Abstract
The hippocampus hosts the continuous addition of new neurons throughout life—a phenomenon named adult hippocampal neurogenesis (AHN). Here we revisit the occurrence of AHN in more than 110 mammalian species, including humans, and discuss the further validation of these data by single-cell RNAseq and other alternative techniques. In this regard, our recent studies have addressed the long-standing controversy in the field, namely whether cells positive for AHN markers are present in the adult human dentate gyrus (DG). Here we review how we developed a tightly controlled methodology, based on the use of high-quality brain samples (characterized by short postmortem delays and ≤ 24h of fixation in freshly prepared 4% paraformaldehyde), to address human AHN. We review that the detection of AHN markers in samples fixed for 24 h required mild antigen retrieval and chemical elimination of autofluorescence. However, these steps were not necessary for samples subjected to shorter fixation periods. Moreover, the detection of labile epitopes (such as Nestin) in the human hippocampus required the use of mild detergents. The application of this strictly controlled methodology allowed reconstruction of the entire AHN process, thus revealing the presence of neural stem cells, proliferative progenitors, neuroblasts, and immature neurons at distinct stages of differentiation in the human DG. The data reviewed here demonstrate that methodology is of utmost importance when studying AHN by means of distinct techniques across the phylogenetic scale. In this regard, we summarize the major findings made by our group that emphasize that overlooking fundamental technical principles might have consequences for any given research field.
Keywords: Adult hippocampal neurogenesis, human, immunohistochemistry, antigen retrieval, autofluorescence
Adult hippocampal neurogenesis (AHN) encompasses the functional integration of new dentate granule cells (DGCs) into the hippocampal trisynaptic circuit throughout life (Zhao et al., 2006). The continuous addition of new neurons confers the adult brain with an extraordinary reserve of plasticity. Indeed, AHN participates in hippocampal-dependent learning (Sahay et al., 2011; Shors et al., 2001) and mood regulation (Hill et al., 2015), and it has been proposed to play a role in memory loss (Akers et al., 2014). The occurrence of AHN is widespread among the > 120 mammalian species in which it has been assessed (see Table 1 for a complete list), including rodents (Altman, 1963), shrews (Gould et al., 1997), sheep (Brus et al., 2013), bats (Chawana et al., 2014; Chawana et al., 2016; Chawana et al., 2020; Gatome et al., 2010), elephants (Patzke et al., 2014), non-human primates (Franjic et al., 2022; Gould et al., 1999; Hao et al., 2022; Kohler et al., 2011), and humans (Boldrini et al., 2018; Eriksson et al., 1998; Flor-Garcia et al., 2020; Knoth et al., 2010; Manganas et al., 2007; Moreno-Jimenez et al., 2019; Moreno-Jimenez et al., 2021; Spalding et al., 2013; Terreros-Roncal et al., 2021). There may be a few exceptions: two studies did not find evidence of AHN in northern minke whales or in harbor porpoises (Patzke et al., 2015), nor in a small number of echolocating microbats captured from the wild (Amrein et al., 2007). However, these results lack further replication to date. Although AHN has been extensively characterized in rodents, technical and ethical aspects may have limited the pace of discoveries in the human AHN field. In this regard, although a few studies failed to detect markers of neurogenesis in the adult human hippocampus (Dennis et al., 2016; Franjic et al., 2022; Sorrells et al., 2018), most literature available supports the occurrence of AHN in our species (Boldrini et al., 2018; Eriksson et al., 1998; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Spalding et al., 2013; Terreros-Roncal et al., 2021; Tobin et al., 2019; W. Wang et al., 2022) (see Table 2 for an extended list). This review addresses the potential overlooking of technical considerations that, in our view, lie behind most controversial aspects and ambiguities related to AHN studies in humans (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2021).
Table 1.
List of studies addressing the occurrence of adult hippocampal neurogenesis (AHN) in distinct mammalian species. Thymidine- H3: Tritiated thymidine. Aβ: Amyloid-β. AHN: Adult hippocampal neurogenesis. BrdU: 5-bromo-2’-deoxyuridine. CB: Calbindin. CA3: Cornu Ammonis 3. CNP: 2’,3’-cyclic nucleotide phosphodiesterase. CR: Calretinin. DCX: Doublecortin. DG: Dentate gyrus. DGC: Dentate granule cell. ETNPPL: Ethanolamine-phosphate phospholyase. GCL: Granule cell layer. GFAP: Glial fibrillary acidic protein. HMGB2: High-mobility group protein B2. IPC: Intermediate Progenitor Cell. MCM2: Minichromosome Maintenance Complex Component 2. ML: Molecular layer. n-3 PUFAS: n-3 polyunsaturated fatty acids. NeuN: Neuronal nuclear antigen. NMDA: N-methyl-D-aspartate. NOS: Nitric oxide synthase. NSC: Neural stem cell. NSE: Neuron-specific enolase. PCNA: Proliferating cell nuclear antigen. PH3: Phospho-Histone H3. PSA-NCAM: Polysialylated-neuronal cell adhesion molecule. RGL: Radial glia-like. RNA: Ribonucleic acid. RNR: Ribonucleotide reductase. S100β: S100 calcium-binding protein β. SGZ: Subgranular zone. Sc-RNA seq: Single-cell RNA sequencing. Sn-RNA seq: single-nucleus RNA sequencing. Sox2: SRY-Box transcription factor 2. TOAD: Turned On After Division. TUC-4: Turned On After Division/Ulip/ CRMP-4. TuJ1: Neuron-specific class III β-tubulin. This table was constructed after performing a manual search of studies that included the following terms (and their combination thereof) in the PubMed database: “Adult hippocampal neurogenesis”, “mammal”, “wild mammals”, “cetacean”, “non-human primate”, “old world primate”, “new-world primate”, “non-placental mammal”, “marsupialia”, “carnivore”, “herbivore”, “primate”, “chiroptera”, “artiodactyla”, “domestic animal”, “eulipotyphla”, “afrosoricida”, “scandentia”, “macroscelidae”, “hyracoidea”, “rodentia”, “rodents”, “didelmorphia”, “pig”, “sheep”, “bat”, “fox”, “mole”, “shrew”, “rabbit”, “wild rodent”, “vole”, “marine mammal”, “prosimian”, “sirenia”, “elephant”, “swine”, “canis”, “bovine”, “ovine”, “felis”, “dolphin”, “whale”, and “seal”. Studies not focused on the hippocampal region or that examined only human subjects were manually excluded.
| INFRACLASS | ORDER | SPECIES | REFERENCE | CONCLUSIONS | |
|---|---|---|---|---|---|
| Marsupialia | Dasyuromorphia | Fat-tailed dunnart (Sminthopsis crassicaudata) | (Harman, Meyer, & Ahmat, 2003) | The occurrence of AHN was demonstrated using thymidine-H3. Stress and age reduced the number of newly generated DGCs. | |
| Tasmanian devil (Sarcophilus harrisii) | (Patzke et al., 2015) | Presence of DCX+ immature neurons in the SGZ and GCL. | |||
| Placental mammals | Eulipotyphla | Hedgehog (Erinaceus concolor) | (Bartkowska et al., 2010) | Presence of DCX+ and Ki-67+ cells in the SGZ and GCL. No colocalization between DCX and glial markers such as vimentin or GFAP. | |
| Mole (Talpa europaea) | |||||
| African giant rat (Cricentomys gambianus) | (Olude, Olopade, & Ihunwo, 2014) | Presence of DCX+ and Ki-67+ cells in the DG. Adults presented lower cell numbers than juveniles. | |||
| (Lavenex, Steele, & Jacobs, 2000) | BrdU incorporation in the DG. No seasonal differences in cell proliferation rate or in total neuron number were observed. | ||||
| Yellow-pine chipmunks (Neotamias amoenus) | (Barker, Wojtowicz, & Boonstra, 2005) | Gray squirrels showed three times the density of Ki-67+ proliferating cells in the DG than chipmunks, which have simple food storage strategies. Both species showed similar density of immature DCX+ neurons. The number of Ki-67+ proliferative cells in gray squirrels and the density of DCX+ immature neurons in chipmunks decreased with age. | |||
| Red squirrel (Tamiasciurus hudsonicus) Cape mole-rats (Georychus capensis) | |||||
| (Wan, Tu, Zhang, Jiang, & Yan, 2019) | BrdU+ cells positive for Ki67, Sp8, and DCX in the adult SGZ. These cell populations peaked at the late gestational stages in comparison to non-pregnant females. | ||||
| (Ormerod & Galea, 2001) | Incorporation of thymidine-H3 and BrdU in the DG. Higher density of proliferative cells in the GCL and the hilus in reproductively inactive females compared to active females. This density correlated negatively with serum estradiol levels. Higher rates of cell survival in the GCL and the hilus in reproductively active females. | ||||
| Prairie vole (Microtus ochrogaster) | (Fowler, Liu, Ouimet, & Wang, 2002) | BrdU incorporation in the DG of females. | |||
| Yellow necked wood mouse (Apodemus flavicollis) Long-tailed wood mouse (Apodemus sylvaticus) | (Amrein, Slomianka, Poletaeva, Bologova, & Lipp, 2004) | AHN was demonstrated in the 4 species, which showed distinct onset of age-driven decrease in AHN. | |||
| Bank vole (Myodes glareolus) | |||||
| Pine vole (Microtus duodecimcostatus) | |||||
| Ultrastructural identification of thymidine-H3-labeled mitotic neuronal precursors in the GCL of rats and mice. | |||||
| (Altman, 1963) | Identification of a proliferative region in the adult rat DG. | ||||
| (Zhu et al., 2003) | Morphological characterization of RNR+ proliferative cells, which do not express markers of differentiated neurons or glial cells, except a fraction that co-expressed GFAP. Colocalization between BrdU and RNR in proliferative cells. | ||||
| (Dawirs, Teuchert-Noodt, Hildebrandt, & Fei, 2000) | Presence of BrdU+ proliferative cells in the DG throughout adult life and aging, despite a decline during juvenile life. | ||||
| (Dawirs, Hildebrandt, & Teuchert-Noodt, 1998) | BrdU incorporation. Haloperidol increased DGC proliferation. | ||||
| Namaqua rock mouse (Micaelamys namaquensis) | (Cavegn et al., 2013) | The numbers of Ki-67+ and DCX+ cells are similar for all the species with a habitat in southern Africa. Lower proliferation but higher neuronal differentiation in rodents from the southern African habitat compared to those from European environments. | |||
| Red veld rat (Aethomys chrysophilus) | |||||
| Highveld gerbil (Tatera brantsii) | |||||
| Spiny mouse (Acomys spinosissimus) Pygmy fieldmice (Apodemus microps) | |||||
| Long-tailed wood mice (Apodemus sylvaticus) | (Hauser, Klaus, Lipp, & Amrein, 2009) | No effect of voluntary running and environmental changes on the number of Ki-67+ proliferative cells and DCX+ immature neurons. | |||
| Stella wood mouse (Hylomyscus stella) Rwenzori striped mouse (Hybomys lunaris) Emin’s pouched rat (Cricetomys emini) | (Patzke et al., 2015) | Presence of KI-67+ proliferative cells and DCX+ immature neurons in the SGZ and GCL. | |||
| Beecroft’s flying squirrel (Anomalurus beecrofti) | |||||
| Target rat (Stochomys longicaudatus) | |||||
| Yellow-spotted brush-furred rat (Lophuromys flavopunctatus) | |||||
| Natal mjultimammate rat (Mastomys natalesis) | |||||
| Lesser egyptian jerboa (Jaculus jaculus) | |||||
| Arabian spinny mouse (Acomys dimidiatus) Cairo spiny mouse (Acomys cahirinus) Wagner’s gerbil (Gerbillys dasyurus) King jird (Meriones libycus) | |||||
| Libyan jird (Meriones libycus) | |||||
| Asian garden dormouse (Eliomys melanurus) Cape ground squirrel (Xerus inauris) | |||||
| Carnivora | |||||
| Feliform banded mongoose (Mungos mungo) | (Pillay et al., 2021) | DCX+ neurons located at the inner border of the GCL and within the SGZ in both species. DCX+ neurons exhibited apical dendrites that traversed the GCL to enter the ML. | |||
| Asian small-clawded otter (Aonyx cinerea) Harp seal (Pagophilus groenlandicus) | (Patzke et al., 2015) | Presence of DCX+ cells with processes that extend into the ML and GCL. | |||
| Nothern fur seal (Callorhinus ursinus) | |||||
| Siberian tiger (Panthera tigris altaica) | |||||
| Chiroptera | Pallas’s long-tongued bat (Glossophaga soricina) Seba’s short-tailed bat (Carollia perspicillata) | ||||
| Pale spear-nosed bat (Phyllostomus discolor) | |||||
| Egyptian slit-faced bat (Nycteris thebaica) | |||||
| Cyclops roundleaf bat (Hipposideros cyclops) Sundevall’s roundleaf bat (Hipposideros caffer) Pseudoromicia rendalli (Neoromicia rendalli) Guinean pipistrelle bat (Pipistrellus guineensis) | (Amrein, Dechmann, Winter, & Lipp, 2007) | AHN was studied by means of the expresion of Ki-67, MCM2, DCX and NeuroD. AHN was present in Chaerephon pumila, Mops condylurus and Hipposideros caffer. In Glossophaga soricina, Carollia perspicillata, Phyllostomus discolor, Nycteris macrotis, Nycteris thebaica, Hipposideros cyclops, Neoromicia rendalli, Pipistrellus guineensis, and Scotophilus leucogaster, no positive cells were detected. Not all the antigens were recognized in all species. | |||
| White-bellied house bat (Scotophilus leucogaster) | |||||
| Little free-tailed bat (Chaerephon pumila) | |||||
| (Patzke et al., 2015) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ and GCL. The processes of the latter cells extended into the ML. | ||||
| (Patzke et al., 2015) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ and GCL. The processes of the latter cells extended into the ML. | ||||
| (Patzke et al., 2015) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ and GCL. The processes of the latter cells extended into the ML. | ||||
| (Chawana et al., 2020) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the DG of adult Egyptian fruit bats from three distinct environments: primary rainforest, subtropical woodland, and fifth-generation captive- bred. No differences in the numbers of proliferative cells, despite higher numbers of immature neurons in wild-caught bats. | ||||
| Trident leaf-nosed bat (Asellia tridens) | (Chawana et al., 2014) | AHN was studied after euthanasia and perfusion following capture. Abundant number of DCX + cells in the DG in animals euthanized and perfused within 15 min of capture. Dramatic drop of AHN between 15 and 30 min post-capture, and absence of DCX+ neurons 30 min post-capture. | |||
| Kuhl’s pipistrelle (Pipistrellus kuhlii) | |||||
| (Chawana et al., 2014) | |||||
| (Patzke et al., 2015) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ. The processes of the latter cells extended into the ML and GCL. | ||||
| Afrosoricida | Hedgehog tenrec (Echinops tefairi) | (Alpar et al., 2010) | Higher numbers of BrdU+ and DCX+ cells in the SGZ of younger than in aged animals. Gradual decrease of AHN with aging. | ||
| (Patzke, Kaswera, Gilissen, Ihunwo, & Manger, 2013) | Presence of DCX+ cells with strongly labeled proceses, presumably axons and dendrites, in the SGZ and GCL. | ||||
| Hottentot golden mole (Amblysomus hottentotus) | (Patzke, LeRoy, et al., 2014) | Presence of DCX+ immature neurons in the SGZ and GCL. The processes of the latter cells extended into the ML. | |||
| Primate | Prosimians | Demidoffs dwarf galago (Galagoides dmidoff), Bosman’s potto (Perodicticus potto) and Ring-tailed lemur (Lemur catta) Gray mouse lemur (Microcebus murinus) | (Fasemore et al., 2018) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ. | |
| (Patzke et al., 2015) | Presence of Ki-67+ proliferative cells and DCX+ immature neurons in the SGZ and GCL. The processes of the latter cells extended into the ML. | ||||
| (Royo et al., 2018) | First evidence of the occurrence of AHN in prosimian primates. Increased number of newborn DGCs after n-3 PUFA supplementation for 21 months. | ||||
| Old World Primates | Vervet monkey (Chlorocebus pygerythrus) | (Patzke et al., 2015) | Presence of DCX+ immature neurons in the SGZ. The processes of the latter cells extended into the ML. | ||
| Olive baboon (Papio anubis) | |||||
| Snow monkey (Macaca fuscata) | (Tonchev, Yamashima, Zhao, Okano, & Okano, 2003) | Incorporation of BrdU and DCX expression in control monkeys. Ischemic damage increased the number o1 BrdU-labeled cells. Newly generated cells expressed Musashi1, Nestin, βIII-tubulin, TUC-4, DCX, Hu, NeuN, S100β, or GFAP. | |||
| (Tonchev & Yamashima, 2006) | BrdU incorporation in the adult DG. Ischemia increased the density of BrdU+ cells. BrdU+ newly generatec cells showed neuronal features 79 days after the insult. Adult-born cells remained in the SGZ and showed immature progenitor phenotype. | ||||
| (Kohler, Williams, Stanton, Cameron, & Greenough, 2011) | Protracted DGC maturation in non-human primates over a minimum of 6 months. Expression βIII-tubulin. DCX, NeuN. Delayed dendritic arborization, and acquisition of mature cell body morphology. | ||||
| Rhesus monkey (Macaca mulatta) | (Ngwenya, Peters, & Rosene, 2006) | Maturation sequence of adult primate DGCs is similar to that of adult rodents. BrdU+ cells expressed TUC- 4. Maturation lasts a minimum of 5 weeks. | |||
| (Franjic et al., 2022) | Reconstruction of the entire AHN trajectory, from RGL cells to mature DGCs in the adult rhesus monkey Profiling of 36,107 hippocampal nuclei using sn-RNA seq. | ||||
| Long-tailed macaque (Macaca fascicularis) | (Hao et al., 2022) | Profiling of 207,785 cells from the adult macaque hippocampus using sc-RNA seq. Reconstruction of the whole AHN trajectory including a heterogeneous pool of RGLs, IPCs and neuroblasts. Identification of HMGB2 as a novel IPC marker. Differences with rodent AHN. | |||
| New World Primates | Common marmoset (Callithrix jacchus) | (Gould, Tanapat, McEwen, Flugge, & Fuchs, 1998) | Production of new neurons in the DG of adult monkeys. Social stress-driven reduction in the number of proliferative cells. | ||
| (Marlatt et al., 2011) | BrdU incorporation and presence of DCX+ immature neurons. Unchanged numbers of BrdU+ or DCX+ cells after 2 weeks of isolation and social defeat stress. | ||||
| (Patzke et al., 2015) | Presence of DCX+ immature neurons in the SGZ. The processes of the latter cells extended into the ML. | ||||
| Squirrel monkey (Saimiri sciureus) | (Lyons et al., 2010) | BrdU incorporation in the DG. Increase in AHN after exposure to intermittent social separation and newpair formation. AHN positively correlated with the expression of genes involved in survival and integration of newly generated DGCs, as well as with enhanced spatial learning performance. | |||
| Macroscelidea | (Slomianka et al., 2013) | Captured sengis show fewer immature DCX+ cells without changes in proliferative PCNA+ cells than other murid species from the same habitat. | |||
| (Patzke et al., 2015) (Patzke, LeRoy, et al., 2014) | Presence of DCX+ immature neurons in the SGZ. The processes of the latter cells extended into the ML. | ||||
| Hyracoidea | Rock hyrax (Procavia capensis) | (Patzke, LeRoy, et al., 2014) | Presence of DCX+ immature neurons in the SGZ. The processes of the latter cells extended into the ML. | ||
| (Patzke et al., 2015) | Presence of DCX+ immature neurons in the SGZ and GCL. The processes of these cells extended into the ML. | ||||
| Artiodactyla | Pig (Sus scrofa) | (Franjic et al., 2022) | Reconstruction of the whole AHN trajectory from RGL cells to mature DGCs in the young adult pig. Profiling of 36,851 hippocampal nuclei using sn-RNA seq. | ||
| Swedish farm sheep (Ovis aries) | |||||
| (Brus et al., 2013) | BrdU incorporation and expression of DCX, NeuN, Sox2 and S100β. Longer maturation process than that in rodents and similar to that in non-human primates. | ||||
| Greater kudu (Tragelaphus strepsiceros) | |||||
| Blue wildbeest (Connochaetes taurinus) Black wildbeest (Connochaetes gnou) Arabian camel (Camelus dromedarius) Affrican buffalo (Syncerus caffer) | |||||
| Nyala (Tragelaphus angasii) | |||||
| Common eland (Taurotragus oryx) Giraffe (Giraffa camelopardalis) Blesbok (Damaliscus pygargus) Springbok (Antidorcas marsumalis) Nubian ibex (Capra nubiana) | |||||
| Simitar-horned oryx (Oryx dammah) | |||||
| Arabian oryx (Oryx leucoryx) | |||||
| Sand gazelle (Gazella marica) River hippopotamus (Hippopotamus amphibius) | |||||
| Domestic pig (Susscrofa) | |||||
| Sirenia | West indian manatee (Trichechus manatus) | ||||
| Cetacea | Northern minke whale (Balaenoptera acutorostrata) Harbour porpoise (Phocoena phocoena) | Small hippocampus size of cetaceans compared to that of other mammals. Lack of DCX+ cells in Northern minke whale and Harbour porpoise. | |||
| Scandentia | Tree shrews (Tupaia belangeri) | (Gould, McEwen, Tanapat, Galea, & Fuchs, 1997) | Incorporation of BrdU in the SGZ. 3 weeks after injection, BrdU+ NSE+ cells with neuronal morphology were incorporated to the GCL. Presence of Vimentin+ RGL cells with their processes extending into the GCL were observed in the SGZ. Negative and positive regulation of AHN by stress and NMDA receptor activation, respectively. | ||
Table 2.
List of studies addressing the occurrence of adult hippocampal neurogenesis (AHN) in humans. ALS: Amyotrophic lateral sclerosis. AD: Alzheimer’s disease. BP: Bipolar disorder. CJD: Creutzfeldt-Jakob disease. DLB: Dementia with Lewy Bodies. FTD: Fronto-temporal dementia. HD: Huntington’s disease. HIE: Hypoxic-ischemic encephalopathy. LWB: Lewy body dementia. MD: Major depression. MDD: Major depressive disorder. MDD/TCA: MDD with tricyclic antidepressant. MDD/SSRI: MDD with selective serotonin reuptake inhibitor. MND: Motor Neuron Disease. MS: Multiple sclerosis. MTLE: Mesial temporal lobe epilepsy. PD: Parkinson’s disease. PDD: Parkinson’s disease with dementia. PD-MCI: Parkinson’s disease mild cognitive impairment. PD-NCI: Parkinson’s disease non-cognitive impairment. SZ: Schizophrenia. TLE: Temporal lobe epilepsy. EM: electron microscopy. ICH: immunohistochemistry. ISH: in-situ hybridization. MRI: magnetic resonance imaging. PLI: polarized light imaging. qPCR: quantitative polymerase chain reaction. snRNA-seq: single nucleus RNA sequencing. AHN: adult hippocampal neurogenesis. DGCs: dentate granule cells. DG: dentate gyrus. DMSO: Dimethyl sulfoxide. F: female. GCL: granule cell layer. gw: gestational weeks. HSV: Hue, Saturation and Value. M: male. NPCs: neural progenitor cells. NSCs: neural stem cells. PBS: phosphate saline buffer. PFA: Paraformaldehyde. PMD: post-mortem delay. SGZ: subgranular zone. SVZ: subventricular zone. AQ4: Aquaporin-4. Ascl1: Achaete-Scute family BHLH transcription factor 1. ATF4: Activating Transcription Factor 4. BLBP: Brain lipid-binding protein. BrdU: bromodeoxyuridine. CB: calbindin. CR: calretinin. DCX: doublecortin. EGFR: epidermal growth factor receptor. GAD1: Glutamate Decarboxylase 1. GFAP: Glial fibrillary acidic protein. Hopx: homeodomain-only protein homeobox. Iba1: Ionized calcium-binding adaptor molecule 1. MAP2: Microtubule-associated protein 2. MBP: Myelin basic protein. MCM2: Minichromosome Maintenance Complex Component 2. METTL7B: methyltransferase-like protein 7B. MHCII: major histocompatibility complex II. NeuN: Neuronal nuclear antigen. NeuroD1: Neuronal Differentiation 1. NF1A: Nuclear Factor I A. NG2: neural-glial antigen 2. Olig2: oligodendrocyte transcription factor 2. OP18: Stathmin. PAX6: Paired box 6. PCNA: proliferating cell nuclear antigen. PH3: Phospho-histone 3. PLP: proteolipid protein. Prox1: Prospero Homeobox 1. PSA-NCAM: Polysialylated-neural cell adhesion molecule. pTau: phospho-Tau. S100β: S100 calcium-binding protein β. Sox1: SRY-Box Transcription Factor 1. Sox2: SRY-Box Transcription Factor 2. STMN1: Stathmin1. Tuj1: Neuron-specific class III β-tubulin. Tbr2: T-box brain protein 2. This table was constructed after performing a manual search of studies that included the following terms (and their combination thereof) in the PubMed database: “Adult hippocampal neurogenesis”, “Human”, and “New neurons”. Studies not focused on the human hippocampal region or that examined only other species were manually excluded.
| Study | Methods | Subject Information | Tissue | PMD | Markers | Main results | Tissue processing | Quantification |
|---|---|---|---|---|---|---|---|
| (Eriksson et al., 1998) | IHC | Undisclosed sex (64.4 ± 2.9 y) | Post-mortem | PMD not reported | BrdU, CB, NSE, GFAP, NeuN | Incorporation of BrdU into the adult human DG. | 4% PFA for 24 h, sectioning on a sliding microtome. | Total number of BrdU+ cells was determined in 5-7 sections from each subject. A semi-automatic image analysis system was used to estimate areas. Cell densities (cells/mm3) were reported. |
| (Blümcke et al., 2001) | IHC | Controls (embryonic, postnatal and adult tissue), undisclosed age and sex. 11F/16M TLE (8 mo–46 y) | Biopsy (TLE and Controls) and postmortem (Controls) | PMD controls (12 h– 3 d) | Nestin, Vimentin, Tuj1, Ki67, MAP1b/5, MAP2a-d, NF, NeuN, S100β, GFAP, Calbindin, CD68, CD45. | Increased neurogenesis in pediatric TLE patients. Delay in hippocampal maturation in a subgroup of TLE patients. | 4% PFA for 24 h (biopsy) or formalin (undisclosed concentration) for ≥ 2 w (autopsies). Vibratome sectioning. Antigen retrieval for Ki67, MAP2a-d and Tuj1. | Positive cells were quantified in 5 regions of interest per DG using a semi-automated imaging analysis software. The same software was used to calculate the hippocampus area. Cell densities (cells/mm2) were reported. |
| (Höglinger et al., 2004) | IHC | 2F/1M Controls (87.7±6.7 y), 2F/1M PD-NCI (71.3±4.9 y), and 2F/3M PD-MCI (85.6±5 y) | Post-mortem | PMD controls (31.5±7.2 h), PD-NCI (25.1±8.2 h), and PD-MCI (19.4±5.9 h) | Nestin, β-III Tubulin | The presence of Nestin+ and β-III Tubulin+neural precursor cells is reduced in the SGZ of PD patients. | Tissue was dissected, fixed and frozen (undisclosed time and fixative). Sectioned in freezing microtome. | Cells were counted in the GCL and SGZ using a semiautomatic stereology system (ExploraNova Mercator) in regularly spaced sections. Cell densities (cells/mm3) were reported. |
| (Jin et al., 2004) | IHC, WB | 3F/8M Controls (46.09±7.4 y), 1F/8M/4 undisclosed AD (76.22±3.2 y) | Post-mortem | PMD Controls (5–12 h) and AD (9–20 h) | DCX, PSA-NCAM, TUC4, NeuroD1 | Increased expression of immature neuron markers in AD patients, measured by means of WB. | Frozen samples (WB), or 4% PFA (unknown fixation time) followed by paraffin embedding. | No quantifications are presented. |
| (Boekhoorn et al., 2006) | IHC | 4F/6M Controls (67.1±2.3 y) and 5F/4M AD (66.2±2.0 y) | Post-mortem | PMD Controls (9.7 h) and AD (5.1 h) | DCX, Ki67, GFAP | Comparison of optimal pH conditions during antigen retrieval for Ki67 detection. Unaltered number of Ki67+ and DCX+ cells in the DG of presenile AD cases. | 10% formalin for 30-646 d. Paraffin-embedding and microtome sectioning. Antigen retrieval was performed for Ki67. | Ki67+ cells were counted in 3-4 sections of each subject. Cell densities (cells/mm2) were reported. For DCX and GFAP, a semi-quantitative approach was used. |
| (Reif et al., 2006) | IHC | 15 Controls (48±10 y), 15 SZ (44±13 y), 15 BP (42±12 y), and 15 MDD (46±9 y). Sex of the subjects is not reported. | Post-mortem | PMD Controls (23±9 h), SZ (33±14 h), BP (32±16 h), and MDD (24±11 h) | Ki67 | Reduced number of Ki67+ cells in SZ patients. | Frozen sections fixed in 4% PFA for 10 min. | Ki67+ cells were counted in the GCL of 7 sections per patient. Cell densities (cells/mm) were reported. |
| (Manganas et al., 2007) | Magnetic resonance spectros copy | 5 healthy subjects (M, F). Three preadolescent (8–10 y) and 3 adolescent (14– 16 y). | Living humans | Proton magnetic resonance spectroscopy (1H-NMR) | Identification of NSCs in the adult human hippocampus. The presence of NSCs decreases with age. | - | Quantification of the signal amplitude (ppm). |
| (Monje et al., 2007) | IHC | Controls (10 mo–63 y) and Cancer (10 mo–61 y) | Post-mortem | PMD controls and Cancer (<24 h) | DCX, Ki67, Olig2, CD68, CD20, CD3 | AHN impairments and increased inflammation after cranial radiotherapy or systemic chemotherapy. | Undisclosed fixation protocol and duration. Embedding in paraffin. | The number of cells positive for each marker/total granule cell nuclei present in DG was reported. |
| (Verwer et al., 2007) | IHC, WB, RT-qPCR | 5F/2M Controls (55–93 y), 15F/9M neurodegenerative diseases (AD, PiD, PSP, LBD, NAD, PD) (57–94 y), and 18F/11M Epilepsy (5–69 y) | Biopsy (Epilepsy) and post-mortem (AD, PiD, PSP, LBD, NAD, PD and Controls) | PMD (9–48 h). | DCX, NeuN, GFAP | Presence of DCX+ immature neurons in the hippocampus of AD patients. | Formalin-fixed tissue (undisclosed fixation time). | Semi-quantitative evaluation by means of a cellular profile scoring. |
| (Li et al., 2008) | IHC and ISH | 9F/6M Controls (83.6±7.4 y) and 7F/7M AD (79.4±10.9 y) | Post-mortem | PMD controls (2.6±0.6 h) and AD (2.4±0.6 h) | MAP2 | Reduced expression of MAP2a,b (mature forms) in the DG of AD patients, which suggests impaired neuronal differentiation. | Fixation in 4% PFA for 24-48 h. | Optical density was measured. |
| (Y. W. Liu et al., 2008) | IHC, WB, RT-PCR | 5F/10M Controls (18–78 y) and 15F/9M TLE (13–57 y) | Biopsy (Blümcke et al.) and post-mortem (Controls) | PMD controls (4.45–18.5 h) | DCX, PCNA, MCM2, PSA-NCAM, Tuj1, NeuN Reelin, CR, CB | The expression of DCX is increased in the hippocampus and temporal cortex of TLE patients. Co-expression of DCX and PCNA, Tuj1 and NeuN. | Perfusion/immersion in 15% formalin (undisclosed duration). | DCX+ cells along the GCL were counted using the Stereoinvestigator software. Cell densities (cells/mm3) were reported |
| (Boldrini et al., 2009) | IHC | 3F/4M Controls (17–53 y), 1F/4M MDD (29–62 y), 1F/2M MDD/TCA (28–61 y), and 2F/2M MDD/SSRI (24–61 y) | Post-mortem | PMD controls (9.5–22 h), MDD (4–22 h), MDD/TCA (9–19 h), MDD/SSRI (6–24 h) | Nestin, Ki67, NeuN, GFAP | Decreased number of NPCs with age. Increased number of NPCs in females. Antidepressant treatment increases the number of NPCs. | Frozen tissue, fixed in 4% PFA for 1w and sectioning in a freezing microtome. | Total number of cells positive for each marker was determined using the optical dissector and fractionator methods. The number of cells x 103 was shown. |
| (Mattiesen et al., 2009) | IHC | 7F/12M Controls (35–81 y) and 9F/13M HIE (35–85 y) | Post-mortem | PMD controls and HIE (0–5 d) | PCNA, TUC4, CR | Increased AHN and apoptosis after HIE. Aging did not impact on the number of proliferating or apoptotic cells in control subjects. | Formalin fixation (undisclosed time and concentration) and embedding in paraffin. | The number of cells positive for each marker was counted manually. Cell densities (cells/mm2) were reported. |
| (Crews et al., 2010) | IHC, qRT-PCR, WB | 3F/2M Controls (87±4.6 y), 3F/4M Early AD (86.1±1.7 y), and 4F/3M Severe AD (80±1.9 y) | Post-mortem | PMD controls (9.5±3.5 h), early AD (11.8±2.8 h), and severe AD (8.2±0.8 h) | BMP6, DCX, Sox2 | Increase in the expression of BMP6 and reduction in the number of DCX+ and Sox2+ cells in AD patients. | Formalin (undisclosed concentration) or 4% PFA (undisclosed duration). Vibratome sectioning. | The number of cells positive for each marker was counted in every sixth section and were multiplied by the reference volume. Total number of cells was reported. |
| (D’Alessio et al., 2010) | IHC | 3F/2M Controls (45.8±14 y) and 6F/3M TLE (40.1±6 y) | Biopsy (Blümcke et al.) and post-mortem (Controls) | PMD not reported. | DCX | Reduced number of DCX+ cells in patients with TLE. | Formalin for 5 d (undisclosed concentration) and embedding in paraffin. Microtome sectioning. | The number of cells positive for each marker was determined by computerized image analysis. Ten fields per section were evaluated. Cell densities (cells/field) were reported. |
| (Geha et al., 2010) | IHC | 3F/2M Surgical Controls (25–66 y), 2F/3M Autopsy Controls (31–64 y), and 6F/4M TLE (22–35 y) | Biopsy (epilepsy and surgical controls) and post-mortem (controls) | PMD not reported | Ki67, MCM2, Mib-1, Tuj1, MAP2, NeuN, Calretinin, GFAP, Nestin, Olig2, NG2. | Fixation time affects the detection of cell cycle- related proteins. Patients with TLE show increased numbers of Ki67+ cells in the SGZ, which acquire glial phenotypes. | Formalin-zinc (formalin 5%; zinc 3g/L; sodium chloride 8g/L) for 3 mo (post-mortem) or 16 h (surgical), and embedding in paraffin. | Cells were counted manually for each of the 10 tissue blocks that made up each hippocampal resection. Cell densities (cells/mm2 or cells/cm2) were reported. |
| (Hong et al., 2010) | IHC | Undisclosed | Undisclosed | DCX, p-TAU (PS396, PT231, PT205). | Co-localization between DCX and p-Tau in patients with AD. | Undisclosed fixation procedure. Freezing microtome sectioning. | - |
| (Knoth et al., 2010) | IHC, ISH, WB | 28F/22M adult (1 d–100 y) and fetal (11,20, and 40 gw) Controls | Post-mortem | PMD: 1–60 h | DCX, PCNA, Ki67, MCM2, Sox2, Nestin, TUC4, Tuj1 Prox1, PSA-NCAM, GFAP, Calretinin, NeuN. | Human AHN shares features identified in rodents. Mild decrease of AHN with age. | Formalin fixation (undisclosed duration and concentration). Paraffin embedding. | DCX+ cells were counted manually in 3 sections of the anterior hippocampus of each patient. The average number of positive cells/mm2 per 3 sections was given. |
| (Lucassen et al., 2010) | IHC | 3F/7M Controls (48–85 y) and 3F/7M MD (45–84 y) | Post-mortem | PMD controls (4–19.15 h) and MD (4–28 h) | MCM2, PH3 | Reduction in the number of MCM2+ cells in MD patients. | 4% Formaldehyde for 4-5 w and embedding in paraffin. | The number of cells positive for each marker was quantified in 5-6 sections. Cell densities (cells/μm2) were reported. |
| (Johnson et al., 2011) | IHC | 5F/3M Controls (71–101 y) and 3F/5M LBD (75–84 y) | Post-mortem | PMD controls (12–84 h) and LBD (51–96 h) | PCNA, GFAP, Musashi, DCX, Nestin, α-synuclein, Aβ | Loss of Musashi and GFAP. Increase in PCNA in the SGZ and GCL, and in the number of DCX+ cells in the GCL of patients with LBD. | Undisclosed fixation protocol. Embedding in paraffin. Microtome sectioning. Antigen retrieval. | The percentage of stained area was analyzed in 5 random images of each region. In addition, DCX+ cells were counted in the whole DG. Data were represented as the percentage of stained area per total area measured. |
| (Low et al., 2011) | IHC | 1F/7M Controls (32–81 y) and 5F/9M HD (35–75 y) | Post-mortem | PMD controls (9–24 h) and HD (3–24 h) | PCNA, Bcl-2, MCM2, Musashi | No differences in SGZ proliferation between control subjects and HD patients. | Perfusion and 24 h fixation with 15% formalin. Microtome sectioning. Antigen retrieval. | Cells were counted manually. Cell density was reported as average number of positive cells per 50 μm, per μm2, and per mm of hippocampal section. |
| (Winner et al., 2012) | IHC | 3F/3M Controls (86.00±10 y) and 1F/5M LBD (81.0±9.1 y). | Post-mortem | PMD controls (12.8±7.2 h) and LBD (9.5±3.1 h) | Sox2, α-synuclein, DCX | Increased α-synuclein and decreased numbers of Sox2+ cells in patients with LBD. | Fixation in 4% PFA (undisclosed duration). | The optical dissector method was used to count positive cells. The reference volume was determined using Stereoinvestigator MicroBrightField software. 3 systematically sampled sections (10 images per section) were analyzed to estimate the average number of immunolabeled cells/mm2. |
| (Boldrini et al., 2012) | IHC | 7F/5M Controls (41.8±14.6 y), 6F/6M MDD (43.6±13.3 y), 4F/2M MDD/TCA (46.2±17.1 y), and 2F/2M MDD/SSRI (38.8 ± 13.8 y) | Post-mortem | PMD controls (15.3±4.8 h), MD (16.0±5.9 h), MD/TCA (12.1±5.2 h), and MD/SSRI (15.8±7.5 h) | Ki67, Nestin, PECAM, Collagen IV | Decreased number of Nestin+ cells with age. SSRI treatment increases the number of Nestin+ cells in the DG of MD patients. | 4% PFA (undisclosed duration) and freezing. Freezing microtome sectioning. | The number of positive cells, area occupied by capillaries, and the volume and length of the DG, CA1 and parahippocampal gyrus were calculated using the optical dissector and fractionator methods. Total number of cells was reported. |
| (Perry et al., 2012) | IHC | 13F/8M Controls (80.9±8.5 y) and 13F/7M AD (81.2±7 y) | Post-mortem | PMD not reported | Musashi, Nestin, PSA-NCAM, DCX, Tuj1, ChAT | Reduced immunoreactivity of Musashi and ChAT, and increased immunoreactivity of Nestin+ and PSA NCAM+ in AD. Higher levels of DCX in the GCL of AD patients. No changes in Tuj1. | 4% Formaldehyde for 4 w and embedding in paraffin. Microtome sectioning. Antigen retrieval | A threshold was established to detect immunopositive signal. The integrated optical density was reported. |
| (Epp et al., 2013) | IHC | 4F/8M Controls (46.8±3.49 y), 5F/7M MDD (42.83±2.92 y), and 6F/6M MDD- psychosis (41.5±3.47 y) | Post-mortem | PMD not reported | DCX, p21, NeuN | Increased number of DCX+/NeuN cells in patients with depression. | Frozen tissue, cryosectioned and fixed in 4% formaldehyde for 15 or 45 min. | The number of cells was counted in the GCL and SGZ of each section. Cell densities (cells/mm2) or the total number of NeuN cells per subject within the region of interest were reported. |
| (Spalding et al., 2013) | C14 dating | 32F/88M Controls (16–92 y) | Post-mortem | PMD not reported | C14 incorporation | Mild decrease in AHN with aging. 700 new neurons are added daily to the human DG. | Frozen tissue. Homogenized for nuclei isolation. | The rate of new neurons added into the human DG was calculated by retrospectively birth dating cells (ΔC14) and a mathematical model based on cell death rate, and the fractions of renewing and non-renewing cells. |
| (Ernst et al., 2014) | IHC, WB | 4 (undisclosed sex) Controls (20–71 y) | Post-mortem | PMD not reported | DCX, PSA-NCAM, MAP2, IdU, caspase-3 | Expression of immature neuron markers, as well as IdU incorporation in the SGZ. | Frozen tissue, sectioned by cryostat and fixed in 2% formaldehyde for 10 min. For IdU, formalin-fixed (undisclosed protocol) and embedding in paraffin sections were used. Antigen retrieval. | Undisclosed. |
| (Gomez-Nicola et al., 2014) | IHC | 5F/5M Controls for CJD variant (20–35 y), 4F/5M Controls for AD (58–79 y), 5F/5M CJD variant(20–34 y), and 5F/5M AD (58–76 y) | Post-mortem | PMD not reported | Ki67, Sox2, CR | Increased number of Ki67+ and CR+, but no changes in that of Sox2+ cells in patients with AD and CJD variant. Decreased numbers of these cell populations in aged controls. | Formalin-fixation (undisclosed protocol) and embedding in paraffin. Antigen retrieval. | The number of positive cells was counted in 4-5 fields per DG sample using ImageJ. Cell densities (cells/mm2) were reported. |
| (Bayer et al., 2015) | IHC | 6F/22M Controls (17–41 y) and 8F/12M heroin users (17–45 y) | Post-mortem | PMD controls (2.4 d) and heroin users (2.6 d) | Musashi, Nestin, Ki67, CR, GFAP, NeuN, Tuj1, DCX | Reduced number of Musashi+ and Nestin+ progenitor and proliferating cells in heroin users. | Fixation in 4% formaldehyde for 48 h-5 y, embedding in paraffin and microtome sectioning. Antigen retrieval. | The number of positive cells was counted manually in at least 3 fields of the GCL, SGZ and CA4. Total cell numbers, percentage of positive cells/total cell numbers, and cell densities (cells/mm2) were reported. |
| (D’Alessio et al., 2015) | IHC | 4F/4M Controls (23-60 y) and 7F/9M TLE (22–51 y) | Biopsy (Blümcke et al.) and post-mortem (Controls) | PMD not reported | Nestin | Reduced number of Nestin+ cells in patients with epilepsy. | Formalin-fixation (undisclosed concentration) for 5 d, embedding in paraffin and microtome sectioning. | The number of Nestin+ cells, mean gray value and reactive area (px2) were determined along the GCL in 10 fields per section. |
| (Ekonomou et al., 2015) | IHC | 5F/7M Braak-Tau stages 0-II (80.3±8.4 y), 8F/3M Braak-Tau stages III-IV (88.9±8.2 y), and 1F/4M Braak-Tau stages V-VI (86.8±5.3 y) | Post-mortem | PMD Braak-Tau stages 0–II (12–28 h), Braak-Tau stages III-IV (7–27 h), and Braak-Tau stages V-VI (9.5–33 h) | HuC/HuD, Nestin, PCNA, GFAP, Iba1 | Reduced number of HuC/HuD+ cells in patients at Braak-Tau V-VI stages. Increased presence of DCX+ cells in patients with dementia at advanced Braak-Tau stages. | Undisclosed fixation protocol. Paraffin embedding. Antigen retrieval. | The hippocampus area was measured on each section. Cell densities (cells/mm) were reported. |
| (Allen et al., 2016) | IHC | 3F/13M Controls (21–81 y) and 5F/5M SZ (55-75 y) | Post-mortem | PMD controls (10–50 h) and SZ (12.5–72 h) | Ki67, NeuN | Reduced number of Ki67+ cells in patients with SZ. | Frozen tissue, fixed in 4% PFA for 10 min at 4°C. | The density of Ki67+ (number of cells/mm2) within the SGZ, GCL and hilus was calculated in 3 slides per case. Stereoinvestigator was used to measure the area on each section. |
| (Dennis et al., 2016) | IHC | 9F/14M Controls (0.2–59 y) | Post-mortem | PMD controls (15–90 h) | Ki67, DCX, Tuj1, Olig2, EGFR, GFAP, PCNA | Marked decrease in proliferation and neuroblasts with age. Among the proliferating cell population, only microglial cells were identified | 15-20% formalin-fixation for 2-3 w. Paraffin embedding. Antigen retrieval. | Ki67+, DCX+ and PCNA+ cells were counted within the entire SVZ and SGZ of each section. Cell densities (cells/mm2) were reported. |
| (Galan et al., 2017) | IHC | 1F/3M Controls (69.50±11.38 y) and 4F/5M ALS (65.60±15.94 y) | Post-mortem | PMD controls and ALS (5±2 h) | Ki67, PCNA, GFAPδ, PSA-NCAM, DCX, Tuj1, GFAP, TDP43 | Proliferation and PSA- NCAM+ markers in the SGZ were decreased in patients with ALS. | Undisclosed fixation protocol. Paraffin embedding and vibratome sectioning. Antigen retrieval. | Cells were counted in ~10 fields for each marker. Cell densities (cells/500 μm2) were reported. |
| (Mathews et al., 2017) | qPCR, IHC | 4F/22M Controls for qPCR and 1F/4M Controls for IHC (18–88 y) | Post-mortem | PMD controls (9–51 h) | Ki67, DCX, GFAPδ, GFAP, Tbr2 | Reduced DCX and Ki67 mRNA expression with aging. | Dissected and fresh frozen tissue (qPCR). Formalin-fixed (undisclosed fixation protocol) and embedded in paraffin (IHC). | 3 randomly selected areas of the hilus were photographed for visual analysis. No cell density values were reported. |
| (Oreja-Guevara et al., 2017) | IHC | 1M Control (undisclosed age) and 1M MS (27 y) | Post-mortem | PMD not reported | GFAPδ, Musashi, Sox2, Pax6, NG2, Ki67, PCNA, Iba1, CD68, MHCII, Tuj1, DCX, PSA-NCAM, MBP, AQ4, Nestin, Olig | Low numbers of NSCs, intermediate progenitors and proliferation. Early AHN impairments in MS. | Fixation in 4% PFA (undisclosed time), paraffin embedding, and microtome sectioning. Antigen retrieval. | 8 random images from each section were analyzed. Cell densities (cells/mm2 or cells/mm) were reported. |
| (J. Liu et al., 2018) | IHC | 5 Developmental Controls (12–13 gw), 6F/9M Adult Controls (28–75 y), and 20F/22M Epilepsy (8–76 y) | Biopsy (epilepsy) and post-mortem (Controls and epilepsy) | PMD not reported | Nestin, DCX, Musashi, Tuj1, NeuN, GFAPδ, MAP2, Olig2, MCM2, AQ4, GS, ZnT3, CD34 | Increased densities of Nestin+ cells in patients with epilepsy. | Formalin-fixed (undisclosed protocol) and embedding in paraffin. | Semiquantitative evaluation of the number of Nestin+ cells. No cell density values were reported. |
| (Le Maitre et al., 2018) | IHC | 6F/11M Controls (24–78 y) and 1F/17M Alcohol consumers (30–67 y) | Post-mortem | PMD controls (11.25–84.5 h) and Alcohol consumers (5.25–64 h) | NeuN, DCX, Sox2, Ki67 | Reduction of AHN markers in alcohol consumers. | Flash-frozen tissue, fixed in 4% formaldehyde for 15-30 min and cryosectioned. | Positive cells for each marker were counted along the entire length of the GCL in the DG, in 5 sections/case. Cell densities (cells/mm2) were reported. |
| (Boldrini et al., 2018) | IHC | 11F/17M Controls (14–79 y) | Post-mortem | PMD controls (4–26 h) | PSA-NCAM, Sox2, Nestin, Ki67, DCX, NeuN, GFAP | Preserved numbers of intermediate progenitors and immature neurons, and decrease in quiescent progenitors throughout aging. | Flash-frozen tissue, fixed in 4% PFA (undisclosed duration) and microtome sectioning. | 10-12 sections per subject were analyzed along the rostro-caudal axis of the hippocampus. The optical dissector with fractionator approach was used with Stereoinvestigator software to estimate total cell numbers in the selected region |
| (Cipriani et al., 2018) | IHC | 39 Controls (13 gw–72 y) and 5 AD (74–89 y). Undisclosed sex. | Post-mortem | PMD controls (4–72 h, 27 subjects undisclosed) and AD (3–23 h) | Nestin, GFAP, DCX, Ki67, MCM2, Sox2, Pax6, Tbr2, Vimentin, Tuj1. | Reduction of RGL, proliferative, and DCX+ cells in the adult human DG. | Frozen tissue was fixed in 4% PFA (undisclosed duration), embedded in paraffin and cryosectioned. | Semiquantitative analysis. No cell densities or numbers were reported. |
| (Sorrells et al., 2018) | IHC, EM | 13F/24M Controls (14 gw–77 y) and 11F/11M Epilepsy (3m–64 y) | Biopsy (epilepsy) and post-mortem (controls) | PMD controls (<48h) | Ascl1, BLBP, DCX, BrdU, GFAP, Hopx, Ki67, MCM2, Nestin, NeuN, NeuroD, Olig2, Pax6, Prox1, PSA-NCAM, Sox1, Sox2, Tbr2, Tuj1, Vimentin, Iba1 | Absence of AHN markers in the adult human DG. | Perfusion with 4% PFA, fixation in either 4% PFA or 10% formalin (undisclosed duration). Additional fixation in 4% PFA for 2 days. | Quantification of 3-5 images across a minimum of 3 randomly selected sampled sections for each age. The DG was subdivided into subregions of interest (GCL, hilus and ML). Cell densities (cells/mm2) and total cells/section were reported. |
| (Stepien et al., 2018) | IHC | 2F/6M Controls (64±10.95 y), 7F/7M Non-hemorrhagic stroke (70±6.03 y), and 2F/6M Hemorrhagic stroke (64.75±12.23 y) | Post-mortem | PMD not reported | GFAP, PH3 | Presence of neural stem cells and NPCs observed in the DG and SVZ. Increased number of PH3+ cells in patients with hemorrhagic stroke. | Undisclosed fixation protocol and embedding in paraffin. Rotary microtome sectioning. Antigen retrieval. | Quantitative analysis was performed using the CellSens software. Cells were counted in the DG and SVZ. Cell densities (cells/mm2) were reported. |
| (Tartt et al., 2018) | IHC, ISH, RNAsco pe | 5 Controls, 5 antidepressant-treated, and 5 untreated subjects with MDD (19–67 y). Undisclosed sex. | Post-mortem | PMD control, antidepressant-treated, and untreated MDD patients (6–27 h) | DCX, NF, Sox2, PSA-NCAM, NeuN | Detection of AHN markers by means of RNAscope. | Flash-frozen tissue. Undisclosed fixation protocol. | Cells expressing DCX mRNA were quantified in 3 sections of the anterior-mid DG. Cell densities (cells/mm3) were reported. |
| (Gatt et al., 2017) | IHC | 15 Controls (80.47±8.48 y) and 41 LBD/PDD (79.98±5.32 y). Sexes were equally represented (52% F 48% M). | Post-mortem | PMD controls (34.26±17.32 h) and LBD/PDD (36.30±25.5149.95±21.57 h) | DCX | Increased presence of DCX+ immature neurons in the SGZ of patients with LBD/PDD. Higher expression of DCX in patients treated with SSRI. Higher DCX expression corelated with higher cognitive scores. | Undisclosed fixation protocol. Paraffin-embedded tissue. Antigen retrieval. | DCX+ cells were counted throughout the entire DG. Cell densities (cells/mm) were reported. |
| (Gomez-Pinedo et al., 2019) | IHC | 4 (undisclosed sex) Controls and 5F/7M MND (ALS or ALS-FTD) (37–87 y). | Post-mortem | PMD controls and ALS (2–6 h) | PCNA, Ki67, GFAPδ, PSA-NCAM, DCX, Tuj1, Iba1, Nestin | Decrease in the proliferative and neurogenic activity, and number of GFAPδ+ cells and neuroblasts in patients with ALS. | Samples were fixed with 10% formalin-(undisclosed time), embedded in paraffin, and microtome sectioned. Antigen retrieval. | The number of cells was determined in 5 slides per patient with 32 fields/slide (CA1, CA2, CA3 and DG). ImageJ was used to measure optical density. The relative number of inclusions was divided by the mean number of neurons per field (cells/mm2). |
| (Moreno-Jimenez et al., 2019) | IHC | 4F/9M Controls (43–87 y) and 19F/26M AD (52–97 y) | Post-mortem | PMD controls (3–38 h) and AD (2.5–10 h) | DCX, PH3, GFAP, Prox1, Tau, NeuN, CR, CB, Tuj1, PSA-NCAM, Aβ | Persistence of AHN markers in neurologically healthy control subjects, mild decrease in AHN with aging, and sharp decrease in AD patients. | Fixed in 4% PFA for 24 h. Vibratome sectioning. Mild and optimized antigen retrieval (limited number of microwave cycles avoiding boiling, 20 min in a commercial citrate buffer at 80°C). | The number of cells was estimated using the physical dissector method adapted to confocal microscopy. Cells were counted on 5–20 stacks of images obtained from 10 sections/subject. The number of cells was divided by the reference volume of the GCL in each image. Cell densities (cells/mm3) were reported. |
| (Seki et al., 2019) | IHC | 6M Controls (16–49 y) and 4F/8M Epilepsy (9–43 y) | Biopsy | PSA-NCAM, Ki67, HuB, DCX, GFAP | Reduced numbers of Ki67+/HuB+/DCX+ cells in the adult DG. | Fixed in 4% PFA for 3 d and frozen; cryostat sectioning. Antigen retrieval. | The number of cells positive for each marker in the GCL was counted in 2-5 sections/subject. Cell densities (cells/mm2) were reported. |
| (Tobin et al., 2019) | IHC | 3F/3M Controls (79–93 y) and 11F/1M AD (85–99 y) | Post-mortem | PMD controls (4.92–20.17 h) and AD (4.42–43.55 h) | DCX, PCNA, Nestin, Sox2, Ki67 | Persistence of AHN markers during aging and AD patients. AHN correlated with cognitive scores. | Undisclosed fixation method. Paraffin embedding. Antigen retrieval. | Cell counts were calculated in 2-4 sections/subject using the optical fractionator workflow of StereoInvestigator. Cell densities (cells/mm3) or total cell numbers were reported. |
| (Flor-Garcia et al., 2020) | IHC | 4F/9M Controls (43–87 y) | Post-mortem | PMD controls (3–38 h) | DCX, NeuN, PSA-NCAM, MAP2, CB, Prox1, CR, PH3, GFAP, Tuj1, Tau, S100β | Reduced expression of NeuN in DCX+ cells as compared to fully mature DGCs. | 4% PFA for 24 h and vibratome sectioning. Mild and optimized antigen retrieval (limited number of microwave cycles avoiding boiling, 20 min in a commercial citrate buffer at 80°C). | A modified physical dissector method coupled to confocal microscopy was used to quantify cell densities inside a reference volume. The number of cells was divided by the reference volume of the GCL in each image. Cell densities (cells/mm3) were reported. |
| (Terreros-Roncal et al., 2021) | IHC | 5F/10M Controls (43–87 y), 6F/6M ALS (48–80 y), 2F/4M HD (47–72 y), 6M DLB (62–89 y), 1F/2M PD (73–82 y), and 2F/4M FTD (56–87 y) | Post-mortem | PMD controls (3–38 h), ALS (5–12 h), HD (6–17 h), DLB (3–10 h), PD (5–10 h), and FTD (3.5–12 h) | S100β, DCX, Nestin, Sox2, PH3, HuC/HuD, PSA-NCAM, CR, NeuN, CB, Iba1, pTau, Tau3R, UEA1, htt, pTDP43, α-synuclein | In patients with neurodegenerative diseases, adult-born DGCs show abnormal morphological development and changes in the expression of differentiation markers. Ratio of quiescent to proliferating hippocampal neural stem cells shifts, and homeostasis of the neurogenic niche is altered. | Samples were fixed in 4% PFA for 24 h, included in 10% sucrose-4% agarose and vibratome-sectioned. Mild and optimized antigen retrieval (limited number of microwave cycles avoiding boiling, 20 min in a commercial citrate buffer at 80°C). | The number of cells was estimated by using the physical dissector method adapted to confocal microscopy. Cells were counted on 5–20 stacks of images obtained from 5-10 sections/subject. The number of cells was divided by the reference volume of the GCL in each image. Cell densities (cells/mm3) were reported.). |
| (Franjic et al., 2022) | IHC; snRNA-seq | 2F/4M Controls for snRNA-seq (44–79 y), 3F/3M Controls for IHC (38–76 y), and 3F/11M AD for IHC (61–89 y) | Post-mortem | PMD controls (2–26.1 h) and AD (8–28 h) | DCX, GAD1, METL7B | Absence of AHN transcriptomic and histological signatures. | Frozen tissue for snRNA-seq. Fresh tissue for IHC, fixed with 4% PFA/PBS (undisclosed duration) followed by 30% sucrose/PBS. High intensity antigen retrieval (20 min in citrate buffer pH 6 at 95°C) for DCX. No antigen retrieval for PSA-NCAM detection. | Undisclosed quantification protocol. |
| (Ammothum kandy et al., 2022) | IHC | 3F/6M Controls (21–56 y) and 12F/6M TLE (20–56 y) | Post-mortem | PMD controls and TLE (6–23 h) | Arc, DCX, c-fos, Ki67, GAD65/67 GFAP, Iba1, Prox1, PSA-NCAM, S100ß, Tuj1, GluR2/3 | Longer duration of epilepsy is associated with a sharp decline in neuronal production. | Fixation in 4% PFA (12 h per mm of tissue thickness) and microtome sectioning. | Quantifications were performed manually on Zeiss blue software. The GCL boundaries were determined using the DAPI channel. Cells were counted in the whole section. Cell densities (cells/mm3) were reported. |
| (Wang et al., 2022) | snRNA-seq, IHC | 2F/4M Controls (52–92 y) | Post-mortem | PMD controls (5–22.7 h) | PCNA, PAX6, Sox2, ETNPPL, NEUROD1, STMN1, STMN2, CB, Ki67, DCX, NeuN, Ascl1, HES6, Prox1, NNAT. | Presence of AHN transcriptomic and histological signatures such as proliferative NSCs, and immature neuron markers. | Blocks were either frozen in liquid nitrogen (snRNA-seq) or fixed overnight in 4% PFA at 4°C (IHC). Sectioned by cryostat. Antigen retrieval. | No quantification protocol reported. Images obtained by confocal microscopy and analyzed with ImageJ software. Cell densities (cells/mm2) were reported. |
| (Zhou et al., 2022) | snRNA-seq, IHC, in vitro slice culture | Sn-RNAseq: 0–2 y, 3M/1F; 3–6 y, 2M/2F; 13–18 y, 2M/2F; 40–60 y, 4M/1F; 86–92 y, 2M/3F; AD, 73–88 y, 4M/4F; and matched controls, 73–88 y, 6M/2F. IHC: 16M/8F (20 gw– 64 y) Slice culture: 5M/5F (2-61 y) | Biopsy (slice culture) and Post-mortem | PMD sn-RNAseq: 2.3–44 h. IHC: 5.9–43 h. | ATF4, CB, Caspase 3, DCX,Iba1, Math3, Ki67, NeuN, NEUROD1, NFIA, OLIG2, OP18, Prox1, S100β, STMN1, Tbr2. | Presence of immature neurons and proliferative progenitors in the human DG. In vitro EdU incorporation. Low-frequency proliferation and extended maturation. Decreased number of immature DGCs in patients with AD. | Blocks were fixed in 4% PFA at 4 °C for 24–48 h and cryoprotected. Forty- μm sections obtained in a frozen sliding microtome. A small proportion of the blocks was formalin-fixed and paraffin-embedded. Incubation with 0.5% NaBH4 for 30 min. Antigen retrieval with target-retrieval solution (DAKO). | PROX1+ DGCs or DAPI+ cells Were counted by semi-automated nuclear staining quantification using Fiji. SGZ and GCL were delineated using Prox1 staining. No quantification protocol reported for other cell counts. |
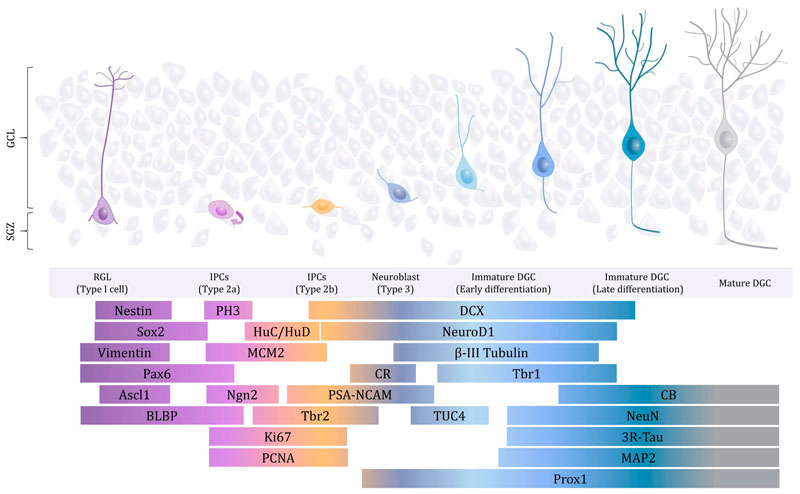
AHN has various sequential stages (Fig. 1) (Kempermann et al., 2004). Two main strategies, namely the birthdating of newly generated proliferative cells (Altman, 1963) and the detection of specific cell markers (Kempermann et al., 2004), have been used to thoroughly characterize AHN. This process relies on the presence of radial glia-like (RGL) neural stem cells (NSCs) in the dentate gyrus (DG) germinative matrix, namely the subgranular zone (SGZ). NSCs give rise to transit-amplifying progenitors and neuroblasts, which show a high proliferative capacity and expand neurogenic cell populations while progressively becoming committed to the neuronal lineage. In contrast, NSCs are mostly quiescent, a phenomenon proposed to be accentuated in aged individuals to preserve the pluripotency of these cells throughout life (Bottes et al., 2021; Harris et al., 2021). Neuroblasts are characterized by an immature neuronal morphology and the expression of proteins related to the cytoskeleton and cell plasticity, such as Doublecortin (DCX) and Polysialylated-neural cell adhesion molecule (PSA-NCAM). After exiting the cell cycle, newborn neurons go through sequential differentiation stages before becoming fully mature. Throughout their maturation, DGCs progressively occupy deeper positions in the granule cell layer (GCL) and show not only more complex dendritic morphologies but also the presence of functional dendritic spines and axons (Zhao et al., 2006).
Fig. 1.
Schematic diagram showing the main stages encompassed by adult hippocampal neurogenesis (AHN). The expression of Nestin, SRY-box 2 (Sox2), Vimentin, Paired box 6 (Pax6), Achaete-Scute family BHLH transcription factor 1 (Ascl1), Brain lipid-binding protein (BLBP), Phospho-Histone 3 (PH3), Human neuronal proteins C and D (HuC/D), Minichromosome maintenance protein 2 (MCM2), Neurogenin 2 (Ngn2), T-box brain protein 2 (Tbr2), Ki67, Proliferating cell nuclear antigen (PCNA), Doublecortin (DCX), Neurogenic differentiation 1 (NeuroD1), β-III Tubulin, Calretinin (CR), Polysialic acid-neural cell adhesion molecule (PSA-NCAM), Prospero Homeobox 1 (Prox1), T-box brain protein 1 (Tbr1), Turned On After Division/Ulip/ CRMP-4 (TUC-4), Calbindin (CB), Neuronal nuclei (NeuN), Three-repeated Tau (3RTau), and Microtubule-associated protein 2 (MAP-2) is shown. GCL: Granule cell layer. SGZ: Subgranular zone. RGL: radial glia-like. IPCs: Intermediate progenitor cells. DGC: dentate granule cell.
The history of human adult hippocampal neurogenesis
As in the rodent AHN field (Altman, 1963), birthdating strategies were initially used to assess the occurrence of AHN in humans. In 1998, Eriksson et al. observed that the peripheral administration of 5-bromo-2’-deoxyuridine (BrdU) results in the uptake of this molecule into discrete proliferative cells of the human DG, thereby supporting the occurrence of AHN in our species (Eriksson et al., 1998). That pioneering study showed that, several weeks/months after BrdU administration, BrdU-labeled cells express neuronal markers. In 2014, Eriksson’s results were further confirmed by Ernst et al. (Ernst et al., 2014), who used a different thymidine analog (5-Iodo-2’-deoxyuridine, IdU) and also observed the incorporation of this molecule into the adult human DG. Their results were further corroborated by a novel methodology, developed by Frisen’s group, to measure the incorporation of C14 into the brain (Spalding et al., 2013). Their studies determined that ~700 new neurons per hemisphere are added daily to the human DG.
In recent decades, numerous studies have used immunohistochemistry (IHC) to assess the occurrence of AHN in the human brain. Most of this work (see Table 2) systematically reported the presence of cells positive for AHN markers throughout human life (Boldrini et al., 2018; Cipriani et al., 2018; Eriksson et al., 1998; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Spalding et al., 2013; Terreros-Roncal et al., 2021). In this regard, despite decreasing throughout physiological aging, AHN remains detectable until the ninth decade of life (Boldrini et al., 2009; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021). Paradoxically, other studies failed to detect cells positive for such markers in the same region (Sorrells et al., 2018), although some of the authors of the aforementioned publication affirmed the existence of human AHN in previous studies (Galan et al., 2017). Given the specific conditions required to perform and validate IHC results on human tissue (Boekhoorn et al., 2006; Flor-Garcia et al., 2020), technical aspects need, more than ever, to be carefully dissected and taken into consideration when addressing these seemingly contradictory results. Indeed, an exhaustive revision of the literature available reveals that tissue processing methodologies differed markedly across the studies (see Table 2). Moreover, several articles do not disclose the criteria used to validate antibodies signal on human tissue, tissue fixation protocols, detailed antigen retrieval procedures, cell counting methods, etc. In this regard, studies by our group quantitatively demonstrate that the fixation time, type of fixative, tissue pre-treatment, and IHC protocols dramatically limit the extent to which AHN markers are visible in the human brain (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021), as will be further discussed throughout this review.
Critical steps to study human AHN by IHC
Tissue fixation
Our studies (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021) demonstrate that prolonged fixation impedes the visualization of numerous AHN markers in the human hippocampus. Most of the antibodies used to detect these markers show optimal performance in samples fixed for ≤ 24 h in 4% freshly prepared PFA at 4°C (Moreno-Jimenez et al., 2019). The use of samples fixed for such short periods allows reconstruction of the main stages encompassed by human AHN, thereby enabling visualization of mature and immature neurons at distinct stages of differentiation (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Moreno-Jimenez et al., 2021; Terreros-Roncal et al., 2021), proliferative cells (Terreros-Roncal et al., 2021), and NSCs (Terreros-Roncal et al., 2021) in the adult human DG until at least the ninth decade of life.
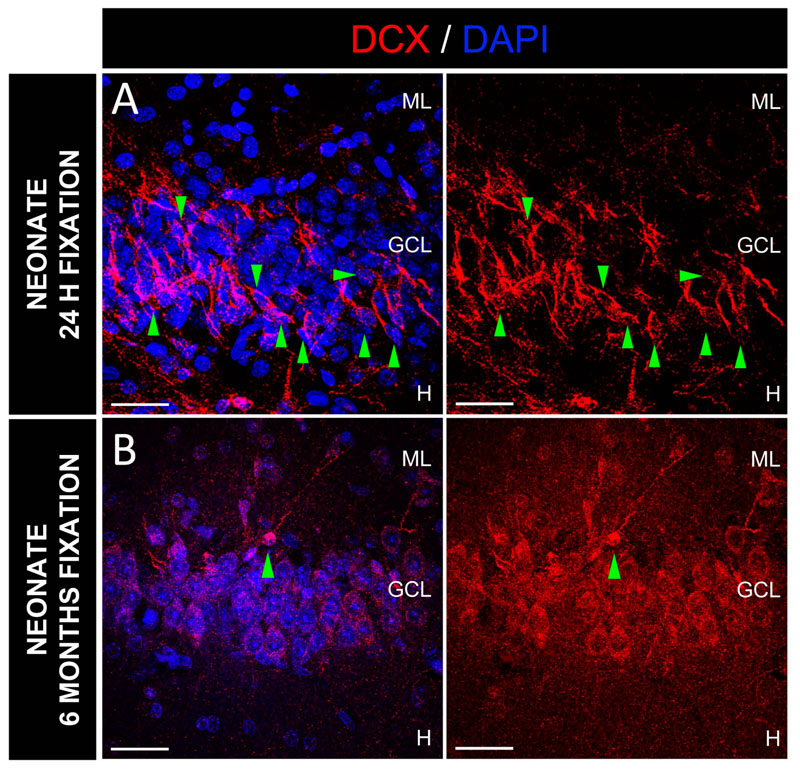
To determine whether the performance of antibodies used to detect AHN markers on human brain tissue depends on the duration of tissue fixation, we obtained the whole hippocampus from several neurologically healthy control subjects (61–87 years of age). Subsequently, we divided these hippocampi into several fragments. Each fragment was fixed for a different period (namely, 1, 2, 6, 12, 24, or 48 h) in 4% freshly prepared PFA at 4°C. We next compared, for each subject, the numbers of DCX+ and PSA-NCAM+ immature neurons that were detected upon distinct fixation times (Extended Data Fig. S2 of (Moreno-Jimenez et al., 2019)). One hour of fixation rendered excessively fragile samples, which also showed diminished signal intensity. Conversely, fixation times between 2 and 12 h increased tissue robustness and allowed smooth vibratome tissue sectioning and the unambiguous observation of DCX+ and PSA-NCAM+ immature neurons. The specific signal detected in these samples was accompanied by very low background intensity. Importantly, no tissue pre-treatment was needed to detect DCX+ or PSA-NCAM+ DGCs in samples fixed up to 12 h. Conversely, prolonged fixation (≥ 24 h) increased background intensity and masked antibody-specific signal, thereby impeding the detection of positive cells. The antigen masking caused by moderate fixation times (24-48 h) was easily reversed by applying mild antigen retrieval, sodium borohydride (NaBH4) incubation, and autofluorescence elimination, as will be discussed in the next sections of this review. The optimized protocols developed by our group (Flor-Garcia et al., 2020) demonstrate the importance of the mildness of the antigen retrieval step. Despite the public availability of these protocols and data, high-intensity antigen retrieval protocols were applied in recent studies, thereby leading to the observation of an unspecific DCX signal (Franjic et al., 2022; Sorrells et al., 2021) (Table 2), as our own experiments predicted (Fig. 4C in (Flor-Garcia et al., 2020)).
Post-mortem delay (PMD)
The PMD, also referred to as the post-mortem interval or the delay to fixation, can be defined as the time elapsed between exitus and sample immersion in fixative. It should not be confounded with the fixation time (namely the time during which a sample is immersed in a fixative). Despite legal issues that unavoidably lengthen the PMD, several studies recommend the use of samples with the shortest PMD possible (de Ruiter, 1983; Eymin et al., 1993; Robinson et al., 2016; Sorensen, 1984). In general terms, proteins and ribonucleic acid (RNA) may be sensitive to post-mortem degradation (Perrett et al., 1988). In fact, several authors (Kempermann et al., 2018; Lucassen, Fitzsimons, et al., 2020; Lucassen, Toni, et al., 2020) pointed to prolonged PMDs as the cause for the putative absence of AHN markers in the human DG reported by Sorrells et al. (Sorrells et al., 2018).
However, subsequent research revealed that, although certain proteins may show particularly rapid post-mortem degradation (Boekhoorn et al., 2006; Sorensen, 1984; Terstege et al., 2022), especially in certain regions of the brain (Siew et al., 2004), the immunodetection of most proteins and enzymes is moderately resistant to this phenomenon (Blair et al., 2016; Ritchie et al., 1986; Schut et al., 2017; Y. Wang et al., 2000). For instance, although NR2A and NR2B subunits appear to be rapidly degraded after death (Y. Wang et al., 2000), most subunits of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors are stable at ~18 h PMD. Studies performed by Boekhoorn et al. (Boekhoorn et al., 2006) and Terstege et al. (Terstege et al., 2022) suggested enhanced susceptibility of DCX to post-mortem degradation. These authors observed that, in rat samples with artificially induced prolonged PMDs (≥ 8 h), DCX staining disappears from the dendrites and is restricted to the soma and nucleus of immature DGCs. Terstege et al. reported that this phenomenon is accentuated in aged animals (Terstege et al., 2022). DCX is detected in the human DG at prolonged PMD intervals (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Moreno-Jimenez et al., 2021; Terreros-Roncal et al., 2021). Our quantitative and qualitative data reveal the stability not only of the number of DCX+ immature neurons in the human DG but also dendritic staining with this marker up to at least 38 h PMD. These results suggest putative interspecies differences in either the sensitivity of DCX protein to degradation or the binding capacity of distinct anti-DCX antibodies to their respective epitopes. However, these hypotheses lack further experimental evidence.
In line with our results that moderate PMDs are compatible with the study of most AHN markers in the human brain using IHC, recent work performed on 556 post-mortem human brains (6–279 h PMD) showed no significant correlation between brain pH, a widely used tissue quality indicator, and the PMD (Robinson et al., 2016). Another study (Blair et al., 2016) elegantly analyzed the intra-individual effects of artificially increasing PMDs. Those authors reported unchanged immunostaining profiles for most of the proteins studied after ≥ 50 h PMD, although degradation patterns were observed for several proteins by means of western blot (Blair et al., 2016). These carefully controlled experiments question the generalized notion that extended PMD is detrimental per se for the study of the human brain using IHC. Nevertheless, the results by Boekhoorn et al. (Boekhoorn et al., 2006) and others (Terstege et al., 2022), together with the fact that the sensitivity of a particular protein to post-mortem degradation cannot be predicted, indicate that performing adequate controls for each protein of interest and reporting individual PMDs should be mandatory in research protocols or scientific studies. Moreover, the use of materials with the shortest PMD possible is recommended when studying novel or putatively labile molecules (Robinson et al., 2016), and when setting up and validating new methodologies, such as single-cell (sc) or single-nucleus (sn) RNA-seq (Kalinina & Lagace, 2022).
Autofluorescence elimination
The aged human brain is particularly enriched in a lipid pigment named lipofuscin (Glees & Hasan, 1976). Neurons accumulating lipofuscin show a characteristic granular morphology that has been described under transmitted light, fluorescence, and electron microscopy (Glees & Hasan, 1976). The presence of lipofuscin granules in the human brain increases with age but starts to be observed during the first decade of life (Goyal, 1982). Indeed, immature and newly generated neurons exhibit less lipofuscin than their developmentally generated counterparts (Ernst et al., 2014; Roeder et al., 2022; Spalding et al., 2013).
In addition to the presence of abundant lipofuscin granules, brain tissue shows a great amount of primary background fluorescence (autofluorescence). It has been known for several decades that the autofluorescence of cell and tissue components depends on the fixative used and fixation time, as well as on excitation wavelength (Del Castillo et al., 1989). In particular, aldehyde fixation causes higher autofluorescence than methanol or ethanol/acetic acid (Del Castillo et al., 1989). Autofluorescence related to aldehyde fixation is attributed to the formation of Schiff’s bases between amines released upon cell death and the aldehydes present in the fixative solution (Willingham, 1983). Importantly, Schiff’s bases show high autofluorescence capacity. In fact, the intense autofluorescence observed in the human brain compromises the visualization and signal specificity of numerous antibodies. Several strategies have been used to decrease or eliminate autofluorescence in this tissue. Clancy et al. showed that a 30-min immersion of free-floating brain tissue sections in a sodium borohydride (NaBH4, 0.1%) solution reduces background fluorescence by ~30% (Clancy & Cauller, 1998), as this compound neutralizes Schiff’s bases by reducing the amine-aldehyde compounds into their corresponding non-fluorescent salts (Clancy & Cauller, 1998; Willingham, 1983). We (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021) and others (Boldrini et al., 2018; Boldrini et al., 2009) have successfully subjected such samples to NaBH4 incubation when studying human AHN. In particular, our data revealed that a 30-min incubation with a 0.5% solution of NaBH4 reduces background autofluorescence by ~40% (Fig. 4E – F in (Flor-Garcia et al., 2020)) and is therefore optimal for the visualization of several AHN markers in the human DG (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021).
Other reagents, such as Sudan black (Baschong et al., 2001; Kajimura et al., 2016), are useful to remove autofluorescence from human brain tissue. In particular, the use of commercial solutions of Sudan Black (such as the Autofluorescence Eliminator reagent (EMD Millipore)) reduces background and lipofuscin granule intensity in human DG samples, thereby facilitating the identification of AHN markers (Fig. 5 in (Flor-Garcia et al., 2020)). Other strategies, such as the use of LED lamps to quench fluorophores, have been assayed to eliminate autofluorescence in predominantly non-mitotic tissues such as the human brain (Sun et al., 2017).
In our hands, the most effective strategy to remove autofluorescence from aldehyde-fixed human brain samples combines a 30-min incubation with a 0.5% solution of NaBH4 before IHC with a brief (5-min) incubation with a commercial solution of Sudan Black (Autofluorescence Eliminator reagent (EMD Millipore)) at the end of the IHC protocol (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Moreno-Jimenez et al., 2021; Terreros-Roncal et al., 2021). Importantly, none of these steps should be confounded with antigen-retrieval protocols, aimed at unmasking antibody-specific signal and which are further discussed below.
Antigen retrieval
Although some epitopes are unaffected by the PMD (Bowers et al., 2003), epitope masking might unavoidably occur after prolonged aldehyde fixation. This process prevents antibodies from detecting and binding the antigens they have been raised against. To unmask epitopes that are especially sensitive to the fixation process, distinct antigen retrieval protocols can be applied to increase the specificity of the signal detected (Smith & Lippa, 1995). We and others have observed that antigen retrieval protocols are necessary to detect only certain antigens and under specific fixation conditions (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Moreno-Jimenez et al., 2021; Terreros-Roncal et al., 2021; Terreros-Roncal et al., 2022a, 2022b). In this regard, the detection of DCX in samples fixed for ≤ 12 h does not require the application of antigen retrieval protocols (Moreno-Jimenez et al., 2019). In contrast, samples fixed for 24-48 h, need to be subjected to a mild heat-mediated citrate buffer antigen retrieval protocol (HC-AR) (Moreno-Jimenez et al., 2019). The mildness of the latter step is crucial to increase the intensity of the specific signal without affecting that of the background (Flor-Garcia et al., 2020). Conversely, our studies show that the application of a harsh antigen retrieval approach cause the appearance of an unspecific antibody signal (Flor-Garcia et al., 2020). We have optimized an antigen retrieval protocol to achieve high-quality performance on human DG samples (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021). This protocol consists of exposing brain samples immersed in a commercial citrate buffer-based solution to short and strictly controlled microwave radiation cycles, followed by a subsequent 20-min incubation in a water bath. Given that aggressive antigen retrieval may lead to lack of signal specificity, our results show that finely adjusting the characteristics of antigen retrieval protocols is an essential step required to perform rigorous AHN studies (Fig. 4C in (Flor-Garcia et al., 2020)).
Other authors have reported that, depending on the duration of tissue fixation, distinct antigen retrieval conditions are optimal for the detection of particular epitopes. In general, long fixation times require high-intensity antigen retrieval (increased heating duration and/or use of lower pH values (Taylor et al., 1994)). For instance, it has been proposed that the detection of the proliferation marker Ki-67 is optimal when antigen retrieval is performed at low pH (Shi et al., 1995), whereas standard pH 6.0 is suboptimal for samples fixed for longer than 24 h (Munakata & Hendricks, 1993). In fact, Boekhoorn et al. (Boekhoorn et al., 2006) compared the adequacy of antigen retrieval under distinct pH conditions, namely pH 1.0 (0.1 M HCL), pH 3.0 (0.01 M citrate buffer), pH 6.0 (0.01 M citrate buffer) and pH 9.0 (0.01 M Tris)), to detect Ki-67+ signal in the human DG. Those authors concluded that the lowest pH renders the highest quality signal on human adult hippocampal tissue. Similar systematic studies are, therefore, needed to optimize antigen retrieval protocols for each antibody intended to be used on human brain samples.
The immunohistochemistry protocol
Antibody signal validation
The validation of primary and secondary antibodies is essential to assess any biological process using IHC. Moreover, given discrepant results reported in the literature, unambiguous signal validation gains further relevance in the context of AHN studies. Although an antibody is theoretically designed to target a protein of interest in a particular species, some antibodies produce unspecific staining, high background, or artifactual detection of other proteins (Flor-Garcia et al., 2020). Moreover, determining whether an observed signal is indeed specific is not straightforward. Despite inter-species differences, a general recommendation to validate a new antibody in a given species is to compare the signal obtained with that described in rodents (whenever this information is available). In this regard, the subcellular distribution of the signal observed should be, a priori, similar in both species (for instance, a microtubule-associated protein is expected to label cytoplasmic structures). Also, the same cell types are expected to be either positive or negative for that marker in both species. In this respect, morphological criteria can be used to identify cell types. However, evaluation of the co-expression of other markers that have been validated previously is advised whenever possible (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2021; Terreros-Roncal et al., 2021). If unexpected cell types appear to show positive staining, further validation with alternative methods (see below) might be required. In particular, in the case of AHN makers, widespread signal is not expected to be observed in non-neurogenic regions of the brain, although there might be exceptions. In this regard, observations of staining with anti-DCX antibodies in non-neurogenic regions have been used to refute the occurrence of human AHN (Alvarez-Buylla et al., 2022; Sorrells et al., 2021). This assumption overlooks two important considerations: first, the existence of DCX+ neurons in non-neurogenic regions of the mouse brain is compatible with the occurrence of rodent AHN and there is no evidence supporting the opposite in humans. Second, a recent study revealed that the DCX+ signal observed in the macaque cortex is not only unspecific but also artifactual (Liu et al., 2020). This finding thus calls for caution when interpreting DCX+ signal in non-neurogenic regions of the primate brain (Terreros-Roncal et al., 2022b).
To assess the specificity of a given antibody, distinct strategies, such as the use of synthetic blocking peptides that mimic the antigen recognition domain by antibodies (Liu et al., 2020; Moreno-Jimenez et al., 2019), can be tested. For instance, the pre-adsorption of an anti-DCX antibody with a specific blocking peptide causes the disappearance of the DCX+ signal by both dot-blot and IHC (Moreno-Jimenez et al., 2019), thereby confirming the authenticity of the DCX signal detected in the human DG. Strikingly, the incubation of anti-DCX antibodies with hippocampal extracts causes the DCX+ signal to disappear in macaque cortices but not in the hippocampi of this animal (Liu et al., 2020). These results question the specificity of certain anti-DCX antibodies used in previous studies (Sorrells et al., 2018), as they might detect other proteins that potentially share structural properties with DCX in some regions of the primate brain (Liu et al., 2020).
It is also important to note that not all the commercially available antibodies raised against a given protein render specific signal even under ideal sample processing conditions (Flor-Garcia et al., 2020). To further validate DCX staining in the human DG, we quantitatively compared the performance of various anti-DCX antibodies raised against distinct domains of the protein. As shown in (Moreno-Jimenez et al., 2019), the quality of the signal, the signal/background ratio, and the working concentrations differed among the distinct commercial antibodies tested. However, all of them rendered comparable numbers of DCX+ immature neurons (Moreno-Jimenez et al., 2019). This finding thus supports the suitability of these antibodies to specifically detect DCX in the human DG. As previously mentioned, none of the nine commercially available antibodies we tested were able to detect DCX+ cells in human hippocampal samples fixed for 6 months in formalin (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019). Importantly, the lack of DCX signal caused by excessive fixation was observed not only in adult samples but also in tissue obtained from juvenile and infantile subjects (Fig 2), in which the occurrence of neurogenesis has not been disputed to date.
Fig. 2. Immature neurons in the infantile human hippocampus.
A: Neonate human hippocampus upon 24 h of sample fixation in 4% freshly prepared paraformaldehyde. The presence of abundant immature neurons is detected. B: Neonate human hippocampus upon 6 months of fixation. The presence of immature neurons is no longer detected in this sample. White scale bar: 50 μm. ML: Molecular layer. GCL: Granule cell layer. H: Hilus. Green triangles: immature neurons. DCX: Doublecortin. DAPI: 4’,6-diamidino-2-phenylindole.
IHC buffer
Additionally, the optimal detection of certain antigens might require particular IHC conditions. In this regard, the choice of the detergent present in wash and incubation buffers is frequently overlooked in the design of antibody validation protocols. Our data show that one of the most used detergents, namely Triton X-100, is suboptimal for the detection of NSC markers such as Nestin in the adult human hippocampus (Terreros-Roncal et al., 2021). Triton X-100 replacement by a milder detergent, such as saponin, enables the detection of cells positive for Nestin in this structure (Terreros-Roncal et al., 2021). The combination of Nestin with astrocyte markers such as S100β has revealed the phenotypic NSC properties of these cells in the human hippocampus (Terreros-Roncal et al., 2021). NSCs express Nestin, Vimentin, Sox2 and GFAP but lack S100β. Moreover, these cells show specific morphological properties (such as their main location at the SGZ and the presence of long, distally-branched processes that transverse the GCL) (Terreros-Roncal et al., 2021; Terreros-Roncal et al., 2022a, 2022b). Although we have found that saponin negatively impacts the detection of other AHN markers, the signal intensity or signal/background ratio may experience slight variations with respect to the use of Triton X-100. In this regard, signal characteristics should be tightly monitored when setting up the use of new antibodies.
Cell count methods
The number of cells positive for AHN markers reported in distinct studies shows apparently dissimilar orders of magnitude, which can be attributed to a variety of technical reasons. In this respect, although unbiased stereological methods were applied in several of these studies (Boldrini et al., 2018; Flor-Garcia et al., 2020; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021; Tobin et al., 2019), the strategies applied to determine cell numbers were not coincident (see Table 2).
The use of stereology frequently involves normalization of the number of cells counted by the reference volume/area (namely the volume/area of the structure in which cells were counted). Therefore, the choice of such a reference structure is the first source of variability. Some studies divided the number of cells counted by the length of the SGZ/GCL boundary or by that length multiplied by the thickness of the section, whereas in other studies, such as ours (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021), the number of cells counted was divided by the volume of the GCL. Therefore, the resulting cell densities calculated in these studies have, indeed, distinct orders of magnitude and units (number of cells/mm or cells/mm2 vs. number of cells/mm3).
Cell counts can be performed using automated cell detection software or can be done manually, which further increases putative sources of variability between studies. Some authors applied semi-automatic systems such as Stereoinvestigator to calculate cell numbers, as well as to detect the reference volume/length (Boldrini et al., 2018; Tobin et al., 2019). Conversely, we performed an unbiased random sampling of the tissue by acquiring several individual stacks of images per subject under a confocal microscope, which was followed by manual cell counts (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021). Automatic vs. manual cell count systems may use distinct criteria to assign a given cell the attribute of “positive” for a certain marker, Moreover, these systems may show variable signal detection thresholds or may present distinct capacity to discriminate signal vs. background, among other factors, thereby accounting for great variations in the number of cells detected.
Moreover, not only does the microscopy equipment used (confocal, conventional, or epifluorescence microscopes) differ between studies but so does the unbiased stereological method applied to estimate cell densities. Several studies used optical dissector and fractionator methods (Boldrini et al., 2018; Tobin et al., 2019), in which a device coupled to an optical microscope goes through the entire thickness of the tissue to detect positive cells. Conversely, other groups, such as ours, used the physical dissector method coupled to confocal microscopy for cell count purposes (Llorens-Martin et al., 2006). In the latter method, extremely thin sections are obtained under a confocal microscope, thereby allowing for accurate Z-axis resolution and furthering the precision of cell counts (Llorens-Martin et al., 2006).
A very small number of human AHN studies have reported total numbers of cells in the entire DG (Boldrini et al., 2018; Tobin et al., 2019), for which previous determination of the DG total volume is required but is not always possible. To circumvent this difficulty, other studies, such as ours (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021), reported relative (normalized) cell densities. Therefore, a direct comparison of normalized vs. absolute numbers of cells is not possible. Similarly, two studies (Boldrini et al., 2018; Tobin et al., 2019) examined the whole rostro-caudal extent of the human DG, whereas only the posterior pole of the anterior hippocampus was examined in (Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021). Consequently, differences in the precise anatomical regions examined may add further disparity to the number of cells reported.
In light of the variety of methodological approaches used to perform cell counts in the available literature (see Table 2), data showing distinct orders of magnitude or units should not be considered contradictory a priori but rather interpreted as being calculated and reported in a dissimilar manner.
Complementary methodologies to the use of IHC
Although most studies addressing the occurrence of human AHN in vivo are based on the use of IHC (see Table 2), several studies explored alternative methodological approaches, as discussed below.
In 2007, the use of functional magnetic spectroscopy by Maletic-Savatic’s group allowed the detection of human and murine hippocampal NSCs in vivo (Manganas et al., 2007). Further research by that group revealed putative metabolites that could account for that specific signal (Kandel et al., 2022). The approach proposed by Maletic-Savatic’s group paved the way for the subsequent development of strategies aimed at non-invasively detecting AHN levels, such as the design of novel ligands and enhanced detection methods for positron emission tomography (PET) brain imaging (Tamura et al., 2016). The potential for further optimization of such non-invasive methodologies lies in their capacity not only to monitor the rate of AHN in living subjects but also to potentially allow the correlation of AHN with, for instance, cognitive scores. Such correlative analyses might be relevant to determine the role played by newly generated neurons in human behavior. Alternatively, some authors have proposed an in vitro approach to correlate proxies for human adult neurogenesis and, for example, cognitive scores (Du Preez, Lefevre-Arbogast, Gonzalez-Dominguez, et al., 2022; Du Preez, Lefevre-Arbogast, Houghton, et al., 2022).
Several years ago, Frisen’s group developed a ground-breaking methodology, based on the measurement of C14 incorporation into the adult brain, which allowed them to demonstrate the addition of new neurons to certain brain regions such as the hippocampus and the striatum (Ernst et al., 2014; Spalding et al., 2013). They corroborated their findings by IHC and lipofuscin quantification, which revealed the presence of newborn immature neurons in these structures. Moreover, using mathematical modeling, they determined that 700 new neurons are added daily to the human DG.
In recent years, scRNAseq/snRNAseq have been used to reconstruct cellular trajectories in various animal species. Regarding human studies, strong discrepancies are found in the literature, even upon the re-analysis of published datasets (Ayhan et al., 2021; Franjic et al., 2022; Habib et al., 2017; Sorrells et al., 2021). Such discrepancies might be partially related to technical dissimilarities between studies (reviewed in (Kalinina & Lagace, 2022)). In this regard, dramatic variations in sample characteristics (including the PMD), tissue dissection, cell/nuclei isolation and purification, and result analysis are found in the literature (Kalinina & Lagace, 2022). Of note, Ayhan et al. (Ayhan et al., 2021) used biopsy samples with ~12 min PMD, which likely rendered high-quality transcriptomes and which sharply contrast with the prolonged 9-12 h PMD used in Franjic et al. (Franjic et al., 2022).
One of the first key steps in sc- and sn-RNAseq protocols is the generation of high-quality single-cell suspensions. sc-RNAseq has been shown to outperform sn-RNAseq as it captures more transcripts per cell (Ding et al., 2020; Habib et al., 2017). Most studies addressing human AHN have used the “Frankenstein” method for cell suspension preparations (Ayhan et al., 2021; Habib et al., 2017; Tran et al., 2021). Conversely, Franjic et al. used sucrose gradient centrifugation to isolate nuclei (Franjic et al., 2022). The number of cells/nuclei analyzed in distinct scRNAseq/snRNAseq human AHN studies presents remarkable variability (14,963 hippocampal cells obtained from 4 male non-diseased donors (40–65 y) and subjected to Drop-seq and DroNc-seq were analyzed by means of Seurat in (Habib et al., 2017); 10,268 cells obtained from 3 male neurotypical donors (40–69 y) were analyzed by means of 10X Genomics, Bioconductor, and MAGMA in (Tran et al., 2021); 109,208 cells isolated from 5 male and female patients (24–60 y) with epilepsy were subjected to 10x Genomics, Seurat, GO, and MAST in (Ayhan et al., 2021); 32,067 DG cells obtained from 6 male and female clinically unremarkable donors (~52 y) were subjected to 10x Genomics and analyzed by means of Seurat, Velocyto, and scVelo in (Franjic et al., 2022); 22,119 cells obtained from 4 male and female subjects (67–92 y) were subjected to 10x Genomics sc-RNAseq and sc-ATAC-seq and analyzed by means of Seurat, Signac, and Cell Ranger in (W. Wang et al., 2022); and 151,701 nuclei obtained from 38 male and female subjects (20 gw–92 y) were subjected to Drop-seq and SPLiT-seq and analyzed by means of Seurat, supervised machine learning approaches in (Zhou et al., 2022)). Noise control strategies and cell exclusion criteria also varied dramatically between these studies.
The aforementioned methodological variability, together with a notable diversity in the depth of analysis of these studies, may account for their distinct resolution capacity to identify various cell populations, especially those that are present in low abundance, such as NSCs. It should be noted that, according to our own quantitative data, it would be necessary to analyze 1,000,000 DGCs to detect a cluster of 200 NSCs in the human DG (Terreros-Roncal et al., 2021). In this regard, Franjic et al. failed to identify NSCs in the DG of three adult human subjects (Franjic et al., 2022). Conversely, Ayhan et al. (Ayhan et al., 2021), Habib et al. (Habib et al., 2017), Wang et al. (W. Wang et al., 2022), and Zhou et al., (Zhou et al., 2022) robustly identified a quantitatively modest but detectable cluster with neural progenitor characteristics in the adult human DG. Moreover, the latter publication provided the first reconstruction of the entire primate AHN trajectory by means of snRNA-seq, thereby identifying a maturation continuum from quiescent and active NSCs to proliferative cells, early and late immature DGCs, and mature DGCs in human beings and macaques (W. Wang et al., 2022), as did another recent publication in the latter species (Hao et al., 2022). The study by Zhou et al., (Zhou et al., 2022) further confirmed the proliferative capacity of the human DG by showing in vitro EdU (5-ethynyl-2’-deoxyuridine) incorporation into this structure. Moreover, proliferative cells expressed neuronal markers after several weeks. The results of these studies thus raise important caveats about the negative results reported in other studies.
Despite the potential usefulness of sc- and sn-RNAseq to reconstruct entire cellular trajectories, important technical aspects, such as the impact of distinct cell isolation methods, the PMD, and other factors, remain unsolved. Moreover, the lack of consensus validation criteria, variations in threshold and clustering strategies, together with putative artifacts derived from tissue processing (Marsh et al., 2022), may underpin the existing controversy between sc/sn-RNAseq studies. Additionally, important numerical considerations, such as the required minimum number of high-quality cells/nuclei required for any intended analysis, need to be carefully examined to adequately interpret results, especially in the context of negative results. Table 3 summarizes the most important methodological aspects that differ between various original publications that aimed to address AHN in primates using sc- and sn-RNAseq.
Table 3.
Comparison between methodologies used in distinct original publications undertaking single-cell (sc)- and single-nucleus (sn)- RNAseq studies on primates. F: Female. M: Male. FACS: Fluorescence-activated cell sorting. cDNA: Complementary deoxyribonucleic acid. This table was constructed after performing a manual search of studies that included the following terms (and their combination thereof) in the PubMed database: “Adult hippocampal neurogenesis”, “Human”, “sc-RNAseq”, and “sn-RNAseq”. Studies not focused on the human hippocampal region or that examined only other species were manually excluded.
| Reference | Age | Sex | Dissociation | PMD (h) | Platform | Analysis | AHN Trajectory | Cell number |
|---|---|---|---|---|---|---|---|---|
| (Tran et al., 2021) | 40.08-54.43 y (human) | 3M | “Frankenstein” (FACS) | 21.5–38 h | 10 X Genomics | Bioconductor, MAGMA | No | 10,268 |
| (Ayhan et al., 2021) | 24–60 y (human) | 2M/3F | “Frankenstein” (FACS) | 0 h | 10 X Genomics | Seurat, GO, MAST | Yes | 129,908 |
| (Hao et al., 2022) | 4–15 y (macaque) | 6M/2F | Specifically developed scRNA-seq pipeline | 0 h | 10x Genomics Chromium sc Kit v3 | Cell Ranger Scrublet, SCANPY, SCENIC, pySCENIC, Cytoscape, UMAP, Louvain, SCCAF, Seurat, MAST, RNA velocyto and scVelo | Yes | 207,785 |
| (Wang et al., 2022) | 67–92 y (human) 0–23 y (macaque) | Human: 3M/1F Macaque: 11M/2F | Tissue minced into <5mm pieces and homogenized. Density gradient centrifugation | Human: 5–20.5 h Macaque: 0 h | 10x Genomics Chromium sc Kit v3 and Chromium Single Cell ATAC v1.0 | Seurat, Signac, Cell Ranger. Clustering with FindNeighbors and FindClusters | Yes | 22,119 (human) and 132,524 (macaque) |
| (Zhou et al., 2022) | 20 gw–92 y (human) | Human: 23M/15F | Modified SPLiT-seq approach for nuclei isolation and snRNA-seq. Snap-frozen tissue | Human: 2.3–44 h | Drop-seq, SPLiT-seq. Nuclei with > 5% UMIs mapped to mitochondrial genes were excluded | Seurat (v.3.6), Supervised learning approach (modified multinomial machine learning (implemented in the LogisticRegression function in scikit-learn60 in Python v3.7) was applied. Multi-class random forest classifier (v4.6.14). cVI40 (v0.6.8) in Python (v3.7). Monocle41 (v2.8). ClueGO (v.2.5.5) plug-in64 in Cytoscape65 (v3.7.2). CellPhoneDB42 tool (v2.1.4) | Yes | Human: 0–2 y, n=4: 15,434 nuclei; 3–6 y, n=4: 24,607 nuclei; 13–18 y, n=4; 16,310 nuclei; 40–60 y, n=5: 29,832 nuclei; 86–92 y, n=5: 16,055 nuclei; AD, 73–88 y, n=8: 27,508 nuclei; and matched controls, 73–88 y, n=8: 21,955 nuclei |
Final considerations
The publication of a study in 2018 proposing the absence of AHN markers in the adult human hippocampus (Sorrells et al., 2018) gave rise to so-called controversy in the field. This controversy revolved around whether cells positive for AHN markers were present in the human DG. We experimentally demonstrated that certain tissue processing methodologies (such as prolonged fixation, or absence/inadequacy of antigen retrieval protocols) used in the previous aforementioned studies are incompatible with the observation of well-validated AHN markers in this structure. Our studies revealed that NSCs, neuroblasts, and immature neurons are present in the adult human DG until at least the ninth decade of life (Flor-Garcia et al., 2020; Moreno-Jimenez et al., 2019; Terreros-Roncal et al., 2021).
Once this fundamental aspect of the original controversy had been resolved, public debate has been diversified into other secondary aspects, mostly unrelated to the original topic, and not supported by experimental evidence either, as previously discussed (Terreros-Roncal et al., 2022a, 2022b). Our strict control experiments reveal that overlooking crucial methodological aspects may potentially have (temporary) consequences for any field of research. Whether slight methodological variations have a similar impact on the study of other processes putatively occurring in the adult human brain (or on the identification of novel neurogenic niches) remains to be fully elucidated. Nevertheless, our results highlight the need for rigorous reporting of methodological details in human studies to facilitate reproducibility between laboratories.
AHN is one of the most extraordinary reserves of plasticity in the adult mammalian brain. We are confident that our systematic studies addressing fundamental biological questions will positively contribute to the overall advance of the AHN field and will enhance our understanding of the mechanisms governing the generation of new neurons during both physiological and pathological aging throughout human life.
Acknowledgments
This study was supported by the following: The European Research Council (ERC) (ERC-CoG-2020-101001916); the Spanish Ministry of Economy and Competitiveness (PID2020-113007RB-I00, SAF-2017-82185-R and RYC-2015-171899); The Alzheimer’s Association (2015-NIRG-340709, AARG-17-528125, and AARG-17-528125-RAPID), The Association for Frontotemporal Degeneration (2016 Basic Science Pilot Grant Award); and the Center for Networked Biomedical Research on Neurodegenerative Diseases (CIBERNED, Spain). Institutional grants from the Fundación Ramón Areces and Banco de Santander to the CBMSO are also acknowledged. The salary of B. M-V was supported by postdoctoral fellowships awarded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) of the Mexican Government (Reference CVU Number: 385084) and the Secretaria de Educación, Ciencia Tecnología e Innovación (SECTEI) of the Regional Government of Ciudad de México (CDMX) (SECTEI/159/2021).
Footnotes
Competing Interests
The authors declare no competing interest.
References
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anatomical Record. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Cebrian-Silla A, Sorrells SF, Nascimento MA, Paredes MF, Garcia-Verdugo JM, Yang Z, Huang EJ. Comment on “Impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science. 2022;376(6590):eabn8861. doi: 10.1126/science.abn8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Dechmann DK, Winter Y, Lipp HP. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS One. 2007;2(5):e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan F, Kulkarni A, Berto S, Sivaprakasam K, Douglas C, Lega BC, Konopka G. Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron. 2021 doi: 10.1016/j.neuron.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49(12):1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- Blair JA, Wang C, Hernandez D, Siedlak SL, Rodgers MS, Achar RK, Fahmy LM, Torres SL, Petersen RB, Zhu X, Casadesus G, et al. Individual Case Analysis of Postmortem Interval Time on Brain Tissue Preservation. PLoS One. 2016;11(3):e0151615. doi: 10.1371/journal.pone.0151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22(4):589–599.:e585. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottes S, Jaeger BN, Pilz GA, Jorg DJ, Cole JD, Kruse M, Harris L, Korobeynyk VI, Mallona I, Helmchen F, Guillemot F, et al. Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nat Neurosci. 2021;24(2):225–233. doi: 10.1038/s41593-020-00759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers DC, Gargan L, Kapur P, Reisch JS, Mulne AF, Shapiro KN, Elterman RD, Winick NJ, Margraf LR. Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol. 2003;21(15):2968–2973. doi: 10.1200/JCO.2003.01.017. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Gheusi G, Keller M, Lledo PM, Levy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J Comp Neurol. 2013;521(1):169–188. doi: 10.1002/cne.23169. [DOI] [PubMed] [Google Scholar]
- Cipriani S, Ferrer I, Aronica E, Kovacs GG, Verney C, Nardelli J, Khung S, Delezoide AL, Milenkovic I, Rasika S, Manivet P, et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb Cortex. 2018;28(7):2458–2478. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- Clancy B, Cauller LJ. Reduction of background autofluorescence in brain sections following immersion in sodium borohydride. J Neurosci Methods. 1998;83(2):97–102. doi: 10.1016/s0165-0270(98)00066-1. [DOI] [PubMed] [Google Scholar]
- Chawana R, Alagaili A, Patzke N, Spocter MA, Mohammed OB, Kaswera C, Gilissen E, Bennett NC, Ihunwo AO, Manger PR. Microbats appear to have adult hippocampal neurogenesis, but post-capture stress causes a rapid decline in the number of neurons expressing doublecortin. Neuroscience. 2014;277:724–733. doi: 10.1016/j.neuroscience.2014.07.063. [DOI] [PubMed] [Google Scholar]
- Chawana R, Patzke N, Alagaili AN, Bennett NC, Mohammed OB, Kaswera-Kyamakya C, Gilissen E, Ihunwo AO, Pettigrew JD, Manger PR. The Distribution of Ki-67 and Doublecortin Immunopositive Cells in the Brains of Three Microchiropteran Species, Hipposideros fuliginosus, Triaenops persicus, and Asellia tridens. Anat Rec (Hoboken) 2016;299(11):1548–1560. doi: 10.1002/ar.23460. [DOI] [PubMed] [Google Scholar]
- Chawana R, Patzke N, Bhagwandin A, Kaswera-Kyamakya C, Gilissen E, Bertelsen MF, Hemingway J, Manger PR. Adult hippocampal neurogenesis in Egyptian fruit bats from three different environments: Are interpretational variations due to the environment or methodology? J Comp Neurol. 2020;528(17):2994–3007. doi: 10.1002/cne.24895. [DOI] [PubMed] [Google Scholar]
- de Ruiter JP. The influence of post-mortem fixation delay on the reliability of the Golgi silver impregnation. Brain Res. 1983;266(1):143–147. doi: 10.1016/0006-8993(83)91317-3. [DOI] [PubMed] [Google Scholar]
- Del Castillo P, Llorente AR, Stockert JC. Influence of fixation, exciting light and section thickness on the primary fluorescence of samples for microfluorometric analysis. Basic Appl Histochem. 1989;33(3):251–257. [PubMed] [Google Scholar]
- Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42(7):621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Adiconis X, Simmons SK, Kowalczyk MS, Hession CC, Marjanovic ND, Hughes TK, Wadsworth MH, Burks T, Nguyen LT, Kwon JYH, et al. Systematic comparison of single-cell and singlenucleus RNA-sequencing methods. Nat Biotechnol. 2020;38(6):737–746. doi: 10.1038/s41587-020-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez A, Lefevre-Arbogast S, Gonzalez-Dominguez R, Houghton V, de Lucia C, Low DY, Helmer C, Feart C, Delcourt C, Proust-Lima C, Pallas M, et al. Impaired hippocampal neurogenesis in vitro is modulated by dietary-related endogenous factors and associated with depression in a longitudinal ageing cohort study. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez A, Lefevre-Arbogast S, Houghton V, de Lucia C, Low DY, Helmer C, Feart C, Delcourt C, Proust-Lima C, Pallas M, Ruigrok SR, et al. The serum metabolome mediates the concert of diet, exercise, and neurogenesis, determining the risk for cognitive decline and dementia. Alzheimers Dement. 2022;18(4):654–675. doi: 10.1002/alz.12428. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Eymin C, Jordan D, Saint-Pierre G, Kopp N. Brain banking for immunocytochemistry and autoradiography. J Neural Transm Suppl. 1993;39:127–134. [PubMed] [Google Scholar]
- Flor-Garcia M, Terreros-Roncal J, Moreno-Jimenez EP, Avila J, Rabano A, Llorens-Martin M. Unraveling human adult hippocampal neurogenesis. Nat Protoc. 2020;15(2):668–693. doi: 10.1038/s41596-019-0267-y. [DOI] [PubMed] [Google Scholar]
- Franjic D, Skarica M, Ma S, Arellano JI, Tebbenkamp ATN, Choi J, Xu C, Li Q, Morozov YM, Andrijevic D, Vrselja Z, et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022;110(3):452–469.:e414. doi: 10.1016/j.neuron.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan L, Gomez-Pinedo U, Guerrero A, Garcia-Verdugo JM, Matias-Guiu J. Amyotrophic lateral sclerosis modifies progenitor neural proliferation in adult classic neurogenic brain niches. BMC Neurol. 2017;17(1):173. doi: 10.1186/s12883-017-0956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatome CW, Mwangi DK, Lipp HP, Amrein I. Hippocampal neurogenesis and cortical cellular plasticity in Wahlberg’s epauletted fruit bat: a qualitative and quantitative study. Brain Behav Evol. 2010;76(2):116–127. doi: 10.1159/000320210. [DOI] [PubMed] [Google Scholar]
- Glees P, Hasan M. Lipofuscin in neuronal aging and diseases. Norm Pathol Anat (Stuttg) 1976;32:1–68. [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17(7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96(9):5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal VK. Lipofuscin pigment accumulation in human brain during aging. Exp Gerontol. 1982;17(6):481–487. doi: 10.1016/s0531-5565(82)80010-7. [DOI] [PubMed] [Google Scholar]
- Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K, Weitz DA, et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods. 2017;14(10):955–958. doi: 10.1038/nmeth.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZZ, Wei JR, Xiao D, Liu R, Xu N, Tang L, Huang M, Shen Y, Xing C, Huang W, Liu X, et al. Single-cell transcriptomics of adult macaque hippocampus reveals neural precursor cell populations. Nat Neurosci. 2022 doi: 10.1038/s41593-022-01073-x. [DOI] [PubMed] [Google Scholar]
- Harris L, Rigo P, Stiehl T, Gaber ZB, Austin SHL, Masdeu MDM, Edwards A, Urban N, Marciniak-Czochra A, Guillemot F. Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell. 2021;28(5):863–876.:e866. doi: 10.1016/j.stem.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology. 2015;40(10):2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura J, Ito R, Manley NR, Hale LP. Optimization of Single-and Dual-Color Immunofluorescence Protocols for Formalin-Fixed, Paraffin-Embedded Archival Tissues. J Histochem Cytochem. 2016;64(2):112–124. doi: 10.1369/0022155415610792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina A, Lagace D. Single-Cell and Single-Nucleus RNAseq Analysis of Adult Neurogenesis. Cells. 2022;11(10) doi: 10.3390/cells11101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel P, Semerci F, Mishra R, Choi W, Bajic A, Baluya D, Ma L, Chen K, Cao AC, Phongmekhin T, Matinyan N, et al. Oleic acid is an endogenous ligand of TLX/NR2E1 that triggers hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2022;119(13):e2023784119. doi: 10.1073/pnas.2023784119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends in Neuroscience. 2004;27(8):447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci U S A. 2011;108(25):10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RX, Ma J, Wang B, Tian T, Guo N, Liu SJ. No DCX-positive neurogenesis in the cerebral cortex of the adult primate. Neural Regen Res. 2020;15(7):1290–1299. doi: 10.4103/1673-5374.272610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Fitzsimons CP, Salta E, Maletic-Savatic M. Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav Brain Res. 2020;381:112458. doi: 10.1016/j.bbr.2019.112458. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Toni N, Kempermann G, Frisen J, Gage FH, Swaab DF. Limits to human neurogenesis-really? Mol Psychiatry. 2020;25(10):2207–2209. doi: 10.1038/s41380-018-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Pronounced individual variation in the response to the stimulatory action of exercise on immature hippocampal neurons. Hippocampus. 2006;16(5):480–490. doi: 10.1002/hipo.20175. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SE, Walker AJ, Kamath T, Dissing-Olesen L, Hammond TR, de Soysa TY, Young AMH, Murphy S, Abdulraouf A, Nadaf N, Dufort C, et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat Neurosci. 2022;25(3):306–316. doi: 10.1038/s41593-022-01022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenez EP, Terreros-Roncal J, Flor-Garcia M, Rabano A, Llorens-Martin M. Evidences for Adult Hippocampal Neurogenesis in Humans. J Neurosci. 2021;41(12):2541–2553. doi: 10.1523/JNEUROSCI.0675-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata S, Hendricks JB. Effect of fixation time and microwave oven heating time on retrieval of the Ki-67 antigen from paraffin-embedded tissue. J Histochem Cytochem. 1993;41(8):1241–1246. doi: 10.1177/41.8.8331288. [DOI] [PubMed] [Google Scholar]
- Patzke N, Olaleye O, Haagensen M, Hof PR, Ihunwo AO, Manger PR. Organization and chemical neuroanatomy of the African elephant (Loxodonta africana) hippocampus. Brain Struct Funct. 2014;219(5):1587–1601. doi: 10.1007/s00429-013-0587-6. [DOI] [PubMed] [Google Scholar]
- Patzke N, Spocter MA, Karlsson KAE, Bertelsen MF, Haagensen M, Chawana R, Streicher S, Kaswera C, Gilissen E, Alagaili AN, Mohammed OB, et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2015;220(1):361–383. doi: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett CW, Marchbanks RM, Whatley SA. Characterisation of messenger RNA extracted post-mortem from the brains of schizophrenic, depressed and control subjects. J Neurol Neurosurg Psychiatry. 1988;51(3):325–331. doi: 10.1136/jnnp.51.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie T, Scully SA, de Vellis J, Noble EP. Stability of neuronal and glial marker enzymes in post-mortem rat brain. Neurochem Res. 1986;11(3):383–392. doi: 10.1007/BF00965012. [DOI] [PubMed] [Google Scholar]
- Robinson AC, Palmer L, Love S, Hamard M, Esiri M, Ansorge O, Lett D, Attems J, Morris C, Troakes C, Selvackadunco S, et al. Extended post-mortem delay times should not be viewed as a deterrent to the scientific investigation of human brain tissue: a study from the Brains for Dementia Research Network Neuropathology Study Group, UK. Acta Neuropathol. 2016;132(5):753–755. doi: 10.1007/s00401-016-1617-2. [DOI] [PubMed] [Google Scholar]
- Roeder SS, Burkardt P, Rost F, Rode J, Brusch L, Coras R, Englund E, Hakansson K, Possnert G, Salehpour M, Primetzhofer D, et al. Evidence for postnatal neurogenesis in the human amygdala. Commun Biol. 2022;5(1):366. doi: 10.1038/s42003-022-03299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schut MH, Patassini S, Kim EH, Bullock J, Waldvogel HJ, Faull RLM, Pepers BA, den Dunnen JT, van Ommen GB, van Roon-Mom WMC. Effect of postmortem delay on N-terminal huntingtin protein fragments in human control and Huntington disease brain lysates. PLoS One. 2017;12(6):e0178556. doi: 10.1371/journal.pone.0178556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Imam SA, Young L, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 1995;43(2):193–201. doi: 10.1177/43.2.7822775. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Siew LK, Love S, Dawbarn D, Wilcock GK, Allen SJ. Measurement of pre-and post-synaptic proteins in cerebral cortex: effects of post-mortem delay. J Neurosci Methods. 2004;139(2):153–159. doi: 10.1016/j.jneumeth.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Smith TW, Lippa CF. Ki-67 immunoreactivity in Alzheimer’s disease and other neurodegenerative disorders. J Neuropathol Exp Neurol. 1995;54(3):297–303. doi: 10.1097/00005072-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Sorensen KV. Rapid post-mortem decomposition of the somatostatin cells in human brain. An immunohistochemical examination. Biomed Pharmacother. 1984;38(9-10):458–461. [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, Page CE, Sandoval K, Knox A, Connolly A, Huang EJ, et al. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J Neurosci. 2021;41(12):2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ip P, Chakrabartty A. Simple Elimination of Background Fluorescence in Formalin-Fixed Human Brain Tissue for Immunofluorescence Microscopy. J Vis Exp. 2017;(127) doi: 10.3791/56188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Takahashi K, Takata K, Eguchi A, Yamato M, Kume S, Nakano M, Watanabe Y, Kataoka Y. Noninvasive Evaluation of Cellular Proliferative Activity in Brain Neurogenic Regions in Rats under Depression and Treatment by Enhanced [18F]FLT-PET Imaging. J Neurosci. 2016;36(31):8123–8131. doi: 10.1523/JNEUROSCI.0220-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Shi SR, Chaiwun B, Young L, Imam SA, Cote RJ. Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol. 1994;25(3):263–270. doi: 10.1016/0046-8177(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Terreros-Roncal J, Moreno-Jimenez EP, Flor-Garcia M, Rodriguez-Moreno CB, Trinchero MF, Cafini F, Rabano A, Llorens-Martin M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2021;374(6571):1106–1113. doi: 10.1126/science.abl5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terreros-Roncal J, Moreno-Jimenez EP, Flor-Garcia M, Rodriguez-Moreno CB, Trinchero MF, Marquez-Valadez B, Cafini F, Rabano A, Llorens-Martin M. Response to Comment on Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2022a;376(6590):eabn7270. doi: 10.1126/science.abn7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terreros-Roncal J, Moreno-Jimenez EP, Flor-Garcia M, Rodriguez-Moreno CB, Trinchero MF, Marquez-Valadez B, Cafini F, Rabano A, Llorens-Martin M. Response to Comment on Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2022b;376(6590):eabo0920. doi: 10.1126/science.abo0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstege DJ, Addo-Osafo K, Campbell Teskey G, Epp JR. New neurons in old brains: implications of age in the analysis of neurogenesis in post-mortem tissue. Mol Brain. 2022;15(1):38. doi: 10.1186/s13041-022-00926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimers Disease Patients. Cell Stem Cell. 2019;24(6):974–982.:e973. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MN, Maynard KR, Spangler A, Huuki LA, Montgomery KD, Sadashivaiah V, Tippani M, Barry BK, Hancock DB, Hicks SC, Kleinman JE, et al. Single-nucleus transcriptome analysis reveals cell-type-specific molecular signatures across reward circuitry in the human brain. Neuron. 2021;109(19):3088–3103.:e3085. doi: 10.1016/j.neuron.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang M, Yang M, Zeng B, Qiu W, Ma Q, Jing X, Zhang Q, Wang B, Yin C, Zhang J, et al. Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell Res. 2022 doi: 10.1038/s41422-022-00678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, TesFaye E, Yasuda RP, Mash DC, Armstrong DM, Wolfe BB. Effects of post-mortem delay on subunits of ionotropic glutamate receptors in human brain. Brain Res Mol Brain Res. 2000;80(2):123–131. doi: 10.1016/s0169-328x(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Willingham MC. An alternative fixation-processing method for preembedding ultrastructural immunocytochemistry of cytoplasmic antigens: the GBS (glutaraldehyde-borohydride-saponin) procedure. J Histochem Cytochem. 1983;31(6):791–798. doi: 10.1177/31.6.6404984. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. Journal of Neuroscience. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li S, Kennedy BC, Zhang DY, Bond AM, Sun Y, Jacob F, Lu L, Hu P, Viaene AN, et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature. 2022 doi: 10.1038/s41586-022-04912-w. [DOI] [PMC free article] [PubMed] [Google Scholar]