Abstract
Marginal zone (MZ) B cells are one of the main actors of T-independent (TI) responses in mice. To identify the B cell subset(s) involved in such responses in humans, we vaccinated healthy individuals with Pneumovax, a model TI vaccine. By high-throughput repertoire sequencing of plasma cells (PCs) isolated 7 days after vaccination and of different B cell subpopulations before and after vaccination, we show that the PC response mobilizes large clones systematically, including an immunoglobulin M component, whose diversification and amplification predated the pneumococcal vaccination. These clones could be mainly traced back to MZ B cells, together with clonally related IgA+ and, to a lesser extent, IgG+CD27+ B cells. Recombinant monoclonal antibodies isolated from large PC clones recognized a wide array of bacterial species from the gut flora, indicating that TI responses in humans largely mobilize MZ and switched B cells that most likely prediversified during mucosal immune responses against bacterial antigens and acquired pneumococcal cross-reactivity through somatic hypermutation.
Introduction
B cell responses are classified as either T-dependent (TD) or T-independent (TI) based on the requirement for T cell help in antibody (Ab) production. TI antigens (Ags) are essentially nonproteins with repeated molecular structures [e.g., bacterial capsular polysaccharides (capPS) of Streptococcus pneumoniae] and generally induce strong B cell receptor (BCR) cross-linking responsible for rapid B cell activation, proliferation, and plasmablastic differentiation (1–4). While being independent of cognate T cell help, TI responses benefit from additional stimuli provided by noncognate T cells, Toll-like receptor agonists, cytokines, and/or signals from accessory cells, which are needed for class switch recombination (CSR) (4–9). In contrast to TD responses, TI responses are generally considered to be unable to generate fully developed germinal centers (GCs) or a memory response characterized by affinity maturation and a stronger, faster response of B cells to Ag rechallenge. Recent data, however, point to some forms of B cell memory in mice arising in response to different model TI Ags and the formation of rapidly collapsing GCs (10–12).
In humans, Pneumovax is a vaccine composed of capPS from 23 pneumococcal serotypes that is considered a model TI Ag. Vaccination with Pneumovax generates serum responses lasting for up to 5 years in humans (13). However, secondary challenges with Pneumovax or other plain polysaccharidic vaccines do not generate an enhanced response and can even face a state of hyporesponsiveness (14–17). Children below 2 years of age and elderly people have poor or no Ab responses to TI Ags (18), and conjugated vaccines composed of polysaccharides linked to peptide moieties have been successfully developed to circumvent this unresponsiveness (19).
Marginal zone (MZ) and B1b B cells are considered to be the main actors of TI responses in the mouse (20), where Abs against model TI Ags are mainly unmutated. In contrast, the anti-capPS Abs generated after vaccination with polysaccharidic vaccines are highly mutated in humans (21–23), being of immunoglobulin M (IgM), IgG, and IgA classes (mostly IgG2 and IgA2) (24, 25). The B cell subset involved in human TI responses is still a debated question, because there is no clear equivalent in humans of the mouse B1b subset (26), and splenic IgM+IgD+CD27+ B cells that have characteristics similar to mouse MZ B cells also display marked differences. They express mutated Ig genes and are able to recirculate in the blood (27, 28). Arguments in favor of their diversification outside classical TD responses came from studies in patients with genetic deficiencies impairing T-B collaboration and GC formation (29). In addition, IgM+IgD+CD27+ B cells are already diversified in infants without obvious marks of Ag-driven selection (30). Together, these findings led us to propose that blood IgM+IgD+CD27+ B cells are circulating splenic MZ B cells harboring a prediversified Ig repertoire (28). However, mutations in the Bcl6 gene as well as clonal relationships between blood IgM+IgD+CD27+ and switched B cells support the view that they are GC-derived memory cells that can participate in TD immune responses (31–33). A small subset of IgM-only B cells also exists, but IgD expression appears insufficient by itself to discriminate IgM memory B cells that might be involved in TD responses from those activated by TI Ags. Both subsets overlap in their expression of surface proteins commonly used to phenotype human B cells (27). In favor of a distinct human MZ lineage as in mice, we have previously identified a distinct precursor capable of differentiating into MZ-like B cells upon Notch2--triggering signals (34). In line with our result, recent work identified a bifurcation of human B cell maturation at the transitional 1 (T1) stage, giving rise to IgMlo and IgMhi T2 cells, the latter being selectively recruited into gut-associated lymphoid tissues (GALTs). The authors proposed a developmental continuum from the IgMhi T2 stage to MZBs, with a stage of repertoire diversification in the GCs of GALTs (35).
Concerning the role of IgM+IgD+CD27+ in TI responses, evidence is largely indirect and mainly based on the correlation of their reduced presence in the blood of young children (<2 years), elderly, or immunodeficient individuals showing increased susceptibility to encapsulated bacteria and/or poor responses to plain polysaccharide vaccines (36–40). Along with these observations, clonal amplification after vaccination (28) and phenotypic analysis of pneumococcal polysaccharide-specific B cells (41) suggest a dominant role of IgM+CD27+ cells in the response to Pneumovax vaccination. However, the study of anti-capPS responses in a humanized severe combined immunodeficient (SCID)/SCID mouse model suggested that IgM+IgD+CD27+ B cells are not the sole contributors of TI immune responses (42).
To further examine TI responses in humans, we took advantage of the vaccination of six healthy individuals with Pneumovax. Our aim was to identify the B cell subset(s) that is responsible for the production of anti-pneumococcal capPS IgM, IgG, and IgA in response to immunization. We observed that the pneumococcal vaccine generated a large plasma cell (PC) response mobilizing pre-diversified and preamplified clones systematically with an IgM component that could in most cases be traced back to MZ B cells, together with IgA and, to a lesser extent, IgG. Pneumococcal-specific clones showed recognition of a wide array of bacterial species from the gut flora, indicating that TI responses in humans largely mobilize MZ and switched B cells that prediversified during mucosal immune responses against commensals harboring cross-reactive glycans.
Results
A major but transient expansion of the plasmablast/plasma cell pool in blood 7 days after vaccination with Pneumovax
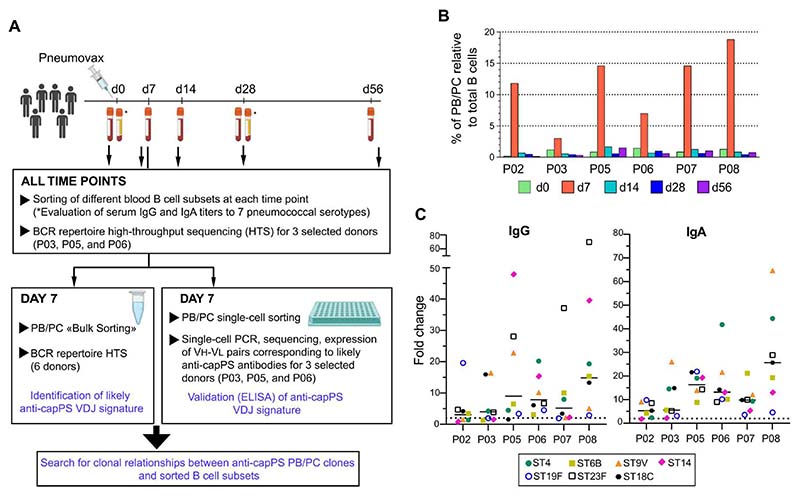
To identify the B cell subset(s) from which anti-capPS–secreting plasmablasts (PBs)/PCs are derived, we isolated day 7–PBs/PCs (PB/PC-d7) along with different B cell subsets sorted before and at days (d) 7, 14, 28, and 56 after vaccination. Whereas high-throughput BCR repertoire sequencing (HTS) was done for PB/PC-d7 of all vaccinees, only three donors were further selected for HTS of their B cell subsets sampled at different time points (Fig. 1A and fig. S1, A and B). In the six vaccinees, Pneumovax induced a strong increase in the proportion of PBs/PCs that ranged from 3 to 19% of total peripheral blood B cells at d7 (average, 11.6%) (Fig. 1B). By d14, however, and for later time points, the proportions of PBs/PCs returned to values that were close to preimmunization levels for most donors. Pre- and post-immunization serum IgG and IgA Ab levels against seven capPS serotypes included in the vaccine were measured, with responses varying greatly among the six vaccinees (Fig. 1C and fig. S1C). Except for P02 (who had an already high preimmunization serum IgG level against serotype 14 and showed a lower overall response), all the vaccinees showed a significant increase in mean IgG and IgA levels after immunization, with at least a twofold increase of Ab levels for most or all the tested serotypes (Fig. 1C). Together, and as suggested by their large PB/PC expansion at d7, the six participants were all responders to the 23-valent vaccine.
Fig. 1. A strong but transient PB/PC response in blood at day 7 after Pneumovax vaccination.
(A) Study design and sample collection (see fig. S1). (B) Percentage of PBs/PCs relative to CD19+ B cells before and at different time points after vaccination with Pneumovax of six individuals. (C) Fold changes in serum levels of serotype-specific anti-capPS IgG and IgA between d0 and d28 after Pneumovax vaccination. The dashed line indicates a fold change of 2 (see fig. S1C).
The PB/PC-d7 pool contains large clonal expansions
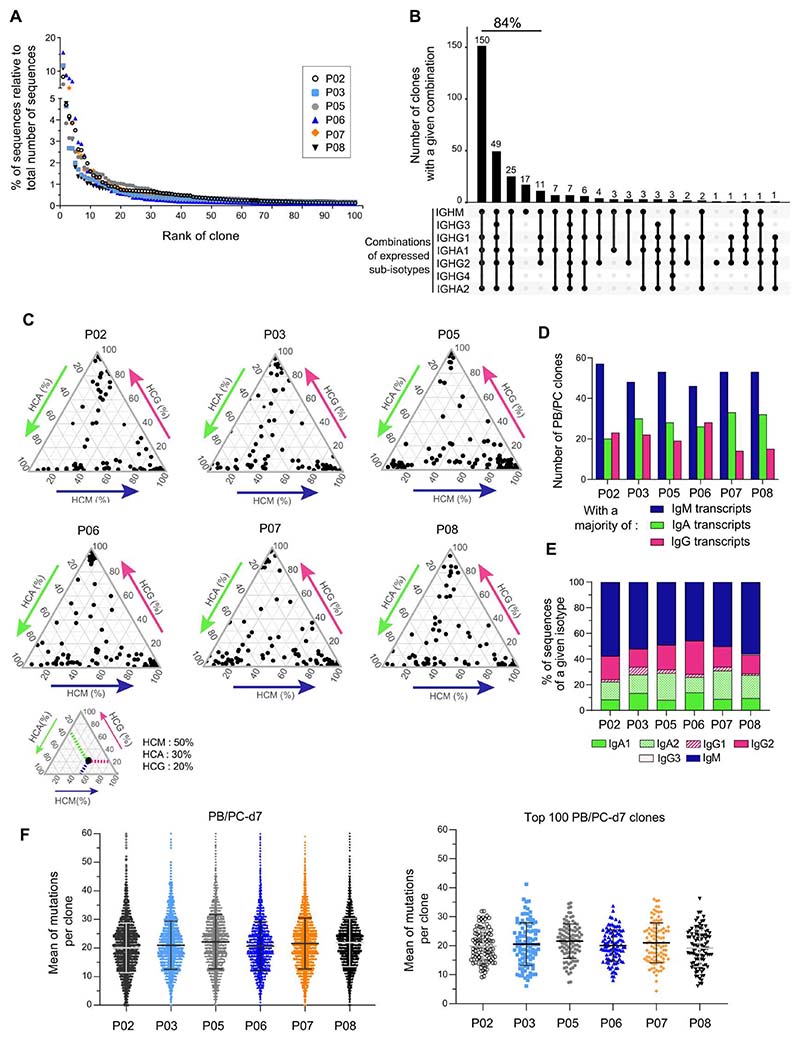
PB/PC-d7 were bulk-sorted for each vaccinee, with the mean number of cells processed for RNA extraction being about 149,000 per sample (45,000 to 275,000) (data file S1). After HTS, the number of Ig sequences ranged from 112,000 to 218,000 (average of 153,000). The sequences were partitioned into clones with a clonal assignment performed on pooled sequences of the three isotypes (IgM, IgA, and IgG). The mean number of PB/PC-d7 clones was 5666 (range, 4947 to 6644). The D50 values (number of clones accounting for 50% of total sequences) ranged from 7 to 46 clones, with an average value of 28 clones, thus revealing large clonal expansions in the PB/PC-d7 repertoire (data file S1). The clones were ranked by size (according to their number of sequences), and the first 100 clones were defined as the “top 100,” which represented on average 70% of the total sequences (Fig. 2A and fig. S2, A and B). Among the top 100 PB/PC clones of each donor, the first 10 clones alone represented at least one-third of the total sequences, and for each vaccinee, the 100th clone had still more than a hundred sequences (mean, 192; range, 120 to 263). We thus assume that the majority of top 100 PB/PC-d7 had been mobilized in specific anti-capPS Ab responses.
Fig. 2. High-throughput sequencing of the Ig repertoire of PB/PC-d7 cells after Pneumovax vaccination shows large clonal expansions, a high load of somatic hypermutations, and a representation of μ, α, and γ isotypes in the majority of the top 100–ranked clones.
(A) Percentages of sequences (relative to the total number of PB/PC-d7 sequences) represented by each of the top 100 PB/PC-d7 clones for each vaccinee (see fig. S2A). (B) Combinations of IgH sub-isotypes expressed by PB/PC-d7 clones. The analysis is done on pooled top 100 PB/PC-d7 clones of P03, P05, and P06. The number on top of each bar represents the number of clones, with the isotype combination indicated below. The total percentage of the five most frequent combinations is indicated above the corresponding histogram bars. (C) Ternary plots depicting the relative proportion of IgA, IgG, and IgM sequences in the top 100 PB/PC-d7 clones of each vaccinee. Each black dot corresponds to one clone. A small ternary plot below shows a theoretical example of a clone with its projected coordinates on the three axes, indicating the proportion of each isotype. (D) Number of PB/PC-d7 clones, among the top 100 clones, that have a majority of IgM, IgA, or IgG sequences. (E) Sub-isotype representation in the top 100 PB/PC-d7 clones of each vaccinee. The relative percentages of sub-isotype sequences were calculated per clone (to allow weighting to clone size) and averaged for the top 100 PB/PC clones. (F) Scatterplots representing the means of Ig-VH mutations per clone for the totality (left) or for the top 100 PB/PC-d7 clones (right) of each vaccinee. Black (or gray) lines and error bars represent means ± SD.
Most of the top 100–ranked PB/PC clones include IgM, IgG, and IgA isotypes with a high mutation load, but IgM sequences have a prominent representation
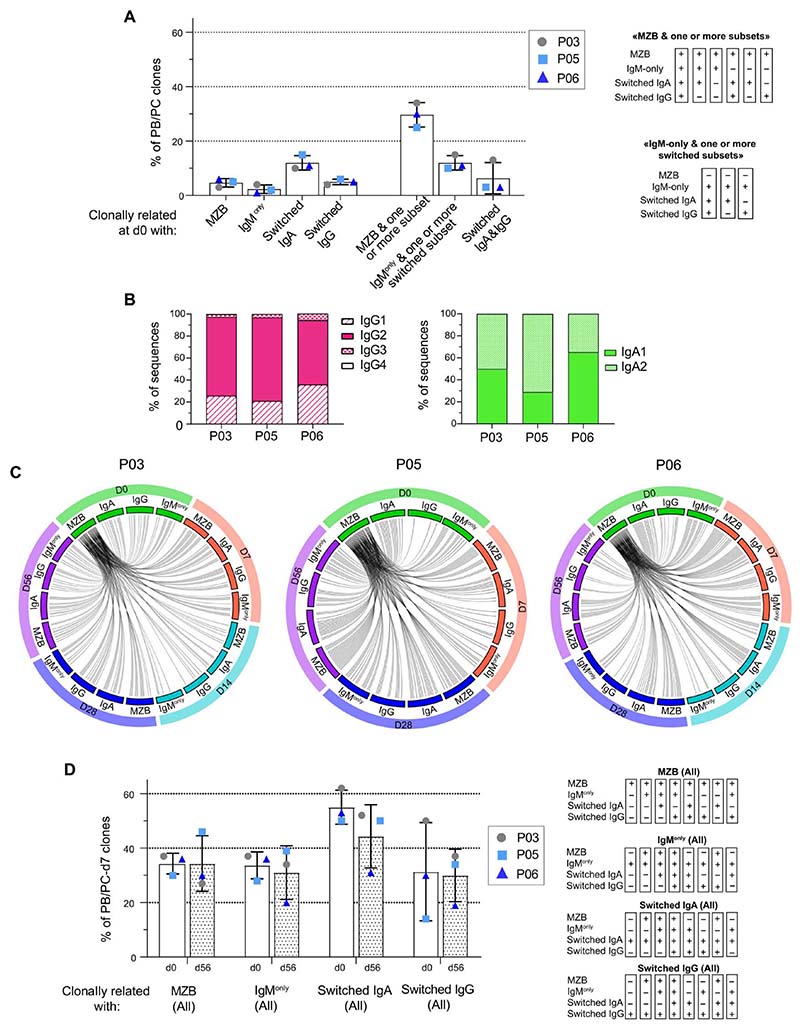
We analyzed the isotype composition of the top 100 PB/PB-d7 clones of each vaccinee. Almost all the clones (from 97 to 100%, depending on the individual) contained IgM sequences, and a mean of 83% of the clones (range, 77 to 91%) were constituted of IgM, IgA, and IgG sequences present in various proportions (Fig. 2, B and C). Clones with a majority of IgM sequences were nevertheless the most numerous in the six vaccinees (Fig. 2D). The five most represented isotype combinations, found in 84% of the clones, all included IgM, either coexpressed with various sub-isotypes or alone (Fig. 2B). For each vaccinee, the relative proportion of sub-isotype sequences was calculated for the top 100 PB/PC-d7 clones (Fig. 2E). IgM accounted for half the sequences, whereas IgG2, IgA2, and IgA1 averaged 18 ± 4.4%, 17 ± 4.1%, and 10 ± 2.6%, respectively, with IgG1 and IgG3 being much less frequent. Together IgM was dominant among the sequences expressed by the top 100 PB/PC-d7 clones, but IgG and IgA were also coexpressed in a large fraction of them.
For PB/PC-d7 clones, the mean mutation number per clone showed a wide distribution, with an average of 21 mutations per clone and very few unmutated ones (Fig. 2F). The top 100 PB/PC-d7 harbored a similar mutation load, but no unmutated clones could be observed. For those comprising IgM, IgA, and IgG sequences, the mean mutations per clone were calculated by isotype (fig. S2C). No significant differences were seen between the means of mutations per clone of IgA- and IgG-associated V-region sequences, both carrying on average ≍21 mutations, whereas, for four of the six donors, IgM sequences were slightly less mutated than IgG. We conclude that the high mutation load of the most expanded PB/PC-d7 clones implies that their direct B cell precursors were already mutated. Moreover, the prominent representation of mutated IgM sequences in the top 100 PB/PC-d7 clones suggests that such precursor B cell clones, whatever their expressed isotype, descended from a common prediversified IgM+ ancestor.
Evidence of recent isotype switch events in the top 100–ranked PB/PC-d7
CSR occurs very rapidly after infection or immunization, during both TD and TI responses (43, 44). Because the majority of the top 100 PB/PC-d7 clones contained the three IgM, IgA, and IgG isotypes, we wondered if they may have undergone CSR recently, before their PB differentiation. We focused on the three vaccinees for which PB/PC-d7 had been also single cell–sorted. Among the unique sequences of the top 100 clones, we searched for strictly identical VDJ sequences associated with different constant regions, suggestive of recent switch events. Evidence of this was numerous, implying mainly switching from IgM to one or several downstream sub-isotypes, with the first three combinations, from IgM toward IgA1 and/or IgA2, accounting for 41% of the switch events (fig. S3A). For IgG sub-isotypes, CSR events leading to IgG2 were the most abundant (18.6%), well ahead of those leading to IgG1 (4.4%). Together, direct or sequential switching events to IgA1 or IgA2 were a majority, accounting for 20 and 55% of all events, respectively. Along with HTS, VDJ-μ, VDJ-α, and VDJ-γ transcripts of PB/PC-d7 sorted as single cells (in 96-well plates) were amplified and sequenced. We analyzed the proportion of wells in which identical VDJ transcripts were associated with two different isotypes (fig. S3B). No wells with a double IgM/IgG isotype were identified for any of the vaccinees. In contrast, a few percent of the single PB/PC-d7 had IgM/IgA, as well as IgG/IgA double transcripts, a substantial frequency considering the transient nature of simultaneous expression of two transcripts after isotype switching.
For P03 and P06, HTS was done on bulk-sorted PB/PC-d14 cells. For each of the top 100 PB/PC-d7 clones, we identified (if present) the corresponding PB/PC-d14 clone belonging to the same clonal group. For d7-d14 clone pairs,the proportion of IgA sequences significantly increased at d14, whereas proportions of IgM and IgG sequences decreased in both donors (fig. S3C). This observation fits well with a preferential switch toward IgA sub-isotypes during the plasmablastic differentiation, with α-transcripts becoming predominant at d14. Another not exclusive possibility is that PBs/PCs originating from already switched IgA+ cells are more persistent in the blood at d14.
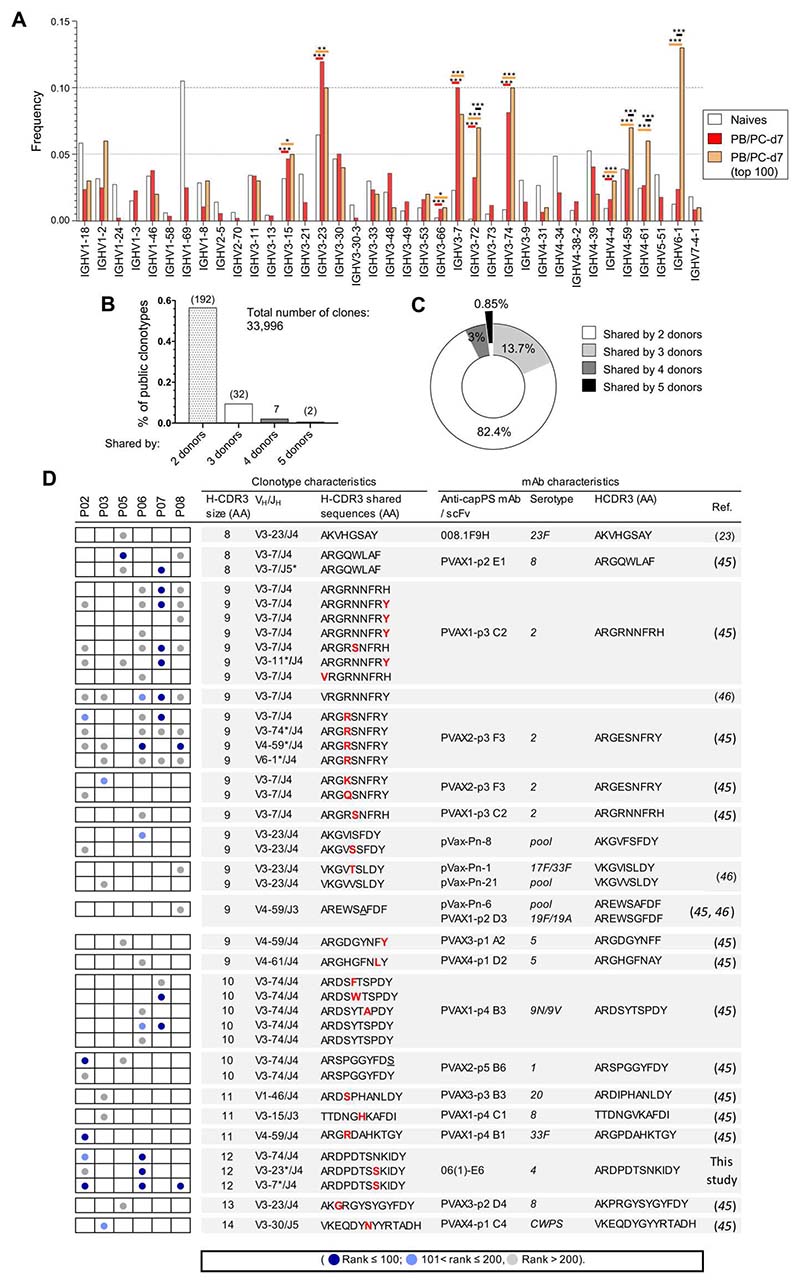
Public clonotypes are shared between Pneumovax vaccinees and match previously described anti-pneumococcal capPS mAbs
The repertoire of published anti-capPS monoclonal Abs (mAbs) shows a bias in VH gene usage for members of the VH3 family, the first five genes in the ranking being VH3-7, VH3-30/33 [frequently used by cell wall polysaccharide (CWPS)–binding Abs (45), VH3-23, VH3-48, and VH3-74 (fig. S4). We thus analyzed the repertoire of both PB/PC-d7 and top 100 PB/PC-d7 clones of donors P03, P05, and P06, for whom heavy chain (HC) sequencing of naive B cells at d0 was also performed. The frequency of VH gene usage, relative to total sequences, was calculated for each repertoire and showed a significant increase in VH3-7, VH3-23, and VH3-74 usage in both PC/PB groups, consistent with previous data on anti-pneumococcal cap PSmAbs (Fig. 3A). We also noticed the increased usage in the top 100 PB/PC-d7 clones of the VH4-59 and VH4-61 genes and especially of the VH6-1 gene. Beyond the observation of a repertoire bias, we searched for public clonotypes shared by two or more of the vaccinated donors (Fig. 3, B and C). Among the PB/PC-d7 clones of the six donors, we identified a total of 233 public clonotypes, most of them shared by two (82%) or three donors (14%). Seven clonotypes were shared by four individuals, and two clonotypes [whoseH-CDR3 sequences were close to a previously described anti-pneumococcal capPS mAb (23, 45, 46)] were shared by five vaccinees (Fig. 3D and data file S2). When the clonotypes were shared between 3, 4, and 5 donors, 45, 71, and 100% of them, respectively, were contributed by one or more of the top 100 PB/PC clones. We further broadened the comparison of anti-pneumococcal mAbs from the literature to all our PB/PC-d7 clones to identify additional shared clonotypes. We found 23 additional clonotypes that matched with known anti-capPS mAbs (Fig. 3D). Among the clonotypes that matched with anti-capPS mAbs both by their H-CDR3 size and amino acid sequences, seven of them expressed different VH genes, which suggests a prominent role of these particular H-CDR3s in defining certain anticapPS specificities (in these particular cases, against serotypes 2 and 4).
Fig. 3. Occurrence of public clonotypes in the repertoire of PB/PC-d7 that are shared by two or more Pneumovax vaccinees, some of these clonotypes being expressed by previously described anti-capPS mAbs.
(A) Frequency of VH gene usage (relative to the total sequences of each subset) for naive (at d0), PB/PCd7, and top 100 PB/PC-d7 (pooled sequences from P03, P05, and P06). Only informative VH genes for the comparison between naive and PB/PC-d7, or between naive and top 100 PB/PC-d7, were plotted, i.e., VH genes either absent or representing less than 0.005% in two of three of the comparison groups were filtered out. Significance was calculated with Fisher’s exact test with a false discovery rate correction. P values are indicated only for VH genes that are significantly more expressed in PB/PC-d7 and/or top 100 PB/PC-d7 versus naive B cells. The color of the lines on top of the histogram bars indicates the comparison groups: red, naive versus PB/PC-d7; orange, naive versus top 100 PB/PC-d7; black, PB/PC-d7 versus top 100 PB/PC-d7. *P < 0.05; **P < 0.005; ***P < 0.0005. (B) Percentages of public clonotypes among the total number of PB/PC-d7 clones. Numbers on top of each histogram bar represent the number of clonotypes. (C) Proportion of public clonotypes, among those identified in the six vaccinees, that were shared by two, three, four, or five vaccinees. (D) Characteristics of the public clonotypes that matched with published anti-capPS mAb. The left part illustrates the sharedness of the clonotypes between the six vaccinees, each column corresponding to one of them, as indicated. The color of the dots in the boxes indicates the rank of the PB/PC-d7 clone associated to the clonotype whose characteristics are indicated on the right (dark blue: rank ≤100; light blue: 101 < rank ≤ 200; gray: rank > 200). Characteristics of the published mAb are indicated in the far right part of the table. “Pool” refers to the cases where a pool of capPS was used to test the specificity of the mAbs/single-chain fragment variable in the cited articles (see fig. S4). *, clonotypes matching with anti-capPS mAbs by both their H-CDR3 size and amino acid sequences, but expressing different VH or JH genes.
Representative VH-VL pairs of largely expanded PB/PC-d7 clones show anti-capPS reactivity that is dependent on somatic hypermutation
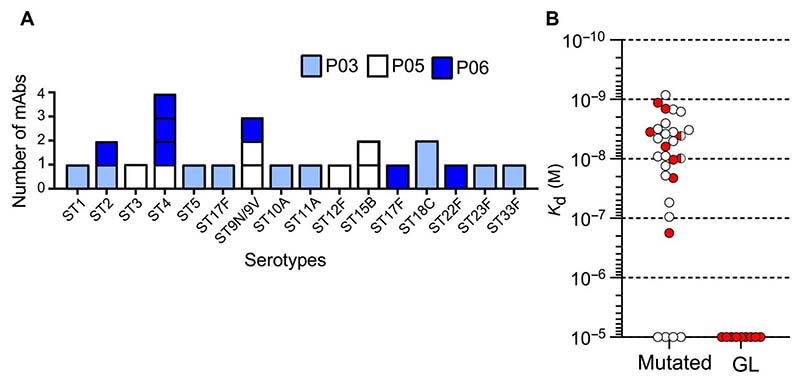
BCR-HTS of bulk-sorted PB/PC-d7 cells highlighted the presence of very large clones. For donors P03, P05,and P06, we amplified and sequenced VDJ-μ, VDJ-α, VDJ-γ, VJ-κ, and VJ-λ transcripts of PB/ PC-d7 cells isolated as single cells in 96-well plates. Among wells for which both productive VH and VL chains were obtained, we selected those whose sequence belonged to a top 100–ranked PB/PC-d7 clone. Corresponding VH and VL pairs (irrespective of the starting HC isotype) were expressed as IgG1 Abs. We produced a total of 28 mAbs (data file S3), of which originally 15 were IgA, 6 were IgG, and 7 were IgM. All were mutated, with on average 21.6 ± 9.5 mutations per VH (ranging from 7 to 40 mutations). Eighty-five percent of the mAbs were reactive against one of the 23 tested serotypes, with one mAb binding both serotypes 9V and 9N, and two capPS having very similar nonbranched structures. Together, a broad range of serotypes contained in the vaccine was recognized by mAbs from the three donors (Fig. 4A). These mAbs displayed relatively high affinity for their cognate Ag, with Kd (dissociation constant) values ranging from 0.8 × 10−9 to 176 × 10−9, with most of them having Kd in the nanomolar range (Fig. 4B). Four mAbs, however, did not recognize any of the tested polysaccharides, including CWPS. Noteworthy, three of these four mAbs without measurable reactivity against any tested capPS were originally IgM. It might be that their affinity for a given serotype could be too weak to be detected when expressed as a soluble IgG1 fraction as opposed to an IgM membrane form. Eight of the 28 mAbs were chosen to be reverted to their VH and VL germline (GL) configuration. After expression and purification, the GL versions tested at the same concentration (1 μg/ml) lost any measurable reactivity toward the capPS serotypes. Together, the majority of expressed mutated VH-VL pairs selected from the top 100 PB/PC-d7 clones bind with relatively high affinity to one of the Pneumovax serotypes, their anti-capPS reactivity relying on the presence of somatic mutations.
Fig. 4. Representative VH-VL pairs of largely expanded PB/PC-d7 clones show anti-capPS reactivity that is dependent on somatic hypermutation.
(A) Pneumococcal serotypes recognized by the mAbs obtained from vaccinees P03, P05, and P06. Each mAb recognized only one serotype (except for 9N/9V). (B) Dissociation constants (Kd, expressed as moles per liter) of the 28 expressed mAbs against their specific serotypes (see data file S2). Red dots correspond to mAbs for which a GL version of the VH/VL pair was expressed. (The two half red circles correspond to the mAb that binds to serotype 9N and 9V with different Kd.) mAbs with no measurable affinity were considered nonbinders (Kd ≥ 10−5).
Top 100 PB/PC-d7 clones are clonally related to large clones that exist before vaccination and include MZB, IgM-only, and switched IgG+ and IgA+ cells
We sought to identify the subset from which top 100 PB/PC-d7 cells originated by examining their clonal relationships with different B cell subsets at d0. For P03, P05, and P06, BCR-HTS was done for eight different B cell fractions: naive, MZB, IgG+, IgA+, and IgM-only B cells from IgD−CD27+ subsets and IgG+, IgA+, or IgM+ from IgD−CD27− subsets [double-negative (DN) cells] (fig. S1B). We found no clonal relationships between the top 100 PB/PC-d7 clones and naive B cells in any of the vaccinees. No or very few relationships were found with DN cells either. Percentages of clonal relationships between PB/PC-d7 and single CD27+ B cell subsets were relatively low (from 2.3 to 5%), except for IgA+CD27+ cells (12% on average) (Fig. 5A). PB/PC-d7 clones were mainly clonally related to complex clonal entities encompassing MZBs and one or several other CD27+ members (IgM-only, IgA+CD27+, and IgG+-CD27+ cells) (fig. S5A). Less frequently, PB/PC-d7 clones were related to d0 clonal entities devoid of MZB cells, but including IgM-only and IgA+ and/or IgG+ cells. However, whereas IgM-only CD27+ is a minor population among sorted IgD−CD27+ cells (14% for P03 and P05, and 11% for P06) (fig. S1A), the Ig library preparation and indexing protocol followed for multiplex HTS resulted in a representation in sequence reads similar to other fractions (data file S1), which artificially increased the clonal relationships observed between PB/PC-d7 clones and the IgM-only subset. Because of the limited sampling (about 20 ml of blood were taken at each time point) and sequencing depth, it is important to note that not finding a clonal relationship does not imply that it does not exist. Conversely, finding relationships with clones present at d0 that did not undergo postvaccine blastic amplification implies that these clones were already large. In conclusion, the majority of PB/PC-d7 originated from preexisting clones encompassing MZB, IgM-only, and switched B cells, confirming that they necessarily descended from a common IgM+ Ag-experienced ancestor and that MZB and IgM-only B cell subsets harbor repertoires with largely overlapping clonal expansions. Moreover, the d0 B cell representation showed a strong TI signature involving 60 to 75% of IgG2 among the IgG isotypes (Fig. 5B).
Fig. 5. The top 100 PB/PC-d7 clones are clonally related to large preexisting clonal entities, including MZB, as well as IgM-only, IgG+, and IgA+ CD27+ B cells that remain stable over time.
(A) Percentage of top 100 PB/PC-d7 clones having clonal relationships at d0 with only one subset or with several subsets. The categories “MZB & one or more subset”and “IgMonly & one or more switched subset”include six and three combinations, respectively (see tables on the right of the graph).“+”means that a PB/PC-d7 clone is clonally linked to a given B cell subpopulation. (B) Isotype representation in the switched IgG and IgA d0 clones that are clonally related to the top 100 PB/PC-d7 clones. The relative percentages of sub-isotype sequences were calculated per clone (to allow weighting to clone size) and averaged. (C) Clonal overlap of d0 MZB clones that are related to the largest PB/PC-d7 clones with all other subsets at different time points, for P03, P05, and P06. For clarity, only the top 50 PB/PC-d7 clones that have clonal relationships with MZB cells at d0 (MZB-d0) were taken into consideration in this analysis, and their relationships with the different subsets are illustrated through an “MZB-d0-centric view” (PB/PC-d7 subsets are not depicted on the plot; see fig. S5). Accordingly, the many intersubset clonal relationships (e.g., between switched IgA, IgG, and IgM-only cells sampled over time) are not shown. (D) Percentage of top 100 PB/PC-d7 clones having clonal relationships with MZBs (All), IgM-only (All), switched IgG (All), or switched IgA (All) B cells at d0 and d56. Each “All”category includes eight possible combinations (see tables on the right of the graph).
The large clones from which the top 100 PB/PC-d7 clones originate remain globally stable 2 months after Pneumovax vaccination
Having shown that top 100– ranked PB/PC-d7 clones originate from large preexisting clones, including MZB, IgM-only, and switched B cells, we next followed these clones at 7, 14 (for P03 and P06), 28, and 56 days after vaccination (Fig. 5, C and D). We found numerous cases of clonal relationships between the subsampled PB/PC-d7 clones and all the other subsets (MZB, IgM-only, IgA+, and IgG+ cells) at all time points (Fig. 5C). Again, considering sampling limitations, this result emphasizes the large size of the preexisting clones mobilized by the Pneumovax vaccine and their persistence after vaccination. We evaluated the percentages of top 100 PB/PC-d7 clones related to MZB, IgM-only, and switched IgG+ or IgA+ clones at d0 and d56. For this calculation, an “All” category was defined for each B cell subset, a category including the clonal relationship with PB/PC-d7 of this subset either alone or in combination with others (Fig.5D). We assumed that, if the size of the pre existing clones that fueled the anti-capPS response did not markedly decrease 2 months after vaccination, the percentage of clonal relationships between these clones and their related PB/PC-d7 clones should remain roughly similar between d0 and d56. Although the results observed individually differ from patient to patient (patients P03 and P06 behaving globally the same and patient P05 showing opposite trends),on average,there was no consistent trend toward a decrease in the percentage of clonal relationships between top 100 PB/PC-d7 clones and the different subsets at d56 compared with d0. Accordingly, we propose that the observed variations (that are of the same order in all subsets) are too small to be compatible with the hypothesis of a strong depletion of the pool of the B clones consecutive to their mobilization in the anti-capPS PC response. This suggests a proliferative maintenance of the preexisting clones while they differentiate into PC.
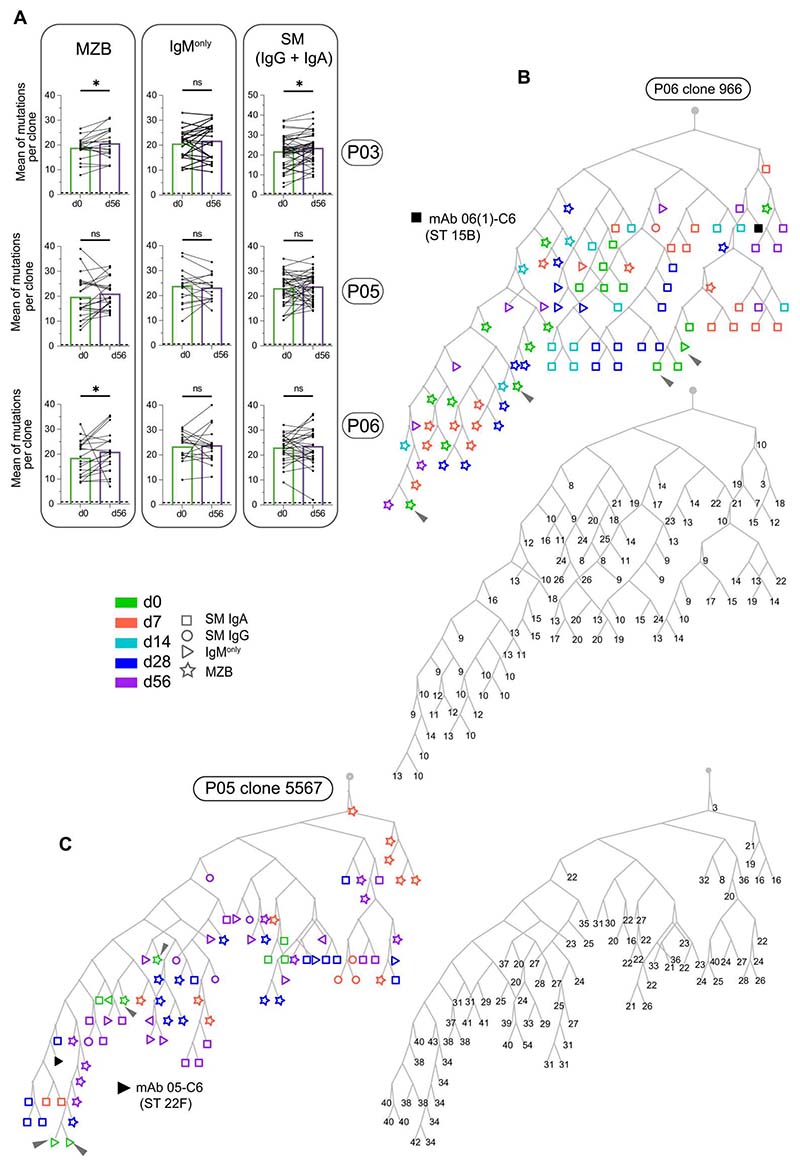
Somatic mutations do not increase after Pneumovax vaccination, and mutation trees of capPS-responding clones do not reveal a time-dependent pattern of mutation accumulation
In a typical TD response, a time-dependent accumulation of somatic mutations can be seen, which results from selection, in GCs, for cells expressing higher-affinity Ab variants. Having found that top 100 PB/PC-d7 clones originate from large B cell clones that include already mutated MZB, IgM-only, and switched cells, we wondered if the load of somatic hypermutation increased in these B cell clones 2 months after vaccination. We thus selected clones encompassing top 100 PB/PC-d7 and for which we had sequences both at d0 and d56 for P03, P05, and P06. Because these clones were generally made of MZB, IgM-only, and switched cells, the mean of mutations (in VH sequences) per clone was calculated separately for each subset [sequences were pooled for switched (IgG + IgA) cells] sorted at d0 and d56. For the three donors before vaccination, the mean mutation levels of MZB cells were lower than those of IgM-only and switched B cells, with 18.8, 22.4, and 22.4 mutations/VH, respectively. Two months after vaccination, a slight increase in mutation level was observed for MZB cells of donor P03 and P06 and for switched (IgG + IgA) cells of donor P03, with differences together modest, whereas, in all the different subsets of P05 and remaining ones of P03 and P06, no significant increase of the mutation levels was observed at d56 (Fig. 6A).
Fig. 6. Somatic mutations in clones related to the top 100 PB/PC-d7 clones do not increase 2 months after Pneumovax vaccination, and Ig mutation trees do not reveal a time-dependent pattern of mutation accumulation.
(A) Mean of mutation numbers (in VH sequences) per clone for clone pairs present at both d0 and d56 in the MZB, IgM-only, and switched (IgG + IgA) subsets, respectively. Dashed lines represent the mean mutation number in VH sequences of the naive cells of each donor. Significance was calculated with the Wilcoxon matched-pairs two-tailed signed-rank test (*P ≤ 0.05; ns, not significant). (B and C) Representative Ig lineage trees of two B cell clones with anti-capPS specificities. The trees were built, using the IgPhyML algorithm, with a subsampling of 10 sequences (or fewer if not available) by B cell subset and by time point. The black symbols correspond to the sequence of the anti-capPS mAb that was isolated from single cell–sorted PCs (see data file S3). The gray rectangle indicates the inferred common progenitor. Each lineage tree has been duplicated to indicate the number of mutations per VH of each sequence. (Arrowheads mark examples of cells sampled at d0 but mapped on terminal leaves.)
We further considered the B cell clones (made of MZB, IgM-only, and switched B cells) from which the top 100 PB/PC-d7 clones were derived, and we focused on those for which one PC-associated VH/VL pair had a validated anti-capPS reactivity. Starting from sets of clonally related Ig sequences, we constructed Ig lineage trees using the IgPhyML algorithm. Figure 6 (B and C) shows representative trees in which, with the exception of the VH sequence of the produced anti-capPS mAbs, PB/PC-d7 sequences are not shown. Starting from the “trunk” originating from an inferred ancestor, it appears that the sequences corresponding to cells from different subsets (MZB, switched IgA and IgG, and IgM-only) were distributed all over the trees, from the first forks and lowest branches to the highest branches and terminal leaves, independently of the time points at which they had been sampled (d0, d7, d14, d28, and d56). Cells sampled at d0 (see arrows on Fig. 6, B and C) often mapped on terminal leaves, where they could also be found in the close vicinity (i.e., attached to the same node) of cells sampled at the latest time points (d28 or d56). In line with this observation, the lineage trees revealed no clear accumulation of somatic mutations with time, with again many examples of d0 cells being more highly mutated than cells sampled at later time points, the reverse being true as well, with examples of d28 or d56 cells being less mutated than cells sampled before the vaccination.
The depicted mutation trees represent clones that included one PC with validated anti-capPS specificity. It is clear that, depending on the impact of mutations, different members of the clone may or may not share this antigenic recognition. To address this question, we included PC sequences in the phylogenetic analysis and, assuming that they largely reflect the anti-capPS response, analyzed their positioning in the tree. Two different examples are shown in fig. S5 (B and C): In the tree depicted in fig. S5C, PCs are distributed all over the different branches, which could correspond to a widely shared anti-capPS specificity by most clone members, irrespective of their intraclonal diversification. In the tree depicted in fig. S5B, PCs (PCs present at d14) are much more clustered, which could correspond in contrast to a case where anti-capPS specificity was acquired or improved by somatic mutations. Together, these results support the conclusion that the process that generated this clonal diversification predated the pneumococcal vaccine response and that no major maturation occurred thereafter.
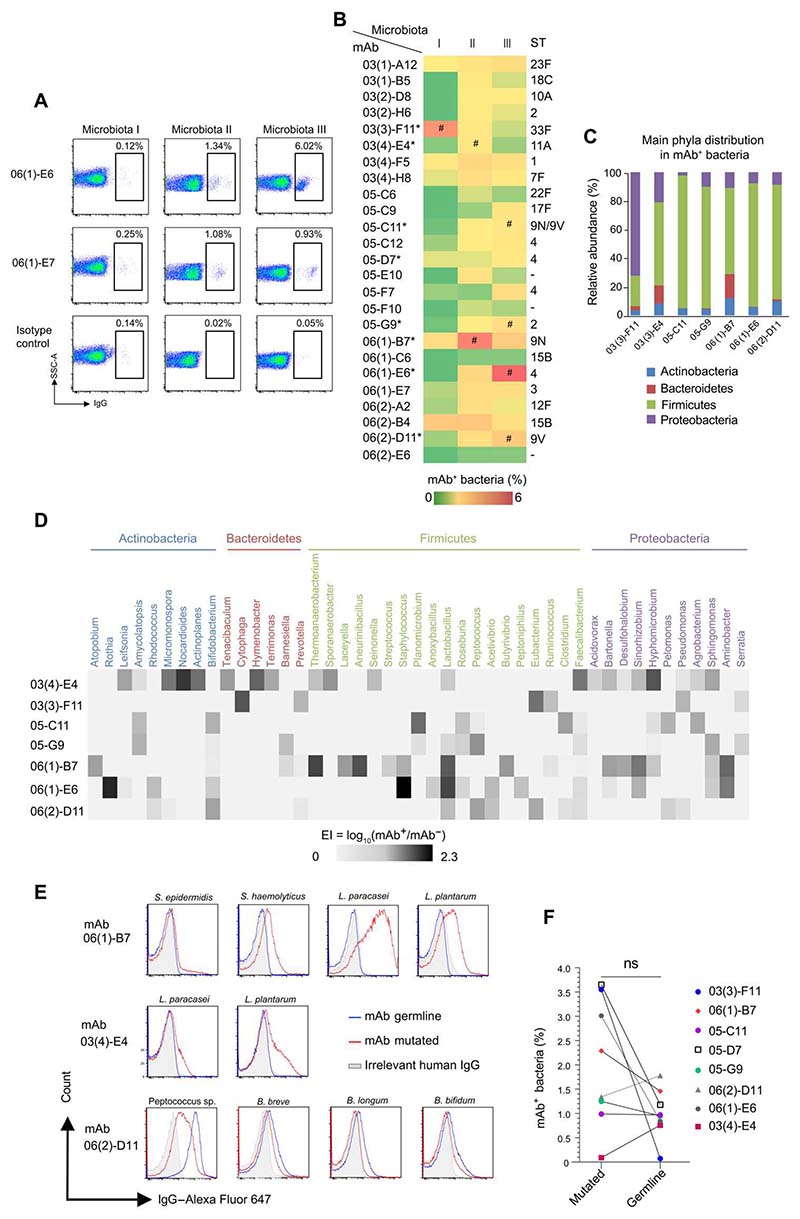
Anti-capPS mAbs cross-react against gut bacterial strains belonging to different genera, with contrasted roles of somatic mutations
We have shown that anti-capPS Abs are produced by PB/PC-d7 clones that differentiate from B cells belonging to large clones encompassing prediversified MZB, IgM-only, and switched B cells. Recently, a high-dimensional analysis has documented the possible diversification of MZ B cells in GCs of GALTs and their dissemination to widely distant sites via blood recirculation (47). We thus wondered if the anti-capPS mAbs that we obtained may also cross-react with bacterial structures present on the gut flora. To this end, we tested the binding of 25 mAb (expressed as IgG1) to three different fecal microbiota obtained from healthy individuals by a flow cytometry assay (Fig. 7, A and B) (48, 49). Two mAbs [06(2)-E6 and 06(1)-C6] had no detectable binding to any of the microbiota tested, and two others (05-F10 and 05-C6) bound only and very weakly to microbiota II, the percentage of labeled bacteria being nevertheless more than three times above the observed value for the isotype control. All the remaining mAbs (i.e., 84%) bound to a small but substantial fraction of one or more of the tested microbiota, fractions whose values ranged from 0.1 to 6% of the total bacteria. To gain more insights into the commensal bacteria bound by the mAbs, we selected eight of them (identified by an asterisk in Fig. 7 and data file S3 and available in their mutated or GL version), and we sorted by fluorescence-activated cell sorting (FACS) the respective mAb+ and mAb− microbiota fractions for 16S ribosomal RNA (rRNA) gene sequencing (Fig. 7B). The sequencing (successfully completed for seven mAbs) showed that they all recognized bacteria from the four major phyla, namely, Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria, but each one exhibited a distinct binding profile (Fig. 7C). The mAbbinding pattern was further characterized by calculating a log-based enrichment index (EI) at the genus level. All mAbs were cross-reactive against a median of 12 genera among the 100 most abundant ones (range, 6 to 21) (Fig. 7D) and thus recognized a wide panel of bacteria. EIs varied among the genera targeted by the mAbs, indicating that they might bind distinct genera with different affinities. Because each of the mAbs may potentially target a unique combination of bacteria belonging to different genera, or may also recognize more species within a genus, we searched for representative bacterial strains that are both cultivable and available (see Materials and Methods). Six mAbs were subsequently evaluated for their binding to isolated strains (fig. S4C), and three of the mutated mAbs showed a low to high binding to specific bacterial strains (Fig. 7E), thus confirming the validity of some of the mAb targets identified by 16S rRNA. No binding was detected for the three remaining mutated mAbs, a result that may rely on the large number of different species present among each genus [e.g., with regard to the Lactobacillus genus only, at least 50 species have been repeatedly detected in the stool of humans (50)]. As mentioned above, the eight mAbs, when reverted to their VH-VL GL configurations, lost their anti-capPS reactivity (data file S3). We thus compared in the same FACS flow experiment the binding of the mutated or GL version of each of the eight mAbs to the same microbiota (I, II, or III) that was used for the 16S RNA sequencing experiment. Whereas the percentages of bacteria stained by GL compared with the mutated mAbs were on average lower (1 ± 0.5% versus 2 ± 1.3%, the difference being not significant), the results were nevertheless heterogeneous: One GL mAb lost almost all its reactivity toward the microbiota, four mAbs showed a marked decrease in their binding, one mAb was unaffected, and two GL mAbs stained a larger bacterial fraction than their mutated counterparts. Among the mAbs tested for their binding to isolated bacterial strains (Fig. 7E), the results were again complex because GL mAb 06(1)-B7 and 03(4)-E04 did not recognize the Staphylococcus and/or Lactobacillus species anymore, whereas GL mAb 06(2)-D11 gained some reactivity against Bifidobacterium species and one Peptococcus sp. Together, these results illustrate that several GL mAbs were already reactive against the gut microbiota, whereas all required further diversification to recognize capPS Ags.
Fig. 7. Anti-capPS mAbs cross-react against gut bacteria from various genera and show a largely preserved cross-reactivity when reverted to their VH-VL GL configuration.
(A) Representative flow cytometry plots of microbiota reactivity of two mAbs toward human gut bacteria. Commensal bacteria were isolated from fecal samples of three healthy individuals (I to III). Data are representative of two independent experiments. (B) Microbiota reactivity of the different mAbs toward human gut microbiota. Frequency of mAb-coated bacteria is represented with a color scale. Data are representative of two independent experiments. Serotype specificity is indicated on the right part of the graph. Asterisks (*) indicate mAbs for which GL versions have been produced. Hash symbols (#) indicate microbiota that have been sorted and analyzed by 16S ribosomal DNA sequencing in (C) and (D). (C) Relative abundance of the four main phyla in mAb+ fractions. (D) Heatmap diagram of EI of the most frequent genera from the three healthy microbiota. Selected genera are recognized by at least one mAb (EI > 0.2) and belong to the 100 most frequent genera in microbiota I, II, and III. Genera are grouped according to their phylum. (E) Representative flow cytometry analysis of mAb (red line) or irrelevant IgG (gray, anti–TNF-α IgG1) staining of pure bacterial strains. (F) Pairwise comparison of microbiota reactivity of mAbs and their respective GL revertants.
Anti-capPS mAbs are mostly non-polyreactive
In a recent work, it was shown that the cross-species binding and high microbiota reactivity of human intestinal IgA did not correlate with Ab polyreactivity (51). We thus screened our 24 anti-capPS mAbs for binding to a panel of four unrelated Ags commonly used to test for polyreactivity (52, 53). As shown in fig. S7, only two of them showed moderate binding to more than three Ags and were thus classified as polyreactive. Together, these findings strongly suggest that the specific binding of our anti-capPS mAbs to the microbiota samples is mediated by gut bacterial glycans.
Discussion
Whereas MZ and B1 B cells are responsible for responses to TI Ags in the mouse, the identity of the B cell subset involved in similar responses in humans remains debated. The notion that IgM+IgD+CD27+ B cells may represent the functional and developmental equivalent of the mouse MZ B cell lineage is still controversial, despite their widely accepted name of MZB cells, and observations in favor of their contribution to TI responses are largely based on correlative evidence. We therefore vaccinated healthy volunteers with Pneumovax, a plain polysaccharide vaccine, and compared the repertoire of d7 PBs/PCs with the repertoire of various B cell compartments analyzed before and after vaccination (with a longitudinal follow-up of up to 2 months) to identify the subset(s) from which the PC amplification originated and the possible repertoire modifications induced in the B cell populations engaged in this response. The strong d7 PB/PC response in blood mobilized very large clones, whose size and Ig mutation level can only be accounted for by the engagement of a preamplified and prediversified B cell population, clones that frequently included a major IgM component together with clonally related IgA (IgA2 more frequently than IgA1) and IgG (mostly IgG2) PCs. Clonal relationships with B cell subsets present in blood before immunization revealed a high frequency of clones present in the MZB and IgA compartments, as well as, but to a lesser extent, IgG. A similar frequency of clonal relatedness was observed between the PB/PC-d7 and the blood CD27+ B cell subsets before and 2 months after vaccination, indicating, at least for this population of young adults (18 to 40 years of age), that the large PC response did not exhaust preexisting clones. Similarly, mutation frequencies of clones observed at these two time points were similar, again supporting the notion of an anti-capPS response taking place through extrafollicular amplification of Ag-experienced cells without contribution of affinity maturation. However, it cannot be excluded that a few additional mutations did occur during the switching from IgM to the other isotypes (9). Nevertheless, in all cases of capPS-specific mAbs analyzed, the mutations of their VH and VL genes appeared mandatory for their specificity. No changes in isotype distribution among CD27+ B cells were either observed with time, whereas, in contrast, the frequency of IgA+ cells among PCs increased at d14 in the two cases where PCs could be analyzed up to this stage, suggesting, together with the identification of single cell–sorted PCs at d7 harboring two isotypes, that isotype switch was occurring during clonal expansion, leading to PC differentiation.
Cloning and reexpression of several Abs expressed by the major PB/PC clones and showing specific recognition of distinct pneumococcal capPS serotypes revealed in most cases a clear binding to components of the gut microbiota. Isolation and identification through 16S sequencing of the bacterial species recognized revealed the capacity of these anti-pneumococcal Abs to bind to diverse types of phyla and genera. Moreover, because the majority of the anti-capPS mAbs (more than 90%) did not harbor any polyreactivity, this binding most likely proceeded through recognition of cross-reactive bacterial glycans. In line with this, it was shown that a reduction of circulating MZ B cells was correlated with a drop in binding to bacterial glycans (39). Although GL-reverted anti-pneumococcal Abs lost their specificity, the impact on their recognition of the gut flora was much more heterogeneous, with binding enhanced, decreased, or maintained upon GL reversion. In a similar experiment, gut IgA Abs were shown to lose their bacterial specificity when reverted to their GL configuration (51). Affinity maturation during gut responses is thus responsible for acquisition of pneumococcal cross-reactivity. Public clonotypes and preferential VH gene usage have been linked to pneumococcal anti-capPS recognition, and we observed similar repertoire convergence among our six vaccinees, as well as with previously published repertoire data. Such public specificities suggest an extensive sharing among individuals of cross-reactive bacterial glycans that elicited and selected these clonotypes. Overall, work thus far supports that diversification against gut-associated Ags, notably glycans, establishes a peripheral IgM B cell compartment, together with clonally related IgA and IgG2 subsets characteristic of a TI signature, with IgG2 representing 60 to 75% of the IgG sub-isotypes. A similar TI polarization is observed upon vaccination during the differentiation of MZ B cells into IgA and IgG PCs.
No clear distinction could be made between MZB and IgM-only CD27+ B cells, this latter subset being much less frequent, suggesting that their repertoire may largely overlap, at least among B cell fractions sorted on the basis of IgD and CD27 surface expression. Moreover, IgA+CD27− B cells have been shown to display some antibacterial binding properties (54), but very few clonal relationships were observed in our study between the PC pool mobilized in the pneumococcal response and the DN compartment. The stability over time of large IgM, IgA, and IgG2 clones circulating in blood has been described recently in a longitudinal follow-up of two healthy donors, showing distinct characteristics from smaller IgG1 clones specific for TD vaccines, and our data fully agree with this observation (55).
The concept of a prediversified MZ B cell repertoire obviously raises the question of the origin and location of such a prediversification step. A recent study reported that MZ B cell precursors differentiated and diversified their BCRs in GCs from GALTs (35). Along with our results of acquisition of capPS recognition through somatic mutations, this suggests a model in which, after an initial commitment at the MZP/T2 stage, MZ B cells can mature their BCRs by somatic hypermutation in GALT, in a process driven by gut commensals. Although less likely in normal physiological conditions, some of this process could also occur in the periphery upon translocation of gut bacterial Ags (56). Moreover, because these responses take place in the specific environment of the gut Peyer’s patches and unexpectedly harbor a typical IgG2 and IgA TI isotypic profile, this would suggest a diversification step triggered by noncanonical T cell help (57). In addition, one cannot formally exclude that asymptomatic carriage of S. pneumoniae may have triggered these diversified MZ B cells (58), but we find this unlikely to have occurred in all six individuals analyzed in this study. Moreover, because immune responses against other types of encapsulated bacteria also mobilize B cells with mutated BCR (59), it would imply the improbable scenario that such silent carriage always occurs.
There remain several questions on the physiology of human MZ B cells particularly concerning their homeostatic regulation and recall properties. TI vaccinations in humans induce the production of protective Abs but do not generate an enhanced Ab response upon a boost, evoking the absence of functional memory. These TI vaccines are inefficient in children below 2 years, and it is generally assumed that this is due to the immaturity of the splenic MZ constituents. Our results on the necessity of a fully diversified repertoire to recognize the pneumococcal serotypes would suggest, as an alternate explanation, an incomplete maturation of the Abs involved. At this young age, the pneumococcal conjugated vaccine gives rise to a classical TD immuneresponse including affinity maturation and B cell memory. Paradoxically, if given in older infants and adults, this conjugated formulation induces, rather than a memory response, a response somehow similar to that obtained with the plain TI vaccine, even if in some reports it seems more robust (60). We propose that the functional splenic MZ B cells could sequester some of these capPS whether they are conjugated or not to a carrier protein, thus preventing them from reaching naive follicular B cells. It would be therefore very instructive to study in a similar way a group of adult participants receiving the conjugated pneumococcal vaccine (Prevnar) to evaluate the B cell repertoire this vaccine mobilizes.
Our study was obviously limited to a small number of volunteers and we were not able to perform analyses beyond 56 days, which would be informative regarding the stability and the persistence of the expanded clones observed. The general relevance of our observations should also be extended to other types of polysaccharide vaccines, for example, against Neisseria meningitidis. In conclusion, we propose that MZ B cells generate a prediversified B cell repertoire in GALT that is driven by commensal bacterial Ags, which thereafter enable the rapid production of opsonizing Abs against highly pathogenic encapsulated bacteria. Many pathogens such as HIV are covered with glycans, which are often the main epitopes recognized by specific neutralizing Abs (61, 62). It is tempting to speculate that a vaccine containing only these glycans may trigger a fast and efficient MZ B cell response in humans (63). Evolutionarily, these results suggest that humans have retained, within a subset of B cells, the strategy used by several species such as chicken, sheep, and rabbit to diversify their pre-immune B cell repertoire through hypermutation and/or gene conversion in GALT to subsequently respond to external challenges (64).
Materials and Methods
Study design
To identify the B cell subset(s) from which anti-capPS–secreting PBs/PCs are derived, and given that the number of serotype-specific PBs/PCs was shown to peak around d7 upon immunization with Pneumovax (65, 66), our strategy was based on the sorting and BCR repertoire sequencing of PB/PC-d7 and of different B cell subsets isolated before and after vaccination. Whereas sequencing was done for PB/PC-d7 of all vaccinees, only three donors (for which higher number of cells could be FACS-sorted) were further selected for HTS of their B cell subsets sampled at different time points. We took advantage of the unique Ig HC VDJ signature expressed by each B cell clone to search for clonal filiations between anti-capPS PB/PC-d7 clones and isolated B cell subsets. Because a direct isolation of anti-capPS PBs/PCs was not possible, likely anticapPS VDJ signatures were first validated through the expression of the corresponding mAbs and their testing against the 23 capPS of the vaccine. Through ad hoc amplification of VDJ-μ, VDJ-α, and VDJ-γ transcripts of the sorted cell fractions and HTS sequencing, the clonal relationships between anti-capPS PBs/PCs and eight different B cell subsets (MZB cells, CD27+ or CD27– IgG+, IgA+, and IgM-only B cells, and last naive B cells) were investigated. Last, we determined the reactivity of pneumococcal-specific mAbs against bacterial species from the gut flora through bacterial flow cytometry assays and 16S rRNA gene sequencing of the mAb-bound microbiota.
Human Pneumovax immunization and sample collection
The study was approved by the Institutional Review Board #00001072 (CPP Ile-de-France II). All the participants gave written informed consent. For details on the Pneumovax-23 vaccine (named Pneumovax throughout this study) and participant inclusion criteria, see the Supplementary Materials. The final six participants are referred as P02, P03, P05, P06, P07, and P08. Serum was obtained before and after vaccination (d0 and d28), and heparinized blood samples (about 25 ml) were collected before vaccination (d0) and at d7, d14, d28, and d56 after vaccination.
Multicolor flow cytometry analysis and cell sorting
Peripheral blood mononuclear cells were isolated by centrifugation on Ficoll and surface-stained for 20 min at 4°C in phosphate-buffered saline (PBS) + 2% fetal calf serum, with Abs listed in Supplementary Materials and Methods. The detailed gating strategy is depicted in fig. S1. FACS-sorted B cell fractions were collected in Eppendorf tubes filled with PBS + 2% fetal calf serum. After centrifugation, the cell pellets were resuspended in 75 μl of reverse transcriptase lysis buffer and RNA extraction was done using the RNeasy Kit (Qiagen). PB/PC-d7 clones were also single cell–sorted into 96-well polymerase chain reaction (PCR) plates (Bio-Rad) containing ice-cold lysis buffer (4 μl per well) as described in (67).
Library preparation and Illumina sequencing
The library preparation for BCR-HTS, designed for paired-end Illumina sequencing, was done as described in (68). Briefly, the method allows the introduction, during the synthesis of double-strand (ds) IgHV-D-J-CH cDNA, of unique molecular identifiers (UMIs) used to reduce PCR amplification biases and sequencing error rate in downstream IgH sequencing analysis. The synthesized ds cDNA was then subjected to a first constant-region multiplex PCR using a mix of CH isotype-specific reverse primers. The resulting PCR product was then used in separate seminested PCRs, with respective inner CH-μ, CH-α,and CH-γ primers (including Illumina adaptor sequences). For more details on library preparation and sequencing, see the Supplementary Materials.
Bioinformatics analysis of IgH sequencing data
UMI Barcoded MiSeq 2×300 reads were processed as described in (68) and in the Supplementary Materials. Clonal assignment and GL reconstruction were performed with Change-O toolkit (69) on sequences having at least two representative reads for PB/PC-d7 and on all sequences for other populations. Clonal assignment was based on the following criteria: sequences that had the same V-gene, J-gene, and junction length, with maximal nucleotide hamming distance in CDR3 of 0.06 for PB/PC-d7 population, 0.15 for switched population, and 0.1 for the other subsets (the optimal threshold determined with the SHazaM R package) (69). For B cell populations (other than PB/PC-d7), clones were assigned by isotype fraction. All the details for the calculation of total sequence number by clone and ranking of PB/PC-d7 clones (Fig. 2 and fig. S2), isotype switch event quantification in top 100 PB/PC-d7 (fig. S3A), and calculation of the proportion of IgM, IgA, and IgG sequences relative to the total of sequences in each PB/PC-d7–d14 clone pair (fig. S3C) are given in the Supplementary Materials, as for the search for public clonotypes among PB/PC-d7 clones of the six vaccinees (Fig. 3). Regarding the public clonotypes shared between the vaccinees of this study, the nucleotide sequences encoding the corresponding H-CDR3 were verified (data file S2) to dispel any PCR contamination issue.
For each vaccinee, we performed clonal relationship analysis between PB/PC-d7 clones having more than 100 sequences and all other populations. The clones having the same V-gene, J-gene, junction length, and minimal hamming nucleotide distance less than 0.15 were considered the same clone. Accordingly, for subsequent analysis, we added to every PB/PC-d7 clone the corresponding clones from MZB, switched IgA, and switched IgG. The anti-PC sequences obtained on a single-cell level were also added to corresponding clones. Lineage trees were built on the basis of the maximum likelihood phylogenetic method using the IgPhyML tool (70, 71). For visibility reason, we subsampled all large fractions to 10 sequences by fraction. When tracing MZB-d0 clones at other time points in Circos plots, we chose only clones related to the top 50–ranked PB/PC-d7 clones and showed for each such clone only one instance of relation, if found, to other populations.
Recombinant Ab production and purification
Starting from single PB/PC-d7 sorted into 96-well PCR plates, single-cell reverse transcription PCR (RT-PCR) was performed and followed by Ig-μ, Ig-γ, Ig-λ, and Ig-κ gene PCR amplifications as described (67). An additional 3'CH1-alpha primer (5′-GAGTGGCTCCTGGGGGAAGA-3′) was designed to amplify also Ig-α chains. Paired IgH and IgL chains from clones of interest were cloned in γ1 HC and κ or λ light chain (LC) expression vectors (67) and transfected into human embryonic kidney (HEK) 293T cells using a jetPRIME kit (Polyplus). Recombinant Abs were purified from supernatants using protein G HP SpinTrap (GE Health-Care) and subsequently dialyzed overnight into PBS [0.01 M sodium phosphate (pH 7.4) and 0.137 M sodium chloride]. To obtain the GL-reverted versions of eight mAbs (marked by anasterisk in data file S2), the GL genes corresponding to each VH-D-JH and Vλ-Jλ (or Vκ-Jκ) pair were synthetized with proper restriction sites (and a codon optimization) and cloned into the γ1 HC and κ or λ LC expression vectors by ProteoGenix (France), transfected, and purified as described above.
Anti-polysaccharide ELISA and affinity measurement
Pre-and post-immunization serum IgG concentrations for seven pneumococcal serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) were measured as described in (72). Purified recombinant mAbs were tested against each of the capPS of Pneumovax. Briefly, 96-well plates were coated with individual capPS. After blocking of wells, purified mAbs diluted at 1μg/ml in enzyme-linked immunosorbent assay (ELISA) buffer containing CWPS (5 μg/ml) were added to the plates. Revelation was done with incubation with horseradish peroxidase–conjugated goat anti-human IgG followed by addition of KPL SureBlue TMB peroxidase substrate, after washing steps. The reaction was stopped by H2SO4, and optical density was measured at 450 nm. Background values given by incubation of PBS alone in coated wells were subtracted. All mAbs were tested in duplicate in two independent experiments.
Ab affinities (Kd) were determined as described in (45) by curve fitting analysis of individual ELISA curves plotted from a dilution series of Ab beginning at 10 μg/ml. Each mAb was tested in duplicate in two experiments whose results were then averaged to obtain the Kd reported in data file S2 (see the Supplementary Materials for details).
Polyreactivity ELISA
mAbs were tested at four consecutive 1:4 dilutions (starting from 1 μg/ml) against dsDNA, keyhole limpet hemocyanin, lipopolysaccharide, and insulin as previously described (52). Abs were considered polyreactive when they recognized at least three structurally different Ags in two distinct experiments. Threshold values for reactivity were determined by using control Abs mGO53 (negative) (53) and ED38 (high positive).
Bacterial strains and fecal bacteria
The different isolated bacterial strains (Fig. 7 and fig. S6) were obtained from the Saint-Antoine Hospital (Paris),the Necker-Enfants Malades Hospital (Paris), the INRA (Jouy en Josas), and the American Type Culture Collection strain collections. Their culture conditions are described in the Supplementary Materials. The fecal bacteria from three healthy individuals were purified by gradient purification under anaerobic conditions as described in (48, 73) and in the Supplementary Materials.
Bacterial flow cytometry
mAb binding to microbiota or bacterial strains was evaluated as previously described (49, 74). All buffers were passed through sterile 0.22-μm filters before use. Thawed microbiota or bacterial strains (106 per condition) were fixed in 500 μl of paraformaldehyde solution (4% in 1× PBS) and stained with Cell Proliferation Dye eFluor 450 (eBioscience) for 25 min at 4°C. After washing with 1× PBS (10 min, 4000g, 4°C), bacteria were suspended in 1× PBS, 2% bovine serum albumin (Sigma-Aldrich), and 0.02% sodium azide (Sigma-Aldrich). mAb or human monoclonal anti–tumor necrosis factor–α (TNF-α) IgG1 (REMICADE; MSD France) was added at a final concentration of 1 μg/ml and incubated for 30 min at 4°C. After washing, goat anti-human IgG–Alexa Fluor 647 or isotype control (both from Jackson ImmunoResearch Laboratories) was incubated for 20 min at 4°C. Then, bacteria were washed and resuspended in sterile PBS. Samples were run using BD FACSCanto II. Analysis was performed with FlowJo software (TreeStar). Medians of fluorescence were used to measure mAb-binding levels for pure strains.
Sorting of IgG-bound microbiota
Gut microbiota were incubated with purified mAbs (at 1 μg/ml) in a 96-well V-bottom plate (106 bacteria per well, 10 wells per mAb) for 30 min at 4°C. After washing in 1 × PBS, live microbiota were stained as described above. Then, sorting was performed using a microbiota-dedicated single laser S3 cell sorter (Bio-Rad Laboratories, USA). Sorted bacteria (9 × 105) were collected in 1× PBS at 4°C, centrifuged (8000g, 10 min, 4°C), and stored at −80°C until DNA extraction. Purity for both fractions was systematically verified after sorting. To check the absence of contaminants in flow cytometer fluid lines, we regularly incubated sheath fluid in Brain-Heart Infusion Broth (BioMérieux) at 37°C for 7 days.
16S rRNA gene sequencing and analysis
DNA extraction, generation of amplicons of V3-V4 regions of 16S rRNA genes, and multiplexed sequencing (MiSeq, Illumina) were done as described in (49) and in the Supplementary Materials. De-multiplexed paired-end 250 nucleotide reads were processed using MG-RAST analysis pipeline. Sequencing artifacts, host DNA contamination, and sequences fewer than 200 base pairs in length were removed. Insufficient quality reads were discarded (<5% of total reads). Sequences were then clustered into operational taxonomic units (OTUs) with a 97% homology using Greengenes database. OTUs containing only a single sequence were discarded. Previously published contaminant sequences (75) were removed if present in only one sorted fraction and absent from paired fractions. OTUs detected at >0.1% relative abundance in at least two samples were finally conserved. The OTU table was rarefied to the minimum sample’s depth (24,785 reads). In calculating the EI, we scored a pseudo-relative abundance equal to 0.0001, which was the lower limit of detection, if a taxon was not detected in a given fraction.
Statistical analyses
Statistics were calculated using GraphPad Prism (v9) and R (version 4.0.4). When a statistical analysis was done on data presented in a given figure, the test used is specified in the corresponding figure legend.
Supplementary Material
Acknowledgments
We thank J. Mégret (Cell Sorting Facility of the SFR Necker) for cell sorting, D. Bagnara for sharing expertise on BCR HTS, P. Villarese and the McIntyre/Asnafi team for access to an Illumina MiSeq sequencer, H. Mouquet and C. Planchais for the gift of mAbs ED38 and MOG53 and helpful discussions, C. Parizot for help in bacterial flow cytometry, S. Storck and P. Chappert for helpful discussions and support, and H. Lecuyer for the gift of bacterial strains.
Funding
This work was supported by a Fondation Princesse Grace grant and by an ERC Advanced Grant (B-response) awarded to J.-C.W. and C.-A.R.
Footnotes
Author contributions: Conceptualization: S.W., J.-C.W., and C.-A.R. Methodology: S.W., J.-C.W., and C.-A.R. Software: T.F. Validation: S.W. and D.S. Investigation: S.W., D.S., E.C., A.V.d.l.A., C.G., R.F., and M.B. Formal analysis: S.W., D.S., T.F., E.C., A.V.d.l.A., C.G., R.F., and M.B. Visualization: S.W., D.S., and T.F. Supervision: S.W., J.-C.W., and C.-A.R. Funding acquisition: J.-C.W. and C.-A.R. Resources: F.B. and G.G. Project administration: S.W., J.-C.W., and C.-A.R. Writing—original draft: S.W., J.-C.W., and C.-A.R. Writing—review and editing: S.W., A.V.d.l.A., C.G., R.F., J.-C.W., and C.-A.R.
Competing interests: J.-C.W. received consulting fees from the Fondation Mérieux outside of this work. The other authors declare that they have no competing interests.
Data and materials availability
The immunoglobulin repertoire sequencing data are available under ArrayExpress accession code E-MTAB-12117. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977;119:1874–1878. [PubMed] [Google Scholar]
- 2.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 3.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 4.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 5.Balázs M, Martin F, Zhou T, Kearney JF. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 6.García de Vinuesa C, MacLennan ICM, Holman M, Klaus GGB. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur J Immunol. 1999;29:3216–3224. doi: 10.1002/(SICI)1521-4141(199910)29:10<3216::AID-IMMU3216>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Jeurissen A, Ceuppens JL, Bossuyt X. T lymphocyte dependence of the antibody response to “T lymphocyte independent type 2” antigens. Immunology. 2004;111:1–7. doi: 10.1111/j.1365-2567.2003.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–336. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zhao Y, Qi H. T-independent antigen induces humoral memory through germinal centers. J Exp Med. 2022;219:e20210527. doi: 10.1084/jem.20210527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musher DM, Manoff SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 14.Cripps AW, Folaranmi T, Johnson KD, Musey L, Niederman MS, Buchwald UK. Immunogenicity following revaccination or sequential vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) in older adults and those at increased risk of pneumococcal disease: A review of the literature. Expert Rev Vaccines. 2021;20:257–267. doi: 10.1080/14760584.2021.1889374. [DOI] [PubMed] [Google Scholar]
- 15.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–722. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- 16.Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–874. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: Is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 18.Siegrist C-A, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 19.Lesinski GB, Westerink MAJ. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47:135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 20.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 21.Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, Westerink MJ. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. doi: 10.1186/1742-4933-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas AH, Moulton KD, Tang VR, Reason DC. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: Variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect Immun. 2001;69:853–864. doi: 10.1128/IAI.69.2.853-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun. 2002;70:4083–4091. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–134. [PMC free article] [PubMed] [Google Scholar]
- 25.Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2subclass antibody responses. J Immunol. 1990;144:3770–3778. [PubMed] [Google Scholar]
- 26.Haas KM. B-1 lymphocytes in mice and nonhuman primates. Ann N Y Acad Sci. 2015;1362:98–109. doi: 10.1111/nyas.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnara D, Squillario M, Kipling D, Mora T, Walczak AM, Da Silva L, Weller S, Dunn-Walters DK, Weill J-C, Reynaud C-A. A Reassessment of IgM memory subsets in humans. J Immunol. 2015;195:3716–3724. doi: 10.4049/jimmunol.1500753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller S, Faili A, Garcia C, Braun MC, Le Deist F, de Saint Basile G, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, Weill J-C, Reynaud C-A. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+IgD+CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangye SG, Good KL. Human IgM+CD27+ B cells: Memory B cells or “memory” B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Budeus B, Schweigle de Reynoso S, Przekopowitz M, Hoffmann D, Seifert M, Küppers R. Complexity of the human memory B-cell compartment is determined by the versatility of clonal diversification in germinal centers. Proc Natl Acad Sci U S A. 2015;112:E5281–E5289. doi: 10.1073/pnas.1511270112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Descatoire M, Weller S, Irtan S, Sarnacki S, Feuillard J, Storck S, Guiochon-Mantel A, Bouligand J, Morali A, Cohen J, Jacquemin E, et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J Exp Med. 2014;211:987–1000. doi: 10.1084/jem.20132203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tull TJ, Pitcher MJ, Guesdon W, Siu JHY, Lebrero-Fernández C, Zhao Y, Petrov N, Heck S, Ellis R, Dhami P, Kadolsky UD, et al. Human marginal zone B cell development from early T2 progenitors. J Exp Med. 2021;218:e20202001. doi: 10.1084/jem.20202001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, Plebani A, Aiuti F, Quinti I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 38.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter H-H, Berner R, Peters A, Boehm T, Plebani A, Quinti I, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maglione PJ, Simchoni N, Black S, Radigan L, Overbey JR, Bagiella E, Bussel JB, Bossuyt X, Casanova J-L, Meyts I, Cerutti A, et al. IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood. 2014;124:3561–3571. doi: 10.1182/blood-2014-07-587824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow M, Valentin A, Little R, Yarchoan R, Pavlakis GN. A splenic marginal zone-like peripheral blood CD27+B220− B cell population is preferentially depleted in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2008;24:621–633. doi: 10.1089/aid.2007.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khaskhely N, Mosakowski J, Thompson RS, Khuder S, Smithson SL, Westerink MAJ. Phenotypic analysis of pneumococcal polysaccharide-specific B cells. J Immunol. 2012;188:2455–2463. doi: 10.4049/jimmunol.1102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol. 2008;181:5306–5312. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- 43.Roco JA, Mesin L, Binder SC, Nefzger C, Gonzalez-Figueroa P, Canete PF, Ellyard J, Shen Q, Robert PA, Cappello J, Vohra H, et al. Class-switch recombination occurs infrequently in germinal centers. Immunity. 2019;51:337–350.:e7. doi: 10.1016/j.immuni.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith K, Muther JJ, Duke AL, McKee E, Zheng N-Y, Wilson PC, James JA. Fully human monoclonal antibodies from antibody secreting cells after vaccination with Pneumovax®23 are serotype specific and facilitate opsonophagocytosis. Immunobiology. 2013;218:745–754. doi: 10.1016/j.imbio.2012.08.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler AS, Mizrahi RA, Spindler MJ, Adams MS, Asensio MA, Edgar RC, Leong J, Leong R, Roalfe L, White R, Goldblatt D, et al. Rare, high-affinity anti-pathogen antibodies from human repertoires, discovered using microfluidics and molecular genomics. MAbs. 2017;9:1282–1296. doi: 10.1080/19420862.2017.1371383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Uduman M, Siu JHY, Tull TJ, Sanderson JD, Wu Y-CB, Zhou JQ, Petrov N, Ellis R, Todd K, Chavele K-M, et al. Spatiotemporal segregation of human marginal zone and memory B cell populations in lymphoid tissue. Nat Commun. 2018;9:3857. doi: 10.1038/s41467-018-06089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fadlallah J, Sterlin D, Fieschi C, Parizot C, Dorgham K, El Kafsi H, Autaa G, Ghillani-Dalbin P, Juste C, Lepage P, Malphettes M, et al. Synergistic convergence of microbiota-specific systemic IgG and secretory IgA. J Allerg Clin Immunol. 2019;143:1575–1585.:e4. doi: 10.1016/j.jaci.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 49.Sterlin D, Fadlallah J, Adams O, Fieschi C, Parizot C, Dorgham K, Rajkumar A, Autaa G, El-Kafsi H, Charuel J-L, Juste C, et al. Human IgA binds a diverse array of commensal bacteria. J Exp Med. 2020;217:e20181635. doi: 10.1084/jem.20181635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi M, Martínez-Martínez D, Amaretti A, Ulrici A, Raimondi S, Moya A. Mining meta-genomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota. Environ Microbiol Rep. 2016;8:399–406. doi: 10.1111/1758-2229.12405. [DOI] [PubMed] [Google Scholar]
- 51.Kabbert J, Benckert J, Rollenske T, Hitch TCA, Clavel T, Cerovic V, Wardemann H, Pabst O. High microbiota reactivity of adult human intestinal IgA requires somatic mutations. J Exp Med. 2020;217:e20200275. doi: 10.1084/jem.20200275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planchais C, Kök A, Kanyavuz A, Lorin V, Bruel T, Guivel-Benhassine F, Rollenske T, Prigent J, Hieu T, Prazuck T, Lefrou L, et al. HIV-1 envelope recognition by polyreactive and cross-reactive intestinal B cells. Cell Rep. 2019;27:572–585.:e7. doi: 10.1016/j.celrep.2019.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Pre-dominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 54.Berkowska MA, Schickel J-N, Grosserichter-Wagener C, de Ridder D, Ng YS, van Dongen JJM, Meffre E, van Zelm MC. Circulating human CD27-IgA+ memory B cells recognize bacteria with polyreactive Igs. J Immunol. 2015;195:1417–1426. doi: 10.4049/jimmunol.1402708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phad GE, Pinto D, Foglierini M, Akhmedov M, Rossi RL, Malvicini E, Cassotta A, Fregni CS, Bruno L, Sallusto F, Lanzavecchia A. Clonal structure, stability and dynamics of human memory B cells and circulating plasmablasts. Nat Immunol. 2022;23:1–10. doi: 10.1038/s41590-022-01230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fine RL, Manfredo Vieira S, Gilmore MS, Kriegel MA. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes. 2020;11:217–230. doi: 10.1080/19490976.2019.1629236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biram A, Shulman Z. T cell help to B cells: Cognate and atypical interactions in peripheral and intestinal lymphoid tissues. Immunol Rev. 2020;296:36–47. doi: 10.1111/imr.12890. [DOI] [PubMed] [Google Scholar]
- 58.Ramos-Sevillano E, Ercoli G, Brown JS. Mechanisms of naturally acquired immunity to Streptococcus pneumoniae. Front Immunol. 2019;10:358. doi: 10.3389/fimmu.2019.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galson JD, Clutterbuck EA, Trück J, Ramasamy MN, Münz M, Fowler A, Cerundolo V, Pollard AJ, Lunter G, Kelly DF. BCR repertoire sequencing: Different patterns of B-cell activation after two Meningococcal vaccines. Immunol Cell Biol. 2015;93:885–895. doi: 10.1038/icb.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weller S, Reynaud C-A, Weill J-C. Vaccination against encapsulated bacteria in humans: Paradoxes. Trends Immunol. 2005;26:85–89. doi: 10.1016/j.it.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Lorin V, Fernández I, Masse-Ranson G, Bouvin-Pley M, Molinos-Albert LM, Planchais C, Hieu T, Péhau-Arnaudet G, Hrebík D, Girelli-Zubani G, Fiquet O, et al. Epitope convergence of broadly HIV-1 neutralizing IgA and IgG antibody lineages in a viremic controller. J Exp Med. 2022;219:e20212045. doi: 10.1084/jem.20212045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shivatare VS, Shivatare SS, Lee C-CD, Liang C-H, Liao K-S, Cheng Y-Y, Saidachary G, Wu C-Y, Lin N-H, Kwong PD, Burton DR, et al. Unprecedented role of hybrid N-glycans as ligands for HIV-1 broadly neutralizing anti-bodies. J Am Chem Soc. 2018;140:5202–5210. doi: 10.1021/jacs.8b00896. [DOI] [PubMed] [Google Scholar]
- 63.Williams WB, Meyerhoff RR, Edwards RJ, Li H, Manne K, Nicely NI, Henderson R, Zhou Y, Janowska K, Mansouri K, Gobeil S, et al. Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies. Cell. 2021;184:2955–2972.:e25. doi: 10.1016/j.cell.2021.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weill J-C, Weller S, Reynaud C-A. A bird’s eye view on human B cells. Semin Immunol. 2004;16:277–281. doi: 10.1016/j.smim.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Heilmann C, Pedersen FK. Quantitation of blood lymphocytes secreting antibodies to pneumococcal polysaccharides after in vivo antigenic stimulation. Scand J Immunol. 1986;23:189–194. doi: 10.1111/j.1365-3083.1986.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 66.Kehrl JH, Fauci AS. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983;71:1032–1040. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crickx E, Chappert P, Sokal A, Weller S, Azzaoui I, Vandenberghe A, Bonnard G, Rossi G, Fadeev T, Storck S, Fadlallah J, et al. Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia. Sci Transl Med. 2021;13:eabc3961. doi: 10.1126/scitranslmed.abc3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta NT, Vander Heiden JA, Uduman M, Gadala-Maria D, Yaari G, Kleinstein SH. Change-O: A toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics. 2015;31:3356–3358. doi: 10.1093/bioinformatics/btv359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoehn KB, Lunter G, Pybus OG. A phylogenetic codon substitution model for antibody lineages. Genetics. 2017;206:417–427. doi: 10.1534/genetics.116.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoehn KB, Vander Heiden JA, Zhou JQ, Lunter G, Pybus OG, Kleinstein SH. Rep-ertoire-wide phylogenetic models of B cell molecular evolution reveal evolutionary signatures of aging and vaccination. Proc Natl Acad Sci U S A. 2019;116:22664–22672. doi: 10.1073/pnas.1906020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerard A-L, Goulenok T, Bahuaud M, Francois C, Aucouturier P, Moins-Teisserenc H, Batteux F, Papo T, Sacre K. Serum IgG2 levels predict long-term protection following pneumococcal vaccination in systemic lupus erythematosus (SLE) Vaccine. 2020;38:6859–6863. doi: 10.1016/j.vaccine.2020.08.065. [DOI] [PubMed] [Google Scholar]
- 73.Juste C, Kreil DP, Beauvallet C, Guillot A, Vaca S, Carapito C, Mondot S, Sykacek P, Sokol H, Blon F, Lepercq P, et al. Bacterial protein signals are associated with Crohn’s disease. Gut. 2014;63:1566–1577. doi: 10.1136/gutjnl-2012-303786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moor K, Fadlallah J, Toska A, Sterlin D, Balmer ML, Macpherson AJ, Gorochov G, Larsen M, Slack E. Analysis of bacterial-surface-specific antibodies in body fluids using bacterial flow cytometry. Nat Protoc. 2016;11:1531–1553. doi: 10.1038/nprot.2016.091. [DOI] [PubMed] [Google Scholar]
- 75.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vander Heiden JA, Yaari G, Uduman M, Stern JNH, O’Connor KC, Hafler DA, Vigneault F, Kleinstein SH. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics. 2014;30:1930–1932. doi: 10.1093/bioinformatics/btu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brochet X, Lefranc M-P, Giudicelli V. IMGT/V-QUEST: The highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–W508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ademokun A, Wu Y-C, Martin V, Mitra R, Sack U, Baxendale H, Kipling D, Dunn-Walters DK. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell. 2011;10:922–930. doi: 10.1111/j.1474-9726.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur J of Immunol. 2000;30:1214–1223. doi: 10.1002/(SICI)1521-4141(200004)30:4<1214::AID-IMMU1214>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 80.Bryson S, Thomson CA, Risnes LF, Dasgupta S, Smith K, Schrader JW, Pai EF. Structures of preferred human IgV genes–based protective antibodies identify how conserved residues contact diverse antigens and assign source of specificity to CDR3 loop variation. J Immunol. 2016;196:4723. doi: 10.4049/jimmunol.1402890. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z, Cox KS, Tang A, Roman J, Fink M, Kaufhold RM, Guan L, Xie A, Boddicker MA, Mcguinness D, Xiao X, et al. Human monoclonal antibodies isolated from a primary pneumococcal conjugate Vaccinee demonstrates the expansion of an antigen-driven Hypermutated memory B cell response. BMC Infect Dis. 2018;18:613. doi: 10.1186/s12879-018-3517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw DR, Kirkham P, Schroeder HW, Roben P, Silverman GJ. Structure-function studies of human monoclonal antibodies to pneumococcus type 3 polysaccharide. Ann N Y Acad Sci. 1995;764:370–373. doi: 10.1111/j.1749-6632.1995.tb55849.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae Type 6B. Infect Immun. 2004;72:3505–3514. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The immunoglobulin repertoire sequencing data are available under ArrayExpress accession code E-MTAB-12117. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.