Abstract
Objective
Neuroimaging findings have been reported in regions of the brain associated with emotion in both adults and adolescents with depression, but few studies have investigated whether such brain alterations can be detected in adolescents with subthreshold depression, a condition at risk for major depressive disorder. In this study, we searched for differences in brain structure at age 14 years in adolescents with subthreshold depression and their relation to depression at age 16 years.
Method
High-resolution structural magnetic resonance imaging was used to assess adolescents with self-reported subthreshold depression (n = 119) and healthy control adolescents (n = 461), all recruited from a community-based sample. Regional gray and white matter volumes were compared across groups using whole-brain voxel-based morphometry. The relationship between subthreshold depression at baseline and depression outcome was explored using causal mediation analyses to search for mediating effects of regional brain volumes.
Results
Adolescents with subthreshold depression had smaller gray matter volume in the ventromedial prefrontal and rostral anterior cingulate cortices and caudates, and smaller white matter volumes in the anterior limb of internal capsules, left forceps minor, and right cingulum. In girls, but not in boys, the relation between subthreshold depression at baseline and high depression score at follow-up was mediated by medial–prefrontal gray matter volume.
Conclusion
Subthreshold depression in early adolescence might be associated with smaller gray and white matter volumes in regions of the frontal–striatal–limbic affective circuit, and the occurrence of depression in girls with subthreshold depression might be influenced by medial–prefrontal gray matter volume. However, these findings should be interpreted with caution because of the limitations of the clinical assessment methods.
Keywords: subthreshold depression, MRI, gray matter, white matter
Subclinical depressive symptoms are frequent in adolescence,1 a critical period for the onset of depressive disorders.2 Adolescents with subclinical, subthreshold depressive symptoms may have clinically relevant depressive symptoms and may experience substantial distress or impairment, without meeting criteria for a diagnosis of major depressive disorder (MDD).3 Despite the different definitions of subthreshold depression used in the literature, based on the number, duration, and impact on functioning of symptoms,3 there is consistent evidence that subthreshold depression strongly predicts MDD in adulthood,1,4,5 with an estimated risk of escalation to full-syndrome disorder of 67%.5 Furthermore, although depressive symptom rates are similar in prepubescent boys and girls, a strong female preponderance in the prevalence of subthreshold and clinical depression emerges after puberty,6,7 suggesting that there might be gender differences in the neural circuitry underlying depression.
Despite the frequency of subthreshold depression in adolescents, with a lifetime prevalence reported as high as 26%,4 only 1 neuroimaging report8 restricted to the rostral anterior cingulate cortex (rACC) showed that boys with subthreshold depressive symptoms, but not girls, had smaller rACC volume than those with no depressive symptoms. The differences are more widespread in adolescents with MDD, as reduced gray matter volumes have been reported in frontal, limbic, and striatal regions such as the prefrontal cortex (PFC),9,10 ACC,11 amygdala,12 hippocampus,13 and caudates.9,14 A gender effect was reported in 1 study demonstrating smaller nucleus accumbens in girls with depression only, and increased growth of the amygdala during adolescence associated with depression in girls and decreased growth in boys.15
A further step requires investigating the morphometry on the whole brain and assessing the clinical outcome of adolescents with subthreshold depression, to shed light on the early brain structure variations involved in such emotional dysregulation and the vulnerability to depressive disorders, including vulnerability related to gender. Therefore, we hypothesized that adolescents with subthreshold depression, especially girls, would exhibit structural variations in these frontal, striatal, and limbic regions implicated in mood disorders, and that the structural variations would indicate vulnerability to depression outcome. We searched for differences in regional volumetry of gray (GM) and white matter (WM) in 14-year-old adolescents with subthreshold depression using T1-weighted magnetic resonance imaging (MRI) and whole-brain voxel-based morphometry (VBM). Based on the previous assumptions, we investigated whether these regional differences would mediate the relation between subthreshold depression at age 14 years and depression at age 16 years.
Method
Participants
The study was approved by the ethics committees of all participating institutions. Written informed assent and consent were obtained, respectively, from all adolescents and their parents after complete description of the study.
Neuroimaging and clinical data were obtained from the Imagen database established across 8 European sites in France, the United Kingdom, Ireland, and Germany, which includes 2,223 adolescents recruited in schools around age 14 years (SD = 0.41 year; age range = 12.9–15.7 years). A detailed description of recruitment and assessment procedures, along with exclusion and inclusion criteria, has been published elsewhere.16 Notably, bipolar disorder, treatment for schizophrenia, and major neurodevelopmental disorders constituted exclusion criteria.
Participants were also followed up 2 years later except for neuroimaging assessment.
Baseline Assessment
Adolescent psychiatric symptoms were assessed with the Development and Well-Being Assessment (DAWBA; www.dawba.com), a self-administered diagnostic questionnaire consisting of both open and closed questions.17 The DAWBA generates the probabilities of having DSM-IV diagnoses that are subsequently validated by experienced clinicians. It is designed to maintain consistency across multiple cultural and language groups, as diagnoses are made by clinical raters who share a common training and who participate in regular cross-language training and consensus meetings. All of the raters were able to read at least 2 of the relevant languages.
Psytools software (Delosis Ltd, London, UK) was used to conduct the following assessments via its Internet-based platform. The assessment battery was self-administered both in participants’ homes and at the neuroimaging facilities. Substance use was reported using the Alcohol Use Disorders Identification Test (AUDIT)18 and the European School Survey Project on Alcohol and Drugs (www.espad.org). The Substance Use Risk Profile Scale (SURPS), which assesses personality risk for pathology along 4 dimensions (Sensation-Seeking, Impulsivity, Anxiety-Sensitivity, Hopelessness), was also used.19 Hopelessness and Anxiety-Sensitivity dimensions have been reported to be risk factors for depressive and anxiety disorders, respectively.20 Other assessments included the Neo Five-Factor Inventory (Neo-FFI),21 the Strengths and Difficulties Questionnaire (SDQ),22 handedness, pubertal status using the Pubertal Development Scale (PDS) questionnaire,23 parental history of depression, using a modified version of the Family Interview for Genetics Studies, the Genetic Screening and Family History of Psychiatric Disorders Interview (GEN),16 and life events using the Life-Events Questionnaire (LEQ).24
The participant selection is described in Figure S1 (available online). Participants with any validated diagnosis (e.g., MDD, bipolar disorder, attention-deficit/hyperactivity disorder), any history of lifetime drug taking, or any symptoms of alcohol abuse or dependence (AUDIT score >4) were excluded from this study, as those disorders have neural correlates that would have biased our neuroimaging investigation.
The group with subthreshold depression included 119 adolescents. Adolescents were included in the group with subthreshold depression if they self-reported having experienced, in the last 4 weeks, at least 3 depressive symptoms including at least 1 core symptom (abnormally depressed, irritable mood, or loss of interest) and 2 or more other DSM-IV depressive symptoms, without fulfilling criteria for MDD in terms of duration, symptom number, or significant impact on functioning, as assessed using the DAWBA.
The control group matched for gender with the group with subthreshold depression included 461 adolescents with fewer than 3 symptoms of depression and a probability of having an MDD diagnosis of less than 0.1% according to the DAWBA. Most participants in the database actually had fewer than 3 symptoms, with an 85.1% prevalence (see Figure S2, available online).
No participant or parent reported being prescribed antidepressants, mood stabilizers, anxiolytics, antipsychotics, or hypnotics.
Follow-Up Assessment
Participants were reassessed using Web-based self-reports 2 years after completion of the baseline study. The assessment was similar to baseline assessment with the addition of the Adolescent Depression Rating Scale (ADRS), a validated 10-item self-rated scale that was introduced to specifically assess adolescent depression25 among the whole cohort. A score of 6 or more has been considered as corresponding to a diagnosis of depression.26 This cutoff provides maximum sensitivity and specificity in screening for MDD according to the DSM-IV, with clinically relevant intensity.25
Subthreshold depression at follow-up was defined using the same criteria as for baseline.
Follow-up of the initial control group and the group with subthreshold depression (Figure S1, available online) retrieved 63.9% (n = 76) and 59.7% (n = 275) of the participants, respectively (χ2[1, N = 580] = 0.54, p = 0.46).
MRI Data
Magnetic resonance imaging at age 14 years was performed on 3 Tesla scanners (General Electric, Siemens, and Philips) from the 8 European sites. High-resolution anatomical MR images were obtained using a standardized 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence based on the ADNI protocol (http://adni.loni.usc.edu/methods/mri-analysis/mri-acquisition/). Acquisition parameters were similar across sites (e.g., sagittal slice plane, repetition time = 2,300 milliseconds: echo time = 2.8 milliseconds, flip angle = 8° ; 256 × 256 × 170 matrix, 1.1 × 1.1 × 1.1 mm voxel size).16 Image preprocessing was performed with Statistical Parametric Mapping 8 software (SPM8) using voxel-based morphometry.27 A detailed description of the MRI data acquisition and preprocessing is provided in Supplement 1, available online. Briefly, T1-weighted images were segmented and normalized using customized tissue probability maps. Then, the normalized, segmented, and modulated GM and WM images were smoothed using a 10-mm full-width at half-maximum (FWHM) Gaussian kernel. Global GM, WM, and cerebrospinal fluid (CSF) volumes were computed for each participant. Total intracranial volume (TIV) was defined as the sum of GM, WM, and CSF volumes. Head size was measured by the volume scaling factor (https://surfer.nmr.mgh.harvard.edu/fswiki/eTIV), computed as the determinant of the affine transform performed during the spatial normalization.
Statistical Analyses
Voxel-Based Analyses
Whole-brain voxelwise comparisons were carried out within the general linear model (GLM) framework using SPM8. We first incorporated group (subthreshold depression/control) and gender as main factors and group-by-gender interaction in the model to explore a potential gender effect. Head size, type of scanner (to account for variance among manufacturers; Table S1, available online) and age were entered as confounding variables. If the interaction term was not statistically significant, only group as main factor was considered, and gender was added as a confounding factor. At the voxel level, statistical significance was set at p < .05 FWE (familywise error) corrected for multiple comparisons. Cluster size was at least 50 voxels. Brain locations were reported as x, y, and z coordinates in Montreal Neurological Institute (MNI) space.
Other Analyses
Group comparisons for socio-demographic and clinical data and global brain volumes were performed within the framework of the GLM using R software (http://cran.r-project.org). As in voxel-based analyses, group-by-gender interactions were tested before considering group as main factor and gender as confounding factor. Age, TIV, and type of scanner were entered as confounding variables to compare global GM, WM, and CSF volumes.
Causal mediation analyses were conducted to determine whether the GM and WM clusters previously identified in SPM between-group analyses could mediate the relation between subthreshold depression at age 14 years and depression at age 16 years. The analyses were performed with a validated algorithm using a set of GLM to derive the mediation and direct effects from the total effect.28 Cut-off ADRS score ≥6 at age 16 years was entered as a dependent factor, and group (subthreshold depression/control) as an independent factor within a logistic regression model. For each SPM cluster, raw volume was extracted from the smoothed, normalized, and modulated images and entered as a mediator variable. To explore a potential gender effect on the mediation model, we added interaction terms for gender in the models to perform a so-called moderated mediation analysis. Type of scanner, TIV, and age were entered as confounding variables. This mediation model was performed using 5000 Monte Carlo draws for nonparametric bootstrap. In causal mediation analysis, a significant mediating effect is defined as a 95% confidence interval that does not include 0. All statistics were corrected for multiple comparisons.
Results
Participant Characteristics
As expected at baseline, adolescents with subthreshold depression had significantly higher scores on the SURPS dimensions of Hopelessness, Anxiety-Sensitivity, and Impulsivity, and on the NEO-FFI dimension of Neuroticism, along with lower scores on Extraversion, Agreeableness, and Conscientiousness (Table 1). They also had higher impact scores on the SDQ and lower LEQ total score, corresponding to a higher level of negative life events. No difference was found between groups regarding age, pubertal status, handedness, or parental history of depression, but adolescents with subthreshold depression were more likely to have at least 1 nonwhite parent. No gender-by-group interaction was found for any clinical characteristics.
Table 1. Clinical Characteristics in Adolescents With Subthreshold Depression Compared With Controls at Baseline and Follow-Up.
| Characteristic | Baseline | Follow-Up | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sDep n = 119 | Controls n = 461 | Statistic 1, n = 580 | p | sDep n = 76 | Controls n = 275 | Statistic 1, n = 351 | p | ||||||||||
| n | % | n | % | χ2 | n | % | n | % | χ2 | ||||||||
| Gender (female) | 78 | 65.55 | 303 | 65.73 | 0.00 | 1 | 53 | 69.74 | 192 | 69.82 | 0.00 | 1 | |||||
| Handedness (right-handed) | 99 | 83.19 | 406 | 88.07 | 1.77 | .18 | 62 | 81.58 | 244 | 88.73 | 2.12 | .15 | |||||
| Parental history of depression | 8 | 6.72 | 30 | 6.51 | 0.00 | 1 | 4 | 5.26 | 17 | 6.18 | 0.00 | .98 | |||||
| Nonwhite ethnicity | 23 | 13.33 | 41 | 8.89 | 9.45 | .002** | 6 | 7.89 | 21 | 7.64 | 0.00 | 1 | |||||
| Subthreshold depression at age 16 | - | - | - | - | - | - | 19 | 25.00 | 26 | 9.45 | 11.52 | <.001*** | |||||
| Depression (ADRS ≥ 6) | - | - | - | - | - | - | 18 | 23.68 | 12 | 4.36 | 26.02 | <.001*** | |||||
| Self-harm since age 14 y | - | - | - | - | - | - | 23 | 30.26 | 27 | 9.82 | 18.09 | <.001*** | |||||
| Mean | SD | Mean | SD | F1,570 | Mean | SD | Mean | SD | F1,341 | ||||||||
| Age, y | 14.45 | 0.36 | 14.40 | 0.41 | 2.40 | .12 | 16.49 | 0.54 | 16.45 | 1.14 | 0.04 | .85 | |||||
| Mean | SD | Mean | SD | F1,569 | Mean | SD | Mean | SD | F1,340 | ||||||||
| Pubertal status (PDS score) | 3.01 | 0.51 | 2.95 | 0.51 | 0.93 | .34 | 3.46 | 0.34 | 3.44 | 0.40 | 0.02 | .88 | |||||
| SDQ impact score | 1.24 | 1.83 | 0.13 | 0.59 | 99.18 | <.001*** | 1.09 | 1.73 | 0.34 | 0.95 | 22.37 | <.001*** | |||||
| Mean | SD | Mean | SD | U | Mean | SD | Mean | SD | U | ||||||||
| NEO-FFI | |||||||||||||||||
| Neuroticism | 28.97 | 7.67 | 21.03 | 6.73 | 12,219 | <.001*** | 27.38 | 7.17 | 20.96 | 7.50 | 9,736 | <.001*** | |||||
| Extraversion | 28.05 | 6.63 | 30.92 | 5.39 | 34,209 | <.001*** | 27.66 | 6.21 | 30.37 | 5.81 | 22,248 | .007** | |||||
| Openness | 26.29 | 6.61 | 26.41 | 5.62 | 28,002 | .73 | 27.88 | 6.29 | 27.47 | 5.87 | 17,351 | .46 | |||||
| Agreeableness | 26.66 | 5.43 | 30.79 | 4.94 | 39,419 | <.001*** | 27.51 | 4.91 | 31.43 | 5.25 | 25,043 | <.001*** | |||||
| Conscientiousness | 26.64 | 7.26 | 28.86 | 6.69 | 32,500 | .002** | 26.80 | 7.88 | 29.72 | 6.96 | 21,941 | .002** | |||||
| SURPS | |||||||||||||||||
| Hopelessness | 14.80 | 3.82 | 12.45 | 2.63 | 17,555 | <.001*** | 14.21 | 4.11 | 12.52 | 2.91 | 13,428 | <.001*** | |||||
| Anxiety-sensitivity | 11.87 | 2.52 | 11.08 | 2.28 | 22,921 | .005** | 11.57 | 2.77 | 10.95 | 2.38 | 15,944 | .06 | |||||
| Impulsivity | 13.08 | 2.31 | 11.52 | 2.01 | 17,035 | <.001*** | 12.03 | 2.03 | 10.87 | 2.04 | 12,466 | <.001*** | |||||
| Sensation-seeking | 16.09 | 2.99 | 15.60 | 3.06 | 25,225 | .17 | 13.86 | 2.63 | 13.72 | 2.73 | 17,836 | .80 | |||||
| LEQ total score | −2.09 | 4.68 | −0.54 | 4.30 | 32,504 | .002** | −1.80 | 4.93 | −0.27 | 4.46 | 19,630 | .13 | |||||
| Mean | SD | Mean | SD | F1,574 | |||||||||||||
| Brain tissue | |||||||||||||||||
| Gray matter (cm3) | 743.62 | 55.81 | 749.21 | 61.49 | 3.41 | .065 | - | - | - | - | - | - | |||||
| White matter (cm3) | 467.42 | 38.60 | 474.44 | 41.21 | 11.51 | <.001*** | - | - | - | - | - | - | |||||
| Cerebrospinal fluid (cm3) | 367.95 | 36.57 | 364.24 | 40.10 | 13.00 | <.001*** | - | - | - | - | - | - | |||||
| Total intracranial volume (cm3) | 1,5791 | 11 | 1,588 | 126 | 0.21 | .64 | – | – | – | – | – | – | |||||
Note: ADRS = Adolescent Depression Rating Scale; F = analysis of variance F value; LEQ = Life-Events Questionnaire; NEO-FFI = NEO Five-Factor Inventory; PDS = Pubertal Development Scale; sDep = adolescents with subthreshold-depression; SDQ = Strengths and Difficulties Questionnaire; SURPS = Substance Use Risk Profile Scale; U = Mann–Whitney–Wilcoxon U value.
p ≤ 0.05;
p ≤ 0.01;
p ≤ .001.
At follow-up, adolescents with subthreshold depression similarly differed from controls except for ethnicity (Table 1). In addition, they reported significantly more self-harm since baseline and were more likely to have subthreshold depression and clinical scores indicating ongoing depression (ADRS ≥6/10).
Because of the longitudinal design of the IMAGEN study, very few follow-up DAWBA diagnoses were validated at the time of analysis. Therefore, it was not possible to retrieve the final diagnoses for all participants. To assess their clinical depression outcome, we used the ADRS cut-off score as a proxy for depression, and considered adolescents having an ADRS score ≥6/10 as adolescents with “depression.”
There was a significant gender-by-group interaction effect regarding depression outcome (F = 8.17; p = .005). Although there was no difference between boys and girls with subthreshold depression (p =.14; odds ratio[OR] = 2.42, 95% CI = 0.74–8.00), control girls were more likely to have an ADRS score 6/10 than control boys (p = .02; OR = 5.30, 95% CI = 1.23–125.27).
There was also a significant gender-by-group interaction effect regarding pubertal status at age 16 (F = 5.30; p = .02). In both groups, girls had higher PDS scores than boys, but this difference was more pronounced in controls (β = 0.46; p < .001 in controls; β = 0.28; p < .001 in adolescents with subthreshold depression).
Lost-to-follow-up participants did not differ from those followed up in any characteristic, but they were more likely to be boys (93 boys/136 girls versus 106 boys/245 girls, respectively, χ2[1, N = 580] = 6.21; p = .01).
Global Brain Tissue
Volumes At baseline, WM volume was significantly smaller, and CSF volume significantly higher, in adolescents with subthreshold depression than in controls (Table 1). In addition, there was a trend toward smaller GM volume in adolescents with subthreshold depression. No between-group difference was found in the TIV measure. No gender-by-group interaction was found regarding global brain tissue volumes.
Voxel-Based Morphometry
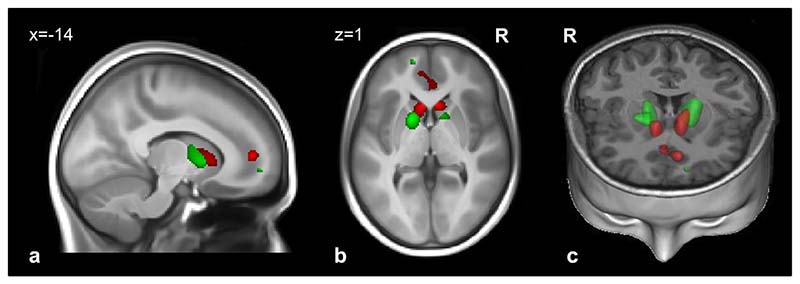
Compared with controls, adolescents with subthreshold depression had significantly smaller GM volume in the medial PFC (mPFC), in a cluster encompassing the left ventromedial prefrontal (vmPFC) and the right rACC cortices, and in the caudate head bilaterally (Figure 1, Table 2). Similarly, adolescents with subthreshold depression had significantly smaller WM volumes in the anterior limb of the internal capsule (ALIC) bilaterally, left forceps minor, and right cingulum (Figure 1, Table 2).
Figure 1.
Regions with smaller gray matter volume (red) and white matter volume (green) in 119 adolescents with subthreshold depression versus 461 controls. Note: voxel level was set at p < .05 (familywise error–corrected for the whole brain). Results are superimposed on a T1-weighted magnetic resonance imaging scan of an adolescent brain from the Imagen database. (a) Sagittal slice; (b) transversal slice; (c) 3-dimensional representation (coronal and transversal slices). R = right.
Table 2. Regions of Smaller Gray and White Matter Volume in 119 Adolescents With Subthreshold Depression Compared With 461 Controls.
| Brain Region | Cluster Level | Voxel Level | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MNI Coordinates (mm) | p a | t | |||||||
| BA | k | P | x | y | z | ||||
| Gray matter | |||||||||
| Left caudate head | – | 400 | .001** | −6 | 18 | 3 | .001** | 5.37 | |
| Right caudate head | – | 234 | .004** | 6 | 20 | 3 | .001** | 5.35 | |
| Left ventromedial PFC | 10 | 369 | .002** | −12 | 50 | 6 | .001** | 5.24 | |
| Right anterior cingulate | 24/32 | 3 | 36 | 0 | .018* | 4.63 | |||
| White matter | |||||||||
| Left forceps minor | – | 69 | .019* | −14 | 57 | −2 | .001** | 5.09 | |
| Left ALIC | – | 1288 | <001*** | −16 | 6 | 3 | .001** | 5.06 | |
| Right ALIC | – | 462 | .002** | 12 | 10 | −2 | .006** | 4.69 | |
| Right cingulum | – | 191 | .008** | 12 | 32 | 25 | .007** | 4.68 | |
Note: Empty cell indicates that region is included in the same cluster as the region immediately above. ALIC = anterior limb of internal capsule; BA = Brodmann Area; k = cluster size in number of voxels (voxel size = 3.375 mm3); MNI = Montreal Neurological Institute coordinates; PFC = prefrontal cortex.
Statistics at voxel level set to p < .05, familywise error corrected for the whole brain, height threshold t = 4.36 (gray matter), t = 4.16 (white matter); extent threshold k = 50 voxels.
p ≤.05;
p ≤.01;
p ≤.001.
No region with larger GM or WM volume was found in adolescents with subthreshold depression.
No gender-by-group interaction was found in any of the whole-brain analyses.
Causal Mediation Analyses
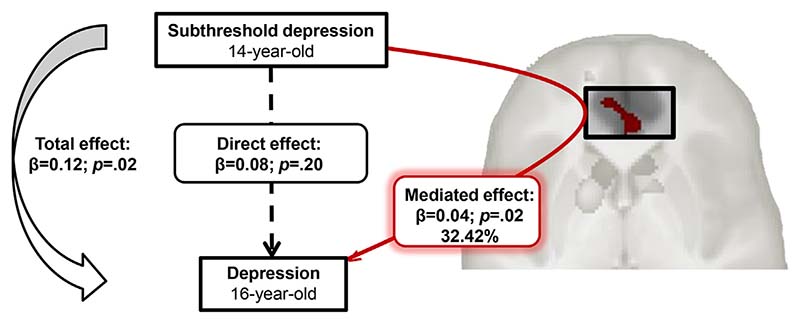
Moderated causal mediation analyses showed that smaller mPFC volume accounted for 32.42% (p = .02) of the total effect in girls (Figure 2; Table S2, available online) but did not mediate the association in boys (p > .99). No mediation effect was found with caudates or WM cluster volumes.
Figure 2.
Mediation of the gray matter medial prefrontal cortex on the relationship between subthreshold depression at age 14 years and depression at age 16 years in girls, using causal mediation analysis.
Discussion
For the first time, to our knowledge, GM and WM volume differences were identified using a whole-brain voxel-based approach in 14-year-old adolescents with subthreshold depression. Smaller GM volumes were detected in the mPFC, including the left vmPFC and right rACC, and in the caudate heads, whereas smaller WM volumes were found in nearby regions, including left forceps minor, right cingulum, and bilateral ALIC. Higher scores on several emotional and personality dimensions both at baseline and at follow-up indicated persistent risk for psychopathology in this group. At follow-up, adolescents with subthreshold depression were more likely to have above-the-cut-off depression scores, indicating ongoing depression. Causal mediation analysis showed that mPFC GM volume at age 14 years explained a significant fraction of the relation between subthreshold depression at age 14 years and depression at age 16 years in girls but not in boys.
The present findings confirm and extend the only previous report in adolescents with subthreshold depressive symptoms showing smaller GM volume in rACC,8 and highlight a cluster of regions central to the recent models of affective processes.29 The mPFC, spanning from the ACC toward the frontal pole, is conceived as an integrative center for affective appraisal. It is unique among cortical regions, as it projects directly to nuclei involved in affect and peripheral regulation, and is essential to integrating functions leading to affective meaning through its subcortical connections.30 Here, the adolescents with subthreshold depression had a smaller volume of perigenual mPFC, a subdivision associated with impaired positive valence.31 Thinner cortical thickness of the vmPFC has been associated with a decreased capacity to appropriately assign affective meaning to sensory and internal cues, which may bias towards negative information, particularly in early adolescence.32 The Hopelessness and Neuroticism dimensions were consistently higher in our group with subthreshold depression. The vmPFC, together with the caudates, is part of the brain reward network that mediates behaviors usually driven by positive valence, such as pleasure and motivation.33 The rACC, conceived as a “transition zone” with connectivity to both regions, would play a role in translating between affective meaning and action.30
Besides GM variations, we found that adolescents with subthreshold depression had smaller WM volumes in nearby tracts that mature through adolescence.34,35 The forceps minor, a bundle constituted by thinner axons in the genu of the corpus callosum, connects the medial surfaces of the frontal lobes, and the cingulum bundle originates in the cingulate cortex and projects rostrally to the ACC and the PFC.36 The ALIC spans between the PFC and the striatum.
Thus, our findings point to both GM and WM variations in regions involved in emotion appraisal and reward systems. The GM regions detected here in adolescents with subthreshold depression have been implicated in the pathophysiology of depression in adults and adolescents,9–11,14,29 and altered WM microstructure within tracts connecting these regions has been reported in adolescents with MDD.37 In addition, functional impairments within the reward system have been hypothesized to contribute to the onset of depressive disorders in adolescence.38 Adolescence is critical for the maturation of these regions, with subcortical structures such as the caudates maturing before cortical prefrontal regions.39 The resulting enhanced bottom–up emotional processing in subcortical regions relative to less effective top–down modulation of affective information in prefrontal regions has been hypothesized to account for greater emotional reactivity and sensitivity during adolescence, which might underlie vulnerability to affective disorder onset.40 Peak cortical thickness is attained in the mPFC between ages 10 and 13 years, followed by cortical thinning through adolescence,41 whereas the caudate volume decreases when the pubertal stage reaches a Tanner score of 2 to 3, corresponding to age 10 to 12 years.42 In this study, both adolescent groups had similar mean PDS score. Thus, our findings would suggest an accelerated GM thinning at both the cortical (mPFC) and subcortical (caudates) levels in the adolescents with subthreshold depression, which might account for emotional dysregulation and lead to enhanced vulnerability to affective disorder onset.40
Furthermore, consistent with the literature, we observed that control girls at age 14 years were at higher risk for depression at age 16 years than control boys.7 Conversely, there was no difference between boys and girls with subthreshold depression regarding depression outcome, in agreement with previous studies.4,5 However, our results further suggest that smaller GM volumes in the mPFC region mediated a substantial part of the relation between subthreshold depression at age 14 years and high depression scores (indicating pathology) at age 16 years in girls only. Thus, the risk of developing depression might be enhanced in girls who have a reduced mPFC volume. This gender difference might be related to the observed gender-by-group interaction effect of pubertal status at age 16 years. However, including PDS score at age 16 years as a confounding covariate in the mediation analyses did not change the result (29.65%; p = .02 in girls and p > .99 in boys). Thus, a distinct developmental pattern in that core emotion regulation region may play an important role in the transition to full-depression disorder during female adolescence.
Thus, overall, our findings suggest that the development of a depressive syndrome in adolescence, particularly in girls, is influenced by early pre-existing mPFC volume changes that may be associated with other structural changes in the striatum.
Of note, the psychometric characteristics of the present group with subthreshold depression were comparable to those reported in the literature on this condition.7 Along with a number of depression symptoms, the adolescents with subthreshold depression had marked indices of negative or unstable emotional valence denoted by high Neuroticism, Hopelessness, Anxiety-Sensitivity, and Impulsivity scores. These characteristics were constant over time with the follow-up 2 years later, denoting the consistency and stability of the definition criteria used in this study. In particular, the follow-up findings confirm that adolescents with subthreshold depression are more likely to convert to depression later on5 and have an increased risk of self-harm.7
These findings should be seen in the light of several limitations. First, due to inclusion/exclusion criteria and cultural differences, the proportions of control adolescents and those with subthreshold depression differed between sites (see Table S3, available online). For instance, fewer adolescents were eligible as controls in London. However, including site instead of scanner as confounding covariate in the analyses did not change the results, either at voxel level or at the cluster level. In addition, even though we controlled for scanner type in the analyses, the among-scanner variability in our measurements (see Table S1, available online) may have underpowered the detection of the effects of interest and needs to be considered as a limitation of our study.
Second, the symptom rating was self-administered, and it cannot be ruled out that some participants might have misunderstood some questions, which could have biased the diagnostic ratings. Moreover, the closed questions in the DAWBA provide information only about the past 4 weeks rather than lifetime depression. Thus, although the DAWBA interview includes open comments from the participant, which allows trained clinicians to check for consistency between ratings and comments and to check for any report of affective episode history, it cannot be ruled out that some adolescents might have experienced a past major depressive episode. If this were the case, the findings would point to early alterations associated with a depressive disorder, and not to vulnerability features in at-risk adolescents.
Third, the use of the DAWBA at age 14 years to assess general adolescent psychiatric symptoms allowed us to select adolescents with subthreshold depression and to exclude those with validated psychiatric diagnoses. At follow-up, the validation of diagnoses was not yet effective, and the ADRS score was used as a proxy for depression. Thus, using different methods of assessing depressive symptoms at baseline and follow-up might have biased the causal mediation analysis. However, the ADRS cut-off score has been shown to discriminate between adolescents defined as having or not having depression according to the DSM-IV. Thus, it is likely that the adolescents with a high ADRS score would have been given the diagnosis had the rating been available. Another limitation is that the ADRS was available only at age 16 years, and it was not possible to assess changes from baseline to follow-up regarding depression scores.
Fourth, the high rate of loss to follow-up could have biased the assessment of depression outcomes. However, this rate did not differ between the control group and the group with subthreshold depression.
Fifth, previous studies of adolescents at familial risk for depression43,44 have reported smaller hippocampal volumes. In contrast, the present adolescents, controls, and those with subthreshold depression had no difference in family history of depression and no between-group difference in hippocampal volumes. This result is in line with other neuroimaging studies in adolescents with MDD that did not report smaller hippocampal volumes either9,12; rather, hippocampal volume reductions in adolescents have been attributed to illness duration.45 Similarly, although studies of pediatric depression have reported reduced amygdala volumes12 or a decreased volume related to comorbid anxiety disorders,13 we did not observe smaller amygdala volumes in adolescents with subthreshold depression. Thus, these negative findings are consistent with studies relating amygdala–hippocampal volume alterations to disease progression, comorbidities, or family load.13,43–45 Finally, the study was not designed to include scanning of the participants at age 16 years, and thus it was not possible to assess the neuroimaging longitudinal correlates of the progressing clinical changes.
Overall, smaller gray and white matter volumes, located in core regions of emotion regulation, were detected in 14-year-old community adolescents with subthreshold depression. Medial–prefrontal volume differences might denote high risk of depression in juveniles, notably in girls. &
Supplementary Material
Clinical Guidance.
In 14-year-old adolescents with self-reported subthreshold depression with no other comorbidity, we observed smaller gray and white matter volumes in frontal–striatal–limbic regions compared to those in control participants. These regions are involved in affective regulation and have consistently been reported as altered in MDD.
Consistent with the literature, we observed that subthreshold depression is a condition at risk for later MDD, notably in girls. Furthermore, in girls with subthreshold depression, transition to MDD at age 16 years might be influenced by smaller medialeprefrontal gray matter volume at age 14 years. A distinct developmental pattern in that core emotion regulation region may play a role in the transition to full-depression disorder during female adolescence.
As adolescents with subthreshold depression are at risk for developing depression, all the more since they have significant changes in emotion regulation brain networks, we suggest that more attention should be paid to subthreshold depression in adolescence, and that intervention strategies should be developed.
Acknowledgments
This work received support from the European Union-funded FP6 Integrated Project IMAGEN (LSHMCT-2007-037286), the FP7 project IMAGEMEND and the Innovative Medicine Initiative Project EUAIMS (115300-2), a Medical Research Council Programme Grant (93558), the Swedish funding agency FORMAS, the Wellcome Trust (University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Department of Health UK, the Bundesministerium für Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the French funding agency ANR (ANR-12-SAMA-0004), an EraneteNeuron grant (project AF12-NEUR0008-01 - WM2NA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris-Descartes-University (collaborative-project-2010), Paris-Sud-University (IDEX-2012), the Fondation de France, and the Mission Interminist erielle de Lutte-contre-la-Drogue-et-la-Toxicomanie (MILDT).
Footnotes
Disclosure: Dr. Goodman is the owner of Youthinmind, Ltd., which provides no-cost and low-cost software and websites related to the Development and Well-Being Assessment and the Strengths-and-Difficulties Questionnaire. Dr. Stringaris has received grant or research support from the Wellcome Trust, the National Institute for Health Research (NIHR), and the Department of Health UK. He has received royalties from Cambridge University Press for his book The Maudsley Reader in Phenomenological Psychiatry. Dr. Barker has received honoraria for teaching from General Electric, acted as consultant of IXICO, and was granted US patent 2015-0123662A1. Dr. Robbins has received compensation as a consultant for Cambridge Cognition. Dr. Paillère-Martinot has received compensation from Janssen-Cilag for CME activities. Drs. Vulser, Lemaitre, Artiges, Penttil€ a, Struve, Fadai, Kappel, Grimmer, Poustka, Conrod, Frouin, Banaschewski, Bokde, Bromberg, Büchel, Flor, Gallinat, Garavan, Gowland, Heinz, Ittermann, Lawrence, Loth, Mann, Nees, Paus, Pausova, Rietschel, Smolka, Schumann, Martinot, and Mr. Miranda report no biomedical financial interests or potential conflict of interest.
Contributor Information
Dr. Hélène Vulser, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France.
Dr. Hervé Lemaitre, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France.
Dr. Eric Artiges, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France; Orsay Hospital, France.
Mr. Ruben Miranda, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France.
Dr. Jani Penttilä, Psychosocial Services Adolescent Outpatient Clinic Kauppakatu 14, Lahti, Finland.
Dr. Maren Struve, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany.
Dr. Tahmine Fadai, Universitaetsklinikum Hamburg Eppendorf, Germany.
Dr. Viola Kappel, Psychosomatics and Psychotherapy, Charité-Universitätsmedizin, Berlin.
Dr. Yvonne Grimmer, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany.
Dr. Robert Goodman, King’s College London Institute of Psychiatry, London.
Dr. Argyris Stringaris, King’s College London Institute of Psychiatry, London.
Dr. Luise Poustka, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Dr. Patricia Conrod, King’s College London Institute of Psychiatry, London; Université de Montréal, Centre Hospitalier Universitaire Sainte-Justine Hospital, Montreal, Canada.
Dr. Vincent Frouin, Neurospin, Commissariat à l’Energie Atomique et aux Energies Alternatives, Paris.
Dr. Tobias Banaschewski, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Dr. Gareth J. Barker, King’s College London Institute of Psychiatry, London.
Dr. Arun L.W. Bokde, Institute of Neuroscience, Trinity College Dublin, Ireland.
Dr. Uli Bromberg, Universitaetsklinikum Hamburg Eppendorf, Germany.
Christian Büchel, Universitaetsklinikum Hamburg Eppendorf, Germany.
Dr. Herta Flor, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany.
Dr. Juergen Gallinat, Campus CharitéMitte, Charité-Universitätsmedizin, Berlin.
Dr. Hugh Garavan, Institute of Neuroscience, Trinity College Dublin, Ireland; University of Vermont, Burlington, VT.
Dr. Penny Gowland, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom.
Dr. Andreas Heinz, Campus CharitéMitte, Charité-Universitätsmedizin, Berlin.
Dr. Bernd Ittermann, Physikalisch-TechnischeBundesanstalt (PTB), Braunschweig und Berlin, Berlin.
Dr. Claire Lawrence, School of Psychology, University of Nottingham.
Dr. Eva Loth, King’s College London Institute of Psychiatry, London.
Dr. Karl Mann, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany.
Dr. Frauke Nees, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Dr. Tomas Paus, Rotman Research Institute, University of Toronto, Toronto.
Dr. Zdenka Pausova, The Hospital for Sick Children, University of Toronto.
Dr. Marcella Rietschel, Central Institute of Mental Health, Medical Faculty Mannheim/Heidelberg University, Germany.
Dr. Trevor W. Robbins, Behavioural and Clinical Neurosciences Institute, University of Cambridge, Cambridge, United Kingdom.
Dr. Michael N. Smolka, Technische Universität Dresden, Dresden, Germany.
Dr. Gunter Schumann, King’s College London Institute of Psychiatry, London; MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, London.
Jean-Luc Martinot, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France.
Dr. Marie-Laure Paillère-Martinot, Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1000, Neuroimaging and Psychiatry Research Unit, Orsay, University Paris-Sud, Orsay, and University Paris Descartes, Sorbonne Paris Cité, France; Hô pitaux de Paris, Maison de Solenn, Cochin Hospital, Paris.
for the IMAGEN Consortium:
J. Dalley, N. Subramaniam, D. Theobald, C. Bach, M. Fauth-Bühler, S. Millenet, R. Spanagel, L. Albrecht, N. Ivanov, M. Rapp, J. Reuter, N. Strache, A. Ströhle, C. Lalanne, J.B. Poline, Y. Schwartz, B. Thyreau, M. Lathrop, J. Ireland, J. Rogers, N. Bordas, Z. Bricaud, I. Filippi, A. Galinowski, F. Gollier-Briant, J. Massicotte, C. Andrew, A. Cattrell, S. Desrivieres, D. Hall, S. Havatzias, T. Jia, C. Mallik, C. Nymberg, L. Reed, B. Ruggeri, L. Smith, K. Stueber, L. Topper, H. Werts, R. Brühl, A. Ihlenfeld, B. Walaszek, T. Hübner, K. Müller, S. Ripke, S. Rodehacke, E. Mennigen, D. Schmidt, N. Vetter, V. Ziesch, D. Carter, C. Connolly, S. Nugent, J. Jones, R. Whelan, J. Yacubian, S. Schneider, K. Head, N. Heym, C. Newman, A. Tahmasebi, and D. Stephens
References
- 1.Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- 3.Bertha EA, Bal azs J. Subthreshold depression in adolescence: a systematic review. Eur Child Adolesc Psychiatry. 2013;22(10):589–603. doi: 10.1007/s00787-013-0411-0. [DOI] [PubMed] [Google Scholar]
- 4.Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62(1):66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- 5.Klein DN, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome depressive disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- 7.Bal azs J, Mikl osi M, Kereszt eny A, et al. Adolescent subthreshold-depression and anxiety: psychopathology, functional impairment and increased suicide risk. J Child Psychol Psychiatry. 2013;54:670–677. doi: 10.1111/jcpp.12016. [DOI] [PubMed] [Google Scholar]
- 8.Boes AD, McCormick LM, Coryell WH, Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol Psychiatry. 2008;63:391–397. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shad MU, Muddasani S, Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J Child Adolesc Psychopharmacol. 2012;22:190–197. doi: 10.1089/cap.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan CL, Moore GJ, Madden R, et al. Prefrontal cortical volume in childhood-onset major depression: preliminary findings. Arch Gen Psychiatry. 2002;59:173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- 11.Pannekoek JN, van der Werff SJ, van den Bulk BG, et al. Reduced anterior cingulate gray matter volume in treatment-naive clinically depressed adolescents. Neuroimage Clin. 2014;4:336–342. doi: 10.1016/j.nicl.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Caetano SC, Fonseca M, Hatch JP, et al. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett. 2007;427:142–147. doi: 10.1016/j.neulet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo K, Rosenberg DR, Easter PC, et al. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. J Child Adolesc Psychopharmacol. 2008;18:121–131. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- 15.Whittle S, Lichter R, Dennison M, et al. Structural Brain Development and depression onset during adolescence: a prospective longitudinal study. Am J Psychiatry. 2014;171:564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- 16.Schumann G, Loth E, Banaschewski T, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psycho-pathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 17.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 18.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 19.Woicik PA, Stewart SH, Pihl RO, Conrod PJ. The Substance Use Risk Profile Scale: a scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav. 2009;34:1042–1055. doi: 10.1016/j.addbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Castellanos-Ryan N, O’Leary-Barrett M, Sully L, Conrod P. Sensitivity and specificity of a brief personality screening instrument in predicting future substance use, emotional, and behavioral problems: 18-month predictive validity of the Substance Use Risk Profile Scale. Alcohol Clin Exp Res. 2013;37(Suppl 1):E281–E290. doi: 10.1111/j.1530-0277.2012.01931.x. [DOI] [PubMed] [Google Scholar]
- 21.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 22.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 23.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb MD, Huba GJ, Bentler PM. A multidimensional assessment of stressful life events among adolescents: derivation and correlates. J Health Soc Behav. 1981;22:400–415. [Google Scholar]
- 25.Revah-Levy A, Birmaher B, Gasquet I, Falissard B. The Adolescent Depression Rating Scale (ADRS): a validation study. BMC Psychiatry. 2007;7:2. doi: 10.1186/1471-244X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revah-Levy A, Speranza M, Barry C, et al. Association between Body Mass Index and depression: the “fat and jolly” hypothesis for adolescents girls. BMC Public Health. 2011;11:649. doi: 10.1186/1471-2458-11-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 28.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial pre-frontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducharme S, Albaugh MD, Hudziak JJ, et al. For the Brain Development Cooperative Group. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–2950. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay LK, Naranjo CA, Graham SJ, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 34.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Giorgio A, Watkins KE, Chadwick M, et al. Longitudinal changes in gray and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 37.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–183. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forbes EE, Dahl RE. Research review: altered reward function in adolescent depression: what, when and how? J Child Psychol Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 40.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao U, Chen L-A, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.