Abstract
Eradicating leukemia requires a deep understanding of the interaction between leukemic cells and their protective microenvironment. The CXCL12/CXCR4 axis has been postulated as a critical pathway dictating leukemia stem cell (LSC) chemoresistance in AML due to its role in controlling cellular egress from the marrow. Nevertheless, the cellular source of CXCL12 in the acute myeloid leukemia (AML) microenvironment and the mechanism by which CXCL12 exerts its protective role in vivo remain unresolved. Here, we show that CXCL12 produced by Prx1+ mesenchymal cells but not by mature osteolineage cells provide the necessary cues for the maintenance of LSCs in the marrow of an MLL::AF9-induced AML model. Prx1+ cells promote survival of LSCs by modulating energy metabolism and the REDOX balance in LSCs. Deletion of CXCL12 leads to the accumulation of reactive oxygen species and DNA damage in LSCs, impairing their ability to perpetuate leukemia in transplantation experiments, a defect that can be attenuated by antioxidant therapy. Importantly, our data suggest that this phenomenon appears to be conserved in human patients. Hence, we have identified Prx1+ mesenchymal cells as an integral part of the complex niche-AML metabolic intertwining, pointing towards CXCL12/CXCR4 as a target to eradicate parenchymal LSCs in AML.
Introduction
Acute myeloid leukemia (AML) is the most common malignant myeloid disorder in adults and displays a particularly high incidence of relapse and poor prognosis. The MLL::AF9 fusion protein, which results from the t(9;11) (p22;q23) translocation, is one of the most common MLL gene translocations, accounting for about 7% of all human AML cases (1).
AML develops from a rare population of leukemia stem cells (LSCs), which are responsible for tumor relapse(2–4). LSCs share a regulatory microenvironment with normal hematopoietic stem and progenitor cells (HSPCs). The contribution of the niche to the development ofhematological malignancies is twofold: 1) alterations in the niche provide the necessary conditions for mutant HSPC clones to arise or flourish(5–7) and 2) neoplastic cells remodel the niche to facilitate their outgrowth (8, 9).
The CXCL12-CXCR4 axis is a mediator of the interplay between LSCs and the bone marrow (BM) microenvironment (10). Elevated expression of CXCR4 is associated with adverse prognosis(11) and targeting of CXCR4 has been shown to sensitize leukemic cells to chemotherapy in AML models. Moreover, AML cells upregulate CXCR4 upon chemotherapy, resulting in increased stromal-mediated survival in vitro, suggesting that the upregulation of surface CXCR4 represents a mechanism of chemoresistance(12).
Cxcl12 is expressed by multiple stromal cell populations in the bone marrow(13, 14). Nevertheless, the source of CXCL12 contributing to AML LSC maintenance remains unknown. Furthermore, the effect exerted by CXCR4 inhibitors is believed to depend on the egress of leukemic cells from the BM, nevertheless, the implication of the CXCL12/CXCR4 axis in LSCs retained in the marrow parenchyma deserves further investigation.
Here, by using a murine model of MLL::AF9 induced AML(4), we demonstrate that early mesenchymal stromal cells (MSCs)are an integral part of the MLL::AF9 derived AML niche and control LSC maintenance. CXCL12 from MSCs regulates the oxidative state of LSCs and modulates energy metabolism. Furthermore, the protective role of the niche through the activation of the CXCL12/CXCR4 axis may also represent a biological hallmark in human pediatric and adult AML.
Material and Methods
Mice
C57BL/6JRj mice were purchased from Janvier Laboratory (bx47363). B6.SJL-Ptprca Pepcb/BoyJ (002014), B6(FVB)-Cxcl12tm1.1Link/J (CXCL12fl/fl)(14) (021773), B6.Cg-Tg(Prrx1-cre)1Cjt/J (Prx1-cre)(15) (005584) and B6.Cg-Tg(Col1a1-cre/ERT2)1Crm/J (Col2.3-creERT2)(16) (016241) mice, all maintained on C57BL/6 genetic background, were purchased from Jackson Laboratory.
Leukemia induction by bone marrow transplant
Leukemia was established as previously described(4, 17) by transplanting 2.5x105 MLL::AF9 leukemic cells (expressing CD45.1) into age and sex-matched non-irradiated recipient mice.
Flow cytometry and Fluorescent-Activated Cell Sorting (FACS)
Immunophenotypic characterization and isolation of hematopoietic populations was performed as previously described(18–20). For details see supplementary information.
Homing assay
2.5x105 MLL::AF9 leukemic cells were intravenously infused through the tail vein and mice were euthanized 1-, 3- and 24-hours post-transplantation. CD45.1 leukemic cells homed to BM were analyzed by flow cytometry.
Seahorse metabolic extracellular flux profiling
Leukemic cells were enriched for Lin- and CD45.2- population by immunomagnetic selection using Miltenyi’s LD columns (Miltenyi Biotec, Bergisch Gladbach, Germany). OCR and ECAR were analyzed following protocols recommended by Seahorse Biosciences using the Seahorse XFp analyzer (Agilent Technologies, Santa Clara, CA, USA) and the XF Cell Mito Stress Test, XF Glycolysis Stress Test and XF Glycolytic Rate Assay Kits (see supplementary information).
Profiling by RNA-Seq
Low input 3´end RNA-Seq was performed following Massively Parallel RNA sequencing (MARS-Seq) protocol adapted for bulk RNA-Seq with minor modifications(21). (see supplementary information)
Results
CXCR4 pathway activity discriminates human AML patients with a transcriptional signature enriched in DNA damage, oxidative stress and metabolic reprogramming
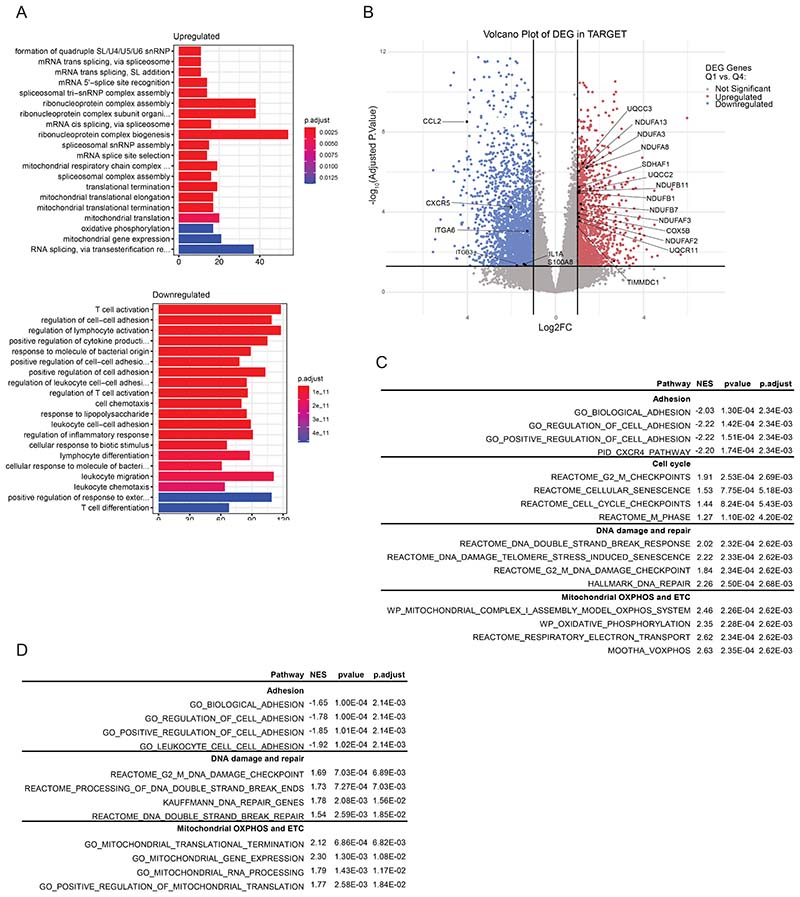
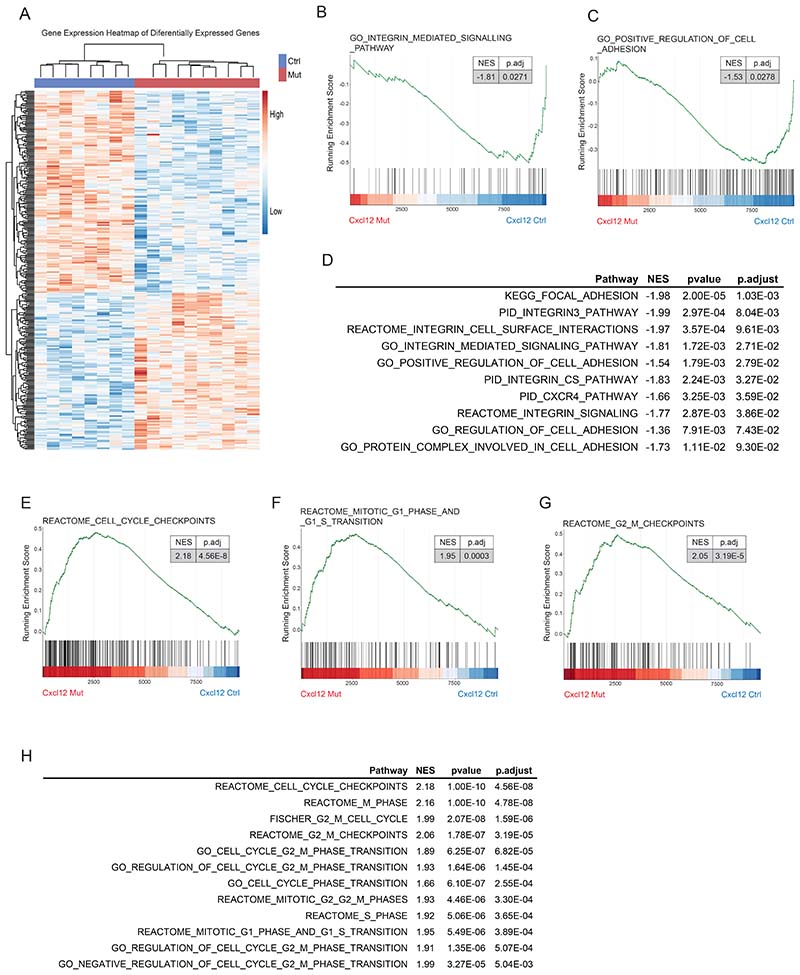
To explore the role of the CXCL12/CXCR4 axis in human AML,we made use of two publicly available datasets that include transcriptional data of adult (Beat AML cohort)(22) and pediatric patients (TARGET initiative)(23). Gene expression was compared between patients showing high (Q4) and low (Q1) transcriptional activity of the CXCR4 pathway (Supp. Fig. 1A, B). Pairwise comparisons between pediatric (TARGET initiative) patients with high versus low CXCR4 activity revealed a total of 4273 differentially expressed genes (adjusted p<0.05, |log2FC|>1) (Supp. Fig. 1C and Supp. Table 1). GO analysis evidenced an enrichment in functions related to mitochondrial integrity, the electron transport chain and energy metabolism in patients with low CXCR4 pathway activity (Fig. 1A). Genes coding for subunits of the mitochondrial Complex I, III IV, as well as Complex I, II and III assembly proteins were found upregulated in the patients with low CXCR4 pathway activation, suggesting a metabolic reprogramming in these patients (Fig. 1B, Supp. Fig. 1D and Supp. Table 1). Patients with low CXCR4 pathway activation exhibited a deficiency in cell adhesion and chemotaxis pathways (Fig. 1A) with downregulation of genes implicated in AML drug resistance, progression and differentiation (Fig. 1B, Supp. Fig. 1E and Supp. Table 1). GSEA revealed a depletion in pathways associated with cell adhesion and an enrichment in cell cycle, genotoxic stress and metabolic reprogramming associated pathways (Fig. 1C and Supp. Table 2). Similarly, analysis of adult leukemia patients (from the BEAT AML cohort) showed a depletion in pathways associated with cell adhesion and an enrichment in pathways associated with metabolic reprogramming and DNA damage (Supp. Fig. 1F and Supp. Table 3). This data suggest that the CXCL12/CXCR4 axis not only modulates cell adhesion but also the metabolic activity of LSCs in AML, potentially protecting leukemic cells from genotoxic stress-resulting mitochondria dysfunction.
Figure 1. CXCR4 pathway activity discriminates human AML patients with a transcriptional signature enriched in DNA damage, oxidative stress and metabolic reprogramming.
(A) Gene Ontology (GO) enrichment analysis showing the top twenty most upregulated and downregulated pathways in leukemic cells with low CXCR4 pathway activation from the TARGET dataset. (B) Volcano plot showing the differentially expressed genes (adjusted p<0.05, |log2FC|>1) in leukemic cells with low CXCR4 pathway activation from the TARGET dataset. (C)Gene set enrichment analysis (GSEA) of the transcriptional signature of leukemic cells showing a deficiency in signatures related with cell adhesion and migration, as well as, an enrichment of pathways related to cell cycle, DNA damage and repair and mitochondrial respiration in patients with low CXCR4 pathway activation from theTARGET dataset.
Deletion of Cxcl12 from Prx1+ mesenchymal cells modulates leukemia dynamics in MLL::AF9-driven AML
In order to explore which cells in the microenvironment are responsible for the production of CXCL12 and its impact on leukemia dynamics in vivo we used the previously described MLL::AF9 retrovirally-induced AML model(4). This model allows for the prospective isolation of leukemia stem/progenitor cells (LSCs). Previous studies have shown that Cxcl12 is expressed by multiple cellular constituents in the bone marrow microenvironment, with mesenchymal stromal cells being the major source of CXCL12 in the BM(13, 14).
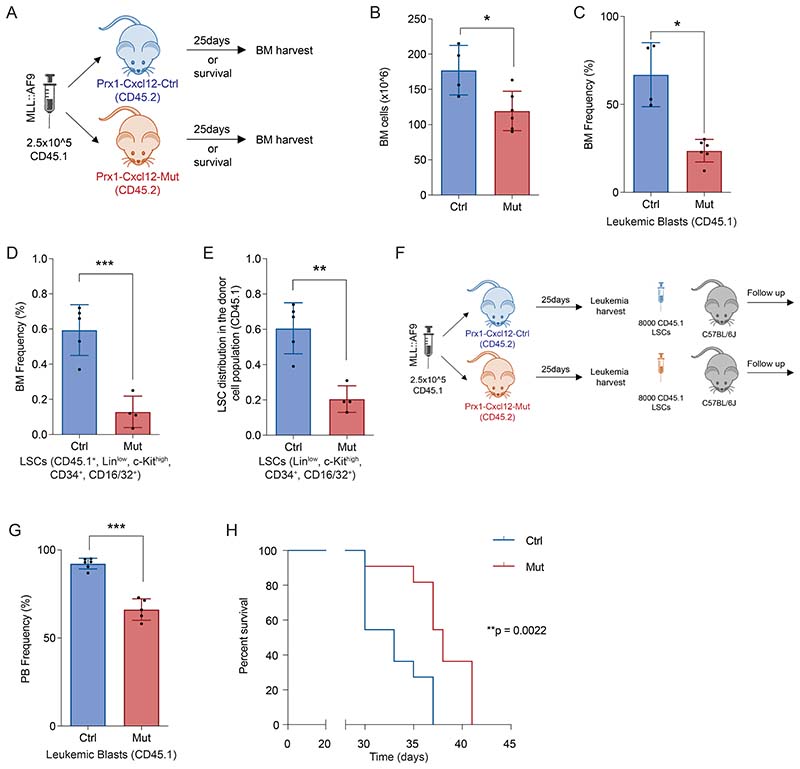
To assess whether CXCL12 expressed by MSCs is necessary for AML leukemia maintenance, we crossed Cxcl12fl/fl mice(14) with mice expressing cre-recombinase under the control of the Prrx1 promoter/enhancer (Prx1-cre)(15) to allow specific deletion of Cxcl12 in Prx1+MSCs. qPCR analysis revealed reduced Cxcl12 expression in MSCs isolated from Prx1-cre+-Cxcl12fl/fl mice (Prx1-Cxcl12-Mut) compared to MSCs from Prx1-cre--Cxcl12fl/fl mice (Prx1-Cxcl12-Ctrl)(Supp. Fig 2A). Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut mice (CD45.2) were transplanted with MLL::AF9 cells (CD45.1) to establish secondary AML (Fig. 2A). A dose of 2.5x105 leukemic blasts per mouse was used in order to achieve 100% leukemia penetrance without the need for irradiation (Supp. Fig. 2B).
Figure 2. Deletion of Cxcl12 from Prx1+ mesenchymal cells modulate leukemia dynamics in MLL::AF9-driven AML.
(A) Schematic overview of the experimental design for AML induction in the Prx1-Cxcl12 mice. (B) Bone marrow analysis showing the frequency of CD45.1leukemic cells(left panel)and the frequency of LSCs (CD45.1+,Linlow, c-Kithigh, Sca1-, CD34+, CD16/32+)(right panel).(C) Kaplan-Meier curve showing survival of C57BL6J mice (n=11/group) transplanted with equal numbers of LSCs from Prx1-Cxcl12-Mut (n=6) and Prx1-Cxcl12-Ctrl (n=6) animals. Data are representative of at least 2 independent experiments. n=5 to 6 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
Deletion of Cxcl12 in Prx1-MSCs revealed that normal hematopoiesis (cells from the recipient origin, CD45.2) in the BM, was significantly preserved when compared to control animals 25 days post leukemia induction (Supp. Fig. 2C-E). Most importantly, BM cellularity (Supp. Fig. 2F), leukemia infiltration (Fig. 2B lef panel) and the frequency of LSCs (Fig. 2B right panel) in the BM of Prx1-Cxcl12-Mut mice were significantly reduced compared to their control littermates. Further characterization of the leukemic clone revealed a change in its composition, with a significantly lower frequency of LSCs (among the leukemic CD45.1 population only) in the Prx1-Cxcl12-Mut mice compared to their littermate controls (Supp. Fig. 2G).
We could not observe differences in survival between leukemic Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut mice (Supp. Fig. 2H). Work described below implicates disbalance of energy and redox pathways in LSCs upon Cxcl12 deletion from Prx1+ stromal cells. LSCs are the most sensitive to these disbalances accounting for the phenotype observed(17), although the relative survival of bulk leukemia may be sufficient to negate any differences in mice survival that could have been seen. In addition, the lack of difference in survival could be attributed to the fact that Pxr1-cre targets cells derived from limb bud mesoderm(15), so the deletion of Cxcl12 only occurred in the long bones but not in the axial skeleton, allowing for normal leukemia growth in those bones. Nevertheless, in order to further demonstrate that the deletion of Cxcl12 has a functional role in AML maintenance, we isolated LSCs or leukemic blasts (CD45.1 cells) from the BM of Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut mice and transplanted them in equal numbers (8000 CD45.1 LSCs or 105 CD45.1 blasts) into non-irradiated C57BL/6J mice (Supp. Fig.2I).
Thirty days post transplantation, the leukemia infiltration in the peripheral blood (PB) of the animals receiving leukemic blasts from the Prx1-Cxcl12-Mut mice showed a significant decrease in the number of leukemic blasts and preserved normal hematopoiesis compared to mice receiving cells from the Prx1-Cxcl12-Ctrl animals (Supp. Fig.2J,K). Moreover, animals receiving LSCs or leukemic blasts from the Prx1-Cxcl12-Mut mice survived significantly longer compared to those animals receiving cells from the Prx1-Cxcl12-Ctrl animals (Fig. 2C and Supp. Fig. 2L). Together, these data demonstrate that CXCL12 produced by Prx1-MSCs regulates LSCs behavior and promotes leukemia progression.
CXCL12 produced by mature osteolineage cells does not affect leukemia dynamics in MLL::AF9-driven AML
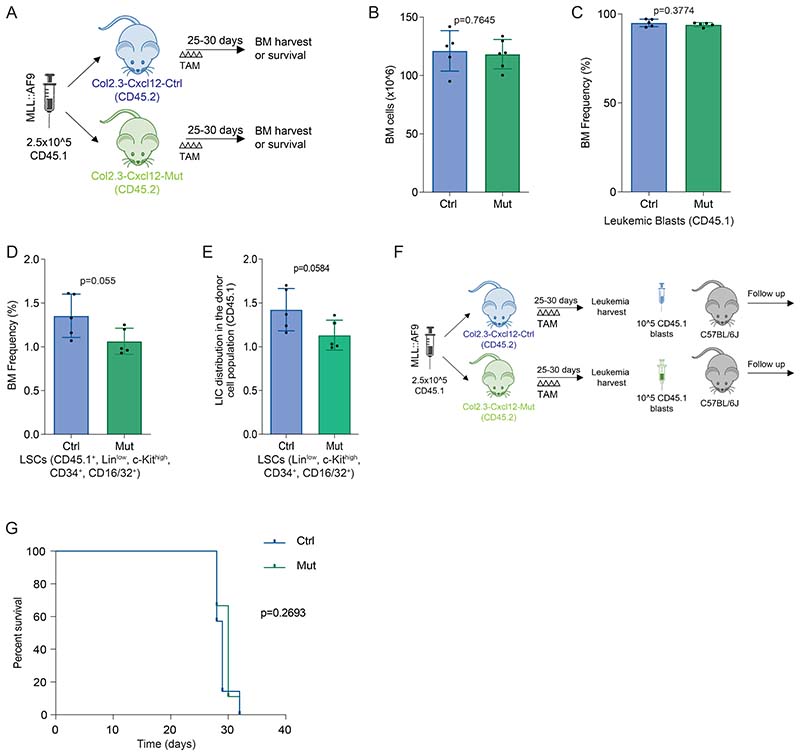
Prx1 is not only expressed in early MSCs but also in their osteolineage cell progeny, which is also known to produce CXCL12(13). In order to resolve whether CXCL12 produced from either of these two BM compartments is necessary for leukemia maintenance, we crossed Cxcl12fl/fl mice(14) with mice expressing cre-recombinase under the control of the Col2.3 promoter (Col2.3-creERT2)(16) to allow specific deletion of Cxcl12 in committed osteoblasts. Six to ten weeks old Col2.3-creERT2--Cxcl12fl/fl mice (Col2.3-Cxcl12-Ctrl) and Col2.3-creERT2+-Cxcl12fl/fl mice (Col2.3-Cxcl12-Mut)were transplanted with MLL::AF9 cells (2.5x105). The cre- recombinase was induced by tamoxifen administration (Fig. 3A).
Figure 3. CXCL12 produced by mature osteolineage cells does not affect leukemia dynamics in MLL::AF9-driven AML.
(A) Schematic overview of the experimental design for AML induction in the Col2.3-Cxcl12 mice. (B) Bone marrow analysis showing: the frequency of CD45.1leukemic cells(left panel) andthe frequency of LSCs (CD45.1+,Linlow, c-Kithigh, Sca1-, CD34+, CD16/32+)(right panel). (C) Kaplan-Meier curve showing survival of C57BL6J mice (n=12/group)transplanted with equal numbers of LSCs from Col2.3-Cxcl12-Mut (n=6) and Col2.3-Cxcl12-Ctrl (n=6) animals. TAM denotes the administration of tamoxifen (100 mg/Kg/day, IP) 2 days after leukemia infusion for 4 consecutive days. Data are representative of at least 2 independent experiments. n=5 to 6 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
Twenty-five to thirty days post leukemia infusion, BM cellularity (Supp. Fig. 3A), leukemia infiltration (Fig. 3B left panel), frequency of LSCs (Fig. 3B right panel) and the leukemic clone composition (Supp. Fig. 3B) were equivalent between the Col2.3-Cxcl12-Mut mice and their littermate controls. We did not observe differences in the residual normal hematopoiesis (Supp. Fig. 3C-E) neither in the survival between the leukemic Col2.3-Cxcl12-Ctrl and Col2.3-Cxcl12-Mut mice (Supp. Fig. 3F). Transplantation of equal numbers of freshly isolated cells (105CD45.1 blasts) from the BM of Col2.3-Cxcl12-Ctrl or Col2.3-Cxcl12-Mut into non-irradiated C57BL/6J mice showed equivalent survival (Fig. 3C and Supp.Fig. 3G), demonstrating that the production of CXCL12 from osteolineage committed cells does not affect LSC cell fate and is dispensable for the evolution of MLL::AF9-derived AML.
Sustained deprivation of CXCL12 produced by Prx1-MSCs does not affect LSC homing nor increase their egressfrom the BM
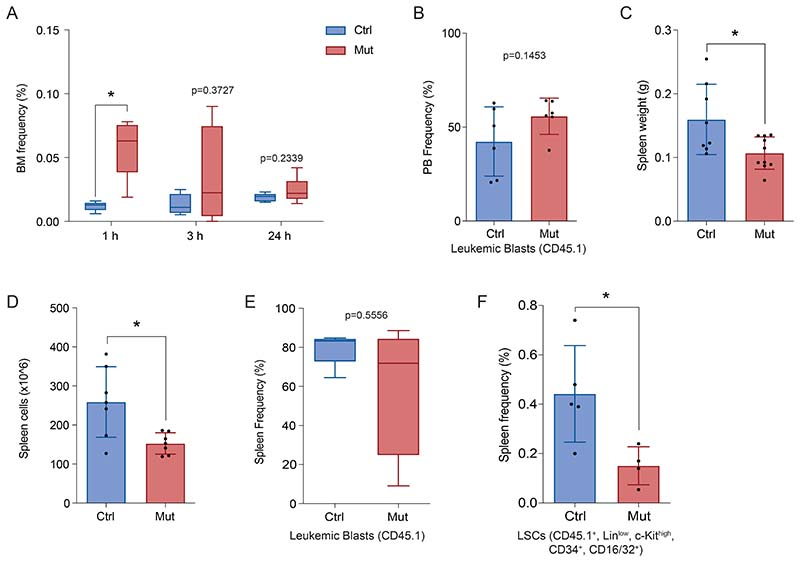
While our data argue that MSC-derived CXCL12 regulates LSC fate regardless of their ability to home to the BM, we explored whether homing of MLL::AF9 cells was impaired by the lack of Prx1+MSCs-produced CXCL12. Leukemic MLL::AF9 cells (2.5x105) were transplanted into non-irradiated Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut mice. Quantification of CD45.1-MLL::AF9 cells in the BM of recipient animals at 1, 3 and 24 hours after leukemia infusion did not reveal a reduced capacity of homing of leukemic cells in the Prx1-Cxcl12-Mut mice (Fig. 4A).
Figure 4. Sustained deprivation of CXCL12 produced by Prx1-MSCs does not affect LSC homing nor their localization in the bone marrow.
(A) Homing assay showing the frequency of CD45.1 leukemic cells in the BM of Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice 1, 3 and 24 hours post infusion of leukemic cells. (B) Leukemia infiltration in the peripheral blood (PB) of Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl 25 days after leukemia induction. (C) Spleen analysis showing: the frequency of CD45.1leukemic cells(left panel) and the requency of LSCs (CD45.1+,Linlow, c-Kithigh, Sca1-, CD34+, CD16/32+), 25 days post-transplant(right panel). Data are representative of at least 2 independent experiments. n=6 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
Besides homing, the CXCL12/CXCR4 axis has been implicated in leukemic cell retention in the BM. Hence, to evaluate whether the changes in LSCs dynamics in the Prx1-Cxcl12-Mut microenvironment are solely a consequence of cell egress from the marrow, we assessed for mislocalization of leukemic cells by examining the peripheral blood and the spleen. Twenty-five days post leukemia induction, mutant and control animals showed equivalent infiltration of leukemic cells in the peripheral blood (Fig. 4B). Importantly, Prx1-Cxcl12-Mut mice had significantly smaller spleen (Supp. Fig. 4A,B). Immunophenotypic characterization of the spleen revealed no changes in the leukemic burden (Fig. 4C left panel) and a substantial reduction in the frequency of LSCs (Fig. 4C right panel) in Prx1-Cxcl12-Mut mice.
Taken together, our results show that deletion of Cxcl12 from Prx1-MSCs does not affect homing or mislocalization of AML-LSCs in the spleen and denote a wider role for the CXCL12/CXCR4 axis in controlling MLL::AF9 driven LSC fate.
Deletion of Cxcl12 from mesenchymal stromal cells leads to cell cycle arrest and cell death in LSCs
We next sought to unravel the molecular re-wiring of LSCs exposed to a mutant microenvironment. To this end, we isolated LSCs from Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut mice (n=4 and 5 mice respectively) 30 days post leukemia induction and performed RNA sequencing (21). Pairwise comparison revealed a total of 359 differentially expressed genes (p.adjust<0.05)(157 upregulated and 202 downregulated) in LSCs exposed to a MSC-derived CXCL12 deficient microenvironment (Supp.Fig. 5A and Supp. Table 4).
Gene set enrichment analysis (GSEA) revealed a downregulation of pathways associated with cellular migration, adhesion and cell-cell communication (including CXCR4 activation) in LSCs isolated from the Prx1-Cxcl12-Mut mice (Fig. 5A and Supp. Table 5). These results suggest that CXCL12 cooperates with multiple other cell adhesion and migration pathways implicated in controlling leukemia-niche interactions,as recently shown in other cancers(24).
Figure 5. Deletion of Cxcl12 from mesenchymal stromal cells leads to cell cycle arrest and cell death in LSCs.
(A) Gene set enrichment analysis (GSEA) of the transcriptional signature of LSCs showing a depletion in signatures related to integrin signaling (left panel) and regulation of cell adhesion (right panel) in LSCs from the Prx1-Cxcl12-Mut mice (NES: Normalized enrichment score). (B) Frequency of LSCs in G0/G1 and S, G2/M phases of the cell cycle of LSCs from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. (C) Cell death measured by Annexin-V, TO-PRO-3 staining in LSCs from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. Data are representative of at least 2 independent experiments. n=6 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
LSC interactions with different niche constituents through adhesion and migration signals in leukemia(25, 26) have been previously shown to regulate cell proliferation. Our transcriptional analysis of LSCs exposed to a CXCL12 deficient microenvironment revealed an enrichment in pathways associated with cell cycle and the activation of cell cycle checkpoints (Supp. Fig. 5B-D and Supp. Table 5). Further, pairwise comparisons evidenced the upregulation of genes implicated in the regulation of cell cycle transition such as Ccnb2, Cdc45 and Lmnb1(27–29), mitotic chromosome organization such as Aurkb and Haus1(30, 31) or control of DNA integrity such as Fancd2 or Clspn(32, 33), among others (Supp. Fig. 5E and Supp. Table 4). Altogether suggesting that these cells are undergoing replication stress. In agreement with this, Ki67-TOPRO-3 staining of LSC from Prx1-Cxcl12-Mut mice suggested the stall of the cell cycle, with LSC accumulation in G0/G1 and a subsequent decrease of cells in S, G2/M phases of the cell cycle (Fig. 5B). Furthermore,cell death in LSCs and leukemic blasts was increased in the Prx1-Cxcl12-Mut mice (Fig. 5C and Supp. Fig. 5F). GSEA revealed an enrichment in pathways associated with programmed cell death, p53-dependent apoptosis mediated by p21 and apoptosis via Trail (Supp. Fig. 5G-I and Supp. Table 5). Further, pairwise comparisons revealed a significant downregulation of Gata2, cKit, Morrbid, Csnk1e and Hoxa5 in LSCs exposed to an MSC-derived CXCL12 deficient microenvironment (Supp. Fig. 5J). These genes regulate survival and their silencing leads to reduced proliferation and apoptosis in AML and other cancers(34–41). In addition, genes such as Cdkn1c, Eif5a and Vdac1, which play a key role in mitochondria mediated apoptosis, were significantly upregulated in LSCs exposed to an MSC-derived CXCL12 deficient microenvironment (Supp. Fig. 5K)(42–45). In agreement, staining for cleaved caspase 3 revealed increased apoptosis of LSCs from the Prx1-Cxcl12-Mut mice (Supp. Fig. 5L).
Altogether,these data suggest that niche engaging pathways are intimately regulated in leukemia and control cell cycle progression and cell death in LSCs.
CXCL12 produced by mesenchymal stromal cells protects LSCs from oxidative stress-induced DNA damage and cell death
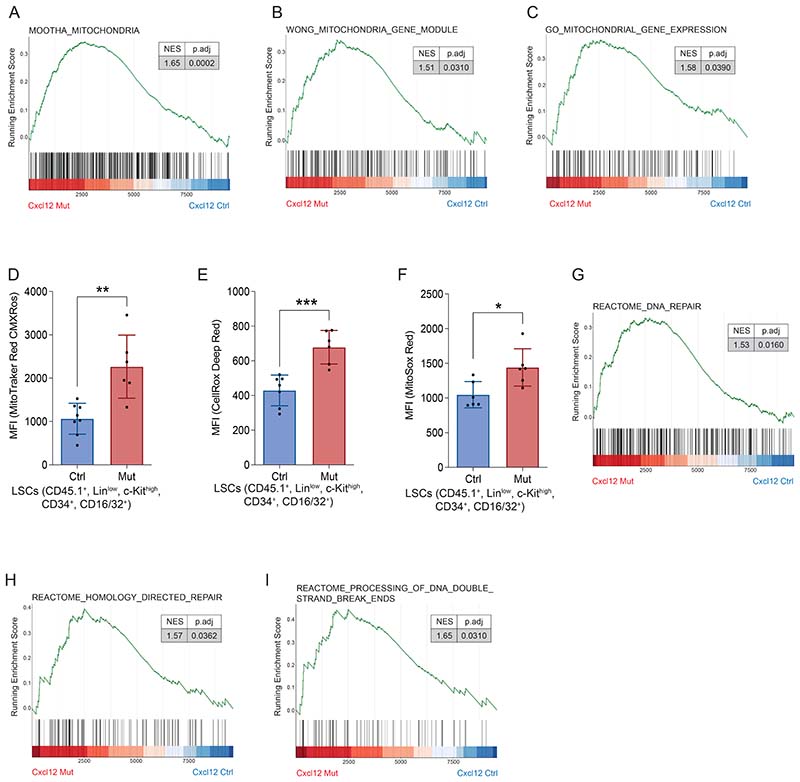
Cell cycle arrest and cell death of LCS upon Cxcl12 deletion from MSCs may result from genotoxic stress(46, 47).
GSEA of LSCs retained in the BM upon CXCL12 deletion from Prx1+ MSCs revealed an enrichment in signatures related to mitochondrial alterations (Supp. Fig. 6A-C and Supp. Table 5). Evaluation of mitochondrial membrane potential revealed a hyperpolarization of the mitochondria in LSCs from the Prx1-Cxcl12-Mut mice, confirming mitochondrial dysfunction (Fig. 6A). Mitochondria hyperpolarization can lead to increased ROS production (48). Importantly, LSCs isolated from Prx1-Cxcl12-Mut mice showed a marked increase in intracellular ROS levels (Fig. 6B) and, in particular, mitochondrial-derived superoxide radicals (Fig. 6C). Similar results were observed in leukemic blasts (Supp. Fig. 5D-F). Accumulation of ROS due to mitochondrial dysfunction often compromises genome integrity, inducing the activation of the DNA damage response (DDR) and DNA repair pathways in normal HSPCs(47). Accordingly, GSEA revealed an enrichment in pathways associated with the DNA damage response DDR and DNA repair in LSCs from the Prx1-Cxcl12-Mut mice (Supp. Fig. 6G-I and Supp. Table 5). Further, levels of γH2AX phosphorylation at serine 139 were significantly increased in LSC from animals deficient in MSC-produced CXCL12 (Fig. 6D).
Figure 6. CXCL12 produced by mesenchymal stromal cells protects LSCs from oxidative stress-induced DNA damage and cell death.
(A) Mitochondrial membrane potential of LSCs isolated from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. (B,C) Quantification of (B) ROS levels and (C) mitochondrial superoxide anion production of LSCs isolated from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. (D) Representative images showing p-γH2AX (ser139) in LSCs isolated from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice and their quantification(right panel). (E) Cell death measured by Annexin-V, TO-PRO-3 staining in LSCs isolated from the Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. (F) Kaplan-Meier curve showing survival of C57BL6J mice (n=12/group) transplanted with equal numbers of LSCs from Prx1-Cxcl12-Ctrl (n=23) and Prx1-Cxcl12-Mut (n=23) animals that were fed NAD or vehicle in the drinking water throughout leukemia development. NAC denotes the addition of N-acetyl-cysteine to the drinking water (1mg/mL), starting 2 days after leukemia infusion. Data are representative of at least 2 independent experiments; n=12 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
In order to test whether excess ROS contributes to the functional defects observed in LSCs from a CXCL12 deficient microenvironment, we fed the ROS scavenger N-acetylcysteine (NAC) in the drinking water to Prx1-Cxcl12-Ctrl and Prx1-Cxcl12-Mut leukemic mice. Treatment with NAC throughout leukemia development decreased the levels of γH2AX phosphorylation at serine 139in LSCs exposed to a CXCL12-deficient microenvironment (Fig.6D) and partially rescued their cell death (Fig.6E), suggesting a direct link in between elevated ROS, genotoxic stress and cell death in the Prx1-Cxcl12-Mut mice. Additionally, NAC was sufficient to restore the leukemogenic capacity of LSCs and leukemic blasts exposed to a CXCL12 deficient microenvironment upon transplantation (Fig. 6F and Supp. Fig. 6J-K).
These results demonstrate that CXCL12 from MSCs regulates the oxidative state of LSCs and preserves their genome integrity in MLL::AF9-induced AML.
Mesenchymal stromal cells control leukemic cells’ energy metabolism through CXCL12 production
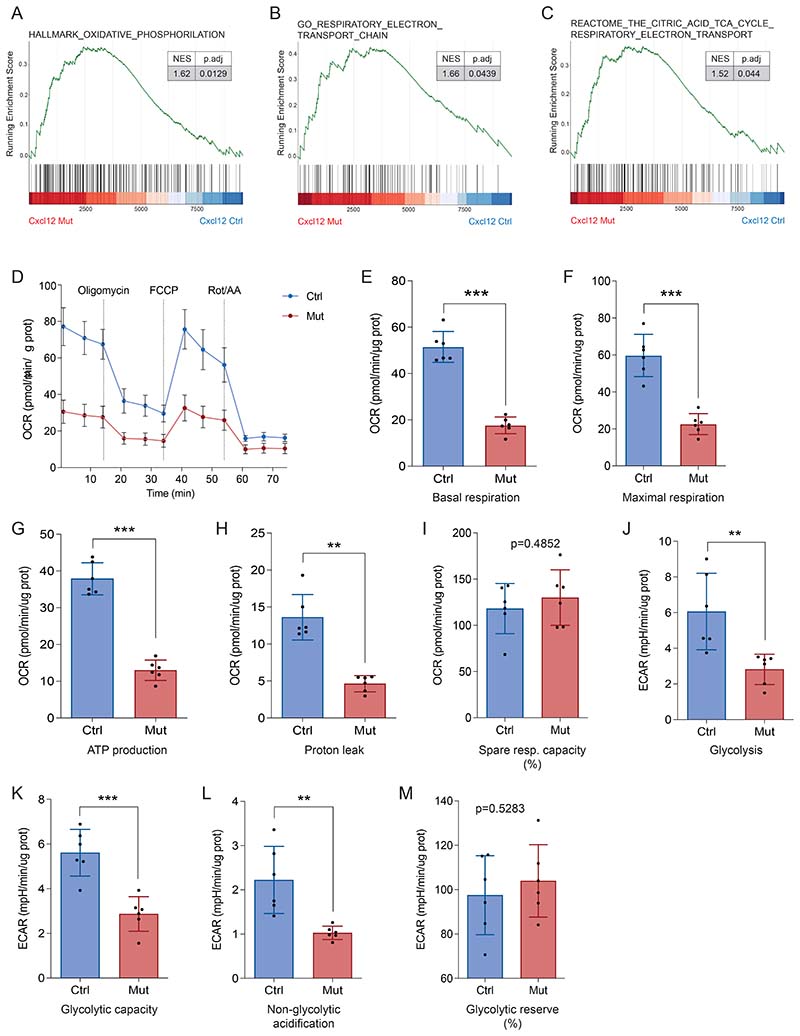
Since the mitochondrial respiratory chain is one of the major sources of ROS, and mitochondrial dysfunction and ROS accumulation is a hallmark of LSCs in the absence of Prx1+ MSC-produced CXCL12, we next evaluated whether LSC’s energy metabolism was altered.
GSEA of LSCs retained in the BM upon Cxcl12 deletion from Prx1+ MSCs revealed an enrichment in pathways associated with the tricarboxylic acid cycle (TCA), OXPHOS and the electron transport chain (ETC) (Supp. Fig. 7A-C and Supp. Table 5), indicative of an altered energy metabolism. To expand this observation, we determined the oxygen consumption rate (OCR), index of OXPHOS, in LSCs-enriched (Lin-, CD45.1+) leukemic cells from the Prx1-Cxcl12-Mut mice. Surprisingly, the OCR was significantly decreased in LSCs-enriched cells from the Prx1-Cxcl12-Mut mice (Fig. 7A). Similarly, basal and maximal respiratory rates (Supp. Fig. 7D, E), ATP production (Fig. 7B) and proton leak (Supp. Fig. 7F), were decreased in LSCs-enriched cells exposed to a CXCL12-deficient microenvironment. The spare respiratory capacity was not changed in LSCs from the Prx1-Cxcl12-Mut mice (Supp. Fig. 7G), suggesting that LSC-enriched cells retain certain ability to respond to high energy demands.
Figure 7. Mesenchymal stromal cells control leukemic cell’s energy metabolism through CXCL12 production.
(A) Oxygen consumption rate (OCR) indicates mitochondrial respiration after oligomycin, FCCP and Rot/AA treatment in LSCs-enriched (Lin-, CD45.1+)cells from Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. (B,C) Seahorse measurement of: (B) ATP production and (C) glycolysis, extracellular acidification rate (ECAR) in LSCs-enriched cells from Prx1-Cxcl12-Mut and Prx1-Cxcl12-Ctrl mice. Data are representative of at least 3 independent experiments; n= 6 mice per genotype per experiment unless otherwise stated. Data are represented as mean and SD. * P< .05; ** P< .01; *** P< .001.
Certain cell types are capable of activating glycolysis, as an alternative to mitochondrial respiration, for energy production(49). LSCs-enriched cells from the Prx1-Cxcl12-Mut mice showed reduced glycolytic activity (Fig. 7C and Supp. Fig.7H) and glycolytic capacity (Supp. Fig. 7I). The non-glycolytic acidification, which measures other sources of extracellular acidification, was much lower in LSC-enriched cells from Prx1-Cxcl12-Mut mice (Supp. Fig. 7J). Additionally, LSC-enriched cells from the Prx1-Cxcl12-Mut mice retain certain ability to respond to high energy demands by activating glycolysis (Supp. Fig. 7K). Evaluation of glycolytic activity taking into consideration the contribution of the mitochondria to CO2 production and acidification confirmed these observations (Supp. Fig. 7L-O).
These results demonstrate that CXCL12 promotes glucose metabolism and energy production in MLL::AF9 leukemic cells.
Discussion
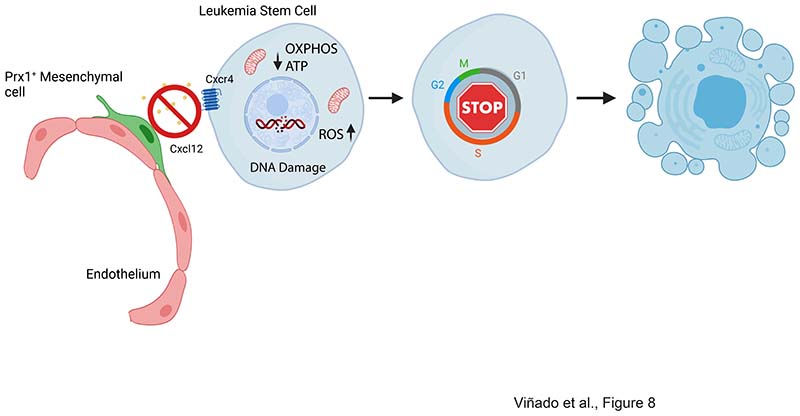
In this study, we demonstrate that mesenchymal stromal cells in the BM microenvironment, characterized by the expression of Prx1-cre, provide the necessary cues for the maintenance of leukemia stem cells (LSC) in a MLL::AF9-induced AML model, a mechanism potentially conserved in human patients. Our work unveiled a previously unrecognized mechanism by which the MSC in the BM microenvironment regulates energy metabolism in LSCs and protects them from the damaging effects of the accumulation of reactive oxygen species (ROS) and genotoxic stress. These findings highlight the importance of the BM microenvironment modulating leukemia maintenance in the absence of pharmacological pressure, allowing the description of parenchymal-mesenchymal interactions during leukemia evolution.
The CXCL12-CXCR4 axis has been proposed as an important mediator of the leukemia-BM microenvironment interaction, yet its role in leukemia biology remains obscure.
CXCR4 expression protect leukemic cells from chemotherapy-induced apoptosis, and correlates with lower disease-free survival(50, 51). These observations have led to the development of small molecules, peptides and antibodies targeting the CXCL12-CXCR4 axis(12). Small molecule antagonists of CXCR4 such as AMD3100 have not fulfilled initial clinical expectations(12), which could be partially explained by AMD3100’s mode of action being limited to blast mobilization, while lacking an effect on the behavior of leukemia stem cells retained in the marrow parenchyma after treatment(51). Beyond inducing mobilization, peptide-like antagonists such as BL-8040 directly induces apoptosis while LY2510924 inhibits proliferation in AML cells(52–55). Antibody based targeting of CXCR4, also pursued clinically, may benefit from Fc domain-mediated effector functions to directly eliminate CXCR4-expressing cells(56, 57). Finally, antagonists of CXCL12 such as CX-01 and NOX-A12 have been developed in order to disrupt the CXCL12-CXCR4 axis and induce chemosensitization(58, 59). Whether any of these drugs modulate energy and redox balance in AML remains to be elucidated. Nevertheless, our observations warrant future pre-clinical and clinical studies further investigating their mechanism of action. Of note, our results highlight that enforced deletion of Cxcl12 from specific stromal populations in the BM has a profound effect on LSC behavior. RNA-seq of parenchymal LSCs revealed a downregulation of adhesion and migration signals in CXCL12 deficient animals. Whether these changes are sufficient to affect the local migratory nature of AML-LSCs remains to be evaluated. Nevertheless, in the absence of Prx-1 cells produced CXCL12, LSCs are unable to engage with their protective niches without evidence of increased egress from the marrow. Altogether suggest that strategies targeting the CXCL12/CXCR4 axis to efficiently target leukemia require the abrogation of parenchymal niche-LSC interactions modulating energy metabolism and REDOX balance. LSC in AML are known to rely on OXPHOS for energy production(49). This metabolic feature of LSCs is, at least in part, controlled by the BM microenvironment. As we demonstrate, CXCL12 produced by Prx1-mesenchymal cells is necessary to maintain energy metabolism in AML, since CXCL12 depletion leads to reduced OXPHOS and ATP production. This is in agreement with a recent report demonstrating that Nestin+ stromal cells increase the bioenergetic capacity and the antioxidant defense of AML cells after chemotherapy(46). Remarkably, these effects are dependent on cell-cell contact, suggesting that abrogating the signals allowing LSCs to engage their niche may overcome these mechanisms of chemoresistance. Importantly, our data demonstrates that the deletion of Cxcl12 from Prx1-MSCs is sufficient to revert theses effects even in the absence of chemotherapy.
But besides energy metabolism, stromal cells in the BM microenvironment also contribute to LSC chemoresistance by modulating pyrimidine biosynthesis. Recently, van Gastel and colleagues demonstrated that LepR+ stromal cells provide glutamine-derived aspartate to LSCs upon chemotherapy to fuel nucleotide biosynthesis, while serum glutamine is uptaken by LSCs to sustain GSH levels(20). While it is currently unknown whether this metabolic dependency also occurs without chemotherapy pressure, the overlap between LepR+ cells and Prx1+ cells and the metabolic shift observed in the latter upon Cxcl12 deletion guarantees future research into this topic.
In summary, our work uncovers a previously unrecognized role of Prx1+ mesenchymal cells controlling AML’s metabolic adaptation in vivo. Novel therapeutics targeting Prx1+ mesenchymal cells or abrogating their ability to produce CXCL12 promises to aid eradication of leukemic cells. Nevertheless, these approaches will require a sustained effect on CXCL12 production, rather than simply inducing LSC mobilization in order to fulfill their expectation.
Supplementary Material
Acknowledgements(291)
We thank P. Garcia-Olloqui, Z. Blasco, E. Petri, E. Santamaría, G. Abizanda and E. Iglesias for their technical assistance. Thanks to S. Sykes and N. van Gastel for their insights and assistance with experiments and the staff of the flow cytometry core, the genomics lab and the animal facility at CIMA . This work was supported by the Instituto de Salud Carlos III (ISCIII) (PI17/01346 and PI20/00152), co-funded by the ERDF (A way to make Europe); FC-AECC (AIO16163636SAEZ); Gobierno de Navarra (0011-3638-2020-000011 and 0011-3597-2020-000005) co-funded by the ERDF through the Operative Program 2014-2020 of Navarra to BS. PI17/00701, and PI20/01308, CIBERONC (CB16/12/00489); ERA-NET Program EraPERMED (MEET-AML); Gobierno de Navarra (AGATA 0011-1411-2020-000010/0011-1411-2020-000011); Fundación La Caixa (GR-NET NORMAL-HIT HR20-00871); and Cancer Research UK [C355/A26819] and FC AECC and AIRC under the Accelerator Award Program to FP. Cancer Research UK [C61367/A26670], MRC [MR/V005421/1] and NHS Blood and Transplant to SM-F and LEL-V. Gobierno de Navarra predoctoral fellowship to ACV. Marie Curie grant (H2020-MSCA-IF-837491) to IAC. AECC predoctoral fellowship to ICA.
Footnotes
Competing Interests statement: Authors declare no conflicts of interest. For a full disclosure of support for this work, please see the acknowledgements section.
Authorship
ACV performed experiments, analyzed and interpreted the data and wrote the manuscript. IAC performed experiments, analyzed data and edited the manuscript. ICA, DO, PSM, JPR, AVZ, LV, MC, PV, EG, FGM, NGC, LPC, LELV, SMF, DO, FPl, IA, TJL LB,PRC and JJR performed experiments and analyzed the data. RZY, APS and FPr interpreted the data, designed experiments and edited the manuscript. BS: conceived the study, performed, analyzed and interpreted the experimental data and wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
References
- 1.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 5.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Zhang H, Rodriguez S, Cao L, Parish J, Mumaw C, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell Stem Cell. 2014;15(1):51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 9.Duarte D, Hawkins ED, Akinduro O, Ang H, De Filippo K, Kong IY, et al. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell. 2018;22(1):64–77.:e6. doi: 10.1016/j.stem.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peled A, Klein S, Beider K, Burger JA, Abraham M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine. 2018;109:11–6. doi: 10.1016/j.cyto.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Ahn JY, Seo K, Weinberg OK, Arber DA. The prognostic value of CXCR4 in acute myeloid leukemia. Appl Immunohistochem Mol Morphol. 2013;21(1):79–84. doi: 10.1097/PAI.0b013e3182606f4d. [DOI] [PubMed] [Google Scholar]
- 12.Cancilla D, Rettig MP, DiPersio JF. Targeting CXCR4 in AML and ALL. Front Oncol. 2020;10:1672. doi: 10.3389/fonc.2020.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Nakashima K, de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004;165(6):1875–82. doi: 10.1016/S0002-9440(10)63240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yusuf RZ, Saez B, Sharda A, van Gastel N, Yu VWC, Baryawno N, et al. Aldehyde dehydrogenase 3a2 protects AML cells from oxidative death and the synthetic lethality of ferroptosis inducers. Blood. 2020;136(11):1303–16. doi: 10.1182/blood.2019001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marcantonio D, Martinez E, Sidoli S, Vadaketh J, Nieborowska-Skorska M, Gupta A, et al. Protein Kinase C Epsilon Is a Key Regulator of Mitochondrial Redox Homeostasis in Acute Myeloid Leukemia. Clin Cancer Res. 2018;24(3):608–18. doi: 10.1158/1078-0432.CCR-17-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saez B, Ferraro F, Yusuf RZ, Cook CM, Yu VW, Pardo-Saganta A, et al. Inhibiting stromal cell heparan sulfate synthesis improves stem cell mobilization and enables engraftment without cytotoxic conditioning. Blood. 2014;124(19):2937–47. doi: 10.1182/blood-2014-08-593426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gastel N, Spinelli JB, Sharda A, Schajnovitz A, Baryawno N, Rhee C, et al. Induction of a Timed Metabolic Collapse to Overcome Cancer Chemoresistance. Cell Metab. 2020;32(3):391–403.:e6. doi: 10.1016/j.cmet.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alameda D, Saez B, Lara-Astiaso D, Sarvide S, Lasa M, Alignani D, et al. Characterization of freshly isolated bone marrow mesenchymal stromal cells from healthy donors and patients with multiple myeloma: transcriptional modulation of the microenvironment. Haematologica. 2020;105(9):e470–3. doi: 10.3324/haematol.2019.235135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526–31. doi: 10.1038/s41586-018-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrar JE, Schuback HL, Ries RE, Wai D, Hampton OA, Trevino LR, et al. Genomic Profiling of Pediatric Acute Myeloid Leukemia Reveals a Changing Mutational Landscape from Disease Diagnosis to Relapse. Cancer Res. 2016;76(8):2197–205. doi: 10.1158/0008-5472.CAN-15-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehghani M, Kianpour S, Zangeneh A, Mostafavi-Pour Z. CXCL12 Modulates Prostate Cancer Cell Adhesion by Altering the Levels or Activities of beta1-Containing Integrins. Int J Cell Biol. 2014;2014:981750. doi: 10.1155/2014/981750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Jaras M, Puram RV, et al. In Vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell. 2013;24(1):45–58. doi: 10.1016/j.ccr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal P, Isringhausen S, Li H, Paterson AJ, He J, Gomariz A, et al. Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell. 2019;24(5):769–84.:e6. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam HJ, van Deursen JM. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol. 2014;16(6):538–49. doi: 10.1038/ncb2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broderick R, Nasheuer HP. Regulation of Cdc45 in the cell cycle and after DNA damage. Biochem Soc Trans. 2009;37(Pt 4):926–30. doi: 10.1042/BST0370926. [DOI] [PubMed] [Google Scholar]
- 29.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25(24):2579–93. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewar H, Tanaka K, Nasmyth K, Tanaka TU. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature. 2004;428(6978):93–7. doi: 10.1038/nature02328. [DOI] [PubMed] [Google Scholar]
- 31.Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol. 2008;181(3):421–9. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharjee S, Nandi S. DNA damage response and cancer therapeutics through the lens of the Fanconi Anemia DNA repair pathway. Cell Commun Signal. 2017;15(1):41. doi: 10.1186/s12964-017-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits VAJ, Cabrera E, Freire R, Gillespie DA. Claspin - checkpoint adaptor and DNA replication factor. FEBS J. 2019;286(3):441–55. doi: 10.1111/febs.14594. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Jia X, Wang J, Li Y, Xie S. Knockdown of homeobox A5 by small hairpin RNA inhibits proliferation and enhances cytarabine chemosensitivity of acute myeloid leukemia cells. Mol Med Rep. 2015;12(5):6861–6. doi: 10.3892/mmr.2015.4331. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Aguilera F, Ramdas B, Daulatabad SV, Srivastava R, Kotzin JJ, et al. Targeting Bim via a lncRNA Morrbid Regulates the Survival of Preleukemic and Leukemic Cells. Cell Rep. 2020;31(12):107816. doi: 10.1016/j.celrep.2020.107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165(2):303–16. doi: 10.1016/j.cell.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang WS, Stockwell BR. Inhibition of casein kinase 1-epsilon induces cancer-cell-selective, PERIOD2-dependent growth arrest. Genome Biol. 2008;9(6):R92. doi: 10.1186/gb-2008-9-6-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menendez-Gonzalez JB, Sinnadurai S, Gibbs A, Thomas LA, Konstantinou M, Garcia-Valverde A, et al. Inhibition of GATA2 restrains cell proliferation and enhances apoptosis and chemotherapy mediated apoptosis in human GATA2 overexpressing AML cells. Sci Rep. 2019;9(1):12212. doi: 10.1038/s41598-019-48589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menendez-Gonzalez JB, Vukovic M, Abdelfattah A, Saleh L, Almotiri A, Thomas LA, et al. Gata2 as a Crucial Regulator of Stem Cells in Adult Hematopoiesis and Acute Myeloid Leukemia. Stem Cell Reports. 2019;13(2):291–306. doi: 10.1016/j.stemcr.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faderl S, Pal A, Bornmann W, Albitar M, Maxwell D, Van Q, et al. Kit inhibitor APcK110 induces apoptosis and inhibits proliferation of acute myeloid leukemia cells. Cancer Res. 2009;69(9):3910–7. doi: 10.1158/0008-5472.CAN-08-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon PM, Dias S, Williams DA. Cytokines secreted by bone marrow stromal cells protect c-KIT mutant AML cells from c-KIT inhibitor-induced apoptosis. Leukemia. 2014;28(11):2257–60. doi: 10.1038/leu.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlachos P, Nyman U, Hajji N, Joseph B. The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ. 2007;14(8):1497–507. doi: 10.1038/sj.cdd.4402158. [DOI] [PubMed] [Google Scholar]
- 43.Magri A, Reina S, De Pinto V. VDAC1 as Pharmacological Target in Cancer and Neurodegeneration: Focus on Its Role in Apoptosis. Front Chem. 2018;6:108. doi: 10.3389/fchem.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Z, Cheng Z, Taylor CA, McConkey BJ, Thompson JE. Apoptosis induction by eIF5A1 involves activation of the intrinsic mitochondrial pathway. J Cell Physiol. 2010;223(3):798–809. doi: 10.1002/jcp.22100. [DOI] [PubMed] [Google Scholar]
- 45.Shoshan-Barmatz V, Krelin Y, Chen Q. VDAC1 as a Player in Mitochondria-Mediated Apoptosis and Target for Modulating Apoptosis. Curr Med Chem. 2017;24(40):4435–46. doi: 10.2174/0929867324666170616105200. [DOI] [PubMed] [Google Scholar]
- 46.Forte D, Garcia-Fernandez M, Sanchez-Aguilera A, Stavropoulou V, Fielding C, Martin-Perez D, et al. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020;32(5):829–43.:e9. doi: 10.1016/j.cmet.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell. 2016;19(5):613–27. doi: 10.1016/j.stem.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Esteras N, Rohrer JD, Hardy J, Wray S, Abramov AY. Mitochondrial hyperpolarization in iPSC-derived neurons from patients of FTDP-17 with 10+16 MAPT mutation leads to oxidative stress and neurodegeneration. Redox Biol. 2017;12:410–22. doi: 10.1016/j.redox.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–41. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–24. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duarte D, Amarteifio S, Ang H, Kong IY, Ruivo N, Pruessner G, et al. Defining the in vivo characteristics of acute myeloid leukemia cells behavior by intravital imaging. Immunol Cell Biol. 2019;97(2):229–35. doi: 10.1111/imcb.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham M, Klein S, Bulvik B, Wald H, Weiss ID, Olam D, et al. The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression. Leukemia. 2017;31(11):2336–46. doi: 10.1038/leu.2017.82. [DOI] [PubMed] [Google Scholar]
- 53.Boddu P, Borthakur G, Koneru M, Huang X, Naqvi K, Wierda W, et al. Initial Report of a Phase I Study of LY2510924, Idarubicin, and Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Front Oncol. 2018;8:369. doi: 10.3389/fonc.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borthakur G, Ofran Y, Tallman MS, Foran J, Uy GL, DiPersio JF, et al. BL-8040 CXCR4 antagonist is safe and demonstrates antileukemic activity in combination with cytarabine for the treatment of relapsed/refractory acute myelogenous leukemia: An open-label safety and efficacy phase 2a study. Cancer. 2021;127(8):1246–59. doi: 10.1002/cncr.33338. [DOI] [PubMed] [Google Scholar]
- 55.Cho BS, Zeng Z, Mu H, Wang Z, Konoplev S, McQueen T, et al. Antileukemia activity of the novel peptidic CXCR4 antagonist LY2510924 as monotherapy and in combination with chemotherapy. Blood. 2015;126(2):222–32. doi: 10.1182/blood-2015-02-628677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu SH, Gu Y, Pascual B, Yan Z, Hallin M, Zhang C, et al. A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies. Blood Adv. 2017;1(15):1088–100. doi: 10.1182/bloodadvances.2016003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bobkov V, Arimont M, Zarca A, De Groof TWM, van der Woning B, de Haard H, et al. Antibodies Targeting Chemokine Receptors CXCR4 and ACKR3. Mol Pharmacol. 2019;96(6):753–64. doi: 10.1124/mol.119.116954. [DOI] [PubMed] [Google Scholar]
- 58.Kovacsovics TJ, Mims A, Salama ME, Pantin J, Rao N, Kosak KM, et al. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018;2(4):381–9. doi: 10.1182/bloodadvances.2017013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludwig H, Weisel K, Petrucci MT, Leleu X, Cafro AM, Garderet L, et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: a Phase IIa Study. Leukemia. 2017;31(4):997–1000. doi: 10.1038/leu.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.