Abstract
Objective
The authors examined whether alterations in the brain’s reward network operate as a mechanism across the spectrum of risk for depression. They then tested whether these alterations are specific to anhedonia as compared with low mood and whether they are predictive of depressive outcomes.
Method
Functional MRI was used to collect blood-oxygenlevel-dependent (BOLD) responses to anticipation of reward in the monetary incentive task in 1,576 adolescents in a community-based sample. Adolescents with current subthreshold depression and clinical depression were compared with matched healthy subjects. In addition, BOLD responses were compared across adolescents with anhedonia, low mood, or both symptoms, cross-sectionally and longitudinally.
Results
Activity in the ventral striatum was reduced in participants with subthreshold and clinical depression relative to healthy comparison subjects. Low ventral striatum activation predicted transition to subthreshold or clinical depression in previously healthy adolescents at 2-year follow-up. Brain responses during reward anticipation decreased in a graded manner between healthy adolescents, adolescents with current or future subthreshold depression, and adolescents with current or future clinical depression. Low ventral striatum activity was associated with anhedonia but not low mood; however, the combined presence of both symptoms showed the strongest reductions in the ventral striatum in all analyses.
Conclusions
The findings suggest that reduced striatal activation operates as a mechanism across the risk spectrum for depression. It is associated with anhedonia in healthy adolescents and is a behavioral indicator of positive valence systems, consistent with predictions based on the Research Domain Criteria.
Alterations in the brain’s reward network are evident in adolescents with depressive disorder (1, 2) as well as in unaffected first-degree relatives of patients with depression (3) and therefore have great potential as risk markers and intervention targets. However, crucial evidence for reward network alterations as a mechanism for depressive disorder is still lacking.
First, it is unclear whether reward network alterations vary across the spectrum of risk for depression. It is important to know whether adolescents with subthreshold depression, who are at high risk of transition to clinical depression (4), have reward network alterations similar to those of adolescents who meet full criteria for a depressive disorder.
Second, the specificity of reward network alterations with depressive symptoms is unclear. Depression has been conceptualized as an imbalance in dual valence systems (5), involving reduced positive and/or increased negative affect. Etiologically, anhedonia is part of a positive valence system, whereas other depressive symptoms, such as low mood, are related to a negative valence system (6). Based on existing evidence, reduced frontostriatal activations during reward anticipation should be specific to anhedonia—defined as diminished motivation or desire to engage in pleasurable activities (7, 8). However, it is not clear whether reduced responses to reward in depression are exclusively associated with anhedonia or low mood, or if they are related to the combined effects of both symptoms. This has important implications, as anhedonia and low mood seem to be associated with differential treatment responses (9, 10) and developmental trajectories (11).
Finally, nearly all work on the brain’s reward network has been cross-sectional (1), and it is unknown whether alterations in reward system function would predict future anhedonia, low mood, or their association, since they are core criteria for depression.
Here we address these outstanding questions by using functional MRI (fMRI) during a reward anticipation experiment (the monetary incentive delay task [12]), in a large community-based sample of adolescents, using both a crosssectional and a longitudinal design. This task is used to investigate reward anticipation and reward feedback (positive and negative outcomes). We focused on reward anticipation because the anticipatory phase, rather than the feedback phase, has been most strongly linked with anhedonia (13), and we examined the feedback phase in exploratory analyses.
We first compared brain responses to reward anticipation between three carefully matched groups: adolescents with clinical depression, adolescents with subthreshold depression, and matched healthy volunteers. This allowed us to investigate how such brain responses vary across the spectrum of risk for depression. We hypothesized reduced activation in frontostriatal regions during reward anticipation in the clinical and subthreshold depression groups relative to the healthy comparison group, in keeping with findings from high-risk studies (3, 14). We further hypothesized that brain activity in anticipation of reward differs in a graded manner across the risk spectrum, with participants suffering from clinical depression having the lowest neural activity. We also tested whether reduced activation in the reward network at baseline predicted clinical and subthreshold depression in previously healthy adolescents 2 years later.
We next investigated whether these reward network alterations are specific to anhedonia, as opposed to low mood, in a general-population sample. So far, no studies have examined this in adolescents, although some data exist from adult samples (15). Previous studies suggested that depressed patients with anhedonia have lower dopaminergic activity in the caudate, putamen, and nucleus accumbens (16) and that anhedonia is associated with exerting less effort toward attaining rewards (7, 13). We hypothesized that alterations in the reward network are also present in adolescents with anhedonia, but not those who have low mood only. Finally, we examined whether reward network alterations differentially predicted development of anhedonia at the 2-year follow up.
Method
Participants
Neuroimaging and clinical data were obtained from the IMAGEN database, which covers eight sites in France, the United Kingdom, Ireland, and Germany and includes 2,223 adolescents recruited in schools around age 14. We used data from the first and second waves of IMAGEN. A detailed description of the recruitment and assessment procedures has been published elsewhere (17). All local ethics research committees approved the study. Written consent was obtained from the parent or guardian, and verbal assent was obtained from the adolescent. After quality control for neuroimaging and behavioral tests, 1,576 adolescents were included in the study. Only a small minority of adolescents in this population-based sample were being treated with psychotropic medications (N=31, 2%).
Measures
Adolescent psychiatric symptoms and their impact were assessed with the Development and Well-Being Assessment (DAWBA), a self-administered diagnostic questionnaire consisting of open and closed questions (18; http://www.dawba.info). The DAWBA is designed to maintain consistency across multiple cultural and language groups, as diagnoses are made by clinical raters who share a common training and participate in regular cross-language training and consensus meetings. The DAWBA generates probabilities of having DSM-IV-TR diagnoses (19), which we used to define the categorical diagnosis of depression.
The Strengths and Difficulties Questionnaire was used to assess general psychopathology and functional impairment (20, 21).
All participants were coded as suffering from loss of interest/anhedonia or low mood if so rated (as “yes” or “no”) by self-report in the screening questions of the depression section of the DAWBA. These items have good face validity (see the data supplement that accompanies the online edition of this article).
Adolescents were included in a clinical depression group if they scored 4 or 5 on the DAWBA depression band (i.e., the highest probability of depression). In addition, following DSM-IV-TR criteria, these adolescents had to report at least five depressive symptoms, including at least one core symptom (abnormally depressed/irritable mood and/or loss of interest), and fulfill criteria for functional impairment and duration. Twenty-two adolescents met criteria for clinical depression at baseline.
Adolescents were included in a subthreshold depression group if in the past 4 weeks they had three or more depressive symptoms, including at least one core symptom and at least two other DSM-IV depressive symptoms, without fulfilling criteria for clinical depression in terms of duration, number of symptoms, or impact on functioning (4). The same criteria were used to define depression groups at follow-up.
Participants with any current psychiatric diagnosis, with any history of depression or bipolar disorder, or with a score >5 on the Alcohol Use Disorders Identification Test were excluded.
At baseline, adolescents with clinical depression (N=22) and subthreshold depression (N=101) were compared with a group of healthy subjects (N=123) matched on age, sex, handedness, and imaging site. The healthy comparison group consisted of adolescents with fewer than three symptoms of depression and a probability of having a diagnosis of major depression of less than 0.1% according to the DAWBA (see Figure S1 in the online data supplement).
Other measures were collected using Psytools (Delosis, London), an online computer platform for self-assessment, including a pubertal status score using the computerized Pubertal Development Scale (22).
Monetary Incentive Delay Task
To study neural responses to reward, we used a modified version of the well-established monetary incentive delay task (12) that included three conditions: reward anticipation and receipt of positive or negative outcomes. A detailed description of the task is provided in the online data supplement.
MRI Data Acquisition
Structural and fMRI data were acquired at eight IMAGEN assessment sites with 3-T scanners from various manufacturers (Siemens, Philips, General Electric, and Bruker). The scanning variables were specifically chosen to be compatible with all scanners. The same scanning protocol was used at all sites. In brief, high-resolution T1-weighted threedimensional structural images were acquired for anatomical localization and coregistration with the functional time series. fMRI blood-oxygen-level-dependent (BOLD) images were acquired with a gradient-echo, echo-planar imaging sequence. For the monetary incentive delay task, 300 volumes were acquired for each subject. Each volume consisted of 40 slices aligned to the anterior commissure-posterior commissure line (2.4-mm slice thickness, 1-mm gap) acquired in a descending order. The echo time was optimized (echo time=30 ms, repetition time=2200 ms) to provide reliable imaging of subcortical areas. A detailed description of the fMRI data acquisition process is provided in the data supplement.
Statistical Analysis
We used coarsened exact matching in Stata, version 11 (StataCorp, College Station, Tex.), to create the matched healthy comparison group (N=123). After matching, no differences were found between the depressed groups (together, N=123) and the comparison subjects in sex, age, handedness, and imaging site.
To test our first hypothesis, the dimensionality of brain response to reward anticipation in adolescents as a risk factor for depression, we used one-way analysis of variance in SPM8 (Wellcome Trust Centre for Neuroimaging, London) to compare the clinical depression group (N=22) and the subthreshold depression group (N=101) with the matched healthy comparison group (N=123) at baseline, controlling for pubertal stage. Analyses were thresholded at an uncorrected p value of 0.001 at the voxel level, with clusters of activated voxels considered statistically significant at p<0.05, corrected for multiple comparisons. We used smallvolume-corrected region-of-interest analyses across a frontostriatal-limbic mask (see the online data supplement). For all comparisons, brain locations were reported in terms of Montreal Neurological Institute coordinates, and the Wake Forest University PickAtlas (23) was used to identify brain regions.
For subsequent analyses, we extracted the averaged beta values from the anatomical regions with significant clusters from the clinical- and subthreshold depression-matched group analyses using the MarsBaR toolbox (http://marsbar.sourceforge.net) (24). Whole regions from the Automated Anatomical Labeling atlas (25) were used, with the exception of the ventral striatum, which we defined according to Martinez et al. (26, 27).
We tested the dimensional hypothesis cross-sectionally, using a trend analysis (28) of the gradual activation change in the significant clusters across unmatched healthy adolescents (N=1,453), adolescents with subthreshold depression (N=101), and adolescents with clinical depression (N=22).
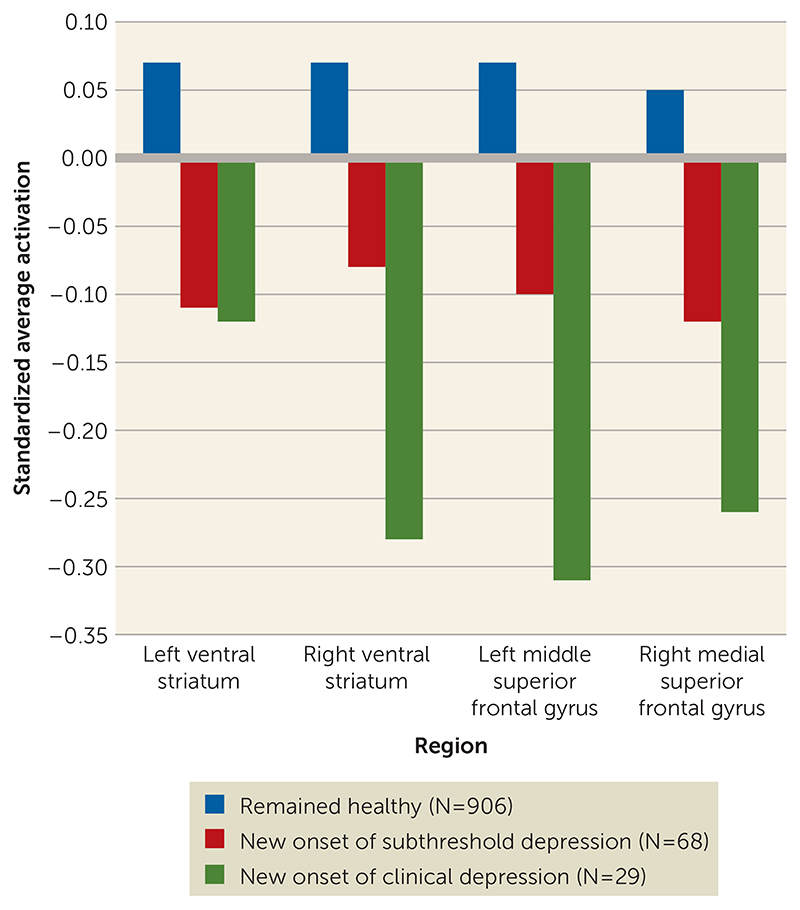
We predicted onset of subthreshold and clinical depression in previously healthy adolescents with logistic regression models with transition to subthreshold or clinical depression as the dependent variable and brain activations as the independent variable, adjusting for baseline depressive symptoms. We performed a trend analysis to examine the gradual change of activation in significant clusters in healthy adolescents across three groups: adolescents who remained healthy (N=906), those who developed subthreshold depression (N=68), and those who developed clinical depression (N=29) at the 2-year follow-up.
We used three methods to test the hypothesis that reduced ventral striatum activation is related to anhedonia but not to low mood. First, we compared brain activations between adolescents who reported anhedonia (N=254) and those who did not (N=1,322), using linear regression. Second, we compared brain activations between adolescents with (N=691) and without low mood (N=885). Third, we compared adolescents with only low mood (N=510), with only anhedonia (N=72), with low mood and anhedonia (N=183), and with no symptoms at all (N=536) using analysis of covariance. Because those with both symptoms—low mood and anhedonia— were more severely impaired (F=26.53, df=2, 762, p<0.001), the modelwas adjusted for functional impairment using the impact subscale of the Strengths and Difficulties Questionnaire.
To examine the specificity of response to reward with depressive symptoms, we used logistic regression models with type of symptom dimensions at 2-year follow-up as the outcome and brain activations as predictor of interest.
Analyses were completed in Stata, adjusting for sex, age, handedness, and pubertal status. The robust cluster option was used to adjust for the effects of imaging site and thus to account for the nonindependence of participants from the same site.
Results
Table 1 provides demographic and clinical information for the three groups at baseline and at 2-year follow-up.
Table 1. Demographic and Clinical Variables for Adolescents With Subthreshold or Clinical Depression and Healthy Matched Comparison Subjects at Baseline and at 2-Year Follow-Up.
| Assessment Point and Measure | Group and Disposition | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1. Healthy matcheda (N=123) | 2. Subthreshold depression (N=101) | 3. Clinical depression (N=22) | 1 versus 2 | 1 versus 3 | 2 versus 3 | |||
| Mean | SD | Mean | SD | Mean | SD | p | p | p | |
| Age (years) | 14.4 | 0.4 | 14.5 | 0.4 | 14.4 | 0.3 | 0.329 | 0.794 | 0.753 |
| Pubertal statusb | 3.7 | 0.6 | 3.8 | 0.6 | 4 | 0.5 | 0.240 | 0.055 | 0.205 |
| General psychopathologyc | 8.4 | 3.8 | 13.9 | 4.7 | 16.4 | 4.5 | <0.001 | <0.001 | 0.025 |
| N | % | N | % | N | % | p | p | p | |
| Female | 90 | 73 | 66 | 65 | 19 | 86 | 0.205 | 0.187 | 0.053 |
| Family history of depressiond | 3 | 3 | 7 | 8 | 4 | 24 | 0.095 | 0.001 | 0.067 |
| Any conduct disorder | 4 | 3 | 13 | 13 | 4 | 18 | 0.007 | 0.005 | 0.513 |
| Any anxiety disorder | 5 | 4 | 33 | 33 | 17 | 77 | <0.001 | <0.001 | <0.001 |
| Two-year follow-up | 1. Remained healthy (unmatched) (N=902) | 2. New subthreshold depression (N=68) | 3. New clinical depression (N=29) | 1 versus 2 | 1 versus 3 | 2 versus 3 | |||
| Mean | SD | Mean | SD | Mean | SD | p | p | p | |
| Age (years) | 16.4 | 0.4 | 16.4 | 0.4 | 16.5 | 0.5 | 0.557 | 0.199 | 0.487 |
| Pubertal status at baselineb | 3.6 | 0.7 | 3.7 | 0.6 | 4.1 | 0.7 | 0.114 | <0.001 | 0.012 |
| General psychopathologyc | 8.9 | 4.6 | 11.9 | 5.1 | 16.6 | 5.4 | <0.001 | <0.001 | <0.001 |
| N | % | N | % | N | % | p | p | p | |
| Female | 446 | 49 | 43 | 63 | 24 | 83 | 0.026 | <0.001 | 0.057 |
| Family history of depressiond | 59 | 8 | 7 | 11 | 3 | 11 | 0.350 | 0.492 | 0.962 |
| Any conduct disorder | 79 | 9 | 10 | 15 | 10 | 34 | 0.098 | <0.001 | 0.028 |
| Any anxiety disorder | 107 | 12 | 16 | 24 | 16 | 55 | 0.005 | <0.001 | 0.002 |
Comparisons with the unmatched healthy group (N=1,453) revealed that adolescents in the subthreshold and clinical depression groups scored higher in pubertal status and general psychopathology, were more likely to be female, had higher rates of anxiety disorders, and in the case of clinical depression, had higher rates of family history of depression.
Pubertal status was assessed with the Pubertal Development Scale.
General psychopathology was assessed with the Strengths and Difficulties Questionnaire (total score).
Information about family history of depression was available for 1,365 individuals (87% of the sample).
Dimensionality of Brain Response to Reward Anticipation in Adolescents
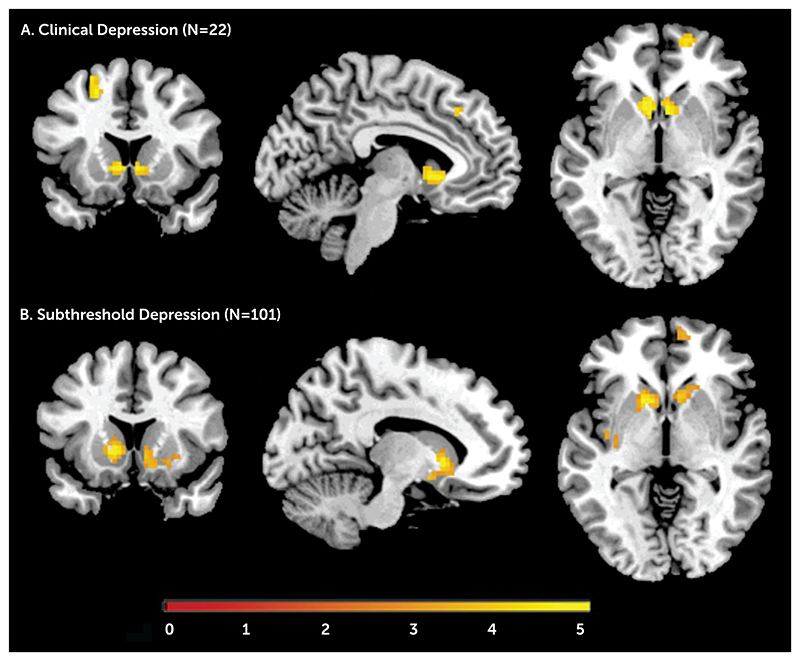
Adolescents with clinical depression showed less activation in the left and right ventral striatum, the right medial superior frontal gyrus, and the left middle superior frontal gyrus relative to the subjects without depression. Similarly, adolescents with subthreshold depression, relative to comparison subjects, had significantly lower activation in the ventral striatum bilaterally (Figure 1; see also Table S1 in the online data supplement). When we used a less conservative threshold (p<0.005 or p<0.01, both uncorrected), adolescents with subthreshold depression also showed reduced activation in the right medial and left superior frontal gyrus.
Figure 1. BOLD Response to Anticipation of Reward in Adolescents With Clinical Depression (N=22) and Subthreshold Depression (N=101)a.
a Blood-oxygen-level-dependent (BOLD) response, overlaid on a T1-weighted structural brain image, is decreased in both groups compared with a matched healthy comparison group (N=123). The image in panel A is centered at x=6, y=15, z=-2, and panel B at x=-12, y=14, z=-2. The color bar represents t values.
Based on these results, we extracted averaged beta values from the left and right ventral striatum, the right medial superior frontal gyrus, and the left middle superior frontal gyrus.
Sex, age, and pubertal status were not related to neural activations.
Trend analyses showed that brain responses to reward anticipation decreased gradually among the three groups (see Figure S3 in the data supplement) for the left (z=23.02, p=0.003) and right ventral striatum (z=22.89, p=0.004) and the right medial superior frontal gryus (z=22.53, p=0.011).
Decreased activation in the ventral striatum predicted transition to subthreshold depression in previously healthy adolescents. Also, decreased activation in the right ventral striatum and the left middle superior frontal gyrus predicted transition to clinical depression 2 years later (Table 2). Reduced ventral striatum response also predicted higher levels of depressive symptoms at follow-up according to the Adolescent Depression Rating Scale (see the data supplement). All these results remained significant after accounting for depressive symptoms at baseline (Table 2). Activations in the right medial superior frontal gyrus did not predict depression.
Table 2. Prediction of Transition to Subthreshold and Clinical Depression at 2-Year Follow-Up in Previously Healthy Adolescents From Baseline Activity in the Ventral Striatum and Left Middle Superior Frontal Gyrus.
| Outcome and Baseline Adjustment | Left Ventral Striatum | Right Ventral Striatum | Left Middle Frontal Gyrus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | |
| Onset of subthreshold depression | |||||||||
| Standarda | 0.81 | 0.69, 0.96 | 0.016 | 0.82 | 0.71, 0.95 | 0.007 | 0.87 | 0.68, 1.12 | 0.284 |
| Symptoms at baseline | 0.82 | 0.69, 0.96 | 0.016 | 0.82 | 0.72, 0.95 | 0.007 | 0.88 | 0.68, 1.12 | 0.297 |
| Onset of clinical depression | |||||||||
| Standarda | 0.80 | 0.58, 1.11 | 0.184 | 0.66 | 0.45, 0.97 | 0.037 | 0.71 | 0.53, 0.96 | 0.027 |
| Symptoms at baseline | 0.81 | 0.60, 1.10 | 0.180 | 0.68 | 0.47, 0.99 | 0.042 | 0.74 | 0.56, 0.98 | 0.035 |
Standard adjustment includes sex, age, handedness, and pubertal status. The robust cluster option was used for scanning site.
Trend analyses in healthy adolescents followed up over a 2-year period showed that activation in the right ventral striatum (z=22.02, p=0.043), the left middle superior frontal gyrus (z=22.38, p=0.017), and the right medial superior frontal gyrus (z=22.02, p=0.044) decreased gradually across adolescents who remained healthy, those who developed subthreshold depression, and those who developed clinical depression (Figure 2).
Figure 2. Standardized BOLD Response in Adolescents Who Were Healthy at Baseline and Either Remained Healthy at 2-Year FollowUp or Developed Subthreshold or Clinical Depression.
Specificity of Brain Response to Reward Anticipation in Adolescents in the Community-Based Sample
Adolescents with anhedonia (N=254) showed significantly decreased activation compared with those without anhedonia (N=1,322) in the ventral striatum bilaterally (left: β=20.20, p<0.001; right: β=20.16, p=0.015) but not in the left middle superior frontal and right medial superior frontal gyri. No significant differences in activation in the extracted regions were found between adolescents who reported low mood (N=691) and those who did not (N=885).
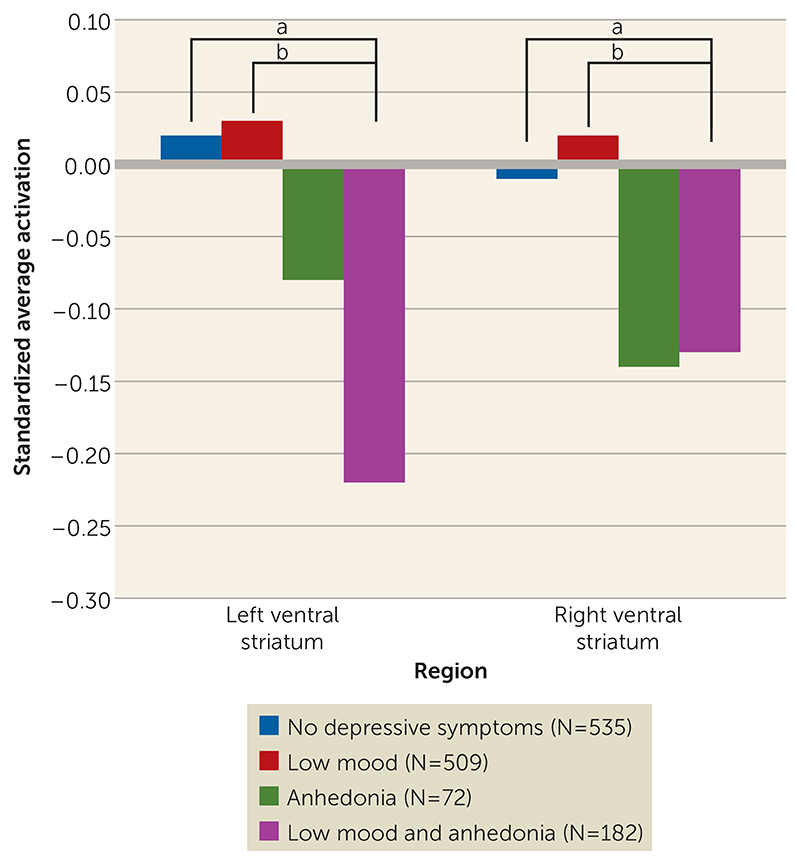
Next, we split the group of adolescents with anhedonia into those with low mood (N=182) and those without low mood (N=72). Adolescents with concurrent anhedonia and low mood symptoms had decreased activation in the ventral striatum bilaterally compared with adolescents with only low mood and adolescents without symptoms (Figure 3). These results remained significant after adjustment for functional impairment. Full comparisons between groups in brain activations as well as in demographic and clinical variables are provided in Tables S2 and S3 in the online data supplement.
Figure 3. Standardized BOLD Response in Left and Right Ventral Striatum in Adolescents From the Community-Based Sample With No Depressive Symptoms, With Low Mood Only, With Anhedonia Only, or With Both Symptoms.
a Left: β=20.28, p=0.006; right: β=20.18, p=0.019.
b Left: β=20.27, p=0.006; right: β=20.15, p=0.027.
In addition, there was a significant gradual decrease across healthy adolescents without anhedonia, healthy adolescents with anhedonia, adolescents with subthreshold depression, and adolescents with clinical depression in all examined regions except the left middle superior frontal gyrus (left ventral striatum: z=-3.51, p<0.001; right ventral striatum: z=-3.16, p=0.002; left middle superior frontal gyrus: β=-1.45, p=0.146; right medial superior frontal gyrus: β=-2.15,p=0.032) (see Figure S4 in the data supplement).
Based on the cross-sectional findings, we tested whether decreased reward network activations predicted anhedonia at follow-up. We found that decreased activation in the left ventral striatum predicted having concurrent anhedonia and low mood over low mood only (odds ratio=0.84, 95% CI=0.73, 0.98, p=0.030). Also, decreased activation in all four significant clusters predicted having both symptoms over no symptoms at all (left ventral striatum: odds ratio=0.79, 95% CI=0.65, 0.97, p=0.027; right ventral striatum: odds ratio=0.81, 95% CI=0.69, 0.94, p=0.007; left middle superior frontal gyrus: odds ratio=0.72, 95% CI=0.63, 0.82, p<0.001; right medial superior frontal gyrus: odds ratio=0.70, 95% CI=0.58, 0.85, p<0.001).
Adjusting the analyses for medication use had no effect on the results (data available on request).
Exploratory Analysis: Association of Depression and Anhedonia With Ventral Striatum Response to Positive and Negative Outcomes
Adolescents with subthreshold depression showed an increased response to positive outcomes on the fMRI task compared with matched healthy comparison subjects (left ventral striatum: β=0.30, p=0.037; right ventral striatum: β=0.36, p=0.019). No significant differences were found for clinical depression, anhedonia, or low mood (see Table S4 in the data supplement).
For negative outcomes, adolescents with subthreshold depression showed enhanced neural response (right ventral striatum: β=0.36, p=0.008). The same was true for adolescents with anhedonia (left ventral striatum: β=0.27, p<0.001; right ventral striatum: β=0.18, p=0.012). No significant differences were found for adolescents with clinical depression or low mood.
Discussion
In a large community-based sample of adolescents, we found that the reduced brain response to anticipation of reward in the ventral striatum varies across the risk spectrum for depression, is specific to anhedonia, and increases the risk for anhedonia and low mood as well as for depressive disorder at 2-year follow-up.
These findings of reduced activation during reward anticipation in frontostriatal regions, both in adolescents with subthreshold depression and in those with clinical depression, are consistent with findings from high-risk studies of depression (3, 29). Our finding of a graded significant decrease in brain activation across groups suggests that frontostriatal response to reward anticipation operates as a mechanism across the spectrum of risk for depression.
Also, low ventral striatum activation was present only in those who had anhedonia, suggesting that this symptom is a behavioral marker of striatal activation. However, it should be noted that adolescents in the anhedonia-only group did not differ significantly in ventral striatal activation from those with low mood only or those without depressive symptoms. There were few participants with anhedonia only, and this may have underpowered our analysis.
The graded increase in the probability of depression with decreasing ventral striatum activation is akin to a doseresponse relationship. Taken together with the specificity of the ventral striatum underactivation for anhedonia, it strengthens the inference that ventral striatum-based reward processing may be a mechanism—and therefore part of the causal chain—leading to depression. However, further steps— for example, the alleviation of depression through intervention targeting the ventral striatum—are required for establishing its role as a causal event, as opposed to a marker, in depressive illness.
We also explored the effects of positive and negative outcomes on the fMRI task. Consistent with previous studies (30, 31), we found little evidence for alterations during positive outcomes. By contrast, we found that adolescents with anhedonia, but not those with low mood, showed increased activation in the ventral striatum during negative outcome. This is in keeping with previous results showing that anhedonia may be associated with sensitivity to punishment (32). As these results were exploratory, they should be further replicated.
Depression is a heterogeneous syndrome with a variety of symptoms among which anhedonia and low mood appear as core features. According to the Research Domain Criteria framework, anhedonia is linked to the positive valence system, whereas low mood is linked to the negative valence system (6). This division is not arbitrary; anhedonia and low mood are associated with different expression of symptoms (33), developmental trajectories (11), and clinical outcomes (9, 10). Moreover, anhedonia has been associated with striatal functional connectivity involving the anterior cingulate cortex, whereas depression severity has been associated with striatal networks involving the ventromedial prefrontal cortex (34). Interestingly, the medial prefrontal cortex, which also showed reduced response to reward anticipation in our depressed group (though it was not linked to anhedonia), is essential to integrating functions leading to “affectivemeaning” through its subcortical connections (35, 36). Indeed, our findings on the ventral striatum and its specific link with anhedonia are consistent with the notion that regulation of positive affect is a critical feature of adolescent depression (5).
Our results showed that a 1-point decrease in standardized ventral striatum activation increased the probability of future subthreshold depression by 20% and clinical depression by 35%, even when accounting for depressive symptoms at baseline. Moreover, a graded decrease was observed across healthy adolescents who, at 2-year follow-up, remained healthy, developed subthreshold depression, or developed clinical depression. In addition, decreased ventral striatum activation also predicted concurrent anhedonia and low mood at 2-year follow-up. Although the contribution to the variance of depression was small, our results suggest that reward circuit alterations precede the clinical expression of depression.
Although adolescents in the depression groups were more likely to be female and had a higher mean pubertal status score, it is unlikely that these differences could account for the neural findings. First, our main analyses were based on a sex-matched group. Second, all analyses in unmatched groups were adjusted for sex and pubertal status. Third, a post hoc analysis revealed that higher pubertal status score was associated with sex but not with depression, and there was no sex-by-depression group interaction. Finally, neither sex nor pubertal status score nor age was correlated with ventral striatum activations, although this may have been because 80% of our sample was between 14 and 15 years old and thus our analyses may have had insufficient variability.
This study should be seen in the light of several limitations. First, our fMRI task was based on monetary rewards rather than social rewards. Future longitudinal studies should distinguish between types of reward and depressive mechanisms. However, altered neural response to reward in depression is a remarkably consistent finding despite differences in fMRI paradigms and depression indices across studies (37). Second, symptoms of anhedonia and low mood were each assessed with a single question, so we were unable to examine different degrees of severity. Third, the DAWBA only provides information about the past 4 weeks rather than lifetime depression. Fourth, because our follow-up data did not span the period of maximum risk for developing depression, we may have underestimated the extent to which neural activation may predict transition.
Supplementary Material
Footnotes
Author and Article Information
From the Department of Child and Adolescent Psychiatry, King’s College London, Institute of Psychiatry, Psychology, and Neuroscience, London; INSERM, UMR 1000, Research Unit Imaging and Psychiatry, Service Hospitalier Frédéric Joliot, Orsay, France; University Paris-Sud 11, Orsay; Paris Descartes University,Sorbonne Paris Cité, Paris; AP-HP, Department of Adolescent Psychopathology and Medicine, Maison de Solenn, Cochin Hospital, Paris; Psychiatry Department 91G16, Orsay Hospital, Orsay; Neurospin, Commissariat à l’Energie Atomique et aux Energies Alternatives, Paris; Department of Social and Health Care, Psychosocial Services Adolescent Outpatient Clinic Kauppakatu 14, Lahti, Finland; Department of Psychiatry, University of Montreal, CHU Ste Justine Hospital, Montreal; Department of Cognitive and Clinical Neuroscience, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany; Universitaetsklinikum Hamburg Eppendorf, Hamburg, Germany; Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Charité-Universitätsmedizin, Berlin, Germany; Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim/ Heidelberg University, Germany; Institute of Neuroscience, Trinity College Dublin, Dublin, Ireland; MRC Social, Genetic, and Developmental Psychiatry Centre, London; Department of Psychiatry and Psychotherapy, Campus Charité Mitte, Charité–Universitätsmedizin, Berlin; Departments of Psychiatry and Psychology, University of Vermont, Burlington; School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom; Physikalisch-Technische Bundesanstalt, Braunschweig and Berlin; Rotman Research Institute, University of Toronto, Toronto; Department of Psychiatry and Psychotherapy and the Neuroimaging Center, Department of Psychology, Technische Universität Dresden, Dresden, Germany.
Dr. Stringaris, Mr. Vidal-Ribas Belil, and Dr. Artiges contributed equally as first authors; Dr. Martinot, Dr. Schumann, and Dr. Paillère-Martinot contributed equally as senior authors. Authors 19–35 are listed alphabetically. Presented in part at the 69th annual meeting of the Society of Biological Psychiatry, New York, N.Y., May 8–10, 2014.
Supported by the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology)(LSHM-CT-2007-037286), the FP7 project IMAGEMEND (Imaging Genetics for Mental Disorders) and the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558),the Swedish funding agency FORMAS, the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the U.K. Department of Health, the Bundesministerium für Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), and NIH (grant RO1 MH085772-01A1). Further support was provided by the French funding agency ANR (ANR-12-SAMA-0004), an Eranet-Neuron grant (project AF12-NEUR0008-01-WM2NA), the Assistance Publique Hôpitaux de Paris and INSERM (interface grant), Paris Descartes University (collaborative project 2010), Paris Sud University (IDEX-2012), the Fondation de France, and the Mission Interministérielle de Lutte Contre la Drogue et la Toxicomanie.
Dr. Stringaris has received funding from the Wellcome Trust and the U.K. National Institutes of Health Research, funds from University College London for a joint project with Johnson & Johnson, and royalties from Cambridge University Press and Oxford University Press. Mr. Vidal-Ribas Belil was supported by the Alicia Koplowitz Foundation. Dr. Goodman is the owner of Youthinmind, Ltd., which provides no-cost and low-cost software and web sites related to the Development and Well-Being Assessment and the Strengths and Difficulties Questionnaire. Dr. Banaschewski has been involved in clinical trials conducted by Shire and Viforpharma, has served as an adviser or consultant for Eli Lilly, Hexal Pharma, Medice, Novartis, Otsuka, Oxford Outcomes, PCM Scientific, Shire, and Viforpharma, and has received conference attendance support and conference support or speaking fees from Eli Lilly, Medice, Novartis, and Shire. Dr. Walter receives an honorarium for his work on the editorial board of Nervenheilkunde and has received a speaking honorarium from Servier. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, the German Science Foundation, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb and speaking fees from AstraZeneca, Bristol-Myers Squibb, and Janssen-Cilag. Dr. Paillère-Martinot has received compensation from Janssen-Cilag for CME activities. The other authors report no financial relationships with commercial interests.
Contributor Information
IMAGEN Consortium members:
JB Poline, Z Pausova, K Mann, GJ Barker, C Lawrence, E Loth, M Rietschel, TW Robbins, L Reed, S Williams, A Lourdusamy, S Costafreda, A Cattrell, C Nymberg, L Topper, L Smith, S Havatzias, K Stueber, C Mallik, TK Clarke, D Stacey, Wong C Peng, H Werts, S Williams, C Andrew, S Desrivieres, S Zewdie, I Häke, N Ivanov, A Klär, J Reuter, C Palafox, C Hohmann, C Schilling, K Lüdemann, A Romanowski, A Ströhle, E Wolff, M Rapp, R Brühl, A Ihlenfeld, B Walaszek, F Schubert, C Connolly, J Jones, E Lalor, E McCabe, A NíShiothcháin, R Spanagel, F Leonardi-Essmann, W Sommer, S Vollstaedt-Klein, S Steiner, M Buehler, E Stolzenburg, C Schmal, F Schirmbeck, N Heym, C Newman, T Huebner, S Ripke, E Mennigen, K Muller, V Ziesch, L Lueken, J Yacubian, J Finsterbusch, N Bordas, Z Bricaud, A Galinowski, C Gourlan, Y Schwartz, C Lalanne, A Barbot, B Thyreau, J Dalley, A Mar, N Subramaniam, D Theobald, N Richmond, M de Rover, A Molander, E Jordan, E Robinson, L Hipolata, M Moreno, M Arroyo, D Stephens, T Ripley, H Crombag, Y Pena, M Lathrop, D Zelenika, S Heath, D Lanzerath, B Heinrichs, T Spranger, B Fuchs, C Speiser, F Resch, J Haffner, P Parzer, R Brunner, A Klaassen, I Klaassen, P Constant, X Mignon, T Thomsen, S Zysset, A Vestboe, J Ireland, and J Rogers
References
- 1.Forbes EE, Dahl RE. Research review: altered reward function in adolescent depression: what, when, and how? J Child Psychol Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerestes R, Davey CG, Stephanou K, et al. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olino TM, McMakin DL, Morgan JK, et al. Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev Cogn Neurosci. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein DN, Shankman SA, Lewinsohn PM, et al. Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome de-pressive disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 7.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paillère-Martinot M-L, Aubin F, Martinot J-L, et al. A prognostic study of clinical dimensions in adolescent-onset psychoses. Schizophr Bull. 2000;26:789–799. doi: 10.1093/oxfordjournals.schbul.a033494. [DOI] [PubMed] [Google Scholar]
- 11.Pine DS, Cohen E, Cohen P, et al. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 12.Knutson B, Adams CM, Fong GW, et al. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treadway MT, Bossaller NA, Shelton RC, et al. Effort-based decisionmaking in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp C, Kim S, Herman L, et al. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J Abnorm Psychol. 2014;123:298–309. doi: 10.1037/a0036191. [DOI] [PubMed] [Google Scholar]
- 15.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bragulat V, Paillère-Martinot M-L, Artiges E, et al. Dopaminergic function in depressed patients with affective flattening or with impulsivity: [18F]fluoro-L-dopa positron emission tomography study with voxel-based analysis. Psychiatry Res. 2007;154:115–124. doi: 10.1016/j.pscychresns.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Schumann G, Loth E, Banaschewski T, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 18.Goodman R, Ford T, Richards H, et al. The Development and WellBeing Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 19.Goodman A, Heiervang E, Collishaw S, et al. The “DAWBA bands” as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Soc Psychiatry Psychiatr Epidemiol. 2011;46:521–532. doi: 10.1007/s00127-010-0219-x. [DOI] [PubMed] [Google Scholar]
- 20.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Stringaris A, Goodman R. The value of measuring impact alongside symptoms in children and adolescents: a longitudinal assessment in a community sample. J Abnorm Child Psychol. 2013;41:1109–1120. doi: 10.1007/s10802-013-9744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 23.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Nymberg C, Banaschewski T, Bokde AL, et al. DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance. Neuropsychopharmacology. 2014;39:2357–2365. doi: 10.1038/npp.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 26.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 27.Mehta MA, Gore-Langton E, Golembo N, et al. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:543–547. doi: 10.1002/sim.4780040416. [DOI] [PubMed] [Google Scholar]
- 29.Forbes EE, Christopher May J, Siegle GJ, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chentsova-Dutton Y, Hanley K. The effects of anhedonia and depression on hedonic responses. Psychiatry Res. 2010;179:176–180. doi: 10.1016/j.psychres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Padrão G, Mallorquí A, Cucurell D, et al. Neurophysiological differences in reward processing in anhedonics. Cogn Affect Behav Neurosci. 2013;13:102–115. doi: 10.3758/s13415-012-0119-5. [DOI] [PubMed] [Google Scholar]
- 33.Buckner JD, Joiner TE, Jr, Pettit JW, et al. Implications of the DSM’s emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159:25–30. doi: 10.1016/j.psychres.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabbay V, Ely BA, Li Q, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–641.:e13. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang WN, Chang SH, Guo LY, et al. The neural correlates of reward-related processing in major depressive disorder: a metaanalysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.