Abstract
In this issue of Molecular Cell, Su et al. (2020) report a cryo-EM structure of the β1-adrenergic receptor (β1AR) in complex with a heterotrimeric Gs protein, which offers novel insights into receptor activation and provides a structural framework to better understand the transducer-coupling mechanism for adrenergic receptors.
The β1-adrenergic receptor (β1AR) belongs to the rhodopsin-like subfamily of G protein-coupled receptors (GPCRs), and it represents one of three subtypes of β-ARs along with β2AR and β3AR (Wachter and Gilbert, 2012). Upon agonist stimulation, the β1AR primarily couples to the stimulatory subtype of heterotrimeric G-proteins (Gs) resulting in increased cAMP levels, and subsequently, it also interacts with multifunctional proteins, β-arrestins (βarrs), which regulate its trafficking and desensitization (Steinberg, 2018; Wachter and Gilbert, 2012). β1AR is widely expressed in heart tissues, and its downstream signaling critically influences multiple physiological processes such as heart rate, contractility, and cardiac output (Wachter and Gilbert, 2012). Antagonists of β-adrenergic receptors, including those of β1AR, referred to as β-blockers, are used as therapeutics for heart failure, hypertension, and myocardial infarction. A number of β1AR crystal structures have been determined in complex with different agonists and antagonists, however, the details of how β1AR interacts with and activates Gs protein upon agonist-stimulation, remains to be directly visualized. In the current issue of Molecular Cell, Su et al. (2020) now report a cryo-EM structure of isoproterenol-bound β1AR in complex with heterotrimeric Gs protein, stabilized by a previously described nanobody against Gs (Su et al., 2020) (Figure 1). This structure provides an important advancement in understanding the commonalities and differences in Gαs-coupling to the two major β-AR subtypes, i.e., β1AR versus β2AR, and also allows a direct comparison between Gαs versus βarr1 interaction with β1AR.
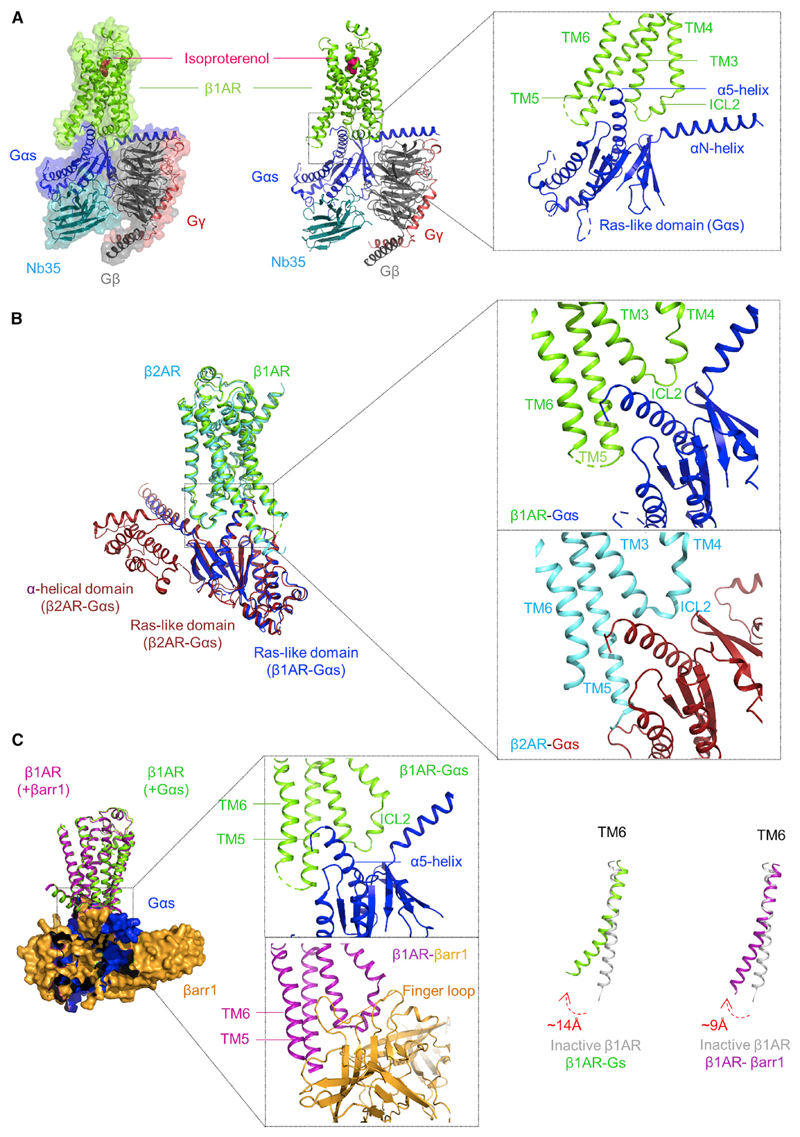
Figure 1. An Overview of β1AR-Gs Structure and Comparison with β2AR-Gs/β1AR-βarr1 Complexes.
(A) A snapshot of the β1AR-Gαs-β1-γ2-Nb35 complex (left panel, surface view; middle panel, ribbon view) generated based on the cryo-EM structure reported by Su et al. (2020) (PDB ID: 7JJO). β1AR is also bound to a synthetic agonist, isoproterenol. The right panel shows a structural snapshot to highlight the overall interaction interface between the β1AR and Gαs, which primarily involves TM3, TM5, TM6, and ICL2 in the receptor, and αN- and α5-helix in the Ras-like domain of Gαs.
(B) Structural comparison of the β1AR-Gs structure with previously determined β2AR-Gs crystal structure (PDB ID: 3SN6) highlights the remarkably similar interaction interface. The α-helical domain in the β1AR-Gs structure is not included here due to its highly dynamic nature resulting in weak density in the cryo-EM map.
(C) Comparison of the β1AR-Gs complex with previously determined β1AR-βarr1 structure (PDB ID: 6TKO) (left panel) reveals an overlapping interface of Gαs and βarr1 on the receptor (middle panel) and also a relatively smaller outward movement of β1AR TM6 helix in the β1AR-βarr1 complex compared to that in β1AR-Gs structure (right panel). A previously determined crystal structure of β1AR in an inactive conformation (PDB ID: 4GPO) is used as a reference.
The interaction interface in the β1AR-Gs structure involves a large surface area comprising of the cytoplasmic side of several transmembrane (TM) helices in the receptor, TM3, TM5, and TM6 in particular, as well as the intracellular loop 2 (ICL2) (Su et al., 2020) (Figure 1A). Gαs subunits of the heterotrimeric G-proteins contain two distinct domains, namely, the Ras-like GTPase domain and the α-helical domain. In the β1AR-Gs structure, the αN-helix and α5-helix from the Ras-like domain are primarily involved in the interaction with the receptor (Figure 1A). This interaction interface observed in the β1AR-Gs complex is mostly similar to that in previously determined GPCR-Gs cryo-EM structures (Safdari et al., 2018; Wang et al., 2020). In addition, a relatively minor interface between the ICL1 of the receptor and the Gβ subunit of the G-protein heterotrimer is also observed.
A major hallmark of GPCR activation is the outward movement of TM6, and it is also prominent in the β1AR-Gs structure where TM6 exhibits a displacement of about 14Å compared to the inactive β1AR structure (Huang et al., 2013; Su et al., 2020). Additionally, an extension of receptor TM5 helix at the cytoplasmic end, and an inward movement of TM7, is also observed in the β1AR-Gs structure (Su et al., 2020). The ionic interaction between Arg139 in TM3 and Glu285 in TM6, referred to as ionic-lock, which restrains the receptor in an inactive conformation, is also disrupted in the β1AR-Gs structure due to the outward movement of TM6 and the insertion of α5-helix of Gαs in the opening on the cytoplasmic side of the receptor. On the Gαs side, the α-helical domain shows a large rearrangement compared to the basal conformation of Gαs alone, potentially displaying a fully open conformation. Such large movement of the α-helical domain is also observed in other GPCR-Gs structures although the dynamic nature of the α-helical domain in these structures, including that in the current β1AR-Gs structure, makes it difficult to accurately model the structural features. In addition, the α5-helix in the Ras-like domain also undergoes a major rearrangement compared to Gαs alone in order to dock itself into the opening created on the intra-cellular side of the receptor. As a result, the α5-helix makes specific interactions with multiple residues in the receptor including those in TM3, TM5, TM6, and ICL2. These major structural changes both in the β1AR and the Gαs provide a possible mechanism for their simultaneous engagement and activation in order to catalyze GDP release from the Gαs nucleotide binding pocket.
The comparison of β1AR-Gs complex with the previously determined β2AR-Gs crystal structure (Rasmussen et al., 2011) reveals an overall similar interaction interface and structural features although some interesting differences are also apparent (Figure 1B). For example, the overall orientation of the α-helical domain of Gαs between the two structures differs in terms of their positioning compared to the Ras-like domain. In the β2AR-Gs crystal structure, the α-helical domain is rotated by about 127° relative to the Ras-like domain, while its rotation in the β1AR-Gs structure is somewhat restricted to about 96°. In order to decipher if this difference reflects a receptor-subtype-specific feature, or simply represents different ensembles captured, further studies are necessary. Importantly, a comparison of the β1AR-Gs structure with that of recently determined β1AR-βarr1 complex (Lee et al., 2020) reveals a significantly overlapping interface on the receptor occupied by Gαs and βarr1, and the engagement of the α5-helix of the Gαs and the finger loop of βarr1 is particularly striking (Figure 1C). An overlapping interface on the receptor occupied by the Gαs and βarr1 provides a mechanistic basis of receptor desensitization by βarrs. It is also interesting to note that the outward movement of TM6 in the β1AR-Gs structure is about 14Å but only about 9Å in β1AR-βarr1. Although some differences in the construct design may partly explain this, it is tempting to speculate that β1AR, and potentially other GPCRs, display a smaller opening on the intracellular side when they couple to βarrs versus G-proteins. Such a mechanism may govern the transducer-coupling preferences, especially in the context of biased agonism (Shukla et al., 2014; Wingler and Lefkowitz, 2020), although it remains to be investigated further.
In conclusion, the β1AR-Gs structure provides an important advance to further our understanding of receptor-G-protein coupling, their interaction interface, and activation mechanisms. Taken together with previously determined structures of adrenergic receptors, it provides a comprehensive structural framework to better understand the mechanism of signal-transduction as well as the differences at the level of receptor subtypes and signal-transducers. The rich structural tapestry of adrenergic receptors available now should facilitate a better design of subtype-specific and pathway-selective ligands going forward.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol. 2013;20:419–425. doi: 10.1038/nsmb.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Warne T, Nehme R, Pandey S, Dwivedi-Agnihotri H, Chaturvedi M, Edwards PC, García-Nafría J, Leslie AGW, Shukla AK, Tate CG. Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Nature. 2020;583:862–866. doi: 10.1038/s41586-020-2419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdari HA, Pandey S, Shukla AK, Dutta S. Illuminating GPCR Signaling by Cryo-EM. Trends Cell Biol. 2018;28:591–594. doi: 10.1016/j.tcb.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Singh G, Ghosh E. Emerging structural insights into biased GPCR signaling. Trends Biochem Sci. 2014;39:594–602. doi: 10.1016/j.tibs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Beta1-Adrenergic Receptor Regulation Revisited. Circ Res. 2018;123:1199–1201. doi: 10.1161/CIRCRESAHA.118.313884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Zhu L, Zhang Y, Paknejad N, Dey R, Huang J, Lee MY, Williams D, Jordan KD, Eng ET, et al. Structural Basis of the Activation of Heterotrimeric Gs-Protein by Isoproterenol-Bound beta1-Adrenergic Receptor. Mol Cell. 2020;80(this issue):59–71. doi: 10.1016/j.molcel.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter SB, Gilbert EM. Beta-adrenergic receptors, from their discovery and characterization through their manipulation to beneficial clinical application. Cardiology. 2012;122:104–112. doi: 10.1159/000339271. [DOI] [PubMed] [Google Scholar]
- Wang J, Hua T, Liu ZJ. Structural features of activated GPCR signaling complexes. Curr Opin Struct Biol. 2020;63:82–89. doi: 10.1016/j.sbi.2020.04.008. [DOI] [PubMed] [Google Scholar]
- Wingler LM, Lefkowitz RJ. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends Cell Biol. 2020;30:736–747. doi: 10.1016/j.tcb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]