Abstract
Sleep plays a role in the consolidation of declarative memories, and has been shown to influence emotional representations more strongly than neutral ones. Although these effects have attracted much attention, few studies have examined their neural correlates. Here, we studied the impact of sleep upon source memory at both behavioural and neural levels. Emotional and neutral sources were retrieved following a twelve hour retention interval including either wake only or wake plus sleep. Our data show reduced forgetting and increased responses in hippocampus and superior parietal cortex during source memory after sleep. Additionally, we reveal a task-specific interaction between sleep and emotional valence, with left amygdala and components of the episodic memory system including right parahippocampus and posterior cingulate more active during emotional memory after sleep. Because these brain responses were not associated with superior memory, and because they were only observed when emotion was specifically relevant to the retrieval task, we interpret them as markers of altered post-retrieval processing. Overall, our findings extend prior work by demonstrating that sleep exerts a protective influence upon source memory, and that this is mediated by enhanced responses in hippocampus and superior parietal cortex. Additionally, we provide novel evidence that sleep can impact upon the manipulation of freshly recalled information when it is relevant to the task at hand.
Keywords: emotional memory, source memory, consolidation, sleep, hippocampus, amygdala
Introduction
Consolidation is the gradual reorganisation and stabilisation of labile, newly encoded memories (McGaugh, 2000). Both lesion work and neuroimaging suggest that consolidation involves alterations in the way memories are represented in the brain such that they cease to depend upon the hippocampus and become increasingly integrated with existing neocortical traces (Frankland and Bontempi, 2005;Eichenbaum, 2000).
A growing literature supports a role for sleep, particularly slow wave sleep, in declarative memory consolidation (Plihal and Born, 1997), but see (Vertes, 2004). This includes work showing reduced episodic memory decay across sleep as compared to wake (Takashima et al., 2006), enhanced episodic memory after sleep (Plihal and Born, 1997), and even enhanced episodic memory after artificial stimulation of slow-waves (Marshall et al., 2006). Furthermore, work in both rats (Wilson and McNaughton, 1994;Hoffman and McNaughton, 2002;Nadasdy et al., 1999) and humans (Maquet et al., 2000;Peigneux et al., 2004;Rasch et al., 2007) has shown that the neural ensembles associated with learning reactivate spontaneously during sleep, and that such reactivation can predict subsequent performance improvements (Peigneux et al., 2004).
Emotional and neutral declarative memories consolidate along separate trajectories (LaBar and Cabeza, 2006). While neutral memories are gradually forgotten (Frankland and Bontempi, 2005;McGaugh, 2000) such decay is less apparent in emotional memories (LaBar and Cabeza, 2006). Work in both rats (McGaugh, 2004) and humans (Ritchey et al., 2008;Dolcos et al., 2004) has suggested that this privileged retention is mediated by interactions between the amygdala and hippocampus at encoding. Such activities are thought to ‘tag’ newly formed emotional memories so they are preferentially strengthened during subsequent consolidation (McGaugh, 2004).
A number of studies (Wagner et al., 2005b;Wagner et al., 2006;Hu et al., 2006;Nishida et al., 2009;Payne et al., 2008;Sterpenich et al., 2007;Sterpenich et al., 2009) suggest that sleep may play a role in this emotion-specific consolidation. This work is largely behavioural, hence the neural correlates of emotion-specific strengthening across sleep as compared to natural wake remain to be established, but see (Sterpenich et al., 2007;Sterpenich et al., 2009) for imaging studies comparing consolidation across nights of sleep and sleep deprivation.
In the current report, we examine these effects at both behavioural and neural levels by using functional magnetic resonance imaging (fMRI) to visualise the impact of retention across a twelve hour period containing sleep plus wake or wake only. Our stimuli included both emotional and neutral source images. Because the task relevance of emotional content in retrieved information modulates activity in the amygdala and connectivity between amygdala and hippocampus (Smith et al., 2006), we examined both emotion-relevant and emotion-irrelevant task variants. Our study therefore followed a 2x2x2 design, with consolidation type (sleep/wake), emotionality (negative/neutral), and retrieval task (emotion-relevant / emotion-irrelevant) as factors.
Based on the above literature, we predicted superior memory performance and stronger responses throughout the episodic memory system during source memory after consolidation across sleep, with all such effects amplified for valenced information.
Materials & Methods
Participants
Twenty-two (12 female) healthy right-handed volunteers aged 19-32 yrs (mean=24.5yrs +-3.3 SD) and free from any history of sleep pathologies, memory difficulties, or other neurological disorders, were recruited. Participants gave written informed consent. The study was approved by the Ethical Review Board at the University of Liverpool.
Task
To investigate the impact of retrieval instructions upon connectivity between amygdala and hippocampus during correct source memory, we used the task developed by Smith and colleagues (Smith et al., 2006). In this task, neutral foreground items (object images) were paired with either negative or neutral backgrounds (source images) at encoding (see below for detail). At test, foreground objects were displayed as cues and participants were asked to retrieve information about the source images with which these had been initially paired. Test consisted of two different task conditions: an emotion-relevant condition (EMOTION) and an emotion-irrelevant condition (PEOPLE). In EMOTION, participants indicated whether the source image associated with each presented object were emotional or neutral. In PEOPLE they indicated whether or not the source image contained people (see below for further detail). The presence/absence of negative emotion and people was balanced across source images.
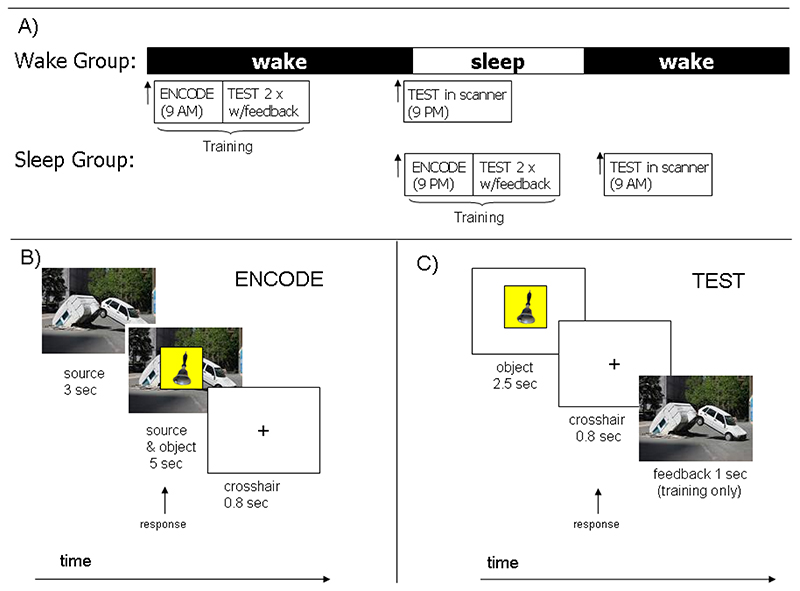
We were interested in consolidation, and therefore introduced a twelve hour retention interval between encoding and the final test session (Figure 1A). To allow examination of the differential influences of retention across a day of normal wakefulness as compared an equivalent interval containing a night of sleep, participants were randomly assigned to two experimental groups: ‘Wake’ and ‘Sleep’, with 6 females and 5 males in each group. In the Wake group, Encoding occurred in the morning (9 AM +/- 1 hour) and Test occurred in the evening (9 PM +/- 1 hour) such that the retention interval included a normal day during which participants did not sleep. In the Sleep groups, however, Encoding occurred in the evening (9 PM +/- 1 hour) and Test occurred in the morning (9 AM +/- 1 hour). For this group, the retention interval included a night of sleep (Figure 1A).
Figure 1. Experimental paradigm and source memory task.
A) Participants were divided into a Wake group (11 participants) who trained in the morning, and a Sleep group (11 participants) who trained in the evening. Each group was tested in the scanner 12 +-1 hours after encoding. The Sleep group therefore consolidated across a night of sleep, while the Wake group consolidated across a day of wakefulness. B) The source memory task (Smith et al., 2006): during Encoding participants viewed a source picture for three seconds before a foreground object was superimposed on it for a further five seconds. Participants formed an association between source and object, then responded with a button press. At Test (C) neutral objects were shown for 2.5 seconds, followed by a crosshair for 0.8 seconds. Testing was divided into two tasks: EMOTION and PEOPLE. In EMOTION, there were 3 possible responses: old originally paired with a negative source, old originally paired with a neutral source, and old but I can’t remember the source. In PEOPLE the response options were equivalent but participants indicated whether the source image contained people or not rather than whether it was negative or neutral. Training included two tests with feedback in which the source image was displayed after the subject had responded. Delayed testing (after the retention period) was carried out in the MRI scanner and did not include feedback. In Delayed testing, learned objects were intermixed with novel unlearned objects, and participants had an additional response option ‘new’ in both tasks.
The combination of the two retrieval tasks, the two retention groups, and the two source valences, produced a 2x2x2 design with the factors task (EMOTION/PEOPLE), group (Sleep/Wake), and source valence (negative/neutral). This allowed comparison of responses associated with the retrieval of negative and neutral memories as a function of both task and retention interval type.
Encoding Procedure
Following the method of Smith et al., (2006), each source image was shown for three seconds during encoding. A foreground object was then superimposed on it for five seconds before both images were replaced with a fixation cross for a further second (Figure 1B). Participants were asked to form an association between object and source images, then press a button. To facilitate learning, the 160 image pairs were separated into two lists of 80, 40 for use in EMOTION and 40 for use in PEOPLE. Encoding lasted twelve minutes for each list.
In order to strengthen memory representations, participants were tested twice with feedback immediately after encoding (immediate tests). In these tests, the learned objects were presented for 2.5 seconds, followed by a 0.8 second inter-stimulus-interval (ISI). Order of presentation was pseudorandom, and stimuli were equally divided between the two retrieval tasks. In EMOTION, there were 3 possible responses: old originally paired with a negative
source, old originally paired with a neutral source, and old but I can’t remember the source. In PEOPLE the response options were equivalent but indicated whether the source image contained people or not rather than whether it was negative or neutral. Once a response had been made, the source image which had been paired with the foreground was displayed for one second as feedback (Figure 1C). The two tasks were interleaved in psuedorandomly ordered blocks of eight items. A screen stating EMOTION or PEOPLE respectively proceeded each block for two seconds and reminded participants of the task and the three possible responses by listing them in terms of finger position. Participants undertook the immediate test twice directly after studying each encoding list. They took a brief break after completing the second immediate test for the first encoding list, then commenced the same procedure of encoding followed by two immediate tests of the second list.
Testing Procedure
Following the 12 hour retention interval, participants performed a Test session in the MRI scanner. This was similar to the immediate tests described in the Encoding section above except that no feedback was given. As in Smith et. al. 2006, stimuli consisted of the 160 studied foreground objects randomly intermixed with 40 new objects and 80 null events. In addition to the three response options used during immediate tests (see above), participants could also respond ‘new’ to indicate that they had not seen an object before. Each object was presented for 2.5 seconds, with a 0.8 seconds ISI during which participants were asked to respond. As with Encoding, the Test session was split into equal halves. Data were collected in two separate fMRI runs, with a brief resting break in between, (participants remained in the scanner).
Stimuli
Our stimulus images were identical to those of Smith et al. (2006), which implemented the same task paradigm. The source images were photographs both taken from the IAPS (Lang, et al., 2005) database and supplemented with images from the internet. Pictures ranged from everyday scenes, to images of injury, violence, decay and contaminated foods. 160 photographs were used as source images and were composed from four equal categories: neutral with people, neutral without people, negative with people, and negative without people. The 320 neutral foreground objects were taken from the Hemera objects collection, http://desktoppub.about.com/cs/stockphotovendors/gr/photoobjects1-2.htm, and appeared on a square yellow background.
Eight unique sets of pairings between foreground objects and background photographs were created pseudo randomly, avoiding pairings where a semantic relationship could easily be produced between the object and source image. The eight encoding lists were presented in a random order, counterbalanced across participants (Smith et al., 2006).
MRI scanning
Scanning was performed using a 3-Tesla Trio MRI scanner (Siemens Vision, Erlangen, Germany) with an 8 channel head coil. Stimuli were back-projected onto a screen at the rear of the magnet bore and viewed via an angled mirror attached to the head-coil. Responses were made using a custom-built button box which recorded these with accuracy of one millisecond.
Imaging parameters
T2-weighted echo planar images (EPI) with BOLD (blood-oxygen-level-dependent) contrast were acquired using a specialized sequence which minimized signal dropout in the medial temporal lobe (Deichmann et al., 2003). We used the following scanning parameters to achieve whole brain coverage: 33 oblique axial slices at a 20 degree tilt in the anterior-posterior axis, TR of 2 seconds, slice thickness of 2 millimetres (80% gap), TE of 30 milliseconds, in plane resolution was 3.5 x 3.5 millimetres. Data were collected in separate sessions (runs). Each session lasted nine minutes, giving 270 volumes. High resolution anatomical whole brain images were obtained using a T1 weighted 3D-gradient-echo pulse sequence, with the following parameters: (T1 190, TR 7.92, TE 2.48, FOV 224x256, matrix 256_256_256 pixels, flip angle 16) acquired in sagittal plane.
FMRI Processing
Functional MRI images were analysed using the statistical parametric mapping (SPM2) software package (Wellcome Trust Centre for Neuroimaging London, UK, http://www.fil.ion.ucl.ac.uk/spm). After the first three volumes of each session were discarded to allow for T1 equilibration effects, images were corrected for head motion by realigning with the first image of the first session and spatially normalised to an EPI template corresponding to the Montreal Neurological Institute (MNI) brain. Normalised images were smoothed using a Gaussian Kernel size with a full width at half-maximum (FWHM) of five millimetres.
FMRI analysis
To characterise functional responses, the data were examined using a two-level random-effects analysis. At the first level, the event-related design matrix contained separate regressors for hits, partially correct items (where the participant correctly recognized an old item but indicated the source incorrectly), and misses for each valence (neutral and negative sources) and for each task (EMOTION and PEOPLE). Correct rejections were also modelled separately for each task. To control for motion artefacts, six rigid body movement parameters were included as regressors of no interest. Parameter estimates reflecting the height of the hemodynamic response function for each regressor were calculated at each voxel. To assess functional responses, contrast images relating to various combinations of correctly classified items were calculated. These included a) direct comparison of source hits (SH) across valences (negative SH >neutral SH), b) negative source hits (neg SH), c) neutral source hits (neut SH), and d) correct rejections (CR). These four contrasts were computed separately for data from EMOTION, data from PEOPLE, and for data pooled across both tasks.
The resulting images were applied in two second-level, random effects ANOVA analyses. The first such analysis (contrast 1) examined the main impact of retention type (sleep vs. wake) upon recognition memory (RM). RM was calculated as RM = [all SH > all CR], and included both neutral and negative items. To determine the impact of retention type, RM was compared across Sleep and Wake groups. Specifically, contrast 1 = Sleep [RM < > Wake RM]. The subtraction of CRs provided two functions in this contrast. First, it controlled for circadian factors by removing activities associated with retrieval at a specific time of day, following the method of (Walker et al., 2005). Second, it allowed isolation of RM related responses. To constrain sleep-related findings to the episodic memory system, the results of this contrast were inclusively masked by the main effect of RM (all SH > all CR) in both Sleep and Wake groups, see Figure S1 and Table S1A of the supplementary material, at p=0.05 uncorrected. The second contrast (contrast 2) isolated brain responses specifically associated with overnight changes in processing negative emotional memories. This was achieved by calculating the interaction: [Sleep (negative SH > neutral SH) < > Wake (negative SH > neutral SH)] (contrast 2). Here, the subtraction of neutral from negative source hits controlled for circadian factors by removing activities associated with retrieval at a specific time of day and isolated effects associated with retrieval of negative emotional items rather than with retrieval in general. To constrain findings to areas associated with emotional processing, the results of this contrast were inclusively masked by the main effect of emotional memory (negative SH > neutral SH, see Figure S2 and Table S1B of the supplementary material) at p=0.05 uncorrected. To examine the influence of retrieval task, both contrasts were calculated first using data pooled across both tasks, next using data from EMOTION, and third using data from PEOPLE. Finally, task-specific effects were tested by comparing contrasts across tasks, e.g. using EMOTION (contrast 1 or 2) < > PEOPLE (contrast 1 or 2). Clusters of 10 or more voxels at p<0.001 were considered significant.
Connectivity Analysis
Functional connectivity was assessed using the psychophysiological interaction (PPI) analysis implemented in SPM2 (Wellcome Trust Centre for Neuroimaging London, UK, http://www.fil.ion.ucl.ac.uk/spm). This analysis evaluates how regional network activity covaries in relation to a source region during task performance (Gitelman et al., 2003;Friston et al., 1997). We used the PPI to determine whether functional connectivity between the parahippocampus (our seed region) and the bilateral amygdala was more strongly modulated by emotion in participants who slept during the retention interval than in participants who remained awake. To do this, we used SPM2 to extract the effects of interest time series for a peak voxel in the right parahippocampus (4 millimeter radius sphere centred on x/y/z/ 24, -16, -20, identified in contrast 2, Figure 4C), this was the physiological effect. Next, we calculated the product of this time course and the contrast (negative SH > neutral SH), (our psychological factor), to create the psychophysiological interaction (PPI) term. A first level design matrix was then constructed for each participant using three regressors: (i) the physiological factor, (ii) the psychological factor, and (iii) the interaction between the first and the second regressors (PPI). To control for motion artefacts, six rigid body movement parameters were included as regressors of no interest. Contrasts for the interaction term (PPI) revealed brain regions considered to covary with the parahippocampal seed region. These connectivity contrasts were then taken through to a second-level, random-effects analysis where we tested for differences between Sleep and Wake groups. Data were thresholded at P=0.001 uncorrected and clusters of 10 or more voxels (K=10) were considered significant. The PPI analysis was performed three times: using data pooled across tasks, using EMOTION task data, and using PEOPLE task data.
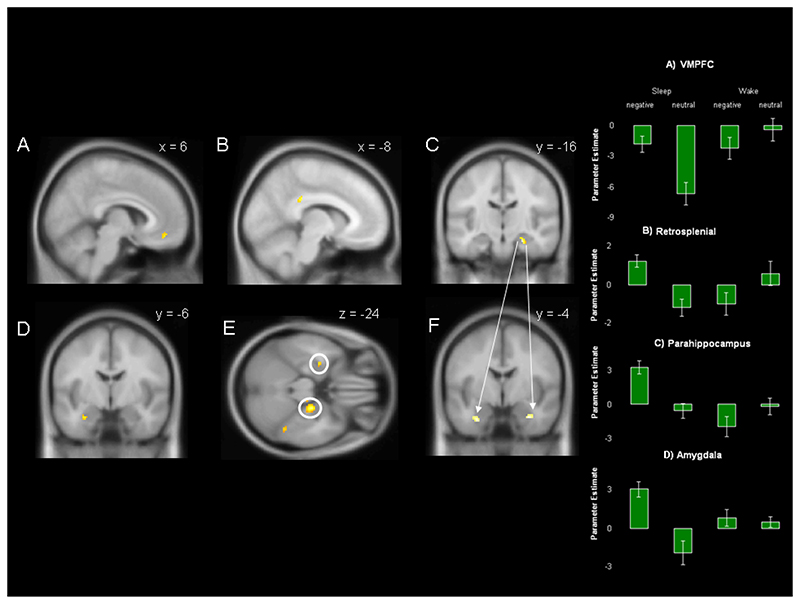
Figure 4. Interaction between sleep and valence.
The figure shows the results of contrast 2 [Sleep (negative SH > neutral SH) > Wake (negative SH > neutral SH)] calculated using data from the EMOTION task. Depicted responses fall in (A) ventromedial prefrontal cortex, (B) posterior cingulate, (C) right hemispheric parahippocampus, and (D) left hemispheric amygdala. (E) Shows an axial view of both parahippocampus and amygdala responses. Parameter estimates for each response are shown to the right, error bars show +-1 SEM. A psychophysiological interaction (PPI) analysis seeded at the peak of parahippocampal response at [24, -16, -20] reveals significantly enhanced functional connectivity between this region and the bilateral amygdala/periamygdala (F). Responses are overlaid on the MNI 152 brain at p<0.001 uncorrected and an extent threshold of K=10.
Results
Behaviour
Pooling data across negative and neutral items in both tasks, mean source memory accuracy was 77% correct (+/- SD 13%) at the immediate test, and 61% correct (+/- SD 16%) following the offline consolidation delay. This was significantly higher than chance (25%) (one-sample T-test P<0.001 in both cases). See Table 1 for details.
Table 1. Source Memory Performance.
This table lists the percentage of correct source memory responses at immediate and delayed test. The standard deviation for each mean percentage is shown in brackets. In the column heads, (-) indicates a negative source, (=) indicates a neutral source, and the final column (All) shows the mean across all 8 conditions.
| Emotion Task | People Task | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Sleep | Wake | Sleep | Wake | |||||
| Valence | - | = | - | = | - | = | - | = | |
| Immediate | 75 (13) | 78 (13) | 74 (14) | 77 (7) | 79 (14) | 80 (13) | 73 (10) | 78 (17) | 77 (13) |
| Delayed | 70 (11) | 64 (17) | 57 (20) | 52 (19) | 71(14) | 61(20) | 60 (13) | 52 (17) | 61 (16) |
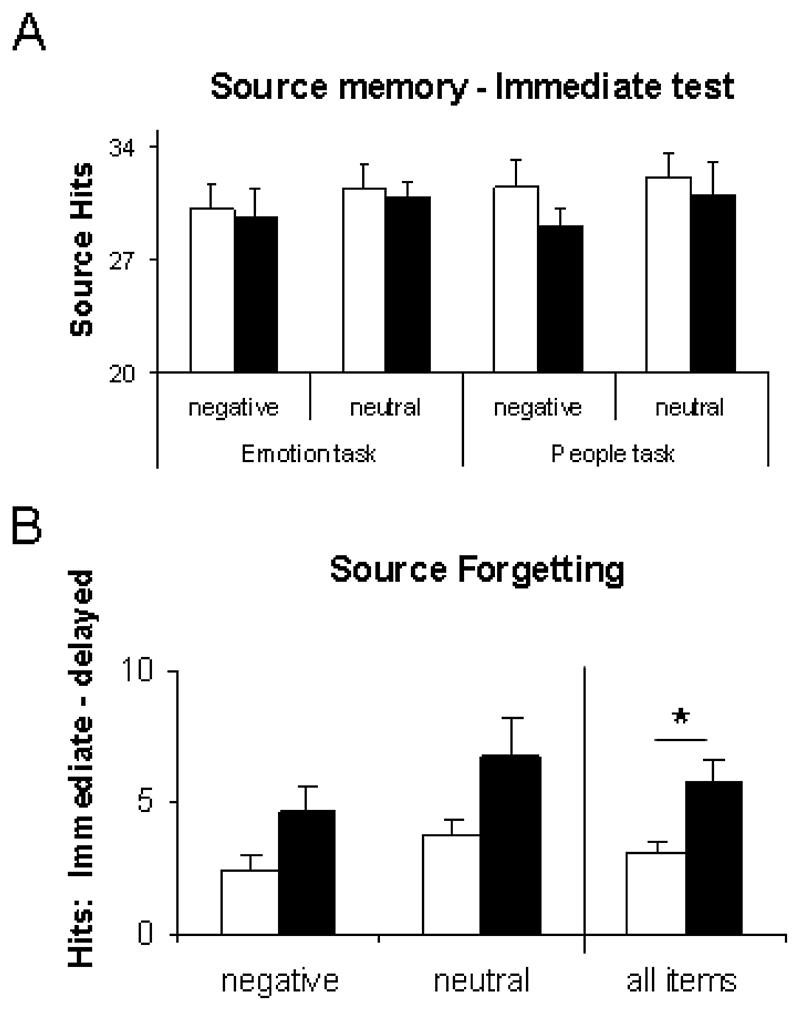
To determine whether performance was equivalent in Sleep and Wake groups prior to consolidation, source memory was examined at the immediate test using a 2x2x2 mixed ANOVA with task (EMOTION/PEOPLE) and source valence (negative/neutral) as the within participant factors, and with group (Sleep/Wake) as the between participant factor. This revealed no significant effects, demonstrating that Sleep and Wake groups performed at equivalent levels before the retention interval (Figure 2A), though there was a trend towards a main effect of valence, with negative sources remembered better than neutral sources (P=0.06). Because the Sleep group encoded in the evening, while the Wake group encoded in the morning, these data demonstrate that the time of day has no significant impact upon source memory responses.
Figure 2. Behavioural performance on the source memory task.
(A) The number of correctly attributed sources at the immediate test did not differ between groups (Sleep/Wake), encoding valences (negative/neutral), or tasks (EMOTION/PEOPLE). (B) Source forgetting, calculated as correct immediate source attributions minus correct delayed source attributions, differed between groups. Participants who slept during the retention interval correctly attributed more sources (main effect of retention type P=0.03). Furthermore, negative sources were more frequently remembered than neutral sources (main effect of valence P=0.02). Error bars show 1 SEM.
To examine how performance changed across the retention interval, the difference between immediate and delayed source memory scores was calculated for each participant, (source forgetting = immediate SH - delayed SH). The 2x2x2 ANOVA described above was then repeated with this measure as dependent variable. This revealed a main effect of valence with negative sources less likely to be forgotten (N=11; F=5.27; P=0.03), and a trend towards reduced forgetting in those who slept (P=0.1). Because there was no effect of retrieval task (N=11; F=0.10; P<0.75) this factor was collapsed in a subsequent ANOVA with the factors valence (negative/neutral) and group (Sleep/Wake). This revealed a main effect of group, with participants who slept during the retention interval forgetting less sources (N=11; F=4.88; P=0.03) than participants who remained awake (Figure 2B). There was also a main effect of valence, with negative sources less likely to be forgotten (N=11; F=5.87; P<0.02).
In summary, source memory performance was equivalent for Sleep and Wake groups immediately after encoding, indicating similar levels of memory at baseline and showing that performance was not influenced by the time of day of training or testing. At the subsequent delayed test however, participants who slept had forgotten less source information than participants who remained awake. There was no interaction between emotional valence and this sleep-related modulation of forgetting.
Functional imaging AST ACTI
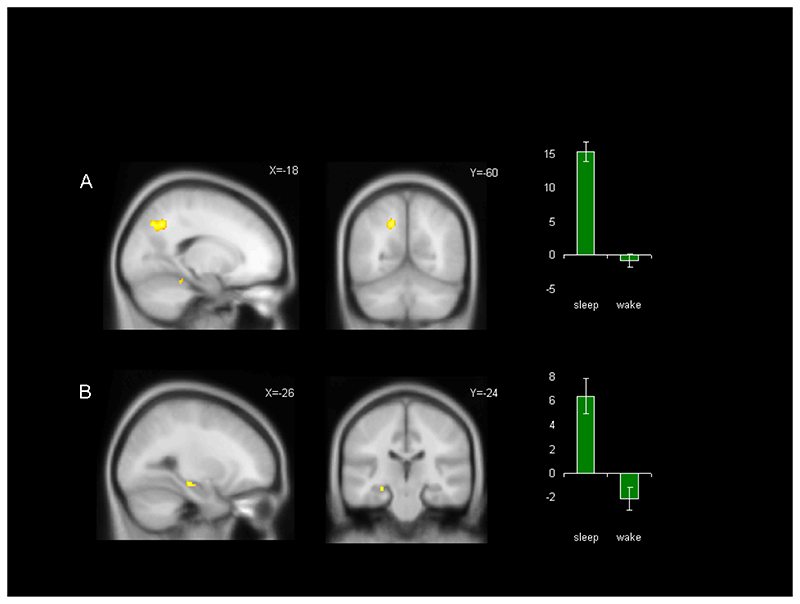
In the functional analysis, we first examined the main effect of retention type (sleep/wake) on correct source memory (contrast 1). Within the EMOTION task, this revealed a robust response in the left hemispheric superior parietal cortex, extending dorsally from the bank of intraparietal sulcus (K=183 voxels), and a more circumscribed response in the left hippocampus (K=33 voxels) Table 2A. No significant result was apparent when this contrast was performed on data from the PEOPLE task alone, however, when data were pooled across both tasks this contrast revealed responses in the same regions of left hemispheric superior parietal (K=154 voxels) and hippocampus (K=13 voxels), indicating that these structures also responded in the PEOPLE task, albeit with reduced significance Table 2B, Figure 3. This was confirmed when a direct comparison of results for the EMOTION task and the PEOPLE task revealed no significant difference. No area was more active in Wake than Sleep in any of the above contrasts.
Table 2. Memory responses modulated by sleep.
Results from contrast 1 (main effect of sleep inclusively masked by recognition memory) were calculated first with data from the EMOTION task only (A), and second with data from both tasks (B). Results from contrast 2 (interaction sleep x valence inclusively masked by emotional source memory) were calculated with data from the EMOTION task (C). Results from a psychophysiological interaction analysis (PPI) seeded at the peak response in C [24, -16, -20] are listed in (D). Clusters of 10 or more voxels were considered significant at P<0.001 uncorrected.
| A) EMOTION TASK: (Sleep RM > Wake RM) masked by RM for both groups | |||||
| # voxels | T | Z | probability | x,y,z {mm} | region |
| 61 | 6.6 | 4.7 | < 0.001 | -12 -32 26 | posterior cingulate |
| 183 | 5.5 | 4.3 | < 0.001 | -18 -60 40 | superior parietal |
| 10 | 5.5 | 4.2 | < 0.001 | 2 -32 -50 | medulla |
| 33 | 5.0 | 4.0 | < 0.001 | -26 -24 -12 | hippocampus |
| B) BOTH TASKS: (Sleep RM > Wake RM) masked by RM for both groups | |||||
| # voxels | T | Z | probability | x,y,z {mm} | |
| 54 | 6.7 | 4.8 | < 0.001 | -12 -34 26 | posterior cingulate |
| 154 | 5.3 | 4.2 | < 0.001 | -18 -60 40 | superior parietal |
| 13 | 4.9 | 4.0 | < 0.001 | -26 -24 -12 | hippocampus |
| C) EMOTION TASK: Sleep x Valence masked by valance in both groups | |||||
| # voxels | T | Z | probability | x,y,z {mm} | |
| 31 | 6.6 | 4.8 | < 0.001 | 24 -16 -20 | parahippocampus |
| 43 | 5.2 | 4.1 | < 0.001 | -8 -34 30 | posterior cingulate |
| 12 | 4.4 | 3.6 | < 0.001 | -30 -6 -24 | amygdala |
| 11 | 4.3 | 3.6 | < 0.001 | 6 44 -14 | ventromedial prefrontal |
| 10 | 3.7 | 3.2 | < 0.001 | 2 -40 38 | precuneus |
| D) BOTH TASKS: PPI seeded on 24 -16 -20, contrast: Sleep x Valence | |||||
| # voxels | T | Z | probability | x,y,z {mm} | |
| 22 | 4.4 | 3.6 | < 0.001 | -28 -6 -32 | amygdala/periamygdala |
| 15 | 4.3 | 3.6 | < 0.001 | 30 -4 -28 | amygdala/periamygdala |
| 12 | 4.1 | 3.4 | < 0.001 | -8 58 26 | dorsomedial prefrontal cortex |
Figure 3. Main effect of sleep on recognition memory.
The figure shows the results of contrast 1 [Sleep (all SH > all CR) > Wake (all SH > all CR)] calculated using data from both EMOTION and PEOPLE tasks. A) shows the superior parietal response while B) shows the left hippocampal response. Both activations are depicted in sagittal and coronal views, with parameter estimates for the peak voxel shown to the right, error bars are +-1 SEM. Responses are rendered onto the MNI 152 brain at p<0.001 uncorrected and an extent threshold of K=10.
The interaction between emotional valence and consolidation across sleep as compared to wake was examined next (contrast 2). Within the EMOTION this analysis revealed a robustly greater response by the Sleep group as compared to the Wake group in four distinct regions. The strongest response fell in right hemispheric anterior parahippocamps (K=31 voxels), however significant responses were also observed in left amygdala (K=12 voxels), ventromedial prefrontal cortex (K=11 voxels), posterior cingulate cortex (K=43 voxels), and precuneus (K=10 voxels), Figure 4, Table 2C. Neither data from PEOPLE nor data pooled across both tasks revealed significant responses; furthermore no region was more active in the Wake group than in the Sleep group.
A number of authors have predicted altered connectivity between the memory-related medial temporal lobe and the amygdala as a result of consolidation across a retention period including sleep (Wagner et al., 2006;Wagner et al., 2005a;Hu et al., 2006). To examine this possibility, we applied a psychophysiological interaction (PPI) analysis, which assesses whether the functional coupling of distant brain regions varies according to experimental parameters (Gitelman et al., 2003;Friston et al., 1997), (see methods). The PPI was seeded in a 4 mm diameter sphere centred on the peak of overnight enhancement in the right parahippocampus (24/-16/-20, Table 2C) and tested for voxels showing greater valence-specific differences in functional connectivity in the Sleep group than in the Wake group using the contrast (emotion SH >neutral SH). This analysis therefore examined connectivity by calculating the interaction Sleep x Valence (contrast 2).
The PPI was first performed using data pooled across both EMOTION and PEOPLE tasks. This revealed significantly greater functional connectivity between the right parahippocampal seed and both left (K=22 voxels, peak Z=3.6) and right (K=15 voxels, peak Z=3.6) amygdala/periamygdala in the Sleep group as compared to the Wake group, Figure 4E, Table 2D. For the purpose of precise localisation, the PPI response was overlaid on a probabilistic cytoarchitectonic maximum probability map (MPM) using the SPM Anatomy toolbox (Version 1.4, http://www.fz-juelich.de/ime/spm_anatomy_toolbox (Eickhoff et al., 2005). This localised the left hemispheric response to basolateral amygdala with a probablility of 60%, and the right hemispheric response to amygdala with a probability of 100%. Within EMOTION, the PPI analysis revealed no significant activation, although a noteworthy sub-threshold response was observed in right amygdala/periamygdala (K=5, Z=3.0) P=0.002 uncorrected, (MPM 70% probability of basolateral amydala) (Eickhoff et al., 2005). Neither analysis of PEOPLE nor direct comparison between results for EMOTION and PEOPLE revealed significant findings. These data demonstrate greater functional coupling between the parahippocampus and amygdala/periamygdala during successful emotional memory retrieval following overnight retention.
Overall our functional data show that sleep-related reduction in decay of source memory is paralleled by enhanced responses in hippocampus and superior parietal cortex. Additionally, these data reveal enhancements in functional responses across the episodic memory system and amygdala, as well as increases in connectivity between these, in association with the interaction between sleep and valence.
Discussion
We examined source memory retrieval after twelve hours of consolidation across either daytime wake or overnight sleep. Our findings extend prior reports by showing that source memory decayed less across sleep than wakefulness, and that this superior performance was linked to stronger responses in the left hemispheric hippocampus and superior parietal cortex. In addition, we show evidence of sleep-related alterations in the way task-relevant emotional information is manipulated after recall. Specifically, correct recall of emotional sources was associated with enhanced responding in the episodic memory system and left amygdala, as well as enhanced connectivity between these structures, in the emotion-relevant task. Our findings are discussed below.
Brain state during retention
We observed significantly less forgetting across a twelve hour retention interval containing a night of sleep than across an equivalent time period of daytime wakefulness (Figure 2 B). This finding reinforces a growing literature suggesting that aspects of declarative memory are preferentially maintained, or even strengthened, across a period of sleep (Born et al., 2006;Walker, 2009) for reviews. Some authors have speculated that elements of sleep physiology such as slow waves and sleep spindles may play an active role in consolidation (Born et al., 2006;Walker, 2009), while others maintain that reduced forgetting observed across sleep is due to the relative absence of interfering stimuli (Ellenbogen et al., 2006). Both possibilities are relevant to the current study, in which the Wake group spent the twelve hour retention interval going about their normal daily activities, while the Sleep group obtained an average of 7.7 hours of sleep. Although we cannot specifically determine the extent to which waking activities may have disrupted consolidation in our participants, previous work has addressed this in skill learning (Walker et al., 2002; Mednick et al., 2003;Huber et al., 2006) and integration of declarative information (Cai et al., 2009), describing deficits rather than enhancements in performance when daytime activities are reduced or completely blocked to mimic the behavioural quiescence of sleep.
In addition to differences in activity levels and amounts of sleep obtained during retention, the diurnal time of testing also differed between Sleep and Wake groups. Specifically, the Sleep group was tested and scanned at approximately 9 AM, while the Wake group was tested and scanned at approximately 9 PM. Could circadian variations in physiology and responsiveness explain our results? We examined this possibility by comparing performance on the immediate test (completed prior to consolidation) in AM (Wake group) and PM (Sleep group). Because the number of correctly attributed sources was equivalent at these two times of day before consolidation (Figure 2A), it is unlikely that the difference observed subsequently (after consolidation) could be due to mere circadian influences. With respect to the functional data, our methodology was specifically designed to control for circadian effects. To this end, our functional contrasts used a two tiered interaction in which items collected in the same experimental session were compared prior to comparisons between Sleep and Wake groups. These same-session comparisons ([source hits > correct rejections] for contrast 1 and [negative source hits > neutral source hits] for contrast 2) removed responses relating to time of day from the analysis. Furthermore, the interaction between functional enhancement and retrieval task, whereby a range of areas in the episodic memory system and amygdala were only activated in the Emotion task, argues strongly against a circadian explanation since diurnal effects would have to be task-specific in order to produce this result.
Main effect of sleep
The hippocampus and superior parietal cortex are core components of the episodic memory system and are both strongly associated with memory retrieval (Cabeza et al., 2008;Cabeza, 2008). The more robust functional responses we observed in these structures post-sleep, when participants also exhibited superior source memory performance compared to post-wake, are therefore very likely associated with altered processing in that system.
A number of studies demonstrate enhanced hippocampal responses during memory retrieval or performance of a learned skill after retention across sleep compared to retention across natural wake (Walker et al., 2005;Albouy et al., 2008) or overnight sleep deprivation (Sterpenich et al., 2007). Such effects may be attributable either to passive protection, whereby hippocampal representations do not decay as dramatically across sleep as they do across equivalent periods of wakefulness, or to active enhancement of such representations across sleep (Ellenbogen et al., 2006). Our current findings extend this literature to include source memory.
Unlike the hippocampal response, the stronger activation we observed in superior parietal cortex after sleep is a novel finding within the literature, and is likely to reflect a specific feature of source memory. Parietal cortex responds more strongly in source memory than in item-memory irrespective of whether information is correctly remembered (Dobbins et al., 2002;Dobbins et al., 2003;Dobbins and Wagner, 2005). These responses may therefore reflect attempts to remember instead of recollection itself. A recent model (Cabeza et al., 2008;Cabeza, 2008) proposes that the dorsal parietal cortex is responsible for top-down direction of attention for mnemonic search in difficult memory problems. This suggests a causal relationship wherein enhanced responses in this region after sleep lead to correspondingly superior memory, potentially as a result of the effortful retrieval of items which might otherwise have been forgotten.
Interaction between sleep and valence
Several studies suggest that sleep exerts a greater influence upon emotional than upon neutral memories (Wagner et al., 2005b;Wagner et al., 2006;Hu et al., 2006;Nishida et al., 2009;Payne et al., 2008;Sterpenich et al., 2007;Sterpenich et al., 2009). Although our behavioural data do not support this proposal, we observed a strong interaction between sleep and source valence at the level of brain response. Specifically, key regions of the episodic memory system responded more strongly during correct recollection of emotional items after sleep. These include the posterior cingulate and parahippocampus, both of which are associated with visual imagery and scene processing (Vann et al., 2009), and the ventromedial prefrontal cortex which is increasingly involved in episodic retrieval as consolidation progresses (Takashima et al., 2006;Takashima et al., 2009;Gais et al., 2007;Sterpenich et al., 2007). The juxtaposition of our null behavioural finding and strongly significant functional result can be interpreted in two different ways.
First, the functional interaction may represent a neural mechanism for the emotion-specific facilitation of retrieval observed by other authors after sleep (Wagner et al., 2005b;Wagner et al., 2006;Hu et al., 2006;Nishida et al., 2009;Payne et al., 2008). This interpretation is consistent with the widely held assumption that the measurement of neural responses is more sensitive than behavioural measures, and that the fMRI data therefore reveal effects which are not apparent from memory scores. Although plausible, this explanation is rendered unlikely by the absence of any trend towards reduced forgetting of emotional items after sleep (sleep x valence interaction P=0.9 in the EMOTION task). Furthermore, although interactions between amygdala and hippocampus are associated with superior encoding (Dolcos et al., 2004) it is less clear how such enhanced connectivity could facilitate retrieval. In fact, greater interaction between these structures at retrieval is more strongly linked to post-retrieval processing (Smith et al., 2006;Dolcos et al., 2005). This leads to a second, more conservative interpretation of our findings, namely that the observed functional interaction results from the manipulation of information once it has been retrieved. Thus, sleep-related enhancements in brain response are equivalent for both emotional and neutral source memory, but the way such information is processed after retrieval differs across valences. This is supported by the marked difference in results from Emotion-relevant and Emotion-irrelevant tasks, with the interaction between sleep and valence only apparent in the EMOTION task.
Studies of emotional memory (Dolcos et al., 2005;Smith et al., 2006) suggest a recursive relationship between amygdala and hippocampus during retrieval, whereby hippocampus-mediated retrieval enhances emotion-related responses in the amygdala, and these emotion-related responses in turn stimulate memory for further detail. Associative retrieval of this type may lead participants to remember additional details after making a correct source attribution. In our study, those who slept had stronger source memory than those who did not (Figure 2) and may therefore have been more likely to retrieve associated information. Furthermore, because the Emotion task required participants to indicate whether sources were negative or neutral, it explicitly encouraged retrieval of valenced information in negative sources. Such, recursive remembering is therefore consistent with the observed interaction between sleep and valence in terms of both localized increases in activity and enhanced connectivity.
Summary
We explored the consolidation of emotional and neutral source memories across a twelve hour interval containing either natural wake or wake plus a night of natural sleep. Source memory decayed more markedly across wake than across sleep, and correct source retrieval was associated with stronger responses in the left hippocampus and right superior parietal cortex after sleep. There was also an interaction between sleep and source valence, with the amygdala and areas of the episodic memory system responding more strongly during emotional memory after sleep when emotion was task relevant. These data indicate that, once it has been recalled, task-relevant information is processed in a distinct manner after consolidation across sleep.
Supplementary Material
Acknowledgement
This work was funded by the University of Liverpool and by a Wellcome Trust VIP Award to PAL. We are grateful to the staff at the Liverpool University MRI unit (MARIARC) for technical assistance and to Andrew Mayes, Matt Walker, Hugo Spiers, and Deborah Talmi for helpful discussions and critical reading of the manuscript.
References
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, ng-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, ng-Vu TT, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, Maquet P, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Sleep on it: cortical reorganization after-the-fact. Trends Neurosci. 2002;25:1–2. doi: 10.1016/s0166-2236(00)02005-1. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotionally arousing declarative memory. Psychological Science. 2006 doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del FG, Degueldre C, Meulemans T, Luxen A, et al. Experiencedependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del FG, Aerts J, Luxen A, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late notcurnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: an event-related FMRI investigation. Cereb Cortex. 2008;18:2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, ng-Vu TT, Desseilles M, D’Argembeau A, Gais S, Rauchs G, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, ng Vu TT, Desseilles M, Phillips C, Degueldre C, Balteau E, Collette F, et al. Sleep promotes the neural reorganization of remote emotional memory. J Neurosci. 2009;29:5143–5152. doi: 10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernandez G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Wagner U, Degirmenci M, Drosopoulos S, Perras B, Born J. Effects of cortisol suppression on sleep-associated consolidation of neutral and emotional memory. Biol Psychiatry. 2005a;58:885–893. doi: 10.1016/j.biopsych.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.