Abstract
Purpose
Inhibition of monocarboxylate transporter (MCT) 1-mediated lactate transport may have cytostatic/cytotoxic effects on tumour cells. We report results from the dose-escalation part of a first-in-human trial of AZD3965, a first-in-class MCT1 inhibitor, in advanced cancer.
Experimental design
This multicentre, Phase 1, dose-escalation and dose-expansion trial enrolled patients with advanced solid tumours or lymphoma and no standard therapy options. Exclusion criteria included history of retinal/cardiac disease, due to MCT1 expression in the eye and heart. Patients received daily oral AZD3965 according to a 3+3 then rolling 6 design. Primary objectives were to assess safety and determine the maximum tolerated dose and/or recommended Phase 2 dose (RP2D). Secondary objectives for dose-escalation included measurement of pharmacokinetics and pharmacodynamic activity. Exploratory biomarkers included tumour expression of MCT1 and MCT4, functional imaging of biological impact and metabolomics.
Results
During dose-escalation, 40 patients received AZD3965 at 5–30 mg once daily or 10 or 15 mg twice daily (BD). Treatment-emergent adverse events were primarily Grade 1/2, most commonly electroretinogram changes (retinopathy), fatigue, anorexia and constipation. Seven patients receiving ≥20 mg daily experienced dose-limiting toxicities (DLTs): Grade 3 cardiac troponin rise (n=1), asymptomatic ocular DLTs (n=5) and Grade 3 acidosis (n=1). Plasma pharmacokinetics demonstrated attainment of target concentrations; pharmacodynamic measurements indicated on-target activity.
Conclusions
AZD3965 is tolerated at doses that produce target engagement. DLTs were on-target and primarily dose-dependent, asymptomatic, reversible ocular changes. An RP2D of 10 mg BD was established for use in dose-expansion in cancers that generally express high MCT1/low MCT4 (not yet published).
Keywords: AZD3965, MCT1, cancer, metabolism, dose-escalation
Introduction
Most tumour cells have increased dependency on the glycolytic pathway for adenosine-5’-triphosphate (ATP) generation (1, 2). The dependence of tumours on glycolysis seems counter-intuitive because rapid cell proliferation, typical of many tumours, has high energetic demands. The glycolytic phenotype, however, offers survival advantages to tumour cells, allowing them to meet their energetic and anabolic requirements and survive in fluctuating oxygen tensions. Key tumour-associated pathways, including oncogenic Myc, loss of p53 function, Ras signalling and hypoxia pathways, drive this phenotype, leading to upregulation of glycolytic enzymes and glucose/lactate transporters in tumour cells (3–5). This phenotype is exploited in [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) imaging for detection and diagnostic staging of many tumour types (6–10).
Intracellular lactate produced by glycolysis is transported out of cells by the monocarboxylate transporter (MCT) family (11, 12). MCT subtypes possess unique biochemical properties, with differential affinities for lactate and divergent sensitivity to known MCT inhibitors (13). High MCT1 expression occurs in a range of solid tumours, where it can function as an influx or efflux transporter of lactate, and it is the normal subtype for efflux under hypoxic conditions (13, 14).
AZD3965 is a selective and potent MCT1 inhibitor with activity against MCT2 but not MCT3/MCT4 and has demonstrated growth inhibition of various tumour cell lines, particularly lymphoma (14, 15). It is hypothesised that when MCT1 is inhibited in normal cells, other MCTs will transport lactate out of cells to maintain normal homeostasis. Accordingly, MCT4 expression has been identified as a resistance mechanism to MCT1 inhibition in tumour cells; introduction of MCT4 into a cell line expressing only MCT1 prevents both lactate accumulation and inhibition of cell growth by MCT1 inhibitors (16). Furthermore, tumour cell line panel data show that cell lines predominantly expressing MCT1 tend to be highly sensitive to AZD3965, whereas those that co-express MCT4 are far less sensitive or resistant (14). MCT1 is expressed in some normal tissues, including the retina and heart (17–20), but the aerobic glycolysis pathway is unlikely to be a major component of energy production in most normal cells, which should be unaffected by selective MCT1 inhibition.
This first-in-human trial of AZD3965 in patients with advanced solid tumours or lymphoma investigated the primary hypothesis that inhibiting MCT1 lactate transport out of cells will result in feedback inhibition of glycolysis and a pH imbalance that may lead to cytostatic/cytotoxic effects. In addition to this potential mechanism, there is evidence that normoxic tumour-associated stromal cells (and possibly hypoxic tumour cells) release lactate that normoxic tumour cells can take up via MCT1 and use as a respiratory substrate (21, 22). This hypothesis is supported by the expression profile of enzymes and transporters in tumour tissues and stromal cells (23), and evidence that tumour cells do not exist as homogeneous populations in vivo. Instead, they are embedded in tumour cell nests surrounded by stromal cells, especially cancer-associated fibroblasts. Thus, stromal fibroblasts could influence adjacent tumour cell metabolism and vice versa (24, 25). This mechanism may also be important in metastasis; tumour cells could couple their metabolism to that of local cells and establish new tumour cell nests. In this model, inhibition of lactate uptake via MCT1 could have anti-tumour effects by breaking the interlinked metabolism of tumour and stromal cells and depriving hypoxic/tumour cells of a key energy source (26). As solid tumours are heterogeneous systems involving multiple cell types across different oxygen and glucose conditions, both roles for MCT1 in lactate transport may co-exist in a single tumour. MCT1 inhibition, by affecting either (or both) lactate influx or efflux, provides a promising anti-cancer strategy.
Here, we report results from the dose-escalation phase (Part 1) of a 2 part (dose-escalation and dose-expansion) first-in-human trial of AZD3965 in advanced cancer.
Materials and Methods
This was a multicentre, open-label, non-randomized, dose-escalation Phase I trial conducted in the United Kingdom (NCT01791595) in two parts. The dose-escalation phase (Part 1; 2 centres) in patients with advanced solid tumours, was designed to determine the RP2D. The RP2D was used in the dose-expansion phase (Part 2; not yet published), in tumour types with high MCT1 and low MCT4 expression, or in which AZD3965 had a demonstrated effect preclinically. The primary objective during dose-escalation was to propose a RP2D and/or MTD of AZD3965 in patients with advanced cancer and establish its safety profile; secondary objectives were to determine the pharmacokinetic profile and pharmacodynamic activity of AZD3965.
The trial was undertaken under the sponsorship and management of the Cancer Research UK Centre for Drug Development, conducted in accordance with International Council for Harmonisation Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki, and approved by the UK Medicines and Healthcare products Regulatory Agency and National Health Service Health Research Authority (12/NE/0345).
Patients recruited during dose-escalation gave written informed consent and had histologically or cytologically-proven advanced solid tumours refractory to conventional treatment or for which no conventional therapy existed and had archival tumour samples available. Other key inclusion criteria were: age ≥18 years, World Health Organization (WHO) performance status 0–1, adequate haematological and biochemical laboratory measurements, glucose (fasting) <7.8 mmol/L, and left ventricular ejection fraction >50%. Key exclusion criteria were known retinal disease or macular degeneration affecting visual acuity (assessed by ophthalmologic tests), and cardiac conditions (including clinically significant cardiovascular event within previous six months and clinically significant arrhythmias).
Treatment
AZD3965 was administered by oral capsule once or twice daily (OD or BD) for six 28-day cycles. Upon data review following six cycles, patients experiencing benefit could continue.
Patients received a single dose of AZD3965 on Day -7 (D-7) to permit single-dose pharmacokinetic characterisation prior to commencing Cycle 1 (C1). In the absence of toxicity after D-7, patients commenced continuous daily dosing on C1 D1. A 14-day interval (from D-7) was observed between recruitment of the first and second patient in each cohort; subsequent patients could be treated in parallel.
AZD3965 binds to human MCT1 with high affinity (Ki 1.6 nM). The 5 mg OD starting dose was chosen based on preclinical observations of eye toxicity and represented approximately 1/20th of the electroretinogram (ERG) no observed adverse effect level, providing a wide safety margin for optical effects, and 1/20th of the dose estimated to result in a 24-hour free plasma concentration above that required to half maximally inhibit the growth of Raji lymphoma cells in vitro (IC50; 12 nM) (14).
The trial initially used 3+3 dose-escalation; a rolling six design (27) was used from Cohort 3. No intra-patient dose-escalation was permitted.
In the event of toxicity, treatment was to be delayed for ≤2 weeks until resolution to Grade ≤1 or baseline. If the toxicity was a dose-limiting toxicity (DLT), AZD3965 was to be reduced to the previous dose level following resolution, with the exception of corrected QT interval prolongation as affected patients were to be withdrawn from the trial. Patients experiencing subsequent DLTs or whose toxicity did not resolve within two weeks were withdrawn.
Assessments
Safety, DLTs and MTD
Safety data were collected from the date of written informed consent until 28 days after the final administration of AZD3965, and included reported adverse events (AEs), physical/vital signs, laboratory toxicities (haematology, biochemistry, urinalysis), and ophthalmic and cardiac assessments. AEs were classified and graded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02 and considered treatment-emergent if they started during or after the first dose of AZD3965.
DLTs were defined as any highly probable or probable AZD3965-related toxicities detailed in Supplemental Methods, including cardiac toxicities, ERG changes, toxicity that caused interruption of treatment for >2 weeks or death. DLTs were evaluated from D-7 to end of C1.
The MTD was defined according to NCI-CTCAE Version 4.02 as the total daily dose level below that at which two out of up to six evaluable patients at the same dose level experienced a DLT.
Pharmacokinetics
Blood samples for pharmacokinetic analysis were collected during C1 and on C2 D1 (refer to Supplemental Methods for timepoints). Plasma was separated by centrifugation at 1,000×g for 10 minutes, frozen at -20°C and analysed to quantify AZD3965 concentrations using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay. Pharmacokinetic parameters were calculated by non-compartmental analysis with a linear/log-trapezoidal model for area under the plasma concentration-time curve (AUC), using WinNonlin™ version 8.1 (Certara, Princeton, NJ, USA).
Pharmacodynamics and Exploratory Biomarkers
Lactate was measured in peripheral blood mononuclear cells (PBMCs) using a validated LC-MS/MS assay. Cell death biomarkers M65, M30, and nucleosomal DNA (nDNA) in plasma were measured using validated M30 Apoptosense® and M65® (Peviva®, VLVbio AB, Nacka, Sweden) and Cell Death Detection (Roche Diagnostics GmbH, Mannheim, Germany) enzyme-linked immunosorbent assays, respectively. Metabolites were detected in plasma and urine using solution state 1H magnetic resonance spectroscopy (MRS); a plasma metabolomics screen was performed using a validated LC-MS/MS protocol (AbsoluteIDQ® p180 kit, Biocrates life sciences AG, Innsbruck, Austria). Imaging pharmacodynamics included assessment of intracellular pH using three-dimensional 31P-MRS (28, 29) and a draft 1H-MRS protocol to detect lactate in the presence of lipid, in addition to [18F]FDG-PET/computerised tomography (CT) imaging using a dynamic PET acquisition performed over a discreet tumour focus and a static whole-body scan. MCT1 and MCT4 expression was assessed by immunohistochemistry (IHC) on archival tumour samples.
Refer to Supplemental Methods for details of procedures and timepoints.
Disease Assessments
Radiological disease assessments were conducted at baseline, at the end of every 2 cycles of AZD3965 and at end-of-treatment. Objective response was assessed according to Response Evaluation Criteria in Solid Tumours (RECIST) Version 1.1 (30).
Statistical Analyses
Eligible patients who received ≥1 dose of AZD3965 were evaluable for safety. The pharmacokinetic population comprised all eligible patients who received AZD3965 on D-7 and had at least a baseline and post-dose sample analysed. Pharmacodynamic/biomarker populations included patients who received AZD3965 and had evaluable pre- and post-treatment samples for blood and urine analyses, or had a tissue sample available for IHC, or had received at least 25% of their first AZD3965 cycle and had suitable tumours for imaging analyses. Data were summarised using descriptive statistics unless otherwise specified.
Results
Patient Characteristics and Treatment
Between April 2013 and October 2017, 42 eligible patients were enrolled during dose-escalation (Part 1); 40 patients received ≥1 dose of AZD3965 and two were withdrawn prior to receiving AZD3965. Enrolled patients had a median age of 64.5 years; all had received prior therapy (Table 1). All patients had advanced (Stage IV) solid tumours at baseline; colorectal adenocarcinoma and mesothelioma were the most common tumour types (20 [47.6%] and five [11.9%] patients, respectively).
Table 1. Patient Characteristics (All Patients).
| Characteristic | Patients (N=42) |
|---|---|
| Sex (male/female), n (%) | 25/17 (59.5/40.5) |
| Age, y, median (range) | 64.5 (18–79) |
| WHO Performance Status, n (%) | |
| 0 | 11 (26.2) |
| 1 | 31 (73.8) |
| Tumour type, n (%) | |
| Adenocarcinoma | 28 (66.7) |
| Colorectal | 20 (47.6) |
| Cholangiocarcinomaa | 3 (7.1) |
| Other adenocarcinomab | 5 (11.9) |
| Mesotheliomac | 5 (11.9) |
| Squamous cell carcinomad | 3 (7.1) |
| Melanomae | 2 (4.8) |
| Otherf | 4 (9.5) |
| Prior therapies, n (%) | |
| Chemotherapy/other therapies | 42 (100) |
| Surgery | 35 (83.3) |
| Radiotherapy | 17 (40.5) |
extra-hepatic bile duct (n=2), intra-hepatic bile duct
adenocarcinoma of the lung, stomach, cervix, urachus, unknown primary
oleura (n=4), paratesticular tissue
lung, oesophagus, nasopharynx (EBV+)
skin, eye
osteosarcoma (humerus), Ewing sarcoma (femur), poorly differentiated carcinoma (cervix), succinate dehydrogenase-deficient renal cell carcinoma (kidney).
Abbreviations: EBV, Epstein Barr virus; n, number; WHO, World Health Organization; y, year.
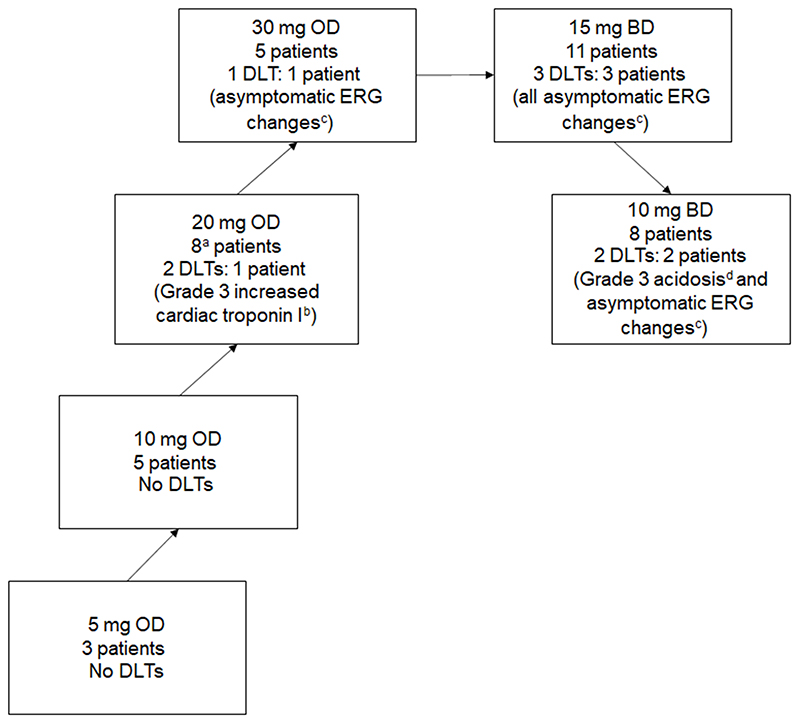
Treated patients (n=40) were included in six dose-escalation cohorts (Figure 1) receiving 5 (n=3), 10 (n=5), 20 (n=8) and 30 mg (n=5) AZD3965 OD, and 10 (n=8) and 15 mg (n=11) AZD3965 BD. Three patients withdrew after D-7 dosing. Patients who received AZD3965 given by continuous daily dosing (n=37) had a median of two cycles (range: 1–6). The most common reason for trial withdrawal was evidence of disease progression (n=23), followed by AEs/serious AEs (SAEs) (n=12), Investigator withdrawal due to clinical reasons not related to AZD3965 (n=2) and other reasons (n=2; one patient decided to stop taking AZD3965 and one patient died due to disease progression).
Figure 1. AZD3965 Dose Escalation.
a An additional two patients were enrolled in the 20 mg OD cohort but were withdrawn prior to receiving AZD3965.
b Two events of Grade 3 increased cardiac troponin I were reported for the same patient.
c ERG change DLTs were reported as AEs of retinopathy or eye disorders – other (ERG changes). All were Grade 1. One event was reported as Grade 2 but it was later confirmed by the Chief Investigator (Principal Investigator at the site) that this should have been recorded as Grade 1 since the patient was asymptomatic and had no change in visual acuity
d The DLT of Grade 3 acidosis occurred after the single dosing day of 20 mg OD at D-7; the patient was withdrawn from the trial prior to commencing daily dosing at 10 mg BD.
Abbreviations: BD, twice daily; DLT, dose limiting toxicity; ERG, electroretinogram; OD, once daily.
DLTs and MTD
Forty patients received ≥1 dose of AZD3965 and were evaluable for DLTs. Eight DLTs were reported in seven patients (Figure 1). The majority (5/8) were asymptomatic protocol-defined DLTs of ERG changes. One patient had two events of Grade 3 increased cardiac troponin I and one patient had Grade 3 acidosis (Figure 1).
ERG changes occurred at daily doses ≥20 mg; none of the ocular events were associated with visual symptoms and were mainly b-wave changes (Supplemental Table 1), indicating changes in bipolar cells. However, significant a-wave changes were seen at 30 mg OD and 15 mg BD AZD3965, indicating subclinical changes at the level of the photoreceptors of the outer retina. The 30 mg OD and 15 mg BD doses were considered intolerable as significant retinal changes were seen in most patients receiving these doses. As DLTs of cardiac troponin I increase were observed at 20 mg OD and the DLT of acidosis occurred after the D-7 20 mg OD dose, the MTD was defined as 10 mg BD AZD3965.
Safety
Of 40 patients evaluable for safety, 38 (95.0%) reported at least one treatment-emergent AE (TEAE), most commonly (≥30% patients) ERG changes (16 patients [40.0%], reported as retinopathy in 15 patients and eye disorders – other [ERG changes] in one patient), fatigue (17 patients [42.5%]), anorexia, constipation (each 14 patients [35.0%]), anaemia and nausea (each 13 patients [32.5%]). TEAEs occurring in ≥10% patients are shown by cohort in Supplemental Table 2. Two patients experienced Grade 5 events (death due to disease progression and hydronephrosis), which were not considered related to AZD3965.
TEAEs considered at least possibly related to AZD3965 were reported by 29 patients (72.5%), most commonly (≥20% patients) ERG changes (16 patients [40.0%]) and fatigue (nine patients; 22.5%) (Table 2). The majority of related TEAEs (97/111; 87.4%) were Grade 1–2, with 13 Grade 3 TEAEs occurring in five patients and one Grade 4 event (myelodysplastic syndrome). Five patients, all treated at AZD3965 daily doses ≥20 mg, experienced six treatment-emergent SAEs considered at least possibly related to AZD3965: ERG changes reported as retinopathy (n=2) and myelodysplastic syndrome, pneumonitis, vomiting and metabolic acidosis (each n=1).
Table 2. All-Grade TEAEs At Least Possibly Related to AZD3965 (All Patients who Received AZD3965).
| CTCAE Body System CTCAE AE Term n (%) | All patients N=40 | ||
|---|---|---|---|
| Grade 1–2 | Grade ≥3 | All Grades | |
| All Related TEAEs | 24 (60.0) | 5 (12.5) | 29 (72.5) |
| Eye disorders | 16 (40.0) | 0 (0.0) | 16 (40.0) |
| Retinopathya | 15 (37.5) | 0 (0.0) | 15 (37.5) |
| Blurred vision | 2 (5.0) | 0 (0.0) | 2 (5.0) |
| Eye disorders – other | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Eye pain | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Metabolism and nutrition disorders | 10 (25.0) | 2 (5.0) | 12 (30.0) |
| Anorexia | 7 (17.5) | 0 (0.0) | 7 (17.5) |
| Metabolism and nutrition disorders – other | 7 (17.5) | 0 (0.0) | 7 (17.5) |
| Hypophosphataemia | 1 (2.5) | 2 (5.0) | 3 (7.5) |
| Acidosis | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Hyponatremia | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Gastrointestinal disorders | 10 (25.0) | 1 (2.5) | 11 (27.5) |
| Nausea | 7 (17.5) | 0 (0.0) | 7 (17.5) |
| Vomiting | 3 (7.5) | 1 (2.5) | 4 (10.0) |
| Mucositis oral | 2 (5.0) | 0 (0.0) | 2 (5.0) |
| Constipation | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Dry mouth | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Dyspepsia | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| General disorders and administration site conditions | 8 (20.0) | 1 (2.5) | 9 (22.5) |
| Fatigue | 8 (20.0) | 1 (2.5) | 9 (22.5) |
| Malaise | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Blood and lymphatic system disorders | 3 (7.5) | 2 (5.0) | 5 (12.5) |
| Anaemia | 3 (7.5) | 2 (5.0) | 5 (12.5) |
| Nervous system disorders | 5 (12.5) | 0 (0.0) | 5 (12.5) |
| Dysgeusia | 2 (5.0) | 2 (5.0) | 2 (5.0) |
| Concentration impairment | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Headache | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Dizziness | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Investigations | 2 (5.0) | 2 (5.0) | 4 (10.0) |
| Alkaline phosphatase increased | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Cardiac troponin I increased | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Cardiac troponin T increased | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Lymphocyte count decreased | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Respiratory, thoracic and mediastinal disorders | 3 (7.5) | 1 (2.5) | 4 (10.0) |
| Dyspnoea | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Epistaxis | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Hoarseness | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Pneumonitis | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Renal and urinary disorders | 3 (7.5) | 3 (7.5) | 3 (7.5) |
| Cystitis noninfective | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Proteinuria | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Urine discolouration | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Injury, poisoning and procedural complications | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Bruising | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Musculoskeletal and connective tissue disorders | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Pain in extremity | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Neoplasms benign, malignant and unspecified | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Myelodysplastic syndrome | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Skin and subcutaneous tissue disorders | 1 (2.5) | 0 (0.0) | 1 (2.5) |
| Dry skin | 1 (2.5) | 0 (0.0) | 1 (2.5) |
Related AEs are those with a causality of possible, probable or highly probable by Investigator assessment
All retinopathy AEs comprised changes on ERGs, and one additional event was reported using a different term (eye disorders – other (ERG changes).
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; TEAE, treatment emergent adverse event.
As eye toxicity had been observed preclinically, ophthalmic tests were carefully monitored, and any vision changes followed up with further assessments. Twenty-five related TEAEs were reported in the eye disorders body system: ERG changes (n=22), blurred vision (n=2), and eye pain (n=1). Ocular events started at doses of 20 mg daily (Supplemental Table 1). All 22 ERG change TEAEs were Grade 1 (asymptomatic), most were reported during C1 (14 patients reported 14 TEAEs) and most resolved during the trial.
Five patients (12.5%) had high cardiac troponin measurements considered clinically significant and reported as TEAEs; in three, this was increased cardiac troponin T, and in two, troponin I. Of these, two patients had cardiac troponin TEAEs considered at least possibly related to AZD3965. The patient with DLTs of Grade 3 cardiac troponin I rise received AZD3965 20 mg OD and had peaks in cardiac troponin I after each dose, indicating a cause of myocyte necrosis related to AZD3965 as opposed to myocardial infarction. The other patient had a Grade 1 cardiac troponin T rise considered probably related to AZD3965.
The patient with a DLT of acidosis had exceptionally high urinary and plasma lactate and ketone levels at baseline, which increased further upon administration of a single 20 mg dose of AZD3965 (D-7) (31). Eligibility criteria were changed to exclude patients with lactate and bicarbonate levels outside normal ranges.
Although MCT1 is expressed on erythrocytes and preclinical studies showed decreases in haemoglobin and haematocrit, no AZD3965-related SAEs of anaemia were observed. The patient with a Grade 4 SAE of myelodysplastic syndrome was withdrawn from the trial; a relationship to AZD3965 could not be excluded but it was considered probably related to previous treatments.
Thirteen patients (37.5%) were withdrawn from the trial due to TEAEs, the majority (n=6) due to ERG changes. Four patients (10.0%) discontinued AZD3965 due to TEAEs but were subsequently withdrawn from the trial due to evidence of disease progression. Twelve patients experienced dose delays, 29 had doses not given, eight experienced dose reductions and one had one extra day of dosing. The majority of dose reductions (six reductions in six patients) occurred in response to ERG changes.
Pharmacokinetics
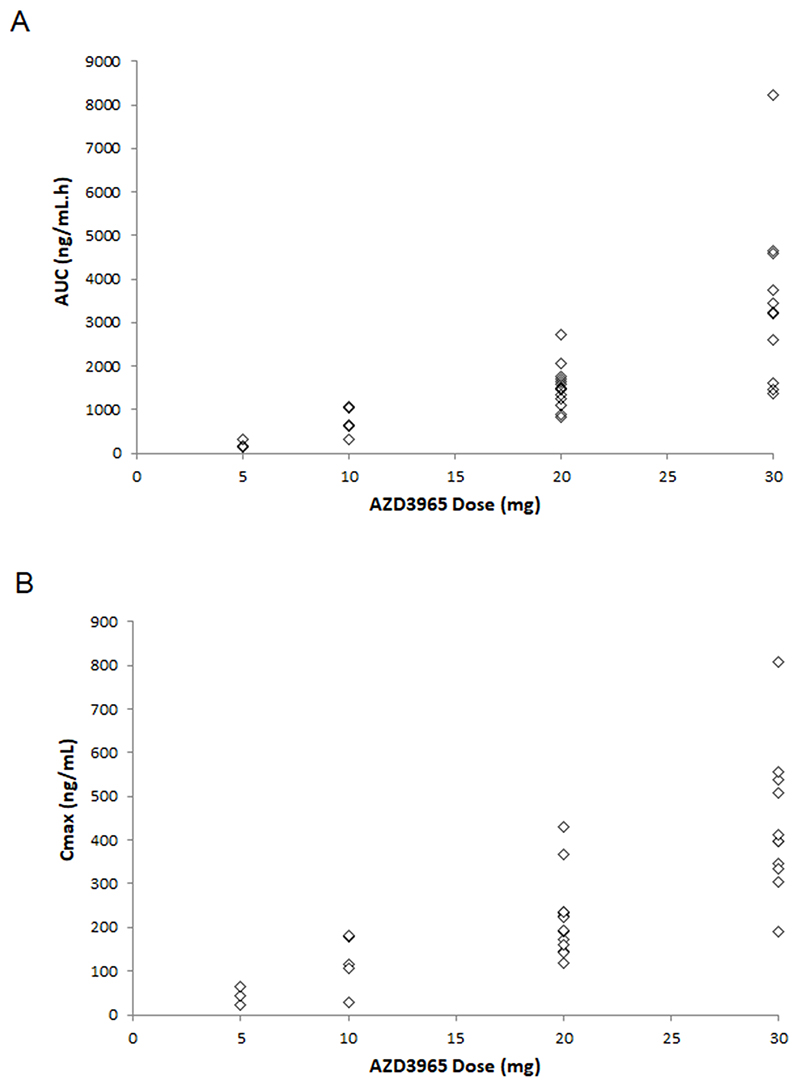
Plasma samples were obtained and analysed for 33 patients. Patients receiving AZD3965 BD received the total dose OD on D-7, before commencing continuous BD dosing on D1; therefore, D-7 pharmacokinetic data (Table 3) reflect the full daily dose. Exposure (maximum plasma concentration [Cmax] and AUC) tended to increase proportionally with dose, although there was high inter-patient variability at all dose levels (Figure 2). Other pharmacokinetic parameters, including terminal half-life, apparent clearance and apparent volume of distribution, were relatively consistent across dose levels. D1 pharmacokinetic data were generally comparable to D-7. AZD3965 half-life was assessed using D-7 data; mean half-life by dose was 36.5–51.9 hours, with shorter half-lives associated with higher doses.
Table 3. Summary Pharmacokinetic Parameters on D-7 by Dose Level of AZD3965.
| Dose Level (mg) | Number of Patients | Cmax (ng/mL) | AUC0-24 (ng/mL•h) | Half-Life (h) | Cl/F (L/h) | Vz/F (L) |
|---|---|---|---|---|---|---|
| 5 | 3 | 21.7–65.6 | 212±85 | 51.9±4.9 | 14.9±3.7 | 1115±290 |
| 10 | 5 | 28.2–182.2 | 731±311 | 45.4±6.4 | 9.5±1.9 | 630±174 |
| 20 | 13 | 117.6–431.2 | 1521±497 | 36.5±5.7 | 11.4±3.8 | 595±206 |
| 30 | 11 | 191.2–808.0 | 3466±1952 | 38.1±10.4 | 9.5±5.2 | 534±363 |
Dose level of 20 mg includes data from AZD3965 20 mg OD and 10 mg BD dose cohorts (both received 20 mg on D-7) and dose level of 30 mg includes data from AZD3965 30 mg OD and 15 mg BD dose cohorts (both received 30 mg on D-7). All values expressed as mean ± standard deviation, except for Cmax, where range is presented.
Abbreviations: AUC0-24, area under the curve during 24 hours; Cmax, maximum observed plasma concentration; Cl/F, apparent clearance of distribution; D=day; Vz/F, apparent volume of distribution.
Figure 2. Summary of Pharmacokinetics: (A) AUC and (B) Cmax versus Dose of AZD3965 at D-7.
Dose level of 20 mg includes data from AZD3965 20 mg OD and 10 mg BD dose cohorts (both received 20 mg on D-7) and dose level of 30 mg includes data from AZD3965 30 mg OD and 15 mg BD dose cohorts (both received 30 mg on D-7). Both panels show individual data per patient.
Abbreviations: AUC, area under the curve; Cmax, maximum plasma concentration; D=day.
The minimum plasma concentration (Cmin) roughly increased with dose, with most patients having Cmin >12 nM (IC50 against Raji lymphoma cells in vitro (14)). BD dosing cohorts, as expected, had higher levels pre-dose D2 than OD dosing cohorts (assessed 12 versus 24 hours post-D1 dose, respectively). Pharmacokinetic data for Cohorts 1–4 indicated that plasma concentrations would be maintained more effectively with BD than OD dosing. There was a trend towards an increase in Cmin across the cycle (pre-dose D2 vs D8, median ratio 1.68), indicating drug accumulation. However, although the Cmin increased with BD dosing, the accumulation ratio did not appear to do so (pre-dose D2 vs D8 median ratio 1.55).
Pharmacodynamics and Exploratory Biomarkers
Extensive pharmacodynamic and exploratory biomarker analyses for this first-in-class agent were undertaken during the dose-escalation phase reported here, to identify optimal assessments and timings for further exploration of target engagement during dose-expansion.
Lactate accumulation and pH changes were assessed to determine if preclinical findings demonstrating AZD3965 target engagement translated to the clinic. In PBMCs, high inter-patient variability was observed with no clear evidence of significant lactate accumulation. To assess tumour pH changes in response to lactate accumulation, 31P-MRS imaging was undertaken, with data for nine patients available (5 mg OD: n=3; 10 mg OD: n=2; 20 mg OD: n=3). No consistent change in pH between pre- and post-AZD3965 values was detected. Ten repeat baseline measurements from seven patients showed reproducibility: the mean difference in measured pH was 0.08 ±0.05 pH units (range 0.01–0.18 pH units). As a pilot, a validated 1H-MRS method for measuring tumour lactate was tested in one patient who received AZD3965 15 mg BD; repeat measurements were considered an indication of reproducibility. Mean lactate concentration was 8.0 ±2.1 mM, with values of 10.71, 7.88, 5.45 and 7.98 mM at baseline and C1 D3, D8 and D28, respectively.
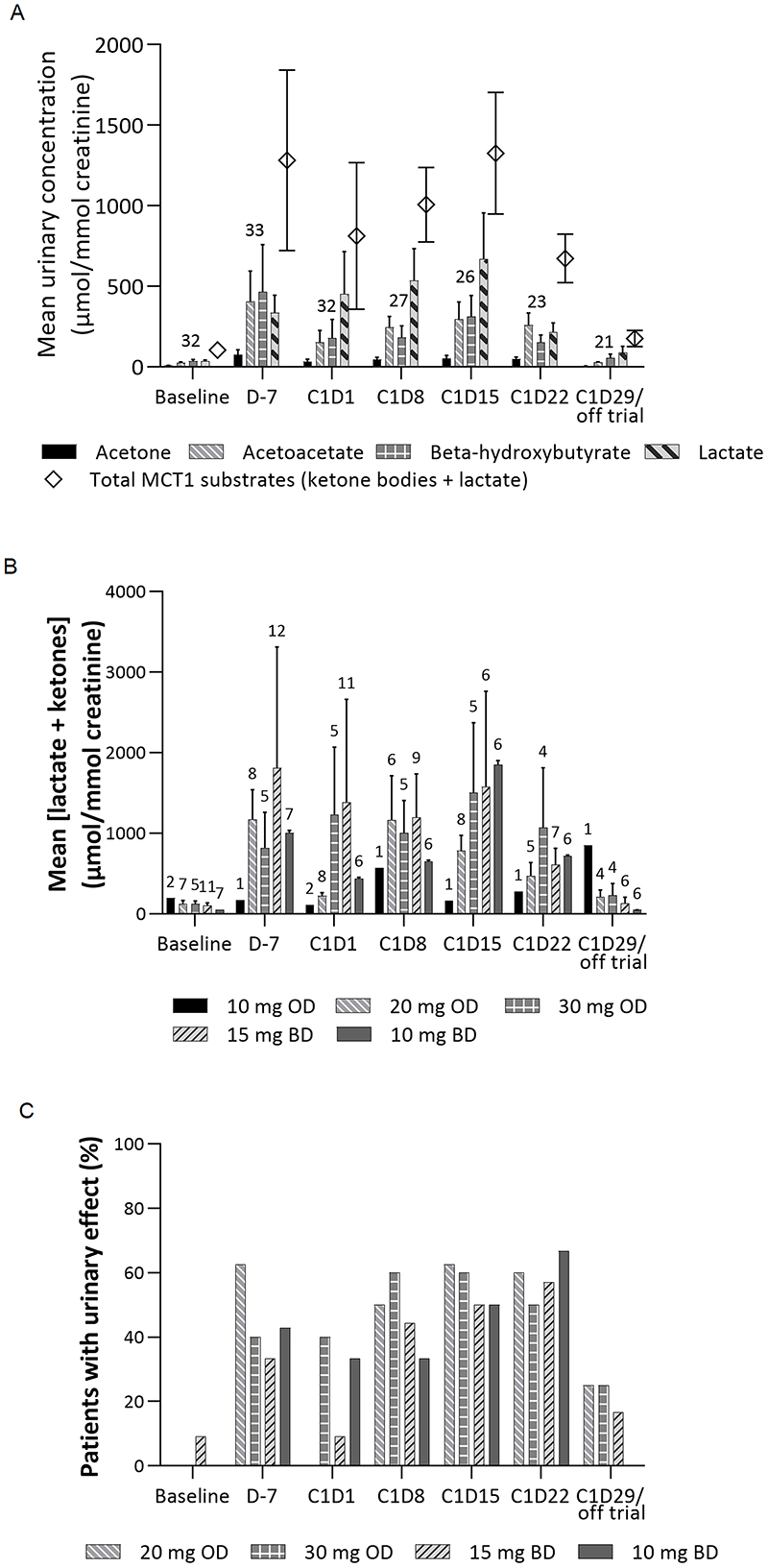
Metabolites in plasma and urine were measured to investigate target engagement. Mean urinary concentration of metabolites indicated significant elevations of lactate and ketone metabolite excretion post-treatment, with recovery to near-baseline at end-of-cycle (Figure 3A). No change in excretion of urinary citrate (not an MCT1 substrate) was seen. Mean values also suggested that the dynamics of lactate response were different from those of ketone metabolites, peaking at a later timepoint (C1 D15 versus D7). However, considerable variation was observed across patients, and a progressive increase in changes with repeated dosing or between cohorts was not clear (Figure 3B). An apparent decrease in changes at C1 D22 suggested possible adaptation to treatment. Using a threshold of 5×median across all patients at baseline (463.9 μmol/mmol creatinine) to define a treatment-related (‘urinary’) effect, an approximately 50% urinary effect rate was observed at multiple timepoints (Figure 3C). Urinary effect rates for all cohorts studied were broadly similar.
Figure 3. Average Urinary Metabolite Excretion (A) Over Time Across All Patients, (B) of Total MCT1 Substrates Over Time Across All Patients by Cohort, and (C) Effect Rates at Each Timepoint According to Urinary Metabolite Excretion Across Cohorts 3–6.
Number of patients for each analysis shown in numerals above relevant bars in panels (A) and (B); the analysis population for panel (C) is the same as for panel (B). Urinary effect defined as 5×median urinary concentration of total MCT1 substrates (lactate and ketone bodies) across all patients at baseline (463.9 μmol/mmol creatinine).
Abbreviations: BD, twice daily; C, cycle; D, day; MCT, monocarboxylate transporter; OD, once daily.
In contrast, no clear AZD3965-related effect could be detected by nuclear magnetic resonance (NMR) spectroscopy analysis of major MCT1 substrates in blood plasma. Targeted metabolite profiling in blood plasma by LC-MS/MS detected minor increases in blood glucose and proline, with concomitant decreases in serine and glycine, after AZD3965 dosing. However, there was no clear rationale linking this specific set of metabolite responses directly to MCT1 function.
As sensitivity to AZD3965 in cell lines in vitro is reliant on high MCT1 and low MCT4 expression, archival tumour samples from 35 patients were analysed for protein expression by IHC. Overall, 34/35 samples showed at least some positive tumour cell staining for MCT1, with a median H-score of 200 (range 6–300); 28/35 showed ≥50% MCT1-positive tumour cells (any intensity). However, 34/35 samples showed at least some positive tumour cell staining for MCT4, with a median H-score of 120 (range 15–300); 25/35 showed ≥50% MCT4-positive tumour cells (any intensity). Within most individual samples, there was spatially heterogeneous expression of both MCT1 and MCT4, with tumour cell staining intensity often ranging from negative to 3+ in different areas of the same section. Striking ‘micro-heterogeneity’ of staining for both MCT1 and MCT4 within individual neoplastic glands was also observed in several samples. In line with the lack of an appropriate phenotype for sensitivity to AZD3965 in solid tumours, there was no clear pattern in cell death marker modulation (M65, M30 or nDNA) in response to AZD3965 (Supplemental Figure 1).
As it was expected that the effect of AZD3965 on lactate utilisation within the tumour microenvironment would lead to increased uptake of glucose/[18F]FDG as an energy source, [18F]FDG-PET/CT scans were used to determine changes in metabolism in normal tissue and tumours in response to AZD3965 in eight patients administered AZD3965 15 mg BD. Amongst normal tissues, no effect of AZD3965 was seen on [18F]FDG uptake in brain, heart or muscle. Uptake of [18F]FDG in the bladder is known to be highly variable; however, a statistically significant increase in urinary [18F]FDG activity in the bladder was observed when comparing immediate follow-up scans to baseline (standardised uptake value [SUV] peak: p=0.050; SUV mean: p=0.004), with a mean change of 5.1 in SUV mean (95% confidence interval 2.3–7.9). In tumours, seven patients had slight increases in [18F]FDG uptake consistent with natural progressive disease; one patient had a 35.1% increase in tumour [18F]FDG uptake (SUV peak) on immediate response scanning (C1 D2), sustained on delayed response scanning (34.3%; C1 D23).
Anti-Tumour Activity
Of 39 eligible patients who received ≥1 dose of AZD3965 and had a baseline disease assessment, nine (23.1%) had a best response of stable disease, 18 (46.2%) had progressive disease, and three (7.7%) had early progression. The remaining nine patients (23.1%) only had a baseline disease assessment and could not be evaluated for response. Median time-to-progression for the 24 patients (60.0%) with available data was 57 days (range: 7–217 days). Three patients dosed at 30 mg OD, 15 mg BD and 10 mg BD had progression-free survival ≥3 months; two had colorectal adenocarcinoma and one had mesothelioma.
Discussion
AZD3965 is a first-in-class selective and orally bioavailable inhibitor of MCT1. We aimed to recommend an RP2D and schedule by evaluating tolerability, profiling pharmacokinetics, studying pharmacodynamics and validating feasibility of using biomarkers predictive of response using the principles of the pharmacological audit trail (32). The safety profile of AZD3965 was consistent with inhibition of MCT1. On-target dose-dependent ocular events were the most common TEAEs during C1, commencing at doses of 20 mg daily and reflecting reversible, asymptomatic ERG changes. Significant a-wave changes were seen in most patients at 30 mg OD and 15 mg BD AZD3965, indicating subclinical changes at the level of the photoreceptors of the outer retina. MCT1 is expressed in the retina and decreased visual acuity has been demonstrated to be a class effect preclinically, with recovery following treatment cessation (17, 18). Ophthalmic examination, including ERG, acuity, visual field assessments and retinal imaging, conducted throughout this trial allowed close monitoring of retinal effects, with dose interruptions and modifications used for management. The reversible, asymptomatic nature of the ERG changes at the RP2D of 10 mg BD allowed DLT criteria to be revised for the dose-expansion phase to enable patients with changes not considered clinically significant to continue dosing and to allow long-term effects to be explored in patients with tumour types selected for high MCT1 and low MCT4 expression, where longer-term dosing was anticipated (not yet published). Although retinal effects were monitored as evidence of on-target effects of AZD3965 in this trial, it is expected that minimal clinical monitoring of retinal effects would be required in practice if vision remains unaffected in further studies.
Cardiac toxicities were monitored closely due to expression of MCT1 in the heart and preclinical toxicity observations (19, 20). Cardiac troponin changes were observed, including two DLTs of troponin I increase in one patient and one additional patient with cardiac troponin changes considered at least possibly related to AZD3965. Additional cardiac troponin monitoring was implemented in the trial following the DLTs, but no other cardiac AEs were reported.
One patient who experienced a DLT of acidosis was found to have had exceptionally high baseline urinary and plasma lactate and ketone levels, which increased further upon a single AZD3965 20 mg administration. The lack of clinical symptoms from the elevated lactate and low blood glucose suggested a diagnosis of ‘hyper-Warburgism’, where the high tumour burden was associated with extensive glucose update and lactate efflux from malignant cells (31). The temporal relationship with AZD3965 administration suggested AZD3965 might have precipitated the patient’s deterioration, but deterioration was likely to have occurred upon disease progression without AZD3965. The patient was withdrawn from the trial due to acidosis; subsequent patients enrolled were required to have lactate and bicarbonate levels within normal ranges and routine laboratory tests for lactate throughout the trial were introduced as a safety measure. No other acidosis of any grade was reported.
The MTD was defined as AZD3965 10 mg BD based on DLTs observed at 30 mg OD, 15 mg BD and 20 mg OD. There was a general trend towards increased AZD3965 Cmax values and exposure with increased dose. Pharmacokinetic data indicated that plasma concentrations would be maintained more effectively with BD than OD dosing, without increased accumulation. Considering safety and pharmacokinetic data, the RP2D was determined to be 10 mg BD.
As AZD3965 is a new treatment modality, extensive assessments were carried out during dose-expansion to determine which preclinical pharmacodynamic measurements translated to the clinic and could be informative as proof-of-principle for target engagement and potential biomarkers of response for further exploration in a population with indications shown to have high MCT1 and low MCT4 expression during dose-expansion. Pharmacodynamic analyses were based on the hypothesis that most tumour cells are dependent on the glycolytic pathway; certain assays were determined to be uninformative, possibly due to assay sensitivities and differing levels and metabolism of lactate in different body compartments.
It was hypothesized that lactate accumulation in PBMCs could provide a direct measure of clinical AZD3965 target engagement, as intracellular lactate had been shown to increase following AZD3965 administration preclinically (14), and it was anticipated these assays would be more sensitive to on-target lactate changes than standard whole-blood assays, which primarily measure extracellular lactate and could be confounded by background noise. However, methodological problems and high inter-patient variability made this assay uninformative, perhaps confounded by the labile nature of lactate as a metabolite. As routine blood lactate assays were introduced for the dose-expansion phase to monitor for toxicities, their utility in measuring AZD3965-related lactate changes can be further explored.
MCT1 inhibition was expected to lead to a rise in the concentration of intracellular lactate, and a corresponding fall in intracellular pH. No consistent change in pH was detected, but this was not surprising given the lack of appreciable anti-tumour effects of AZD3965 in a population unselected for MCT4/MCT1 expression. Furthermore, preclinical studies completed after commencement of this trial showed no detectable change in the intracellular pH of Raji lymphoma xenograft tumour cells (high MCT1, low MCT4) following treatment with AZD3965 despite tumour lactate accumulation (33). The one patient assessed by 1H-MRS for lactate concentration had a tumour unlikely dependent on MCT1; however, the method was shown to be reproducible. Results suggested that changes of >50% in lactate concentration may be regarded as significant and should be used to assess AZD3965-related changes in cohorts of sensitive tumours.
Preclinical metabolomic studies suggested exposure to AZD3965 produced an increase in lactate, ketone metabolites and citrate in plasma and urine and a decrease in blood lipids in tumour-bearing and non-tumour-bearing animals (34). MCT1 is expressed in the kidney, where it is involved in reabsorbing lactate (35); therefore, the changes in total urinary excretion of lactate and ketone metabolites after AZD3965 administration in this trial indicated target engagement in the clinic, making these markers of interest for further exploration as biomarkers of response to AZD3965.
Immunohistochemistry in tumour samples was shown to be robust and reproducible in detecting MCT1 and MCT4 expression and all but one patient with MCT1 expression also had MCT4 expression. These assays were implemented for patient selection for the dose-expansion part of the trial to identify patients who were more likely to respond to AZD3965. Of note, the patient with an immediate and sustained increase in [18F]FDG uptake following a single dose of AZD3965 had one of the highest MCT1 scores (H-score 300, 100% positive) and lowest MCT4 scores (H-score 90, 40% positive) seen in the trial. While this was a finding in a single patient, these data were encouraging for the possible utility of [18F]FDG PET imaging in dose-expansion.
There is now increased understanding of the altered metabolic pathways that cancer cells exploit to fulfil their energy needs. These differences from normal cells offer promising targets for therapeutics albeit with a narrow therapeutic window. However, the flexibility shown by tumour cells to adapt their metabolism to fulfil energy demands and the complexity of the tumour microenvironment hamper success and there is a lack of agents with successful translation to the clinic (36–38).
Target engagement by AZD3965 was demonstrated through on-target retinal and cardiac effects and acidosis, together with metabolic changes, but was conducted in an unselected patient population found to rarely express the high MCT1/low MCT4 phenotype considered required for response to AZD3965. After evaluating and demonstrating manageability of the on-target toxicities during dose-escalation as described here, the RP2D has now been administered during dose-expansion in patients with tumour types shown to express high MCT1/low MCT4 as proof-of-principle for MCT1 inhibition as an anti-tumour strategy.
Supplementary Material
Statement of Translational Relevance.
The increased dependence of tumour cells on glycolysis compared with normal cells provides an opportunity to target cell metabolism as an anti-tumour strategy.
Monocarboxylate transporters (MCTs) have a key role in transport of intracellular lactate produced by glycolysis in tumour cells. Preclinically, differential expression of MCT subtypes in tumour cells can be successfully targeted using a potent and selective first-in-class MCT1 inhibitor, AZD3965, with anti-tumour effects observed.
Here, we demonstrate that predicted target engagement concentrations were reached at tolerable doses of AZD3965 in patients with advanced solid tumours in the dose-escalation part of a first-in-human Phase I trial. Assessment of exploratory biomarkers demonstrate proof-of-principle for target engagement and support further exploration of AZD3965 in cancer types expressing high MCT1 and low MCT4.
Acknowledgments
This trial was sponsored by Cancer Research UK. GP was funded by Cancer Research UK and EPSRC Cancer Imaging Centre Grants C1060/A10334 and C1060/A16464. UB was funded by NIHR RP 2016-07-028 and an infrastructural grant from National Institute of Health Biomedical Research Centre initiative.
Immunohistochemistry was performed by the MRC/EPSRC Newcastle Molecular Pathology Node (now renamed NovoPath).
The authors would like to acknowledge the following individuals for their contributions to the this clinical trial: Melanie Griffin for pharmacokinetic analyses, Dr Alexandros Siskos (Lead Analyst), Dr ChungHo Lau, Dr Eirini Kouloura and Dr Arti Sikka for their involvement with the metabolomics analyses; Professor Anthony Moore, Clinical Pediatrics, University of California San Franscisco (UCSF), San Francisco, United States and Professor Graham Holder, Moorfields Eye Hospital, London, United Kingdom for contributions on ophthalmology, and Dr Jonathan Tugwood and team for their work on the cell death biomarker analyses.
Finally, the authors thank all of the patients and clinical research team members who contributed to this research.
Financial support
The trial was sponsored by Cancer Research UK. Infrastructure for this research was supported by funding from Cancer Research UK and the Departments of Health to Experimental Cancer Medicine Centres (ECMCs), including the Newcastle, Institute of Cancer Research and The Royal Marsden, and Imperial College ECMCs. This work was also supported by the National Institute for Health Research (NIHR), Imperial Biomedical Research Centre (BRC).
Footnotes
Conflict of interest disclosure statement: SH, ID, KH, SP and BMS are employees of Cancer Research UK. SRW: Former employee and stockholder of AstraZeneca; receives research funding from Cancer Research UK and Astex Pharmaceuticals; secondee of Cancer Research UK. PS: AMLo Biosciences (employment); MCP: Honoraria from Merck, Eisai and Incyte. AG: Honoraria for advisory boards and/or speaker fees for Bayer, Roche, Amgen, Janssen, MSD (Merck Sharp and Dohme), Bristol Myers Squibb, AstraZeneca, Boehringer-Ingelheim, Pfizer, Takeda and Novartis. Research grants from AstraZeneca. CP: Honoraria for delivery of educational talks or chairing educational meetings from Abbott, Amgen, Celgene, Incyte, Ipsen, Medtronic, Novartis, Pfizer, and Roche. GP: Honoraria for delivery of educational talks or chairing educational meetings by GE Healthcare and Gilead sciences. HCK: Research grants from AstraZeneca. UB: Honoraria for attending advisory boards for Boehringer Ingelheim, Jannssen and Pegacy. Clinical trial funding in clinical trials from Onyx, BTG, Carrick Therapeutics, Chugai and Verastem Oncology. RP: Honoria for attending advisory boards from Pierre Faber, Bayer, Novartis, Biosceptre, BMS, Cybrexa, Ellipses, CV6 Therapeutics, Astex Therapeutics, Medivir, GammaDelta Therapeutics, and Sanofi Aventis; honoraria for delivery of educational talks or chairing educational meetings by AstraZeneca, Novartis, Bayer, Tesaro and BMS and received funds to support attendance at conferences from MSD and BMS. GJV, GSP, CMB, ECH, WAI, KM, MR: No conflicts of interest to declare.
Data Availability
Data from this trial and the final clinical study protocol have been submitted to ClinicalTrials.gov and will be available immediately following publication, with no end date, at https://clinicaltrials.gov/ct2/show/results/NCT01791595. Individual deidentified patient data that underlie the results reported in this article (text, figures, tables and supplementary information) will be shared with researchers whose proposed use of the data is approved by a review committee of the Sponsor. All requests made within 5 years from end-of-trial will be considered; requests made subsequently will be considered where possible. Requests should be submitted to drugdev@cancer.org.uk.
References
- 1.Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:113. doi: 10.1038/nrclinonc.2017.1. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Yu L, Chen X, Sun X, Wang L, Chen S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J Cancer. 2017;8:3430–40. doi: 10.7150/jca.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–99. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesney J, Telang S. Regulation of glycolytic and mitochondrial metabolism by ras. Curr Pharm Biotechnol. 2013;14:251–60. doi: 10.2174/1389201011314030002. [DOI] [PubMed] [Google Scholar]
- 6.Gallamini A, Zwarthoed C, Borra A. Positron Emission Tomography (PET) in Oncology. Cancers (Basel) 2014;6:1821–89. doi: 10.3390/cancers6041821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanmugam M, McBrayer SK, Rosen ST. Targeting the Warburg effect in hematological malignancies: from PET to therapy. Curr Opin Oncol. 2009;21:531–6. doi: 10.1097/CCO.0b013e32832f57ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailly C, Carlier T, Jamet B, Touzeau C, Moreau P, Kraeber-Bodere F, et al. (18)F-FDG PET/CT in multiple myeloma: critical insights and future directions. Eur J Nucl Med Mol Imaging. 2019;46:1048–50. doi: 10.1007/s00259-019-04279-7. [DOI] [PubMed] [Google Scholar]
- 9.El-Galaly TC, Villa D, Gormsen LC, Baech J, Lo A, Cheah CY. FDG-PET/CT in the management of lymphomas: current status and future directions. J Intern Med. 2018;284:358–76. doi: 10.1111/joim.12813. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD. PET/CT in Lymphoma: Current Overview and Future Directions. Semin Nucl Med. 2018;48:76–81. doi: 10.1053/j.semnuclmed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Fisel P, Schaeffeler E, Schwab M. Clinical and Functional Relevance of the Monocarboxylate Transporter Family in Disease Pathophysiology and Drug Therapy. Clin Transl Sci. 2018;11:352–64. doi: 10.1111/cts.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halestrap AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. 2013;34:337–49. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP. The monocarboxylate transporter family—Structure and functional characterization. IUBMB life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 14.Curtis NJ, Mooney L, Hopcroft L, Michopoulos F, Whalley N, Zhong H, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget. 2017;8:69219–36. doi: 10.18632/oncotarget.18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble RA, Bell N, Blair H, Sikka A, Thomas H, Phillips N, et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2017;102:1247–57. doi: 10.3324/haematol.2016.163030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Floch R, Chiche J, Marchiq I, Naiken T, Ilk K, Murray CM, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011;108:16663–8. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen AE, Martin EA, Greenwood K, Grant C, Vince P, Lucas RJ, et al. Effects of a monocarboxylate transport 1 inhibitor, AZD3965, on retinal and visual function in the rat. British journal of pharmacology. 2020;177:4734–49. doi: 10.1111/bph.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhart DZ, Leino RL, Drewes LR. Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience. 1999;92:367–75. doi: 10.1016/s0306-4522(98)00699-x. [DOI] [PubMed] [Google Scholar]
- 19.Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86:6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT. Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol. 1997;80:17A–25A. doi: 10.1016/s0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- 21.Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat. 2015;38:117–22. doi: 10.1159/000375435. [DOI] [PubMed] [Google Scholar]
- 22.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate Metabolism in Human Lung Tumors. Cell. 2017;171:358–71.:e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon N, Skinner AM, Pommier RF, Schillace RV, O’Neill S, Peckham JL, et al. Gene expression signatures of breast cancer stem and progenitor cells do not exhibit features of Warburg metabolism. Stem Cell Res Ther. 2015;6:157. doi: 10.1186/s13287-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ubaldo E, Martinez-Outschoorn, Pavlides S, Howell A, Pestell GR, Tanowitz BH, Sotgia F, Lisanti PM. Stromal-epithelial metabolic coupling in cancer: Integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem and Cell Biol. 2011;43:1045–51. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 28.Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. The Journal of biological chemistry. 1973;248:7276–8. [PubMed] [Google Scholar]
- 29.Rata M, Giles SL, deSouza NM, Leach MO, Payne GS. Comparison of three reference methods for the measurement of intracellular pH using 31P MRS in healthy volunteers and patients with lymphoma. NMR in biomedicine. 2014;27:158–62. doi: 10.1002/nbm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.McNeillis R, Greystoke A, Walton J, Bacon C, Keun H, Siskos A, et al. A case of malignant hyperlactaemic acidosis appearing upon treatment with the monocarboxylase transporter 1 inhibitor AZD3965. British Journal of Cancer. 2020;122:1141–5. doi: 10.1038/s41416-020-0727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji U, Workman P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin Oncol. 2016;43:436–45. doi: 10.1053/j.seminoncol.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Beloueche-Babari M, Wantuch S, Casals Galobart T, Koniordou M, Parkes HG, Arunan V, et al. MCT1 Inhibitor AZD3965 Increases Mitochondrial Metabolism, Facilitating Combination Therapy and Noninvasive Magnetic Resonance Spectroscopy. Cancer Res. 2017;77:5913–24. doi: 10.1158/0008-5472.CAN-16-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siskos A. Exploratory Metabolomics of Urine and Plasma to Identify Novel Pharmacodynamic Biomarkers in a Phase I Clinical Trial of AZD3965. [cited 2022];MSACL 2019. 2019 Available from: https://www.msacl.org/program/view_abstract_selection.php?topic=Metabolites+%26+Metabolomics&event=2019%20EU. [Google Scholar]
- 35.Halestrap AP, Wilson MC. The monocarboxylate transporter family—Role and regulation. IUBMB Life. 2012;64:109–19. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 36.Sukjoi W, Ngamkham J, Attwood PV, Jitrapakdee S. Targeting Cancer Metabolism and Current Anti-Cancer Drugs. Advances in experimental medicine and biology. 2021;1286:15–48. doi: 10.1007/978-3-030-55035-6_2. [DOI] [PubMed] [Google Scholar]
- 37.Montrose DC, Galluzzi L. Drugging cancer metabolism: Expectations vs reality. International review of cell and molecular biology. 2019;347:1–26. doi: 10.1016/bs.ircmb.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Eu JQ, Kong LR, Wang L, Lim YC, Goh BC, et al. Targeting Metabolism in Cancer Cells and the Tumour Microenvironment for Cancer Therapy. Molecules (Basel, Switzerland) 2020;25 doi: 10.3390/molecules25204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this trial and the final clinical study protocol have been submitted to ClinicalTrials.gov and will be available immediately following publication, with no end date, at https://clinicaltrials.gov/ct2/show/results/NCT01791595. Individual deidentified patient data that underlie the results reported in this article (text, figures, tables and supplementary information) will be shared with researchers whose proposed use of the data is approved by a review committee of the Sponsor. All requests made within 5 years from end-of-trial will be considered; requests made subsequently will be considered where possible. Requests should be submitted to drugdev@cancer.org.uk.