Abstract
It is nowadays well-accepted that the extracellular matrix (ECM) is not a simple reservoir for growth factors but is an organization center of their biological activity. In this review, we focus on the ability of the ECM to regulate the biological activity of BMPs. In particular, we survey the role of the ECM components, notably the glycosaminoglycans and fibrillary ECM proteins, which can be promoters or repressors of the biological activities mediated by the BMPs. We examine how a process called mechano-transduction induced by the ECM can affect BMP signaling, including BMP internalization by the cells. We also focus on the spatio-temporal regulation of the BMPs, including their release from the ECM, which enables to modulate their spatial localization as well as their local concentration. We highlight how biomaterials can recapitulate some aspects of the BMPs/ECM interactions and help to answer fundamental questions to reveal previously unknown molecular mechanisms. Finally, the design of new biomaterials inspired by the ECM to better present BMPs is discussed, and their use for a more efficient bone regeneration in vivo is also highlighted.
Keywords: BMPs, Extracellular matrix, bioinspiration, biomaterials, mechanotransduction
1. Introduction
Bone morphogenetic proteins (BMPs) are secreted cytokines, part of the transforming growth factors-β superfamily, with pivotal roles during embryogenesis [1, 2], tissue homeostasis [3], cellular proliferation [4, 5], migration [6, 7] and differentiation [8, 9]. BMPs signaling is widely investigated for vertebral skeletal development and homeostasis [10] but it has fundamental roles in the development of the majority of tissues [11]. The main BMPs involved in the skeletal development are BMP-2,-4,-5,-6,-7 as well as their antagonist Noggin and Gremlin (reviewed in [10]). The conditional ablation of these BMP genes selectively in the limb bud or the total deficiency of these genes generated defects at the cell level (osteoblasts, chondrocytes) and at the tissue level (joint defect) [10]. BMPs have different physiological and pathological roles. BMP-2 plays an important role in the development of the musculoskeletal system [12], notably cartilage and bone formation. BMP-2 and BMP-4 have an important role in the regulation of normal and cancer stem cells, in particular in the hematopoietic system [13], in brain cancer and neurological diseases [11], BMP-5 in nephrogenesis [14] and lung carcinoma [15], BMP-6 in the regulation of ion metabolism [16] and BMP-7 in inflammation, macrophage polarization and differentiation of fat cells [17]. BMP-9 and BMP-10 have a key role in vascular development and angiogenesis [18, 19]. Their role in bone formation in vivo has emerged very recently [20]
BMPs bind to BMP receptors (BMPR) which are composed by a complex of type-1 (BMPR1) also called Activin receptor-like kinase (ALK)- 1, 2, 3, or 6 and type-2 (BMPR2), Activin A receptor, type-2 A (ACVR2A) and ACVR2B) [21, 22]. They are serine/threonine kinase receptors which trigger the phosphorylation of SMAD 1/5/8 and together with SMAD 4 translocate to the nucleus to activate the transcription of target genes [23]. BMPs signaling induces also a SMAD-independent (non-canonical) pathway via the phosphorylation of MAPK which also leads to osteogenic differentiation [24].
The extracellular matrix (ECM) is composed of several fibrillar proteins (fibronectin, collagen, fibrillins) and proteoglycans. The fibrillar ECM proteins have not only the structural role of supporting organs and tissues allowing cellular adhesion via integrins but they have the role of binding and delivery signaling molecules like growth factors [25]. Finally, the physical properties such as the stiffness are also important to guide cellular fate [26]. The fibrillar proteins described in this review can bind BMPs and/or their prodomains to regulate BMP-mediated signaling. Fibronectin, collagen type IIA and IV and fibrillin are known to have specific BMPs and/or BMP prodomains binding sites, as reviewed elsewhere [25] and, close to them, interaction sites for other cell membrane receptors such as integrins or heparin binding sites [27, 28].
Proteoglycans (PGa) are glycosylated proteins composed by core-protein on which one or more glycosaminoglycans (GAGs) chain are covalently attached to a serine residue of the core protein. GAGs are polysaccharides chains of repeated disaccharides anchored via their reducing end to the core proteins [29]. In particular, a tetrasaccharide primer is elongated by adding disaccharides repetitions in the Golgi apparatus where other enzymes as sulfotransferases and N-deacetylases modify the GAG chain. They are then expressed at the cell membrane. There are four families of GAGs: heparin/heparan sulfate (HS), chondroitin/ dermatan sulfate (DS), keratan sulfate and hyaluronic acid (HA). For the first three families, the GAGs are synthetized in the Golgi apparatus while hyaluronic acid is synthetized directly at the plasma membrane by transmembrane enzymes and immediately secreted [30]. The ectodomains of cell-surface proteoglycans (PGs) can be released by the action of sheddases generating extracellular PGs.
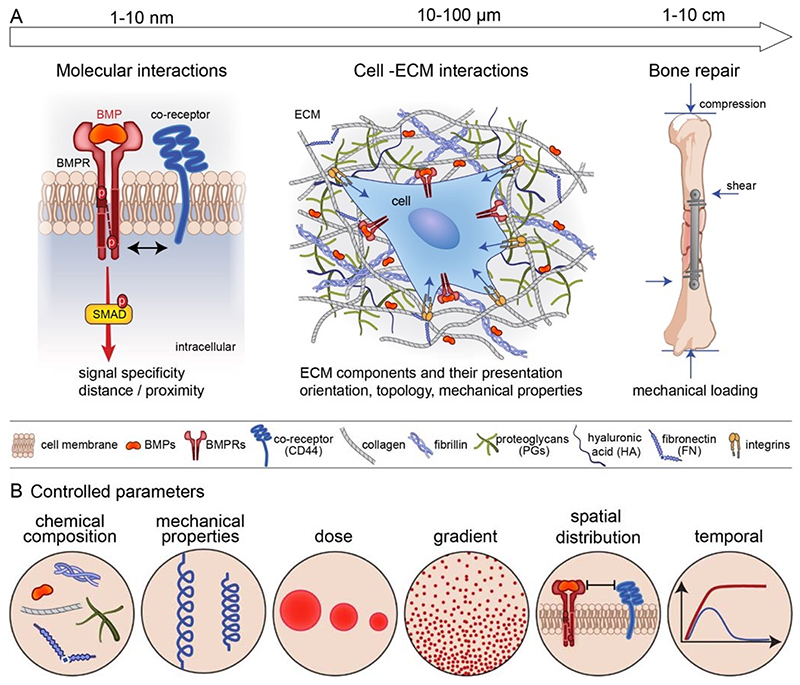
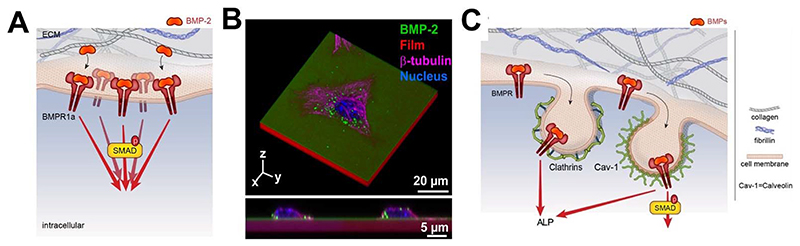
It is now widely accepted that the ECM is not simply the reservoir for these BMPs but is a direct regulator of the biological activities of BMPs [31]: by providing the binding site for BMPs, regulating their dose and availability, and providing a mechanical signal to the cells. In terms of length scale, the BMPs may act at different length scales (FIG. 1A). At the nanoscale, which is the level of the BMP receptors, there are different types of co-receptors engaged by the ECM proteins and GAGs. At the cellular level, the cell is embedded in the ECM proteins and GAGs that present and orient the BMPs to the cells and provide mechanical forces on the receptors. At the bone tissue level, mechanical forces are playing a key role on bone repair induced by the bone progenitors and environmental factors. Whatever the length scale considered (nanoscale, cellular and tissue scale), the chemical composition and mechanical properties of the ECM, the dose and the gradient of BMPs, their spatial and temporal availability will impact the responses of the receptors, cells and tissue (FIG. 1B).
Fig. 1. Different lengths of BMP-mediated biological activity and common biophysical parameters.
(A) At the level of the BMP receptors (nanoscale), there are different types of co-receptors engaged by the ECM proteins and glycosaminoglycans. At the cellular level, the cell is embedded in the ECM proteins and GAGs that present and orient the BMPs to the cells provide mechanical forces on the receptors and permit cellular adhesion. At the bone tissue level, mechanical forces are playing a key role in bone repair induced by bone progenitors and modulated by environmental factors (mechanical loading: compression and shear forces). (B) At each of this level (nanoscale for the receptors, cellular for the cells and tissular for the bone tissue), several common parameters are impacting the biological responses: chemical composition and mechanical properties, dose of BMPs, spatial gradient of BMPs, spatial distribution of the cell receptors and temporal availability of the BMPs.
In this review, we aim to show the effect of the ECM as an important regulator of the activity of BMPs. We first summarize the role of the ECM components, notably the glycosaminoglycans (part 2) and fibrillary ECM (part 3), which are important for the binding and presentation of BMPs to the BMPRs. ECM components can be either promoters or inhibitors of BMP bioactivity. We then present how the forces transmitted from the ECM and converted in biochemical signals into the cell, the so-called mechano-transduction, affects BMP signaling (part 4) and internalization (part 5). We also show the effect of the spatio-temporal regulation of BMP release from the ECM (part 6). For each aspect, we report how biomaterial approaches can be used in vitro to answer fundamental biological questions and help to reveal previously unknown mechanisms. Finally, we show how biomaterials inspired from ECM/BMP interactions can enable a better presesentation of BMPs for a more efficient bone regeneration in vivo.
2. Glycosaminoglycans and BMP signaling
After their synthesis and secretion, BMPs interact with proteoglycans, which are present both at the cell surface and within the ECM. Indeed, proteoglycans decorate both ECM proteins such as collagen, fibronectin, fibrillin and other receptors present at the cell membrane, including MuSK (muscle-specific kinase) and BAMBI (BMP and activin membrane-bound inhibitor), which are reviewed somewhere else [32]. By interacting with the cell surface and ECM, cell-secreted BMPs have a constrained range of action in the pericellular space.
GAGs binding sites and BMPs recognition motifs
BMP-2 and 7 can bind to GAGs [33, 34], mainly to heparin and heparan sulfate-GAG family [35, 36]. However, their binding to other GAGs with a lower affinity than to heparin has also been demonstrated [37]. For example, BMP-2 binding affinity to heparin has been measured to be in the range of 2 and 20 nM [35, 37] while the binding affinity to dermatan sulfate was 10 fold lower with a slower association and a faster dissociation rate [37]. BMP-9 and 10 do not present GAG binding sites. However in the pericellular space, they are not free to circulate since they can bind to cell-surface co-receptors such as endoglin [19].
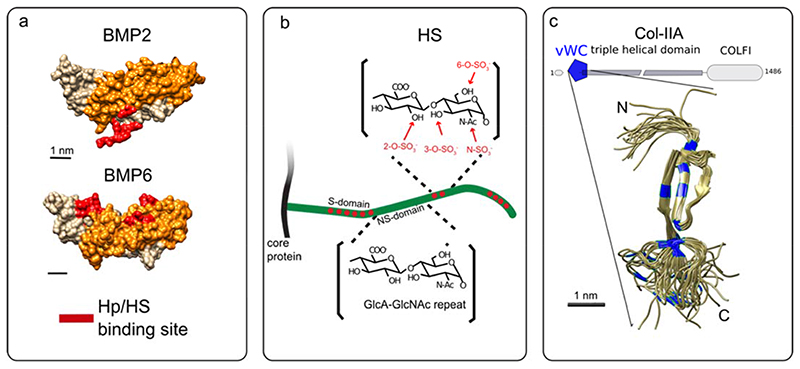
The heparin binding site on BMP-2 has been revealed by Ruppert et al. in 1996 [35]. This sequence of alternated basic amino acids is located at the N-terminal of the protein is called Cardin-Weintraub motif (FIG. 2A). This motif is generally composed by a sequence like XBBXBX or XBBBXXBX where B are the basic residues and X corresponds to hydropathic residues [38]. Molecular studies presented Cardin-Weintraub motifs located at the N-terminus of BMP-2 and BMP-4 (FIG. 2A) and at the C-terminus of BMP-5/6/7 [34] (FIG. 2A) while no heparin-binding site is presented on BMP-9 and BMP-10 [39]. The BMP homolog in Drosophila decapentaplegic (Dpp) also presents the heparin binding site at the N-terminal domain [40]. It is not clear whether the Cardin-Weintraub motif mediates the binding of BMPs to other GAGs such as dermatan sulfate (CS) [41] and to chemically sulfated hyaluronic acid (HA) [42].
Fig. 2. Structures of selected BMPs and GAGs and ECM proteins.
(A) Structure of BMP-2 (PDB 6OMN) and BMP-6 (PDB 6OMO) [120] as example of BMPs presenting the Heparin/Heparan sulfate-binding site (Hp/HS) at the N and C-terminal domains, respectively. (B) Scheme of HS grafted to core proteins to form heparan sulfate proteoglycans (HSPGs). Non-sulfated domains composed by repetitive subunits of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA) are represented. The sulfated domains are represented in red with the possible positions of the sulfation motifs. Adapted from [45]. C) Collagen IIA with the structure of the vWC motif (PDB 1U5M) [213]. Cysteine residues are labeled in blue. Adapted from [156]
BMPs bind to HS specifically at the sulfated regions of the polysaccharides (FIG. 2B). In these regions, HS presents specific sulfation motifs (the number and distribution of N- or O-sulfate groups along the chain) that work as molecular recognition elements [43, 44]. An extensive analysis of HS structure and function has shown that the cell type-specific pattern of sulfation determines the binding affinity of HS to growth factors, cytokines, and other extracellular proteins [43, 45–47], thereby modeling their distribution and activity in the extracellular space [29]. The basis of the biochemical interaction and the importance of a specific sulfation pattern on BMPs bioactivity, have not been deciphered at the molecular level. Indeed, characterization of BMP-2 binding to specific HS-sulfation motifs has so far relied on molecular indirect studies on enzymatically de-sulfated heparin on specific positions, chemically sulfated HA or sulfated chitosan [42, 48, 49]. More recently, it has been proposed, by enzymatical removal of specific sulfations of HS decasaccharides, that the N-sulfations have an important role on the binding of BMP-2 followed by the 6-O-sulfation while 2-O-sulfation has a secondary role in binding BMP-2 [48]. By these indirect methods, the sequence and sulfation pattern of HS motifs recognized by growth factors cannot be revealed and the binding affinity of growth factors to the different HS motifs cannot be directly quantified. The saccharides analysis of long HS fragments is difficult to achieve due to the complexity of the HS polymer while progress on the synthetic heparin and HS are expected to be the solution to determine the structure-function relationship between HS sulfation pattern and BMP-2 bioactivity [50].
Computational data with highly sulfated GAGs derivatives as sulfated hyaluronic acid and chondroitin sulfate proved a conformational change of the N-terminal region of BMP-2 upon binding. This conformational change reduces the affinity of BMP-2 to BMPR1A in a sulfation-dependent manner [42]. This SPR measurement is in contradiction with previous results showing that BMP-2–HS interaction helped the BMP-2/BMPR1A recognition, [51] leaving this finding still under debate.
Regarding HA, since it has no sulfated group, it might not be expected to interact with BMPs at neutral pH. However, a study showed that HA-based matrices could retain and then deliver BMP-2 over 4 weeks [52]. Using the Van der Waals forces between the BMPs and HA, an innovative approach was used to improve the retention of BMP-2 on HA-based hydrogel by changing the protonation state of the carboxylic acid residues of the GAG [53]. Future studies may use HA to retain more efficiently BMP and may also aim to understand the underlying mechanism of BMP/HA molecular interactions.
Role of GAGs on BMPs signaling
After the discovery of the heparin binding-site on BMP-2, the majority of studies regarding the roles of GAGs on BMPs bioactivity were concentrated on heparin and HS. The importance of HS in BMPs signaling has been demonstrated in Drosophila where the abrogation of heparin synthesis was found to disrupt the Dpp signaling pathway [54, 55] and on a rare paediatric disease called hereditary multiple exostoses which causes bone masses at the side of long-bones [56, 57]. This disease is due to mutations of Ext1 glycosyltransferase responsible for the elongation of the HS chain, in the Golgi apparatus by adding to the initial tetrasaccharide primer alternating units of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA). It has been reported that Ext1-deficient cells in the cartilaginous region of endochondral bones, present an increased BMP signaling at early tumour stages [58]. This implies that the deficiency of HS at the cellular surface enhances BMP-2 bioactivity. In line with that other studies have supported the inhibitory effect of Heparin/HS on BMP-SMAD signaling. The mutated form of BMP-2 for the heparin binding site presented 15–20 fold increased SMAD 1/5/9 phosphorylation as compared to the wild type BMP-2 [35]. However, this variant leads to a reduction in osteoinductivity of BMP-2 in vivo [59]. On the other hand, addition of basic amino acids at the N-terminal region of BMP-2 induces an increase in the binding to heparin, leading to an enhancement in the osteoinductive properties and resulting in higher and faster bone formation [59].
Another study has shown that the injection of soluble heparin inhibits the ALP activity mediated by BMP-6 in vitro on C2C12 cells and consequently in vivo bone and cartilage formation in rat [60].
Exogenous HS injected in solution was also found to enhance BMP-2-mediated SMAD 1/5/9 phosphorylation [51] in C2C12 cells. In vivo soluble HS seems to improve bone regeneration, suggesting the use of this GAG in future regenerative medicine applications [61, 62]. This controversial result is likely due to a different but unknown function of cell-surface and extracellular HS-proteoglycans (FIG. 3A). It has been shown that the exogenous soluble HS improves BMP-2-mediated chondrogenic differentiation as well as heparitinase treatment of cell-surface HSPGs, while the up-regulation of syndecan-3 (cell-surface HSPGs) suppresses BMP-2-mediated SMAD phosphorylation [63]. Studies in Drosophila showed that Dally, a cell-surface HSPG, potentiates Dpp signaling and acts as a co-receptor [64].
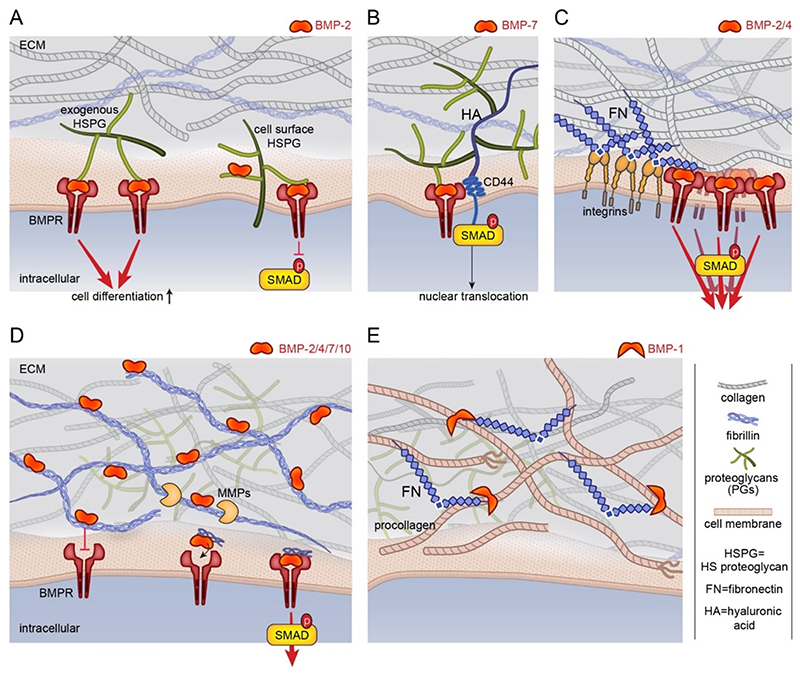
Fig. 3. Schematic representation of the role of ECM components on BMP bioactivity.
(A) HSPGs bind and present BMPs to cellular receptors, namely the BMP receptors (BMPR). Their role of HSPGs on BMP-SMAD signaling is highly debated but it seems to depend on their location, either in the ECM or at the cell surface. HSPG localized in the ECM may enhance signaling while HSPG localized at the cell surface may inhibit BMP-SMAD signaling [63]. (B) The non-sulfated GAG hyaluronic acid (HA) enhances BMP-7-mediated chondrogenic differentiation since its receptor CD44 interacts with SMAD1 [75]. (C). The ECM protein fibronectin (FN) presents BMPs and integrin binding sites in close proximity, which induces a synergy between BMPRs and integrin to modulate SMAD signaling [83]. (D) The ECM proteins fibrillins bind the prodomains of BMP-2, 4, 7, 10 [88, 89, 91]. The binding of BMP-7 prodomain to fibrillin-1 induces a fibrillin conformational change that prevents BMP-7 from binding to its BMP receptors [91]. BMPs can later be released in the ECM when the ECM is remodeled by metalloproteases. Their release in the matrix enables their binding to the BMPRs. (E) Collagen fibrillogenesis depends on fibronectin (FN) assembly in the ECM since FN fibrils provide binding sites for BMP-1/tolloidin (a metalloprotease) to facilitate procollagen processing [107].
The studies on exogenous HS used a soluble form of it. However, in the natural environment, HS is not in solution but rather grafted on core proteins forming HSPGs. To mimic this presentation mode, novel bioinspired 2D surfaces have been developed to graft HS as it is in nature by its reducing end [65–68]. They consist in streptavidin platforms on which biotinylated HS is grafted and oriented (iHS) thanks to the site-specific biotinylation [68]. Once BMP-2 is adsorbed iHS and presented to cells the p-SMAD 1/5/9 signaling is sustained over time and up-regulated with respect to the soluble form [65]. It has been suggested that the antagonist Noggin is not able to inhibit BMP-2 once it is adsorbed to iHS, which is not the case for soluble HS [65]. To clarify the mechanism of action of cell-surface and extracellular HS on BMPs signaling, it would be important to compare the effect of these biomimetic platforms on HS-deficient cells.
Although less studied, some evidence has shown that other GAGs including chondroitin and dermatan sulfate (CS and DS) impact BMP-SMAD signaling [69] through their binding to different BMPs [37, 41]. CS is structurally closely related to HS but contains N-acetylgalactosamine (GalNAc) instead of GlcNAc. It is sulfated at specific positions generating different type o CS called CS-A (sulfation on carbon 4 of GalNAc) CS-B (dermatan sulfate DS composed by sulfation on carbon 4 of GalNAc and iduronic acid) CS-C (sulfation on carbon 6 of the GalNAc), CS-D (sulfation on carbon 2 of GlcNAc) and CS-E (sulfation on carbon 4 and 6 of GalNAc). Studies using soluble of CS-E bound to BMP-2 and BMP-4 provided opposite results on BMPs bioactivity. In one study soluble CS-E inhibited p-SMAD 1/5/9 in presence of BMP-2 [70] while in the second study soluble CS-E enhanced BMP-4-mediated osteogenic differentiation [71]. The treatment of primary chondrocytes with chondroitinase reduced the bioactivity of BMP-2 showing that cell-surface CS enhances BMP-2 bioactivity [69].
Another type of ECM PGs, the small leucine-rich proteoglycans (SLRP) consists of a protein core with leucine rich-repeat motifs that is covalently linked to GAG side chains of CS, dermatan and keratan sulfate [72]. Generally, SLRP presents an inhibition effect on BMPs bioactivity [72].
Sulfated GAGs were used to form biomaterials based on a multilayer assembly of chitosan as polycation and oxidized HS and CS as polyanion, together with adsorbed BMP-2. These biomaterials with oxidized GAGs act as a reservoir for the sustained release of BMP-2 and promote cell adhesion, migration and osteogenic differentiation [73].
The non-sulfated GAG HA has also an important function on bone growth. Indeed, it is a main component of the bone ECM and it has been isolated on archeological human skeleton as well as CS [74]. It has been reported that in the chondrocyte response to BMP-7, SMAD1 might immunoprecipitate with the HA receptor CD44 (FIG. 3B) and that this complex is needed for SMAD1 and 5 nuclear translocation [75]. Soluble HA enhances BMP-2-mediated SMAD 1/5/9 phosphorylation and ALP expression [76]. In this study, the authors proposed that HA enhances BMP-2 signaling by blocking the cellular expression of Noggin and Follistatin, another BMP antagonist [76]. A HA-based hydrogel with modified protonation state of the carboxylic acid residues enables the binding of BMP-2 and promotes bone formation in vivo, in comparison to a non-modified HA hydrogel [53]. This result is in line with our studies on Poly(L-lysine) (PLL)/HA polyelectrolyte multilayer films showing that the incorporation of BMP-2 was potentiated at pH 3 in the absence of salt, in comparison to a neutral salt-containing solution (pH 7.4 at 150 mM NaCl) [77]. Such (PLL/HA) films present BMP-2 to cells in a matrix-bound manner, due to the physico-chemical (non-covalent) interactions of BMP-2 with the film. Matrix-bound BMP-2 was shown to increase p-SMAD 1/5/9 activity and osteogenic differentiation [77] in comparison to soluble BMP-2 [78]. In addition, soft films enabled to reveal a so-far hidden role of BMP-2 on cell adhesion and migration [7]. To investigate the possibility of enhanced BMP-2 binding using sulfated GAGs, heparin was added to HA to form mixed PLL/HA-heparin films [79]. The thickness of the LbL was significantly lower for (PLL/heparin) in comparison to the (PLL/HA) films. Moreover, the presence of heparin was not improving the amount of incorporated BMP-2 nor the ALP activity on C2C12 cells [79], which was solely BMP-2-dose dependent and not dependent on the presence of sulfated groups. Thus, HA by itself may also be a good way to retain BMP-2.
3. Fibrillar ECM and BMP signaling
Fibrillar ECM as a dynamic modulator of BMP signaling
Fibrillar proteins from ECM include multiple, independently folded domains. Some of these domains bind adhesion receptors such as integrins that mediate cell-matrix adhesion. Other domains found in fibronectin, collagen and fibrillin are capable of binding directly to BMP and/or BMP signaling molecules [31, 80]. They act as dynamic modulators of BMP bioavailability and activity [25, 81–83]. This property inspired the field of bioengineering. Indeed, different engineered material surfaces have been designed to mimic the BMP-2 microenvironment and tune cellular as already described in our previous review [84]. It is known that Fibronectin is able to bind BMP-2/4 through the FN 12-14 domain which is localized very close to the FN 7-11 domain known to interact with integrins [83], providing functional proximity between integrins and BMPRs (FIG. 3C). Structural analyses have also revealed that the N-terminal prodomain of collagen type IIA binds BMP-2 [85] (FIG. 2C), whereas the C-terminal part of collagen IV binds BMP-4 [86]. A mechanism based on type IIA procollagen mRNA splicing provides an interesting model for BMP reservoir establishment and induction and differentiation of bone. At its N terminus, this major collagen of cartilage, contains a chordin-like the von Willebrand factor type C (VWC) domain (FIG. 2C) that binds BMP-2 and TGF-β1 two chondrogenic growth factors. The VWC domain is alternatively spliced, included in prechondrogenic mesoderm and early developing cartilage but excluded in mature cartilage when the cells differentiate into chondrocytes [85]. This VWC or chordin domain is found in many ECM proteins acting as a negative regulator of BMP functions [87]. Collagen IV, the universal constituent of basement membranes, restricts Dpp/BMP signaling range in Drosophila model [86]. The Dpp-binding motif identified in the C-terminal domains of the two Drosophila collagen IV subunits is highly conserved across phyla, suggesting that this interaction may be important in other contexts [86].
Fibrillins are other major components of the ECM that bind via their N-terminal part to BMP prodomain in the case of BMP-2, -4, -7, and -10 [81, 88, 89] (FIG. 3D). Fibrillins 1, 2 and 3 are self-assembling glycoproteins. They are critically involved in determining tissue elasticity and stiffness [90], which can eventually impact BMP diffusion and gradient. Findings from Nistala et al. [91] identify fibrillins as critical regulators of bone formation through the modulation of endogenous BMP signaling. BMP/fibrillin interactions have been also described as crucial for limb digit formation patterning [92]. However, sequestration of BMPs by fibrillin can inhibit binding to BMPR. The binding of the BMP-7 prodomain to fibrillin-1 causes a conformational change that prevents BMP-7 from binding to BMP receptors (FIG. 3D) [91]. However, BMPs can later be released through matrix remodeling, so that BMP sequestration functions to provide a reservoir [91]. BMPs assemble non-covalently with fibrillins [88], but BMP-propeptide association does not prevent necessarily the ligand from interacting with its receptors [93]. It has been suggested that the elevation of osteoinductive BMP signals in Fibrillin1-/- osteoblast cultures overrides the inhibitory action of improper TGF-β activity. As such, fibrillin-1 microfibrils might act as negative regulators of TGF-β and BMP bioavailability in the forming bone. Interestingly, BMPR2-deficient cells express genes indicative of altered biophysical properties, including up-regulation of ECM such as Fibrillin1 and of the adhesion receptors integrins [94]. Finally, dynamic properties of ECM are important not only for BMP sequestration but also for BMP protection from neutralization by soluble inhibitors.
BMP signaling as regulator of matrix remodeling
BMP and BMP-related molecules participate in matrix remodeling. BMPs promote the production of ECM proteins in vitro [7, 95–97]. Analysis of BMPR1aCKO and BMPR1b –/– embryos reveals an essential role of BMP signaling in the production and remodeling of cartilage ECM proteins in vivo which likely impact the cartilage-to-bone transition through a defect of chondrocyte proliferation and/or survival [95]. Furthermore, the mammalian tolloid (TLD) family of metalloproteinases (BMP-1/TLD) is essential for tissue patterning and ECM assembly. The BMP-1/TLD family is conserved in species ranging from Drosophila to humans and their importance is highlighted by the embryonic lethal phenotype of BMP-1/Tll1 homozygous null mice, which display heart malformations and abnormal procollagen processing. In vertebrates, BMP-1/TLD proteinases are involved in the biosynthetic processing of a wide range of ECM precursors including collagens, the crosslinking enzymes lysyl oxidase (LOX) and LOX-like, laminin basement membrane proteins and SLRPG osteoglycin and probiglycan [98, 99]. As an example, assembly of collagen requires proteolytic processing of procollagen by distinct proteinases ADAMTS-2 and of BMP-1 that cleave the N- and C-terminal propeptides, respectively [100–103]. However, it is worth to note that cleavage of collagen by BMP-1 induces the production of endotrophin known to be highly inflammatory and to be present in fibrosis and tumoral tissue [104]. BMP-1 and its enhancer PCPE-1 have been shown to bind to FN [105, 106]. More recent results indicate that collagen fibrillogenesis depends on the FN matrix at least in part since FN fibrils provide binding sites for BMP-1 to facilitate procollagen processing [107]. An increased rate of processing is also observed in presence of heparin. This supports that the activity, bioavailability and diffusion of extracellular BMP signaling agonists and antagonists are further regulated by ECM components [82]. More investigations would be helpful to discriminate whether, how and in what circumstances ECM-bound BMPs need to be released to soluble form or can act as solidphase ligands given that fragments of ECM proteins can be released by proteolysis (FIG. 3E).
4. BMPs signaling in a biomechanical contest
Several reviews have already been dedicated to growth factor signaling and its relation to the biomechanical environment. Kopf et al. [96] have reviewed more specifically the synergy between the integrin adhesion receptors and BMPs-mediated signaling. Several other recent reviews highlighted the fact that growth factors signaling and mechano-transduction mediated via integrin receptors can be combined by engineering cellular microenvironments [108–110], including a very recent review published in bone [111]. At the tissue scale, it is already known that mechanical loading is important for bone growth and regeneration [112] and that BMP signaling and mechano-transduction pathways are tightly interconnected and represent an elaborate signaling network that is active during development [96]. At the cellular scale, cells interpret the biophysical properties of their microenvironment via cell surface mechanoreceptors that are coupled to the contractile cytoskeleton. Signalling is achieved via direct and indirect pathways that control cell fate via nuclear organization and gene expression [113, 114].
Involvement of BMPR in mechanosensing
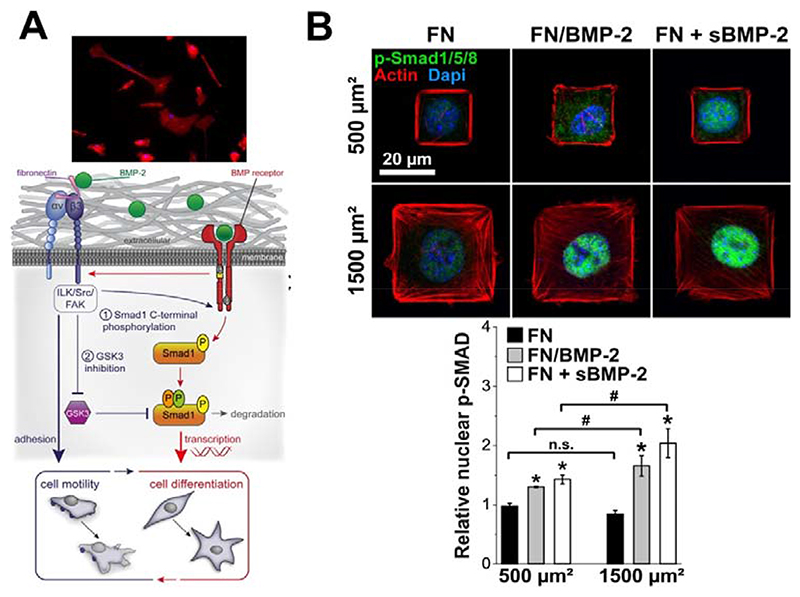
The proximity between FN 12-14 and FN 7-11 domains of FN is crucial to favor the cross-talk between BMP receptors and integrins. Using the (PLL/HA) biopolymeric films presenting bound BMP-2 (bBMP-2), we have shown that the level of SMAD phosphorylation in the presence of BMP-2 on stiff films was similar to that of soluble BMP-2 (sBMP-2), but higher phosphorylation was observed for bBMP-2 on soft films [78] in comparison to sBMP-2. An unexpected finding was that, besides BMP-2-mediated signaling, bBMP-2 is able to induce cell adhesion and spreading of soft films (FIG. 4A) and to potentialize cell migration on both soft and stiff films, the effects being stronger for soft films. This BMP-2 induced cell spreading was found to be mostly mediated by β3 integrins [7]. and to depend on the FN that is already present in the pericellular coat of C2C12 skeletal myoblasts. BMPR and β3 integrins work together to control SMAD signaling and cell adhesion/migration through a LIM kinase pathway involving Cdc42–Src–FAK–ILK, independently of ROCK. β3 integrin was found to regulate a multistep process to control first the BMP receptor activity and second the inhibitory role of GSK3 on SMAD signaling (FIG. 4A).
Fig. 4. BMP-mediated cell signaling is influenced by its presentation via a biomaterial and by the engagement of BMP receptors and co-receptors.
(A) Schematic view of β3 integrin–GSK3β and BMP-2–SMAD cooperation. The interaction between BMP-2 and BMP-2 receptors activates αvβ3 integrin and mediate cell spreading and cell migration thanks to BMP-2/FN interactions. In turn, αvβ3 is required first to allow the C-terminal phosphorylation of SMAD by BMPR and second to inhibit GSK3 activity via the Src–FAK–ILK pathway. BMP receptors and β3 integrin signaling converge to control both focal adhesion dynamics and SMAD signaling to couple cell migration and fate commitment. Reproduced from Fourel et al., [7], copyright from Rockefeller University press. (B) Phosphorylation and translocation of SMAD 1/5/8 to the nucleus depends on cell spreading: immunofluorescence images of C2C12 myoblasts spread on small (500 μm2) and large (1500 μm2) micropatterns of FN/BMP-2 and FN without or with BMP-2 in solution after 4 h of culture (FN alone being a negative control while FN in the presence of soluble BMP-2 (sBMP-2) is a positive control). Actin is in red, nuclei in blue and p-SMAD 1/5/8 in green and corresponding quantification of the relative nuclear p-SMAD 1/5/8 (n > 100 cells) in function of the size and composition of the micropatterns. *p < 0.01 versus negative control; #p < 0.01 between small and large micropatterns. n.s. stands for non-significant (i.e. p > 0.01). Adapted with permission from Fitzpatrick et al., [97], copyright Nature 2017.
Interestingly, BMP-2 can also be printed onto the layer-by-layer biopolymeric films and remain bioactive, which can be used to spatially control cell differentiation [115]. Mixed fibronectin/BMP-2 single-cell micropatterns on soft films were used to study the mechanism underlying BMP-2-mediated mechano-transduction [97]. Cell spreading itself potentiated BMP-2-dependent phosphorylation and nuclear localization of pSMAD 1/5/8 (FIG. 4B). This SMAD signaling was found to depend on LIM kinase 2 and ROCK, rather than myosin II activation. This result was different from the effect of cell spreading induced by sBMP-2 on mesenchymal stem cells cultured on stiff substrates, which was found to be regulated solely by RhoA/ROCK [116].
The same type of collaboration is illustrated in the case of Drosophila collagen IV which binds Dpp (a BMP homolog) and enhances its interactions with BMPR to regulate the dorsoventral axis and the numbers of germinal stem cells in the ovary through integrin activation [117, 118]. However, integrin can also act as an inhibitor of BMP signaling. Indeed, in a specific cellular context and experimental conditions, integrins can act as inhibitors. It has been shown that upon interaction with collagen type II, β1 integrin compete with BMPR to bind with SMAD1 and then inhibit SMAD1 activation and nuclear import [119]. This helps to suppress helping articular chondrocytes hypertrophy and osteoarthritis progression. The diversity of BMP signaling may also result from the combination of BMP ligands. Recently, a BMP-2/BMP-6/activin A chimera with increased binding affinity to BMP receptors was developed, notably to interact with the ALK2 receptor involved in bone formation [120]. These examples illustrate the capacity of conserved elements of ECM proteins to regulate, either positively or negatively, the functions of various BMPs. However, to date, the ability of ECM to bind a combination of BMP ligand has not been systematically investigated yet. It would be important to better characterized and understand ECM/BMP ligand interactions.
Conversely, the ECM may be affected by BMPR mutations. Shore et al [121] showed that fibroblasts mimicking the Fibrodysplasia Ossificans Progressiva (FOP) pathology, a mutation in ALK2 (R206H/+), have an altered collagen content and organization within the fibroproliferative tissue. Basal activation of BMP signaling pathways, even in the absence of ligand, has also been shown to regulate cell contractility in mesenchymal stem cells [122]. When cultured on tissue culture plastic (a stiff substrate), Rho activation was more robustly increased in Acvr1R206H/+ cells compared with controls and increased levels of phospho-cofilin, of phospho-myosin light chain 2 and nuclear stiffness, and a sensitivity to the ROCK inhibitor Y27, indicating the over-activation of the mechanotransduction pathway Rho/ROCK in mutant cells cultured on stiff substrates. When cultured on polyacrylamide gels of different stiffness, the Acvr1R206H/+ cells exhibited significantly greater cell areas and spreading compared with control cells, especially on the soft gels. In addition, the nuclear localization of the transcription factor RUNX2 was also higher in Acvr1R206H/+ compared with Acvr1+/+ control cells on soft gels. All these data suggested that Acvr1R206H/+ cells misinterpret their biophysical microenvironment, responding to soft substrates similarly to stiff ones, which leads to important changes in key factors that direct cellular differentiation and extracellular matrix production.
More recently, the same team further studied the interplay between Rho/ROCK and YAP pathways in the Acvr1R206H/+ cells [123]. The YAP/TAP complex is a key intracellular regulator of cell differentiation [124] that is known to be regulated by ECM stiffness and cell geometry. YAP/TAZ proteins shuttle between the cytoplasm and the nucleus in response to substrate stiffness and other environmental cues [125]. Cytoplasmic localization of YAP/TAZ is associated with a soft ECM and adipogenic condition, whereas YAP translocation to the nucleus occurs with a stiffer ECM, proliferation and osteogenic conditions [124]. YAP1 was recently found to influence the osteogenic differentiation of MC3T3-EA cells through the regulation of ID1 [126]. A recent study showed that YAP1 governs the initiation of fracture repair by inhibition of cartilaginous callus tissue formation and that it can interact with RunX2 [127]. Interestingly, Shore et al. [123] found that YAP1 has a nuclear localization in Acvr1R206H/+ cells on soft substrates, which was inhibited by the LDN-193189 drug targeting Acvr1 receptors. RhoA expression was increased in mutant cells and this was associated with an increased cellular adhesion and focal adhesion formation. The mutant cells also exerted a higher force compared to normal cells. The ACVR1R206H/+ cells were also poised to chondro/osteogenic differentiation even in the absence of inductive factors. Their results established RhoA and YAP 1 signaling as modulators of mechanotransduction in FOP and showed that the increased BMP pathway signaling through mutant ACVR leads to a higher sensitivity to mechanical stimuli and to increased chondro/osteo differentiation. This synergistic increase in BMP signaling and mechanical signaling promotes heterotopic ossification formation.
An additional step to understand the interaction between cells and the ECM would be to recapitulate the viscoelasticity of the native microenvironment. Viscoelastic matrices enable cell-mediated reorganization of the matrix nanoarchitecture, which in turn modifies local mechanics. Interestingly, in these 3D matrices, stiffness appears to be less important than the stress relaxation rate and fiber topography and remodeling [128–130]. The use of newly-developed viscoelastic materials, possibly as surface coatings, may enable to decouple the effects of stiffness, stress relaxation, fiber remodeling, and ligand density. Indeed, the spatio-temporal control of mechanical properties and the presentation of adhesive ligands might direct cell attachment and migration, or even modulate the mechano-active state of the cell through mechanical priming.
Contribution of microgravity in BMP-2-induced cytoskeleton organization
Recent interesting findings were obtained from experiments performed under simulated microgravity, since bone loss is the most prominent and well recognized phenomenon occurring during spaceflight [131]. One of the consequences of the microgravity is to disrupt the organization of the actin cytoskeleton. Dai et al. [132] showed using drug disruption of the actin cytoskeleton, that the cytoskeleton is mediating the Cbfa1 (RunX2) responsiveness to BMP-2. On the contrary, stabilization of the cytoskeleton by Jasplakinolide can reverse the responsiveness of RunX2 to BMP-2. The same team later demonstrated that integrin αvβ3 participated in the synergetic regulation of RunX2 activity by insulin growth factor 1 under simulated microgravity [133] via PI3K signaling. The role of the actin cytoskeleton in the BMP-2 mediated SMAD signaling studied under microgravity was recently shown to involve calponin 1 [134], an actin-binding protein that promotes and sustain actin polymerization and is crucial for cell contractility. In fact, calponin 1 appears to decrease BMP-2 induced p-SMAD signaling and to block SMAD translocation to the nucleus. Calponin 1 can interact directly with SMAD and p-SMAD. These studies provide a new piece of information on the mechanisms of mechanotransduction under simulated microgravity conditions, which probably contributes to bone formation decrease induced by this experimental condition.
The primary cilium has recently emerged as a sensory organelle in signal transduction in microgravity and electromagnetic field sensing involved in bone disease and cancers [135]. Indeed, Xie et al. [136] have proposed that BMP-2-SMAD 1/5/8 signaling plays a key role in primary cilium-dependent electromagnetic field sensation. They found that pulsed electromagnetic fields stimulated the osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPR2, which is localized at the base of the primary cilium.
All these studies highlight the importance of mechanical stimuli in BMP-mediated cell response and the mechanical sensitivity of BMP receptors.
5. BMP internalization to modulate BMP signaling
While BMP receptor and BMP ligand endocytosis has been proposed to promote receptor turnover and regulate BMP signal transduction [137], it is important to consider the ability of BMP to be internalized or not. Previous studies have indicated that clathrin/dynamin-dependent endocytosis of BMP type I receptors is conserved in various models, including C. Elegans, Drosophila, mouse, and human fibroblasts [138]. It is worth to note that the level of BMPR expression impacts BMP internalization. Indeed overexpression of BMPR1A induces an increase of BMP-2 uptake [139] and higher expression of BMPR2 short form at the plasma membrane results in enhanced activation of SMAD signaling (FIG. 5A), stressing the potential importance of the multilayered regulation of BMPR2 expression at the plasma membrane [140]. The endocytic machinery can drive specific BMP signaling or attenuate retrograde BMP signaling [141–144]. The (PLL/HA) films of controlled stiffness were used to study the internalization of matrix-bound BMP-2 (i.e. non-covalently bound BMP-2) and its dependence on film stiffness [141]. Interestingly, bBMP-2 internalization was found to be strongly stiffness-dependent: the amount of internalized BMP-2 is 8 fold higher and the internalization kinetics 5 times faster when BMP-2 is internalized from a soft film in comparison to a stiff film. BMP-2 internalization was independent of the presentation mode (soluble BMP-2 versus bound BMP-2) for low crosslinked films (soft) in striking contrast with high crosslinked (stiff) films where internalization was much lower and slower for bBMP-2. BMPR1A (ALK3) and BMPR-II receptor receptors are both involved in the BMP-2 internalization process in C2C12 cells. Caveolin-mediated internalization was found to be related to both SMAD and ALP signaling while clathrin-mediated internalization was only related to ALP signaling (FIG. 5B). To note, the scission of the endocytic vesicles was not required for SMAD or ALP signaling, highlighting the fact that signaling can still occur at the plasma membrane even in the absence of BMP-2 internalization.
Fig. 5. Influence of BMP receptor overexpression and of BMP internalization on cell signaling.
(A) Schematic showing that the overexpression of BMPR1A induces an increase of BMP-2 uptake and higher expression of the BMPR2 short form at the plasma membrane (not shown here), resulting in enhanced activation of SMAD signalling (adapted from [139]). (B) Internalization of matrix-bound BMP-2 by C2C12 skeletal muscle cells. The so-called matrix-bound BMP-2 corresponds to the presentation of BMP-2 by a thin matrix made by a layer-by-layer assembly of poly(L-lysine) and hyaluronic acid) (PLL/HA films). This (PLL/HA) film made of 24 pairs of layers has a total thickness of ~4-5 μm. 3D reconstruction of a confocal section (top view) and transverse section (bottow view) showing the cell lying on top of the film (BMP-2Carboxyfluorescein being used to visualize BMP-2 in the film (green), while the film itself is labeled using a rhodamine dye (red) (C) In these experimental conditions, calveolin-1 mediates both SMAD and ALP signaling while clathrin mediates solely ALP signaling. Panel B and C are reproduced with permission from Gilde et al., [141], Copyright Elsevier 2016.
BMP-2 internalization on soft ECM might be considered as an induction mechanism of stem cell differentiation by ECM elasticity [7, 141, 145]. More recently, it has been shown that retrograde BMP signaling including micropinocytosis, a mode of clathrin- and dynamin-independent endocytosis, might be also a key instructive regulator of BMP-dependent bone development [146]. This mode of endocytosis is indispensable for signaling-dependent downregulation of BMPRs at the developing neuro-muscular junction synapse. However, BMP internalization might be affected by BMP antagonists in different ways. While gremlin and Noggin strongly increased BMP-2 internalization, chordin blocked BMP-2 uptake explaining some of the selective biological functions of BMP antagonists [139, 147]. Since BMP-2 is not significantly degraded upon internalization, sorting to late endosomal particles could also provide a reservoir for later use or may be associated with other cellular functions.
In line with the fact that BMP internalization is not required to trigger downstream signaling, several studies used BMPs covalently immobilized to the surface [148–152]. BMP-2 and BMP-6 were covalently coupled to gold nanoparticles with the heterobifunctional linker MU-(N-hydroxysuccimide) NHS [148–150]. Both BMPs were still active after covalent coupling and enabled SMAD 1/5/9 phosphorylation. Moreover, the effect of immobilized BMPs vs soluble BMPs on SMAD 1/5/9 phosphorylation was significant at low BMP-6 doses (1 ng) [148]. Site-specific immobilization of BMP-2 ensures a good orientation of the BMP toward BMPRs that permits a reduction of the BMP dose in comparison to non-specific immobilization. Site-directed immobilization of BMP-2 by click chemistry has been applied on beads and it promotes ALP expression of C2C12 cells in vitro [151, 152]. Nevertheless, BMP2 internalization should be considered in all biomaterials since BMP endocytosis is highly regulated. Besides, BMP signal transduction may depend on the context-specific endocytic routes taken by the receptors.
6. Spatio-temporal control of BMP signaling
The development and homeostasis of multicellular organisms require the coordinated interaction between different biomolecules and receptors. These mechanisms allowing communication between cells within and between tissues are required to confirm the proper assignment of cell types during development and repair. By nature, the signaling by morphogens, like BMPs, is closely related to their spatial position and dose, so that signaling varies across a tissue depending on the amount of ligand, the presence and concetnration of antagonists, and the receptors that are present in the cells of this tissue. The function and efficiency of cell signaling pathways are highly dependent on their coordinated organization both in space and time.
Spatial regulation of BMP signaling
Signaling components in cells are organized to transmit information across cells in nanomter/micrometer size-compartments. This spatial organization of signaling pathways depends on molecular interactions that occur at the nanoscale between the cellular components.
BMP interactions with ECM components facilitate their localization and spatially-regulated signaling, thanks to the high affinity and promiscuous ECM protein-binding, as described in detail in the previous parts. BMPs are recognized to have numerous interactions with GAGs [35, 36] and fibrillar ECM proteins [25, 28, 83, 85, 86, 88, 91, 97, 104, 105, 153–157]. Thus, the ECM components contribute to control the availability of BMPs, since they act as a localized reservoir [91]. ECM proteins regulate cell signaling via their interactions with the adhesive cellular receptors (integrins and others). The adhesive receptors engaged by the ECM act in close proximity to BMP receptors and have an important role as BMP co-receptors. Via their interactions, ECM proteins can thus have a role of enhancers or inhibitors of BMP signaling, depending on the context. For instance, the presence of metalloproteases (BMP-1/TLD) can establish an antagonist sink regulating the bioavailability of BMPs, which is considered as the primary mechanism to drive the formation of BMP gradients [97, 158].
In vivo, the spatial organization of BMP signaling has fundamental roles in both embryonic development and postnatal bone homeostasis [3]. Much of our understanding of spatially-distinct signaling comes from studies on murine and avian limb buds where BMP-2 and BMP-7 were localized to the distal tip of the limb bud [159]. The growth factor gradient appears to be crucial for cell fate decisions. A gradient in genes related to BMP-2 expression has been observed across the cartilage growth plate, likely playing a role in zonal differentiation [160], the gradients for BMP-2 and 6 signaling being mainly present within the hypertrophic zones. This suggests that BMP signaling gradients exist across both growth plate and articular cartilage. These spatial gradients favor the differentiation of chondrocytes in the endochondral skeleton. Furthermore, BMP signaling is one of the key pathways regulating craniofacial development and the formation of mineralized structures. It is involved in the early patterning of the head, development of cranial neural crest cells, and facial patterning [161].
The presence of BMP gradients demonstrates that BMP can be confined to a specific volume, due to a spatially restricted pattern of expression. A recent study elaborated on a model to propose different mechanisms to explain how components work together to create and maintain BMP signaling gradient in the embryo [162]. This model relies on a restricted diffusion BMP antagonists acting as a sink that drives BMP signaling through a dorsal gradient. This data suggests that the spatio-temporal patterns of chordin and BMPs gene expression are dominant drivers of shape in 3D [162]. Another mathematical model [163] demonstrated the importance of BMPR localization in human embryonic stem cells in vitro and mouse embryos in vivo, to shape morphogen signaling during embryogenesis. It shows that the receptor localization facilitates a robust BMP signaling gradient in the mouse embryo. The BMP receptor signaling during embryogenesis depends on the restricted, basolateral localization of BMPR, since the BMP receptor localization at the apical side (mislocalization) results in ectopic BMP signaling in the mouse epiblast in vivo.
Several steps of embryonic development are also found during bone healing [164], indicating that there is a spatio-temporal pattern of BMP-2 expression through the bone fracture. Indeed, the spatially and temporally restricted patterns of BMP expression during bone regeneration appear to be necessary for determining the sites of coupled angiogenesis and osteogenesis that underlie bone regeneration.
Temporal regulation of BMP signaling
Fracture healing involves a synchronized discrete temporal association of cell types and many local and systematic regulatory factors. In the case of bone fracture, bone healing presents five temporal phases that are in chronological order: i) hematoma formation, ii) inflammation, iii) angiogenesis, iv) cartilage formation with subsequent calcification and cartilage removal, and finally v) bone formation and bone remodeling [165]. Furthermore, the expression of BMPs is temporally distinct during fracture healing [160], BMP-2 being expressed at all stages of bone repair.
Besides, the intensity, duration, and fluctuation of signaling pathway stimulation are important in determining the output from the downstream transduction network. Improving our understanding of how these differences in the input are converted into distinct outputs will be essential to unravel the mechanisms of cell fate decisions [166]
A recent review recapitulates the different strategies for targeted and control bone formation in vivo [167]. The ideal carrier should protect the BMPs from degradation, maintain its bioactivity whilst releasing the protein in a spatially and timely-controlled manner to promote the formation of new bone at the treatment site, avoiding secondary and undesirable effects. Traditionally, the immobilization of BMPs on material surfaces is achieved using various methods: via adsorption, entrapment or by covalent binding [168]. While spatial control has been developed by engineering gradients [115] and surface patterning [97], developing a temporal control is more challenging.
A few strategies imply temporal control over BMPs presentation on the substrate or degradation of the links to release BMPs in a time-controlled manner. This strategy also protects BMPs from rapid degradation. Controlled degradation can be achieved using degradable substrates or degradable chemical tethers. These approaches allow researchers to tightly control temporal release kinetics simply by increasing or decreasing the concentration of the cleavable element. The release of BMPs in vitro may be via active degradation, such as a macrophage-mediated release system coupled with the bone repair inflammatory stage [169]. Other examples of active delivery include the release of the BMPs through rupture hydrolysis [170] or proteolytic release [171] induced by the cells, or by external stimuli, such as heat in the case of thermosensitive hydrogels [172]. BMP-2 delivery via the matrix-metallo protease-degradable linker is used to induce osteogenic differentiation in C2C12 cells and mesenchymal stem cells [173]. As a perspective, the control of BMPs release over time is a promising approach to mimic the time-pattern of BMP expression during development and bone repair [174]. Biomaterials may be combined with genetic manipulation as already illustrated in the case of mesenchymal stem cells [175]. Indeed, genetically engineered BMP expressing cells can release the growth factors in situ at the site of injury over extended periods of time at a physiological concentration [176]. To deliver the BMP-plasmids, the coupling with the material-based strategies allows generating BMP signaling over a specific period [177]. This can be accomplished by assembling functional genetic modules to control intrinsic gene expression patterns and similarly, assemble functional ECM domains to create custom-made microenvironments for directing cell behavior. The use of degradable matrices containing the expression plasmids [178] and particularly the use of alginate has been successfully used for the transfer of BMP plasmid [179]. The BMP-genes may also be activated by a pharmacological approach, heat or light to offer a robust and time-tunable protein expression [180]. Optogenetic systems may enable a more precise bone regeneration, inducing the gene BMP-2 on mesenchymal stem cells in a specific location of the bone defect area [181]. It remains to demonstrate whether such regulatory issues raised by too complex systems are not counterproductive.
7. Bone regeneration in vivo using biomaterials inspired from ECM /BMP interactions
As we have seen above that BMP naturally exert their morphogenetic functions within the context of the ECM microenvironment, the interactions between BMPs and other ECM components may be used to optimize the delivery of growth factors for human clinics [182–186]. Overall, biomaterial engineering explores the concept that ECM proteins bind and present growth factors as solid-phase ligands. Cellular responses to nanometer scale signals provided by biomaterial may be exploited to ensure effective osteointegration and osteoinduction of orthopedic devices. Three major interrelated-parameters may be optimized thanks to ECM-inspired biomaterials: i) the dose of BMP-2 that is delivered, which should be as low as possible to avoid side effects (like inflammation and osteolysis) while being as efficient as possible [187, 188]; ii) the temporal control of BMP-2 release, which should as controlled as possible to avoid the so-called burst release since a very fast release at the beginning often leading to an overdose and side effect [189]; iii) the spatial control of BMP-2 to deliver BMP-2 at the right place and avoid its diffusion in fluids or other tissues [187]. Some strategies use surface coatings or nanoparticles to trap or encapsulate BMP-2 and deliver it in a spatially-controlled manner [190, 191].
In their recent review, Hachim et al. [192] described how GAGs-based biomaterials can also be used to reduce the dose of BMPs, given their ability to bind and slowly release BMPs, to their natural composition recognized by remodeling ECM-enzymes and to their anti-inflammatory effect [193]. Heparin has been proposed as carriers for BMPs in several types of biomaterials: microparticles [194–196], hydrogels [197] and scaffolds [198, 199]. The release of BMP-2 from heparin-based biomaterials is in the range between 13 days [200] and 28 days [201], depending on the type of biomaterial used: fibrin hydrogel or microparticles, respectively. Due to its anticoagulant effect other GAGs as CS and HS have been proposed as an alternative candidate for BMPs delivery [51, 61, 71, 202, 203]. Stupp’s group proposed to enhance bone regeneration by developing peptide amphiphile nanofibers able to bind HS and a low dose of BMP-2 [204]. These hybrid materials released only the ~ 34% of BMP-2 after 8 days and enhanced bone repair in a rat critical-size femoral defect (FIG. 6A). It has been shown that CS scaffolds can release BMP-2 for two weeks [205]. GAGs-mimetics has also been developed to promote osteogenic differentiation. Some examples are RGTA© (ReGeneraTing Agents) molecules [206], which consist of a saccharides backbone chemically modified with specific sulfate and carboxyl groups [207]. On the contrary of HS they are not recognized by glycanases and once complexed with collagen sponges they promote bone repair in vivo [206]. 6-O and 2-N-sulfated chitosan has also been proposed as an alternative to HS to promote osteogenic differentiation and angiogenesis in vitro and in vivo [208].
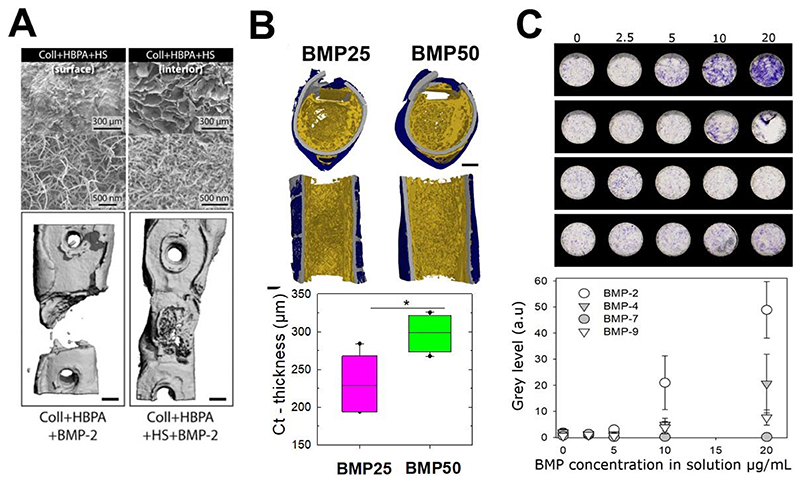
Fig. 6. Bone repair in vivo using BMP-2 delivering biomaterials and future high throughput solutions for in vitro cell studies.
(A) Top: scanning electron microscopy image the surface and the interior of a collagen scaffold infiltrated with amphiphile heparin peptide (HBPA) and heparan sulfate (HS) solution. Bottom: Femur reconstructions from micro-computed tomography showing the effect of HBPA scaffold presenting BMP-2 without and with HS. Adapted with permission from Lee et al [214], Copyright 2013, Elsevier. (B) Representative images of high-resolution 3D images of bone regeneration, showing transversal and sagittal sections. These images were taken using synchrotron radiation micro-computed tomography eight weeks after implantation of film-coated polymeric tubes at two BMP-2 doses of BMP-25 and BMP50 (scale bars, 1 mm). The film used to locally deliver the BMP-2 from the surface of the implant are of similar chemical composition (PLL/HA) than those used in Figure 5B to study BMP-2-mediated cell internalization, but the amont of BMP-2 loaded in the films is adapted to studies in vivo for bone regeneration (higher doses of BMP-2 are loaded in the film). The polymer is poly(lactic)glycolic acid (PLGA), a widely-used polymer in bone repair surgeries. Reproduced with permission from Bouyer et al., [184], Copyright 2016, Elsevier. (C) ALP staining of periosteum-derived stem cells cultured on biomimetic films that contain increased amounts of matrix-bound BMPs (BMP-2, 4, 7, 9) and corresponding quantification ALP expression using a scanner. Images are taken for cells cultured for two weeks on the BMP-containing films. Reproduced with permission from Machillot et al., [212] Copyright 2018, John Wiley and Sons.
Other GAGs or ECM proteins can also be exploited. Recently, Salmeron-Sanchez and coworkers [209] proposed a controlled material-based approach to enhance the activity of BMP-2 during tissue healing using fibrillary fibronectin driven by adsorption onto the polymer poly(ethyl acrylate) (PEA). This fibrillar fibronectin adsorbed onto PEA, but not a fibronectin globular conformation obtained on control polymers, promoted a synergistic presentation of integrin-binding sites and bound BMP-2, which enhanced mesenchymal stem cell osteogenesis in vitro and enabled full regeneration of a non-healing bone defect in vivo at low BMP-2 concentrations. Using this approach, it was possible to reduce the BMP-2 dose by adsorbing it at 50 μg/mL in comparison to 1.5 mg/mL for the concentration used with the collagen sponge using currently in clinics. In their latest study [210], the same team translated this technology and used plasma-polymerized PEA on 2D and 3D substrates to enhance osteogenic differentiation in vitro, proving that this combined fibrillar FN /BMP-2 presentation via the PEA is able to heal a critical-sized bone injury in mice as well as a non-healing humerus fracture in dogs.
A surface coating of implantable materials made of the (PLL/HA) films of controlled stiffness, for which an integrin/ BMPR receptor crosstalk was otherwise revealed [7], was used as osteoinductive coatings of orthopedic and maxillo-facial implants: the (PLL/HA coatings was deposited on ceramics [211], titanium alloy [185], or on the biodegradable polymer polylactic-co-glycolic acid (PLGA) [184] (FIG. 6B). The film-coated implants were tested in rats for their capacity to be osteoinductive and to trigger bone formation in muscle [185, 211]. When coated at the surface of a 3D polymeric hollow tube, they could regenerate a femoral bone defect in rats [184]. The amount of BMP-2 loaded in the film was controlled by tuning the initial concentration of BMP-2 in solution (used for BMP-2 loading in the film), while the BMP-2 released from the film was controlled via film crosslinking level: the low crosslinked film releasing the highest amount of BMP-2. By tuning these parameters, the amount of newly formed bone and the bone repair kinetics could be modulated: an effective and fast repair was obtained in 1 to 2 weeks in the best conditions, including complete defect bridging, the formation of vascularized and mineralized bone tissue. Histological staining and high-resolution computed tomography revealed the presence of bone regeneration inside and around the tube with a spatially distinct organization for trabecular-like and cortical bones. Notably, the amount of cortical bone and its thickness increased with the BMP-2 dose delivered via the polyelectrolyte film [184].
Altogether, these results show that ECM protein and GAG can be used to potentiate and optimized BMP-2 induced bone regeneration.
8. Conclusions and Perspectives
Clearly, the ECM appears to play a significant role in the presentation of BMP to cells to control BMP-mediated signaling. BMP signaling is ECM-dependent and the cellular context depends on the BMPR repertoire and the co-receptor involvement to potentiate or inhibit the BMP signaling. The role of BMP inhibitors, which are also tissue and fluid specific should not be overlooked. In addition, BMP signaling also depends on the BMP dose, spatial presentation time scale and pattern (duration, presence of gradients…). The contribution of biophysicists is and will be useful to design simplified experimental approaches to control the accurate parameters to decorticate the complex biological phenomena that occur in vivo during development and tissue regeneration. The future biomaterials for in vivo applications are also likely to be inspired by the natural ECM. The new paradigm of these future engineered biomaterials is to reduce the BMP dose and to spatially and temporally control the BMP delivery to repair bones in an optimized manner.
Last but not least, technological developments regarding the automated fabrication of biomaterials to enable high content screening of cell behaviors in vitro could boost the time needed to design and test the biomaterials in a parallelized manner. In this context, our team recently developed a process to deposit the biomimetic layer-by-layer films directly in cell culture microplates using a commercial liquid handling robot to study BMP-mediated cellular processes, including cell adhesion and differentiation in 96-well plates in [212]. Being optical transparent, the biomimetic coatings are compatible with microplate readers and common optical microscopes. A proof-of-concept was made to assess the differentiation of skeletal progenitors into bone cells at high content using the film-coated microplates with different BMP proteins (FIG. 6C). Using such BMPs loaded film-coated plates, it could also be envisioned to study the combined effects of film stiffness and bioactive molecules on stem cell differentiation, to unravel signaling pathways associated with cell fate and thus to propose new strategies to further optimize bone regeneration.
The future research for skeletal regeneration will need to be strongly interdisciplinary at the frontier of biology, synthetic biology, genetics, and material engineering to offer the tools to recapitulate the in vivo ECM patterns that are the most appropriate for bone repair in a sophisticated but not too complex way.
Acknowledgements
C. Picart is a senior member of the Institut Universitaire de France, whose financial support is acknowledged. A Guevara is supported by a fellowship from the mexican gouvernment CONACYT - (CVU: 532484)). This work was funded by the Fondation Recherche Médicale (DEQ20170336746 to CP and DEQ20170336702 to CAR), by the ANR (CO-DECIDE, grant ANR-17-CE13-022 and GlyCON, grant ANR-19-CE13-0031-01 PRCI), by the European Research Council (ERC Biomim, GA 259370, POC OSCODI 334966 and POC BioactiveCoatings, GA692924), and by Initiative de Recherche Stratégique, University Grenoble Alps (IDEX-IRS 2018-2021).
Bibliographic references
- [1].Hegarty SV, O’Keeffe GW, Sullivan AM. BMP-Smad 1/5/8 signalling in the development of the nervous system. Progress in neurobiology. 2013;109:28–41. doi: 10.1016/j.pneurobio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- [2].Hashiguchi M, Mullins MC. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development (Cambridge, England) 2013;140(9):1970–1980. doi: 10.1242/dev.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chau M, Lui JC, Landman EBM, Späth S-S, Vortkamp A, Baron J, Nilsson O. Gene Expression Profiling Reveals Similarities between the Spatial Architectures of Postnatal Articular and Growth Plate Cartilage. PloS one. 2014;9(7):e103061. doi: 10.1371/journal.pone.0103061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ning J, Zhao Y, Ye Y, Yu J. Opposing roles and potential antagonistic mechanism between TGF-β and BMP pathways: Implications for cancer progression. EBioMedicine. 2019;41:702–710. doi: 10.1016/j.ebiom.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Joppé SE, Hamilton LK, Cochard LM, Levros L-C, Aumont A, Barnabé-Heider F, Fernandes KJL. Bone morphogenetic protein dominantly suppresses epidermal growth factor-induced proliferative expansion of adult forebrain neural precursors. Front Neurosci. 2015;9:407. doi: 10.3389/fnins.2015.00407. 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fiedler J, Röderer G, Günther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. Journal of cellular biochemistry. 2002;87(3):305–12. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- [7].Fourel L, Valat A, Faurobert E, Guillot R, Bourrin-Reynard I, Ren K, Lafanechere L, Planus E, Picart C, Albiges-Rizo C. beta3 integrin-mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. The Journal of cell biology. 2016;212(6):693–706. doi: 10.1083/jcb.201508018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harbor Perspectives in Biology. 2016;8(5) doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reddi AH. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine & growth factor reviews. 2005;16(3):249–50. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [10].Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nature reviews Endocrinology. 2016;12(4):203–21. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- [11].Obradovic Wagner D, Sieber C, Bhushan R, Börgermann JH, Graf D, Knaus P. BMPs: From bone to body morphogenetic proteins. Science Signaling. 2010;3(107):mr1-mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- [12].Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS genetics. 2006;2(12):e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Toofan P, Wheadon H. Role of the bone morphogenic protein pathway in developmental haemopoiesis and leukaemogenesis. Biochemical Society transactions. 2016;44(5):1455–1463. doi: 10.1042/BST20160104. [DOI] [PubMed] [Google Scholar]
- [14].Bramlage CP, Müller GA, Tampe B, Bevanda J, Maatouk I, Koziolek M, Lange K, Ahrens K, Schmid H, Cohen CD, Bramlage P, et al. The role of bone morphogenetic protein-5 (BMP-5) in human nephrosclerosis. Journal of nephrology. 2011;24(5):647–55. doi: 10.5301/JN.2011.6330. [DOI] [PubMed] [Google Scholar]
- [15].Deng T, Lin D, Zhang M, Zhao Q, Li W, Zhong B, Deng Y, Fu X. Differential expression of bone morphogenetic protein 5 in human lung squamous cell carcinoma and adenocarcinoma. Acta biochimica et biophysica Sinica. 2015;47(7):557–63. doi: 10.1093/abbs/gmv037. [DOI] [PubMed] [Google Scholar]
- [16].Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nature genetics. 2009;41(4):482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grgurevic L, Christensen GL, Schulz TJ, Vukicevic S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine & growth factor reviews. 2016;27:105–18. doi: 10.1016/j.cytogfr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- [18].Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H, Roose-Girma M, Zhang G, Shou W, Yan M. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(29):11887–92. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–61. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- [20].Salmon RM, Guo J, Wood JH, Tong Z, Beech JS, Lawera A, Yu M, Grainger DJ, Reckless J, Morrell NW, Li W. Molecular basis of ALK1-mediated signalling by BMP9/BMP10 and their prodomain-bound forms. Nature communications. 2020;11(1):1621. doi: 10.1038/s41467-020-15425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Molecular and cellular biology. 1994;14(9):5961–74. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth factors (Chur, Switzerland) 2004;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- [23].Liu F, Hata A, Baker JC, Doody J, Carcamo J, Harland RM, Massague J. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996;381(6583):620–3. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- [24].Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine & growth factor reviews. 2009;20(5-6):343–55. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [25].Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY) 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [27].Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Science translational medicine. 2011;3(100):100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- [28].Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4563–8. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sarrazin S, Lamanna WC, Esko JD. Heparan Sulfate Proteoglycans. Cold Spring Harbor Perspectives in Biology. 2011;3(7) doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. The Journal of biological chemistry. 1999;274(35):25085–92. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- [31].Sedlmeier G, Sleeman JP. Extracellular regulation of BMP signaling: welcome to the matrix. Biochemical Society transactions. 2017;45(1):173–181. doi: 10.1042/BST20160263. [DOI] [PubMed] [Google Scholar]
- [32].Nickel J, Ten Dijke P, Mueller TD. TGF-beta family co-receptor function and signaling. Acta biochimica et biophysica Sinica. 2018;50(1):12–36. doi: 10.1093/abbs/gmx126. [DOI] [PubMed] [Google Scholar]
- [33].Billings PC, Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect Tissue Res. 2015;56(4):272–80. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Billings PC, Yang E, Mundy C, Pacifici M. Domains with highest heparan sulfate-binding affinity reside at opposite ends in BMP2/4 versus BMP5/6/7: Implications for function. The Journal of biological chemistry. 2018;293(37):14371–14383. doi: 10.1074/jbc.RA118.003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. European journal of biochemistry / FEBS. 1996;237(1):295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- [36].Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Current biology : CB. 2002;12(3):205–9. doi: 10.1016/s0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- [37].Kisiel M, Klar AS, Ventura M, Buijs J, Mafina MK, Cool SM, Hilborn J. Complexation and sequestration of BMP-2 from an ECM mimetic hyaluronan gel for improved bone formation. PloS one. 2013;8(10):e78551. doi: 10.1371/journal.pone.0078551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis (Dallas, Tex) 1989;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- [39].Lawera A, Tong Z, Thorikay M, Redgrave RE, Cai J, van Dinther M, Morrell NW, Afink GB, Charnock-Jones DS, Arthur HM, Ten Dijke P, et al. Role of soluble endoglin in BMP9 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(36):17800–17808. doi: 10.1073/pnas.1816661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Groppe J, Rumpel K, Economides AN, Stahl N, Sebald W, Affolter M. Biochemical and biophysical characterization of refolded Drosophila DPP, a homolog of bone morphogenetic proteins 2 and 4. The Journal of biological chemistry. 1998;273(44):29052–65. doi: 10.1074/jbc.273.44.29052. [DOI] [PubMed] [Google Scholar]
- [41].Sasaki N, Hirano T, Ichimiya T, Wakao M, Hirano K, Kinoshita-Toyoda A, Toyoda H, Suda Y, Nishihara S. The 3’-phosphoadenosine 5’-phosphosulfate transporters, PAPST1 and 2, contribute to the maintenance and differentiation of mouse embryonic stem cells. PloS one. 2009;4(12):e8262. doi: 10.1371/journal.pone.0008262. e8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hintze V, Samsonov SA, Anselmi M, Moeller S, Becher J, Schnabelrauch M, Scharnweber D, Pisabarro MT. Sulfated glycosaminoglycans exploit the conformational plasticity of bone morphogenetic protein-2 (BMP-2) and alter the interaction profile with its receptor. Biomacromolecules. 2014;15(8):3083–92. doi: 10.1021/bm5006855. [DOI] [PubMed] [Google Scholar]
- [43].Zhang X, Wang F, Sheng J. “Coding” and “Decoding”: hypothesis for the regulatory mechanism involved in heparan sulfate biosynthesis. Carbohydrate research. 2016;428:1–7. doi: 10.1016/j.carres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- [44].El Masri R, Seffouh A, Lortat-Jacob H, Vives RR. The “in and out” of glucosamine 6-O-sulfation: the 6th sense of heparan sulfate. Glycoconjugate journal. 2017;34(3):285–298. doi: 10.1007/s10719-016-9736-5. [DOI] [PubMed] [Google Scholar]
- [45].Connell BJ, Lortat-Jacob H. Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition. Frontiers in immunology. 2013;4:385. doi: 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grobe K, Ledin J, Ringvall M, Holmborn K, Forsberg E, Esko JD, Kjellen L. Heparan sulfate and development: differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochimica et biophysica acta. 2002;1573(3):209–15. doi: 10.1016/s0304-4165(02)00386-0. [DOI] [PubMed] [Google Scholar]
- [47].Ashikari-Hada S, Habuchi H, Kariya Y, Itoh N, Reddi AH, Kimata K. Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. The Journal of biological chemistry. 2004;279(13):12346–54. doi: 10.1074/jbc.M313523200. [DOI] [PubMed] [Google Scholar]
- [48].Smith RAA, Murali S, Rai B, Lu X, Lim ZXH, Lee JJL, Nurcombe V, Cool SM. Minimum structural requirements for BMP-2-binding of heparin oligosaccharides. Biomaterials. 2018;184:41–55. doi: 10.1016/j.biomaterials.2018.08.056. [DOI] [PubMed] [Google Scholar]
- [49].Zhang F, McLellan JS, Ayala AM, Leahy DJ, Linhardt RJ. Kinetic and structural studies on interactions between heparin or heparan sulfate and proteins of the hedgehog signaling pathway. Biochemistry. 2007;46(13):3933–41. doi: 10.1021/bi6025424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tsai CT, Zulueta MML, Hung SC. Synthetic heparin and heparan sulfate: probes in defining biological functions. Current opinion in chemical biology. 2017;40:152–159. doi: 10.1016/j.cbpa.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [51].Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, van Wijnen AJ, Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50(4):954–64. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. Journal of biomedical materials research. 2002;59(3):573–84. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- [53].Yan HJ, Casalini T, Hulsart-Billstrom G, Wang S, Oommen OP, Salvalaglio M, Larsson S, Hilborn J, Varghese OP. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials. 2018;161:190–202. doi: 10.1016/j.biomaterials.2018.01.041. [DOI] [PubMed] [Google Scholar]
- [54].Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development (Cambridge, England) 2004;131(9):1927–38. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- [55].Nishihara S. In: Methods in Enzymology. Fukuda M, editor. Academic Press; 2010. Chapter Fifteen - Glycosyltransferases and Transporters that Contribute to Proteoglycan Synthesis in Drosophila: Identification and Functional Analyses Using the Heritable and Inducible RNAi System; pp. 323–351. [DOI] [PubMed] [Google Scholar]
- [56].Pacifici M. Hereditary Multiple Exostoses: New Insights into Pathogenesis, Clinical Complications, and Potential Treatments. Current osteoporosis reports. 2017;15(3):142–152. doi: 10.1007/s11914-017-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pacifici M. The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses. Matrix biology : journal of the International Society for Matrix Biology. 2017 doi: 10.1016/j.matbio.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Huegel J, Enomoto-Iwamoto M, Sgariglia F, Koyama E, Pacifici M. Heparanase stimulates chondrogenesis and is up-regulated in human ectopic cartilage: a mechanism possibly involved in hereditary multiple exostoses. The American journal of pathology. 2015;185(6):1676–85. doi: 10.1016/j.ajpath.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wurzler KK, Emmert J, Eichelsbacher F, Kubler NR, Sebald W, Reuther JF. [Evaluation of the osteoinductive potential of genetically modified BMP-2 variants] Mund-, Kiefer- und Gesichtschirurgie : MKG. 2004;8(2):83–92. doi: 10.1007/s10006-004-0528-x. [DOI] [PubMed] [Google Scholar]
- [60].Brkljacic J, Pauk M, Erjavec I, Cipcic A, Grgurevic L, Zadro R, Inman GJ, Vukicevic S. Exogenous heparin binds and inhibits bone morphogenetic protein 6 biological activity. International orthopaedics. 2013;37(3):529–541. doi: 10.1007/s00264-012-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Murali S, Rai B, Dombrowski C, Lee JL, Lim ZX, Bramono DS, Ling L, Bell T, Hinkley S, Nathan SS, Hui JH, et al. Affinity-selected heparan sulfate for bone repair. Biomaterials. 2013;34(22):5594–605. doi: 10.1016/j.biomaterials.2013.04.017. [DOI] [PubMed] [Google Scholar]
- [62].Smith RAA, Chua RJE, Carnachan SM, Tan CLL, Sims IM, Hinkley SFR, Nurcombe V, Cool SM. Retention of the structure and function of heparan sulfate biomaterials after gamma irradiation. Tissue engineering Part A. 2017 doi: 10.1089/ten.TEA.2017.0263. [DOI] [PubMed] [Google Scholar]
- [63].Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix biology : journal of the International Society for Matrix Biology. 2006;25(1):27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [64].Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development (Cambridge, England) 2003;130(8):1515–22. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- [65].Migliorini E, Horn P, Haraszti T, Wegner S, Hiepen C, Knaus P, Richter P, Cavalcanti-Adam E. Enhanced biological activity of BMP-2 bound to surface-grafted heparan sulfate. Advanced Biosystems. 2017;1(4):1600041. doi: 10.1002/adbi.201600041. [DOI] [PubMed] [Google Scholar]
- [66].Migliorini E, Thakar D, Sadir R, Pleiner T, Baleux F, Lortat-Jacob H, Coche-Guerente L, Richter RP. Well-defined biomimetic surfaces to characterize glycosaminoglycan-mediated interactions on the molecular, supramolecular and cellular levels. Biomaterials. 2014;35(32):8903–15. doi: 10.1016/j.biomaterials.2014.07.017. [DOI] [PubMed] [Google Scholar]
- [67].Thakar D, Dalonneau F, Migliorini E, Lortat-Jacob H, Boturyn D, Albiges-Rizo C, Coche-Guerente L, Picart C, Richter RP. Binding of the chemokine CXCL12alpha to its natural extracellular matrix ligand heparan sulfate enables myoblast adhesion and facilitates cell motility. Biomaterials. 2017;123:24–38. doi: 10.1016/j.biomaterials.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Thakar D, Migliorini E, Coche-Guerente L, Sadir R, Lortat-Jacob H, Boturyn D, Renaudet O, Labbe P, Richter RP. A quartz crystal microbalance method to study the terminal functionalization of glycosaminoglycans. Chemical communications (Cambridge, England) 2014;50(96):15148–51. doi: 10.1039/c4cc06905f. [DOI] [PubMed] [Google Scholar]
- [69].Bachvarova V, Dierker T, Esko JD, Hoffmann D, Kiellen L, Vortkamp A. Chondrocytes respond to an altered heparan sulfate composition with distinct changes of heparan sulfate structure and increased levels of chondroitin sulfate. Matrix biology : journal of the International Society for Matrix Biology. 2020 doi: 10.1016/j.matbio.2020.03.006. [DOI] [PubMed] [Google Scholar]
- [70].Büttner M, Möller S, Keller M, Huster D, Schiller J, Schnabelrauch M, Dieter P, Hempel U. Over-sulfated chondroitin sulfate Derivatives induce osteogenic differentiation of hMSC independent of BMP-2 and TGF-β1 signalling. Journal of cellular physiology. 2013;228(2):330–340. doi: 10.1002/jcp.24135. [DOI] [PubMed] [Google Scholar]
- [71].Miyazaki T, Miyauchi S, Tawada A, Anada T, Matsuzaka S, Suzuki O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. Journal of cellular physiology. 2008;217(3):769–77. doi: 10.1002/jcp.21557. [DOI] [PubMed] [Google Scholar]
- [72].Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) Journal of cell communication and signaling. 2009;3(3-4):323–35. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Anouz R, Repanas A, Schwarz E, Groth T. Novel Surface Coatings Using Oxidized Glycosaminoglycans as Delivery Systems of Bone Morphogenetic Protein 2 (BMP-2) for Bone Regeneration. Macromolecular bioscience. 2018;18(11):1800283. doi: 10.1002/mabi.201800283. [DOI] [PubMed] [Google Scholar]
- [74].Coulson-Thomas YM, Coulson-Thomas VJ, Norton AL, Gesteira TF, Cavalheiro RP, Meneghetti MC, Martins JR, Dixon RA, Nader HB. The identification of proteoglycans and glycosaminoglycans in archaeological human bones and teeth. PloS one. 2015;10(6):e0131105. doi: 10.1371/journal.pone.0131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, Thomas RO, Hollingsworth RE, Knudson CB. CD44 modulates Smad1 activation in the BMP-7 signaling pathway. The Journal of cell biology. 2004;166(7):1081–91. doi: 10.1083/jcb.200402138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kawano M, Ariyoshi W, Iwanaga K, Okinaga T, Habu M, Yoshioka I, Tominaga K, Nishihara T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochemical and biophysical research communications. 2011;405(4):575–80. doi: 10.1016/j.bbrc.2011.01.071. [DOI] [PubMed] [Google Scholar]
- [77].Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Layer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: controlled differentiation of myoblasts to osteoblasts. Small (Weinheim an der Bergstrasse, Germany) 2009;5(5):598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- [78].Crouzier T, Fourel L, Boudou T, Albiges-Rizo C, Picart C. Presentation of BMP-2 from a soft biopolymeric film unveils its activity on cell adhesion and migration. Advanced materials (Deerfield Beach, Fla) 2011;23(12):H111-8. doi: 10.1002/adma.201004637. [DOI] [PubMed] [Google Scholar]
- [79].Crouzier T, Szarpak A, Boudou T, Auzély-Velty R, Picart C. Polysaccharide-Blend Multilayers Containing Hyaluronan and Heparin as a Delivery System for rhBMP-2. Small (Weinheim an der Bergstrasse, Germany) 2010;6(5):651–662. doi: 10.1002/smll.200901728. [DOI] [PubMed] [Google Scholar]
- [80].Paralkar VM, Weeks BS, Yu YM, Kleinman HK, Reddi AH. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. Journal of Cell Biology. 1992;119(6):1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Current opinion in cell biology. 2009;21(5):616–22. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development (Cambridge, England) 2009;136(22):3715–28. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factorbinding domain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(12):4711–21. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- [84].Migliorini E, Valat A, Picart C, Cavalcanti-Adam EA. Tuning cellular responses to BMP-2 with material surfaces. Cytokine & growth factor reviews. 2016;27:43–54. doi: 10.1016/j.cytogfr.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. The Journal of cell biology. 1999;144(5):1069–80. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–7. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- [87].Garcia Abreu J, Coffinier C, Larrain J, Oelgeschlager M, De Robertis EM. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene. 2002;287(1-2):39–47. doi: 10.1016/s0378-1119(01)00827-7. [DOI] [PubMed] [Google Scholar]
- [88].Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. The Journal of biological chemistry. 2008;283(20):13874–88. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. The Journal of biological chemistry. 2011;286(7):5087–99. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jensen SA, Robertson IB, Handford PA. Dissecting the fibrillin microfibril: structural insights into organization and function. Structure (London, England : 1993) 2012;20(2):215–25. doi: 10.1016/j.str.2011.12.008. [DOI] [PubMed] [Google Scholar]
- [91].Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, Sakai LY, et al. Fibrillin-1 and -2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. The Journal of cell biology. 2010;190(6):1107–21. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. Regulation of limb patterning by extracellular microfibrils. The Journal of cell biology. 2001;154(2):275–81. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sengle G, Ono RN, Lyons KM, Bachinger HP, Sakai LY. A new model for growth factor activation: type II receptors compete with the prodomain for BMP-7. Journal of molecular biology. 2008;381(4):1025–39. doi: 10.1016/j.jmb.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hiepen C, Jatzlau J, Hildebrandt S, Kampfrath B, Goktas M, Murgai A, Cuellar Camacho JL, Haag R, Ruppert C, Sengle G, Cavalcanti-Adam EA, et al. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics. PLoS biology. 2019;17(12):e3000557. doi: 10.1371/journal.pbio.3000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5062–7. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kopf J, Paarmann P, Hiepen C, Horbelt D, Knaus P. BMP growth factor signaling in a biomechanical context. BioFactors (Oxford, England) 2014;40(2):171–87. doi: 10.1002/biof.1137. [DOI] [PubMed] [Google Scholar]
- [97].Fitzpatrick V, Fourel L, Destaing O, Gilde F, Albiges-Rizo C, Picart C, Boudou T. Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Scientific reports. 2017;7:41479. doi: 10.1038/srep41479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vadon-Le Goff S, Hulmes DJ, Moali C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix biology : journal of the International Society for Matrix Biology. 2015;44-46:14–23. doi: 10.1016/j.matbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- [99].Muir A, Greenspan DS. Metalloproteinases in Drosophila to humans that are central players in developmental processes. The Journal of biological chemistry. 2011;286(49):41905–11. doi: 10.1074/jbc.R111.299768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current opinion in cell biology. 2008;20(5):495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Moali C, Font B, Ruggiero F, Eichenberger D, Rousselle P, Francois V, Oldberg A, Bruckner-Tuderman L, Hulmes DJ. Substrate-specific modulation of a multisubstrate proteinase. C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. The Journal of biological chemistry. 2005;280(25):24188–94. doi: 10.1074/jbc.M501486200. [DOI] [PubMed] [Google Scholar]
- [102].Prockop DJ, Hulmes DJ. Assembly of Collagen Fibrils, Extracellular matrix assembly and structure. 1994;9:47. [Google Scholar]
- [103].Greenspan DS. Biosynthetic processing of collagen molecules, Collagen. Springer; 2005. pp. 149–183. [Google Scholar]
- [104].Heumuller SE, Talantikite M, Napoli M, Armengaud J, Morgelin M, Hartmann U, Sengle G, Paulsson M, Moali C, Wagener R. C-terminal proteolysis of the collagen VI alpha3 chain by BMP-1 and proprotein convertase(s) releases endotrophin in fragments of different sizes. The Journal of biological chemistry. 2019;294(37):13769–13780. doi: 10.1074/jbc.RA119.008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Huang G, Zhang Y, Kim B, Ge G, Annis DS, Mosher DF, Greenspan DS. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. The Journal of biological chemistry. 2009;284(38):25879–88. doi: 10.1074/jbc.M109.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Weiss T, Brusel M, Rousselle P, Kessler E. The NTR domain of procollagen C-proteinase enhancer-1 (PCPE-1) mediates PCPE-1 binding to syndecans-1, -2 and -4 as well as fibronectin. The international journal of biochemistry & cell biology. 2014;57:45–53. doi: 10.1016/j.biocel.2014.09.023. [DOI] [PubMed] [Google Scholar]
- [107].Saunders JT, Schwarzbauer JE. Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing. Molecular biology of the cell. 2019;30(17):2218–2226. doi: 10.1091/mbc.E19-03-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Monteiro AI, Kollmetz T, Malmstrom J. Engineered systems to study the synergistic signaling between integrin-mediated mechanotransduction and growth factors (Review) Biointerphases. 2018;13(6):06d302. doi: 10.1116/1.5045231. [DOI] [PubMed] [Google Scholar]
- [109].Cipitria A, Salmeron-Sanchez M. Mechanotransduction and Growth Factor Signalling to Engineer Cellular Microenvironments. Advanced healthcare materials. 2017;6(15) doi: 10.1002/adhm.201700052. [DOI] [PubMed] [Google Scholar]
- [110].Dalby MJ, García AJ, Salmeron-Sanchez M. Receptor control in mesenchymal stem cell engineering. Nature Reviews Materials. 2018;3(3):17091 [Google Scholar]
- [111].da Silva Madaleno C, Jatzlau J, Knaus P. BMP signalling in a mechanical context - Implications for bone biology. Bone. 2020;137:115416. doi: 10.1016/j.bone.2020.115416. [DOI] [PubMed] [Google Scholar]
- [112].Boerckel JD, Kolambkar YM, Stevens HY, Lin AS, Dupont KM, Guldberg RE. Effects of in vivo mechanical loading on large bone defect regeneration. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(7):1067–75. doi: 10.1002/jor.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Discher DE, Smith L, Cho S, Colasurdo M, Garcia AJ, Safran S. Matrix Mechanosensing: From Scaling Concepts in ‘Omics Data to Mechanisms in the Nucleus, Regeneration, and Cancer. Annual review of biophysics. 2017;46:295–315. doi: 10.1146/annurev-biophys-062215-011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science (New York, NY) 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Almodovar J, Guillot R, Monge C, Vollaire J, Selimovic S, Coll JL, Khademhosseini A, Picart C. Spatial patterning of BMP-2 and BMP-7 on biopolymeric films and the guidance of muscle cell fate. Biomaterials. 2014;35(13):3975–85. doi: 10.1016/j.biomaterials.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wang YK, Yu X, Cohen DM, Wozniak MA, Yang MT, Gao L, Eyckmans J, Chen CS. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem cells and development. 2012;21(7):1176–86. doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- [118].Sawala A, Scarcia M, Sutcliffe C, Wilcockson SG, Ashe HL. Peak BMP responses in the drosophila embryo are dependent on the activation of integrin signaling. Cell reports. 2015;12(10):1584–93. doi: 10.1016/j.celrep.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lian C, Wang X, Qiu X, Wu Z, Gao B, Liu L, Liang G, Zhou H, Yang X, Peng Y, Liang A, et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1–SMAD1 interaction. Bone Research. 2019;7(1):8. doi: 10.1038/s41413-019-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Seeherman HJ, Berasi SP, Brown CT, Martinez RX, Juo ZS, Jelinsky S, Cain MJ, Grode J, Tumelty KE, Bohner M, Grinberg O, et al. A BMP/activin A chimera is superior to native BMPs and induces bone repair in nonhuman primates when delivered in a composite matrix. Science translational medicine. 2019;11(489) doi: 10.1126/scitranslmed.aar4953. [DOI] [PubMed] [Google Scholar]
- [121].Haupt J, Stanley A, McLeod CM, Cosgrove BD, Culbert AL, Wang L, Mourkioti F, Mauck RL, Shore EM. ACVR1(R206H) FOP mutation alters mechanosensing and tissue stiffness during heterotopic ossification. Molecular biology of the cell. 2019;30(1):17–29. doi: 10.1091/mbc.E18-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Heo SJ, Han WM, Szczesny SE, Cosgrove BD, Elliott DM, Lee DA, Duncan RL, Mauck RL. Mechanically Induced Chromatin Condensation Requires Cellular Contractility in Mesenchymal Stem Cells. Biophysical journal. 2016;111(4):864–874. doi: 10.1016/j.bpj.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Stanley A, Heo SJ, Mauck RL, Mourkioti F, Shore EM. Elevated BMP and Mechanical Signaling Through YAP1/RhoA Poises FOP Mesenchymal Progenitors for Osteogenesis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2019;34(10):1894–1909. doi: 10.1002/jbmr.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- [125].Dasgupta I, McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. The Journal of biological chemistry. 2019;294(46):17693–17706. doi: 10.1074/jbc.REV119.007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yang B, Sun H, Chen P, Fan N, Zhong H, Liu X, Wu Y, Wang J. YAP1 influences differentiation of osteoblastic MC3T3-E1 cells through the regulation of ID1. Journal of cellular physiology. 2019;234(8):14007–14018. doi: 10.1002/jcp.28088. [DOI] [PubMed] [Google Scholar]
- [127].Deng Y, Wu A, Li P, Li G, Qin L, Song H, Mak KK. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell reports. 2016;14(9):2224–2237. doi: 10.1016/j.celrep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- [128].Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nature communications. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature materials. 2016;15(3):326–34. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Matellan C, Del Río Hernández AE. Engineering the cellular mechanical microenvironment - from bulk mechanics to the nanoscale. Journal of cell science. 2019;132(9) doi: 10.1242/jcs.229013. [DOI] [PubMed] [Google Scholar]
- [131].Nagaraja MP, Risin D. The current state of bone loss research: data from spaceflight and microgravity simulators. Journal of cellular biochemistry. 2013;114(5):1001–8. doi: 10.1002/jcb.24454. [DOI] [PubMed] [Google Scholar]
- [132].Dai Z, Wu F, Chen J, Xu H, Wang H, Guo F, Tan Y, Ding B, Wang J, Wan Y, Li Y. Actin microfilament mediates osteoblast Cbfa1 responsiveness to BMP2 under simulated microgravity. PloS one. 2013;8(5):e63661. doi: 10.1371/journal.pone.0063661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Dai Z, Guo F, Wu F, Xu H, Yang C, Li J, Liang P, Zhang H, Qu L, Tan Y, Wan Y, et al. Integrin alphavbeta3 mediates the synergetic regulation of core-binding factor alpha1 transcriptional activity by gravity and insulin-like growth factor-1 through phosphoinositide 3-kinase signaling. Bone. 2014;69:126–32. doi: 10.1016/j.bone.2014.09.018. [DOI] [PubMed] [Google Scholar]
- [134].Xu H, Wu F, Zhang H, Yang C, Li K, Wang H, Yang H, Liu Y, Ding B, Tan Y, Yuan M, et al. Actin cytoskeleton mediates BMP2-Smad signaling via calponin 1 in preosteoblast under simulated microgravity. Biochimie. 2017;138:184–193. doi: 10.1016/j.biochi.2017.04.015. [DOI] [PubMed] [Google Scholar]
- [135].Shi W, Ma Z, Zhang G, Wang C, Jiao Z. Novel functions of the primary cilium in bone disease and cancer. Cytoskeleton (Hoboken, NJ) 2019;76(3):233–242. doi: 10.1002/cm.21529. [DOI] [PubMed] [Google Scholar]
- [136].Xie YF, Shi WG, Zhou J, Gao YH, Li SF, Fang QQ, Wang MG, Ma HP, Wang JF, Xian CJ, Chen KM. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone. 2016;93:22–32. doi: 10.1016/j.bone.2016.09.008. [DOI] [PubMed] [Google Scholar]
- [137].Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Molecular and cellular biology. 2006;26(20):7791–805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ehrlich M. Endocytosis and trafficking of BMP receptors: Regulatory mechanisms for fine-tuning the signaling response in different cellular contexts. Cytokine & growth factor reviews. 2016;27:35–42. doi: 10.1016/j.cytogfr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- [139].Alborzinia H, Schmidt-Glenewinkel H, Ilkavets I, Breitkopf-Heinlein K, Cheng X, Hortschansky P, Dooley S, Wolfl S. Quantitative kinetics analysis of BMP2 uptake into cells and its modulation by BMP antagonists. Journal of cell science. 2013;126(Pt 1):117–27. doi: 10.1242/jcs.109777. [DOI] [PubMed] [Google Scholar]
- [140].Amsalem AR, Marom B, Shapira KE, Hirschhorn T, Preisler L, Paarmann P, Knaus P, Henis YI, Ehrlich M. Differential regulation of translation and endocytosis of alternatively spliced forms of the type II bone morphogenetic protein (BMP) receptor. Molecular biology of the cell. 2016;27(4):716–30. doi: 10.1091/mbc.E15-08-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gilde F, Fourel L, Guillot R, Pignot-Paintrand I, Okada T, Fitzpatrick V, Boudou T, Albiges-Rizo C, Picart C. Stiffnessdependent cellular internalization of matrix-bound BMP-2 and its relation to Smad and non-Smad signaling. Acta biomaterialia. 2016;46:55–67. doi: 10.1016/j.actbio.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].O’Connor-Giles KM, Ho LL, Ganetzky B. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron. 2008;58(4):507–18. doi: 10.1016/j.neuron.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rodal AA, Blunk AD, Akbergenova Y, Jorquera RA, Buhl LK, Littleton JT. A presynaptic endosomal trafficking pathway controls synaptic growth signaling. The Journal of cell biology. 2011;193(1):201–17. doi: 10.1083/jcb.201009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gui J, Huang Y, Shimmi O. Scribbled Optimizes BMP Signaling through Its Receptor Internalization to the Rab5 Endosome and Promote Robust Epithelial Morphogenesis. PLoS genetics. 2016;12(11):e1006424. doi: 10.1371/journal.pgen.1006424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9466–71. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kim N, Kim S, Nahm M, Kopke D, Kim J, Cho E, Lee MJ, Lee M, Kim SH, Broadie K, Lee S. BMP-dependent synaptic development requires Abi-Abl-Rac signaling of BMP receptor macropinocytosis. Nature communications. 2019;10(1):684. doi: 10.1038/s41467-019-08533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. The Journal of cell biology. 2009;184(4):597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Posa F, Grab AL, Martin V, Hose D, Seckinger A, Mori G, Vukicevic S, Cavalcanti-Adam EA. Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling. Cells. 2019;8(12) doi: 10.3390/cells8121646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Schwab EH, Pohl TL, Haraszti T, Schwaerzer GK, Hiepen C, Spatz JP, Knaus P, Cavalcanti-Adam EA. Nanoscale control of surface immobilized BMP-2: toward a quantitative assessment of BMP-mediated signaling events. Nano letters. 2015;15(3):1526–34. doi: 10.1021/acs.nanolett.5b00315. [DOI] [PubMed] [Google Scholar]
- [150].Pohl TL, Boergermann JH, Schwaerzer GK, Knaus P, Cavalcanti-Adam EA. Surface immobilization of bone morphogenetic protein 2 via a self-assembled monolayer formation induces cell differentiation. Acta biomaterialia. 2012;8(2):772–80. doi: 10.1016/j.actbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- [151].Tabisz B, Schmitz W, Schmitz M, Luehmann T, Heusler E, Rybak J-C, Meinel L, Fiebig JE, Mueller TD, Nickel J. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. Biomacromolecules. 2017;18(3):695–708. doi: 10.1021/acs.biomac.6b01407. [DOI] [PubMed] [Google Scholar]
- [152].Siverino C, Tabisz B, Luhmann T, Meinel L, Muller T, Walles H, Nickel J. Site-Directed Immobilization of Bone Morphogenetic Protein 2 to Solid Surfaces by Click Chemistry. Journal of visualized experiments : JoVE. 2018;(133) doi: 10.3791/56616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hiepen C, Jatzlau J, Hildebrandt S, Kampfrath B, Goktas M, Murgai A, Cuellar Camacho JL, Haag R, Ruppert C, Sengle G, Cavalcanti-Adam EA, et al. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFbeta responses and altered cell mechanics. PLoS biology. 2019;17(12):e3000557. doi: 10.1371/journal.pbio.3000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials. 2013;34(3):704–12. doi: 10.1016/j.biomaterials.2012.10.015. [DOI] [PubMed] [Google Scholar]
- [155].Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999;14(7):1075–83. doi: 10.1359/jbmr.1999.14.7.1075. [DOI] [PubMed] [Google Scholar]
- [156].Xu ER, Blythe EE, Fischer G, Hyvonen M. Structural analyses of von Willebrand factor C domains of collagen 2A and CCN3 reveal an alternative mode of binding to bone morphogenetic protein-2. The Journal of biological chemistry. 2017;292(30):12516–12527. doi: 10.1074/jbc.M117.788992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sanyal A, Oursler MJ, Clemens VR, Fukumoto T, Fitzsimmons JS, O’Driscoll SW. Temporal expression patterns of BMP receptors and collagen II (B) during periosteal chondrogenesis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(1):58–65. doi: 10.1016/S0736-0266(01)00078-X. [DOI] [PubMed] [Google Scholar]
- [158].Zinski J, Bu Y, Wang X, Dou W, Umulis D, Mullins MC. Systems biology derived source-sink mechanism of BMP gradient formation. eLife. 2017;6 doi: 10.7554/eLife.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Badugu A, Kraemer C, Germann P, Menshykau D, Iber D. Digit patterning during limb development as a result of the BMP-receptor interaction. Scientific reports. 2012;2(1):991. doi: 10.1038/srep00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cho T-J, Gerstenfeld LC, Einhorn TA. Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily During Murine Fracture Healing. Journal of Bone and Mineral Research. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- [161].Graf D, Malik Z, Hayano S, Mishina Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine & growth factor reviews. 2016;27:129–39. doi: 10.1016/j.cytogfr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Li L, Wang X, Mullins MC, Umulis DM. Evaluation of BMP-mediated patterning in a 3D mathematical model of the zebrafish blastula embryo. Journal of mathematical biology. 2020;80(1-2):505–520. doi: 10.1007/s00285-019-01449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhang Z, Zwick S, Loew E, Grimley JS, Ramanathan S. Mouse embryo geometry drives formation of robust signaling gradients through receptor localization. Nature communications. 2019;10(1):4516. doi: 10.1038/s41467-019-12533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nature genetics. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- [165].Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- [166].Housden BE, Perrimon N. Spatial and temporal organization of signaling pathways. Trends in biochemical sciences. 2014;39(10):457–64. doi: 10.1016/j.tibs.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].White KA, Olabisi RM. Spatiotemporal Control Strategies for Bone Formation through Tissue Engineering and Regenerative Medicine Approaches. Advanced healthcare materials. 2019;8(2):1801044. doi: 10.1002/adhm.201801044. [DOI] [PubMed] [Google Scholar]
- [168].Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2004;58(2):197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [169].Annamalai RT, Turner PA, Carson WFt, Levi B, Kunkel S, Stegemann JP. Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials. 2018;161:216–227. doi: 10.1016/j.biomaterials.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cabanas-Danes J, Landman E, Huskens J, Karperien M, Jonkheijm P. Hydrolytically Labile Linkers Regulate Release and Activity of Human Bone Morphogenetic Protein-6. Langmuir : the ACS journal of surfaces and colloids. 2018;34(31):9298–9306. doi: 10.1021/acs.langmuir.8b00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Metzger S, Blache U, Lienemann PS, Karlsson M, Weber FE, Weber W, Ehrbar M. Cell-Mediated Proteolytic Release of Growth Factors from Poly(Ethylene Glycol) Matrices. Macromolecular bioscience. 2016;16(11):1703–1713. doi: 10.1002/mabi.201600223. [DOI] [PubMed] [Google Scholar]
- [172].Liu X, Yu B, Huang Q, Liu R, Feng Q, Cai Q, Mi S. In vitro BMP-2 peptide release from thiolated chitosan based hydrogel. International Journal of Biological Macromolecules. 2016;93:314–321. doi: 10.1016/j.ijbiomac.2016.08.048. [DOI] [PubMed] [Google Scholar]
- [173].Metzger S, Blache U, Lienemann PS, Karlsson M, Weber FE, Weber W, Ehrbar M. Cell-Mediated Proteolytic Release of Growth Factors from Poly(Ethylene Glycol) Matrices. Macromolecular bioscience. 2016;16(11):1703–1713. doi: 10.1002/mabi.201600223. [DOI] [PubMed] [Google Scholar]
- [174].Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46(3):841–851. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [175].Mesure B, Menu P, Venkatesan JK, Cucchiarini M, Velot E. Biomaterials and Gene Therapy: A Smart Combination for MSC Musculoskeletal Engineering. Current stem cell research & therapy. 2019;14(4):337–343. doi: 10.2174/1574888X14666181205121658. [DOI] [PubMed] [Google Scholar]
- [176].Raftery RM, Walsh DP, Castaño IM, Heise A, Duffy GP, Cryan S-A, O’Brien FJ. Delivering Nucleic-Acid Based Nanomedicines on Biomaterial Scaffolds for Orthopedic Tissue Repair: Challenges, Progress and Future Perspectives. Advanced Materials. 2016;28(27):5447–5469. doi: 10.1002/adma.201505088. [DOI] [PubMed] [Google Scholar]
- [177].Weisenberger MS, Deans TL. Bottom-up approaches in synthetic biology and biomaterials for tissue engineering applications. Journal of industrial microbiology & biotechnology. 2018;45(7):599–614. doi: 10.1007/s10295-018-2027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Loozen LD, Kruyt MC, Kragten AHM, Schoenfeldt T, Croes M, Oner CF, Dhert WJA, Alblas J. BMP-2 gene delivery in cell-loaded and cell-free constructs for bone regeneration. PloS one. 2019;14(7):e0220028. doi: 10.1371/journal.pone.0220028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wilson CG, Martín-Saavedra FM, Vilaboa N, Franceschi RT. Advanced BMP gene therapies for temporal and spatial control of bone regeneration. Journal of dental research. 2013;92(5):409–17. doi: 10.1177/0022034513483771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Wang W, Huang D, Ren J, Li R, Feng Z, Guan C, Bao B, Cai B, Ling J, Zhou C. Optogenetic control of mesenchymal cell fate towards precise bone regeneration. Theranostics. 2019;9(26):8196–8205. doi: 10.7150/thno.36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Salmerón-Sánchez M, Dalby MJ. Synergistic growth factor microenvironments. Chemical Communications. 2016;52(91):13327–13336. doi: 10.1039/c6cc06888j. [DOI] [PubMed] [Google Scholar]
- [183].Garcia JR, Clark AY, Garcia AJ. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. Journal of biomedical materials research Part A. 2016;104(4):889–900. doi: 10.1002/jbm.a.35626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Bouyer M, Guillot R, Lavaud J, Plettinx C, Olivier C, Curry V, Boutonnat J, Coll JL, Peyrin F, Josserand V, Bettega G, et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016;104:168–81. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Guillot R, Gilde F, Becquart P, Sailhan F, Lapeyrere A, Logeart-Avramoglou D, Picart C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials. 2013;34(23):5737–46. doi: 10.1016/j.biomaterials.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science (New York, NY) 2014;343(6173):885–8. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- [187].Schmidt-Bleek K, Willie BM, Schwabe P, Seemann P, Duda GN. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine & growth factor reviews. 2016;27:141–8. doi: 10.1016/j.cytogfr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- [188].Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue engineering Part A. 2011;17(9-10):1389–99. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Begam H, Nandi SK, Kundu B, Chanda A. Strategies for delivering bone morphogenetic protein for bone healing. Materials science & engineering C, Materials for biological applications. 2017;70(Pt 1):856–869. doi: 10.1016/j.msec.2016.09.074. [DOI] [PubMed] [Google Scholar]
- [190].Huang Y, Luo Q, Zha G, Zhang J, Li X, Zhao S, Li X. Biomimetic ECM coatings for controlled release of rhBMP-2: construction and biological evaluation. Biomaterials science. 2014;2(7):980–989. doi: 10.1039/c3bm60254k. [DOI] [PubMed] [Google Scholar]
- [191].Wang Z, Dong L, Han L, Wang K, Lu X, Fang L, Qu S, Chan CW. Self-assembled Biodegradable Nanoparticles and Polysaccharides as Biomimetic ECM Nanostructures for the Synergistic effect of RGD and BMP-2 on Bone Formation. Scientific reports. 2016;6:25090. doi: 10.1038/srep25090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Hachim D, Whittaker TE, Kim H, Stevens MM. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. Journal of controlled release : official journal of the Controlled Release Society. 2019;313:131–147. doi: 10.1016/j.jconrel.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Yanamoto S, Babazada H, Sakai S, Higuchi Y, Yamashita F, Hashida M. Anti-inflammatory Effect of Self-assembling Glycol-Split Glycosaminoglycan-Stearylamine Conjugates in Lipopolysaccharide-Stimulated Macrophages. Biological & pharmaceutical bulletin. 2017;40(4):540–545. doi: 10.1248/bpb.b16-00928. [DOI] [PubMed] [Google Scholar]
- [194].Hettiaratchi MH, Krishnan L, Rouse T, Chou C, McDevitt TC, Guldberg RE. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Science advances. 2020;6(1):eaay1240. doi: 10.1126/sciadv.aay1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Dang PN, Dwivedi N, Phillips LM, Yu X, Herberg S, Bowerman C, Solorio LD, Murphy WL, Alsberg E. Controlled Dual Growth Factor Delivery From Microparticles Incorporated Within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification. Stem Cells Transl Med. 2016;5(2):206–17. doi: 10.5966/sctm.2015-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Gong Y, Zhang Y, Cao Z, Ye F, Lin Z, Li Y. Development of CaCO(3) microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration. Biomaterials science. 2019;7(9):3614–3626. doi: 10.1039/c9bm00463g. [DOI] [PubMed] [Google Scholar]
- [197].He C, Ji H-F, Qian Y, Wang Q, Liu X, Zhao W, Zhao C. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. Journal of Materials Chemistry B. 2019;7 doi: 10.1039/c8tb02671h. [DOI] [PubMed] [Google Scholar]
- [198].Yang HS, La W-G, Cho Y-M, Shin W, Yeo G-D, Kim B-S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Experimental & Molecular Medicine. 2012;44(5):350–355. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Kim IG, Hwang MP, Du P, Ko J, Ha CW, Do SH, Park K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials. 2015;50:75–86. doi: 10.1016/j.biomaterials.2015.01.054. [DOI] [PubMed] [Google Scholar]
- [200].Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue engineering Part A. 2010;16(4):1225–33. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- [201].Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35(25):7228–38. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Wang Z, Wang K, Lu X, Li M, Liu H, Xie C, Meng F, Jiang O, Li C, Zhi W. BMP-2 encapsulated polysaccharide nanoparticle modified biphasic calcium phosphate scaffolds for bone tissue regeneration. Journal of biomedical materials research Part A. 2015;103(4):1520–32. doi: 10.1002/jbm.a.35282. [DOI] [PubMed] [Google Scholar]
- [203].Bai X, Lü S, Cao Z, Ni B, Wang X, Ning P, Ma D, Wei H, Liu M. Dual crosslinked chondroitin sulfate injectable hydrogel formed via continuous Diels-Alder (DA) click chemistry for bone repair. Carbohydrate polymers. 2017;166:123–130. doi: 10.1016/j.carbpol.2017.02.062. [DOI] [PubMed] [Google Scholar]
- [204].Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, Shah RN, Stupp SI. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34(2):452–459. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Andrews S, Cheng A, Stevens H, Logun MT, Webb R, Jordan E, Xia B, Karumbaiah L, Guldberg RE, Stice S. Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration. Stem Cells Transl Med. 2019;8(6):575–585. doi: 10.1002/sctm.18-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Barritault D, Gilbert-Sirieix M, Rice KL, Siñeriz F, Papy-Garcia D, Baudouin C, Desgranges P, Zakine G, Saffar JL, van Neck J. RGTA(®) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: from concept to curing patients. Glycoconjugate journal. 2017;34(3):325–338. doi: 10.1007/s10719-016-9744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Papy-Garcia D, Barbier-Chassefière V, Rouet V, Kerros M-E, Klochendler C, Tournaire M-C, Barritault D, Caruelle J-P, Petit E. Nondegradative Sulfation of Polysaccharides. Synthesis and Structure Characterization of Biologically Active Heparan Sulfate Mimetics. Macromolecules. 2005;38(11):4647–4654. [Google Scholar]
- [208].Pan Y, Chen J, Yu Y, Dai K, Wang J, Liu C. Enhancement of BMP-2-mediated angiogenesis and osteogenesis by 2-N,6-O-sulfated chitosan in bone regeneration. Biomaterials science. 2018;6(2):431–439. doi: 10.1039/c7bm01006k. [DOI] [PubMed] [Google Scholar]
- [209].Llopis-Hernandez V, Cantini M, Gonzalez-Garcia C, Cheng ZA, Yang J, Tsimbouri PM, Garcia AJ, Dalby MJ, Salmeron-Sanchez M. Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Science advances. 2016;2(8):e1600188. doi: 10.1126/sciadv.1600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Cheng ZA, Alba-Perez A, Gonzalez-Garcia C, Donnelly H, Llopis-Hernandez V, Jayawarna V, Childs P, Shields DW, Cantini M, Ruiz-Cantu L, Reid A, et al. Nanoscale Coatings for Ultralow Dose BMP-2-Driven Regeneration of Critical-Sized Bone Defects. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2019;6(2):1800361. doi: 10.1002/advs.201800361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Crouzier T, Sailhan F, Becquart P, Guillot R, Logeart-Avramoglou D, Picart C. The performance of BMP-2 loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer film coating. Biomaterials. 2011;32(30):7543–54. doi: 10.1016/j.biomaterials.2011.06.062. [DOI] [PubMed] [Google Scholar]
- [212].Machillot P, Quintal C, Dalonneau F, Hermant L, Monnot P, Matthews K, Fitzpatrick V, Liu J, Pignot-Paintrand I, Picart C. Automated buildup of biomimetic films in cell culture microplates for high-throughput screening of cellular behaviors. Advanced materials (Deerfield Beach, Fla) 2018;30(27):e1801097. doi: 10.1002/adma.201801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].O’Leary JM, Hamilton JM, Deane CM, Valeyev NV, Sandell LJ, Downing AK. Solution structure and dynamics of a prototypical chordin-like cysteine-rich repeat (von Willebrand Factor type C module) from collagen IIA. The Journal of biological chemistry. 2004;279(51):53857–66. doi: 10.1074/jbc.M409225200. [DOI] [PubMed] [Google Scholar]
- [214].Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, Shah RN, Stupp SI. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34(2):452–9. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]