Abstract
Neuropsychological disturbances in the sense of limb ownership provide unique opportunities to study the neurocognitive basis of body ownership. Previous small sample studies that showed discrete cortical lesions cannot explain why multisensory, affective and cognitive manipulations alter disownership symptoms. We tested the novel hypothesis that disturbances in the sense of limb ownership would be associated not only with discrete cortical lesions, but also with disconnections of white-matter tracts supporting specific functional networks. We drew on an advanced lesion-analysis and Bayesian statistics approach in 49 right-hemisphere patients (23 with and 26 without limb disownership). Our results reveal that disturbances in the sense of ownership are associated with lesions in the supramarginal gyrus and disconnections of a fronto-insular-parietal network, involving the frontal-insular and frontal inferior longitudinal tracts, confirming previous disconnection hypotheses. Together with previous behavioural and neuroanatomical results, these findings lead us to propose that the sense of body ownership involves the convergence of bottom-up, multisensory integration and top-down monitoring of sensory salience based on contextual demands.
Keywords: Asomatognosia, Disownership, Embodiment, Somatoparaphrenia, White matter disconnection
Introduction
A fundamental aspect of self-consciousness is the sense of body ownership (SBO), or the experience of one’s physical body as belonging to the unitary self (Gallagher 2000). The SBO is considered distinct from the attribution of body parts to a conceptual self (Neisser and Jopling 1994) typically studied by projecting one’s body parts as images, or on video displays and testing their self-recognition in extrapersonal space (e.g. Saxe et al. 2006). A different tradition of studies has investigated the SBO as a more intuitive and less doxastic sense of the body (Tsakiris 2010; de Vignemont 2011) using experimental manipulations to identify the mechanisms by which sensations are combined to give rise to the SBO in different temporal, spatial and affective conditions (Tsakiris 2010; Blanke et al. 2015; Kilteni et al. 2015). In this tradition, the SBO is regarded less as a fixed mental representation of the body in the brain and more as a dynamic process of multisensory integration, subject to both learned top-down factors, and bottom-up sensory signals. Consistently with these insights, functional neuroimaging studies have shown that the SBO is supported by a network of multimodal areas, composed of premotor, temporoparietal, and occipital areas, as well as the insular cortex (Blanke et al. 2015; Tsakiris 2017 for reviews). Early stroke lesion work pointed to the insular cortex as a main component in the sensation of ownership of own extremities (e.g., Cereda et al., 2002; Baier and Karnath, 2008). The functional role of the insula in the SBO seems to relate both to its contribution to the salience network of the brain (Menon and Uddin 2010) and to its fundamental role in the central processing of interoceptive signals from the body (Craig 2009). Indeed, several studies have shown that the degree to which certain sensations are integrated to give rise to the SBO relies on their salience and reliability in given spatial, temporal and even affective and social contexts (Apps and Tsakiris 2014; Fotopoulou 2015; Northoff, 2016; Qin et al., 2020;). Moreover, it has been increasingly understood that the SBO not only involves the integration of exteroceptive modalities such as vision and touch, but also interoceptive modalities such as cardiac and respiratory systems (Azzalini et al., 2021; Walla et al., 2008; Monti et al. 2020), as well as affective touch (Jenkinson et al. 2020). Finally, in accordance to more general computational insights about dynamic brain functions, the SBO has been recently understood as the consequence of probabilistic inferences of the most likely cause of one’s multisensory experience (Apps and Tsakiris 2014; Fotopoulou 2015; et al. 2019;Samad et al. 2015).
Despite progress in understanding the SBO through such experimental and computational efforts, it remains difficult to tease apart the phenomenally elusive components of body ownership (Longo et al. 2009; de Vignemont 2011). By contrast, neurological patients with symptoms such as asomatognosia (i.e., the lack of recognition regarding the existence or ownership of one’s limbs, Vallar and Ronchi 2009) show a clear and long-lasting, subjective experience of body disownership, denying ownership of their affected body parts (Jenkinson et al. 2018). Sometimes this denial is accompanied by delusions about the affected arm (i.e. somatoparaphrenia; Gerstmann 1942) and it may take several clinical forms (Jenkinson et al. 2018;see Vallar and Ronchi 2009; Romano and Maravita 2019 for reviews). The term ‘disturbed sensation of limb ownership’ (DSO; Baier and Karnath 2008; Martinaud et al. 2017; Jenkinson et al. 2018) captures any behavioural manifestation that involves abnormal feelings and beliefs regarding the recognition, ownership and sense of belonging of ones’ limbs in the first person and in peripersonal space.
DSO has been studied primarily via small samples and in standard symptom-lesion mapping analyses (Feinberg et al. 2010; Invernizzi et al. 2013; see Vallar and Ronchi 2009; Gandola et al. 2012; Romano and Maravita 2019 for discussions), with the co-occurrent neuropsychological symptoms being considered in the clinical comparison between DSO and control patients, but not directly analysed as covariate during the anatomical investigation (Moro et al., 2016; Invernizzi et al., 2013; Gandola et al., 2012). As such, the neuroanatomical correlates of DSO are not clearly distinguished from those accounted for by concurrent symptoms such as neglect or anosognosia for hemiplegia”. Furthermore, although these studies were not focused on specific regions of interest, and investigated the whole brain, the results identified discreet cortical lesions to areas such as the supramarginal gyrus, or the ventral prefrontal cortex (Moro et al. 2016) or the insula (Baier and Karnath 2008), thereby encouraging modular explanations of SBO. However, more recent studies show that symptoms cannot be explained by discrete lesions as they are subject to modulation by multisensory, affective and cognitive manipulations (Fotopoulou et al. 2011; Zeller et al. 2011; Jenkinson et al. 2013, 2020; Romano et al. 2014; Salvato et al. 2016; Martinaud et al. 2017; Romano and Maravita 2019) and linked to damage to wider, cortical networks and white matter tracts (Feinberg et al. 2010; Gandola et al. 2012; Invernizzi et al. 2013; Moro et al. 2016). These observations have led some authors to suggest the original hypothesis that lesions prevent the processing and the integration of different afferent information arising from the affected body part (bottom-up processes) with higher-order and pre-existing body representations normally computed in higher-order cortices. However, this disconnection hypothesis has not been investigated beyond the aforementioned symptom lesion-mapping methods. In this study, we aimed to examine this hypothesis using advanced methods for identification of grey and white matter damage (Foulon et al., 2018; Thiebaut de Schotten et al., 2014; 2015; Fox, 2018; Pacella et al., 2019; Monai et al., 2020) and controlling for other neuropsychological symptoms.
Based on the insights of the aforementioned studies on the disconnection hypothesis of DSO and our recent understanding of the disconnections underlying another right hemisphere syndrome, namely anosognosia for hemiplegia (Pacella et al. 2019; Kirsch, L. P., Mathys, C., Papadaki, C., Talelli, P., Friston, K., Moro, V., & Fotopoulou 2021; Klingbeil et al. 2020),we predicted that DSO would be associated with damage not only to discrete frontal, parietal and insular cortical regions, but also with disconnections of fronto-parietal (superior longitudinal fasciculus) and fronto-insular white matter tracts supporting functional networks for multisensory integration and body awareness (Pacella et al. 2019).
To study this hypothesis and overcome the aforementioned methodological limitations in the field, we drew on a relatively large cohort of right hemisphere stroke patients (N = 135), from which we selected patients with a reliable diagnosis of DSO (n = 23; DSO+ group) and compared them to a group of control patients, matched on all key demographic and clinical variables, apart from a complete absence of DSO (n = 26; DSO- group; see Table 1). To our knowledge, this is the largest sample of patients with DSO ever studied. First, we used an advanced lesion analysis method (BCBtoolkit; Foulon et al. 2018), which generates a probabilistic map of disconnections from each patient’s brain lesion to identify the disconnections that are associated with given neuropsychological deficits at the group level, and has been successfully used to examine the brain disconnections implicated in several other neuropsychological disorders (Thiebaut de Schotten et al., 2014; 2015; Fox, 2018; Pacella et al., 2019; Monai et al., 2020). Second, in order to understand the contribution of each single lesion (grey matter) or tract disconnection in the syndrome, beyond the potential effects of the clinical variables, we used advanced Bayesian modelling comparisons to contrast each single lesion site (grey matter) or tract disconnection against clinical variables alone (Kaufman L, Rousseeuw PJ. 1990; Hartigan JA, Wong MA. 1979). Finally, we applied cluster analyses on the resultant lesion patterns, to identify whether the grey and white matter lesions associated with DSO symptoms can be classified in different networks of brain areas and to determine the degree of grey matter damage and probability of damage to white matter connections in each area of each network.
Table 1. Results of the clinical and neuropsychological assessment for DSO and Control groups.
| DSO (N=23) | Controls (N=26) | BF10b | |
|---|---|---|---|
| Age (years) | 66.22 ± 15.33a | 67.42 ± 13.26 | 0.29H0* |
| Education (years) | 11.43 ± 3.5 | 10.43 ± 3.46 | 0.12H0 |
| Interval (days) | 32.17 ± 43.35 | 28.62 ± 41.52 | 0.59 |
| Lesion Size (n. voxels) | 166833.57 ± 115216.26 | 117802.18± 122448.57 | 0.01H0 |
| DSO (range 0-6) | 4.49 ± 0.73 | 0.0 ± 0.0 | > 150H1 |
| MRC-UUL | 0.0 ± 0.0 | 0.0 ± 0.0 | Scores are equal H0 |
| Proprioception (max = 9) | 4.96 ± 2.79 | 4.65 ± 3.01 | 0.02H0 |
| Bisiach scale (max = 3) | 1.63 ± 1.14 | 1.67 ± 1.25 | 0.03H0 |
| Comb (right bias, cut-off = -0.11) | -0.39 ± 0.32 | -0.23 ± 0.41 | 0.02H0 |
| Line cancellation crossing (max = 36) | 19.91 ± 11.26 | 21.19 ±13.24 | 0.27H0 |
| Digit span backward | 3 ± 1.54 | 3.46 ± 1.7 | 0.06H0 |
Mean and ± standard deviation of demographic and clinical variables, neurological and neuropsychological assessments are reported.
Bayes Factors (BF10) were calculated, with BF10 > 3 = alternative hypothesis (there are differences between the groups); a BF10 < 1/3 = null hypothesis (no differences between groups); results within 3 and 1/3 are inconclusive. All comparisons show no differences between the two groups (BF10 < 1/3, acceptance of the null hypothesis), with one exception (indicated in italic within the table) in the Interval since lesion onset in days. In this latter case, BF10 does not allow to accept the null hypothesis, but it represents anecdotal evidence towards the null hypothesis36.
H1 = the scores between the two groups are different; H0 = the scores between the two groups are equal.
Materials and methods
Patients
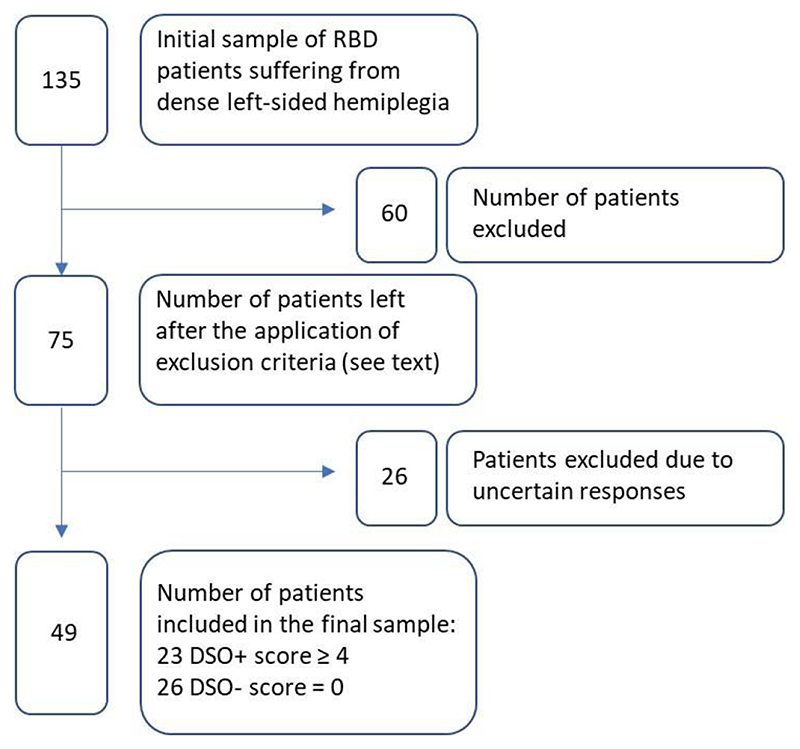
To explore the neural correlates of the sense of body (dis)ownership, a consecutive sample of 135 right hemisphere stroke patients, with dense left-sided hemiplegia, were screened at two stroke units located in Italy (Rehabilitation Department, IRCSS Sacro Cuore Hospital in Negrar, Verona, Italy) and the United Kingdom (Acute Stroke Rehabilitation Unit, St. Thomas Hospital, London). These patients included 132 who had previously taken part in two studies on anosognosia for hemiplegia (Pacella et al. 2019; Moro et al. 2021). Seventy-five patients met the inclusion criteria: (i) unilateral right hemisphere damage, secondary to a first-ever ischemic stroke, as confirmed by clinical neuroimaging (MRI or CT); (ii) severe plegia of contralesional upper limb (Medical Research Council - MRC scale (O'Brien 1976) = 0). Exclusion criteria were: (i) previous history of neurological or psychiatric illness; (ii) medication with severe cognitive or mood side-effects; (iii) severe language, general cognitive impairment, or mood disturbance that precluded completion of the assessment. Structural neuroimaging and clinical data were collected from the included patients, who were subsequently divided into two groups according to the presence/absence of DSO. DSO group classification (presence/DSO+ vs. absence/DSO-) was established according to an interview adapted from previous studies (Feinberg et al. 2010; Moro et al. 2016; Jenkinson et al. 2020) and widely used in the clinical setting. Three questions were asked with reference to the patient’s left hand (moved to the ipsilesional field to reduce neglect): i) “Is this your hand?”; ii) “Do you ever feel as if this was not your hand?”; iii) “Does it belong to someone else?”. Patients’ responses to each question were scored by two expert clinicians, with 0 = patient recognizes the arm as belonging to him/her; 1 = uncertain answers indicating doubts about ownership; 2 = responses indicating disownership and/or attribution of the arm to somebody else (min score = 0; max score = 6). Patients scoring ≥ 4 were diagnosed as DSO, while only patients scoring ‘0’ were considered for the control group. This allowed the exclusion of any uncertain cases, where patients did not show clear limb disownership, but gave unclear responses, for example due to somatosensory disorders or affective components (e.g., “I would like the arm was not mine”; “I don’t feel like it is mine as I don’t experience any physical sensations from it”).
The resulting sample (N = 49) comprised 23 DSO+ patients and 26 DSO- controls. A summary of the selection process of the patients is shown in Figure 1. All patients gave written, informed consent and the research was conducted in accordance with the guidelines of the Declaration of Helsinki (2013) and approved by the Local Ethical Committees of each location.
Figure 1.
A schematic representation of the patient’s selection process by applying the inclusion and exclusion criteria. Uncertain responses were considered when patients did not show clear limb disownership but gave unclear responses that did not allow patients to be clearly classified as DSO+ or DSO-.
Neurological and neuropsychological assessment
There were no differences in age and education between the two groups. As the data were collected from different units, only the scores in the neuropsychological tests that were administrated in both the centers were considered (Table 1).
Proprioception was assessed by asking the patients to state whether or not they felt a passive movement administered to an upper limb joint (i.e. index finger, wrist, and elbow; Vocat et al. 2010). Personal neglect was assessed by means of the ‘Comb’ subtest, of the Comb and Razor test (McIntosh et al. 2000), while patients’ score on the Line cancellation crossing subtest of the Behavioral Inattention Test (BIT) was considered as a measure of extra-personal neglect (Wilson et al. 1987). The backward digit span scores (Baddeley et al. 1975) served as index of working memory. Anosognosia for hemiplegia (AHP) was assessed via the Bisiach’s scale (Bisiach et al. 1986; D’Imperio et al. 2017 for scoring).
Lesions drawing and disconnection maps
Neuroimaging data were acquired via Computerized Tomography and Magnetic Resonance (CT = 89%; MRI = 11%) and lesions were segmented and co-registered using an established manual procedure (Moro et al. 2016; Pacella et al. 2019; Jenkinson et al. 2020). The lesion drawing was independently performed by two experts, who were blind to the patients’ group classification. In cases of disagreement on the extension or the site of a lesion, a third anatomist’s opinion was consulted.
Scans were registered on the ICBM152 template of the Montreal Neurological Institute, furnished with the MRIcron software (ch2, http://www.mccauslandcenter.sc.edu/mricro/mricron/). First, the standard template was rotated on the three plans (size: 181 x 217 x 181 mm, voxel resolution: 1 mm2) to match the orientation of the patient’s MRI or CT scan. Lesions were outlined on the axial slices of the rotated template. The resulting lesion volumes were then rotated back into the canonical orientation, to align the lesion volumes of each patient to the same stereotaxic space. Finally, to remove voxels of lesions outside the white and grey matter brain tissue, lesion volumes were filtered by means of custom masks based on the ICBM152 template.
Disconnectome maps were computed with the ‘disconnectome map’ tool of the BCBToolkit software (Foulon et al. 2018). The first step of the procedure is the tracking of white matter fibres passing through each patient’s lesion, by means of the registration of lesions on the diffusion-weighted imaging dataset of 10 healthy controls (Thiebaut de Schotten et al. 2017). This produces a percentage overlap map that takes into account the inter-individual variability of tractography in a healthy controls’ dataset. Therefore, in the resulting disconnectome maps computed for each lesion, voxels show the probability of disconnection (from 0 to 100%; Thiebaut de Schotten et al. 2015). These disconnection probabilities of each patient were used as a predictor variable in subsequent analyses (see Statistical analyses section).
Statistical analyses
Clinical and neuropsychological variables
A comparison of the clinical and neuropsychological variables between the two groups was executed by means of Bayesian linear models. Unlike traditional frequentist statistics which only allow inferences to be made on the basis of rejecting the null hypothesis, these Bayesian models allow both the null (i.e. no differences between groups) and alternative (i.e. differences between groups) hypotheses to be accepted or rejected (Gelman et al. 2013). Bayes Factors (BF10) were calculated, with BF10 > 3 indicating that the alternative hypothesis should be accepted, a BF10 < 1/3 indicating that the null hypothesis should be accepted, and results within 3 and 1/3 being inconclusive. In this latter case, it is considered as valid to accept the null (simpler) model (for the principle of the Ockham’s razor; Jefferys and Berger 1992). To compare results that are expressed in different scoring ranges, all the scores from neuropsychological tests were transformed to z-scores, with higher scores corresponding to better performances. Missing data (11% of the total clinical and neuropsychological data) were replaced by means of multivariate imputation by chained equations using the mice package (van Buuren and Groothuis-Oudshoorn 2011) in R ver. 4.0.0. This algorithm is able to estimate the missing data starting from the existing data, exploiting correlations among variables. The missing values were present in: the Bisiach test, which correlates with the DSO score (Moro et al. 2021); the personal neglect, the line crossing and the proprioception tests, which correlate with each other (Committeri et al. 2018; Fisher et al. 2020); the digit span backward score, which correlates with age (Hester et al. 2004); and the interval between the lesion onset and neuroimaging recording, that correlates with the gravity of the symptom. In order to guarantee that the missing data imputation did not introduce biases, a series of Kolmogorov-Smirnov tests were applied to all the variables with missing data, comparing the data distribution with and without the imputed data. In all cases the distributions were no different (all D < 0.06 and all p > 0.99).
Sites of lesion and tract disconnections
Two separate linear regression analyses were used to explore differences in lesion sites and tract disconnections between the two groups, both using the same procedure via the tool “randomize”, part of FSL package (http://www.fmrib.ox.ac.uk/fsl/, version 5.0), which performs nonparametric statistics on neuroimaging data. Data on lesion size, lesion onset-neuroimaging interval (de Haan and Karnath 2018), anosognosia, personal and extrapersonal neglect, working memory, and proprioception were used as covariates. Threshold-Free Clusters Enhancement option was applied to boost cluster-like structures of voxels, and results that survived the 5000 permutations testing were controlled for family-wise error rate (P > 0.95).
To identify the grey matter structures emerging as significant from the regression analysis, results were compared with the probability maps of the correspondent anatomical structures (thresholded at 60%) in the Harvard-Oxford Atlas (Desikan et al. 2006). This allowed us to compute the proportion of grey matter structures affected by each patient’s lesion. Similarly, to identify the disconnected white matter tracts on the disconnection results, the tracts probability maps (thresholded at 80%) of an atlas of human brain connections (Rojkova et al. 2016) were used. The masks were also used to extract the probabilities of disconnection for each tract from each patient’s disconnectome map.
Contribution of grey and white matter structures in DSO
With the purpose of understanding if, in addition to the clinical variables, each single lesion site (grey matter) or tract disconnection was able to explain the DSO symptomatology better than the clinical variables alone, Bayesian Bernoulli models (BBMs) were used. For each structure and tract separately, a null model was fitted with DSO symptoms (1 = present, 0 = absent) regressed on the clinical variables scaled on a [-1, 1] range (lesion size, lesion-onset/neuroimaging interval, anosognosia, visuo-spatial neglect – Line Crossing test –, personal neglect – Comb test –, working memory – Digit Span Backward – and proprioception scores). The null model was compared with other models where the DSO symptoms were regressed on the probability of disconnection of the white matter tract or on the proportion of the lesioned grey matter structure ([0, 1] range) plus the clinical variables (alternative hypothesis models). When the Bayes Factor was in favour of the alternative hypothesis model (i.e., the structure considered + the clinical variables explained the DSO symptoms better than the clinical variables alone), that white or grey matter structures were used in the following step.
Here, the possibility that the damaged structures found to occur in DSO are organized in neural networks was investigated. For this, the optimal number of clusters was calculated by employing the Average Silhouette method (Kaufman and Rousseeuw 1990) on the grey matter lesions (PrCG, PsCG, SMG) and disconnection data (AS, SLFIII, SLFII, FIL and FIT5) that had resulted to be associated with DSO (and thus using only data from DSO patients).
Then, the clusters of lesion and disconnection data of DSO patients were determined via the K-means algorithm (Hartigan and Wong 1979). The rationale behind this procedure was the necessity to verify if the DSO patients were grouped by different patterns of lesion (grey and white matter) independently from the DSO severity. Then, in order to understand whether the clusters represented a different degree of severity of the DSO symptom, a Bayesian Gaussian Model was fitted, with the raw DSO severity score as dependent variable (range: {4, 5, 6}) and the division in clusters and the clinical variables as regressors (scaled in the [-1, 1] range). This model was compared to a null model with the same dependent variable and the scaled clinical variables, but not the cluster factor, as regressors. Finally, the proportion (grey matter) or the probability (white matter) of each damaged structure was compared between the two clusters (controlling for the scaled clinical variables) by means of Bayesian Gaussian Models (again, each alternative model – i.e. cluster and control clinical variables - was compared to the null model - control clinical variables alone). The goal of this analysis was to understand which structures were damaged in an equivalent degree in both clusters. A summary of the statistical procedure is shown in SM A.
Cluster and Bayesian analyses were computed in R ver. 4.0.0 (R Core Team 2020). Bayesian models were fitted by means of the brms package (Bürkner 2017), with 4 chains of 100,000 iterations each one and 10,000 burn-in steps, for a total of 360,000 samplings. Comparisons between Bayesian models were carried out using BF10 (Kass and Raftery 1995). BF10 were computed by using the bridgesampling package (Gronau et al. 2020). Bayesian Bernoulli models had Cauchy distributions with mean 0 and scale 10 as priors for the regressors, and the Bayesian Gaussian models used Cauchy distribution, with mean 0 and scale 10 for the regressors, and mean 0 and scale 1 for the intercept, as prior distributions. For the average silhouette method, the Factoextra package (Kassambara and Mundt 2020) was used.
Results
Results from the clinical and neuropsychological assessment, with comparison between the two groups, are shown in Table 1. The two groups differed only in their level of DSO, as expected.
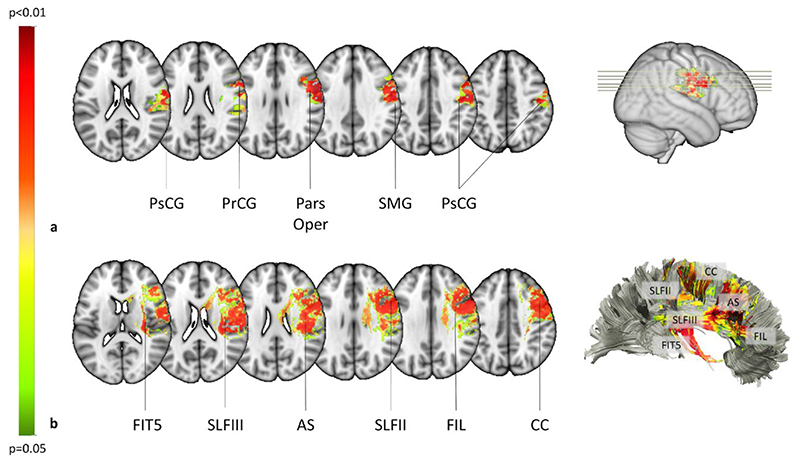
In a first step of analysis, we examined the grey and white matter structures involved in DSO, using two separate linear regressions, and controlling for extraneous demographic, clinical and neuropsychological variables. We found that DSO was associated with right-hemisphere grey matter damage to the Pars Opercularis of the Inferior Frontal gyrus (IFG; BA 6, 45; P = 0.003), the Pre-central gyrus (PrCG; BA 4; P = 0.003), the Post-central gyrus (PsCG; BA 4; P = 0.003), and the Supramarginal gyrus (SMG; BA 40; P = 0.003) (Fig. 1a). Additionally, damage to several white matter tracts were associated with DSO, comprising the Frontal Inferior Longitudinal Fasciculus (FIL; P = 0.008), the Fronto-Insular tract 5 (FIT5; P = 0.008), the Anterior Segment (AS) of the Arcuate Fasciculus (P = 0.009), the second (P = 0.009) and third (P = 0.008) branches of the Superior Longitudinal Fasciculus (SLFII; SLFIII), and the Corpus Callosum (CC; P = 0.009) (Fig. 1 and Fig. 2 a-c).
Figure 1. Mapping of lesions and brain disconnections in DSO when compared with the control group.
a) Statistical mapping of the lesions in the grey matter. PsCG: postcentral gyrus; PrCG: precentral gyrus; Pars Oper: pars opercularis of the inferior frontal gyrus; SMG: supramarginal gyrus. b) Statistical mapping of the brain disconnections in DSO when compared with the control group. FIT5: fronto-insular, tract 5. SLFIII: third branch of the superior longitudinal fasciculus. AS: anterior segment of the arcuate fasciculus; SLFII: second branch of the superior longitudinal fasciculus. FIL: frontal inferior longitudinal tract of the frontal longitudinal system. CC: corpus callosum. Colour bar represents the p-value statistic, only significant voxels are shown (P<.05).
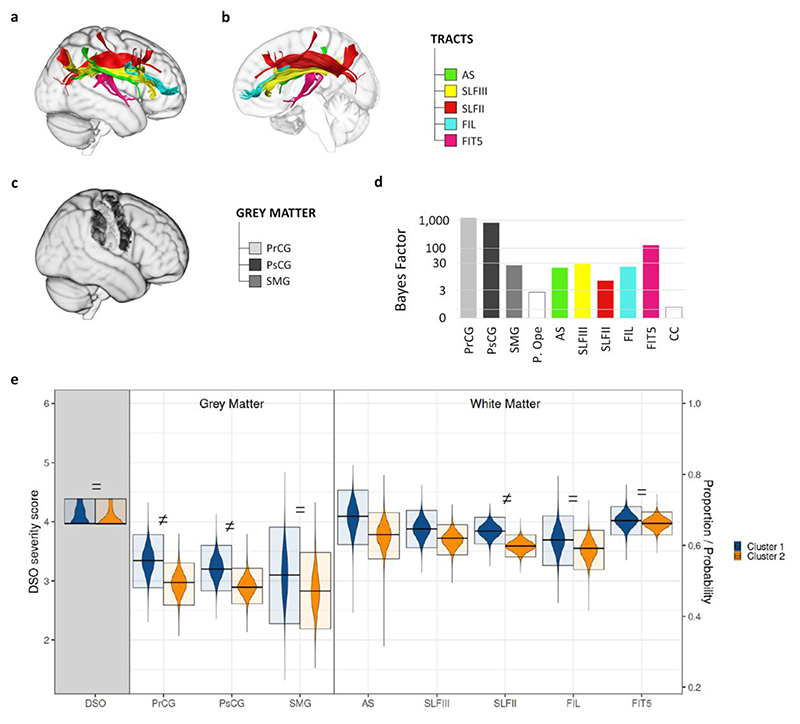
Figure 2. The white matter tract that contribute to DSO symptoms.
a) Lateral and b) medial view of the reconstruction of the white matter tracts contributing to DSO (BF10>3). AS = anterior segment of the arcuate fasciculus. SLFIII = third branch of the superior longitudinal fasciculus. SLFII = second branch of the superior longitudinal fasciculus. FIL = frontal inferior longitudinal tract. FIT5 = fronto-insular tract 5. c) Identification on a brain template of the grey matter structure contributing to DSO (BF10>3). PrCG = precentral gyrus. PsCG = postcentral gyrus. SMG = supramarginal gyrus. d) Bayes Factor for each anatomical result’s model representing the hypothesis that the damage to grey matter structure/tract disconnection contributes to the emergence of DSO, against the null model. e) Posterior Distributions (PD) from the Bayesian Analysis of DSO patients’ grouping along the 2 clusters obtained from the Silhouette analysis. The violin plots represent the PDs obtained from the Bayesian Analysis; the upper and lower boundaries of the box are the boundaries of the 95% Highest Density Interval of the PD; the midline is the Mode of the PD. The first variable on the x-axis (grey-shaded background) represents the DSO mean severity score for each cluster (range 0 - 6, left-side y-axis). On the right part of the plot, the proportions of grey matter structures and the disconnection probability of the tracts involved in each cluster are represented (range 0 - 1, right-side y-axis). = stands for BF10< 1/3; ≠ stands for BF10 > 3.
In a second step of analysis, we aimed to identify which of these structures account for DSO symptoms, over and above explaining concurrently observed clinical and neuropsychological deficits. To do this, Bayesian Bernoulli Models (BBMs) of each cortical area and white matter tract were compared with a null model containing only the clinical variables (see methods for details). All structures except the CC (BF10 = 0.795) and Pars Opercularis (BF10 = 2.692), provided a better explanation for DSO symptoms rather than simply accounting for the concurrent clinical variables (BFs10 > 3, minimum BF10 = 7.044; Fig. 2d). These two lesion sites were not, therefore, considered in the next step of our analyses, in which we aimed to identify the network of structures that form the neuroanatomical basis of core DSO symptoms.
Silhouette analysis (Kaufman and Rousseeuw 1990) was used to identify two clusters as the optimal number of clusters needed to explain DSO symptoms. K-means clustering (Hartigan and Wong 1979) then identified the components of these two clusters, both of which contained the same anatomical areas, as detailed in Fig. 2e. A Bayesian Gaussian Model subsequently showed that the two clusters explained equally well the observed severity of DSO symptoms (BF10 = 0.3; Fig. 2e, left/grey-shaded panel). Thus, as a final step we used Bayesian Gaussian Models to assess the level of agreement between the two clusters in terms of proportion of damage in each grey matter lesion and probability of each of the white matter disconnections. Results of this analysis indicated that FIT5 (BF10 = 0.0519), FIL (BF10 = 0.198) and SMG (BF10 = 0.253) had equal contributions across the clusters (Fig. 2e). By contrast, results for SLFIII and AS were not conclusive (BF10 = 0.459 and 1.099, respectively), and there are differences between the two clusters in the involvement of SLFII (BF10 = 20.140), of PsCG (BF10 = 3.420) and of PrCG (BF10 = 5.622) (Fig.2e).
Discussion
The current study drew on recent ideas that the sense of body ownership (SBO) is a dynamic process relying not only on cortical premotor and parietal areas associated with sensory processing and multisensory integration (Blanke et al. 2015; Kilteni et al. 2015; Tsakiris 2017), but also on wider brain networks responsible for salience monitoring and belief updating (Apps and Tsakiris 2014; Fotopoulou 2015), supported by fronto-parietal and fronto-insular connections(Gandola et al., 2012; Invernizzi et al., 2013; Moro et al., 2016; Romano & Maravita, 2019; Azzalini et al., 2021; Walla et al., 2008; Northoff, 2016; Qin et al., 2020) as recently found in other areas of body awareness (Pacella et al., 2019; Kirsch et al., 2021). To provide causal evidence for this hypothesis we used advanced lesion mapping analyses and examined the brain disconnections associated with disturbed sensation of body ownershp (DSO) in a large sample of patients with damage to the right-hemisphere. To go beyond the limitations of previous neuroanatomical studies of DSO, we applied Bayesian model comparisons to identify the model that best explains the syndrome over and above clinical scores alone and then we applied cluster analyses on the resultant lesion patterns to identify the possibly dissociable network(s) of structures, and the degree of involvement of each area in each network associated with DSO symptoms and not with other neuropsychological symptoms.
Our results revealed a major role of white matter disconnections in DSO that, when combined with direct grey matter lesions to the parietal cortex (mainly Supramarginal gyrus, SMG), revealed that DSO is the result of disruption to a fronto- insular-parietal- network, involving several necessary lesioned brain areas, such as SMG, precentral and postcentral gyri, and disconnections of the anterior segment of the arcuate fasciculus (AS), the second and third branches of the superior longitudinal fasciculus (SLFII, SLFIII), the frontal inferior longitudinal tract (FIL) and the tract 5 of the fronto-insular tract (FIT5). These structures combine in at least two critical ways to cause the syndrome. More specifically, we found strong evidence for the involvement of SMG and disconnections of the fronto-insular (FIT5) and the Frontal Inferior Longitudinal (FIL) tracts to this network. We also found evidence that damage to central gyrus areas and disconnections of the SLFII is of smaller, additional contribution to DSO, while the degree of involvement of the anterior segment of the Arcuate Fasciculus (AS) and the third branch of the superior longitudinal fasciculus (SLFIII) remains inconclusive. Correspondingly, we conclude that the SBO requires the convergence (i.e., integration) of a number of cognitive processes, rather than being purely the functional role of a segregated cortical area. Indeed, this interpretation is consistent with previous studies on the syndrome that have used standard lesion-mapping approaches and revealed white matter disconnections (Gandola et al., 2012; Invernizzi et al., 2013; Moro et al., 2016). The involvement of the SMG is also consistent with previous results (Feinberg, 1990; Martinaud et al., 2017), while in our sample the insula (Baier and Karnath, 2008; Feinberg et al., 2010) and the ventral prefrontal cortex (Gandola et al., 2012; Martinaud et al., 2013) result to be involved in the symptoms because of disconnections rather than direct lesions. Methodological differences may contribute to these divergent results. First, previous studies controlled for clinical and neuropsychological variables based on the comparison of clinical scores between the DSO and control groups, while in this study these variables have been directly controlled in the analysis of neuroanatomical results. Second, previous evidence regarding white matter disconnection was deduced from lesion mapping approaches and not specifically investigated by means of a tractography as done in the current study.
Our findings are also consistent with the dynamic features of DSO, revealed by experimental manipulations that allow patients to temporarily recognise their own arms, or dummy arms as belonging to the self (Fotopoulou et al. 2011; Jenkinson et al. 2013; Martinaud et al. 2017), including experimental studies that manipulated the salience and emotional value of sensory stimulations (Jenkinson et al. 2013, 2020; Romano et al. 2014; D’Imperio, Bulgarelli, et al. 2017). Our hypothesis on the role of multisensory integration and executive functions in DSO is indeed speculative, as unfortunately these functions were not directly assessed in our patients. However, this possibility is supported by the finding that frontal and insular disconnections play a crucial role in the emergence of DSO. The role of the insula in the sense of body ownership is well known (Baier and Karnath 2008; Craig 2009; Moro et al. 2016), interpreted either as contributing to the integration of interoceptive sensations with other signals about the body (Craig 2009), or as regulating the salience of sensory signals (Menon and Uddin 2010; Allen et al. 2016), including during belief updating about sensory states (Wang et al. 2020) and self-awareness (Kirsch et al. 2020). Indeed, the insula is also part of the salient network, a large-scale brain network identified in resting state fMRI studies and connectivity analyses and thought also to relate to the ventral attention network (VAN; Corbetta et al. 2008), involving the temporoparietal junction and the ventral frontal cortex, connected by the ventral branch of the SLF. While damage to this later track was found to be part of the core disconnections of another right hemisphere disruption of the bodily self, namely motor unawareness, or anosognosia for hemiplegia (Pacella et al. 2019), the present study produced inconclusive results regarding the degree of its involvement in DSO. Instead, we found stronger evidence that DSO is predicted by damage to the FIT5, which links the sub-central gyrus to the anterior long gyrus of the insula (Rojkova et al. 2016) and the FIL that connects the PrCG to the ventral part of the middle frontal gyrus and the superior part of the inferior frontal gyrus, reaching anteriorly the frontal pole (Catani et al. 2012).
The SMG, in the inferior parietal lobe, and to a lesser degree the precentral and postcentral gyrus, were the only grey matter areas involved in DSO symptoms when the contribution of the anatomical structures was compared with that of the remaining clinical variables (null model). The SMG is implicated in multisensory integration and particularly the spatial coding of incoming sensations (Bolognini et al. 2014; D’Imperio et al. 2017) and actions (Daprati et al. 2010). In addition, the SMG is involved in implicit mentalising (Wiesmann et al. 2020) and the self-other distinction, and particularly the inhibition of emotional egocentricity (Besharati et al. 2016) as also demonstrated by the experimental induction of out-of-body experiences (e.g. Blanke et al. 2002). However, although necessary, SMG damage does not appear sufficient to explain DSO symptoms in isolation, and the involvement of the precentral and postcentral gyrus appears even weaker.
Moreover, our clustering analyses indicated that the grey matter lesions and white matter disconnections associated with DSO symptoms and their severity can cluster together in two ways, with some areas contributing equally to each cluster and other not. These differences were not associated with differences in symptom severity in the present study, but more detailed characterization of patients’ behaviour in future studies using these advanced neuroanatomical and statistical methods may examine whether these differences are associated with different types of DSO phenomenology.
A limitation of our study, and all structure-function inferences derived from lesion mapping work, is the indirect nature of our functional inferences, which are based on structural brain damage and probabilistic white matter disconnection information. Another limitation regards the manual lesion delineation and registration methods and the sensitivity level of neuroimaging techniques that do not depict the full extent of damage produced by stroke lesions (Hillis et al. 2000). However, these limitations mainly apply to small sample studies, while here the relatively large number of patients investigated, the strict criteria of inclusion in the two groups (that excluded patients that could not be diagnosed with sufficient certainty) and the control for neuropsychological variables reduce these risks. Future studies would benefit from applying functional imaging methods to study DSO patients, although such studies are difficult to perform and, to our knowledge, there is no such group study on the syndrome and thus no corresponding functional connectivity analyses.
We did not control for patients’ age, gender and education in the analyses, although previous studies reported effects of aging (Giorgio et al., 2010; Zhao et al., 2015), gender (Kanaan et al., 2012; Cosgrove et al., 2007) and education (Umarova et al., 2021) on structural anatomy. These covariates were not added because the over-correction of lesional and disconnection analyses is shown to lead to biases and/or type II errors (Sperber et al., 2020). Thus, we limited the number of control covariates to the lesion volume, which has been found to strongly correlate with general cognitive impairment (Zhang et al., 2014; de Haan and Karnath, 2018), and the main clinical variables (Romano & Maravita, 2019) as the disentanglement of their neural correlates from DSO is within the main aim of the study. Finally, a more in-depth clinical assessment of neuropsychological variables, interoception and multisensory integration would be useful in defining the neuropsychological patterns of these patients. However, the neuroanatomical approach and statistical methodologies used in the study require the choice of key but limited, behavioural data as any variables that are entered as co-variates have a cost in terms of statistical power (de Haan & Karnath, 2018). Thus, we prioritized recruiting a larger sample of patients and presenting only the neuropsychological tests administered to the majority of patients. In conclusion, on the basis of an advanced grey and white matter symptom-lesion and disconnection mapping study, we demonstrate a core fronto-insular-parietal network for the sense of body ownership (SBO). Combining these results with previous experimental findings, we suggest that the SBO is not limited to bottom-up processes of multisensory integration, but it involves the convergence between such processes, and top-down control and monitoring of sensory salience in different contexts.
Supplementary Material
Funding
This study was supported by a European Research Council Consolidator Award (METABODY: [ERC- 2018-COG-818070], to A.F.); Fondation pour la Recherche Médicale (FRM DEQ20150331725, to V.P.) and by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Italy (PRIN 2017 - 2017N7WCLP to V.M. and M.S.). Also, by a Commonwealth Scholarship, an Oppenheimer Memorial Trust Fellowship, and a Neuropsychology International Fellowship Award from the British Psychological Society in conjunction with the British Neuropsychological Society to S.B.
Notes
We thank Sara Bertagnoli and Cristina Bulgarelli for their help in data collection. We are grateful to all the patients and relatives for their willingness to participate in the study. The datasets generated during and analysed during the current study are available in the OSF repository, https://osf.io/7yxh5/.
Conflict of interest: None declared.
References
- Allen M, Fardo F, Dietz MJ, Hillebrandt H, Friston KJ, Rees G, Roepstorff A. Anterior insula coordinates hierarchical processing of tactile mismatch responses. Neuroimage. 2016;127:34–43. doi: 10.1016/j.neuroimage.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Tsakiris M. The free-energy self: A predictive coding account of self-recognition. Neurosci Biobehav Rev. 2014;41:85–97. doi: 10.1016/j.neubiorev.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzalini D, Buot A, Palminteri S, Tallon-Baudry C. Responses to Heartbeats in Ventromedial Prefrontal Cortex Contribute to Subjective Preference-Based Decisions. J Neurosci. 2021;41(23):5102–5114. doi: 10.1523/JNEUROSCI.1932-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Thomson N, Buchanan M. Word length and the structure of short-term memory. J Verbal Learning Verbal Behav. 1975;14:575–589. [Google Scholar]
- Baier B, Karnath HO. Tight Link Between Our Sense of Limb Ownership and Self-Awareness of Actions. 2008;39(2):486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Besharati S, Forkel SJ, Kopelman M, Solms M, Jenkinson PM, Fotopoulou A. Mentalizing the body: Spatial and social cognition in anosognosia for hemiplegia. Brain. 2016;139:971–985. doi: 10.1093/brain/awv390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E, Vallar G, Perani D, Papagno C, Berti A. Unawareness of disease following lesions of the right hemisphere: anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia. 1986;24:471–482. doi: 10.1016/0028-3932(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419(6904):269–70. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Blanke O, Slater M, Serino A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron. 2015;88:145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Ronchi R, Casati C, Fortis P, Vallar G. Multisensory remission of somatoparaphrenic delusion: My hand is back! Neurol Clin Pract. 2014;4:216–225. doi: 10.1212/CPJ.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner P-C. brms: An R Package for Bayesian Multilevel Models UsingStan. J Stat Softw. 2017;80:1–28. [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59(12):1950–5. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- Committeri G, Piervincenzi C, Pizzamiglio L. Personal neglect: A comprehensive theoretical and anatomo—clinical review. Neuropsychology. 2018;32:269–279. doi: 10.1037/neu0000409. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crucianelli L, Serpell L, Paloyelis Y, Ricciardi L, Robinson P, Jenkinson P, Fotopoulou A. The effect of intranasal oxytocin on the perception of affective touch and multisensory integration in anorexia nervosa: Protocol for a double-blind placebo-controlled crossover study. BMJ Open. 2019 doi: 10.1136/bmjopen-2018-024913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Imperio D, Bulgarelli C, Bertagnoli S, Avesani R, Moro V. Modulating anosognosia for hemiplegia: The role of dangerous actions in emergent awareness. Cortex. 2017;92:187–203. doi: 10.1016/j.cortex.2017.04.009. [DOI] [PubMed] [Google Scholar]
- D’Imperio D, Tomelleri G, Moretto G, Moro V. Modulation of somatoparaphrenia following left-hemisphere damage. Neurocase. 2017;23:162–170. doi: 10.1080/13554794.2017.1329444. [DOI] [PubMed] [Google Scholar]
- Daprati E, Sirigu A, Nico D. Body and movement: Consciousness in the parietal lobes. Neuropsychologia. 2010;48:756–762. doi: 10.1016/j.neuropsychologia.2009.10.008. [DOI] [PubMed] [Google Scholar]
- de Haan B, Karnath HO. A hitchhiker’s guide to lesion-behaviour mapping. Neuropsychologia. 2018;115:5–16. doi: 10.1016/j.neuropsychologia.2017.10.021. [DOI] [PubMed] [Google Scholar]
- de Vignemont F. Embodiment, ownership and disownership. Conscious Cogn. 2011;20:82–93. doi: 10.1016/j.concog.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Venneri A, Simone AM, Fan Y, Northoff G. The neuroanatomy of asomatognosia and somatoparaphrenia. J Neurol Neurosurg Psychiatry. 2010;81:276–281. doi: 10.1136/jnnp.2009.188946. [DOI] [PubMed] [Google Scholar]
- Fisher G, Quel de Oliveira C, Verhagen A, Gandevia S, Kennedy D. Proprioceptive impairment in unilateral neglect after stroke: A systematic review. SAGE Open Med. 2020;8:205031212095107. doi: 10.1177/2050312120951073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulou A. The virtual bodily self: Mentalisation of the body as revealed in anosognosia for hemiplegia. Conscious Cogn. 2015;33:500–510. doi: 10.1016/j.concog.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Jenkinson PM, Tsakiris M, Haggard P, Rudd A, Kopelman MD. Mirrorview reverses somatoparaphrenia: Dissociation between first-and third-person perspectives on body ownership. Neuropsychologia. 2011;49:3946–3955. doi: 10.1016/j.neuropsychologia.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M, Volle E, Thiebaut de Schotten M. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. 2018;7 doi: 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med. 2018;379:2237–2245. doi: 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gandola M, Invernizzi P, Sedda A, Ferrè ER, Sterzi R, Sberna M, Paulesu E, Bottini G. An anatomical account of somatoparaphrenia. Cortex. 2012;48:1165–1178. doi: 10.1016/j.cortex.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. Third. ed. New York, NY, USA: Chapman and Hall/CRC; 2013. [Google Scholar]
- Gerstmann J. Problem of imperception of disease and of impaired body territories with organic lesions: Relation to body scheme and its disorders. Arch Neurol Psychiatry. 1942;48:890–913. [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronau QF, Singmann H, Wagenmakers EJ. Bridgesampling: An R package for estimating normalizing constants. J Stat Softw. 2020;92 [Google Scholar]
- Hartigan JA, Wong MA. Algorithm AS 136: A K-Means Clustering Algorithm. Appl Stat. 1979;28 [Google Scholar]
- Hester RL, Kinsella GJ, Ong B. Effect of age on forward and backward span tasks. J Int Neuropsychol Soc. 2004;10:475–481. doi: 10.1017/S1355617704104037. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Barker PB, Beauchamp NJ, Gordon B, Wityk RJ. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55:782–788. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- Invernizzi P, Gandola M, Romano D, Zapparoli L, Bottini G, Paulesu E. What is mine? Behavioral and anatomical dissociations between somatoparaphrenia and anosognosia for hemiplegia. Behav Neurol. 2013;26:139–150. doi: 10.3233/BEN-2012-110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys WH, Berger JO. Ockham’s Razor and Bayesian Analysis. Am Sci. 1992;80:64–72. [Google Scholar]
- Jenkinson PM, Haggard P, Ferreira NC, Fotopoulou A. Body ownership and attention in the mirror: Insights from somatoparaphrenia and the rubber hand illusion. Neuropsychologia. 2013;51(8):1453–62. doi: 10.1016/j.neuropsychologia.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Jenkinson PM, Moro V, Fotopoulou A. Definition: Asomatognosia. Cortex. 2018;101:300–301. doi: 10.1016/j.cortex.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Jenkinson PM, Papadaki C, Besharati S, Moro V, Gobbetto V, Crucianelli L, Kirsch LP, Avesani R, Ward NS, Fotopoulou A. Welcoming back my arm: affective touch increases body ownership following right-hemisphere stroke. Brain Commun. 2020;22(1):fcaa034. doi: 10.1093/braincomms/fcaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Allin M, Picchioni M, Barker GJ, Daly E, Shergill SS, McGuire PK. Gender differences in white matter microstructure. PloS one. 2012;7(6):e38272. doi: 10.1371/journal.pone.0038272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, Raftery AE. Bayes Factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- Kassambara A, Mundt F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Package Version 107 R Packag version. 2020;1 [Google Scholar]
- Kaufman L, Rousseeuw PJ. Finding Groups in Data: An Introduction to Cluster Analysis. Eepe.Ethz.Ch; 1990. (Wiley Series in Probability and Statistics). [Google Scholar]
- Kilteni K, Maselli A, Kording KP, Slater M. Over my fake body: Body ownership illusions for studying the multisensory basis of own-body perception. Front Hum Neurosci. 2015;24(9):141. doi: 10.3389/fnhum.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch LP, Mathys C, Papadaki C, Talelli P, Friston K, Moro V, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil J, Wawrzyniak M, Stockert A, Karnath H-O, Saur D. Hippocampal diaschisis contributes to anosognosia for hemiplegia: Evidence from lesion network-symptom-mapping. Neuroimage. 2020;208:116485. doi: 10.1016/j.neuroimage.2019.116485. [DOI] [PubMed] [Google Scholar]
- Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. Self awareness and the body image. Acta Psychol (Amst) 2009;132:166–172. doi: 10.1016/j.actpsy.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Martinaud O, Besharati S, Jenkinson PM, Fotopoulou A. Ownership illusions in patients with body delusions: Different neural profiles of visual capture and disownership. Cortex. 2017;87:174–185. doi: 10.1016/j.cortex.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RD, Brodie EE, Beschin N, Robertson IH. Improving the Clinical Diagnosis of Personal Neglect: A Reformulated Comb and Razor Test. Cortex. 2000;36:289–292. doi: 10.1016/s0010-9452(08)70530-6. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5-6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monai E, Bernocchi F, Bisio M, Bisogno AL, Salvalaggio A, Corbetta M. Multiple Network Disconnection in Anosognosia for Hemiplegia. Front Syst Neurosci. 2020;14:21. doi: 10.3389/fnsys.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Porciello G, Tieri G, Aglioti SM. The “embreathment” illusion highlights the role of breathing in corporeal awareness. J Neurophysiol. 2020;123:420–427. doi: 10.1152/jn.00617.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro V, Besharati S, Scandola M, Bertagnoli S, Gobbetto V, Ponzo S, Bulgarelli C, Fotopoulou A, Jenkinson PM. The Motor Unawareness Assessment (MUNA): A new tool for the assessment of Anosognosia for hemiplegia. J Clin Exp Neuropsychol. 2021;43:91–104. doi: 10.1080/13803395.2021.1876842. [DOI] [PubMed] [Google Scholar]
- Moro V, Pernigo S, Tsakiris M, Avesani R, Edelstyn NMJ, Jenkinson PM, Fotopoulou A. Motor versus body awareness: Voxel-based lesion analysis in anosognosia for hemiplegia and somatoparaphrenia following right hemisphere stroke. Cortex. 2016;83:62–77. doi: 10.1016/j.cortex.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Neisser U, Jopling DA. The Conceptual Self in Context: Culture Experience Self Understanding. Cambridge University Press; 1994. [Google Scholar]
- Northoff G. Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain’s spontaneous activity. Cogn Neurosci. 2016;7(1-4):203–22. doi: 10.1080/17588928.2015.1111868. [DOI] [PubMed] [Google Scholar]
- O’Brien M. Aids to the examination of the peripheral nervous system. London (UK); 1976. [Google Scholar]
- Pacella V, Foulon C, Jenkinson PM, Scandola M, Bertagnoli S, Avesani R, Fotopoulou A, Moro V, Thiebaut de Schotten M. Anosognosia for hemiplegia as a tripartite disconnection syndrome. Elife. 2019;8:1–13. doi: 10.7554/eLife.46075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Wang M, Northoff G. Linking bodily, environmental and mental states in the self-A three-level model based on a meta-analysis. Neurosci Biobehav Rev. 2020;115:77–95. doi: 10.1016/j.neubiorev.2020.05.004. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020.
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell’Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2016;221:1751–1766. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Romano D, Gandola M, Bottini G, Maravita A. Arousal responses to noxious stimuli in somatoparaphrenia and anosognosia: Clues to body awareness. Brain. 2014;137:1213–1223. doi: 10.1093/brain/awu009. [DOI] [PubMed] [Google Scholar]
- Romano D, Maravita A. The dynamic nature of the sense of ownership after brain injury. Clues from asomatognosia and somatoparaphrenia. Neuropsychologia. 2019;132:107119. doi: 10.1016/j.neuropsychologia.2019.107119. [DOI] [PubMed] [Google Scholar]
- Salvato G, Gandola M, Veronelli L, Agostoni EC, Sberna M, Corbo M, Bottini G. The spatial side of somatoparaphrenia: a case study. Neurocase. 2016;22(2):154–60. doi: 10.1080/13554794.2015.1077257. [DOI] [PubMed] [Google Scholar]
- Samad M, Chung AJ, Shams L. Perception of body ownership is driven by Bayesian sensory inference. PLoS One. 2015;10(2):e0117178. doi: 10.1371/journal.pone.0117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex. 2006;16(2):178–82. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Sperber C, Nolingberg C, Karnath HO. Post-stroke cognitive deficits rarely come alone: Handling co-morbidity in lesion-behaviour mapping. Hum Brain Mapp. 2020;41:1387–1399. doi: 10.1002/hbm.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Ratiu P, Leslie A, Howells H, Cabanis E, Iba-Zizen MT, Plaisant O, Simmons A, Dronkers NF, Corkin S, et al. From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting Disconnection Syndromes. Cereb Cortex. 2015;25:4812–4827. doi: 10.1093/cercor/bhv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut De Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, Bartolomeo P, Doricchi F. Damage to white matter pathways in subacute and chronic spatial neglect: A group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex. 2014;24:691–706. doi: 10.1093/cercor/bhs351. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Batrancourt B, Levy R, Dubois B, Cerliani L, Volle E. Rostro-caudal Architecture of the Frontal Lobes in Humans. Cereb Cortex. 2017;27:4033–4047. doi: 10.1093/cercor/bhw215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. The multisensory basis of the self: From body to identity to others. Q J Exp Psychol. 2017;70:597–609. doi: 10.1080/17470218.2016.1181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umarova RM, Schumacher LV, Schmidt CSM, Martin M, Egger K, Urbach H, Hennig J, Klöppel S, Kaller CP. Interaction between cognitive reserve and age moderates effect of lesion load on stroke outcome. Sci Rep. 2021;11:4478. doi: 10.1038/s41598-021-83927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Ronchi R. Somatoparaphrenia: a body delusion. A review of the neuropsychological literature. Exp Brain Res. 2009;192:533–551. doi: 10.1007/s00221-008-1562-y. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- Vocat R, Staub F, Stroppini T, Vuilleumier P. Anosognosia for hemiplegia: a clinical-anatomical prospective study. Brain. 2010;133:3578–3597. doi: 10.1093/brain/awq297. [DOI] [PubMed] [Google Scholar]
- Walla P, Duregger C, Greiner K, Thurner S, Ehrenberger K. Multiple aspects related to self-awareness and the awareness of others: An electroencephalography study. J Neural Transm. 2008;115:983–992. doi: 10.1007/s00702-008-0035-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zou Q, Ao Y, Liu Y, Ouyang Y, Wang X, Biswal B, Cui Q, Chen H. Frequency-dependent circuits anchored in the dorsal and ventral left anterior insula. Sci Rep. 2020;10(1):16394. doi: 10.1038/s41598-020-73192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann CG, Friederici AD, Singer T, Steinbeis N. Two systems for thinking about others’ thoughts in the developing brain. Proc Natl Acad Sci U S A. 2020;117 doi: 10.1073/pnas.1916725117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68:98–102. [PubMed] [Google Scholar]
- Zeller D, Gross C, Bartsch A, Johansen-Berg H, Classen J. Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: A lesion study. J Neurosci. 2011;31:4852–4857. doi: 10.1523/JNEUROSCI.5154-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z. Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp. 2014;35:5861–5876. doi: 10.1002/hbm.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Cao M, Niu H, Zuo X-N, Evans A, He Y, Dong Q, Shu N. Age-related changes in the topological organization of the white matter structural connectome across the human lifespan. Hum Brain Mapp. 2015;36:3777–3792. doi: 10.1002/hbm.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.