Abstract
The potential importance of the methylation cycle during embryonic development necessitates the establishment of methodology to detect alterations in the relative abundance of SAM and SAH in an embryonic experimental system. We have developed a precise and sensitive method for measurement of SAM and SAH based on liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) in single neurulation-stage mouse embryos. Use of a penta-fluorinated HPLC stationary phase gave enhanced sensitivity due to optimal ionisation in organic mobile phase and increased retention time compared to standard reversed-phase separation. Calibration curves suitable for the analysis of neurulation-stage mouse embryos (SAM 0.02-25.0 µM, SAH 0.01-10.0 µM) were linear (r2 > 0.997) with limits of detection for SAM and SAH of 10 and 2.5 nmol/L, respectively.
1. Introduction
Methylation of biomolecules including DNA, RNA, lipids and proteins, is essential for a range of cellular processes including epigenetic control of gene expression [1] and regulation of protein function [2;3]. The methyl donor for methyltransferase-catalysed reactions is S-adenosylmethionine (SAM), which is concomitantly converted to s-adenosylhomocysteine (SAH). In turn, SAH acts as a product inhibitor of methyltransferases [4] such that the ratio of SAM to SAH is crucial for regulation of methylation. SAH must be efficiently recycled, via the production of homocysteine and methionine, for methylation potential to be maintained [5;6]. The relative abundance of SAM to SAH is not only important for methylation, but can also influence flux through the folate cycle, which is interlinked to the methylation cycle, since SAM is able to inhibit 5,10-methylene tetrahydrofolate reductase (MTHFR; EC1.7.99.5).
Integrity of the folate and methylation cycle appear to be crucial during embryonic development, impairment being associated with the occurrence of neural tube defects (NTD), a group of severe birth defects [6;7]. Risk factors for NTD include sub-optimal levels of folate in maternal serum, elevated homocysteine and reduced levels of vitamin B12 [8-10], all of which could be associated with reduced flux through the methylation cycle and a change in the levels of SAM and SAH. Indeed provision of SAM for DNA methylation does appear essential for neural tube closure, since NTD are observed in mouse embryos that are homozygous null for DNA methyltransferase 3b [11].
The potential importance of the methylation cycle during embryonic development necessitates the establishment of methodology for an accurate and sensitive assay of SAM and SAH in an embryonic experimental system such as mouse embryos. Recently, liquid chromatography coupled to mass spectrometry (LC-MS) has been utilised for determination of SAM and SAH owing to the high sensitivity of detection and the ability to use stable-isotope-labelled internal standards for precise quantification [12-14]. The previously reported LC-MS method allows assay of tissues (rat liver and lung) as well as fluids (whole blood, plasma, serum and urine) [12]. In comparison, tandem mass spectrometry (LC-MS/MS) provides enhanced selectivity, less time-consuming sample preparation and has been utilised for plasma and cerebrospinal fluid samples [13;14]. Here, we report a modified LC-MS/MS method that allows precise and simultaneous quantification of SAM and SAH in low abundance tissue samples, in this case neurulation-stage mouse embryos (at embryonic day 9.5 and 10.5). In particular, we made use of the increased retention of these analytes using novel pentafluorophenylpropyl HPLC stationary phase and thereby minimised sample preparation and enhanced sensitivity, which is critical when tissue quantities are limiting.
2. Experimental Procedures
2.1. Materials
SAM and SAH were purchased from Sigma-Aldrich (Dorset, UK). The internal standard, [2H3]-SAM, was purchased from CDN Isotopes (Pointe-Claire, Quebec, Canada). Ammonium acetate, formic acid and heptafluorobutyric acid were purchased from Sigma-Aldrich (Dorset, UK). Solvents and water were all of HPLC grade (Fisher Scientific UK Ltd., Loughborough, UK).
2.2. Samples
Non-mutant random-bred CD1 mice were purchased from Charles River Laboratories Inc. (Kent, UK). Experimental litters were generated by timed matings in which females were paired with males overnight and checked for a copulation plug the following morning, this being designated embryonic day 0.5 (E0.5). At E9.5 or E10.5, each female mouse was killed by cervical dislocation and the uterus was explanted into Dulbecco’s Modified Eagle’s medium (DMEM; Sigma-Aldrich, Dorset, UK) containing 10% fetal calf serum (Sigma-Aldrich). Embryos were explanted from the maternal decidua and separated from the visceral yolk sac and Reichert’s membrane. Embryos were subsequently rinsed in phosphate-buffered saline, immediately frozen and stored at –80oC until required. Genomic DNA for PCR was prepared from individual yolk sacs as described previously [15], allowing determination of the sex of the corresponding embryo by PCR using sex-specific primers to amplify the Smcx and Smcy genes [16].
2.3. Preparation of Samples
Embryos at E9.5 or E10.5 were suspended in 200 μl of ice-cold aqueous mobile phase (100% B, see below) containing 1 μM [2H3]-SAM and sonicated immediately at 12 micron amplitude for 10 seconds on ice, to produce a homogeneous solution. A 10 μl aliquot was retained for determination of protein concentration using the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL). The remaining solution was heated at 80oC for 5 minutes to precipitate endogenous proteins, cooled immediately on ice for 2 minutes and centrifuged for 15 minutes at 12,000 g to remove any particulate. Samples were analysed immediately by LC-MS/MS.
2.4. Calibration and quantification
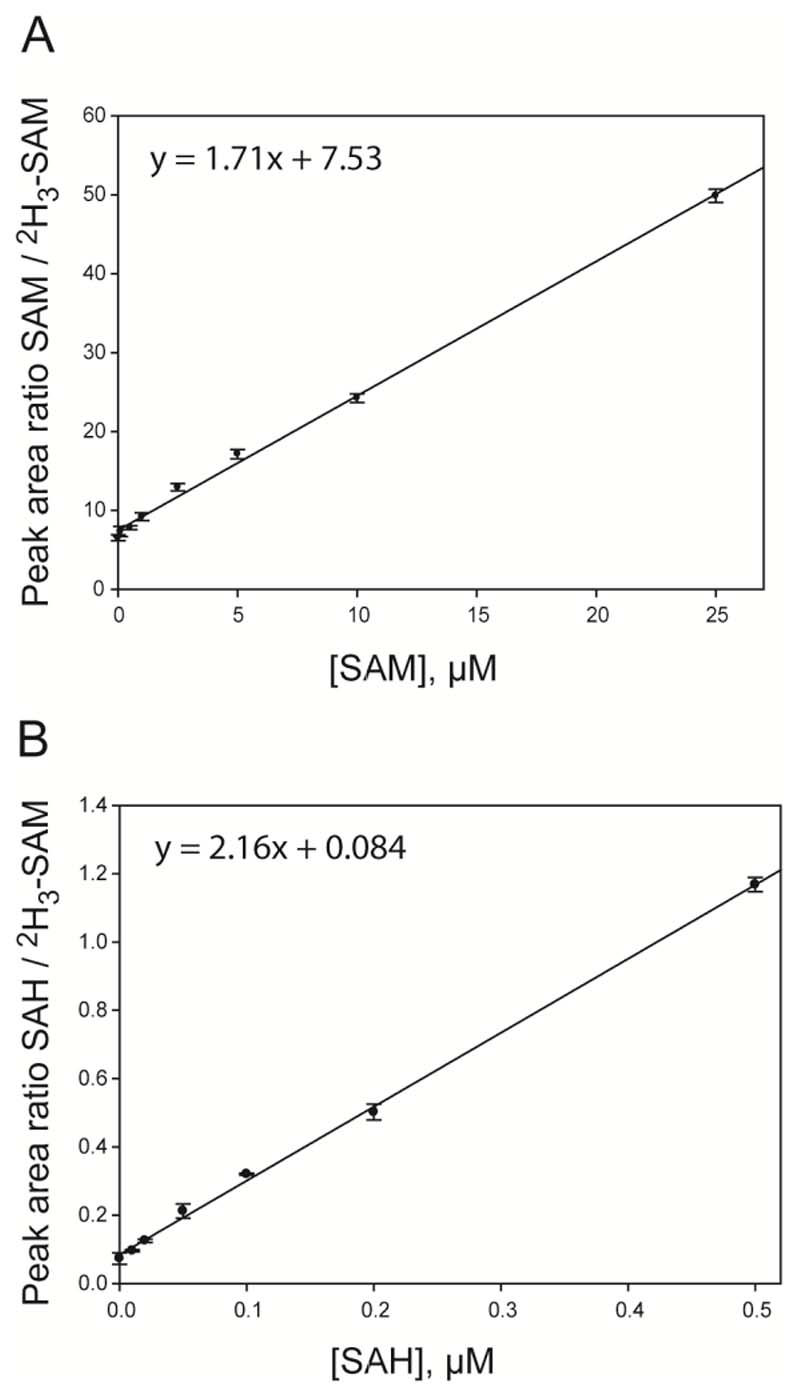
In order to quantify endogenous levels of SAM and SAH in whole embryos, the “calibration curve method” was implemented using combined SAM and SAH calibrators made up in a matrix of pooled mouse embryos. To generate a calibration curve, the “peak area of the calibrator / peak area of the internal standard” was plotted against increasing concentrations of calibrator spiked into the matrix of pooled embryos (Figure 3). This calibration curve was then used to deduce the endogenous level of SAM or SAH in the pooled embryo matrix by determining the intercept on the x-axis when y = 0. The endogenous level of SAM or SAH was subtracted from each of the calibrators to create a curve that passed through zero on the x-axis. These working calibration curves were then used to quantify the levels of SAM or SAH in the embryo samples. Linear regression analysis (un-weighted) was performed using Sigma Plot Version 7.0 (SPSS Inc., Chicago, Illinois). For neurulation stage (E9.5 – E10.5) embryos, calibrators containing 0.5, 1, 2.5, 5, 10, 25 µM of SAM and 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 µM of SAH were utilised (Figure 3). All calibrators contained 1 μM of the internal standard, [2H3]-SAM.
Fig. 3.
Calibration curves of peak-area ratios plotted against SAM (a) and SAH (b) concentration. Calibrators were made up in a pooled sample of homogenised embryos with 1.0 µmol/L of [2H3]-SAM (internal standard) added to each sample. SAM data points correspond to 0.5, 1, 2.5, 5, 10, 25 µM of SAM and the SAH data points correspond to 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 µM. Each sample was run in triplicate (n=3).
2.5. LC-MS/MS Method
Prior to analysis by mass spectrometry SAM, SAH and [2H3]-SAM were separated on a pentafluorophenylpropyl (PFPP)-bonded silica column (Discovery HS F5; 50 x 2.1 mm (i.d.); 5 µm bead size; Supelco, Sigma-Aldrich) using a 2795XE high performance liquid chromatography (HPLC) unit with solvent divert valve (Waters, Manchester, UK). Solvents for HPLC were: A, 100% methanol; B, 4 mM ammonium acetate, 0.1% formic acid, 0.1% heptafluorobutyric acid (pH2.5). The column was equilibrated with 40% A: 60% B. The sample injection volume was 40µl. The HPLC protocol consisted of 40% A: 60% B for 2 minutes, followed by a gradient of 40-100% A over a 2 minute period. The column was then washed with 100% A for 4 minutes before re-equilibration for 7 minutes. The flow rate was 0.5 ml/min and was diverted to waste for the first 72 seconds after sample injection, to minimise accumulation of endogenous compounds on the ionisation source. The HPLC was coupled to a MicroMass Quattro triple quadrupole tandem mass spectrometer (Waters) operating in positive-ion mode using the following settings: capillary 3.86 kV, source temperature 150°C, desolvation temperature 350°C, cone voltage 20 V, collision energy 26V, cone gas flow rate 50 L/hr and desolvation gas flow rate 650 L/hr. The Selected Reaction Monitoring (SRM) mode was used for quantification and data were acquired and processed using MassLynx software (version 4.0, Waters).
2.6. Statistical Analysis
Analyte concentrations were compared by One Way ANOVA. Ratios of SAM:SAH (overall and within each sex) were compared by t-test. Statistical tests were computed using SigmaStat Version 2.03 (SPSS Inc.).
3. Results
3.1. Mass Spectra
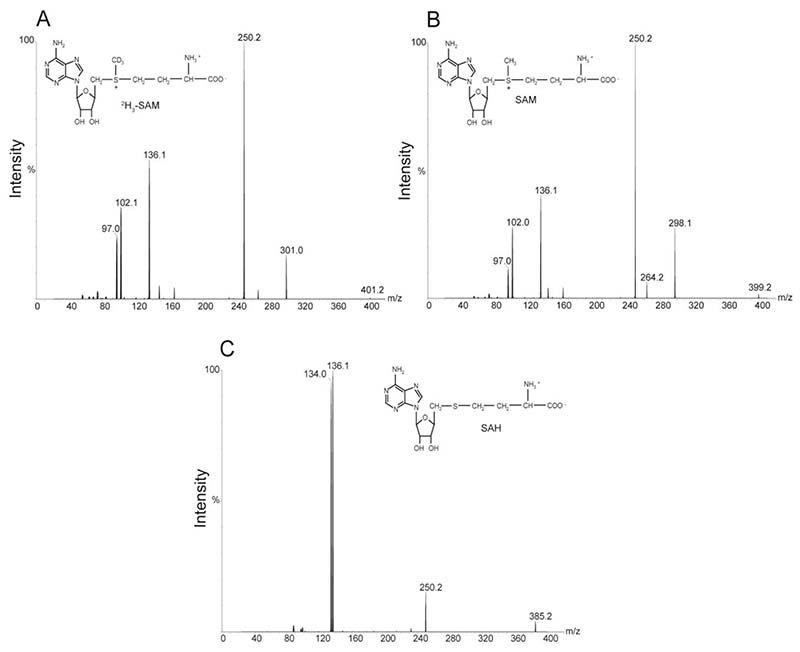
The expected [M+H]+ protonated molecules (precursor ions) were m/z 399.2, 402.2 and 385.2 for SAM, 2H3-SAM and SAH, respectively (Figure 1). Observed experimental results matched the theoretical masses. Product ion spectra for each of the three molecules yielded a fragment of m/z 136.1 (Figure 1), believed to be derived from the adenosine backbone [13]. Thus, the final SRM transitions were as follows: SAM, 399.2 → 136.1; 2H3-SAM, 402.2 → 136.1; SAH 385.2 → 136.1.
Fig. 1.
Product ion spectra of protonated molecules used for quantification. Precursor ions were at m/z 401.2 for [2H3]-SAM (a), 399.2 for SAM (b) and 385.2 for SAH (c). In each case the MS/MS conditions were optimised to favour the transition to a major product ion at m/z 136.1, thought to correspond to adenine [13].
3.2. Sample Preparation
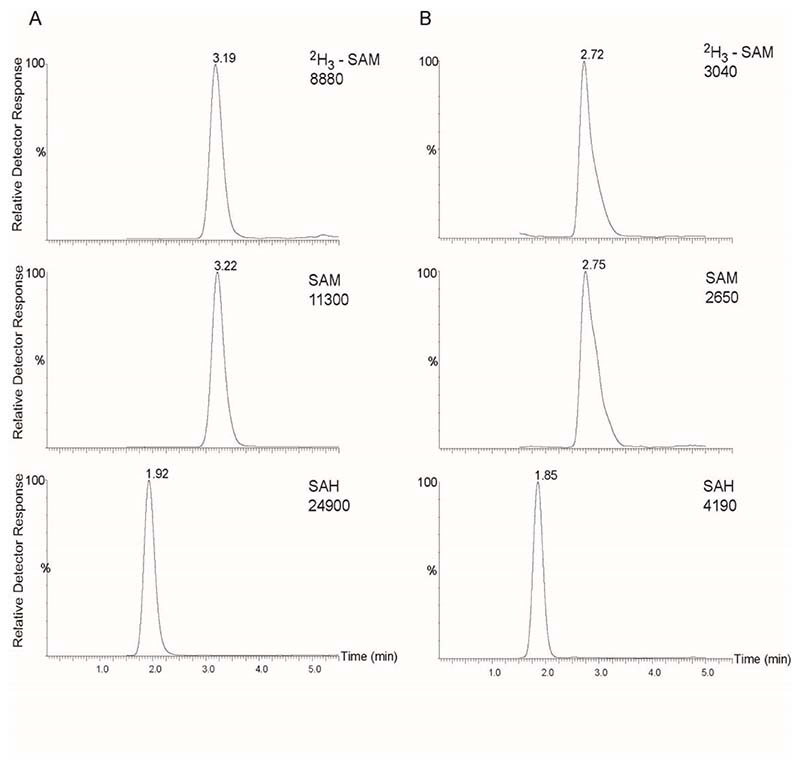
Recent reports indicate that, unless acidified, SAM in plasma may be unstable during storage at − 20 °C [12;14]. For this reason, tissue samples were stored at −80 °C and homogenised in acidified mobile phase (solution B) with no apparent degradation of SAM. These embryo tissue samples contained approximately 100 or 400 µg protein for E9.5 and E10.5 embryos respectively. Several procedures were evaluated for preparation of samples for LC-MS/MS following homogenisation. These included acetone precipitation, acetonitrile precipitation, ethanol precipitation, heat precipitation, acidification with perchloric acid and filtration on spin-columns (YM-3 Centricon, Millipore). In all cases there was a change in the retention time of SAM, SAH and 2H3-SAM resulting in earlier elution of the three compounds in embryo samples under analysis compared to aqueous standards. Similarly, the spiking of calibrators into pooled embryo matrix resulted in the same change of retention compared to analysis of standards in aqueous solution. A change in retention of all three compounds when analysed in an embryo matrix compared to aqueous solution suggests that this is due either to ion-pairing type reactions or to an interaction with unidentified compounds present in the embryo matrix that results in slight changes in the retention time of SAM/SAH on the HPLC column. However, the change in retention time had no effect on quantification as retention in samples and calibrators are the same. Moreover, mass spectra for SAM and SAH were identical irrespective of retention time. Filtration on spin-columns did not sufficiently recover the SAM and SAH from the homogenised sample. Solid phase extraction was not suitable due to low sample volume (200 µl), inherent in the analysis of individual mouse embryos (or other low abundance tissue samples). Therefore, heat precipitation was the method of choice due to better chromatographic peak shape observed with this method of protein precipitation (Figure 2). To evaluate potential loss of SAM due to heat treatment, control standard samples were divided and analysed with or without the heat step. Using this brief heat treatment and immediate cooling there was no change in signal intensity for SAM, SAH or [2H3]-SAM compared to non-heat treated samples, indicating that degradation was not caused by heating (data not shown). The minimal sample handling using this approach minimises recovery losses as indicated by linearity of calibration curves, CV and recovery values (see below).
Fig. 2.
LC-MS/MS chromatograms obtained in SRM mode. Representative chromatograms are shown for, (a) 1 µmol/L aqueous standards and, (b) endogenous SAM and SAH in an E10.5 mouse embryo, containing measured concentrations of 0.766 µmol/L SAM and 0.121 µmol/L SAH. Retention times are indicated above peaks.
The degree of ionisation of an analyte can be suppressed or enhanced by the residual components in the matrix. To determine possible matrix effects, the signal for all analytes in extracted spiked embryo samples was compared to non-extracted aqueous standards [17]. Matrix components slightly enhanced the signal (data not shown), which could be a direct result of endogenous material present in the embryo samples or due to mobile phase additives used during the preparation of embryo samples.
3.3. Chromatography
Representative chromatograms of SAM and SAH in both aqueous solution and in an analytical sample, comprising an E9.5 mouse embryo, are shown in Figure 2. The PFPP column was chosen over standard reverse-phase columns due to the additional ion-exchange properties that help overcome the minimal retention of SAM in reversed phase HPLC [18]. Prolonged retention on the column is particularly beneficial in analysis of tissue samples in order to avoid co-elution with early eluting endogenous compounds that produce ion suppression. Addition of 0.1% heptafluorobutyric acid to the mobile phase resulted in greater retention on the column due to ion pairing interactions and without having any effect on ion suppression.
3.4. Linearity, Limits of Detection and Precision
The internal standard, [2H3]-SAM, was used for quantification of both SAM and SAH. The minor structural difference from SAH, which lacks a methyl group, had minimal effect on linearity and precision. Moreover, as CVs for SAH were comparable to SAM and within acceptable limits it was unnecessary to synthesise an additional SAH standard. Calibration curves, made up in a matrix of pooled homogenised embryos, were linear throughout a concentration range of 0.5-25 µM and 0.01-0.5 µM for SAM and SAH, respectively (Figure 3). This range of concentrations was found to be suitable for the analysis of individual mouse embryos at E9.5 and E10.5, encompassing the period when neural tube closure is completed in the cranial and spinal regions. The coefficient of linear correlation (r2) was 0.997 and 0.998 for SAM and SAH, respectively. The limits of detection (determined at a signal-to-noise ratio of 5:1) for SAM and SAH were 10 nmol/L and 2.5 nmol/L, respectively, corresponding to 0.4 pmoles and 0.1 pmoles in the 40 μl injection. The lower and upper limits of quantification for SAM and SAH using this method were 0.02-25 µM and 0.01-10 µM, respectively.
To determine the precision for analysis of tissue samples, experiments were performed by pooling homogenised whole embryo samples either from E9.5 embryos at the 24-26 somite stage or from late stage E10.5 embryos at the 37 somite stage. All samples contained 1 μM of the internal standard, [2H3]-SAM. Overall, intra-assay coefficients of variation (CVs) ranged from 4.09-4.13% for SAM and 4.64-5.68% for SAH and inter-assay CVs ranged from 6.34-7.74% for SAM and 11.56-11.93% for SAH (Table 1).
Table 1. Inter- and Intra-assay precision of the LC-MS/MS method for embryonic samples.
The precision was determined by repeated assay of embryos sample, which consisted of pools of homogenised embryos collected at E9.5 and E10.5. CV, coefficient of variation.
| SAM | SAH | ||||
|---|---|---|---|---|---|
| Sample | Mean concentration (μM ±SD) | CV(%) | Mean concentration, (μM ±SD) | CV(%) | |
| E9.5 mouse embryo | Intra-assay (n=12) | 2.52 ± 0.10 | 4.13 | 0.044 ± 0.002 | 4.64 |
| E10.5 mouse embryo | Intra-assay (n=12) | 11.72 ± 0.48 | 4.09 | 0.21 ± 0.01 | 5.68 |
| E9.5 mouse embryo | Inter-assay (n=8) | 2.55 ± 0.20 | 7.74 | 0.040 ± 0.005 | 11.93 |
| E10.5 mouse embryo | Inter-assay (n=8) | 12.18 ± 0.77 | 6.34 | 0.20 ± 0.02 | 11.56 |
Accuracy was evaluated by spiking whole embryo homogenates with increasing concentrations of SAM or SAH, and quantifying the total SAM or SAH (Table 2). The measured concentration was expressed as a percentage of the predicted ‘nominal’ concentration, which was the sum of the added concentration and endogenous level (determined in the 0 μM added sample). In the majority of cases the measured value was greater than 90% of the predicted nominal value (Table 2). Recovery was assessed by comparing the response of pre-extracted spiked embryo to post-extracted spiked embryo samples [19]. Mean recoveries for SAM and SAH from E9.5 and E10.5 pooled mouse embryo matrices were 97 % and 92 %, respectively (Table 2).
Table 2. Accuracy and recovery data for the LC-MS/MS quantification of SAM and SAH in mouse embryos.
Embryo samples consisted of pools of homogenised embryos collected at E9.5 and E10.5. Accuracy (% nominal concentration) was determined by comparing the measured concentration of SAM of SAH against the nominal concentration (the predicted value equating to the baseline concentration with 0 pM SAM or SAH added + spiked concentration). Thus, %Nominal concentration = Mean Measured Conc.]/[Mean Baseline conc. + Spiked conc.]. Recovery was determined by comparing the assay results for embryo samples spiked pre- and post- extraction (n = 3 for each condition). % Recovery = ([Response of Pre-Extracted Spiked Sample] / [Response of Post-Extracted Spiked Sample]) x 100 [as ref. 22]. Concentration values are given as mean ± standard deviation.
| Sample | Spiked conc. added (μM) | Measured conc. (μM) | Accuracy (% Nominal Conc.) | Measured conc. (μM) | Accuracy (% Nominal Conc.) | Recovery (%) |
|---|---|---|---|---|---|---|
| SAM Added | Spiked pre-extraction | Spiked post-extraction | ||||
| E9.5 mouse embryo | 0 | 0.08 ± 0.020 | ||||
| 0.5 | 0.57 ± 0.030 | 98 | 0.57 ± 0.02 | 98 | 100 | |
| 1.0 | 1.06 ± 0.020 | 98 | 1.09 ± 0.06 | 101 | 97 | |
| 2.0 | 2.08 ± 0.004 | 100 | 2.12 ± 0.09 | 102 | 98 | |
| E10.5 mouse embryo | 0 | 0.79 ± 0.01 | ||||
| 0.5 | 1.21 ± 0.01 | 94 | 1.19 ± 0.17 | 92 | 101 | |
| 1.0 | 1.82 ± 0.04 | 102 | 2.03 ± 0.28 | 113 | 90 | |
| 2.0 | 2.64 ± 0.04 | 95 | 2.83 ± 0.27 | 101 | 94 | |
| SAH Added | ||||||
| E9.5 mouse embryo | 0 | 0.11 ± 0.009 | ||||
| 0.05 | 0.13 ± 0.002 | 81 | 0.14 ± 0.003 | 88 | 97 | |
| 0.1 | 0.19 ± 0.001 | 86 | 0.21 ± 0.009 | 100 | 87 | |
| 0.2 | 0.33 ± 0.027 | 106 | 0.35 ± 0.030 | 113 | 92 | |
| E10.5 mouse embryo | 0 | 0.20 ± 0.030 | ||||
| 0.05 | 0.23 ± 0.001 | 92 | 0.24 ± 0.006 | 96 | 90 | |
| 0.1 | 0.30 ± 0.070 | 100 | 0.34 ± 0.030 | 113 | 91 | |
| 0.2 | 0.38 ± 0.040 | 95 | 0.42 ± 0.050 | 105 | 95 | |
3.5. Mouse embryo samples
A series of embryos were collected from the non-mutant CD-1 mouse strain, and analysed to establish reference intervals of SAM and SAH. Levels were normalised to protein concentration and correlated with developmental stage (Table 3). In embryos at E9.5 and E10.5 mean levels of SAM were 1.98 and 2.78 nmol/mg protein respectively, while mean levels of SAH were 0.031 and 0.057 nmol/mg protein (Table 3). Thus, SAM levels are typically 50-70 fold higher than SAH levels in mouse embryos at these stages of development. At a particular stage, comparison of male and female embryos did not indicate any sex difference in SAH or SAM levels (Table 3). Moreover, within litters there was no apparent effect of position of the embryo within the uterus as levels were comparable for conceptuses close to the cervix compared with those close to the ovary (data not shown). On the other hand, there were significant changes in the concentration of both SAM and SAH as development proceeds (Table 3). Thus, at E10.5 the abundance of both SAM and SAH had increased compared to a day earlier in development. As the abundance of SAH increased to a greater extent than SAM, the SAM:SAH ratio decreased significantly at E10.5 compared to E9.5.
Table 3. SAM and SAH abundance in mouse embryos determined by LC-MS/MS.
SAM and SAH were quantified in individual CD1 embryos at E9.5 (Mean number of somites 25.7 (standard deviation 1.4)) and E10.5 (Mean number of somites 33.6 (standard deviation 2.4)). Values are given as mean ± standard deviation.
| Embryonic stage | No. samples | SAM ng/mg protein | SAH ng/mg protein | SAM:SAH Ratio |
|---|---|---|---|---|
| E9.5 | Total (n=24) | 1.98 ± 0.71 | 0.031 ± 0.011 | 67.8 ± 23.2 |
| Male (n=13) | 2.23 ± 0.64 | 0.034 ± 0.012 | 71.6 ± 24.3 | |
| Female (n=11) | 1.69 ± 0.71 | 0.027 ± 0.009 | 63.4 ± 22.2 | |
| E10.5 | Total (n=26) | 2.78 ± 0.67 | 0.057 ± 0.029 | 53.0 ± 13.9 |
| Male (n=15) | 2.75 ± 0.65 | 0.051 ± 0.021 | 46.8 ± 13.3 | |
| Female (n=11) | 2.83 ± 0.65 | 0.065 ± 0.023 | 57.6 ± 12.8 |
4. Discussion
SAM and SAH are key metabolites in one-carbon metabolism, determining the methylation potential in a tissue [6;20]. Since sub-optimal methylation capacity is associated with various diseases as well as developmental abnormalities, it is necessary to develop methodology for measurement of the relative quantities of SAM and SAH, potentially in limiting amounts of sample. For this reason, we have applied LC-MS/MS methodology for the simultaneous quantification of SAM and SAH in tissue samples. The sample preparation method allows analysis of tissues quantities (less than 1 mg) considerably lower than used in methods that utilise solid phase extraction (typically 100-300 mg wet weight [18]).
Previously, UV detection following HPLC or capillary electrophoresis has been used for detection of SAM and SAH in tissue samples as well as in urine, red blood cells and lymphocytes [21-23]. Increased sensitivity can be achieved by the use of coulometric electrochemical detection [24] or through derivatisation of SAH and SAM followed by fluorescent detection, although this is time-consuming and requires additional sample preparation steps [25;26]. Here, we developed a method that exploits the selectivity and sensitivity of tandem mass spectrometry, yielding a method with limits of detection of 10 nmol/L and 2.5 nmol/L for SAM and SAH respectively. Sample preparation is minimised compared to previous MS-based methods, which require solid phase extraction [13;14]. Instead, the method that we report involves homogenisation of samples in acidified mobile phase and heat precipitation of proteins, which increases throughput and reduces sample loss (with a resulting increase in sensitivity), without degradation of SAM. A single internal standard was used for both assays, since although [2H3]-SAM is technically a type II internal standard when used in the quantitation of SAH, the inter- and intra-coefficients of variation observed were within acceptable limits. Presumably, this is due to the superior ability of the HS F5 column to retain small, polar metabolites over conventional reverse phase columns, resulting in fewer co-eluting and interfering compounds that can lead to ionic suppression.
In mouse embryos the concentration of SAM that we detected (2-2.8 nmol/mg protein) are approximately 20-40 times higher than values reported for adult liver and kidney in rats [12;21;22]. However, the values for adult tissue were expressed per wet weight of tissue, which is not practical with small tissue samples such as embryos. We estimate that SAM levels in embryonic and adult tissue are approximately comparable, based on comparison of protein content and wet weight for a limited number of embryos. SAH concentrations reported in adult rats vary widely between tissues [12;21;22], and are similar or up to 10 times lower than in mouse embryos, although direct comparison is again complicated by normalisation to wet weight rather than protein. However, the concentrations of SAM and SAH that we detect in neurulation-stage mouse embryos are similar to the levels (also normalised to protein content) measured in a recent study of pooled chick embryos [27]. We detect a significant increase in the concentration of both SAM and SAH in the period from E9.5 to E10.5, despite the fact that there is a concomitant dramatic increase in the protein content of the embryo. At the same developmental period there is a decrease in the SAM:SAH ratio, since the magnitude of the increase in SAH concentration is greater than that for SAM, perhaps due to increased demand for SAM in transmethylation reactions or as precursor in polyamine synthesis.
In summary, the use of LC-MS/MS enables sensitive, precise and accurate quantification of the picomole quantities of SAM and SAH in neurulation-stage mouse embryos. Although, the method was particularly designed for the analysis of individual mouse embryos, it could potentially be applied to any tissue where limited amount of sample is available depending on the matrix and the levels of SAM and SAH in the tissue to be analysed.
Acknowledgments
We thank Simon Eaton, Bryan Winchester and Peter Clayton for helpful comments and discussion. The authors are grateful for financial support from the Birth Defects Foundation, the Wellcome Trust, Trustees of Great Ormond Street Hospital, Genzyme and Great Ormond Street Hospital for Children NHS Trust.
Abbreviations
- HPLC
high performance liquid chromatography
- LC-MS
liquid chromatography coupled to mass spectrometry
- SRM
Selected Reaction Monitoring
- MS/MS
tandem mass spectrometry
- NTD
neural tube defects
- PFPP
pentafluorophenylpropyl
- SAM
sadenosylmethionine
- SAH
s-adenosylhomocysteine
References
- [1].Friso S, Choi SW. J Nutr. 2002;132:2382S–2387S. doi: 10.1093/jn/132.8.2382S. [DOI] [PubMed] [Google Scholar]
- [2].Vafai SB, Stock JB. Fed Eur Biochem Soc Lett. 2002;518:1–4. doi: 10.1016/s0014-5793(02)02702-3. [DOI] [PubMed] [Google Scholar]
- [3].Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. J Biol Chem. 2000;275:17605–17610. doi: 10.1074/jbc.C000079200. [DOI] [PubMed] [Google Scholar]
- [4].De Cabo SF, Santos J, Fernandez-Piqueras J. Cytogenet. Cell Genet. 1995;71:187–192. doi: 10.1159/000134104. [DOI] [PubMed] [Google Scholar]
- [5].Finkelstein JD. EurJ Pediatr. 1998;157(Suppl 2):S40–S44. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- [6].Scott JM. ProcNutrSoc. 1999;58:441–448. [Google Scholar]
- [7].Copp AJ, Greene NDE, Murdoch JN. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- [8].Kirke PN, Daly LE, Molloy A, Weir DG, Scott JM. Lancet. 1996;348:67–68. doi: 10.1016/s0140-6736(05)64402-9. [DOI] [PubMed] [Google Scholar]
- [9].Steegers-Theunissen RPM, Boers GHJ, Trijbels FJM, Finkelstein JD, Blom HJ, Thomas CMG, Borm GF, Wouters MGAJ, Eskes TKAB. Metabolism. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- [10].Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. QJM. 1993;86:703–708. [PubMed] [Google Scholar]
- [11].Okano M, Bell DW, Haber DA, Li E. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- [12].Stabler SP, Allen RH. ClinChem. 2004;50:365–372. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- [13].Struys EA, Jansen EE, De Meer K, Jakobs C. Clin Chem. 2000;46:1650–1656. [PubMed] [Google Scholar]
- [14].Gellekink H, Oppenraaij-Emmerzaal D, van Rooij A, Struys EA, Den Heijer M, Blom HJ. Clin Chem. 2005;51:1487–1492. doi: 10.1373/clinchem.2004.046995. [DOI] [PubMed] [Google Scholar]
- [15].Copp AJ, Checiu I, Henson JN. Dev Biol. 1994;165:20–29. doi: 10.1006/dbio.1994.1230. [DOI] [PubMed] [Google Scholar]
- [16].Agulnik AI, Longepied G, Ty MT, Bishop CE, Mitchell M. Mamm. Genome. 1999;10:926–929. doi: 10.1007/s003359901116. [DOI] [PubMed] [Google Scholar]
- [17].Mallet CR, Lu Z, Mazzeo JR. Rapid Commun. Mass Spectrom. 2004;18:49–58. doi: 10.1002/rcm.1276. [DOI] [PubMed] [Google Scholar]
- [18].Needham SR, Jeanville PM, Brown PR, Estape ES. J Chromatography B. 2000;748:77–87. doi: 10.1016/s0378-4347(00)00271-1. [DOI] [PubMed] [Google Scholar]
- [19].Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- [20].Cook RJ. Homocysteine in Health and Disease. CUP; Cambridge: 2001. pp. 113–134. [Google Scholar]
- [21].She QB, Nagao I, Hayakawa T, Tsuge H. Biochem Biophys Res Commun. 1994;205:1748–1754. doi: 10.1006/bbrc.1994.2871. [DOI] [PubMed] [Google Scholar]
- [22].Delabar U, Kloor D, Luippold G, Muhlbauer B. J Chrom B. 1999;724:231–238. doi: 10.1016/s0378-4347(98)00580-5. [DOI] [PubMed] [Google Scholar]
- [23].Uthus EO. Electrophoresis. 2003;24:1221–1226. doi: 10.1002/elps.200390157. [DOI] [PubMed] [Google Scholar]
- [24].Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. Clin Chem. 2000;46:265–272. [PubMed] [Google Scholar]
- [25].Weir DG, Molloy AM, Keating JN, Young PB, Kennedy S, Kennedy DG, Scott JM. Clin Sci (Lond) 1992;82:93–97. doi: 10.1042/cs0820093. [DOI] [PubMed] [Google Scholar]
- [26].Capdevila A, Wagner C. Anal Biochem. 1998;264:180–184. doi: 10.1006/abio.1998.2839. [DOI] [PubMed] [Google Scholar]
- [27].Afman LA, Blom HJ, Drittij M-J, Brouns MR, van Straaten HWM. Brain Res Dev Brain Res. 2005;158:59–65. doi: 10.1016/j.devbrainres.2005.06.002. [DOI] [PubMed] [Google Scholar]