Abstract
Accurate measurement of central blood pressure (BP) using upper arm cuff-based methods is associated with several factors, including determining the level of systolic BP (SBP) amplification. This study aimed to determine the agreement between cuff-based and invasively measured SBP amplification.
Patients undergoing coronary angiography had invasive SBP amplification (brachial SBP – central SBP) measured simultaneously with cuff-based SBP amplification using a commercially available central BP device (device 1: Sphygmocor Xcel; n = 171, 70% men, 60 ± 10 years) and a now superseded model of a central BP device (device 2: Uscom BP+; n = 52, 83% men, 62 ± 10 years).
Mean difference (±2SD, limits of agreement) between cuff-based and invasive SBP amplification was 4 mmHg (−12, +20 mmHg, P < 0.001) for device 1 and −2 mmHg (−14, +10 mmHg, P = 0.10) for device 2. Both devices systematically overestimated SBP amplification at lower levels and underestimated at higher levels of invasive SBP amplification, but with stronger bias for device 1 (r = −0.68 vs. r = −0.52; Z = 2.72; P = 0.008). Concordance of cuff-based and invasive SBP amplification across quartiles of invasive SBP amplification was low, particularly in the lowest and highest quartiles. The root mean square errors from regression between cuff-based central SBP and brachial SBP were significantly lower (indicating less variability) than from invasive regression models (P < 0.001).
Irrespective of the difference from invasive measurements, cuff-based estimates of SBP amplification showed evidence of proportional systematic bias and had less individual variability. These observations could provide insights on how to improve the performance of cuff-based central BP.
Keywords: angiography, blood pressure determination, pulse wave analysis
Abbreviations
- AIx
augmentation index
- BP
blood pressure
- PP
pulse pressure
Introduction
High blood pressure (BP) is a leading modifiable risk factor for cardiovascular morbidity and mortality worldwide [1]. Accurate measurement of BP is, therefore, critical to enable correct diagnosis and best clinical practice to lower high BP [2]. Although cuff BP is the principal measurement method, it does not always reflect individual intra-arterial BP, either at the aortic or brachial arteries [3]. In an attempt to provide more accurate and clinically relevant assessment of BP, noninvasive devices have been developed with the goal of estimating central aortic BP as distinct from standard cuff BP [4,5]. These devices employ pulse wave analysis techniques to derive central aortic BP from a BP waveform recorded at a peripheral artery [6–8]. This technology has now been incorporated into upper arm cuff-based BP devices with operation similar to standard cuff BP to enable ease of use in clinical practice.
Accurate estimation of central aortic BP relative to intra-arterial aortic BP using upper arm cuff-based methods is associated with several factors, including accurately determining the level of intra-arterial systolic blood pressure (SBP) amplification (brachial SBP - aortic SBP) [9]. Although invasive SBP amplification is highly variable between individuals [10], there is an assumption that central BP devices calibrated with standard cuff SBP and diastolic BP (DBP) estimate SBP amplification relatively accurately compared to intra-arterial SBP amplification [4,11]. However, to our knowledge only one study has assessed this in a mostly male, high-risk group of 45 patients, and found a low level of agreement between cuff-based estimation of SBP amplification and invasively measured SBP amplification [12]. Our study aimed to extend on this by determining the level of agreement between cuff and invasive SBP amplification in a larger study sample using two central BP devices, comprising one currently available device (Sphygmocor, Xcel, hereafter ‘device 1’) and a superseded device (Uscom BP+, hereafter ‘device 2’). This study has not evaluated the Uscom BP+ device that is currently available.
Methods
Participants
Four hundred and twenty-five patients scheduled to undergo coronary angiography at the Royal Hobart Hospital catheterization laboratory were approached for study involvement. Participants recruited until 15 May 2018 had cuff BP measurements with device 1 and after this date device 2 was used. The flow of participants excluded from the study and the analysis is depicted in Figure S1, Supplemental Digital Content. Briefly, exclusion criteria included inter-arm cuff BP difference ≥5 mmHg between cuff SBP and/or DBP (n = 23), aortic valve disease or arrhythmia (n = 57), intra-arterial access via femoral artery (n = 21), medical issues arising during the clinical procedure that prevented the research protocol (n = 41) or technical issues that prevented recording of cuff or intra-arterial BP (n = 20). A further 20 people declined to participate, leaving 243 eligible participants. From these, 20 had negative intra-arterial SBP amplification ≤-5 mmHg and were excluded. Although some level of negative amplification occurs during invasive measurement [13,14], SBP amplification is typically positive when estimated by noninvasive devices. For the purpose of this paper, participants with invasive SBP amplification values ≤-5 mmHg were excluded because this threshold is outside the bounds of random BP variation from zero [15]. In total, there were 223 participants (device 1: n = 171, 70% men, aged 60 ± 10 years; device 2: n = 52, 83% men, aged 62 ± 10 years) with complete data for analysis. Participant clinical characteristics were extracted from hospital medical records. The study protocols were approved by the University of Tasmania Health and Medical Human Research Ethics Committee (H0010566 and H0016939).
Intra-arterial (invasive) SBP amplification
Intra-arterial BP acquisition was conducted according to ARTERY guidelines [4] and the details have been described elsewhere [16]. In brief, a fluid-filled catheter [e.g., 5F or 6F, Judkins Left, multipurpose (Cordis Corporation, Hialeah, Florida, USA) or TIG (Terumo Corporation, Somerset, New Jersey, USA)] was advanced from the right radial artery access site and positioned in the ascending aorta within 1–5 cm of the aortic valve, with confirmation by fluoroscopy. The transducer (Meritrans DTXPlus, model DT-4812; Merit Medical, South Jordan, Utah, USA) was maintained on the catheter table at a height equivalent of the heart, and this system was calibrated and flushed before every acquisition of invasive central and brachial BP waveforms. To measure intra-arterial BP, the catheter was first positioned in the ascending aorta to capture invasive central BP waveforms and then pulled back to the mid-humeral level in the right brachial artery to record invasive brachial BP waveforms. All intra-arterial BP waveform signals were acquired at a sample rate of 1000 Hz via an analog-to-digital converter (PowerLab ML870; AD Instruments, Bella Vista, Australia) and recorded using acquisition software (LabChart 7, AD Instruments). Markers were inserted on the LabChart recording at the precise time each BP recording commenced at each arterial site and at the time of cuff BP measurement. Waveform signals were converted from Volts to mmHg via an offline 2-point calibration procedure as previously described [17]. The SBP was taken as the peak of the ensembled waveform and DBP as the nadir. SBP amplification was calculated as brachial SBP – central SBP, and pulse pressure (PP) amplification was calculated as brachial PP – central PP. Invasive aortic and brachial augmentation index (AIx) was calculated as the difference between the second (P2) and the first (P1) aortic and brachial systolic peak pressure and was presented as percentage of the corresponding PP, Aix (%) = [(P2 – P1)/PP] × 100.
Cuff-based (noninvasive) SBP amplification
Noninvasive brachial BP and waveforms used to estimate central BP and SBP amplification were captured using two commercial BP devices. Cuff measurements taken at time of invasive brachial BP for these devices were closely aligned with invasive amplification. For the currently available device 1 (Sphygmocor Xcel, model EM4C; Atcor Medical, Sydney, Australia), an appropriately sized cuff was placed around the patient’s left upper arm prior to the clinical procedure. One cuff measurement was performed simultaneously with invasive brachial BP. Standard brachial BP was measured during the first inflation and was immediately followed by reinflation of the cuff to a sub-diastolic pressure level. The cuff was held inflated at this sub-diastolic pressure for a period of 5 s while noninvasive BP waveforms were recorded. Details on the performance of device 1 have been previously reported [6,18]. Device 2 (BP+, version 2; Uscom, Sydney, Australia) was used on different participants as described above. It was also operated simultaneously with invasive brachial BP to have oscillometric measurement of brachial BP recorded conventionally. No more than 3 s after cuff deflation, the cuff automatically reinflated and held for 10 s at a suprasystolic pressure approximately 30 mmHg above the measured brachial SBP. During this held-inflation period, suprasystolic pressure signals were recorded. Details on the performance of device 2 have been previously reported [17,19]. Waveforms from both devices were calibrated with the corresponding cuff SBP and DBP measured by each device respectively, and proprietary methods were automatically applied to estimate central BP waveforms. The model of device 2 used in this analysis was recently superseded because the noninvasive BP measurement componentry used to measure the brachial BP has been changed. The updated model of device 2 uses identical componentry to record suprasystolic waveforms and pulse wave analysis algorithms to estimate central BP and SBP amplification. Therefore, this change is not expected to alter the main conclusions of the study.
Statistical analysis
Data are presented as mean ± SD or n (%). Differences between continuous clinical characteristics and BP measures were assessed by t tests or one-way ANOVA with post hoc Tukey HSD test to quantify the statistical significance of any differences. Agreement between cuff-based and invasive SBP amplification was assessed by mean difference and SD of the mean difference. Pearson correlation and linear regression within Bland-Altman plots were used to determine magnitude and direction of any proportional systematic bias. The magnitude of any proportional systematic bias between cuff-based and invasively measured SBP amplification was compared using Fisher’s z (comparing correlation coefficients). Variability of the relationship between cuff-based central SBP and cuff brachial SBP compared to variability of the relationship between invasive central SBP and brachial SBP was assessed using univariable and multivariable linear regression adjusting for potential confounders including age, sex, height, and heart rate. Adjusted R2 and root mean square errors (RMSE) were used to quantify variability between regression models. To do this, 95% confidence intervals for adjusted R2, RMSE and their differences were calculated by bootstrapping approach, as suggested by Kilian et al. [20]. Bootstrapping was performed with 2000 replications using the command provided by STATA 16.0 [21]. Statistical analyses were performed identically for both devices, P values ≤0.05 were considered statistically significant.
Results
Clinical characteristics
Participants were predominantly male, middle-to-older age, overweight, and the majority had at least one diseased coronary artery. Participants who had BP measured by device 1 or device 2 had similar cuff brachial SBP and heart rate, invasive central and brachial SBP, invasive SBP amplification, invasive central heart rate, and invasive central AIx (Table 1). Cuff heart rate, invasive central and brachial AIx were significantly different across invasive SBP amplification quartiles for both devices (Ptrend ≤ 0.047 for all, Table 2).
Table 1. Participant characteristics and clinical measures stratified by blood pressure devices.
| Variables | Device 1 (n = 171) | Device 2 (n = 52) | P values |
|---|---|---|---|
| Participant characteristics | |||
| Male sex [n (%)] | 119 (70) | 43 (83) | 0.063 |
| CAD [n (%)] | 111 (65) | 42 (81) | 0.031 |
| Age (years) | 60 ± 10 | 62 ± 10 | 0.446 |
| Weight (kg) | 86.8 ± 18.8 | 92.8 ± 13.4 | 0.037 |
| Height (cm)a | 170.8 ± 9.4 | 173.9 ± 10.9 | 0.052 |
| BMI (kg/m2) | 29.7 ± 5.3 | 30.6 ± 3.7 | 0.270 |
| eGFR (ml/min per 1.73 m2) | 89.5 ± 25.9 | 83.9 ± 24.3 | 0.169 |
| Noninvasive measures (mmHg) | |||
| Estimated aortic SBP | 119 ± 16 | 127 ± 18 | 0.002 |
| Cuff brachial SBP | 131 ± 18 | 133 ± 17 | 0.489 |
| Estimated SBP amplification | 12 ±4 | 6 ± 4 | <0.001 |
| Cuff heart rate (bpm) | 66 ± 12 | 65 ± 12 | 0.508 |
| Invasive measures (mmHg) | |||
| Aortic SBP | 130 ± 21 | 128 ± 21 | 0.715 |
| Brachial SBP | 138 ± 21 | 136 ± 20 | 0.554 |
| SBP amplification | 8 ± 8 | 8 ± 7 | 0.542 |
| Aortic heart rate (bpm) | 64 ± 12 | 66 ± 11 | 0.307 |
| Aortic Alx (%) | 23.2 ± 20.0 | 25.2 ± 17.8 | 0.519 |
| Brachial Alx (%) | –14 ± 20.6 | 1.0 ± 16.2 | 0.437 |
Sphygmocor Xcel and Uscom BP+ were defined as device 1 and device 2. respectively.Data are mean ± standard deviation or n (%).SBP amplification = brachial SBP - aortic SBP; PP amplification = brachial PP - aortic PP.AIx. augmentation index; eGFR, estimated glomerular filtration rate: PP. pulse pressure; SBP. systolic blood pressure.
Height data were available in n = 164 (Xcel device) and n = 40 (BP+ device) due to missing.
Table 2. Clinical measures stratified by BP devices and invasive SBP amplification quartiles.
| Device 1 (n = 171) | Device 2 (n = 52) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Q1 | Q2 | Q3 | Q4 | P trend | Q1 | Q2 | Q3 | Q4 | P trend |
| Noninvasive measures | ||||||||||
| Esta aortic SBP | 119 ± 12 | 122 ± 15 | 117 ± 16 | 116 ± 19 | 0.317 | 131 ± 20 | 128 ± 15 | 117 ± 19 | 132 ± 15 | 0.106 |
| Cuff brachial SBP | 130 ± 13 | 134 ± 17 | 128 ± 18 | 131 ± 21 | 0.471 | 134 ± 19 | 134 ± 13 | 123 ± 19 | 140 ± 15 | 0.094 |
| Esta SBP amplification | 11 ± 3 | 12 ± 4 | 11 ± 4 | 14 ± 3 | <0.001 | 3 ± 2 | 6 ± 4 | 7 ± 4 | 8 ± 4 | 0.027 |
| Cuff heart rate (bpm) | 63 ± 11 | 64 ± 12 | 69 ± 13 | 69 ± 13 | 0.032 | 62 ± 9 | 65 ± 14 | 60 ± 11 | 72 ± 11 | 0.042 |
| Invasive measures | ||||||||||
| Aortic SBP | 133 ± 18 | 136 ± 18 | 126 ± 25 | 124 ± 21 | 0.021 | 136 ± 24 | 132 ± 15 | 115 ± 19 | 131 ± 18 | 0.033 |
| Brachial SBP | 132 ± 18 | 141 ± 18 | 135 ± 25 | 144 ± 22 | 0.047 | 136 ± 23 | 137 ± 15 | 124 ± 18 | 148 ± 19 | 0.027 |
| SBP amplification | -1 ± 2 | 5 ± 1 | 10 ± 2 | 20 ± 5 | <0.001 | -1 ± 1 | 5 ± 2 | 9 ± 2 | 17 ± 3 | <0.001 |
| Aortic heart rate (bpm) | 62 ± 11 | 63 ± 12 | 67 ± 13 | 66 ± 11 | 0.138 | 63 ± 9 | 65 ± 9 | 62 ± 9 | 76 ± 11 | 0.003 |
| Aortic AIx (%) | 32.8 ± 12.1 | 26.0 ± 18.1 | 25.0 ± 21.9 | 9.0 ± 19.1 | <0.001 | 37.5 ± 12.2 | 20.4 ± 21.3 | 22.2 ± 16.4 | 20.6 ± 16.0 | 0.034 |
| Brachial Aix (%) | 9.0 ± 9.6 | 5.5 ± 20.3 | -2.7 ± 19.6 | -17.5 ± 20.3 | <0.001 | 10.2 ± 10.9 | 2.9 ± 12.6 | -7.4 ± 19.8 | -1.8 ± 16.4 | 0.035 |
Sphygmocor Xcel and Uscom BP+ were defined as device 1 and device 2, respectively-Data are mean ± standard deviation.
Est: estimated.AII blood pressure variables are reported in mmHg Aix. augmentation index; PP, pulse pressure; SBP amplification = brachial SBP - aortic SBP: SBP. systolic blood pressure.
Systolic blood pressure amplification
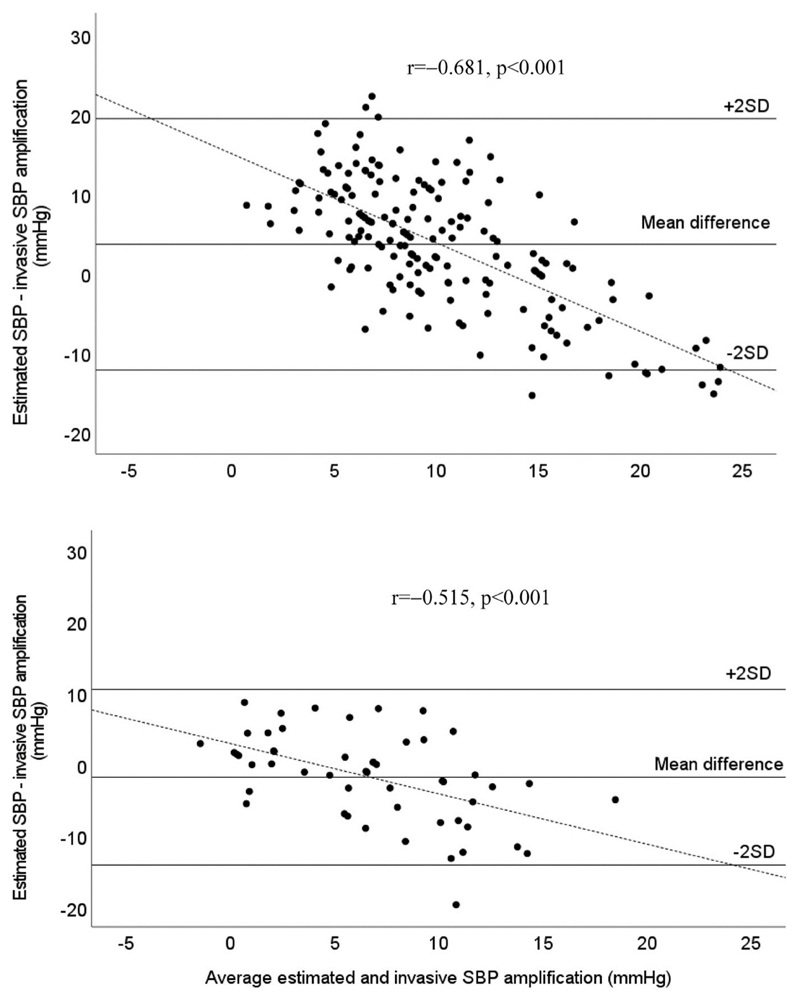
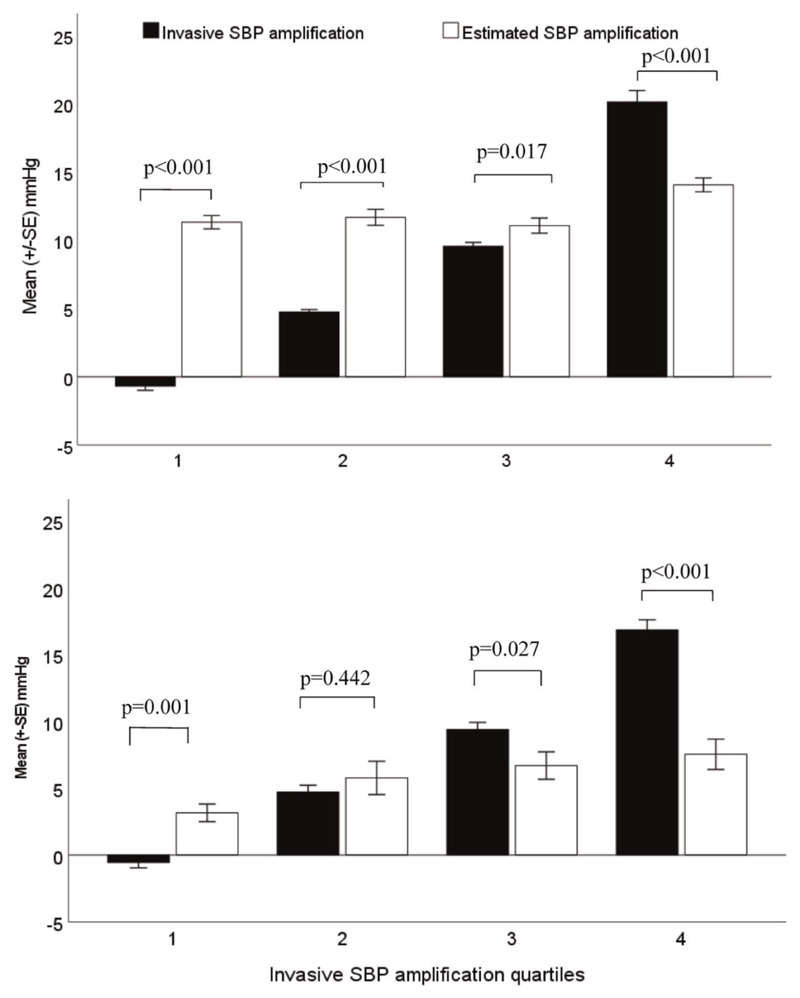
Mean difference (±2SD, limits of agreement) between cuff-based and invasive SBP amplification from device 1 was 4 mmHg (−12 to +20 mmHg, P < 0.001), whereas this value from device 2 was −2 mmHg (−14 to +10 mmHg, P = 0.10) (Fig. 1). Similar patterns were found for cuff-based and invasive PP amplification (Table S1, Supplemental Digital Content). For both devices, Bland–Altman plots revealed cuff SBP amplification overestimated invasive SBP amplification at lower levels of SBP amplification and underestimated at higher levels of SBP amplification (Fig. 1). A stronger association (more bias) was observed for device 1 compared to device 2, and there was a significant difference in correlation coefficients (r = −0.68 vs. r = −0.52; z = 2.72; P = 0.008). There were similar findings for PP amplification (Figure S2, Supplemental Digital Content). In addition, across quartiles of invasive SBP amplification, while there was a stepwise increase in mean invasive SBP amplification (P < 0.001), the same increase in cuff-based SBP amplification was not evident for device 1, and less pronounced for device 2 (Table 2, Fig. 2). Cuff-based SBP amplification was overestimated at lower invasive SBP amplification quartiles (P ≤ 0.001, first quartile) but underestimated at higher invasive SBP amplification quartiles (P < 0.001, fourth quartile; Fig. 2). The concordance between cuff-based and invasive SBP amplification was calculated for each quartile of invasive SBP amplification (i.e. the number of cuff-based SBP amplification cases that were concordant with the corresponding invasive SBP amplification cases over the total number of invasive SBP amplification cases). For device 1, from quartile 1 to 4, these proportions were 0% (0/43), 9% (4/43), 63% (27/43), and 64% (27/42), respectively. For the device 2, they were 38% (5/13), 54% (7/13), 38% (5/13), and 8% (1/13), respectively. Cuff-based PP amplification was also underestimated at the highest invasive PP amplification quartile (P < 0.001) but the overestimation at the lower invasive PP amplification quartile was not statistically significant for device 2 (P ≥ 0.37 for first and second quartiles, Table S1, Supplemental Digital Content, Figure S3, Supplemental Digital Content).
Figure 1.
Bland–Altman plot of difference between estimated SBP and invasive SBP amplification from device 1 (Sphygmocor Xcel, top) and device 2 (Uscom BP+, bottom). Dashed line is the line of best fit. Solid lines are mean difference and ±2 SDs of the difference between estimated and invasive SBP amplification. Bland-Altman plots indicate evidence of systematic bias for greater underestimation of SBP amplification with increasing level of SBP amplification, but with stronger bias observed for device 1 (r = −0.68 vs. r = −0.52; z = 2.72; P = 0.008).
Figure 2.
Bar plots (mean ± SE) of estimated SBP amplification (white bars) and invasive SBP amplification (black bars) per invasive SBP amplification quartiles from device 1 (Sphygmocor Xcel, top) and device 2 (Uscom BP+, bottom). From these figures, there was a stepwise increase in mean invasive SBP amplification for each of elevated invasive SBP quartile (Ptrend < 0.001) whilst estimated SBP amplification was overestimated at the first quartiles (P ≤ 0.001, for all) and underestimated at the highest quartile (P < 0.001, for all).
Variability of cuff-based SBP amplification compared to invasive SBP amplification
Table 3 shows the regression of cuff-based central SBP on cuff brachial SBP, and of invasive central SBP on invasive brachial SBP to compare variability between cuff-based and invasive SBP amplification. With or without adjustment, invasive and cuff-based measurements were strongly associated (P < 0.001, for all). Notably, R2 values from invasive models were lower than those from noninvasive models for device 1 (0.85 vs. 0.96, P < 0.001) and device 2 (0.89 vs. 0.95, P≤0.001). The R2 values remained similar after adjusting for age, sex, height, and heart rate. Moreover, RMSE from regression models was substantially greater for invasive, compared with cuff-based models for either device 1 (8.04 vs. 3.13, P < 0.001) or device 2 (6.74 vs. 4.01, P < 0.001). After adjusting for potential confounders including age, sex, height, and heart rate, RMSE remained similar (device 1: 7.09 vs. 3.19, P < 0.001; device 2: 7.16 vs. 3.63, P < 0.001). These RMSE observations were similar for the association between cuff-based central PP and cuff brachial PP, and between invasive central PP and invasive brachial PP (Table S2, Supplemental Digital Content).
Table 3. Regression of central aortic SBP on brachial SBP measured via noninvasive cuff and invasive recordings.
| n | β (95% Cl) | P | R2b (95% Cl)c | P | RMSE (95% Cl)c | P | ||
|---|---|---|---|---|---|---|---|---|
| Device 1 - unadjusted models | ||||||||
| Estimated aortic SBP ~ cuff SBP | 171 | 0.89 (0.86; 0.92) | <0.001 | 0.96 (0.95; 0.98) | 3.13(2.80; 3.46) | |||
| Invasive aortic SBP ~ brachial SBP | 171 | 0.91 (0.86; 0.97) | <0.001 | 0.85 (0.81; 0.90) | 8.04(7.22; 8.86) | |||
| Difference c | 0.11 (0.07; 0.15) | <0.001 | –4.91 (–5.74; –4.08) | <0.001 | ||||
| - Adjusted modelsa | ||||||||
| Estimated aortic SBP ~ cuff SBP | 164 | 0.89 (0.86; 0.92) | <0.001 | 0.96 (0.95; 0.97) | 3.19(2.86; 3.52) | |||
| Invasive aortic SBP ~ brachial SBP | 164 | 0.89 (0.83; 0.94) | <0.001 | 0.88 (0.84; 0.92) | 7.09(6.29; 7.90) | |||
| Difference c | 0.08 (0.04; 0.11) | <0.001 | –3.90 (–4.70; –3.10) | <0.001 | ||||
| Device 2 - unadjusted models | ||||||||
| Estimated aortic SBP ~ cuff SBP | 52 | 1.03 (0.96; 1.09) | <0.001 | 0.95 (0.92; 0.98) | 4.01 (3.28; 4.75) | |||
| Invasive aortic SBP ~ brachial SBP | 52 | 0.95 (0.86; 1.05) | <0.001 | 0.89 (0.85; 0.94) | 6.74 (5.80; 7.69) | |||
| Difference c | 0.06 (0.02; 0.09) | ≤0.001 | –2.73 (–3.81;–1.65) | <0.001 | ||||
| - Adjusted modelsa | ||||||||
| Estimated aortic SBP I cuff SBP | 40 | 1.05 (0.98; 1.12) | <0.001 | 0.97 (0.95; 0.99) | 3.63(2.79; 4.46) | |||
| Invasive aortic SBP I brachial SBP | 40 | 0.95 (0.84; 1.07) | <0.001 | 0.89 (0.84; 0.95) | 7.16(5.74; 8.58) | |||
| Difference c | 0.08 (0.03; 0.13) | 0.002 | –3.53 (–5.02; –2.04) | <0.001 | ||||
Sphygmocor Xcel and Uscom BP+ were defined as device 1 and device 2. respectively. Data are unstandardized beta (95% confident interval).
Models are adjusted for age. sex, height, and invasive aortic heart rate.
Adjusted R2.
Adjusted R2 (95% Cl) and RMSE (95% Cl) differences between noninvasive and invasive models calculated by bootstrapping with 2000 replications Cl. confidence interval, RMSE. root mean square error: SBP systolic blood pressure.
Discussion
This study aimed to determine the agreement between cuff-based and invasive SBP amplification derived from two central BP devices operating in a type I function [4]. The main finding was that irrespective of the difference between cuff-based and invasive SBP amplification, both devices systematically overestimated SBP amplification at lower levels and underestimated at higher levels of invasive SBP amplification. This resulted in low concordance between cuff-based and invasive SBP amplification across quartiles of invasive SBP amplification, particularly in the lowest and highest quartiles due to significantly less individual variability of cuff-based SBP amplification than invasive measures. Although there are no guidelines on the acceptable level of accuracy, our finding does not support the previously held assumption that type I central BP devices always provide appropriate estimations of SBP amplification [4,11]. This observation may be helpful towards improving the performance of cuff-based central BP measurement.
Mean arterial pressure and DBP are relatively similar between central and peripheral arterial sites, whereas systolic BP may increase substantially across the aorta-to-brachial arterial segments [10]. The magnitude of SBP amplification is highly variable between individuals and can range from −5 to >30 mmHg [3]. Accurately measuring the level of SBP amplification is critically important because it can help refine BP risk stratification and clinical management. As an example, two people with similar peripheral (brachial) SBP may have vastly different central SBP due to different levels of SBP amplification. In this situation, the person with higher central SBP has theoretically greater cardiovascular risk but this cannot be discerned using standard cuff measurement methods [10,22,23]. Despite this, few clinical trials have to date attempted to assess the implications of this theory using targeted central BP management [24,25], nor confirmed in large trials with hard clinical outcomes.
There are many cuff-based devices available that aim to noninvasively estimate central SBP relative to brachial SBP and purport to accurately estimate SBP amplification using a type I function [4–6,8,26]. Alternative calibration modes or algorithms using a type II function can be used to estimate central SBP [4], but this function was not available for either of the devices tested in this study. In any case, the type II device function is not relevant to this study as the process can provide central SBP values that are higher than the standard cuff SBP and does not seek to determine the true level of SBP amplification. The two devices tested in this study both overestimated SBP amplification in the lowest quartiles of invasive SBP amplification but underestimated SBP amplification in the highest quartiles (Figs. 1 and 2). The concordance of cuff-based SBP amplification with true SBP amplification was highly variable, ranging from 0% to 64% concordance across different quartiles of invasive SBP amplification. Overall, the findings indicate that modification of central BP device operation is needed, particularly to accurately discern individuals with low or high levels of SBP amplification.
The average invasive SBP amplification in this study (8 mmHg) is the same as the average value reported in a large individual level invasive data meta-analysis among 515 people from 13 independent studies [3]. However, to our knowledge, only one other study has specifically set out to determine the concordance between invasive and cuff-based SBP amplification. Using the Mobil-O-Graph central BP device in 45 patients during elective coronary angiography [12], cuff based SBP amplification was the same average value as device 1 in our study (12 mmHg). However, average invasive values (16 mmHg) in that study were much higher than observed by us and those reported in the above meta-analysis. This anomaly may be due to most of the study cohort (69%) having high BP, including many with high-grade hypertension (average invasive brachial SBP, 164 mmHg) and thus potentially higher propensity towards elevated SBP amplification. One other recent study measured invasive and noninvasive SBP amplification (using the Mobil-O-Graph device) in 303 individuals, but the focus of that study was on the clinical impacts of mismatch between cuff and invasive brachial BP, and results were not provided for study population averages of SBP amplification [27]. Extrapolated data from a study that used high-fidelity catheters for invasive BP and the Sphygmocor Xcel device in 36 patients had virtually identical SBP amplification results to this current study [7]. The above results from independent studies tend to support wider generalizability of our findings, albeit accepting there may be device-specific variability.
Device 1 performed poorly for predicting low SBP amplification (0% concordance), whereas device 2 was poor at predicting high SBP amplification (8% concordance). Reasons underlying these differences cannot be verified but may be due to the different device measurement functions. For example, device 1 employs a generalized transfer function to derive central SBP, which is a population averaged algorithm that when applied to a dampened peripheral waveform (as occurs with measurement at subdiastolic pressure with device 1 [28]) may not have sufficient sensitivity to capture the individual variability in SBP amplification [29]. On the other hand, device 2 records brachial cuff waveforms amplified to provide a highly featured waveform with complete cuff occlusion of the artery. The impedance of the occluding cuff amplifies the pressure wave reflections and a time-domain, physics-based model is then applied to estimate central aortic pressure waveforms [19]. Since SBP can amplify significantly along the brachial and radial arterial segments [30], the suprasystolic cuff BP used for waveform measurement for device 2 could be a factor contributing to underestimation of SBP amplification at higher values relative to device 1. Future refinement for better accuracy could use the best features of each device, for example, peripheral waveform estimation from sub-diastolic BP and central waveform estimation from supra-systolic waveforms. Combining these approaches could lead to better identification of the true level of SBP amplification.
There are some limitations. First, the study population comprised patients with characteristics and clinical indications to undergo coronary angiography including being of older age; therefore, the results may not be generalizable to other populations or younger cohorts. Second, fluid-filled catheters were used to record invasive BP, which if handled incorrectly, could lead to inaccurate measures. However, the study was performed in accordance with the Artery taskforce recommendations [4] and following a standardized protocol for the measurement of invasive central BP, including removal of bubbles and regular flushing. Other researchers have used high-fidelity catheters to record invasive SBP amplification, and their measurements are highly consistent with our invasive values [3].
In summary, this study found that cuff-based central BP devices had a proportional systematic bias for estimating SBP amplification compared with invasive values. Concordance of cuff-based SBP amplification across quartiles of invasive SBP amplification was highly variable, particularly in the lowest and highest quartiles. These cuff-based devices also provided SBP amplification with significantly less individual variability than invasive measures. These findings help understand some device specific factors that may contribute to the accurate estimation of central BP and may be useful to achieve future refinement of the methods.
Supplementary Material
Acknowledgements
The authors wish to thank all staff from the Royal Hobart Hospital Cardiology Department and Cardiac Catheterization Laboratory for their assistance in facilitating this study.
Sources of Funding
This work is supported by Royal Hobart Hospital Research Foundation grants (references 19-202 and 21-006) and was supported by a Vanguard Grant from the National Heart Foundation of Australia (reference 101836). D.S.P. is supported by a Postdoctoral Fellowship (reference 104774) from the National Heart Foundation of Australia. M.G.S. is supported by a Future Leadership Fellowship (reference 102553) from the National Heart Foundation of Australia. M.K.A. is supported by a National Institutes of Health Cardiovascular Interdisciplinary Research Fellowship grant (reference T32DK007690). X.P. is supported by the National Natural Science Foundation of China (NSFC-82000399).
Footnotes
Disclosures: University of Tasmania (who employs T.V.B., D.S.P., M.G.S., and J.E.S.) has received equipment and research funding from manufacturers of BP devices, including AtCor Medical, and Pulsecor (Uscom). None of the authors have personal, financial or commercial interests related to BP device companies. The remaining authors report no conflicts.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–2712. doi: 10.1016/S0140-6736(16)31134-5. [DOI] [PubMed] [Google Scholar]
- 3.Picone DS, Schultz MG, Otahal P, Aakhus S, Al-Jumaily AM, Black JA, et al. Accuracy of cuff-measured blood pressure: systematic reviews and meta-analyses. JAm Coll Cardiol. 2017;0:572–586. doi: 10.1016/j.jacc.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 4.Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, et al. Validation of noninvasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38:2805–2812. doi: 10.1093/eurheartj/ehw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millasseau S, Agnoletti D. Noninvasive estimation of aortic blood pressures: a close look at current devices and methods. Curr Pharm Des. 2015;21:709–718. doi: 10.2174/1381612820666141023163748. [DOI] [PubMed] [Google Scholar]
- 6.Schultz MG, Picone DS, Armstrong MK, Black JA, Dwyer N, Roberts-Thomson P, et al. Validation study to determine the accuracy of central blood pressure measurement using the Sphygmocor Xcel cuff device. Hypertension. 2020;76:244–250. doi: 10.1161/HYPERTENSIONAHA.120.14916. [DOI] [PubMed] [Google Scholar]
- 7.Shoji T, Nakagomi A, Okada S, Ohno Y, Kobayashi Y. Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure. J Hypertens. 2017;35:69–75. doi: 10.1097/HJH.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 8.Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313. [DOI] [PubMed] [Google Scholar]
- 9.Picone DS, Schultz MG, Peng X, Black JA, Dwyer N, Roberts-Thomson P, et al. Intra-arterial analysis of the best calibration methods to estimate aortic blood pressure. J Hypertens. 2019;37:307–315. doi: 10.1097/HJH.0000000000001902. [DOI] [PubMed] [Google Scholar]
- 10.Picone DS, Schultz MG, Peng X, Black JA, Dwyer N, Roberts-Thomson P, et al. Discovery of new blood pressure phenotypes and relation to accuracy of cuff devices used in daily clinical practice. Hypertension. 2018;71:1239–1247. doi: 10.1161/HYPERTENSIONAHA.117.10696. [DOI] [PubMed] [Google Scholar]
- 11.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35:3122–3133. doi: 10.1093/eurheartj/ehu293. [DOI] [PubMed] [Google Scholar]
- 12.Nakagomi A, Okada S, Shoji T, Kobayashi Y. Comparison of invasive and brachial cuff-based noninvasive measurements for the assessment of blood pressure amplification. Hypertens Res. 2017;40:237–242. doi: 10.1038/hr.2016.132. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RP, Gibbs HH, O’Rourke MF, Daley JE, Mang K, Morgan JJ, et al. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990;11:138–144. doi: 10.1093/oxfordjournals.eurheartj.a059669. [DOI] [PubMed] [Google Scholar]
- 14.Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–1198. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]
- 15.Stergiou GS, Lourida P, Tzamouranis D. Replacing the mercury manometer with an oscillometric device in a hypertension clinic: implications for clinical decision making. J Hum Hypertens. 2011;25:692–698. doi: 10.1038/jhh.2010.107. [DOI] [PubMed] [Google Scholar]
- 16.Peng X, Schultz MG, Picone DS, Black JA, Dwyer N, Roberts-Thomson P, et al. Arterial reservoir characteristics and central-to-peripheral blood pressure amplification in the human upper limb. J Hypertens. 2017;35:1825–1831. doi: 10.1097/HJH.0000000000001400. [DOI] [PubMed] [Google Scholar]
- 17.Costello BT, Schultz MG, Black JA, Sharman JE. Evaluation of a brachial cuff and suprasystolic waveform algorithm method to noninvasively derive central blood pressure. Am J Hypertens. 2015;28:480–486. doi: 10.1093/ajh/hpu163. [DOI] [PubMed] [Google Scholar]
- 18.Gotzmann M, Hogeweg M, Seibert FS, Rohn BJ, Bergbauer M, Babel N, et al. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J Hypertens. 2020;38:235–242. doi: 10.1097/HJH.0000000000002237. [DOI] [PubMed] [Google Scholar]
- 19.Lowe A, Harrison W, El-Aklouk E, Ruygrok P, Al-Jumaily AM. Noninvasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech. 2009;42:2111–2115. doi: 10.1016/j.jbiomech.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Kilian R, Matschinger H, Löeffler W, Roick C, Angermeyer MC. A comparison of methods to handle skew distributed cost variables in the analysis of the resource consumption in schizophrenia treatment. J Ment Health Policy Econ. 2002;5:21–31. [PubMed] [Google Scholar]
- 21.Bittmann F. Bootstrapping: an integrated approach with Python and Stata. De Gruyter. 2021 [Google Scholar]
- 22.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 23.Picone DS, Schultz MG, Hughes AD, Sharman JE. Cuff under pressure for greater accuracy. Curr Hypertens Rep. 2020;22:93. doi: 10.1007/s11906-020-01103-8. [DOI] [PubMed] [Google Scholar]
- 24.Sharman JE, Marwick TH, Gilroy D, Otahal P, Abhayaratna WP, Stowasser M. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertension. 2013;62:1138–1145. doi: 10.1161/HYPERTENSIONAHA.113.02001. [DOI] [PubMed] [Google Scholar]
- 25.Borlaug BA, Olson TP, Abdelmoneim SS, Melenovsky V, Sorrell VL, Noonan K, et al. A randomized pilot study of aortic waveform guided therapy in chronic heart failure. J Am Heart Assoc. 2014;3:e000745. doi: 10.1161/JAHA.113.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharman JE, Laurent S. Central blood pressure in the management of hypertension: soon reaching the goal? J Hum Hypertens. 2013;27:405–411. doi: 10.1038/jhh.2013.23. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski C, Yang K, Charron T, Doucet M, Hatem R, Kouz R, et al. Inaccuracy of brachial blood pressure and its potential impact on treatment and aortic blood pressure estimation. J Hypertens. 2021;39:2370–2378. doi: 10.1097/HJH.0000000000002943. [DOI] [PubMed] [Google Scholar]
- 28.Peng X, Schultz MG, Picone DS, Dwyer N, Black JA, Roberts-Thomson P, et al. Noninvasive measurement of reservoir pressure parameters from brachial-cuff blood pressure waveforms. J Clin Hypertens (Greenwich) 2018;20:1703–1711. doi: 10.1111/jch.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MM, Cheng HM, Sung SH, Liao CF, Chen YH, Huang PH, et al. Estimation of central aortic systolic pressure from the second systolic peak of the peripheral upper limb pulse depends on central aortic pressure waveform morphology. J Hypertens. 2012;30:581–586. doi: 10.1097/HJH.0b013e3283501354. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong MK, Schultz MG, Picone DS, Black JA, Dwyer N, Roberts-Thomson P, et al. Brachial and radial systolic blood pressure are not the same: evidence to support the Popeye phenomenon. Hypertension. 2019;73:1036–1041. doi: 10.1161/HYPERTENSIONAHA.119.12674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.