Introduction
Depression is a serious public health concern, both socially and economically, and is increasing in prevalence (World Health Organization, 2017). In 2017, more than 322 million people suffered from depression globally, and depression is predicted to be the leading cause of the total disease burden by 2030 (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Mathers et al., 2008). In order to address this growing problem, there is a need to better understand the features of our modern lifestyles which may be contributing to this disease. The incidence of depressive episodes rises sharply during adolescence (Malhi and Mann, 2018). This increase is thought to be the result of cognitive changes related to the development of brain circuitry, hormones, and accompanying social changes (Thapar et al., 2012). Defined as a cluster of specific symptoms and associated impairment, depression in adolescents may exhibit as irritability and mood reactivity, as well as anhedonia and low mood (Thapar et al., 2012). One factor which may contribute to risk for development of depression is sleep duration. Although the association between sleep duration and depression is well supported in adults, with those sleeping too little and too much at risk for subsequent depression (Zhai et al., 2015), much less is known about this association in adolescence. As sleep duration in childhood and adolescence appears to be decreasing in the population by an average of 0.75 mins per year (Matricciani et al., 2012), advancing knowledge on the causal relationship between sleep and subsequent depression is of urgent concern.
Previous studies in adolescents have examined cross-sectional associations between sleep duration and depressive symptoms (Berger et al., 2019; Dickinson et al., 2018; Wahlstrom et al., 2017) as well as longitudinal associations of sleep duration (Conklin et al., 2018; Fredriksen et al., 2004; Orchard et al., 2020; Roberts and Duong, 2014) and sleep timing (Orchard et al., 2020; Tochigi et al., 2016) with subsequent depressive symptoms. In a UK adolescent population, a longer sleep duration on school days at age 15 was found to be associated with reduced odds of depression diagnosis at age 17y or 24y and fewer depressive symptoms at age 21y (Orchard et al., 2020). Similarly, in a sample from Texas, US, short sleep duration (<6h) (across all nights or on week nights alone) in adolescence was found to be associated with increased depressive symptoms and increased odds of major depression 12 months later (Roberts and Duong, 2014). There is less evidence available for sleep timing. A longitudinal study of Japanese adolescents reported that later bedtime was associated with an increased depression/anxiety score the following year (Tochigi et al., 2016). However, a UK study reported that sleep onset time was not a predictor of subsequent depression diagnosis or depressive symptoms (Orchard et al., 2020). Several studies have found the longitudinal association of sleep duration or sleep timing with depressive symptoms to be stronger in girls than in boys (Conklin et al., 2018; Goldstone et al., 2020; Tochigi et al., 2016). For example, Conklin et al. (2018) found that cumulative sleep deprivation (defined as < 8h of sleep across 2 out of 3 waves of data collection) was associated with increased levels of depressive symptoms at the third wave, among female, but not male adolescents. To improve evidence for healthy sleep habits in adolescents, these disparate findings need clarifying. We note that while research findings for depressive symptoms and depressive disorder are generally comparable (Thapar et al, 2012), depressive symptoms may be subthreshold to clinical depression. Therefore our precise terminology aligns with what was reported in the literature.
To our knowledge, all studies to date which have investigated the longitudinal associations between sleep duration or timing and subsequent depressive symptoms in adolescence have used self-reported measures of sleep timings. Self-reported sleep is prone to recall and reporting bias as it is difficult for adolescents to accurately recall time spent sleeping. Together with the necessarily subjective nature of the outcome measure this may bias previously reported findings. In this study we used data from a cohort of adolescents in which both device-measured and self-reported data on sleep timings was collected at baseline to investigate the associations between sleep duration and timing and subsequent development of depressive symptoms. We address the following research questions:
-
1)
What is the cross-sectional association between sleep duration, sleep onset time and depressive symptoms in UK adolescents?
-
2)
What is the association between sleep duration, sleep onset time and development of depressive symptoms over time, controlling for baseline symptoms?
-
3)
Are these associations different in girls and boys?
Methods
Participants
The ROOTS study recruited 1,283 adolescents from secondary schools in Cambridgeshire, United Kingdom, from 2005 to 2007 (Goodyer et al., 2010; Lewis et al., 2016). This longitudinal study aimed to characterise risk factors for psychopathology in adolescence, a particularly vulnerable time. Baseline data collection at mean age 14.5 years (SD=0.3; T1) included a participant-completed questionnaire covering demographic and psychosocial measures and a semi-structured interview which included assessment of psychiatric diagnoses. Six months after baseline, all participants were invited to take part in a sub-study focusing on diet, physical activity, sleep and body composition, and 909 (73.4%) participated. All participants were followed up and the questionnaire administered at 16.0 years (SD=0.3; T2) and 17.5 years (SD=0.3; T3). All students aged between 14 and 14 years 11 months during the 2 week interview period in participating schools were eligible to take part.
Ethical approval was provided by the Cambridgeshire 2 Research Ethics Committee. Written informed consent from participants and their parents/legal guardians was obtained prior to the start of the study.
Measures
Sleep onset time and sleep duration
Device-measured sleep was assessed using a combined heart rate and movement sensor (Actiheart©,CamNtech Ltd., Cambridge, UK). Actigraphy shows good validity and reliability in assessing sleep, both in healthy populations and populations with abnormal sleep patterns, and has been validated in adolescents (Sadeh, 2011). Measuring heart rate and uniaxial acceleration, the monitor was set to record data every 30 seconds. The full calibration and monitoring protocol for the combined sensor is reported elsewhere (Brage et al., 2005). Two ECG electrodes joined by a single wire are attached to the participant’s chest, one medially at the base of the sternum and one laterally on the left. For each individual, the Actiheart accelerometer was calibrated using an 8 minute sub-maximal step test (150 mm step) based on estimated exercise intensity. Participants were requested to wear the small waterproof monitor continuously for 4 days and nights without interruption, which included both weekend and weekdays, as all devices were fitted on a Thursday or Friday and retrieved the following week.
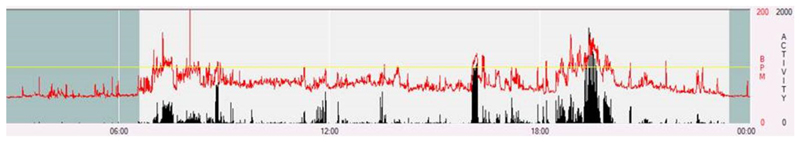
A single researcher, blind to all other data, reviewed plots of the sensor time-series data and marked sleep onset and termination times by the following criteria. Sleep onset was defined as the beginning of sustained absence of movement accompanied by a steady decline in heart rate; sleep termination was defined as re-initiation of movement after a long period of little-to-no movement, together with an abrupt rise in heart rate (figure 1). This method has been published previously (Collings et al., 2015). Cases were excluded where sleep onset and termination were unclear. For those whose sleep onset started the following day (e.g. at 1am), 24 hours were added to account for this. Sleep duration was calculated as the time from sleep onset to termination. In order to obtain an overall picture of adolescents’ sleep patterns, average duration and timing across measured days were obtained by weighting the average weekday values (5/7) and weekend values (2/7), which were used in all further analyses. To examine weekday night and weekend night sleep separately, we also created sleep measures averaged across only weekday nights or weekend nights. All included participants had data on both weekend and weekday nights.
Figure 1.
An example participant’s heart rate and accelerometry traces from Actiheart data during waking and sleeping hours. Red indicated heart rate, black indicates acceleration. The darker shaded area indicates sleep time.
Participants also reported the time that they usually got up and went to bed on school nights and weekend nights separately, as defined in the Sleep Habits Survey for Adolescents, which has been validated against sleep diaries and actigraphy [22]. Subjective sleep duration and onset time were obtained from these questions; weekly averages were then calculated in the same manner as for device-measured sleep to facilitate comparison with previous studies.
Depressive Symptoms
The Mood and Feelings Questionnaire (MFQ) is a 33-item self-report questionnaire assessing depressive symptoms over the previous two weeks in children and adolescents (Costello and Angold, 1988). The MFQ has shown moderate to high criterion validity as a screen for adolescents with unipolar depression (Burleson Daviss, 2006). For each item, participants reported their mood over the previous two weeks on a four-point scale (never/sometimes/mostly/always). The categories ‘mostly’ and ‘always’ were collapsed to give a 3-point scale, aligning with previous literature (Costello and Angold, 1988). For the present study, two items assessing sleep quality were excluded to avoid artificial correlation with the exposure variable. A sensitivity analysis which included these items found no difference in the results. Each MFQ item ranged from a score of 0-2, and for this analysis, rather than summing MFQ scores, we averaged across item scores to obtain a final score ranging from 0-2, with a higher MFQ score indicating more depressive symptoms, as this improved model fit. MFQ was obtained at T1-T3. For inclusion at each time point, we required MFQ responses to be ≥85% complete.
Covariates and inclusion criteria
Models were grouped by gender, and the sample were of a very similar age at each time point (SD=0.3y), so age was not found to be a significant confounder. We tested a wide range of further covariates for inclusion in this study (e.g. socioeconomic status, stressful life events, adversity, friendships, see Supplementary Table A1), however we found no covariates which showed associations with both exposure (sleep duration or sleep onset) and outcome (intercept or slope of the MFQ growth model, and thus would meet the definition of being a confounder. The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-PL) was used to establish DSM-IV axis 1 diagnoses at baseline, allowing us to exclude those with current mental disorders at baseline and focus on an initially healthy population. The K-SADS-PL is a semi-structured interview, and was conducted by fully trained researchers (Joan Kaufman et al., 2016). In addition to the requirement of no current mental health disorder at baseline, participants were required to have sleep data for at least one weekday and one weekend night, and at least one measure of MFQ across the 3 time points.
Statistical analyses
The analysis sample constituted all participants with data available on the exposure (device-measured and self-reported sleep) at baseline, the outcome (MFQ score) at at least one time-point, and reporting no mental disorder at baseline.
Descriptive analyses were performed in Stata version 16, with latent growth modelling performed in R version 4.0.2 (R Core Team, 2019), using the lavaan package for latent variable modelling (Rosseel, 2012). Student’s t tests and χ2 tests were used to assess differences in sociodemographic and anthropometric variables between those included and excluded from the current analysis.
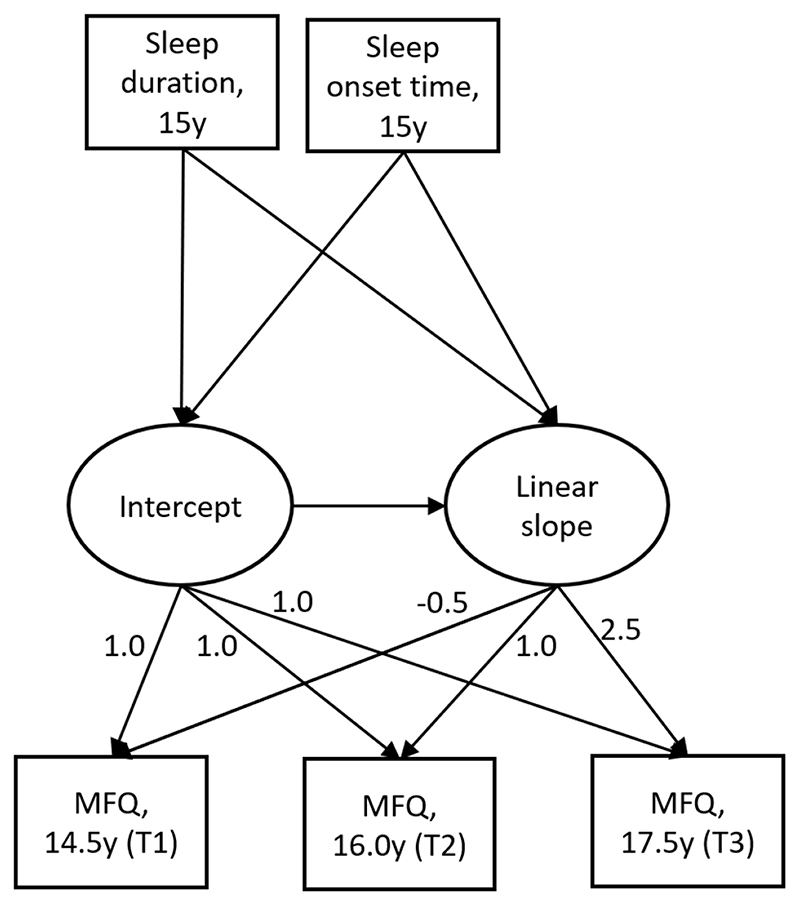
We used latent growth curve modelling (Figure 2) to model change in depressive symptoms from ages 14.5 to 17.5 and investigated associations with baseline sleep duration and sleep onset time. Sleep duration and sleep onset time were first analysed as exposures in separate models, and also combined in a model which included both exposures. Sleep duration and onset time were moderately correlated using either device-measured and self-reported sleep measures, r = -0.623, r = -0.672 respectively. Full information maximum likelihood (FIML) (Ferro, 2014) was used to account for missing outcome data, and a maximum likelihood estimator with robust standard errors (MLR) was used to account for the skew in MFQ data. Models were also grouped by gender, given previously reported gender differences in sleep duration and depression by gender (Conklin et al., 2018; Goldstone et al., 2020; Matamura et al., 2014; Tochigi et al., 2016). The time scores for MFQ were set relative to the time at which sleep was measured, at -0.5 years, 1 year, and 2.5 years. Both device-measured and self-reported sleep measures were used as exposures, to examine whether differences in association were seen according to the method of sleep assessment. We further examined the association of device-measured sleep with change in depressive symptoms, split by week night and weekend night.
Figure 2.
Growth model showing parameterisation of MFQ latent growth factors (intercept and linear slope as function of age), with explanatory variables (sleep duration and sleep onset time). Numbers by arrows (factor loadings) for linear slope indicate elapsed time in years from sleep exposure assessment.
The Akaike Information Criteria (AIC), Bayesian Information Criteria (BIC), Comparative Fit Index (CFI), Tucker-Lewis Index (TLI) and Root Mean Square Error of Approximation (RMSEA) were used to assess model fit. Acceptable fit was determined to be the model with the lowest AIC and BIC values, a CFI and TLI greater than 0.95 and an RMSEA of less than 0.05 (Xia and Yang, 2019).
Results
Of 1,283 recruited participants, 688 were included in the present analysis (n=388 girls). Those with missing data on all three MFQ scores (n=93), missing sleep measures (n=429), or prevalent mental disorder at baseline (n=73) were excluded. Excluded participants reported higher MFQ at all waves, poorer perceived family functioning and friendship support, and greater adversity and stressful events. Moreover, there was greater representation from minority ethnicities (Table 1). Of the 688 participants with sleep data, 98.5% (n=678) had MFQ data at baseline, 84. 9% (n=584) at T2, and 89.2% (n=614) at T3.
Table 1. Descriptive characteristicsa and exposure and outcome data on excluded and included participants.
| Excluded (n=595 (46.38%)) | Included (n=688 (53.62%)) | |||
|---|---|---|---|---|
| Overall | Male | Female | ||
| Baseline Age Mean (SD) (n = 1,147 (89.4%)) | 14.49 (0.286) | 14.48 (0.275) | 14.47 (0.29) | 14.47 (0.27) |
| Gender (n = 1,283 (100%)), N (%) | Male:264 (46.8%) | |||
| Female: 286 (42.43%) | n/a | 300 (43.60%) | 388 (56.40%) | |
| Ethnicity (n = 1,283, (100%)), N (%) | ||||
| Minority | 144 (24.20%) | 52 (7.56%) | 17(5.67%) | 35 (9.02%) |
| Not minority | 451 (75.80%)* | 636 (92.44%) | 283 (94.33%) | 353 (90.98%) |
| Acorn SES, n = 1,207 (94.1%), N (%) | ||||
| Wealthy Achievers | 274 (52.79%) | 388 (56.40 %) | 163 (54.33%) | 225 (57.99%) |
| Urban Prosperity | 36 (6.94 %) | 44 (6.40%) | 25 (8.33%) | 19 (4.90%) |
| Comfortably Off | 129 (24.86%) | 162 (23.55 %) | 68 (22.67%) | 94 (24.23%) |
| Moderate Means | 27 (5.20 %) | 28 (4.07%) | 11 (3.67%) | 17 (4.38%) |
| Hard Pressed | 53 (10.21 %) | 66 (9.59 %) | 33 (11.00%) | 33 (8.51%) |
| Device-measured Sleep Duration (n = 786 (61.3%)), Mean (SD) (hours:mins) | 7:44 (0:50) | 7:45 (0:48) | 7:35 (0:49)*** | 7:53 (0:46) |
| Device-measured Sleep Onset time (n = 786 (61.3%)) Mean (SD) (hours:mins) | 23:44 (1:10) | 23:38 (0:49) | 23:40 (0:47) | 23:37 (0:51) |
| Self-reported sleep duration n = 931 (72.6%) Mean (SD) (hours:mins) | 9:58 (0:57) | 9:09 (0:50) | 9:09 (0:48) | 9:07 (0:44) |
| Self-reported sleep onset time n = 931 (72.6%) Mean (SD) (hours:mins) | 22:41 (0:48)* | 22:34 (0:47) | 22:32 (0:41) | 22:35 (0:42) |
| MFQ Score (t1) n = 1160 (90.4%) Mean (SD) | 0.531 (0.339)*** | 0.403 (0.276) | 0.342 (0.23)*** | 0.451 (0.30) |
| MFQ Score (t2) n = 888 (69.29%) Mean (SD) | 0.504 (0.376)*** | 0.377 (0.305) | 0.280 (0.23)*** | 0.455 (0.33) |
| MFQ Score (t3) n = 1009 (78.6%) Mean (SD) | 0.461 (0.351)*** | 0.383 (0.290) | 0.311 (0.25)*** | 0.437 (0.31) |
| Stressful life events n = 1156 (90.1%) Mean (SD) | 0.663 (1.01)* | 0.521 (0.895) | 0.286 (0.57) | 0.700 (1.04) |
| Adversity n = 1140 (88.8%) N (%) | ||||
| None or mild | 282 (60.91%)** | 477 (70.46%) | 212 (71.14%) | 265 (69.92%) |
| Moderate/Severe | 181 (39.09%)** | 200 (29.54%) | 86 (28.86%) | 114 (30.08%) |
| Poor perceived family functioning n = 1,034 (80.6%) Mean (SD) | 21.47 (4.76)** | 20.62 (4.71) | 20.62 (4.56) | 20.62 (4.83) |
| Perceived friendship support = 1,133 (88.3%) Mean (SD) | 23.31 (4.27)** | 23.70 (4.15) | 23.77 (4.22) | 23.64 (4.11) |
Measure details (beyond exposures and outcome) are found in Supplementary table A1 missingness on covariates ranged from 38.7%-5.9% in included sample
p < 0.05
p < 0.01
p < 0.001, differences between included and excluded participants are shown in the excluded column, between males and females are shown in the males column MFQ scores presented are averaged item scores, ranging from 0-2.
Descriptive characteristics of the sample
Sample characteristics along with means and standard deviations of sleep measures and MFQ are listed in Table 1. Based on device data, average sleep duration was 7 hours and 44 minutes, and average sleep onset time was 23:44. Device-measured sleep duration was longer among girls than boys by an average of 18 mins (p < 0.001). No difference was found in self-reported sleep (p = 0.668). On MFQ, females scored significantly higher than males at all waves (t-test, T1: mean difference = 3.49, p < 0.001; T2: mean difference = 5.36, p <0.001; T3: mean difference = 3.82, p< 0.001).
Growth models of change in depressive symptoms with age
Our growth models revealed a linear growth model of acceptable fit (Supplementary Tables B1-B3). Due to a non-significant quadratic term (p=0.814) we did not pursue the quadratic model. Model fit improved when models were grouped by sex, allowing a different intercept and slope for males and females.
In the full sample and in males when separated by gender, slope and intercept were negatively associated (full sample: 0.973 decrease in slope per unit increase in intercept, p = 0.004; males: 1.360 decrease in slope per unit increase in intercept, p = 0.016), indicating that those with higher depressive symptoms at age 14.5 were more likely to have these symptoms decline with time and vice versa (regression to the mean). Thus, in order to control for this phenomenon in models including sleep variables, slope was allowed to regress on the intercept as well as the explanatory variables. The inclusion of this control improved model fit so that all adjusted models in Table 2 had acceptable fit (Supplementary Tables B1-B3). Models where the effect of the intercept was not controlled for are presented in Supplementary Table C1. Without this control, females who slept longer had a significantly greater increase in MFQ, otherwise findings are comparable to those reported in Table 3. Models with sleep predictors used mean centred MFQ scores as this improved model fit.
Table 2. Average MFQ score at sleep assessment (intercept) and change per year of age (slope) in males and females with and without adjusting for intercept.
| Estimate (95% CI) | P value | |||
|---|---|---|---|---|
| MFQ score without adjusting for intercept | Males | Intercept | 0.328 (0.302, 0.353) | < 0.001 |
| Slope | -0.013 (-0.023, -0.003) | 0.010 | ||
| Intercept-slope covariance | -0.004 (-0.008, -0.000) | 0.109 | ||
| Females | Intercept | 0.451 (0.424, 0.478) | < 0.001 | |
| Slope | -0.005 (-0.017, 0.007) | 0.372 | ||
| Intercept-slope covariance | -0.004 (-0.012, 0.004) | 0.234 | ||
| Full sample | Intercept | 0.398 (0.020, 0.059) | < 0.001 | |
| Slope | -0.008 (-0.016, -0.000) | 0.049 | ||
| Intercept-slope covariance | -0.005 (-0.010, -0.001) | 0.044 | ||
| MFQ score after adjusting for intercept | Males | Intercept | 0.328 (0.302, 0.353) | < 0.001 |
| Slope | 0.027 (-0.010, 0.064) | 0.155 | ||
| Females | Intercept | 0.451 (0.424, 0.478) | < 0.001 | |
| Slope | 0.030 (-0.017, 0.077) | 0.222 | ||
| Full sample | Intercept | 0.398 (0.020, 0.059) | < 0.001 | |
| Slope | 0.029 (0.000, 0.058) | 0.048 |
MFQ scores presented are averaged item scores, ranging from 0-2.
Table 3. Growth models of sleep duration and onset time (age 15) predicting MFQ ages 14.5-17.
| Univariate sleep variable predictor models* | Both sleep variables as predictors in model* | |||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |||
| Device-measured Sleep | ||||||
| Sleep Duration (hours) | Males | Intercept | -0.032 (-0.061, -0.003) | 0.035 | -0.009 (-0.046, 0.028) | 0.614 |
| Slope | -0.009 (-0.023, 0.005) | 0.242 | -0.005 (-0.021, 0.011) | 0.500 | ||
| Females | Intercept | -0.053 (-0.090, -0.016) | 0.005 | -0.042 (-0.101, 0.017) | 0.154 | |
| Slope | 0.017 (-0.001, 0.035) | 0.054 | 0.003 (-0.019, 0.025) | 0.767 | ||
| Sleep Onset Time (hours) | Males | Intercept | 0.044 (0.015, 0.073) | 0.004 | 0.038 (0.001, 0.075) | 0.046 |
| Slope | 0.008 (-0.006, 0.022) | 0.226 | 0.005 (-0.012, 0.021) | 0.500 | ||
| Females | Intercept | 0.041 (0.012, 0.070) | 0.008 | 0.016 (-0.031, 0.063) | 0.511 | |
| Slope | -0.021 (-0.035, -0.007) | 0.002 | -0.019 (-0.037, -0.001) | 0.044 | ||
| Self-reported Sleep | ||||||
| Sleep Duration (hours) | Males | Intercept | -0.040 (-0.067, -0.013) | 0.005 | -0.005 (-0.040, 0.030) | 0.766 |
| Slope | 0.001 (-0.012, 0.013) | 0.811 | 0.010 (-0.006, 0.026) | 0.231 | ||
| Females | Intercept | -0.051 (-0.090, -0.012) | 0.010 | -0.014 (-0.069, 0.041) | 0.608 | |
| Slope | -0.003 (-0.019, 0.013) | 0.731 | -0.010 (-0.032, 0.012) | 0.352 | ||
| Sleep Onset Time (hours) | Males | Intercept | 0.067 (0.032, 0.102) | <0.001 | 0.064 (0.019, 0.109) | 0.006 |
| Slope | 0.007 (-0.009, 0.023) | 0.349 | 0.015 (-0.007, 0.037) | 0.170 | ||
| Females | Intercept | 0.067 (0.028, 0.106) | 0.001 | 0.057 (0.002, 0.112) | 0.044 | |
| Slope | -0.004 (-0.022, 0.014) | 0.667 | -0.012 (-0.038, 0.013) | 0.355 | ||
In order to control for regression to the mean in MFQ, intercept was included as a covariate when modeling slope.
MFQ scores presented are averaged item scores, ranging from 0-2.
Based on previous research showing a differential association of depressive symptoms and sleep by gender (Calhoun et al., 2019; Orchard et al., 2020; Tochigi et al., 2016), we tested models grouped by gender. A gender-grouped model revealed no significant decline in MFQ separately in males or females from ages 14.5-17, once MFQ slope was regressed on the intercept (Table 2).
Associations with sleep onset time and duration
In both males and females, shorter sleep duration and later sleep onset time were associated with more depressive symptoms cross-sectionally (a higher MFQ intercept, Table 3). No longitudinal associations were seen between sleep duration and change in depressive symptoms over time. In females but not males, later sleep onset was associated with a decrease in depressive symptoms over time (a decrease in MFQ slope). Very similar coefficients were seen for device-measured and self-reported sleep measures across all the associations tested, with overlapping confidence intervals observed between estimates. However, there was no association between later sleep onset and change in depressive symptoms over time seen using the self-reported sleep measure in either gender.
In order to ascertain the independent association of the two sleep exposures, we also specified models with sleep duration and onset time as simultaneous predictors of MFQ growth models. There was no effect of sleep duration in any of these models (Table 3). A later sleep onset time was associated with a higher MFQ intercept in males for device-measured sleep onset and in all participants using self-reported sleep onset. For device-measured sleep in females, a later sleep onset time was associated with a reduced MFQ slope.
Investigation of the separate associations between weekday sleep and weekend device-measured sleep with depressive symptoms, showed similar associations for weekday sleep as for weekday and weekend sleep combined. There was little association of weekend sleep duration with depressive symptoms. The cross-sectional association of weekend sleep onset time with depressive symptoms appeared smaller than for weekday sleep, and remained significant only in males.
Device-measured and self-reported sleep
Device-based and self-reported sleep duration and onset time were correlated (respectively, r=0.248, r=0.466), with significant differences between the two types of measurement (t-test, respectively, p < 0.001, p < 0.001). Compared with self-reported measures, device-based measures revealed a shorter mean sleep duration by 1:24 (hours:mins), and onset time was 1:04 (hours:mins) later. By gender, significant differences between the types of measurement persisted in both sleep duration and onset time (male: t-test, respectively, mean difference = 4:46, 13:98, p < 0.001, p <0.001; Female: respectively, mean difference = 4:64, 13:94, p < 0.001, p < 0.001).
Discussion
Summary of main findings
In this study of associations between sleep duration and sleep onset time in mid-adolescence and development of depressive symptoms from mid to late adolescence, we found cross-sectional associations suggesting that shorter sleep duration and later sleep onset time were associated with a higher level of depressive symptoms. Although these differences appear small, they are large enough that they may be relevant for public health. For example, in females, a difference of -0.053 points in MFQ score associated with a one hour longer sleep duration, is more than 10% of the mean baseline MFQ score reported in this group (0.451 points). Cross-sectionally, we found that associations between sleep onset time and depressive symptoms persisted after adjustment for sleep duration, suggesting an independent association.
We did not find longitudinal associations between baseline sleep duration and the development of depressive symptoms over time. The only significant longitudinal association observed showed that later device-based sleep onset time in females was associated with a decrease in depressive symptoms over time but coming down from a higher baseline level of depressive symptoms.
In general, findings were fairly consistent between device-measured and self-reported sleep, despite device-measured sleep being on average shorter than self-reported sleep. Associations with MFQ based on device-based measures were stronger for sleep duration but weaker for sleep onset time. This may highlight either a misreporting issue or alternatively the greater difficulty in discerning the difference between the time of lying still trying to fall asleep and actually falling asleep. Examination of sleep data from week nights and weekend night separately revealed that the associations seen were primarily driven by weekday rather than weekend sleep duration and onset time.
Comparison with previous research
Previous studies have found associations between self-reported sleep and depressive symptoms, reporting that sleeping longer (Conklin et al., 2018; Fredriksen et al., 2004; Goldstone et al., 2020; Raudsepp, 2019) and going to bed earlier (Tochigi et al., 2016) were associated with lower level of depressive symptoms both cross-sectionally and longitudinally. In this study, we found that cross-sectionally, our findings agreed with previous research. However, unlike many previous studies we did not find a longitudinal association of sleep duration with depressive symptoms using either device-based or self-report measures of sleep duration. Our findings therefore disagree with those of previous studies, but not due to differences in sleep data collection methods. Similar to a previous UK-based study (Orchard et al., 2020), we also did not find that earlier sleep onset time was associated with lower depressive symptoms longitudinally, again using either device-based or self-report measures of sleep duration.
We found that MFQ slope unadjusted for intercept declined significantly overall and in males but not in females. This is consistent with some previously studied cohorts, which also saw a decrease in depressive symptoms over time as adolescents aged (Calhoun et al., 2019; Fan et al., 2017; Tochigi et al., 2016), although other studies have found an increase in depressive symptoms through adolescence (Matamura et al., 2014; Raudsepp, 2019). Most studies did not investigate sex differences. Following accounting for regression to the mean by adjusting MFQ slope by the intercept, average MFQ scores increased significantly over time overall, although no significant trend was found by gender.
In device-measured sleep duration models, without adjusting for baseline level of depressive symptoms, females who slept longer had a significantly greater increase in MFQ (Supplementary Table C1). However, when the intercept was controlled for, this effect disappeared, indicating potential regression to the mean. Even when adjusting for MFQ intercept, females who went to sleep earlier experienced a greater increase in depressive symptoms over time as compared to those who went to sleep later, contrary to previous research (Calhoun et al., 2019; Conklin et al., 2018; Fredriksen et al., 2004; Goldstone et al., 2020; Matamura et al., 2014; Orchard et al., 2020; Roberts and Duong, 2014; Tochigi et al., 2016). This effect was not observed when self-reported sleep variables were used. Although sleep onset time did not differ by gender, it is possible that 15-year-old females who went to bed earlier felt increasingly excluded from social activities over time, which had a negative influence on their mood. This is congruent with the association of peer rejection and the development of depressive symptoms (Platt et al., 2013). While we did not find an association of sleep onset time and supportive friendships at age 14.5, more detailed data could help reveal whether sleep patterns predict differential peer socialization, perceived friendship support, and subsequent depressive symptoms.
In models which included both sleep predictors, sleep onset time generally remained a significant predictor of MFQ intercept cross-sectionally whilst associations with sleep duration became non-significant. A later sleep onset time, therefore, appeared a more important correlate of contemporaneous depressive symptoms. Power may be of concern in the stratified model, as the estimates are similar in both sets of analyses, although the significance differs. One possible reason for sleep onset time being a more robust correlate of depressive symptoms than sleep duration may be that sleep onset time is a reflection of circadian rhythm (Dickinson et al., 2018) and so is a measure of how far off the adolescent’s natural rhythm is from the rhythm they are forced to live. Later circadian rhythms in adolescents have been associated with a reduction in sleep on weekdays, and a compensatory increase in sleep on weekends, and has been associated with increased depressive symptoms (Knapen et al., 2018; Wittmann et al., 2006). Indeed, in this study, when we assessed the association of weekday and weekend sleep separately, we found that our results were mostly driven by weekday sleep, particularly when considering sleep duration. This corresponds to findings in previous studies (Orchard et al., 2020; Roberts and Duong, 2014).
Among other studies who compared actigraphy with self-reported measures of sleep, agreement in these measures was found in sleep efficiency among adolescents with and without chronic pain (r = .37, p < .05) (Palermo et al., 2007). In the present cohort study, device-measured sleep was obtained using a previously published protocol (averaging sleep duration and onset time based on heart rate and movement data continuously monitored over four days and four nights) (Collings et al., 2015), which revealed that only 3.34% of participants met the NHS recommendation of 9 hours of sleep for those aged 14-16 (NHS, 2018), adding to prior findings of sleep deprivation in adolescents (Matricciani et al., 2012).
Our findings indicated significantly longer self-reported sleep compared to device-measured sleep by a mean of 1:23 hours. Our device-measured sleep onset time was over an hour later than our self-reported measurement, indicating that over-estimates of sleep duration may be due to participants going to bed but not commencing sleep for some time. Compared to previous studies, our device measured sleep duration of 7 hours and 45 minutes was comparable to four studies (Fredriksen et al., 2004; Matamura et al., 2014; Roberts and Duong, 2014; Tochigi et al., 2016), but was much less than in other samples (Conklin et al., 2018; Fan et al., 2017; Goldstone et al., 2020; Orchard et al., 2020), all of which used self-reported sleep. Our self reported sleep, however, was comparable to those previous cohorts which found longer sleep duration (Conklin et al., 2018; Fan et al., 2017; Goldstone et al., 2020; Orchard et al., 2020). Our self-reported sleep onset time was about half an hour earlier than previous studies (Matamura et al., 2014; Orchard et al., 2020; Tochigi et al., 2016), while our device measured sleep onset time was about half an hour later. It should be taken into consideration, however, that our self-report measure of sleep was a measure of usual sleep times, not sleep times on the specific recorded nights.
Strengths and limitations
The strengths of our study include comprehensive measures of exposure and outcome, testing for a wide range of potential confounders and the longitudinal study design, allowing examination of both cross-sectional and prospective associations. Device-measured assessment of sleep timings is more objective and likely to be more accurate than self-reported timings which are subject to reporting biases. The MFQ score is a well-established and validated method of assessing depressive symptoms in this age group (Burleson Daviss, 2006). Although as a self-report measure the MFQ is subject to reporting biases, use of the same instrument at baseline and follow-ups in the present study will ameliorate the impact of any time-invariant biases on prospective associations.
The ROOTS cohort was more economically advantaged and ethnically white than the rest of the UK (Goodyer et al., 2010). Additionally, the subsample in the present analysis appears less impaired than the original cohort (fewer depressive symptoms, stressful events, adversities, more social support), which may reduce generalisability of the findings. Participants were recruited into this study in 2005-7, and these data may therefore not reflect the sleep habits of contemporary adolescents. We excluded participants with mental health disorders at baseline, but the presence of current sleep disorders was not assessed and we were therefore unable to exclude participants with sleep disorders.
In this study we calculated total sleep duration from initial sleep and final wake times without taking into account wake after sleep onset. This is a limitation as it means that participants may have slept for a shorter time period than that was recorded by their total sleep duration, however it does make our device-measured and self-reported sleep measures more comparable. In addition, device-measured sleep was only collected for 4 days and nights (2 weekday and 2 weekend nights), which may limit our ability to capture habitual sleeping patterns. Self-reported sleep was reported in terms of sleep on a usual night, as opposed to sleep on the specific nights actigraphy was measured, which may have influenced the results if the nights measured differ from the participants’ usual pattern of sleep.
In the present study, sleep was only measured at baseline, whereas other studies have measured it longitudinally (Conklin et al., 2018; Fan et al., 2017; Matamura et al., 2014; Tochigi et al., 2016). Thus, we could not test the association of changing sleep patterns with changing depressive symptoms over time. Since this study is an observational study and not a random controlled trial, causal inference is limited and associations may still be subject to residual confounding.
Conclusion and Implications
Cross-sectional associations with both sleep duration and sleep onset time were found, where sleeping less and later were associated with more depressive symptoms. Sleep onset time was a more robust predictor of contemporaneous depressive symptoms than sleep duration, accentuating the particular importance of adolescents going to sleep in a timely manner. Our cross-sectional findings highlight the importance of good sleep hygiene in adolescents, but also the need for a detailed investigation into the possibility and implications of reverse causality (whereby mental health predicts sleep). Longitudinally, our results suggest that in early adolescence a shorter sleep and going to sleep later does not have a detrimental effect on the development of depressive symptoms through adolescence, although given the limitations of our study further study of the association of device-measured sleep with longitudinal depressive symptoms is recommended.
Supplementary Material
Table 4.
Growth models of sleep duration and onset time (device-measured at age 15) predicting MFQ ages 14.5-17. Sleep duration and onset time are examined by weekday sleep and weekend sleep, and compared to weekday and weekend sleep combined.
| Weekday and weekend sleep | Weekday sleep | Weekend sleep | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | |||
| Sleep Duration (hours) | Males | Intercept | -0.032 (-0.061, -0.003) | 0.035 | -0.028 (-0.055, -0.001) | 0.046 | -0.008 (-0.027, 0.012) | 0.399 |
| Slope | -0.009 (-0.023, 0.005) | 0.242 | -0.005 (-0.016, 0.007) | 0.412 | -0.002 (-0.010, 0.006) | 0.572 | ||
| Females | Intercept | -0.053 (-0.090, -0.016) | 0.005 | -0.048 (-0.079, -0.017) | 0.003 | -0.007 (-0.027, 0.013) | 0.513 | |
| Slope | 0.017 (-0.001, 0.035) | 0.054 | 0.012 (-0.002. 0.026) | 0.106 | 0.006 (-0.002, 0.014) | 0.112 | ||
| Sleep Onset Time (hours) | Males | Intercept | 0.044 (0.015, 0.073) | 0.004 | 0.033 (0.006, 0.060) | 0.022 | 0.027 (0.007, 0.047) | 0.008 |
| Slope | 0.008 (-0.006, 0.022) | 0.226 | 0.008 (-0.004, 0.020) | 0.205 | 0.001 (-0.009, 0.011) | 0.789 | ||
| Females | Intercept | 0.041 (0.012, 0.070) | 0.008 | 0.034 (0.007, 0.061) | 0.016 | 0.019 (-0.004, 0.042) | 0.113 | |
| Slope | -0.021 (-0.035, -0.007) | 0.002 | -0.018 (-0.030, -0.006) | 0.002 | -0.007 (-0.017, 0.003) | 0.163 | ||
In order to control for regression to the mean in MFQ, intercept was included as a covariate when modeling slope.
MFQ scores presented are averaged item scores, ranging from 0-2.
Acknowledgements
The authors would like to thank all study participants as well as all those who contributed to the data collection. In particular we would like to thank the physical activity technical team at the MRC Epidemiology Unit (in particular Kate Westgate and Stephanie Hollidge) for their help processing the activity and sleep data.
Abbreviations
- K-SADS-PL
Kiddie Schedule for Affective Disorders and Schizophrenia
- MFQ
Mood and Feelings Questionnaire
- MLR
maximum likelihood estimator with robust standard errors
- NHS
national health service
Footnotes
Declarations of interest:
none
References
- Berger AT, Wahlstrom KL, Widome R. Relationships between sleep duration and adolescent depression: a conceptual replication. Sleep Health. 2019;5:175–179. doi: 10.1016/j.sleh.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59:561–570. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- Daviss Burleson. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. J Child Psychol Psychiatry. 2006 doi: 10.1111/j.1469-7610.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- Calhoun BH, Ridenour TA, Fishbein DH. Associations between Child Maltreatment, Harsh Parenting, and Sleep with Adolescent Mental Health. J Child Fam Stud. 2019;28:116–130. doi: 10.1007/s10826-018-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings PJ, Wijndaele K, Corder K, Westgate K, Ridgway CL, Sharp SJ, Atkin AJ, Bamber D, Goodyer I, Brage S, Ekelund U. Prospective associations between sedentary time, sleep duration and adiposity in adolescents. Sleep Med. 2015;16:717–722. doi: 10.1016/j.sleep.2015.02.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin AI, Yao CA, Richardson CG. Chronic sleep deprivation and gender-specific risk of depression in adolescents: a prospective population-based study. BMC Public Health. 2018;18:724. doi: 10.1186/s12889-018-5656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Angold A. Scales to assess child and adolescent depression: checklists, screens, and nets. J Am Acad Child Adolesc Psychiatry. 1988;27:726–737. doi: 10.1097/00004583-198811000-00011. [DOI] [PubMed] [Google Scholar]
- Dickinson DL, Wolkow AP, Rajaratnam SMW, Drummond SPA. Personal sleep debt and daytime sleepiness mediate the relationship between sleep and mental health outcomes in young adults. Depress Anxiety. 2018;35:775–783. doi: 10.1002/da.22769. [DOI] [PubMed] [Google Scholar]
- Fan F, Zhou Y, Liu X. Sleep Disturbance Predicts Posttraumatic Stress Disorder and Depressive Symptoms: A Cohort Study of Chinese Adolescents. J Clin Psychiatry. 2017;78:882–888. doi: 10.4088/JCP.15m10206. [DOI] [PubMed] [Google Scholar]
- Ferro MA. Missing data in longitudinal studies: cross-sectional multiple imputation provides similar estimates to full-information maximum likelihood. Ann Epidemiol. 2014;24:75–77. doi: 10.1016/j.annepidem.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: Tracking the Effects of Adolescent Sleep Loss During the Middle School Years. Child Dev. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond Engl. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A, Javitz HS, Claudatos SA, Buysse DJ, Hasler BP, de Zambotti M, Clark DB, Franzen PL, Prouty DE, Colrain IM, Baker FC. Sleep Disturbance Predicts Depression Symptoms in Early Adolescence: Initial Findings From the Adolescent Brain Cognitive Development Study. J Adolesc Health Off Publ Soc Adolesc Med. 2020;66:567–574. doi: 10.1016/j.jadohealth.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Croudace T, Dunn V, Herbert J, Jones PB. Cohort Profile: Risk patterns and processes for psychopathology emerging during adolescence: the ROOTS project. Int J Epidemiol. 2010;39:361–369. doi: 10.1093/ije/dyp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman Joan, Birmaher Boris, Axelson David, Perepletchikova Francheska, Brent David, Ryan Neal. K-SADS-PL DSM-5. 2016.
- Knapen SE, Riemersma-van der Lek RF, Antypa N, Meesters Y, Penninx BWJH, Schoevers RA. Social jetlag and depression status: Results obtained from the Netherlands Study of Depression and Anxiety. Chronobiol Int. 2018;35:1–7. doi: 10.1080/07420528.2017.1374966. [DOI] [PubMed] [Google Scholar]
- Kobel S, Wartha O, Dreyhaupt J, Kettner S, Steinacker JM. Cross-sectional associations of objectively assessed sleep duration with physical activity, BMI and television viewing in German primary school children. BMC Pediatr. 2019;19 doi: 10.1186/s12887-019-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G, Jones PB, Goodyer IM. The ROOTS study: a 10-year review of findings on adolescent depression, and recommendations for future longitudinal research. Soc Psychiatry Psychiatr Epidemiol. 2016;51:161–70. doi: 10.1007/s00127-015-1150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Mann J. Depression. The Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- Matamura M, Tochigi M, Usami S, Yonehara H, Fukushima M, Nishida A, Togo F, Sasaki T. Associations between sleep habits and mental health status and suicidality in a longitudinal survey of monozygotic twin adolescents. J Sleep Res. 2014;23:290–294. doi: 10.1111/jsr.12127. [DOI] [PubMed] [Google Scholar]
- Mathers C, Fat DM, Boerma JT, World Health Organization, editors. The global burden of disease: 2004 update. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16:203–211. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- NHS. How much sleep do children need? 2018. [accessed 2.7.20]. [WWW Document]. nhs.uk. URL https://www.nhs.uk/live-well/sleep-and-tiredness/how-much-sleep-do-kids-need/
- Orchard F, Gregory AM, Gradisar M, Reynolds S. Self-reported sleep patterns and quality amongst adolescents: cross-sectional and prospective associations with anxiety and depression. J Child Psychol Psychiatry. 2020 doi: 10.1111/jcpp.13288. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and Subjective Assessment of Sleep in Adolescents with Chronic Pain Compared to Healthy Adolescents. Clin J Pain. 2007;23:812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Cohen Kadosh K, Lau JYF. The role of peer rejection in adolescent depression. Depress. Anxiety. 2013;30:809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. [Google Scholar]
- Raudsepp L. Brief report: Problematic social media use and sleep disturbances are longitudinally associated with depressive symptoms in adolescents. J Adolesc. 2019;76:197–201. doi: 10.1016/j.adolescence.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37:239–244. doi: 10.5665/sleep.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R Package for Structural Equation Modeling. J Stat Softw. 2012;1(2) 2012. [Google Scholar]
- Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochigi M, Usami S, Matamura M, Kitagawa Y, Fukushima M, Yonehara H, Togo F, Nishida A, Sasaki T. Annual longitudinal survey at up to five time points reveals reciprocal effects of bedtime delay and depression/anxiety in adolescents. Sleep Med. 2016;17:81–86. doi: 10.1016/j.sleep.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Wahlstrom KL, Berger AT, Widome R. Relationships between school start time, sleep duration, and adolescent behaviors. Sleep Health. 2017;3:216–221. doi: 10.1016/j.sleh.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the Validity of a Sleep Habits Survey for Adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders Global Health Estimates. World Health Organization; Geneva: 2017. [Google Scholar]
- Xia Y, Yang Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation methods. Behav Res Methods. 2019;51:409–428. doi: 10.3758/s13428-018-1055-2. [DOI] [PubMed] [Google Scholar]
- Zhai L, Zhang H, Zhang D. Sleep Duration and Depression Among Adults: A Meta-analysis of Prospective Studies. Depress Anxiety. 2015;32:664–670. doi: 10.1002/da.22386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.