Abstract
Understanding the neurobiology of major depressive disorder (MDD) remains one of the major challenges in neuroscience. The disease is heterogeneous in nature and patients present with a varied symptom profile. Studies seeking to identify biomarkers for MDD diagnosis and treatment have not yet found any one candidate which achieves sufficient sensitivity and specificity. In this article, we consider whether neuropsychological impairments, specifically affective biases, could provide a behavioural biomarker. Affective biases are observed when emotional states influence cognitive function. These biases have been shown to influence a number of different cognitive domains with some specific deficits observed in MDD. It has also been possible to use these neuropsychological tests to inform the development of translational tasks for non-human species. The results from studies in rodents suggest that quantification of affective biases is feasible and may provide a reliable method to predict antidepressant efficacy as well as pro-depressant risk. Animal studies suggest that affective state-induced biases in learning and memory operate over a different time course to biases influencing decision-making. The implications for these differences in terms of task validity and future ideas relating to affective biases and MDD are discussed. We also describe our most recent studies which have shown that depression-like phenotypes share a common deficit in reward-related learning and memory which we refer to as a reward-induced positive bias. This deficit is dissociable from more typical measures of hedonic behaviour and motivation for reward and may represent an important and distinct form of reward deficit linked to MDD.
Keywords: affective bias, antidepressant, pro-depressant, predictive validity, reward, animal model
Introduction
Major depressive disorder (MDD) has a lifetime prevalence of approximately 10-15% with the incidence of the disorder increasing in modern society (Kessler, Berglund et al. 2003, Lépine and Briley 2011). As current predictions suggest that this debilitating psychiatric disorder will soon become the leading cause of disability adjusted life years (Wittchen, Jacobi et al. 2011, 2017), strategies which can improve diagnosis and treatment are needed. In psychiatry, diagnosis is almost always made based on the classification of symptoms reported by the patients. The diagnostic statistical manual (DSM-V 2013) provides details of how to interpret the different symptom clusters to categorise and inform diagnosis and treatment. For a diagnosis of MDD, a patient must present with one of the core symptoms, low mood and/or loss of pleasure in daily activities, and at least 4 other symptoms during the same 2-week period (DSM-V, 2013). These can include additional psychological symptoms and changes in homeostatic processes including sleep, appetite and body weight. Most of the other measures used in the assessment of mood disorders (e.g. ICD-10, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale, Beck Depression Inventory; (Hamilton 1959, Hamilton 1960, World Health 1992, Beck, Steer et al. 1996) are based on similar methods where the patients self-reported experience of their symptoms is used to assess type of illness, severity of illness and response to treatment. This approach poses some major challenges to the field. These subjective self-report measures lack the precision and dynamics of an objective biological marker and identification of robust biomarkers could greatly improve diagnosis and treatment. A summary of the nature of these challenges in the field of MDD is presented in table 1.
| Research area | Problem | Benefits of a biomarker |
|---|---|---|
| Diagnosis | Limited capacity to categorise subtypes of disease and link to the best treatment | Improved diagnosis of distinct patient populations which may be linked to treatment options |
| Response to treatment | Delayed onset of action can mean patients require 4-6 weeks of treatment before changing to an alternative medication | Early detection of response to antidepressant medication |
| Aetiology | Lack of consistent findings across populations and animal studies | Early diagnosis, identification of at risk populations |
| Animal models | Limited translational validity as current clinical methods cannot be translated to animal studies and vice versa | Improved cross species validation |
| Drug development | Limited knowledge of disease aetiology | Identification of novel drug targets |
| Clinical trials | High placebo responses and limited efficacy leading to phase II and II failures Loss of investment from pharmaceutical companies |
Improved early detection of efficacy Less likely to be influenced by placebo response |
A biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” Biomarkers Definitions Working Group, [(2001)]. An ideal disease biomarker would be sufficiently specific to enable a differential diagnosis and sensitive enough to provide a measure of treatment response (Jentsch, Van Buel et al. 2015). The heterogeneous nature of MDD and its presentation as a cluster of varied emotional and physical symptoms, means that the current symptom-based evaluation is limited. Most treatments have a delayed onset of action when assessed using these subjective methods, requiring several weeks of treatment before any decision about efficacy can be made. It is clear that the symptom-based, subjective measures used clinically to diagnose and monitor treatment for MDD are insufficient and therefore alternative, more objective methods are necessary.
There have been ongoing efforts to try to address the need for biomarkers for MDD research. To date, these attempts have not been able to identify any one method which delivers both scientific and diagnostic merit (Jentsch, Van Buel et al. 2015, Strawbridge, Young et al. 2017). An ideal biomarker would be something which could be detected through a simple blood test. Most biomarker-based research has concentrated on areas linked to the different hypotheses about the cause of MDD (Jentsch, Van Buel et al. 2015, Strawbridge, Young et al. 2017). The monoamine hypothesis is perhaps the most widely researched and has developed from early psychopharmacology studies which linked the monoamine transmitters with regulation of mood (Schildkraut 1965, Hirschfeld 2000, Ruhé, Mason et al. 2007). Further support for a key role for serotonin came from the development of the serotonin specific re-uptake inhibitors which were first licensed in the 1990s (Stahl 1998). Despite good pre-clinical data and some clinical evidence, studies have generally failed to find a consistent deficit in terms of a genetic risk factor or peripheral markers related serotonin e.g. the major metabolite of 5-HT, peripheral 5-HT receptors, in blood, urine or CSF. This may be due to the limitations of peripheral markers in terms of their relationship to the central nervous system, but even measures of cerebral spinal fluid (CSF) have not provided a consistent picture (Uddin 2014, Jentsch, Van Buel et al. 2015, Strawbridge, Young et al. 2017). More recently, other hypotheses relating to the aetiology of MDD have gained traction. These include dysregulation of the stress response and altered feedback via the hypothalamic-pituitary-adrenal (HPA) axis, neurotrophic deficits associated with neuronal, dendritic and/or synapse loss within key regions such as the hippocampus and prefrontal cortex and a role of neuro-inflammatory processes (see (Strawbridge, Young et al. 2017) for recent review). Although discussed as separate theories, these hypotheses may not be mutually exclusive and there are obvious links between these different systems. Both clinical and preclinical studies have identified potential molecular biomarkers associated with the stress and neurotrophic hypotheses e.g. brain derived neurotrophic factor (BDNF) and cortisol/corticosterone. Like the monoaminergic markers, consistent deficits which can provide the level of sensitivity and reliability needed for a biomarker have not been forthcoming.
In their recent review, Jentsch et al (2016) carried out a systematic evaluation of the different proposed biomarkers for depression considering studies based on imaging methods, molecular markers measured in blood, urine or CSF, genetic markers and animal models. This work considered evidence relating to a wide variety of markers related to genetic risk factors, the monoamine transmitters, neurotrophic factors and pro-inflammatory processes. They also reviewed the evidence relating to functional brain imaging studies and activity changes within key neural circuits e.g. increased amygdala and subgenual cingulate. In their conclusions, they propose that no measure evaluated to date has met the criteria for a suitable biomarker for MDD and further investment is needed to try to address this area of unmet clinical need (Strawbridge, Young et al. 2017). In this article, we consider the questions about biomarkers for MDD from the perspective of our recent animal research and consider whether new research, developing from translational methods to study neuropsychological deficits in MDD, supports the idea of a behavioural biomarker for MDD. It should be noted that the idea of mapping behavioural deficits in psychiatric disorders is not new and endophenotypes in psychiatry (Hasler, Drevets et al. 2004) and recently the Research Domain of Criteria (RDoC) project (Insel, Cuthbert et al. 2010, Cuthbert and Insel 2013, Insel 2014, Nusslock and Alloy 2017) have shown how it is possible to breakdown these complex psychiatric disorders into more objective symptom clusters.
Limitations associated with conventional animal models of depression
Studies in animals can be particularly useful for research into the brain because they can provide a more readily manipulated system in which to test arising hypotheses. Animal research is less constrained by practical and ethical considerations than studies in patients and has the benefit of being able to look at both normal and disease models before, during and after a manipulation. Despite the obvious benefits of using an animal model, studies investigating psychiatric disorders in non-human species are particularly challenging. The major problem researchers have faced is being able to recapitulate in an animal a disorder which is ultimately defined clinically by its subjective, self-report symptoms. The limitations of animal models for psychiatry research, particularly for the study of emotional disorders, has been reviewed elsewhere by ourselves and others (Willner and Mitchell 2002, Willner 2005, Cryan and Slattery 2007, Nestler and Hyman 2010, Neumann, Wegener et al. 2011, Berton, Hahn et al. 2012, Hales, Stuart et al. 2014, Commons, Cholanians et al. 2017, Slattery and Cryan 2017) and is therefore only considered briefly here. Additionally, this section only considers methods used to detect a depression-related behavioural deficit and has not considered methods used to induce a disease model or their associated validity. Review articles relating to disease models include (Willner 1995, Willner and Mitchell 2002, Nestler and Hyman 2010, Berton, Hahn et al. 2012, Slattery and Cryan 2014).
Following the serendipitous discovery of the first antidepressant drugs, researchers sought to develop methods which they could use in animals to predict whether a novel compound may have similar clinical efficacy. Porsolt (Porsolt, Le Pichon et al. 1977, Porsolt, Anton et al. 1978) first published work showing that the antidepressant drug, imipramine, could change the behaviour of a rodent when exposed to an inescapable stressor. The animal is first given a pre-test where they experience the stressor (e.g. placement in a container of water from which they cannot escape), followed 24hrs later by re-exposure to the same apparatus. Pre-treatment with an antidepressant before the re-test resulted in the animal exhibiting a reduction in immobility time i.e. they showed increased escape behaviours. The effect was relatively selective for antidepressant drugs although psychomotor stimulants were reported to yield false positive results. The forced swim test (FST) (Porsolt, Le Pichon et al. 1977, Porsolt, Anton et al. 1978, Porsolt, Bertin et al. 1979) and a subsequent modification of this model for mice, the tail suspension test (TST) (Steru, Chermat et al. 1985), have been shown to be sensitive to monoaminergic antidepressant drugs. They have provided a valuable tool for the development of the second-generation antidepressants but concerns about exactly what the FST/TST is measuring and its validity as a model of depression have been raised (for discussion about animal model validity (Geyer 1995, Cryan and Slattery 2007, Der-Avakian, Barnes et al. 2016). Specifically, these models have some predictive validity but this may be limited to monoaminergic drugs. They also have some face validity as the immobility time is thought to be analogous to behavioural despair, comparable to the hopelessness exhibited by depressed patients. However, recent work suggests that the behaviour of animals in this task may be more relevant to stress-coping mechanisms than to MDD (de Kloet and Molendijk 2016, Commons, Cholanians et al. 2017). There are several examples of novel agents which have been shown to have an antidepressant effect in these assays but that have subsequently failed in the clinic or, in the case of rimonabant, were found to increase negative mood and induce suicidal ideation and behaviour in some patients (Stuart, Butler et al. 2014). An additional criticism of the FST/TST is their failure to predict the time course of effects of antidepressant drugs, with both conventional antidepressants and ketamine having similar rapid onsets of action in this model, which is not seen clinically. The relevance of these assays, in terms of construct validity, is also difficult to interpret as the underlying cause(s) of MDD in people are not understood.
An alternative approach to testing for depression-related behaviour in animals has been to study reward-processing using tests of anhedonia (Willner, Towell et al. 1987, Zacharko and Anisman 1991, Der-Avakian, Barnes et al. 2016, Slattery and Cryan 2017). Anhedonia is defined as a ‘loss or interest in pleasurable activities’ and is one of the core symptoms of MDD. (Der-Avakian, Barnes et al. 2016). The most commonly used method is the sucrose preference test (SPT) although other methods including intracerebral self-stimulation thresholds and operant methods to look at reward motivation and effort or learning have also been used (Slattery, Markou et al. 2007, Der-Avakian, D’Souza et al. 2013, Der-Avakian, Barnes et al. 2016, Slattery and Cryan 2017). In the SPT, animals are given access to a low concentration sucrose or saccharin solution and their intake of this versus water in recorded. Normal animals show a preference for the sweet solution but this is reduced in stress-models of depression. In the first studies using the SPT, animals exposed to chronic mild stress were shown to exhibit an impairment in their ability to detect and respond to a low concentration of sucrose (Willner, Towell et al. 1987). This deficit was reversed by chronic but not acute treatment with antidepressants suggesting that the assay could better predict clinical outcomes over a timescale which more closely reflected the clinical effects. The problem for the SPT and other measures of reward sensitivity has been that patients with depression do not show similar deficits when tested using methods which measure ‘in the moment’ pleasure (Amsterdam, Settle et al. 1987, Berlin, Givry-Steiner et al. 1998, Scinska, Sienkiewicz-Jarosz et al. 2004, Swiecicki, Zatorski et al. 2009, Dichter, Smoski et al. 2010). Instead, they show an impaired ability to cognitively value reward in questionnaire measures of prospective, retrospective or hypothetical experiences (McFarland and Klein 2009, Watson and Naragon-Gainey 2010, Strauss and Gold 2012). Reward learning, quantified using methods such as the probabilistic learning task (PRL), seems to be more sensitive to deficits in MDD and patient’s ability to use both positive and negative feedback to adapt their behaviour and learn about reward (Pizzagalli, Jahn et al. 2005, Pizzagalli, Iosifescu et al. 2008, Pechtel, Dutra et al. 2013, Vrieze, Pizzagalli et al. 2013). PRL tasks has been developed and tested for rodents, including a version involving serial reversals, but only a small number of studies have been published to date and therefore there is limited evidence as to how well the human and animal versions of the tasks compare (Bari, Theobald et al. 2010, Der-Avakian, D’Souza et al. 2013, Der-Avakian, D’Souza et al. 2017). The PRL tasks require animals to learn which response is associated with the better outcomes overall but they must learn this whilst also receiving false feedback i.e. the ‘rich’ stimulus is rewarded 80% of the time and the ‘lean’ stimulus 20%. In both human and animal versions of the task, the subject must learn to ignore false feedback and continue to respond to the stimulus which gives the better outcome overall. A detailed analyses of the behavioural responses to positive or negative feedback and how the subject adapts their subsequent choice behaviour can also be investigated.
The lack of valid animal models with which to evaluate depression-related behaviour has further limited our understanding of the aetiology of MDD. As neither the tests for behavioural despair nor anhedonia directly map onto clinical measures in people or, in the case of the SPT, an analogous task failed to find a deficit in patients with MDD, translational studies are similarly restricted. This has led to a situation where the progress made in the pre-clinical field has failed to translate to clinical benefits. Additionally, it has not been possible to evaluate, in an objective way, hypotheses about the aetiology of MDD and potential biomarkers identified from clinical studies.
Why neuropsychological deficits in MDD could provide a translational biomarker?
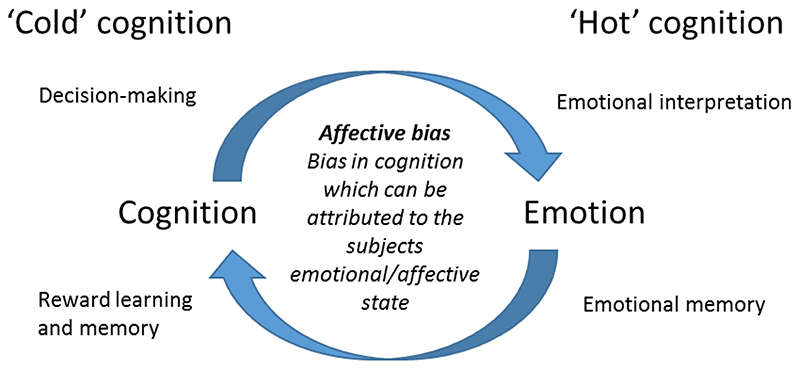
To try to address the issues relating to a lack of translation between clinical and pre-clinical research into MDD, our group has sought to develop and validate novel approaches to study depression-related behaviour in non-human species. Alongside the more traditional use of subjective self-report measures to quantify the symptoms of MDD, researchers are also developing neuropsychological tests which could measure behavioural deficits objectively. This work builds on the ideas that negative schema could contribute to the development and perpetuation of mood disorders as first discussed by Beck in the 1960s (Beck 1967). The types of tasks and neuropsychological assessments used are discussed in (Roiser, Elliott et al. 2012, Robinson and Roiser 2016). The primary objective has been to try to understand the impact of MDD on behaviours which can be measured using an objective method and most commonly, a computer-based task which tests a specific aspect of emotional or cognitive behaviour. These approaches therefore present new opportunities for translational studies (Paul, Harding et al. 2005). As computer-based assessment methods have developed, researchers have been able to show that patients with depression exhibit deficits in a range of cognitive domains as well as changes in the way they interpret emotional information (Gur, Erwin et al. 1992, Surguladze, Young et al. 2004, Mathews and MacLeod 2005, Leppänen 2006, Ressler and Mayberg 2007, Williams, Barnhofer et al. 2007, Gotlib and Joormann 2010, Elliott, Zahn et al. 2011, Roiser, Elliott et al. 2012). These deficits have been categorised as ‘hot’ = emotional and ‘cold’ = non-emotional deficits in cognition (Roiser, Elliott et al. 2012, Robinson and Roiser 2016), Figure 1). MDD has been shown to be associated with deficits in both hot and cold cognition and there is now increasing evidence to suggest that these different cognitive domains can be modulated by the emotional state of the individual. The terms ‘affective biases’ or ‘cognitive affective biases’ have been used to describe these modulatory effects of emotion and both positive and negative biases can be observed dependent on the state of the individual. Recent clinical work in this field has focused on affective biases in relation to tasks where emotional stimuli are used (e.g. faces, words and pictures), however, for the purposes of our translational work, we have interpreted the term affective biases more broadly and suggest that this term can usefully be applied to describe any cognitive behaviour where a specific effect of the individuals emotional or affective state can be observed, human or animal (Figure 1).
Figure 1.
The term ‘affective bias’, has been used to describe the biases in emotional processing observed in patients with affective disorders such as anxiety or major depressive disorder. In our attempts to develop a translational approach to studying these neuropsychological mechanisms in non-human species, we have expanded our definition of an affective bias as shown above. We propose that affective biases are not limited to domains of emotional or ‘hot’ cognition but are also relevant to other cognitive domains sometimes referred to as ‘cold’ cognition.
One of the most commonly reported deficits in MDD is a change in the way emotional information is processed. Specifically, there is evidence that both depressed individuals and at-risk populations exhibit increased negative affective biases compared to healthy individuals (Clark, Chamberlain et al. 2009, McCabe, Woffindale et al. 2012, Roiser, Levy et al. 2012). Affective biases can occur at different stages of information processing, for example, in attention, interpretation, memory or decision-making (Mathews and MacLeod 2005). Depression has mainly been linked to explicit memory biases and interpretation biases (Mathews and MacLeod 2005, Mogg and Bradley 2005, Gotlib and Joormann 2010). Memory biases have been reported in both recent (e.g. word recall) and remote (e.g. autobiographical memory) recall tasks (Matt, Vázquez et al. 1992, Mathews and MacLeod 2005, Williams, Barnhofer et al. 2007). For example, individuals with MDD preferentially recall negative compared to positive material, remembering ~10% more negative words than positive words (Matt, Vázquez et al. 1992, Gotlib and Joormann 2010). Additionally, patients with depression exhibit enhanced memory for negative compared to positive autobiographical material (Williams and Scott 1988, Brittlebank, Scott et al. 1993). There is also evidence of biases in the interpretation of ambiguous information, with depressed individuals being more likely to interpret neutral or ambiguous expressions as negative (Bourke, Douglas et al. 2010). Attentional biases in depression are less robust, but when they have been reported, individuals with depression may display difficulties disengaging attention from negative material (Mogg and Bradley 2005, Rinck and Becker 2005, Caseras, Garner et al. 2007). This suggests that individuals with MDD display negative biases in emotion processing, particularly in explicit memory and interpretation.
To assess whether affective biases could provide a useful biomarker for MDD, it is important to consider (1) specificity of these biases to the disorder and (2) their sensitivity to treatment. Affective biases reported in MDD may be dissociable from those reported in other disorders such as generalised anxiety disorder (GAD). Specifically, memory biases but not attentional biases have consistently been reported in depression, whereas the converse is found in GAD (Coles and Heimberg 2002, Mogg and Bradley 2005, Marchetti, Everaert et al. 2018). Unlike MDD, individuals with GAD display a bias towards threat stimuli (Mogg and Bradley 2005). This suggests that whilst affective biases have been reported across affective disorders, the nature of these biases may differ (Gotlib and Joormann 2010). Future research investigating multiple affective biases, and the interplay between them, in both anxiety and depression is needed to gain a better understanding of the similarities and differences in affective biases across disorders and to determine whether a clear dissociation can be found (Klein, de Voogd et al. 2017, Salem, Winer et al. 2017). To the best of our knowledge, the field lacks a comprehensive systematic review that synthesizes prior work on affective biases across different disorders. Affective biases may also be sensitive to treatment response. For example, acute administration of an antidepressant drug can reverse objective negative affective biases, without changes in subjective mood (Harmer, O’Sullivan et al. 2009, Pringle, Browning et al. 2011). Interestingly, one study in depressed individuals reported that early changes in affective biases predicted later clinical outcome (Tranter, Bell et al. 2009). This is important as it may provide an earlier prediction of the antidepressant efficacy or pro-depressant risk associated with novel treatments. Overall, there is accumulating evidence to suggest that affective biases may provide a useful behavioural biomarker for MDD
Development and validation of translational tasks to study affective biases in non-human species
Studies in animals cannot be directly based on the aforementioned emotional processing methods as they use stimuli which cannot be readily translated to use in non-human species. The underlying principles of affective bias are however translatable if we consider that the ability of positive or negative emotions to bias cognitive processes extends beyond only those associated with innately emotional stimuli. The approach used for the animal work has been to look at how affective biases may modulate cognitive behaviours where animals have been trained to associate specific, previously neutral cues, with positively or negatively valenced outcomes e.g. reward or punishment (Hales, Stuart et al. 2014). Behavioural studies, using learnt association between a novel stimuli and prediction of either a reward or punishment are commonly used in animal research. Models such as fear conditioning have been used to study emotional learning and memory whilst reward-based tasks involving operant responses can be used to study a wide range of cognitive processes including anticipation of reward and reward motivation. Combining these two areas of research, two different types of affective bias task have been developed in the non-human literature. These methods specifically look at decision-making behaviour in response to ambiguity and reward learning and memory.
Affective biases and decision-making
The first example of a cognitive affective bias in a non-human species was published by Mendl and colleagues in 2004 (Harding, Paul et al. 2004). In a decision-making task, animals were trained to associate specific tone cues with positively or negatively valenced outcomes. Once the animals had learnt these associations, they were presented with intermediate, ambiguous cues and their response selection was recorded. Animals in a putative negative affective state made less responses in anticipation of reward suggesting a negative bias or ‘pessimism’. Several groups including our own have now replicated and extended this work and affective biases have been reported across a wide range of species from insects to humans (Hales, Stuart et al. 2014). The underlying principle of the judgement bias task (JBT, also sometimes referred to as an ambiguous cue interpretation task) is that an animal’s affective state biases its decision-making behaviour when they are presented with an ambiguous cue, intermediate between the two reference cues they have learnt to associate with positive or negative/less positive outcomes. Different research groups have tested versions of this task including methods using spatial cues, textures, tones and visual stimuli (for review see (Hales, Stuart et al. 2014). The most commonly reported method utilises an operant chamber and tone cues which predict the response required to either obtain reward or avoid punishment, or an adaptation to this task where the reference cues predict high or low reward.
There have been a number of different studies now published where animals in a putative negative affective state have been trained and tested in the JBT (Hales, Stuart et al. 2014, see table 2 for summary). These studies have found a broadly consistent picture where the depression-like state of the animal is associated with an increase in pessimistic choices. Similar to the findings of Mendl and colleagues, Enkel et al., 2009 reported that a genetic model of depression, the learned helplessness rat, exhibited a negative bias in this task. They could also replicate the effect by treating normal animals with a pharmacological stressor. Studies from Rygula’s group have reported similar negative biases in rats exposed to chronic stress (Rygula, Abumaria et al. 2005, Papciak, Popik et al. 2013). In the high versus low reward version of the task, we have also observed that exposure to a chronic mild stress manipulation induced a negative bias (Hales, Robinson et al. 2016). These findings suggest that a depression-like phenotype results in an increase in anticipation of negative events and/or a reduction in anticipation of positive events which compares favourably with the clinical scenario. There have also now been two publications where healthy human participants have been tested using a similar task (Anderson, Hardcastle et al. 2012, Aylward, Hales et al. 2017). Although the effects observed in these studies appear to relate more closely to anxiety than MDD, studies in clinical populations have yet to be undertaken.
Table 2. Summary of results obtained from the judgement bias task illustrate the effects of acute treatments on decision-making behaviour in this task.
| Judgement bias task | ||
|---|---|---|
| Treatment | Dose | Effects |
| Amphetamine | 0.1mg/kg | Neu b,h |
| 0.3mg/kg | Pos h | |
| 0.5mg/kg | Neu b | |
| 1.0mg/kg | Pos b | |
| AM2511 | 1.0mg/kg | Neu f |
| AM6302 | 1.0mg/kg | Neu f |
| Citalopram | 1.mg/kg | Neu b |
| 5.0mg/kg | Neu b | |
| 10.0mg/kg | Neu b | |
| Cocaine | 1.0mg/kg | Neu c,h |
| 2.0mg/kg | Neu c,h | |
| 5.0mg/kg | Neu c,h | |
| Diazepam | 0.3mg/kg | Neu d |
| 1.0mg/kg | Neu d | |
| Desipramine | 1.0mg/kg | Neu b |
| 2.0mg/kg | Neu b | |
| 5.0mg/kg | Neu b | |
| FG7142 | 3.0mg/kg | Neg g |
| 5.0mg/kg | Neg g | |
| Fluoxetine | 0.1mg/kg | Neu d |
| 0.3mg/kg | Neu d,h | |
| 1.0mg/kg | Neu d,h | |
| Ketamine | 0.3mg/kg | Neu h |
| 1.0mg/kg | Pos h | |
| 3.0mg/kg | Neu h | |
| Lithium | 10.0mg/kg | Neu e |
| 50.0mg/kg | Pos e | |
| 100.0mg/kg | Neu e | |
| Mazindol | 0.5mg/kg | Neg c |
| 1.0mg/kg | Neg c | |
| 2.0mg/kg | Neg c | |
| Phencyclidine | 0.3mg/kg | Neu h |
| 1.0mg/kg | Neu h | |
| 3.0mg/kg | Neu h | |
| Reboxetine | 0.3mg/kg | Neu d,h |
| 1.0mg/kg | Neu d,h | |
| 3.0mg/kg | Neu d | |
| URB5973 | 0.1mg/kg | Neu f |
| 0.3mg/kg | Neu f | |
| 1.0mg/kg | Pos f | |
| Valproic acid | 100.0mg/kg | Neu e |
| 200.0mg/kg | Neu e | |
| 400.0mg/kg | Neu e | |
| Venlafaxine | 1.0mg/kg | Neu h |
| 3.0mg/kg | Neu h | |
| Manipulation | Effects | |
| Restraint stress and social isolation | Neu g | |
| Tickling | Pos a |
Rygula et al. 2012
Anderson et al. 2015
Rygula 2015
Kregiel et al. 2016
cannabinoid receptor type 1 (CB1) inverse agonist
cannabinoid receptor type 2 (CB2) inverse agonist
irreversible anandamide hydrolysis inhibitor
Animal studies provide a useful model for evaluating novel drug targets, but require a high degree of predictive validity. Using a range of different pharmacological manipulations, we have tested whether decision-making behaviour in the JBT is sensitive to changes in affective state following acute or chronic administration (Anderson, Munafo et al. 2013, Hales, Robinson et al. 2016, Hales, Houghton et al. 2017). The results for acute treatments with conventional antidepressants suggest that they have no initial impact on decision-making (Hales, Houghton et al. 2017). We have observed positive biases with acute amphetamine whereas induction of an anxiety-like state with FG7142 induces a negative bias (Hales, Robinson et al. 2016, Hales, Houghton et al. 2017). The only other publications where antidepressant treatments have been tested acutely have also failed to observe a consistent positive bias with antidepressants but did observe similar, positive biases with amphetamine (Rygula, Papciak et al. 2014, Rygula, Szczech et al. 2014). In contrast, we have observed that chronic antidepressant treatment does induce a positive bias and this develops over time (Hales, Robinson et al. 2016). Furthermore, we have recently reported that the rapid onset antidepressant, ketamine, induces a positive bias following acute administration (Hales, Houghton et al. 2017). Taken together, these findings suggest that affective biases linked to decision-making behaviour in the JBT may provide an animal model which can predict the efficacy and rate of onset of an antidepressant. This may be useful for drug development but it is more difficult to know if this task achieves construct validity. Studies in humans have suggested that interpretation biases are modulated acutely by conventional antidepressants (Harmer, O’Sullivan et al. 2009, Pringle, Browning et al. 2011) suggesting the rat JBT likely involves different cognitive mechanisms. This may arise from the different types of stimuli being used for the animal versus human work and back translation of this rodent task and further studies in patients may help explain these differences.
Affective biases and reward learning and memory
The method developed by our research group, the affective bias test (ABT), has specifically focused on reward-learning using a bowl digging task where animals are required to learn the association between a specific cue (a digging substrate) and a rewarding outcome (a food reward). The task is designed to test how reward associated with two, independently learnt experiences is valued by the animal and whether this can be modulated by the animal’s affective state at the time of learning (see figure 2). Unlike the JBT, the ABT assay requires a within-subject design where the animal receives the two independent rewarding experiences of equal value but with one learnt during an affective state manipulation and the other learnt under control conditions. To test for any bias, animals are tested in a final session where both previously rewarded substrates are presented at the same time and the animals choices are recorded over a series of randomly reinforced trials. Because the absolute value of the rewards is the same, the animals should not exhibit any preference, however, if the manipulation has induced a bias in terms of the memory of that experience, this would be apparent during the choice test. Consistent with our prediction, induction of a negative affective state using either acute stress or pharmacological treatments, resulted in animals biasing their choices away from the treatment paired substrate (Stuart, Butler et al. 2013, Refsgaard, Haubro et al. 2016, Hinchcliffe, Stuart et al. 2017, Stuart, Wood et al. 2017). We have now evaluated a large number of treatments including drugs from different pharmacological classes which have been identified as increasing the risk of depression in patients. In contrast, pre-treatment with conventional antidepressant drugs or social enrichment induced a positive bias in the ABT. The biases induced in this assay appear to be specific to manipulations which change affective state, as drugs of abuse and other control compounds failed to induce any effects. Table 3 provides a summary of the findings in the ABT to date. Comparing the effects of acute treatments with these manipulations in the ABT and results for emotional processing biases in humans, the results are remarkably consistent (Pringle, Browning et al. 2011). These data also suggest that the ABT can predict the longer-term effects on mood in patients based on the outcome of an acute treatment in this assay.
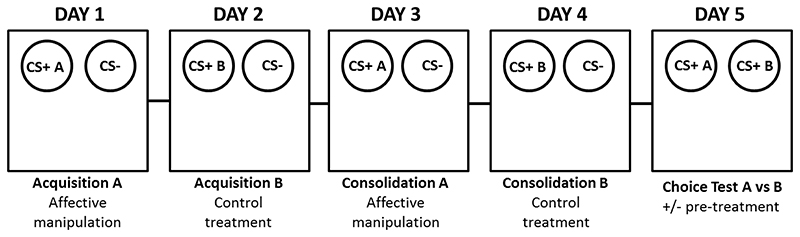
Figure 2.
Schematic representation of the affective bias test protocol illustrating the 5 day procedure which would be used for a single animal. The animals learn to associated substrates CS+A or CS+B with finding a food reward. The CS- is unrewarded. Following a counter-balanced design where one substrate is paired during treatment and other is paired during control conditions, the animals are presented with both previously rewarded substrates and their choices recorded. Animals can also receive a treatment before the choice test to determine if the previously acquired biases can be subsequently modulated.
Table 3. Summary of results obtained for the affective bias test illustrating the effects of different acute treatments on reward learning and memory.
| Affective bias test | ||
|---|---|---|
| Treatment | Dose | Effects |
| Agomelatine | 0.1mg/kg | Neu a |
| 0.3mg/kg | Neu a | |
| 1.0mg/kg | Pos a | |
| Amphetamine | 0.3mg/kg | Neu a |
| Aprepitant | 0.1mg/kg | Neu a |
| 0.3mg/kg | Neu a | |
| 1.0mg/kg | Neu a | |
| 10.0mg/kg | Neu a | |
| 30.0mg/kg | Neu a | |
| Carbamazepine | 3.0mg/kg | Neu c |
| 10.0mg/kg | Neu c | |
| 30.0mg/kg | Neu c | |
| Citalopram | 0.1mg/kg | Neu a |
| 0.3mg/kg | Neu a | |
| 1.0mg/kg | Pos a | |
| 3.0mg/kg | Neu a | |
| Clomipramine | 1.0mg/kg | Pos a |
| Cocaine | 3.0mg/kg | Neu a |
| Corticosterone | 0.1mg/kg | Neu d |
| 1.0mg/kg | Neg d | |
| 10.0mg/kg | Neg b,c | |
| 30.0mg/kg | Neg c | |
| Diazepam | 0.3mg/kg | Neu a |
| 1.0mg/kg | Neu a | |
| 3.0mg/kg | Neu a | |
| Duloxetine | 1.0mg/kg | Pos b |
| 3.0mg/kg | Pos b | |
| 10.0mg/kg | Pos b | |
| Ethanol | 800.0mg/kg | Neu a |
| FG7142 | 1.0mg/kg | Neu a |
| 3.0mg/kg | Neg a,d | |
| 5.0mg/kg | Neg a | |
| 6.0mg/kg | Neg d | |
| Fluoxetine | 0.3mg/kg | Pos a |
| 1.0mg/kg | Pos a | |
| 3.0mg/kg | Pos a | |
| Idazoxan | 1.0mg/kg | Neu b |
| 3.0mg/kg | Pos b | |
| 10.0mg/kg | Neu b | |
| Interferon alpha | 10.0U/kg | Neu c |
| 100.0U/kg | Neg c | |
| LPS | 0.01mg/kg | Neu c |
| 0.1 mg/kg | Neu c | |
| 1.0 mg/kg | Neg c | |
| 10.0 mg/kg | Neg c | |
| Mirtazapine | 0.3mg/kg | Pos a |
| Montelukast | 1.0mg/kg | Neu c |
| 3.0mg/kg | Neu c | |
| Morphine | 5.0mg/kg | Neu a |
| Nicotine | 0.06mg/kg | Neu a |
| Reboxetine | 0.1mg/kg | Neu a |
| 0.3mg/kg | Pos a | |
| 1.0mg/kg | Pos a | |
| Retinoic Acid | 1.0mg/kg | Neu a |
| 3.0mg/kg | Neg a | |
| 10.0mg/kg | Neg a | |
| Rimonabant | 1.0mg/kg | Neu a |
| 3.0mg/kg | Pos a | |
| 10.0mg/kg | Pos a | |
| Sertaline | 1.0mg/kg | Pos d |
| 3.0mg/kg | Pos d | |
| 10.0mg/kg | Pos d | |
| Tetrabenazine | 0.1mg/kg | Neu c |
| 0.3mg/kg | Neu c | |
| 1.0mg/kg | Neg c | |
| Varenicline | 0.03mg/kg | Neu c |
| 0.1mg/kg | Neu c | |
| 0.3mg/kg | Neu c | |
| 1.0mg/kg | Neu c | |
| Venlafaxine | 1.0mg/kg | Pos a |
| 3.0mg/kg | Pos a,d | |
| 10.0mg/kg | Pos a,d | |
| Vortioxetine | 1.0mg/kg | Neu b |
| 3.0mg/kg | Pos b | |
| 10.0mg/kg | Pos b | |
| Manipulation | Effects | |
| High value reward | Pos a,d | |
| Social play | Pos a,d | |
| Restraint stress and social isolation | Neg a,d |
To improve our understanding of whether the ABT is also able to quantify relevant neuropsychological substrates involved in MDD, we have undertaken more extensive mechanistic studies. Our initial hypothesis behind developing the ABT was the observation that patients with MDD attribute reduced value to what should be rewarding experiences. For example, they are less motivated to re-engage in rewarding activities and become increasingly withdrawn from social and environmental interactions which should have positive outcomes. If affective biases in humans can modulate reward learning in the way we have observed in our ABT, this could contribute to the development of these symptoms. We were interested to see if the ABT could be used to test a theory about the role neuropsychological mechanisms play in the delayed onset of antidepressant drugs first proposed by Harmer et al (Harmer, Goodwin et al. 2009, Harmer, Duman et al. 2017). In their model, antidepressant drugs act acutely to remediate negative affective biases linked to emotional processing, particularly in relation to the interpretation and memory associated with social cues. These effects reveal that acute and short term changes in relevant neuropsychological processes can be detected if the assessment method used does not depend on a self-report measure. In fact, Harmer and colleagues have been able to demonstrate that the same individual can show changes in emotional processing but not any subjective experience of a change in mood. The subjective effects are delayed because time and new learning are required to overcome the effects of the previously learnt and negatively biased experiences. If a similar model of affective bias applies to reward-learning and memory in the ABT, then this could potentially be studied in our animal task. The first studies we undertook were to compare delayed versus rapid onset antidepressants to determine if they would differentially interact with the learning aspect of the task versus the memory of the experience i.e. have effects on recall (Stuart, Butler et al. 2015). We found that systemic treatment with ketamine blocked the negative bias induced by stress or a pharmacologically-induced negative state. However, ketamine did not bias new learning. In contrast, the monoaminergic antidepressant, venlafaxine, did not attenuate the negative biases once it had been learnt but could modify new learning. We therefore proposed that this dissociation in effects on learning versus memory, could explain the differences in the rate of onset of clinical benefit. This theory is further supported by evidence linking these different neuropsychological mechanisms to different brain areas. The effects of venlafaxine could be blocked by lesions to the amygdala whilst ketamine’s effects were localised to the medial pre-frontal cortex and interestingly, they could be replicated by an infusion of the GABAA agonist, muscimol, into the same area. Although more detailed studies are needed, the ABT seems to involve brain regions strongly linked to emotional processing in humans (Ressler and Mayberg 2007, Murphy, Norbury et al. 2009). The temporal differences between conventional antidepressants and ketamine could be explained by their differential effects on the neuropsychological mechanisms which are involved in this task. Perhaps the most important observation from the perspective of a behavioural biomarker is the sensitivity of the assay in predicting clinical outcomes. Sensitivity has been observed in terms of antidepressant efficacy, time course of effects and pro-depressant risk.
Reward-learning deficits in a modified ABT
One obvious challenge with the ABT is that it uses a within subject study design and does not readily lend itself to studies in disease models. The assay requires the animal to make a relative assessment of the value it attributes to two different experiences during the recall test and this can only be achieved if the animal receives both treatments in a counter-balanced design. However, we have routinely used a modified version of the ABT during training to check that each new cohort of rats is able to perform the task before we progress to testing novel manipulations. In the modified ABT, animals receive two rewards during one pairing session and one reward in the other session (Stuart, Wood et al. 2017). This means that each substrate becomes associated with a different absolute value of reward and the animal should bias its choices during the preference test towards the higher value reward-paired substrate. We have called this a reward-induced positive bias. As part of our interests in how affective states influence different aspects of cognition, we have now also tested whether reward-learning in this modified ABT is impaired in animals in putative negative affective states. We hypothesised that the acute negative biases associated with the pro-depressant manipulations may, if experienced chronically, induce a more generalised impairment in reward learning and memory which could be measured using this modified assay. We compared how animals performed in the modified ABT with more typical measures of anhedonia by also carrying out an SPT in the same cohort. We have now used this assessment in animals in putative negative affective states, induced by chronic treatment with interferon-alpha and retinoic acid, and found that these treatments result in an attenuated reward-induced positive bias without affecting consummatory anhedonia in the SPT (Stuart, Wood et al. 2017). We have tested animals with chronic neuropathic pain, exposure to early life adversity or chronic stress (unpublished observations) and observed similar results. All the manipulations attenuated the reward-induced positive bias in the ABT but only chronic stress also affected sucrose preference. Studies in animals exposed to sub-chronic phencyclidine (PCP) to induce a model of schizophrenia have also observed a similar deficit in reward-learning (Sahin, Doostdar et al. 2016). Previous studies using the PCP model have also generally failed to observe any changes in performance in the SPT, although there are some exceptions (Vardigan, Huszar et al. 2010, Neill, Harte et al. 2014). This raises the possibility that the deficit we observe in reward learning is independent of changes in consummatory anhedonia. It also suggests that this is a form of affective bias, where the deficit is related to the ability of the animal to appropriately learn about the reward-cue association and then use this information to guide subsequent behaviour and choices when re-presented with those cues. If we reconsider the biases observed in the JBT, they may concur with this observation as the reduced anticipation of reward seen in this task occurred over a delayed time course, possibly due to a mechanism involving learning.
Impaired ability to learn about reward and use this information to guide behaviour has been discussed in relation to the clinical literature (Treadway and Zald 2011, Treadway and Zald 2013, Romer Thomsen, Whybrow et al. 2015, Thomsen 2015, Whitton, Treadway et al. 2015) but not specifically considered from the perspective of an affective bias. However, if the deficits are related to learning and memory for reward rather than the perception of reward, as our animal data suggests, then this would mean that the anhedonia in MDD is potentially more about cognitive processes related to reward rather than hedonic mechanisms. The ability to quantify anhedonia in animals using tests which measure perception of reward (SPT) or motivation for reward (progressive ratio tasks) has made these appealing models for depression research. As discussed earlier however, the SPT seems to be particularly sensitive to impairments induced by chronic stress, but is not always observed for other models of depression. Our findings suggest that the modified ABT is sensitive to impairments in reward learning and memory resulting from different biological or psychological substrates.
Summary of pre-clinical affective bias studies and their relationship to clinical findings
The field of affective biases in non-human subjects has developed over the last 13 years following Mendl et al first publication in 2004. The studies in the JBT suggest that decision-making behaviour in a wide range of species is biased by the animal’s affective state. The response to antidepressants is not yet fully understood as studies are limited but they appear to mirror the clinical time course of subjective effects on mood rather than the more immediate effects seen in the clinic when using emotionally valenced stimuli. This may be a consequence of the differences in methodology as the animal studies use cues paired with positive or negatively valenced outcomes rather than emotional cues. Results in the ABT have shown that reward learning is biased by acute changes in both positive and negative affective states. The task can predict potential antidepressant efficacy and pro-depressant risk. We have also identified a novel deficit in reward processing using a modification to the ABT and suggest that learning about the value of reward and using this information to guide subsequent behaviour is impaired. This effect is distinct from consummatory or motivational forms of anhedonia more traditionally measured in animals, but how this relates to measures of anhedonia in patients requires further investigation. There have also been advances in clinical research into affective biases suggesting that these may provide an objective and dynamic method to categorise disease and detect early responses to antidepressants. These early responses may also predict longer term outcomes. Taken together, these data suggest that studies investigating the impact of affective state on different cognitive domains may be a useful approach for translational studies into MDD. Although the tasks used in animals are not identical to those used in patients, because of species differences, they have shown that cognitive processes in non-human species are sensitive to the animals’ affective state. The data for phenotypic models and pharmacological studies support improved validity over conventional models such as the FST/TST.
Conclusions: Affective biases as a behavioural biomarker for translational studies into MDD
1. Specificity to disease state
Evidence to date suggests that certain cognitive processes are modulated by affective states in both human and non-human species. It is not yet possible to fully evaluate how specific these deficits are to MDD as most studies only look at a limited number of tasks and in a single disease state. However, more detailed analyses involving assessment of biases across different cognitive domains and in different psychiatric disorders could reveal a pattern of deficits which provides a biomarker. For example, a meta-analytic study comparing anxiety versus MDD suggests that attentional biases are associated with anxiety whereas emotional memory is negatively biased in MDD (Marchetti, Everaert et al. 2018). In their analysis they found that memory biases were reliably and strongly related to depression whereas attentional biases were not. They also found no overlap between attentional biases and memory biases. In contrast Mogg et al., (2005) reported attentional biases in generalised anxiety disorder but did not find similar deficits in MDD. Our animal studies have also shown that biases in reward learning are observed in animals where the depression-like phenotype has been induced using a variety of different methods including social and pharmacological. Further developments in both fields, and particularly studies where the methods used are more closely aligned, will help determine the validity of this approach.
2. Sensitivity to treatment
Acute effects of antidepressants in emotional processing tasks and the rodent ABT suggests that early positive biases in these tasks may predict longer term clinical outcomes. Of the pharmacological agents tested to date, evidence strongly supports specificity to antidepressant and pro-depressant effects. The effects of antidepressants in the JBT do not directly mirror the interpretation biases observed when acute antidepressants are tested in people, however, there are potential benefits from a preclinical drug development perspective. If the preliminary data obtained to date are replicated with other antidepressants, then this assay may provide a valuable tool for predicting the rate of onset of antidepressants. The current findings suggest that rapid onset antidepressants such as ketamine induce an immediate effect on decision-making behaviour whereas the effects of conventional antidepressants develop more slowly following chronic treatment.
Future directions
The similarities between the methods used to study affective biases in humans and animals has enabled researchers to more closely align the two fields and has also revealed the potential value of these behavioural deficits as a biomarker. Future validation of this approach would benefit from a wider evaluation of the cognitive domains which are modified by affective biases and how these differ between affective disorders such as anxiety and MDD, and other disorders such as schizophrenia. As non-human studies are limited in terms of using emotional stimuli, studies focusing more on tasks involving cognitive processes linked to associative learning between novel cues and positive or negatively valenced outcomes would be a priority. The complementarity between these animal and human methodologies will also make studies to investigate the relationship between affective biases and the aetiology of MDD feasible.
Acknowledgments
ESJR currently has research funding from the MRC, BBSRC, Wellcome Trust and academic research grants from Boehringer Ingelheim and Eli Lilly. She has also previously received research funding from MSD, Pfizer and GSK. Previous support which has contributed to the development of this work includes funding from RCUK and the British Pharmacological Society Integrative Pharmacology Fund. CS is funded by a Wellcome Trust doctoral training studentship. JKH is funded by a University of Bristol PhD Studentship.
References
- Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22(12):1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Anderson MH, Hardcastle C, Munafò MR, Robinson ES. Evaluation of a novel translational task for assessing emotional biases in different species. Cogn Affect Behav Neurosci. 2012;12(2):373–381. doi: 10.3758/s13415-011-0076-4. [DOI] [PubMed] [Google Scholar]
- Anderson MH, Munafo MR, Robinson ES. Investigating the psychopharmacology of cognitive affective bias in rats using an affective tone discrimination task. Psychopharmacology (Berl) 2013;226(3):601–613. doi: 10.1007/s00213-012-2932-5. [DOI] [PubMed] [Google Scholar]
- Aylward J, Hales C, Robinson E, Robinson OJ. Back-Translating A Rodent Measure Of Negative Bias Into Humans: The Impact Of Induced Anxiety And Unmedicated Mood And Anxiety Disorders. bioRxiv. 2017:143453. doi: 10.1017/S0033291718004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35(6):1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Depression : clinical, experimental, and theoretical aspects. Hoeber Medical Division; 1967. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio: 1996. pp. 12–15. [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13(6):303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Berton O, Hahn CG, Thase ME. Are we getting closer to valid translational models for major depression? Science. 2012;338(6103):75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R. Processing of Facial Emotion Expression in Major Depression: A Review. Australian New Zealand Journal of Psychiatry. 2010;44(8):681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Brittlebank AD, Scott J, Williams JM, Ferrier IN. Autobiographical memory in depression: state or trait marker? The British journal of psychiatry : the journal of mental science. 1993;162:118–121. doi: 10.1192/bjp.162.1.118. [DOI] [PubMed] [Google Scholar]
- Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;116(3):491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Memory biases in the anxiety disorders: current status. Clinical psychology review. 2002;22(4):587–627. doi: 10.1016/s0272-7358(01)00113-1. [DOI] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20(1):1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Barnes SA, Markou A, Pizzagalli DA. Translational Assessment of Reward and Motivational Deficits in Psychiatric Disorders. Curr Top Behav Neurosci. 2016;28:231–262. doi: 10.1007/7854_2015_5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Potter DN, Chartoff EH, Carlezon WA, Jr, Pizzagalli DA, Markou A. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology (Berl) 2017;234(9-10):1603–1614. doi: 10.1007/s00213-017-4584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27(9):859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-V. DSM-5 Task ForceDiagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; Washington, D.C: 2013. [Google Scholar]
- Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36(1):153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MAMA. Animal models of psychiatric disorders Psychopharmacology: the fourth generation of progress K D Bloom FE. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology. 2010;6(1):285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42(3):241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hales CA, Houghton CJ, Robinson ESJ. Behavioural and computational methods reveal differential effects for how delayed and rapid onset antidepressants effect decision making in rats. Eur Neuropsychopharmacol. 2017 doi: 10.1016/j.euroneuro.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CA, Robinson ES, Houghton CJ. Diffusion Modelling Reveals the Decision Making Processes Underlying Negative Judgement Bias in Rats. PLoS One. 2016;11(3):e0152592. doi: 10.1371/journal.pone.0152592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CA, Stuart SA, Anderson MH, Robinson ES. Modelling cognitive affective biases in major depressive disorder using rodents. Br J Pharmacol. 2014;171(20):4524–4538. doi: 10.1111/bph.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A RATING SCALE FOR DEPRESSION. J Neurol Neurosurg Psychiat. 1960;23 doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MAX. THE ASSESSMENT OF ANXIETY STATES BY RATING. British Journal of Medical Psychology. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Harding EJ, Paul ES, Mendl M. Animal behaviour: cognitive bias and affective state. Nature. 2004;427(6972):312. doi: 10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- Harmer C, Goodwin G, Cowen P. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195(2):102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4(5):409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of Acute Antidepressant Administration on Negative Affective Bias in Depressed Patients. American Journal of Psychiatry. 2009;166(10):1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe JK, Stuart SA, Mendl M, Robinson ESJ. Further validation of the affective bias test for predicting antidepressant and pro-depressant risk: effects of pharmacological and social manipulations in male and female rats. Psychopharmacology (Berl) 2017 doi: 10.1007/s00213-017-4687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(Suppl 6):4–6. [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jentsch MC, Van Buel EM, Bosker FJ, Gladkevich AV, Klein HC, Oude Voshaar RC, Ruhe EG, Eisel UL, Schoevers RA. Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark Med. 2015;9(3):277–297. doi: 10.2217/bmm.14.114. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Klein AM, de Voogd L, Wiers RW, Salemink E. Biases in attention and interpretation in adolescents with varying levels of anxiety and depression. Cognition and Emotion. 2017:1–9. doi: 10.1080/02699931.2017.1304359. [DOI] [PubMed] [Google Scholar]
- Lépine J-P, Briley M. The increasing burden of depression. Neuropsychiatric disease and treatment. 2011;7(Suppl 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Marchetti I, Everaert J, Dainer-Best J, Loeys T, Beevers CG, Koster EHW. Specificity and overlap of attention and memory biases in depression. Journal of Affective Disorders. 2018;225:404–412. doi: 10.1016/j.jad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive Vulnerability to Emotional Disorders. Annual Review of Clinical Psychology. 2005;1(1):167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review. 1992;12(2):227–255. [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry. 2012;72(7):588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26(2):117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional Bias in Generalized Anxiety Disorder Versus Depressive Disorder. Cognitive Therapy and Research. 2005;29(1):29–45. [Google Scholar]
- Murphy S, Norbury R, O’Sullivan U, Cowen P, Harmer C. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194(6):535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol. 2014;24(5):822–835. doi: 10.1016/j.euroneuro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J, Olivier JD, Mathe AA. Animal models of depression and anxiety: What do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(6):1357–1375. doi: 10.1016/j.pnpbp.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB. Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3–16. doi: 10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papciak J, Popik P, Fuchs E, Rygula R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav Brain Res. 2013;256:305–310. doi: 10.1016/j.bbr.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Paul ES, Harding EJ, Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci Biobehav Rev. 2005;29(3):469–491. doi: 10.1016/j.neubiorev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47(12):1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57(2-3):201–210. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(7):1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Refsgaard LK, Haubro K, Pickering DS, Stuart SA, Robinson ES, Andreasen JT. Effects of sertraline, duloxetine, vortioxetine, and idazoxan in the rat affective bias test. Psychopharmacology (Berl) 2016;233(21–22):3763–3770. doi: 10.1007/s00213-016-4407-6. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, Becker ES. A Comparison of Attentional Biases and Memory Biases in Women With Social Phobia and Major Depression. Journal of Abnormal Psychology. 2005;114(1):62–74. doi: 10.1037/0021-843X.114.1.62. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Roiser JP. Affective Biases in Humans and Animals. Curr Top Behav Neurosci. 2016;28:263–286. doi: 10.1007/7854_20105_5011. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Goldman D, Hodgkinson CA, Hasler G, Sahakian BJ, Drevets WC. Serotonin transporter genotype differentially modulates neural responses to emotional words following tryptophan depletion in patients recovered from depression and healthy volunteers. J Psychopharmacol. 2012;26(11):1434–1442. doi: 10.1177/0269881112442789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162(1):127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R, Papciak J, Popik P. The effects of acute pharmacological stimulation of the 5-HT, NA and DA systems on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Eur Neuropsychopharmacol. 2014;24(7):1103–1111. doi: 10.1016/j.euroneuro.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Rygula R, Szczech E, Papciak J, Nikiforuk A, Popik P. The effects of cocaine and mazindol on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Behav Brain Res. 2014;270:206–212. doi: 10.1016/j.bbr.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Sahin C, Doostdar N, Neill JC. Towards the development of improved tests for negative symptoms of schizophrenia in a validated animal model. Behav Brain Res. 2016;312:93–101. doi: 10.1016/j.bbr.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Salem T, Winer ES, Nadorff MR. Combined behavioural markers of cognitive biases are associated with anhedonia. Cognition and Emotion. 2017:1–9. doi: 10.1080/02699931.2017.1307808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Scinska A, Sienkiewicz-Jarosz H, Kuran W, Ryglewicz D, Rogowski A, Wrobel E, Korkosz A, Kukwa A, Kostowski W, Bienkowski P. Depressive symptoms and taste reactivity in humans. Physiol Behav. 2004;82(5):899–904. doi: 10.1016/j.physbeh.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. The ups and downs of modelling mood disorders in rodents. Ilar j. 2014;55(2):297–309. doi: 10.1093/ilar/ilu026. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology (Berl) 2017;234(9-10):1451–1465. doi: 10.1007/s00213-017-4552-6. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Cryan JF. Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 2007;190(4):555–568. doi: 10.1007/s00213-006-0630-x. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Young AH, Cleare AJ. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat. 2017;13:1245–1262. doi: 10.2147/NDT.S114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Munafo MR, Nutt DJ, Robinson ES. A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology. 2013;38(9):1625–1635. doi: 10.1038/npp.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Munafò MR, Nutt DJ, Robinson ES. Distinct Neuropsychological Mechanisms May Explain Delayed-Versus Rapid-Onset Antidepressant Efficacy. Neuropsychopharmacology. 2015:1–10. doi: 10.1038/npp.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Robinson ES. Animals models of risk factors for suicidal ideation and behaviour. Springer International Publishing; Switzerland: 2014. [Google Scholar]
- Stuart SA, Wood CM, Robinson ESJ. Using the affective bias test to predict drug-induced negative affect: implications for drug safety. Br J Pharmacol. 2017;174(19):3200–3210. doi: 10.1111/bph.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, Scinska A. Gustatory and olfactory function in patients with unipolar and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):827–834. doi: 10.1016/j.pnpbp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Thomsen KR. Measuring anhedonia: impaired ability to pursue, experience, and learn about reward. Front Psychol. 2015;6:1409. doi: 10.3389/fpsyg.2015.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. Journal of Affective Disorders. 2009;118(1-3):87–93. doi: 10.1016/j.jad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr Dir Psychol Sci. 2013;22(3):244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. Blood-based biomarkers in depression: emerging themes in clinical research. Mol Diagn Ther. 2014;18(5):469–482. doi: 10.1007/s40291-014-0108-1. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95(2):223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73(7):639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2010;30(7):839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychol Bull. 2007;133(1):122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Scott J. Autobiographical memory in depression. Psychological medicine. 1988;18(3):689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- Willner P. Animal models of depression: validity and applications. Adv Biochem Psychopharmacol. 1995;49:19–41. [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13(3):169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- World Health, O. The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. World Health Organization; 1992. [Google Scholar]
- Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev. 1991;15(3):391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]