Abstract
Neurodegenerative disorders are debilitating and largely untreatable conditions that pose a significant burden to affected individuals and caregivers. Overwhelming evidence supports a crucial preclinical role for endosomal dysfunction as an upstream pathogenic hub and driver in Alzheimer‘s disease (AD) and related neurodegenerative disorders. We present recent advances on the role of endosomal acid-base homeostasis in neurodegeneration and discuss evidence for converging mechanisms. The strongest genetic risk factor in sporadic AD is the ε4 allele of Apolipoprotein E (ApoE4), which potentiates pre-symptomatic endosomal dysfunction and prominent amyloid beta (Aβ) pathology, although how these pathways are linked mechanistically has remained unclear. There is emerging evidence that the Christianson syndrome protein NHE6 is a prominent ApoE4 effector linking endosomal function to Aβ pathologies. By functioning as a dominant leak pathway for protons, the Na+/H+ exchanger activity of NHE6 limits endosomal acidification and regulates β-secretase (BACE)-mediated Aβ production and LRP1 receptor- mediated Aβ clearance. Pathological endosomal acidification may impact both Aβ generation and clearance mechanisms and emerges as a promising therapeutic target in AD. We also offer our perspective on the complex role of endosomal acid-base homeostasis in the pathogenesis of neurodegeneration and its therapeutic implications for neuronal rescue and repair strategies.
Keywords: Alzheimer’s disease, Amyloid, ApoE4, Endosomal pH, Na+/H+ exchanger, NHE6
1. A Balancing Act: pH Regulation in the Endosome
The endosome is a busy station handling cargo traffic at the degradation-recycling crossroads. There is widespread interest in deciphering basic mechanisms of endosomal traffic not only to understand a fundamental cellular process but also to identify new diagnostic and therapeutic targets in disease (Afghah et al. 2019; Casey et al. 2010; Maxfield 2014). Endosomes play important roles in membrane and protein turnover, nutrient uptake, receptor-ligand uncoupling, antigen presentation, enzyme activation, vesicle budding and exosome formation, and as crucial regulators of cellular signaling pathways (Afghah et al. 2019; Casey et al. 2010; Maxfield 2014). Despite this multipartite function of the endosome, relatively few studies have addressed the contribution of endosomes to human health and disease. However, recent studies have begun to shed light on the mechanistic roles of endosomes in a host of developmental and degenerative disorders of the brain, including autism and Alzheimer’s disease, and in oncogenesis, including glioblastoma (Kondapalli et al. 2014, 2015; Nixon 2005, 2017; Ko et al. 2020). Although very diverse at first sight, pathophysiologic commonalities between these disorders emerge upon closer analysis. Compelling evidence points to vesicle trafficking defects as a unifying mechanism underlying these disorders, which in turn critically depend on luminal pH within the endosomal-lysosomal compartment (Kondapalli et al. 2014; Prasad and Rao 2015a).
A precise balance of luminal pH and endosomal ion flux is required for endosomal trafficking, and a thorough dissection of pathways regulating endosomal pH may be a promising strategy to reveal pathophysiological mechanisms (Kondapalli et al. 2014; Prasad and Rao 2015a). Traditionally, research on endosomal acidification in human health and disease has focused on the vacuolar-type ATPase (V-ATPase) that pumps protons into the endosomes. Altered V-ATPase activity and defective lysosomal acidification have been linked to cellular aging and neurodegenerative disorders including Parkinson’s and Alzheimer’s disease (Colacurcio and Nixon 2016). However, a growing number of studies are highlighting the role of multiple ion transporters that act in concert with the V-ATPase for luminal pH regulation (Scott and Gruenberg 2011) (Fig. 1a). The compensatory movement of Cl− ions is critical to allow acidification of the endolysosomal lumen by dissipating the membrane potential resulting from unidirectional pumping of H+ by the V-ATPase. The CLC family includes plasma membrane Cl− channels as well as vesicular transporters, CLC3 through CLC7 in mammals, which function as Cl−/H+ exchangers. The stoichiometry of exchange, 2Cl−/H+, results in three negative charges introduced into the vesicle lumen per transport cycle, albeit with loss of one H+, to counter the V-ATPase. Gene disruptions in mouse models result in severe neurodegeneration, including loss of hippocampus in Clcn3−/− null mouse and pathological similarity to lysosomal storage disorders such as neuronal ceroid lipofuscinosis in various targeted Clcn7−/− models, reviewed elsewhere (Jentsch and Pusch 2018; Poroca et al. 2017). Potential players in regulating endosomal pH also include the mucolipin subfamily of transient receptor potential (TRPML) calcium channels found on endosomes, particularly the pH-regulated TRPML3 isoform, overexpression of which is known to increase endosomal pH (Martina et al. 2009). Activation of two-pore Ca2+ channels (TPCs) residing in endosomal-lysosomal compartments may also regulate luminal pH (Morgan and Galione 2007) (Fig. 1a).
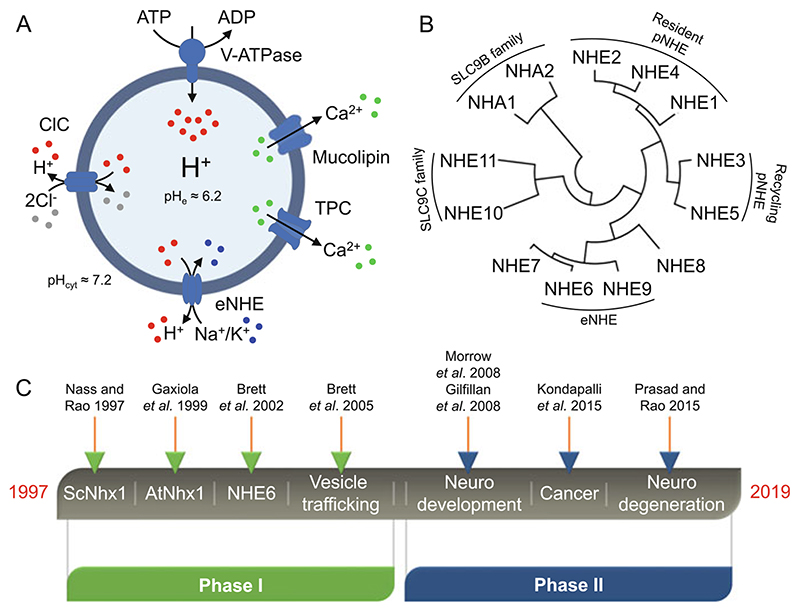
Fig. 1. Endosomal pH regulation by Na+/H+ exchangers.
(a) Under physiological conditions, the endosomal pH is acidic (≈6.2) as compared to the cytoplasmic pH (≈7.2). According to the “pumpleak” model, conserved from yeast to plants and mammals, the exchange of luminal protons for Na+ or K+ ions by eNHE counters the activity of the H+ pumping V-ATPase to precisely tune the endosomal pH. Also depicted in the figure are ClC transporters and Ca2+ channels (mucolipins and TPC) also reported to regulate endosomal pH. The pH values indicated in the figure were obtained from experimental observations (Prasad and Rao 2018a). (b) SLC9 phylogenetic tree constructed using the Mega5 program depicting three major subclasses of SLC9A, SLC9B, and SLC9C in mammals. There are nine NHE isoforms within the SLC9A subclass, which are further subdivided as resident or recycling plasma membrane (pNHE), and intracellular including the endosomal NHE (eNHE) isoforms, NHE6 and NHE9. (c) Timeline of two decades of research on eNHE highlighting some relevant publications. eNHE endosomal Na+/H+ exchangers, ClC Cl−/H’ antiporters, TPC two-pore channels
Recent clinical reports and genetic studies have underscored the contribution of endosomal Na+/H+ exchangers in the pathophysiology of a plethora of human disorders (Kondapalli et al. 2014; Prasad and Rao 2015a; Zhao et al. 2016). Encoded by the SLC9 gene family of solute carriers, Na+/H+ exchangers (NHE) are grouped into three subfamilies: (1) SLC9A that has nine isoforms, SLC9A1–9, encoding NHE1–9 proteins; (2) SLC9B that has two isoforms, SLC9B1–2, encoding NHA1–2 proteins; and (3) SLC9C that has two isoforms, SLC9C1–2, encoding NHE10–11 proteins (Brett et al. 2005a; Donowitz et al. 2013; Fuster and Alexander 2014; Pedersen and Counillon 2019) (Fig. 1b). The SLC9A subfamily consists of electroneutral Na+/H+ exchangers that reside on the plasma membrane (NHE1, NHE2 and NHE4), recycle between plasma membrane and endosomal compartments (NHE3 and NHE5), or function within intracellular compartments, including the Golgi (NHE8), trans Golgi network (NHE7), and the early and recycling endosomes (NHE6 and NHE9) (Brett et al. 2005a; Donowitz et al. 2013; Fuster and Alexander 2014; Pedersen and Counillon 2019) (Fig. 1b). Cation selectivity of the NHE transporters reflects the availability of ions in the surrounding milieu: whereas plasma membrane isoforms are restricted to Na+ (and Li+), intracellular isoforms can additionally transport K+ ions (Pedersen and Counillon 2019). As a result, cation/H+ exchange can be driven by both proton and cation gradients. All NHE members have a cytoplasmic C-terminal domain, which is significantly unstructured and binds to a number of regulatory factors, including calcineurin homology protein (CHP) and the PDZ-domain scaffold protein NHERF (Pedersen and Counillon 2019). The C-terminal tail mediates scaffolding to the cytoskeleton and activation of transport in response to signaling cascades, although much less is known about regulation and trafficking of endosomal NHE isoforms (Kondapalli et al. 2014; Kagami et al. 2008).
Endosomal NHE (eNHE) activity was originally described in the tonoplast membranes of plant vacuoles (Barkla and Blumwald 1991; Blumwald and Poole 1985). The gene was first cloned in yeast, where it was recognized as an evolutionarily ancient subtype, distinct from the well-characterized plasma membrane NHE (Nass et al. 1997; Nass and Rao 1998; Brett et al. 2005a), and subsequently multiple isoforms were identified in plants, mammals, and other organisms (Gaxiola et al. 1999; Brett et al. 2002). Early research on eNHE (Fig. 1c) focused on fundamental questions relating to their role in vesicle trafficking using model organisms, first in yeast and soon after in plants and metazoans, including mammalian cells (Brett et al. 2002, 2005b; Gaxiola et al. 1999; Nass et al. 1997; Nass and Rao 1998; Bowers et al. 2000). In the last decade (Fig. 1c), there has been an exponential rise in interest due to the emerging links between eNHE and a growing list of human diseases, including neurodevelopmental disorders such as autism and intellectual disability (Christianson Syndrome), attention deficit hyperactivity disorder, addiction, several types of cancers (Ko et al. 2020), and more recently to neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease (Gilfillan et al. 2008; Kerner-Rossi et al. 2019; Kondapalli et al. 2014, 2015; Morrow et al. 2008; Prasad and Rao 2015b, 2018a; Ullman et al. 2018). While a fraction of eNHE patient variants reported in the literature may be benign polymorphisms, a significant proportion is deleterious and causal to disease phenotypes (Ilie et al. 2016; Kondapalli et al. 2013; Prasad et al. 2017). As a major source of proton leak activity in endosomes, the eNHE functions as an endosomal gatekeeper that must be tightly regulated and controlled (Kondapalli et al. 2014). Given that Na+/H+ exchangers are estimated to have exceptionally high transport rates of ~1,500 ions/s, even small perturbations in expression or activity may result in dramatic consequences within the limited confines of endosomal space (Lee et al. 2013). Dysregulation of endosomal pH could potentially impact a plethora of downstream events including alterations in cargo sorting and trafficking, protein processing, and receptor turnover and thus represents a previously unidentified pathogenic mechanism in neurodegeneration. Here, we review evidence linking endosomal pH and eNHE function to Alzheimer’s disease and discuss how this knowledge might help us understand the broader role for endosomal acid-base homeostasis in neurodegenerative diseases. We also briefly address the potential role of endosomal pH in other neurodegenerative disorders and discuss translational prospects.
2. Lessons from Baker’s Yeast
Simple organisms such as yeast can powerfully inform our understanding of genetic players in neurological disorders such as Alzheimer’s disease and aid in discovery of new therapies (Treusch et al. 2011). Much of what we know about specific functions and raison d’etre of eNHE in humans has come from rigorous studies on the yeast ortholog Nhx1. Beginning in the mid-1990s, Nhx1 was cloned, localized to the prevacuolar compartment in yeast, and defined as the founding member of an evolutionarily conserved, phylogenetic cluster of endosomal Na+/H+ exchangers with proposed roles in vesicle trafficking, ion homeostasis, and biogenesis of lysosomes (Bowers et al. 2000; Brett et al. 2005a; Nass et al. 1997; Nass and Rao 1998). Extension of these studies to plants also revealed a critical role in determining flower color and salt tolerance (Gaxiola et al. 1999; Kondapalli et al. 2014). The “pump-leak” model of pH regulation is conserved from yeast to plants and humans, with the exchange of luminal protons for sodium or potassium ions by eNHE acting as a brake that counters H+ pumping activity of the V-ATPase to precisely tune the endosomal pH (Kondapalli et al. 2014) (Fig. 1a). Loss of this leak pathway leads to over-acidification of early and recycling endosomes, missorting of cargo, defects in protein processing, and receptor turnover (Kondapalli et al. 2014). Importantly, trafficking defects in Nhx1-null mutants could be corrected with weak base or exacerbated by weak acids (Brett et al. 2005b). Furthermore, a genome-wide analysis of vacuolar pH in ~4,600 yeast null mutants revealed a reciprocal link between luminal pH and vesicle trafficking (Brett et al. 2011). Hyperacidification of the endolysosomal compartment was observed in the null mutant of Ncr1, the yeast ortholog of the human gene linked to Niemann-Pick type C (NPC) disease, resulting in the endosomal trapping of unesterified cholesterol and establishing a link between dysregulation of luminal pH and neurodegeneration (Brett et al. 2011). Deletion of Nhx1 (also known as Vps44) results in enlarged endosomes, hyperacidic luminal pH, enhanced proteolysis of the chaperone protein Vps10 (an ortholog of the AD susceptibility factor SORL1/sortilin-related receptor 1), mistrafficking of lysosomal hydrolases, and profound deficiencies in lysosomal cargo sorting and delivery (Bowers et al. 2000; Brett et al. 2005b; Nass and Rao 1998). Interestingly, these cellular phenotypes are strikingly reminiscent of preclinical stages of Alzheimer disease, as discussed below, and point to a role for eNHE in AD. Although the contribution of the V-ATPase in acidification of endosomal-lysosomal compartments in well established, the role of eNHE is remains largely unexplored in mammalian cells, including neurons. Much of the early evidence concerning endosomal NHE involvement in vesicle trafficking is based on studies performed using simple yeast models and non-neuronal mammalian systems; the direct extrapolation of such observations to explain complex events such as synapse formation and turnover, however, is not straightforward and further research is necessary to elucidate mechanisms that link disrupted neuronal function to changes in endosomal pH in AD.
3. Endosomopathy Is a Preclinical Hallmark of Alzheimer’s Disease
The number of people living with dementia worldwide is currently estimated at 47 million and is projected to triple by 2050. Alzheimer’s disease (AD) is the leading cause of dementia and contributes up to 70% of case (Baumgart et al. 2015; Tarawneh and Holtzman 2012). A majority of AD cases occur sporadically, with 40–65% of patients carrying at least one copy of the E4 allele of Apolipoprotein E (ApoE4), the strongest known genetic risk factor for AD. Indeed, the presence of two copies of the E4 allele is known to increase risk of AD by ~12-times as compared to E3 isoform (Corder et al. 1993; Verghese et al. 2013; Yamazaki et al. 2016). Unfortunately, the underlying cellular pathology that leads to neurodegeneration occurs long before clinical symptoms emerge, resulting in abysmal clinical failure (up to 99.6%) of drugs to reverse advanced pathology of amyloid plaques, neurofibrillary tangles, and neuronal death (Cummings et al. 2014). Therefore, early detection and intervention in preclinical stages may be key to treatment of this devastating disease. In this context, it is critical to identify preclinical AD features in high-risk populations and cognitively normal seniors that are associated with future cognitive decline and mortality.
Endosomal abnormalities, as early features of AD that precede the clinical onset of the disease by several decades, were proposed as early as 1998 by Troncoso et al. (1998). More recently, there have been several studies documenting endosomal dysfunction and trafficking defects in AD; however, the underlying molecular mechanisms and cellular consequences of endosomal deficits largely remains to be elucidated. Notably, in 2017, Nixon coined the term “endosomopathy” in relation to AD, and Petsko and colleagues proposed endosomal “traffic jams” as an upstream pathogenic hub in AD, suggesting that interventions designed to “unjam” the endosome may have far-reaching therapeutic implications (Nixon 2017; Small et al. 2017). Indeed, the earliest and most prominent brain pathology known to precede cognitive impairment is the marked enlargement and amplification of the endosomal pool, pointing to underlying dysfunction of the endosomal-lysosomal pathway (Nixon 2005, 2017). More importantly, inheritance of ApoE4 is known to potentiate prominent pre-symptomatic endosomopathy in AD brains (Cataldo et al. 2000). Although this observation was made around two decades ago, the absence of known molecular effectors and druggable targets has precluded translation from bench to bedside.
The importance of endosomal function in AD is strengthened by several recently identified genes in genome-wide association studies (GWAS) of AD risk, including BIN1, PICALM, CD2AP, EPHA1, and SORL1, that are known to regulate endosomal mechanisms (Karch and Goate 2015). Understanding the abnormalities in the endosomal-lysosomal system in AD could potentially be a new “lamp post” that lights the path to new promising therapeutic targets. AD-related endosomal dysfunction manifests functionally as abnormal endocytic activity, mistrafficking of lysosomal hydrolases, and abnormal accumulation of unesterified cholesterol (Nixon 2005, 2017). One well-studied endosomal molecular player is Rab5, a regulatory guanosine triphosphatase (GTPase) that is associated with the early or sorting endosome and participates in early endosomal biogenesis and cellular signaling reactions. The family of Rab proteins regulates multiple steps in membrane trafficking, from vesicle formation to fusion. Abnormal activation of Rab5 is observed in AD and Down syndrome where it is thought to contribute to prominent early endosomal dysfunction (Jiang et al. 2010; Kim et al. 2016; Nixon 2005, 2017). Several AD-related risk factors, including amyloid precursor protein (APP) dosage, increased β-secretase BACE1 (beta-site APP cleaving enzyme 1) expression, accumulation of β-carboxyl-terminal fragment of APP (β-CTF), inheritance of the ApoE4 allele, reduced retromer/Vps35 expression, and abnormal cellular accumulation of cholesterol, potentiate Rab5-mediated dysfunction of early endosomes (Cataldo et al. 2000; Jiang et al. 2010; Kim et al. 2016).
In comparison, the role of recycling endosomes is relatively understudied and underappreciated as a component of AD. Synaptic function critically depends on recycling endosomal function and surface proteostasis of membrane proteins, which includes the mechanisms by which synaptic proteins are endocytosed and recycled back to the plasma membrane to regulate their surface expression (Park et al. 2004; Schmidt and Haucke 2007). Rab11 is a member of the Rab family of proteins that localizes to recycling endosomes where it mediates membrane traffic. Recently, β-CTF-mediated Rab11 impairment was reported in AD, leading to the dysfunction of recycling endosomes that could contribute to synapse dysfunction and defective long-term potentiation (LTP) seen in this disorder (Park et al. 2004; Woodruff et al. 2016). Other studies have documented impaired endocytic recycling of glutamate and insulin receptors in brains of ApoE4 carriers (Chen et al. 2010; Zhao et al. 2017). The pan-functional role of endosomal pH in plasma membrane protein recycling and turnover has recently received much attention in the context on autism and brain cancer, with the accumulation of evidence showing that recycling endosomal alkalization promotes receptor recycling and prolonged oncogenic signaling, whereas endosomal hyperacidification enhances turnover and thereby reduces lifetime of membrane proteins (Kondapalli et al. 2013, 2015; Prasad and Rao 2015a). These and many other observations have been consolidated into a unifying hypothesis centering on vesicle trafficking dysfunction that is particularly germane to our understanding of AD pathogenesis.
4. Protons to Patients: Linking Endosomal pH and Neurodegeneration
We hypothesize that endosomal pH is central to the mechanism of AD. Notably, APP, its proteolytic fragments (Aβ and β-CTF), and key clipping enzymes (β and γ-secretases) localize to endosomes. Pathological endosomal acidification promotes amyloid pathology at all key steps: production, aggregation, and deposition (Fig. 2).
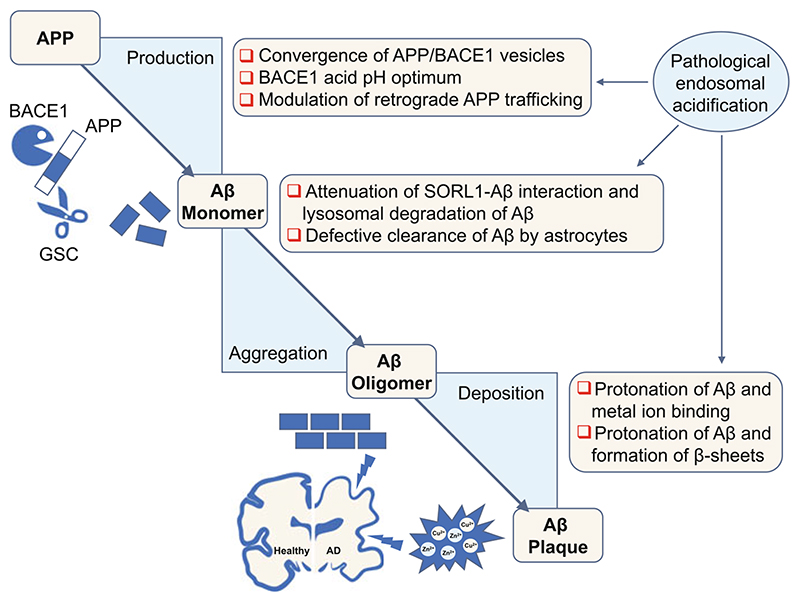
Fig. 2. Acidic endosomal pH promotes Alzheimer’s disease pathology.
Pathological endosomal acidification promotes amyloid pathology at all key steps of amyloidogenesis, including cleavage of APP to produce Aβ, monomer aggregation, and oligomer deposition, culminating in plaques complexed with metal ions. APP amyloid precursor protein, BACE1 β-secretase, GSC gamma secretase complex, SORL1 Sortilin Related Receptor 1, Aβ amyloid β
The processing of APP and production of Aβ itself is linked to endosomal pH and function at multiple levels: First, the endosomal lumen must be acidic for the obligatory and rate-limiting convergence of APP/BACE1 (substrate/enzyme) vesicles in neurons (Das et al. 2013). Second, BACE1 protease has an acid pH optimum at pH 4.5 and its active site is in the lumen of the vesicle (Vassar et al. 1999; Vassar and Kandalepas 2011). Notably, there exists a roughly bell-shaped relationship between pH and BACE activity with optimum at pH 4.5, and both very low levels and very high levels depress enzyme activity. Importantly, biochemical studies determining the pH dependence of BACE activity around the functional pH range of endosomes showed roughly linear relationship between acidity and BACE activity. At pH 6, which broadly corresponds to early endosomal pH, the BACE activity is low, i.e., relative activity below 20% that increases markedly in conditions resembling pathological endosomal acidification to ~50% at pH 5.5 and ~80% at pH 5.0 (Vassar et al. 1999). The prominent pH dependence of the BACE enzyme predicts that conditions that hyperacidify endosomes could promote BACE1 mediated clipping of APP. Notably, reducing BACE1 levels ameliorates neuropathology and behavioral defects in AD and Down syndrome mouse models, pointing to its importance as a promising therapeutic target (Cai et al. 2001; Jiang et al. 2016).
Third, endosomal pH regulates retrograde APP trafficking from endosomes and trans-Golgi network to aid in physical approximation of APP and BACE1 (Prasad and Rao 2015b). Furthermore, APP shows an acidic pH-dependent conformational switch, although the functional consequence remains to be determined (Hoefgen et al. 2015). Next, defective Aβ cellular clearance and degradation processes can lead to the accumulation and aggregation of Aβ to form neurotoxic oligomers. Hyperacidic endosomal pH could impact this aggregation step of Aβ by at least two distinct mechanisms. First, acidic endosomal pH disrupts SORL1-Aβ interaction and attenuates lysosomal targeting of Aβ for degradation in neurons (Caglayan etal. 2014). Second, pathological endosomal acidification reduces the capacity of astrocytes to clear Aβ (Prasad and Rao 2018a). Finally, acidic endosomal pH could aggravate Aβ deposition into neurotoxic plaques by enhancing protonation of three histidine residues (His6, His13 and His14) in Aβ to promote metal ion binding and formation of β-sheets and fibril assembly (Olubiyi and Strodel 2012) (Fig. 2).
In parallel to these observations, it has been recognized for over two decades that drugs causing endosomal alkalization including V-ATPase inhibitors (e.g., bafilomycin and concanamycin), alkalizing drugs (e.g., chloroquine and ammonium chloride), and ionophore drugs that mediate Na+(K+)/H+ exchange (e.g., monensin and nigericin) have beneficial roles in reducing APP processing and Aβ production (Caporaso et al. 1992; Haass et al. 1995; Lahiri 1994; Schrader-Fischer and Paganetti 1996; Urmoneit et al. 1998). However, V-ATPase inhibitors, alkalinizing drugs and ionophores have potent and multiple compartmental effects. As a result, they cause undesired changes in vesicle trafficking, Golgi and lysosomal function, and impact the mammalian target of rapamycin (mTOR) and autophagy pathways. Therefore, they have therefore not been exploited for managing AD (Mauvezin and Neufeld 2015; Zoncu et al. 2011). Thus far, there are no clinical agents in AD therapy available to specifically and effectively target endosomal pH. In this context, the discovery of endosomal Na+/H+ exchangers and recognition of their key roles in precisely tuning endosomal pH and vesicle trafficking provides a unique opportunity for compartment-specific targeting of early and recycling endosomal pathology in AD.
5. Acid Indigestion in the Endosome: The Role of ApoE4
Key cellular pathologies associated with ApoE4 include endosomal dysfunction, lysosomal leakage, translocation of lysosomal cathepsin D, and neuronal cell death (Cataldo et al. 2000; Ji et al. 2002; Persson et al. 2017). As discussed earlier, the endolysosomal network is central to the recycling and turnover of cellular components, and the pH within this network plays a critical role in receptor-mediated endocytosis and vesicular trafficking (Casey et al. 2010). Thus, reliable measurement of intracellular pH is increasingly important in studies of disease mechanisms. To gain a comprehensive understanding of the cellular “pH-stat,” more recently, flow-cytometry and confocal microscopy-based tools have been developed for precise, ratiometric fluorescence-based quantification of endosomal, lysosomal, and cytoplasmic pH. Using these techniques on ApoE genotyped patient fibroblasts and murine astrocytes with human ApoE variants, we demonstrated that endosomes were hyperacidic (~1 pH unit lower), whereas lysosomes were hyperalkaline (~1 pH unit higher), in disease-associated ApoE4, compared to normal ApoE3 cells (Prasad and Rao 2018a). Evidence from lower eukaryotes, e.g., yeast, shows that acidic endosomes also result in more acidic lysosomes (Brett et al. 2005b). However, only a single eNHE isoform (Nhx1) and single Cl−/H+ transporter (Gef1) exist in yeast, whereas mammalian cells have multiple isoforms with discrete localizations to various endosomal and lysosomal compartments. As a result, pH homeostasis in each organelle may be regulated independently and uniquely adapted to specific needs, and alterations in luminal pH in endosomes might not extend to lysosomes. This might also explain why ectopic expression of NHE6 does not alter lysosomal pH (Prasad and Rao 2018a). Significant downregulation of expression of V-ATPase might underlie lysosomal alkalinization in ApoE4 astrocytes (Prasad and Rao 2018a). Previously, abnormal alkalization of lysosomal pH was also reported in a genetic model of presenilin 1-deficiency in AD, in cell culture and neurons (Lee et al. 2010). A comprehensive overview of lysosomal pH dysregulation in AD is available elsewhere, so we will focus on the regulation of endosomal pH in AD. The recent identification of intracellular NHE isoforms in genome-wide association studies of risk factors in AD further strengthens the link with endosomal pH (Martinelli-Boneschi et al. 2013; Meda et al. 2012; Perez-Palma et al. 2014). We suggest that defective pH regulation in the endosomal-lysosomal system may be a broad, unifying mechanism and a new paradigm for understanding the pathogenesis of familial and sporadic late onset AD.
6. NHE6 Is an ApoE4 Effector
Although several genetic studies have identified links between eNHE to a host of neurological disorders, including autism, intellectual disability, attention deficit hyperactivity disorder, epilepsy, Parkinson’s disease, brain cancer, multiple sclerosis, and more recently to late-onset AD, a mechanistic basis for the contribution of Na+/H+ exchange to these disorders is still emerging (Kondapalli et al. 2014; Prasad and Rao 2015a). Mutations in SLC9A6, encoding NHE6, are causal to Christianson syndrome, a monogenic X-linked disorder with neurodevelopmental hallmarks of syndromic autism and intellectual disability. Although ubiquitous in tissue distribution, NHE6 is highly expressed in the brain, particularly in the hippocampus and Purkinje cell layer of the cerebellum, where it has been implicated in neuronal spine dynamics, dendritic arborization, and synaptic strength (Deane et al. 2013; Gilfillan et al. 2008; Kondapalli et al. 2014; Ouyang et al. 2013). As summarized in Table 1, patients with loss-of-function mutations in NHE6 also present with striking and progressive neurodegenerative pathology including (1) early-onset, severe degeneration of cortex and cerebellum, (2) loss of neurons, (3) gliosis, and (4) deposition of hyperphosphorylated tau in neurons and astrocytes (Garbern et al. 2010; Mignot et al. 2013). Notably, NHE6 deletion in mice leads to cellular phenotypes reminiscent of AD, including endosomal hyperacidification, endolysosomal dysfunction, accumulation of unesterified cholesterol in endosomes, and neurodegeneration (Ouyang et al. 2013; Stromme et al. 2011). These observations point to a more widespread role for NHE6 in neurodegenerative disorders. Given the link between ApoE genotype and endosomal pathology, we hypothesized that the Christianson syndrome protein NHE6 is a potential ApoE effector and that dysfunction of NHE6 in disease-associated ApoE4 variants is causal to a subset of pathologies and phenotypes associated with AD.
Table 1. Summary of genetic evidence supporting the role of NHE6 and NHE9 in neurodegenerative disorders.
| Gene name | Study type | Nucleotide change | Protein change | Phenotype | Reference |
|---|---|---|---|---|---|
| SLC9A6/NHE6 | Linkage analysis, electron microscopy, histopathological and biochemical investigations | c.1109_1117delGGAGTACCT | P.W370_T372del | Corticobasal degeneration and tau deposition | Garbem et al. (2010) |
| Serial brain MRI, clinical and mutational analysis | c.916C > T | p.Q306X | Progressive degeneration of cortex and cerebellum | Mignot et al. (2013) | |
| Brain MRI, clinical and mutational analysis | c.1560dupT | p.T521YfsX23 | Parkinsonism in female carriers | Riess et al. (2013) a | |
| Clinical and mutational analysis | c.190G > T | p.E64X | Corticobasal degeneration syndrome and parkinsonism in female carriers | Sinajon et al. (2016) | |
| SLC9A9/NHE9 | GWAS | Multiple SNPs | Alzheimer’s disease | Perez-Palma et al. (2014) | |
| GWAS | rs17636071 | Alzheimer’s disease, response to cholinesterase inhibitors | Martinelli-Boneschi et al. (2013) | ||
| GWAS | rs9828519 | Multiple sclerosis, response to inter- feron-ß | Esposito et al. (2015) |
GWAS genome-wide association studies, MRI magnetic resonance imaging, del deletion, dup duplication, fs frameshift, X stop codon, SNPs single nucleotide polymorphisms; Amino acids are represented by their single letter codes in protein (p) sequence
Mutation referred in relation to longer NHE6.1 isoform (NP_001036002.1)
6.1. NHE6 Is Downregulated in Alzheimer’s Disease
Our analysis of publicly available patient databases revealed that NHE6 is one ofthe top 100 downregulated genes in late-onset AD, predictive of endosomal hyperacidification (Prasad and Rao 2018a). Furthermore, NHE6 downregulation was proportional to disease severity as determined by cognitive and pathological scores (Prasad and Rao 2015b). Importantly, NHE6 expression strongly correlated with average expression of set of synapse genes found to be downregulated in AD. These correlations suggest that reduced NHE6 levels and, by extrapolation, pathological endosomal acidification may underlie synaptic pathology in AD. Indeed, NHE6 protein was low in sporadic AD brains relative to control (Prasad and Rao 2015b). Female carriers of loss-of-function NHE6 mutations have learning difficulties and behavioral problems and some present in late adulthood with low Mini-Mental Status Exam (MMSE) scores indicating early cognitive decline and susceptibility to neurodegeneration (Sinajon et al. 2016). Consistent with these clinical data, transcriptomic analysis of brains areas involved in cognition revealed that NHE6 is among the most highly downregulated genes (up to sixfold) in old (70 years) brain, compared to adult (40 years) (Naumova et al. 2012). NHE6 was also identified as a top hub transcript in AD with 202 network connections that control a plethora of downstream effects (Webster et al. 2009). Another study employing knowledge-based algorithms predicted NHE6 as a molecular player contributing to early-stage AD (Mayburd and Baranova 2013). A network analysis of the metastable subproteome associated with AD identified NHE6 as a top hub gene regulating trafficking and clearance mechanisms necessary for safeguarding cellular proteostasis (Kundra et al. 2017). Although not identified in GWAS studies on AD risk, a more recent deep-learning approach that involved construction of an integrated heterogeneous omics profile using gene expression and methylation data identified NHE6 as one ofthe 35 genes that improved the accuracy ofAD prediction (Park et al. 2019). Together, these observations provide compelling evidence supporting a more widespread role for NHE6 and endosomal acid-base homeostasis in AD. Central to our hypothesis, ApoE4-associated downregulation of NHE6 expression was observed in post-mortem brains and cellular models (Prasad and Rao 2018a; Xu et al. 2006).
6.2. Co-expression Clues in Alzheimer’s Disease
Genetic architecture and gene co-expression networks have been beneficial in understanding mechanisms of disease pathogenesis and progression in AD (Zhang et al. 2013). Clustering analysis of RNA-seq data from 524 normal human brains from the Allen Brain Atlas was used to gather functional insights from the inherent patterns of co-expression (Miller et al. 2014). This approach, also used in cancer research, is based on the premise that genes that are expressed together are likely to function together in a common pathway (Dang et al. 2019; Prasad and Rao 2015b; Zhang et al. 2013). Gene expression clustering works on the principle of “guilt by association” and could provide clues to functional interactions and form the basis for new hypothesis-driven research as evidenced by the close association of enzymesubstrate-receptor group (BACE1, APP, SORL1), receptor-ligand group (TREM2, TYROBP), and members of same family such as MS4A/CD20 proteins (MS4A4A, MS4A6A), and apolipoproteins (APOE, APOJ/clusterin) (Karch and Goate 2015) (Fig. 3). Recent studies have validated functional links between other close associations observed from this clustering analysis: (TYROBP, CD33, TREM2; Haure- Mirande et al. 2017), (ADAM10, SIRT1; Theendakara et al. 2013), (CD2AP, FERMT2; Shulman et al. 2014), (BIN1, APOJ/clusterin; Zhou et al. 2014), and (PICALM, PSEN1; Kanatsu et al. 2014). Thus, gene co-expression analysis may not only identify novel disease-related genes but also may point to previously unrecognized gene functions (Fig. 3).
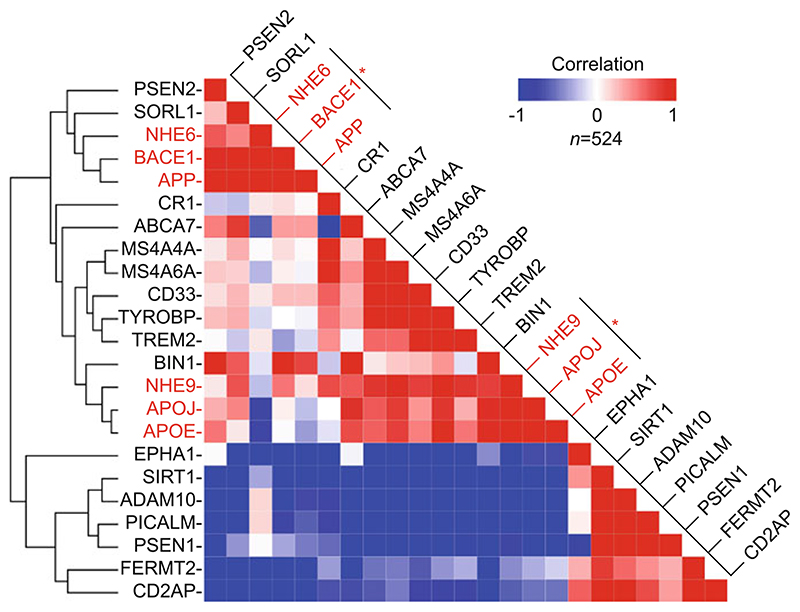
Fig. 3. Co-expression of eNHE isoforms with Alzheimer disease genes.
Clustering analysis of RNA-seq data from 524 normal human brains from the Allen Brain Atlas (low correlation, blue; high correlation, red). Note the strong correlation of NHE6 with APP and BACE1 and of NHE9 with APOE and APOJ (red asterisks)
NHE6 expression was tightly linked with amyloid precursor protein APP and β-secretase enzyme BACE1, with a prominent linear correlation for APP and NHE6 (Pearson correlation 0.75; n = 524; p = 7.0 × 10−96). Indeed, NHE6 ranked among the top 0.5% of human brain transcriptome transcripts (of 52,376) whose expression was most correlated with APP. This observation prompted an investigation of the role of NHE6 in APP metabolism and also uncovered a significant association of the closely related endosomal isoform NHE9 with APOE and APOJ, two lipid carrier proteins that are central to cholesterol homeostasis in brain. The APOE-NHE9 cluster also had a prominent linear correlation (Pearson correlation: 0.78, n: 524, p value: 1.7 × 10−108), suggesting a functional link that warrants future investigation. Intriguingly, NHE9 has been associated with late-onset AD and poor response to cholinesterase inhibitor treatment in AD (Martinelli-Boneschi et al. 2013; Perez-Palma et al. 2014). Furthermore, NHE9 is among the top 1% of human brain transcriptome (out of 52,376 transcripts) whose expression was most correlated with the expression of APOE across 524 samples. Another interesting observation from our analysis is the differential clustering of presenilin proteins (PSEN1 and PSEN2) with the two sheddases, α- and β-secretase. Presenilin proteins constitute the catalytic subunits of the gamma-secretase complex, which participates in both non-amyloidogenic and amyloidogenic APP processing pathways, in addition to its essential role in processing of Notch and other substrates (Karch and Goate 2015). Intriguingly, during normal brain development, PSEN2 clustered with β-secretase (BACE1), and, in contrast, PSEN1 clustered with α-secretase (ADAM10), pointing at potential hitherto unnoticed differences in functions of the two presenilin proteins that could form the basis for new hypothesis-driven research (Fig. 3).
6.3. NHE6 Blocks Amyloid Buildup
Tripartite synapses are sites of cell-cell contact specialized to transmit and compute information in the brain, which, as the name suggests, consists of three essential components: presynaptic neuron, postsynaptic neuron, and the surrounding astrocyte process. Synaptic transmission involves translating the presynaptic information in form of neurotransmitters into various postsynaptic events, ranging from alterations in resting membrane potential, downstream biochemical cascades, and gene expression (Pereda 2014) (Fig. 4). Growing evidence points to deficits in synaptic transmission and loss of synapses in Aβ toxicity that occurs early in AD pathogenesis and precedes neuronal loss by several decades (Masliah et al. 2001; Sheng et al. 2012). Aβ production at the synapses is mediated by clathrin-dependent endocytosis of surface APP at presynaptic terminals into endosomes followed by physical approximation of APP and BACE1 positive vesicles, proteolytic cleavage of APP, and release of Aβ into the synaptic cleft (Cirrito et al. 2005; Das et al. 2013) (Fig. 4). One way enlarged and amplified endosomes seen in AD brains could impact amyloidogenesis is by providing increased surface area for BACE1-mediated APP cleavage. Here, we discuss how hyperacidic pH within these dysfunctional endosomes in neurons could regulate the BACE1 function. Specific targeting of microenvironment within endosomes has the advantage of dampening BACE1- mediated cleavage of APP and not of non-amyloid substrates that are processed in an endocytosis-independent manner, thus preventing mechanism-based toxicities and adverse events (Ben Halima et al. 2016).
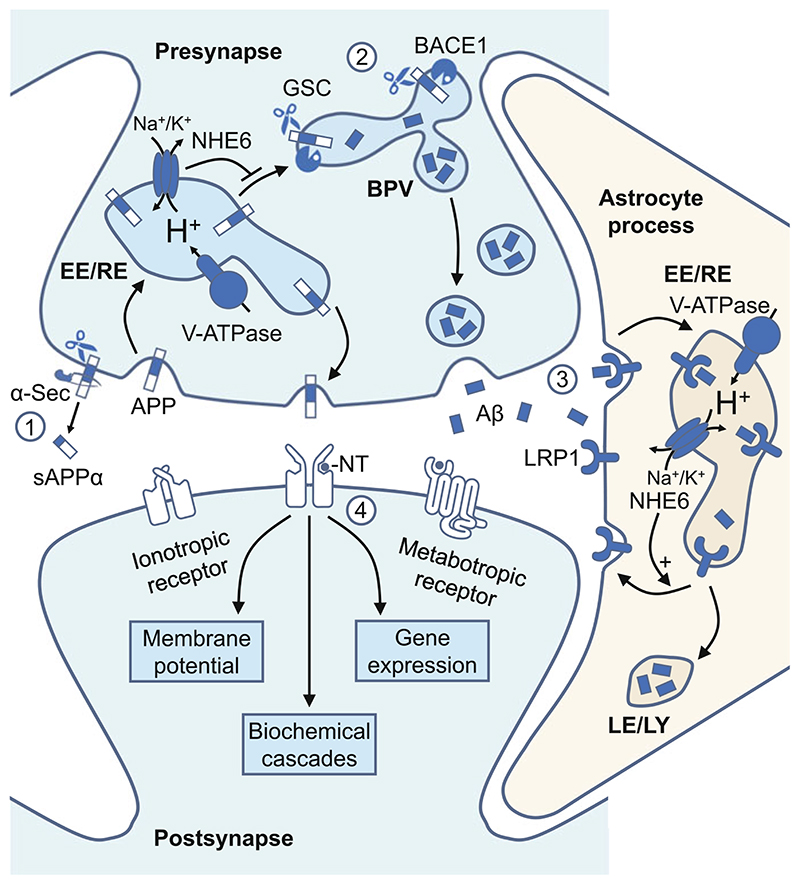
Fig. 4. Model for endosomal pH regulation of synaptic Aβ production and clearance.
Endosomes are involved at multiple steps in the production and clearance of Aβ. At the cell surface (Step 1), APP can be cleaved by α- and γ-secretases (non-amyloidogenic pathway). Alternatively, APP is internalized by clathrin-dependent endocytosis at presynaptic terminals, where it undergoes amyloidogenic processing by β- and γ-secretases (Step 2). NHE6 alkalinizes the endosomal lumen, blocks trafficking of APP from the endosome to BACE1 positive vesicles, and limits Aβ production by neurons. Astrocytes clear Aβ peptides from the synaptic cleft by LRP1 receptor-mediated endocytosis (Step 3). NHE6 activity stabilizes surface expression of LRP1 and promotes Aβ clearance by astrocytes. An imbalance between Aβ production and clearance results in pathological accumulations of Aβ that could disrupt synaptic transmission and cause synapse loss (Step 4). APP amyloid precursor protein, α-Sec α-secretase, BACE1 β-secretase, GSC γ-secretase complex, BPV BACE1-positive vesicles, Aβ amyloid β, LRP1 LDL receptor-related protein 1, EE/RE early and recycling endosomes, LE/LY late endosome and lysosome, NT neurotransmitter
Using neuronal and non-neuronal cell models, NHE6 was shown to regulate trafficking and BACE1-mediated processing of APP (Prasad and Rao 2015b). First, endogenous APP was co-localized with NHE6 in Rab11 positive endosomal compartments. Next, in a well-characterized HEK293-derived cell line stably expressing human APP, NHE6 activity blocked retrograde trafficking of APP from endosomes to the trans-Golgi network. These changes were correlated to luminal pH of sorting endosomes, and causality established using monensin, an ionophore and Na+/H+ exchange mimetic (Prasad and Rao 2015b). It is important to note that neurons have developed an efficient mechanism of restricting substrate (APP) and enzyme (BACE1) to separate organelles and the APP-BACE1 convergence, intriguingly, is known to occur in acidic microdomains (Das et al. 2013). We therefore hypothesized that by limiting excess endosomal acidification, NHE6 in neurons blocks trafficking and physical approximation of APP from endosome and BACE1-positive vesicles, thus limiting biogenesis of Aβ under normal physiology (Fig. 4). This idea was validated in cell culture model by documenting significant reduction in substrateenzyme (APP-BACE1) overlap and diminished Aβ production with NHE6 expression. In contrast, BACE1-mediated APP processing and Aβ production was elevated upon NHE6 depletion (Prasad and Rao 2015b). These observations linking NHE6 dysregulation to Aβ pathology were supported by (1) significantly lower brain weight and (2) elevated Aβ levels in brain homogenates from 7-month old NHE6KO mice, relative to wild-type mice (Prasad and Rao 2018a).
6.4. NHE6 Promotes Amyloid Clearance
Astrocytes are integral functional elements of the tripartite synapse, responding to neuronal activity and regulating overall brain homeostasis and housekeeping. Astrocytes are specifically dysfunctional in neurodegenerative diseases, including AD (Phatnani and Maniatis 2015). It is increasingly evident from studies in cell culture, humans, and mouse models that ApoE4 genotype negatively affects uptake and clearance of secreted Aβ by astrocytes, a major central nervous system cell type (Castellano et al. 2011; Mawuenyega et al. 2010; Verghese et al. 2013). Using murine astrocytes that produce, lipidate, package, and secrete human ApoE variants in a brain-relevant physiological fashion, significant time-dependent deficit in Aβ clearance was observed in E4 variants, compared to E3. Consistent with data from human brain transcriptome, NHE6 transcript and protein expression was downregulated in ApoE4 astrocytes, correlating with endosomal hyperacidification and ~50%. reduced surface levels of Aβ receptor LRP1 (low-density lipoprotein receptor-related protein 1) (Prasad and Rao 2018a). These findings were independently recapitulated by AD patient-derived ApoE4/4 fibroblasts compared with ApoE3/3 fibroblasts from age-matched control (Prasad and Rao 2018a).
Loss-of-function mutation or downregulation of eNHE hyperacidifies endosomes and reduces surface expression of membrane proteins, and, conversely, overexpression of eNHE alkalinizes endosomes and stabilizes membrane proteins; both mechanisms have been previously shown to play causal roles in human diseases, including autism and brain cancer (Kondapalli et al. 2014; Prasad and Rao 2015a). Therefore, hyperacidification of early and recycling endosomes in ApoE4 astrocytes was proposed to cause loss of LRP1 surface expression and Aβ clearance. Consistent with this hypothesis, correction of endosomal pH by ectopic, lentiviral-mediated expression of NHE6 resulted in robust restoration of surface LRP1 levels and correction of defective Aβ clearance by ApoE4 cells. Similar findings were observed with monensin, establishing causality. Thus, NHE6, by localizing to early and recycling endosomes, significantly alkalinizes luminal pH, stabilizes surface expression of LRP1, and elevates clearance of Aβ peptides (Prasad and Rao 2018a) (Fig. 4). Furthermore, given that LRP1 is a receptor for multiple ligands, these observations, if extended to other ligands and receptors, have the potential to explain number of ApoE4 defects besides Aβ clearance (Prasad and Rao 2018a). Taken together, the findings from these studies, summarized in Table 2, support the following unifying hypothesis: loss of NHE6 function in AD is central to the endosomal pathology observed in pre-symptomatic AD brains and results in over-acidification of early and recycling endosomes, which creates an imbalance between Aβ production and clearance, culminating in Aβ plaques and neurodegeneration (Fig. 5).
Table 2. Summary of experimental studies documenting the role of endosomal acid-base homeostasis in neurodegenerative diseases.
| Experimental system | Key findings | Reference |
|---|---|---|
| Post-mortem human brain tissue | Significantly lower NHE6 transcript and protein levels in AD brains relative to control | Prasad and Rao (2015b) |
| NHE6 downregulation in AD correlated with disease severity as determined by cognitive and pathological scores | ||
| NHE6 expression strongly correlated with synapse genes down regulated in AD | ||
| NHE6KO mice | Endolysosomal dysfunction, accumulation of unesterified cholesterol in endosomes and neurodegeneration | Stromme etal. (2011) |
| Endosomal hyperacidification, diminished neuronal arborization and synapse number | Ouyang et al. (2013) | |
| Lower brain weight and elevated Aβ levels in the brain | Prasad and Rao (2018a) | |
| Cell culture model | NHE6 blocks trafficking of APP from endosome to trans-Golgi network | Prasad and Rao (2015b) |
| Expression of NHE6 regulates physical approximation of substrate (APP) and enzyme (BACE1) | ||
| BACE1-mediated APP processing and Aβ production was elevated upon NHE6 depletion | ||
| Astrocytes | Endosomal hyperacidification and reduced surface levels of Aβ receptor LRP1 in ApoE4 astrocytes relative to ApoE3 astrocytes | Prasad and Rao (2018a) |
| Significantly lower NHE6 transcript and protein levels in ApoE4 astrocytes relative to ApoE3 astrocytes | ||
| Restoring NHE6 expression by lentiviral expression or HDACi treatment corrected surface LRP1 levels and defective Aβ clearance deficit in ApoE4 astrocytes | ||
| Neurons | Reduced surface levels of LRP8 in neurons treated with ApoE4 | Xian et al. (2018) |
| NHE6 lentiviral knockdown restores normal trafficking of LRP8 in the presence of ApoE4 | ||
| Non-selective NHE inhibitor EMD87580 restores normal trafficking of LRP8 in the presence of ApoE4 | ||
| PBMCs | Significantly lower NHE9 transcript levels in PBMCs in MS patients with an active disease course | Esposito et al. (2015) |
| NHE9 expression regulates polarization and differentiation of T cells | ||
| Proinflammatory IFNγ expression was elevated upon NHE9 depletion |
AD Alzheimer’s disease, MS multiple sclerosis, HDACi histone deacetylase inhibitor, PBMCs peripheral blood mononuclear cells, LRP1 LDL receptor related protein 1, LRP8 LDL receptor related protein 8, IFNγ interferon gamma
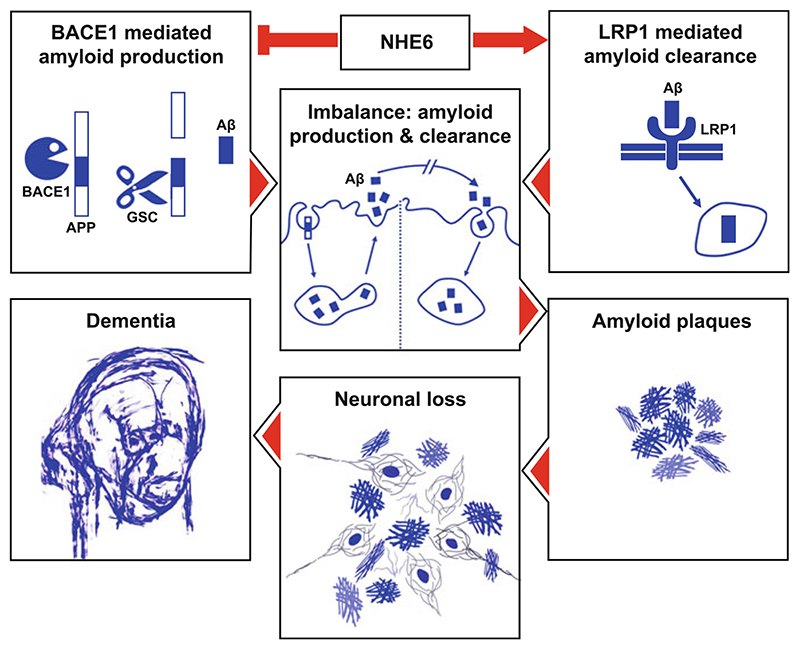
Fig. 5. Loss of NHE6 function is central to the endosomal pathology in Alzheimer’s disease.
NHE6 blocks BACE1-mediated amyloid production and promotes LRP1-mediated Aβ clearance. Downregulation of NHE6 expression or activity contributes to the endosomal pathology observed in pre-symptomatic AD brains by causing over-acidification of early and recycling endosomes, which creates an imbalance between Aβ production and clearance pathways, culminating in Aβ plaques, neurodegeneration, and dementia. Depiction of dementia is adapted from the self-portrait of Mr. William Utermohlen, an artist and AD patient. APP amyloid precursor protein, BACE1 β-secretase, GSC gamma secretase complex, LRP1 LDL receptor-related protein 1, Aβ amyloid β
Given the pan-functional role of eNHE on recycling of endocytosed proteins to the plasma membrane (Kondapalli et al. 2014; Prasad and Rao 2015a), we suggest that loss of NHE6 and pathological luminal acidification may be an upstream event causal to dysfunction of recycling endosomes and defective recycling of multiple proteins, in addition to LRP1, including glutamate and insulin receptors known to be reduced in brains of ApoE4 carriers (Chen et al. 2010; Zhao et al. 2017). Further experimentation will determine the precise mechanism of pH-sensitive regulation of vesicular traffic. A slight alteration in the pH even as little as 0.2 pH units can have profound sequelae (Musgrove et al. 1987). Notably, under physiological conditions, each compartment within the endosomal network has a unique pH range that is one of the defining characteristics of compartmental identity and function (Casey et al. 2010; Pedersen and Counillon 2019). Modulation of acid-base homeostasis within these endosomal compartments may affect cargo fate, redirecting content to or from the degradation pathway. Thus, it is attractive to speculate that changes in luminal pH alone are sufficient to drive endosomal recycling, with modest alkalization of the endosomal pH favoring the recycling pathway, rather than sorting cargo for degradation, and vice versa. This hypothesis, however, requires experimental validation.
6.5. The Goldilocks Scenario: Just the Right pH?
Humans with loss-of-function mutations in NHE6 and mouse lines for NHE6 deletion show progressive neurodegeneration, and this aspect of their phenotype provides crucial evidence to support the role for NHE6 inhibition/downregulation and endosomal hyperacidification in AD (Tables 1 and 2) (Garbern et al. 2010; Mignot et al. 2013; Stromme et al. 2011). The phenotypes of lower brain weight and elevated Aβ levels in the brain observed in knockout mice suggest a protective role for NHE6 activity in Aβ metabolism, neuronal health, and function (Prasad and Rao 2018a). Supporting this notion, studies have demonstrated that NHE6 deletion and endosomal hyperacidification diminish neuronal arborization and can cause synapse loss in mice (Ouyang et al. 2013). Progressive changes in pathology of postnatal brain in two separate mouse models of NHE6 deletion revealed mixed neurodevelopmental and neurodegenerative changes (Xu et al. 2017). Furthermore, disruption of Ca2+ entry, a known ApoE4-related pathology, has been recently described in NHE9 null neurons, which show endosomal hyperacidification and impairment in neurotransmitter release (Chen et al. 2010; Ullman et al. 2018).
In contrast, studies by Xian et al. have reported neuroprotective effects of NHE6 knockdown and NHE inhibitor EMD87580 that reversed Apoer2/LRP8 surface trafficking deficits in neurons treated with ApoE4, proposed to result from a molten globule-like state of ApoE4 induced in low pH (Xian et al. 2018). More recent studies, however, showed enhanced stability at acidic pH relative to neutral pH for all ApoE isoforms, contradicting earlier reports of molten-globule confirmation of ApoE4 in a low pH environment (Garai et al. 2011). Because ApoE4 aggregates and shows enhanced binding to lipids in a low pH environment, it seems probable from a biophysical perspective that pathological aggregation of ApoE4 should be exacerbated in hyperacidic endosomal pH (Garai et al. 2011). Another biophysical property for consideration is that protonation of three histidine residues in Aβ under conditions of hyperacidic endosomal pH drives formation of β-sheets and metal ion binding, simulating the process of amyloid aggregation and plaque deposition (Olubiyi and Strodel 2012). Similarly, protonation of glutamate or aspartate resides which are buried in the four-helix bundle of the amino-terminal domain is thought to provide enhanced stability for ApoE in acidic environment (Garai et al. 2011). Several key questions should be considered to resolve the conflicting conclusions. Use of a non-selective NHE inhibitor EMD87580 could have confounding effects by acting on multiple NHE isoforms, including the recycling NHE5 isoform, which is enriched in neuronal endosomes and works to acidify, rather than alkalinize endosomal lumen (Diering et al. 2013); thus inhibition of NHE5 will lead to endosomal alkalization. Furthermore, it is known that depletion of NHE6 results in compensatory increase in NHE9 isoform, which shows overlapping endosomal distribution with NHE6, to delay or diminish endosomal acidification processes (Kondapalli et al. 2013; Prasad and Rao 2015b). Similarly, downregulation of NHE6 levels associated with reciprocal NHE9 upregulation was found in brains of autistic patients (Schwede et al. 2014). In the absence of direct endosomal pH measurements to confirm pH changes in response to gene knockdowns or inhibitor treatment, it is difficult to conclude how these manipulations lead to the observed cellular phenotypes.
Although similar in function, NHE6 and NHE9 have distinct and non-redundant roles as evidenced by disease pathologies in patients and cell phenotypes (Kondapalli et al. 2014). A key unresolved question in the field is whether eNHE isoforms have differential effects on the trafficking of specific membrane proteins. Answering this question will be critical to predict the effects of changes in endosomal pH on surface expression of different membrane proteins. For instance, the evidence in the literature suggests that NHE6, but not NHE9, regulates LRP1 trafficking, and on the other hand, epidermal growth factor receptor (EGFR) trafficking was altered by NHE9 expression and not by NHE6, pointing to isoformspecific functions in receptor trafficking (Ilie et al. 2016; Kondapalli et al. 2015; Prasad and Rao 2018a). It is important to note that NHE9 is also downregulated in ApoE4 models and has been previously linked to AD (Martinelli-Boneschi et al. 2013; Perez-Palma et al. 2014; Prasad and Rao 2018a). Thus, one potential mechanism, although it remains to be characterized, to explain beneficial effects observed with NHE6 knockdown could be that concurrent upregulation of NHE9 isoform enhances recycling of a subset of receptors, such as LRP8 reported in ApoE4 neurons.
Nevertheless, the intriguing observations made by Xian et al. may highlight an emerging Goldilocks scenario in biology, wherein cellular functions depend on an optimal condition, with higher or lower extremes potentially hampering them. It is possible that there exists a bell-shaped relationship between endosomal pH and vesicle trafficking in which intermediate levels of endosomal acidification potentiate endocytic recycling; both very low levels and very high levels depress surface expression. This paradigm could explain the protective effects of NHE6 activation and inhibition in ApoE4 cells, reported in the literature. This seemingly conflicting role for endosomal acid-base homeostasis emerging in AD could add an additional layer of complexity to this dauntingly complex syndrome.
7. Big Data Could Fuel Big Progress
There has been an effort to identify disease mechanisms and facilitate repurposing of drugs to restore gene expression in a given disease by trawling through transcriptome databases (Pessetto et al. 2017; Prasad et al. 2019). This in silico prediction approach has the advantage of being quick and inexpensive. Yeast microarray datasets remains a valuable, yet relatively untapped resource as compared to mammalian data for discovery-driven mining efforts. An unbiased bioinformatics approach, based on data from 284 microarray studies comprising a wide range of experimental conditions, was used to identify evolutionarily conserved mechanism for regulation of eNHE expression and to identify candidate molecules to enhance/restore NHE6 levels in AD (Prasad and Rao 2018b). A predictive model for regulation of Nhx1 expression via the histone deacetylase (HDAC) Rpd3 acting on transcription factor Abf1 to regulate Nhx1 expression was derived from top hits in gene expression (both up- and downregulation) that was then experimentally validated in yeast. The Rpd3 inhibitor, trichostatin A (TSA), increased Nhx1 expression by approximately threefold and alkalinized luminal pH, consistent with the in silico derived model (Prasad and Rao 2018b).
To extend these findings to mammalian cells, a panel of HDAC inhibitors was tested on ApoE4 astrocytes to identify candidates, including TSA and vorinostat that restored NHE6 expression but not that of closely related NHE isoforms. These HDAC inhibitors also normalized endosomal pH and corrected Aβ clearance defects. In contrast, HDAC inhibitors that resulted in minimal changes in NHE6 expression (MC1568, tubacin) failed to correct Aβ clearance defects (Prasad and Rao 2018a). As predicted by this model, increased nuclear translocation of HDAC4 was observed in ApoE4 astrocytes, resulting in suppression of CREB (cAMP response element-binding protein) and NHE6 expression (Prasad and Rao 2018a, b). A translatable finding from these studies is that pharmacological HDAC inhibition (by TSA, vorinostat) or CREB activation (by Forskolin, Rolipram) selectively elevate endosomal pH and have the potential to correct human pathologies such as AD and autism resulting from aberrant endosomal hyperacidification (Prasad and Rao 2018a, b). Apart from CREB, HDACs have been previously shown to interact and inhibit myocyte enhancer factor 2A (MEF2A) transcription factor activity in neurons (Li et al. 2012). MEF2A is a key regulator of activity-dependent gene program that controls synapse development and function. Intriguingly, NHE6 is a known downstream target of MEF2A in neurons (Flavell et al. 2008). Thus, as an additional/alternative mechanism, we propose that ApoE4-mediated nuclear translocation of HDACs could potentially downregulate MEF2A-NHE6 axis resulting in endosomal hyperacidification that favors amyloidogenic processing of APP and production of Aβ in neurons.
8. Extending the Endosomal Acid-Base Paradigm Beyond Alzheimer’s Disease
Evidence for an ever-expanding role for endosomes in neurodegeneration has led to the notion that the endosomal dysfunction could trigger multiple effects affecting diverse pathways. Thus, it appears likely that the disturbances in endosomal pH, by inducing complex patterns of synaptic dysfunction and alterations in neural circuitry, could play a role in multiple neurodegenerative disorders beyond AD. Similar to what has been shown in AD, endosomal aberrations and enlarged endosomes are also the earliest neuronal pathology described in Niemann-Pick type C (NPC), Parkinson’s disease, Down syndrome, and other neurodegenerative disorders (Nixon 2005, 2017). NPC is an inherited lysosomal storage disorder associated with impairment in cholesterol trafficking and excessive glycosphingolipid storage (Alam et al. 2016). We have previously observed a direct link between hyper-acidic endosomal-lysosomal pH and disruption of cholesterol trafficking and accumulation of free cholesterol, quantified by filipin staining, in yeast and fibroblast models of NPC disease (Brett et al. 2011). This link between pathological endosomal acidification and cholesterol mistrafficking is strengthened by in vivo studies on NHE6KO mice, showing hyperacidic endosomes and positive labeling with filipin in neurons (Ouyang et al. 2013; Stromme et al. 2011). In both yeast and mammalian cell culture models of NPC disease, we showed that these trafficking phenotypes could be rescued by mild alkalization of endosomal-lysosomal compartments using low concentrations (10 nm) nigericin, a K+/H+ ionophore (Brett et al. 2011). Similarly, endosomal alkalization might explain reduced cholesterol accumulation reported in NPC patient fibroblasts treated with alexidine dihydrochloride, an antimicrobial compound with V-ATPase inhibitory activity (Chan et al. 2012; Pugach et al. 2018). Given our observation of increased NHE6 expression and endosomal alkalization with HDAC inhibition, we suggest that correction of endosomal hyperacidification pathology could potentially contribute to well-documented therapeutic effects of HDAC inhibitor drugs in Niemann-Pick type C disease (Alam et al. 2016; Prasad and Rao 2018a).
Recent studies suggest that endosomal-lysosomal dysfunction may be a primary defect in Parkinson’s disease (PD). For instance, mutations in Vps35/retromer, a master regulator of endosome sorting, have been linked to familial PD, highlighting the importance of endosomal pathway in the pathogenesis (Kett and Dauer 2016). More recently, deficiency of Vps35 has been also reported in primary tauopathies, including progressive supra-nuclear palsy and Picks’ disease, suggesting a mechanistic overlap with PD (Vagnozzi et al. 2019). A genome-wide analysis of vacuolar pH in ~4,600 yeast null mutants identified dysregulation of luminal pH in Vps35 deletion yeast, although the extrapolation of these findings to humans remain to be validated (Brett et al. 2011). More compelling evidence to link endosomal acid-base dysregulation in PD emerged from recent clinical reports of patients with NHE6 mutations, summarized in Table 1. Riess et al. reported a family with NHE6 mutation where affected males had Christianson syndrome and obligate carrier females showed signs of Parkinsonism (Riess et al. 2013). Similar observations have been reported more recently wherein female carriers of NHE6 mutations showed corticobasal degeneration syndrome (CBDS) and atypical parkinsonism (Sinajon et al. 2016). These observations are further strengthened by a clinical report of a ΔWST372 in-frame deletion of three amino acids in NHE6 that was described by Garbern et al. in patients with corticobasal degeneration, severe intellectual disability, autistic symptoms accompanied by deposition of hyperphosphorylated tau in the brain (Garbern et al. 2010). Loss of function was demonstrated by structure-function evaluation of this mutation (Prasad and Rao 2015b). NHE6 inactivation is associated with tauopathy, characterized by neuronal and glial tau inclusions and a preponderance of pathological 4R tau isoform (Garbern et al. 2010). Consistent with this observation, our analysis of postmortem brains from normal and AD dataset revealed that decreased NHE6 expression was correlated with greater tau pathology assessed via neurofibrillary tangle scores (Prasad and Rao 2015b). Furthermore, NHE6 expression was found downregulated in substantia nigra in patients with Parkinson’s disease (Hauser et al. 2005). Taken together, these observations provide compelling clues for the links between NHE6 function in Parkinson’s disease and tauopathy, and future studies are awaited to define this broader role for endosomal pH in neurodegeneration.
More recently, a pharmacogenetic study by Esposito et al. identified an intriguing link between multiple sclerosis (MS) and endosomal pH (Esposito et al. 2015). MS is a chronic inflammatory and demyelinating condition of the central nervous system characterized with prominent neurodegeneration, and this newly discovered link may lead to new therapies. Intriguingly, chloroquine, a weakly basic anti-malarial drug that accumulates in acidic organelles such as endosomes and alkalinizes luminal pH, was long found to have therapeutic effect in MS (Thome et al. 2013). Furthermore, polymorphisms in NHE9 are linked to N-glycosylation alterations, an important molecular mechanism in MS (Huffman et al. 2011; Mkhikian et al. 2011). Moreover, NHE9 downregulation was found in a model of Down syndrome, a developmental disorder with Alzheimer’s-related endosome dysfunction and early-onset neurodegeneration (Hibaoui et al. 2014; Jiang et al. 2010). By using an approach that integrated GWAS with experimental studies, Esposito et al. showed a significant association between an intronic variant in NHE9 (rs9828519) and non-response to interferon-β (IFNβ) therapy in MS patients (Table 1). Downregulation of NHE9 levels was observed in peripheral blood mononuclear cells (PBMCs) in MS patients with an active disease course. Furthermore, NHE9 expression was induced in PBMCs with IFNβ treatment. NHE9 was found to influence polarization and differentiation of T cells and knockdown of this protein showed increased expression of proinflammatory molecule interferon gamma (IFNγ) (Esposito et al. 2015) (Table 2). A follow-up study Liu et al. identified significant association between rs9828519 variant and dysregulation of NHE9 expression in three brain regions, including occipital cortex, intralobular white matter, and substantia nigra (Liu et al. 2017). Further detailed mechanistic studies in well-defined animal models are warranted to understand the link between NHE9 and MS.
Taken together, available literature reviewed here and elsewhere raises the possibility that a significant proportion of deficits in patients with neurodegenerative disorders are due to endosomal trafficking defects that act as an upstream pathogenic hub. The precise mechanisms by which disturbances in endosomal pH cause neuronal dysfunction and ultimately death in different neurodegenerative disorders remain to be defined and potential therapeutic interventions aimed at endosomal pH need to be determined. This concept has far-reaching therapeutic implications. While the reversal of structural defects in endosomes such as amplifications in its size and number remains a challenge, the correction of endosomal pH deficits, the re-establishment of vesicle trafficking and an improvement of network function appear to be within plausible reach.
9. Summary, Key Questions, and Translational Prospects
What are the translational prospects of the complex endosomal acid-base alterations in neurodegenerative diseases reviewed herein? Most well-known genetic risks for sporadic AD are endosomal proteins, yet targeting these has not provided effective treatments. Notably, an earliest preclinical hallmark of the AD and other neurode-generative disorders is dysfunction of endolysosomal system that manifests morphologically as enlarged and amplified compartments, biochemically as hyperacidic luminal pH, and functionally as trafficking defects that promotes amyloid pathology (Nixon 2017; Prasad and Rao 2018a; Small et al. 2017). There is a clear potential for development of interventions to exploit the disease-modifying benefit of endosomal pH. An integrated approach involving data mining and in silico co-expression analysis combined with wet lab studies using NHE6KO mice, post-mortem brains, patient fibroblasts, cultured neuronal and astrocyte lines, and simple yeast models demonstrated a novel ApoE-regulated cellular mechanism and identified a druggable target in AD. By regulating endosomal pH in amyloid pathology, NHE6 is a prominent effector of ApoE4 (Prasad and Rao 2015b, 2018a, b). The fact that acidic endosomal pH is linked to AD at multiple levels makes endosomal pH an attractive target for “disease-modifying” drugs for AD therapy. Significant mechanism-based toxicity has been reported with strong inhibition of APP processing enzymes (β- and γ-secretases), which has directed efforts to identify novel regulators/modulators of APP processing (Ben Halima et al. 2016). Moderate secretase inhibition coupled with novel therapies targeting cellular microenvironment hold promise to benefit in CNS and limit mechanism-based toxicities.
Endosomal Na+/H+ exchangers bring new focus to endosome biology, a neglected area in neurodegenerative disorders, and forge a new link between autism/intellectual disability and AD. Endosomal pH emerges as a critical mechanistic link between neurodevelopmental and neurodegenerative disorders. We suggest that in both genetic and sporadic AD, there is disease-initiated perturbation of endosomal-lysosomal pH that may represent the mechanism for relentless disease progression. Consistent with this idea, amphipathic drugs such as bepridil and amiodarone which partition into acidic compartments and alkalinize endosomes also correct Aβ pathology in cell culture and animal models (Mitterreiter et al. 2010). Similarly, endosomal alkalization might explain APP redistribution and reduced Aβ production reported in APP stable cells treated with destruxin E, a natural cyclic hexadepsipeptide with V-ATPase inhibitory activity (Itoh et al. 2009). Our research provides a rational basis for such screening and repurposing of existing US Food and Drug Administration (FDA)-approved drugs and natural product derivatives, known to have off-label activity of endosomal alkalization, as “disease-modifying” drugs to target the cellular micro-environment in AD. Membrane transporters like NHE6 are generally useful “druggable” targets of therapeutic potential. A compelling example is of cystic fibrosis transmembrane conductance regulator (CFTR), one of the most widely studied membrane transporters that has been successfully targeted using small molecule potentiators and correctors as a therapeutic approach to cystic fibrosis. Development of small molecular activators of NHE6 to enhance endosomal pH therefore has the potential to reduce Aβ pathology. Targeting NHE6 has the advantage of specific β-secretase inhibition within endosomes, enhancing their potential as AD therapeutics without undesired mechanism-based toxicities. In addition to regulating luminal pH, endosomal NHE could modulate ionic composition of the endosomal lumen (e.g., K+ and Na+) that may directly impact inter- and intra-endosomal functions and dynamics, including osmotic effects and altering membrane curvature and microdomains (Scott and Gruenberg 2011). Further experiments are needed to determine if any such non-pH roles are perturbed in AD.
An emerging pathophysiology of AD is transsynaptic dissemination of the pathological proteins by altering the content of exosomes (Rajendran et al. 2006). The luminal pH within endosomal-lysosomal compartment might play a role in exosome content, biogenesis, and release. In this context, it is important to note that the ionophore monensin that mediates Na+/H+ exchange, alkalinize luminal pH, and mimics constitutively activated NHE6 is known to stimulate the endosomal recycling pathway and exosome secretion (Muro et al. 2006; Savina et al. 2003). We speculate that NHE6 downregulation in AD might disrupt vesicular traffic and alter the number of intraluminal vesicles in the multivesicular body and/or alter the content of exosomes. Further studies are needed to characterize and validate this mechanism. Pathological events, such as epileptiform activity, and normal physiological processes, such as the sleep-wake cycle, are known to regulate Aβ production and if such diverse stimuli also regulate expression/activity of NHE6, a known epilepsy linked protein, remain to be determined (Cirrito et al. 2005; Kang et al. 2009; Kondapalli et al. 2014). Previously, we have shown that endosomal hyperacidification in astrocytes could result in reduced surface levels of glutamate uptake transporter, resulting in aberrant increase in levels of glutamate in and around the synaptic cleft, which could cause epileptiform phenotype and neurodegeneration seen in autism (Kondapalli et al. 2013). Similar mechanism could also explain, in part, AD-related excitotoxicity. While the evidence to link NHE6 downregulation and synapse dysfunction is compelling, it must be acknowledged that the overall contribution of endosomal pH and endosomal NHE to synaptic protein expression, in the context of normal physiology and in pathologies such as AD, remains unclear and more research is warranted. Thus, for example, although enhanced recruitment of NHE6 into dendritic spines during N-methyl D-aspartate (NMDA)-dependent LTP has been reported in the literature, the accumulation of NHE6 at dendritic spines and presynaptic terminals alone cannot be taken as unequivocal evidence for its role in synapse formation and maintenance (Deane et al. 2013). Given the emerging role for recycling endosomal dysfunction in AD (Woodruff et al. 2016), therapeutic enhancement of pH of recycling endosomes might result in better endocytic recycling of membrane proteins, including adhesion molecules and neurotransmitter receptors and transporters, and provide neuroprotection independently of protection against plaque formation. Targeting this inside-out control of surface proteostasis by eNHE therefore may provide new treatments for sporadic AD and related disorders.
Lastly, based on our data, we propose that amyloid pathologies may contribute to autism and intellectual disability phenotypes seen in patients with NHE6 mutations that could have important prognostic implications for early intervention to limit regression and marked neurodegeneration seen in Christianson syndrome patients (Mignot et al. 2013). Notably, alterations in APP processing and Aβ production have been reported in autism, Fragile X syndrome and 15q duplication in patients and animal models (Wegiel et al. 2012). An understanding of the mechanistic overlap between developmental and aging disorders is urgently needed to expand therapeutic options for these disorders. Consistent to our reports of lower NHE6 levels in AD, downregulation of NHE6 gene expression has been documented in post-mortem autism brains, marking the possibility of a mechanistic overlap between autism and AD pathologies (Prasad and Rao 2015b, 2018a; Schwede et al. 2014). A long-term goal of our research is to determine if a subset of autism patients with dysregulated NHE6 activity, either by loss-of-function mutations or by downregulated gene expression, have a higher risk of premature aging and developing neurodegenerative disorders, thereby providing a rational basis to stratify patients for targeted therapies. In summary, studies on NHE6 have proven to be instrumental for appreciating the role of endosomal acid-base homeostasis in neurodegenerative diseases and have provided us an opportunity through which we can begin to study and understand the role of other ion transporters regulating endosomal pH in human pathologies. Restoring endosomal acid-base disturbance that fuels the progression of AD holds promise to slow and ultimately even prevent cognitive impairment in this syndrome. Future research will hopefully further define additional molecular players and pathways regulating critical endosomal acidification process and in doing so may identify promising targets for therapies for Alzheimer’s disease and related neuro- degenerative disorders.
Acknowledgments
H.P. is an Early Career Fellow of the DBT Wellcome Trust India Alliance (IA/E/17/1/503665). R.R. is supported by grants from the NIH (NIDDK R01DK108304) and BSF (130440).
Abbreviations
- AD
Alzheimer’s disease
- ApoE4
Apolipoprotein E4
- APP
Amyloid precursor protein
- eNHE
Endosomal Na+/H+ exchanger
- HDAC
Histone deacetylase
- V-ATPase
Vacuolar H+-ATPase
Contributor Information
Hari Prasad, Email: hariprasad@iisc.ac.in, Department of Molecular Reproduction, Development and Genetics, Indian Institute of Science, Bangalore, India, Department of Physiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Rajini Rao, Department of Physiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- Afghah Z, Chen X, Geiger JD. Role of endolysosomes and inter-organellar signaling in brain disease. Neurobiol Dis. 2019;134:104670. doi: 10.1016/j.nbd.2019.104670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MS, Getz M, Haldar K. Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann-pick type C disease in a mouse model. Sci Transl Med. 2016;8(326):326ra323. doi: 10.1126/scitranslmed.aad9407. [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Blumwald E. Identification of a 170-kDa protein associated with the vacuolar Na +/H+ antiport of Beta vulgaris. Proc Natl Acad Sci U S A. 1991;88(24):11177–11181. doi: 10.1073/pnas.88.24.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Ben Halima S, Mishra S, Raja KMP, Willem M, Baici A, Simons K, Brustle O, Koch P, Haass C, Caflisch A, Rajendran L. Specific inhibition of beta-secretase processing of the Alzheimer disease amyloid precursor protein. Cell Rep. 2016;14(9):2127–2141. doi: 10.1016/j.celrep.2016.01.076. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ. Na/H antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Levi BP, Patel FI, Stevens TH. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2000;11(12):4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282(5):C1031–C1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005a;288(2):C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005b;16(3):1396–1405. doi: 10.1091/mbc.e04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Kallay L, Hua Z, Green R, Chyou A, Zhang Y, Graham TR, Donowitz M, Rao R. Genome-wide analysis reveals the vacuolar pH-stat of Saccharomyces cerevisiae. PLoS One. 2011;6(3):e17619. doi: 10.1371/journal.pone.0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan S, Takagi-Niidome S, Liao F, Carlo AS, Schmidt V, Burgert T, Kitago Y, Fuchtbauer EM, Fuchtbauer A, Holtzman DM, Takagi J, et al. Lysosomal sorting of amyloidbeta by the SORLA receptor is impaired by a familial Alzheimer’s disease mutation. Sci Transl Med. 2014;6(223):223ra220. doi: 10.1126/scitranslmed.3007747. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4(3):233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Gandy SE, Buxbaum JD, Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157(1):277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Prudom C, Raines SM, Charkhzarrin S, Melman SD, De Haro LP, Allen C, Lee SA, Sklar LA, Parra KJ. Inhibitors of V-ATPase proton transport reveal uncoupling functions of tether linking cytosolic and membrane domains of V0 subunit a (Vph1p) J Biol Chem. 2012;287(13):10236–10250. doi: 10.1074/jbc.M111.321133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A. 2010;107(26):12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the riskof Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DK, Makena MR, Llongueras JP, Prasad H, Ko M, Bandral M, Rao R. A Ca(2+)- ATPase regulates e-cadherin biogenesis and epithelial-mesenchymal transition in breast cancer cells. Mol Cancer Res. 2019;17(8):1735–1747. doi: 10.1158/1541-7786.MCR-19-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Scott DA, Ganguly A, Koo EH, Tang Y, Roy S. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 2013;79(3):447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane EC, Ilie AE, Sizdahkhani S, Das Gupta M, Orlowski J, McKinney RA. Enhanced recruitment of endosomal Na+/H+ exchanger NHE6 into Dendritic spines of hippocampal pyramidal neurons during NMDA receptor-dependent long-term potentiation. J Neurosci. 2013;33(2):595–610. doi: 10.1523/JNEUROSCI.2583-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Numata Y, Fan S, Church J, Numata M. Endosomal acidification by Na+/H+ exchanger NHE5 regulates TrkA cell-surface targeting and NGF-induced PI3K signaling. Mol Biol Cell. 2013;24(21):3435–3448. doi: 10.1091/mbc.E12-06-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Asp Med. 2013;34(2-3):236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Sorosina M, Ottoboni L, Lim ET, Replogle JM, Raj T, Brambilla P, Liberatore G, Guaschino C, Romeo M, Pertel T, et al. A pharmacogenetic study implicates SLC9a9 in multiple sclerosis disease activity. Ann Neurol. 2015;78(1):115–127. doi: 10.1002/ana.24429. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60(6):1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466(1):61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- Garai K, Baban B, Frieden C. Self-association and stability of the ApoE isoforms at low pH: implications for ApoE-lipid interactions. Biochemistry. 2011;50(29):6356–6364. doi: 10.1021/bi2006702. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, Friez MJ, Schwartz CE, Stevenson R, Sima AA. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010;133(Pt 5):1391–1402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci U S A. 1999;96(4):1480–1485. doi: 10.1073/pnas.96.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, Sjoholm H, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82(4):1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Capell A, Citron M, Teplow DB, Selkoe DJ. The vacuolar H(+)-ATPase inhibitor bafilomycin A1 differentially affects proteolytic processing of mutant and wild-type betaamyloid precursor protein. J Biol Chem. 1995;270(11):6186–6192. doi: 10.1074/jbc.270.11.6186. [DOI] [PubMed] [Google Scholar]
- Haure-Mirande JV, Audrain M, Fanutza T, Kim SH, Klein WL, Glabe C, Readhead B, Dudley JT, Blitzer RD, Wang M, Zhang B, et al. Deficiency of TYROBP, an adapter protein for TREM2 and CR3 receptors, is neuroprotective in a mouse model of early Alzheimer’s pathology. Acta Neuropathol. 2017;134(5):769–788. doi: 10.1007/s00401-017-1737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MA, Li YJ, Xu H, Noureddine MA, Shao YS, Gullans SR, Scherzer CR, Jensen RV, McLaurin AC, Gibson JR, Scott BL, et al. Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Arch Neurol. 2005;62(6):917–921. doi: 10.1001/archneur.62.6.917. [DOI] [PubMed] [Google Scholar]
- Hibaoui Y, Grad I, Letourneau A, Santoni FA, Antonarakis SE, Feki A. Data in brief: Transcriptome analysis of induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. Genom Data. 2014;2:226–229. doi: 10.1016/j.gdata.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefgen S, Dahms SO, Oertwig K, Than ME. The amyloid precursor protein shows a pH-dependent conformational switch in its E1 domain. J Mol Biol. 2015;427(2):433–442. doi: 10.1016/j.jmb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Huffman JE, Knezevic A, Vitart V, Kattla J, Adamczyk B, Novokmet M, Igl W, Pucic M, Zgaga L, Johannson A, Redzic I, et al. Polymorphisms in B3GAT1, SLC9A9 and MGAT5 are associated with variation within the human plasma N-glycome of 3533 European adults. Hum Mol Genet. 2011;20(24):5000–5011. doi: 10.1093/hmg/ddr414. [DOI] [PubMed] [Google Scholar]
- Ilie A, Gao AY, Reid J, Boucher A, McEwan C, Barriere H, Lukacs GL, McKinney RA, Orlowski J. A Christianson syndrome-linked deletion mutation ((287)ES(288)) in SLC9A6 disrupts recycling endosomal function and elicits neurodegeneration and cell death. Mol Neurodegener. 2016;11(1):63. doi: 10.1186/s13024-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Okochi M, Tagami S, Nishitomi K, Nakayama T, Yanagida K, Fukumori A, Jiang J, Mori K, Hosono M, Kikuchi J, et al. Destruxin E decreases Beta-amyloid generation by reducing colocalization of beta-amyloid-cleaving enzyme 1 and beta-amyloid protein precursor. Neurodegener Dis. 2009;6(5-6):230–239. doi: 10.1159/000236902. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev. 2018;98(3):1493–1590. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 potentiates amyloid beta peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002;277(24):21821–21828. doi: 10.1074/jbc.M112109200. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2010;107(4):1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Rigoglioso A, Peterhoff CM, Pawlik M, Sato Y, Bleiwas C, Stavrides P, Smiley JF, Ginsberg SD, Mathews PM, Levy E, et al. Partial BACE1 reduction in a Down syndrome mouse model blocks Alzheimer-related endosomal anomalies and cholinergic neurodegeneration: role of APP-CTF. Neurobiol Aging. 2016;39:90–98. doi: 10.1016/j.neurobiolaging.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami T, Chen S, Memar P, Choi M, Foster LJ, Numata M. Identification and biochemical characterization of the SLC9A7 interactome. Mol Membr Biol. 2008;25(5):436–447. doi: 10.1080/09687680802263046. [DOI] [PubMed] [Google Scholar]
- Kanatsu K, Morohashi Y, Suzuki M, Kuroda H, Watanabe T, Tomita T, Iwatsubo T. Decreased CALM expression reduces Abeta42 to total Abeta ratio through clathrin-mediated endocytosis of gamma-secretase. Nat Commun. 2014;5:3386. doi: 10.1038/ncomms4386. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner-Rossi M, Gulinello M, Walkley S, Dobrenis K. Pathobiology of Christianson syndrome: linking disrupted endosomal-lysosomal function with intellectual disability and sensory impairments. Neurobiol Learn Mem. 2019;165:106867. doi: 10.1016/j.nlm.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett LR, Dauer WT. Endolysosomal dysfunction in Parkinson’s disease: recent developments and future challenges. Mov Disord. 2016;31(10):1433–1443. doi: 10.1002/mds.26797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, Jiang Y, Nixon RA. Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer’s disease. Mol Psychiatry. 2016;21(5):707–716. doi: 10.1038/mp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Quiñones-Hinojosa A, Rao R. Emerging links between endosomal pH and cancer. Cancer Metastasis Rev. 2020;39(2):519–534. doi: 10.1007/s10555-020-09870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli KC, Hack A, Schushan M, Landau M, Ben-Tal N, Rao R. Functional evaluation of autism-associated mutations in NHE9. Nat Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli KC, Prasad H, Rao R. An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front Cell Neurosci. 2014;8:172. doi: 10.3389/fncel.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli KC, Llongueras JP, Capilla-Gonzalez V, Prasad H, Hack A, Smith C, Guerrero-Cazares H, Quinones-Hinojosa A, Rao R. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun. 2015;6:6289. doi: 10.1038/ncomms7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra R, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M. Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc Natl Acad Sci U S A. 2017;114(28):E5703–E5711. doi: 10.1073/pnas.1618417114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK. Effect of ionophores on the processing of the beta-amyloid precursor protein in different cell lines. Cell Mol Neurobiol. 1994;14(4):297–313. doi: 10.1007/BF02088713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Kang HJ, von Ballmoos C, Newstead S, Uzdavinys P, Dotson DL, Iwata S, Beckstein O, Cameron AD, Drew D. A two-domain elevator mechanism for sodium/proton antiport. Nature. 2013;501(7468):573–577. doi: 10.1038/nature12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A, Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat Med. 2012;18(5):783–790. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang F, Hu Y, Jiang Y, Gong Z, Liu S, Chen X, Jiang Q, Hao J. Genetic variants and multiple sclerosis risk gene SLC9A9 expression in distinct human brain regions. Mol Neurobiol. 2017;54(9):6820–6826. doi: 10.1007/s12035-016-0208-5. [DOI] [PubMed] [Google Scholar]
- Martina JA, Lelouvier B, Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic. 2009;10(8):1143–1156. doi: 10.1111/j.1600-0854.2009.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli-Boneschi F, Giacalone G, Magnani G, Biella G, Coppi E, Santangelo R, Brambilla P, Esposito F, Lupoli S, Clerici F, Benussi L, et al. Pharmacogenomics in Alzheimer’s disease: a genome-wide association study of response to cholinesterase inhibitors. Neurobiol Aging. 2013;34(6):1711.e1717–1711.e1713. doi: 10.1016/j.neurobiolaging.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56(1):127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Mauvezin C, Neufeld TP. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy. 2015;11(8):1437–1438. doi: 10.1080/15548627.2015.1066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR. Role of endosomes and lysosomes in human disease. Cold Spring Harb Perspect Biol. 2014;6(5):a016931. doi: 10.1101/cshperspect.a016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayburd A, Baranova A. Knowledge-based compact disease models identify new molecular players contributing to early-stage Alzheimer’s disease. BMC Syst Biol. 2013;7:121. doi: 10.1186/1752-0509-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Narayanan B, Liu J, Perrone-Bizzozero NI, Stevens MC, Calhoun VD, Glahn DC, Shen L, Risacher SL, Saykin AJ, Pearlson GD. A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer ‘s disease in the ADNI cohort. NeuroImage. 2012;60(3):1608–1621. doi: 10.1016/j.neuroimage.2011.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot C, Heron D, Bursztyn J, Momtchilova M, Mayer M, Whalen S, Legall A, Billette de Villemeur T, Burglen L. Novel mutation in SLC9A6 gene in a patient with Christianson syndrome and retinitis pigmentosum. Brain and Development. 2013;35(2):172–176. doi: 10.1016/j.braindev.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterreiter S, Page RM, Kamp F, Hopson J, Winkler E, Ha HR, Hamid R, Herms J, Mayer TU, Nelson DJ, Steiner H, et al. Bepridil and amiodarone simultaneously target the Alzheimer’s disease beta-and gamma-secretase via distinct mechanisms. J Neurosci. 2010;30(26):8974–8983. doi: 10.1523/JNEUROSCI.1199-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkhikian H, Grigorian A, Li CF, Chen HL, Newton B, Zhou RW, Beeton C, Torossian S, Tatarian GG, Lee SU, Lau K, et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun. 2011;2:334. doi: 10.1038/ncomms1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007;402(2):301–310. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- Musgrove E, Seaman M, Hedley D. Relationship between cytoplasmic pH and proliferation during exponential growth and cellular quiescence. Exp Cell Res. 1987;172(1):65–75. doi: 10.1016/0014-4827(87)90093-0. [DOI] [PubMed] [Google Scholar]
- Nass R, Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J Biol Chem. 1998;273(33):21054–21060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- Nass R, Cunningham KW, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272(42):26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- Naumova OY, Palejev D, Vlasova NV, Lee M, Rychkov SY, Babich ON, Vaccarino FM, Grigorenko EL. Age-related changes of gene expression in the neocortex: preliminary data on RNA-Seq of the transcriptome in three functionally distinct cortical areas. Dev Psychopathol. 2012;24(4):1427–1442. doi: 10.1017/S0954579412000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurode-generative diseases. Neurobiol Aging. 2005;26(3):373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 2017;31(7):2729–2743. doi: 10.1096/fj.201700359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olubiyi OO, Strodel B. Structures of the amyloid beta-peptides Abeta1-40 and Abeta1-42 as influenced by pH and a D-peptide. J Phys Chem B. 2012;116(10):3280–3291. doi: 10.1021/jp2076337. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80(1):97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park C, Ha J, Park S. Prediction of Alzheimer’s disease based on deep neural network by integrating gene expression and DNA methylation dataset. Expert Syst Appl. 2019;140:112873 [Google Scholar]
- Pedersen SF, Counillon L. The SLC9A-C mammalian Na(+)/H(+) exchanger family: molecules, mechanisms, and physiology. Physiol Rev. 2019;99(4):2015–2113. doi: 10.1152/physrev.00028.2018. [DOI] [PubMed] [Google Scholar]
- Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci. 2014;15(4):250–263. doi: 10.1038/nrn3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Palma E, Bustos BI, Villaman CF, Alarcon MA, Avila ME, Ugarte GD, Reyes AE, Opazo C, De Ferrari GV Alzheimer’s Disease Neuroimaging I Group N-LNFS. Overrepresentation of glutamate signaling in Alzheimer’s disease: network-based pathway enrichment using meta-analysis of genome-wide association studies. PLoS One. 2014;9(4):e95413. doi: 10.1371/journal.pone.0095413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson T, Lattanzio F, Calvo-Garrido J, Rimondini R, Rubio-Rodrigo M, Sundstrom E, Maioli S, Sandebring-Matton A, Cedazo-Minguez A. Apolipoprotein E4 elicits lysosomal cathepsin D release, decreased thioredoxin-1 levels, and apoptosis. J Alzheimers Dis. 2017;56(2):601–617. doi: 10.3233/JAD-150738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessetto ZY, Chen B, Alturkmani H, Hyter S, Flynn CA, Baltezor M, Ma Y, Rosenthal HG, Neville KA, Weir SJ, Butte AJ, et al. In silico and in vitro drug screening identifies new therapeutic approaches for Ewing sarcoma. Oncotarget. 2017;8(3):4079–4095. doi: 10.18632/oncotarget.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6):a020628. doi: 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poroca DR, Pelis RM, Chappe VM. ClC channels and transporters: structure, physiological functions, and implications in human chloride channelopathies. Front Pharmacol. 2017;8:151. doi: 10.3389/fphar.2017.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Rao R. Applying knowledge of autism to brain cancer management: what do we know? Future Oncol. 2015a;11(13):1847–1850. doi: 10.2217/fon.15.93. [DOI] [PubMed] [Google Scholar]
- Prasad H, Rao R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J Biol Chem. 2015b;290(9):5311–5327. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc Natl Acad Sci U S A. 2018a;115(28):E6640–E6649. doi: 10.1073/pnas.1801612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Rao R. Histone deacetylase-mediated regulation of endolysosomal pH. J Biol Chem. 2018b;293(18):6721–6735. doi: 10.1074/jbc.RA118.002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Osei-Owusu J, Rao R. Functional analysis of Na(+)/H(+) exchanger 9 variants identified in patients with autism and epilepsy. Matters (Zur) 2017. 2017 doi: 10.19185/matters.201704000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Dang DK, Kondapalli KC, Natarajan N, Cebotaru V, Rao R. NHA2 promotes cyst development in an in vitro model of polycystic kidney disease. J Physiol. 2019;597(2):499–519. doi: 10.1113/JP276796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach EK, Feltes M, Kaufman RJ, Ory DS, Bang AG. High-content screen for modifiers of Niemann-pick type C disease in patient cells. Hum Mol Genet. 2018;27(12):2101–2112. doi: 10.1093/hmg/ddy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103(30):11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess A, Rossier E, Kruger R, Dufke A, Beck-Woedl S, Horber V, Alber M, Glaser D, Riess O, Tzschach A. Novel SLC9A6 mutations in two families with Christianson syndrome. Clin Genet. 2013;83(6):596–597. doi: 10.1111/j.1399-0004.2012.01948.x. [DOI] [PubMed] [Google Scholar]
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calciumdependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Haucke V. Recycling endosomes in neuronal membrane traffic. Biol Cell. 2007;99(6):333–342. doi: 10.1042/BC20070007. [DOI] [PubMed] [Google Scholar]
- Schrader-Fischer G, Paganetti PA. Effect of alkalizing agents on the processing of the betaamyloid precursor protein. Brain Res. 1996;716(1-2):91–100. doi: 10.1016/0006-8993(96)00002-9. [DOI] [PubMed] [Google Scholar]
- Schwede M, Garbett K, Mirnics K, Geschwind DH, Morrow EM. Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol Psychiatry. 2014;19(3):277–279. doi: 10.1038/mp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Gruenberg J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays. 2011;33(2):103–110. doi: 10.1002/bies.201000108. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer ‘s disease. Cold Spring Harb Perspect Biol. 2012;4(5):a005777. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Imboywa S, Giagtzoglou N, Powers MP, Hu Y, Devenport D, Chipendo P, Chibnik LB, Diamond A, Perrimon N, Brown NH, et al. Functional screening in Drosophila identifies Alzheimer’s disease susceptibility genes and implicates Tau-mediated mechanisms. Hum Mol Genet. 2014;23(4):870–877. doi: 10.1093/hmg/ddt478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinajon P, Verbaan D, So J. The expanding phenotypic spectrum of female SLC9A6 mutation carriers: a case series and review of the literature. Hum Genet. 2016;135(8):841–850. doi: 10.1007/s00439-016-1675-5. [DOI] [PubMed] [Google Scholar]
- Small SA, Simoes-Spassov S, Mayeux R, Petsko GA. Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 2017;40(10):592–602. doi: 10.1016/j.tins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, Micsenyi MC, Stephney G, Ellevog L, Klungland A, Walkley SU. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134(Pt 11):3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. 2012;2(5):a006148. doi: 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theendakara V, Patent A, Peters Libeu CA, Philpot B, Flores S, Descamps O, Poksay KS, Zhang Q, Cailing G, Hart M, John V, et al. Neuroprotective Sirtuin ratio reversed by ApoE4. Proc Natl Acad Sci U S A. 2013;110(45):18303–18308. doi: 10.1073/pnas.1314145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome R, Moraes AS, Bombeiro AL, Farias Ados S, Francelin C, da Costa TA, Di Gangi R, dos Santos LM, de Oliveira AL, Verinaud L. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS One. 2013;8(6):e65913. doi: 10.1371/journal.pone.0065913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, et al. Functional links between Abeta toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science. 2011;334(6060):1241–1245. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Cataldo AM, Nixon RA, Barnett JL, Lee MK, Checler F, Fowler DR, Smialek JE, Crain B, Martin LJ, Kawas CH. Neuropathology of preclinical and clinical late-onset Alzheimer’s disease. Ann Neurol. 1998;43(5):673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- Ullman JC, Yang J, Sullivan M, Bendor J, Levy J, Pham E, Silm K, Seifikar H, Sohal VS, Nicoll RA, Edwards RH. A mouse model of autism implicates endosome pH in the regulation of presynaptic calcium entry. Nat Commun. 2018;9(1):330. doi: 10.1038/s41467-017-02716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urmoneit B, Turner J, Dyrks T. Pulse-chase experiments revealed beta-secretase cleavage from immature full-length amyloid precursor protein harboring the Swedish mutation. Implications for distinct pathways. J Mol Neurosci. 1998;11(2):141–150. doi: 10.1385/JMN:11:2:141. [DOI] [PubMed] [Google Scholar]
- Vagnozzi AN, Li JG, Chiu J, Razmpour R, Warfield R, Ramirez SH, Pratico D. VPS35 regulates tau phosphorylation and neuropathology in tauopathy. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0453-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vassar R, Kandalepas PC. The beta-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res Ther. 2011;3(3):20. doi: 10.1186/alzrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110(19):E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, Holmans P, Rohrer K, Zhao A, Marlowe L, Kaleem M, McCorquodale DS, 3rd, Cuello C, et al. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84(4):445–458. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Frackowiak J, Mazur-Kolecka B, Schanen NC, Cook EH, Jr, Sigman M, Brown WT, Kuchna I, Wegiel J, Nowicki K, Imaki H, et al. Abnormal intracellular accumulation and extracellular Abeta deposition in idiopathic and Dup15q11.2-q13 autism spectrum disorders. PLoS One. 2012;7(5):e35414. doi: 10.1371/journal.pone.0035414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G, Reyna SM, Dunlap M, Van Der Kant R, Callender JA, Young JE, Roberts EA, Goldstein LS. Defective transcytosis of APP and lipoproteins in human iPSC-derived neurons with familial Alzheimer’s disease mutations. Cell Rep. 2016;17(3):759–773. doi: 10.1016/j.celrep.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Pohlkamp T, Durakoglugil MS, Wong CH, Beck JK, Lane-Donovan C, Plattner F, Herz J. Reversal of ApoE4-induced recycling block as a novel prevention approach for Alzheimer’s disease. Elife. 2018;7:e40048. doi: 10.7554/eLife.40048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PT, Li YJ, Qin XJ, Scherzer CR, Xu H, Schmechel DE, Hulette CM, Ervin J, Gullans SR, Haines J, Pericak-Vance MA, et al. Differences in apolipoprotein E3/3 and E4/4 allele-specific gene expression in hippocampus in Alzheimer disease. Neurobiol Dis. 2006;21(2):256–275. doi: 10.1016/j.nbd.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Xu M, Ouyang Q, Gong J, Pescosolido MF, Pruett BS, Mishra S, Schmidt M, Jones RN, Gamsiz Uzun ED, Lizarraga SB, Morrow EM. Mixed neurodevelopmental and neurodegenera-tive pathology in Nhe6-null mouse model of Christianson syndrome. eNeuro. 2017;4(6):0388. doi: 10.1523/ENEURO.0388-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Painter MM, Bu G, Kanekiyo T. Apolipoprotein E as a therapeutic target in Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2016;30(9):773–789. doi: 10.1007/s40263-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Carney KE, Falgoust L, Pan JW, Sun D, Zhang Z. Emerging roles of Na(+)/H(+) exchangers in epilepsy and developmental brain disorders. Prog Neurobiol. 2016;138-140:19–35. doi: 10.1016/j.pneurobio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Liu CC, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, Painter MM, Sullivan PM, Bu G. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96(1):115–129.:e115. doi: 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hayashi I, Wong J, Tugusheva K, Renger JJ, Zerbinatti C. Intracellular clusterin interacts with brain isoforms of the bridging integrator 1 and with the microtubule-associated protein Tau in Alzheimer’s disease. PLoS One. 2014;9(7):e103187. doi: 10.1371/journal.pone.0103187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]