Abstract
Cognitive abilities such as Theory of Mind (ToM), and more generally mentalizing competences, are central to human sociality. Neuroimaging has associated these abilities with specific brain regions including temporo-parietal junction, superior temporal sulcus, frontal pole, and ventromedial prefrontal cortex. Previous studies have shown both that mentalizing competence, indexed as the ability to correctly understand others’ belief states, is associated with social network size and that social group size is correlated with frontal lobe volume across primate species (the social brain hypothesis). Given this, we predicted that both mentalizing competences and the number of social relationships a person can maintain simultaneously will be a function of grey matter volume in these regions associated with conventional theory of mind. We used voxel-based morphometry of Magnetic Resonance Images (MRIs) to test this hypothesis in humans. Specifically, we regressed individuals’ mentalizing competences and social network sizes against grey matter volume. This revealed that grey matter volume in bilateral posterior frontal pole and left temporoparietal junction and superior temporal sucus varies parametrically with mentalizing competence. Furthermore, grey matter volume in the medial orbitofrontal cortex and the ventral portion of medial frontal gyrus, varied parametrically with both mentalizing competence and social network size, demonstrating a shared neural basis for these very different facets of sociality. These findings provide the first fine-grained anatomical support for the social brain hypothesis. As such, they have important implications for our understanding of the constraints limiting social cognition and social network size in humans, as well as for our understanding of how such abilities evolved across primates.
Keywords: Intentionality, Social Brain, Theory of Mind, Voxel Based Morphometry, Ventromedial Prefrontal Cortex
Introduction
The ability to infer the mental states of other individuals, commonly known as Theory of Mind, is widely accepted as a crucial cognitive basis for sociality in humans (Frith and Frith, 2003; Leslie, 1987; Leslie, 1994; Wimmer and Perner, 1983). The neural basis of Theory of Mind (ToM) has received considerable attention in recent years and a number of core brain structures have been implicated in solving false belief tasks, the generally accepted benchmark for Theory of Mind (Wimmer and Perner, 1983). These include regions of the medial prefrontal cortex (mPFC) (Gallagher, 2003), temporo-parietal junction (TPJ) (Saxe and Kanwisher, 2003), superior temporal sulcus (STS) (Frith and Frith, 2003), and frontal pole (FP) (Decety and Lamm, 2007; Gallagher et al., 2000; Saxe, 2006; Saxe et al., 2004; Spreng et al., 2009). ToM is functionally equivalent to second order intentionality (“I believe that you suppose…”), and is acquired by the age of about 4-5 years (Astington, 1993; Leslie, 1987). As such, ToM poses no challenges for normal adult humans, who are usually able to cope with mentalizing tasks up to 4th or 5th order intentionality (Kinderman et al., 1998; Stiller and Dunbar, 2007), with only rare individuals performing well at 6th order. Performance at these higher orders of intentionality has recently been shown to predict prefrontal volume (Powell et al. 2010). However, this work used large regions of interest encompassing the entire dorsal and orbital prefrontal cortices and thus provides little information as to the specific subregions in which these quantitative differences in neurobiology may occur (Dunbar, 2009; 2011).
At the other end of the scale, the well-documented cross-species positive correlation between social group size and neocortex volume in primates, otherwise known as the social brain hypothesis (Dunbar, 1998; Dunbar and Shultz, 2007; Pérez-Barbería et al., 2007; Shultz and Dunbar, 2007; Shultz and Dunbar in press), implies that the social cognitive skills underpinning this relationship must have some kind of neurophysiological representation (Shultz and Dunbar, in press). To date, it has proved difficult to integrate the social brain hypothesis with any underlying cognitive and neurophysiological substrates, and this weakness has attracted some criticism (Healy and Rowe, 2007). Nonetheless, at least in humans, individual competences on high order intentionality tasks correlate positively with the size of personal social networks (Stiller and Dunbar, 2007), and there is strong circumstantial evidence to suggest that social cognitive competences might correlate positively with some aspects of brain size (notably frontal lobe volume) across primate species (Dunbar, 2003).
These observations raise two obvious questions. First, do those who can compute higher levels of intentionality have access to greater processing power in the brain networks required for mentalizing? In other words, is there a quantitative relationship between the level of intentionality at which a healthy individual can habitually work and the volume of neural matter in the classic Theory of Mind regions that the individual can bring to bear on the problem? Second, do either of these correlate with the size of the person’s social network (i.e. the number of individuals they list as personal friends)?
Here, we used voxel-based morphometry (VBM) of high resolution magnetic resonance imaging (MRI) brain scans to test these hypotheses. On the basis of several recent meta-analyses (Frith and Frith, 2003; Spreng et al., 2009; Van Overwalle, 2009), we identified four principal regions of the brain that are commonly associated with conventional Theory of Mind: mPFC, TPJ, STS, and FP. We first tested for a quantitative relationship between individuals’ social cognitive competences (indexed as the intentionality fail level) and the volume of grey matter in these four regions. We then tested for a relationship between the size of each individual’s personal social network and grey matter volume in these same regions. Finally, we tested for areas correlating with both high order intentionality abilities and social network size. We chose the fine-grained
analysis offered by VBM in order to determine whether or not those regions known to be involved in Theory of Mind (i.e. conventional second order intentionality) exhibit a parametric relationship with individual differences in mentalizing competences (higher order intentional competences) and/or network size. Such a relationship would imply that higher order intentionality is computationally more demanding, and that social competence is linked to neural recruitment within quite tightly defined brain regions. Since we aimed to test the role of information processing, rather than information exchange between processing units, we focus explicitly on grey matter volume rather than white matter volume.
Materials and Methods
We sampled 45 individuals, 26 females and 19 males aged between 18 and 50 years (mean 25.6 years for both females and males, SEM 2 and 1 years respectively). We used subjects who: (1) reported no history of neurological problems, (2) were native English speakers, and (3) had not previously been involved in research on Theory of Mind. All subjects gave written fully informed consent of their willingness to participate. The study was approved by the appropriate local ethics committee.
We determined competence on intentionality and memory tasks using a written questionnaire. This consisted of a series of five short stories, based on revised versions of those used by Stiller & Dunbar (Stiller and Dunbar, 2007), and identical to those used in Powell et. al. 2010 (Powell et al. 2010) (see supplementary materials) which were designed to test subjects’ ability to correctly infer the mind states, i.e. the beliefs, of the characters in the story. Each story was approximately 200 words in length and described a social interaction involving several individuals. Subjects answered 20 questions immediately after reading each story, 10 mentalizing questions varying from 2nd to 6th order intentionality and 10 factual (memory) questions varying from 2 to 6 facts. The original stories were revised to a standard length, composition, and number of questions at each complexity level. The subject’s own mind state was defined as first order intentionality, and the mind state of each protagonist from the story included in a question added successive levels of intentionality. A 6th order intentionality question thus involved tracking the mind states of five individuals in the story, as well as the subject’s own mind state. The number of words used and the number of people mentioned were balanced across intentionality and memory questions (t-test P>0.05 in both cases). Participants were asked to read the stories to themselves twice and then proceed to the questions which they answered by specifying T or F. Subjects were not cued as to which were mentalizing and which memory questions, and the two kinds of questions were randomly interspersed. Performance was assessed in an identical manner for both intentionality and memory for facts. In both cases, following (Stiller and Dunbar, 2007), we calculated the mean ‘fail point’ by using a re-scaled weighted mean of performance at the 5 levels of complexity examined (levels 2-6).
Personal social networks consist of a series of concentric layers that include progressively more individuals, with the size of the layers scaling with each other with a constant scaling ratio of about 3 (Hill & Dunbar 2003; Zhou et al. 2005; Hamilton et al. 2007). Because the layers scale so closely with each other, which layer is used as an index of an individual’s social engagement is largely a matter of convenience. For present purposes, we have focused on the second layer, sometimes known as the sympathy group (Lars & Buysen 1977) because it maximizes the range of variation across individuals while at the same time minimizing the time and effort required to complete the questionnaire, as well as reducing the risk of individuals being overlooked (Roberts et al. 2009). We derived information about social network size using a second written questionnaire completed immediately after the IMT (see online supplementary material). Here, following prior reports (Dunbar and Spoors, 1995; Stiller and Dunbar, 2007; Powell et al. 2010),
participants were asked to list the initials of every individual with whom they had had personal contact or communication over the previous 30 days. The instructions were: In the spaces below, please list the INITIALS of everyone with whom you had some kind of social contact (a) during the last 7 days and (b) during the rest of the last month (i.e. approx. 30 days). Contact means some form of interaction, including face-to-face, phone call, email or text-messaging, or a letter. Please DO NOT INCLUDE people whom you contacted for professional reasons (e.g. your doctor, lawyer, hairdresser, priest, employer or supervisor, plumber or DIY consultant etc) UNLESS you considered that interaction to have been of a mainly SOCIAL nature at the time. Please also indicate Males [M] and Females [F] after all initials. You can look at a list of names in your phone / address book if this helps.’
MRI data were acquired using a Siemans Trio 3.0 Tesla, whole body MRI system, with an eight channel head coil. High resolution (1 mm isotropic) anatomical whole brain images were obtained using a T1 weighted 3D-gradient-echo pulse sequence, with the following parameters (T1 190, TR 7.92, TE 2.48, FOV 224x256, matrix 256x256x256 pixels, flip angle 16) acquired in sagittal plane. After MR acquisition, datasets were imported into BrainVoyager for realignment. Pre-processing required reformatting the image, and orienting it to a standardized saggital plane orthogonal to the bicommissural plane.
A voxel-based morphometry protocol was performed on the anatomical scans using SPM2 (available at http://www.fil.ion.ucl.ac.uk/spm/). First, a customized whole-brain T1-weighted template image and prior probability maps for grey matter, white matter, and cerebrospinal fluid were created from the anatomical scans of our 45 participants. These customised images were used as priors during subsequent image segmentation. The original T1 scans were then normalised and segmented using the optimized VBM approach developed by Good et al. (Good et al., 2001; Good et al., 2002). Finally, the optimized parameters from the latter normalization step were applied to the original anatomical scans which were segmented to obtain grey matter, white matter, and cerebrospinal fluid images in MNI space. These segments were modulated, smoothed with an 8 mm Gaussian kernel, and used for the statistical analysis.
We constructed two separate design matrices. The first tested for correlations between grey matter volume and intentionality performance using intentionality fail point as the regressor of interest. Memory fail point, age, and sex were included as covariates of no interest. The second tested for correlations between grey matter volume and social network size using network size as the main regressor, with age and sex again included as covariates of no interest. The neural correlates for each regressor of interest were examined using a simple t-test, with positive and negative relationships examined independently. Because the widespread use of overly stringent corrections for multiple comparisons in neuroimaging has recently been criticised as a known source of Type II errors (false negatives) (Lieberman and Cunningham 2009) we used the more liberal threshold of p=0.001 uncorrected, with an extent threshold of k>5 voxels within our a priori regions of interest (the mPFC, TPJ, STS, and FP). For added statistical rigor, small volume corrections at p=0.05 were performed on uncorrected findings using 8 mm radius spheres centred on coordinates taken from (Spreng et al., 2009).
In order to isolate areas where grey matter volume correlated with both intentionality and social network size, we then performed a conjunction analysis using the masking function of SPM2. First, a one-sample t-test was computed for one of the contrasts of interest (i.e. the contrast for areas varying parametrically with intentionality fail point) and the suprathreshold voxels from this analysis were used to form a mask. A second one-sample t-test was then computed for the other contrast of interest (i.e. the contrast for areas varying parametrically with social network size) and the mask from the first analysis was applied such that the resulting conjunction revealed regions of conjoint significance. The individual one-sample t-tests were thresholded at p < 0.01, such that the conjoint probability of the conjunction analysis, estimated using Fisher's method (Fisher, 1950; Lazar et al., 2002) was p < 0.001. As above, statistical results in the mPFC, TPJ, STS, and FP were considered significant at p=0.001 uncorrected, with an extent threshold of k>5 voxels.
Results
The mean number of social contacts reported was 36.7 +/- SEM 2.7. As found by Stiller & Dunbar (2007), there was a significant Pearson correlation (r=0.254, N=45, p<0.05) between social network size and intentionality fail point. In order to control for the potential contribution of memory abilities, we also ran this analysis as a partial correlation with memory fail point partialed out. This showed that the relationship between network size and intentionality remained significant and was, if anything, slightly stronger (rp= 0.330, N=42, p=0.024 2-tailed) when memory was partialled out.
To identify brain areas where cortical volume correlates with individuals’ social cognitive competences, we conducted a whole brain voxel-based-morphometry (VBM) analysis by regressing grey matter volume against subjects’ individual fail-points, as calculated using data from all 50 Theory of Mind questions. To control for the contribution of memory to these results, our model included a measure of memory performance calculated in a parallel manner using the 50 memory questions.
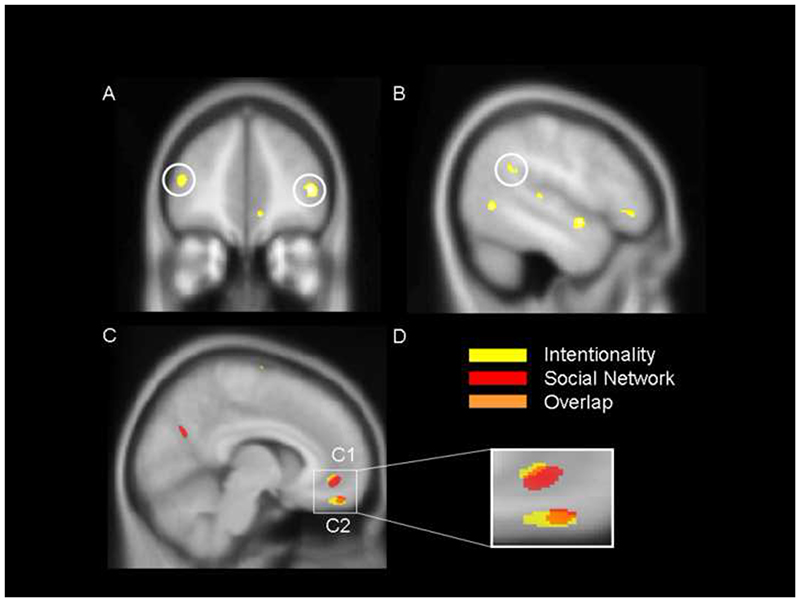
Our parametric regression analysis revealed that performance on high-level intentionality questions predicts grey matter volume in the left TPJ as well as in the posterior FP bilaterally (see Table 1A for a full list or areas & Figure 1 A&B for illustrations). The observation that individuals who can perform more complex intentionality calculations have more grey matter in these brain regions, which are known to activate during ToM tasks, suggests that grey matter volume may relate directly to processing power in this task. As a test of our a priori hypothesis that there would be an overlap between the areas in which grey matter volume correlated with intentionality ability and with social network size, we next performed a formal conjunction analysis (see Methods) which searched specifically for regions where grey matter volume correlated with both intentionality competence and social network size. This revealed a conjoint relationship of that description in two regions of the vMPFC: one in the medial orbitofrontal cortex and a more circumscribed area in the ventral medial frontal gyrus (figure 1C table 1C). Notably, the inverse contrasts, which tested for areas showing a negative correlation between grey matter volume and intentionality fail point, and between grey matter volume and social network size, revealed no significant findings at p=0.001 uncorrected.
Table 1.
Areas within the regions of interest in mPFC, TPJ, STS, and FP in which statistical findings survived thresholding at p=0.001 uncorrected with an extent threshold of k>5 voxels (A) when grey matter volume was correlated with intentionality competence and (B) when grey matter was correlated with social network size, (C) when grey matter was correlated with both intentionality and social network size (conjunction analysis). The number of surviving voxels, peak Z value, uncorrected probability level, and peak coordinates are shown for each region. Results surviving small volume correction at p=0.05 are indicated with an asterisk.
| # | maximum | uncorrected | ||
|---|---|---|---|---|
| Voxels | Z value | probability | x,y,z | Brain region |
| A) Intentionality | ||||
| 241 | 3.7 | <0.001 | 43 46 6 | frontal pole |
| 126 | 3.5 | <0.001 | -50 -1 -11 | superior temporal gyrus |
| 24 | 3.1 | 0.001 | -49 -63 1 | middle temporal gyrus |
| 6 | 3.1 | 0.001 | -48 -48 25 | supramarginalgyrus (TPJ)* |
| 16 | 3.1 | 0.001 | -43 42 12 | middle frontal gyrus |
| 8 | 3.0 | 0.001 | -52 -28 7 | frontal pole |
| B) Social network | ||||
| 11 | 3.3 | 0.001 | 10 51 25 | ventromedialfrontal gyrus* |
| C) Conjunction of Intentionality and Social Network Size | ||||
| 103 | - | 0.001 | 8 50 -23 | medial orbitofrontalgyrus |
| 14 | - | 0.001 | 8 44 -7 | ventromedialfrontal gyrus |
Figure.
There was a significant positive correlation between the volume of grey matter in the bilateral frontal pole (FP), an area commonly associated with Theory of Mind, and performance on high order intentionality tasks (A). A similar correlation was observed in the left temporo-parietal junction (TPJ) (B). These results are rendered on the MNI 152 brain at a visualisation threshold of p=0.005. In C) a conjunction analysis reveals regions of shared positive correlation with both intentionality performance and social network size in the (ventral) medial frontal gyrus (C1) and in the medial orbitofrontal cortex (C2). Here, correlations with intentionality are depicted in yellow, and correlations with social network size are depicted in red, both at p=0.01. Areas of overlap, depicted in orange, are significant at p=0.001 (Fisher, 1950; Lazar et al., 2002) and suggest a common neural underpinning for these two socio-cognitive abilities.
The observation that both subjects with the ability to process higher orders of intentionality and subjects having larger than average social groups, have greater grey matter volume in vMPFC substantiates the suggestion that these aspects of social cognition are closely related.
The social brain hypothesis holds that the primate brain has evolved to support the demands of increasingly large numbers of dynamically complex relationships in bonded social groups (Dunbar, 1998; Shultz and Dunbar, 2007). A simple interpretation of this hypothesis would predict that computational power in the same brain regions correlates both with intentionality processing ability and with social network size. Our current findings support this both behaviourally (by showing a significant positive relationship between intentionality fail level and network size) and neuroanatomically (by showing that grey matter volume in two areas of VMPFC predicts both measures: Fig. 1C and Table 1C).
Discussion
Our data support a previous finding that there is a significant correlation between the size of an individual’s social network and their level of social cognitive competence (indexed as their ability to correctly understand others’ belief states). We extend this by showing that these measures predict the grey matter volume of brain regions used in mentalizing and emotional processing. The quantitative relationship that we demonstrate between the levels of intentionality at which an individual can habitually work (an index of social cognitive competence) and the volume of neural material in the brain regions known to underpin Theory of Mind suggests that grey matter volume in the ToM network itself may serve as an index of processing power in these tasks. This is in line with a recent study which used the IMT task to show that ability on high order intentionality is predictive of gross volume in the orbital prefrontal cortex (Powell et al. 2010), as well as a clinical study (Herold et al., 2009) which demonstrated that performance on a faux pas test predicted grey matter deterioration in the frontal pole and orbitofrontal cortices of schizophrenic patients. Our data are more difficult to interpret when compared with findings from studies of autism, partly because these are somewhat inconsistent (Brieber et al., 2007; Girgis et al., 2007; Hardan et al., 2006; Ke et al., 2008), partly because some of them have used different anatomical measures, and also because the extent to which studies of such clinical cases can inform us about about quantitative individual differences among normal adults has yet to be determined.
Of the Theory of Mind regions in which we observed enhanced grey matter volume in people who exhibited a higher fail point on our intentionality task (the IMT), the left TPJ is the most strongly linked to ToM, see Van Overwalle, 2009 for a recent meta-analysis of over 200 neuroimaging studies of social cognition. Observation of greater grey matter volume in this region in high performers is in particularly good keeping with the literature because the IMT is a test of belief inference (see supplementary information for sample questions). Although left TPJ is not quite so frequently active during ToM processing as right TPJ, which is widely acknowledged as playing a key role in belief inference (Saxe et al., 2004), it is nevertheless active during identification of goals and intentions in a wide range of tasks including cartoons (Gallagher et al., 2000), videos (German et al., 2004) and text-based stories (Perner et al., 2006; Saxe, 2006; Saxe and Kanwisher, 2003; Saxe and Powell, 2006). Medial prefrontal cortex (mPFC) is also commonly activated during these tasks, but instead of belief inference, it appears to be involved in decoupling the perspectives of other people from one’s own (Frith and Frith, 2001; Gallagher, 2003). This distinction between TPJ and mPFC is illustrated by observation of stronger responses in the former, but not the latter, during inferred beliefs compared to true beliefs (Sommer et al., 2007). Furthermore, patients with lesions to the prefrontal cortex are unimpaired on simple false belief tasks, but markedly impaired on more difficult ones (Stuss et al., 2001). Achieving a high fail point on the IMT requires inference about multiple layers of belief. For instance, a typical True/False question in the task reads "Sam thought that Henry knew the Post Office was in Bold Street and hence that Henry must have intended to mislead Sam’. To answer correctly, participants have to infer the beliefs of several other individuals, while suppressing their own belief. We conjecture that the observed correlation between this task and mPFC grey matter volume may therefore relate to a superior ability to separate out these various layers.
In addition to findings in the TPJ and vMPFC, our data showed correlations between intentionality performance and grey matter volume in the frontal pole and STS, both areas that have been linked to calculation of what a person is likely to do or think (Gallager & Frith 2003). The frontal polar result is particularly interesting because it is in keeping with findings of (Herold et al., 2009) regarding grey matter deterioration and Theory of Mind impairment in schizophrenic patients. Prefrontal cortex is involved in planning for the future and in anticipating the future state of the world, including the state of the minds of others (Coltheart, 1989; Walter et al., 2004). The particular region of frontal pole in which we observed this correlation is also associated with prospective memory (Burgess et al., 2001). Within theory of mind tasks, this region is believed to be involved in prediction of thoughts and feelings (Frith and Frith, 2006; Walter et al., 2004), and has been shown to activate during prospective intentionality calculations (den Ouden et al., 2005).
Turning to the STS, the specific portion of this structure in which we observed a correlation between grey matter volume and intentionality falls towards the rear of the anterior temporal lobe. Because the anterior temporal lobe is critical for semantic memory (Patterson et al., 2007) as well as for Theory of Mind, some authors have suggested that it may be important for predicting what a person is likely to do or think (Frith and Frith, 2006). This area has recently been observed to activate during a task in which mental state is judged using the eyes only (Castelli et al.2010), and was also more active during the social emotion of embarrassment, in which one typically metalizes about the thoughts and reactions of others, than during the less social emotion of guilt (Takahashi et al., 2004). Our current data add to these findings by showing that greater grey matter volume in this area is associated with a superior score on our mentalizing task.
Moving to the results of our conjunction analysis, the correlation which we observed between mPFC grey matter volume and both IMT performance and social network size suggests that the cognitive abilities underpinning these two behavioural phenomena draw upon common computational resources. This in turn suggests that there may be a deep biological basis for individual differences in both social skills and sociality. Appropriately, this shared resource falls in a region which is known to be important for mentalizing about the emotional states of others. The orbitofrontal cortex plays a well established role in emotional processing, for recent reviews, see (Rempel-Clower, 2007; Rolls, 2008; Rudebeck et al., 2008; Szily and Keri, 2008), and is also important for various forms of empathy (Farrow et al., 2001; Moll et al., 2002; Shamay-Tsoory et al., 2009). Furthermore, both medial orbitofrontal cortex and medial prefrontal gyrus have been shown to respond selectively to mentalizing about emotional states (Vollm et al., 2006; Walter et al., 2004), a capacity which might reasonably be expected to impact upon one’s ability to maintain close friendships.
Taken together, these findings add weight to the social brain hypothesis which states that, in primates as a whole (i.e. including humans), the ability to handle social complexity (as reflected in social group size) is a function of neocortical volume and, in particular, of frontal neocortex volume (Dunbar, 2003; Dunbar, 2011). The current results present the strongest support for this hypothesis to date since, rather than looking at large scale cortical volume, we use the high spatial precision of Voxel Based Morphometry to show the precise cortical regions in which grey matter volume correlates with performance on a complex social task, while prior studies have examined gross anatomy of much larger brain regions, i.e. the entire dorsal and orbital prefrontal cortices (Powell et al. 2010). Furthermore, the current results show that these regions of increased volume overlap with the network of brain structures which are functionally associated with social network size. Importantly, these results also refine the claim of the social brain hypothesis (1) by demonstrating that this relationship is sufficiently fine-tuned to be evident at the within-species (i.e. between-individual) level and not just at the between-species level, and (2) by suggesting that the region most critical to this shared processing falls in the ventral portion of medial frontal cortex. While previous behavioural studies have shown that social cognition is especially taxing compared to conventional executive function cognition (Kinderman et al., 1998), the present findings provide the first neurophysiological evidence to support this claim. In this respect, we provide the first clear evidence as to why, in broader evolutionary terms, sociality has required significant investment in the neural matter, as implied by the social brain hypothesis.
From an evolutionary perspective, our demonstration that humans exhibit a quantitative relationship between grey matter volume in selected brain regions and social cognitive abilities suggests an underlying biological continuum which might reasonably be expected to reach back into the nonhuman primates, and perhaps to mammals more generally. Quite how this continuum might be instantiated in these species remains to be seen. We do not know enough about primate (or even mammalian) social cognition, let alone about the functional differences in neurophysiology between species, to do more than speculate at this stage (Dunbar, 2009). However, our findings should stimulate a search for correlations between these same regions and socio-cognitive skills in non-human primates in order to test this claim. Understanding how such relationships relate to cognition in general and the scale of our social world may go some way to allowing us to understand exactly why it is that humans differ from other species of animals (Dunbar, 2008; Dunbar, 2009).
Supplementary Material
Acknowledgments
This research was funded by the University of Liverpool, and MRI scans were paid for by a Wellcome VIP award to PAL. PAL is currently supported by the University of Manchester, and by a New Investigator award from the BBSRC. RIMD is supported by the University of Oxford and the British Academy Centenary Research Project and, at the time the experiments were carried out, by a British Academy Research Professorship. We are grateful to the staff at MARIARC for technical support, to Pia Rotstien for help with the analysis, and to Chris Frith, Sarah-Jayne Blakemore, Rebecca Elliott, and Roland Zahn for critical reading of the manuscript.
Contributor Information
Penelope A. Lewis, School of Psychological Sciences, University of Manchester, Manchester, Manchester, UK
Roozbeh Rezaie, University of Texas Health Science Center at Houston; Houston, Texas 77030, USA.
Rachel Browne, School of Biological Sciences, University of Liverpool, Liverpool L69 3BX, UK.
Neil Roberts, Division of Medical and Radiological Sciences, School of Clinical Sciences and Community Health, University of Edinburgh, Edinburgh, UK.
R.I.M. Dunbar, British Academy Centenary Research Project, Institute of Cognitive & Evolutionary Anthropology, University of Oxford, 64 Banbury Rd, Oxford OX2 6PN, UK
References
- Astington JW. The Child’s Discovery of the Mind. Cambridge University Press; Cambridge (MA): 1993. [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Fink GR, Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Castelli I, Baglio F, Blasi V, Alberoni M, Falini A, Liverta-Sempio O, Nemni R, Marchetti A. Effects of aging on mindreading ability through the eyes: an fMRI study. Neuropsychologia. 2010;48:2586–2594. doi: 10.1016/j.neuropsychologia.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Tim Shallice. xvi. Cambridge University Press; New York: 1989. From Neuropsychology to Mental Structure; p. 462. 1988 illus. $59.50; paper, $24.95. Science 246, 827-828. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. [Google Scholar]
- Dunbar RIM. In: Primate Life Histories and Socioecology. Kappeler P, Pereira M, editors. Chicago University Press; Chicago: 2003. Why are apes so smart; pp. 285–298. [Google Scholar]
- Dunbar RIM. Mind the gap: or why humans aren’t just great apes. Proc Brit Acad. 2008;154:403–423. [Google Scholar]
- Dunbar RIM. Darwin and the ghost of Phineas Gage: neuro-evolution and the social brain. Cortex. 2009;45:1119–1125. doi: 10.1016/j.cortex.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. In: Oxford Handbook of Social Neuroscience. Decety J, Cacioppo J, editors. Oxford University Press; Oxford: 2011. Evolutionary basis of the social brain. [Google Scholar]
- Dunbar RIM, Shultz S. Understanding primate brain evolution. Phil Trans R Soc Lond. 2007;362B:649–658. doi: 10.1098/rstb.2006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM, Spoors M. Social networks, support cliques and kinship. Human Nature. 1995;6:273–290. doi: 10.1007/BF02734142. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, Griffiths PD, Woodruff PW. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for research workers. Oliver and Boyd; London: 1950. [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001:151–155. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Phil Trans R Soc Lond B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- German TP, Niehaus JL, Roarty MP, Giesbrecht B, Miller MB. Neural correlates of detecting pretense: automatic engagement of the intentional stance under covert conditions. J Cogn Neurosci. 2004;16:1805–1817. doi: 10.1162/0898929042947892. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Minshew NJ, Melhem NM, Nutche JJ, Keshavan MS, Hardan AY. Volumetric alterations of the orbitofrontal cortex in autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:41–45. doi: 10.1016/j.pnpbp.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RS. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006;163:1290–1292. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SD, Rowe C. A critique of comparative studies of brain size. Proceedings of the Royal Society of London. 2007;274B:453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Herold R, Feldmann A, Simon M, Tenyi T, Kover F, Nagy F, Varga E, Fekete S. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatr Scand. 2009;119:199–208. doi: 10.1111/j.1600-0447.2008.01297.x. [DOI] [PubMed] [Google Scholar]
- Hill RA, Dunbar RIM. Social network size in humans. Human Nature. 2003;14(640):53–72. doi: 10.1007/s12110-003-1016-y. [DOI] [PubMed] [Google Scholar]
- Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, Zhou Z, Ruan Z, Lu Z, Tao G, Liu Y. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport. 2008;19:921–925. doi: 10.1097/WNR.0b013e328300edf3. [DOI] [PubMed] [Google Scholar]
- Kinderman P, Dunbar RIM, Bentall RP. Theory-of-mind deficits and causal attributions. British J Psych. 1998;89:191–204. [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Leslie AM. Pretence and representation in infancy: the origins of theory of mind. Psych Rev. 1987;94:84–106. [Google Scholar]
- Leslie AM. Pretending and believing: issues in the theory of ToM. Cognition. 1994;50:211–238. doi: 10.1016/0010-0277(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: rebalancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and nonmoral social judgments. Neuroimage. 2002;16:696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pérez-Barbería J, Shultz S, Dunbar RIM. Evidence for intense coevolution of sociality and brain size in three orders of mammals. Evolution. 2007;61:2811–2821. doi: 10.1111/j.1558-5646.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G. Thinking of mental and other representations: the roles of left and right temporo-parietal junction. Soc Neurosci. 2006;1:245–258. doi: 10.1080/17470910600989896. [DOI] [PubMed] [Google Scholar]
- Powell JL, Lewis PA, Dunbar RI, Garcia-Finana M, Roberts N. Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia. 2010;48:3554–3562. doi: 10.1016/j.neuropsychologia.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Ann N Y Acad Sci. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Roberts SBG, Dunbar RIM, Pollet T, Kuppens T. Exploring variations in active network size: constraints and ego characteristics. Social Networks. 2009;31:138–146. [Google Scholar]
- Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung. 2008;95:131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Saxe R. Why and how to study Theory of Mind with fMRI. Brain Res. 2006;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Ann Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporoparietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shultz S, Dunbar RIM. The evolution of the social brain: Anthropoid primates contrast with other vertebrates. Proc R Society Lond B. 2007;274B:2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Dunbar RIM. Encephalisation is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc Nat Acad Sci USA. doi: 10.1073/pnas.1005246107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Dohnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G. Neural correlates of true and false belief reasoning. Neuroimage. 2007;35:1378–1384. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative metaanalysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Stiller J, Dunbar RIM. Perspective-taking and memory capacity predict social network size. Social Networks. 2007;29:93–104. [Google Scholar]
- Stuss DT, Gallup GG, Jr, Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Szily E, Keri S. Emotion-related brain regions. Ideggyogy Sz. 2008;61:77–86. [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. J Cogn Neurosci. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Zhou W-X, Sornette D, Hill RA, Dunbar RIM. Discrete hierarchical organization of social group sizes. Proc R Soc Lond. 2005;272B:439–444. doi: 10.1098/rspb.2004.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.