Abstract
CRISPR-Cas adaptive immune systems in bacteria and archaea utilize short CRISPR RNAs (crRNAs) to guide sequence-specific recognition and clearance of foreign genetic material. Multiple crRNAs are stored together in a compact format called a CRISPR array that is transcribed and processed into the individual crRNAs. While the exact processing mechanisms vary widely, some CRISPR-Cas systems including those encoding the Cas9 nuclease rely on a trans-activating crRNA (tracrRNA). The tracrRNA was discovered in 2011 and was quickly co-opted to create single-guide RNAs as core components of CRISPR-Cas9 technologies. Since then, further studies have uncovered processes extending beyond the tracrRNA’s traditional role in crRNA biogenesis, revealed Cas nucleases besides Cas9 that are dependent on tracrRNAs, and established new applications based on tracrRNA engineering. In this review, we describe the biology of the tracrRNA and how its ongoing characterization has garnered new insights into prokaryotic immune defense and enabled key technological advances.
Keywords: crystal structure, immune defense, sgRNA, RNA processing, RNase III
Introduction
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and their CRISPR-associated (Cas) proteins protect bacteria and archaea against invading nucleic acids (3, 67). As the only known adaptive immune systems in prokaryotes, the systems store nucleic acid information in CRISPR arrays as short spacers in between direct repeats. To enact immunity, the CRISPR array is transcribed into a long precursor CRISPR RNA (pre-crRNA) that is processed into individual mature CRISPR RNAs (crRNAs). The crRNAs then direct Cas effector nucleases to cleave DNA or RNA sequences complementary to the “guide” portion of the crRNA and flanked by a protospacer-adjacent motif (PAM) (for DNA targets) or a sequence lacking extensive complementarity to the crRNA repeat (for RNA targets) (59, 70). Because target selection is dictated by the acquired spacer, CRISPR-Cas systems can specifically clear future infections by the same invader. The spacer sequence can also be readily changed to direct Cas nucleases to other nucleic-acid targets with appropriate flanking sequences, opening a range of applications (extensively reviewed in (79)). In turn, the ongoing characterization of CRISPR-Cas immune systems has not only advanced our understanding of the ongoing arms race between prokaryotes and mobile genetic elements but also provided numerous benefits to society.
Arguably the best-studied and mostly widely harnessed CRISPR-Cas systems are Type II systems encoding the effector nuclease Cas9. As the first characterized single-effector nuclease, Cas9 was primed to be co-opted for genome editing and other applications (12, 15, 18, 35, 44, 47, 49, 68, 90, 102). However, completing the jump from biology to technology required a fundamental understanding of how the system’s crRNAs are generated to guide DNA targeting. The discovery of the trans-activating crRNA (tracrRNA) as a core component of crRNA biogenesis became the missing piece that enabled the invention of the single-guide RNA (sgRNA) and the adoption of Cas9 as the centerpiece of CRISPR technologies (23). Accordingly, the discovery of the tracrRNA is central to the awarding of the 2020 Nobel Prize in Chemistry to Dr. Emmanuelle Charpentier and Dr. Jennifer Doudna for their development of CRISPR-Cas9 for genome editing (72). Since the discovery of the tracrRNA and its use for genome editing almost a decade ago, there have been numerous discoveries that have advanced our understanding of its biological functions and have expanded the list of systems involving tracrRNAs. There have also been numerous technological advances that have improved and expanded the reach of tracrRNAs in CRISPR technologies. In this review, we describe the role of the tracrRNA in immune defense of CRISPR-Cas9 and beyond, non-traditional functions involving tracrRNAs, and how the tracrRNA has enabled an expanding set of CRISPR technologies.
THE tracrRNA IN CRISPR RNA BIOGENESIS
Understanding the role of the tracrRNA in CRISPR biology first requires a perspective on the remarkable diversity of CRISPR-Cas systems and how each system confers adaptive immunity. As adaptive immune systems, all CRISPR-Cas systems store invader-derived spacers into CRISPR arrays, process individual crRNAs from a transcribed pre-crRNA, and use the crRNAs to guide the Cas nucleases to recognize and clear complementary nucleic acids. However, the commonalities largely end there. The Cas proteins associated with CRISPR-Cas systems vary widely and confer different mechanisms for each step of adaptive immunity. This diversity drove the field to create a hierarchical classification scheme that underwent multiple rounds of revision as new systems were discovered. The current scheme comprises two classes (1 and 2), six types (I - VI), over 30 subtypes (e.g. I-E, II-A), and a scattering of subtype variants (e.g. I-F1, II-C2) (67). Because these proteins enact the different steps of adaptive immunity, many of the associated mechanisms partition with the CRISPR-Cas type and subtype. For example, in the early days of characterizing CRISPR biology, Type I and III systems were shown to rely on their encoded endoribonuclease Cas6 to cleave each repeat as part of crRNA biogenesis (8, 10, 11, 39, 67). This protein is absent in other CRISPR-Cas types including Type II systems, spurring efforts to elucidate how the different groups of systems process a pre-crRNA into individual crRNAs.
The tracrRNA was first reported in 2011 from a seminal study with the human pathogen Streptococcus pyogenes and its endogenous Type II-A CRISPR-Cas system (23) (Figure 1a). The tracrRNA was identified during a period when next-generation sequencing was first being applied to identify small RNAs missed by standard annotation algorithms (23, 93). One technique (differential RNA-seq, or dRNA-seq) (23, 89, 93) was applied to identify small RNAs and determine whether they possessed a dedicated promoter or were processed from a larger transcript. The sequencing analyses revealed the tracrRNA as one of the most abundant small RNAs in the cell. The RNA was also encoded adjacent and divergent to the cas9 gene (then called csn1), suggesting some role in the flanking CRISPR-Cas system. The tracrRNA further appeared as not one but three different transcripts with a shared 3′ end: ~171 and ~89 nt products representing primary transcripts and a ~75 nt species processed from both longer transcripts. The processing site was within a 24-nt stretch bearing extensive complementarity to the CRISPR repeats, indicating that the tracrRNA is directly involved in crRNA biogenesis.
Figure 1. The tracrRNA in crRNA biogenesis and immune defense by Type II CRISPR-Cas systems.
(a) Genomic architecture and crRNA biogenesis pathway for the Type II-A CRISPR-Cas system from S. pyogenes. The CRISPR array comprising conserved repeats (R, black rectangles) with intervening invader-derived spacers (S, colored rectangles) is transcribed into a long pre-crRNA. The tracrRNA then base pairs with each repeat, driving cleavage by RNase III and binding by Cas9. The processed crRNA:tracrRNA duplex then directs bound Cas9 to DNA sequences matching the crRNA guide sequence (protospacer) and flanked by a protospacer adjacent motif (PAM). Gray arrows: processing/cleavage sites. A few steps in crRNA biogenesis remain unknown, including how the 5′ end of each crRNA is trimmed and the fate of the first repeat in the array, which lacks a corresponding spacer. (b) Comparing crRNA biogenesis for II-A and II-C CRISPR-Cas systems. Type II-A systems initiate transcription of the array through an upstream promoter encoded within the leader region. Type II-C systems initiate transcript of the array within each spacer, which is initiated through promoter elements encoded in each repeat. The two systems also differ in the side of the array in which new spacers are added. The outside repeat is copied as part of acquisition to maintain the pattern of the array. (c) Predicted secondary structures capturing the vast majority of identified tracrRNAs. The structures were grouped into ten clusters identified in (26).
The ensuing experiments conducted in S. pyogenes informed our primary view of how the tracrRNA participates in crRNA biogenesis (Figure 1a) (23). The CRISPR array is transcribed into a pre-crRNA containing multiple repeats. Each repeat then base pairs with the 24-nt stretch of the tracrRNA termed the anti-repeat domain. The AT-rich sequence within the repeat:anti-repeat duplex presents a preferred substrate for the host endoribonuclease RNase III (75), where cleavage forms a 2-nt overhang on the 3′ end of the repeat. The cleavage event also divides the pre-crRNA into individual immature crRNAs consisting of a full-length spacer flanked by either half of the repeat. The RNA duplex is bound by Cas9, while the 5′ end of the immature crRNA is trimmed ~10 nucleotides (nts) into the spacer. The trimming is presumably conducted by host ribonucleases, although these enzymes remain to be identified. The exact order of events (e.g. duplex formation, Cas9 binding, RNase III cleavage) was also unclear, although in vitro experiments with the homologous II-A system from Streptococcus thermophilus reported that Cas9 promoted pre-crRNA:tracrRNA hybridization but did not exhibit measurable binding to the tracrRNA (54). Regardless of the exact mechanisms though, the resulting ribonucleoprotein effector complex comprises Cas9 bound to an ~40-nt mature crRNA and an ~75-nt processed tracrRNA. The duplex of the mature crRNA and processed tracrRNA can be fused with a short tetraloop to create the sgRNA, which simplifies the characterization and implementation of Cas9 nucleases (48).
Subsequent efforts to characterize tracrRNAs and crRNA biogenesis upheld these basic principles while revealing variations on the theme. In line with the original work, the tracrRNA is considered a ubiquitous feature of Type II CRISPR-Cas systems and essential for crRNA biogenesis (16, 34, 41, 57). Various bioinformatics search tools have also been developed for identifying tracrRNAs (Sidebar). The variations have been principally associated with how crRNAs are generated and the structures of the crRNA:tracrRNA complex. The mode of crRNA generation currently depends on the CRISPR-Cas subtype (Figure 1b). Type II-A systems initiate transcription from the leader region and proceed through the entire CRISPR array, generating one pre-crRNA transcript. In contrast, characterized Type II-C systems contain -10 promoter elements near the 3′ end of each repeat (27, 113). Transcription therefore initiates within each downstream spacer, creating different lengths of pre-crRNA transcripts. RNase III processing was also shown to be dispensable for immune defense by the II-C system in Neisseria meningitidis--at least through the last spacer in the array (113). Spacer acquisition also appears to occur on opposite sides of the array (Figure 1b), where Cas9 and the tracrRNA are involved in this process (40, 103, 113). For II-B systems, only the system present in Francisella novicida has been well characterized (1), although the site(s) of spacer acquisition and transcription initiation remain unknown. Beyond acquisition and transcription initiation, the structure of the crRNA:tracrRNA complex varies widely across Type II systems. One comprehensive bioinformatics search for tracrRNAs predicted 10 main groups based on predicted secondary structure (Figure 1c) (26). These assigned groups could be differentiated by the bulge between the RNA duplex as well as the secondary structure downstream of the tracrRNA anti-repeat.
Sidebar. Bioinformatic identification of tracRNAs.
Predicting tracrRNAs is important for the characterization of new Cas9 proteins and their development as genome editing tools (34, 55). As the tracrRNA can be distinct among different CRISPR systems in terms of sequence, secondary structure, and placement within the CRISPR-Cas loci (67, 110), its computational identification is not trivial. Current prediction methods often consist of first identifying Cas9 through protein homology followed by searching for nearby CRISPR arrays. The CRISPR repeat sequence is then used to search for anti-repeat sequences in the vicinity, while the end of the tracrRNA is determined by scanning for a downstream rho-independent terminator (7, 17, 26). These steps can be achieved using individual online or local tools step-by-step (7, 16), although there are also bundled algorithm codes that allow for batch analyses (17, 26). The identified tracrRNA is recommended to be confirmed through RNA-seq analyses either in the native microorganism or through heterologous expression in a lab strain. These methods can be modified for predicting tracrRNAs for the type V CRISPR-Cas systems possessing tracrRNAs, although more adjustments would be needed particularly for predicting the V-C and V-D scoutRNAs given the lack of extensive complementarity to the crRNA repeat.
THE tracrRNA IN Cas9 RECOGNITION AND TRANSCRIPTIONAL REGULATION
Crystal structures of SpyCas9 have proven invaluable for understanding the molecular details of how Cas9 specifically recognizes the crRNA:tracrRNA duplex (2, 43, 45, 46, 50, 77, 115). The processed crRNA:tracrRNA complex bound by SpyCas9 can be divided into seven domains: guide; upper stem, bulge, and lower stem forming the repeat:anti-repeat duplex; and a stem-loop termed the nexus (6), linker, and two additional stem-loops forming the tracrRNA tail (Figure 2a). SpyCas9 forms numerous contacts with all domains except the upper stem, freeing the upper stem for unhindered cleavage by RNase III (Figure 2b). These interactions are principally based on RNA secondary structure through recognition of the sugar-phosphate backbone, although base-specific interactions occur through the bulge and the nexus as well as the bottom of the lower stem and second stem-loop and the loop region of the third stem-loop. Both lobes of SpyCas9 are also involved in binding the crRNA:tracrRNA complex, with the repeat:anti-repeat duplex bound by the REC lobe, linker and last two stem-loops bound by the NUC lobe, and the nexus and linker bound by both lobes. Both structural and sequence-specific recognition allows Cas9 to differentiate this complex from the vast array of other RNAs present in a cell. Upon sgRNA binding, the REC lobe of Cas9 undergoes substantial conformational rearrangement typified by the formation of a central channel for target DNA binding (50). This rearrangement represents the first of many for SpyCas9 to proceed from a holoenzyme to a ribonucleoprotein complex bound to cleaved DNA.
Figure 2. Recognition of the crRNA:tracrRNA duplex by Cas9.
(a) Anatomy of the crRNA:tracrRNA complex. (b) Structure and processing of the crRNA:tracrRNA complex bound to Cas9 from the Type II-A system in S. pyogenes (left) and the Type II-C system in C. jejuni (right). The crRNA:tracrRNA duplex undergoes processing by RNase III (yellow circle).
Besides SpyCas9, a number of other Cas9 nucleases have been crystalized in complex with an sgRNA that highlight the shared and distinct features across these proteins. Within II-A systems, the small Cas9 from Streptococcus aureus (SauCas9) represents one of the few additional Cas9 nucleases that have been crystallized. Contrasting with SpyCas9, the REC lobe and a distinct WED domain present in the NUC lobe interacts with the repeat:anti-repeat duplex non-specifically through its sugar-phosphate backbone. The nexus is recognized by the REC lobe similar to SpyCas9, while the second and only other stem-loop in the tracrRNA tail was dispensable to crystallize the ribonucleoprotein complex. Structural studies of Cas9 proteins from II-B and II-C systems revealed notable structural differences in their sgRNA scaffolds as well as diverse features of domain interaction and arrangement (41, 97, 108). Most notably, the Cas9 from the II-C system in Campylobacter jejuni (CjeCas9) recognizes a complicated structure in the tracrRNA tail comprising a pseudoknot and triple helix (108) (Figure 2b). Given the distinct modes of recognition, it is not surprising that phylogenetically distant Cas9 nucleases cannot utilize each other’s sgRNAs for DNA targeting, even when the cognate PAM is present (6, 30). The tracrRNA tail also appears to be the primary determinant of specificity, as swapping these tails allowed recognitzed of the sgRNA by the non-cognate Cas9 (6).
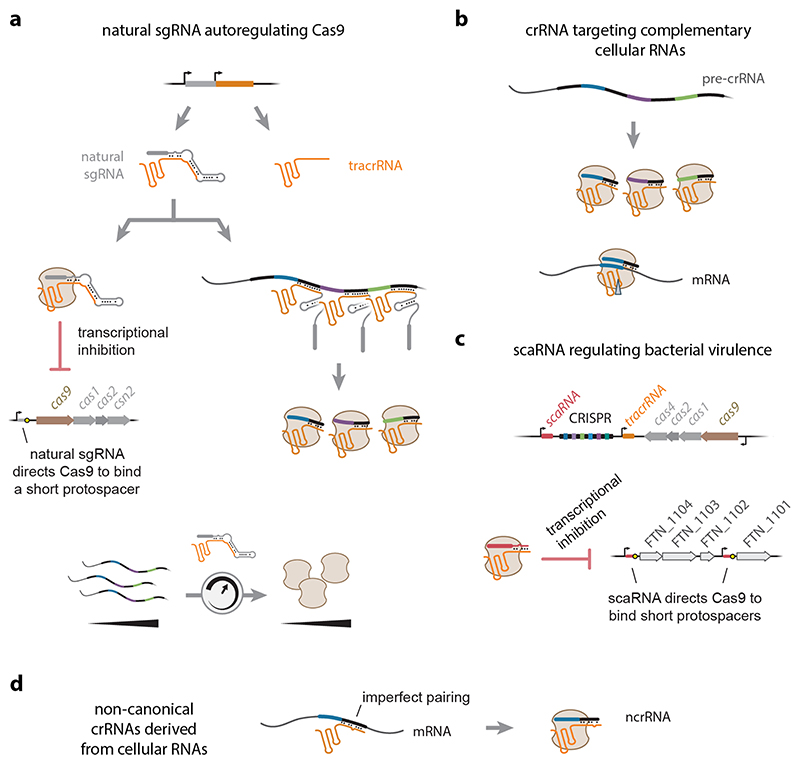
Apart from playing key roles in crRNA biogenesis and recognition by Cas9, the tracrRNA was recently shown to also regulate Cas9 expression (105) (Figure 3a). This discovery came from exploring the longer version of the tracrRNA in S. pyogenes (23). Both the shorter (89 nts) and longer (171 nts) versions encode the anti-repeat that duplexes with a pre-crRNA repeat and undergo processing by RNase III to yield a mature crRNA. However, the extended 5′ end allows the longer version to fold into a structure mimicking an sgRNA, albeit with a 13-nt guide sequence, an imperfect stem that does not undergo processing by RNase III, and a large structure protruding from the stem. Despite these differences, this “natural sgRNA” binds Cas9 and directs it to recognize an 11-bp sequence flanked by a PAM in Cas9’s own promoter. The limited pairing allows DNA binding but not cleavage, resulting in transcriptional repression. Because higher Cas9 levels would lead to stronger repression, the longer version of the tracrRNA provides feedback control of Cas9 levels. Furthermore, because the longer version of the tracrRNA either forms the natural sgRNA or interacts with a pre-crRNA repeat, the longer version of the tracrRNA adjusts Cas9 levels based on the abundance and length of the pre-crRNA. The feedback loop appeared to mitigate autoimmunity by preventing the acquisition of self-targeting spacers caused by overexpression of the cas genes. The natural sgRNA was predicted in almost half of assessed II-A systems.
Figure 3. Alternative roles of the tracrRNA in Cas9 biology.
(a) A long form of the tracrRNA directs autoregulation of Cas9 expression. The long form folds into a natural single-guide RNA (sgRNA). The natural sgRNA complexes with Cas9 and directs the nuclease to bind a PAM-flanked protospacer downstream of the promoter driving expression of the cas operon. The protospacer is sufficiently short to allow DNA binding but not cleavage, while binding blocks transcription of the operon. Alternatively, the natural sgRNA preferentially hybridizes to a repeat within the pre-crRNA. The long form of the tracrRNA then undergoes processing by RNase III to generate a mature crRNA. The natural sgRNA therefore serves as a feedback controller by tuning Cas9 levels to the abundance and length of the pre-crRNA. (b) The crRNA processed with the tracrRNA can direct binding and cleavage of cellular RNAs. These RNAs encode sequences with extensive complementarity to the crRNA guide but do not require the presence of a PAM. At least one Cas9 (i.e. SpyCas9) binds complementary RNAs but does not cleave the RNA. (c) A small CRISPR-associated RNA (scaRNA) downregulates target genes to promote bacterial virulence. The scaRNA is encoded adjacent to the CRISPR array and hybridizes with the tracrRNA similar to a mature crRNA. The RNA duplex then directs Cas9 to bind shorter PAM-flanked protospacers located downstream of two different promoters. Downregulating these genes promotes virulence in the human pathogen Francisella novicida. (d) Generation of non-canonical crRNAs (ncrRNAs) from cellular RNAs. The RNAs contain a sequence resembling the CRISPR repeat, promoting hybridization to the anti-repeat portion of the tracrRNA and recognition of the RNA duplex by Cas9. The RNA duplex can then direct DNA targeting. To date, b and d have no known biological functions.
THE tracrRNA BEYOND IMMUNE DEFENSE
Type II CRISPR-Cas systems consistently use the crRNA:tracrRNA complex to recognize and cleave double-stranded DNA as part of adaptive immunity. However, there are growing examples of Cas9 nucleases performing other functions with the help of the tracrRNA. One established example involves some Cas9 nucleases recognizing and cleaving RNA (Figure 3b). Recognition occurs through pairing between the RNA and the guide sequence in the duplexed crRNA:tracrRNA, although the rules for recognition and cleavage vary. For instance, RNA binding and cleavage by SpyCas9 required a flanking PAM presented as double-stranded DNA or a DNA:RNA duplex. While absent in natural RNAs, the duplex could be artificially created by annealing a PAM-presenting ssDNA (PAMmer) (78). Later work showed that deactivating the HNH and RuvC endonuclease domains in SpyCas9 allowed RNA binding even in the absence of a PAM or PAMmer (4, 74). The Cas9 nucleases from Neisseria meningitidis (NmeCas9), S. aureus, and C. jejuni were also reported to bind and cleave targeted RNA (28, 85, 96), but without a PAM or PAMmer. Evidence also exists that the Cas9 from Francisella novicida (FnoCas9) can also bind and cleave RNA (81). The study with CjeCas9 in particular provided evidence for RNA targeting through the endogenous Type II-C CRISPR-Cas system (28). By identifying RNAs in C. jejuni bound by the endogenous Cas9, the authors showed the CRISPR-Cas system’s crRNAs were directing RNA binding and cleavage of many cellular transcripts. It remains to be seen whether targeting of endogenous transcripts by Cas9 represents a biological function or a background activity tolerated by the cells.
Whether targeting RNA or DNA, Cas9 utilized a crRNA derived from the CRISPR array. However, prior work showed that a separate RNA encoded in the vicinity of the CRISPR array can also direct Cas9. This small CRISPR-Cas-associated RNA (scaRNA) was identified in the intracellular pathogen F. novicida as an essential component of Cas9-mediated repression of genes related to immune avoidance (87) (Figure 3c). The ~58-nt scaRNA is encoded immediately upstream of the CRISPR array and resembles a crRNA, with a guide sequence and repeat-like sequence complementary to the tracrRNA anti-repeat. To exert its regulatory function, the scaRNA hybridizes with the tracrRNA, and the scaRNA:tracrRNA complex is bound by Cas9. The scaRNA then directs Cas9 to two DNA targets with ~15 nts complementary to the scaRNA guide and flanked by a recognized PAM in the F. novicida genome (82). The limited base pairing allows DNA binding but not cleavage similar to the natural sgRNA, resulting in the transcriptional repression of the four downstream virulence genes. Given that a scaRNA would be hard to distinguish from the first repeat in a CRISPR array, more work will be needed to determine the prevalence of scaRNAs and the roles they play in microbial physiology.
Beyond CRISPR arrays and the scaRNA, recent work demonstrated that Cas9 can be guided by cellular RNAs encoded outside of CRISPR-Cas loci (5). These non-canonical RNAs (ncrRNAs) were identified in C. jejuni as a subset of transcript fragments bound by Cas9, similar to the RNA targets of the crRNAs (28). Unlike the crRNA-targeted RNAs though, the transcript fragments shared a motif complementary to the tracrRNA anti-repeat. Further interrogation revealed that these ncrRNAs were derived through base pairing with the tracrRNA and subsequent processing presumably by host RNases (Figure 3d). The extent of base pairing was often imperfect although sufficient to mediate tracrRNA binding and recognition of the complex by Cas9. In a few cases, combining the ncrRNA-encoding mRNA and the tracrRNA drove sequence-specific cleavage of the corresponding DNA target by Cas9. While no phenotype was associated with the assessed ncrRNAs in C. jejuni, the finding shows that Cas9 can be guided by RNAs unrelated to CRISPR-Cas systems. It also shows the potential impact of base pairing between a cellular transcript and the tracrRNA anti-repeat--for better or for worse--that could impact the horizontal transfer and evolution of CRISPR-Cas systems utilizing tracrRNAs.
THE tracrRNA BEYOND Cas9
While the tracrRNA was originally considered a unique feature of Type II CRISPR-Cas systems, efforts to expand the known repertoire of CRISPR-Cas systems revealed Type V systems requiring tracRNAs (67) (Figure 4a). Type V systems encode Cas12 effector nucleases that tend to target dsDNA similar to Cas9, although Cas12 nucleases create a 5′ overhang as part of target DNA cleavage and drive collateral degradation of ssDNA upon target recognition (14, 112). The mature crRNA also comprises a repeat preceding the guide sequence. While all Type II systems rely on a tracrRNA, some Cas nucleases like Cas12a or the Cas12i process the crRNA repeat without accessory factors (31, 110). Of the Type V systems with tracrRNAs, the first reported example came from a bioinformatics search for novel Class 2 CRISPR-Cas systems (9, 91). Characterization of one system later classified as Type V-B revealed a highly abundant small RNA encoded between cas2 and the CRISPR array. The 3′end of the small RNA exhibited extensive complementarity to the CRISPR repeat, underwent processing within the repeat:anti-repeat duplex, and was indispensable for DNA targeting by the C2c1/Cas12b nuclease. This work also identified C2c3/Cas12c from Type V-C systems but without a tracrRNA. Later work showed that a tracrRNA was present and required for DNA targeting, although limited complementarity between the crRNA repeat and tracrRNA anti-repeat prevented initial annotation (38, 110). Similar issues prevented the identification of tracrRNAs in Type V-D systems, although later work showed that CasY/Cas12d relied on a tracrRNA for DNA targeting (38). Separate searches for novel Class 2 nucleases within metagenomic sequences and uncultivated microorganisms identified the smaller CRISPR nuclease CasX/Cas12e representing Type V-E systems and even smaller nuclease Cas14a/Cas12f1 representing Type V-F1 systems, which both required a tracrRNA for DNA targeting (37, 64). Cas12f1 nucleases preferentially cleave ssDNA but can also weakly cleave dsDNA flanked by a PAM (37, 53). A broader investigation of Cas12 nucleases revealed Cas12g from Type V-G systems, the only Type V systems shown to target RNA, which were also dependent on a tracrRNA (110). Finally, one of the newly discovered CRISPR-associated transposons, which guide DNA transposition through a CRISPR effector, is classified as a Type V-K system and relies on a tracrRNA (95). Other subtypes and variants with Type V systems have been identified through bioinformatics searches but remain uncharacterized experimentally, leaving open the possibility that additional Type V systems encode tracrRNAs (67).
Figure 4. tracrRNAs associated with Type V CRISPR-Cas systems.
(a) List of currently classified Type V subtypes and variants along with the name of the effector nuclease and whether (Y) or not (n) the system includes a tracrRNA. Dashes indicate systems where no tracrRNAs are predicted, but the systems have not undergone experimental characterization. (b) Structure and processing for crRNA:tracrRNA complexes bound to the cognate Cas12 nuclease. The structures associated with Cas12b, Cas12e, and Cas12f1 are based on crystal structures, while the structure for Cas12c/d is based on folding predictions and experimental evidence. Cas12f1 forms a dimer that binds one crRNA:tracrRNA complex. The crRNA:tracrRNA duplex associated with Cas12b, Cas12e, and Cas12f1 are presumed to undergo processing by RNase III (yellow oval). Processing of the crRNA guide by Cas12c/d is established for Cas12c but remains circumstantial for Cas12d.
All of these Type V subtypes rely on a tracrRNA that hybridizes through its 3′ end to the crRNA repeat, although few additional shared properties exist (Figure 4b). For instance, the length of the processed tracrRNA varies from ~78 (for V-B) to ~216 nts (for V-K), while the extent of predicted base pairing between the crRNA repeat and unprocessed tracrRNA anti-repeat varies from 5 (for V-C/D) to 20 bps (for V-E) (38, 64, 91). The types of structures formed between the crRNA repeat and tracrRNA anti-repreat and how these structures are processed to form the final mature crRNA ranged widely and often deviate from the paradigm set by Type II systems. Type V-B, V-E, V-F1, and V-G systems represent the closest analogs to Type II systems, as the tracrRNA for these systems forms an extended duplex with the crRNA repeat that presumably undergoes processing by RNase III (64, 91, 110). All but the V-G systems represent notable deviations though because the repeat:antirepeat duplex is divided into two regions of pairing. For V-B and V-F1 systems, the two regions are separated by an intervening pseudoknot and, for V-F1 systems, additional stem-loops. The crRNA:tracrRNA duplex for V-F1 systems may also be processed by a host ribonuclease besides RNase III, as the reconstructed host genome lacks this gene and no duplex processing was observed with Cas12f1 in vitro (37). Within Type V-E systems, the repeat:anti-repeat duplex includes a triple-helix formed between two strands of the tracrRNA and one strand of the crRNA repeat (64). V-C and V-D systems represent the largest deviation from Type II systems to-date. For these systems, the 5’ end of the tracrRNA is predicted to form a large hairpin with a bulge near the base of the stem (38). Mutational analyses and sequence conservation within the crRNA and tracrRNA suggested that a short stretch within this bulge base pairs with the crRNA repeat to form a pseudoknot, although further structural analyses are needed. Cas12c was further shown to process the crRNA repeat a fixed distance from the proposed crRNA:tracrRNA pseudoknot. This mode of processing reflects nucleus-mediated processing of the repeat by Cas12a and Cas12i, while the tracrRNA aids in crRNA recognition by the nuclease (38). In vitro processing of the crRNA by Cas12d was inconclusive. Based on the unique proposed structure of the tracrRNA and its involvement in crRNA processing, the authors proposed the tracrRNA for these systems instead be called a short-complementarity untranslated RNA (scoutRNA) (38). How the crRNA:tracrRNA duplex is formed and processed remains to be elucidated for V-G and V-K systems.
The few available crystal structures of Cas12 nucleases bound to a guide RNA have begun to reveal how these nucleases uniquely interact with the crRNA:tracrRNA complex. To-date, crystal structures are available for Cas12b, Cas12e, and Cas12f1 bound to an sgRNA and target DNA (64, 65, 98, 109). Cas12b consists of two lobes that both interact extensively with the crRNA:tracrRNA complex, reflecting sequence-specific as well as non-specific interactions (109). Most of the interactions occur through the region of the repeat:anti-repeat stem adjacent to the guide as well as two of the stem loops within the rest of the tracrRNA. The pseudoknot within the tracrRNA minimally contacts the nuclease and instead likely helps form the structure of the crRNA:tracrRNA complex recognized by Cas12b. Cas12e interacts with the triple helix formed between the crRNA repeat and tracrRNA as well as with a stem-loop within the rest of the tracrRNA (64). Beyond the triple helix though, the traditional duplex formed between the crRNA repeat and the tracrRNA projected away from the nuclease without any direct contacts. Finally, Cas12f1, as a miniature version of other Cas12 nucleases, dimerizes to form the typical bilobed architecture that recognizes one crRNA:tracrRNA complex (98, 106). Both lobes interact extensively with the sgRNA but to varying degrees. For instance, the pseudoknot interacts with only one nuclease monomer, while the stem-loop sandwiched between the divided regions of the crRNA:tracrRNA duplex interacts with both monomers. These few structures highlight the diversity of mechanisms Cas12 nucleases employ to recognize the crRNA:tracrRNA complex, and new modalities likely await discovery as other Cas12 nucleases are crystallized.
THE tracrRNA IN CRISPR TECHNOLOGIES
Beyond its natural functions, CRISPR-Cas systems have been a seemingly unlimited source of CRISPR technologies. The technological potential of these immune systems was clear as the original mechanisms emerged around 2010. However, much of the early characterization work focused on Type I and III systems that would require three to eight proteins along with designed crRNAs for any application (67). Studies of Type II systems instead offered the most expedient path to CRISPR technologies, as these systems rely on one Cas protein for immune defense, and the next discovered single-effector nuclease (Cas12a) came much later (112). Utilizing Cas9 initially required two RNAs as well as RNase III (or an equivalent ribonuclease). While processed crRNA:tracrRNA complexes have been widely used with delivered ribonucleoprotein complexes, expressing these various components in cells represented a notable but surmountable hurdle (18). Fortunately, a simple workaround was immediately apparent: fusing the processed repeat:anti-repeat duplex with a stable tetraloop (Figure 5a) (48). The resulting single-guide RNA, or sgRNA, still contained the final processed crRNA:tracrRNA complex bound by Cas9 but as one RNA species. With this addition, RNA-directed DNA targeting by Cas9 could be achieved with only two components. The Cas9-sgRNA combination quickly became the standard for implementing CRISPR technologies. The concept of fusing the processed crRNA:tracrRNA complex through its duplex also readily applied to the tracrRNA-dependent Cas12 nucleases, allowing many of these nucleases to also be harnessed as two-component CRISPR technologies (53, 64, 94, 100).
Figure 5. tracrRNA technologies.
(a) Creation of the single-guide RNA (sgRNA). The minimal region of the crRNA:tracrRNA duplex is fused with a short tetraloop. The resulting sgRNA only needs to be paired with Cas9 to achieve targeted DNA binding and cleavage. In contrast, using Cas9 and a CRISPR array also requires the tracrRNA and RNase III. (b) Co-expressing multiple sgRNAs. sgRNAs lose the inherent multiplexing capabilities of CRISPR arrays because the tracrRNA is fused to the crRNA. However, different workarounds have been developed to co-express multiple sgRNAs, including arrayed expression constructs and single transcripts with multiple sgRNAs and intervening cleavage domains. (c) Extensively modifying the sgRNA sequence. Almost all of the sgRNA can be altered as long as the overall secondary structure is maintained. These sequence alterations can improve DNA targeting by improving folding or allowing the synthesis of linear DNA encoding extremely long sgRNA arrays. Different domains within the sgRNA can also be extended without interfering with Cas9 binding or DNA targeting. (d) Extending the tracrRNA to localize different effector domains to Cas9 targets. Aptamer domains are fused to the 3′ end of the tracrRNA and the cognate protein ligands are fused to the effector domains. The guide sequence therefore determines which effector domains are localized to a specific DNA locus. (e) sgRNA switches engineered by extending the tracrRNA tail. Binding of the sensed RNA trigger (brown) drives the sgRNA switch into a conformation incompatible with Cas9 recognition and subsequent DNA targeting. (f) Reprogramming the tracrRNA to create RNA-derived non-canonical crRNAs (ncrRNAs). The reprogrammed tracrRNAs (Rptrs) contain an altered anti-repeat domain designed to base pair with an RNA-of-interest, recreating the standard crRNA:tracrRNA duplex. Cas9 recognizes the complex and utilizes the resulting ncrRNAs for DNA targeting. As a result, DNA targeting occurs only if the RNA-of-interest is present. (g) The Rptr-based platform for multiplexable RNA detection. The platform, called LEOPARD (Leveraging Engineering tracrRNAs and On-target DNAs for Parallel RNA Detection), combines Rtprs and associated DNA targets with a pool of RNAs, and the DNA targets are monitored to determine whether the RNAs-of-interest are present in the sample.
While the critical contribution of sgRNAs to advancing CRISPR technologies cannot be overstated, their implementation came with an immediate drawback: loss of multiplexing inherent to CRISPR arrays. CRISPR arrays encode and produce large numbers of crRNAs from a singular compact locus. For Type II and many Type V systems, the tracrRNA serves as the processing element to proceed from a long pre-crRNA to individual crRNAs. As a result, fusing the crRNA:tracrRNA duplex prevented the architecture of the CRISPR array from being readily used to express multiple sgRNAs. This challenge gave way to numerous workarounds that could achieve multiplexing with sgRNAs (Figure 5b) (see (69) for an in-depth review). One common approach has been fusing sgRNA expression constructs, with each construct encoding a promoter, sgRNA, and transcriptional terminator (52, 86). The resulting constructs were much larger than the standard spacer-repeat subunit of a CRISPR array, although they could be spliced together using established cloning approaches. An alternative and more compact approach involved separating multiple sgRNAs with RNA cleavage sites. Different types of cleavage sites have been employed, including ribozymes that undergo self-cleavage without accessory factors (33), the repeat hairpin from I-F CRISPR-Cas systems cleaved by the Csy4/Cas6 processing enzyme (101), and immature tRNAs processed by RNase P and RNase Z (80, 107). While these approaches have become standard for CRISPR-based multiplexing in eukaryotes, CRISPR arrays and the tracrRNA are still regularly employed in bacteria (19, 47, 60).
The ability to multiplex with an expanding collection of CRISPR technologies spurred interest in implementing multiple technologies in the same cellular environment. However, using different versions of the same Cas nuclease (e.g. Cas9 and dCas9) faced immediate complications, as the two nucleases cannot differentiate between sgRNAs intended for different functions. Insights into the orthogonal recognition of the crRNA:tracrRNA complex became the immediate solution. By utilizing two phylogenetically distant Cas9 nucleases, more than one function could be implemented at one time. Examples have included achieving DNA cleavage and gene regulation in the same cell (29, 61), enhancing editing through proximal binding (13), and performing combinatorial genetic screens (73). Later work established other innovative workarounds centered around engineering the sgRNA so one Cas nuclease could be used for multiple functions (21, 56, 63), although use of orthogonal Cas nucleases remains a popular approach.
THE tracrRNA AS AN ENGINEERING PLATFORM
The original suite of CRISPR technologies relied heavily on sgRNAs directly derived from the fused crRNA:tracrRNA complex. However, new generations of these technologies treated the sgRNA not as a fixed scaffold for guide sequences but as an engineering platform that could be extensively modified. One common insight from structural studies and mutational analyses was that the crRNA-tracrRNA complex is bound by few sequence restrictions as long as the general secondary structure is maintained and all domains were present (6, 42, 77). One study combined these insights along with machine learning and three rounds of testing to create design rules for synthetic sgRNA scaffolds (84). The resulting set of scaffolds maintained recognition and DNA targeting by SpyCas9 but bore little resemblance to each other (Figure 5c). By combining different sgRNA scaffolds, a large set of sgRNA expression constructs could be synthesized as a single piece of DNA--a feat that would have been impossible with unmodified sgRNAs given the repetitiveness of the resulting DNA. In some cases, altered scaffolds exhibited enhanced DNA targeting for sgRNAs as well as crRNA:tracrRNA duplexes (22, 84, 88). Similar approaches are now being applied to guide RNA scaffolds utilized by other Cas nucleases (24).
Beyond modifying the sequence of the sgRNA scaffold, the scaffold can accommodate extensions to imbue the sgRNA with new functionalities. These extensions could be added to locations on the crRNA or tracrRNA portions of the sgRNA represented highly flexible regionsor extending beyond the minimal region bound by Cas9, including the 5′ end of the sgRNA guide, the upper stem of the repeat:anti-repeat duplex, the top of the nexus, and the 3′ end of the tracrRNA tail (Figure 5c). Most extensions had little impact on DNA targeting (25, 66, 76, 111), although extending the 5′ end of the sgRNA guide even a few nts has been associated with reduced targeting activity (71). The most widely adopted approach involves extending the sgRNA with RNA aptamers that recruit proteins to a target DNA locus (Figure 5d). The proteins principally involve fusions of an aptamer’s peptide ligand fused to an effector module, such as an epigenetic or transcriptional modifier for gene regulation or a fluorescent protein for DNA or RNA imaging (25, 66, 111). Because different aptamers can be fused with different copy numbers and combinations, individual sgRNAs can dictate functionally and quantitatively different effects at a given locus, even with the same Cas nuclease. Beyond introducing aptamers, sgRNAs have been extended to render DNA targeting by Cas9 dependent on the presence of another RNA (Figure 5e) (32, 36, 51, 92). This “RNA trigger” base pairs with the extended portion of the engineered sgRNA “switch,” driving a conformational change that either allows or prevents Cas9 recognition or DNA targeting. A similar concept was applied to fuse sgRNAs with aptazymes, rendering DNA targeting dependent on a small molecule (58, 99).
These examples highlight opportunities to engineer the tracrRNA for CRISPR technologies, although they all rely on an sgRNA or crRNA:tracrRNA complex. In contrast, the discovery of ncrRNAs (Figure 3d) offered a distinct opportunity for tracrRNA engineering (5). Specifically, reprogramming the tracrRNA anti-repeat to base pair with a selected cellular RNA drove the generation of an ncrRNA and subsequent sequence-specific DNA targeting by Cas9 (Figure 5f) (5). These reprogrammed tracrRNAs, or Rptrs, open numerous applications in which a CRISPR technology can be rendered fully dependent on the presence of a selected RNA. The most immediate application involved cleaving a matching DNA target as a specific readout of the corresponding RNA in a sample, forming a multiplexable diagnostic platform called LEOPARD (Leveraging Engineered tracrRNAs and On-target DNAs for Parallel RNA detection). LEOPARD was used to sense multiple respiratory viruses including SARS-CoV-2, while the sequence-specificity of Cas9 allowed LEOPARD to readily differentiate a single point mutation distinguishing SARS-CoV-2 and its dominant D614G variant in patient samples.
tracrRNA BIOLOGY AND TECHNOLOGIES GOING FORWARD
Clearly, our understanding of tracrRNA biology and its use in CRISPR technologies has advanced tremendously since the tracrRNA’s discovery roughly a decade ago. At the same time, there are ample opportunities to further explore the biology of tracrRNAs and how they can be further harnessed for CRISPR technologies. One opportunity is within crRNA biogenesis. While the basic steps were elucidated with the original discovery of the tracrRNA, there are still fundamental aspects that remain poorly understood. For instance, while the tracrRNA, crRNA, Cas9, RNase III, and other host RNases participate in formation of the final effector complex, the order of events remains largely unknown. The abundance of crRNAs from an array also varies widely, likely impacting the extent of immune defense conferred by each crRNA. However, the underlying mechanisms remain poorly explored (20, 62, 83). Another opportunity centers around the structures of Cas9 and Cas12 nucleases bound to the crRNA:tracrRNA complex. The few available structures (Table S1) have revealed varying shapes and folds that allow the nuclease to selectively distinguish the crRNA:tracrRNA complex from the rest of the RNA milieu. These structures cannot be readily predicted with standard RNA folding algorithms, requiring additional structural studies. The few characterized Cas12 nucleases also suggest an incredible range of structures that could be found within the diversity of Cas9 nucleases (34). Finally, the combination of RNA targeting by Cas9, the regulatory role of the scaRNA in by Francisella, and the generation of ncrRNAs in Campylobacter suggest that the tracrRNA could participate in roles extending beyond immune defense (104). Beyond further uncovering tracrRNA biology, the inherent importance of the tracrRNA in sgRNAs lends to any new insights being translated into new and improved CRISPR technologies. The engineering of Rptrs opens up additional opportunities beyond multiplexable RNA detection and could be extended to the growing set of tracrRNA-dependent Cas12 nucleases. Overall, the study and engineering of tracrRNAs remains a fruitful source for biological discovery and beneficial technologies.
Supplementary Material
Acknowledgments
We thank Natalia Luise Peeck for help illustrating the Cas12 structures. This work was supported in part through a European Research Council Consolidator Award (865973 to C.L.B.) and the SPP2141 priority program of the Deutsche Forschungsgemeinschaft (BE 6703/1-1 to C.L.B.).
Footnotes
Conflicts Of Interest
C.L.B. is a co-founder and member of the scientific advisory board for Locus Biosciences and a member of the scientific advisory board for Benson Hill. C.L.B. has filed multiple patent applications related to CRISPR technologies.
References
- 1.Acharya S, Mishra A, Paul D, Ansari AH, Azhar M, et al. Cas9 interrogates genomic DNA with very high specificity and can be used for mammalian genome editing. Proc Natl Acad Sci U S A. 2019;116(42):20959–68. doi: 10.1073/pnas.1818461116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513(7519):569–73. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–12. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 4.Batra R, Nelles DA, Pirie E, Blue SM, Marina RJ, et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell. 2017;170(5):899–912.:e10. doi: 10.1016/j.cell.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao C, Sharma S, Dugar G, Peeck NL, Bischler T, et al. Non-canonical crRNAs derived from host transcripts enable multiplexable RNA detection by Cas9. doi: 10.1126/science.abe7106. (revision submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briner AE, Donohoue PD, Gomaa AA, Selle K, Slorach EM, et al. Guide RNA functional modules direct Cas9 activity and orthogonality. Mol Cell. 2014;56(2):333–39. doi: 10.1016/j.molcel.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Briner AE, Henriksen ED, Barrangou R. Prediction and validation of native and engineered Cas9 guide sequences. Cold Spring Harb Protoc. 2016;2016(7) doi: 10.1101/pdb.prot086785. [DOI] [PubMed] [Google Scholar]
- 8.Brouns SJJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJH, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321(5891):960–64. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein D, Harrington LB, Strutt SC, Probst AJ, Anantharaman K, et al. New CRISPR-Cas systems from uncultivated microbes. Nature. 2017;542(7640):237–41. doi: 10.1038/nature21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carte J, Pfister NT, Compton MM, Terns RM, Terns MP. Binding and cleavage of CRISPR RNA by Cas6. RNA. 2010;16(11):2181–88. doi: 10.1261/rna.2230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22(24):3489–96. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang N, Sun C, Gao L, Zhu D, Xu X, et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 2013;23(4):465–72. doi: 10.1038/cr.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Ding X, Feng Y, Seebeck T, Jiang Y, Davis GD. Targeted activation of diverse CRISPR-Cas systems for mammalian genome editing via proximal CRISPR targeting. Nat Commun. 2017;8:14958. doi: 10.1038/ncomms14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–39. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho SW, Kim S, Kim JM, Kim J-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology. 2013;31(3):230–32. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 16.Chylinski K, Le Rhun A, Charpentier E. The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol. 2013;10(5):726–37. doi: 10.4161/rna.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chyou T-Y, Brown CM. Prediction and diversity of tracrRNAs from type II CRISPR-Cas systems. RNA Biol. 2019;16(4):423–34. doi: 10.1080/15476286.2018.1498281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong L, Ran FA, Cox D, Lin S, Barretto R, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cress BF, Toparlak ÖD, Guleria S, Lebovich M, Stieglitz JT, et al. CRISPathBrick: modular combinatorial assembly of Type II-A CRISPR arrays for dCas9-mediated multiplex transcriptional repression in E. coli. ACS Synth Biol. 2015;4(9):987–1000. doi: 10.1021/acssynbio.5b00012. [DOI] [PubMed] [Google Scholar]
- 20.Creutzburg SCA, Wu WY, Mohanraju P, Swartjes T, Alkan F, et al. Good guide, bad guide: spacer sequence-dependent cleavage efficiency of Cas12a. Nucleic Acids Res. 2020;48(6):3228–43. doi: 10.1093/nar/gkz1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlman JE, Abudayyeh OO, Joung J, Gootenberg JS, Zhang F, Konermann S. Orthogonal gene knockout and activation with a catalytically active Cas9 nuclease. Nat Biotechnol. 2015;33(11):1159–61. doi: 10.1038/nbt.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang Y, Jia G, Choi J, Ma H, Anaya E, et al. Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol. 2015;16(1):1–10. doi: 10.1186/s13059-015-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–7. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeWeirdt PC, Sanson KR, Sangree AK, Hegde M, Hanna RE, et al. Optimization of AsCas12a for combinatorial genetic screens in human cells. Nat Biotechnol. 2021;39(1):94–104. doi: 10.1038/s41587-020-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C, Fontana J, Patel A, Carothers JM, Zalatan JG. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat Commun. 2018;9(1):2489. doi: 10.1038/s41467-018-04901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooley SK, Baken EK, Moss W, Howe A, Young JK. Identification and evolution of Cas9 tracrRNAs. BioRxiv. 2020 doi: 10.1101/2020.09.02.279885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugar G, Herbig A, Förstner KU, Heidrich N, Reinhardt R, et al. High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates. PLoS Genet. 2013;9(5):e1003495. doi: 10.1371/journal.pgen.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dugar G, Leenay RT, Eisenbart SK, Bischler T, Aul BU, et al. CRISPR RNA-dependent binding and cleavage of endogenous RNAs by the Campylobacter jejuni Cas9. Mol Cell. 2018;69(5):893–905.:e7. doi: 10.1016/j.molcel.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, Church GM. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods. 2013;10(11):1116–21. doi: 10.1038/nmeth.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonfara I, Le Rhun A, Chylinski K, Makarova KS, Lécrivain A-L, et al. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42(4):2577–90. doi: 10.1093/nar/gkt1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 2016;532(7600):517–21. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- 32.Galizi R, Duncan JN, Rostain W, Quinn CM, Storch M, et al. Engineered RNA-Interacting CRISPR guide RNAs for genetic sensing and diagnostics. CRISPR J. 2020;3(5):398–408. doi: 10.1089/crispr.2020.0029. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Zhao Y. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol. 2014;56(4):343–49. doi: 10.1111/jipb.12152. [DOI] [PubMed] [Google Scholar]
- 34.Gasiunas G, Young JK, Karvelis T, Kazlauskas D, Urbaitis T, et al. A catalogue of biochemically diverse CRISPR-Cas9 orthologs. Nat Commun. 2020;11(1):5512. doi: 10.1038/s41467-020-19344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194(4):1029–35. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanewich-Hollatz MH, Chen Z, Hochrein LM, Huang J, Pierce NA. Conditional guide RNAs: programmable conditional regulation of CRISPR/Cas function in bacterial and mammalian cells via dynamic RNA nanotechnology. ACS Cent Sci. 2019;5(7):1241–49. doi: 10.1021/acscentsci.9b00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma E, et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362(6416):839–42. doi: 10.1126/science.aav4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrington LB, Ma E, Chen JS, Witte IP, Gertz D, et al. A scoutRNA Is required for some Type V CRISPR-Cas systems. Mol Cell. 2020;79(3):416–24.:e5. doi: 10.1016/j.molcel.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329(5997):1355–58. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heler R, Samai P, Modell JW, Weiner C, Goldberg GW, et al. Cas9 specifies functional viral targets during CRISPR-Cas adaptation. Nature. 2015;519(7542):199–202. doi: 10.1038/nature14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirano H, Gootenberg JS, Horii T, Abudayyeh OO, Kimura M, et al. Structure and engineering ofFrancisella novicidaCas9. Cell. 2016;164(5):950–61. doi: 10.1016/j.cell.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–32. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huai C, Li G, Yao R, Zhang Y, Cao M, et al. Structural insights into DNA cleavage activation of CRISPR-Cas9 system. Nat Commun. 2017;8(1):1375. doi: 10.1038/s41467-017-01496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature Biotechnology. 2013;31(3):227–29. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, et al. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016;351(6275):867–71. doi: 10.1126/science.aad8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015;348(6242):1477–81. doi: 10.1126/science.aab1452. [DOI] [PubMed] [Google Scholar]
- 47.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnology. 2013;31(3):233–39. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343(6176):1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin M, Garreau de Loubresse N, Kim Y, Kim J, Yin P. Programmable CRISPR-Cas repression, activation, and computation with sequence-independent targets and triggers. ACS Synth Biol. 2019;8(7):1583–89. doi: 10.1021/acssynbio.9b00141. [DOI] [PubMed] [Google Scholar]
- 52.Kabadi AM, Ousterout DG, Hilton IB, Gersbach CA. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014;42(19):e147. doi: 10.1093/nar/gku749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karvelis T, Bigelyte G, Young JK, Hou Z, Zedaveinyte R, et al. PAM recognition by miniature CRISPR-Cas12f nucleases triggers programmable double-stranded DNA target cleavage. Nucleic Acids Res. 2020;48(9):5016–23. doi: 10.1093/nar/gkaa208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karvelis T, Gasiunas G, Miksys A, Barrangou R, Horvath P, Siksnys V. crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA Biol. 2013;10(5):841–51. doi: 10.4161/rna.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karvelis T, Young JK, Siksnys V. A pipeline for characterization of novel Cas9 orthologs. Methods Enzymol. 2019;616:219–401. doi: 10.1016/bs.mie.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Kiani S, Chavez A, Tuttle M, Hall RN, Chari R, et al. Cas9 gRNA engineering for genome editing, activation and repression. Nat Methods. 2015;12(11):1051–54. doi: 10.1038/nmeth.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koonin EV, Makarova KS. CRISPR-Cas: evolution of an RNA-based adaptive immunity system in prokaryotes. RNA Biol. 2013;10(5):679–86. doi: 10.4161/rna.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kundert K, Lucas JE, Watters KE, Fellmann C, Ng AH, et al. Controlling CRISPR-Cas9 with ligand-activated and ligand-deactivated sgRNAs. Nat Commun. 2019;10(1):1–11. doi: 10.1038/s41467-019-09985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leenay RT, Beisel CL. Deciphering, communicating, and engineering the CRISPR PAM. J Mol Biol. 2017;429(2):177–91. doi: 10.1016/j.jmb.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leenay RT, Vento JM, Shah M, Martino ME, Leulier F, Beisel CL. Genome editing with CRISPR-Cas9 inLactobacillus plantarumrevealed that editing outcomes can vary across strains and between methods. Biotechnol J. 2019;14(3):e1700583. doi: 10.1002/biot.201700583. [DOI] [PubMed] [Google Scholar]
- 61.Lian J, HamediRad M, Hu S, Zhao H. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system. Nat Commun. 2017;8(1):1688. doi: 10.1038/s41467-017-01695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao C, Ttofali F, Slotkowski RA, Denny SR, Cecil TD, et al. Modular one-pot assembly of CRISPR arrays enables library generation and reveals factors influencing crRNA biogenesis. Nat Commun. 2019;10(1):2948. doi: 10.1038/s41467-019-10747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G, Yin K, Zhang Q, Gao C, Qiu J-L. Modulating chromatin accessibility by transactivation and targeting proximal dsgRNAs enhances Cas9 editing efficiency in vivo. Genome Biol. 2019;20(1):145. doi: 10.1186/s13059-019-1762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J-J, Orlova N, Oakes BL, Ma E, Spinner HB, et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature. 2019;566(7743):218–23. doi: 10.1038/s41586-019-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L, Chen P, Wang M, Li X, Wang J, et al. C2c1-sgRNA complex structure reveals RNA-guided DNA cleavage mechanism. Mol Cell. 2017;65(2):310–22. doi: 10.1016/j.molcel.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 66.Ma H, Tu L-C, Naseri A, Chung Y-C, Grunwald D, et al. CRISPR-Sirius: RNA scaffolds for signal amplification in genome imaging. Nat Methods. 2018;15(11):928–31. doi: 10.1038/s41592-018-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nature Reviews Microbiology. 2020;18(2):67–83. doi: 10.1038/s41579-019-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mali P, Yang L, Esvelt KM, Aach J, Guell M, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–26. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarty NS, Graham AE, Studená L, Ledesma-Amaro R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat Commun. 2020;11(1):1281. doi: 10.1038/s41467-020-15053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meeske AJ, Marraffini LA. RNA guide complementarity prevents self-targeting in Type VI CRISPR systems. Mol Cell. 2018;71(5):791–801.:e3. doi: 10.1016/j.molcel.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullally G, van Aelst K, Naqvi MM, Diffin FM, Karvelis T, et al. 5′ modifications to CRISPR–Cas9 gRNA can change the dynamics and size of R-loops and inhibit DNA cleavage. Nucleic Acids Research. 2020;48(12):6811–23. doi: 10.1093/nar/gkaa477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mullard A. CRISPR pioneers win Nobel prize. Nat Rev Drug Discov. 2020;19(12):827. doi: 10.1038/d41573-020-00198-7. [DOI] [PubMed] [Google Scholar]
- 73.Najm FJ, Strand C, Donovan KF, Hegde M, Sanson KR, et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol. 2018;36(2):179–89. doi: 10.1038/nbt.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nelles DA, Fang MY, O’Connell MR, Xu JL, Markmiller SJ, et al. Programmable RNA tracking in live cells with CRISPR/Cas9. Cell. 2016;165(2):488–96. doi: 10.1016/j.cell.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicholson AW. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip Rev RNA. 2014;5(1):31–48. doi: 10.1002/wrna.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishimasu H, Cong L, Yan WX, Ran FA, Zetsche B, et al. Crystal structure ofStaphylococcus aureusCas9. Cell. 2015;162(5):1113–26. doi: 10.1016/j.cell.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156(5):935–49. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Connell MR, Oakes BL, Sternberg SH, East-Seletsky A, Kaplan M, Doudna JA. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516(7530):263–66. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20(8):490–507. doi: 10.1038/s41580-019-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Port F, Bullock SL. Augmenting CRISPR applications in Drosophila with tRNA-flanked sgRNAs. Nat Methods. 2016;13(10):852–54. doi: 10.1038/nmeth.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price AA, Sampson TR, Ratner HK, Grakoui A, Weiss DS. Cas9-mediated targeting of viral RNA in eukaryotic cells. Proc Natl Acad Sci U S A. 2015;112(19):6164–69. doi: 10.1073/pnas.1422340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ratner HK, Escalera-Maurer A, Le Rhun A, Jaggavarapu S, Wozniak JE, et al. Catalytically active Cas9 mediates transcriptional interference to facilitate bacterial virulence. Mol Cell. 2019;75(3):498–510.:e5. doi: 10.1016/j.molcel.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reimann V, Alkhnbashi OS, Saunders SJ, Scholz I, Hein S, et al. Structural constraints and enzymatic promiscuity in the Cas6-dependent generation of crRNAs. Nucleic Acids Res. 2017;45(2):915–25. doi: 10.1093/nar/gkw786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reis AC, Halper SM, Vezeau GE, Cetnar DP, Hossain A, et al. Simultaneous repression of multiple bacterial genes using nonrepetitive extra-long sgRNA arrays. Nat Biotechnol. 2019;37(11):1294–1301. doi: 10.1038/s41587-019-0286-9. [DOI] [PubMed] [Google Scholar]
- 85.Rousseau BA, Hou Z, Gramelspacher MJ, Zhang Y. Programmable RNA cleavage and recognition by a natural CRISPR-Cas9 system from Neisseria meningitidis. Mol Cell. 2018;69(5):906–14.:e4. doi: 10.1016/j.molcel.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakuma T, Nishikawa A, Kume S, Chayama K, Yamamoto T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci Rep. 2014;4:5400. doi: 10.1038/srep05400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sampson TR, Saroj SD, Llewellyn AC, Tzeng Y-L, Weiss DS. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497(7448):254–57. doi: 10.1038/nature12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott T, Urak R, Soemardy C, Morris KV. Improved Cas9 activity by specific modifications of the tracrRNA. Sci Rep. 2019;9(1):16104. doi: 10.1038/s41598-019-52616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma CM, Vogel J. Differential RNA-seq: the approach behind and the biological insight gained. Curr Opin Microbiol. 2014;19:97–105. doi: 10.1016/j.mib.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 90.Shen B, Zhang J, Wu H, Wang J, Ma K, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23(5):720–23. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, et al. Discovery and functional characterization of diverse Class 2 CRISPR-Cas systems. Mol Cell. 2015;60(3):385–97. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siu K-H, Chen W. Riboregulated toehold-gated gRNA for programmable CRISPR-Cas9 function. Nat Chem Biol. 2019;15(3):217–20. doi: 10.1038/s41589-018-0186-1. [DOI] [PubMed] [Google Scholar]
- 93.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43(6):880–91. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strecker J, Jones S, Koopal B, Schmid-Burgk J, Zetsche B, et al. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10(1):212. doi: 10.1038/s41467-018-08224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strecker J, Ladha A, Gardner Z, Schmid-Burgk JL, Makarova KS, et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;365(6448):48–53. doi: 10.1126/science.aax9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strutt SC, Torrez RM, Kaya E, Negrete OA, Doudna JA. RNA-dependent RNA targeting by CRISPR-Cas9. Elife. 2018;7:e32724. doi: 10.7554/eLife.32724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun W, Yang J, Cheng Z, Amrani N, Liu C, et al. Structures ofNeisseria meningitidisCas9 complexes in catalytically poised and anti-CRISPR-inhibited states. Mol Cell. 2019;76(6):938–52.:e5. doi: 10.1016/j.molcel.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeda SN, Nakagawa R, Okazaki S, Hirano H, Kobayashi K, et al. Structure of the miniature type V-F CRISPR-Cas effector enzyme. Mol Cell. 2021;81(3):558–70.:e3. doi: 10.1016/j.molcel.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 99.Tang W, Hu JH, Liu DR. Aptazyme-embedded guide RNAs enable ligand-responsive genome editing and transcriptional activation. Nat Commun. 2017;8:15939. doi: 10.1038/ncomms15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teng F, Cui T, Feng G, Guo L, Xu K, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018;4:63. doi: 10.1038/s41421-018-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, et al. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol. 2014;32(6):569–76. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–18. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei Y, Terns RM, Terns MP. Cas9 function and host genome sampling in Type II-A CRISPR-Cas adaptation. Genes Dev. 2015;29(4):356–61. doi: 10.1101/gad.257550.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wimmer F, Beisel CL. CRISPR-Cas systems and the paradox of self-targeting spacers. Front Microbiol. 2019;10:3078. doi: 10.3389/fmicb.2019.03078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Workman RE, Pammi T, Nguyen BTK, Graeff LW, Smith E, et al. A natural single-guide RNA repurposes Cas9 to autoregulate CRISPR-Cas expression. Cell. 2021;184(3):675–88.:e19. doi: 10.1016/j.cell.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 106.Xiao R, Li Z, Wang S, Han R, Chang L. Structural basis for the dimerization-dependent CRISPR-Cas12f nuclease. BioRxiv. 2020 doi: 10.1101/2020.12.22.424058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie K, Minkenberg B, Yang Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci U S A. 2015;112(11):3570–75. doi: 10.1073/pnas.1420294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamada M, Watanabe Y, Gootenberg JS, Hirano H, Ran FA, et al. Crystal structure of the minimal Cas9 from Campylobacter jejuni reveals the molecular diversity in the CRISPR-Cas9 systems. Mol Cell. 2017;65(6):1109–21.:e3. doi: 10.1016/j.molcel.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 109.Yang H, Gao P, Rajashankar KR, Patel DJ. PAM-Dependent target DNA recognition and cleavage by C2c1 CRISPR-Cas endonuclease. Cell. 2016;167(7):1814–28.:e12. doi: 10.1016/j.cell.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan WX, Hunnewell P, Alfonse LE, Carte JM, Keston-Smith E, et al. Functionally diverse type V CRISPR-Cas systems. Science. 2019;363(6422):88–91. doi: 10.1126/science.aav7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1-2):339–50. doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–71. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, Heidrich N, Ampattu BJ, Gunderson CW, Seifert HS, et al. Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. Mol Cell. 2013;50(4):488–503. doi: 10.1016/j.molcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y, Zhang H, Xu X, Wang Y, Chen W, et al. Catalytic-state structure and engineering of Streptococcus thermophilus Cas9. Nature Catalysis. 2020;3(10):813–23. [Google Scholar]
- 115.Zuo Z, Zolekar A, Babu K, Lin VJ, Hayatshahi HS, et al. Structural and functional insights into the catalytic state of Cas9 HNH nuclease domain. Elife. 2019;8:e46500. doi: 10.7554/eLife.46500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.