinostat, (Fig. 2M-O and movie S1). Overall, panobinostat treatment resulted in a reduction in the ratio between glomeruli with crescent over glomeruli containing new podocytes (Fig. 2P). Finally, we obtained pure primary cultures of mouse Pax2-labelled renal progenitors by sorting GFP+ cells from Pax2.rtTA;TetO.Cre;R26.mT/mG mice (Fig. 2Q, R and Fig. S8A, B). Single cell RNAseq showed that after treatment with panobinostat primary cultures of mouse GFP+ renal progenitors upregulated podocyte markers (Fig. 2S, T). These data suggest that panobinostat reduces crescent formation and promotes de novo generation of podocytes.
Panobinostat improves 3D glomerular structure and restores slit diaphragm integrity
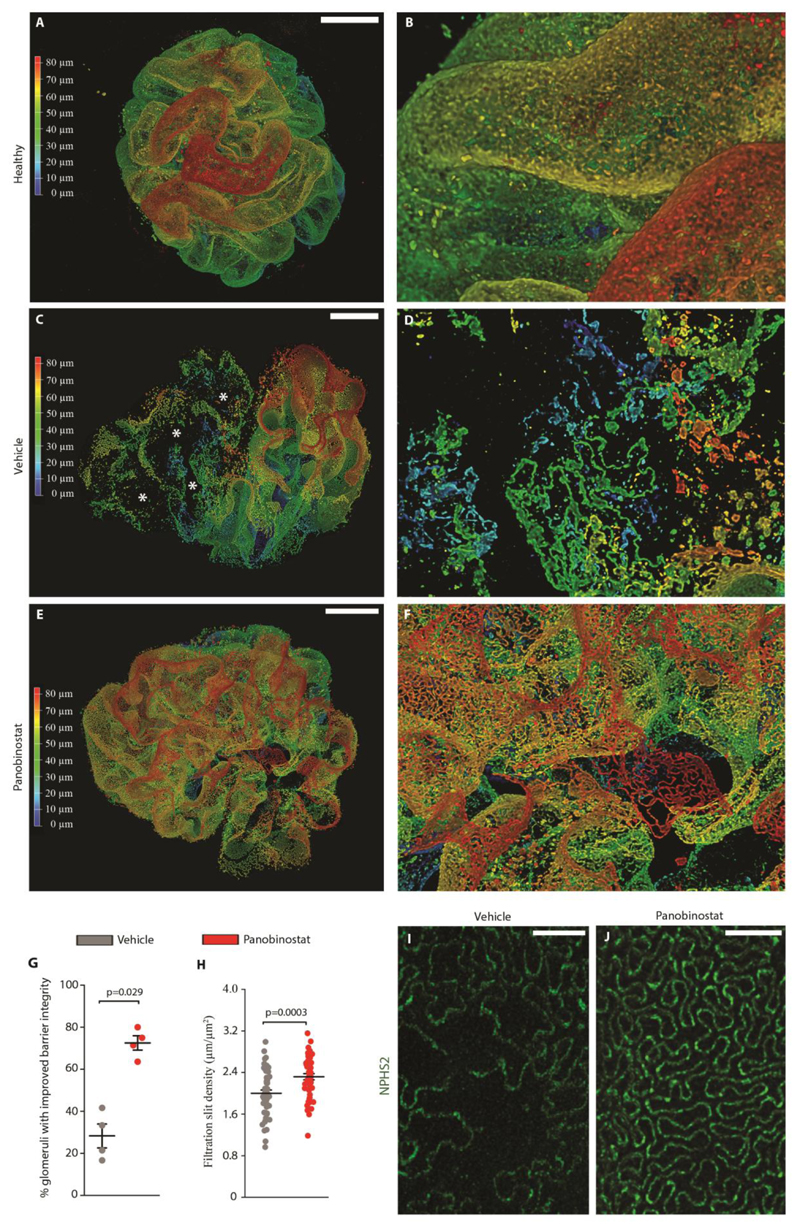
In order to gain further insight into how panobinostat influenced the integrity of the glomerular filtration barrier, we used STED super-resolution microscopy imaging for NPHS2, a slit diaphragm component, in optically cleared kidney tissues to produce volumetric representations of the glomerular slit ultrastructure at the nanometer scale (29). Three-dimensional rendering of confocal z-stacks showed the complex slit diaphragm structure with interdigitating foot processes in healthy glomeruli (Fig. 3A, a’), but simplified structure in mice treated with anti GBM serum (Fig. 3B, b’), consistent with foot process effacement or podocyte loss in crescent areas and in areas of podocytes surrounding the crescents. Podocyte loss as well as reduction of foot process density were less evident in panobinostat treated animals (Fig. 3C, c’). Overall, panobinostat treatment resulted in a high increase of glomeruli with an improved barrier integrity from 28.27.0% ± 5.68 to 72.52% ± 3.44 (Fig. 3D). Quantitative analysis showed that panobinostat treatment increased the filtration slit density as a marker of podocyte foot process density in comparison to vehicle treated mice (Fig. 3E-G). These results show that panobinostat turns the uncontrolled hyperplasia of immature PECs into a coordinated differentiation into new mature podocytes that repair the injured glomerular filtration barrier and favor restoration of the slit diaphragm density in crescentic glomerulonephritis.
Fig. 3. Panobinostat improves 3D glomerular structure and slit diaphragm integrity.
(A-C, a’-c’) 3D reconstructions of whole glomeruli using optical tissue clearing. The panel shows main patterns of glomerular lesion based on NPHS2 expression in healthy mice (A, a’) and in crescentic glomerulonephritis mice treated with vehicle (B, b’) or panobinostat (C, c’). NPHS2 expression is shown with a depth coding profile to preserve z-information. Bars=20 μm. (D) Percentage of glomeruli presenting an improved slit diaphragms integrity in vehicle (n=4) and panobinostat treated mice (n=4), assessed on fluorescently labelled optically cleared kidney tissue. (E) Density of slit diaphragm was assessed as a marker of terminal podocyte differentiation by using STED-super resolution microscopy. The quantification was carried out for 5 randomly selected areas for each glomerulus in at least 5 glomeruli per mouse (vehicle n=4 and panobinostat n=4 mice per group). (F-G) Representative images of podocyte foot processes by using STED-super resolution microscopy upon tissue clearing. NPHS2 is stained in green. Bars= 2 μm.
In dot plots (D and E) bars indicate mean values. Individual scores are shown. Statistical significance in D and E was calculated by Mann-Whitney test; numbers on graph represent p-values. GBM, glomerular basement membrane. NPHS2, podocin.
Delayed treatment with panobinostat maintains its renoprotective capacity and avoids chronic kidney disease
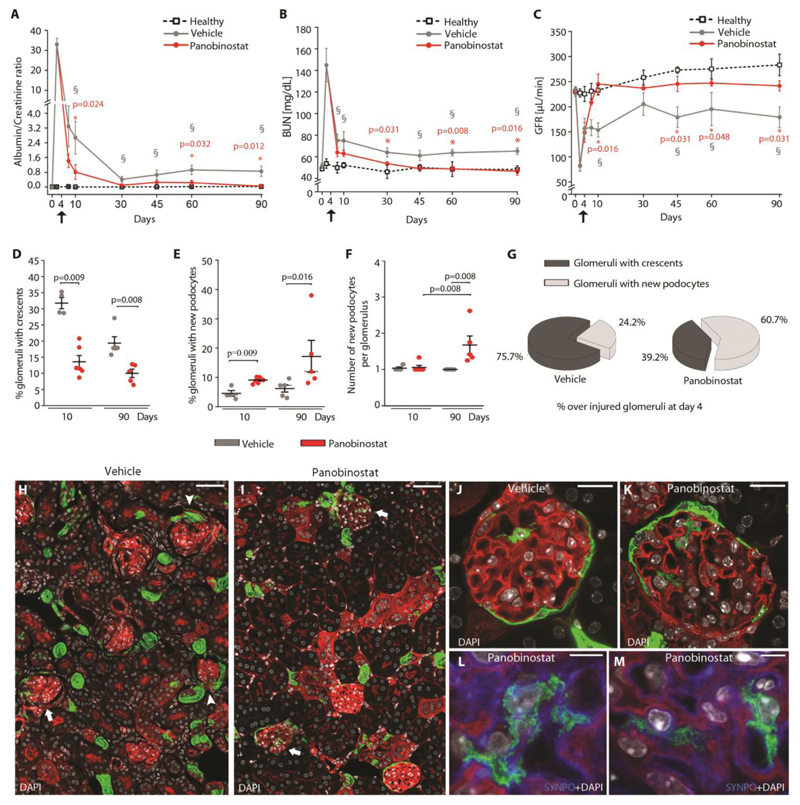
We then evaluated the capacity of panobinostat to exert a renoprotective effect even once the injury had already established and prevent kidney failure, even after apparent acute phase remission. To this aim, we administered the drug to mice on day 4 after the induction of injury, and we followed-up the mice for 90 days measuring proteinuria and kidney function at different time points (Fig. 4A-C, and Fig. S9A). Starting treatment with panobinostat at day 4, i.e. once the peak of proteinuria had passed and crescents were already present, maintained its efficacy in reducing proteinuria and recovering kidney function (Fig. 4A-C). The difference was already visible at day 10, 1 week after the start of treatment (Fig. 4A-C) and was comparable to the effect observed when the treatment was started at day 1 (Fig. S9B-D). At day 90, mice treated with vehicle achieved a partial remission, but with persistent proteinuria, elevated BUN and reduced GFR. In contrast, mice treated with panobinostat underwent complete remission of proteinuria and BUN showing normal GFR (Fig. 4A-C), hence, did not develop kidney failure (Fig. 4A-C).
Fig. 4. Panobinostat ameliorates crescentic glomerulonephritis and prevents kidney failure.
(A) Time course of urine albumin/creatinine ratio determined in healthy (n=4), vehicle (n=9 at day 4 and 10, n=5 at day 30, 45, 60 and 90) and panobinostat (n=11 at day 4 and 10, n=5 at day 30, 45, 60 and 90) treated mice. (§) Significance of vehicle versus healthy mice: day 10 p=0.004, day 30, 45, 60 and 90 p=0.016). (*) Significance of panobinostat versus vehicle. (B) Time course of BUN determined in healthy (n=4), vehicle (n=9 at day 4 and 10, n=5 at day 30, 45, 60 and 90) and panobinostat (n=11 at day 4 and 10, n=5 at day 30, 45, 60 and 90) treated mice. (§) Significance of vehicle versus healthy mice: day 7, 10 p=0.019, day 45, 60 and 90 p=0.032). (*) Significance of panobinostat versus vehicle. (C) GFR determined in healthy (n=4), vehicle (n=5) and panobinostat (n=4) treated mice. Black arrows indicate the starting point for drug treatment. Data are mean ±SEM. (§) Significance of vehicle versus healthy mice: day 10, 45 and 90 p=0.016, day 60 p=0.032). (*) Significance of panobinostat versus vehicle. (D) Percentage of glomeruli presenting crescents at day 10 (vehicle n=4, panobinostat n=6) and day 90 (vehicle n=5, panobinostat n=5). (E) Percentage of glomeruli with new podocyte(s) over total number of glomeruli at day 10 (vehicle n=4, panobinostat n=6) and day 90 (vehicle n=5, panobinostat n=5). (F) Dot plot showing the mean number of Pax2+ cells observed within glomeruli at day 10 (vehicle n=4, panobinostat n=6) and day 90 (vehicle n=5, panobinostat n=5). (G) Percentage of glomeruli with crescent and glomeruli with new podocytes observed at day 90 (vehicle n=5, panobinostat n=5). Percentages were calculated among glomeruli presenting crescent at day 4 after injury. (H, I) Representative images of kidneys from vehicle (H) and panobinostat (I) treated Pax2.rtTA;TetO.Cre;R26.mT/mG mice sacrificed at day 90, showing the presence of Pax2+ derived cells inside the glomerular tuft in panobinostat treated mice (arrows) and the presence of crescent in vehicle treated mice (arrowheads). Bars=50 μm. (J, K) Representative image of a glomerulus showing Pax2+ derived cells inside the glomerular tuft in vehicle (J) and panobinostat (K) treated Pax2.rtTA;TetO.Cre;R26.mT/mG mice sacrificed at day 90. Bars=20 μm. (L, M) Higher magnification showing foot processes and synaptopodin (SYNPO) expression (blue) in the Pax2+ derived cell in panobinostat treated Pax2.rtTA;TetO.Cre;R26.mT/mG mice sacrificed at day 90. DAPI counterstains nuclei (white). Bars=5 μm.
Signals for fluorescent mT/mG proteins are GFP, green, and tdTomato, red. In dot plots (D-F) bars indicate mean values. Individual scores are shown. Statistical significance in A-F was calculated by Mann-Whitney test. Numbers on graph represent p-values.
BUN, blood urea nitrogen; DAPI, 4',6-diamidino-2-phenylindole; SYNPO, synaptopodin.
Analysis of the kidneys showed a reduction of the percentage of glomeruli with crescent at day 10 and an almost total disappearance at day 90 (Fig. 4D). This associated with an increase in the percentage of glomeruli that contained new podocytes at day 10, that had further increased at day 90 (Fig. 4E). Strikingly, at day 90 we also observed an increase of the number of newly generated podocytes per glomerulus in panobinostat treated mice in comparison not only to vehicle, but also to panobinostat treated mice at day 10 (Fig. 4F), suggesting a persistently active repair process over time. Consistently, at day 90, glomeruli with newly generated podocytes represented 60.7% of glomeruli presenting crescents on day 4 (at start of treatment) in panobinostat treated mice vs. only 24.3% in vehicle treated mice (Fig. 4G). Representative pictures are shown in Fig. 4H-M. Taken together, these results show that panobinostat exerts long-term nephroprotective effects by persistently promoting the production of new podocytes over time for a continued glomerular repair.
Expansion within crescents of a PEC subset expressing CD133 and stratifin associates with patient outcome
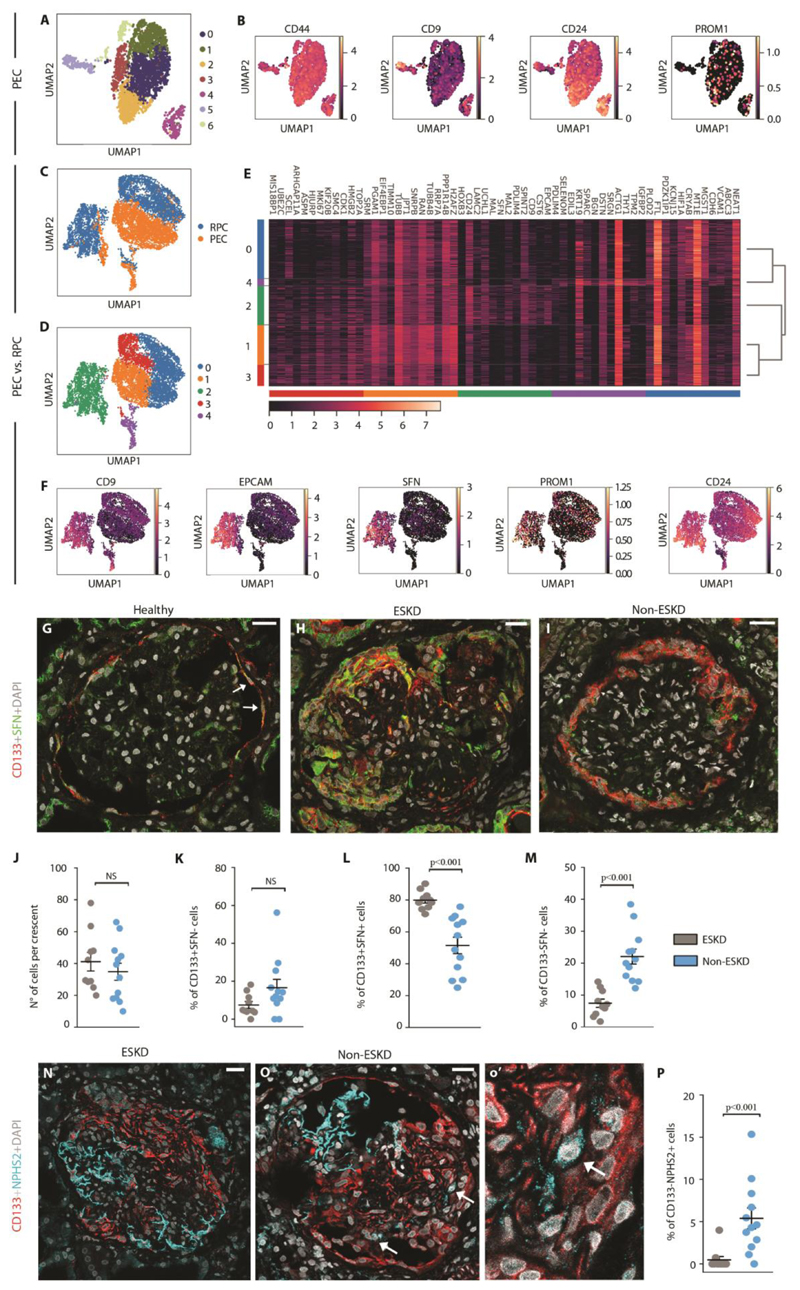
To identify the cell subset generating crescents also in human, we obtained PEC cultures from outgrowth of glomeruli of healthy human kidney (30) and processed them by 10X Genomics Chromium system combined with Illumina sequencing. Clustering of the entire pooled dataset identified seven transcriptionally distinct populations (Fig. 5A) and for each of them we looked for the 50 most differentially expressed genes (Table S1). By cross-referencing genes that were enriched in these clusters with the published literature, we observed that cluster 4 and 5 were highly enriched in previously reported crescent markers (8) (Fig. 5B). In addition, cluster 4 was enriched for genes with roles in cellular pathways intimately linked to crescents, stem cell niches (6, 31, 32) and cancer-stem cell (33-35) such as EPCAM, CD9, CD24, SFN, LAMC2, CRNDE, HOXB9, CDA (Table S1). To verify the progenitor signature of cluster 4, we analyzed the PEC outgrowth dataset together with a previously described renal progenitor dataset (36) (Fig. 5C-E). By matching them through the mutual nearest neighbors (MNN) batch correction algorithm, we obtained a matrix of 7443 cells that showed a high correspondence between some PEC clusters and RPCs. Louvain clustering revealed a large overlap between the cluster 4 present in PEC dataset and cluster 2 of RPC/PEC dataset (Fig. S10A). Consistently, cluster 2 was enriched for genes with roles in cellular pathways linked to crescents such as CD44, CD9, to cancer-stem cells, such as EPCAM and SFN (32) as well as the RPC markers CD133 (PROM1) and CD24 (Fig. 5F). Interestingly, stratifin, encoded by SFN, is a cell cycle checkpoint protein involved in stem cell proliferation/differentiation (37) that showed a virtually selective expression in cluster 2 of RPCs/PECs. We then analyzed stratifin expression in healthy human glomeruli and in the glomeruli of 22 patients with crescents at kidney biopsies, 13 patients with ANCA-associated vasculitis (AAV) and 9 patients with lupus nephritis (LN) selected following the flow chart shown in Fig. S11. Tables S2 and S3 list the clinical characteristics of AAV and LN patients, respectively. Double immunolabeling for CD133 and stratifin (SFN) in glomeruli of healthy patients confirmed the existence of a subset of CD133+ progenitors of Bowman capsule expressing stratifin and representing the 15.0 ± 1.5% of CD133+ PEC (Fig. 5G), while in crescents they were enriched to 64.3 ± 2.5% (Fig. 5H, I). Comparison between patients on the basis of their progression or not to end-stage kidney disease (ESKD) after two years of follow-up (Table S2 and S3) showed that while the percentage of glomeruli with crescents, crescent dimension and percentage of CD133+SFN- was similar (Fig. 5J-K and Table S2, S3), the percentage of CD133+stratifin+ cells constituting the crescents was lower in the group with a good outcome (Fig. 5L and Table S2, S3). In addition, patients with a good prognosis showed a higher percentage of CD133-stratifin- cells (Fig. 5M), which was at least in part related to a higher podocyte amount within the crescents (Fig. 5N-P). Although in a small number of patients, these results suggest that crescents mostly result from the amplification of CD133+stratifin+ PECs, and that the extent of involvement of this cell population associates with clinical outcome.
Fig. 5. Expansion within crescents of a PEC subset expressing CD133 and stratifin associates with patient outcome.
(A) UMAP projection of PEC culture single cell RNAseq dataset colored by cluster. (B) UMAP visualization of expression of progenitor and crescent main markers. (C, D) UMAP projection of matched RPC/PEC dataset splitted and colored by sample (C) and cluster (D), respectively. (E) Heatmap representing the expression of the first 12 main markers for each cluster. (F) UMAP visualization of stem cell and cancer-stem cell marker expression. Color bar indicates log2 normalized expression. (G-I) Representative images showing CD133 (red) and SFN (green) expression and localization in healthy (G) and pathological human tissues obtained from patients with diagnosis of ANCA-associated vasculitis and progressing (H) or not to ESKD (I). Arrows indicate region with CD133 and SFN co-expression. h’ depicts a magnification of the splitted channels of H. DAPI (white) counterstains nuclei. Bars=25 μM. (J) Graph representing the number of cells per crescent in patients progressing (grey dots, n=10) or not progressing (blue dots, n=12) to ESKD. (KM) Graphs representing the percentage of CD133+SFN+ cells (K), CD133+SFN- cells (L) and CD133-SFN- cells (M) over the total number of cells within the crescent of patients progressing (n=10) or not progressing to ESKD (n=12). (N, O) Representative images showing CD133 (red) and NPHS2 (cyan) expression and localization in pathological tissues obtained from patients with AAV from the ESKD (N) and the non ESKD group (O). A detail is shown in o’. Arrows point CD133-NPHS2+ cells in a crescent. DAPI (white) counterstains nuclei. Bars=25 μM. (P) Quantification of percentage of CD133- NPHS2+ cells over the total number of cells within the crescent in ESKD (n=10) and not ESKD patients (n=12).
In dot plots (J-M and P) bars indicate mean values. Individual scores are shown. Statistical significance was calculated by Mann-Whitney test; numbers on graph represent p-values. PEC, parietal epithelial cells; UMAP, uniform manifold approximation and projection; PROM1, prominin 1 (CD133); RPC, renal progenitor cell; EPCAM, epithelial cell adhesion molecule; SFN, stratifin, NPHS2, podocin; DAPI, 4',6-diamidino-2-phenylindole, ESKD, end-stage kidney disease.
Panobinostat inhibits RPC proliferation and promotes their differentiation into podocytes also in humans
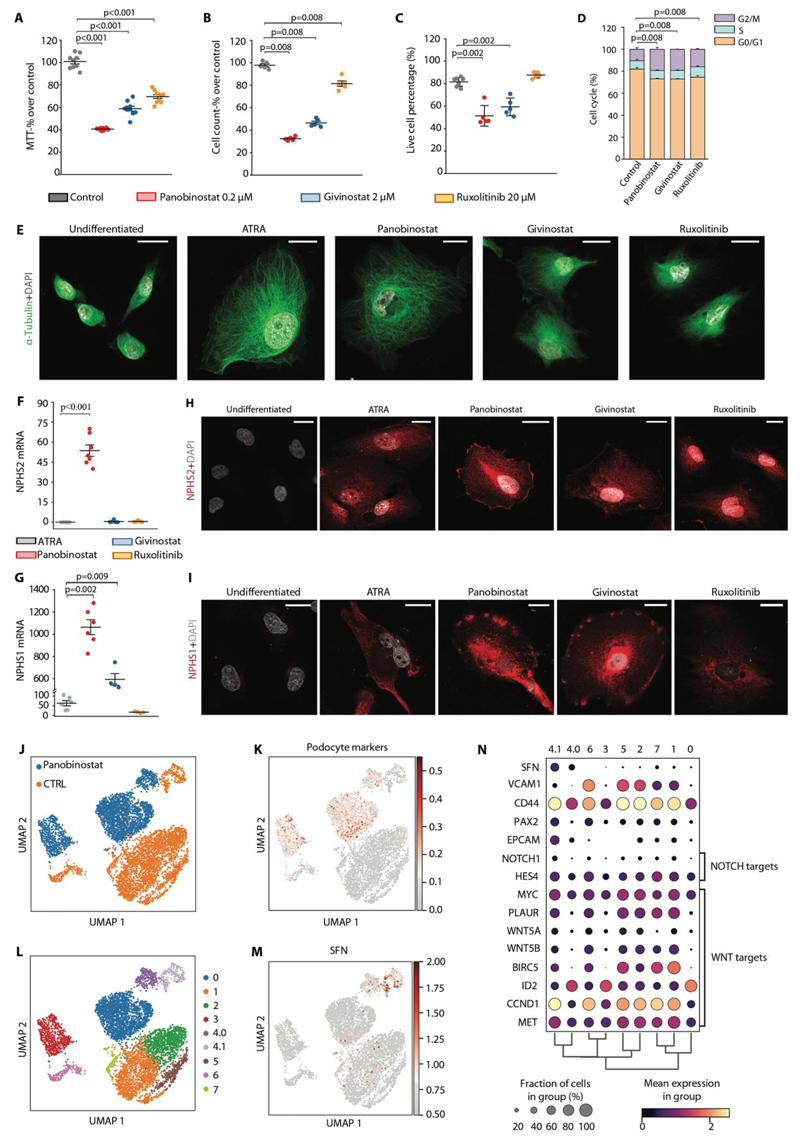
The results obtained in human biopsies, suggesting CD133+SFN+ PEC as the cells of origin of crescents, prompted us to verify the effects of panobinostat observed in the mouse model of crescentic glomerulonephritis also in human RPC cultures. Givinostat and ruxolitinib were tested as additional controls. All the three drugs reduced metabolic activity of RPCs, with panobinostat being the most effective, as assessed by the MTT test (Fig. 6A). This associated with a sharp reduction in the number of cells, i.e., an anti-proliferative effect (Fig. 6B). Panobinostat and givinostat also induced a reduction in the percentage of live cells, as detected by the annexin-V/PI assay (Fig. 6C and Fig. S12A). However, cell cycle analysis by flow cytometry also highlighted a doubling of the percentage of RPCs in the G2/M phase following panobinostat, givinostat, and ruxolitinib treatment, suggesting all the three drugs promoted cell cycle arrest (Fig. 6D and Fig. S12B). Taken together, drugs that inhibit hematopoietic progenitor proliferation can also inhibit the proliferation of RPCs. We then evaluated the capacity of the aforementioned compounds to modulate RPC differentiation into podocytes. We used all-trans retinoic acid (ATRA) as positive control given its well-defined effect as podocyte differentiation inducer (10, 38). Panobinostat was much more efficient than all-trans retinoic acid (39) in promoting RPC differentiation toward podocytes, as demonstrated by the change in cell morphology shown by tubulin staining and super-resolution microscopy (Fig. 6E), and the high expression of NPHS2 and NPHS1 mRNAs (Fig. 6F, G) as well as proteins (Fig. 6H, I). Givinostat only increased NPHS1, while NPHS2 expression was not detectable, suggesting a limited effect of progenitor differentiation (Fig. 6F-I). Ruxolitinib did not induce NPHS2 or NPHS1 (Fig. 6F-I).
Fig. 6. Panobinostat inhibits RPC proliferation and promotes their differentiation also in humans.
(A-I) In vitro drugs screening on RPC following 48 hours stimuli with panobinostat, givinostat and ruxolitinib. Each dot represents the mean of technical triplicates. (A) MTT assay on primary human RPC treated with panobinostat, givinostat and ruxolitinib for 48 hours. Data were obtained from 3 different primary cultures in 3 independent experiments. (B) Cell count following 48 hours treatment. Data were obtained from 3 different primary cultures in 2 independent experiments. (C) Quantification of live cells of Annexin-V/Propidium Iodide FACS staining for assessment of apoptosis induction after treatment with the three compounds. Data were obtained from 3 different primary cultures in 3 independent experiments. (D) Distribution of RPCs in cell cycle phases assessed by flow cytometry. Data were obtained from 3 different primary cultures in 2 independent experiments. (E) Representative STED images showing cytoskeleton changes based on tubulin expression after treatment with the three compounds. Untreated cells and ATRA treated cells were used as negative and positive control, respectively. One representative of 3 independent experiments is shown. DAPI counterstains nuclei (white). Bars=20 μm. (F, G) Podocyte marker expression in RPCs after treatment with ATRA, panobinostat, givinostat and ruxolitinib. mRNA expression of NPHS2 (F) and NPHS1 (G) were determined by real-time RT-PCR and reported as fold increase over untreated cells. Data were obtained from 3 different primary cultures in 3 independent experiments. (H, I) Representative confocal microscopy images showing expression of podocyte markers (NPHS2 and NPHS1 in red) following treatment with the three compounds. Untreated and ATRA treated cells were used as negative and positive control, respectively. One representative of 3 independent experiments is shown. DAPI counterstains nuclei (white). Bars=25 μm. (J) UMAP projection of control and panobinostat treated PEC culture scRNAseq dataset splitted by sample. (K) UMAP visualization of podocyte markers expression. (L) UMAP projection of control and panobinostat treated PEC culture scRNAseq dataset splitted by clusters. (M) UMAP visualization of stratifin expression. (N) Dot plot showing the expression of genes related to crescent, NOTCH or WNT pathways affected by panobinostat treatment.
In dot plots (A-C, F and G), bars indicate mean values. Individual scores are shown. Statistical significance was calculated by Mann-Whitney test; numbers on graph represent p-values. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ATRA, all-trans retinoic acid; DAPI, 4',6-diamidino-2-phenylindole; NPHS2, podocin; NPHS1, nephrin, UMAP, uniform manifold approximation and projection; SFN, stratifin.
Comparison of the single-cell transcriptome of control- and panobinostat treated PEC culture obtained from outgrowth of glomeruli of healthy human kidney (Fig. 6J), confirmed that panobinostat induced upregulation of podocyte-specific markers NPHS1, NPHS2, MAFB, KFL14, KFL15, PLA2G7, PTPRO, SYNPO, in different PEC subsets including stratifin-expressing RPCs (Fig. 6K-M). Interestingly, panobinostat treatment also downregulated expression of stratifin as well as of genes of the Notch and of the β-catenin pathways (Fig. 6N), involved in promoting renal progenitor proliferation and inhibiting their differentiation into podocytes, respectively. Altogether, panobinostat is a powerful inhibitor of podocyte progenitor proliferation and inducer of their differentiation into podocytes also in humans with potential for treatment of rapidly progressive glomerulonephritis.
Discussion
Crescent formation compromises glomerular filtration and urine outflow independent from the upstream pathomechanisms of loop necrosis and treatments specifically targeting the causative uncontrolled hyperplasia of PECs are not available. In this study, we show that 1. Crescents form via clonal proliferation of single cells from a particular immature subset of PECs along Bowman capsule; 2. panobinostat, an inhibitor of clonal proliferation used in primary myelofibrosis, successfully reduces proteinuria and recovers kidney function in models of crescentic glomerulonephritis; 3. The beneficial effect of panobinostat relates to its capacity to turn the uncontrolled hyperplasia of immature RPCs into a controlled differentiation into new podocytes and thereby restoring the injured glomerular filtration barrier.
According to the traditional concept, crescents represent an overshooting epithelial healing response of the PECs lining along Bowman capsule occurring in response to ruptures of the glomerular basement membrane during capillary loop necrosis (1, 2). Using lineage tracing analysis of Pax2+ podocyte progenitors, clonal analysis with the Confetti reporter and single cell RNAseq we show that a distinct PEC subpopulation forms the crescent. This reflects heterogeneity among PECs in terms of both differentiative potential and proliferative capacity, as known for other stem cell compartments (13, 40). We identified the subset of PECs that forms the crescent by clonal expansion by an expression pattern similar to stem cells of other organs and tissues, including the hematopoietic stem cells. This further underlines the similarities between the stem cells of the kidney and of the bone marrow, as already previously suggested (40, 41). Indeed, the bone marrow stem cell niche of hematopoietic stem cells involves endothelial cells producing CXCL12, which keeps hematopoietic stem cells largely in an immobile and quiescent state (13). The glomerulus where RPCs reside among the PECs along the inner aspect of Bowman capsule has a similar structure. Of note, in healthy conditions podocytes produce high amounts of CXCL12 to keep RPCs among the PECs quiescent and undifferentiated (5). Other similarities relate to the expression of CD44 (7) and VCAM1 (42) by RPCs and their role in RPC activation. In addition, the local presence of high amounts of VEGF (43) and TGF-β (12), also contribute to RPCs quiescence and survival, like in the bone marrow stem cell niche (44). The results of this study add another crucial similarity. Crescents develop from a clonal proliferation of an immature PEC subset, a podocyte progenitor, that expands but without differentiating into podocyte conceptually like other diseases driven by clonal proliferation of resident progenitor or stem cell populations not only in the bone marrow (14, 15) but also in the gut (45, 46) or the skin (47). Of note, we recently reported that injury of kidney tubules stimulates progenitors scattered within such tubules to also undergo hyperplasia and form papillary adenomas and carcinomas in a similar clonal fashion (36). As such, tubule adenomas may be the tubular equivalent to glomerular crescents. In the crescent, stimuli for PEC proliferation likely include mitogenic serum factors reaching Bowman space upon capillary loop necrosis and GBM rupture and the consequent inflammation (48). Even if all PECs are similarly exposed to this mitogenic micromilieu, a particular PEC subset with phenotypic and functional features of podocyte progenitors has a higher capacity to proliferate consistent with its stem cell features, becoming the cell of origin for a clonal crescent. The crescent-forming PEC subset is characterized by expression of stratifin, also known as 14-3-3σ. The 14-3-3 proteins are 28- to 33-kDa polypeptides found in all eukaryotic organisms (49). Seven members (b, c, e, g, s, h/t and f) are found in mammals (50). Through binding to at least 200 proteins including enzymes, transcription factors, cytoskeletal proteins, signaling molecules, apoptosis factors and tumor suppressors (50), 14-3-3 proteins are pivotal drivers of proliferation and differentiation (50) and essential regulators of stem cell function (51-53) and cancer development (32, 54) in multiple organs and tissues. For example, 14-3-3 interfere with the adaptor protein LNK, a critical inhibitor of JAK2, thereby limiting hematopoietic stem and progenitor cell self-renewal (52). LNK deficiency promotes myeloproliferative neoplasm development in mice (55), and LNK loss-of-function mutations are found in human myeloproliferative disorders (56), proposing 14-3-3 as crucial players in these diseases (52). The 14-3-3 protein stratifin expression determines the choice between proliferation and differentiation also in embryonic stem cells (51). In particular, it induces PI3K/Akt activation and triggers dissociation from the APC/axin/GSK-3b complex of β-catenin, which translocates into the nucleus to drive the transcription of mitogenic genes (51). Interestingly, β-catenin induces PEC proliferation, while its deletion promotes PEC differentiation into podocytes (57). In addition, retinoid acid, a crucial driver of progenitor differentiation into podocytes (39), selectively suppresses stratifin (51). Finally, stratifin+ RPCs show a low expression of VCAM1. Like hematopoietic cells (58), VCAM1 expression characterizes immature RPCs that exhibit stem cell potential and are quiescent in the glomerular niche (42). Once VCAM1+ RPCs are committed to podocyte differentiation they first start to express podocalyxin (42) and then downregulate VCAM1 before upregulating podocyte markers and differentiating into podocytes (42). The results of this study suggest that stratifin expression characterizes a VCAM low RPC subset functionally committed to proliferate that undergoes clonal expansion like bone marrow myeloproliferative disorders (Fig. S13). Stratifin+RPCs are characterized also by expression of CD44 (59) and CD9 (8), crucial drivers of crescent formation (8, 59). Clonal amplification of this subset promotes crescent formation, as confirmed also by the association between the percentage of stratifin-expressing PECs within crescents and a poor outcome in patients with crescentic glomerulonephritis.
Patients with crescentic glomerulonephritis have a high risk of progressing toward ESKD, despite therapy (1, 3). Current treatments target upstream autoimmune and inflammatory pathways but do not directly target crescent formation itself (3), albeit cyclophosphamide that is used for some of these diseases elicits antiproliferative effects not only on immune cells but also on PECs (1). These results provided the rationale for the evaluation of drugs already in use for disorders of clonal stem cell hyperplasia, such as myeloma or primary myelofibrosis (18, 19, 60, 61), in crescentic glomerulonephritis. All the three drugs showed anti-inflammatory and immunosuppressive effects and reduced viability and proliferation of RPCs, suggesting common mechanisms mediating clonal amplification in stem cell disorders across different organs, including the kidney. However, when administered to models of crescentic glomerulonephritis in vivo, only panobinostat promoted crescent and proteinuria reduction as well as kidney function recovery, an effect that was consistent with its strong and unique capacity to promote differentiation into podocytes of a PEC subset with phenotypic and functional features of podocyte progenitors. Consistently, the reduction in crescent number associated with a downregulation of stratifin as well as β-catenin target genes in stratifin expressing human PECs and with their shift from crescent formation to generation of new podocytes in vivo. These results suggest that inhibition of crescent formation alone cannot efficiently treat crescentic glomerulonephritis. By contrast, drug-induced differentiation of podocyte progenitors into podocytes can solves two problems a) hyperplasia of the immature PECs, b) repair of visceral filtration barrier, and is therapeutically more efficient. Strikingly, delayed treatment with panobinostat administered only after the peak of proteinuria and crescents had formed still induced proteinuria remission and avoided long-term development of kidney failure, an effect associated to a continued generation of new podocytes and crescent regression over time.
The results reported herein should be considered in the light of some limitations. First, the number of biopsies of crescentic glomerulonephritis analyzed is relatively small, owing to the rarity of this disease (1). Further studies are needed to confirm the association of CD133+Stratifin+ PECs in the crescent with the risk of ESKD progression. Second, crescentic glomerulonephritis in human may involve also other mechanisms in addition to those targeted in the anti-GBM model used in this study (62). However, studies in patients with different type of myeloproliferative disorders and lymphomas suggest that panobinostat exerts wide immunosuppressive effects that could also be useful for treatment of the disorder (17, 18, 63).
Taken altogether, these results offer mechanistic insights into crescent formation suggesting that this lesion is caused by fast clonal proliferation of single immature PECs and by a lack of their differentiation toward a fully differentiated podocyte phenotype upon severe glomerular injury. More importantly, in analogy with other disorders caused by dysregulation of the stem cell response, development of this lesion can be interrupted by using drugs that have already been developed and successfully used to block abnormal clonal stem cell growth in other organs and tissues.
Materials and Methods
Study design
The purpose of this study was to examine the contribution of renal progenitors among the PEC to the generation of crescents and to identify the mechanism involved in this process. These objectives were addressed by (i) evaluating renal progenitors as the cell of origin of crescents in transgenic mouse models and evaluating crescent clonality, (ii) analyzing the effect of drugs currently used in haematological stem cell disorders on crescentic glomerulonephritis outcome in mouse models, (iii) identifying the crescent-forming PEC subset in human by single cell RNAseq and immunofluorescence analysis on human biopsies of crescentic glomerulonephritis patients (iv) evaluating the mechanisms of panobinostat effects on human renal progenitors.
Human kidney biopsies
A total of 22 native kidney biopsies of patients with AAV (9 women and 4 men, mean age 47.5 ± 2Ũ.7 years, Table S2) and with LN (8 females and 1 male, mean age 33.5 ± 13.3 years, Table S3) were selected for the analysis and retrospectively reviewed in agreement with the Ethical Committee on human experimentation of the Meyer Children University Hospital and of the Careggi University Hospital, Florence, Italy. As controls, normal kidney fragments were obtained from three patients who underwent nephrectomy for localized renal tumors (1 woman and 2 men, mean age 64.67 ± 8.45 years). These fragments were obtained from the pole opposite to the tumor. All biopsies were cut into multiple sections and were stained with Jones methenamine silver, periodic acid Schiff, hematoxylin and eosin (H&E) and trichrome for clinical purposes. The cohort size was limited by the availability of both clinical data and representative kidney biopsy material, therefore sample size calculation was not applicable. Initial (time of biopsy) and 2 years follow-up clinical data were recorded (Table S2, S3), and the outcome was assigned based on the estimated GFR at 2 years (eGFR < 15 ml/min/1.73m2, ESKD; eGFR >15 ml/min/1.73m2, non ESKD).
Animal studies
Mice were randomly assigned to experimental and control groups, but investigators were not blinded. Sample size was calculated on the basis of the primary endpoint rate of crescent formation and other assumptions based on preliminary experiments. No data were excluded from studies in this manuscript. Pathology analysis was performed in a blinded fashion. All procedures were performed in accordance with institutional protocols approved by the Institutional Animal Care and Use Committee of the University of Florence, Italy.
Statistical analysis
Human studies
Results are reported as means ± SEM unless otherwise stated. Between-group comparisons for continuous variables were performed by Mann-Whitney test according to a nonparametric distribution (Table S2, S3). A two-sided p value of <0.05 was considered statistically significant.
Animal and in vitro studies
Results are reported as means ± SEM unless otherwise stated. The number of mice used, numbers of replicates, and statistical values (where applicable) are provided in the Figure legends. Comparison between groups was performed by the Mann-Whitney test or through the analysis of variance (ANOVA) for multiple comparisons (ANOVA for repeated measures) with Bonferroni post hoc analysis. A p value <0.05 was considered statistically significant. Statistical analysis was performed using OriginPro (RRID:SCR_015636) statistical software.
Supplementary Material
Single sentence summary.
Glomerular crescents derive from hyperplasia of single progenitors and a drug enhancing their differentiation into podocytes attenuates crescentic glomerulonephritis
Acknowledgments and Funding
This study was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 101019891). This research was also funded by Tuscany Region Bando Ricerca Salute 2018, progetto NIKE. M.E.M. was supported by a FIRC-AIRC fellowship for Italy. A.J.P. was the recipient of the Fondazione Umberto Veronesi fellowship. H.-J.A. was supported by the Deutsche Forschungsgemeinschaft (AN372/14-4, 27-1, 30-1).
Footnotes
Author contributions: P.R. designed the study with the contribution of L.L and M.E.M. and all of them interpreted the data. M.E.M. performed or supervised all the experiments. R.S. analysed the data from the scRNA-seq analysis. M.L.A, G.A. C.C. and V.R. designed and performed immunofluorescence and confocal microscopy. B.M. carried out all scRNA-seq and assisted with data analysis. S.L. validated and sequenced the single-cell libraries. L.D.C., M.D. and S.S. performed flow cytometry analysis. A.B. provided the Pax2.rtTA mouse. A.J.P., G.L.R. and A.Molli carried out mouse experiments. L.M. performed cell sorting experiments. G.L., F.R. and F.G. organized patient tissue collection, assisted with statistical analysis and scored the human samples blinded. G.L.R. and A.Molli performed mouse genotyping and assisted with mouse experiments. G.L.R. and N.B. helped with in vitro experiments. A.Magi helped with scRNA-seq analysis. F.A. assisted and advised on flow cytometry data interpretation. E.L. and H.J.A. critically revised and edited the manuscript and advised on data interpretation. P.R. wrote the manuscript with the contribution of L.L. and M.E.M. All authors read and approved the final manuscript.
Competing interests: H.J.A. reports paid consulting from AstraZeneca, Bayer, GSK, Novartis, Kezar and Vifor. All other authors declare no competing interests.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding authors upon reasonable request. Single cell RNAseq data are available in NCBI's Gene Expression Omnibus repository as GSE195784, GSE195785 and GSE195797.
References
- 1.Anguiano L, Kain R, Anders HJ. The glomerular crescent: triggers, evolution, resolution, and implications for therapy. Curr Opin Nephrol Hypertens. 2020;29:302–309. doi: 10.1097/MNH.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SK, Jeansson M, Quaggin SE. New insights into the pathogenesis of cellular crescents. Curr Opin Nephrol Hypertens. 2011;20:258–262. doi: 10.1097/MNH.0b013e32834583ec. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. 2019;15:91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 4.Segelmark M, Hellmark T. Anti-glomerular basement membrane disease: an update on subgroups, pathogenesis and therapies. Nephrol Dial Transplant. 2019;34:1826–1832. doi: 10.1093/ndt/gfy327. [DOI] [PubMed] [Google Scholar]
- 5.Romoli S, Angelotti ML, Antonelli G, Kumar S, Vr, Mulay SR, Desai J, Anguiano Gomez L, Thomasova D, Eulberg D, Klussmann S, Melica ME, et al. CXCL12 blockade preferentially regenerates lost podocytes in cortical nephrons by targeting an intrinsic podocyte-progenitor feedback mechanism. Kidney Int. 2018;94:1111–1126. doi: 10.1016/j.kint.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, Becherucci F, Mazzinghi B, Sisti A, Romoli S, Burger A, et al. Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Remission and Can Be Pharmacologically Enhanced. Stem Cell Reports. 2015;5:248–263. doi: 10.1016/j.stemcr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roeder SS, Barnes TJ, Lee JS, Kato I, Eng DG, Kaverina NV, Sunseri MW, Daniel C, Amann K, Pippin JW, Shankland SJ. Activated ERK1/2 increases CD44 in glomerular parietal epithelial cells leading to matrix expansion. Kidney Int. 2017;91:896–913. doi: 10.1016/j.kint.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazareth H, Henique C, Lenoir O, Puelles VG, Flamant M, Bollee G, Fligny C, Camus M, Guyonnet L, Millien C, Gaillard F, et al. The tetraspanin CD9 controls migration and proliferation of parietal epithelial cells and glomerular disease progression. Nat Commun. 2019;10:3303. doi: 10.1038/s41467-019-11013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazareth H, Lenoir O, Tharaux PL. Parietal epithelial cells role in repair versus scarring after glomerular injury. Curr Opin Nephrol Hypertens. 2020;29:293–301. doi: 10.1097/MNH.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 10.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazzeri E, Angelotti ML, Peired A, Conte C, Marschner JA, Maggi L, Mazzinghi B, Lombardi D, Melica ME, Nardi S, Ronconi E, et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun. 2018;9:1344. doi: 10.1038/s41467-018-03753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014;20:154–165. doi: 10.1016/j.molmed.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Mead AJ, Mullally A. Myeloproliferative neoplasm stem cells. Blood. 2017;129:1607–1616. doi: 10.1182/blood-2016-10-696005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Kao YR, Sun D, Todorova TI, Reynolds D, Narayanagari SR, Montagna C, Will B, Verma A, Steidl U. Myelodysplastic syndrome progression to acute myeloid leukemia at the stem cell level. Nat Med. 2019;25:103–110. doi: 10.1038/s41591-018-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romagnani P, Rinkevich Y, Dekel B. The use of lineage tracing to study kidney injury and regeneration. Nat Rev Nephrol. 2015;11:420–431. doi: 10.1038/nrneph.2015.67. [DOI] [PubMed] [Google Scholar]
- 17.Schieber M, Crispino JD, Stein B. Myelofibrosis in 2019: moving beyond JAK2 inhibition. Blood Cancer J. 2019;9:74. doi: 10.1038/s41408-019-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evrot E, Ebel N, Romanet V, Roelli C, Andraos R, Qian Z, Dolemeyer A, Dammassa E, Sterker D, Cozens R, Hofmann F, et al. JAK1/2 and Pandeacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin Cancer Res. 2013;19:6230–6241. doi: 10.1158/1078-0432.CCR-13-0905. [DOI] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 20.Chen A, Lee K, D'Agati VD, Wei C, Fu J, Guan TJ, He JC, Schlondorff D, Agudo J. Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J Clin Invest. 2018;128:3413–3424. doi: 10.1172/JCI97879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitching AR, Alikhan MA. CD8+ cells and glomerular crescent formation: outside-in as well as inside-out. J Clin Invest. 2018;128:3231–3233. doi: 10.1172/JCI122045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moll S, Angeletti A, Scapozza L, Cavalli A, Ghiggeri GM, Prunotto M. Glomerular Macrophages in Human Auto-and Allo-Immune Nephritis. Cells. 2021;10 doi: 10.3390/cells10030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odobasic D, Gan PY, Summers SA, Semple TJ, Muljadi RC, Iwakura Y, Kitching AR, Holdsworth SR. Interleukin-17A promotes early but attenuates established disease in crescentic glomerulonephritis in mice. Am J Pathol. 2011;179:1188–1198. doi: 10.1016/j.ajpath.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitching AR, Holdsworth SR, Tipping PG. Crescentic glomerulonephritis--a manifestation of a nephritogenic Th1 response? Histol Histopathol. 2000;15:993–1003. doi: 10.14670/HH-15.993. [DOI] [PubMed] [Google Scholar]
- 25.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 26.Luque Y, Cathelin D, Vandermeersch S, Xu X, Sohier J, Placier S, Xu-Dubois YC, Louis K, Hertig A, Bories JC, Vasseur F, et al. Glomerular common gamma chain confers B-and T-cell-independent protection against glomerulonephritis. Kidney Int. 2017;91:1146–1158. doi: 10.1016/j.kint.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Pusey CD. Mechanisms of glomerular crescent formation. UpToDate. 2020 [Google Scholar]
- 28.Anders HJ. Diagnosis and management of crescentic glomerulonephritis: state of the art. Saudi J Kidney Dis Transpl. 2000;11:353–361. [PubMed] [Google Scholar]
- 29.Unnersjo-Jess D, Scott L, Blom H, Brismar H. Super-resolution stimulated emission depletion imaging of slit diaphragm proteins in optically cleared kidney tissue. Kidney Int. 2016;89:243–247. doi: 10.1038/ki.2015.308. [DOI] [PubMed] [Google Scholar]
- 30.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 31.Safarikia S, Carpino G, Overi D, Cardinale V, Venere R, Franchitto A, Onori P, Alvaro D, Gaudio E. Distinct EpCAM-Positive Stem Cell Niches Are Engaged in Chronic and Neoplastic Liver Diseases. Front Med (Lausanne) 2020;7:479. doi: 10.3389/fmed.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiba-Ishii A, Kano J, Morishita Y, Sato Y, Minami Y, Noguchi M. High expression of stratifin is a universal abnormality during the course of malignant progression of early-stage lung adenocarcinoma. Int J Cancer. 2011;129:2445–2453. doi: 10.1002/ijc.25907. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Zhang D, Fan Y, Chao Y, Chang J, Li N, Han L, Han C. Regulation of Cancer Stem Cell Self-Renewal by HOXB9 Antagonizes Endoplasmic Reticulum Stress-Induced Melanoma Cell Apoptosis via the miR-765-FOXA2 Axis. J Invest Dermatol. 2018;138:1609–1619. doi: 10.1016/j.jid.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Bhatlekar S, Fields JZ, Boman BM. Role of HOX Genes in Stem Cell Differentiation and Cancer. Stem Cells Int. 2018;2018:3569493. doi: 10.1155/2018/3569493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 36.Peired AJ, Antonelli G, Angelotti ML, Allinovi M, Guzzi F, Sisti A, Semeraro R, Conte C, Mazzinghi B, Nardi S, Melica ME, et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aaw6003. [DOI] [PubMed] [Google Scholar]
- 37.Lazzeri E, Angelotti ML, Conte C, Anders HJ, Romagnani P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol Med. 2019;25:366–381. doi: 10.1016/j.molmed.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Dai Y, Chen A, Liu R, Gu L, Sharma S, Cai W, Salem F, Salant DJ, Pippin JW, Shankland SJ, Moeller MJ, et al. Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor alpha. Kidney Int. 2017;92:1444–1457. doi: 10.1016/j.kint.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol. 2013;24:1756–1768. doi: 10.1681/ASN.2012090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romagnani P. Toward the identification of a "renopoietic system"? Stem Cells. 2009;27:2247–2253. doi: 10.1002/stem.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sainio K, Raatikainen-Ahokas A. Mesonephric kidney--a stem cell factory? Int J Dev Biol. 1999;43:435–439. [PubMed] [Google Scholar]
- 42.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 43.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200. doi: 10.1681/ASN.2018080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saw S, Weiss A, Khokha R, Waterhouse PD. Metalloproteases: On the Watch in the Hematopoietic Niche. Trends Immunol. 2019;40:1053–1070. doi: 10.1016/j.it.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014;15:62–69. doi: 10.1002/embr.201337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 47.Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 48.Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J Pathol. 2012;228:482–494. doi: 10.1002/path.4046. [DOI] [PubMed] [Google Scholar]
- 49.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 50.Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang TC, Liu CC, Hsing EW, Liang SM, Chi YH, Sung LY, Lin SP, Shen TL, Ko BS, Yen BL, Yet SF, et al. 14-3-3sigma regulates beta-catenin-mediated mouse embryonic stem cell proliferation by sequestering GSK-3beta. PLoS One. 2012;7:e40193. doi: 10.1371/journal.pone.0040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang J, Balcerek J, Rozenova K, Cheng Y, Bersenev A, Wu C, Song Y, Tong W. 14-3-3 regulates the LNK/JAK2 pathway in mouse hematopoietic stem and progenitor cells. J Clin Invest. 2012;122:2079–2091. doi: 10.1172/JCI59719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cianfarani F, Bernardini S, De Luca N, Dellambra E, Tatangelo L, Tiveron C, Niessen CM, Zambruno G, Castiglia D, Odorisio T. Impaired keratinocyte proliferative and clonogenic potential in transgenic mice overexpressing 14-3-3sigma in the epidermis. J Invest Dermatol. 2011;131:1821–1829. doi: 10.1038/jid.2011.137. [DOI] [PubMed] [Google Scholar]
- 54.Fan X, Cui L, Zeng Y, Song W, Gaur U, Yang M. 14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA, Chikwava KR, Tong W. Lnk constrains myeloproliferative diseases in mice. J Clin Invest. 2010;120:2058–2069. doi: 10.1172/JCI42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baran-Marszak F, Magdoud H, Desterke C, Alvarado A, Roger C, Harel S, Mazoyer E, Cassinat B, Chevret S, Tonetti C, Giraudier S, et al. Expression level and differential JAK2-V617F-binding of the adaptor protein Lnk regulates JAK2-mediated signals in myeloproliferative neoplasms. Blood. 2010;116:5961–5971. doi: 10.1182/blood-2009-12-256768. [DOI] [PubMed] [Google Scholar]
- 57.Grouls S, Iglesias DM, Wentzensen N, Moeller MJ, Bouchard M, Kemler R, Goodyer P, Niggli F, Grone HJ, Kriz W, Koesters R. Lineage specification of parietal epithelial cells requires beta-catenin/Wnt signaling. J Am Soc Nephrol. 2012;23:63–72. doi: 10.1681/ASN.2010121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eymael J, Sharma S, Loeven MA, Wetzels JF, Mooren F, Florquin S, Deegens JK, Willemsen BK, Sharma V, van Kuppevelt TH, Bakker MA, Ostendorf T, van der Vlag J, et al. CD44 is required for the pathogenesis of experimental crescentic glomerulonephritis and collapsing focal segmental glomerulosclerosis. Kidney Int. 2018;93:626–642. doi: 10.1016/j.kint.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Rambaldi A, Iurlo A, Vannucchi AM, Noble R, von Bubnoff N, Guarini A, Martino B, Pezzutto A, Carli G, De Muro M, Luciani S, et al. Safety and efficacy of the maximum tolerated dose of givinostat in polycythemia vera: a two-part Phase Ib/II study. Leukemia. 2020 doi: 10.1038/s41375-020-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mascarenhas J. Rationale for combination therapy in myelofibrosis. Best Pract Res Clin Haematol. 2014;27:197–208. doi: 10.1016/j.beha.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Odobasic D, Ghali JR, O'Sullivan KM, Holdsworth SR, Kitching AR. Glomerulonephritis Induced by Heterologous Anti-GBM Globulin as a Planted Foreign Antigen. Curr Protoc Immunol. 2014;106:15 26 11–15 26 20. doi: 10.1002/0471142735.im1526s106. [DOI] [PubMed] [Google Scholar]
- 63.Tan D, Phipps C, Hwang WY, Tan SY, Yeap CH, Chan YH, Tay K, Lim ST, Lee YS, Kumar SG, Ng SC, et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol. 2015;2:e326–333. doi: 10.1016/S2352-3026(15)00097-6. [DOI] [PubMed] [Google Scholar]
- 64.Baggiolini A, Varum S, Mateos JM, Bettosini D, John N, Bonalli M, Ziegler U, Dimou L, Clevers H, Furrer R, Sommer L. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16:314–322. doi: 10.1016/j.stem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 65.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Mort RL, Ford MJ, Sakaue-Sawano A, Lindstrom NO, Casadio A, Douglas AT, Keighren MA, Hohenstein P, Miyawaki A, Jackson IJ. Fucci2a: a bicistronic cell cycle reporter that allows Cre mediated tissue specific expression in mice. Cell Cycle. 2014;13:2681–2696. doi: 10.4161/15384101.2015.945381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedemann J, Heinrich R, Shulhevich Y, Raedle M, William-Olsson L, Pill J, Schock-Kusch D. Improved kinetic model for the transcutaneous measurement of glomerular filtration rate in experimental animals. Kidney Int. 2016;90:1377–1385. doi: 10.1016/j.kint.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 68.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Susztak K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360:758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lun AT, Bach K, Marioni JC. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 2016;17:75. doi: 10.1186/s13059-016-0947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 72.Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haghverdi L, Lun ATL, Morgan MD, Marioni JC. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol. 2018;36:421–427. doi: 10.1038/nbt.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Cheng F, Woan K, Sahakian E, Merino O, Rock-Klotz J, Vicente-Suarez I, Pinilla-Ibarz J, Wright KL, Seto E, Bhalla K, et al. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. J Immunol. 2011;186:3986–3996. doi: 10.4049/jimmunol.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann Rheum Dis. 2012;71:424–431. doi: 10.1136/ard.2011.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huarte E, Peel MT, Verbist K, Fay BL, Bassett R, Albeituni S, Nichols KE, Smith PA. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates Cytokine Storm in Experimental Models of Hyperinflammation Syndrome. Front Pharmacol. 2021;12:650295. doi: 10.3389/fphar.2021.650295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo G, Luc S, Marco E, Lin TW, Peng C, Kerenyi MA, Beyaz S, Kim W, Xu J, Das PP, Neff T, et al. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell Stem Cell. 2013;13:492–505. doi: 10.1016/j.stem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dawson MI, Elstner E, Kizaki M, Chen DL, Pakkala S, Kerner B, Koeffler HP. Myeloid differentiation mediated through retinoic acid receptor/retinoic X receptor (RXR) not RXR/RXR pathway. Blood. 1994;84:446–452. [PubMed] [Google Scholar]
- 79.Triviai I, Stubig T, Niebuhr B, Hussein K, Tsiftsoglou A, Fehse B, Stocking C, Kroger N. CD133 marks a stem cell population that drives human primary myelofibrosis. Haematologica. 2015;100:768–779. doi: 10.3324/haematol.2014.118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding authors upon reasonable request. Single cell RNAseq data are available in NCBI's Gene Expression Omnibus repository as GSE195784, GSE195785 and GSE195797.