Abstract
Folic acid supplementation can prevent many cases of neural tube defects (NTDs) whereas sub-optimal maternal folate status is a risk factor, suggesting that folate metabolism is a key determinant of susceptibility to NTDs. Despite extensive genetic analysis of folate cycle enzymes, and quantification of metabolites in maternal blood, neither the protective mechanism nor the relationship between maternal folate status and susceptibility are understood in most cases. In order to investigate potential abnormalities in folate metabolism in the embryo itself, we derived primary fibroblastic cell lines from fetuses affected by NTDs and subjected them to the dU suppression test, a sensitive metabolic test of folate metabolism. Significantly, a subset of NTD cases exhibited low scores in this test, indicative of abnormalities in folate cycling that may be causally linked to the defect. Susceptibility to NTDs may be increased by suppression of the methylation cycle, which is interlinked with the folate cycle. However, reduced efficacy in the dU suppression test was not associated with altered abundance of the methylation cycle intermediates, s-adenosylmethionine and s-adenosylhomocysteine, suggesting that a methylation cycle defect is unlikely to be responsible for the observed abnormality of folate metabolism. Genotyping of samples for known polymorphisms in genes encoding folate-associated enzymes did not reveal any correlation between specific genotypes and the observed abnormalities in folate metabolism. These data suggest that as yet unrecognised genetic variants result in embryonic abnormalities of folate cycling that may be causally related to NTDs.
Keywords: Neural tube defects, anencephaly, spina bifida, folate, s-adenosylhomocysteine, sadenosylmethionine
Abbreviations
- dTMP
deoxythymidine monophosphate
- dU
deoxyuridine
- MTHFR
5,10-methylenetetrahydrofolate reductase
- MTRR
methionine synthase reductase
- NTDs
neural tube defects
- SAH
s-adenosylhomocysteine
- SAM
s-adenosylmethionine
Introduction
Despite their high frequency (1 per 1,000 pregnancies) and clinical severity, the cause of neural tube defects (NTDs) in human pregnancies is poorly understood in most cases. Population and family studies indicate a genetic component in human NTDs, while more than 100 genetic mutations are known to cause NTDs in mice (Copp et al., 2003; Boyles et al., 2005). However, the relative scarcity of familial cases and failure to identify single gene mutations as major causes of NTDs suggests that most cases result from summation of multiple genetic and/or environmental factors. Among these factors, maternal nutritional status is a key determinant of pregnancy outcome and attention has focussed on folic acid, a vitamin whose biological derivatives are integral to the interlinked folate and methionine cycles (Scott, 1999; Blom et al., 2006). Clinical trials demonstrate that supplementation with folic acid prior to and during early pregnancy reduces the risk of NTDs in the developing fetus (Wald et al., 1991; Czeizel and Dudás, 1992). Conversely, reduced folate levels or elevated homocysteine levels (an inverse indicator of folate status) in maternal serum or plasma are risk factors for NTDs (Kirke et al., 1993; Steegers-Theunissen et al., 1994; Mills et al., 1995).
Despite compelling evidence for an involvement of folate metabolism, neither the protective mechanism nor the relationship between maternal folate status and susceptibility to NTDs are well defined. One possibility is that folic acid acts to overcome insufficient maternal folate levels. However, in most cases, mothers of affected fetuses have either normal folate status or are, at most, mildly folate-deficient, arguing against maternal folate deficiency as a major causative factor (Mills et al., 1996; Scott1999; Van der Put et al., 2001). Alternatively, it is postulated that supplemental folic acid may act to overcome an underlying defect in folate metabolism that results from a genetic mutation in mother or fetus. For this reason proteins that mediate, or are functionally associated with, folate metabolism have provided candidates for genetic analysis in human NTDs (Boyles et al., 2005). Attention has particularly focussed on polymorphisms in the gene encoding 5,10-methylenetetrahydrofolate reductase (MTHFR), with the C677T polymorphism conferring increased risk of NTDs in some, but not all, populations (Van der Put et al., 2001; Kirke et al., 2004; Boyles et al., 2005). In those populations in which an association between MTHFR genotype and NTDs has been detected, estimates of the proportion of NTDs that can be attributed to this genetic polymorphism range from 11 to 26% (Ou et al., 1996; Kirke et al., 2004). Analysis of other MTHFR polymorphisms or different folate-related genes has not revealed consistent associations with NTDs (Boyles et al., 2005). Therefore, the extent to which the genetic component of NTDs involves folate-related genes is currently unclear.
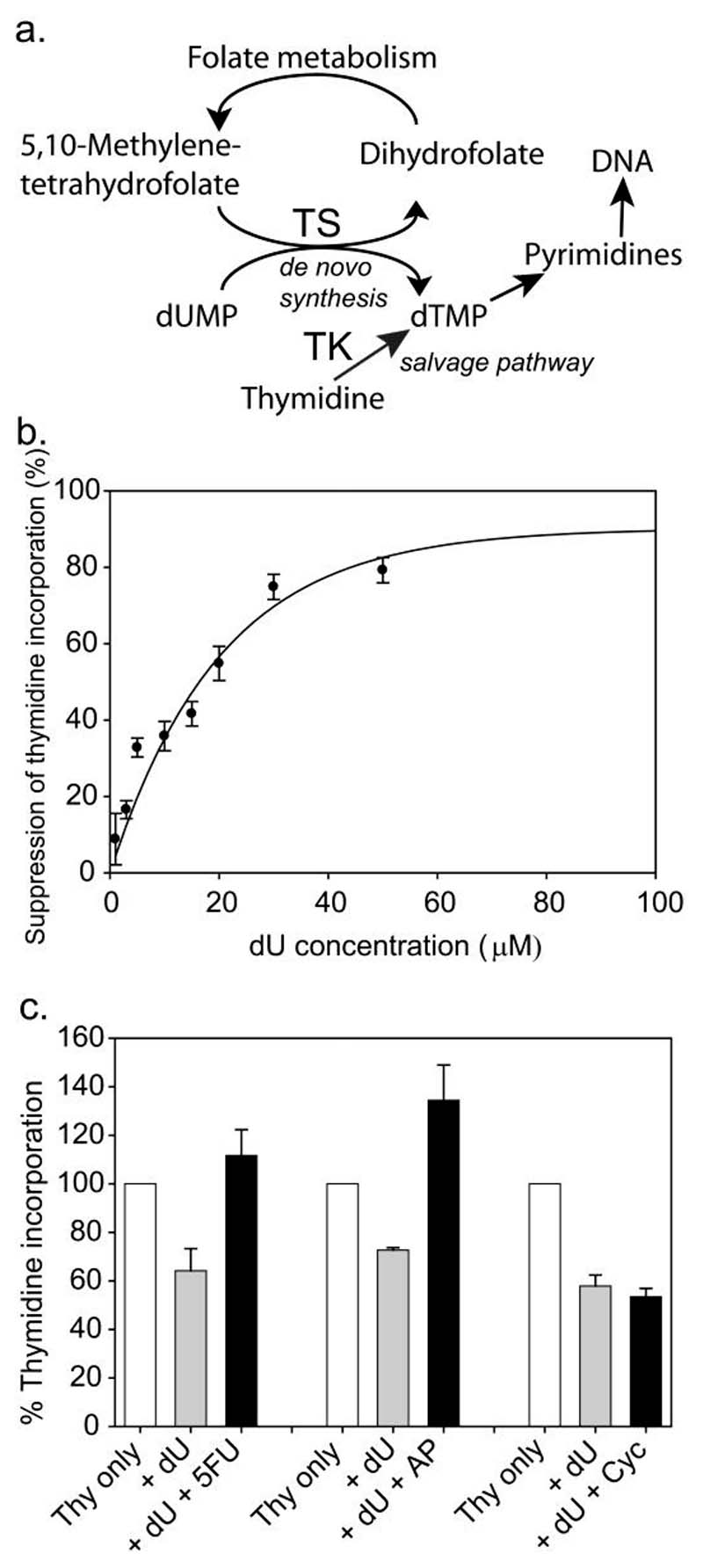
As an alternative approach to screening for genetic mutations we decided to directly test the possibility that abnormalities in folate metabolism may be associated with increased risk of NTDs, by analysis of fibroblastic cell lines generated from fetuses affected by NTDs. As a means of investigating the integrity of the folate cycle we utilised the deoxyuridine (dU) suppression test (Killman, 1964; Fleming and Copp, 1998), which depends on the ability of deoxyuridine monophosphate (dUMP) to suppress the incorporation of thymidine into DNA. Deoxythymidine monophosphate (dTMP), the precursor of pyrimidines, can be generated from dUMP (de novo synthesis catalysed by thymidylate synthase) or by phosphorylation of thymidine (the salvage pathway effected by thymidylate kinase) (Fig. 1a). Exogenous dUMP stimulates the de novo pathway and thereby suppresses incorporation of thymidine into genomic DNA, provided that the key intermediate 5,10-methylene tetrahydrofolate is available as part of normal folate metabolism (Fig. 1a). Therefore, the ability of dUMP to suppress thymidine incorporation into DNA provides a sensitive metabolic indicator of the integrity of the folate cycle.
Figure 1. Deoxyuridine suppression test can detect folate cycle inhibition in human fibroblastic cell lines.
(a) Addition of dU stimulates de novo synthesis of dTMP and suppresses incorporation of thymidine via the salvage pathway. (b-c) Effect of dU and exogenous inhibitors on thymidine incorporation in a human primary fibroblast cell line derived from a normal control pregnancy. (b) Dose-dependent suppression of thymidine incorporation by exogenous dU. (c) Inclusion of 5-fluorouracil (5FU; 25 µM) or aminopterin (AP; 10 µM) reversed the suppression of thymidine incorporation by 10 µM dU, whereas cycloleucine (cyc; 15 mM) had no detectable effect (n = 3 for each treatment group). Thy, Thymidine; TK, thymidine kinase; TS, thymidylate synthase.
Methods
Clinical samples and cell culture
Samples were collected at University College London Hospital, at 12-21 weeks of pregnancy, with informed consent and ethical permission. Tissue samples (skin or cartilage) were collected at termination of pregnancy following ultrasound diagnosis of a fetal abnormality, and amniotic fluid was collected at amniocentesis (performed for diagnostic purposes). A total of 85 patients were recruited and primary cell lines were successfully established for 76 samples using standard techniques (Boyle and Griffin, 2001). These included pregnancies in which the fetus was diagnosed with NTDs (spina bifida and/or anencephaly), diagnosis being confirmed following examination by a fetal pathologist. Controls included pregnancies in which an abnormality other than NTD was detected in the fetus (abnormal controls) and apparently normal pregnancies undergoing amniocentesis. Since dU suppression test results did not differ between these two control groups, they were pooled for comparison with the NTD group. All subsequent culture and experimentation was performed ‘blind’ to sample category. Among these cell lines 33 were viable at the fifth passage when sufficient material was available for the dU suppression test to be applied. Primary human fibroblasts and mouse 3T3 cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% v/v fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin, at 37°C in 5% CO2.
Deoxyuridine suppression test
Cells were seeded in tissue culture plates at 0.25 x106 cells per well and incubated overnight. Cells were then treated (1% v/v) with varying concentrations of deoxyuridine (dU; Sigma) in phosphate buffered saline (PBS) or PBS alone (n = 3 for each treatment). After 2 hours [3H]-thymidine (0.5 μCi/ml; Amersham Biosciences) was added and culture was continued for 24 hours. Cells were harvested by trypsinisation, resuspended in 0.3M sodium hydroxide in PBS, lysed by freeze-thawing and analyzed for [3H]thymidine incorporation into DNA and for total protein content (bicinchoninic acid protein assay reagent; Pierce). The percentage suppression of incorporation was calculated as the difference between [3H]-thymidine incorporation in the presence of dUMP (suppressed) or PBS only (maximal incorporation) expressed as a percentage of the incorporation with PBS only.
Quantification of s-adenosylmethionine and s-adenosylhomocysteine
Metabolites were quantified in individual cell lines by liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) as described previously (Burren et al., 2006).
Genotyping for known folate-related polymorphisms
Genomic DNA was prepared and genotyped for polymorphisms in MTHFR (C677T and A1298C)(Frosst et al., 1995;Van der Put et al., 1998), MTHFD1 (G1958A)(Brody et al., 2002), DHFR (IVS1del19bp)(Johnson et al., 2004), GCPII (C1561T)(Vieira et al., 2002), MTR (A2756G)(Zhu et al., 2003) and MTRR (A66G)(Zhu et al., 2003), using published methods.
Results
Parameters of the dU suppression test, previously established for mouse embryos in culture (Fleming and Copp,1998), were optimised for application to cultured fibroblasts using mouse NIH3T3 cells (data not shown) and a primary human fibroblast cell line from a normal control pregnancy. Incorporation of [3H] thymidine into genomic DNA of cultured cells was suppressed by exogenous dU in a dose-dependent manner, reaching a maximum of approximately 80% at doses of 50 μM and above (Fig. 1b). In order to test the ability of the dU suppression test to detect abnormal folate metabolism, pharmacological inhibitors of the folate cycle or the inter-linked methylation cycle were added to the cultures together with varying concentrations (10, 15 and 50 μM) of dU. We evaluated 5-fluorouracil, an inhibitor of thymidylate synthase, aminopterin, an inhibitor of dihydrofolate reductase and cycloleucine which inhibits methionine adenosyl transferase, a key enzyme of the methylation cycle. In each experiment, addition of dU alone suppressed the incorporation of thymidine, as expected, whereas 5-fluorouracil and aminopterin completely reversed this effect (data for cell line from control human pregnancy shown in Fig. 1c). Indeed, addition of inhibitor frequently caused thymidine incorporation in the presence of dU to exceed that in its absence (Fig. 1c), suggesting that flux through the thymidylate synthase or dihydrofolate reductase-mediated reactions was suppressed below baseline levels. As 5-fluorouracil acts directly to inhibit conversion of dUMP to dTMP, reversal of dU suppression was predicted. Aminopterin acts at a more distant part of the folate cycle, but its effect was nevertheless detectable by the dU suppression test. This finding provided confidence that, were a defect in the folate cycle to be present in a primary fetal cell line, then it would be detectable.
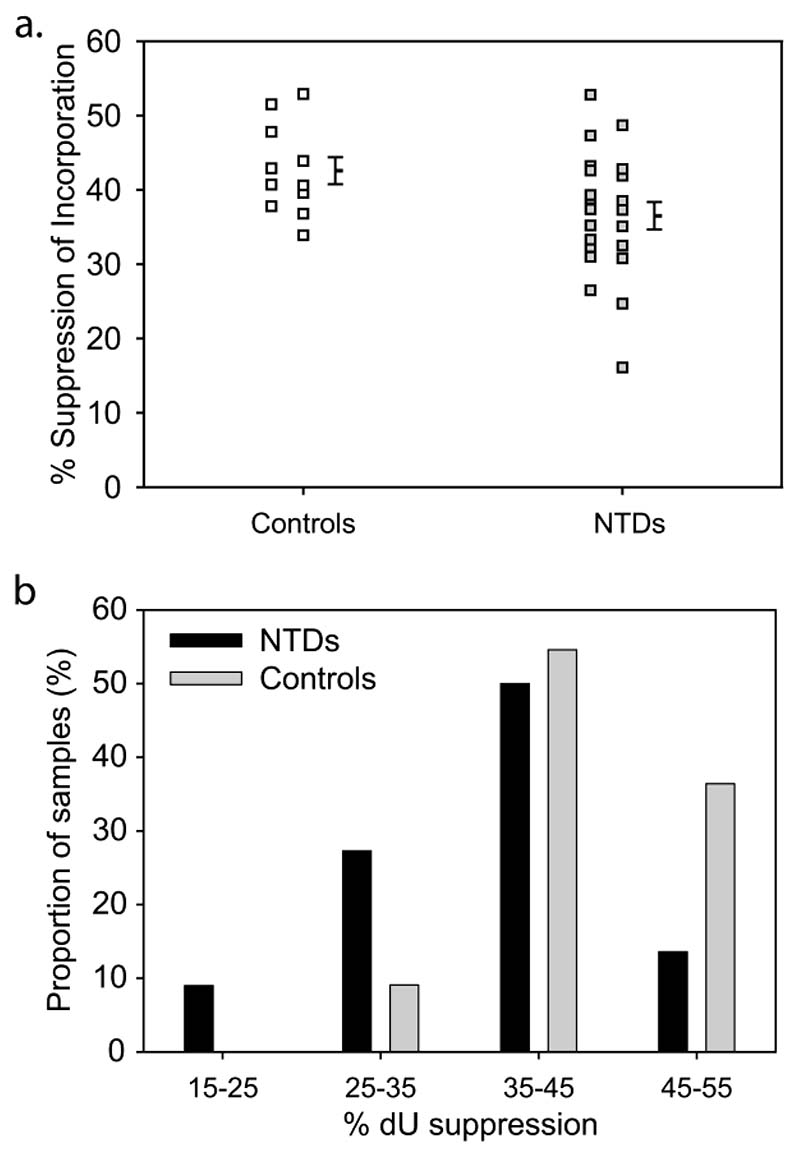
We next tested primary human fibroblast cell lines derived from fetuses with NTDs and from controls (Table 1). A dose of 10 μM dU was applied, since this concentration produces significant suppression of thymidine incorporation but is within the dose-dependent range, in order to optimise sensitivity to variation in response between samples. Control cell lines exhibited suppression of thymidine incorporation of 34-53%, whereas NTD cell lines exhibited a greater variation between samples (16-53%; Fig. 2a). Notably, the mean suppression of thymidine incorporation by dU was significantly lower in NTD cell lines than in controls (Table 1, Fig. 2a). Of particular interest, a subset of NTD cell lines displayed low suppression values, indicative of reduced efficacy of folate metabolism (Fig. 2b). Thus, when data for all cell lines are considered in combination, there are significantly more NTD than control cell lines with dU suppression scores in the lowest quartile of values (8 NTD lines compared to 0 controls; p<0.05, Fisher Exact Test). The 8 cell lines with lowest dU suppression scores included 6 spina bifida, 1 anencephaly and 1 spina bifida with anencephaly. Although, low scores appear more prevalent in cell lines derived from spina bifida than anencephaly cases, this may reflect the greater proportion of spina bifida among the overall sample set (Table 1). Thus, there was no significant difference between mean dU suppression scores for the NTD sub-types.
Table 1. Fetus-derived cell lines used for dU suppression test.
Among primary cell lines initially established, 33 were viable at the fifth passage when sufficient cells were available for performance of the dU suppression test. The mean gestational age of fetuses did not significantly differ between control and NTD cell lines. Sex was determined by karyotyping or PCR using primers to X and Y chromosome-specific genes. * indicates significantly lower score in NTDs than controls (t-test, p<0.05).
| Sample type | Cell lines tested | Type of abnormalities | Mean gestational age (weeks) | Sex of fetus | Mean dU test (%) |
|---|---|---|---|---|---|
| NTDs | 22 | Spina Bifida (15), | 17.34 ± 0.98 | 12 Male, 9 | 36.5 ± 1.8* |
| Anencephaly (6), Spina | Female, 1 Not | ||||
| Bifida & Anencephaly (1) | Determined | ||||
| Controls | 11 | Ventriculomegaly (1) | 18.43 ± 0.66 | 7 Male, | 42.6 ± 1.8 |
| Kidney defect (1) | 3 Female, | ||||
| Pena-Shokeir (1) | 1 Not | ||||
| OEIS complex (1) | determined |
Figure 2. Cell lines derived from NTD fetuses exhibit abnormal folate metabolism.
(a) Score (mean of 3 experiments) in the dU suppression test is plotted for each cell line with the overall mean (± SEM) shown for NTDs and controls. (b) The distribution of dU suppression test scores for NTDs is skewed towards lower values compared to controls.
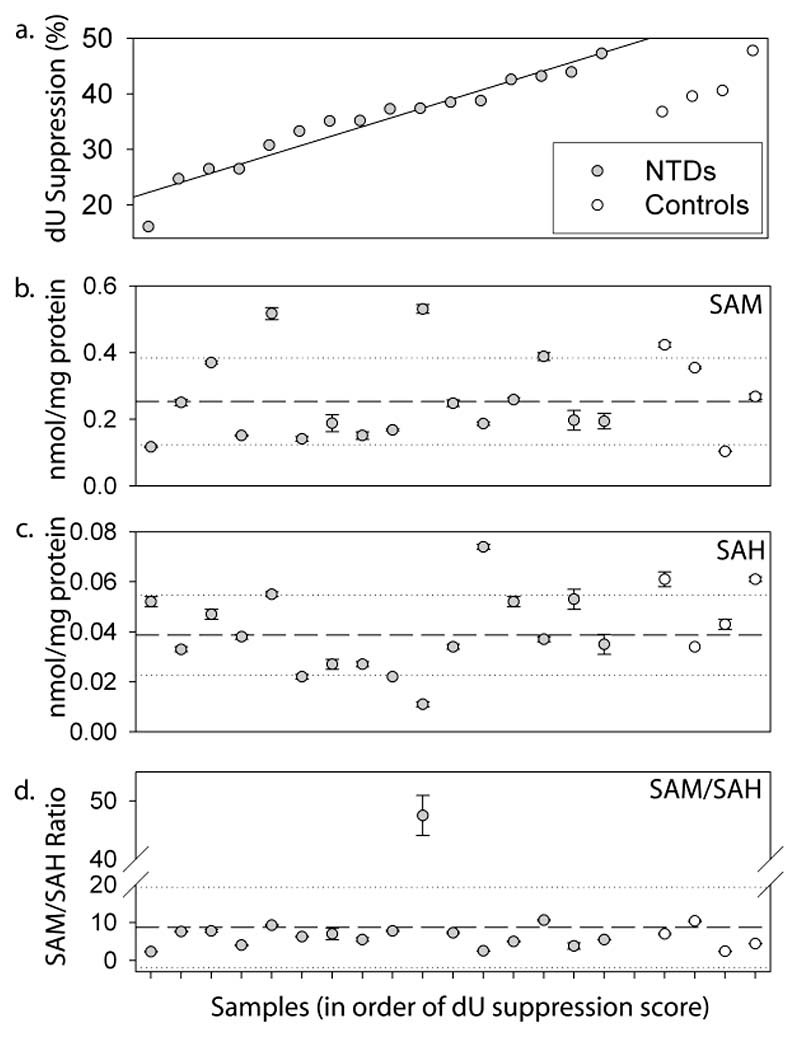
Remethylation of homocysteine by methionine synthase, which depends on 5-methyl tetrahydrofolate as co-factor, has been implicated as a potential site for folate-related defects in NTDs, owing to identification of elevated maternal homocysteine and reduced vitamin B12 levels as risk factors (Kirke et al., 1993; Mills et al., 1995; Lucock et al., 1998). A potential role for the methylation cycle is also indicated by experimental studies in which suppression of the methylation cycle causes NTDs in mouse embryos (Dunlevy et al., 2006a; Dunlevy et al., 2006b). However, the abnormal dU suppression results obtained for NTD cell lines in this study appear unlikely to result from a defect in the methylation cycle, as we were unable to detect inhibition of the methylation cycle by cycloleucine treatment using the dU suppression test (Fig. 1c). This was despite the fact that cycloleucine treatment did inhibit the methylation cycle, as shown by a significant reduction in the abundance of the intermediate s-adenosylmethionine in a control cell line (0.694 nmoles/mg protein compared to 0.496 nmoles/mg protein in treated cells; p<0.005). In addition, there was no apparent correlation between the abundance (or the ratio of abundance) of s-adenosylmethionine (SAM) or s-adenosylhomocysteine (SAH) and the results of the dU suppression test in the fetal cell lines (Fig. 3). In fact, the level of SAH (Fig. 3c) and the SAM/SAH ratio (Fig.3d), which are thought to be the key indicators of methylation cycle flux (Caudill et al., 2001), differed from the overall mean by less than one standard deviation in the majority of cell lines. In one individual the SAM/SAH ratio was unusually high corresponding to a very high SAM concentration although this sample exhibited a ‘normal’ result in the dU suppression test. The biological significance of this finding is unclear, as a high SAM/SAH ratio would be predicted to correlate with enhanced flux through the methylation cycle, whereas it is reduced flux that has been associated with NTDs in mouse models (Dunlevy et al., 2006a; Dunlevy et al., 2006b).
Figure 3. Abundance of methylation cycle intermediates.
Metabolites were quantified in individual cell lines from NTD cases and a subset of controls, and the data plotted in order of dU suppression test score (a). Abundance (mean ± SEM) of s-adenosylmethionine (b) and s-adenosylhomocysteine (c) and the SAM/SAH ratio (d) are shown. In each case the overall mean value (dashed line)and 1 standard deviation (dotted lines) are indicated.
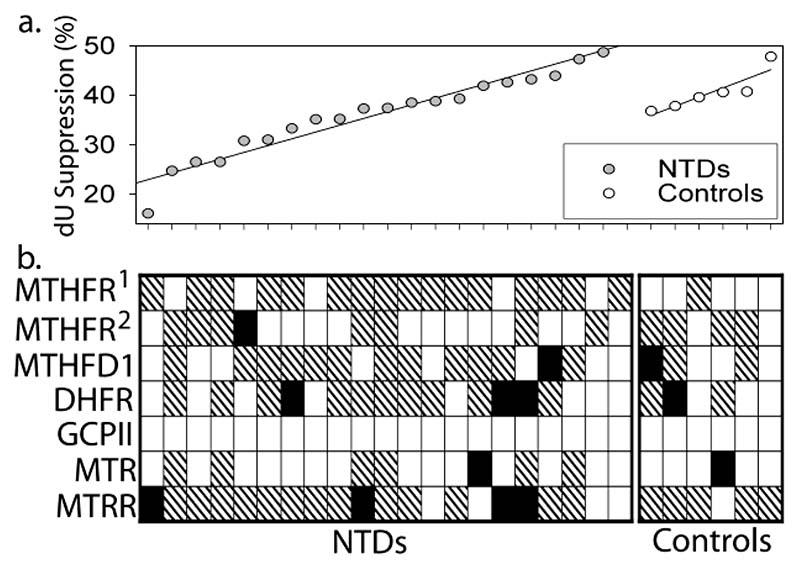
In order to test whether abnormal dU suppression response resulted from genetic lesions in the folate cycle, samples were genotyped for known polymorphisms in folate-related genes (Fig. 4), including 5,10-methylene tetrahydrofolate reductase (MTHFR), methylene tetrahydrofolate dehydrogenase/cyclohydrolase (MTHFD1), dihydrofolate reductase (DHFR), methionine synthase (MTR), methionine synthase reductase (MTRR) and glutamate carboxypeptidase II (GCPII). To date, the polymorphism most frequently linked to NTDs has been MTHFR C677T (Boyles et al., 2005). However, in this study neither occurrence of NTDs nor abnormal dU suppression response can be attributed to the TT genotype as every cell line carried at least one C allele. Apart from MTHFR, the A66G polymorphism in MTRR has been most consistently found to show an association with NTDs (Pietrzyk et al., 2003; Wilson et al., 1999; Guéant-Rodriguez et al., 2003), albeit mainly in combination with low cobalamin (Wilson et al., 1999) or the CC MTHFR genotype (Gueant-Rodriguez et al., 2003). Among NTDs in our study, four were homozygous for the MTRR A66G polymorphism, including the sample that exhibited the lowest score in the dU suppression test. However, MTRR genotype cannot solely explain the abnormal test results as the scores for the remaining cases are within the ‘normal’ range. This does not rule out the possibility that the GG genotype contributed to development of NTDs in these cases by a mechanism that does not involve significant suppression of the folate cycle. However, the frequency of GG genotype among the NTD cases (4 out of 21) does not significantly differ from the frequency in a control population (44 out of 184), analysed in a parallel study (data not shown). Overall, no obvious correlation between genotype and dU suppression score or occurrence of NTDs was detected (Fig. 4).
Figure 4. Abnormal folate metabolism does not correlate with known polymorphisms in folate-related genes.
Cell lines from NTD cases and a subset of controls were genotyped for polymorphisms in MTHFR (C677T1 and A1298C2), MTHFD1 (G1958A), DHFR (IVS1del19bp), GCPII (C1561T), MTR (A2756G) and MTRR (A66G). Cell lines are shown in order of score in the dU suppression test (a); genotypes (b) are indicated by white boxes for homozygous wild-type, hatched boxes for heterozygous and black boxes for homozygosity for the polymorphic allele.
Discussion
In this study, we observed diminished response in the dU suppression test, which indicates abnormal folate metabolism, in cell lines from a subset of fetuses affected by NTDs. Previous studies have investigated potential defects in folate metabolism in NTDs by quantification of metabolites in maternal blood. Interpretation is complicated, however, by the large number of folate derivatives, numerous feedback regulatory mechanisms and variation in findings between whole blood lysates and erythrocytes (Lucock et al.,1998; Lucock et al., 1997). Measuring the response to perturbation of folate metabolism may be more likely to reveal underlying defects. For example, cell lysates derived from blood of women who had previously experienced an NTD pregnancy were able to utilise exogenous folate derivatives less effectively than control lysates (Lucock, 2000). However, it is unclear how observations in non-gestational maternal blood extrapolate to pregnancy and to any effect on the fetus. One study utilised trophoblast cells, on the assumption that metabolism in these cells may be more relevant to the fetus. However, trophoblast is a highly specialised cell lineage that separates from the fetal component very early in development, only a few days after fertilisation. In a limited number of samples derived from placentae of control and NTD pregnancies (at mid-gestation or term), a short delay in incorporation of exogenous 5-methyl tetrahydrofolate into nucleic acid was observed in association with NTDs (Habibzadeh et al., 1993). In the present study, we assessed folate metabolism in fibroblasts of fetal origin and used the dU suppression test to provide a global test of the efficacy of the folate cycle, rather than relying on a single reaction of folate metabolism. We found an abnormal dU suppression result in approximately one-third of NTD samples, whereas the remaining NTD cell lines gave dU suppression values in the normal range. In the NTD cases that showed apparently normal dU suppression results it seems likely that the underlying causes are unrelated to folate metabolism, although we cannot exclude the possibility that such cases would be preventable by folic acid at high dose (4-5 mg per day). It is known, for example, that a proportion of NTDs are not preventable by folic acid (Wald et al., 1991; Blom et al., 2006). Larger scale studies will be needed, in future, to confirm the proportion of NTD fetuses in which a folate metabolic defect exists, and to determine the cause of NTDs that exhibit a normal dU suppression test.
In our study the dU suppression test was not sensitive to inhibition of the methylation cycle and, therefore we quantified s-adenosylmethionine and s-adenosylhomocysteine in controls and NTDs. Despite evidence from human (Blom et al., 2006) and mouse studies (Dunlevy et al., 2006b) that impairment of the methylation cycle may increase the risk of NTDs, we did not detect alterations in metabolite levels that would suggest such an effect in the cases examined in this study. However, as this study was based on cultured cells this does not rule out a role for NTD risk being increased by impaired methylation which results from an environmental effect, such as diet.
Currently, it is not possible to specify which step(s) of the folate cycle are sub-optimal leading to reduced availability of 5,10-methylene tetrahydrofolate, the cause of the abnormal dU suppression results in the NTD samples. Indeed, the underlying defect may differ between cases. However, as the fetal cell lines were cultured under identical conditions, and split several times prior to testing, variation in response to dU is presumably determined by genetic factors. Folate metabolism is regulated by a complex network of interactions in which abundance or ratios of metabolites influence activity of key enzymes. It is therefore likely that flux through the folate cycle and, hence, availability of 5,10-methylene tetrahydrofolate, is influenced by multiple gene products, some of which are direct mediators of folate cycle reactions. As a step towards identification of the genes responsible for abnormal folate metabolism we screened known polymorphisms. The lack of correlation between folate cycle efficacy and the known polymorphisms in folate-related genes suggests that other, as yet unidentified, mutations are responsible for abnormal folate metabolism in these NTD cases. Such changes could be in suspected ‘risk factor’ genes such as MTHFR, or may be in other genes that have not been associated with NTDs to date.
In summary, folic acid is known to reduce susceptibility to NTDs and our data suggest strongly that an apparently genetically determined abnormality in fetal folate metabolism, may be causally related to NTDs.
Acknowledgements
We thank the staff of the NE London Regional Cytogenetics Laboratory, Great Ormond St Hospital for establishing cell lines for our use. The authors are grateful to Kevin Mills for technical advice and to the Medical Research Council UK, SPARKS, BDF Newlife and the Wellcome Trust for financial support.
References
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle T, Griffin D. In: Human cytogenetics: constitutional analysis. 3rd edition. Rooney DE, editor. Oxford: Oxford University Press; 2001. The cytogenetics of pregnancy. [Google Scholar]
- Boyles AL, Hammock P, Speer MC. Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135:9–23. doi: 10.1002/ajmg.c.30048. [DOI] [PubMed] [Google Scholar]
- Brody LC, Conley M, Cox C, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71:1207–1215. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burren KA, Mills K, Copp AJ, Greene ND. Quantitative analysis of sadenosylmethionine and s-adenosylhomocysteine in neurulation-stage mouse embryos by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 doi: 10.1016/j.jchromb.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill MA, Wang JC, Melnyk S, et al. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Dunlevy LPE, Burren KA, Chitty LS, Copp AJ, Greene NDE. Excess methionine suppresses the methylation cycle and inhibits neural tube closure in mouse embryos. FEBS Lett. 2006a;580:2803–2807. doi: 10.1016/j.febslet.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Dunlevy LPE, Burren KA, Mills K, Chitty LS, Copp AJ, Greene NDE. Integrity of the methylation cycle is essential for mammalian neural tube closure. Birth Defects Res A Clin Mol Teratol. 2006b;76:544–552. doi: 10.1002/bdra.20286. [DOI] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nature Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- Gueant-Rodriguez RM, Rendeli C, Namour B, et al. Transcobalamin and methionine synthase reductase mutated polymorphisms aggravate the risk of neural tube defects in humans. Neurosci Lett. 2003;344:189–192. doi: 10.1016/s0304-3940(03)00468-3. [DOI] [PubMed] [Google Scholar]
- Habibzadeh N, Schorah CJ, Seller MJ, Smithells RW, Levene MI. Uptake and utilization of DL-5-[methyl-14C] tetrahydropteroylmonoglutamate by cultured cytotrophoblasts associated with neural tube defects. Proc Soc Exp Biol Med. 1993;203:45–54. doi: 10.3181/00379727-203-43571. [DOI] [PubMed] [Google Scholar]
- Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Genet. 2004;124A:339–345. doi: 10.1002/ajmg.a.20505. [DOI] [PubMed] [Google Scholar]
- Killman S-A. Effect of deoxyuridine on incorporation of tritiated thymidine: difference between normoblasts and megaloblasts. Acta Med Scand. 1964;175:483–488. doi: 10.1111/j.0954-6820.1964.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Kirke PN, Mills JL, Molloy AM, et al. Impact of the MTHFR C677T polymorphism on risk of neural tube defects: case-control study. Br Med J. 2004;328:1535–1536. doi: 10.1136/bmj.38036.646030.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–708. [PubMed] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Lucock MD, Daskalakis I, Lumb CH, Schorah CJ, Levene MI. Impaired regeneration of monoglutamyl tetrahydrofolate leads to cellular folate depletion in mothers affected by a spina bifida pregnancy. Mol Genet Metab. 1998;65:18–30. doi: 10.1006/mgme.1998.2738. [DOI] [PubMed] [Google Scholar]
- Lucock MD, Wild J, Lumb CH, et al. Risk of neural tube defect-affected pregnancy is associated with a block in maternal one-carbon metabolism at the level of N-5-methyltetrahydrofolate:homocysteine methyltransferase. Biochem Mol Med. 1997;61:28–40. doi: 10.1006/bmme.1997.2580. [DOI] [PubMed] [Google Scholar]
- Mills JL, McPartlin JM, Kirke PN, et al. Homocysteine metabolism in pregnancies complicated by neural- tube defects. Lancet. 1995;345:149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- Mills JL, Scott JM, Kirke PN, et al. Homocysteine and neural tube defects. J Nutr. 1996;126:756S–760S. doi: 10.1093/jn/126.suppl_3.756S. [DOI] [PubMed] [Google Scholar]
- Ou CY, Stevenson RE, Brown VK, et al. 5,10 methylenetetrahydrofolate reductase genetic polymorphism as a risk factor for neural tube defects. Am J Med Genet. 1996;63:610–614. doi: 10.1002/(SICI)1096-8628(19960628)63:4<610::AID-AJMG15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Pietrzyk JJ, Bik-Multanowski M, Sanak M, Twardowska M. Polymorphisms of the 5,10-methylenetetrahydrofolate and the methionine synthase reductase genes as independent risk factors for spina bifida. J Appl Genet. 2003;44:111–113. [PubMed] [Google Scholar]
- Scott JM. Folate and vitamin B12. Proc Nutr Soc. 1999;58:441–448. doi: 10.1017/s0029665199000580. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RPM, Boers GHJ, Trijbels FJM, et al. Maternal hyperhomocysteinemia: A risk factor for neural tube defects. Metabolism. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Van der Put NMJ, Gabreëls F, Stevens EMB, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects. Am J Hum Genet. 1998;62:1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Put NMJ, Van Straaten HWM, Trijbels FJM, Blom HJ. Folate, homocysteine and neural tube defects: An overview. Proceedings of the Society for Experimental Biology and Medicine. 2001;226:243–270. doi: 10.1177/153537020122600402. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Trembath D, Vandyke DC, et al. Studies with His475Tyr glutamate carboxipeptidase II polymorphism and neural tube defects. Am J Med Genet. 2002;111:218–219. doi: 10.1002/ajmg.10568. [DOI] [PubMed] [Google Scholar]
- Wald N, Sneddon J, Densem J, Frost C, Stone R, MRC Vitamin Study Res Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Wilson A, Platt R, Wu Q, et al. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab. 1999;67:317–323. doi: 10.1006/mgme.1999.2879. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wicker NJ, Shaw GM, et al. Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol Genet Metab. 2003;78:216–221. doi: 10.1016/s1096-7192(03)00008-8. [DOI] [PubMed] [Google Scholar]