Abstract
Background
Previous Mendelian randomization (MR) studies on obesity and risk of breast cancer adopted a small number of instrumental variables and focused mainly on the crude total effect. We aim to investigate the independent causal effect of obesity on breast cancer susceptibility, considering the distribution of fat, covering both early and late life.
Methods
Using an enlarged set of female-specific genetic variants associated with adult general [body mass index (BMI)] and abdominal obesity [waist-to-hip ratio (WHR) with and without adjustment for BMI, WHR and WHRadjBMI] as well as using sex-combined genetic variants of childhood obesity (childhood BMI), we performed a two-sample univariable MR to re-evaluate the total effect of each obesity-related exposure on overall breast cancer (Ncase = 133 384, Ncontrol = 113 789). We further looked into its oestrogen receptor (ER)-defined subtypes (NER+ = 69 501, NER– = 21 468, Ncontrol = 105 974). Multivariable MR was applied to estimate the independent causal effect of each obesity-related exposure on breast cancer taking into account confounders as well as to investigate the independent effect of adult and childhood obesity considering their inter-correlation.
Results
In univariable MR, the protective effects of both adult BMI [odds ratio (OR) = 0.89, 95% CI = 0.83–0.96, P = 2.06 × 10−3] and childhood BMI (OR = 0.78, 95% CI = 0.70–0.87, P = 4.58 × 10−6) were observed for breast cancer overall. Comparable effects were found in ER+ and ER− subtypes. Similarly, genetically predicted adult WHR was also associated with a decreased risk of breast cancer overall (OR = 0.87, 95% CI = 0.80–0.96, P = 3.77 × 10−3), restricting to ER+ subtype (OR = 0.88, 95% CI = 0.80–0.98, P = 1.84 × 10−2). Conditional on childhood BMI, the effect of adult general obesity on breast cancer overall attenuated to null (BMI: OR = 1.00, 95% CI = 0.90–1.10, P = 0.96), whereas the effect of adult abdominal obesity attenuated to some extent (WHR: OR = 0.90, 95% CI = 0.82–0.98, P = 1.49 × 10–2; WHRadjBMI: OR = 0.92, 95% CI = 0.86–0.99, P = 1.98 × 10–2). On the contrary, an independent protective effect of childhood BMI was observed in breast cancer overall, irrespective of adult measures (adjusted for adult BMI: OR = 0.84, 95% CI = 0.77–0.93, P = 3.93 × 10–4; adjusted for adult WHR: OR = 0.84, 95% CI = 0.76–0.91, P = 6.57 × 10–5; adjusted for adult WHRadjBMI: OR = 0.80, 95% CI = 0.74–0.87, P = 1.24 × 10–7).
Conclusion
Although successfully replicating the inverse causal relationship between adult obesity-related exposures and risk of breast cancer, our study demonstrated such effects to be largely (adult BMI) or partly (adult WHR or WHRadjBMI) attributed to childhood obesity. Our findings highlighted an independent role of childhood obesity in affecting the risk of breast cancer as well as the importance of taking into account the complex interplay underlying correlated exposures.
Keywords: Childhood adiposity, general obesity, abdominal obesity, Mendelian randomization, breast cancer
Key Messages.
In this study, we conducted a comprehensive multivariable Mendelian randomization to revisit the causal role of multiple obesity-related traits on breast cancer susceptibility, considering the distribution of fat, covering both early and late life.
Using data from the hitherto largest genome-wide association studies, we successfully replicated the total effect of both adult and childhood obesity on lowering the risk of breast cancer.
Conditional on childhood body mass index (BMI), we further identified that the total effect of adult general obesity (adult BMI) on breast cancer was largely attributed to childhood obesity, whereas that of adult abdominal obesity [waist-to-hip ratio (WHR) with and without adjustment for BMI, WHR and WHRadjBMI] was at least partly attributed to childhood obesity. Biological mechanisms underlying such findings warrant attention in future research.
These findings indicate a predominantly independent effect of childhood BMI in affecting breast cancer onset and highlight the importance of taking into account the complex interplay underlying correlated exposures.
Introduction
Obesity, a widely recognized public health challenge, plays a complex role in the development of female breast cancer,1 with associations differing depending on their distribution (e.g. general vs abdominal) and timing (e.g. childhood vs adulthood). Traditional epidemiological studies have consistently observed an increased risk of post-menopausal breast cancer and a decreased risk of pre-menopausal breast cancer to be associated with adult body mass index [body mass index (BMI), a measure of adult general obesity], whereas the results for waist-to-hip ratio (WHR, a measure of adult abdominal obesity) and childhood BMI remain conflicting.2–4 These intricate associations motivate the need for understanding the causality and interaction of multiple obesity-related traits on breast cancer risk.
Mendelian randomization (MR) is a powerful tool that uses genetic variants [single-nucleotide polymorphisms (SNPs)] as instrumental variables (IVs) to make causal inference5 and has been widely applied to determine the causal association between obesity and breast cancer risk.6–10 Using 15 748 breast cancer cases and a limited number of IVs (77 SNPs for adult BMI; 15 SNPs for childhood BMI), Gao et al. conducted a univariable MR, which found a suggestive protective effect of general obesity (adult BMI: OR = 0.66, 95% CI = 0.57–0.77; childhood BMI: OR = 0.71, 95% CI = 0.60–0.80) on breast cancer overall.7 In an enlarged MR study conducted by Ooi et al. using 122 977 breast cancer cases and the same number of IVs (77 SNPs for adult BMI as in Gao et al.), a consistent protective effect of BMI on breast cancer overall was identified (OR = 0.81, 95% CI = 0.74–0.89). Such an effect remained in subtypes defined by oestrogen receptor (ER) status (ER+: OR = 0.81, 95% CI = 0.74–0.89; ER−: OR = 0.78, 95% CI = 0.67–0.91).10 Expanding the number of IVs into 166 or ∼700 BMI-associated SNPs, another two studies drew similar conclusions.6,8 Opposite to general obesity, MR studies on abdominal obesity remain sparse. A null association was detected for WHR,7 whereas a decreased risk of breast cancer (OR = 0.85, 95% CI = 0. 79–0.91) was reported by Shu et al. for BMI-adjusted WHR (WHRadjBMI, representing abdominal body fat independent of general body fat) using 54 WHRadjBMI-associated SNPs.6 Furthermore, to understand the independent effect of correlated exposures on outcome, multivariable MR11 (an extension to univariable MR) has been developed. In the hitherto only available multivariable MR study,9 which modelled simultaneously adult and childhood body size using composite IVs (191 SNPs for adult body size plus 124 SNPs for childhood body size), a protective effect of childhood body size with breast cancer was observed conditioning on adult body size, whereas the protective effect of adult body size turned to null conditioning on childhood body size, highlighting the importance of taking into account multiple obesity-related traits over the life course simultaneously.
Despite existing MR studies having advanced our knowledge on an intrinsic link underlying obesity and breast cancer, a few gaps need to be filled. First, most studies did not use female-specific IVs to match with a female disease—heterogeneity derived from sex-combined IVs would lead to a biased MR estimate.12 Second, existing studies using a handful of IVs were of poor statistical power—the most updated genome-wide association study (GWAS) of BMI and WHR has identified a 4-fold enlarged number of female-specific IVs, which would greatly improve the statistical power and the accuracy of estimates. Third, the only available multivariable MR study to date used retrospective questionnaire-based categorized data for perceived childhood obesity, yielding potentially to measurement error. Last but not least, despite breast cancer being a complex disease with distinct subtypes, most studies did not phenotype the disease by ER status.
As the sample size of GWAS(s) continues to grow and the data continue to accumulate,13,14 it is timely to conduct a comprehensive reassessment of the causal role of obesity in breast cancer through an MR design. Therefore, in this study, we used a largely increased set of sex-specific IVs derived from the hitherto largest GWAS(s) conducted for exposure and outcome13–16 to (i) re-evaluate the total effect of obesity-related traits (general and abdominal obesity, adult and childhood obesity) on breast cancer overall and its ER-defined subtypes; (ii) estimate the independent effect of each obesity-related trait, accounting for the confounding effects from four major risk factors, including smoking, drinking, age at menarche and age at natural menopause; (iii) investigate the independent effect of adult and childhood obesity taking into account their inter-correlation.
Methods
Data sources
Exposure GWAS(s)
The hitherto largest GWAS(s)14 of general obesity (BMI) and abdominal obesity (WHR and WHRadjBMI) in adults were conducted via a collaboration of UK Biobank and the Genetic Investigation of Anthropometric Traits consortium in 2019, including ∼700 000 individuals of European ancestry. Anthropometric parameters, including height, weight, waist and hip circumferences, were measured according to standard protocols. BMI was calculated dividing weight by height squared and WHR was calculated by dividing waist circumference by hip circumference. WHRadjBMI was generated from the regression of WHR on BMI by including BMI as an additional independent variable. Due to the large sample size, sex-specific analysis was performed based on 434 794 women for BMI, 381 152 women for WHR and 379 501 women for WHRadjBMI.
As for childhood BMI, the latest and the largest GWAS was conducted by the Early Growth Genetics consortium in 202013 combining data of 41 studies involving 55 354 children aged 6–10 years and of European ancestry. Unfortunately, sex-specific results of childhood BMI were not available due to data restrictions.
Outcome GWAS(s)
Summary-level data were available for three phenotypes—the overall breast cancer, ER+ and ER– subtypes. For breast cancer overall, we retrieved data from the most updated GWAS conducted in 2020 involving 133 384 cases and 113 789 controls of European ancestry combining results from 82 studies participating in the Breast Cancer Association Consortium and 11 other breast cancer genetic studies.15 This GWAS expanded upon a previous GWAS from the Breast Cancer Association Consortium16 (2017) with an additional 10 407 cases and 7815 controls (10% increase) and identified 32 novel susceptibility loci upon the previously detected 153 loci.
For breast cancer subtypes, we used data from a previous GWAS from the Breast Cancer Association Consortium16 (2017) including 69 501 ER+ cases, 21 468 ER− cases and 105 974 controls, which is the hitherto largest GWAS performed for ER subtypes.
Other GWAS(s)
We included four risk factors (age at menarche, age at natural menopause, smoking and drinking) as potential confounders to be controlled for in our MR study. For age at menarche, we used the GWAS from the Reproductive Genetics Consortium published in 2017 comprising 329 345 European women from 40 participating studies (N = 179 117), 23andMe (N = 76 831) and UK Biobank (N = 73 397).17 For age at natural menopause, we used the most updated GWAS from the Reproductive Genetics Consortium in 2021 involving data of 201 323 European women.18 For smoking and drinking, we used data published in 2019 from the GWAS & Sequencing Consortium of Alcohol and Nicotine use, with 1 232 091 participants for smoking initiation and 941 280 participants for drinks per week,19 all of European ancestry.
Instrument selection
We extracted genome-wide significant and uncorrelated (i.e. not in linkage disequilibrium) genetic variants from the original GWASs as our IVs for each exposure. Specifically, this yielded 281 independent top-associated SNPs for BMI, 203 independent top-associated SNPs for WHR, 266 independent top-associated SNPs for WHRadjBMI (all restricted to females, P < 5 × 10−9, < 0.05) and 25 independent top-associated SNPs for childhood BMI (both sexes combined, P < 5 × 10−8, < 0.20). In addition, we also extracted independent top-associated SNPs for the confounders, resulting in 375 SNPs for age at menarche, 290 SNPs for age at natural menopause (P < 5 × 10−8, < 0.05), as well as 378 and 99 SNPs for smoking and drinking (P < 5 × 10−9, < 0.10). We then matched and harmonized these SNPs with the outcome GWAS. For details, please also see Supplementary Table S1 (available as Supplementary data at IJE online).
Bias from weak instruments can result in seriously misleading estimates of causal effects. We therefore calculated F-statistics to measure the strength of instruments, using the proportion of variance in the phenotype explained by genetic variants (), sample size (N) and number of instruments (K) via the formula F = . An F-statistic of >10 indicates a strong instrument and sufficient strength to ensure the validity of instrumental variable methods. We extracted , N and K from the original GWASs. When not reported by the original GWAS, was calculated using (estimated genetic association of each SNP with the trait) and MAF (minor allele frequency) via the formula R2 = .
Statistical analysis
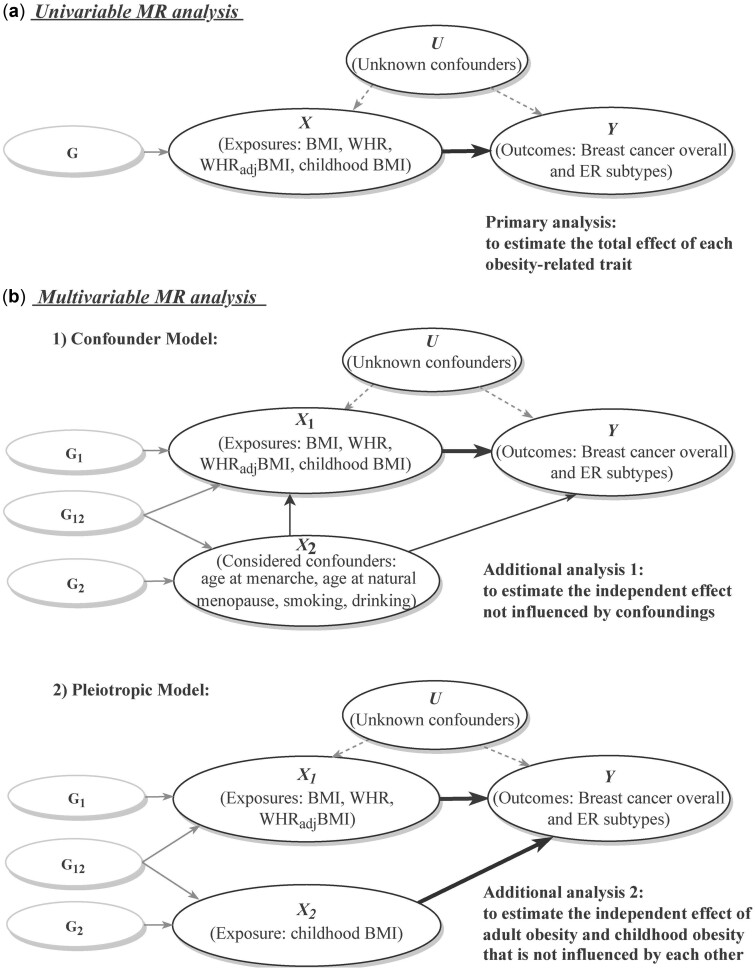
A comprehensive two-sample MR analysis was performed to evaluate a putative causal relationship between exposures (BMI, WHR, WHRadjBMI, childhood BMI) and outcomes (breast cancer overall, ER+ and ER− subtypes), with an analytical schematic diagram presented in Figure 1.
Figure 1.
Analytical schematic diagram of the Mendelian randomization (MR) analysis implemented in this study
(a) Univariable MR analysis; (b) multivariable MR analysis, including two models: (i) confounder model; (ii) pleiotropic model.
G represents genetic variants (single-nucleotide polymorphisms, SNPs) that reliably predict the exposure variable (X) and are used as instrumental variables to represent exposure. G1 and G2 represent SNPs that specifically affect X1 and X2, respectively, whereas G12 represents SNPs that affect both X1 and X2 simultaneously. Thick lines illustrate the causal effect confirmed by the current analysis.
BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; ER, oestrogen receptor.
Univariable MR analysis
To investigate the total effect of each obesity-related trait on breast cancer, univariable MR was conducted as our primary analysis. We first employed an inverse-variance weighted (IVW) approach to estimate the causal effect by regressing the outcome effect coefficient on the exposure effect coefficient with no intercept term.20 Considering the potential bias derived from horizontal pleiotropy of instruments, we complemented IVW with MR–Egger regression21 and the weighted-median approach.22 MR–Egger regression is largely similar to IVW except its regression model contains an intercept to reflect directional pleiotropy. The weighted-median approach is more robust to invalid IVs compared with IVW and MR–Egger regression. Moreover, we also implemented MR-PRESSO (Mendelian Randomization Pleiotropy Residual Sum and Outlier) to evaluate the presence of horizontal pleiotropy and to re-evaluate the causal effect after removing the detected outlying SNPs.23 A putative total causal effect was considered if the exact P-value was <0.05 in any of these four methods and the estimates maintained directional consistency across methods.
Several sensitivity analyses were conducted to assess the robustness of results, including (i) analysis using IVs excluding palindromic SNPs with strand ambiguity; (ii) analysis using IVs excluding pleiotropic SNPs that were associated with the potential confounding traits (age at menarche, age at natural menopause, smoking and drinking) according to GWAS Catalog; (iii) leave-one-out analysis in which each SNP was removed sequentially to identify outliers that might bias the MR estimates.24
In addition, a bidirectional MR analysis was also performed to evaluate whether genetic predisposition to breast cancer would influence obesity. We collected all previously reported IVs reaching genome-wide significance (P < 5 × 10−8) in the breast cancer GWAS.16
Multivariable MR analysis
To further evaluate whether the causal effects of obesity on breast cancer are affected by major confounders and whether the casual effects of childhood and adult obesity on breast cancer are independent of each other, we conducted two additional analyses in the framework of multivariable MR.11,25
Four risk factors (age at menarche, age at natural menopause, smoking and drinking), believed as important confounders of the obesity–breast cancer association, were incorporated together with the exposures, one at a time as well as simultaneously to estimate the independent effect of each exposure after accounting for the confounding effects. Considering that there might be overlapping or correlated SNPs in composite IVs (the sum of IVs from different traits or exposures), we thus removed SNPs in linkage disequilibrium (> 0.001) to obtain a list of independent SNPs, by applying the ‘clump_data’ function of the ‘TwoSampleMR’ package (clump_r2 = 0.001, clump_kb = 500). Stratified analysis on ER subtypes was performed following the same procedure.
Considering the inter-correlation between adult and childhood obesity, childhood BMI was incorporated with each adult obesity trait (BMI, WHR, WHRadjBMI) to examine their independent effect on breast cancer. Three sets of composite IVs after a linkage disequilibrium clumping with > 0.001 were used.26 These included composite IVs involving 270 SNPs for BMI and childhood BMI, 208 SNPs for WHR and childhood BMI and 266 SNPs for WHRadjBMI and childhood BMI. Stratified analysis on ER subtypes was performed following the same procedure.
In our MR analysis, P-values were transformed to q-values to account for the false discovery rate in multiple tests. We conducted univariable MR using the package ‘TwoSampleMR’ (version 0.5.6) and multivariable MR using the package ‘MendelianRandomization’ (version 0.5.1) in software R (version 4.1.0).
Genetic correlation analysis
To understand the shared genetic basis between exposures and outcomes, a genome-wide genetic correlation analysis was further conducted. Full-set GWAS summary data were used to estimate genome-wide genetic correlations (), which quantifies the intrinsic average sharing of the genetic effect between pairs of traits that is independent of environmental factors.27 An algorithm implemented in software linkage disequilibrium score regression (LDSC) was adopted to perform regression on the product of z-scores across any two traits leveraging SNPs across the whole genome.28
Results
The basic characteristics of each GWAS data set and IVs are shown in Table 1. Current IVs explained ∼4% of the phenotypic variance of each exposure (4.0% for adult BMI with 281 index SNPs; 4% for WHR with 203 index SNPs; 3.6% for WHRadjBMI with 266 index SNPs; 3.6% for childhood BMI with 25 index SNPs). F-statistics for these IVs ranged from 53 to 78, suggesting strong instruments.
Table 1.
Description of the genome-wide association study data sets and instrumental variables used in our study
| Phenotype | IV | Sample size | Ethnicity | Consortium | R 2 | F-statistic | Author, year |
|---|---|---|---|---|---|---|---|
| Exposures | |||||||
| BMI | 281 | 434 794 females | European | Genetic Investigation of ANthropometric Traits (GIANT) and UK Biobank | 0.040 | 63.799 | Pulit, 2019 |
| WHR | 203 | 381 152 females | European | Genetic Investigation of ANthropometric Traits (GIANT) and UK Biobank | 0.040 | 78.191 | Pulit, 2019 |
| WHRadjBMI | 266 | 379 501 females | European | Genetic Investigation of ANthropometric Traits (GIANT) and UK Biobank | 0.036 | 53.242 | Pulit, 2019 |
| Childhood BMI | 25 | 55 354 | European | Early Growth Genetics (EGG) | 0.036 | 58.975 | Vogelezang, 2020 |
| Outcomes | |||||||
| BC overall | 170 | 133 384 cases/113 789 controls | European | Breast Cancer Association Consortium (BCAC) | 0.302 | 608.642 | Zhang, 2020 |
| ER+ | NA | 69 501 cases/105 974 controls | European | Breast Cancer Association Consortium (BCAC) | NA | NA | Michailidou, 2017 |
| ER– | NA | 21 468 cases/105 974 controls | European | Breast Cancer Association Consortium (BCAC) | NA | NA | Michailidou, 2017 |
| Confounders | |||||||
| AAM | 375 | 329 345 | European | Reproductive Genetics (ReproGen) | 0.074 | 67.656 | Day, 2017 |
| ANM | 290 | 201 323 | European | Reproductive Genetics (ReproGen) | 0.130 | 103.187 | Ruth, 2021 |
| Smoking | 378 | 1 232 091 | European | GWAS & Sequencing Consortium of Alcohol and Nicotine (GSCAN) | 0.023 | 77.222 | Liu, 2019 |
| Drinking | 99 | 941 280 | European | GWAS & Sequencing Consortium of Alcohol and Nicotine (GSCAN) | 0.002 | 17.811 | Liu, 2019 |
GWAS, genome-wide association study; IV, instrumental variable; BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; BC, breast cancer; ER, oestrogen receptor; AAM, age at menarche; ANM, age at natural menopause.
The intrinsic average genome-wide sharing between pairs of exposure and outcome was evaluated. The genetic correlation analysis showed a negative genetic correlation between breast cancer and childhood BMI (overall: = −0.06, P = 4.98 × 10−2; ER+: = −0.08, P = 1.82 × 10−2) but not for BMI, WHR and WHRadjBMI (Supplementary Figure S1 and Supplementary Table S2, available as Supplementary data at IJE online).
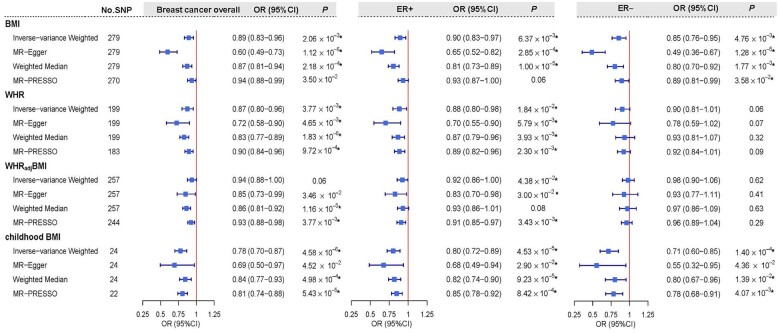
Motivated by these findings, we continued to investigate the putative causal relationship between exposures and outcomes. As shown in Figure 2, using univariable MR, genetically predicted female-specific BMI presented an inverse association with the risk of breast cancer overall (OR = 0.89, 95% CI = 0.83–0.96, P = 2.06 × 10−3). Such a relationship remained consistent in both ER+ (OR = 0.90, 95% CI = 0.83–0.97, P = 6.37 × 10−3) and ER− subtypes (OR = 0.85, 95% CI = 0.76–0.95, P = 4.76 × 10−3) and all survived the false discovery rate correction. Similar findings were observed for genetically predicted female-specific WHR on a decreased risk of breast cancer overall (OR = 0.87, 95% CI = 0.80–0.96, P = 3.77 × 10−3), as well as for ER+ subtype (OR = 0.88, 95% CI = 0.80–0.98, P = 1.84 × 10−2), but not for ER− subtype. When the effect of BMI was removed from WHR (WHRadjBMI), the observed inverse association of WHR reduced to some extent in breast cancer overall (OR = 0.94, 95% CI = 0.88–1.00, P = 0.06) and in ER+ subtype (OR = 0.92, 95% CI = 0.86–1.00, P = 4.38 × 10−2). As for childhood BMI, strong evidence of a protective effect was observed consistently across all breast cancer phenotypes (overall: OR = 0.78, 95% CI = 0.70–0.87, P = 4.58 × 10−6; ER+: OR = 0.80, 95% CI = 0.72–0.89, P = 4.53 × 10−5; ER−: OR = 0.71, 95% CI = 0.60–0.85, P = 1.40 × 10−4). All these aforementioned results derived from IVW were further supported by the weighed-median approach and the MR–Egger regression, with estimates consistent in direction and without apparent sign of horizontal pleiotropy (Supplementary Table S3, available as Supplementary data at IJE online). Results from MR-PRESSO using the outlier-corrected method were also highly consistent with those from IVW.
Figure 2.
Estimated total effects of obesity-related traits on the risk of breast cancer using univariable Mendelian randomization
Boxes denote the point estimates of causal effects, and error bars denote 95% confidence intervals. Asterisks (*) denote the tests survived false discovery rate (FDR) correction (PFDR < 0.05). Inverse-variance weighted approach was used as primary analysis; MR–Egger, weighted-median and MR-PRESSO were used as sensitivity analyses.
BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; ER, oestrogen receptor; No. SNP, number of instrumental variables; OR, odds ratio.
Sensitivity analyses excluding pleiotropic SNPs or palindromic SNPs, as well as the leave-one-out analysis, showed similar findings, demonstrating the robustness of the results (Supplementary Figures S2 and S3, available as Supplementary data at IJE online). Additionally, bidirectional MR did not find a genetic predisposition to breast cancer overall to affect any obesity trait (Supplementary Table S4, available as Supplementary data at IJE online).
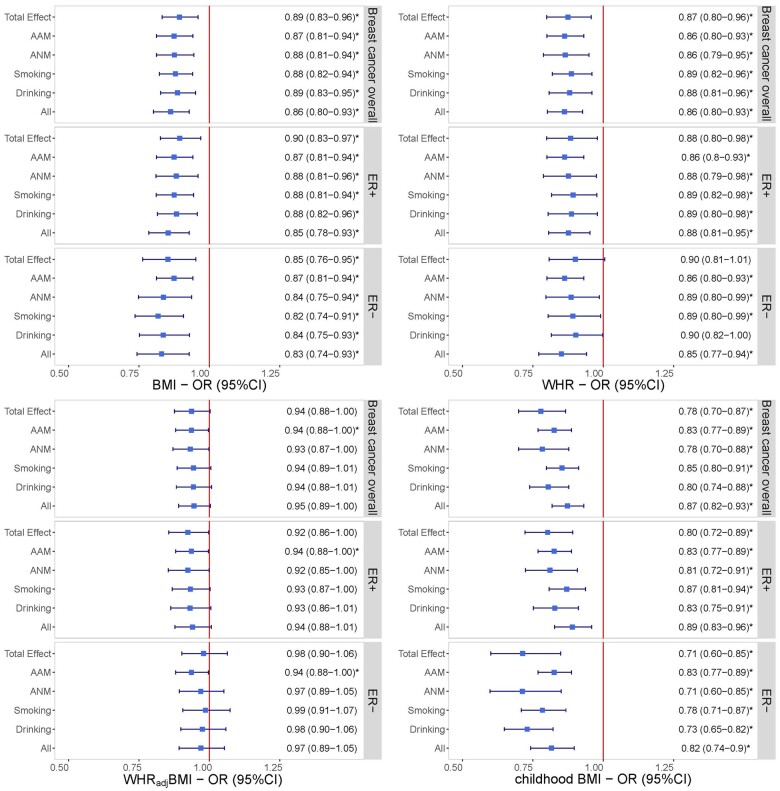
Considering important mediatory phenotypes or risk factors that may affect the relationship between obesity and breast cancer, we performed multivariable MR by incorporating each exposure with confounders (age at menarche, age at natural menopause, smoking and drinking), separately and together. The effect of each obesity trait on breast cancer remained consistent in both direction and magnitude after adjusting for confounders and all survived multiple testing corrections (Figure 3).
Figure 3.
Independent effects of genetically predicted obesity-related traits on the risk of breast cancer after adjusting for each confounder separately and together using multivariable Mendelian randomization
The y-axis details the genetically predicted confounder(s) for which adjustment was made and the x-axis details the odds ratios and 95% confidence intervals per 1-standard deviation increase in exposure. Asterisks (*) denote the tests survived false discovery rate (FDR) correction (PFDR < 0.05). Total effect refers to the estimate derived from univariable Mendelian randomization.
BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; ER, oestrogen receptor; AAM, age at menarche; ANM, age at natural menopause; OR, odds ratio.
Despite our prior results providing evidence that both childhood and adult obesity contribute to a decreased risk of breast cancer, their independent effects remain unclear. We conducted a series of multivariable MR to examine whether the casual effects of childhood and adult obesity on breast cancer are independent of each other (Table 2). Notably, the effect of adult BMI on breast cancer overall attenuated to null in multivariable MR after adjusting for childhood BMI (OR = 1.00, 95% CI = 0.90–1.10, P = 0.96), suggesting the effect of adult general obesity on breast cancer is influenced by childhood obesity. On the contrary, the decreased risk of adult abdominal obesity (WHR and WHRadjBMI) with breast cancer overall attenuated slightly when conditional on childhood BMI (WHR: OR = 0.90, 95% CI = 0.82–0.98, P = 1.49 × 10–2; WHRadjBMI: OR = 0.92, 95% CI = 0.86–0.99, P = 1.98 × 10–2). Similar results were also identified in ER+ (WHR: OR = 0.90, 95% CI = 0.81–1.00, P = 4.29 × 10–2; WHRadjBMI: OR = 0.91, 95% CI = 0.84–0.98, P = 1.92 × 10–2), but not in ER− subtype. These results indicated the effect of adult abdominal obesity on breast cancer to be partially independent of childhood obesity, suggesting multiple distinct pathways influencing breast cancer susceptibility. On the contrary, a strong independent effect of childhood BMI was consistently observed in breast cancer overall when conditional on each adult obesity trait (adjusted for adult BMI: OR = 0.84, 95% CI = 0.77–0.93, P = 3.93 × 10–4; adjusted for adult WHR: OR = 0.84, 95% CI = 0.76–0.91, P = 6.57 × 10–5; adjusted for adult WHRadjBMI: OR = 0.80, 95% CI = 0.74–0.87, P = 1.24 × 10–7) and the effect remained across both ER+ subtype (adjusted for adult BMI: OR = 0.86, 95% CI = 0.77–0.95, P = 4.05 × 10–3; adjusted for adult WHR: OR = 0.86, 95% CI = 0.78–0.95, P = 2.87 × 10–3; adjusted for adult WHRadjBMI: OR = 0.82, 95% CI = 0.75–0.90, P = 3.40 × 10–5) and ER− subtype (adjusted for adult BMI: OR = 0.83, 95% CI = 0.72–0.96, P = 1.43 × 10–2; adjusted for adult WHR: OR = 0.76, 95% CI = 0.68–0.85, P = 6.10 × 10–7; adjusted for adult WHRadjBMI: OR = 0.74, 95% CI = 0.67–0.82, P = 6.09 × 10–9).
Table 2.
Independent effect of adult obesity and childhood obesity on the risk of breast cancer using multivariable Mendelian randomization analysis
| BC overall |
ER+ |
ER– |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Model 1 | ||||||
| BMI | 1.00 (0.90–1.10) | 0.96 | 0.99 (0.88–1.11) | 0.88 | 0.96 (0.82–1.12) | 0.58 |
| Childhood BMI | 0.84 (0.77–0.93) | 3.93 × 10–4* | 0.86 (0.77–0.95) | 4.05 × 10–3* | 0.83 (0.72–0.96) | 1.43 × 10–2* |
| Model 2 | ||||||
| WHR | 0.90 (0.82–0.98) | 1.49 × 10–2* | 0.90 (0.81–1.00) | 4.29 × 10–2 | 0.93 (0.84–1.04) | 0.20 |
| Childhood BMI | 0.84 (0.76–0.91) | 6.57 × 10–5* | 0.86 (0.78–0.95) | 2.87 × 10–3* | 0.76 (0.68–0.85) | 6.10 × 10–7* |
| Model 3 | ||||||
| WHRadjBMI | 0.92 (0.86–0.99) | 1.98 × 10–2* | 0.91 (0.84–0.98) | 1.92 × 10–2* | 0.96 (0.88–1.05) | 0.35 |
| Childhood BMI | 0.80 (0.74–0.87) | 1.24 × 10–7* | 0.82 (0.75–0.90) | 3.40 × 10–5* | 0.74 (0.67–0.82) | 6.09 × 10–9* |
Model 1: independent effect of adult BMI and childhood BMI on BC; Model 2: independent effect of adult WHR and childhood BMI on BC; Model 3: independent effect of adult WHRadjBMI and childhood BMI on BC. Asterisks (*) denote the tests survived false discovery rate (FDR) correction (PFDR < 0.05).
BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; BC, breast cancer; ER, oestrogen receptor; OR, odds ratio.
Discussion
Our MR study revisited the causal role of multiple obesity-related traits in the development of breast cancer overall as well as its ER-defined subtypes, utilizing data from the hitherto largest GWAS(s) conducted for each trait. By incorporating a set of 4-fold enlarged female-specific IVs, both the precision and the accuracy of our MR estimates were substantially improved. We successfully replicated the protective effects of genetically predicted adult BMI, adult WHR, adult WHRadjBMI and childhood BMI on breast cancer. Integrating obesity-related traits together, we further found the effect of adult BMI on breast cancer was largely attributed to childhood BMI, whereas the effect of adult WHR (or WHRadjBMI) was partly dependent on childhood BMI. On the contrary, childhood BMI consistently showed an independent protective effect on breast cancer irrespective of adult measures. Additionally, subtype-specific analyses suggested that the protective effects of adult and childhood BMI held true for both ER+ and ER− subtypes, whereas the effects of WHR and WHRadjBMI were only restricted to ER+ subtypes.
Despite several studies that have applied an MR approach to discover associations between genetically predicted general obesity and breast cancer,6–10 our work presents a comprehensive reconsideration of these associations. First, compared with previous MR, we used an enlarged set of female-specific instruments involving 281 adult BMI-associated variants explaining 4.0% of the phenotypic variance, greatly enhancing the statistical power. Second, we considered potential influence from important confounders, which previous MR did not have the opportunity for. The consistent protective effect of adult BMI on breast cancer overall with and without conditioning on confounders provided convincing evidence of a putative causal relationship. Third, using multivariable MR, we further controlled for the effect of childhood BMI and found mitigation on the effect of adult BMI, indicating the identified putative causal relationship to be largely attributed to a high childhood BMI. These findings were supported by a previous multivariable MR9 conducted based on data from UK Biobank and the Breast Cancer Association Consortium (adult body size: ORunivariable = 0.82, P = 8.04 × 10–4 vs ORmultivariable = 1.08, P = 0.32)—whereas they used questionnaire-based perceived obesity at age 10 years, we used actual measured obesity among children, minimizing the likelihood of misclassification. Collectively, these findings suggest a complex interplay underlying multiple obesity-related traits over the life course, highlighting the importance of taking multiple traits into consideration simultaneously.
Two previous univariable MRs attempted to examine the role of genetically predicted abdominal obesity in breast cancer. One used 14 sex-combined instruments of WHR and concluded a null association,7 whereas the other used 54 sex-combined instruments of WHRadjBMI and reported a decreased effect on breast cancer overall.6 Our univariable MR, using an expanded set of IVs involving 203 WHR-associated female-specific SNPs, confirmed a protective effect on breast cancer overall. This protective effect of WHR remained even after adjusting for confounders and adult BMI (WHRadjBMI), whereas it reduced to some extent after adjusting for childhood BMI. Notably, such a relationship—increased abdominal obesity associated with a decreased risk of breast cancer—conflicts with observational studies that identified a positive association for post-menopausal breast cancer29,30 and an inconsistent association for pre-menopausal breast cancer.31–33 One potential interpretation could be that genetically predicted WHR and WHRadjBMI primarily reflect excessive visceral adipose tissue deposition by affecting genetic predisposition in early life, rather than in late adulthood. To the best of our knowledge, the effect of adult abdominal obesity on breast cancer in observational studies was often modified by the obesogenic environment,34 such as sugar-sweetened beverages, fried foods and physical inactivity, the majority of which were unlikely to be captured by our study using genetic instruments as proxies. Further experimental studies are warranted to clarify the precise molecular mechanism underlying this finding.
Our study highlights a non-trivial role of childhood BMI in the development of breast cancer. The protective effect from univariable MR was largely in line with previous work,7,9 whereas results of multivariable MR provided strong evidence for an independent causal association of childhood obesity with breast cancer overall irrespective of adult measures. Furthermore, genetic correlation analysis confirmed a negative shared genetic basis, indicating a higher genetically predicted childhood BMI to correlate with a decreased susceptibility to breast cancer carcinogenesis. Our results corroborate findings of prospective cohort studies showing an inverse relationship between childhood BMI and breast cancer.35,36 Potential mechanisms include a decreased frequency of ovulatory cycles37 and earlier breast differentiation due to higher levels of oestrogens derived from adipose tissues in obese children,38 terminally decreasing the susceptibility to malignant transformation.
Subtype-specific analyses provide implications for understanding the biological mechanisms linking genetically predicted obesity with breast cancer risk. In our study, although the effect of BMI in both childhood and adulthood on breast cancer did not differ across ER-defined subtypes (regardless of conditional analysis), the protective effects of genetically predicted adult WHR and WHRadjBMI were restricted to ER+ subtype (consistent across all conditional analysis adjusting for childhood obesity and confounders). Obesity is known to profoundly affect oestrogen metabolism and fat-derived oestrogens are considered a principal biological mechanism through which abdominal obesity mainly impacts the risk of ER+ but not ER− subtype.39
This is a comprehensive MR conducted to interrogate the independent role of multiple correlated obesity traits in breast cancer using the hitherto largest female-specific data with an almost four-times increased number of instruments and a doubled phenotypic variance explained compared with previous studies, substantially improving statistical power.40 Although reverse causality is always a potential concern for MR, our findings from bidirectional MR indicated that the obesity–breast cancer causal effect was unlikely to be biased by reverse causation. Nevertheless, we also need to acknowledge several limitations. First, although we adopted female-specific instruments for each adult obesity-related trait to match with female-specific cancer, we were unable to estimate the sex-specific effect of childhood obesity due to data restrictions. Given that the sex instrumental heterogeneity has been recently confirmed to have a non-ignorable impact on the estimates of two-sample MR,12 future investigations would benefit from developing girl-specific IVs of childhood obesity. Second, pleiotropy derived from undetected confounders might bias the causal estimates. However, we tried to reduce such bias to the best of our ability. The directional consistent results derived from multiple ‘pleiotropy-robust’ methods41 supported the validity of our MR results. Lastly, as the two-sample MR approach is typically based on linear assumption, we could not examine the nonlinear relationship that has been extensively evaluated in the traditional epidemiological studies.42,43 Future one-sample MR studies using semi-parametric methods44 are perhaps warranted.
To conclude, our comprehensive MR study with an enlarged sample size successfully replicated the inverse relationship of obesity with the risk of breast cancer. We further identified that the total effect of adult general obesity on breast cancer was largely attributed to childhood obesity, whereas that of adult abdominal obesity was at least partly attributed to childhood obesity. Finally, we demonstrated a predominantly independent effect of childhood BMI in affecting breast cancer onset, irrespective of adult measures. Nevertheless, our results do not advocate weight gain as a preventative intervention against breast cancer, but rather highlight the importance of considering together multiple exposures of obesity at different time points across the life course. We anticipate that understanding the biological mechanisms underlying the inverse association of early-life obesity with breast cancer might motivate the identification of additional modifiable risk factors.
Ethics approval
All analyses were conducted using summary-level data generated by previous studies that have described their relevant ethical approvals.
Supplementary Material
Acknowledgements
We would like to thank the Genetic Investigation of Anthropometric Traits consortium, the Early Growth Genetics consortium, the Breast Cancer Association Consortium, the Reproductive Genetics Consortium, the GWAS & Sequencing Consortium of Alcohol and Nicotine use and UK Biobank for making available the summary statistics of their GWAS meta-analyses.
Conflict of interest
None declared.
Contributor Information
Yu Hao, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Jinyu Xiao, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Yu Liang, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Xueyao Wu, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Haoyu Zhang, Division of Cancer Epidemiology and Genetics, Department of Health and Human Services, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Chenghan Xiao, Department of Maternal, Child and Adolescent Health, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Li Zhang, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Stephen Burgess, MRC Biostatistics Unit, Cambridge Institute of Public Health, University of Cambridge, Cambridge, UK.
Nan Wang, Department of Maternal, Child and Adolescent Health, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Xunying Zhao, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Peter Kraft, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Jiayuan Li, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Xia Jiang, Department of Epidemiology and Biostatistics, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China; Department of Nutrition and Food Hygiene, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China.
Data availability
Publicly available GWAS data on breast cancer were obtained from https://bcac.ccge.medschl.cam.ac.uk/bcacdata/. Adult BMI and WHR GWAS data are publicly available at https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consor-tium_data_files. Childhood BMI GWAS data are publicly available at https://egg-consortium.org/. Age at menarche and age at natural menopause GWAS data are publicly available at https://www.reprogen.org/data_download.html. Smoking and drinking data are publicly available at https://genome.psych.umn.edu/index.php/GSCAN.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
Y.H., J.X., X.J. and J.L. designed the study; Y.L., X.W., H.Z., C.X. and L.Z. provided code and analytical advice; S.B., N.W., X.Z. and P.K. provided analytical advice and critical interpretation of findings; Y.H. and J.X. conducted all analyses and drafted the manuscript; all authors edited and approved the manuscript.
Funding
This study was supported by funds from the National Natural Science Foundation of China (No. 81874282), the National Key R&D Program of China (2020YFC2006505), the Health Commission of Sichuan Province (20PJ093) and the Key R&D Program of Sichuan, China (2022YFS0055).
References
- 1. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol 2021;17:350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freisling H, Arnold M, Soerjomataram I et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer 2017;116:1486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pischon T, Nimptsch K, eds. Obesity and Cancer, Vol. 208. Cham, Switzerland: Springer International Publishing, 2016. [Google Scholar]
- 4. Norat T, Chan D, Vingeliene S, et al. World Cancer Research Fund International systematic literature review: the associations between food, nutrition and physical activity and the risk of breast cancer. Imperial College London, World Cancer Research Fund International, 2017. https://www.wcrf.org/wp-content/uploads/2021/02/Breast-cancer-report.pdf (7 December 2021, date last accessed).
- 5. Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 6. Shu X, Wu L, Khankari NK et al. ; Breast Cancer Association Consortium. Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol 2019;48:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao C, Patel CJ, Michailidou K et al. ; the Colorectal Transdisciplinary Study (CORECT); Discovery, Biology and Risk of Inherited Variants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI); and Transdisciplinary Research in Cancer of the Lung (TRICL). Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol 2016;45:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson KE, Siewert KM, Klarin D et al. ; the VA Million Veteran Program. The relationship between circulating lipids and breast cancer risk: a Mendelian randomization study. PLOS Med 2020;17:e1003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ 2020;369:m1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ooi BNS, Loh H, Ho PJ et al. The genetic interplay between body mass index, breast size and breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol 2019;48:781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Y, Zhang J, Zhao H, Guan F, Zeng P. Instrumental heterogeneity in sex-specific two-sample Mendelian randomization: empirical results from the relationship between anthropometric traits and breast/prostate cancer. Front Genet 2021;12:651332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogelezang S, Bradfield JP, Ahluwalia TS et al. ; Early Growth Genetics Consortium. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLOS Genet 2020;16:e1008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pulit SL, Stoneman C, Morris AP et al. ; GIANT Consortium. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet 2019;28:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michailidou K, Lindström S, Dennis J et al. ; ConFab/AOCS Investigators. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551:92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Ahearn TU, Lecarpentier J et al. ; GEMO Study Collaborators. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet 2020;52:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Day FR, Thompson DJ, Helgason H et al. ; The LifeLines Cohort Study. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet 2017;49:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruth KS, Day FR, Hussain J et al. ; 23andMe Research Team. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021;596:393–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu M, Jiang Y, Wedow R et al. ; HUNT All-In Psychiatry. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019;51:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 2017;28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics 2017;207:481–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess S, Zuber V, Valdes‐Marquez E, Sun BB, Hopewell JC. Mendelian randomization with fine‐mapped genetic data: choosing from large numbers of correlated instrumental variables. Genet Epidemiol 2017;41:714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bulik-Sullivan B, Finucane HK, Anttila V et al. ; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47:1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulik-Sullivan BK, Loh P-R, Finucane HK et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015;47:291–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heo M, Kabat GC, Strickler HD et al. Optimal cutoffs of obesity measures in relation to cancer risk in postmenopausal women in the Women’s Health Initiative Study. J Womens Health (Larchmt) 2015;24:218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tehard B, Clavel-Chapelon F. Several anthropometric measurements and breast cancer risk: results of the E3N cohort study. Int J Obes (Lond) 2006;30:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses’ Health Study II. J Natl Cancer Inst 2011;103:273–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fagherazzi G, Chabbert-Buffet N, Fabre A et al. Hip circumference is associated with the risk of premenopausal ER–/PR– breast cancer. Int J Obes 2012;36:431–39. [DOI] [PubMed] [Google Scholar]
- 33. Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg LA. Prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 2007;16:1795–802. [DOI] [PubMed] [Google Scholar]
- 34. Walter S, Mejía-Guevara I, Estrada K, Liu SY, Glymour MM. Association of a genetic risk score with body mass index across different birth cohorts. JAMA 2016;316:63–69. [DOI] [PubMed] [Google Scholar]
- 35. Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 2010;171:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aarestrup J, Bjerregaard LG, Meyle KD et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes 2020;44:1546–60. [DOI] [PubMed] [Google Scholar]
- 37. Baer HJ, Colditz GA, Willett WC, Dorgan JF. Adiposity and sex hormones in girls. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2007;16:1880–88. [DOI] [PubMed] [Google Scholar]
- 38. Hilakivi-Clarke L, Shajahan A, Yu B, de Assis S. Differentiation of mammary gland as a mechanism to reduce breast cancer risk. J Nutr 2006;136:2697S–9S. [DOI] [PubMed] [Google Scholar]
- 39. Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction: implications for pulmonary arterial hypertension. Pulm Circ 2020;10:2045894020952019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith GD, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho Y, Haycock PC, Sanderson E et al. Exploiting horizontal pleiotropy to search for causal pathways within a Mendelian randomization framework. Nat Commun 2020;11:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Byun D, Hong S, Ryu S et al. Early-life body mass index and risks of breast, endometrial, and ovarian cancers: a dose–response meta-analysis of prospective studies. Br J Cancer 2022;126:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet 2014;384:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure‐outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol 2017;41:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available GWAS data on breast cancer were obtained from https://bcac.ccge.medschl.cam.ac.uk/bcacdata/. Adult BMI and WHR GWAS data are publicly available at https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consor-tium_data_files. Childhood BMI GWAS data are publicly available at https://egg-consortium.org/. Age at menarche and age at natural menopause GWAS data are publicly available at https://www.reprogen.org/data_download.html. Smoking and drinking data are publicly available at https://genome.psych.umn.edu/index.php/GSCAN.