Abstract
The genetic and phenotypic variability of life history traits determines the demographic attributes of tree populations and, thus, their responses to anthropogenic climate change. Growth- and survival-related traits have been widely studied in forest ecology, but little is known about the determinism of reproductive traits.
Using an elevation gradient experiment in the Pyrenees we assessed the degree to which variations in reproductive effort along climatic gradients are environmentally or genetically driven, by comparing oak populations (Quercus petraea) growing under field and common garden conditions.
In situ monitoring revealed a decline in reproductive effort with increasing elevation and decreasing temperature. In common garden conditions, significant genetic differentiation was observed between provenances for reproduction and growth: trees from cold environments (high elevations) grew more slowly, and produced larger acorns in larger numbers. Our observations show that genetic and phenotypic clines for reproductive traits have opposite signs (counter-gradient) along the environmental gradient as opposed to growth, for which genetic variation parallels phenotypic variation (co-gradient).
The counter-gradient found here for reproductive effort reveals that genetic variation partly counteracts the phenotypic effect of temperature, moderating the change in reproductive effort according to temperature. We consider the possible contribution to this counter-gradient in reproductive effort as an evolutionary trade-off between reproduction and growth.
Keywords: Counter-gradient, Elevation gradient, Local adaptation, Quercus petraea, Reproduction
Introduction
Studies of the responses of life history traits to environmental changes have repeatedly demonstrated the important contribution of such traits to the current and future geographic distribution of multiple tree species over local and large geographic scales (Aitken et al., 2008; Benito-Garzón et al., 2013; Reich and Oleksyn, 2008). In ecology, assessments of life history trait variations provide vital information about the fitness of an individual, its ability to survive, grow and reproduce in an environment. At landscape level, the phenotypic variation of such traits in response to environmental changes depends on (1) genetic variation within and between populations and (2) phenotypic plasticity (i.e. the ability of an individual to produce different phenotypes in response to different environmental conditions) of each individual (Aitken et al., 2008; Nicotra et al., 2010). High levels of genetic diversity and phenotypic plasticity favor local adaptation to environmental changes (Kremer et al., 2014; Lindner et al., 2010). Investigations of the environmental and genetic determinism of life history traits are therefore essential, to predict the process of population adaptation and, indirectly, to predict species distributions in response to environmental changes (Chevin et al., 2010).
Common garden experiments are widely used in ecology, to assess local adaptation and to account for the contributions of genetic and environmental sources of phenotypic variation to the response to environmental changes (de Villemereuil et al., 2016; Linhart and Grant, 1996). Common garden experiments have revealed genetic clines for phenotypic life history traits, such as phenology, seedling regeneration, survival and growth along gradients of latitude (Hall et al., 2007; Kawakami et al., 2011; Ducousso et al., 1996) and elevation (Alberto et al., 2013; Firmat et al. 2017; Sáenz-Romero et al., 2006; Vitasse et al., 2009a). Life history traits are closely related to tree fitness, so their variation in common gardens may be seen as signatures of adaptation to local environmental pressures. The genetic clines observed in common gardens are, to a lesser extent, reflected in the phenotypic clines observed in situ, indicating that the adaptive response can be enhanced by plasticity. However, in rare cases, plasticity may constrain local adaptation, as phenotypic variation along an environmental gradient may act in the opposite direction to the genetic variation along the same gradient, leading to a so-called “counter-gradient” (Kremer et al., 2014). Counter-gradient variation may be interpreted as a genetic evolution to compensate to significant environmental pressure that negatively impacts individual fitness (Conover et al., 2009; Grether, 2005).
In plants, examples of counter-gradients are scarce and most of them have been observed for phenology-related rather than morphological traits (Radersma et al., 2020). This is the case in beech (Fagus sylvatica) where in the Pyrenees, along an elevation gradient, leaf unfold earlier at low than at high elevation while in common garden experiments, population from high elevation are unfolding earlier (Vitasse et al., 2009a). At high elevation, beech populations compensate for low fitness by increasing growing season length instead of providing a safety margin against late spring frost. However, counter-gradient variation of phenology related traits is not consistent across species: in the same experimental design, oak (Quercus petraea) and ash (Fraxinus excelsior) display on the contrary co-gradient patterns.
In forest tree species, phenotypic variation in response to selective pressure has been widely studied for growth and survival, two of the main components of tree fitness (Conkle, 1973; Namkoong and Conkle, 1976). However, only a few studies (Caignard et al. 2019, Alexandre et al., 2020) have considered the extent to which variations of reproduction, a key component of plant fitness, are driven by environmental and/or genetic influences. Reproduction related traits, such as seed production or age at first reproduction have a significant importance for forest persistence, regeneration and migration (Aitken et al, 2008), an investment to reproduction at a younger age maximise for example plant fitness (Kawecki 1993, Wenk and Falster, 2015). Thus, the lack of knowledge in the variability of reproduction related traits, represents a limit nowadays in our global understanding of tree population dynamics in response to environmental changes (Kunstler et al. 2021).
Reproduction has been shown to respond to environmental variation. Changes in temperature affect the timing of flowering (Fitter and Fitter, 2002; Sparks et al., 2000) and, to a lesser extent, the timing of fruiting (Lechowicz, 1995). Furthermore, in oak species, combinations of temperature variation and drought may have either a negative (Pérez-Ramos et al., 2010; Sanchez-Humanes and Espelta, 2011) or a positive effect on seed production (Caignard et al., 2017). Due to the difficulties of measuring reproduction in long-lived species, very few studies have investigated the genetic differentiation of reproduction between populations. Santos-del-Blanco et al. (2012) reported genetic variation for size at first reproduction between populations of Pinus pinaster. Zhuk and Goroshkevich (2018) compared reproduction and growth in Pinus sibirica populations spread along a latitudinal gradient. Few studies have been performed in grassland ecosystems, and those published have reported conflicting results: no local adaptation was observed in three species in the Alps (Frei et al. 2014), whereas significant genetic differentiation for reproductive investment was found in Poa alpina (Hautier et al. 2009, Fischer et al. 2011), Arabis alpina (de Villemereuil et al. 2018) and Festuca eskia (Gonzalo-Turpin and Hazard 2009).
We quantified the extent to which differences in reproductive effort were related to environmental and genetic variations in a temperate European white oak (Quercus petraea), through long-term in situ monitoring along an elevation gradient. We compared seed production in trees growing in situ and under common garden conditions in Southern France over successive years, to determine whether genetic variation paralleled or counteracted in situ phenotypic variation. One of our main objectives was to estimate the genetic determinism of the variation of seed production, seed size and the proportion of trees producing seeds as a proxy of age at first reproduction. We also explored whether the differentiation of reproductive traits between populations was due to divergent selection. Finally, we investigated whether the evolutionary patterns observed for reproductive traits mimicked those of other fitness-related traits, such as growth.
Materials & Methods
Experimental design
Since 2005, we have made systematic recurrent observations of life history traits in Q. petraea populations located along an elevation gradient in the French Pyrenees (in situ - IS), to monitor tree responses to temperature variations (see Dantec et al., 2015, 2014; Vitasse et al., 2009b). The temperature changes markedly over very short distances in such gradients, decreasing by 0.5 - 0.6°C with every 100 m increase in elevation (Laiolo and Obeso, 2017; Vitasse et al., 2009b). The elevation gradient was replicated in two parallel valleys: Ossau (latitude 42.88 to 43.22; longitude -0.73 to -0.40) and Gaves (latitude: 42.78 to 43.75; longitude: -0.41 to 0.21), in which we sampled 10 (five per valley) natural mature populations of Q. petraea for monitoring. These populations are located at various elevations, from 131 to 1630 m asl (Table S1, see also (Vitasse et al., 2009b)).
In 2006, acorns from 152 adult trees were collected from the 10 natural populations along the elevation gradient. These acorns were sown and grown at the INRAE nursery in Pierroton (44° 44°N, 00°46W), and, in 2008, the saplings were transplanted to a common garden located at the INRAE research station in Toulenne (lowland common garden, 9 m asl; 44°34’N, 00°16’W). Mean annual temperature in this common garden from 2014 to 2019 was 13.64°C, and mean total annual precipitation was 792.3 mm. In total, 3448 seedlings from 150 families were planted in a complete randomized block design with five blocks. Trees were planted 1.5 m apart, in rows 3.5 m apart (Alberto et al., 2011). In 2016, the experiment included a total of 1435 surviving trees from 131 families, of which 296 trees from 71 families produced at least one acorn during the three years of monitoring (Table S1 and Fig. S1). The trees began to reproduce in 2014 and were 10 years old in 2016.
We assessed the impact of elevation on growth, in existing reciprocal transplant experiments (RTEs), in which three populations from the same elevation gradient, originating from sites 427, 803 and 1235 m above sea level in the Gaves valley, were transplanted to five planting sites at different elevations (131, 488, 833, 1190 and 1533) in the same valley (Vitasse et al., 2010). The trees from these RTEs were 12 or 13 years old in 2017. We monitored a mean of 30.4 ± 7.2 individuals per site (Table S2).
Trait measurements
Seed production
We monitored the reproductive traits of trees along the elevation gradient in situ (IS) and in the common garden (CG) over six years (from 2014 to 2019). In situ, large nets were attached 1 m above the ground under the entire tree canopy in August, to catch the acorns, which start to fall in September. In total, 25 adult trees were monitored in 2014, and from 2015 to 2019, 30 trees were monitored each year (Table S1). The trees had a mean height of 19.2 ± 9.4 (SD) meters and a mean diameter of 377 ± 196 mm. Acorn predation was minimized by harvesting the litter (organic components falling from the trees: leaves, branches, fruits) from the nets every two weeks from mid-September until the beginning of December. The litter was then sorted in the laboratory.
In the common garden, acorn fall from each tree was monitored weekly over the same period as for IS observations. Acorn fall was facilitated by gently shaking the tree, and acorns were harvested directly from the ground. For both designs, we assessed the total number of acorns produced, total dry mass and mean acorn mass (in grams) per tree and per year.
Growth traits
Height and diameter were measured in the RTEs in 2011 and 2017, and in the common garden of Toulenne (CG) every year from 2014 to 2019. For both designs, we estimated the annual height and diameter increments, between 2011 and 2017 for each tree of the RTEs, and between 2006 and 2019 for each tree of the common garden. We also estimated the projected area of the canopy on the ground for each tree in situ. For the adult trees in situ, we first determined the point at the center of the canopy (O) and measured the distances from O to the outer limit of the tree canopy (Bi) at eight points 45° apart (OB1-8). The projected canopy area was calculated as the sum of the areas of the eight sections . The projected surface area was then used to normalize acorn production per m2 and to estimate seed production per square meter in situ. In the common garden, the diameter and surface area of the canopy were estimated in 2017 for 299 trees. Based on the diameter obtained during the last year of measurement (2019) we estimated the projected canopy area for every tree of this same year by modeling the relationship between diameter and surface area in 2017 with a power model. We used the estimated area (m2) for 2019 to standardize seed production between trees.
Climatic data
Air temperature and relative humidity were recorded hourly, for each population of the elevation gradient (IS), at each planting site in the RTE and in the common garden of Toulenne. Data loggers (HOBO Pro RH/Temp, Onset Computer 230 Corporation, Bourne, Massachusetts, USA) were used for these measurements. Missing data for a particular station were inferred by a gap-filling method based on a linear regression fitted with the climatic variables for the nearest alternative station, with R2 > 0.90.
Statistical analysis
Correlations between life history traits and environmental variables along the elevation gradient
We assessed the variability of reproductive traits with elevation, using a generalized linear mixed-effects model for the mean mass of one acorn and a negative binomial log-link random regression model for the number of seeds produced per m2, as seed count data were highly overdispersed relative to a Poisson distribution. For both models, we define elevation as a fixed effect and valley-level and year as random intercepts, with the mean mass of one acorn and the number of seeds per m2, respectively, as responses. We assessed the variability of growth increments in height and diameter along the gradient and within the RTE, using a linear mixed-effect model with elevation as a fixed factor and block as a random intercept, with height increment as the response. A similar model but with a quadratic term for diameter increment as a response was also used.
Slope/exposure can influence the local microclimate along the elevation gradient. We therefore investigated whether mean annual temperature at the study site made a greater contribution to the variability of the mean mass of one acorn, height increment and diameter increment and whether mean April temperature made a greater contribution to the variability of seed production per m2 than elevation per se. We selected mean April temperature as the temperature during this period is determinant for oak pollination and acorn production (Schermer et al. 2019). We therefore performed analyses similar to those described above, but with temperature replacing elevation. Mean annual temperature at the study site from 2007 to 2018 was used to explain the variability of the mean mass of one acorn, height increment and diameter increment, and mean April temperature over the same time period was used to explain the variability of the number of seeds per m2.
Genetic variability of life history traits between environments (common garden)
• Genetic clines
We assessed the genetic clines within the common garden for the mean mass of one acorn, height increment and diameter increment with a generalized linear mixed-effects model and a negative binomial log-link random regression model for the number of seed per m2, as seed count data were highly overdispersed relative to a Poisson distribution. For both models, elevation was considered as a fixed effect, with block, valley-level and year as random intercepts. For the mean mass of one acorn and the number of acorns per m2, only trees producing at least one acorn during the six years of monitoring were taking into account in the analysis. The population analyzed consisted of 539 trees. Similar models with annual mean temperature of provenance origin for the mean mass of one acorn, height increment and diameter increment and mean April temperature for the number of seeds produced per m2as independent variables were also used.
We also assessed the variability, with elevation of provenance origin, of the proportion of trees producing and not producing acorns during the six years of measurement. We used a generalized linear model (GLM) to regress the percentages against elevation and temperature with a binomial family, logit link model.
We fitted curves to all the linear mixed-effect models and negative binomial random regression models by the restricted maximum likelihood (REML) method, with the glmmTMB R package (Brooks et al., 2017). These analyses were performed in R version 1.3.959 (R core team 2014).
Results
Phenotypic variations in situ
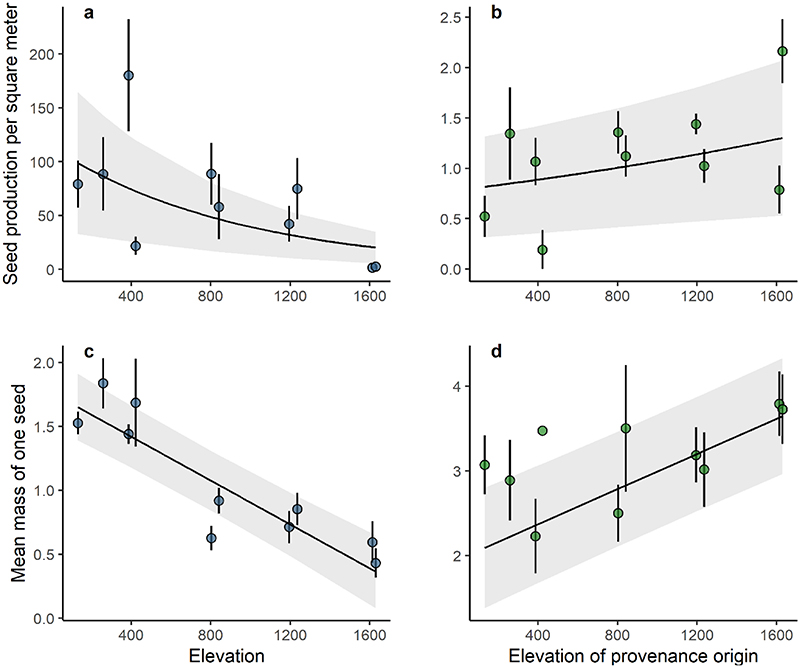
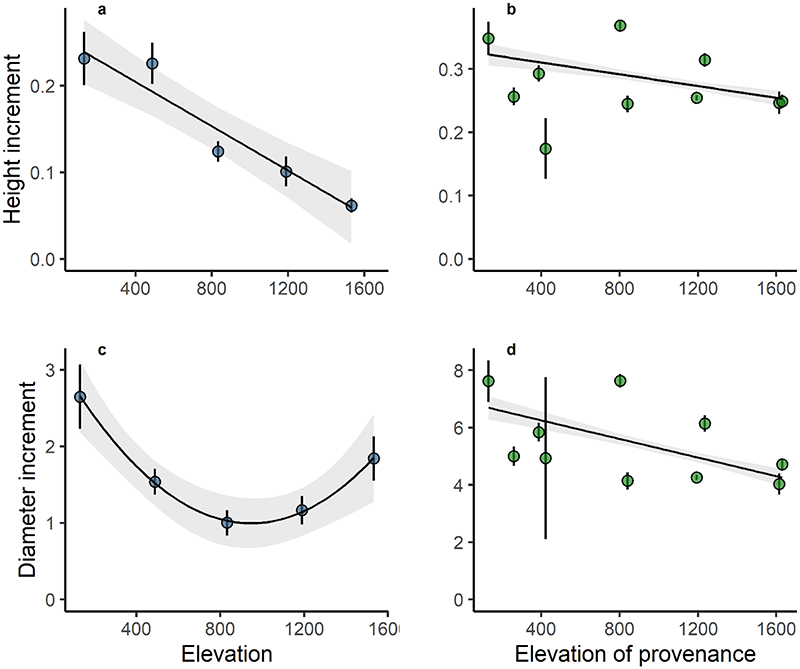
Seed production and seed weight decreased significantly with elevation (Figs. 1a and 1c), but with considerable year-to-year variability of seed production for all populations of the gradient (Figs. S2a). A similar significant change with elevation was observed for height increment (Figs. 2a), with a loss of 0.013 [0.009, 0.017] m for every 100 m increase in elevation (Table 1). The relationship between diameter increment and elevation was quadratic, with larger increments at low and high elevations (Figs. 2c). Temperature explained as much of the variation of reproduction and growth as elevation (Table 1), suggesting that temperature was one of the main drivers of the phenotypic variation observed along the gradient (Fig. S3 a and c, Fig. S4, a and c)
Figure 1.
Variation in seed production (in seeds.m-2) and variation of the mean mass of one acorn (in g) with elevation in situ (a and c) and with elevation of provenance origin in the common garden (b and d), respectively. Each dot represents mean annual acorn production or the mean mass of one acorn of each population estimated overs six years for both experimental designs. The bars represent the standard errors for each population. Negative binomial random regression models were used for experiments in situ and in the common garden for seed production per m2, and linear mixed-effect models were used for experiments in situ and in the common garden for mean mass of one acorn.
Figure 2.
Variation of mean annual height (in m) and diameter (in mm) increments between 2011 and 2017 with elevation in situ (a and c) and elevation of provenance origin in the common garden (b and d). Each dot represents the mean value for the population. The bars represent the standard errors for each population. Linear mixed-effect models were used for experiments in situ for height increment and in the common garden, and a quadratic term was added for diameter increment in situ.
Table 1.
Regression slopes (estimated) and their confidence intervals, estimated for all traits assessed along the elevation gradient (in situ) and in the common garden. The total number of acorns produced (Ntot) and the total number of acorns produced per unit ground area (Ntot_area) in situ and in the common garden were regressed against elevation and mean April temperature in a negative binomial random regression model. The mean mass of one acorn (Ma) and the mean annual height (ΔH) and diameter (ΔD) increments were regressed against elevation and annual mean temperature in a linear mixed-effect model. The estimates as a function of elevation are shown per 100 m increase. The standard deviation (SD), confidence interval and the p-value are indicated for each trait.
| Trait | Estimate | SD | p.value | ||||
|---|---|---|---|---|---|---|---|
| In situ | Elevation | ||||||
| log(Ntot-area)/100 m | -0.10 [-0.14, -0.07] | 0.016 | <0.001 | *** | |||
| log(Ntot)/100 m | -0.12 [-0.15, -0.09] | 0.015 | <0.001 | *** | |||
| Ma/100 m | -0.086[-0.104, -0.067] | 0.0095 | <0.001 | *** | |||
| ΔH/100 m | -0. 013 [-0. 017, -0. 009] | 0.00212 | <0.001 | *** | |||
| ΔD/100m | quadratic term | 0.025 [0.014, 0.036] | 0.006 | <0.001 | *** | ||
| linear term | -0.47 [-0.66, -0.28] | 0.095 | <0.001 | *** | |||
| Temperature | log(Ntot-area) | 0.23 [0.15, 0.32] | 0.044 | <0.001 | *** | ||
| log(Ntot-) | 0.25[0.18,0.33] | 0.039 | <0.001 | *** | |||
| Ma | 0.22 [0.17, 0.27] | 0.023 | <0.001 | *** | |||
| ΔH | 0. 024 [0. 016, 0. 033] | 0.004 | <0.001 | *** | |||
| ΔD | quadratic term | 0.13 [0.07, 0.19] | 0.029 | <0.001 | *** | ||
| linear term | -2.34 [-3.45, -1.24] | 0.566 | <0.001 | *** | |||
| common garden | Elevation | log(Ntot-area)/100 m | 0.027 [0.009, 0.046] | 0.009 | 0.003 | ** | |
| log(Ntot)/100 m | 0.012 [-0.004, 0.036] | 0.008 | 0.14 | ns | |||
| Ma/100 m | 0.10 [0.08, 0.12] | 0.011 | <0.001 | *** | |||
| ΔH/100 m | -0.004 [-0.006, -0. 003] | 0.0009 | <0.001 | *** | |||
| ΔD/100 m | -0.16 [-0.20, -0.11] | 0.021 | <0.001 | *** | |||
| Temperature | log(Ntot-area) | -0.054 [-0.09, -0.019] | 0.018 | 0.003 | ** | ||
| log(Ntot) | -0.005 [-0.036, 0.026] | 0.016 | 0.75 | ns | |||
| Ma | -0.28 [-0.33, -0.22] | 0.028 | <0.001 | *** | |||
| ΔH | 0. 014 [0. 010, 0. 018] | 0.002 | <0.001 | *** | |||
| ΔD | 0.48 [0.38, 0.58] | 0.051 | <0.001 | *** | |||
Units for each trait, for elevation and temperature, respectively: log(Ntot-area) in seed.m-2.100 m-1 and seed. °C-1; Ma in g. 100 m-1 and g. °C-1; ΔH in m.year-1.100 m-1 and m.year-1. °C-1; ΔD in mm.year-1.100 m-1 and mm.year-1. °C-1.
Genetic differentiation in the common garden
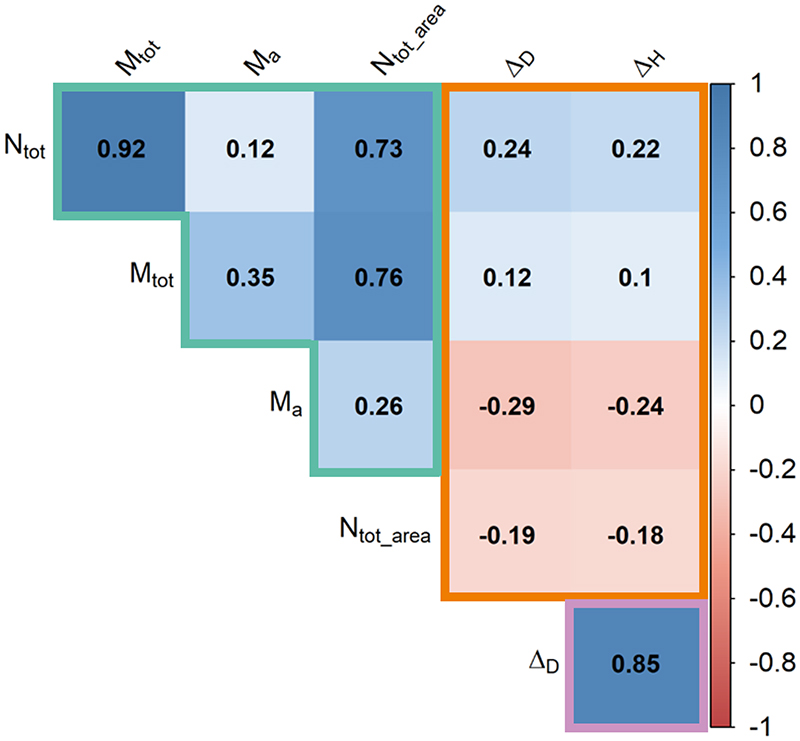
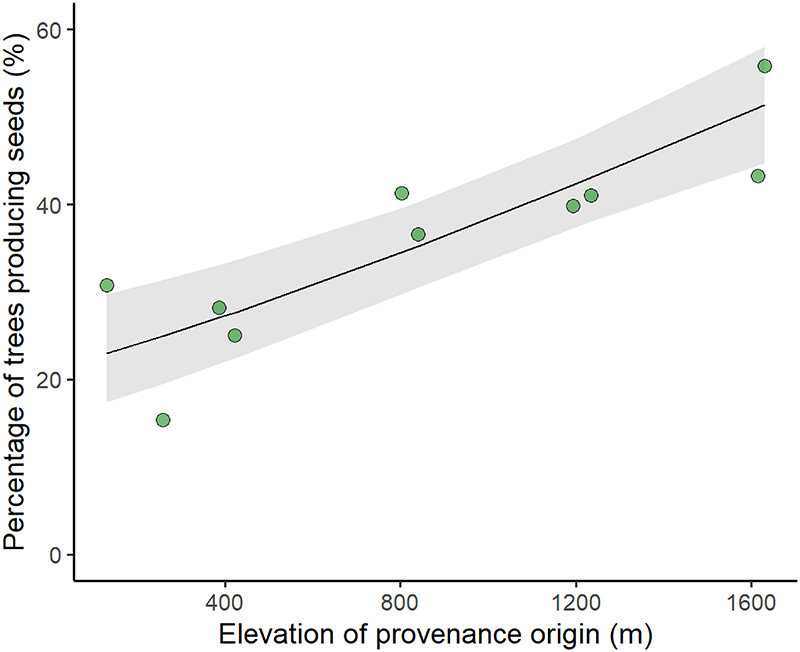
In the lowland common garden, we observed a significant increase in seed production and mean seed weight with elevation of provenance origin (Table 1, Fig. 1b and 1d), whereas there was a trend towards a negative relationship with temperature of provenance origin for both these traits (Fig. S3 b and d). Provenances from high elevations (lower temperatures) produced larger acorns, in larger numbers, than populations from low elevations (higher temperatures). Standard deviations were large for the reproductive traits, with a mean and standard deviation of 1.33 ± 3.98 seeds m-2 for seed production and 3.42 ± 1.38 g for mean seed weight, potentially contributing to the dispersion of the population mean values shown in Fig. 1b. By contrast, the genetic clines observed for growth traits were significant but negative with respect to elevation of provenance origin (Table 1, Figs. 2b, 2d). Thus, in the common garden, provenances originating from low elevations grew faster, reaching greater heights and diameters than those from high elevations. Although the genetic correlation between related traits such as the increment in height and diameter or the total seed production and the total seed mass produced were relatively high, the genetic correlations between growth and reproductive traits were weak (Fig. 3). In addition, the proportion of trees producing acorns per provenance also increased significantly with increasing elevation of provenance origin (Fig. 4, Table 2). This increase was consistent every year (Figs. S5, Table 2) and over the six years studied (Fig. 4).
Figure 3.
Genetic correlations between traits measured in the common garden experiment during the most productive year in acorns (2019) using the coefficient of Spearman. Correlations between reproductive traits, between growth traits and between growth and reproductive traits are highlighted in green, purple and orange, respectively.
Ntot: Total number of acorns produced | Mtot: Total mass of acorns produced in g | Ma: Mean mass of one acorn in g | ΔD: Tree diameter increment in mm | ΔH: Tree height increment in m | Ntot_area: Total number of acorns produced per square meter.
Figure 4.
Variation of the proportion of reproductive trees with elevation of provenance origin in the common garden experiment, for all years. The proportion of reproductive trees is assessed as the percentage of trees producing at least one acorn during the 6 years of measurement. We used a generalized linear model (GLM) to regress the percentages against temperature, using a binomial family, logit link model.
Table 2.
Regression slopes (estimated) and their confidence intervals, estimated for the proportion of trees producing acorns assessed in the common garden according to the elevation and annual mean temperature of provenance of origin. We used a generalized linear model (GLM) to regress the percentages against elevation and temperature, using a binomial family, logit link model.
| Year | Estimate | SD | p.value | ||
|---|---|---|---|---|---|
| Elevation | All years | 0.08 [0.05, 0.11] | 0.015 | 0.000 | *** |
| 2014 | 0.13 [0.08, 0.18] | 0.025 | 0.000 | *** | |
| 2015 | 0.14 [0.08, 0.19] | 0.029 | 0.000 | *** | |
| 2016 | 0.10 [0.05, 0.15] | 0.025 | 0.000 | *** | |
| 2017 | 0.04 [0.01, 0.07] | 0.018 | 0.018 | * | |
| 2018 | 0.06 [0.01, 0.11] | 0.025 | 0.017 | * | |
| 2019 | 0.08 [0.05, 0.12] | 0.018 | 0.000 | *** | |
| Temperature | All years | -0.22 [-0.30,-0.14] | 0.04 | 0.000 | *** |
| 2014 | -0.30 [-0.43, -0.18] | 0.07 | 0.000 | *** | |
| 2015 | -0.34 [-0.49, -0.20] | 0.07 | 0.000 | *** | |
| 2016 | -0.24 [-0.37, -0.11] | 0.06 | 0.000 | *** | |
| 2017 | -0.12 [-0.21, -0.03] | 0.05 | 0.011 | * | |
| 2018 | -0.14 [-0.27, -0.02] | 0.06 | 0.027 | * | |
| 2019 | -0.23 [-0.32, -0.14] | 0.05 | 0.000 | *** |
Discussion
Phenotypic variation along elevation gradients is known to be due to conditions becoming increasingly harsh at higher elevations due to lower temperatures, topography and a lower availability in soil nutrients (Laiolo and Obeso, 2017, Friend and Woodward, 1990, Vitasse et al. 2009b). With increasing elevation, trees become smaller and their seed production declines (Körner, 2012). Our observations for Q. petraea are consistent with these widely observed patterns. The main goal of this study was to identify the factors underlying these phenotypic patterns. We provide here the first evidence for genetically driven effects on the reproductive traits of trees, in addition to the genetically driven effects on growth traits. We found that genetic variations of seed production and size counteracted the phenotypic influence of temperature, decreasing phenotypic variation along the elevation gradient. The genetic and phenotypic clines along the gradient thus operated in opposite directions, forming a counter-gradient. By contrast, for growth traits, genetic variation paralleled the phenotypic variation measured in situ (co-gradient).
Genetic differentiation
The genetic clines along the elevation gradient observed for seed production and seed weight suggest significant genetic differentiation between provenances for these two traits. These results are consistent with those for other functional traits measured in the same experimental design. For example, genetic differences were previously observed between these same populations for leaf unfolding, leaf senescence and canopy duration, despite considerable variability within populations (Alberto et al., 2011; Firmat et al., 2017). In these previous studies, population differentiation for the date of bud burst (as measured by determining QST) was greater than the differentiation estimated for neutral markers (FST), suggesting that the differences between populations were probably due to diversifying selection (Leinonen et al., 2013; Whitlock, 2008). The observed differentiation of seed production between populations at different elevations probably results from divergent selection favoring reproduction at higher elevations. Previous assessments of the genetic variation of reproductive success of Q. petraea within natural populations have suggested that there is substantial variation that may fuel natural selection (Alexandre et al., 2020). Our data show that both acorn production per se, and the age at first reproduction in this long-lived oak species display genetic differentiation according to elevation. Together, these traits contribute collectively to securing recruitment at higher elevations and allow populations to persist (Anderson, 2016; Obeso, 2002).
Phenotypic and genetic clines
Opposing signs of phenotypic and genetic clines along environmental gradients have been reported for many organisms (Conover et al., 2009, Laiolo and Obeso, 2017), but have rarely been observed in plants (Radersma et al., 2020), particularly forest trees (Kremer et al., 2014). Previous studies in temperate oaks have shown that phenotypic traits, such as bud burst, leaf senescence and growth, display co-gradient variation (Alberto et al. 2013; Vitasse et al. 2009a; Kremer et al. 2014). Cases of counter-gradient variation in trees have been observed for the timing of bud burst in Fagus sylvatica (Vitasse et al. 2009a), and in some conifers, such as Pinus jeffreyi (Martínez-Berdeja et al., 2019), Abies lasiocarpa, Abies amabilis (Worrall, 1983) and Pseudotsuga sp. (Acevedo-Rodríguez et al., 2006), for which provenances from higher latitudes/elevations flush earlier in common gardens than populations from lower latitudes/elevation, the opposite trend being observed in situ (Gauzere et al., 2020, Vitasse et al., 2009a). Zhuk and Goroshkevitch (2018) recently identified trends in the variation of reproductive traits in Pinus sibirica along latitudinal gradients that resembled counter-gradient patterns.
The counter-gradient observed here highlights the contrasting contributions of environmental and genetic components to the expression of reproduction. Environmental conditions become harsher at high elevations, increasing the contribution of the environment to the phenotypic value of reproductive traits. Conversely, natural selection for survival favors early and effective reproduction in trees at high elevations. In this context, plastic and genetic responses act in opposing directions. Unlike co-gradients, which enhance local adaptation because selection and plasticity act in the same direction to drive populations towards higher fitness, counter-gradients can be interpreted as corresponding to situations in which the plastic response to the environment is non-adaptive. This situation is similar to that described by Conover et al. (2009) as “genetic compensation”, in which genetic trends in variation counteract environmental constraints that have a negative effect on fitness (Grether, 2005). However, it remains unknown whether plasticity is under genetic control. If it is, plasticity may also evolve, ultimately reversing the gradient. This possibility was suggested by King and Hadfield (2019), and Scheiner (2013), who showed that plasticity can evolve under spatially and temporally contrasting environments, a scenario that cannot be excluded in the context of current environmental changes.
This study has several limitations linked to the difficulties of measuring reproductive traits due to (1) the long generation times of trees and (2) the masting pattern characteristic of some tree species, such as oaks (Bogdziewicz et al., 2017, Caignard et al. 2017). Assessments of the phenotypic and genetic variability of reproductive effort require long-term experiments. In our study, for example, we could not work with all the trees in the common garden because not all the trees had reached the age of reproductive maturity. Age at first reproduction is, nevertheless, an interesting trait to analyze, because, as pointed out above, it also affects the fitness of the individual. The method used to analyze the reproductive effort of each tree in situ is highly accurate, as all the acorns produced each year were counted. However, this method is time-consuming, limiting the size of the sample for analysis. Nevertheless, the repetition of measurements over a period of six years and the accuracy of the measurements obtained provided strong insight into overall reproductive effort along the gradient.
Evolutionary trade-off between growth and reproduction
Studies investigating the environmental and genetic determinism of reproduction in forest trees are scarce, with most previous investigations focusing on other life history traits characterizing tree fitness, such as growth. Studies addressing the genetic and phenotypic gradients for both categories of traits in trees are even rarer, the most notable exception being the study by Zhouk and Goroshkevitch (2018). Here, we aimed to perform a joint dissection of the environmental and genetic sources of variation for both reproductive and growth traits and to demonstrate the existence of significant genetic differentiation for both types of trait along the elevation gradient. Growth differences between provenances are conserved as the trees age. Unlike reproductive traits, vegetative growth traits follow a co-gradient pattern with elevation. For example, in the highest peripheral populations, the lack of resources and the environmental constraints applying at high elevations may result in lower levels of phenotypic vegetative growth. In this case, lower levels of growth increase fitness, either due to a negative genetic correlation with reproduction or because limited vegetative growth per se increases viability. Indeed, faster growth may expose trees to a higher risk of lethal damage due to the breakage of branches and stems by snow or wind (Körner, 2012). Conversely, a negative genetic correlation may reflect a trade-off between growth and reproduction. We generally distinguish two types of trade-off between life history traits: functional trade-offs based on phenotypic values and genetic trade-offs based on the genetic values and excluding the environmental effect (Obeso, 2002). We were unable to assess the phenotypic trade-off here, as phenotypic records of both traits (growth and reproduction) were not available for individual trees growing in situ. However, the contrasting genetic clines observed in the common garden clearly indicate a genetic trade-off. This genetic trade-off may be explained by either a pleiotropic effect, affecting both reproductive and growth traits, or by statistical associations in the population (due to linkage disequilibrium) between different genes that affect each other. However, in our study we observed a relatively weak negative genetic correlation between growth and reproductive traits at individual level (Fig. 3). Previous study on a closely related species (Quercus robur) have shown an absence of co-localization between QTLs identified for growth and reproduction which could have suggested a genetic linkage (Caignard et al. 2019). In addition, along the elevation gradient, the genetic trade-off probably operates at population rather than individual level, and may be the outcome of past selection pressures along the elevation gradient. We assume here that, along the gradient, the difference in clines between growth and reproductive traits are not due to mechanistic effects, such as pleiotropy or genetic linkage, but to the action of different selective pressures on the two phenotypic traits. Whatever the mechanism, natural selection at high elevations decreases the genetic component of vegetative growth, just as plasticity reduces the environmental component. In a broader context, considering populations over the whole gradient, the opposing directions of the genetic contributions of growth and reproductive traits along the gradient suggest that trees from high and low elevations may respond differently. At low elevation and warmer temperatures, natural selection favors vegetative growth over reproduction, whereas, at higher elevations, the harsher conditions favor greater fruiting fitness and slow growth. Such contrasting responses to natural selection have also often been interpreted as reflecting different life history strategies corresponding to a trade-off between vegetative growth and reproduction. At low elevation, there is more competition between trees (Alexander et al., 2015; Kunstler et al., 2011), potentially explaining their greater investment in growth than in reproduction. Conversely, populations growing at higher elevations (Coomes and Allen, 2007) are subject to lower levels of competition and may adopt life history strategies typical of pioneer species, with a greater investment in reproduction than in growth (Marchand and Roach, 1980).
Conclusion
The differences in adaptive strategies between growth and reproduction observed here highlight the importance of investigating the genetic determinism of reproduction together with other life history traits. These results raise new questions about the life history strategies adopted by a given species along environmental gradients. In the context of climate change, assessment of the determinism of reproductive effort in many species remains a major challenge for ecologists, but one that we must take up if we are to understand forest regeneration and dynamics. In this context, this study sheds new light on the local adaptation of forest tree species to environmental changes, and should encourage further investigations into reproductive traits.
Supplementary Material
Acknowledgments
This research was supported by the European Research Council through the Advanced Grant Project TREEPEACE (#FP7-339728), the French national research agency through the ANR FOREPRO (#ANR-19-CE32-0008). We thank Benjamin Dencausse and Nicolas Cheval for their contributions to the monitoring of seed crops and growth traits at the forest sites. We thank the experimental units of both Pierroton (UE 0570, INRA, 69 route d’Arcachon, 33612 CESTAS, France) and Toulenne (UE 0393 INRA, Domaine des Jarres 33210 Toulenne, France) for technical support. TC received a PhD grant from TREEPEACE and the Initiative of Excellence program (IdEX-03-02) of Bordeaux University.
Footnotes
Authors’ contributions
T.C., A.K. and S.D. conceived the idea for this work; T.C., J.M.L and J.P assembled the dataset; T.C and X.B. analyzed the data; T.C., A.K. and S.D., wrote the manuscript and S.V. and X.B. revised the manuscript.
Data availability statement
Data upon which this study is based are available through the Data INRAE Repository: https://doi.org/10.15454/TZGRBR (Caignard et al. 2021).
References
- Acevedo-Rodríguez R, Vargas-Hernández JJ, López-Upton J, Velázquez Mendoza J, Acevedo-Rodríguez R, Vargas-Hernández JJ, López-Upton J, VelázquezMendoza J. Effect of geographic origin and nutrition on shoot phenology of mexican Douglas-Fir (Pseudotsuga sp.) seedlings. Agrociencia. 2006;40:125–137. [Google Scholar]
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations: Climate change outcomes for tree populations. Evolutionary Applications. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberto F, Bouffier L, Louvet J-M, Lamy J-B, Delzon S, Kremer A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient: Variation of phenological traits in Q. petraea. Journal of Evolutionary Biology. 2011;24:1442–1454. doi: 10.1111/j.1420-9101.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- Alberto FJ, Derory J, Boury C, Frigerio J-M, Zimmermann NE, Kremer A. Imprints of natural selection along environmental gradients in phenology-related genes of Quercus petraea. Genetics. 2013;195:495–512. doi: 10.1534/genetics.113.153783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Diez JM, Levine JM. Novel competitors shape species’ responses to climate change. Nature. 2015;525:515–518. doi: 10.1038/nature14952. [DOI] [PubMed] [Google Scholar]
- Alexandre H, Truffaut L, Ducousso A, Louvet J-M, Nepveu G, Torres-Ruiz JM, Lagane F, Firmat C, Musch B, Delzon S, Kremer A, et al. In situ estimation of genetic variation of functional and ecological traits in Quercus petraea and Q. robur. Tree Genet Genomes. 2020;16:32. doi: 10.1007/s11295-019-1407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT. Plant fitness in a rapidly changing world. New Phytol. 2016;210:81–87. doi: 10.1111/nph.13693. [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal. 2017;9(2):378–400. https://journal.r-project.org/archive/2017/RJ-2017-066/index.html . [Google Scholar]
- Benito-Garzón M, Ruiz-Benito P, Zavala MA. Interspecific differences in tree growth and mortality responses to environmental drivers determine potential species distributional limits in Iberian forests. Global Ecology and Biogeography. 2013;22:1141–1151. doi: 10.1111/geb.12075. [DOI] [Google Scholar]
- Bogdziewicz M, Szymkowiak J, Kasprzyk I, Grewling Ł, Borowski Z, Borycka K, Kantorowicz W, Myszkowska D, Piotrowicz K, Ziemianin M, Pesendorfer MB. Masting in wind-pollinated trees: system-specific roles of weather and pollination dynamics in driving seed production. Ecology. 2017;98:2615–2625. doi: 10.1002/ecy.1951. [DOI] [PubMed] [Google Scholar]
- Caignard T, Kremer A, Bouteiller XP, Parmentier J, Louvet J-M, Delzon S. Growth and reproduction data of sessile oak forests (Quercus petraea) distributed along a gradient of elevation (French Pyrenees - in situ) and in a common garden experiment (Toulenne, France) 2021 doi: 10.15454/TZGRBR. [DOI] [Google Scholar]
- Caignard T, Delzon S, Bodénès C, Dencausse B, Kremer A. Heritability and genetic architecture of reproduction-related traits in a temperate oak species. Tree Genet Genomes. 2019;15:1. doi: 10.1007/s11295-018-1309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caignard T, Kremer A, Firmat C, Nicolas M, Venner S, Delzon S. Increasing spring temperatures favor oak seed production in temperate areas. Scientific Reports. 2017;7:8555. doi: 10.1038/s41598-017-09172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkle MT. Growth data for 29 years from the California elevational transect study of ponderosa pine. Forest Science. 1973;19:31–39. doi: 10.1093/forestscience/19.1.31. [DOI] [Google Scholar]
- Conover DO, Duffy TA, Hice LA. The covariance between genetic and environmental influences across ecological gradients. Annals of the New York Academy of Sciences. 2009;1168:100–129. doi: 10.1111/j.1749-6632.2009.04575.x. [DOI] [PubMed] [Google Scholar]
- Coomes DA, Allen RB. Effects of size, competition and altitude on tree growth. Journal of Ecology. 2007;95(5):1084–109. doi: 10.1111/j.1365-2745.2007.01280.x. [DOI] [Google Scholar]
- Dantec CF, Ducasse H, Capdevielle X, Fabreguettes O, Delzon S, Desprez-Loustau M-L. Escape of spring frost and disease through phenological variations in oak populations along elevation gradients. Journal of Ecology. 2015;103(4):1044–1056. doi: 10.1111/1365-2745.12403. [DOI] [Google Scholar]
- Dantec CF, Vitasse Y, Bonhomme M, Louvet J-M, Kremer A, Delzon S. Chilling and heat requirements for leaf unfolding in European beech and sessile oak populations at the southern limit of their distribution range. International Journal of Biometeorology. 2014;58:1853–1864. doi: 10.1007/s00484-014-0787-7. [DOI] [PubMed] [Google Scholar]
- Ducousso A, Guyon JP, Kremer A. Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt.) Liebl.) Annales des Sciences Forestières. 1996;53(2/3):775–782. [Google Scholar]
- de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I. Common garden experiments in the genomic era: new perspectives and opportunities. Heredity. 2016;116:249–254. doi: 10.1038/hdy.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmat C, Delzon S, Louvet J-M, Parmentier J, Kremer A. Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. Journal of Evolutionary Biology. 2017;30(30):2116–2131. doi: 10.1111/jeb.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Weyand A, Rudmann-Maurer K, Stöcklin J. Adaptation of Poa alpina to altitude and land use in the Swiss Alps. Alpine Botany. 2011;121:91. doi: 10.1007/s00035-011-0096-2. [DOI] [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Frei ER, Ghazoul J, Pluess AR. Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PLoS ONE. 2014;9:e98677. doi: 10.1371/journal.pone.0098677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend AD, Woodward FI. In: Advances in Ecological Research. Begon M, Fitter AH, Macfadyen A, editors. Academic Press; 1990. Evolutionary and ecophysiological responses of mountain plants to the growing season environment; pp. 59–124. [DOI] [Google Scholar]
- Gauzere J, Teuf B, Davi H, Chevin L-M, Caignard T, Leys B, Delzon S, Ronce O, Chuine I. Where is the optimum? Predicting the variation of selection along climatic gradients and the adaptive value of plasticity. A case study on tree phenology. Evol Lett. 2020;4:109–123. doi: 10.1002/evl3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Turpin H, Hazard L. Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia. Journal of Ecology. 2009;97:742–751. doi: 10.1111/j.1365-2745.2009.01509.x. [DOI] [Google Scholar]
- Grether GF. Environmental change, phenotypic plasticity, and genetic compensation. The American Naturalist. 2005;166:E115–E123. doi: 10.1086/432023. [DOI] [PubMed] [Google Scholar]
- Hall D, Luquez V, Garcia VM, St Onge KR, Jansson S, Ingvarsson PK. Adaptive population differentiation in phenology across a latitudinal gradient in European aspen (Populus tremula, L.): a comparison of neutral markers, candidate genes and phenotypic traits. Evolution. 2007;61:2849–2860. doi: 10.1111/j.1558-5646.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Randin CF, Stöcklin J, Guisan A. Changes in reproductive investment with altitude in an alpine plant. Journal of Plant Ecology. 2009;2:125–134. doi: 10.1093/jpe/rtp011. [DOI] [Google Scholar]
- Kawakami T, Morgan TJ, Nippert JB, Ocheltree TW, Keith R, Dhakal P, Ungerer MC. Natural selection drives clinal life history patterns in the perennial sunflower species, Helianthus maximiliani. Molecular Ecology. 2011;20:2318–2328. doi: 10.1111/j.1365-294X.2011.05105.x. [DOI] [PubMed] [Google Scholar]
- Kawecki TJ. Age and Size at Maturity in a Patchy Environment: Fitness Maximization versus Evolutionary Stability. Oikos. 1993;66:309–317. doi: 10.2307/3544819. [DOI] [Google Scholar]
- King JG, Hadfield JD. The evolution of phenotypic plasticity when environments fluctuate in time and space. Evol Lett. 2019;3:15–27. doi: 10.1002/evl3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. Alpine Treelines. Springer Basel; Basel: 2012. [DOI] [Google Scholar]
- Kremer A, Potts BM, Delzon S. Genetic divergence in forest trees: understanding the consequences of climate change. Functional Ecology. 2014;28:22–36. doi: 10.1111/1365-2435.12169. [DOI] [Google Scholar]
- Kunstler G, Guyennon A, Ratcliffe S, Rüger N, Ruiz-Benito P, Childs DZ, Dahlgren J, Lehtonen A, Thuiller W, Wirth C, Zavala MA, et al. Demographic performance of European tree species at their hot and cold climatic edges. J Ecol. 2021;109:1041–1054. doi: 10.1111/1365-2745.13533. [DOI] [Google Scholar]
- Kunstler G, Albert CH, Courbaud B, Lavergne S, Thuiller W, Vieilledent G, Zimmermann NE, Coomes DA. Effects of competition on tree radial growth vary in importance but not in intensity along climatic gradients. Journal of Ecology. 2011;99:300–312. doi: 10.1111/j.1365-2745.2010.01751.x. [DOI] [Google Scholar]
- Laiolo P, Obeso JR. Life-history responses to the altitudinal gradient. SpringerLink; 2017. pp. 253–283. [DOI] [Google Scholar]
- Lechowicz MJ. Seasonality of flowering and fruiting in temperate forest trees. Canadian Journal of Botany. 1995;73:175–182. doi: 10.1139/b95-021. [DOI] [Google Scholar]
- Leinonen T, McCairns RJS, O’Hara RB, Merilä J. QST-FST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics. 2013;14:179–190. doi: 10.1038/nrg3395. [DOI] [PubMed] [Google Scholar]
- Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolström M, Lexer MJ, et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management. 2010;259:698–709. doi: 10.1016/j.foreco.2009.09.023. [DOI] [Google Scholar]
- Linhart YB, Grant MC. Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics. 1996;27:237–277. [Google Scholar]
- Marchand PJ, Roach DA. Reproductive strategies of pioneering alpine species: seed production, dispersal, and germination. Arctic and Alpine Research. 1980;12:137–146. doi: 10.2307/1550511. [DOI] [Google Scholar]
- Martínez-Berdeja A, Hamilton JA, Bontemps A, Schmitt J, Wright JW. Evidence for population differentiation among Jeffrey and ponderosa pines in survival, growth and phenology. For Ecol Manag. 2019;434:40–48. doi: 10.1016/j.foreco.2018.12.009. [DOI] [Google Scholar]
- Namkoong G, Conkle MT. Time trends in genetic control of height growth in ponderosa pine. Forest Science. 1976;22:2–12. doi: 10.1093/forestscience/22.1.2. [DOI] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytol. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Ramos IM, Ourcival JM, Limousin JM, Rambal S. Mast seeding under increasing drought: results from a long-term data set and from a rainfall exclusion experiment. Ecology. 2010;91:3057–3068. doi: 10.1890/09-2313.1. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. URL http://www.R-project.org/ [Google Scholar]
- Radersma R, Noble DWA, Uller T. Plasticity leaves a phenotypic signature during local adaptation. Evol Lett. 2020;4:360–370. doi: 10.1002/evl3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Oleksyn J. Climate warming will reduce growth and survival of Scots pine except in the far north: Scots pine growth and survival following climate transfer. Ecology Letter. 2008;11:588–597. doi: 10.1111/j.1461-0248.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- Sáenz-Romero C, Guzmán-Reyna RR, Rehfeldt GE. Altitudinal genetic variation among Pinus oocarpa populations in Michoacán, Mexico. Forest Ecology and Management. 2006;229:340–350. doi: 10.1016/j.foreco.2006.04.014. [DOI] [Google Scholar]
- Sanchez-Humanes B, Espelta JM. Increased drought reduces acorn production in Quercus ilex coppices: thinning mitigates this effect but only in the short term. Forestry. 2011;84:73–82. doi: 10.1093/forestry/cpq045. [DOI] [Google Scholar]
- Santos-del-Blanco L, Climent J, González-Martínez SC, Pannell JR. Genetic differentiation for size at first reproduction through male versus female functions in the widespread Mediterranean tree Pinus pinaster. Annals of Botany. 2012;110:1449–1460. doi: 10.1093/aob/mcs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner SM. The genetics of phenotypic plasticity. XII. Temporal and spatial heterogeneity. Ecol Evol. 2013;3:4596–4609. doi: 10.1002/ece3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer É, Bel-Venner M-C, Fouchet D, Siberchicot A, Boulanger V, Caignard T, Thibaudon M, Oliver G, Nicolas M, Gaillard J-M, Delzon S, et al. Pollen limitation as a main driver of fruiting dynamics in oak populations. Ecology Letter. 2019;22:98–107. doi: 10.1111/ele.13171. [DOI] [PubMed] [Google Scholar]
- Sparks TH, Jeffree EP, Jeffree CE. An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. International Journal of Biometeorology. 2000;44:82–87. doi: 10.1007/s004840000049. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Bresson CC, Kremer A, Michalet R, Delzon S. Quantifying phenological plasticity to temperature in two temperate tree species: Quantifying plasticity of leaf phenology. Functional Ecology. 2010;24:1211–1218. doi: 10.1111/j.1365-2435.2010.01748.x. [DOI] [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research. 2009a;39:1259–1269. doi: 10.1139/X09-054. [DOI] [Google Scholar]
- Vitasse Y, Delzon S, Dufrêne E, Pontailler J-Y, Louvet J-M, Kremer A, Michalet R. Leaf phenology sensitivity to temperature in European trees: do within-species populations exhibit similar responses. Agricultural and Forest Meteorology. 2009b;149:735–744. doi: 10.1016/j.agrformet.2008.10.019. [DOI] [Google Scholar]
- Wenk EH, Falster DS. Quantifying and understanding reproductive allocation schedules in plants. Ecol Evol. 2015;5:5521–5538. doi: 10.1002/ece3.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock MC. Evolutionary inference from QST. Molecular Ecology. 2008;17:1885–1896. doi: 10.1534/genetics.108.099812. [DOI] [PubMed] [Google Scholar]
- Worrall J. Temperature — Bud-burst relationships in amabilis and subalpine fir provenance tests replicated at different elevations. Temp — Bud-Burst Relatsh Amabilis Subalp Für Proven Tests Replicated Differ Elev. 1983;32:203–209. [Google Scholar]
- Zhuk E, Goroshkevich S. Growth and reproduction in Pinus sibirica ecotypes from Western Siberia in a common garden experiment. New For. 2018;49:159–172. doi: 10.1007/s11056-017-9611-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data upon which this study is based are available through the Data INRAE Repository: https://doi.org/10.15454/TZGRBR (Caignard et al. 2021).