Abstract
Osteoporosis care has evolved markedly over the last 50 years, such that there are now an established clinical definition, validated methods of fracture risk assessment and a range of effective pharmacological agents. However, it is apparent that both in the context of primary and secondary fracture prevention, there is a considerable gap between the population at high fracture risk and those actually receiving appropriate antiosteoporosis treatment. In this narrative review article, we document recent work describing the burden of disease, approaches to management and service provision across Europe, emerging data on gaps in care, and existing/new ways in which these gaps may be addressed at the level of healthcare systems and policy. We conclude that although the field has come a long way in recent decades, there is still a long way to go, and a concerted, integrated effort is now required from all of us involved in this field to address these urgent issues at all levels to ensure the best possible outcomes for our patients.
Keywords: osteoporosis, epidemiology, fracture, treatment gap, policy
Introduction
Over the last 50 years, there have been major advances in the management of osteoporosis, encompassing its diagnosis, the assessment of fracture risk, the development of therapies to reduce the risk of fractures, and the production of clinical guidelines (1, 2). However, despite this evolution, a minority of men and women at high fracture risk actually receive treatment (3–5). This is true even in patients who have already sustained a fragility fracture, with some studies documenting fewer than 20% receiving therapies to reduce the risk of a further fracture in the year following the index fracture event (6, 7). Rates of treatment are particularly poor for older women and people who live in long-term care. Large gaps in service provision exist, as indicated by disparities in the use of fracture risk assessment tools such as FRAX®, which vary one thousand-fold worldwide (8). This variability is far greater than the 30-fold variation in crude, or 10-fold variation in age-standardised hip fracture incidence globally (9, 10). These differences may only partially be explained by limitations in access to the internet, lack of national assessment guidelines for osteoporosis in many countries, and the availability of alternative assessment algorithms (9). The under-assessment and treatment of those at very high risk of further fracture is concerning, but even more worrying is the apparent downward trend in treatment with antiosteoporosis medications after hip fracture, demonstrated both in the USA, European and UK populations (11, 12). In this review, we give an overview of the burden of disease and the reasons for suboptimal osteoporosis care, or treatment gaps, at all levels; we conclude with a discussion of possible approaches to remedy this situation.
Burden of disease
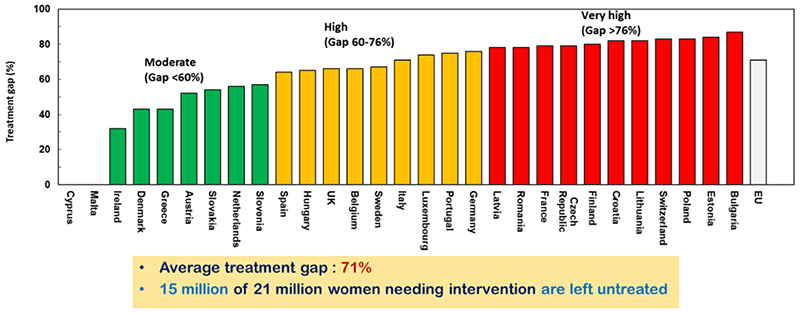
Recent work led by the International Osteoporosis Foundation (IOF) has comprehensively updated our understanding of the burden of disease consequent to osteoporosis and associated fragility fractures, service provision, care gaps and national policy, across Europe (8). The Scorecard for Osteoporosis in Europe thus documented information derived from a variety of sources across the 27 countries of the European Union plus the UK and Switzerland (termed EU27+2). The total cost, in 2019, of new fragility fractures, existing fragility fractures and pharmacological interventions was €56.9 billion. This equated to an average direct cost of osteoporotic fractures of €109.12 for each individual in the EU27+2, a notable increase from 2010 when the average for the EU27 was €82.77 (after adjusting for inflation). Overall, the total spend on healthcare in the EU27+2 amounted to €1.6 trillion, and the cost of osteoporotic fractures thus accounted for approximately 3.5% of healthcare spending, indicating a very substantial impact of fragility fractures on the present healthcare budgets of the EU countries. Using WHO criteria for its densitometric definition, there were approximately 32.0 million individuals with osteoporosis in the EU27+2 in 2019, of whom 6.5 million were men and 25.5 million were women. There were estimated to be 4.3 million new fragility fractures in the EU27+2 in 2019, equivalent to 11,705 fractures/day (or 487 per hour). About twice as many fractures occurred in women compared to men. Hip, vertebral, forearm and other fractures accounted for 19, 16, 15 and 50% of all fractures, respectively. Major osteoporotic fractures are associated with reduced relative survival (13), there were estimated to be 248,487 deaths causally related to fractures in 2019, which is comparable to, or exceeds, the number consequent to other common causes such as lung cancer, diabetes, chronic lower respiratory diseases. It is apparent in that this health burden is only going to increase, giving the increasingly ageing demographic and thus the annual number of osteoporotic fractures in the EU27+2 is projected to increase by 1.06 million from 4.28 million in 2019 to 5.05 million in 2034 (+24.8%). In this study, the treatment gap was clearly apparent (Figure 1), with the proportion of women at high fracture risk, but not receiving therapy for osteoporosis, being on average 71% but ranging from 32% to 87%, which appeared somewhat worse than the average 55% in 2010. In terms of numbers rather than percentages, the burden is even more alarming with, overall, 10.6 million women who were eligible for treatment being untreated in 2010, rising to 14.0 million in 2019.
Figure 1.
The treatment gap across Europe. The figure shows the percentage of women at high fracture risk who do not receive appropriate antiosteoporosis medication. Reproduced with permission from (8).
Such findings are highly congruent with previous data from both Europe and elsewhere globally, which also demonstrate potentially declining rates of treatment. Thus, for example in the UK, despite increases in use of antiresorptive over the preceding decade (14, 15), fewer than 50% of hip fracture patients receiving treatment, from around 2011, there was a plateau and a possible decrease in prescriptions occurred in the UK (16), with substantial heterogeneity by geographic region (17). Data from the GLOW study, a prospective observational study of over 60,000 older women recruited from primary care practices in 10 countries across US, Europe and Australia, demonstrated that more than 80% of women with a fragility fracture did not receive osteoporosis treatment (18). In another international prospective study of 1,795 patients who sustained a low-energy hip fractures in ten countries (Australia, Austria, Estonia, France, Italy, Lithuania, Mexico, Russia, Spain, and the UK), only 27% were prescribed pharmacological fracture prevention after the hip fracture (19).
In the U.S., a large retrospective analysis was conducted, based on U.S. administrative insurance claims data of nearly 100,000 men and women aged 50 years or more who were hospitalized for hip fracture (11). The uptake of osteoporosis medication within 12 months after discharge from hospital was examined; the estimated probability of receiving osteoporosis medication within 12 months after discharge from hospital was 28.5% over this time period but showed a significant decline over a 10-year interval, from 40.2% in 2002 to 20.5% in 2011 (11). Analysis of the US Medical Expenditure Panel Survey support these findings, demonstrating a marked reduction in the prevalence of bisphosphonate use particularly markedly amongst women, from 2007 onwards (20).
This it is clearly apparent that despite major advances in every area of the management of osteoporosis, a major care gap persists which requires attention at every level from patient to physician to policymaker. In the remainder of this article, we will review the potential reasons for the care gap and existing and novel approaches to its closure.
Potential origins of the care gaps in osteoporosis
Awareness and perception by patients and physicians
As shown by the studies cited above, there was a successful increase in antiresorptive treatment rates, both in primary and secondary fracture prevention until around ten years ago. The clinical situation was bolstered by advances in risk assessment and policy, for example through the use of risk calculators such as FRAX® (21, 22), guidance on intervention (23), together with the availability of generic bisphosphonates. In the context of this prospering field it is tragic that treatment rates, both before and after a fracture, have declined in recent years, despite inexorable expansion of the population at risk (24).
Many factors appear to contribute to the poor rates of treatment for osteoporosis. Insufficient implementation of strategies at a national and international level to effect primary and secondary prevention is one such reason. For patients and clinicians alike, primary fracture prevention is always made difficult by the concept of managing a future “risk”, rather than treating a disease event which has already happened. It is clear that musculoskeletal diseases are viewed both by patients and policymakers as a lower priority than outcomes such as myocardial infarction and cancer (3), despite the fact that the Global Burden of Disease initiative has demonstrated musculoskeletal disease to be a leading cause of disability worldwide (25).
There is a clear mismatch between the severity of the condition, and associated perceptions. Many do not recognise that a hip fracture, for example, is a devastating life event, with a 20% associated reduced survival compared with non-fracture peers (13). When compared with a parallel event such as an acute myocardial infarction, it would be impossible to imagine a situation in a higher-income country in which it would be acceptable for less than 50% of such cardiac patients to receive risk-reducing treatments such as aspirin, statins and antihypertensives (26). This misperception of risk is well documented in the large international GLOW cohort, in which many women underestimated their fracture risk compared with their peers (27). Perhaps, in a world where many populations are ageing and physicians and patients are dealing with multimorbidity, osteoporosis treatment falls to the bottom of the priority list.
Physicians’ perceptions of osteoporosis and efficacy of treatments have been further confused by inaccurate and harmful conclusions about the treatment of osteoporosis such as the articles from Järvinen et al. in the British Medical Journal and Journal of Internal Medicine (28, 29). These articles, claiming, for example that “the dominant approach to hip fracture prevention is neither viable as a public health strategy nor cost effective” and that “the main ways to prevent these fractures have not changed in nearly 25 years: stop smoking, be active and eat well” are frankly incorrect, unbalanced and refuted by overwhelming evidence (as stated by international and national societies such as the International Osteoporosis Foundation and the American Society for Bone and Mineral Research); nonetheless, such “fake news” presented in high impact journals has traction, and clear damage has been done (30).
Concerns regarding medication adverse effects
There are abundant data showing that alarming reports about osteoporosis medication in the media have been followed by a reduction in use of these medications, despite evidence that the benefits of treatment clearly outweigh the risks for the vast majority of users (31). In order to better understand patients’ concerns regarding medication safety, Jha et al. examined relationships between medication use (data from the Medical Expenditure Panel Survey and National Inpatient Sample in the US), internet search activity for alendronate between 2006 and 2010, and media reports of safety concerns (20). There were marked spikes of internet search activity corresponding to events such as a 2006 lawsuit filed against Merck for Fosamax allegedly causing osteonecrosis of the jaw, a major ABC World News feature on Fosamax and atypical femoral fractures (AFFs) in 2010, and several other media reports of such rare, but serious side effects, set against the backdrop of a decline in bisphosphonate use by more than 50% between 2008 and 2012. The Australian Longitudinal Study on Women’s Health findings were consistent with the US data; total use of antiosteoporosis medications increased over the period 2000 to 2007 but then decreased from 2007-2010. In Australia, indications for bone density testing had been relaxed and a subsidy for antiosteoporosis medications was introduced, but despite these interventions, the most marked declines in prescriptions coincided with adverse media stories such as a major report on osteonecrosis of the jaw (ONJ) in 2007 (32).
The serious long-term adverse side-effects of bisphosphonates are very rare in absolute terms (with incidences in the range of 1/100,000 to 1/10,000 per year) (33). However, the approach to risk/benefit communication has largely been on the side of declaring risk, amongst the media (as demonstrated above), physicians and policymakers. The fact that the underlying disease is associated with substantial morbidity and increased mortality, with fracture risk markedly reduced by antiosteoporosis medications, seems generally under-articulated in these discussions. For example, the recent UK National Institute for Health and Care Excellence (NICE) guidance on multi-morbidity (34) specifically targeted bisphosphonates for review after 3 years treatment despite evidence for longer term efficacy and safety being more reliable than for other treatments considered.
A comparison of the benefits vs. risks for BP therapy (35) indicated that the benefits for fracture reduction for short-term therapy for 3 to 5 years far outweigh any risks of AFFs. Under the most likely set of assumptions about AFF risk [relative risk of 1.7 for any BP use (36)], treating 10,000 osteoporotic women for 3 years, would lead to the prevention of 1000 fractures, including 110 hip fractures, whilst causing only 0.08 AFFs. Another way of stating this would be that for one AFF associated with 3 years of BP treatment, 1200 fractures (including about 130 hip and 850 vertebral fractures) would be prevented, indicating that the benefits of treatment far outweigh any AFF risks (37).
In more likely scenario of longer term use, the concerns regarding the rare side effects of AFFs and ONJ are compounded by studies suggesting that longer therapy duration increases these risks. This has led to the view that patients on long-term treatment with bisphosphonates or denosumab should always be offered a treatment holiday: however this is not well supported by the existing evidence. For example, rapid bone loss has been described following denosumab discontinuation with an incidence of vertebral fractures of around 5% (38). Reassuringly in terms of the risk-benefit ratio in longer term users of bisphosphonates, a study in the Danish population has demonstrated that users of alendronate still have a reduced risk of fracture compared with matched controls even after 10 years use, and that the number of hip fractures prevented is still substantially greater than the number of subtrochanteric femoral fractures occurring even by the end of a decade of bisphosphonate treatment (39). A new systematic review led by the International Osteoporosis Foundation has concluded that drug holidays should only be considered in patients at low risk of fracture (40), and recent international guidelines emphasise that osteoporosis treatment is a lifelong consideration, with treatment likely continued in those at highest risk(41). Thus, the field needs to dramatically improve its approach to communicating the risks and benefits of treatments, and to robustly counter ill-informed adverse media stories in a timely fashion.
Policies in healthcare and osteoporosis assessment
In comparison with comparable non-communicable diseases, osteoporosis has rarely attracted proportionate levels of attention from healthcare providers and governments. An individual nation’s policy on access to bone densitometry with dual-energy x-ray absorptiometry (DXA) and its reimbursement will greatly influence the assessment and treatment of this disease. Various regional audits have been published by the International Osteoporosis Foundation (IOF) (https://www.iofbonehealth.org/regional-audits) covering the European Union, Eastern Europe and Central Asia, Latin America, North America, the Middle East and Africa, Asia Pacific. These have demonstrated large variations in terms of epidemiology, burden and costs of osteoporosis. For example, in the Asia Pacific region, whilst Australia, Hong Kong, Japan, New Zealand, Republic of Korea and Singapore had 12-24 DXA machines per million of population, China, India, Indonesia, Pakistan, Philippines, Sri Lanka and Vietnam were greatly under-resourced with less than 1 DXA machine per million of population. The audits demonstrated that BMD testing and osteoporosis treatment were not fully reimbursed by insurance or healthcare policies in many countries, which served as a barrier to accessing treatment. Similar inequalities were seen in Europe, where it was assumed that 11 DXA machines per million of population were needed to provide adequate osteoporosis care (8). In North America (though no official IOF audit is available), reimbursement for treatment also varies greatly, depending on each individual patient’s health insurance plan. Healthcare reform is evolving in the USA from a “fee for service” system to supporting improved disease prevention and care coordination, with financial incentives to encourage healthcare professionals or systems to improve patient outcomes. The number of DXA providers has fallen following a major drop in reimbursement for DXA scans in the office setting, resulting in more than 1 million fewer DXA scans performed per annum (42). This coincides with a plateau in the secular decline in age and sex adjusted hip fracture rates which had been present up until 2012 (43).
Existing and novel approaches to closing osteoporosis care gaps
Secondary prevention: risk minimisation following an index fracture
As indicated by the evidence detailed above, osteoporotic fractures place a huge burden on societies across the world. As osteoporosis is a silent disease until a fracture occurs, and patient perception of fracture risk is often underestimated (44, 45), initiation of primary prevention is usually reliant on health care practitioners. Secondary prevention (identifying individuals for treatment based on a low trauma fragility fracture occurring) is therefore the approach usually taken as the starting point for fracture prevention.
Several methods have been explored to enable fracture risk assessment and initiation of appropriate treatment, some staff based, some IT-based and others a combination of the two. The most successful systems have been shown to focus on a multi-disciplinary Fracture Liaison Service (FLS) (46, 47), incorporating orthogeriatricians, rheumatologists, other physicians and clinical nurse specialists. Members of the FLS multidisciplinary team work together to ensure that medical management of patients admitted with fracture is optimised, both whilst in hospital and for future fracture prevention, ideally with a lead clinician responsible for coordinating the team (48). The Capture the Fracture® initiative, instituted by the International Osteoporosis Foundation (http://www.capturethefracture.org/) is “a global campaign to facilitate the implementation of coordinated, multi-disciplinary models of care for secondary fracture prevention.” This initiative has provided guidance on secondary fracture prevention, and also a global map, with a quality grading scheme, on which, subject to application, secondary fracture prevention services can be documented (49, 50). Huge variation in the availability, scope and quality of secondary prevention facilities has been observed, not only within, but also between countries. The aim of the Capture the Fracture initiative is to raise the quality and coverage of fracture liaison services providing secondary prevention for osteoporosis, providing a clinically valuable and cost-effective contribution to service improvement (51, 52).
Vertebral fracture case finding is an additive approach to secondary fracture prevention as many such events go undetected – around 12% of postmenopausal women with osteoporosis have at least one vertebral deformity, with less than a third of these individuals coming to clinical attention (53). Screening strategies based in primary care (54), and history-taking strategies distinguishing back pain likely to relate to vertebral fracture from other types of back pain may facilitate detection of these fractures (55). Different methods for radiological assessment of vertebral fractures exist, consistent reporting of radiographs, CT scans and the incorporation of vertebral fracture assessment in DXA scans (56), using quantitative and qualitative morphometric techniques and algorithms, plus the use of artificial intelligence technologies on standard CT scans will help with secondary fracture prevention in individuals with prevalent osteoporotic vertebral fracture (57, 58).
Primary prevention: commencing treatment in individuals at high fracture risk
Whilst DXA screening is officially a standard policy in the US (at the age of 65 years in women, and age 70 in men, and in individuals over the age of 50 years who have suffered an adult fracture) (59), in the majority of countries, primary prevention is focused more on opportunistic case-finding, triggered by the presence of clinical risk factors (23, 60–62). However, based on evidence from recent randomised controlled trials, there is increasing support for introduction of a screening based approach to systematic identification of individuals at high fracture risk in the community or primary care setting, led by the International Osteoporosis Foundation internationally, and in the UK by the Royal Osteoporosis Society Osteoporosis and Bone Research Academy (63).
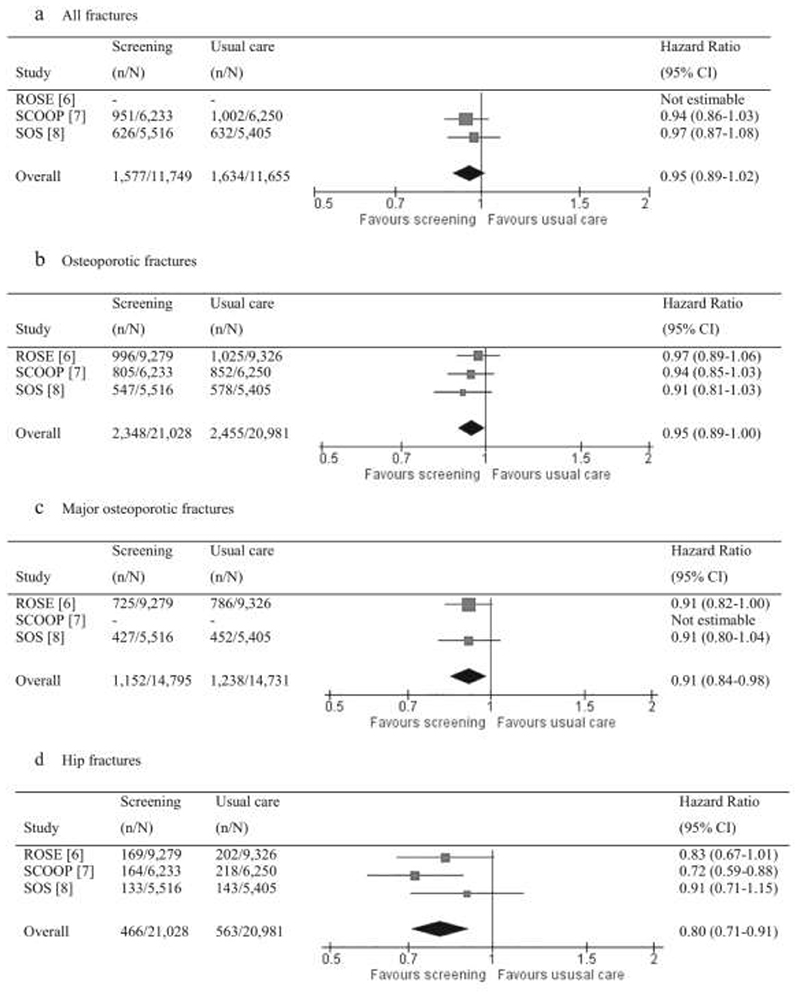
Thus, in the UK, a seven-centre randomised controlled trial (the UK SCOOP study) investigated the clinical and cost-effectiveness of screening older women in primary care for the prevention of fractures. Approximately 12,500 older women were randomised to either normal care or screening and subsequent treatment (stratified using FRAX hip fracture probability). The trial demonstrated that this intervention led to a 28% reduction in hip fracture risk (64, 65). Screening appeared most effective in those at highest baseline fracture risk (as would be expected, since these were the individuals targeted for treatment) (66), and importantly, was shown to be cost-effective (67): the findings suggested that screening 1000 patients prevents 9 hip fractures and 20 non-hip fractures over the remaining lifetime (mean 14 years), compared with usual management. In total, the screening arm saved costs (£286) and gained 0.015 quality adjusted life years per patient (68). The finding that women who were identified by FRAX as moderate or high risk of fracture benefited most from a screening programme was supported by the Danish Risk Stratified Osteoporosis Strategy Evaluation (ROSE) study, though this study found no overall effect on fracture incidence of a screening strategy (69). However, a systematic review (Figure 2) combining data from these two trials together with a further trial in the Netherlands (SALT) demonstrated a reduction in both major osteoporotic fractures [hazard ratio 0.91 (95% CI: 0.84, 0.98)] and hip fractures [0.80 (0.71, 0.91)] (70). A recent evidence report and systematic review for the US Preventive Services Task Force concluded that screening to prevent osteoporosis in women may reduce hip fractures (71).
Figure 2.
Meta-analysis of results from the SCOOP, ROSE and SOS trials of screening for fracture risk. Reused with permission from (70).
Personalised medicine in osteoporosis
Treatment targeted according to low, high and very high fracture risk
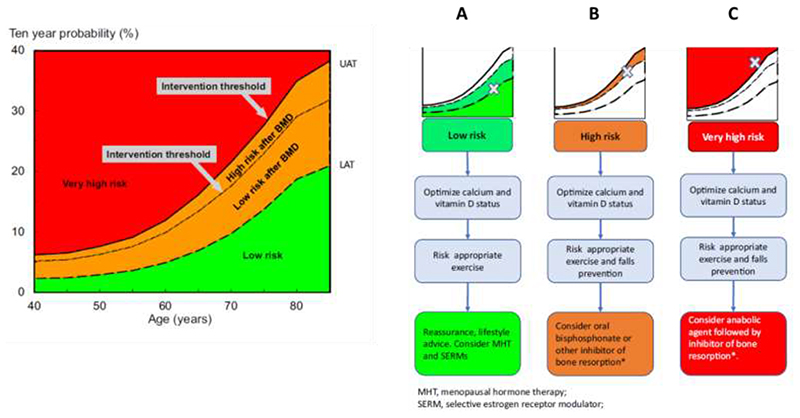
Whilst there is clearly a major issue with treating patients at all, there is also increasing evidence to suggest that stratification of treatment according to baseline fracture risk may permit targeting of the most effective treatments to patients at the highest fracture risk (72, 73). Such a strategy would ensure greatest rates of fracture risk reduction in those most likely to fracture and thus contribute to addressing the current treatment gap as well as maximising benefits for the most vulnerable individuals. Some treatments for osteoporosis, for example oral bisphosphonates, menopausal hormonal therapy (MHT) and selective oestrogen receptor modulators (SERMs) have suboptimal efficacy; studies of goal-directed treatment in osteoporosis have highlighted difficulties in meeting treatment goals with such therapies in the highest fracture risk patients (74). Consequently, the IOF and European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) published guidance for the diagnosis and management of osteoporosis in 2019, with subsequent recommendations (Figure 3) on treatment stratification in 2020 (75), stating that, in patients at the highest risk of fracture, treatment initiation with an anabolic (bone-forming) agent such as teriparatide, abaloparatide or romosozumab, followed by an antiresorptive to maintain the gains in bone mineral density, appears now a highly appropriate strategy to achieve a rapid and sustained reduction in fracture risk (41, 76).
Figure 3.
Personalisation of antiosteoporosis treatment according to baseline fracture risk. Initial risk assessment is performed using FRAX with clinical risk factors alone. FRAX probability in the red zone indicates very high risk, in which pathway C may be appropriate (anabolic agent followed by an inhibitor of bone resorption). FRAX probability in the green zone suggests low risk, in which pathway A should be followed, with advice to be given on lifestyle, calcium and vitamin D nutrition and menopausal hormonal treatment considered. FRAX probability in the orange zone (intermediate, between the upper assessment threshold, UAT, and lower assessment threshold, LAT) should be followed by BMD assessment and recalculation of FRAX probability including femoral neck BMD. After recalculation, risk may therefore be in the red zone (very high risk), orange zone (high risk, pathway B, which suggests initial antiresorptive therapy), reproduced with permission from (96).
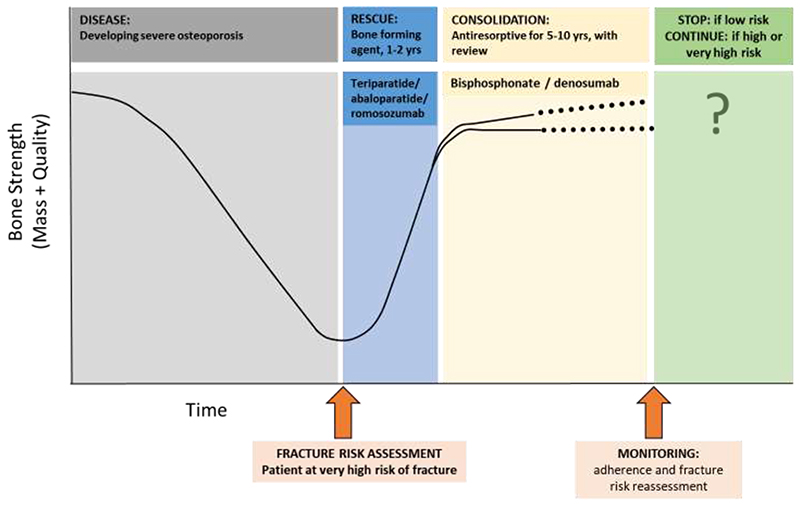
This recommendation has strong evidential support from recent studies comparing anabolic with antiresorptive medications, demonstrating a more rapid and greater fracture risk reduction with the former, compared with antiresorptive treatments alone (77–80). This was formalised as a management strategy in a recent position paper, again deriving from an ESCSEO working group (Figure 4), in which the clinical approach was described (73). Whilst a number of approaches have been developed (81, 82) to address the characterisation of individuals at very high fracture risk, the best developed is that based on the FRAX® fracture risk calculator (73, 75).
Figure 4.
Outline of a recommended approach to sequential therapy: in a patient with severe osteoporosis at high imminent risk of fracture following fracture risk assessment, a bone forming agent for 1-2 years is recommended (duration according to prescribing guidelines). Following this, bone-forming therapy, a consolidation period of antiresorptive therapy (such as a bisphosphonate or denosumab) is recommended. Monitoring, including assessment of treatment adherence and reassessment of fracture risk, is required. Reproduced with permission from (73).
Consistent with the age-dependent approach to the intervention threshold (that is, the probability conferred by a prior fracture in the absence of other risk factors or BMD, and with an average BMI), in the IOF-ESCEO approach, very high risk can be defined as a fracture probability that lies above the upper assessment threshold (1.2 times the intervention threshold) after a FRAX assessment, with or without the inclusion of BMD, i.e. where BMD testing is unavailable, the same probability threshold can be used (75). A similar approach has been applied nationally in the UK National Osteoporosis Guideline Group recommendations (83), with the threshold adapted to incorporate the constant probability threshold above the age of 70 years in this hybrid setting (84). The next question to address is what attributes and clinical risk factors are associated with FRAX probabilities in low, high and very high fracture risk categories. In this setting, it is apparent that the presence of a single clinical risk factor rarely leads to very high fracture risk categorisation but a combination of risk factors, particularly older age, recent fracture and glucocorticoid use, more frequently result in this high fracture risk outcome (83).
Modification of fracture risk calculation
The trajectory of risk associated with the recent prior fracture appears to be a particularly important, but by no means exclusive, contributor to very high fracture risk categorisation. To this end, several studies have demonstrated that fracture risk is acutely elevated immediately after an index fracture and that this elevated risk wanes over the succeeding 2 years (this transiently elevated risk can be termed “imminent” risk), but does not return to baseline and subsequently increases with age (85–88). Thus, a fracture at any time in the past is associated with increased risk of an incident fracture event, but an index fracture is associated with a marked excess fracture risk over and above this in the next two years (81, 89). It is also apparent that fracture probability varies according to the site of a prior fracture, with probability being greater following a vertebral or hip than a radius fracture (89). This pattern has been most comprehensively assessed in the Iceland Reykjavík cohort (85, 89), and further data from the Reykjavik Study have shown that, in individuals who sustained a recurrent fracture, 31-45% of fractures occurred within one year of the first (sentinel) fracture, depending on the fracture site (89). Further work using this cohort has demonstrated that the transient risk increase following an index fracture is of sufficient magnitude to materially alter the 10-year probability of fracture generated by the FRAX tool (89). Importantly, the currently available tool does not incorporate recency of fracture, or indeed a different risk associated with different fracture sites and therefore will underestimate 10-year fracture probability in the context of a prior fracture in the last 2 years. To address this situation, multipliers specific to age, sex and fracture site have been generated to enable the physician to accommodate the excess risk associated with recency and particular fracture types (90). The multiplier decreases with age, partly because of the competing effect of mortality with which recency is also associated. However, because fracture probability is so strongly dependent upon age, the final adjusted absolute probability is almost always greater at older compared with younger ages (90). It is also apparent that the magnitude of the absolute fracture probability is always greater when viewed over a 10 year, than over a 2-year, time horizon (91). Development of a platform enabling the easy incorporation of the multiplier as a modifier of the FRAX calculator online is ongoing and will be available as FRAXPLUS. A key advantage of this approach is that recency and site of fracture, along with other modifiers of FRAX probability, for example dose of glucocorticoids, history of diabetes, trabecular bone score and lumbar spine-femoral neck BMD disparities, can be used to modify FRAX probability in a way that is immediately interpretable in the context of current national guidelines which are based on 10-year FRAX probability (22, 41, 73, 92). The limitation of calculators and algorithms estimating fracture risk over the next 2 years is that, at present, there is generally no guideline infrastructure through which the outputs can be directly incorporated into clinical practice and there are few data to support their generalisability into other country settings. On a practical note, the key message in terms of fracture recency is that a fracture event requires urgent assessment of fracture risk and intervention with antiosteoporosis medications.
Conclusion
Osteoporosis has undergone a dramatic transformation over the last five decades or so, from having been viewed as an inevitable consequence of ageing, to now being a well characterised chronic non-communicable disease, with diagnostic criteria, validated methods of risk assessment and a range of effective therapeutic medications. Despite this backdrop, however, there is evidence from the UK, US and continental Europe that treatment rates have declined substantially in recent years. With ageing populations and overstretched health services, osteoporosis may often fall off the bottom of the list of priorities for patients and clinicians alike. The rare but serious side effects of antiresorptives have become a disproportionately (and inappropriately) major concern, compounded by dramatic and widespread media reports, which have usually been inadequately countered by the clinical community. Indeed, in many cases doctors, dentists and patients now appear more frightened of the treatment than they are of the disease itself (7).
The clear imperative to tackle this issue has been recognised by key organisations such as the International Osteoporosis Foundation and the American Society for Bone and Mineral Research, leading to the publication of recommendations and roadmaps to address the critical care gap in osteoporosis treatment (4, 93), reflected in national positions from societies such as the UK Royal Osteoporosis Society (63). Improved public awareness and public health strategies to optimise bone health from a young age will also contribute to prevention of osteoporosis in future generations (94). Novel strategies to implement systematic identification of individuals at high fracture risk in primary care, and to personalise management by targeting the most effective interventions to those at the highest fracture risk, are likely to be essential components in optimisation of osteoporosis care. Given the rapid ageing of the global population and the importance of good musculoskeletal health in old age, we must come together to ensure that during the coming decade, 2020-2030, hailed by the WHO and others as the “Decade of Healthy Ageing”, bone health and fracture prevention become the priority they so urgently need to be.
Practice points.
Osteoporosis and associated fragility fractures are common and are a leading cause of disability and mortality.
Despite major advances in recent decades, there are substantial treatment gaps and thus most individuals at high fracture risk do not receive appropriate treatment for osteoporosis.
Evidence is accumulating to support systematic primary care focused identification of those at high fracture risk in a screening-based approach to fracture prevention.
Recommendations such as European guidelines, and those from the UK National Osteoporosis Guideline Group, based on the FRAX® fracture risk calculator, can be used to assess fracture risk.
Emerging work has demonstrated that treatment decisions can be refined further based on calculated fracture risk using FRAX. Anabolic and parenteral treatments can therefore be directed to those at very high fracture risk.
Recent fracture is an indication of very high fracture risk and requires urgent assessment and treatment with antiosteoporosis medications to reduce the risk of a further fracture.
Major gaps in osteoporosis management and service provision require urgent attention at every level from public awareness to healthcare systems to national policy.
Research priorities.
Evaluation of barriers and facilitators to the systematic identification of individuals at high fracture risk in primary care.
Investigation of technological solutions to automated screening for high fracture risk in populations.
Demonstration of impact and cost effectiveness of new approaches to antiosteoporosis treatment stratified according to baseline fracture risk.
Studies to increase understanding and optimisation of communication of risk between physicians and patients.
Acknowledgements
We would like to thank the UK Medical Research Council (MC_PC_21003; MC_PC_21001), UK Medical Research Foundation (MRF-145-0011-DG-HARV-C0913), National Institute for Health Research, Wellcome Trust, Versus Arthritis, Royal Osteoporosis Society Osteoporosis and Bone Research Academy and International Osteoporosis Foundation for supporting this work. This article includes text reproduced/adapted, with permission from (1, 2, 5, 8, 63, 73, 95).
Footnotes
Disclosures
EC reports honoraria/travel support from Eli Lilly, UCB and Amgen outside the submitted work. EMD reports no conflicts of interest. CC reports personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare, UCB, Kyowa Kirin and Internis Pharma, outside the submitted work.
Ethical Approval
This review article does not present any previously unpublished original research, and ethical approval is therefore not applicable.
References
- 1.Curtis EM, Woolford S, Holmes C, Cooper C, Harvey NC. General and Specific Considerations as to why Osteoporosis-Related Care Is Often Suboptimal. Current osteoporosis reports. 2020;18(1):38–46. doi: 10.1007/s11914-020-00566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. doi: 10.1016/j.bone.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, et al. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2017;28(5):1507–29. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey NC, McCloskey E. Gaps and solutions in bone health: A global framework for improvement. 2016. [Google Scholar]
- 5.Liu J, Curtis EM, Cooper C, Harvey NC. State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest. 2019;42(10):1149–64. doi: 10.1007/s40618-019-01041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Seminars in arthritis and rheumatism. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Svedbom A, Harvey N, McCloskey EV. The osteoporosis treatment gap. J Bone Miner Res. 2014;29(9):1926–8. doi: 10.1002/jbmr.2301. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Archives of osteoporosis. 2021;16(1):82. doi: 10.1007/s11657-020-00871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanis JA, Johansson H, Oden A, Cooper C, McCloskey EV. Worldwide uptake of FRAX. Archives of osteoporosis. 2014;9(1):166. doi: 10.1007/s11657-013-0166-8. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–56. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–37. doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velde RY, Wyers CE, Teesselink E, Geusens PP, van den Bergh JP, de Vries F, et al. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: Variation by age, sex, geographic location and ethnicity. Bone. 2016;94:50–5. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6(2):99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 14.Klop C, Welsing PM, Cooper C, Harvey NC, Elders PJ, Bijlsma JW, et al. Mortality in British hip fracture patients, 2000-2010: a population-based retrospective cohort study. Bone. 2014;66:171–7. doi: 10.1016/j.bone.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 15.van der Velde RY, Wyers CE, Teesselink E, Geusens PP, van den Bergh JP, de Vries F, et al. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: Variation by age, sex, geographic location and ethnicity. Bone. 2017;94:50–5. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis. J Bone Miner Res. 2016;31(11):2008–15. doi: 10.1002/jbmr.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah A, Prieto-Alhambra D, Hawley S, Delmestri A, Lippett J, Cooper C, et al. Geographic variation in secondary fracture prevention after a hip fracture during 1999-2013: a UK study. Osteoporos Int. 2017;28(1):169–78. doi: 10.1007/s00198-016-3811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan SL, Wyman A, Hooven FH, Adami S, Gehlbach S, Anderson FA, Jr, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. Journal of the American Geriatrics Society. 2012;60(3):455–61. doi: 10.1111/j.1532-5415.2011.03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Archives of osteoporosis. 2013;8(1–2):137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996-2012: An Ecological Analysis. J Bone Miner Res. 2015;30(12):2179–87. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, et al. Interpretation and use of FRAX in clinical practice. OsteoporosInt. 2011;22(9):2395–411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV. A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Archives of osteoporosis. 2016;11(1):25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26(9):2243–8. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 25.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Tu JV, Ko DT, Alter DA. Factors associated with the use of evidence-based therapies after discharge among elderly patients with myocardial infarction. CMAJ. 2008;179(9):901–8. doi: 10.1503/cmaj.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siris ES, Gehlbach S, Adachi JD, Boonen S, Chapurlat RD, Compston JE, et al. Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW) Osteoporos Int. 2011;22(1):27–35. doi: 10.1007/s00198-010-1211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Järvinen TL, Michaëlsson K, Jokihaara J, Collins GS, Perry TL, Mintzes B, et al. Overdiagnosis of bone fragility in the quest to prevent hip fracture. BMJ: British Medical Journal. 2015;350:h2088. doi: 10.1136/bmj.h2088. [DOI] [PubMed] [Google Scholar]
- 29.Järvinen TLN, Michaëlsson K, Aspenberg P, Sievänen H. Osteoporosis: the emperor has no clothes. Journal of internal medicine. 2015;277(6):662–73. doi: 10.1111/joim.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compston J. Overdiagnosis of osteoporosis: fact or fallacy? Osteoporos Int. 2015;26(8):2051–4. doi: 10.1007/s00198-015-3220-0. [DOI] [PubMed] [Google Scholar]
- 31.Cipriani C, Pepe J, Minisola S, Lewiecki EM. Adverse effects of media reports on the treatment of osteoporosis. J Endocrinol Invest. 2018;41(12):1359–64. doi: 10.1007/s40618-018-0898-9. [DOI] [PubMed] [Google Scholar]
- 32.Peeters G, Tett SE, Duncan EL, Mishra GD, Dobson AJ. Osteoporosis medication dispensing for older Australian women from 2002 to 2010: influences of publications, guidelines, marketing activities and policy. Pharmacoepidemiology and drug safety. 2014;23(12):1303–11. doi: 10.1002/pds.3703. [DOI] [PubMed] [Google Scholar]
- 33.Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer C, Fenu E, O’Flynn N, Guthrie B. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016;354:i4843. doi: 10.1136/bmj.i4843. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. The New England journal of medicine. 2016;374(3):254–62. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 36.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N. Atypical Femur Fractures: Review of Epidemiology, Relationship to Bisphosphonates, Prevention, and Clinical Management. Endocrine reviews. 2019;40(2):333–68. doi: 10.1210/er.2018-00001. [DOI] [PubMed] [Google Scholar]
- 38.Brown JP, Roux C, Törring O, Ho P-R, Beck Jensen J-E, Gilchrist N, et al. Discontinuation of denosumab and associated fracture incidence: Analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) Trial. Journal of Bone and Mineral Research. 2013;28(4):746–52. doi: 10.1002/jbmr.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. Bmj. 2016;353:i3365. doi: 10.1136/bmj.i3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennison EM, Cooper C, Kanis JA, Bruyere O, Silverman S, McCloskey E, et al. Fracture risk following intermission of osteoporosis therapy. Osteoporos Int. 2019 doi: 10.1007/s00198-019-05002-w. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overman RA, Farley JF, Curtis JR, Zhang J, Gourlay ML, Deal CL. DXA Utilization Between 2006 and 2012 in Commercially Insured Younger Postmenopausal Women. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2015;18(2):145–9. doi: 10.1016/j.jocd.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewiecki EM, Adler RA, Curtis J, Gagel RF, Saag KG, Singer A, et al. Hip fractures and declining DXA testing: at a breaking point? J Bone Miner Res. 2016 [Google Scholar]
- 44.Grover ML, Edwards FD, Chang YH, Cook CB, Behrens MC, Dueck AC. Fracture risk perception study: patient self-perceptions of bone health often disagree with calculated fracture risk. Women’s health issues: official publication of the Jacobs Institute of Women’s Health. 2014;24(1):e69-75. doi: 10.1016/j.whi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Gregson CL, Dennison EM, Compston JE, Adami S, Adachi JD, Anderson FA, Jr, et al. Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int. 2014;25(1):85–95. doi: 10.1007/s00198-013-2438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Current osteoporosis reports. 2013;11(1):52–60. doi: 10.1007/s11914-012-0130-3. [DOI] [PubMed] [Google Scholar]
- 48.Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteoporos Int. 2016;27(5):1719–27. doi: 10.1007/s00198-015-3452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24(8):2135–52. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Javaid MK, Kyer C, Mitchell PJ, Chana J, Moss C, Edwards MH, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture(R) Best Practice Framework tool. Osteoporos Int. 2015;26(11):2573–8. doi: 10.1007/s00198-015-3192-0. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell P, Akesson K, Chandran M, Cooper C, Ganda K, Schneider M. Implementation of Models of Care for secondary osteoporotic fracture prevention and orthogeriatric Models of Care for osteoporotic hip fracture. Best practice & research Clinical rheumatology. 2016;30(3):536–58. doi: 10.1016/j.berh.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Javaid MK, Sami A, Lems W, Mitchell P, Thomas T, Singer A, et al. A patient-level key performance indicator set to measure the effectiveness of fracture liaison services and guide quality improvement: a position paper of the IOF Capture the Fracture Working Group, National Osteoporosis Foundation and Fragility Fracture Network. Osteoporos Int. 2020;31(7):1193–204. doi: 10.1007/s00198-020-05377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7(2):221–7. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 54.Clark EM, Gould V, Morrison L, Ades AE, Dieppe P, Tobias JH. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(3):664–71. doi: 10.1002/jbmr.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark EM, Gooberman-Hill R, Peters TJ. Using self-reports of pain and other variables to distinguish between older women with back pain due to vertebral fractures and those with back pain due to degenerative changes. Osteoporos Int. 2016;27(4):1459–67. doi: 10.1007/s00198-015-3397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lems WF, Paccou J, Zhang J, Fuggle NR, Chandran M, Harvey NC, et al. Vertebral fracture: epidemiology, impact and use of DXA vertebral fracture assessment in fracture liaison services. Osteoporos Int. 2021;32(3):399–411. doi: 10.1007/s00198-020-05804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oei L, Koromani F, Breda SJ, Schousboe JT, Clark EM, van Meurs JB, et al. Osteoporotic Vertebral Fracture Prevalence Varies Widely Between Qualitative and Quantitative Radiological Assessment Methods: The Rotterdam Study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2018;33(4):560–8. doi: 10.1002/jbmr.3220. [DOI] [PubMed] [Google Scholar]
- 58.Muehlematter UJ, Mannil M, Becker AS, Vokinger KN, Finkenstaedt T, Osterhoff G, et al. Vertebral body insufficiency fractures: detection of vertebrae at risk on standard CT images using texture analysis and machine learning. European radiology. 2019;29(5):2207–17. doi: 10.1007/s00330-018-5846-8. [DOI] [PubMed] [Google Scholar]
- 59.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23(9):2257–76. doi: 10.1007/s00198-012-1958-1. [DOI] [PubMed] [Google Scholar]
- 61.Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Archives of osteoporosis. 2012;7(1–2):25–30. doi: 10.1007/s11657-012-0070-7. [DOI] [PubMed] [Google Scholar]
- 62.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Archives of osteoporosis. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harvey NC, Poole KE, Ralston SH, McCloskey EV, Sangan CB, Wiggins L, et al. Towards a cure for osteoporosis: the UK Royal Osteoporosis Society (ROS) Osteoporosis Research Roadmap. Archives of osteoporosis. 2022;17(1):12. doi: 10.1007/s11657-021-01049-7. [DOI] [PubMed] [Google Scholar]
- 64.Shepstone L, Fordham R, Lenaghan E, Harvey I, Cooper C, Gittoes N, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23(10):2507–15. doi: 10.1007/s00198-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 65.Shepstone L, Lenaghan E, Cooper C, Clarke S, Fong-Soe-Khioe R, Fordham R, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018;391(10122):741–7. doi: 10.1016/S0140-6736(17)32640-5. [DOI] [PubMed] [Google Scholar]
- 66.McCloskey E, Johansson H, Harvey NC, Shepstone L, Lenaghan E, Fordham R, et al. Management of Patients With High Baseline Hip Fracture Risk by FRAX Reduces Hip Fractures-A Post Hoc Analysis of the SCOOP Study. J Bone Miner Res. 2018;33(6):1020–6. doi: 10.1002/jbmr.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner DA, Khioe RFS, Shepstone L, Lenaghan E, Cooper C, Gittoes N, et al. The Cost-Effectiveness of Screening in the Community to Reduce Osteoporotic Fractures in Older Women in the UK: Economic Evaluation of the SCOOP Study. J Bone Miner Res. 2018;33(5):845–51. doi: 10.1002/jbmr.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Söreskog E, Borgström F, Shepstone L, Clarke S, Cooper C, Harvey I, et al. Long-term cost-effectiveness of screening for fracture risk in a UK primary care setting: the SCOOP study. Osteoporos Int. 2020;31(8):1499–506. doi: 10.1007/s00198-020-05372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubin KH, Rothmann MJ, Holmberg T, Hoiberg M, Moller S, Barkmann R, et al. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int. 2018;29(3):567–78. doi: 10.1007/s00198-017-4326-3. [DOI] [PubMed] [Google Scholar]
- 70.Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM. Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2020;31(2):251–7. doi: 10.1007/s00198-019-05226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viswanathan M, Reddy S, Berkman N, Cullen K, Middleton JC, Nicholson WK, et al. Screening to Prevent Osteoporotic Fractures: Updated Evidence Report and Systematic Review for the US Preventive Services Task ForceUSPSTF Evidence Report: Screening to Prevent Osteoporotic FracturesUSPSTF Evidence Report: Screening to Prevent Osteoporotic Fractures. Jama. 2018;319(24):2532–51. doi: 10.1001/jama.2018.6537. [DOI] [PubMed] [Google Scholar]
- 72.McClung MR. Role of bone-forming agents in the management of osteoporosis. Aging clinical and experimental research. 2021;33(4):775–91. doi: 10.1007/s40520-020-01708-8. [DOI] [PubMed] [Google Scholar]
- 73.Curtis EM, Reginster JY, Al-Daghri N, Biver E, Brandi ML, Cavalier E, et al. Management of patients at very high risk of osteoporotic fractures through sequential treatments. Aging clinical and experimental research. 2022 doi: 10.1007/s40520-022-02100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC, Black DM, et al. Goal-Directed Treatment for Osteoporosis: A Progress Report From the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2017;32(1):3–10. doi: 10.1002/jbmr.3039. [DOI] [PubMed] [Google Scholar]
- 75.Kanis JA, Harvey NC, McCloskey E, Bruyére O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanis JA, Cooper C, Rizzoli R, Abrahamsen B, Al-Daghri NM, Brandi ML, et al. Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int. 2017;28(7):2023–34. doi: 10.1007/s00198-017-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40. doi: 10.1016/S0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 78.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017;377(15):1417–27. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 79.Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, et al. Efficacy of Pharmacological Therapies for the Prevention of Fractures in Postmenopausal Women: A Network Meta-Analysis. The Journal of clinical endocrinology and metabolism. 2019;104(5):1623–30. doi: 10.1210/jc.2019-00192. [DOI] [PubMed] [Google Scholar]
- 80.Díez-Pérez A, Marin F, Eriksen EF, Kendler DL, Krege JH, Delgado-Rodríguez M. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis. Bone. 2019;120:1–8. doi: 10.1016/j.bone.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 81.McCloskey EV, Borgstrom F, Cooper C, Harvey NC, Javaid MK, Lorentzon M, et al. Short time horizons for fracture prediction tools: time for a rethink. Osteoporos Int. 2021;32(6):1019–25. doi: 10.1007/s00198-021-05962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Javaid MK, Harvey NC, McCloskey EV, Kanis JA, Cooper C. Assessment and management of imminent fracture risk in the setting of the fracture liaison service. Osteoporos Int. 2022 doi: 10.1007/s00198-021-06284-9. In press. [DOI] [PubMed] [Google Scholar]
- 83.Kanis JA, Johansson H, Harvey NC, Lorentzon M, Liu E, Vandenput L, et al. An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines: A report for the National Osteoporosis Guideline Group (NOGG) Osteoporos Int. 2021;32(10):1951–60. doi: 10.1007/s00198-021-05942-2. [DOI] [PubMed] [Google Scholar]
- 84.McCloskey E, Kanis JA, Johansson H, Harvey N, Oden A, Cooper A, et al. FRAX-based assessment and intervention thresholds--an exploration of thresholds in women aged 50 years and older in the UK. Osteoporos Int. 2015;26(8):2091–9. doi: 10.1007/s00198-015-3176-0. [DOI] [PubMed] [Google Scholar]
- 85.Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, et al. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28(3):775–80. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–9. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 87.Ahmed LA, Center JR, Bjørnerem Å, Bluic D, Joakimsen RM, Jørgensen L, et al. Progressively increasing fracture risk with advancing age after initial incident fragility fracture: The Tromsø Study. Journal of Bone and Mineral Research. 2013;28(10):2214–21. doi: 10.1002/jbmr.1952. [DOI] [PubMed] [Google Scholar]
- 88.van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Annals of the rheumatic diseases. 2009;68(1):99–102. doi: 10.1136/ard.2008.092775. [DOI] [PubMed] [Google Scholar]
- 89.Kanis JA, Johansson H, Oden A, Harvey NC, Gudnason V, Sanders KM, et al. Characteristics of recurrent fractures. Osteoporos Int. 2018;29(8):1747–57. doi: 10.1007/s00198-018-4502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, et al. Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures. Osteoporos Int. 2020;31(10):1817–28. doi: 10.1007/s00198-020-05517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, et al. The use of 2-, 5-, and 10-year probabilities to characterize fracture risk after a recent sentinel fracture. Osteoporos Int. 2021;32(1):47–54. doi: 10.1007/s00198-020-05700-w. [DOI] [PubMed] [Google Scholar]
- 92.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Archives of osteoporosis. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. The Lancet Diabetes & Endocrinology. 2017;5(11):898–907. doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harvey N, Dennison E, Cooper C. Osteoporosis: a lifecourse approach. J Bone Miner Res. 2014;29(9):1917–25. doi: 10.1002/jbmr.2286. [DOI] [PubMed] [Google Scholar]
- 95.Harvey NCW, McCloskey EV, Rizzoli R, Kanis JA, Cooper C, Reginster J-Y. In: Encyclopedia of Endocrine Diseases (Second Edition) Huhtaniemi I, Martini L, editors. Academic Press; Oxford: 2019. Osteoporosis: Treatment Gaps and Health Economics; pp. 288–95. [Google Scholar]
- 96.Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2019 doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]