Abstract
Current dogma dictates that during adulthood, endothelial cells (ECs) are locked in an immutable stable homeostatic state. By contrast, herein we show that maintenance of EC fate and function are linked and active processes, which depend on the constitutive cooperativity of only two ETS-transcription factors (TFs) ERG and Fli1. While deletion of either Fli1 or ERG manifest subtle vascular dysfunction, their combined genetic deletion in adult EC results in acute vasculopathy and multiorgan failure, due to loss of EC fate and integrity, hyperinflammation, and spontaneous thrombosis, leading to death. ERG and Fli1 co-deficiency cause rapid transcriptional silencing of pan- and organotypic vascular core genes, with dysregulation of inflammation and coagulation pathways. Vascular hyperinflammation leads to impaired hematopoiesis with myeloid skewing. Accordingly, enforced ERG and FLI1 expression in adult human mesenchymal stromal cells activates vascular programs and functionality enabling engraftment of perfusable vascular network. GWAS-analysis identified vascular diseases are associated with FLI1/Erg mutations. Constitutive expression of ERG and Fli1 uphold EC fate, physiological function, and resilience in adult vasculature; while their functional loss can contribute to systemic human diseases.
Introduction
Endothelial cells (ECs) perform a wide array of physiological functions, maintaining tissue circulation and homeostasis, while constantly adjusting and conforming to inflammatory, metabolic, and hypoxic stressors, as well as accommodating infrastructural, and morphological demands. In adult organs, organotypic ECs coordinate transportation of key nutrients and oxygen, and regulate tissue-specific stem and progenitor cells, while simultaneously fine-tuning coagulation and inflammatory output, through angiocrine signaling1,2. Transcription factors (TFs) that define diversified EC function for several organs have been identified3–5. However, whether the homeostatic functions of tissue-specific adult ECs, including sustaining an EC fate, are executed by a pan-EC set of TFs remains unknown.
The evidence for potential cooperative functions of ERG and Fli1 has emerged from several studies. We have shown that direct conversion of non-vascular embryonic mid-gestation human and mouse cells and of pluripotent stem cell derived epithelium into ECs can be achieved by transient expression of the TF ETV2 followed by sustained overexpression of both ERG and Fli16–8. In these studies, transduction with either ERG or Fli1 individually was not sufficient to convert embryonic cells into ECs. We have also shown that ERG regulates several key EC markers and functions9–12 in addition to being essential for EC fate during development13,14 and stabilizing EC fate during adulthood15. EC-specific deletion of ERG results in embryonic lethality with angiogenic defects in the developing embryo and neonates16,17. During adulthood ERG exerts multiple roles, controlling vascular permeability, hemostasis, endothelial-to-mesenchymal transition, and inflammation15,16,18. Despite its wide-ranging control of vascular homeostasis, at steady state conditions, deletion of ERG in adult mouse ECs does not result with acute loss of vascular integrity and mortality, suggesting cooperativity or compensation by other TFs. Fli1 is the closest homologue to ERG, also constitutively expressed in most ECs in homeostatic conditions. Loss of Fli1 in mouse ECs also does not obliterate EC identity and is well tolerated both in vitro and in vivo, with subtle alterations in specific EC functions in the context of injury, inflammation, and fibrosis19–21. Furthermore, the synergistic developmental murine global deletion of a single allele copy of both ERG and Fli1 in mice propagates a lung inflammatory phenotype22. Thus, we hypothesized that in the adult mammalians, ERG and Fli1 might have synergistic and complementary functions in maintaining global multi-organ EC fate and homeostasis.
To test this hypothesis, we generated a vascular-specific double knock-out deletion model in adult mice by crossing floxed ERGflox/flox mice17 with floxed Fli1flox/flox mice23. Endothelial-specific gene deletion was achieved in adult mice by crossing these double floxed mice with a transgenic EC-specific-Cre Cdh5-CreERT2 mouse line24. We show that conditional deletion of both ERG and Fli1 in adult mice results in a rapid loss of vascular fate, disruption of EC physiological functions, including dysregulation of the homeostatic coagulation balance, breach of barrier function, and excessive inflammatory response, which results in multi-organ failure and death.
Furthermore, we demonstrate that EC genomic program activation and EC-like functions, including balanced expression of key vascular and coagulation factors, and manifestation of proper inflammatory response, can be induced by introduction of both ERG and FLI1 into human adult non-vascular mesenchymal stromal cells. Therefore, during adulthood, mutual constitutive expression of both ERG and Fli1 is necessary to enforce definitive global vascular cell fate and function, maintaining organ cardiovascular homeostasis.
Results
ERG and Fli1 maintain physiological endothelial cell function
Deletion of either ERG or Fli1 during early development results in the disruption of vascular homeostasis leading to fetal lethality. By contrast, while in the adult mice deletion of either ERG or Fli1, results in some organotypic angiogenic aberrations, the mice survive without manifesting acute fatal events15,18,19,22. However, the synergistic contributions of ERG and Fli1 in mammalians to physiological vascular homeostatic functions are unknown. Thus, as we hypothesized that ERG and Fli1 could cooperate in driving essential vascular functions, we deleted both ERG and Fli1 in adult mice, by crossing ERGflox/floxFli1flox/flox with VE-cadherin (Cdh5)-CreERT2/-. EC-specific deletion in adult mice was induced by administration of tamoxifen at 12 weeks of age to generate ERGΔECFli1ΔEC double knock out (dKO) in adult mice (Fig. 1a). We demonstrate a robust EC deletion efficiency for all four ERG and Fli1 alleles in dKO mice (Extended Data Fig. 1, 2).
Figure 1. ERG and Fli1 are essential for the maintenance of in vivo vascular and circulatory homeostatic functions.
a, Schematic representation of the experimental setup for the analysis of ERGΔECFli1ΔEC dKO deletion in adult mice at 12 weeks of age (Control=Ctl). b, Survival curve after the induction of ERGΔECFli1ΔEC dKO in mice using tamoxifen. n = 11 Ctl mice and 10 dKO mice. Student t-test two sided ±SEM. c, Number of circulating platelets (PLT) in control and ERGΔECFli1ΔEC dKO mice at day 12, as determined by HESKA veterinary hematology system. n = 10 mice. Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice. d-f, Hematoxylin and eosin (H&E) staining of brain, kidney, and heart from Ctl and ERGΔECFli1ΔEC dKO mice. Arrows indicate thrombosis and microanatomical defects in the endothelium. n = 5 mice. Bar size represents 200μm. g, H&E staining of liver from Ctl and ERGΔECFli1ΔEC dKO mice. n = 5 mice, bar size represents 500μm. h-k, Quantification of Evans Blue dye leakage in the brain, kidney, heart, and liver of Ctl and ERGΔECFli1ΔEC dKO mice. n = 5 mice. Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM. l, Echography M-mode image of Control and ERGΔECFli1ΔEC dKO mice showing cardiac contraction across time. m, Fractional shortening of cardiac function measure as percentage of blood volume ejected from the heart. n = 6 Ctl mice and 8 dKO mice. Student t-test two sided analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM.
Notably, EC-specific ERG and Fli1 deletion results in rapid mortality between day 10 and day 15 post first tamoxifen injection (Fig. 1b). This rapid demise was multifactorial and manifested in abnormal hematological and biochemical indices. An increase in hemoglobin (HGB), hematocrit (HCT) and total red blood cell (RBC) counts was noted in the blood (Extended Data Fig. 3a-c), indicating an increase in blood viscosity possibly due to vascular leakiness and dehydration. RBCs exhibited a decreased size indicated by their mean corpuscular volume (MCV) and increased corpuscular hemoglobin (MCHC) (Extended Data Fig. 3d-e).
Furthermore, serum analysis revealed liver and kidney dysfunction (Extended Data Fig. 3f-q) in dKO mice. Reduced levels of serum Alkaline Phosphatase (ALP), and elevated Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) (Extended Data Fig. 3f-h), decreased Albumin levels and Albumin/Globulin ratio (Extended Data Fig. 3i-k); indicated impairment of liver function, along with elevated BUN (Blood Urea Nitrogen) to Creatinine ratio (Extended Data Fig. 3l-n), reflecting dehydration and kidney filtration dysfunction. We also noted \ metabolic alterations measured by the changes in Lactate dehydrogenase (LDH), cholesterol and glucose levels (Extended Data Fig. 3o-q).
Blood analysis at day 12 revealed areduction in platelets, that was most probably attributed to platelet consumption (Fig. 1c). Notably, histological analyses of dKO mice organs, just before demise at day 12 post-tamoxifen, revealed macro- and micro-vessels intravascular thrombi and microanatomical defects (Figure 1d-g). These microangiopathies were manifested as vessel occlusion in the brain, RBC infiltration within the heart tissue associated with hemorrhage from vessels with disrupted barrier function, kidney glomerular compaction, and liver vascular congestion due to portal vein and capillary microthrombi (Fig. 1d-g). To assess vascular barrier function in dKO mice, 12 days after tamoxifen injection, we intravenously infused mice with Evans Blue Dye (EBD). A significant increase in vessel permeability was observed in the brain, heart, kidney and liver, confirming in vivo loss of vascular integrity (Fig. 1h-k and Extended Data Fig. 3r).
Alterations in the myocardial EC barrier have been associated with myocardial dysfunction25. Echocardiographic analysis of dKO mice showed decompensation of systolic cardiac function as measured by fractional shortening and end systolic volume (Fig 1l, m and Extended Data Figure 4a-e). The observed onset of multiorgan failure, thrombocytopenia, along with diffused large and small vessel thrombosis, enhanced vascular leakiness, and abridged cardiac function, indicate that the demise of dKO mice might be triggered by a vascular collapse combined with a severe disseminated intravascular coagulopathy (DIC).
ERG and Fli1 sustain vascular programs in adult ECs
To uncover the early events by which loss of ERG and Fli1 leads to global vascular collapse, we performed RNA-seq analysis on isolated ECs from dKO mice at 10 days post tamoxifen induction, from the heart, liver and lung (Fig. 2a). Analysis of the principal component differences across all tissues and samples identified a common differential expression pattern separating control samples from dKO samples in PC3 (Fig. 2b). Gene ontology analysis of the signaling pathways enriched upon these differences identified an enrichment of vascular pathways in the control ECs (Fig. 2c), contrary to an enrichment in blood coagulation and inflammatory signaling in ECs derived from dKO mice (Fig. 2c).
Figure 2. ERG and Fli1 enforce multiorgan and tissue-specific vascular transcriptional programs.
a, Control (Ctl) and dKO mice were treated with tamoxifen as indicated before. At day 10, lung, heart and livers were harvested, and ECs were sorted based on CD45neg, CD31+, VE-cadherin+ markers expression. b, Principal component analysis identifying PC3 as the main driver contributing to the differences between Control and dKO samples. c, Gene ontology analysis of the pathways contributing to the global differences between Control (Right) and dKO (Left) mice, based on the genes composing PC3. d, Gene set enrichment analysis (GSEA) of the vascular enriched list of genes in lung, heart and liver. Results show a decreased expression of vascular set of genes in the dKO mice compared to the Controls across tissues. e, GSEA of the vascular organ specific list of genes set in lung, heart and liver. Results show a decreased expression of these unique sets of genes in the dKO mice compared to the Controls across all tissues. f, Heatmaps representation of pan (upper panel) and organotypic (lower panel) specific vascular genes taken from the endothelial cell enriched and organotypic specific gene set lists. Results exhibit a decreased expression of most pan and tissue specific genes, although a level of heterogenic organotypic response to ERG/Fli1 deletion is noted for distinct tissues.
The mechanism by which deletion of ERG and Fli1 results in multi-organ vascular collapse could be due to an aberrant control of the molecular determinants and responses of EC fate core program, as suggested from gene ontology analysis. To determine to what extent ERG and Fli1 control the pan-EC cell fate and homeostatic programs, we generated a vascular core gene set common to ECs derived from heart, lung, liver, kidney and fat ECs and compared it to a non-hematovascular cell type (fat derived non-EC stromal cells) in order to exclude common housekeeping genes. The EC core gene set was generated based on a log2 fold change >4.5 and FDR<0.001 of the EC genes common across all tissues compared to the non-vascular cell transcriptome and was found to contain 114 genes (Supplementary Table 1 and Supplementary Dataset 1). Notably, this EC enriched gene list contains most of the previously described EC genes, such as VEGFR2 (Kdr), VE-cadherin (Cdh5), Pecam1 (CD31), and Claudin5 (Cldn5), including the TFs ERG and Fli1 (Supplementary Table 1 and Supplementary Dataset 1). Analysis of the expression of pan vascular enriched genes across multiple tissues exhibited decreased enrichment in the dKO samples compared to controls (Fig 2d), confirming that ERG and Fli1 are essential for the maintenance of a vascular program.
Although all ECs share a common vascular program, ECs from each organ specialize and adapt to the requirements of each tissue displaying tissue-specific signatures. . The individual signatures were identified based on a log2 fold change >4.5 and FDR<0.001 of the EC genes uncommon across all tissues. Comparison of the expression of the specific organotypic vascular genes set across multiple organs revealed the loss of vascular organotypic specialization in dKO mice (Fig. 2e). Notably, heatmap analysis of vascular enriched and organ specific genes demonstrates intra-organ heterogeneity in the response to ERG and Fli1 deletion. For example, an opposing increased expression of Clnd5, Gja4 and Kit was observed uniquely in the heart versus other tissues (Fig. 2f). Thus, our analyses confirm that ERG and Fli1 are essential for the maintenance of both vascular and organotypic programs in tissue-specific endothelium.
ERG and Fli1 maintain vascular homeostasis and coagulation
To further explore the extent of dysregulation of the vascular program and collapse after ERG and Fli1 deletion, we performed RNA-seq at earlier and later timepoints, following tamoxifen administrationin the liver endothelium. Principal component analysis of samples from days 7, 10 and 12 show a differential clustering based on PC1 separating control from dKO samples (Fig. 3a and Supplementary Table 2). Gene ontology analysis of the differential pathways enriched in control as compared to dKO mice showed similar trends across distinct time points, with increasing dysregulation of vascular homeostatic pathways over time, measured by increased integrin signaling pathways, chemokine- and cytokine-mediated inflammation signaling pathways, as well as an increase in blood coagulation pathways (Fig. 3b). Analysis of the intra-daily dysregulated gene ontology pathways identified a differential activation response, with blood coagulation and glycolysis pathways within the most significantly altered genes in dKO ECs across all time points (Fig. 3c). Further analysis of the vascular homeostasis pathway identified a continuous decrease in the expression of genes associated with endothelial cell membrane and gap junctions such as Tek, Gja4, Gja5, Kit and Tie1 (Fig. 3d and Extended Data Fig. 5a-c). Similarly, the expression of other vascular homeostatic signaling pathways were also altered such as permeability, tip cell markers, Notch and Igfbps, and alteration of shear stress related genes, including Klf26 and Mef227 TFs (Extended Data Fig 5). Notably, this dysregulation was further affected by an enhanced expression of multiple integrin and matrix regulatory genes, as well as pro-inflammatory signaling pathways with activation of glycolytic pathways (Figure 3d, Extended Data Fig. 5d-o). The increase in several pro-inflammatory mediators, including Bmp1, Gdf10 and Mmp9 (Fig 4c and Extended Data Fig. 5) could explain the observed anomalous inflammatory response and emergence of vascular collapse.
Figure 3. ERG and Fli1 maintain vascular homeostasis and coagulation.
a, Control (Ctl) and dKO mice were treated with tamoxifen as indicated before. At days 7, 10 and 12 livers were collected, and ECs were sorted based on CD45neg, CD31+, VE-cadherin+ markers expression. Principal component analysis shows PC1 separating the samples between Ctl and dKO mice. b, Gene ontology analysis of the pathways contributing to the global differences between Control (Left) and dKO (Right) mice, based on the genes composing PC1. c, Gene ontology analysis of the differentially expressed pathways at days 7, 10 and 12, between Controls and dKO mice. Graphs show pathways enriched in the dKO mice. d, Heatmap analysis presenting the expression pattern of vascular integrity and remodeling genes, glycolysis pathway, and coagulation genes (split as: pro-thrombosis and anti-thrombosis genes), differentially expressed between Ctl and dKO mice. e, Schematic image illustrating the model for the altered signaling pathways between Control and dKO mice, that contribute to dysregulated thrombosis and coagulation cascades.
Figure 4. ERG and Fli1 safeguard vascular chromatin accessibility.
ATAC-seq analysis was performed in control (Ctl) and dKO mice at day 10 after been treated with tamoxifen as indicated before. a, Pie representation of overlapping and uniquely accessible elements among Ctl (blue) and dKO (red) ATAC-seq combined replicates. b, Distribution of peak annotations using ChlPseeker in Ctl and dKO across different genomic locations. Student t-test *p<0.05, **p<0.01, ***p<0.001. c, Top 6 identified enriched domains by HOMER in Ctl and dKO differentially enriched peaks (P-adjusted < 0.01). d, Top 4 Molecular functions associated to Ctl and dKO differentially accessible peaks using GREAT. e, Volcano plot demonstrating highlighted factors from footprinting analysis of dKO samples versus Ctl. The threshold is set at transcription factors with -log10(p-value) above the 95% quantile, a differential binding scores smaller than the 5% quantile, or a differential binding score greater than the 95% quantile. Note increased predicted activity of inflammatory AP-1 TF family members Jun:Fos. f, Aggregate footprint signal plot of Jun:Fos family of genes that are associated with an overall increase inflammatory signaling of flanking chromatin activation in dKO samples (red) and Ctl samples (blue). g, Representative IGV plots demonstrating peaks appearance from RNA-seq, ATAC-seq, and ATAC-seq based footprinting analysis over the genomic locus of the inflammatory induced vascular gene Serpine1. Area labeled with a dotted line mark predicted regulatory enhancer regions upstream to the transcriptional start site (TSS). Note an increased chromatin accessibility for enhancer regions predicted with a footprint target of Fos:Jun motif in dKO samples vs. Ctl samples.
Further analysis of coagulation pathways revealed an unbalanced response of anti-coagulant relative to pro-coagulant signaling pathways starting at day 7, with further enhancement by day 12 (Fig. 3d, e and Extended Data Fig. 5f). In the dKO mice, we observed decreased expression of vascular components contributing to the anti-coagulation and anti-thrombotic signaling pathways, such as Nos3 (eNOS), Thbd, and Tfpi. In parallel, there was an increase in the expression of pro-coagulant and pro-thrombotic response genes, such as Par1-proteinase activator receptor 1 (F2r) and thrombospondin 1 (Thbs1) (Fig. 3d, e). Thus, the morbidity in ERG/Fli1 deficient mice can be also triggered by the profound disruption of the anti-inflammatory and anti-coagulant pathways leading to a systemic physiological collapse. Therefore, ERG and Fli1 are required for the maintenance of multi-organ homeostatic EC functions and interference with the function of these two TFs leads to an imbalanced and inadequate EC physiological control over coagulation, inflammation, and vascular integrity, which culminate in vascular collapse and mortality (Extended Data Fig. 5p).
ERG and Fli1 deficiency augments pro-inflammatory programs
To study the mechanism by which depletion of ERG and Fli1 results in multiorgan loss of vascular attributes, we performed ATAC-seq analysis of control and dKO mice at day 10 post tamoxifen administration, to assess chromatin accessibility rearrangement. Analysis of the global open chromatin peaks identified in control and dKO mice revealed almost 2.5-fold more unique peaks to newly accessible regions in dKO ECs versus unique peaks to accessible chromatin in WT ECs (Fig 4a), associated with a differential clustering of the samples (Extended Data Fig 6a, b). Overall, a global chromatin relaxation was observed in the absence of ERG/Fli1, as we noted enhanced peak signals in dKO ECs (Fig. 4b). Detailed analysis of the regions associated with differential chromatin accessibility shows an increased prevalence for ATAC-seq peak enrichment within the distal intergenic regions (abundant with enhancers) in the dKO ECs (Fig. 4b). This is in line with a previous report demonstrating ERG enrichment at distal genomic regions, in ECs, regulating enhancers and super-enhancers28. Analysis of the transcription factor consensus motifs enriched in the differential peaks using Homer, shows increased abundance of generic EC motifs (i.e. ETS factors) in Control compared to dKO samples; with the latter being enriched for motifs associated with stress response and inflammatory genes (e.g. Atf3, and Jun/Fos) (Fig. 4c). Analysis of the molecular pathways associated with the differential genomic regions show alterations in the vascular signature of dKO ECs (Fig. 4d). Next, to predict alternative TF activity in the absence of ERG/Fli1, we performed “footprinting” analysis of differential ATAC-seq data and detected an increased activity signature of the inflammatory TFs, including AP1, Jun, and Fos family members (Fig. 4e, f). This finding was further confirmed by individual footprinting analysis of FosJunB at the EC inflammatory gene Serpine1. This gene locus exhibited a FosJunB footprint at regulatory enhancer regions upstream of the TSS that were accessible only in dKO ECs, with RNA-seq analysis demonstrating increased transcription at this site (Fig. 4g).
Jun and Fos are downstream targets of multiple pro-inflammatory and pro-coagulation signaling cascades29,30. Notably, upregulated Jun was revealed as a vascular aging promoting factor31, suggesting the abnormal repression of ERG and Fli1 in aged ECs. Jun and Fos compete with ERG for DNA binding; and that decreased ERG levels lead to an increased accessibility for Jun and Fos32–33. Our ATAC-seq data is in agreement with these findings and suggests that loss of Fli1 may compound the effect of ERG loss on chromatin accessibility for Jun and Fos. These findings, in combination with the transcriptome profile of dKO ECs, suggest that Fli1 serves as a secondary safeguard TF for ERG-dependent pathways, without which there is a complete loss of endothelial identity, function, and regulatory functions over inflammatory and coagulation responses.
ERG and Fli1 maintain vascular wall integrity
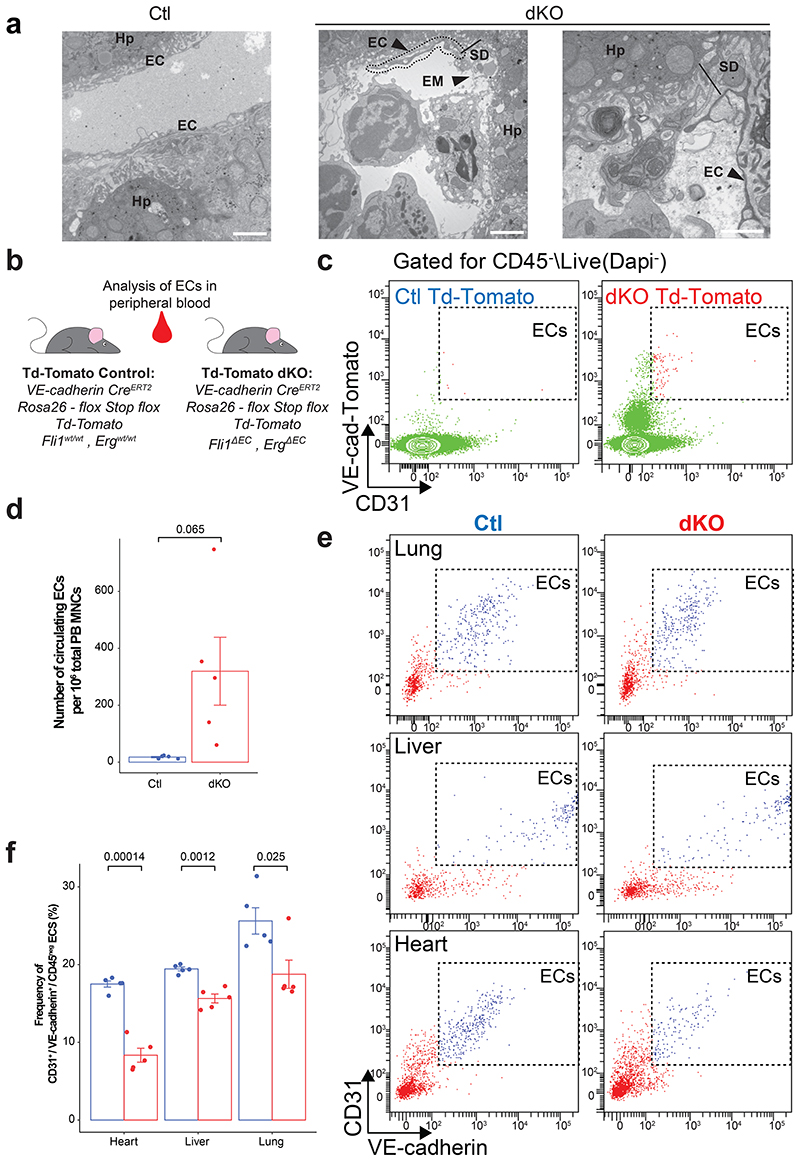
Electron microscopy analysis of the liver vasculature showed that the blood vessels within dKO mice manifested vascular leakiness with prominent gaps along the vessel wall, exposing extracellular matrix to circulation (Fig. 5a). Aberrant adherence of leukocytes to the denuded vessel wall was observed (Fig. 5a), confirming an increased inflammatory phenotype associated with alterations in liver metabolic profile (Extended Data Fig 3f-h). Hence, we hypothesized that ECs in dKO mice lose their intercellular and juxtacellular adhesion integrity and are dislodged into the circulation.
Figure 5. ERG and Fli1 are necessary to maintain in vivo vascular and circulatory integrity.
a, Transmission electron microscopy (TEM) images of Control (Ctl) and ERGΔECFli1ΔEC dKO mouse livers. Hp = hepatocyte, EC = endothelial cells, EM = extracellular matrix exposition, SD = Space of Disse. n = 3, bar size represents 1μm. b, Schematic representation of the experimental setup to generate Td-tomato-ERGAECFli1AEC dKO mice. c-d, Flow cytometry analysis and quantification of circulating ECs following deletion of ERG and Fli1 (Td-tomato-ERGΔECFli1ΔEC dKO mice). n = 5 mice per genotype group. c, Representative flow dot plots cells double positive for CD31 and TdTomato. d, Number of circulating ECs per million of peripheral blood (PB) mononuclear cells (MNCs) as quantified by flow cytometry. Student t-test two sided analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM. e-f, Representative dot plots for tissue resident ECs and quantification of EC frequency per tissue following ERG and Fli1 deletion, as determined by flow cytometry. n = 5 mice per genotype group. Student t-test two sided analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM.
To track the fate of ERG/Fli1 deficient ECs, we backcrossed Cdh5-CreERT2 ERGflox/floxFli1flox/flox mice with a Rosa26-flox-Stop-flox-TdTomato reporter mouse line, to label and track ECs simultaneously following gene deletion induction with tamoxifen (Fig. 5b). Notably, on day 10 post ERG and Fli1 deletion, we observed a significant increase in the numbers of detached, circulating CD31+/Td-Tomato+ ECs in the peripheral blood of dKO mice (Fig. 5c, d). Another population of CD31negative/loW/TdTomatoloW/+ cells, that was observed in the dKO mice, might represent dislodged ECs or ECs exhibiting downregulation of CD31 secondary to ERG/Fli1 deletion (Fig. 5c, d). Quantification of the frequency of CD31+VE-cadherin+ ECs per tissue, revealed a decrease in EC frequency in multiple dKO mice organs (Fig. 5e, f). Therefore, in dKO mice, loss of key adherent and tight junctional molecules, leads to detachment and dissemination of ECs from the blood vessel lumen into circulation. Accordingly, following EC denudation, exposure of extracellular matrix combined with pro-inflammatory and pro-coagulant EC profile, trigger the excessive immune cell infiltration and the observed DIC-like syndrome.
ERG and FLI1 maintain hematopoietic vascular niche function
As the observed physiological dysfunctions in inflammation and coagulation can be also attributed to impaired performance and trafficking of hematopoietic cells, we next examined the bone marrow (BM). We and others have previously shown, that genetic, pharmacologic, or stress-related pathophysiologic peril of vascular barrier function in the BM perturbs vascular niche regulation of hematopoiesis34,35. Increased barrier permeability is associated with altered BM architecture, reduced numbers of hematopoietic stem and progenitor cells (HSPCs), and excessive mobilization to peripheral blood (PB), and skewed myelopoiesis of immune cells. Indeed, the BM of dKO mice show increased vascular barrier permeability at day 12 post-tamoxifen treatment (Fig. 6a). Mobilization of HSPCs was observed in the PB of dKO mice (Fig. 6b-e), and examination of the BM revealed profound architectural destruction accompanied with leakage of red blood cells (RBCs) into the BM parenchyma, along with clot formations in BM vasculature (Fig. 6f). Notably, although PB platelet numbers were dramatically decreased at day 12 (Fig. 1c), BM megakaryocytes maintained their quantitative perivascular location and normal morphology in dKO mice (Fig. 6f), suggesting that platelet numbers are decreased due to coagulopathy-mediated consumption. At day 7 in dKO mice, BM architecture was preserved although RBC leakage into the parenchyma and mini-clot formation were already detected (Extended Data Fig. 7a), with accelerated mobilization to the PB and enhanced circulating platelet levels (Extended Data Fig. 7b, c). We also noted reduced numbers of total BM cells and subsets of HSPCs, beginning at an early point of day 7 and persisting to day 12 (Fig. 6 g-j). Additionally, mature hematopoietic cells in the PB exhibited a significant skewing from lympho- to myelopoiesis at early and late time points (Fig. 6 k-m), associated with unscheduled excessive infiltration of myeloid cells, into peripheral tissues (Extended Data Fig. 7d-g). Therefore, ERG and Fli1 enforce programs for proper vascular niche functions, maintaining tissue homeostasis via proper shepherding of tissue resident HSPCs.
Figure 6. Maintenance of proper BM hematopoiesis by the vascular niche requires endothelial ERG and Fli1 expression.
Samples from control (Ctl) mice are labeled in blue and from dKO mice are labeled in red. n = 5 mice. Student t-test two sided analysis was performed ±SEM. a, Bone tissues of Control and dKO mice at day 12 injected i.v. with EBD. EBD Quantification in the BM of Ctl and dKO mice. b, Number of PB white blood cells (WBC) at day 12 as determined by HESKA veterinary hematology system. c, Number of colony forming units (CFU) per 2X105 PB WBC plated in methylcellulose as scored after 1 week. d, Frequency of PB LSK HSPC was determined by flow cytometry and numbers were calculated relatively to PB WBC counts per mouse. e, Frequency of PB SLAM LSK HSPC was determined by flow cytometry and numbers were calculated relatively to PB WBC counts per mouse. Representative flow dot plot images for the SLAM markers CD150 and CD48 after pre-gating for Ter-119negLSK cells. Red labeled dots represent CD150+CD48neg LSK HSPCs in the PB. f, BM H&E images of of Control and dKO mice at day 12. Enlarged areas display perivascular megakaryocytes. Scale bar = 100 μm. g, Number of BM white blood cells (WBC) as determined by haematocytometer counting using Turk dye. h, Frequency of BM LSK HSPC was determined by flow cytometry and numbers were calculated relatively to BM WBC counts per mouse. i, Frequency of BM SAM LSK HSPC was determined by flow cytometry and numbers were calculated relatively to BM WBC counts per mouse. j, Representative flow density plot images for the SLAM markers CD150 and CD48 after pre-gating for Ter-119negLSK cells. Green labeled areas surrounded by a dotted line represent CD150+CD48neg LSK HSPCs in the BM. k-m, Frequencies of PB lymphocytes, monocytes, and granulocytes as determined by HESKA veterinary hematology system.
ERG and Fli1 uphold physiological vascular EC signature
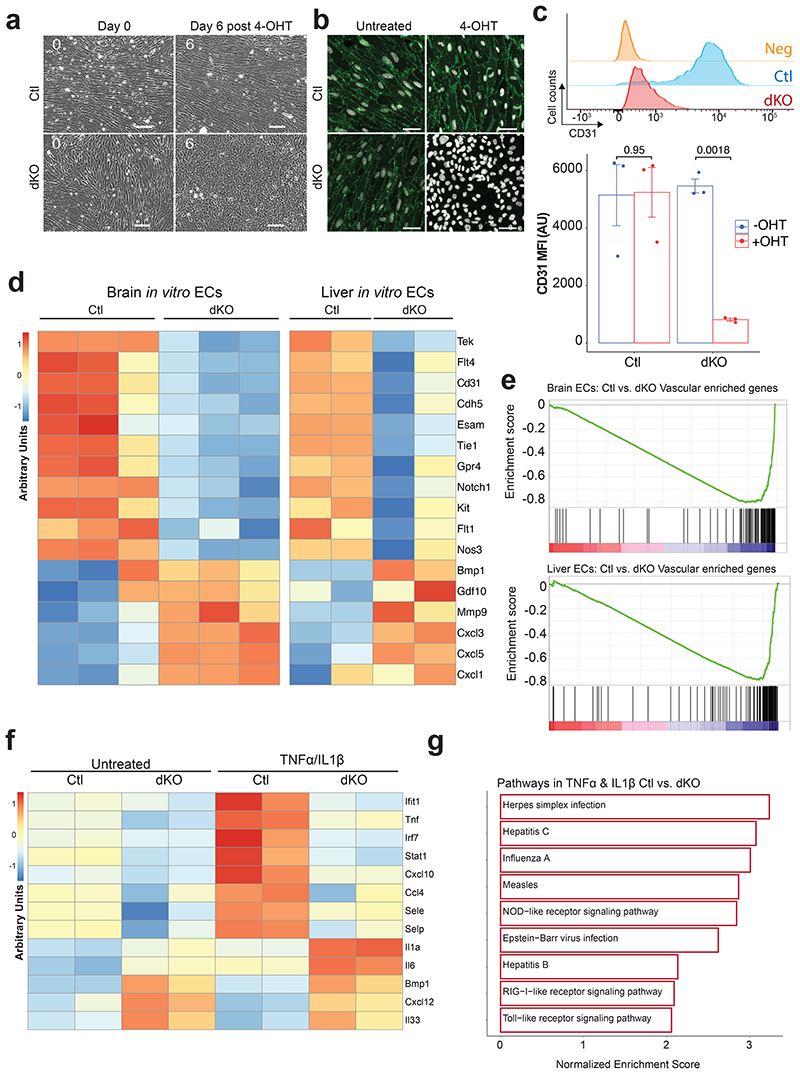
Due to early mortality of dKO mice, it was not feasible to assess whether ECs over time transition to a non-vascular cell fate. To this end, we designed an in vitro culture model, whereby primary CD31+CD45neg ECs from brain and liver of control (Ctl, WT), and dKO mice were purified and cultured ex vivo. Deletion of ERG and Fli1 in vitro was induced by adding 4-hydroxy-Tamoxifen (4-OHT) to the cell culture for two days followed by phenotypic cell analysis at days 6 to 7. Time-lapse image microscopy analysis revealed immediate morphological changes in dKO cultured ECs. A profound decrease in cellular spindle shape appearance and network disorganization was observed among ECs isolated from dKO mice at day 2 (Supplementary Video 1). A complete disruption of vascular EC morphology evolved in dKO ECs by day 6 post-tamoxifen induction (Fig. 7a and Supplementary Video 1). To further investigate the influence of ERG and Fli1, we performed immunofluorescence, flow cytometry, and RNA-seq analysis in Ctl and WT cells in vitro. Significant reduction in CD31, and other vascular specific markers, were observed only after deletion of both ERG and Fli1 in brain and liver ECs (Fig. 7b-d). Thus, ERG and Fli1 have cooperative functions in constitutively enforcing the proper expression of vital vascular specific factors.
Figure 7. ERG and Fli1 are essential for in vitro vascular programs and function maintenance.
a, Time-lapse analysis of the morphological changes observed in ERGΔECFli1ΔEC dKO cells compared to control (Ctl) during 6 days of culture. Images were acquired from the same spatial spots in wells for 6 days. Representative images of ECs isolated from n=3 mice. Scale bar = 100 μm. b, Immunofluorescence of CD31 in ECs from Ctl and ERGΔECFli1ΔEC dKO mice treated with tamoxifen (4-OHT). Representative images of ECs isolated from n=3 mice. Scale bar = 50 μm. c, Bar plots indicating CD31 mean fluorescent intensity (MFI) as measured by flow cytometry and representative flow cytometry histogram plots analysis for the expression levels of CD31 after Tamoxifen (4-OHT) treatment. Ctl and dKO ECs were isolated from n=3 mice. Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM. d, Analysis of vascular gene expression levels in Ctl and dKO brain and liver ECs by RNA-seq, 7-days post 4-OHT treatment. Data is represented as a heatmap of the averaged normalized raw counts per row from n=3 biological replicates per group in the brain samples and n=2 in the liver samples. e, GSEA analysis for the expression levels of EC enriched vascular genes set (see Figure 2), performed on 4-OHT treated Ctl and ERGΔECFli1ΔEC dKO brain and liver ECs. f, Heatmap representation of the average expression levels of vascular inflammatory genes in Ctl and dKO liver ECs following stimulation with 4-OHT, 10 ng/mL IL1β, and 10 ng/mL TNFα. n=2 biological repeats per sample. g, Cultured Ctl and dKO liver ECs were in vitro treated with 10 ng/mL IL1β and 10 ng/mL TNFα for 16 hours and analyzed by RNA-seq. KEGG GO pathway analysis of differentially expressed genes enriched in Ctl versus dKO liver ECs is presented.
RNA-seq analysis also revealed downregulation of EC genes, including Tek and Cldn5, as previously observed in vivo, in dKO cells harvested from brain and liver (Fig. 7d). Notably, in vitro deletion of both TFs in brain and liver ECs resulted in a vascular fate loss when compared to EC enriched gene list (Fig. 7e).
Next, to determine if ERG and Fli1 expression is required to mount a pre-programmed vascular immune response, ECs were stimulated with IL1β and TNFα and assessed for the alterations in their inflammatory response through RNA-seq analysis. Activation of inflammatory pathways was suppressed in dKO cells due to their inability to respond to IL1β and TNFα stimulation. Gene ontology analysis of WT versus dKO cells revealed upregulation of interferon and TNF response signaling pathways involved in the antiviral response and the Toll-like receptor signaling pathway in WT naive ECs, in contrast to dKO cells (Fig. 7f, g). This defect in vascular immune response can be explained by the loss of vascular core EC programs, which executes modular inflammatory-induced responses. Indeed, expression of numerous differentially expressed genes, including the EC-specific inflammatory response genes E-Selectin (Sele) and P-Selectin (Selp), did not increase in TNFα/IL1β stimulated dKO cells as was induced in TNFα/IL1β stimulated WT ECs (Fig. 7f). Hence, ERG and Fli1 duo are required not only for the maintenance of the EC enriched EC signature and proper inflammatory regulation, but also for the physiological activation of EC pre-ordained inflammatory programs, which is lost alongside the EC fate maintenance programs.
To uncover if ERG and/or FLI-1 dysregulation contributes to development of human cardiovascular related disorders, we analyzed the SNPs linked to ERG and FLI1 within ±100kb of their genomic regions in two different GWAS databases: The FinnGen and the UK Biobank. We identified multiple human disorders present in both datasets for each gene (Extended Data Figure 8a, b). Notably, many of these pathologies were directly or indirectly associated to cardiovascular diseases, among them: Diseases of veins, lymphatic vessels and lymph nodes for ERG, or nontraumatic intracranial hemorrhage for Fli 1, having thrombocytopenia and several heart diseases shared by mutations in both genes (Extended Data Figure 8a, b). This data is further supported by previous results observed for Erg motifs associated SNP mutations linked to cardiovascular disorders28. Therefore, ERG and Fli1 could confer potential key roles in the maintenance of human vascular physiological integrity and regulatory in vivo function over inflammation and coagulation.
ERG/FLI1 induce an EC program in mesenchymal stromal cells
Transient transduction of the embryonically expressed ETV2 gene, followed by constitutive expression of both ERG and FLI1, induce an EC fate in human and mouse embryonic fetal stromal and epithelial cells6,7. To test whether ERG and FLI1 by themselves are sufficient to induce a vascular program in adult non-EC stromal cells, we transduced human adult BM-derived mesenchymal stromal cells (MSC) in vitro with lentiviral vectors overexpressing ERG and FLI1. We performed single cell RNA-seq analysis on MSCs overexpressing ERG and FLI1, a week post transfection. Combining both control and ERG/FLI1 treated cells, we detected 7 clusters with differential RNA expression profile (Fig. 8a, b). Analysis of the contribution of each sample to the clusters, revealed a change in the proportions across clusters, with a 100% contribution of ERG/FLI1 overexpressing cells to Cluster 7 (Fig. 8c). Unbiased characterization/classification of the clusters using SingleR reveal the detection of an EC cluster among the cells overexpressing ERG and FLI1 (Fig. 8d). Analysis of EC marker genes across all clusters revealed the ERG/FLI1 unique cluster 7 to be the EC cluster (Fig. 8e). Flow cytometry analysis of CD31 and VE-cadherin EC markers expression demonstrates that over a 21-day period of ERG and FLI1 overexpression, 12-15% of the transduced cells exhibit an EC phenotype (Fig. 8f). Bulk RNA-seq analysis at the end of a 3-weeks culture on sorted ERG/Fli1 overexpressing VE-cadherin+ cells, control MSCs, and human umbilical ECs (HUVECs), showed a very similar expression pattern of genes, including vascular core gene markers, between CD31+ VE-cadherin+ converted cells and HUVECs, as well as a decrease expression of MSCs genes such as TGFp (Fig. 8g, and Supplementary Table 3). Moreover, ERG and FLI1 overexpression in MSCs induced higher levels of pivotal anti-coagulation regulatory gene pathways (Figure 8h, Extended Data Fig. 9a), further supporting the cooperative role of these TFs in the regulation of EC physiological hemostatic function. Thus, overexpression of ERG and FLI1 converted a cluster of mesenchymal cells to a prototypical EC signature. Additionally, to examine if ERG/FLI1-induced MSCs, enable MSCs to mount an inflammatory immune response, we leveraged the transwell hematopoietic migration model. Here, we assessed the ability of mobilized human HSPCs to transmigrate through a monolayer of stromal cells barrier towards a gradient of the chemokine CXCL12/SDF-1, resembling the physiological process of HSPC transendothelial migration and peripheral mobilization. Spontaneous and passive diffusion of FITC-Dextran through a monolayer formed by MSCs or ERG/FLI1 expressing MSCs was used as a control assay for active HSPC migration (Extended Data Fig. 9b). We observed that MSCs with ERG/FLI1 overexpression formed a more restrictive barrier to HSPC migration, compared to control MSCs, and mild inflammatory stimulation could mount the proper immune response in ERG/FLI1-induced MSCs to enhance HSPC transmigration, with no evident effect on control MSC (Extended Data Fig. 9c). Passive permeability assay revealed that this inflammatory stimulation was not optimal to increase barrier permeability of ERG/FLI1-induced MSCs, but it did for control MSCs (Extended Data Fig. 9d). Imaging of stromal monolayers revealed an adherent monolayer formed by ERG/FLI1-induced MSCs, while control MSCs formed a discontinuous layer with gaps in between cells (Extended Data Fig. 9e).
Figure 8. ERG and FLI1 overexpression induces a vascular transcriptional program in adult human mesenchymal stromal cells.
Human BM derived MSC (n=4 healthy donors) were transfected with ERG and FLI1 (ERG/FLI1) or with “empty”-cassette (Ctl) Lentivectors. a, UMAP analysis plot for distinct MSC sub-populations at day 4. b, Identification of genes differentially expressed by each cluster. c, proportions plot showing the differential contribution of Ctl and ERG/FLI1 samples to each cluster. d, SingleR analysis identifies distinct populations of cell types associated to each sample. e, Dot plot analysis demonstrating that cluster 7 has an increased and preferential expression signature of vascular EC genes. f, Representative and Frequencies of VE-cadherin and CD31 expression in treated MSCs. Student t-test two sided analysis was performed comparing Ctl and ERG/Fli1 overexpressing MSCs ±SEM. n = 4 biological samples from independent donors. g, RNA-seq heatmap analysis performed for Ctl and ERG/FLI1 transfected MSCs and for HUVECs at week 3. n = 4 donors each. n = 3 for HUVEC donors. h, Gene Ontology analysis of signaling pathways differentially expressed between ERG/FLI1 MSCs vs. Ctl MSCs. i-k Representative images from n = 3 donors exhibiting tube formation in fibrin gel assay embedded with ERG/FLI1 MSCs and introduced in microfluidics chip devices for 3 days. Bars = 100 μm. (h) Stained with UEA-1 (purple), (i) Stained with anti V-cadherin (BV9 clone, red), and (j) bright field image of blood perfused tubes. l, Transplantation of Matrigel plugs containing Ctl and ERG/FLI1 transfected MSCs into NSG mice. Mice were i.v. injected with anti-human-VE-cadherin antibody (clone BV9, blue color). Matrigel plugs were stained with anti-human-CD31 antibody (green color). Images represent plugs embedded with either Ctl or ERG/FLI1 transfected cells from n=4 donors. Bars = 1000 μM.
To further confirm vascular functionality of converted MSCs, we performed an in vitro tubulogenesis Matrigel assay. ERG/FLI1-induced MSCs self-assembled into vascular networks, while naive MSC failed to do so (Extended Data Fig. 9f). Moreover, once embedded in fibrin gel, and introduced into a microfluidic chip device, MSCs with ERG and FLI1 overexpression self-organized into lumenized tubes, displaying vascular markers, including UEA-1 lectin and VE-cadherin binding. (Fig. 8i, j and Supplementary Video 2). Remarkably, the tubes formed by ERG/FLI1 overexpressing MSCs, were perfusable and able to transport adult human blood (Fig. 8k and Supplementary Videos 3, 4).
Next, we examined the in vivo capacity of ERG/FLI1-induced MSCs to establish vascular networks that can functionally anastomose with the hosts vasculature. Examination of Matrigel plugs, 1-month post-transplantation, showed human ERG/FLI1-induced MSCs vascular networks anastomosed with the mouse circulation being perfused with hosts blood stream (Fig. 8l and Supplementary Video 5). These results are in agreement with the reports indicating that ERG/FLI1 induce EC fate during embryogenesis36. Hence, enforced expression of ERG and FLI1 are sufficient to convert MSCs into cells with vascular EC transcriptional profile and functional vascular capacity.
Discussion
Vascular EC fate determination has been proposed to be acquired and established during development, however little is known about the maintenance of homeostatic and inflammatory EC programs and vascular physiological functionality in adult mammalian organisms. Genetic models have shown that either ERG or Fli1 specify EC lineage during development17,37. Nonetheless, these two factors are not individually essential to maintain the function of adult ECs and can compensate for each other’s loss. While EC Fli1 deficiency is well tolerated without compromise of homeostatic functions19, ERG deletion in ECs causes vascular abnormality with some organ-specific dysfunctions, spontaneous systemic coagulopathy, enhanced inflammation and evidence of endothelial to mesenchymal transition (EndoMT)15. However, EC-specific deletion of ERG in adult ECs does not lead to a dramatic loss of EC physiological functions, or subsequent acute spontaneous mortality. In contrast, we show combined deletion of ERG and Fli1 in adult mice results with the rapid death of these mice, setting forth the essential roles of these two TFs in enforcing vascular fate in adult organs. (Extended Data Fig. 10).
Although the transcriptional control orchestrated by ERG and Fli1 is essential to maintain multi- organ EC programs, the regulation of ERG and Fli1 might be predominantly at the protein level and could serve as an important deregulatory pathway in the onset of cardiovascular diseases. Recent reports have shown a very similar phenotype when Erk1/2 deficient ECs, with mice dying by the 5th week post induction, exhibiting vascular collapse, fibrosis and EndoMT38. The regulation of ERG and Fli1 proteins by Erk and PKC has been previously reported39,40, suggesting that the phenotype observed in EC-specific Erk1/2 deficient mice might be attributed to inactivation of ERG and Fli1. Moreover, inflammatory mediators are involved in regulation of endothelial ERG and Fli1 protein levels32,33. Moreover, maintenance of vascular EC quiescence is an active metabolically regulated process41, which also regulates EndoMT via balancing glycolysis versus fatty acid production within the TCA cycle42, confirming our observations of increased glycolysis following ablation of ERG and Fli1. Manipulation of signaling and metabolic pathways, which balance ERG and Fli1 protein activation and stabilization might be a potential therapeutic avenue for the treatment of vascular disorders.
Our study demonstrates that ERG and Fli1 act constitutively and cooperatively to maintain EC fate that is essential for conserving generic multi-organ vascular functions and integrity of the circulation. This may be relevant to human diseases, where ERG EC expression is decreased or lost, in atherosclerosis43 and chronic liver diseases15. However, in the absence of both ERG and Fli1, no other EC TF is able to compensate for the loss of this duo and thus the vasculature undergoes aberrant activation, damage, and detachment with consequent acute vascular collapse and systemic coagulopathy, resulting in rapid mortality.
Studies have shown that EC shear-dependent TFs Klf2 and Klf4 are also important for the regulation of coagulation and vascular gene expression, and their EC depletion resulted in cardiac failure44. Notably, ERG is required for Klf2 transcriptional activity in the low-shear microvasculature, suggesting a vascular cooperative role between these sets of TFs18. Vascular shear stress dependency on KLF2 and KLF4 are critical for ECs function26. Furthermore, the combined deletion of the TFs Mef2A/C/D27 leads to activation of coagulation cascades similarly to that observed in Klf2/Klf4 double KO, resulting in acute lethality. Temporal analysis on dKO mice have not found to decrease the expression of all these core vascular TFs as a group. Implying for an independent role for these sets of TFs in mandating vascular physiological programs, without the ability to compensate for the loss of each set. Furthermore, contrary to what we observed in dKO mice, Klf2/Klf4 deficient endothelium exhibits decreased glycolytic enrichment, suggesting that the metabolic mechanisms driving both phenotypes are different.
During embryonic development the ephemeral expression of the pioneer transcription factor ETV2, induces EC fate in mesodermal precursor cells45. However, ETV2 is abruptly silenced around mid-gestation and the question of how EC fate is maintained after ETV2 silencing has been under scrutiny. In this study, we have deciphered that EC vascular cell fate maintenance during adulthood is not predetermined by ETV2 but rather is dynamic and requires active cooperation of ERG and Fli1 to sustain the constitutive expression of a pro-EC core gene sets essential for maintenance of global homeostatic EC vascular functions (Extended Data Fig. 10). Furthermore, our in vitro studies show how ERG and Fli1 deletion disrupts ECs both phenotypically and functionality, congruent with the phenotypes observed in siRNA knock down of Fli1 and ERG in human ECs46.
This notion is further supported by our ability to generate EC-like cells by the partial induction of an EC transcriptional program in adult human non-EC mesenchymal stromal cells following introduction of human ERG and FLI1 genes without enforced expression of additional TFs.
The identification of TFs that enforce adult transcriptional and functional EC fate offer insight into the key pathways and programs required for maintaining ECs both in vitro and in vivo (Extended Data Fig. 10). Additionally, it holds merit for the development of tractable regenerative platforms to sustain faithful EC fate in the context of vascular failure. Proper induction of both ERG and FLI1 warrants large scale “reprogramming” protocols for conversion of stromal cells into vascular ECs, with the potential of applying this approach for cardiovascular therapeutics to treat vascular-associated diseases.
Methods
Animal experiments
All animal experiments were performed under the approval of Weill Cornell Medicine Animal Care and Use Committee which provided ethical approval. Fli1 flox/flox mice were generated and obtained from Franqois Morle’s laboratory. Erg flox/flox mice were generated and obtained from Anna Randi’s laboratory. These mice were crossed with the VE-Cadherin (CDH5)-CreERT2 mouse line generated and obtained from Ralf Adams’s laboratory. The ROSA-TdTomato reporter mouse line was acquired from Jackson labs. All these lines were maintained on a C57BL/6 background. The generation of Ergflox/flox Fliflox/flox VE-Cadheirn-CreERT2 mouse line was obtained by crossing homozygotes of each of these floxed lines and by crossing the F1 progeny. Control mice were considered Ergflox/flox Fliflox/flox negative for the VE-Cadheirn-CreERT2 or transgenic mice carrying only the VE-Cadherin-CreERT2 transgene. The induction of Cre induced gene deletion was performed by the administration of 6 doses of 40mg/kg of Tamoxifen for 9 days: 3 days ON, 3 days OFF, 3 days ON, when the mice age was 12 weeks old. Both male and female mice were induced and analyzed. Mice were usually analyzed at 10 or 12 days after Tamoxifen administration unless indicated otherwise in the text.
Mice used for RNAseq analysis were intravitally labeled using a VE-Cadherin Alexa-647 antibody (clone BV13, Biolegend). Mice were euthanize using a CO2 chamber and processed immediately after. All mice were perfused with 20-30 mL of PBS. Tissues were extracted and stained for flow cytometry and described ahead. Sample tissues, from the bigger liver lobe and other organs, were extracted and processed for flow cytometry, after that, mice were perfused with 10 mL of 4% PFA and tissues were harvested for further histological analysis.
Evans Blue dye analysis was performed as previously described47. Briefly, 100 μl solution of 1% Evans blue was intravenously administrated. Mice were analyzed 30 minutes later. We used 3 control mice without Evans blue as negative controls for the basal measurements. For analysis, small piece of each organ was collected, weight and incubated in 500 μl formamide at 55°c overnight. Supernatants were analyzed at 620nm and 740nm, as control for heme-containing proteins. We used a standard curve of known concentration of Evans blue as a normalization reference for our calculations. Total Evans blue concentration was determined normalizing to total protein measurements using a BSA standard curve (diluted in formamide) as a reference.
Echocardiography Studies
Cardiac function and dimensions were analyzed by transthoracic echocardiography using a Vevo 770 Imaging System (VisualSonics). All mice underwent echocardiography at 10 days after injection with tamoxifen. Mice were minimally anesthetized with inhaled isoflurane (less than 0.5% in O2). M-mode was used to assess the left ventricle, all measurements were obtained from at least three consecutive cardiac cycles; average values were used for analysis. LVIDd and LVIDs dimensions were measured from the M-mode traces, and fractional shortening was calculated using the following formula: [(LVDd – LVDs)/LVDd]. Diastolic measurements were taken at the point of maximum cavity dimension, and systolic measurements were made at the point of minimum cavity dimension, using the leading-edge method of the American Society of Echocardiography48. The investigators performing and reading the echocardiograms were blinded to the KO status of the mice.
Blood and Serum analysis
The analysis of blood and serum parameters from Control WT or Ergflox/flox Fliflox/flox dKO mice was performed by the retro-orbital extraction of blood. Blood tests and parameter analysis were performed using ADVIA120 and HESKA Hematrue hematology systems. For serum analysis blood was spun down twice at 500G for 5 minutes to eliminate all red blood cells and submitted to the Laboratory of Comparative Pathology at Memorial Sloan Kettering Cancer Center for a full mouse chemical panel analysis.
Histology
All organs were fixed over-night in 4% PFA at 4°C, washed 3 times with PBS for 10 minutes each time. Next, organs for Paraffined histology were kept in 70% Ethanol and were sent to Histoserv, Inc. to be processed for: Hematoxylin and Eosin. The tissues used for immunofluorescence were maintained for 3 days in a 30% Sucrose solution at 4°C. After this time, organs were embedded in OCT.
For electron microscopy analysis tissues were fixed in a solution of glutaraldehyde overnight and samples were submitted to the Microscopy and Image Analysis Core Facility at Weill Cornell Medicine. Images were acquired in a JEOL JEM 1400 Transmission Electron Microscope.
Immunofluorescence protocol
Mouse tissues embedded in OCT were sectioned using a cryostat at 20 μm thickness and kept at -80°c. Sections were thawed at room temperature and washed 3 times with PBS solution for 5 minutes. Tissues were permeabilized using 0.1% Triton solution for 10 minutes and washed with PBS 3 times for 5 minutes. Afterwards samples were incubated for 30 minutes with blocking solution (1X PBS, 5% Dondey Serum, 0.1% Triton). Next, tissues were incubated for 2 days at 4°C with primary antibodies, diluted 1:100 in blocking solution. Primary antibodies were washed 3 times with PBS for 5 minutes. Secondary antibody staining was performed for 3 hours at room temperature in blocking solution. Secondary antibodies were washed 3 times in PBS for 10 minutes. Samples were mounted on Fluoroshield with Dapi (F6057, Sigma) and imaged on a Zeiss 710 confocal microscope. The following antibodies were used: VE-cadherin/CDH5 – R&D Systems – AF938; Endomucin – Santa Cruz Biotechnology – sc-65495; CD45-488 – Biolegend – 103122. Anti-donkey secondary antibodies from Life Technologies were applied for non-conjugated primary antibodies.
Bone tissue processing for hematopoietic analysis
Femur and tibia bones were harvested from Control WT or Ergflox/flox Fliflox/flox dKO mice. For further flow cytometry analysis, bones were flushed and crushed with MACS buffer media. Collected cells were filtered and washed prior to further procedures. Counts and hematopoietic parameter analysis was performed as described above using a fraction of cells from flushed and crushed femur. Some bones were cleaned from external tissue and placed for histological analysis as described above. Femurs from mice injected with Evans Blue Dye were flushed and crushed in 1 mL formamide and further analyzed for EBD permeability as described above.
Flow cytometry and Cell Sorting for mouse endothelial and hematopoietic cells
Isolation of ECs for flow cytometry and cell sorting analysis was performed as follow. Endothelial cell sorting for RNA-seq experiments were performed based on CD45-, CD31+ or CD45-, CD31+, VE-cadherin+ markers as indicated in the figures. For VE-cadherin staining mice were intravitally label with 25 μg of anti-VE-cadherin-AF647 antibody (clone BV13, Biolegend) retro-orbitally. Processing of tissues for flow cytometry or cell sorting was performed as follows: Mouse tissues were chopped and minced and incubated with a solution containing Collagenase A (25mg/ml) and Dispase II (25mg/mL) at 37°C for 10-45 min (depending on the processed tissue). Cells were filtered using a 100 zm filter and spun down at 300g for 5 minutes. Samples were RBC lysed for 5 minutes on ice using RBC lysis solution (Biolegend). After 5 minutes, samples were rinsed with PBS and spun down. Samples were stained on 1X MACS buffer solution. Cells were first incubated with the FC-quenching (blocking) antibody before following with the staining of other surface markers. The following antibodies were used for staining: CD45 – BV421 – 103134 – Biolegend; CD31 – 488 – 102414 – Biolegend; VE-cadherin – Alexa 647 (conjugated in house) – 138002 – Biolegend; TrueStain fcX – 101320 – Biolegend. Antibodies were used at a 1:100 dilution. Samples were acquired using a BD LSR II machine for flow cytometry or sorted using a BD ARIA 2 sorter machine. Endothelial cells were identified as CD45- CD31+ VE-cadherin+. For cell sorting we also included Dapi to exclude dead cells.
Isolation of hematopoietic stem and progenitor cells (HSPCs) for flow cytometry analysis was performed as follow. Bone marrow cells were filtered, RBC lysed, and blocked as described above. The following antibodies were used for staining: Ter-119 – APC – 116212 – Biolegend; Lineage – FITC – 78022 – Biolegend; Sca-1 – PE – 108108 – Biolegend; c-Kit – APC/Cy7 – 105826 – Biolegend; CD150 – PE/Cy7 – 115914 – Biolegend; CD48 – BV421 – 103427 – Biolegend. Dapi was included to exclude dead cells. Samples were acquired using a BD ARIA 2 sorter machine. LSK HSPCs were identified as Lineage- Sca-1+ c-Kit+. SLAM LSK HSPCs were identified as CD150+ CD48- Lineage- Sca-1+ c-Kit+.
For analysis of HSPCs or TdTomato+ circulating ECs in mouse peripheral blood, blood was collected from the heart using a 1mL 26G syringe (BD) and transferred into heparinized tubes. Blood was further diluted 1:1 with PBS. Next the mononuclear cell (MNC) fraction was isolated from the RBC fraction by performing a gradient based centrifugation protocol using Ficoll-Paque™ Plus solution (17-1440-03, GE Healthcare). MNC cell fraction was isolated and washed with PBS twice. Samples were then RBC lysed to eliminate any residual RBCs as described above. Cells were stained for flow analysis and acquired as described above.
Methylcellulose assay (CFU-C assay) for peripheral blood progenitors
A total of 200,000 PB MNCs were seeded per 35 mm dish with MethoCult media (M3434, STEMCELL Technologies) and cultured at 20% oxygen for 14 days. The number of colony-forming units was determined and scored using a dissection microscope (Nikon SMZ1500) and using an automated colony counter, STEMvision (STEMCELL technologies).
RNA sequencing
Mouse endothelial cells from control WT and ErgΔECFli1ΔEC dKO were sorted as indicated above under the Flow cytometry and Sorter description. RNA from sorted from adult mouse ECs was isolated using the RNA isolation Mini Kit from Qiagen (74104 - Quiagen), following manufacturer’s instructions. At least 100 ng of total RNA from freshly harvested cells was isolated and purified using Qiagen’s RNeasy Mini Kit. RNA quality was verified using an Agilent Technologies 2100 Bioanalyzer prior to sequencing. RNA library preps were generated and multiplexed using Illumina’s TruSeq RNA Library Preparation Kit v2 (non-stranded and poly-A selection). 10 nM of cDNA was used as input for high-throughput sequencing via Illumina’s HiSeq 4000 producing 51 bp paired-end reads. For analysis related methodology see ahead in human MSC methods section.
ATAC sequencing
Mouse endothelial cells from control WT and ErgΔECFli1ΔEC dKO were sorted as indicated above under the Flow cytometry and Sorter description. We have followed the previously described Omni-ATAC-seq protocol49. Briefly, sorted cells were spun down for at 500rcf for 5 min at 4°c in a fixed angle. Next, cells were resuspended in RSB buffer and pipetted 3 times, followed up by 3 min incubation on ice. Cell lysate was washed, and nuclei pellet was acquired after spin at 500rcf for 10 min at 4°c. Nuclei pellet was resuspended in transposition mixture at incubated at 37°c for 30 min in a thermomixer with 1000 rpm mixing speed. Cleanup reaction was performed with a Zymo DNA clean and concentrator-5 kit (Zymo). DNA was eluted and amplified using NEBNext X2 MasterMix (NEB). Following additional amplification and cleanup, library concentration was determined using the KAPA library quantification kit (KAPA). DNA library was sequenced on Illumina, using HiSeq4000, PE read, 50 cycles. Raw read quality control, adapter trimming, alignment and filtering, and peak calling were performed using the nf-core ATAC-seq workflow (v. 1.2.1). Venn diagrams were generated using the ChIPpeakAnno R library package after combining peaks from biological replicates using default setting for finding overlapping peaks. Alignment files across biological replicates were merged and duplicates re-removed. BigWig files were generated using deeptools normalized to reads per genomic content (1x normalization). Differential peak analysis was performed with DESeq2 R package. Peaks were re-called on the merged alignment files and footprinting was performed using TOBIAS with the JASPAR 2020 core vertebrates non-redundant position frequency matrix. Aggregate signal and the volcano plot were generated using the TOBIAS command line tools on merged alignment files. Peak annotations were performed using the ChIPSeeker R package and functional enrichment was performed using Genomic Regions Enrichment of Annotations Tool (GREAT) on differentially accessible elements with p-adjusted value < 0.01 in both the double knockout and control regions. HOMER was used for motif enrichment in genomic regions with argument -size given.
Mouse Endothelial Cell cultures
Mouse endothelial cells were isolated from the brains and liver of mice with the corresponding genotype. Brains and livers were chopped, minced and incubated with a digestion solution composed of Collagenase A (25mg/ml) and Dispase II (25mg/mL) at 37°c for 10-15 min. Cells were filtered using a 100 zm filter and spin down at 300g for 5 minutes. Cell pellets were washed for 3 times in MACS buffer and spun down at 500g for 5 minutes each time. Next, pellets were resuspended and plated on fibronectin coated plates using the following medium formulation: DMEM/F12 basal medium (10-092-CV Corning) supplemented with 20% Fetal Calf Serum (Corning), Endothelial Cell Growth Supplement (J64516 Alfa Aesar), 1X Corning glutagro Supplement (25-015-CI Corning), 1X Hepes (25-060-Cl Corning), 1X Antimicrobial antifungal (2020/11 Corning), 100 μg/mL Heparin (H3393 – Sigma), 20 mg/mL bFGF (100-18B Peprotech), 10mg/mL VEGF (400-32 Peprotech) and TGFb signaling inhibitor – SB431542 (10μM, Tocris). For the selection of mouse brain endothelium, cells were cultured with 4μg/mL of puromycin for 5 days. For the selection of liver endothelium, we performed sorting of CD31+, VE-cadherin+ cells twice. Medium was changed every 2 to 3 days.
The induction of the deletion of Erg and/or Fli1 in cultured ECs was achieved by the addition of 4-hydroxy-Tamoxifen (4-OHT, 1ng/mL) for 48 hours to the culture medium. Cells were cultured for an additional 7 days following the initial addition of tamoxifen before final harvest and analysis. To study the iniluflammatory response of the endothelial cells we added a concentration of 10ng/ml of both IL1 and TNFα at day 7 after tamoxifen administration and lysed the cells 16 hours afterwards for RNA-seq analysis, as described above.
Analysis of SNPs surrounding FLU and ERG regions
The phenotypes from the top associated variant in the genes FLI1 and ERG ±100Kqqb for were downloaded from the FinnGen website at http://r5.finngen.fi/. Variants were then filtered depending if they had associated matching results in the UK biobank GWAS study at http://www.nealelab.is/uk-biobank/ and then the relative p-values of the associations were then plotted in a volcano plot displaying log10 p-values for each study.
Human Mesenchymal Stromal Cell (MSC) Cultures Isolation of human MSC
Human MSC were obtained from healthy bone marrow donors following a bone marrow aspiration procedure performed at Weill Cornell Medicine (NYP hospital). Informed consent was obtained from human bone marrow donors. Donor samples arrived from the Department of Pathology and were approved by the institutional review board at Will Cornell Medicine as part of the IRB protocol. Bone marrow samples were digested as previously described for mouse endothelial cultures, and plated O/N on a plastic culture dish with MesenCult™ MSC (Human) media (Cat# 05401, 05402 - STEMCELL Technologies). The following day, media and cells in suspension were removed and the adherent fraction was further cultured in MesenCult™ media. After achieving full confluency cells were stained using antibodies against human VE-cadherin (BV9 clone, Biolegend) conjugated to AF647 (in house conjugation), human CD31 PE/CY7 (WM59 clone, Biolegend), and human CD45 PacBlue (HI30 clone, Biolegend). Triple negative cells for the former markers were sorted using BD ARIA II and then frozen (Recovery™ Cell Culture Freezing Medium, Gibco) in aliquots for future experiments.
ERG/FLU lentiViral transduction strategy
Lentiviral vectors for ERG1 and FLI1 were produced as previously described6. Thawed MSCs in MesenCult™ were cultured (at 37°C 5% CO2 5% O2 incubator) on 6-wells plates until 60-70% confluency was achieved. Next, lentivectors for ERG1 and FLI1 at MOI of 5 each or a control empty vector at MOI of 10 were introduced with fresh media. After 48 hours media was exchanged to human endothelial cell full culture media containing: M199 basal media (HyClone), 20% Fetal Calf Serum (Corning), Endothelial Cell Growth Supplement (J64516 Alfa Aesar), 1X Corning glutagro Supplement (25-015-CI Corning), 1X Hepes (25-060-Cl Corning), 1X Antimicrobial antifungal (2020/11 Corning), 100 μg/mL Heparin (H3393 – Sigma), 20 mg/mL bFGF (100-18B Peprotech), 10mg/mL VEGF (400-32 Peprotech), and TGFb signaling inhibitor – SB431542 (10μM, Tocris). The day of media exchange is considered as day 0 for further conversion experiments and studies. After 7 days post primary transduction, a similar 2ndery transduction was performed. At day 7, 14, and 21 cells were stained using previously described antibodies to assess the frequency of EC-like converted cells in culture. Cells were submitted for bulk RNAseq analysis on day 21 or for single cell RNAseq analysis on day 4 post media exchange.
Matrigel in vitro tubulogenesis assay
To assess the capacity of MSCs to form tubular networks following ERG/Fli1 transduction, control and ERG/Fli1 transduced MSCs were isolated at day 7 and 50,000 cells were seeded on Matrigel (Sigma)-coated 96wells and examined after 24 hours in culture conditions (37°C, 5% CO2, 5% O2). Images were acquired using a digital inverted microscope (EVOS).
Fibringel in vitro perfusable tube formation assay
To study the potential of ERG/FLI1 transduced MSCs to establish a tubular perfusable network, 300K of ERG/FLI1 MSCs or HUVECs were resuspended in 36 μL of Fibrin (3 mg/mL, Sigma), mixed with additional 4 μL of Thrombin (2 U/mL), and immediately introduced into a lane of microchip μ-Slide VI 0.4 IbITreat device (Ibidi). TB Syringes (1 mL, BD) were placed in the wells at the ends of the lanes and 1mL of EC-medium was placed in the entrance well syringe and 100 μL of EC-media was placed in the exit well syringe. After 3-4 days of incubation in 37°C, 5% CO2, 20% O2, upon establishment of liquid equilibrium in both syringes (indicating tube formation and perfusion), lanes were perfused with either antibodies: anti-human-VE-cadherin antibodies (clone BV9, Biolegend), anti-human-UEA1 (Vector laboratories); or with heparinized diluted human blood. Images and videos were acquired using Zeiss Cell Observer spinning disk confocal microscope equipped with Photometrics Evolve EMCCD camera.
Matrigel plug in vivo vessel formation assay
To assess the capacity of MSCs to form functional anastomosed blood vessels following ERG/FLI1 transduction, control and ERG/FLI1 transduced MSCs were isolated at day 21 and 2.5 million cells were embedded in 250 μL of Matrigel (Sigma), followed by subcutaneous injection into NSG mice (each mouse had both plugs with control and ERG/FLI1 cells on different sides). One-month post-transplant, mice were i.v. injected with AF647 anti-human-VE-cadherin antibodies (clone BV9, Biolegend), and 10-15 min later plugs were harvested and immediately transferred into 4% PFA/PBS solution for O/N fixation. Plug samples were washed, permeabilized, stained with AF488 anti-human-CD31 antibodies (Biolegend), washed, and whole mounted on a slide with aqueous mounting media. Images were acquired using Zeiss Cell Observer spinning disk confocal microscope equipped with Photometrics Evolve EMCCD camera.
Transwell migration and permeability assays
Control and ERG/FLI1 MSCs were plated over 0.4 {M (permeability assay) and 8 {M (migration assays) pores transwells (cell culture inserts, Costar) precoated with gelatin or in plate wells (Costar) pre coated with gelatin, and cultured in EC-media (37°C, 5% CO2, 5% O2). Cells in plate wells were monitored under a digital inverted microscope (EVOS) and imaged once full confluency was achieved. Upon achievement of confluency cells in transwells were incubated O/N with fresh EC media (control) or with fresh EC media supplemented with both IL-1 (10 ng/mL) and TNFα (10 ng/mL). For migration assays, sorted CD34+ hematopoietic stem and progenitor cells from mobilized peripheral blood donors, were placed in the upper well and also in index control tube. CXCL12/SDF-1 (125 ng/mL, Prospec Bio) was added to the lower well to induce a migration gradient. Hematopoietic cells were allowed to migrate for 4 hours following which media from the lower well and index were collected, and cell number was determined by flow cytometry. Frequency of migration was evaluated relatively to the index acquisition. For permeability assay, 1 μg/mL of FITC-Dextran 70 kDa (Sigma) was added to the upper transwell and allowed to diffuse for 4 hours. Media from the lower well was collected and FITC-Dextran concentration was determined following measurements with a fluorescent plate reader (SpectraMax) after plotting into a standard curve defined by measuring known concentrations.
Bulk RNA sequencing
RNA was isolated using the RNA isolation Mini Kit from Qiagen (74104 - Quiagen), following the instructions from the manufacturer. At least 100 ng of total RNA from freshly harvested was isolated and purified using Qiagen’s RNeasy Mini Kit. RNA quality was verified using an Agilent Technologies 2100 Bioanalyzer prior to sequencing. RNA library preps were generated and multiplexed using Illumina’s TruSeq RNA Library Preparation Kit v2 (non-stranded and poly-A selection). 10 nM of cDNA was used as input for high-throughput sequencing via Illumina’s HiSeq 4000 producing 51 bp paired-end reads. FASTQ files were checked for quality (FastQC v0.11.5) and processed using the Digital Expression Explorer 2 (DEE2) workflow. Adapter trimming was performed with Skewer (v0.2.2). Further quality control was done with Minion, part of the Kraken package. Reads were mapped to reference genome GRCm38 for mouse or GRCh38 for human using STAR aligner and gene-wise expression counts generated using the “-quantMode GeneCounts” parameter. After further filtering and quality control, R package edgeR was used to calculate RPKM and Log2 counts per million (CPM) matrices as well as perform differential expression analysis. Ranked by log fold change GSEA analysis was performed with software from the Broad Institute and UC San Diego (v.4.0.3) using log fold change. Heatmaps were generated using the pheatmap (Pretty Heatmaps) R package. R package version 1.0.12. with dendrograms calculated by Euclidean distance. Gene ontology analysis was performed in David Bioinformatics Database and plot using GO Plot (v1.0.2). Pathway analysis was performed using the WebGestalt and represented using ggplot2 R package. For analysis of timeseries between days 7, 10 and 12 samples were first normalized using the controls from each day to avoid differences derived from independent sequencing times.
Single cell RNA-seq analysis
Human mesenchymal stromal cells transduce with an empty control lentivirus or with ERG/Fli1 overexpressing lentiviruses were sorted as indicated above and transferred to the Genomics Core Facility at Weill Cornell Medicine to proceed with Illumina Bio-Rad SureCell WTA 3’ Library Prep kit using Bio-Rad ddSEQ Single-Cell Isolator system. Briefly, according to manufacturer’s instruction (Illumina, cat # 20014280), the sorted cells were washed with 1x PBS + 0.1% BSA, counted by Bio-Rad TC20 Cell Counter, and cell viability was assessed. A total of 10,000 cells and barcode mixes were loaded into each channel of the cartridge to generate the droplets on ddSeq Single Cell Isolator. After the first strand were synthesized in droplets, individual droplets were disrupted and the short fragments – unbound barcodes were removed from the first strand products. The second strand cDNA synthesis was carried out and RNA template was removed. Using Illumina Nextera SureCell transposome kit, cDNA was fragmented simultaneously and tagged with adapter sequences in a single step. Followed by PCR amplification, cDNA libraries were assessed by Agilent Technology 2100 Bioanalyzer and sequenced on Illumina NextSeq 500 sequencer using the high output mode with 150 cycle kit. FASTQ files were then generated in the Illumina BaseSpace SureCell Single-Cell System.
Seurat version 3.1.0 was used to perform quality control, count normalization and clustering on the single cell transcriptomic data using standard methods as follows: Unique molecular identifiers (UMIs) that barcode each individual mRNA molecule within a cell during reverse transcription were used to remove PCR duplicates. Cells expressing less than 400 or greater than 7000 genes were filtered out to exclude non-cells or cell aggregates and cells expressing greater than 40000 UMIs were also filtered out. Seurat standard pipeline was used for normalization and scaling and after ascertainment using jackstraw and elbow plots the first 20 principal components were selected for dimensionality reduction. The shared nearest neighbor modularity optimization-based clustering algorithm was used to group cells into clusters with cluster resolution set at 0.8. Differential gene expression for gene marker discovery across the clusters was performed using the Wilcoxon rank sum test from the Seurat package.
Statistical analysis
Statistics were calculated using Graphpad Prism Software or R software for the bioinformatic analysis from the single cell or bulk RNA samples. Groups of 2 different conditions were compared using T-test. Groups bigger than 2 were compared using ONE WAY ANOVA. We used TWO WAY ANOVA when multiple conditions where compared. Statistical significance is shown as: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Extended Data
Extended Data Figure 1. Endothelial in vivo deletion of ERG is achieved at a multiorgan level.
a-h, Immunofluorescence analysis of ERG (green) expression at day 12 after tamoxifen administration in the endothelium counterstained with VE-cadherin (magenta), Endomucin (Emcn, cyan) and Dapi (white), a-b, brain, c-d, heart, e-f, kidney, g-h, liver, ERGΔECFli1ΔEC dKO mice are represented as dKO, Control WT mice are represented as Ctl, Representative image of n=5 mice stained in the liver, Bar size = 50μm.
Extended Data Figure 2. Endothelial in vivo deletion of Fli1 is achieved at a multiorgan level.
a-h, Immunofluorescence analysis of Fli1 (green) expression at day 12 after tamoxifen administration in the endothelium counterstained with VE-cadherin (magenta), Endomucin (Emcn, cyan) and Dapi (white), a-b, brain, c-d, heart, e-f, kidney, g-h, liver, ERGΔECFli1ΔEC dKO mice are represented as dKO, Control WT mice are represented as Ctl, Representative image of n=5 mice stained in the liver, Bar size = 50μm.
Extended Data Figure 3. Endothelial deletion of ERG and Fli1 leads to multiorgan alterations.
a-e, Blood parameters were analyzed in Control and Fli1ΔECERGΔEC dKO mice 12 days post tamoxifen administration, Hematocrit – HCT (a), Red blood cells – RBC (b), Hemoglobin – HGB (c), Mean corpuscular volume – MCV (d), mean corpuscular hemoglobin concentration – MCHC (e), Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM, f-q, Plasma parameters were measured in Control and ERGΔECFli1ΔEC dKO mice 12 days post tamoxifen administration, Alanine aminotransferase – ALT (f), Aspartate aminotransferase – AST (g), Alkaline phosphatase – ALP (h), Albumin – ALB (i), Globin – GLOB (j), Albumin / Globin ratio – A/G ratio (k), Blood urea nitrogen – BUN (l), Creatinine – CREA (m), Blood urea nitrogen/ Creatinine ratio – BUN/CREA ratio (n), Lactic acid dehydrogenase – LDH (o), Cholesterol – CHOL (p), Glucose – GLU (q), Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM, r, Brain tissue of Control and Fli1ΔECERGΔEC dKO mice 12 days post tamoxifen administration injected IV with Evans Blue, Representative image of n = 5, Blood and serum parameters were measured in n = 10 mice per group.
Extended Data Figure 4. Expression of ERG and Fli1 in endothelium is essential for proper cardiac and circulatory function.
a, b, Left ventricular internal dimension in diastole (LVIDd) or systole (LVIDs), c, d, Left ventricular cardiac volume in diastole (LV Vol d) or systole (LV Vol s), e, Ejection fraction, measured as the percentage of ejected blood (EF), Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM from n = 6 Ctl mice and 8 dKO mice.
Extended Data Figure 5. Endothelial deletion of ERG and Fli1 disrupts the vascular program in favor of a maladapted inflammatory phenotype.
a, Endothelial cells from Control (Ctl) and ERGΔECFli1ΔEC dKO mice were collected at day 7, 10 and 12 following tamoxifen administration as indicated before, Cells were sorted based on CD45neg, CD31+, and VE-cadherin$ markers followed by RNA-seq analysis, b-n, Representation of genes involved in EC signaling pathways based on their expression pattern in ERGΔECFli1ΔEC dKO versus Ctl ECs, All genes with an FDR < 0,05 were colored based on their Fold induction < ±2 or > ±2, as indicated in the color legend at the top right, b, Cell membrane and cell junction genes, c, Gap junction genes, d, Vascular permeability genes, e, Tip cell markers, f, Coagulation genes, g, Integrin genes, h, Matrix genes classified in the subfamilies of MMPs, Collagens, Adam family and Crosslinkers, i, Inflammation, j, BMP/TGFp, k, Lamins, l, Glycolysis, m, Notch, n, Igfbp genes, o, Shear stress, p, Graphical representation of the physiological model based on the alterations observed in ERGΔECFli1ΔEC dKO versus Ctl ECs.
Extended Data Figure 6. Deletion of ERG and Fli1 alters chromatin accessibility landscape.
a, Principal component analysis of ATAC-seq analysis of control (Ctl) and dKO mice at day 10 after been treated with tamoxifen as indicated before, b, ATAC-seq distance matrix showing association between subclusters.
Extended Data Figure 7. In vivo deletion of ERG and Fli1 induces early hematopoietic mobilization and multiorgan myeloid infiltration.
a, BM H&E images of Control and Fli1ΔECERGΔEC dKO mice 7 days post tamoxifen administration, Representative image of n = 5 mice, Enlarged areas display perivascular megakaryocytes, Scale bar = 100 μm, b, c, Number of PB white blood cells (WBC)(b) and platelets (c) at day 7, as determined by HESKA veterinary hematology system, n = 5 mice, Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice ±SEM, d, Immunofluorescence of VE-cadherin (magenta) and CD45 (cyan) in control ERGAECFli1AEC dKO mice in liver, heart and kidney, Representative images of n = 5 mice, bar represents 100μm, e-g, Flow cytometry quantification for the frequency of total CD45+ cells (hematopoietic), myeloid (CD45+Gr-1+CD11b+), B-cells (CD45+B220+) or T-cells (CD45+CD3+) present in the heart (e), kidneys (f) and liver (g) of control and ERGΔECFli1ΔEC dKO mice. n = 5 mice per group. Student t-test analysis was performed comparing Ctl and ERGΔECFli1ΔEC dKO mice.
Extended Data Figure 8. ERG and FLI1 are associated to diverse cardiovascular disorders in GWAS.
a-b, Analysis of the FinnGen and Uk Biobank GWAS associated pathologies common to both studies, Several vascular and vascular associated pathologies are label, Identification of associated GWAS was performed by selectin ±100kb of ERG and FLI1 genes and significantly enriched pathologies within the FinnGen were included and compared to the Uk biobank.
Extended Data Figure 9. ERG and FLI1 cooperatively induce a vascular EC program in non-vascular human MSCs.
a, RNA-seq analysis was performed after 3 weeks in culture for isolated Ctl and ERG/FLI1 MSCs and for cultured HUVECs, Heatmaps of normalized raw counts expression levels of coagulation pathway related genes, n = 4 donors per each MSC group and n = 3 HUVEC donors, b, Schema of experimental design for panels c, d, After 3 weeks of culture control (Ctl) and VE-cadherin+ ERG/FLI1 MSCs were isolated, plated in transwells (8 fM pore size for transmigration assays (e) and 0,4 fM pore size for permeability assays (f)) and in regular culture plate wells, and incubated in EC media until confluency was observed in all culture plate wells, Media in lower well and upper transwell was exchanged for O/N with fresh EC media (control) or with fresh EC media supplemented with both IL-1 (10 ng/mL) and TNFα (10 ng/mL), c, Following incubation, for migration assays, lower well media was replaced with fresh serum free media supplemented with CXCL12/SDF-1 (125 ng/mL), and upper transwell media was exchanged with serum free media containing isolated CD34+ HSPCs from mobilized peripheral blood donors, n = 3 BM MSC donors and n = 2 HSPC donors, HSPCs were allowed to migrate for 4 hours and then collected for flow analysis to determine frequency of migration in lower well relatively to loaded HSPCs in the upper transwell, Two independent experiments were performed each time with a different HSPC donor, Each mark represents an average of n = 3 technical repeats, Two-way ANOVA statistical analysis was performed comparing all groups, d, For permeability assays, lower well media was replaced with fresh serum free media, and upper transwell media was exchanged with serum free media containing FITC-dextran 70 kDa (1ng/mL), n = 3 BM MSC donors, FITC-dextran was allowed to diffuse for 4 hours and then collected for analysis by fluorescent plate reader to determine FITC-dextran concentration in lower well, Two independent experiments were performed, Each mark represents an average of n = 3 technical repeats, Two-way ANOVA statistical analysis was performed comparing all groups, e, Representative stromal monolayer images from n = 3 MSC donors displaying each experimental condition at the time point of beginning of either transmigration or permeability assays, after achieving full confluency, Bars indicate 1000 μm, f, Representative images from n = 4 donors of tube network formation assay 24h post seeding in Matrigel, Ctl and ERG/FLI1 MSCs were isolated after 7 days in culture, Bars indicate 1000 μm.
Extended Data Figure 10. Cooperative ERG and Fli1 co-expression is essential for sustaining and induction of endothelial genetic core programs and physiological vascular functions.
a, In vivo deletion of ERG and Fli1 (dKO) induces acute systemic coagulopathy and death in adult mice, associated with a decrease in vascular junctional proteins, EC detachment, increase in vascular leakiness, aberrant expression of cytokines/chemokines and abnormal inflammatory response, b, ERG and Fli1 (in vitro) sustain the expression of the EC core program and ERG/Fli1 combined genetic deletion leads to acquisition of a non-endothelial cell fate which exhibits inadequate response to inflammatory stimulation, c, Overexpression of ERG and FLI1 is sufficient for the induction of a transcriptional vascular EC core programs in human adult non-vascular mesenchymal stromal cells, conferring them with functional vascular tubulogenic network capacity in vitro and in vivo, with the acquisition of an anti-coagulation gene signature, and with the capacity to mount an immune response to facilitate transmigration of hematopoietic cells following inflammatory stimulation.
Supplementary Material
Acknowledgments
JM Gomez-Salinero was a New York Stem Cell Foundation - Druckenmiller Fellow and is currently supported by Weill Cornell Medicine Fund for the Future program and JumpStart career development program. The New York Stem Cell Foundation (NYSCF), the New York State Stem Cell Science (NYSTEM), and the British Heart Foundation (BHF) supported this research. S Rafii is funded by Hartman Institute for Therapeutic Organ Regeneration, Ansary Stem Cell Institute, grants from the National Institute of health (NIH), R35 HL150809, RC2 DK114777, U01AI138329, the Empire State Stem Cell Board NYSTEM) (C030160), Daedalus Fund for Innovation and iSelma and Lawrence Ruben Science to Industry Bridge Fund from Weill Cornell Medicine, the Starr Foundation stem cell core project and initiatives TRI-SCI #2019-029. Anna M. Randi, Graeme M. Birdsey, Neil Dufton, Claire Peghaire were funded by British Heart Foundation grant numbers RG/11/17/29256 and RG/17/4/32662.Viktoria Kalna was funded by British Heart Foundation PhD Studentship FS/15/65/32036.
Footnotes
Author contributions statement:
JMG-S, TI, AMR, CB, and SR designed and initiated Fli1/ERG dKO studies. JMG-S, TI, AMR, and SR designed and completed experimental studies described in all figures. JMG-S, TI, and CB performed the initial characterization, electron microscopy of the phenotype and blood abnormalities in double KO mice. JMGS, CB, TI and TML collected data for in vivo RNAseq analysis. JMGS and TI performed data collection for in vitro RNAseq analysis. JMG-S, YL and GL carried out functional characterization of the Fli1, ERG, VE-cadherin CREERT2 mice for EC barrier functions and immunofluorescence analysis. TI along with JMG-S and YL executed the breeding, crossing and phenotypic characterization and analysis of Td-Tomato Fli1, ERG, VE-cadherin CREERT2. SH, DR, JMG-S and TI performed bioinformatic analysis of the samples for the bulk RNAseq analysis and single cell RNAseq analysis. JMG-S, along with TI and YL performed EC isolation and in vitro characterization and analysis of ECs. TI carried out human in vitro and in vivo ERG and FLI1 MSC-EC conversion, and murine hematopoiesis relevant studies. GMB, VK, ND, CRP assisted with in vitro design and experiments and bioinformatic analysis. MY, MW performed and interpret the echocardiography analysis. JZX helped in the processing analyzing of all RNAseq bulk and single cell samples. Y-MSH provided samples and technical support for the in vitro human conversion studies. RS carried out acquisition and analysis of time lapse imaging and technical support for the immunofluorescence experiments. JMG-S, TI, AMR, GMB and SR wrote, edited, compiled, and revised the manuscript. All authors read and approved the final manuscript.
Competing Interest Statement:
Shahin Rafii is the co-founder and non-paid consultant to Angiocrine Bioscience, CA.
Data availability
Data from bulk RNA-seq, ATAC-seq and scRNA-seq has been deposited in Gene Expression Omnibus (GEO) under the accession number GSE210119. Additional data of this study is included in the main article, supplementary material and associated source data.
References
- 1.Rafii S, Butler J, Ding B. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Salinero JM, Rafii S. Endothelial cell adaptation in regeneration. Science. 2018;362:1116–1117. doi: 10.1126/science.aar4800. [DOI] [PubMed] [Google Scholar]
- 3.Nolan D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabbagh Mv, et al. Transcriptional and Epigenomic Landscapes of CNS and non-CNS Vascular Endothelial Cells. Elife. 2018;7 doi: 10.7554/eLife.36187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry Dv, et al. Molecular Determinants of Nephron Vascular Specialization in the Kidney. Nat Commun. 2019;10 doi: 10.1038/s41467-019-12872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsberg Mv, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schachterle Wv, et al. Sox17 drives functional engraftment of endothelium converted from non-vascular cells. Nat Commun. 2017;8:13963. doi: 10.1038/ncomms13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu TM, et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2016950118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birdsey GM, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperone A, et al. The transcription factor Erg inhibits vascular inflammation by repressing NF-kappaB activation and proinflammatory gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:142–150. doi: 10.1161/ATVBAHA.110.216473. [DOI] [PubMed] [Google Scholar]
- 11.Birdsey GM, et al. The transcription factor Erg regulates expression of histone deacetylase 6 and multiple pathways involved in endothelial cell migration and angiogenesis. Blood. 2012;119:894–903. doi: 10.1182/blood-2011-04-350025. [DOI] [PubMed] [Google Scholar]
- 12.Dryden NH, et al. The transcription factor Erg controls endothelial cell quiescence by repressing activity of nuclear factor (NF)-kappaB p65. J Biol Chem. 2012;287:12331–12342. doi: 10.1074/jbc.M112.346791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AV, Birdsey GM, Randi AM. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. Vascul Pharmacol. 2016;86:3–13. doi: 10.1016/j.vph.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolova-Krstevski V, et al. ERG is required for the differentiation of embryonic stem cells along the endothelial lineage. BMC Dev Biol. 2009;9:72. doi: 10.1186/1471-213X-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufton NP, et al. Dynamic regulation of canonical TGFbeta signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nat Commun. 2017;8:895. doi: 10.1038/s41467-017-01169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AV, et al. The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of Notch signalling and vascular stability. Nat Commun. 2017;8:16002. doi: 10.1038/ncomms16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birdsey GM, et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/beta-catenin signaling. Dev Cell. 2015;32:82–96. doi: 10.1016/j.devcel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peghaire C, et al. The transcription factor ERG regulates a low shear stress-induced anti-thrombotic pathway in the microvasculature. Nat Commun. 2019;10:5014. doi: 10.1038/s41467-019-12897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asano Y, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennard Richard ML, et al. Acetylation impacts Fli-1-driven regulation of granulocyte colony stimulating factor. Eur J Immunol. 2016;46:2322–2332. doi: 10.1002/eji.201646315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou N, Lennard Richard ML, Yu J, Kindy M, Zhang XK. The Fli-1 transcription factor is a critical regulator for controlling the expression of chemokine C-X-C motif ligand 2 (CXCL2) Mol Immunol. 2017;81:59–66. doi: 10.1016/j.molimm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Looney A, et al. Synergistic Role of Endothelial ERG and FLI1 in Mediating Pulmonary Vascular Homeostasis. Am J Respir Cell Mol Biol. 2017;57:121–131. doi: 10.1165/rcmb.2016-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starck J, et al. Inducible Fli-1 gene deletion in adult mice modifies several myeloid lineage commitment decisions and accelerates proliferation arrest and terminal erythrocytic differentiation. Blood. 2010;116:4795–4805. doi: 10.1182/blood-2010-02-270405. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 25.Chistiakov D, Orekhov A, Bobryshev Y. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front Physiol. 2015;6 doi: 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangwung P, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight. 2017;2:e91700. doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu YW, et al. MEF2 (Myocyte Enhancer Factor 2) Is Essential for Endothelial Homeostasis and the Atheroprotective Gene Expression Program. Arterioscler Thromb Vasc Biol. 2021;41:1105–1123. doi: 10.1161/ATVBAHA.120.314978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalna V, et al. The Transcription Factor ERG Regulates Super-Enhancers Associated With an Endothelial- Specific Gene Expression Program. Circ Res. 2019;124:1337–1349. doi: 10.1161/CIRCRESAHA.118.313788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahmy RG, et al. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 2006;24:856–863. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor alpha synergistically stimulate tissue factor expression in human endothelial cells: regulation through c-Fos and c-Jun. J Biol Chem. 2004;279:36142–36147. doi: 10.1074/jbc.M405039200. [DOI] [PubMed] [Google Scholar]
- 31.Gou B, et al. Single-Cell Analysis Reveals Transcriptomic Reprogramming in Aging Cardiovascular Endothelial Cells. Front Cardiovasc Med. 2022;9:900978. doi: 10.3389/fcvm.2022.900978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzotta C, et al. FLI1 and ERG protein degradation is regulated via Cathepsin B lysosomal pathway in human dermal microvascular endothelial cells. Microcirculation. 2020:e12660. doi: 10.1111/micc.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan N, et al. Transcriptional networks specifying homeostatic and inflammatory programs of gene expression in human aortic endothelial cells. Elife. 2017:e22536. doi: 10.7554/eLife.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itkin T, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohde D, et al. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nature Cardiovascular Research. 2022;1:28–44. doi: 10.1038/s44161-021-00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergiers I, et al. Single-cell transcriptomics reveals a new dynamical function of transcription factors during embryonic hematopoiesis. Elife. 2018;7 doi: 10.7554/eLife.29312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abedin MJ, et al. Fli1 acts downstream of Etv2 to govern cell survival and vascular homeostasis via positive autoregulation. Circ Res. 2014;114:1690–1699. doi: 10.1161/CIRCRESAHA.1134303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricard N, et al. Endothelial ERK1/2 signaling maintains integrity of the quiescent endothelium. J Exp Med. 2019;216:1874–1890. doi: 10.1084/jem.20182151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fish J, et al. Dynamic regulation of VEGF-inducible genes by an ERK/ERG/p300 transcriptional network. Development. 2017;144:2428–2444. doi: 10.1242/dev.146050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, et al. A screen for Fli-1 transcriptional modulators identifies PKC agonists that induce erythroid to megakaryocytic differentiation and suppress leukemogenesis. Oncotarget. 2017;8:16728–16743. doi: 10.18632/oncotarget.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falkenberg KD, Rohlenova K, Luo Y, Carmeliet P. The metabolic engine of endothelial cells. Nat Metab. 2019;1:937–946. doi: 10.1038/s42255-019-0117-9. [DOI] [PubMed] [Google Scholar]
- 42.Xiong J, et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol Cell. 2018;69:689–698.:e687. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sperone A, et al. The transcription factor Erg inhibits vascular inflammation by repressing NF-kappaB activation and proinflammatory gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:142–150. doi: 10.1161/ATVBAHA.110.216473. [DOI] [PubMed] [Google Scholar]
- 44.Sangwung P, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight. 2017;2:e91700. doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Val S, et al. Combinatorial Regulation of Endothelial Gene Expression by Ets and Forkhead Transcription Factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagai N, et al. Downregulation of ERG and FLI1 expression in endothelial cells triggers endothelial-to- mesenchymal transition. PLoS Genet. 2018;14:e1007826. doi: 10.1371/journal.pgen.1007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulos M, et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest. 2017;127:4163–4178. doi: 10.1172/JCI93940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donner D, Kiriazis H, Du X, Marwick T, McMullen J. Improving the quality of preclinical research echocardiography: observations, training, and guidelines for measurement. Am J Physiol Heart Circ Physiol. 2018;315:58–70. doi: 10.1152/ajpheart.00157.2018. [DOI] [PubMed] [Google Scholar]
- 49.Corces MR, et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from bulk RNA-seq, ATAC-seq and scRNA-seq has been deposited in Gene Expression Omnibus (GEO) under the accession number GSE210119. Additional data of this study is included in the main article, supplementary material and associated source data.