Abstract
Purpose of Review
The purpose of this article is to review alterations in microbiota composition, diversity, and functional features in the context of chronic inflammation and comorbidities associated with HIV infection.
Recent Findings
The gut microbiome is an important mediator of host immunity, and disruption of gut homeostasis can contribute to both systemic inflammation and immune activation. Ageing and HIV share features of intestinal damage, microbial translocation and alterations in bacterial composition that contribute to a proinflammatory state and development of age-related comorbidities. One such inflammatory pathway reviewed is the NAD+ producing Kynurenine Pathway (KP). Kynurenine metabolites regulate many biological processes including host-microbiome communication, immunity and oxidative stress and the KP in turn is influenced by the microbiome environment. Age-associated decline in NAD+ is implicated as a driving factor in many age-associated diseases, including those seen in PWH. Recent studies have shown that KP can influence metabolic changes in people with HIV (PWH), including increased abdominal adiposity and cardiovascular disease (CVD). Furthermore, KP activity increases with age in the general population, but it is elevated in PWH at all ages compared to age-matched controls. Host or microbiome-mediated targeting of this pathway has merits to increase healthy longevity and has potential therapeutic applications in PWH.
Summary
As a growing proportion of PWH age, many face increased risks of developing age-related comorbidities. Chronic inflammation, a pillar of geroscience, the science of ageing and of age-related disease, is influenced by the gut microbiome and its metabolites. Combined, these contribute to a systemic inflammatory signature. Advances in geroscience-based approaches and therapeutics offer a novel paradigm for addressing age-related diseases and chronic inflammation in HIV infection. Whether targeted inhibition of KP activity alleviates pathological conditions or promotes successful ageing in PWH remains to be determined.
Keywords: HIV, microbiome, microbiota, dysbiosis, kynurenine, geroscience, senotherapeutics
Introduction
Since the introduction of combination antiretroviral therapy (ART), PWH are now achieving the life expectancy of the general population (1). Evidence suggests that treated HIV infection is associated with development of age-related complications at a higher rate, and in some cases at an earlier age than the HIV-negative population (2). The disproportionately greater prevalence of ageing-related comorbidities in PWH extends across both resource-rich and limited care settings (3). This has led to the application of the geroscience hypothesis to PWH, which proposes that the root cause of most ageing-related chronic diseases and conditions is the ageing process itself. Multiple components, including environmental, socioeconomic, psychological as well as biological factors, contribute to ageing. One significant contributor in relation to chronic disease is unresolved inflammation and chronic immune activation (4). Indeed, late-life, chronic, low-grade inflammation and altered signal transduction pathways in metabolism have been identified as two key pillars of geroscience. One such signal transduction pathway is Tryptophan (TRP) metabolism through the kynurenine pathway (KP), which plays essential roles in energy production and ageing.
The gut microbiome is an important mediator of host immunity, and disruption of gut homeostasis can result in systemic inflammation and immune activation (5). HIV infection is associated with alterations in microbiota composition, microbial metabolites, disruption of the gut endothelial barrier, and the production of microbial toxins such as lipopolysaccharide (LPS) (6). These changes may contribute to greater microbial translocation and the persistence of a pro-inflammatory state even after restoration of circulating CD4+ T cell counts under successful ART(5). Combined with chronic innate immune activation, these processes play a role in the development of comorbid conditions in PWH (7,8). The study and management of these comorbid conditions has focused on the unique signatures related to each condition. Yet, extending from the geroscience hypothesis, therapeutically targeting fundamental aging processes along a common pathway of inflammation could have a greater impact on alleviating or delaying aging-associated comorbidities than addressing each disease individually. The use of pharmacological agents, or “Senotherapeutics” to combat age-related diseases, have been proposed as a Geroscience treatment approach.
In this review, we aim to focus on recent publications about microbial translocation, the kynurenine pathway, and the development of comorbidities in PWH. Furthermore, we will discuss how the geroscience theory could be applied in the management of these comorbidities and the role senotherapeutics could have in PWH. Such an understanding can inform prevention strategies, management, and subsequent research directions.
HIV and microbiome compositional changes
HIV infection is associated with a disruption of microbial composition, decreased microbial diversity and shifts in composition, including increases in pathogenic and decreases in beneficial gut microbial species. Some of these compositional patterns are unique to PWH, with enrichment of potential pathobioints Fusobacteria, Enterobacteriaceae, Prevotella, and Proteobacteria species, and depletion of beneficial short-chain fatty acid-producing bacteria that are anti-inflammatory such as Bacteroides, Ruminococcus, Lactobacillus, and Bifidobacterium species (9). These compositional changes are associated with an overall increase in inflammation, systemic immune activation, and microbial translocation in PWH that have been linked to HIV disease progression and the development of non-AIDS-related comorbidities (8)(9)(10).
For instance, gut microbial compositional changes are seen in PWH with cardio-metabolic comorbidities, with decreased abundance of Bifidobacterium, Bacteroides and Akkermansia seen in PWH with type 2 diabetes (T2DM)(10). These changes in composition extend even to those with prediabetes as described in a recent cross-sectional analysis of 40 PWH, 20 of whom had prediabetes and 20 who were normoglycaemic. This study found that alpha-diversity, or the measure of microbiome diversity applicable to a single sample, and beta-diversity, a measure of the similarity or dissimilarity of two communities, was significantly lower in PWH with prediabetes than in those with normoglycemia and that relative abundance of two genera in Firmicutes (Streptococcus and Anaerostignum) were significantly higher in the prediabetes group (11).
In PWH and the general population, there is an overall reduction in gut microbial diversity in obese compared to lean participants, with higher levels of microbes from phylum Firmicutes and lower proportions of Bacteroidetes (12,13). This relative abundance of Firmicutes could be responsible for an increase in the capacity to digest polysaccharides, giving rise to increases in monosaccharides and short-chain fatty acids (SCFA) capable of being absorbed by the host, thus leading to increased caloric intake and weight gain. In PWH, a two-fold risk of metabolic syndrome was found in the microbiome profiles that had a high HIV-related microbiota index, mainly driven by enrichment of Desulfovibrionaceae, hydrogen sulfide-producing bacteria which can cause toxic effects, and decrease in several Clostridia species, known butyrate-producers (14).
Gut microbiome and Butyrate
Microbial metabolites, byproducts produced by intestinal bacteria, are also important in influencing immune responses(15). Butyrate is one of the SCFAs produced as end-products of intestinal microbial fermentation. Butyrate plays an important role in immune regulation through several mechanisms. It can support the integrity of the intestinal epithelial barrier by regulating the expression of tight junctional proteins, it assists with gut motility and has anti-inflammatory properties by downregulation of the NF-κB signalling pathway which is involved in gene transcription of inflammatory cytokines (16). Butyrate production also correlates with a decrease in monocyte activation and inflammation as measured by circulating soluble CD14 (sCD14) and C-reactive protein (CRP)(17). Lower levels of butyrate-producing bacteria (BPB) are associated with inflammation and insulin resistance (18). In PWH, decreased abundance of BPB is seen in untreated PWH compared to HIV-uninfected subjects and this was correlated with microbial translocation, immune activation and vascular inflammation (19). Furthermore, lower BPB in those with T2DM, metabolic syndrome and dyslipidaemia is seen in the general population as well as PWH (12,20,21).
HIV, microbial translocation and clinical comorbidities
PWH have increased risk of developing cardiovascular disease (CVD) and metabolic disease beyond that explained by traditional risk factors. CVD is now the leading cause of death in PWH on ART and PWH have an approximate two-fold increased risk of myocardial infarction(22). Furthermore, compared to the general population, prediabetes and T2DM in PLWH are more prevalent(23). Chronic systemic inflammation resulting in dysregulation of glucose and lipid trafficking, utilization, storage, alongside elevations in biomarkers of inflammation are thought to contribute to increased CVD and metabolic risk in PWH. The gut microbiome may additionally impact metabolic syndrome and CVD through effects on intestinal barrier function, leading to microbial translocation and systemic inflammation(24). Microbial markers, such as LPS and sCD14 have been linked to innate immune activation, atherosclerosis, coronary artery calcification and clinical vascular disease in PWH on ART(25–27).
Fungal mass constitutes the second player after bacterial mass in the composition of gut microbiota and so markers of fungal translocation are also associated with immune activation and systemic inflammation in PWH. Intestinal fatty-acid binding protein (I-FABP) levels, a marker of enterocyte damage, are consistently higher in people chronically infected with HIV compared to HIV-negative controls(28) and higher circulating I-FABP is associated with increased mortality and lower CD4+ T-cell counts(29). The role of fungal translocation on metabolic alterations and inflammation in HIV is beginning to be realised. I-FABP was found to be inversely related to BMI and adiposity in chronic HIV infection(28) and Beta-D-Glucan (BDG), a marker of fungal translocation, was associated with increased adiposity over 96 weeks following ART initiation. This association remained when accounting for confounders such as age, physical activity, smoking, alcohol and drug history, race, and sex. (30). Following this, a study in 2021 showed an association between plasma BDG levels and subclinical coronary atherosclerosis plaque in 93 ART-treated PLWH but not uninfected controls(31). All participants included were over the age of 40 and had a 10-year Framingham risk score ranging from 5% to 20%. It is interesting to note that the commonly used marker of microbial translocation LPS was not elevated in either of these BDG studies, however, LPS is not always a reliable marker of bacterial translocation given its short half-life and interference by other factors. Thus, although BDG appears as a new marker associated with non-AIDS comorbidities, current observations rely on associations only. More studies are thus needed to determine the mechanism linking fungal translocation and comorbidities.
Taken together, these changes in microbiome composition and function in PWH contribute to a chronic inflammatory state in some PWH that persists even after restoration of circulating CD4+ T cell counts under successful ART(5). Moreover, ageing and HIV share these features of intestinal damage and alterations in bacterial composition, each contributing to this pro-inflammatory state and development of age-related comorbidities(32). These shared characteristics could be targeted as a therapeutic strategy, as introduced by the geroscience theory, which proposes to target the ageing process itself. One interesting target worth discussing is the kynurenine (KP) metabolic pathway.
Kynurenine pathway and age-related comorbidities in HIV
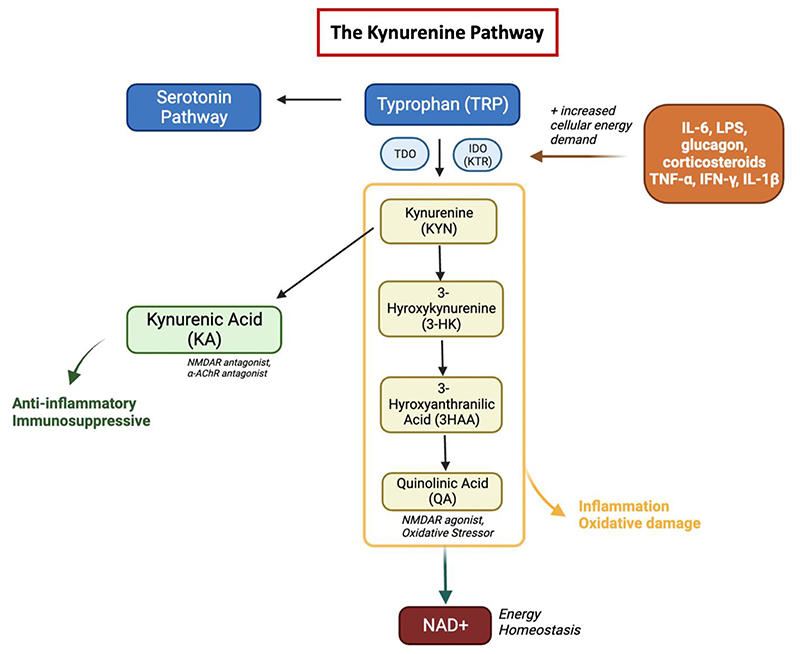
The KP is the sole de novo nicotinamide adenine dinucleotide (NAD+) biosynthetic pathway, generating NAD+ from ingested tryptophan (TRP)(33) (See Figure 1). NAD+ is an essential cofactor that plays a critical role in energy production as well as many enzymatic redox reactions. The first and rate-limiting step of the kynurenine pathway is made by tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO), the activity of which can be measured using the kynurenine/ tryptophan ratio (KTR) as a surrogate marker. IDO activity levels, measured by KTR, increase with age in a general population cohort without known infections and is reflected by an age-associated decline in NAD+ levels and energy production (34). This age-associated decline in NAD+ is implicated as a driving factor in many age-associated conditions, including metabolic and neurodegenerative disease (35–37).
Figure 1. The Kynurenine Pathway.
In the kynurenine pathway, TRP is metabolized to kynurenine (KYN) by tryptophan 2,3-dioxygenase (TDO), found mostly in hepatic tissue or indoleamine 2,3-dioxygenase (IDO), an enzyme found in most tissues that is stimulated by steroid hormones, cytokines and growth factors. Kynurenine is then either metabolized to kynurenic acid (KA) or to 3-hydroxykynurenine (3-HK). Under basal conditions, most of kynurenine is metabolized to KA, a N-methyl-D-aspartate (NMDA) and α7-nicotinic acetylcholine (α7nACh) receptor antagonist. However, inflammation, oxidative stress and pro-inflammatory cytokines will shift kynurenine metabolism to 3-HK. Further metabolism through this pathway results in quinolinic acid (QA), a metabolite that is a NMDA receptor agonist and an oxidative stressor. The KP produces several other biologically active metabolites, including the redox cofactors oxidized NAD+. NAD is a common mediator of various biological processes, including energy metabolism, mitochondrial function, calcium homeostasis and generation of oxidative stress. TRP metabolites like serotonin, quinolinic acid, and kynurenic acid have important implications in neurotransmission, growth and inflammation. IDO, indoleamine 2,3-dioxygenase; NAD, nicotinamide adenine dinucleotide.
Gut bacteria can alter the rate and availability at which tryptophan metabolism occurs through the KP and this can both protect the host from excess tryptophan, as well as produce beneficial kynurenine metabolites. Thus, the KP is a double-edged sword; it has key roles in cell growth, cell maintenance and neurotransmission but can also drive inflammation and systemic immune responses. Since many microbial organisms rely on the essential amino acid TRP, its’ degradation through IDO/TDO activity induced by exogenous pathogens can limit infection through TRP starvation. Thus, by reducing available substrate for microorganisms, the KP acts to inhibit growth during acute infections(33). This pathway constitutes a delicate balance between pathogen defense and host protection and is influenced by various stressors including infection, inflammation, and oxidative stress. Altered levels of KP metabolites, such as kynurenic acid (KA) or quinolinic acid (QA) have been reported in numerous disease processes, including metabolic syndrome (38), CVD(39), obesity (40), frailty, and neurologic diseases such as mental health disorders (36). Indeed Kynurenic acid (KA) can serve as an early biomarker for diabetes and some other metabolic diseases (41). Given these diverse physiological functions, metabolites of the KP are emerging as key targets in diseases such as diabetes, atherosclerosis, and more recently in HIV (42).
The link between KP and HIV infection has been known since 1998 when increased KTR was seen in PWH thus suggesting the link between increased KP activity and HIV immune dysfunction (43). The KTR represents an as independent marker of CD4 T-cell counts, with a higher KTR associated with lower CD4 T-cell counts and more advanced stages of disease (44). Furthermore, microbiota alterations in PWH have been linked with tryptophan catabolism and systemic inflammation (45). Increased KTR corresponds with detectable levels of microbial translocation from the gastrointestinal tract and gut microbiota changes showing a dysbiotic mucosal-adherent community enriched in Proteobacteria and depletion of Bacteroidia. Despite early initiation of ART, we see little improvement in this mucosal dysfunction or KP activity in PWH (46), enabling a self-sustaining feedback loop of inflammation. This constitutes a vicious cycle of immune activation, immune tolerance, senescence, and exhaustion. This immunophenotype of PWH and KP metabolism is similar to that seen in older people.
The KP has been closely linked with HIV-specific comorbidities such as HIV-associated neurogenerative disease (HAND) as well as both CVD and T2DM in PWH (47,48,49). Elucidating this link between inflammation in PWH and metabolic clinical outcomes has been an expanding area of research. The Copenhagen Comorbidity in HIV infection (COCOMO) study is an ongoing, longitudinal, observational study with the aim of assessing the burden of non-AIDS comorbidities in PWH. In a 2019 study they looked at 864 PWH and 75 uninfected controls to assess possible associations between KP metabolites and serum lipids, hypertension, and diabetes. They found that an increase in abdominal adipose tissue was associated with increased KTR in PWH (42). In 2022 they went one step further, and a new analysis of the COCOMO cohort that included 383 PWH who were mostly male, virologically suppressed on stable ART and showed that HIV-related gut microbiota alterations were associated with increased KTR. This in turn was associated with indices of visceral abdominal tissues (VAT)(50).
These patterns of inflammation induced by the KP in PWH share features with those seen in ageing (51). A recent retrospective multi-site cohort study examined the relationship of KTR, bacterial translocation, and ageing in 205 PWH on ART that had a median age of 52 years. They showed that although KTR increases with age in the general population, it is elevated in PWH at all ages compared to age-matched controls(52). This study provides an accurate reflection of the impact age has on KP activity in PWH, as they excluded subjects with comorbidities such as malignancy, hepatitis, and autoimmune disease as well as those on immunosuppressive medication. Unlike previous studies, they did not appreciate a relationship between KP activity and microbial translocation, but they only used LPS as an isolated marker of bacterial translocation. These findings suggest that a disturbed gut microbiota composition and tryptophan catabolism could represent pathogenic pathways potentially interacting in HIV infection and age-related comorbidities.
The Geroscience approach to gut microbiome modification in HIV
Efforts to target drivers of chronic inflammation in PWH through microbiome modification have been met with modest success. Interventions targeting microbial translocation with the use of probiotics, prebiotics and when combined in the form of synbiotics, have had varying results (17,53). Other studies have tried to target microbial translocation in PWH by reducing circulating LPS or sCD14 levels with the use of drugs such as sevelamer (54) and rifaxmin (55), but these treatment strategies failed to reduce levels of markers of inflammation. Clinical trials assessing the clinical outcomes of fecal transplantation in PWH have had limited results, but suggest good tolerability and feasibility (56).
Recently, attention has turned to the geroscience hypothesis, which proposes that therapeutically targeting fundamental mechanisms that underly ageing will have a significantly greater impact on overall human disease burden than an individual disease treatment approach. Hallmarks of ageing include inflammation, impaired adaptation to stress, mitochondrial dysfunction, altered metabolism and immunosenescence. These processes have been viewed historically as distinct types of biological processes, but evidence suggests that these pillars of ageing are intricately linked (57).
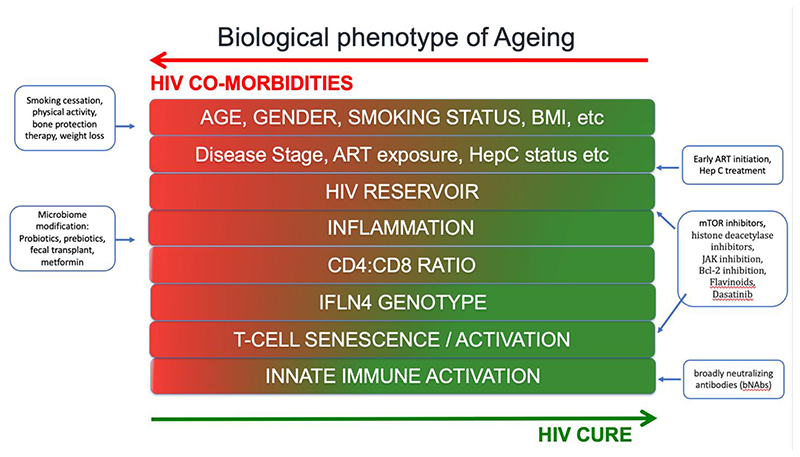
The use of pharmacological agents to combat age-related diseases, termed senotherapeutics, have been proposed as a geroscience treatment approach. These pharmacologic agents act either by selectively promoting the death of senescent cells (‘senolytics’) or modifying senescent phenotype (‘senomorphics’)(58). Cellular senescence is an age-related phenotype, related to HIV disease progression, ART and comorbidities in PWH (59). Several senolytics have been explored as part of investigational HIV cure strategies by targeting the HIV viral reservoir and include the flavinoid Quercetin, the BCL-2 antagonist Venetoclax, the mTOR inhibitor Rapamycin, the JAK 1/2 inhibitor Ruxolitinib and the histone deacetylase inhibitor Panobinostat (58) (See Figure 2). Dasatinib, a tyrosine kinase inhibitor with some antiviral properties, showed some promise as a HIV cure strategy when it was shown to reduce the viral reservoir of PWH with CML who were on simultaneous treatment with ART (60). Other senotherapeutics have been evaluated for potential geroprotective effects. One such drug examined in PWH is metformin, an anti-diabetic agent with anti-inflammatory properties. Historically used with caution in PWH due to risk of lactic acidosis and weight loss, recent studies in PWH have found that metformin benefits microbiota composition, promotes gut barrier integrity and reduces inflammation in diabetic and non-diabetic PWH(61,62). Nevertheless, to date, no clinical trials employing senotherapeutics to target age-related diseases have been undertaken in PWH. Encouragingly, a recent trial has been approved for development by the AIDS Clinical Trial Group (ACTG) to look at Dasatinib and Quercetin in PWH.
Figure 2. Biological phenotype of ageing and therapeutic modification strategies.
The Kynurenine pathway as a senotherapeutic target
The KP has recently been identified as a promising target to increase healthy longevity in age-related diseases. Therapies targeting the KP so far have focused on cancer-related therapeutics through the use of IDO inhibitors, which weaken the immunosuppressive functions by suppressing the KP, starving the cell of energy (63). Therapeutic targets from a senotherapy perspective takes the opposite approach, increasing energy production. Given the role NAD+ has in the ageing process, efforts to increase NAD+ levels by increasing the availability of NAD+ have been key targets of research. Some studies have shown that raising intracellular NAD+ levels with NAD+ precursors, nicotinamide mononucleotide (NMN), and nicotinamide riboside (NR) may offer a promising therapeutic strategy for age-associated degenerative diseases in general and to extend lifespan. Achieving this goal is challenging due to inter-individual variations in NAD+ levels that are affected by age, gender, diet, exercise, genetic factors and individual health status (64).
CD38 is another attractive target as a therapy to treat NAD+ age-related metabolic dysfunction. CD38, a glycoprotein widely expressed in immune cell types, is involved in T-cell activation and can be used as a predictor of HIV disease progression, as well as being linked to monocyte activation and CVD outcomes in PWH (47,67). Functionally, CD38 is mainly a NADase, degrading circulating NAD and its precursor NMN, preventing NAD synthesis and regulating T-cell responses(68). Thus, increases in CD38 have been hypothesised as the main regulator of age-related NAD+ decline in ageing and metabolic disorders. Studies looking at CD38 inhibitors (CD38i) have shown anti-ageing properties of the thiazoloquin(az)olin(on)e 78c, reversing age-related NAD+ decline in mice. Other agents such as the CD38 monoclonal antibody daratumumab, have shown promising results in multiple myeloma patients (69).
As well as altering the mechanisms of ageing, targeting KP signalling networks, or its metabolic pathways harbors high potential to prevent and treat metabolic diseases. NMN was recently shown to improve insulin sensitivity in prediabetic women (70). In addition, a recently developed oral antidiabetic medication, imeglimin, can enhance glucose-stimulated ATP generation and induce the synthesis of NAD+ in pancreatic islets derived from diseased rodents with type 2 diabetes (71). Currently, there are over 15 clinical trials underway (72) that aim to assess the clinical efficacy of NAD+ precursors as a therapeutic strategy to attenuate markers for metabolic dysfunction. To date, however, none of these clinical trials are investigating the application of these therapies in PWH.
Gaps in knowledge and future perspectives
Few therapeutic trials targeting the KP pathway or NAD+ precursors have looked specifically at HIV-related inflammation. However, one recent clinical trial investigated whether treatment with the neurokinin-1 receptor antagonist, aprepitant, alters tryptophan metabolism in PWH. They found that aprepitant decreased both kynurerine and tryptophan levels in ART-naive subjects but in PWH on ART, it caused a significant decreased the KTR (73). Targeting the KP has vast translational potential to target, prevent and treat age-related comorbidities in PWH and beyond. Additional studies will be needed to further delineate the role between the KP and the gut microbiome in PWH and whether the KP could be targeted by specific therapies to prevent or treat age-related comorbidities in PWH.
Conclusion
With a growing aging population of PWH come new challenges with the management of age-related comorbidities. Understanding the link between gut dysfunction, inflammation, the KP and these comorbidities is important to not only optimise health outcomes, but also help predict those most at risk. Advances in Geroscience-based approaches and senotherapeutics offer a novel paradigm for addressing age-related disease in chronic HIV infection, refocusing the goals of successful ageing in PWH.
Key points.
HIV infection is associated with persistent inflammation with translocation of microbes and/or microbial products from the gastrointestinal tract to the circulation; this process persists even with successful antiretroviral treatment.
HIV infection is also associated with a reduction in diversity and alteration of the microbial composition with reduced abundances of beneficial bacteria and enrichment of potentially proinflammatory bacteria.
The KP is involved in the control of immune responses and inflammation and has been associated with HIV disease progression and metabolic outcomes in PWH.
Geroscience-based approaches, through the use of senotherapeutics could prove beneficial for improving health outcomes in age-related diseases seen in PWH.
Treatment strategies targeting the KP pathway using NAD+ precursors and CD38ases could present a promising therapeutic target and further studies are needed in PWH.
Acknowledgements
This work was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning and the Health and Social Care, Research and Development Division, Northern Ireland.
Footnotes
Conflicts of Interest
None
References
- 1.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017 Aug;4(8):e349–56. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins KL, Brown TT, Margolick JB, Erlandson KM. Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS. 2017 Jun 1;31(Supplement 2):S137–46. doi: 10.1097/QAD.0000000000001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: A crosssectional study. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2018 May;70:30–5. doi: 10.1016/j.ijid.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Pelchen-Matthews A, Ryom L, Borges ÁH, Edwards S, Duvivier C, Stephan C, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. 2018 Oct 23;32(16):2405–16. doi: 10.1097/QAD.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 5.Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017 Oct 2;5(4):e1373208. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyboh K, Jenabian MA, Mehraj V, Routy JP. HIV and the Gut Microbiota, Partners in Crime: Breaking the Vicious Cycle to Unearth New Therapeutic Targets. J Immunol Res. 2015;2015:1–9. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis. 2014 Oct 15;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim JH, Mukerji SS, Russo SC, Lo J. Gastrointestinal Dysfunction and HIV Comorbidities. Curr HIV/AIDS Rep. 2021 Feb;18(1):57–62. doi: 10.1007/s11904-020-00537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects. Relman DA, editor. PLoS Pathog. 2014 Feb 20;10(2):e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JY, Zolnik CP, Wang Z, Qiu Y, Usyk M, Wang T, et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 2018 Nov;37:392–400. doi: 10.1016/j.ebiom.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayanama K, Phuphuakrat A, Pongchaikul P, Prombutara P, Nimitphong H, Reutrakul S, et al. Association between gut microbiota and prediabetes in people living with HIV. Curr Res Microb Sci. 2022;3:100143. doi: 10.1016/j.crmicr.2022.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelpi M, Vestad B, Hansen SH, Holm K, Drivsholm N, Goetz A, et al. Impact of Human Immunodeficiency Virus-Related Gut Microbiota Alterations on Metabolic Comorbid Conditions. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Nov 5;71(8):e359–67. doi: 10.1093/cid/ciz1235. [DOI] [PubMed] [Google Scholar]
- 13.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 14.Ijssennagger N, van der Meer R, van Mil SWC. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol Med. 2016 Mar;22(3):190–9. doi: 10.1016/j.molmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016 Jun;16(6):341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui MT, Cresci GAM. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021;14:6025–41. doi: 10.2147/JIR.S300989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Villar S, Vázquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando-Martínez S, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017 Sep;10(5):1279–93. doi: 10.1038/mi.2016.122. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open. 2021 Jul 1;4(7):e2118811. doi: 10.1001/jamanetworkopen.2021.18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS Lond Engl. 2017 Feb 20;31(4):511–21. doi: 10.1097/QAD.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva-Millán MJ, Pérez-Matute P, Recio-Fernández E, Lezana Rosales JM, Oteo JA. Characterization of gut microbiota composition in HIV-infected patients with metabolic syndrome. J Physiol Biochem. 2019 Aug;75(3):299–309. doi: 10.1007/s13105-019-00673-9. [DOI] [PubMed] [Google Scholar]
- 21.Arora T, Tremaroli V. Therapeutic Potential of Butyrate for Treatment of Type 2 Diabetes. Front Endocrinol. 2021;12:761834. doi: 10.3389/fendo.2021.761834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. Epidemiological Evidence for Cardiovascular Disease in HIV-Infected Patients and Relationship to Highly Active Antiretroviral Therapy. [cited 2022 Sep 6];Circulation. 2008 Jul 8;118(2) doi: 10.1161/CIRCULATIONAHA.107.189624. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phuphuakrat A, Nimitphong H, Reutrakul S, Sungkanuparph S. Prediabetes among HIV-infected individuals receiving antiretroviral therapy: prevalence, diagnostic tests, and associated factors. AIDS Res Ther. 2020 Dec;17(1):25. doi: 10.1186/s12981-020-00284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011 Jun 1;121(6):2126–32. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallio KAE, Hätönen KA, Lehto M, Salomaa V, Männistö S, Pussinen PJ. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015 Apr;52(2):395–404. doi: 10.1007/s00592-014-0662-3. [DOI] [PubMed] [Google Scholar]
- 26.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011 Mar 15;203(6):780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 Apr 24;28(7):969–77. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheru LT, Park EA, Saylor CF, Burdo TH, Fitch KV, Looby S, et al. I-FABP Is Higher in People With Chronic HIV Than Elite Controllers, Related to Sugar and Fatty Acid Intake and Inversely Related to Body Fat in People With HIV. Open Forum Infect Dis. 2018 Nov;5(11):ofy288. doi: 10.1093/ofid/ofy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014 Oct 15;210(8):1228–38. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirajlal-Fargo S, Moser C, Rodriguez K, El-Kamari V, Funderburg NT, Bowman E, et al. Changes in the Fungal Marker β-D-Glucan After Antiretroviral Therapy and Association With Adiposity. Open Forum Infect Dis. 2019 Nov;6(11):ofz434. doi: 10.1093/ofid/ofz434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isnard S, Fombuena B, Sadouni M, Lin J, Richard C, Routy B, et al. Circulating β-d-Glucan as a Marker of Subclinical Coronary Plaque in Antiretroviral Therapy-Treated People With Human Immunodeficiency Virus. Open Forum Infect Dis. 2021 Jun;8(6):ofab109. doi: 10.1093/ofid/ofab109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, et al. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci. 2015 Oct 1;129(7):515–27. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]
- 33.Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol. 2020 Apr;132:110841. doi: 10.1016/j.exger.2020.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004 Aug;37(8):684–7. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Teunis C, Nieuwdorp M, Hanssen N. Interactions between Tryptophan Metabolism, the Gut Microbiome and the Immune System as Potential Drivers of Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Diseases. Metabolites. 2022 Jun 2;12(6):514. doi: 10.3390/metabo12060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorgdrager FJH, Vermeiren Y, Van Faassen M, van der Ley C, Nollen EAA, Kema IP, et al. Age-and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J Neurochem. 2019 Dec;151(5):656–68. doi: 10.1111/jnc.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ala M, Eftekhar SP. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int J Tryptophan Res. 2022 Jan;15:117864692210966. doi: 10.1177/11786469221096643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017 Jul 28;357(6349):eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Chen S, Zhong J, Teng K, Yin Y. Crosstalk between Tryptophan Metabolism and Cardiovascular Disease, Mechanisms, and Therapeutic Implications. Oxid Med Cell Longev. 2017;2017:1602074. doi: 10.1155/2017/1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, et al. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology. 2017 May 1;158(5):1049–63. doi: 10.1210/en.2016-1839. [DOI] [PubMed] [Google Scholar]
- 41.Oxenkrug GF. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol Neurobiol. 2015 Oct;52(2):805–10. doi: 10.1007/s12035-015-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelpi M, Ueland PM, Trøseid M, Mocroft A, Lebech AM, Ullum H, et al. Abdominal Adipose Tissue Is Associated With Alterations in Tryptophan-Kynurenine Metabolism and Markers of Systemic Inflammation in People With Human Immunodeficiency Virus. J Infect Dis. 2020 Jan 14;221(3):419–27. doi: 10.1093/infdis/jiz465. [DOI] [PubMed] [Google Scholar]
- 43.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998 Apr;44(4):858–62. [PubMed] [Google Scholar]
- 44.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010 May 19;2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vesterbacka J, Rivera J, Noyan K, Parera M, Neogi U, Calle M, et al. Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Sci Rep. 2017 Dec;7(1):6269. doi: 10.1038/s41598-017-06675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenabian MA, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, et al. Immunosuppressive Tryptophan Catabolism and Gut Mucosal Dysfunction Following Early HIV Infection. J Infect Dis. 2015 Aug 1;212(3):355–66. doi: 10.1093/infdis/jiv037. [DOI] [PubMed] [Google Scholar]
- 47.Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, et al. Plasma Tryptophan-Kynurenine Metabolites Are Altered in Human Immunodeficiency Virus Infection and Associated With Progression of Carotid Artery Atherosclerosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018 Jul 2;67(2):235–42. doi: 10.1093/cid/ciy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010 Jan 14;115(2):161–7. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forsythe P, Kunze W, Bienenstock J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis? BMC Med. 2016 Dec;14(1):58.:s12916-016-0604-8. doi: 10.1186/s12916-016-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelpi M, Vestad B, Raju SC, Hansen SH, Høgh J, Midttun Ø, et al. Association of the Kynurenine Pathway of Tryptophan Metabolism With Human Immunodeficiency Virus-Related Gut Microbiota Alterations and Visceral Adipose Tissue Accumulation. J Infect Dis. 2022 Jun 1;225(11):1948–54. doi: 10.1093/infdis/jiac018. [** This study demonstrates a novel link between HIV-related gut microbiota alterations and an a increased KTR with indices of visceral abdominal tissues (VAT) in a population of older people with HIV.] [DOI] [PubMed] [Google Scholar]
- 51.Al Saedi A, Chow S, Vogrin S, Guillemin GJ, Duque G. Association Between Tryptophan Metabolites, Physical Performance, and Frailty in Older Persons. Int J Tryptophan Res IJTR. 2022;15:11786469211069952. doi: 10.1177/11786469211069951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baer SL, Colombo RE, Johnson MH, Wakade S, Pacholczyk G, Newman-Whitlow C, et al. Indoleamine 2,3 dioxygenase, age, and immune activation in people living with HIV. J Investig Med Off Publ Am Fed Clin Res. 2021 Aug;69(6):1238–44. doi: 10.1136/jim-2021-001794. [** This study shows that KTR increases with age, independent of comorbidties and other risk factors and is higher in PWH compared to uninfected controls across all ages. This demonstrates that PWH have an inflamed or aged phenotype despite effective ART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu YS, Chu QS, Ashuro AA, Di DS, Zhang Q, Liu XM, et al. The Effect of Probiotics, Prebiotics, and Synbiotics on CD4 Counts in HIV-Infected Patients: A Systematic Review and Meta-Analysis. BioMed Res Int. 2020;2020:7947342. doi: 10.1155/2020/7947342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014 Nov 15;210(10):1549–54. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenorio AR, Chan ES, Bosch RJ, Macatangay BJC, Read SW, Yesmin S, et al. Rifaximin has a Marginal Impact on Microbial Translocation, T-cell Activation and Inflammation in HIV-Positive Immune Nonresponders to Antiretroviral Therapy-ACTG A5286. J Infect Dis. 2015 Mar 1;211(5):780–90. doi: 10.1093/infdis/jiu515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caira-Chuquineyra B, Fernandez-Guzman D, Soriano-Moreno DR, Fernandez-Morales J, Flores-Lovon K, Medina-Ramírez SA, et al. Fecal Microbiota Transplantation for People Living with Human Immunodeficiency Virus: A Scoping Review. AIDS Res Hum Retroviruses. 2022 May 24; doi: 10.1089/AID.2022.0016. [DOI] [PubMed] [Google Scholar]
- 57.Robinson AR, Yousefzadeh MJ, Rozgaja TA, Wang J, Li X, Tilstra JS, et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol. 2018 Jul;17:259–73. doi: 10.1016/j.redox.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szaniawski MA, Spivak AM. Senotherapeutics for HIV and aging. Curr Opin HIV AIDS. 2020 Mar;15(2):83–93. doi: 10.1097/COH.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen J, Torres C. HIV-associated cellular senescence: A contributor to accelerated aging. Ageing Res Rev. 2017 Jul;36:117–24. doi: 10.1016/j.arr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigón L, Martínez-Román P, Rodríguez-Mora S, Torres M, Puertas MC, Mateos E, et al. Provirus reactivation is impaired in HIV-1 infected individuals on treatment with dasatinib and antiretroviral therapy. Biochem Pharmacol. 2021 Oct;192:114666. doi: 10.1016/j.bcp.2021.114666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isnard S, Lin J, Fombuena B, Ouyang J, Varin TV, Richard C, et al. Repurposing Metformin in Nondiabetic People With HIV: Influence on Weight and Gut Microbiota. Open Forum Infect Dis. 2020 Sep;7(9):ofaa338. doi: 10.1093/ofid/ofaa338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouyang J, Isnard S, Lin J, Fombuena B, Marette A, Routy B, et al. Metformin effect on gut microbiota: insights for HIV-related inflammation. AIDS Res Ther. 2020 Mar 10;17(1):10. doi: 10.1186/s12981-020-00267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Günther J, Däbritz J, Wirthgen E. Limitations and Off-Target Effects of Tryptophan-Related IDO Inhibitors in Cancer Treatment. Front Immunol. 2019;10:1801. doi: 10.3389/fimmu.2019.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braidy N, Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp Gerontol. 2020 Apr;132:110831. doi: 10.1016/j.exger.2020.110831. [DOI] [PubMed] [Google Scholar]
- 65.Ebata T, Shimizu T, Fujiwara Y, Tamura K, Kondo S, Iwasa S, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 inhibitor navoximod (GDC-0919) as monotherapy and in combination with the PD-L1 inhibitor atezolizumab in Japanese patients with advanced solid tumours. Invest New Drugs. 2020 Apr;38(2):468–77. doi: 10.1007/s10637-019-00787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Cherney EC, Zhu X, Linan T, Gullo-Brown J, Maley D, et al. Discovery of Imidazopyridines as Potent Inhibitors of Indoleamine 2,3-Dioxygenase 1 for Cancer Immunotherapy. ACS Med Chem Lett. 2021 Mar 11;12(3):494–501. doi: 10.1021/acsmedchemlett.1c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 Antigen Expression on CD8+ T Cells Is a Stronger Marker for the Risk of Chronic HIV Disease Progression to AIDS and Death in the Multicenter AIDS Cohort Study Than CD4+ Cell Count, Soluble Immune Activation Markers, or Combinations of HLA-DR and CD38 Expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Oct;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 68.Li W, Liang L, Liao Q, Li Y, Zhou Y. CD38: An important regulator of T cell function. Biomed Pharmacother Biomedecine Pharmacother. 2022 Sep;153:113395. doi: 10.1016/j.biopha.2022.113395. [DOI] [PubMed] [Google Scholar]
- 69.Shallis RM, Terry CM, Lim SH. The multi-faceted potential of CD38 antibody targeting in multiple myeloma. Cancer Immunol Immunother. 2017 Jun;66(6):697–703. doi: 10.1007/s00262-017-1990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021 Jun 11;372(6547):1224–9. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallakou-Bozec S, Vial G, Kergoat M, Fouqueray P, Bolze S, Borel AL, et al. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021 Mar;23(3):664–73. doi: 10.1111/dom.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.NAD+ precursor therapies. [cited 2022 Oct 21]. [Internet]. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NAD%2B+precursors&cntry=&state=&city=&dist=
- 73.Spitsin S, Pappa V, Kinder A, Evans DL, Rappaport J, Douglas SD. Effect of aprepitant on kynurenine to tryptophan ratio in cART treated and cART naïve adults living with HIV. Medicine (Baltimore) 2021 Jun 11;100(23):e25313. doi: 10.1097/MD.0000000000025313. [** this is the first clinical trial to look at modifying tryptophan metabolism in PWH in both ART-naive subjects as well as those on ART. Aprepitant affected tryptophan metabolism in all PWH but particularly in those on ART, suggesting a role for novel therapeutic targets that reduce inflammation in chronic, treated HIV.] [DOI] [PMC free article] [PubMed] [Google Scholar]