Abstract
In outbred mice, susceptibility or resistance to diet-induced obesity is associated with rapid changes in hypothalamic proopiomelanocortin (POMC) levels. Here, we evaluated three hypotheses that potentially explain the development of the different obesity phenotypes in outbred Swiss mice. First, rapid and differential changes in the gut microbiota in obese-prone (OP) and obese-resistant (OR) mice fed on a high-fat diet (HFD) might cause differential efficiencies in fatty acid harvesting leading to changes in systemic fatty acid concentrations that in turn affect POMC expression and processing. Second, independently of the gut microbiota, OP mice might have increased blood fatty acid levels after the introduction of a HFD, which could affect POMC expression and processing. Third, fatty acids might act directly in the hypothalamus to differentially regulate POMC expression and/or processing in OP and OR mice. We evaluated OP and OR, male Swiss mice using 16S rRNA sequencing for determination of gut microbiota; gas chromatography for blood lipid determination; immunoblot and real-time PCR for protein and transcript determination and indirect calorimetry. Some experiments were performed with human pluripotent stem cells differentiated into hypothalamic neurons. We did not find evidence supporting the first two hypotheses. However, we found that in OP but not in OR mice, palmitate induces a rapid increase in hypothalamic POMC, which is followed by increased expression of proprotein convertase subtilisin/kexin type 1 (PCSK1, PC1/3). Lentiviral inhibition of hypothalamic PC1/3 increased caloric intake and body mass in both OP and OR mice. In human stem cell-derived hypothalamic cells, we found that palmitate potently suppressed the production of POMC-derived peptides. Palmitate directly regulates PC1/3 in OP mice, and likely has a functional impact on POMC processing.
Keywords: Food intake, Hypothalamus, Leptin
Introduction
The consumption of large amounts of dietary fats is an important contributing factor in the development of obesity (1). Studies have shown that dietary saturated fats can trigger hypothalamic inflammation that damages key neurons involved in the regulation of caloric intake and energy expenditure (2–5). Recent studies have shown that both inflammation and anomalous hypothalamic neuronal function occur as early as one day after the introduction of large portions of dietary fats (5, 6).
In a recent study, we explored the question of whether hypothalamic inflammation or defective neuronal function would be the earliest hypothalamic abnormality resulting from the consumption of a high-fat diet (HFD) (6). Using an outbred mouse model that allows the prediction of predisposition or resistance to development of diet-induced obesity (7–10), we observed that defective regulation of POMC transcript expression preceded hypothalamic inflammation. Obesity-prone (OP) mice showed an early reduction (12 h) followed by a rapid (24 h) increase of POMC transcript an effect that was not observed in obesity-resistant (OR) mice. The correction of abnormal hypothalamic POMC regulation could attenuate the OP phenotype (6).
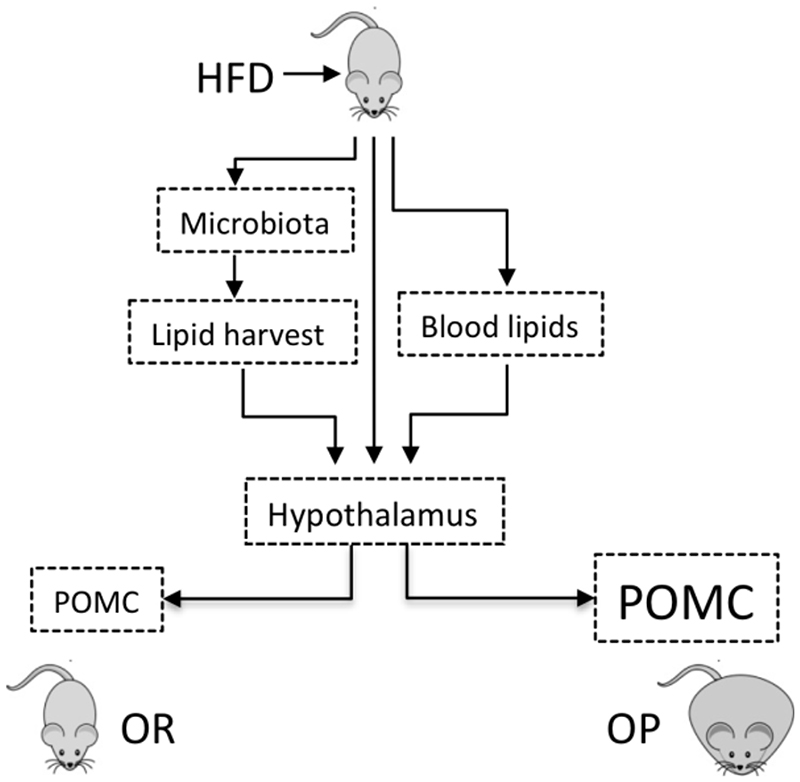
Here, as a follow-up of our previous report (6), we explored factors potentially involved in the defective regulation of hypothalamic POMC in OP mice exposed to saturated fats in the diet. Despite the fact that POMC is modulated as early as 12 h after the introduction of a HFD, we wanted to explore why in OP mice there is an increased expression of POMC after 24 h. We initially hypothesized that hypothalamic POMC transcript levels in OP mice could be affected by one of the following mechanisms: i, changes in the gut microbiota leading to increased fatty acid harvesting; ii, changes in blood fatty acid levels not related to gut microbiota; iii, direct action of fatty acids on hypothalamic POMC expression or processing (Fig. 1).
Figure 1. Schematic representation of the hypotheses tested in this study.
In hypothesis #1, we evaluated if, upon high-fat diet (HFD) feeding, obesity-prone (OP) and obesity-resistant (OR) mice would undergo different changes in the gut microbiota, which would result in different lipid harvesting from the diet impacting on the hypothalamus to produce the different phenotypes. In hypothesis #2, we evaluated if, independently of the gut microbiota, OP and OR mice would present differences in blood lipids, which would impact on the hypothalamus to produce the different phenotypes. In hypothesis #3, we evaluated if saturated fat could directly impact on the hypothalamus to produce the different phenotypes. POMC, proopiomelanocortin.
In the first part of the study, we discarded hypotheses i and ii because none of the parameters were significantly modified when OP and OR mice were compared. However, when testing hypothesis iii, we observed that the systemic infusion of palmitate (palmitic acid, PA) produced significant increase of POMC and PC1/3 gene expression in OP but not in OR mice. Moreover, lentivirus-mediated inhibition of hypothalamic PC1/3 in the hypothalamus was sufficient to promote increased caloric intake and body mass in OR mice. To further explore the clinical relevance of fatty acids on POMC-processing to human biology, we extended this study to look at the effects of fatty acids in human POMC neurons. We added palmitate to human stem-cell derived hypothalamic neurons and observed similar effects on PC1/3 expression at a transcriptional level, and a strong downregulation of POMC products α-MSH and b-endorphin at the peptide level. These results suggest that PC1/3 is directly regulated by palmitate, and this regulation occurs differently in OP and OR mice. Thus, palmitate can rapidly alter melanocortin production in both mice and humans.
Materials and methods
Reagents and kits
Palmitic acid (cat.no P0500), linoleic acid (cat.no L1376), fatty acid-free bovine serum albumin (cat.no A0281), and GW9508 (cat. no 9797), were purchased from Sigma Aldrich. Furin inhibitor I (cat. no 14965) was purchased from Cayman Chemical Company, and protein convertase inhibitor 537076 (cat. no US1537076) was purchased from Calbiochem. Chemically defined lipid concentrate (cat. no 11905031) was purchased from Thermo Fisher Scientific (Suppl. Table 1). The iTaqMan™ Universal PCR Master Mix (cat.no 4444963) was purchased from Thermo Fisher Scientific. The primers for human POMC (Hs01596743_m1), PCSK1 (Hs01026107_m1), PCSK2 (Hs00159922_m1), CPE (Hs00175676_m1), PRCP (Hs00234607_m1), SOCS3 (Hs02330328_s1), GAPDH (Hs99999905_m1), and β-Actin (Hs01060665_g1) and for mice POMC (Mm00435874_m1), PC1/3 (Mm00479023_m1), NPY (Mm03048253_m1), AgRP (Mm00475829_g1), CART (Mm00489086_m1), ORE (Mm01964031_s1), MCH (Mm01242886_g1), TRH (Rn00564880_m1), CRH (Mm01293920_s1), F4/80 (Mm00802529_m1) IL6 (Mm00434228_m1), IL10 (Mm01288386_m1), IL1β (Mm00434228_m1), TNFα (Mm0043258_m1), CD11b (Mm00434455_m1) CX3CL1 (Mm00436454_m1), and GAPDH (4352339E) were purchased from Thermo Fisher Scientific. The antibodies ab3532 (anti-PC1/3) was purchased from Abcam and t5168 (anti-alpha tubulin) was purchased from Sigma-Aldrich.
Experimental animals
Five-week old Swiss mice were housed in individual cages at 22 °C on a 12-h light/dark cycle with food and water access ad libitum. The Ethics Committee at the University of Campinas (protocol #3580-1) approved all experimental procedures involving mice. Investigators were not blinded for performing this study. The number of mice employed in each experiment was five per group, this information is provided in the legends for the Figures. In all experiments using the mice hypothamalus the tissue dissection was performed considering the following anatomical limits: apex of the hypothalamic third ventricle as the superior limit, the optical tracts as the lateral limit, the mammillary bodies as the posterior limit and the optic chiasm as the anterior limit.
Obesity prone and resistant protocol
Five-week-old male Swiss mice were divided into obesity-prone (OP) and obesity-resistant (OR) groups as described previously (6). Briefly, mice were fed a high-fat diet (HDF) for 24 hours and grouped into quartiles for total food intake. The top 25% were defined as OP, whereas the bottom 25% were defined as OR. Thereafter, mice were subdivided into groups fed on chow, HFD, or chow plus the use of a micro-osmotic pump for palmitate infusion. The macronutrient composition of the diets is presented in Supplementary Table 2.
Osmotic infusion of palmitate
Micro-osmotic pump model 1002 (Alzet-Osmotic Pumps, Cupertino-CA) were loaded with palmitate (2.0 mM) or vehicle, and subcutaneously implanted between the scapulae of mice according to the manufacturer’s instructions. Palmitate was pre-diluted in ethanol (4%) and then brought to final concentration in saline; saline used in control infusion contained the same proportion of ethanol as in the palmitate solution. Pump weight was determined before and after delivery (0.450±0.00037 and 0.425±0.00027, respectively). Delivery of palmitate was 0.25 μL/h.
Mice were fed on chow, and 24 h after the beginning of osmotic infusion, the hypothalamus was dissected and processed for mRNA or protein expression analysis.
Stereotaxic surgery and viral injection
Eight-week-old OP and OR mice were stereotaxically injected using a Stoelting (Wood Dale, IL, USA) stereotaxic apparatus. A shRNA to inhibit mouse PC1/3 (SHCLNV-NM_013628) or Scramble (ShC001V) were purchased from Sigma-Aldrich and injected bilaterally into the hypothalamic arcuate nucleus. The hypothalamic PC1/3 inhibition was evaluated by protein content. The stereotaxic coordinates relative to the Bregma were: anteroposterior, −1.50 mm; lateral, +/− 0.3 mm; and depth, 5 mm. Accuracy of the injection site was evaluated by the injection of trypan blue. After recovery from surgery, mice were fed a high-fat diet or chow for 4 weeks. Body mass gain and food intake were measured once per week, and indirect calorimetry was performed at the end of the fourth week.
Immunoblot
For the immunoblot experiments, hypothalamus was homogenized in solubilization buffer (1% Triton X-100, 100 mM Tris (pH 7.4), 100 mM sodium pyrophosphate, 100 mM 4 sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mM PMSF and 0.1 mg/mL 5 aprotinin) at 4°C in a Polytron PTA 20S Generator (Brinkmann Instruments mode EN 6 10/35). The protein levels were measured by biuret assay and 80 micrograms of protein per samples was separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 3% BSA solution in TBST (1× TBS and 0.1% Tween 20) for 2 h, washed tree times with TBST, and incubated with primary antibodies overnight at 4 °C. HRP-coupled secondary antibodies were used for detection of the chemiluminescence, and visualization was achieved by exposure to an Image Quant LAS4000 (GE Healthcare, Life Sciences), using α-tubulin as loading control.
Indirect calorimetry
Oxygen consumption and carbon dioxide production were measured in an LE405 Gas Analyzer (Panlab—Harvard Apparatus, Holliston, MA, USA) over a 24-h period in OP and OR mice transfected with shRNA to inhibit mouse PC1/3 or scramble sequence. Real-time PCR. Mouse hypothalamic total RNA was extracted using TRIzol (Invitrogen cat. #15596026) or using the Nucleospin RNA XS kit (cat. #740902250) for human cells following the recommendations of the manufacturer. An amount of 0.5–2.0 μg of RNA was used to synthesize cDNA by employing the High Capacity cDNA Reverse Transcription Kit (Life Technologies cat. #4368814). Each qPCR reaction contained 40 ng of reverse-transcribed RNA, specific primers, and TaqMan PCR Master Mix and was performed by using the Step One Plus or QuantiStudio 12k Flex (Applied Biosystem).
Serum lipid profile
Serum lipids were directly methylated using a method described elsewhere (11). Fatty acid methyl esters (FAME) were analyzed with a gas chromatograph-mass spectrometer (model GCMS-QP2010 Ultra; Shimadzu) with a Stabilwax column (length, 30 m; internal diameter, 0.25 mm; thickness, 0.25 μm; Restek, USA). High-grade pure helium (He) was used as the carrier gas with a constant flow rate of 1.0 mL/min and specific GC column temperature program. A 1.0 μl sample was injected, the split ratio of the injector being 1:50 with an injection temperature of 80 °C. Mass conditions were as follows: ionization voltage, 70 eV; ion source temperature, 200 °C; full scan mode in the 35–500 mass range with 0.2 s/scan velocity. Oven temperature initially 80 °C for 2 min, was increased up to 150 °C and after 180 °C, with 10 min at holding time. Thereafter, temperature was increased up to 240 °C with 50 min at holding time.
Gut microbiota analysis
The gut microbiota from OP and OR mice fed on chow or high-fat diet for 24 h were measured by sequencing 16S rRNA from feces. Fecal gDNA was extracted from 100 mg of feces per animal using a QIAmp Fast DNA Stool Mini Kit (Qiagen) according to the manufacturer’s instructions and quantified using the Quant-iT Picogreen dsDNA Assay Kit. The 16S rRNA V4-V5 regions were amplified using the Platinum Taq DNA Polymerase High Fidelity enzyme. For the PCR reaction, 100 ng of gDNA was used in conjunction with 10 μM of primers V4F (5’-TCG GCA GCG TGC MGC CGC GGT AA-3’) and V5R (5’-GTCTCG TGG GCT CTT YMT TTR AGT TT-3’), in a total reaction volume of 25 μL. The PCR cycling program was 30 s at 94 °C; 30 cycles of 15 s at 94 °C, 30 s at 62 °C, 45 s at 68 °C; hold at 4 °C. The product was purified with AMPURE XP reagent and quantified with the Quant-iT Picogreen dsDNA Assay Kit. The samples were prepared for 16S ribosomal RNA gene amplicons for the Illumina MiSeq System, and 4 nmol of the samples were used for Miseq Illumina analyses using the V2 2x250bp kit according to the manufacturer’s instructions.
Human hypothalamic differentiation
Human hypothalamic neurons were differentiated from human pluripotent stem cells (hPSCs). The methods employed to prepare the cells are described in detail in a report published by some of us (12). For hypothalamic differentiation, hPSCs were replated onto Geltrex-coated 24 well plates at approximately Day 30 post-differentiation, once POMC-immunopositive neurons could be clearly detected.
Fatty acid stimulus in human hypothalamic cells
Hypothalamic neurons from human pluripotent stem cells (HUES9) tested for mycoplasma contamination were treated at Day 35 post-differentiation with palmitate (4.8 mM), linoleate (4.8 mM), chemically defined lipid concentrate (1:50) (Suppl. Table 1), or controls (vehicle, BSA or vehicle + BSA) for 8 h. BSA was used at 5% in N2B27 medium as described elsewhere(12),43, and the vehicle was 0.2% ETOH in N2B27 medium with 5% BSA. After treatment, the cells were collected in TRIzol and prepared for qPCR analyses. Cell morphology and viability were tested at the beginning and end of the experimental protocols as detailed elsewhere (12).
Mass spectrometry
Human hypothalamic neurons were treated at D29 with palmitate (4.8 mM in 0.2% ethanol + 5% BSA), GW9508 (100 μM), furin inhibitor I (25 μM), protein convertase inhibitor 537076 (25 μM), and controls (0.2% ethanol + 5% BSA or 0.1% DMSO in medium) for 24 h. After treatment, the cells were lysed in 80% acetonitrile (ACN) to enrich for peptides and prepared for liquid chromatography-tandem mass spectroscopy (LC-MS/MS) analysis as described in detail elsewhere (13). Peptide quantification was performed by taking the area under the curve for peaks with m/z and column retention times corresponding to the peptides of interest.
Statistical analysis
All results are presented as mean ± SEM. Levene’s test for the homogeneity of variances was employed to check the required assumptions for parametric ANOVA. The results were analyzed by two-way ANOVA followed by Tukey’s or Bonferroni’s post test to determine the significance of individual differences when three or more groups were compared and by Student’s t-test when two groups were compared. The level of significance was set at P < 0.05. Comparison of peptide quantification groups was performed by t-test with Holm-Sidak correction for multiple comparisons, with alpha set at 0.05.
Results
The gut microbiota is affected similarly by dietary fats in OP and OR mice
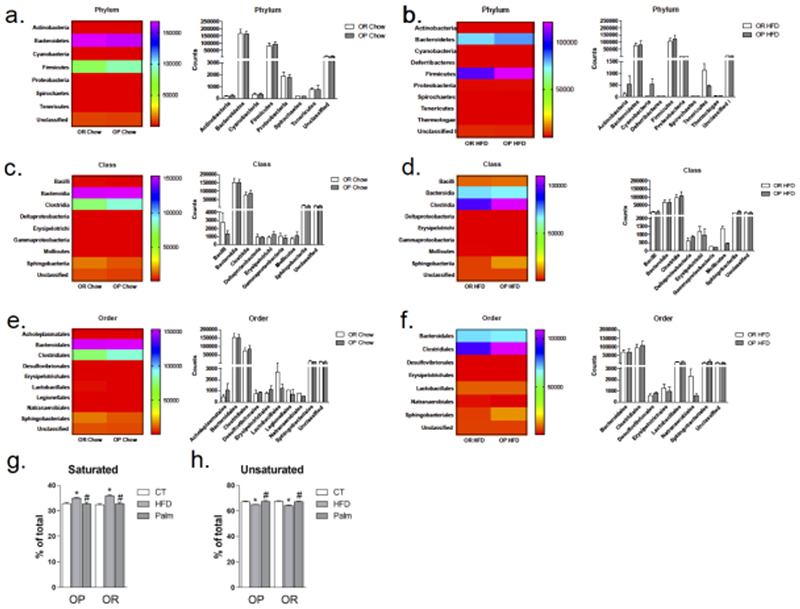
To test the hypothesis that gut microbiota would be affected differently when OP and OR mice were exposed to large portions of dietary fats, mice were fed on chow or HFD (composition of diets in Suppl. Table 2) for 24 h, and feces were collected for 16S rRNA sequencing. There were no significant differences in food intake and body mass between the groups. Under high-fat feeding there was a decrease in Bacterioidetes, but this occurred similarly in OP and OR mice (Suppl. Fig. 1). In addition, gut bacterial taxonomy identification at the Phylum, Class, and Order levels revealed that the consumption of a HFD resulted in a modification in the general landscape of the gut microbiota; however, in no condition was there a significant difference between OP and OR mice (Fig. 2a-2f).
Figure 2. Specific impact of dietary intervention on gut bacterial Phylum, Class, Order and graphic representation of the relative amounts of fatty acids in the blood of mice.
The gut microbiota was analyzed by sequencing V4-V5 regions of 16S rRNA in feces samples. In a, c and e white bars represent OR fed on chow and grey bars OP fed on chow; In b, d, and f white bars represent OR fed on HFD for 24 h and grey bars OP fed on HFD for 24 h; Phylum, Class, and Order were quantified and represented graphically and by heat map. Saturated (g) and unsaturated (h) fatty acids were determined in blood samples collected from OP and OR mice fed on chow (CT) or a HFD for 24 h or infused with palmitate (Palm) for 24 h. The control group for Palm received saline through the osmotic pump; results were statistically similar to the control groups of mice fed chow; therefore the graphs (g and h) display chow control, only. In all conditions n = 5; *p < 0.05 vs. respective CT; #p<0.05 vs. respective HFD. CT, control fed on chow; OR, obesity-resistant mice; OP, obesity-prone mice; HFD, high-fat diet; Palm, palmitate infused.
Blood levels of fatty acids are similar between OP and OR mice
Gas chromatography revealed that mice fed on a HFD presented a significant increase in saturated and a significant decrease of unsaturated blood fatty acids (Fig. 2g-2h and Suppl. Tables 3-4). However, changes occurred similarly in OP and OR mice.
Palmitate increases hypothalamic POMC in OP but not OR mice
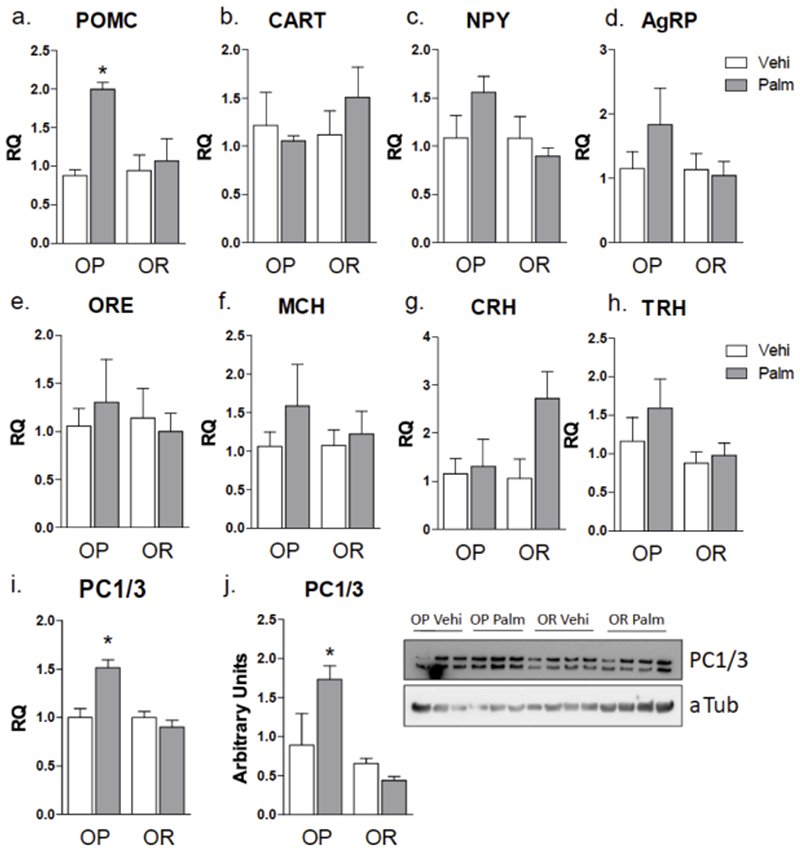
In order to test the effect of saturated fatty acids on the hypothalamus, OP and OR mice were implanted with an osmotic micro-pump loaded with either vehicle or palmitate, and experiments were performed after 24 h. Palmitate infusion did not modify 24-h caloric intake and body mass (not shown), as well as the systemic blood levels of fatty acids (Fig, 2g-2h and Suppl. Tables 3-4); however, in OP but not OR mice, palmitate infusion led to an increase in hypothalamic POMC and PC1/3 expression (Fig. 3a, 3i and 3j). When PC1/3 was measured comparing mice fed on chow vs. HFD without previous sorting by obesity predisposition, only a trend for increasing PC1/3 was found in HDF (not shown). Palmitate infusion resulted in no significant changes in the expressions of CART, NPY, AgRP, Orexin, MCH, CRH, and TRH (Fig. 3b-3h). These results are in concert with the ones obtained when OP and OR mice are fed for short period on a HFD, as previously shown (6).
Figure 3. Graphic representation of transcript expression in the hypothalamus of mice.
Obese-prone (OP) and obese-resistant (OR) mice were implanted subcutaneously with a micro-osmotic pump loaded with vehicle or palmitate, which were delivered for 24 h. The hypothalamus was extracted for RNA preparation and used for real-time PCR determination of transcript expression of POMC (a), CART (b), NPY (c), AgRP (d), orexin (ORE) (e), MCH (f), CRH (g), THR (h), and PC1/3 (i); in addition protein extract was employed in immunoblot determination of PC1/3 expression (j). In all experiments n = 5; * p < 0.05 vs. respective vehicle treated control. Palm, palmitate; vehi, vehicle.
Palmitate induces hypothalamic inflammation in OP but not OR mice
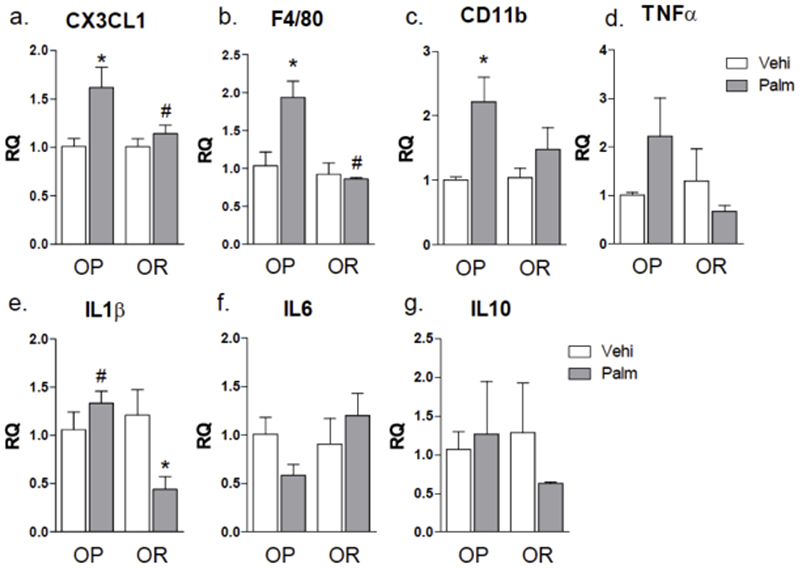
The infusion of palmitate resulted in increased hypothalamic expressions of CX3CL1 (Fig. 4a), F4/80 (Fig. 4b) and CD11b (Fig. 4c) in OP but not OR mice. Moreover, there were significant differences between OP and OR mice receiving palmitate in the hypothalamic expression of IL-1β (Fig. 4e). Neither palmitate nor obesity predisposition impacted in the hypothalamic levels of TNFα (Fig. 4d), IL-6 (Fig. 4f), and IL-10 (Fig. 4g).
Figure 4. Graphic representation of transcript expression in the hypothalamus of mice.
Obese-prone (OP) and obese-resistant (OR) mice were implanted subcutaneously with a micro-osmotic pump loaded with vehicle or palmitate, which were delivered for 24 h. The hypothalamus was extracted for RNA preparation and used for real-time PCR determination of transcript expression of CX3CL1 (a), F4/80 (b), CD11b (c), TNFα (d), IL1β (e), IL6 (f), IL10 (g). In all experiments n = 5; * p < 0.05 vs. respective vehicle treated control; #p<0.05 vs. OR treated with palmitate. Palm, palmitate; vehi, vehicle.
Inhibition of hypothalamic PC1/3 increases caloric intake and body mass in mice
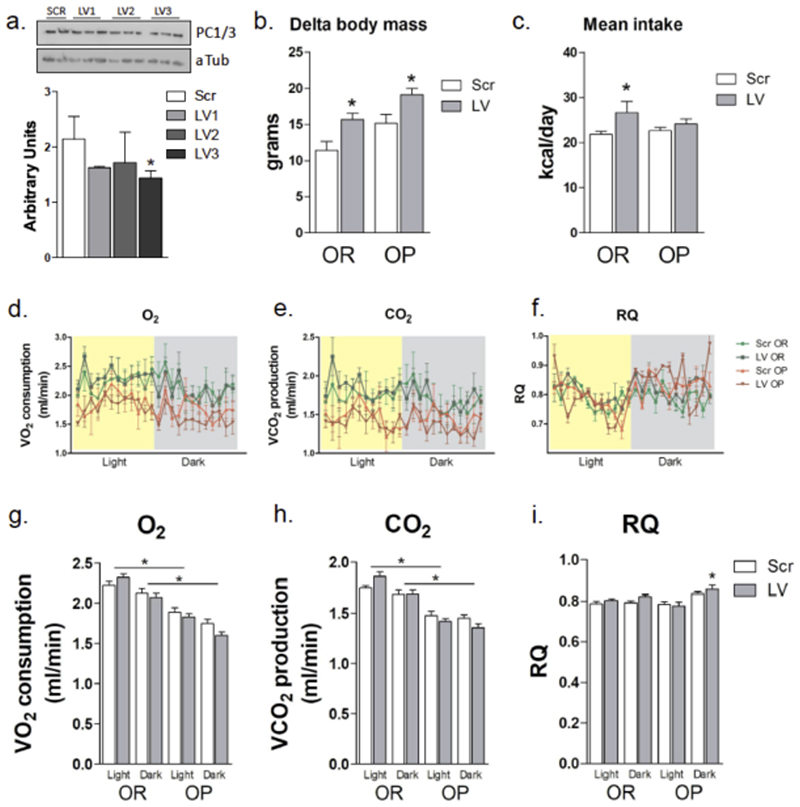
In order to disturb the pattern of regulation of hypothalamic PC1/3 expression, we inhibited PC1/3 gene expression using a lentivirus expressing shRNA targeting PCSK1. As depicted in Fig. 5a, the lentivirus #3 (LV3) was capable of reducing PC1/3 expression to 65% of control levels in the hypothalamus. As a consequence of hypothalamic PC1/3 inhibition, OR mice presented increased body mass, becoming phenotypically similar to their OP counterparts (Fig. 5b). Body mass gain was accompanied by increased caloric intake in OR mice (Fig. 5c) but not by a reduction of energy expenditure (Fig. 5d-5i). In fact, OR mice submitted to hypothalamic inhibition of PC1/3 consumed more O2 and produced more CO2 during the light cycle, suggesting that most of the body mass gain was due to increased caloric intake. The inhibition of PC1/3 in the hypothalamus of OP mice was also efficient at increasing body mass (Fig. 5a). This was accompanied by a non-significant increase in caloric intake (Fig. 5b) and by no major changes in energy expenditure (Fig. 5c-5h) except for an increase in the respiratory quotient (RQ) during the dark cycle of the day (Fig. 5h). During calorimetry, inhibition of PC1/3 resulted in changes neither in spontaneous physical activity nor in resting metabolic rate (not shown).
Figure 5. Phenotype of mice under hypothalamic inhibition of PC1/3.
Lentivirus particles carrying three different sequences aimed at inhibiting the expression of PC1/3 (LV1-LV3) and a scramble sequence (Scr) were injected bilaterally into the arcuate nucleus (stereotaxic coordinates: anteroposterior, -1.50 mm; lateral, +/- 0.3 mm; and depth, 5 mm) of obese-prone (OR) mice, and protein expression was determined by immunoblot (a). Groups of obese-prone (OP) and OR mice were treated with the scramble or LV3 lentivirus and fed on a high-fat diet for 4 weeks (b-i). Total body mass gain (b) and mean daily food intake (c) were measured. At the end of the experimental period, mice were submitted to indirect calorimetry for determination of O2 consumption (d, g), CO2 production (e, h), and calculation of respiratory quotient (f, i). In all experiments n = 5; in a, * p < 0.05 vs. Scr; in b-c, *p < 0.05 vs. respective Scr; in g-h, *p < 0.05 vs. respective OP; in I, *p<0.05 vs. respective Scr. aTub, alpha-tubulin, protein loading control.
Palmitate increases human hypothalamic POMC and POMC processing enzymes
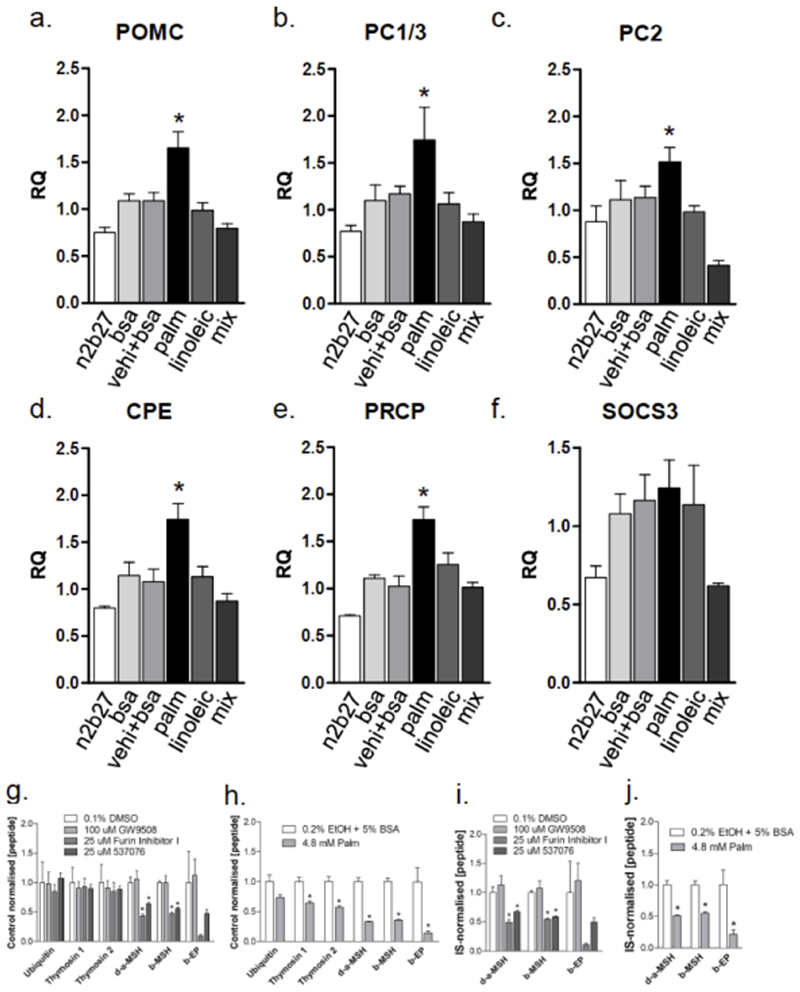
In order to test the capacity of palmitate to directly regulate POMC neurons and, particularly, to evaluate if this regulation could occur in human cells, we employed a recently discovered method to differentiate hPSCs into human hypothalamic neurons. This method is fully described in a previous publication (12). As depicted in Fig. 6, palmitate, but not linoleic acid or a mixture of lipids (containing 0.8 μM palmitate; complete composition in Suppl. Table 1), was capable of inducing the expression of POMC (Fig. 6a), the enzymes that process POMC into active peptide neurotransmitters PC1/3 (Fig. 6b), PC2 (Fig. 6c), and CPE (Fig. 6d), and the enzyme that inactivates some of the products of POMC, PRCP (Fig. 6e). None of the conditions modified the expression of SOCS3 (Fig. 6f). None of the treatment protocols produced cell damage.
Figure 6. Transcript expression in human hypothalamic neurons and palmitate treatment effects on POMC processing in human neurons.
For transcript expression analyses human hypothalamic neurons were maintained in medium (n2b27) with no treatment or treated with bovine serum albumin (bsa), vehicle plus bsa (vehi + bsa), palmitate (palm), linoleic acid (linoleic), or a chemically defined lipid concentrate (mix) for 8 h and then harvested for preparation of RNA and determination of transcript expression of POMC (a), PCSK1 (b), PCSK2 (c), CPE (d), PRCP (e), and SOCS3 (f) using real-time PCR. To test the effects of palmitate on POMC processing (g-j), human hypothalamic neurons were maintained in N2B27 medium and switched to medium with drug vehicle (0.1% DMSO) or drugs, or palmitate (PA) vehicle (0.2% ethanol + 5% BSA) or 4.8 mM PA for 24 h before being harvested for peptide identification and quantification by LC-MS/MS. g) Small molecule prohormone convertase inhibitors specifically reduced the measured concentration of POMC-derived peptide, whereas the GPR40/120 agonist GW9508 had no detectable effect. h) 4.8mM PA treatment led to a significant reduction in peptide expression for both broadly-expressed (thymosin) and POMC-derived peptides i and j) Normalization of POMC-derived peptides to internal standards confirmed the sensitivity of these peptide concentrations to both prohormone convertase inhibitors (i) and to palmitate (j). In all experiments n = 4; *p < 0.05 vs. all other conditions.
Palmitate reduces the production of POMC-derived neuropeptides in human hypothalamic neurons
Since palmitate led to consistent changes in the expression level of prohormone convertases in both mice in vivo and human stem cell-derived hypothalamic neurons in vitro, we hypothesized that palmitate might alter the processing of POMC into the peptides that alter feeding behavior by acting on MC4R, thereby mediating the phenotypic differences observed between OP and OR mice. This effect might be mediated by the binding of palmitate to the free fatty acid receptors GPR40 or GPR120, or via some other mechanism. To test this hypothesis, we applied quantitative peptidomic methods to measure the effects of palmitate on the concentration of the human POMC-derived peptides desacetyl-alpha-melanocyte stimulating hormone (d-α-MSH(1-13)) and beta-MSH (β-MSH(1-18)), and beta-endorphin (β-EP(1-31)). LC-MS/MS is highly sensitive and specific, and provides information on thousands of peptides in parallel, providing internal controls for global, nonspecific changes in peptide concentration. We first added small molecule inhibitors of prohormone convertases that are required for POMC processing (25 uM Furin inhibitor 1, or 25 uM 537076), and found that these compounds reduced the concentration of POMC-derived peptides (Fig. 6g), suggesting that this method might be sufficiently sensitive to detect changes induced by physiological levels of palmitate. In contrast, treatment of hPSC-derived neurons with 100 μM GW9508, a small molecule agonist of GPR40 and GPR120, had no significant effect on the expression of any peptides we analysed, suggesting that activation of these receptors is not likely to directly affect the expression of POMC-derived peptides. We next exposed cells to 4.8 mM palmitate or vehicle controls, and found that palmitate lead to a significant reduction in the broadly expressed peptides thymosin to approximately 65% of control levels, and to an significant reduction in d-α-MSH(1-13), β-MSH(1-18), and β-EP(1-31) to approximately 30% of control levels (Fig. 6h). To test whether POMC-derived peptides were more strongly affected by palmitate than other peptides, we normalized POMC-derived peptide concentrations to ubiquitin- and thymosin-derived peptides. We found that even after normalizing for nonspecific changes in peptide concentration, palmitate induced a significant approximately 50% reduction in the concentration of POMC-derived peptides (Fig. 6j). These reductions were similar in magnitude to those observed with pharmacological inhibition of prohormone convertases (Fig. 6i).
Discussion
The hypothalamic melanocortin system provides the most robust neural signals to inhibit caloric intake (14). In humans, mutations in the MC4R gene are the most frequent monogenic causes of obesity in subjects with a body mass index greater than 40 (15–17). However, in non-monogenic obesity, the epidemiological and mechanistic involvement of the melanocortin system in the abnormal regulation of caloric intake and energy expenditure is not clear.
Experimental studies performed during the last 10 years have shown that in diet-induced obesity, hypothalamic inflammation triggered by dietary fats can affect POMC neurons of the medio-basal hypothalamus (2–5, 18). The role of abnormal POMC neuronal function in obesity has been tested in a number of experimental studies employing models submitted to obesity-inducing methods for prolonged periods of time (19–22). However, until recently, little was known about the regulation of this system in rodents exposed to excessive dietary fats for very short periods of time, and particularly if POMC abnormal regulation could impact on different phenotypes of outbred rodents with resistance or susceptibility to obesity. Thaler and coworkers were the first to show that hypothalamic inflammation is triggered as early as one day after the introduction of a HFD (5). With this concept in mind, we asked what the earliest hypothalamic abnormality induced by the consumption of dietary fats segregating obese prone and obese resistant outbred mice would be (6). In a previous work, we showed that the early effects of dietary fats to disturb physiological patterns of regulation of food intake and energy expenditure occurred predominantly through the melanocortin system and that dopaminergic/hedonic system was affected at a later time-point (6). Also, we confirmed that hypothalamic inflammation is induced a few hours after the introduction of dietary fats, but we found that an abnormal regulation of POMC expression and processing into β-endorphin and α-MSH occurs even earlier than inflammation and segregates the different phenotypes regarding propensity and resistance to obesity (6).
In the present study, we tested three hypotheses that could explain the differential regulation of POMC neurons in OP and OR mice. Importantly, as hypothalamic abnormalities generated by the consumption of large amounts of dietary fats is a very early phenomenon, preceding by far the actual development of obesity, we employed an animal model that is not obese, but only pre-selected for their predisposition to develop obesity, as previously described (6). The first hypothesis was that excessive dietary fats could promote different changes in the gut microbiota in OP and OR mice, leading to increased lipid harvesting in OP, which, in turn, could promote changes in POMC neuron activity. Obesity, in both humans and rodents, is accompanied by changes in the gut microbiome landscape (23, 24). One of the most dramatic consequences of the acquisition of an obesity-related gut microbiota is the increased capacity to harvest lipids from the diet (24). In the present study, despite the fact that increased dietary fat consumption promoted changes in the gut microbiome, the changes were similar in OP and OR mice, refuting hypothesis #1. This is strongly supported by recent studies showing that environment (diet in this case) and not genetics explain most of the changes gut microbiota associated with different phenotypes (25).
Hypothesis #2 predicted that even in the absence of different microbiome landscapes, OP and OR mice could still have intrinsic capacities to differentially harvest dietary lipids, and this could in turn promote differential regulation of POMC neurons. In living organisms, most of the long-chain fatty acids in the body are stored as esterified lipids inside the cells. Importantly, FFAs present in the blood reach the hypothalamus, and the consumption of large amounts of dietary fats impacts not only on the blood levels of lipids but also on the amount and composition of the lipids reaching the hypothalamus (26). Here we used gas chromatography to determine the composition of blood lipids in OP and OR mice fed chow and a HFD for one day. Despite the considerable impact promoted by the increased consumption of dietary fats on the composition of blood FFAs, the changes occurred similarly between OP and OR mice, making hypothesis #2 null.
The third hypothesis predicted that even in the presence of similar amounts and composition of blood FFAs, OP and OR mice would differentially regulate POMC neurons when fed a HFD. The results obtained by several experimental approaches supported this hypothesis. The saturated fatty acid palmitate could increase hypothalamic POMC in OP mice only. Moreover, this was accompanied by increased expression of the POMC-processing enzyme PC1/3 and by increased expression of inflammatory cytokines. Of note, Schwinkendorf and coworkers (27) found no changes in rat hypothalamic POMC expression treated with a single icv bolus injection of palmitate; however, experimental animals were not separated by obesity prone/obesity resistance phenotypes, which seems to be an important experimental requirement in this context. POMC is a precursor peptide that is processed by sequential enzymatic digestion to produce distinct hormones and neurotransmitters (28, 29). In the neurons of the medio-basal hypothalamus, α-MSH and β-endorphin are the most abundant products of POMC (29). Previously, it was believed that POMC neurons of the medio-basal hypothalamus would provide only anorexigenic signals by producing α-MSH to be released in projections to the paraventricular and lateral hypothalamic nuclei (30, 31). However, a recent study has shown that depending on the stimulus, POMC neurons can provide both orexigenic and anorexigenic signals depending on the balance between α-MSH and β-endorphin (32).
The finding that PC1/3 expression was increased in OP mice raised the suspicion that POMC could be anomalously processed, which could contribute for the development of the obesity prone phenotype. Surprisingly, upon inhibition of PC1/3 both OP and OR mice increased body mass gain. Particularly, in the case of OR mice, this phenotype change was sufficient to promote an increase in body mass similar to that in OP mice. In both humans and experimental animals, mutations of PC1/3 are known to promote changes in body mass (33, 34). The obese phenotype caused by mutations of PC1/3 is mostly due to changes in caloric intake (29), similar to the findings in our model in the present study. Previously, only few studies have evaluated the regulation of PC1/3 by dietary factors or hormones/signals involved in energy homeostasis. Perello and coworkers have shown that the arcuate nucleus expression of PC1/3 is reduced during fasting, and the infusion of leptin can restore its levels (35). Ethanol and morphine have also been shown to reduce the expression of PC1/3 (36, 37). The present study is the first to demonstrate that dietary fats can also modulate the expression of hypothalamic PC1/3 differently in OP and OR mice. Because POMC processing can result in the production of both orexigenic and anorexigenic neurotransmitters, which could explain the phenotype obtained as a result of the inhibition of PC1/3, we decided to employ a cell system to further explore the effects of palmitate in POMC neurons.
In 2015, two groups reported the generation of hypothalamic neurons from hPSCs (38, 39), providing an unprecedented opportunity to test whether the results obtained in mouse models might be relevant to humans, and to gain insight into the molecular and cellular mechanisms underlying phenotypes observed in vivo. In order to determine if dietary fats acted directly on hypothalamic neurons to regulate PC1/3 expression and POMC processing, we performed experiments on hPSC-derived hypothalamic neurons, which contain a substantial fraction of POMC neurons (12).
In our study we showed that the long-chain saturated fatty acid palmitate induces increased expression of the transcripts for POMC and the POMC processing enzymes PC1/3, PC2, CPE, and PRCP in human hypothalamic neurons. This finding not only provides the first demonstration that a nutrient can directly affect POMC synthesis and processing in hypothalamic neurons, but it also shows that a long-chain saturated fatty acid known to be strongly associated with the abnormal function of the hypothalamus in obesity (4) can disrupt the complex processing system required to produce α-MSH and β-endorphin. Indeed, we observed significantly reduced expression of the neuropeptides α-MSH, β-MSH and β-endorphin from human hypothalamic cultures in response to palmitate. Pharmacological stimulation of the unsaturated fatty acid receptors GPR40 and GPR120, which are expressed in the hypothalamus (40), had no detectable effect on POMC processing, suggesting that palmitate alters POMC processing by a method other than GPR40- and GPR120-mediated signaling. In addition, other compositions of fatty acids, such as the one in the lipid mixture, could not reproduce the findings with palmitate, reinforcing the lipid-specific nature of this regulation. At first, it could sound counterintuitive that increased expression of POMC processing enzymes resulted in reduction of the products, α-MSH and β-endorphin. However, the fact that PRCP catalyzes the inactivation of α-MSH suggests that the whole catalytic chain of POMC is accelerated, explaining the fact that the amount of the final products is reduced. The enzymatic system that inactivates β-endorphin is currently unknown; thus, at this time, we cannot be sure this rational applies for both α-MSH and β-endorphin.
In conclusion, this study has shown that in an outbred model of diet-induced obesity, changes in the gut microbiome landscape and lipid harvesting do not segregate with the distinct obese predisposition phenotypes. We showed that a saturated fat can directly modulate the expression of POMC and its processing enzymes, an effect that segregates with the obese phenotype. Moreover, in human hypothalamic neurons, palmitate can directly regulate POMC expression and the expression of its enzymes and products. Thus, the direct regulation of POMC by dietary saturated fat emerges as an important mechanism involved in the development of diet-induced obesity.
Abbreviations
- AgRP
Agouti-related protein
- CPE
Carboxypeptidase E
- HFD
High-fat diet
- IL-10
Interleukin 10
- IL-1⍰
Interleukin 1 beta
- IL-6
Interleukin 6
- NPY
Neuropeptide Y
- OP
Obesity-prone
- OR
Obesity-resistant
- PC1/3
Prohormone convertase 1/3
- PC2
Prohormone convertase 2
- PCR
Polymerase-chain reaction
- POMC
Proopiomelanocortin
- PRCP
Prolyl carboxypeptidase
- TNF-⍰
Tumor necrosis factor alpha
Supplementary Material
Research in context section.
Evidence before this study
In experimental obesity the abnormal regulation of hypothalamic POMC expression and processing into α-MSH and β-endorphin is an important factor defining obese prone and obese resistance phenotypes. However, little is known about the mechanisms linking the consumption of a high fat diet and the regulation of POMC.
Added value of this study
The study shows that in an outbred strain of mice, obesity predisposition is related neither to differences in diet-induced modification of gut microbiota nor to differences in dietary fat harvesting from the diet. The main difference explaining the phenotypes is the direct action of dietary saturated fat to regulate POMC processing. The study provides the first evidence that saturated fat can directly regulate the expression of POMC processing enzymes resulting in different productions of α-MSH and β-endorphin.
Implications of all the available evidence
The development of methods that correct the abnormal regulation of POMC processing in obese prone subjects can be potentially useful as strategy to treat obesity.
Acknowledgments
Daniela Razolli received financial support from the São Paulo Research Foundation (FAPESP − 2014/00742-0). The authors thank Erika Roman and Gerson Ferraz for laboratory management. The authors also thank Magdalena Jura for assistance with hypothalamic differentiation. The study was supported by grants from the São Paulo Research Foundation (2013/07607-8) and Conselho Nacional de Pesquisa e Desenvolvimento Cientifico. Florian Merkle was supported by funds from the Medical Research Council (MR/P501967/1), and the Academy of Medical Sciences (SBF001\1016).
Funding
São Paulo Research Foundation (FAPESP − 2013/07607-8)
Disclaimer
Accepted, unedited article not yet assigned to an issue. The statements, opinions and data contained in this publication are solely those of the individual authors and contributors and not of the publisher and the editor(s). The publisher and the editor(s) disclaim responsibility for any injury to persons or property resulting from any ideas, methods, instructions or products referred to in the content.
Author Contributions
DSR and LAV designed the experiments; DSR, TMA, MRS, PK and DEC performed the experiments; DSR, FTM and LAV discussed and organized results; DSR and LAV wrote the paper; LAV was also responsible for funding acquisition. All authors contributed to the editing and discussion of the manuscript.
Ethics approval
The animal experiments performed in the present study were approved by the Institutional Animal Care and Use Committee (CEUA #3580-1).
Competing interests
The authors declare that they have no competing interests.
Consent for publication under Declaration
Not applicable
Data Availability
Please contact author for data requests
References
- 1.Goris AH, Westerterp KR. Physical activity, fat intake and body fat. Physiol Behav. 2008;94(2):164–8. doi: 10.1016/j.physbeh.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–9. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza GF, Solon C, Nascimento LF, De-Lima-Junior JC, Nogueira G, Moura R, et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci Rep. 2016;6:29290. doi: 10.1038/srep29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, et al. Diet-induced changes in uncoupling proteins in obesity-prone and obesityresistant strains of mice. Proc Natl Acad Sci U S A. 1998;95(7):4061–5. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS. Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res. 2002;34(11–12):691–7. doi: 10.1055/s-2002-38251. [DOI] [PubMed] [Google Scholar]
- 9.Huang XF, Han M, Storlien LH. Differential expression of 5-HT(2A) and 5-HT(2C) receptor mRNAs in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2004;127(1–2):39–47. doi: 10.1016/j.molbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 11.Shirai N, Suzuki H, Wada S. Direct methylation from mouse plasma and from liver and brain homogenates. Anal Biochem. 2005;343(1):48–53. doi: 10.1016/j.ab.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Kirwan P, Jura M, Merkle FT. Generation and Characterization of Functional Human Hypothalamic Neurons. Curr Protoc Neurosci. 2017;81(3):331–324. doi: 10.1002/cpns.40. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan P, Kay RG, Brouwers B, Herranz-Perez V, Jura M, Larraufie P, et al. Quantitative mass spectrometry for human melanocortin peptides in vitro and in vivo suggests prominent roles for beta-MSH and desacetyl alpha-MSH in energy homeostasis. Mol Metab. 2018 doi: 10.1016/j.molmet.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 15.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed S, Bonnefond A, Manzoor J, Shabir F, Ayesha H, Philippe J, et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity (Silver Spring) 2015;23(8):1687–95. doi: 10.1002/oby.21142. [DOI] [PubMed] [Google Scholar]
- 17.Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab. 2005;90(1):219–24. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 18.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Bumaschny VF, Yamashita M, Casas-Cordero R, Otero-Corchon V, de Souza FS, Rubinstein M, et al. Obesity-programmed mice are rescued by early genetic intervention. J Clin Invest. 2012;122(11):4203–12. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorfman MD, Krull JE, Scarlett JM, Guyenet SJ, Sajan MP, Damian V, et al. Deletion of Protein Kinase C lambda in POMC Neurons Predisposes to Diet-Induced Obesity. Diabetes. 2017;66(4):920–34. doi: 10.2337/db16-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, et al. A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology. 2015;156(5):1701–13. doi: 10.1210/en.2014-1961. [DOI] [PubMed] [Google Scholar]
- 22.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107(33):14875–80. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 25.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 26.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7(1):e30571. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG. Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes (Lond) 2011;35(3):336–44. doi: 10.1038/ijo.2010.159. [DOI] [PubMed] [Google Scholar]
- 28.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9(1):159–79. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 29.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660(1):213–9. doi: 10.1016/j.ejphar.2010.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293(1):E121–31. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- 32.Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519(7541):45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson RS, Creemers JW, Farooqi IS, Raffin-Sanson ML, Varro A, Dockray GJ, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112(10):1550–60. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15(11):1884–93. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- 35.Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab. 2007;292(5):E1348–57. doi: 10.1152/ajpendo.00466.2006. [DOI] [PubMed] [Google Scholar]
- 36.Nie Y, Ferrini MG, Liu Y, Anghel A, Paez Espinosa EV, Stuart RC, et al. Morphine treatment selectively regulates expression of rat pituitary POMC and the prohormone convertases PC1/3 and PC2. Peptides. 2013;47:99–109. doi: 10.1016/j.peptides.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerma-Cabrera JM, Carvajal F, Alcaraz-Iborra M, de la Fuente L, Navarro M, Thiele TE, et al. Adolescent binge-like ethanol exposure reduces basal alpha-MSH expression in the hypothalamus and the amygdala of adult rats. Pharmacol Biochem Behav. 2013;110:66–74. doi: 10.1016/j.pbb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merkle FT, Maroof A, Wataya T, Sasai Y, Studer L, Eggan K, et al. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development. 2015;142(4):633–43. doi: 10.1242/dev.117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Meece K, Williams DJ, Lo KA, Zimmer M, Heinrich G, et al. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J Clin Invest. 2015;125(2):796–808. doi: 10.1172/JCI79220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dragano NRV, Solon C, Ramalho AF, de Moura RF, Razolli DS, Christiansen E, et al. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J Neuroinflammation. 2017;14(1):91. doi: 10.1186/s12974-017-0869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact author for data requests