Abstract
Background
Cellular insulin resistance is the hallmark of type 2 diabetes and predominantly affects adipose and muscle cells. The saturated free fatty acid palmitate is elevated in insulin-resistant states and may directly contribute to cellular insulin resistance. A spectrum of renal disease is associated with increased markers of insulin resistance, although direct causal mechanisms are not known. In the kidney, glomerular podocytes are novel insulin-sensitive cells that have the ability to rapidly transport glucose. In this study, we tested the hypothesis that palmitate would induce insulin resistance in podocytes.
Methods
Conditionally immortalized human podocytes were cultured for up to 24 h with 375–750 μM palmitate. Functional effects on glucose uptake and ceramide production were measured. Gene expression was investigated using a focused gene array, and protein signalling and trafficking were studied with Western blotting and immunofluorescence.
Results
We found that palmitate blocked insulinstimulated glucose uptake in human podocytes. This was associated with increased ceramide production, and use of the ceramide inhibitors myriocin and fumonisin B1 partially recovered the insulin sensitivity. At the level of transcription, palmitate downregulated genes associated with several pathways involved in insulin signalling. At the protein level, phosphorylation of the insulin receptor, IRS1 and PKB was reduced and there was impaired translocation of GLUT4 to the cell surface.
Conclusion
This is the first study to demonstrate a direct effect of saturated fatty acids on podocyte function. These findings may represent a novel link between systemic insulin resistance and the development of nephropathy.
Introduction
The glomerular filtration barrier comprises fenestrated endothelial cells, glomerular basement membrane and podocytes. At this interface, plasma filtration is tightly controlled to conserve macromolecules and allow the passage of small solutes and water. Disruption of the barrier results in loss of larger molecules, and if the disruption is persistent, there is associated glomerular damage and reduction in renal function. Diabetes mellitus is strongly associated with disruption of the glomerular filtration barrier, and type 2 diabetes is a leading cause of renal failure worldwide. The global incidence of type 2 diabetes is increasing dramatically in parallel with the increasing prevalence of obesity [1]. In addition to type 2 diabetes, insulin resistance is evident in non-diabetic, obese patients [2] who are prone to renal lesions such as focal segmental glomerulosclerosis (FSGS) [3]. Increased measures of insulin resistance have also been reported in patients with mild renal impairment and this has been termed the renal insulin resistance syndrome [4]. Furthermore, insulin resistance is increased in adults with active nephrotic syndrome [5].
Therefore, the relevance of insulin resistance applies across a wide spectrum of renal disease. However, the underlying links between insulin resistance and glomerular filtration barrier dysfunction are not yet known. Free fatty acids (FFA) are elevated in insulin-resistant states and are thought to play a critical role in the progression to type 2 diabetes. Palmitate is the predominant circulating saturated FFA. It is elevated in the insulin-resistant states [6] and known to induce insulin resistance in vitro [7]. Furthermore, high levels of palmitate correlate with increased ceramide production, and both endogenous and exogenous ceramide have been associated with the development of cellular insulin resistance [8,9]. There is evidence that the podocyte is a direct target in diabetes [10,11] with podocyte loss and widening of foot processes [12]. In addition, a reduction in expression of the key podocyte protein nephrin is described in diabetic patients [13]. We have recently shown that podocytes are insulin-sensitive cells [14] and are able to rapidly transport glucose via GLUT1 and GLUT4. We have also shown that this function is dependent upon the expression of nephrin in podocytes [15], thus conferring a unique specificity to the mechanism of insulin sensitivity in these cells.
In this study, we investigated the effect of the FFA palmitate on human podocytes by studying aspects of podocyte function, gene expression, protein signalling and trafficking.
Research design and methods
Cell culture plastics were from Greiner (Gloucestershire, England) and all reagents were from Sigma (Poole, Dorset, UK), unless otherwise stated.
Cell lines
Wild-type human podocytes [16] additionally transfected with a telomerase construct [17] were used. The cells differentiated for 14 days at the non-permissive temperature (37°C) in the RPMI-1640 medium with glutamine and glucose (11 mM) (R-8758; Sigma, St. Louis, MO, USA) that was supplemented with 10% FCS (Life Technologies, Grand Island, NY, USA) and insulin–transferrin–sodium selenite (Sigma I-1184; 1 ml/ 100 ml).
Palmitate stimulation of cells
Palmitate treatment was based on a published method [9]. Briefly, 20% fatty acid-free BSA was heated to 37°C before the addition of palmitate dissolved in ethanol. The solution was heated to 37°C until clear and diluted with RPMI to give a final concentration of 5% BSA, 750 μM palmitate and 1% ethanol. The solutions were filter sterilized before being added onto the cells. The control for palmitate was 5% BSA and 1% ethanol, and the dose of palmitate was based on previous in vitro studies [9,18]. This is two to three times higher than normal circulating levels of palmitate [19] and more representative of elevated NEFA levels seen in subjects with insulin resistance [20]. With ceramide inhibitors, dose ranges were based on previous studies [21–23], and podocytes were pre-treated with either 10 μM myriocin (a serine palmitoyl-transferase inhibitor) or 10 μM fumonisin B1 (a ceramide synthase inhibitor) for 30 min before being incubated with 750 μM palmitate and 10 μM myriocin or 10 μM fumonisin B1 for 24 h.
2 deoxy-glucose uptake
The cells in six-well plates were incubated for 24–48 h with control or treatment medium containing 10% FCS before being serum starved in the corresponding medium for 2 h. For the final 15 min, the cells were incubated in a pre-warmed Krebs–Ringer phosphate (KRP) buffer after washing three times in PBS. Glucose uptake assays were performed as previously described [14].
Cytotoxicity assays
Differentiated wild-type podocytes were treated with control or palmitate over a 24 h time course. The MTT reagent in PBS was added to the cells in a 12-well plate (final concentration of 0.5 mg/ml) and the cultures were incubated for 4 h at 37°C. The reaction was stopped by the addition of 100% DMSO, and the tetrazolium crystals were dissolved by mixing on a Titertek plate shaker for 20 min at room temperature. The samples were measured on a Biorad 450 plate reader at a test wavelength of 595 nm and a reference wavelength of 650 nm. The ATPLite assay (PerkinElmer, Boston, MA, USA) involved adding a 50 μl lysis solution to each well of a 96-well plate followed by a 50 μl substrate solution. The plates were dark-adapted, and luminescence was measured giving comparative adenosine triphosphate (ATP) levels. The Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, USA) involved incubating the cellular supernatant in the 96-well plates with the substrate mixture before reading in a colorimetric plate reader giving comparative LDH activity.
Ceramide production
Differentiated podocytes were stimulated for 24 h with control or medium containing either 375 μM or 750 μM and with the addition of 14C-radiolabelled palmitate. Lipid extraction was performed using a Folch extraction procedure [24], and lipid extracts were then separated by thin layer chromatography on silica plates. The plates were dried and the silica scraped from the Rf region 0.6–0.7 (which has been shown to contain D-e-C16:0/D-e-C18:0 ceramide phosphate and D-e-C16:0/D-e-C18:0 dihydroceramide phosphate using this solvent system [25]). The silica from each treatment was then thoroughly mixed with 5 ml of scintillation fluid before 14C counts were determined using a Beckman LS 6500 multipurpose scintillation counter. The final counts were corrected for the quantity of the total cellular protein in each sample.
Protein extraction and Western blotting
Wild-type podocytes were incubated with control or treatment medium, serum starved and stimulated for 5–15 min with insulin (100 nM) and glucose (25 μM) as required. The cells were washed twice with ice cold PBS and scraped into a NP40 extraction buffer [50 mM Tris/HCl, pH 7.5, containing 1 mM EDTA, 120 mM NaCl, 50 mM NaF, 1 mM benzamidine, 1% NP40, 1 μM microcystin, 7.2 mM 2-mercaptoethanol, 5 mM orthovanadate and 10 μl/ml protease and phosphatase inhibitor cocktails (Sigma)]. The cell extracts were centrifuged at 10000 g for 10 min at 4°C and following protein quantification, as determined by a bicinchoninic acidbased (BCA) assay (Pierce, Rockford, IL, USA), the supernatants were stored at –80°C. SDS–PAGE using 7.5% acrylamide gels was performed, and the protein was transferred onto polyvinylidene fluoride membranes (Millipore, MA, USA). The membranes were blocked with 5% BSA and incubated overnight with the following primary antibodies: PI3 kinase, Nck1 (Cell Signaling Technology, MA, USA), VEGF-A (R&D systems, Abingdon, UK), phospho-PKB serine 473 (Cell Signaling Technology, Danvers, MA, USA) and 4G10 (Upstate, Billerica, MA, USA). Secondary HRP antibodies (Amersham Biosciences, Little Chalfont, UK) were used and luminescence was created with Femto SuperSignal luminal (Pierce, Rockford, IL, USA) before imaging and analysis with a ChemiDoc-It imaging system (UV products, Upland, CA, USA).
Analysis of gene expression by focused gene array
Messenger RNA was extracted from differentiated podocytes treated for 24 h with control or palmitate using an Array Grade mRNA purification kit (SuperArray Inc., Bethesda, MD, USA). Gene expression was determined using the human insulin signalling pathway gene array kit (GEArray Q series, HS-030, SuperArray Inc.). Each array was a 3.8 cm × 4.8 cm nylon membrane spotted with cDNA of 96 marker genes in quadruplicate. In addition, there were four positive controls including glyceraldehyde-3- phosphate dehydrogenase (GAPDH) and a negative control. The protocol used 0.5 μg of mRNA as a template for synthesis of a biotinylated cDNA probe. mRNA was reverse transcribed using gene-specific primers, and the cDNA was amplified and labelled with biotin-16-deoxyuridine triphosphate using an AmpoLabelling-LPR kit (SuperArray Inc.). Biotinylated cDNA probes were denatured and hybridized to the prepared array membranes overnight. The membranes were then washed, blocked and then incubated with alkaline phosphate-conjugated streptavidin. Chemiluminescence was produced by incubating membranes with CDP-Star (SuperArray Inc.), and images were acquired and analysed using a ChemiDoc-It imaging system (UVP, Upland CA, USA).
Statistical analysis
For glucose uptake, ceramide production and densitometry with more than one group, one-way ANOVA was performed and the groups were compared with the post hoc Bonferroni multiple comparison test. For densitometry of two groups, an unpaired t-test was used. The GraphPad Prism 4 program was used for analysis. P-values <0.05 were deemed significant. The standard error of the mean is shown for all experiments.
Results
Palmitate blocked insulin-stimulated glucose uptake in podocytes
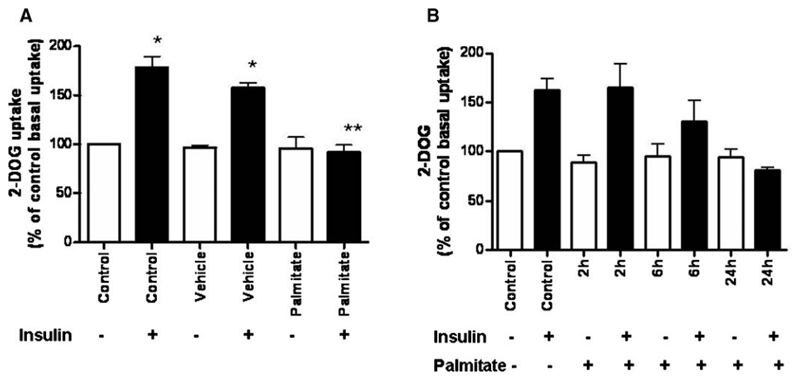
Conditionally immortalized human podocytes had significantly increased glucose uptake in response to insulin as demonstrated previously [14]. The same effect was observed with vehicle-treated cells. However, after 24 h of treatment with 750 μM palmitate, insulin-stimulated glucose uptake was blocked (Figure 1A). This finding demonstrates that palmitate has a direct effect on podocyte insulin responsiveness. The effect of palmitate on glucose uptake over a time course is shown in Figure 1B, which also shows a gradual reduction in insulin sensitivity with a block in insulin-stimulated glucose uptake at 24 h.
Fig. 1.
(A) 2-D0G uptake in differentiated, wild-type human podocytes. Cells were cultured in six-well plates supplemented with 5% BSA (vehicle) or 750 μM palmitate for 24 h followed by 2 h in the corresponding serum-free medium. 2-DOG uptake over 5 min was measured following stimulation with or without 100 nM insulin for 15 min. Control and vehicle-treated cells showed a significant increase in glucose uptake in response to insulin* and this effect was blocked when the cells were treated with palmitate** (one-way ANOVA with post hoc Bonferroni, p < 0.001). A time-course is shown in panel (B). Data are expressed as % of control basal uptake and the SEM is shown of n = 6 independent experiments each performed in triplicate.
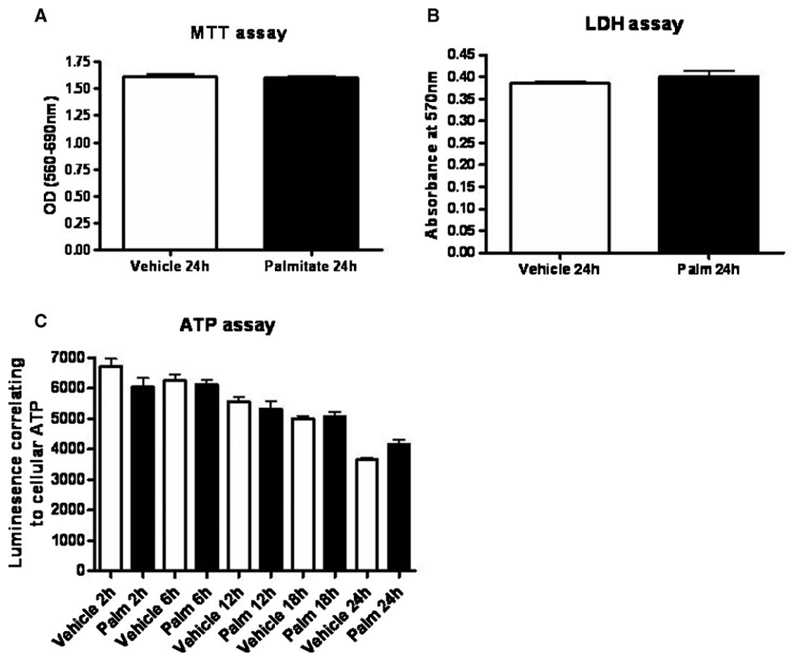
Palmitate did not increase cytotoxicity
To assess palmitate, cytotoxicity MTT, lactate dehydrogenase (LDH) and ATP assays were performed. At 24 h, there was no difference in cell viability between vehicle- and palmitate-treated cells using the MTT assay (Figure 2A). In addition, the LDH concentration in the supernatant was the same with vehicle and palmitate treatment (Figure 2B) indicating no enhanced cell death. Furthermore, ATP assays demonstrated no differences between vehicle and palmitate treatments (Figure 2C). There was a decline in comparative ATP levels over a 24 h time course in both groups that may have been due to the serum-free conditions used in this assay.
Fig. 2.
MTT, LDH and ATP assays were used to assess toxicity. Differentiated podocytes were cultured in 96-well plates supplemented with 5% BSA (vehicle) or 750 μM palmitate in the serum-free medium. Data are representative of n = 2 experiments each with eight repeats for each condition. (A) There was no difference in cell viability measured by the MTT assay. (B) There were no significant differences in LDH production between palmitate- and vehicle-treated cells. (C) There was a reduction in ATP over 24 h in both vehicle- and palmitate-treated cells. This may have been due to the length of time in serum-free conditions.
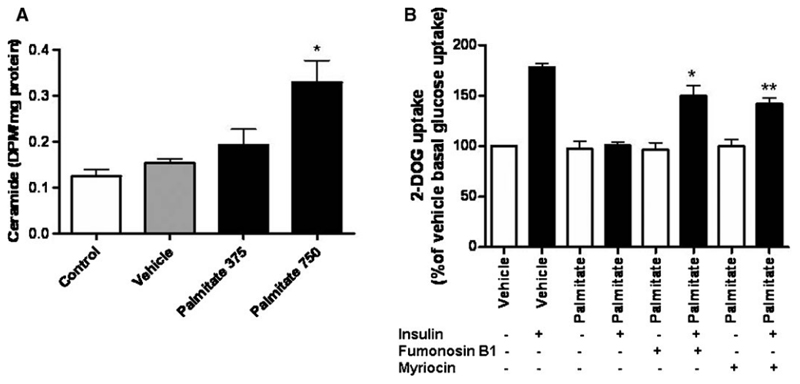
Palmitate-induced ceramide production in podocytes
There was a significant increase in the production of intracellular ceramide in podocytes following treatment with 750 μM palmitate for 24 h (P < 0.05) (Figure 3A), and this effect was dose dependent. Furthermore, the inhibitors of ceramide production, myriocin and fumonisin B1 resulted in a significant recovery of insulin-stimulated glucose uptake at 24 h in cells treated with palmitate (P < 0.001 and P < 0.01, respectively) (Figure 3B).
Fig. 3.
(A) Differentiated podocytes were cultured in the control medium or supplemented with 5% BSA (vehicle), 375 μM or 750 μM palmitate for 24 h. Ceramide production was determined by lipid extraction and thin layer chromatography. There was a significant increase in ceramide production in podocytes treated with 750 μM palmitate compared to control (one-way ANOVA, overall P = 0.0137, with post hoc Bonferroni, P < 0.05*). These data represent n = 2 repeats with each experiment performed in triplicate. (B) Differentiated podocytes were treated for 24 h with 5% BSA (vehicle), 750 μM palmitate or a combination of 750 μM palmitate with either fumonisin B1 or myriocin. 2-DOG uptake over 5 min was measured following stimulation with or without 100 nM insulin for 15 min. Both fumonisin B1 and myriocin significantly increased insulin-stimulated glucose uptake in palmitate-treated cells (one-way ANOVA, overall P < 0.0001 with post hoc Bonferroni, P < 0.001 with palmitate and fumonisin Bl* and P < 0.01 with palmitate and myriocin**). Data are expressed as % of vehicle basal uptake and the SEM is shown of n = 3 independent experiments each performed in triplicate.
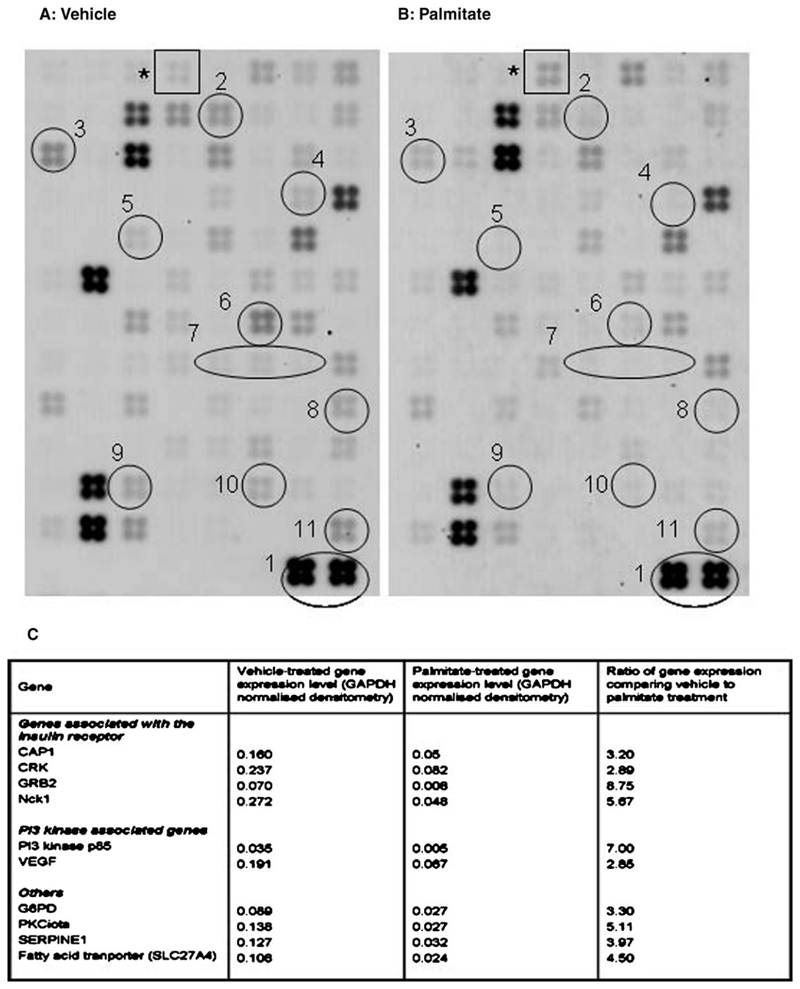
Palmitate reduced the expression of genes associated with insulin sensitivity
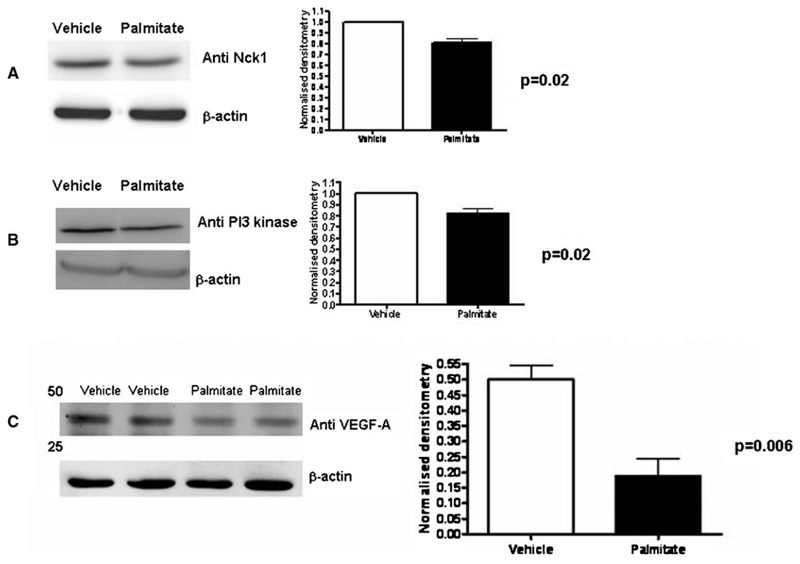
Using a focussed gene array, messenger RNA analysis demonstrated broadly similar patterns of gene expression with vehicle and palmitate treatment (Figure 4), and this reproducibility has also been seen in four further insulinsignalling arrays performed from podocytes (data not shown). The analysis quantified the difference between vehicle and palmitate treatment. Palmitate reduced the expression of genes associated with insulin sensitivity including those associated with insulin receptor signalling (CAP1, CRK). These molecules have been described in an alternative insulin-signalling pathway leading to translocation of GLUT4 [26]. The adaptor protein Nck1 was also downregulated with palmitate and this molecule has recently been described in the context of nephrin and actin interactions [27]. This finding was also validated by Western blotting (Figure 5A). Two targets of PI3 kinase signalling showed reduced expression, namely SERPINE1 (also known as plasminogen activator inhibitor-1 or PAI-1) and vascular endothelial growth factor A (VEGF-A). The downregulation of both PI3 kinase and VEGF-A gene expression was further validated by Western blotting (Figure 5B and C). The fatty acid transporter SL27A4 was reduced by palmitate, and this may represent a negative feedback mechanism. Beta-3-adrenergic receptor (ADRB3) was the only gene upregulated >2 fold by palmitate, and this gene has been associated with obesity [28] and type 2 diabetes [29]. These findings indicate that palmitate treatment interferes at the RNA level with a wide range of molecules involved in insulin signalling.
Fig. 4.
Focused gene array images obtained from mRNA extracted from podocytes treated with 5% BSA (vehicle) (A) or 750 μM palmitate (B) for 24 h. Each square contains four replicates of 96 genes associated with insulin signalling. The overall pattern of expressed mRNA is similar. Densitometry of each square was normalized to the control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1). Palmitate treatment resulted in a 2- to 8-fold reduction in expression of 10 genes associated with insulin signalling. These include CAPI (2), CRK (3), G6PD (4), GRB2 (5), Nckl (6), PI3 Kinase (7), PRKCI (8), SERPINE 1 (9), VEGF (10) and the fatty acid transporter SL27A4(ll). ADRB3 (*) was upregulated by palmitate. A comparison of relative expression is shown in (C).
Fig. 5.
Differentiated podocytes were treated with 5% BSA (vehicle) or 750 μM palmitate for 24 h. Expression of Nck-1 (A), PI3-kinase (B) and VEGF-A (C) was significantly reduced, validating the gene expression data. The blots are representative images, and normalized densitometry was calculated from n = 3 independent experiments and the SEM is shown.
Palmitate reduced activation of insulin signalling molecules
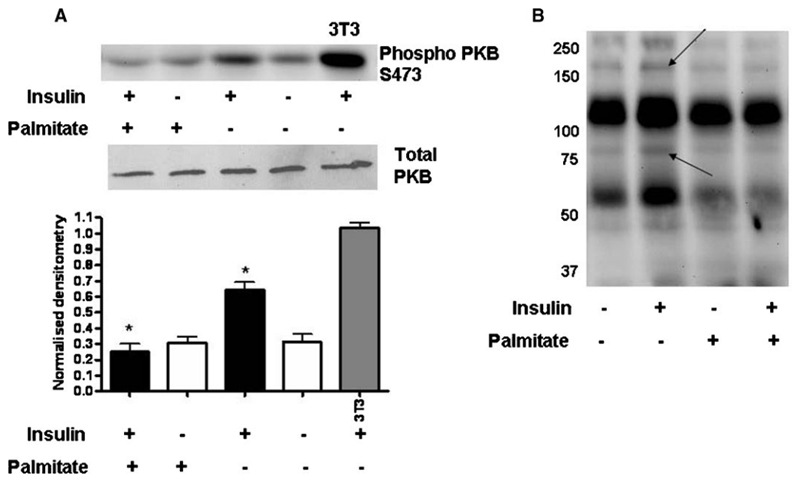
There was a significant reduction in insulin-stimulated phosphorylation of PKB at the serine 473 (S473) phoshorylation site following palmitate treatment (Figure 6A). Similarly, there was a reduction in tyrosine phosphorylation following insulin stimulation in cells treated with palmitate. In particular, this was evident at the molecular weight of the insulin receptor and insulin receptor substrate 1 (IRS1) suggesting a proximal interruption of insulin signalling in cells treated with palmitate.
Fig. 6.
Differentiated podocytes were treated with 5% BSA (vehicle) or 750 μM palmitate for 24 h followed by 2 h in the corresponding serum-free medium. Cells were then stimulated with or without 100 nM insulin and 11 mM glucose for 5 min. Cells were lysed and prepared for Western blotting. (A) Phosphorylation of PKB at serine 473 (S473) increased 2-fold in response to insulin in vehicle-treated podocytes, and this effect was significantly reduced in palmitate-treated cells (one-way ANOVA, overall P < 0.0001 with post hoc Bonferroni, P < 0.001 between vehicle with insulin and palmitate with insulin*). This is a representative blot for n = 6 independent experiments and the densitometry SEM is shown. (B) Tyrosine phosphorylation was upregulated in vehicle compared to palmitate-treated cells, in particular at the level of IRS 1 (180 kD) and the insulin receptor (90 kD) as indicated by arrows.
Palmitate impairs GLUT4 translocation in podocytes
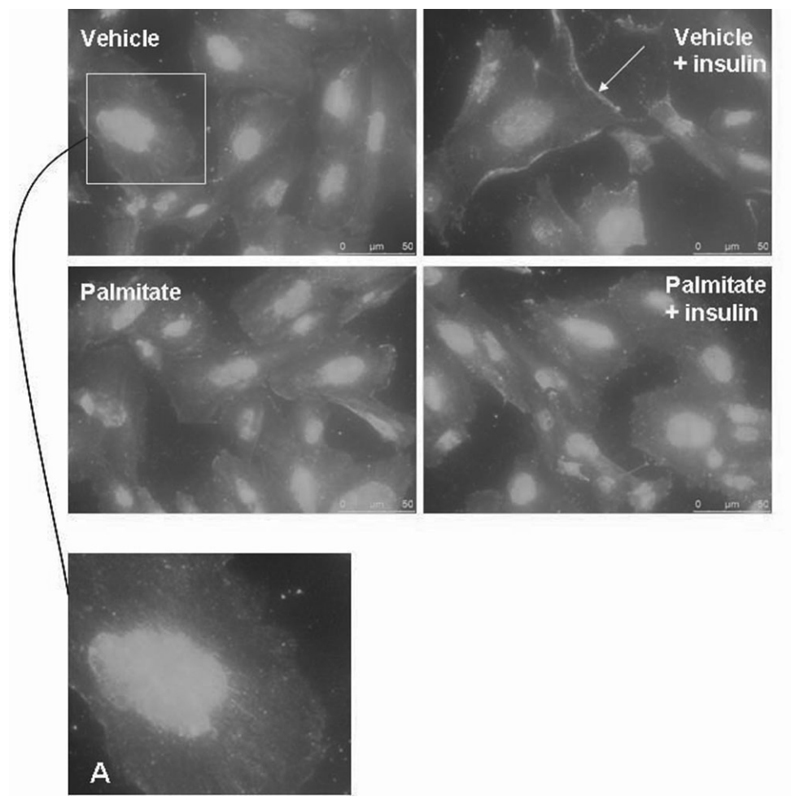
Vehicle-treated cells in the basal state showed cytoplasmic GLUT4 (Figure 7A). Following 15 min of stimulation with insulin and glucose (11 mM), the vehicletreated cells demonstrated increased GLUT4 translocation with a greater distribution at the cell periphery (Figure 7). Palmitate-treated cells in the basal state also demonstrated cytoplasmic GLUT4 (Figure 7). Notably, in the palmitate-treated cells after insulin and glucose stimulation, GLUT4 translocation was less marked at the cell periphery. These findings suggest that the pathway leading to GLUT4 translocation in podocytes is impaired when cells are treated with palmitate.
Fig. 7.
Differentiated podocytes were treated with 5% BSA (vehicle) or 750 μM palmitate for 24 h, followed by 2 h in the corresponding serum-free medium before stimulation with or without 100 nM insulin and 11 mM glucose for 15-30 min. Vehicle cells in the basal state demonstrated cytoplasmic GLUT4 (A). Upon insulin stimulation, GLUT4 became more peripheral and increased at the leading edges of cells (arrow). Palmitate-treated cells in basal conditions also demonstrated diffuse cytoplasmic GLUT4; however, following insulin stimulation, there was minimal GLUT4 relocalization.
Discussion
The role that podocytes play in maintaining the integrity of the glomerular filtration barrier was highlighted by the discovery of the podocyte protein nephrin in 1998. Mutations of NPHS1 encoding nephrin result in congenital nephrotic syndrome of the Finnish type [30]. Since then, a number of mutated genes have been found in human nephrotic syndromes or animal models, and these discoveries have given significant insights into disease mechanisms [31]. We recently reported the novel finding that human podocytes are insulin-sensitive cells [14] and this function depends upon nephrin expression [15]. The development of insulin resistance in podocytes would therefore represent a direct link between insulin action and the slit diaphragm. Further study of the factors that control insulin sensitivity in podocytes would lead to a better understanding of podocyte dysfunction in insulin-resistant states.
Insulin resistance is associated with renal disease and also with significant changes in glucose and lipid homeostasis. Although one link between circulating adipokines and the development of cellular insulin resistance has been proposed [32], the direct links between insulin resistance and nephropathy are unknown. Palmitate is the predominant circulating and are strongly implicated in the causation of insulin resistance [33]. In skeletal muscles, palmitate has been shown to decrease insulin signalling [34] through the generation of ceramide. Our study demonstrates a novel functional effect of palmitate on podocytes. Insulin-stimulated glucose uptake was abolished in podocytes treated with palmitate for 24 h, and using similar doses of palmitate, other studies have reported a reduction in insulin-stimulated glucose uptake in insulin-sensitive cells [7,35,36].
We went on to confirm that palmitate was associated with a dose-dependent increase in ceramide production in podocytes, and using the ceramide inhibitors myriocin and fumonisin B1, it was possible to partially recover insulinstimulated glucose uptake. The association between ceramide and insulin resistance has been reported previously in insulin-sensitive cells [18,21,23], and in keeping with these studies, we found that palmitate reduced the phosphorylation of PKB at serine 473, which is a key signalling step in the cellular insulin response. Furthermore, Fabry disease that results in cellular accumulation of the glycolipid ceramide trihexoside, leads to glomerular proteinuria renal failure with podocyte pathology [37]. Our study has demonstrated that ceramide accumulation in podocytes is associated with insulin resistance, and this may also be relevant in the development of proteinuria in Fabry disease.
Palmitate treatment also reduced the tyrosine phosphorylation of proteins at the level of the insulin receptor and IRS1. This suggests a proximal interruption of insulin signalling. Indeed, reduced tyrosine phosphorylation of both insulin receptor and IRS proteins has been associated with the development of insulin resistance [38] using in vitro and in vivo models. In our study, we also demonstrated altered translocation of GLUT4 in palmitate-treated cells, representing interruption in the distal insulin-signalling pathway. However, it is unclear from our study whether the insulin-signalling pathway is interrupted by one or several mechanisms at different levels, and this will be important to clarify in subsequent studies. In addition, we have not assessed the mechanism of action of palmitate and although we have used albumin to transport palmitate across the plasma membrane, we have not studied the effect of palmitate on plasma membrane proteins.
Analysis of gene expression demonstrated that genes associated with human insulin signalling were significantly reduced with palmitate treatment. These involved a variety of genes associated with insulin receptor signalling, the alternative and PI-3 kinase insulin signalling pathways. The downregulation of VEGF-A gene expression was further validated at the protein level where downregulation was evident and significant at the molecular weight corresponding to the VEGF-A145 isoform. The gene and protein expression of the adaptor protein Nck1 was also downregulated by palmitate, and the importance of this molecule in podocyte cell biology has been shown by a series of recent studies that demonstrate that the Nck adaptor proteins link the podocyte protein nephrin to the actin cytoskeleton [27,39,40]. Our own work demonstrated that nephrin is required for podocyte insulin sensitivity [15], and perhaps the interaction between nephrin and Nck is necessary for podocyte insulin responsiveness. We speculate that this may occur by impairing actin reorganization needed forsaturated FFA, and elevated plasma FFAs have been identified in both adult and childhood obesity recycling of glucose transporters to the plasma membrane. Overall, gene expression data suggest that palmitate affects podocyte gene expression across several pathways involved in insulin signalling, although a full investigation of the effect of these changes on protein translation needs to be undertaken.
The spectrum of renal disease associated with insulin resistance is broad and ranges from nephrotic syndrome [5], mild renal dysfunction [4] and FSGS in obesity [3] to diabetic nephropathy. Palmitate-induced insulin resistance in podocytes may be a novel link between insulin resistance and nephropathy. Indeed, if podocytes require optimal insulin sensitivity for normal function, insulin-sensitizing agents such as PPARγ agonists may have direct beneficial effects on podocytes. These agents have already been shown to reduce albuminuria [41] and podocyte loss [42] in patients with type 2 diabetes. Therefore, there may well be a role for these insulin-sensitizing agents across a wider spectrum of renal disease.
Palmitate is just one of many molecules that correlate with insulin resistance, and this study indicates a direct relationship between high levels of palmitate and podocyte dysfunction. Further study of other factors associated with insulin resistance and their role in podocyte injury is required to identify pathways that could potentially be targeted to reduce disease progression in proteinuric states.
Acknowledgements
R.L. was funded by the Wellcome Trust for this work. R.J.C. is funded by the Medical Research Council. We also acknowledge the Medical Research Council for providing an Infrastructure Award and Joint Research Equipment Initiative Grant to establish the School of Medical Sciences Cell Imaging Facility that was used for cell imaging. Finally, we thank North Bristol NHS Trust for funding that supported this work.
References
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Mohn A, Marcovecchio M, Chiarelli F. Validity of HOMA-IR as index of insulin resistance in obesity. J Pediatr. 2006;148:565–566. doi: 10.1016/j.jpeds.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 5.Dogra GK, Herrmann S, Irish AB, et al. Insulin resistance, dyslipidaemia, inflammation and endothelial function in nephrotic syndrome. Nephrol Dial Transplant. 2002;17:2220–2225. doi: 10.1093/ndt/17.12.2220. [DOI] [PubMed] [Google Scholar]
- 6.Groop L, Ekstrand A, Forsblom C, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (noninsulin-dependent) diabetes mellitus. Diabetologia. 1993;36:642–647. doi: 10.1007/BF00404074. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos N, Watson M, Sakamoto K, et al. Differential effects of palmitate on insulin action and glucose utilization in rat L6 skeletal muscle. Biochem J. 2006;399:473–48. doi: 10.1042/BJ20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jebailey L, Wanono O, Niu W, et al. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent rac-activation and actin remodeling in muscle cells. Diabetes. 2007;56:394–403. doi: 10.2337/db06-0823. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 10.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 11.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 12.White KE, Bilous ROB. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440. doi: 10.1093/ndt/gfh129. [DOI] [PubMed] [Google Scholar]
- 13.Patari A, Forsblom C, Havana M, et al. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes. 2003;52:2969–2974. doi: 10.2337/diabetes.52.12.2969. [DOI] [PubMed] [Google Scholar]
- 14.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 15.Coward RJ, Welsh GI, Koziell A, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 16.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 17.O’Hare MJ, Bond J, Clarke C, et al. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci USA. 2001;98:646–65. doi: 10.1073/pnas.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabin MA, Stewart CE, Crowne EC, et al. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol. 2007;211:244–252. doi: 10.1002/jcp.20922. [DOI] [PubMed] [Google Scholar]
- 19.Sabin MA, De Hora M, Holly JM, et al. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics. 2007;120:e1426–e1433. doi: 10.1542/peds.2007-0189. [DOI] [PubMed] [Google Scholar]
- 20.Mook S, Halkes CJM, Bilecan S, et al. In vivo regulation of plasma-free fatty acids in insulin resistance. Metabolism. 2004;53:1197–1201. doi: 10.1016/j.metabol.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 22.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 23.Powell DJ, Turban S, Gray A, et al. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitateinduced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal Biochem. 2001;298:141–150. doi: 10.1006/abio.2001.5342. [DOI] [PubMed] [Google Scholar]
- 26.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones N, Blasutig IM, Eremina V, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 28.Marvelle AF, Lange LA, Qin L, et al. Association of FTO with obesityrelated traits in the Cebu Longitudinal Health and Nutritional Survey (CLHNS) cohort. Diabetes. 2008;57:1987–1991. doi: 10.2337/db07-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch CP, Hegele RA. Genetic determinants of type 2 diabetes mellitus. Clin Genet. 2001;60:243–254. doi: 10.1034/j.1399-0004.2001.600401.x. [DOI] [PubMed] [Google Scholar]
- 30.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 31.Patari-Sampo A, Ihalmo P, Holthofer H. Molecular basis of the glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med. 2006;38:483–492. doi: 10.1080/07853890600978149. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 33.Javor ED, Moran SA, Young JR, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89:3199–3207. doi: 10.1210/jc.2003-032140. [DOI] [PubMed] [Google Scholar]
- 34.Storz P, Doppler H, Wernig A, et al. Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem. 1999;266:17–25. doi: 10.1046/j.1432-1327.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 35.Van Epps-Fung M, Williford J, Wells A, et al. Fatty acid-induced insulin resistance in adipocytes. Endocrinology. 1997;138:4338–4345. doi: 10.1210/endo.138.10.5458. [DOI] [PubMed] [Google Scholar]
- 36.Hunnicutt JW, Hardy RW, Williford J, et al. Saturated fatty acidinduced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 37.Torra R. Renal manifestations in Fabry disease and therapeutic options. Kidney Int Suppl. 2008;111:s29–s32. doi: 10.1038/ki.2008.522. [DOI] [PubMed] [Google Scholar]
- 38.Jiang G, Zhang BB. Modulation of insulin signalling by insulin sensitizers. Biochem Soc Trans. 2005;33:358–361. doi: 10.1042/BST0330358. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Zhu J, Aoudjit L, et al. Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem Biophys Res Commun. 2006;349:310–316. doi: 10.1016/j.bbrc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 40.Verma R, Kovari I, Soofi A, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24:2047–2055. doi: 10.1097/01.hjh.0000244955.39491.88. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Ushiyama C, Osada S, et al. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001;50:1193–1196. doi: 10.1053/meta.2001.26703. [DOI] [PubMed] [Google Scholar]