Abstract
Background
Short-term weight loss may lead to remission of type 2 diabetes but the effect of maintained weight loss on cardiovascular disease (CVD) is unknown. We quantified the associations between changes in weight 5 years following diabetes diagnosis, and incident CVD events and mortality up to 10 years after diagnosis.
Methods
Observational analysis of the ADDITION-Europe trial of 2,730 adults with screen-detected type 2 diabetes from the UK, Denmark, and the Netherlands. We defined weight change based on the maintenance at 5-years of weight loss achieved in the year after diabetes diagnosis, and as 5-year overall change in weight. Incident CVD events (n=229) and all-cause mortality (n=225) from years 5-10 follow-up were ascertained from medical records.
Findings
Gaining >2% weight in the year after diabetes diagnosis was associated with higher hazard of all-cause mortality vs maintaining weight [Hazard Ratio(HR)(95%CI): 3.18(1.30-7.82)]. Losing ≥5% weight 1 year after diagnosis was also associated with mortality, whether or not weight loss was maintained at 5 years [2.47(0.99-6.21) and 2.72(1.17-6.30), respectively]. Losing ≥10% weight over 5 years was associated with mortality among those with BMI<30kg/m2 [4.62(1.87-11.42)]. Associations with CVD incidence were inconclusive.
Interpretation
Both weight loss and weight gain after screen-detected diabetes diagnosis were associated with higher mortality, but not CVD events, particularly among participants without obesity. The clinical implications of weight loss following diabetes diagnosis are likely to depend on its magnitude and timing, and may differ by BMI status. Personalisation of weight loss advice and support may be warranted.
Introduction
Weight management is recognized as critical in controlling cardio-metabolic risk factors, however there is little evidence about the long-term effects of weight loss on diabetes complications. Prospective observational studies have suggested that short-term weight loss may lead to remission of diabetes and reduction in 10-year incidence of CVD events among people with type 2 diabetes [1], particularly if weight loss occurs soon after diagnosis [2]. Weight loss occurring soon after diabetes diagnosis may be representative of intentional weight loss, motivated by the recent diagnosis. However, other research has shown null or inverse associations between weight loss and CVD among people with diabetes [3–5]. Long-term weight loss or weight loss later in the course of diabetes, especially among older adults, may not be protective against complications as this may be indicative of unintentional weight loss due to illness or frailty, which is more common among people with diabetes [6].
The effects of intentional weight loss achieved through behavioural intervention programmes on CVD remain unclear. The Action for Health in Diabetes (Look AHEAD) trial of an intensive lifestyle intervention among adults with obesity and type 2 diabetes was stopped after 9 years of follow-up as the intervention did not demonstrate a reduction in CVD events [7]. The lack of effect on long-term outcomes may be due to heterogeneity in intervention effects [8] or the fact that behaviour-based weight management programmes, while effective for short-term weight loss [9, 10], are typically followed by weight regain [11–13].
Despite weight regain, weight loss early in the course of the disease may still yield long-term reductions in cardio-metabolic risk [14, 15]. For example, in Look AHEAD, participants who had the largest 1-year weight loss but fully regained weight, had larger improvements in glycemic control at 4 years than participants with no initial weight loss [16], suggesting a potential legacy effect of large initial weight loss on glucose metabolism. However, the extent to which weight loss regain and weight loss maintenance are associated with incidence of cardiovascular disease (CVD) events has not been assessed. In light of recent results from the Diabetes Remission Clinical Trial (DiRECT) showing that substantial weight loss leads to sustained remission of type 2 diabetes [17], there is a need for evidence on the long-term effects of weight loss among people with diabetes.
We aimed to assess whether maintenance of weight loss following diabetes diagnosis by screening, and longer-term changes in weight, are associated with incidence of CVD events and all-cause mortality among participants in the Anglo–Danish–Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION-Europe).
Methods
This study includes adults with screen-detected type 2 diabetes from 343 general practices in Denmark, the Netherlands, Cambridge, UK, and Leicester, UK enrolled in the ADDITION-Europe trial. Details on the enrollment and data collection procedures have been reported previously [18] and additional information is available on the study website (http://www.addition.au.dk/). Adults aged 40-69 registered at participating general practices were invited to attend a stepwise diabetes screening programme from 2001-2006. Criteria for invitation to the screening programmes varied across the centres, as follows: a diabetes risk score based on medical records (Cambridge), self-administered questionnaires (Denmark and the Netherlands), or invitation to attend screening with no prior diabetes risk assessment (Leicester). During the screening programme, 3,057 people were diagnosed with type 2 diabetes based on World Health Organization criteria [19] and 3,055 of them consented to participate in the ADDITION-Europe trial [18]. Practices were cluster-randomised to either multifactorial treatment (n=167 practices) or routine care (n=176 practices) [18]. Briefly, physicians at the practices randomised to multifactorial treatment were invited to attend academic sessions where they were encouraged to consider prescribing medication for control of glycemia, blood pressure and blood lipids early on. The patients at these practices were also given educational materials on diabetes management. Physicians at practices in the routine care arm were encouraged to follow standard care procedures according to the national recommendations in each country [18]. As most study participants had overweight or obesity at the time of diabetes diagnosis, as part of standard care they would have routinely received weight management advice, however no specific behavioural interventions were administered as part of the trial. While treatment targets across the study centres were generally similar, we addressed potential confounding by differences in standard care across the centres by adjusting for study centre in all analyses. The present study is an observational analysis pooling participants from the two trial arms.
Weight and waist circumference were measured at the date of diabetes screening, 1 year later (at the UK and Netherlands centres), and at 5 years (at all study centres) by trained staff. Body weight was measured in light clothing, according to standard operating procedures as described previously [18]. Waist circumference was estimated as the average of two measurements taken with a tape measure halfway between the lowest point of the rib cage and the anterior superior iliac crests. During the baseline, 1 year and 5 year visits, participants completed questionnaires regarding demographics, lifestyle, and medication use, among other health-related factors. The EuroQol three-level index score (EQ-5D) which assesses health in the domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. EQ-5D was also administered at baseline. Incidence of CVD events and all-cause mortality was ascertained from national registers and medical records for the 10-year period following diabetes diagnosis.
Exposure definition
Change in weight between baseline, 1 year and 5 years was defined in two ways in order to separately assess maintenance of 1-year weight loss and overall 5-year weight change.
1-year weight change
At study centres in the UK and the Netherlands, changes in weight from baseline to 1 year were determined by subtracting the weight at baseline from the weight at 1 year. Among those with ≥5% weight loss at 1 year, maintenance of this weight loss at 5 years was defined by the amount of weight regained, where ≥50% regain at 5 years distinguished weight maintenance from weight regain. Categories of weight changes at 1 year were definedFfi as follows: >2% weight gain; ≤2% gain or <2% loss (no change); 2-5% loss; ≥5% loss with regain at 5 years; ≥5% loss with maintenance at 5 years.
5-year weight change
Overall 5-year weight change was determined by subtracting the weight measured at baseline from the weight measured at 5 years. Percentage weight change was then categorized as follows: >5% gain, >2% to ≤5% gain; ≤2% gain or <2%loss (maintained weight); ≥2% to <5% loss; ≥5% to <10% loss; ≥10% loss.
We also considered changes in waist circumference as a measure of central adiposity. Five-year changes in waist circumference were determined by subtracting waist circumference at baseline from waist circumference at 5 years. Change in waist circumference was categorized based on quintile cut-points of the distribution.
Outcome definition
The outcomes were a composite CVD endpoint and all-cause mortality occurring from years 5-10 of follow-up (mean duration of follow-up 5.0 years). The composite CVD endpoint includes nonfatal myocardial infarction, nonfatal stroke, fatal CVD events, non-traumatic amputation, peripheral vascular revascularization, and invasive cardiovascular revascularization. Information on outcome events was ascertained from national registers, medical records, electrocardiographs, hospital discharge summaries, and death certificates, among other sources, and each event was independently adjudicated [18].
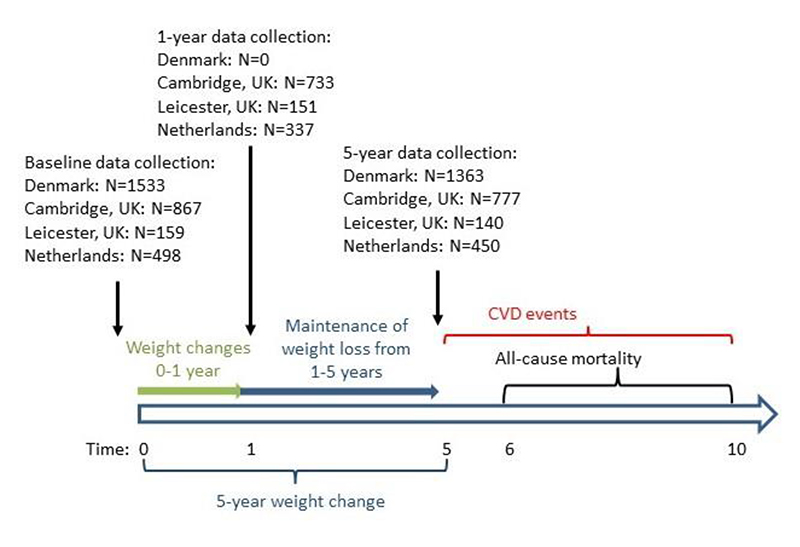
As weight change was assessed during the 5 years following diabetes diagnosis, the follow-up period for incident CVD events began after the 5-year study visit (Figure 1). For individuals with a CVD event occurring during the period when weight was measured (within the first 5 years after diabetes diagnosis), the first subsequent event occurring after the 5 year study visit was counted. Participants who did not attend a 5-year study visit (n=327) were excluded from the analyses.
Figure 1.
Timeline of assessment of weight at 0 (baseline), 1 and 5 years, and incident cardiovascular disease and all-cause mortality from 5-10 years. ADDITION-Europe 2002-2014. Among those with ≥5% weight loss at 1 year, maintenance of this weight loss at 5 years was defined by the amount of weight regained, where ≥50% regain at 5 years distinguished weight maintenance from weight regain. Five-year weight change was defined as the difference in weight at baseline (year 0) and 5 years.
Among the 2,730 people included in the study, there were 229 incident CVD events and 249 deaths during the 5-year follow-up period. Among the participants with incident CVD events, 44 reported having had a history of myocardial infarction or stroke prior to their diabetes diagnosis. We excluded 24 individuals who died within one year after weight change was assessed (i.e. in the 6th year after diabetes diagnosis) in order to reduce the chance that weight loss may be due to underlying disease. Therefore we included a total of 229 CVD events and 225 deaths.
Statistical analyses
We used Cox proportional hazards regression models to estimate associations between weight change categories and 5-year incidence of CVD and all-cause mortality. The time scale was time since the 5-year final study visit. In analyses in which mortality was the outcome of interest, time at risk began 6 years after diabetes diagnosis because deaths in the year following the 5-year study visit were not counted (Figure 1). Models were adjusted for trial arm, study centre, and confounders identified a priori using a causal diagram [20]: sex (male, female), age at diabetes diagnosis (continuous), weight at baseline (continuous), smoking status at baseline (current, former, never), CVD event within 5 years following diabetes diagnosis (yes, no), anti-hypertensive medication use prior to diabetes diagnosis (yes, no), lipid-lowering medication use prior to diabetes diagnosis (yes, no), changes in each of anti-hypertensive, lipid-lowering, and glucose-lowering medication use between baseline and 5 years (initiated medication use, stopped medication use, no change), and age left full-time education (<16 years, ≥16 years).We did not adjust for glucose-lowering medication use prior to baseline (the time of diabetes diagnosis) because few participants reported this. We accounted for clustering of individuals by general practice using a cluster robust variance estimator. We tested the proportional hazards assumption by plotting the survival function versus time, and by modelling an interaction term between the natural log of time and each covariate. The tests indicated no departures from proportional hazards. All analyses were performed using Stata version 15.1 (StataCorp, College Station, TX. 2019).
Models for 1-year weight change included participants only from study centres that measured weight at baseline, 1 year and 5 years (UK and Netherlands). Models for 5-year weight changes included participants from all centres.
Effect measure modification
We assessed effect measure modification by BMI category at the time of diabetes diagnosis. We modelled interaction terms between a binary variable for BMI status at baseline (≥30 kg/m2 vs <30 kg/m2) and weight change. Few participants had BMI <25 kg/m2 at baseline so we were not able to consider this group separately. There was no evidence of interaction with BMI for the associations of 1-year weight change and CVD or mortality, but there was interaction for the association of 5-year weight change and mortality (Wald test p-value 0.04). Therefore we present hazard ratios for 5-year weight change stratified by BMI. We also assessed modification by age at diagnosis (≥65 vs <65 years), by modelling an interaction term between a variable for age at baseline (≥65 vs <65 years) and weight change. There was no evidence of interaction with age in any models (Wald test p-values >0.05).
Sensitivity analyses
We separately excluded individuals with a self-reported history of myocardial infarction or stroke prior to diabetes diagnosis (n=243). We also performed separate analyses adjusting for HbA1c at baseline. As the Denmark centre was not included in the analyses of 1-year weight change and CVD, we performed analyses of 5-year weight change separately among the Danish participants (n= 1,363) and then among participants from the remaining study centres (n=1,367).
Results
Descriptive analyses
This study includes 2,730 participants followed for a mean of 5.0 years (SD= 1.1 years) for incident CVD events, accumulating a total of 13,549 person-years of follow-up. The mean change in weight from baseline to 1 year was -3.3 kg (SD= 6.4 kg) (UK and Netherlands centres) and the mean change from baseline to 5 years was -2.1 kg (SD= 7.4 kg) (all centres) (Table 1 and Figure 2). Overall 88.2% of participants who had their weight measured at baseline also had their weight measured at 5 years. People who had missing information on weight at 5 years were similar with respect to weight at baseline, age, sex, and CVD risk factors compared to participants not missing weight at 5 years; however, they were more likely to be current smokers at baseline (36.7% vs 25.6%) (data not shown). There were no substantial differences in weight change or CVD risk factors across the study centres and the analyses were based on data from all centres combined.
Table 1.
Descriptive characteristics at baseline (unless otherwise noted) by study centre. ADDITION-Europe 2002-2014
| Total (N=3057) | Denmark (N= 1533) | Cambridge (N=867) | Leicester (N=159) | Netherlands (N=498) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total | Mean | SD | n | Mean | SD | Mean | n | SD | n | Mean | SD | n | Mean | SD | |||||
| Age at diagnosis | 3057 | 60.2 | 6.9 | 1533 | 59.9 | 6.9 | 867 | 61.1 | 7.1 | 159 | 57.7 | 8.0 | 498 | 60.5 | 5.2 | |||||
| BMI (kg/m2) | 2957 | 31.6 | 5.6 | 1450 | 30.8 | 5.4 | 861 | 33.5 | 5.7 | 156 | 30.8 | 5.4 | 490 | 30.9 | 5.2 | |||||
| Weight (kg) | 2959 | 90.6 | 17.6 | 1450 | 89.2 | 17.6 | 863 | 94.4 | 17.8 | 156 | 85.8 | 17.1 | 490 | 89.5 | 16.1 | |||||
| Weight at 1yr (kg) | 1221 | 89.3 | 17.5 | -- | -- | -- | 733 | 91.2 | 17.7 | 151 | 84.6 | 19.0 | 337 | 87.4 | 15.7 | |||||
| Change weight 0-1yr | 1209 | -3.3 | 6.4 | -- | -- | -- | 730 | -3.6 | 5.5 | 148 | -2.2 | 5.9 | 331 | -3.1 | 8.2 | |||||
| Percent change in weight 0-1yr (%) | 1209 | -3.5 | 6.7 | -- | -- | -- | 730 | -3.7 | 5.7 | 148 | -2.7 | 6.0 | 331 | -3.2 | 8.7 | |||||
| Weight at 5yr (kg | 2683 | 88.8 | 18.0 | 1326 | 88.5 | 18.0 | 773 | 90.5 | 18.3 | 139 | 83.1 | 18.3 | 445 | 88.4 | 17.0 | |||||
| Change weight 0-5yr | 2611 | -2.1 | 7.4 | 1266 | -1.1 | 7.1 | 770 | -4.0 | 7.3 | 137 | -3.0 | 6.4 | 438 | -1.4 | 7.7 | |||||
| Percent change in weight 0-5yr (%) | 2611 | -2.2 | 7.8 | 1266 | -1.1 | 7.7 | 770 | -4.1 | 7.5 | 137 | -3.5 | 7.1 | 438 | -1.5 | 8.2 | |||||
| Alcohol (units/week) | 2675 | 9 | 12 | 1372 | 10 | 11 | 853 | 8 | 12 | 90 | 11 | 9 | 360 | 9 | 16 | |||||
| Systolic BP (mmHg) | 2963 | 149 | 22 | 1451 | 148 | 20 | 865 | 142 | 20 | 158 | 146 | 17 | 489 | 165 | 23 | |||||
| Diastolic BP (mmHg) | 2964 | 86 | 11 | 1451 | 88 | 11 | 865 | 82 | 10 | 158 | 89 | 10 | 490 | 89 | 11 | |||||
| Total cholesterol (mmol/1) | 2893 | 5.6 | 1.1 | 1410 | 5.6 | 1.1 | 848 | 5.4 | 1.1 | 159 | 5.6 | 1.2 | 476 | 5.6 | 1.1 | |||||
| LDL-cholesterol (mmol/l) | 2749 | 3.4 | 1.0 | 1324 | 3.4 | 1.0 | 817 | 3.3 | 1.0 | 142 | 3.5 | 1.0 | 466 | 3.7 | 1.0 | |||||
| 2730 | 5.0 | 1.1 | 1363 | 4.9 | 1.0 | 777 | 5.1 | 1.2 | 140 | 5.1 | 0.7 | 450 | 4.9 | 1.0 | ||||||
| n | Med. | Q1 | Q3 | n | Med. | Q1 | Q3 | n | Med | Q1 | Q3 | n | Med. | Q1 | Q3 | n | Med. | Q1 | Q3 | |
| HbA1c (%) | 2889 | 6.6 | 6.1 | 7.3 | 1477 | 6.4 | 6.0 | 7.0 | 846 | 6.8 | 6.4 | 7.6 | 159 | 6.8 | 6.4 | 7.6 | 407 | 6.8 | 6.3 | 7.6 |
| HbA1c (mmol/mol) | 2889 | 48.6 | 43.2 | 56.3 | 1477 | 46.4 | 42.1 | 53.0 | 846 | 50.8 | 45.4 | 60.7 | 159 | 50.8 | 46.4 | 59.6 | 407 | 50.8 | 45.4 | 59.6 |
| 2874 | 1.7 | 1.2 | 2.4 | 1391 | 1.6 | 1.1 | 2.3 | 847 | 1.8 | 1.1 | 2.5 | 159 | 1.8 | 1.1 | 2.5 | 477 | 1.6 | 1.2 | ||
| Total n | % | n | Total n | % | n | N | % | n | N | % | n | N | % | n | ||||||
| Sex | ||||||||||||||||||||
| Women | 3057 | 42 | 1286 | 1533 | 43 | 660 | 867 | 39 | 337 | 159 | 37 | 59 | 498 | 46 | 230 | |||||
| Smoking | ||||||||||||||||||||
| Current | 2996 | 27 | 819 | 1513 | 34 | 518 | 865 | 18 | 158 | 159 | 16 | 25 | 459 | 26 | 118 | |||||
| Former | 2996 | 40 | 1200 | 1513 | 37 | 554 | 865 | 44 | 383 | 159 | 27 | 43 | 459 | 48 | 220 | |||||
| Never | 2996 | 33 | 977 | 1513 | 29 | 441 | 865 | 37 | 324 | 159 | 57 | 91 | 459 | 26 | 121 | |||||
| Medication use prior to diabetes diagnosis | ||||||||||||||||||||
| Antihypertensive | 2949 | 45 | 1337 | 1466 | 43 | 634 | 865 | 58 | 499 | 159 | 42 | 67 | 459 | 30 | 137 | |||||
| Lipid-lowering | 2949 | 16 | 480 | 1466 | 13 | 185 | 865 | 24 | 204 | 159 | 21 | 34 | 459 | 12 | 57 | |||||
| Left full-time education ≤16 years old | 2154 | 42 | 899 | 1039 | 13 | 131 | 657 | 72 | 474 | 139 | 65 | 91 | 319 | 64 | 203 | |||||
| History of CVD prior to diabetes diagnosis | 2857 | 9 | 243 | 1460 | 8 | 112 | 855 | 12 | 99 | 104 | 7 | 7 | 438 | 6 | 25 | |||||
| Incident CVD events† | 2730 | 8 | 229 | 1363 | 8 | 114 | 777 | 10 | 75 | 140 | 6 | 8 | 450 | 13 | 32 | |||||
| Incident all-cause mortality‡ | 2706 | 8 | 225 | 1353 | 9 | 119 | 766 | 9 | 69 | 140 | 2 | 3 | 447 | 8 | 34 | |||||
Incident CVD events are defined as first event occurring during the follow-up period, beginning 5 years after diabetes diagnosis
Incident all-cause mortality events exclude events occurring in the first year of the follow-up period, therefore follow-up for all-cause mortality begins 6 years after diabetes diagnosis.
Figure 2.
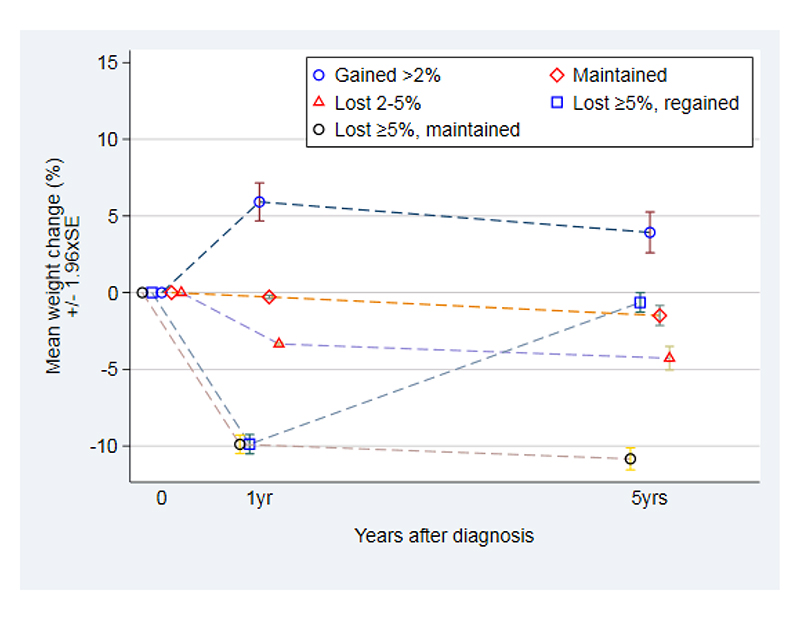
Patterns of percentage weight change over 5 years following diabetes diagnosis, by category of 1-year weight change. ADDITION-Europe 2002-2014.
At 1-year follow-up, weight was measured only among participants at UK and NL study centers.
Weight loss maintenance was determined among 1,209 participants from the UK and Netherlands study centres who had their weight measured at baseline, 1 year, and 5 years: among the 413 (34%) participants who lost ≥5% weight in the year following diabetes diagnosis, 165 (40%) regained >50% of the weight lost, and 248 (60%) maintained the weight loss. Baseline weight was similar among those who maintained, gained or lost weight. The participants who maintained weight loss at 5 years had similar average weight at 1 year and 5 years, however participants who regained their weight had an average weight at 5 years similar to their baseline weight (Table 2; Supplemental Figure S1).
Table 2.
Distributions of weight at baseline, 1 year, 5 years and 10 years in study, by category of weight loss maintenance. ADDITION-Europe
| 1-year weight change category: | Gained >2% at 1 yr | Maintained (gained≤2% or lost >2%) at 1 yr | Lost 2-5% at 1 yr | Lost >5% at 1 yr and regained† at 5 yr | Lost >5% at 1 yr maintained‡ at 5 yr | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=158 | n=358 | n=280 | n=165 | n=248 | ||||||||||||||
| Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | ||||
| BMI at baseline | 158 | 31.3 | 5.7 | 358 | 32.1 | 5.2 | 278 | 32.4 | 5.4 | 165 | 33.0 | 5.5 | 247 | 33.3 | 5.6 | |||
| Weight, kg | ||||||||||||||||||
| Baseline | 158 | 90.2 | 17.9 | 358 | 92.0 | 16.4 | 280 | 92.6 | 16.6 | 165 | 93.6 | 18.2 | 248 | 93.3 | 18.2 | |||
| 1 year | 158 | 95.4 | 19.6 | 358 | 91.8 | 16.3 | 280 | 89.5 | 16.1 | 165 | 84.3 | 16.6 | 248 | 84.0 | 16.1 | |||
| 5 years | 139 | 93.3 | 19.3 | 323 | 90.3 | 15.6 | 265 | 88.5 | 16.8 | 132 | 93.0 | 17.8 | 248 | 83.1 | 16.2 | |||
| 10 years | 102 | 89.7 | 18.6 | 250 | 88.0 | 16.4 | 216 | 87.4 | 16.3 | 109 | 89.0 | 16.9 | 186 | 83.3 | 16.9 | |||
| Weight change, % | ||||||||||||||||||
| 0-1 year | 158 | 5.9% | 7.9 | 358 | -0.3% | 1.1 | 280 | -3.3% | 0.8 | 165 | -9.9% | 4.1 | 248 | -9.9% | 4.9 | |||
| 0-5 years | 139 | 3.9% | 8.0 | 323 | -1.5% | 6.0 | 265 | -4.3% | 6.4 | 132 | -0.6% | 3.7 | 248 | -10.8% | 5.8 | |||
| 5-10 years | 99 | -3.8% | 7 | 242 | -2.7% | 8.1 | 210 | -2.6% | 9.0 | 101 | -2.8% | 6.3 | 186 | -0.3% | 9.7 | |||
| 5-year weight change category: | Gained >5% | Gained >2 to ≤5% | Maintained (gained ≤2% or lost <2%) | Lost ≥2% to <5% | Lost 5 to <10% | Lost ≥10% | ||||||||||||
| n=378 | N=324 | n=594 | n=462 | n=512 | n=341 | |||||||||||||
| Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | |
| BMI at baseline | 378 | 30.6 | 5.7 | 324 | 31.0 | 5.4 | 594 | 31.3 | 5.2 | 462 | 31.4 | 5.2 | 510 | 32.1 | 5.2 | 340 | 33.1 | 6.1 |
| Weight, kg | ||||||||||||||||||
| Baseline | 378 | 89.0 | 18.3 | 324 | 89.9 | 17.6 | 594 | 90.5 | 16.8 | 462 | 91.1 | 16.8 | 512 | 91.7 | 17.2 | 341 | 92.0 | 19.2 |
| 1 year | 114 | 91.7 | 17.0 | 110 | 90.5 | 17.0 | 224 | 90.4 | 16.1 | 224 | 90.3 | 16.9 | 246 | 86.9 | 16.1 | 189 | 85.2 | 17.8 |
| 5 years | 378 | 97.7 | 20.3 | 324 | 92.9 | 18.1 | 594 | 90.4 | 15.8 | 462 | 88.0 | 16.2 | 512 | 85.1 | 16.0 | 341 | 78.0 | 15.9 |
| 10 years | 233 | 94.7 | 20.5 | 200 | 90.5 | 19.0 | 392 | 88.5 | 15.9 | 323 | 87.1 | 16.5 | 358 | 84.3 | 16.1 | 218 | 80.7 | 16.6 |
| Weight change, % | ||||||||||||||||||
| 0-1 years | 114 | 2.2% | 6.0 | 110 | -1.3% | 4.7 | 224 | -2.5% | 4.2 | 224 | -3.3% | 4.5 | 246 | -5.3% | 4.8 | 189 | -7.7% | 9.9 |
| 0-5 years | 378 | 9.7% | 5.1 | 324 | 3.4% | 0.8 | 594 | -0.1% | 1.2 | 462 | -3.4% | 0.9 | 512 | -7.2% | 1.5 | 341 | -15.1% | 5.5 |
| 5-10 years | 233 | -2.8% | 7.2 | 200 | -3.3% | 6.5 | 392 | -2.8% | 6.9 | 323 | -2.5% | 6.9 | 358 | -1.4% | 7.0 | 218 | 1.4% | 10.6 |
Weight regain defined as regaining ≥50% of weight lost in the first year in study
Weight loss maintenance defined as regaining <50% of weight lost in the first year in study
5-year weight change was measured among 2,611 participants at all study centres. Among them, 341 (13%) had ≥10% weight loss, 512 (20%) had 5-10% weight loss, 462 (18%) had 2-5% weight loss, 594 (23%) maintained their weight, 324 (12%) gained 2-5% weight, and 378 (14%) gained >5% weight. Baseline weight was similar across the groups. Among participants who lost ≥10% weight at 5 years, average weight change in the year after diabetes diagnosis was -7.7% (UK and NL centres only), and average weight change at 5 years was -15.1%. Therefore, participants in this group had progressive weight loss across the study period (Table 2; Supplemental Figure S2). In contrast, among those who lost ≥5% weight in the year after diabetes diagnosis, there was no further weight loss from 1-5 years (Supplemental Figure S1).
Maintenance of weight loss following diabetes diagnosis and incidence of CVD and mortality
Compared with those who maintained their weight, those who lost ≥5% weight at one year, whether it was maintained or regained, had a similar lower hazard of CVD at 10 years, but the confidence intervals around these associations were wide and overlapped the null [HR (95%CI) ≥5% weight loss followed by regain: 0.62 (0.23, 1.62); ≥5% weight loss without regain: 0.65 (0.29, 1.46)]. Weight loss in the year after diabetes diagnosis, as well as weight gain >2%, was associated with higher hazard of all-cause mortality [HR (95%CI) for weight loss ≥5% with regain: 2.72 (1.17, 6.30); HR (95%CI) for weight gain >2%: 3.18 (1.30, 7.82)]. Weight loss ≥5% that was maintained was suggestively associated with all-cause mortality [HR (95%CI) 2.47 (0.99, 6.21)] (Table 3). Among participants without a history of CVD, the associations between weight changes, CVD and mortality were similar (Supplemental Table S1), where the association between weight loss ≥5% and mortality was stronger among those who regained weight compared to those who maintained their weight loss.
Table 3. Hazard ratios for the associations of 1-year and 5-year weight change following diabetes diagnosis and incident CVD and mortality over 5 years of follow-up.
| N Cases/Total | HR[95% CI] CVD† | N Cases/Total | HR[95% CI] Allcause mortality† | |
|---|---|---|---|---|
| 1-year weight change: UK and NL study centres (N=934) | ||||
| >2% weight gain | 8/106 | 0.86 [0.42, 1.76] | 10/105 | 3.18 [1.30, 7.82] |
| Maintained weight | 28/272 | 1 | 10/271 | 1 |
| 2-5% weight loss | 21/232 | 0.98 [0.50, 1.90] | 15/230 | 1.81 [0.84, 3.86] |
| Lost ≥5% and regained‡ | 7/115 | 0.62 [0.23, 1.62] | 11/115 | 2.72 [1.17, 6.30] |
| Lost ≥5% and maintained§ | 11/209 | 0.65 [0.29, 1.46] | 17/208 | 2.47 [0.99, 6.21] |
| 5-year weight change: All study centres (N=1,990) | ||||
| Full cohort | ||||
| Gained >5% | 16/276 | 0.92 [0.50, 1.70] | 21/274 | 1.27 [0.72, 2.22] |
| Gained 2-5% | 23/247 | 1.35 [0.81, 2.24] | 21/246 | 1.31 [0.79, 2.20] |
| Maintained | 33/471 | 1 | 30/469 | 1 |
| >2 to <5% weight loss | 34/361 | 1.44 [0.87, 2.39] | 26/360 | 1.12 [0.66, 1.92] |
| ≥5 to <10% weight loss | 28/393 | 1.05 [0.62, 1.80] | 22/392 | 0.85 [0.47, 1.54] |
| ≥10% weight loss | 20/242 | 1.50 [0.85, 2.66] | 27/239 | 2.04 [1.17, 3.55] |
| BMI ≥30 kg/m2 at diagnosis (n=1,222) | ||||
| Gained >5% | 7/140 | 0.87 [0.35, 2.15] | 12/139 | 1.42 [0.68, 2.93] |
| Gained 2-5% | 19/139 | 2.34 [1.27, 4.32] | 13/138 | 1.37 [0.70, 2.69] |
| Maintained | 18/277 | 1 | 19/276 | 1 |
| >2 to <5% weight loss | 21/216 | 1.63 [0.85, 3.13] | 18/215 | 1.22 [0.62, 2.40] |
| ≥5 to <10% weight loss | 19/273 | 1.10 [0.56, 2.15] | 15/273 | 0.73 [0.36, 1.46] |
| ≥10% weight loss | 14/179 | 1.49 [0.70, 3.14] | 15/176 | 1.27 [0.61, 2.63] |
| BMI <30 kg/m2 at diagnosis (n=766) | ||||
| Gained >5% | 9/127 | 0.87 [0.36, 2.08] | 9/135 | 1.05 [0.39, 2.80] |
| Gained 2-5% | 4/108 | 0.46 [0.16, 1.38] | 8/108 | 1.25 [0.46, 3.37] |
| Maintained | 15/194 | 1 | 11/193 | 1 |
| >2 to <5% weight loss | 13/145 | 1.24 [0.57, 2.70] | 8/145 | 0.95 [0.38, 2.34] |
| ≥5 to <10% weight loss | 9/122 | 1.05 [0.46, 2.42] | 7/121 | 1.10 [0.41, 2.96] |
| ≥10% weight loss | 6/63 | 1.63 [0.59, 4.53] | 12/63 | 4.62 [1.87, 11.42] |
95% CI: 95% confidence interval. CVD: Cardiovascular disease. HR: Hazard ratio.
Deaths occurred between 5 and 10 years in study
Models are adjusted for age, gender, baseline weight, education, smoking, trial group, study centre, baseline antihypertensive or lipid-lowering medication use, changes in medication use between baseline and 5 years, and having a CVD event within 5 years after diabetes diagnosis.
Regained ≥50% of weight lost in the first year after diabetes diagnosis
Maintenance of weight loss at 5 years classified as regaining <50% of initial loss
Sample sizes are where all covariates included in the model are nonmissing
5-year weight change and incidence of CVD and mortality
Large weight loss (≥10%) in the 5 years following diabetes diagnosis was associated with higher hazard of all-cause mortality [2.04 (1.17, 3.55)]. This association was modified by BMI at baseline. Stratifying by BMI at baseline, the association between large weight loss and mortality was strong among participants with BMI <30 kg/m2 [4.62 (1.87, 11.42)], while there was no association among participants with BMI ≥30 kg/m2 [1.27 (0.61, 2.63)]. Moderate weight loss (5-10%) was not associated with CVD or all-cause mortality, nor was weight gain (Table 3).
Results were similar among participants from Denmark vs the other study centres (Supplemental Table S2) and after adjusting for baseline HbA1c (Supplemental Table S3). There were no differences in cause death between categories of weight change (Supplemental Table S4).
5-year waist circumference change
There were no associations between decreases in waist circumference and incidence of CVD or all-cause mortality (Supplemental Table S5).
Discussion
In this observational study of long-term follow-up of participants in the ADDITION trial whose diabetes was diagnosed by screening, we have shown that hazard of all-cause mortality was three times higher in participants who gained 2% or more in weight between baseline and 1 year compared to those whose weight was stable. Hazard of mortality was similarly elevated among participants who lost more than 5% of weight initially but subsequently regained it. Large weight loss among those who did not have obesity at baseline was associated with a 4.6-fold higher mortality compared to the group who maintained weight, whereas there was no association among those who had obesity at baseline. This is the first research to suggest that weight loss may differently affect people with type 2 diabetes dependent on BMI.
Few studies have considered the associations between weight loss and long-term incidence of CVD and mortality among people with diabetes. Observational analyses in the Look AHEAD trial showed that participants who achieved ≥10% weight loss in 1 year had a 20% lower 10-year hazard of CVD compared to those who had stable weight or gained weight [1]. In the ADDITION-Cambridge study, more moderate 5% weight loss in the year after diabetes diagnosis was associated with similar reductions in CVD events [2]. No studies have considered how maintenance of this weight loss is associated with outcomes.
Our study did not find evidence of an association between maintenance of weight loss in the year after diabetes diagnosis and CVD; however, participants who lost weight had a higher hazard of all-cause mortality compared to those who maintained their weight. It is unclear whether maintenance of weight loss, or weight loss followed by regain, showed different associations with mortality as the hazard ratios were very similar, although the confidence interval on the estimate for maintained weight loss included the null. These findings are somewhat in contrast to results from the Look AHEAD trial that showed that people with type 2 diabetes who lost weight and then regained had improvements in cardiovascular risk factors compared to participants who had never lost weight [16]. However, a study in the Scottish Care Information Diabetes Collaboration showed that weight variability was associated with increased risk of mortality [21]. In the DiRECT trial, large weight loss was associated with remission of diabetes after 2 years [17], but the study has not yet reported follow-up for CVD events or mortality. In contrast to other studies, our study included people with a history of CVD. After excluding individuals with a history of CVD prior to diabetes diagnosis, we saw marginally stronger protective associations between maintenance of weight loss and CVD, but the adverse associations with mortality remained. We were unable to perform analyses of cause-specific mortality due to small numbers of events.
Loss of ≥10% weight over 5 years was associated with higher hazard of mortality. However, this association was not apparent when we considered changes in waist circumference as a measure of central adiposity (Supplemental Table S5). Unintentional weight loss due to underlying disease may have contributed to the observed associations. Information on intention to lose weight is not available in the ADDITION study, but among Cambridge participants only (N=867) we collected questionnaire data on health behaviours. We previously showed that reductions in alcohol use and total calorie intake were associated with lower 10-year hazard of CVD [22], but participants who made these changes did not necessarily lose weight. We did not count deaths in the year after weight change was assessed to reduce the chance of confounding by unintentional weight loss. Other research has estimated the potential impacts of confounding due to unintentional weight loss, and concluded that this bias may be small. In the Nurses’ Health Study there was no association between weight loss and mortality, and results were unchanged in a sensitivity analysis applying lag times to account for weight loss due to undiagnosed chronic disease or frailty [23]. However, other research has shown that weight loss among people with type 2 diabetes may increase risk of mortality if weight loss is unintentional [24], and frailty may be higher in the diabetes population [6].
Weight loss occurring in the year after diabetes diagnosis may be more representative of intentional weight loss, which may be motivated by the recent diabetes diagnosis and associated recommendations from practitioners. Participants in our study were diagnosed via screening and most had overweight or obesity. Consequently, they may have been more motivated by clinician advice to lose weight compared to other cohorts of patients that had a longer duration of diabetes. Among participants from the Cambridge centre, where data are available on changes in diet and physical activity following diabetes diagnosis, over 90% of participants reported making changes to their lifestyle that would facilitate weight loss. However, we have no information concerning whether weight losses were intentional or not. We explored the causes of death by category of weight loss, but there was no difference in cause of death by weight loss group (Supplemental Table S4). Other research on weight loss following bariatric surgery has shown that large weight loss reduces risk of CVD and mortality [25, 26]. However, metabolic changes following bariatric surgery may play a role in these associations, independent of weight loss [27]. It is possible that large weight loss is beneficial for some people with diabetes but not for others. In post hoc analyses in the Look AHEAD trial, the intensive lifestyle intervention helped some patients to reduce their risk of CVD while patients with well-controlled diabetes but poor self-rated health were harmed [8, 28]. While we did not see any clear differences in baseline cardiovascular risk factors between the weight change groups, there were small differences in EQ-5D scores by category of weight change, where participants who lost ≥10% weight over 5 years had a lower baseline median EQ-5D compared to the other groups (Supplemental Table S6). Participants who lost the most weight may have had poorer underlying health and therefore weight loss may be more likely to have been unintentional in this group.
In our study we also observed heterogeneity, where large weight loss was strongly associated with mortality among adults who had BMI <30 kg/m2 and there was no apparent harm, or benefit, among participants with BMI ≥30 kg/m2. There were little baseline differences in cardiovascular risk factors between the obese vs non-obese groups, so it is unlikely that the disparities in outcomes are purely driven by underlying health differences (Supplemental Table S7). Furthermore, baseline EQ-5D scores were higher among participants without obesity compared to participants with obesity, suggesting higher health-related quality of life among participants without obesity. This information does not support that heterogeneity in the effects of weight loss across BMI groups would be due to poorer health among participants with lower baseline BMI. Furthermore, moderate 5-10% weight loss over 5 years was not associated with CVD or mortality. Results suggest that the amount of weight loss, as well as the timing of weight loss in the course of the disease, may differentially affect risk of CVD and mortality. The observed heterogeneity by BMI shows that among individuals who are overweight rather than obese, weight loss may be associated with higher hazard of mortality. However, we note that the estimated hazard ratios had low precision, reflected by wide confidence intervals, and therefore should be interpreted with caution.
There are several limitations that may affect the inferences from this study. As in any observational study of weight change, we cannot distinguish between intentional and unintentional weight loss. However, among people with diabetes, intentional weight loss may be most common during the period following diabetes diagnosis [24]. Furthermore, we do not know in which component (bone, fat, muscle) the weight loss is occurring when we consider weight change. Loss of muscle mass and bone mass are components of frailty, and people with diabetes experience more frailty with ageing compared to the general population [6]. Our finding of less marked effects in analyses focused on waist circumference compared to weight support that weight loss in the cohort may have occurred in non-fat components. If the primary compartments of weight loss differed between the obese and non-obese participants, this may have contributed to the observed heterogeneity in associations by BMI. While we saw heterogeneity in associations by baseline BMI, differences in health across BMI groups may influence these results. BMI was part of the diabetes risk assessment used to identify individuals for screening in the Netherlands, Denmark and Cambridge centres. Therefore, study participants with lower BMI would have had other indicative risk factors, such as family history of diabetes or older age in order to be selected. However, as noted above, we did not observe differences in blood pressure, lipid levels, or HbA1c between the BMI groups and EQ-5D was somewhat higher in the non-obese groups. At baseline, many participants reported antihypertensive medication use, and smoking was somewhat common in the cohort (27% current and 33% former smokers). The strong effects of smoking and hypertension on CVD incidence may have reduced our ability to detect an effect of weight change on CVD; however, we adjusted for smoking status and medication use in all analyses. Weight at 1 year was not measured among participants in Denmark, which substantially reduced the number of participants included in our analyses of weight loss maintenance. For this reason, we discuss our results in light of not only the magnitude of associations but precision of the estimates, reflected in the confidence intervals. Results may have been sensitive to our definition of weight regain as 50% regain of weight lost in the year after diabetes diagnosis. However, our results were unchanged when using an alternative 25% weight regain definition which has been used previously (data not shown) [29]. Although the study had a relatively small number of events resulting in wide confidence intervals around the estimated hazard ratios, results are hypothesis generating for future studies to address this question among larger cohorts with longer follow-up and among trials of weight loss interventions.
The ADDITION-Europe trial was a pragmatic population-based cardiovascular outcomes trial in which people with diabetes were diagnosed by screening [30]. There were high rates of attendance at screening and high rates of enrollment, and therefore the study sample is likely representative of the underlying target population of adults with newly diagnosed type 2 diabetes from the regions included in the study. However, the majority of the study population was white European with overweight or obesity, and results may not be generalizable to other groups. This is the first study to assess maintenance of weight loss following diabetes diagnosis and CVD events and mortality. Since the study featured multiple measurements of weight following diabetes diagnosis, we were able to assess weight changes early in the course of the disease. Furthermore, since participants were diagnosed by screening, they were earlier in the diabetes progression trajectory than would be if they had received a clinical diagnosis. The study also features near complete ascertainment of CVD outcomes and mortality due to linkage with national registers, and all outcomes were independently adjudicated.
In summary, losing weight in the year after diabetes diagnosis may be associated with a lower hazard of CVD events, but a higher hazard of all-cause mortality, regardless of whether or not weight loss was maintained. One-year weight gain was associated with all-cause mortality. Large weight loss over 5 years was also associated with mortality, except among participants with BMI ≥30 kg/m2 at diagnosis. In contrast, moderate (5-10%) 5-year weight loss was not associated with mortality. The results highlight the heterogeneity of the effects of weight loss on long-term outcomes among people with type 2 diabetes. While future studies in larger populations should address this question more definitively, this study raises questions as to whether the current one-size-fits-all approach to weight loss is appropriate for all people with type 2 diabetes, particularly among those who do not have obesity at the time of diagnosis. Though recent results from the DiRECT trial showed that large weight loss can lead to diabetes remission [17], there is little evidence supporting long-term cardiovascular benefits of weight loss among people with diabetes. Our results highlight the need for larger studies of weight loss interventions with longer follow-up periods to investigate the varying impact of different magnitudes of weight loss occurring earlier as opposed to later in the course of the disease, and effects on CVD events and mortality. The findings from this study emphasize the relevance of heterogeneity in the health effects of weight loss among people with type 2 diabetes. Further research is warranted to improve understanding of this heterogeneity to inform more personalized strategies and recommendations.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contribution of all participants, practice nurses and general practitioners in the ADDITION-Europe study. We are grateful to the independent endpoint committee in Denmark (Professors Kristian Thygesen, Hans Ibsen, Ole Færgeman and Birger Thorsteinsson), the United Kingdom (Professor Jane Armitage and Dr Louise Bowman) and the Netherlands (Professors Cees Tack and Jaap Kappelle), and the Independent Data Monitoring and Safety Committee (Christian Gluud, Per Winkel and Jørn Wetterslev). We thank Daniel Barnes for contributions to data cleaning.
We are grateful to the ADDITION-Cambridge independent trial steering committee (Nigel Stott (Chair), John Weinman, Richard Himsworth, and Paul Little). Aside from the authors, the ADDITION-Cambridge study team has included Amanda Adler, Judith Argles, Gisela Baker, Rebecca Bale, Roslyn Barling, Daniel Barnes, Mark Betts, Sue Boase, Clare Boothby, Ryan Butler, Parinya Chamnan, Kit Coutts, Sean Dinneen, Pesheya Doubleday, Mark Evans, Tom Fanshawe, Francis Finucane, Philippa Gash, Julie Grant, Wendy Hardeman, Robert Henderson, Garry King, Ann-Louise Kinmonth, Joanna Mitchell, Richard Parker, Nicola Popplewell, A. Toby Prevost, Richard Salisbury, Lincoln Sargeant, Rebecca Simmons, Megan Smith, Stephen Sutton, Fiona Whittle and Kate Williams. We thank the following groups within the MRC Epidemiology Unit: data management (Clare Boothby and Adam Dickinson), information technology (Iain Morrison and Rich Hutchinson), technical (Matt Sims) and field epidemiology (James Sylvester, Gwen Brierley, Richard Salisbury and Kit Coutts).
Funding
This research was funded by the Medical Research Council. ADDITION-Denmark was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and South Jutland; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Center for Evaluation and Health Technology Assessment; the diabetes fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. Parts of the grants from the Novo Nordisk Foundation and the Danish Council for Strategic Research were transferred to the other centres. ADDITION-Cambridge was supported by the Wellcome Trust (grant reference no: G061895), the Medical Research Council (grant reference no: G0001164), the National Institute for Health Research (NIHR) Health Technology Assessment Programme (grant reference no: 08/116/300), and National Health Service research and development support funding (including the Primary Care Research and Diabetes Research Networks), and the NIHR under its Programme Grants for Applied Research scheme (RP-PG-0606-1259 to M.J.D. and K.K.). Bio-Rad provided ADDITION-Cambridge with the equipment for HbA1c testing during the screening phase. ADDITION-Netherlands was supported by the Julius Centre for Health Sciences and Primary Care, University Medical Centre, Utrecht. ADDITION-Leicester was supported by the Department of Health and ad hoc Support Sciences, the NIHR Health Technology Assessment Programme (grant reference no: 08/116/300), and National Health Service research and development support funding (including the Primary Care Research and Diabetes Research Networks Leicestershire, NIHR CLAHRC-East Midlands, ARC-East Midlands and the Leicester Lifestyle BRC).
The funding sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Conflicts of Interest
The authors declare that they have no relevant conflicts of interest to disclose.
Author’s Contributions
JS designed the study and the analysis plan, analyzed the data, and wrote the manuscript. SJS and SJG also contributed to the design of the study and the analysis plan. SJS, KK, RCV, GEHMR, DRW, AS, DRW, NJW, and SJG contributed to the interpretation of the results and edited the manuscript.
Simon J Griffin is the guarantor of this work.
References
- 1.Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. The lancet Diabetes & endocrinology. 2016;4(11):913–21. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strelitz J, Ahern AL, Long GH, Hare MJL, Irving G, Boothby CE, et al. Moderate weight change following diabetes diagnosis and 10 year incidence of cardiovascular disease and mortality. Diabetologia. 2019;62(8):1391–402. doi: 10.1007/s00125-019-4886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koster-Rasmussen R, Simonsen MK, Siersma V, Henriksen JE, Heitmann BL, de Fine Olivarius N. Intentional Weight Loss and Longevity in Overweight Patients with Type 2 Diabetes: A Population-Based Cohort Study. PloS one. 2016;11(1):e0146889. doi: 10.1371/journal.pone.0146889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodegard J, Sundstrom J, Svennblad B, Ostgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes & metabolism. 2013;39(4):306–13. doi: 10.1016/j.diabet.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Xing Z, Pei J, Huang J, Peng X, Chen P, Hu X, et al. Weight change is associated with increased all-cause mortality and non-cardiac mortality among patients with type 2 diabetes mellitus. Endocrine. 2019;64(1):82–9. doi: 10.1007/s12020-019-01892-2. [DOI] [PubMed] [Google Scholar]
- 6.Aguayo GA, Hulman A, Vaillant MT, Donneau AF, Schritz A, Stranges S, et al. Prospective Association Among Diabetes Diagnosis, HbA1c, Glycemia, and Frailty Trajectories in an Elderly Population. Diabetes care. 2019;42(10):1903–11. doi: 10.2337/dc19-0497. [DOI] [PubMed] [Google Scholar]
- 7.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries TI, Dorresteijn JAN, van der Graaf Y, Visseren FLJ, Westerink J. Heterogeneity of Treatment Effects From an Intensive Lifestyle Weight Loss Intervention on Cardiovascular Events in Patients With Type 2 Diabetes: Data From the Look AHEAD Trial. Diabetes care. 2019;42(10):1988–94. doi: 10.2337/dc19-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JCG, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet. 2017;389(10085):2214–25. doi: 10.1016/S0140-6736(17)30647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the look ahead trial. Archives of internal medicine. 2010;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neiberg RH, Wing RR, Bray GA, Reboussin DM, Rickman AD, Johnson KC, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring, Md) 2012;20(10):2048–56. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama. 2018;320(11):1172–91. doi: 10.1001/jama.2018.7777. [DOI] [PubMed] [Google Scholar]
- 13.Hensrud DD. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes. Obes Res. 2001;9(Suppl 4):348s–53s. doi: 10.1038/oby.2001.141. [DOI] [PubMed] [Google Scholar]
- 14.Hamdy O, Mottalib A, Morsi A, El-Sayed N, Goebel-Fabbri A, Arathuzik G, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ open diabetes research & care. 2017;5(1):e000259. doi: 10.1136/bmjdrc-2016-000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldstein AC, Nichols GA, Smith DH, Stevens VJ, Bachman K, Rosales AG, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes care. 2008;31(10):1960–5. doi: 10.2337/dc08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Espeland MA, Clark JM, Hazuda HP, Knowler WC, Pownall HJ, et al. Association of Weight Loss Maintenance and Weight Regain on 4-Year Changes in CVD Risk Factors: the Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes care. 2016;39(8):1345–55. doi: 10.2337/dc16-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. The lancet Diabetes & endocrinology. 2019;7(5):344–55. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 18.Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–67. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine : a journal of the British Diabetic Association. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999;10(1):37–48. [PubMed] [Google Scholar]
- 21.Aucott LS, Philip S, Avenell A, Afolabi E, Sattar N, Wild S. Patterns of weight change after the diagnosis of type 2 diabetes in Scotland and their relationship with glycaemic control, mortality and cardiovascular outcomes: a retrospective cohort study. BMJ open. 2016;6(7):e010836. doi: 10.1136/bmjopen-2015-010836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strelitz J, Ahern AL, Long GH, Boothby CE, Wareham NJ, Griffin SJ. Changes in behaviors after diagnosis of type 2 diabetes and 10-year incidence of cardiovascular disease and mortality. Cardiovascular diabetology. 2019;18(1):98. doi: 10.1186/s12933-019-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danaei G, Robins JM, Young JG, Hu FB, Manson JE, Hernan MA. Weight Loss and Coronary Heart Disease: Sensitivity Analysis for Unmeasured Confounding by Undiagnosed Disease. Epidemiology (Cambridge, Mass) 2016;27(2):302–10. doi: 10.1097/EDE.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Trying to lose weight, losing weight, and 9-year mortality in overweight U.S. adults with diabetes. Diabetes care. 2004;27(3):657–62. doi: 10.2337/diacare.27.3.657. [DOI] [PubMed] [Google Scholar]
- 25.Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care. PLoS medicine. 2015;12(12):e1001925. doi: 10.1371/journal.pmed.1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliasson B, Liakopoulos V, Franzen S, Naslund I, Svensson AM, Ottosson J, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. The lancet Diabetes & endocrinology. 2015;3(11):847–54. doi: 10.1016/S2213-8587(15)00334-4. [DOI] [PubMed] [Google Scholar]
- 27.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. The lancet Diabetes & endocrinology. 2014;2(2):152–64. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- 28.Baum A, Scarpa J, Bruzelius E, Tamler R, Basu S, Faghmous J. Targeting weight loss interventions to reduce cardiovascular complications of type 2 diabetes: a machine learning-based post-hoc analysis of heterogeneous treatment effects in the Look AHEAD trial. The lancet Diabetes & endocrinology. 2017;5(10):808–15. doi: 10.1016/S2213-8587(17)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger SE, Huggins GS, McCaffery JM, Lichtenstein AH. Comparison among criteria to define successful weight-loss maintainers and regainers in the Action for Health in Diabetes (Look AHEAD) and Diabetes Prevention Program trials. The American journal of clinical nutrition. 2017;106(6):1337–46. doi: 10.3945/ajcn.117.157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons RK, Borch-Johnsen K, Lauritzen T, Rutten GE, Sandbaek A, van den Donk M, et al. A randomised trial of the effect and cost-effectiveness of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with screen-detected type 2 diabetes: the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION-Europe) study. Health technology assessment (Winchester, England) 2016;20(64):1–86. doi: 10.3310/hta20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.