Abstract
Cancer therapy with accelerated charged particles is one of the most valuable biomedical applications of nuclear physics. The technology has vastly evolved in the past 50 years, the number of clinical centers is exponentially growing, and recent clinical results support the physics and radiobiology rationale that particles should be less toxic and more effective than conventional X-rays for many cancer patients. Charged particles are also the most mature technology for clinical translation of ultra-high dose rate (FLASH) radiotherapy. However, the fraction of patients treated with accelerated particles is still very small and the therapy is only applied to a few solid cancer indications. The growth of particle therapy strongly depends on technological innovations aiming to make the therapy cheaper, more conformal and faster. The most promising solutions to reach these goals are superconductive magnets to build compact accelerators; gantryless beam delivery; online image-guidance and adaptive therapy with the support of machine learning algorithms; and high-intensity accelerators coupled to online imaging. Large international collaborations are needed to hasten the clinical translation of the research results.
Keywords: Particle therapy, Medical accelerators, Gantry, FLASH, Moving targets, Radioactive ions
1. Introduction
For many millennia, infections and neonatal diseases have been the leading causes of human mortality worldwide. Only in the XXI century, cardiovascular diseases and cancer got the first positions [1], as already was the case in the industrialized countries since many years. The main risk factors for these diseases is age, i.e. they become prevalent when life expectancy increase. Cancer is the 2nd most common cause of death worldwide and cancer therapy is therefore obviously in the forefront of medical research [2,3]. Even if enormous progress in systemic pharmacological approaches have been accomplished recently, local therapies – surgery and radiotherapy – remain indispensable. Radiotherapy is indeed used for over 50% of the cancer patients, sometimes as single treatment option but more often as a part of the multimodal cancer care.
The ultimate goal of curative radiotherapy is to kill the tumor cells with acceptable toxicity for the surrounding normal tissues. The effectiveness in killing malignant and normal cells primarily depends on the ionizing radiation dose i.e. the energy deposited per unit target mass (measured in gray; 1 Gy = 1 J/kg). Every tumor can be destroyed if the dose is high enough, but the prescribed dose is limited by the normal tissues surrounding the malignancy. Treatments of malignancies with radiation started only months after the discovery of X-rays by Wilhelm Röntgen in 1895, and in over a century of technological progress underwent many modifications and increasing complexity and automation [4,5]. Currently over 80% of the patients eligible for radiotherapy are treated with external beams (teletherapy) of X-rays produced by bremsstrahlung of electrons accelerated in linacs to about 6 MeV. The others are treated with specialized treatments such as gamma knife or brachytherapy, and less than 1% is treated with accelerated charged particles (protons or heavier ions) [6]. This number is, however, steadily increasing [7].
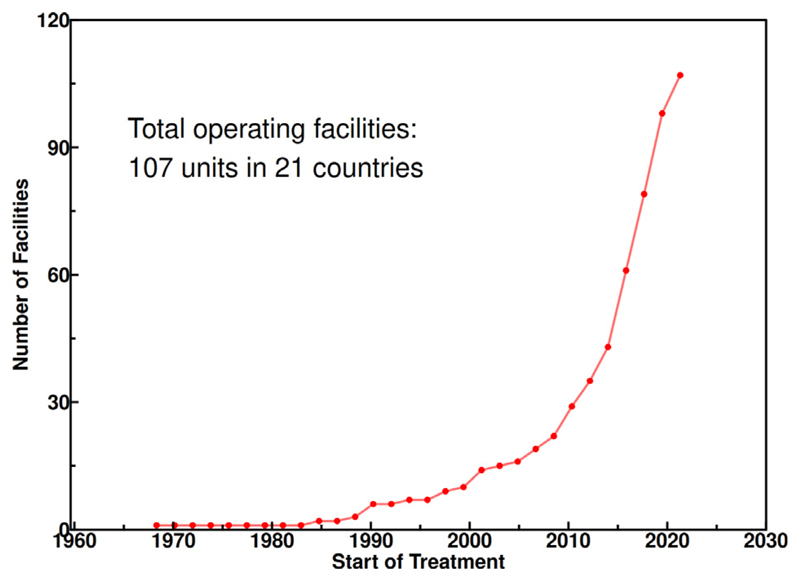
According to the Particle Therapy Co-Operative Group (PTCOG) database [8], 324.586 patients were treated with charged particles until the end of 2021. Around 86% of those patients were treated with protons. The number of facilities in operation is also rapidly increasing in the past years (Fig. 1) [9]. Whilst most of the facilities are in Japan and in the USA, many other countries are in rapid expansion such as Spain (11 centers are planned in addition to the two in operation in Madrid) or China [10].
Fig 1. The growth of the particle therapy facilities worldwide.
Source: Data from PTCOG [9].
The rationale for particle therapy lies in its physical properties. Unlike X-rays, the energy deposited per unit track length increases with depth (Fig. 2), therefore for a single beam the dose to the normal tissue will be much lower for ions than for photons when delivering the same dose to the tumor. Particle therapy is therefore intrinsically more conformal than X-ray therapy, i.e. can provide excellent high dose distributions in tumors of complex shape with relatively low dose to the surrounding normal tissue (Fig. 3). The basic physics of particle therapy has been reviewed many times [11–18] and is not debated. For many years, however, the cost effectiveness of particle therapy remained controversial because the increased costs compared to X-rays was not matched by level-1 (i.e. derived from randomized phase-III clinical trials) clinical evidence of superiority [19–21].
Fig 2. Physical advantages of charged particle therapy.
A, depth-dose distributions of high-energy X-rays and monoenergetic beams of protons or carbon ions. At the same range, C-ions have lower straggling than protons, but a tail of fragments is visible beyond the Bragg peak. The curves were generated with TOPAS [34]/Geant4 simulations. In clinical applications, the Bragg peak must be extended to cover the whole tumor (Spread-Out-Bragg-Peak, SOBP) (B). This can be done by overlapping different pristine beams at different energy and intensity, as shown for protons.
Source: Data generated with TRiP98 [35].
Fig 3. Comparison of treatment plans for a chordoma patient with a large central tumor for A.
IMRT (9 fields) or B. carbon ions (2 fields). The IMRT plan was generated with the MatRad [36] open treatment planning toolkit, the carbon plan was generated with TRiP98 [35]. The substantial reduction in the integral dose to the normal tissue and the sparing of critical structures is clear using particles.
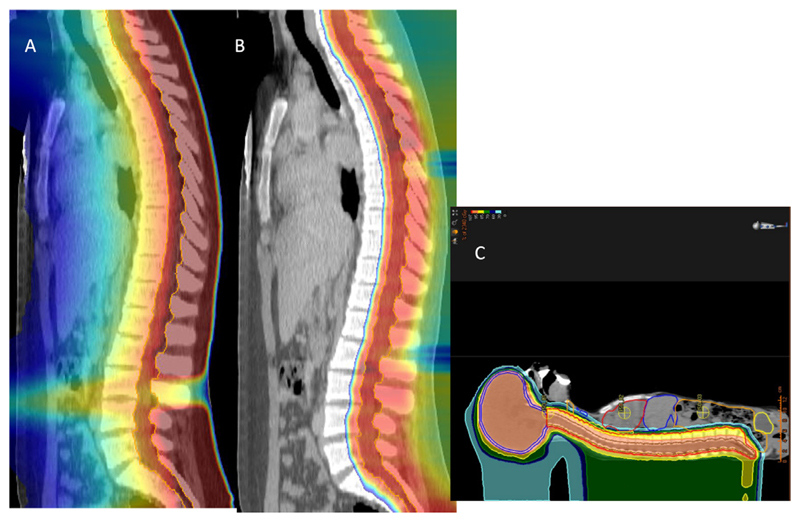
In recent years, this criticism became weaker. First, cost has been reduced with compact superconducting cyclotrons and single-room treatment facilities [22,23]. Second, evidence showing superiority of charged particles compare to photons in comparative studies is rapidly emerging. Reduced toxicity of protons in head-and-neck tumors has been shown in retrospective analysis in different clinical centers [24–27]. A randomized trial comparing protons to intensity modulated radiotherapy (IMRT) with X-rays for locally advanced esophageal cancer has demonstrated reduced risk and severity of adverse events when particles are used [28]. Finally, a recent prospective trial has shown increased effectiveness of proton beam therapy in patients treated for leptomeningeal metastasis [29]. Patients with leptomeningeal metastasis, i.e when cancer spreads to the membranes lining the brain and spinal cord, have a dismal prognosis with a survival of only 3–6 months [30]. Patients treated with protons had improved progression free survival and overall survival compared to those receiving focal X-ray irradiation. This clinical success is entirely due to the physics of protons. With protons, it is possible to have high-dose full craniospinal irradiation that is very toxic with X-rays, because the whole body would be irradiated. Proton craniospinal irradiation is typically employed for treatment of pediatric medulloblastoma (Fig. 4) [31], and can be now a key method to improve survival of patients with leptomeningeal metastasis [32], especially those with reduced genomic variability in circulating tumor DNA [33].
Fig 4. Craniospinal irradiation of a pediatric medulloblastoma patient.
A. IMRT plan, B. proton plan. C. full proton plan including the brain and the countering of the internal organs at risk.
Nevertheless, substantial challenges remain for proton or heavy ion therapy physics. First, even if the cost has been reduced with single-room facilities, it remains substantially higher than photons. Therefore, more compact and cheaper machines remain absolutely necessary to further expand particle therapy [37–39]. Second, even if the sophisticated pencil beam scanning provides excellent coverage of the target, there is a substantial uncertainty on the beam range in the patient [40], entailing large margins around the target that somehow jeopardize the Bragg peak accuracy [41]. The problem is complicated by the organ motion, especially for thoracic tumors, because the movement disrupts the dose distribution. Finally, patient irradiation takes several minutes and is delivered in several daily fractions. Recently, it has been shown in pre-clinical studies that ultra-high dose rates (FLASH radiotherapy) can substantially reduce normal tissue toxicity without affecting tumor control [42]. Beyond the potential biological advantages, ultra-high dose rate can improve the comfort and patient workflow and contribute to solve the organ motion problem. The physical challenge will be to produce particle accelerators and beam delivery systems able to cope with this high-intensity while maintaining conformality.
In short, the current goal is to make particle therapy cheaper, more conformal and faster. Emerging approaches toward these goals will be summarized here.
2. Smaller and cheaper systems
The high investment cost of particle therapy is caused by the complexity of the accelerator, beam transport and delivery technology. The large footprint of these devices brings along high construction costs to satisfy the radiation protection requirements. For this reason, cheaper is almost equivalent to smaller [43]. The most bulky technological components are the accelerator itself (presently either cyclotron plus energy selection system or synchrotron; see Table 1) and the gantry, i.e. the rotating nozzle that allows the delivery of the beam at any angle prescribed in the treatment plan. The size of these machines is determined by the gyroradius ρ in the Lorentz’s formula:
| (1) |
where B is the magnetic field, p the momentum and q the charge of the accelerated particle. In the relativistic expression of the momentum, m0 is the particle rest mass, β the relative velocity, c the speed of light and γ the Lorentz factor. This means that size increases with the particle kinetic energy per nucleon and the mass/charge ratio. Accelerators are designed to reach a desired range in (water-equivalent) tissue, typically 30 cm, which corresponds to a particle-specific kinetic energy, such as 430 MeV/n for carbon or 220 MeV for protons. As shown in Fig. 5, the corresponding magnetic rigidity Bρ is around 3 times higher for C-ions than for protons. Therapy with ions heavier than protons is therefore associated to larger and more expensive accelerators and gantries.
Table 1. Characteristics of the main accelerators potentially used in particle therapy.
In parenthesis are the objectives that are planned but not yet reached.
| Accelerator | Average size (m) | Energy modulation | Repetition rate (Hz) | Energy range (MeV/n) | Ion species | In use today |
|---|---|---|---|---|---|---|
| Linacs | 50 | Modules on/off | ∼200 | <20 (200) | 1 (2) | Yes |
| Cyclotrons | 5 | Absorbers | ∼106 | 70–250 | 1 | Yes |
| Superconducting cyclotrons | 1.5b | Absorbers | ∼106 | 70–250 (400) | 1 (multiple) | Yes |
| Synchrotrons | 20 | Ramp | 0.5–1 | 70–430 | Multiple | Yes |
| RCMSc | 20 | Ramp | 30 | 70–430 | Multiple | No |
| Cyclinacs | 30 | Modules on/off | 300 | 70 (430) | 2 | No |
| FFAGd | 18 | Ramp | 1000 | (70–430) | Multiple | No |
| DWAe | 2 | Modules on/off | 50 | (200) | 1 (2) | No |
| LDPAf | 2–3 | Selection from full spectrum | ≪1 (10) | 100 (400) | Multiple | No |
Superconducting.
Approximately 6.3 m diameter for the C-ions prototype C400.
Rapid cycling medical synchrotron.
Fixed field alternating gradient.
Dielectric wall accelerator.
Laser-driven particle accelerators.
Fig 5. Magnetic rigidity as a function of the particle energy for protons and carbon ions.
2.1. Current accelerators
The accelerators presently in use in particle therapy are produced by different companies [44,45]. All of these companies are implementing effort to reduce the size of the accelerators. According to Eq. (1), this means increasing B, which in turn means using superconducting magnets [46]. Table 2 gives a summary of the main accelerators currently on the market for therapy with protons or carbon ions. Clearly the market focuses on cyclotrons, characterized by a quasi-continuous beam with high intensity and stability; and synchrotrons, whose main advantage is the possibility of actively changing the energy.
Table 2. Characteristics of the main accelerators currently in use for particle therapy.
| Name | Company | Accelerator type | Particle (max energy) | Diameter (m) | Magnet type | Max field (T) |
|---|---|---|---|---|---|---|
| C230 | IBA | Isochronous cyclotron | p (230 MeV) | 4.3 | Normal conducting | 1.7 |
| S2C2 | IBA | Synchrocyclotron | p (250 MeV) | 2.2 | Superconducting (NbTi) | 5.7 |
| Probeam | Varian | Isochronous cyclotron | p (250 MeV) | 3.1 | Superconducting (NbTi) | 2.4 |
| SC360 | ProNova | Isochronous cyclotron | p (250 MeV) | 2.8 | Superconducting (NbTi) | 4 |
| SC230 | Sumitomo | Isochronous cyclotron | P (230 MeV) | 2.8 | Superconducting (NbTi) | 4 |
| S250 | Mevion | Synchrocyclotron | p (250 MeV) | 1.5 | Superconducting (Nb3Sn) | 9 |
| PROBEAT | Hitachi | Synchrotron | p (220 MeV) | 5.1 | Normal conducting | 1.8 |
| Conforma 3000 | Optivus | Synchrotron | P (250 MeV) | 8 | Normal conducting | 1.4 |
| Radiance | ProTom | Synchrotron | p (330 MeV) | 4.1 | Normal conducting | 1.9 |
| Toshiba | Toshiba | Synchrotron | C (430 MeV/n) | 20 | Normal conducting | 1.5 |
| PIMMS | CERN | Synchrotron | C (400 MeV/n) | 25 | Normal conducting | 1.5 |
| PTS | SIEMENS | Synchrotron | C (430 Mev/n) | 20 | Normal conducting | 1.43 |
| HITACHI | Hitachi | Synchrotron | C (430 MeV/n) | 17 | Normal conducting | 1.5 |
| C400 | IBA | Isochronous cyclotron | C (400 MeV/n) | 6.6 | Superconducting | 4.5 |
Cyclotrons for therapy need to reach high energies, where relativistic effects cannot be neglected. The angular frequency ω of the electric field for a particle of charge q and rest mass m0 is:
| (2) |
To cope with the variability of the accelerating frequency in Eq. (2), two strategies are used [47]. In synchrocyclotrons, the angular frequency ω of the RF is modified to account for the variation of γ with increasing velocity. In isochronous cyclotrons, ω is kept constant but the magnetic field B in Eq. (2) is changed. Synchrocyclotrons are smaller and more compact than isochronous cyclotrons, but they have lower output beam current and duty cycle.
Synchrotrons generally use single energy extraction, which requires 1–4 s switching times between energies. Multiple energy extraction, originally developed at NIRS in Japan for C-ions [48], decelerates the beam in discrete steps between short extraction phases so that several energy layers can be delivered in each accelerator spill [49]. This technology reduces the beam deliver time and allows rapid range adaptation without any additional passive energy degrader. Multiple energy extraction reduces beam delivery time also in proton therapy [50] and is indeed implemented in the PROBEAT proton synchrotron (Table 2).
The more stable magnetic field of cyclotrons is more favorable to superconductivity, and it is indeed employed in all modern cyclotrons [45]. While both cyclotrons and synchrotrons are used for protons, until now, only synchrotrons are in use for heavy ions (14 in operation with C-ions by the end of 2022 [9]). However, the C400 IBA compact cyclotron for carbon ions [51] is about to be commissioned in Caen (France) [52].
Proton machines are smaller than those able to accelerate C-ions, and clearly superconducting magnets, usually NbTi, result in more compact accelerators. Yet superconductivity in a hospital-based center comes with a cost. To achieve high fields (B > 3 T), a large quantity of superconducting material is needed, resulting in high cost and challenging cooling. Magnets in synchrotrons in addition need field ramp rates >1 T/s, a curved shape and usually quadrupole integration [53]. For multiple-energy extraction, fast cycling (>10 Hz) magnets are needed to change energy in small steps, corresponding to the different tumor slices. However, alternating current in superconducting magnets generates eddy currents in the coil and the iron yoke that lead to increased losses and transition to a normal state [54]. High-temperature superconductors can provide a breakthrough in this direction [55], but they are still in experimental testing. Two main candidates are Bi-2212 round wires [56] and rare-earth barium copper oxide (REBCO) coated conductors [57], both theoretically able to reach intensities around 20 T, much higher than those currently achieved with cold superconductors.
2.2. Future accelerators
In Table 1 we show other accelerator concepts that may help reducing cost and footprint of future particle therapy facilities.
Radiofrequency linear accelerators were proposed already over 30 years ago by Ugo Amaldi at TERA Foundation and CERN [59,60]. The initial concept was a cyclinac, with a commercial 30 MeV-cyclotron as injector into a 3 GHz linac. However, cyclotrons and linacs have different time structures and the injection is therefore very complex and leads to a large intensity loss (around 95% beam loss in the longitudinal phase space). An all-linac solution can theoretically reach 100% transmission with clean beam dynamics. The TULIP all-linac concept developed at CERN is shown in Fig. 6 [58]. The system is based on the CERN 750 MHz radiofrequency quadrupole (RFQ) that injects 5 MeV particles into an interdigital H-mode cavity. The 10 MeV beam is then accelerated in a 3 GHz drift-tube linac up to 70 MeV followed by a couple-cavity linac up to 230 MeV. The company AVO-ADAM has recently completed a 230 MeV, 16-m long proton linac at the STFC Daresbury laboratory in UK. The accelerator, called LIGHT, is similar to TULIP [61]. Other companies are also working on linac concepts for proton therapy. In principle, linacs can also be used for heavier ions, but their length becomes increasingly long. A project for an advanced compact heavy ion linac with real-time image-guided capability has been recently proposed by the Argonne National Laboratory [62]. An issue of the linacs is the high HF power demand, leading to a low macro cycle of 200 Hz for the current design (Table 1). The AVO-ADAM first hospital-based installation at the Harley Street Proton Therapy Center (Marylebone, London) will show if this leads to problems in clinical application. As the number of involved cavities can be changed from pulse to pulse, in principle very fast energy changes are possible, offering the possibility of online range adaptation and to operate in FLASH regime.
Fig 6. Sketch of the TULIP all-linac accelerator.
Source: Image from Ref. [58], reproduced with permission.
Other concepts described in Table 1, such as the rapid cycling medical synchrotron (RCMS) [63], fixed-field alternating gradient (FFAG) accelerators [64], and the dielectric wall accelerators [65] have been proposed since several years but have not reached the level of industrial production as linacs. The big promise for the future is certainly in laser-driven particle accelerators (LDPA) that have the potential to provide ultra-compact high-intensity systems for medical applications [66]. The investment in this technology is very high worldwide but, despite many recent efforts for designing beam transport and delivery in therapeutic scenarios [67,68], large problems remain. This includes [69] increasing the intensity and repetition rate; increasing the maximum particle energy; shielding for secondary radiation, especially the very abundant low-energy ions, which is likely to be bulky and expensive; target stability; improving shot-to-shot reproducibility (at least to the few % level); addressing quality-assurance and patient-safety aspects. To date, the highest proton energy (∼100 MeV) were achieved at the Vulcan laser of the Rutherford Appleton Laboratory in the UK [70]. Pre-clinical radiobiology studies have been demonstrated at the Draco PW laser in Dresden (Germany), where a mouse ear tumor was treated with ∼60 MeV protons [71]. Full laser-driven ion medical accelerators remain far in the future, but low-energy linear injectors in ring accelerators could be laser-driven. The current plans for miniaturized synchrotrons in Japan include the use of a short laser-driven particle accelerator as injector for a high-B field superconducting synchrotron [72].
While these new accelerator concept are interesting and have the potential to provide a breakthrough to reduce the footprint of medical accelerators, for the short- and medium-term future it is likely that most of the progress will come from the superconductive magnets.
2.3. Beam delivery
Pencil beam scanning is now universally accepted as the beam delivery system for particle therapy (Supplementary video 1). Scanning is essential for intensity-modulated particle therapy (IMPT) and provides a dose conformity which is impossible to obtain with the classical passive scattering method initially used in particle therapy [73]. As we will see in the next section, it brings the problem of interplay between beam and organ motion [74], a problem that still hampers the application of particle therapy to moving organs, especially thoracic tumors. Moreover, 3D volumetric scanning with energy changes is relatively slow and reduces the speed of the treatment.
From the volume/mass point of view, the most massive and expensive element in beam delivery is the rotating gantry. In conventional radiotherapy, the linac itself rotates around the patient. In particle therapy, the gantry is a magnetic system that deflects the beam and can rotate 360° around the patient’s couch [47]. Only the Mevion cyclotron (Table 2) is designed to rotate itself around the patient, without additional gantry magnets. A typical proton gantry is about 10 m × 10 m with a weight around 170 tons. As shown in Fig. 5, the size of a C-ion rotating gantry must be much bigger. The first C-ion gantry was installed at the Heidelberg Ion Therapy facility and is a gigantic 25 m × 13 m structure with a weight of 670 tons. The superconductive C-ion gantry at NIRS in Chiba (Japan) is still very big (19 m × 13 m) but significantly lighter (300 tons) [83], and the first commercial C-ion Toshiba gantry in operation at Yamagata is still lighter (around 200 tons) [84]. It is possible to further reduce the gantry size using curved canted cosine-tetha superconducting magnets [85], which should be able to reach size comparable to proton gantries [86]. Other projects are studying fixed magnets around the patient, where the beam can be directed on the target from any angle by changing the field strength and beam position [75,87,88] thus eliminating the need of rotating magnets (Fig. 7).
Fig 7. Sketch of a toroidal fixed gantry, with 16 superconducting coils, able to deliver the beam from any angle without rotation.
Source: Image from Ref. [75], reproduced with permission.
Even with the introduction of superconducting technology, gantries remain one of the most massive and expensive components. Certainly an upright positioning system, where the patient is sitting on a rotating chair, would allow gantryless systems still saving the option of irradiation from multiple angles [89,90]. There is a long history of chairs in particle therapy (Fig. 8), but their use was hampered by imaging, which is normally done in horizontal position. If a patient is planned on a couch, obviously the anatomic changes that occur when moving in vertical position can jeopardize the conformality of the treatment. For these reason, radiation oncologists always included a rotating gantry among the essential requirements for particle therapy, especially for extracranial targets. The Shanghai Proton and Heavy Ion Center is currently irradiating some patients with cranial tumors on a hexapod chair [80]. The use of the chairs is currently expanding thanks to the introduction of vertical CT imaging [91], which is now commercially available (Leo Cancer Care and p-Cure). Irradiation in upright position has the main advantage of being gantryless and therefore substantially cheaper in particle therapy, but an additional advantage is the reduction of organ motion. For lung cancers, MRI [92] and CT [93] studies of healthy volunteers have demonstrated reduced cranio-caudal lung motion and increased lung volume in upright compared to supine position. This suggests that the upright position can lead to a lower mean lung dose. Moreover, a similar reduction in cranio-caudal movement for upper abdomen tumors is expected. Tests of upright position devices in head-and-neck [94] and pelvic [82] cancer patients have demonstrated good reproducibility and improved patient control. A couch will probably remain necessary in clinical practice (e.g. for pediatric patients), but it is likely that irradiation in upright position can reduce the number of gantries needed in a particle therapy center and therefore eventually reduce the costs.
Fig 8. Historical overview over different upright positioning systems used for particle therapy.
a upright positioner for the pioneering studies at Lawrence Berkeley National Lab (Source: imaging archive of the Lawrence Berkeley National Laboratory, © 2010–2019 The Regents of the University of California, Lawrence Berkeley National Laboratory). b Chair positioner and vertical CT scanner at the Hyogo HIMAC, Japan [76]. c Chair prototype at the GSI carbon ion therapy pilot project [77]. d and e Chair positioners used at the Indiana University Health Proton Therapy Center and the Oklahoma Proton Center, respectively [78]. f Chair system in operation at Northwestern Medicine Chicago Proton Center. Image courtesy to Northwestern Medicine Chicago Proton Center. g Chair system of the ‘PROMETHEUS’ proton therapy center at P.N. Lebedev Physical Institute of the Russian Academy of Sciences (Protvino, Russia) [79]. h Chair system at the Shanghai Proton and Heavy Ion Center (Shanghai, China) [80]. i Chair design with soft-robotics immobilization [81]. j Leo Cancer Care upright positioner [82].
3. Highly conformal particle therapy
From the physics point of view, radiotherapy is relatively simple: the goal is to provide the maximum possible dose to the target at the lowest possible dose to the surrounding normal tissue. Dose escalation extends progression-free survival and reduces local recurrence rates, but is limited by normal tissue complications: radiotherapy is not conformal enough. The prescribed dose is indeed directed to the planned target volume (PTV), which accounts for the various geometrical uncertainties, such as patient re-positioning and intra- and inter-fraction organ motion. ICRU [95] defines the malignancy visible in the CT as gross-tumor volume (GTV) and its extension to account for microscopic extensions as clinical target volume (CTV). The PTV is an extension of the CTV affected by significant delineation uncertainty [96]. Its contouring is only based on statistical analysis of a small patient population and isotropic extension of the volume of interest [97], not personalized on the individual patient [98]. Ideally, the margin PTV-CTV should be reduced to zero to ensure maximum effectiveness of the treatment.
3.1. Range uncertainty
Thanks to the Bragg peak (Fig. 2), particle therapy is in principle more conformal than X-ray therapy, even in its advanced delivery technologies such as IMRT, tomotherapy or volumetric arc-therapy (VMAT). However, this potential advantage is partly jeopardized by range uncertainty [99]. The prediction of the particle range in the patient is indeed associated with considerable uncertainties due to imaging, patient setup, beam delivery and dose calculation. Table 3 summarizes some of the sources of range uncertainties and their magnitude for proton therapy [40]. The issues are very similar for heavy ions. Some uncertainties depend on dose calculation, while others do not. A major source of uncertainty is the Brag peak degradation, caused by tissue inhomogeneities that lead to a wider distal fall-off [100]. This eventually causes an underdosage of the target volume and an overdosage of normal tissue distal to the target volume [101]. A second major contributor is the uncertainty on the mean excitation value I, which enters directly in the calculation of the beam energy deposition and transport [102,103]. The standard value recommended by ICRU [104] is 75 eV, but values ranging 74.6–81.8 eV are reported in the literature, with an average value I = 79.2 ± 1.6 eV [105].
Table 3. Estimated proton range uncertainties, their sources, and the potential of Monte Carlo for reducing the uncertainty.
| Source of uncertainty | Uncertainty type | Range uncertainty |
|---|---|---|
| Commissioning | Measurement uncertainty in water | ±0.3 mm |
| Beam delivery | Compensator design | ±0.2 mm |
| Beam reproducibility | ±0.2 mm | |
| Positioning | Patient setup | ±0.7 mm |
| Biology | Biological range extension | ≈0.8% (always positive) |
| Dose calculation | CT imaging and calibration | ±0.5% |
| CT conversion to tissue | ±0.5% | |
| CT grid size | ±0.3% | |
| Mean excitation value (I-value) in tissue | ±1.5% | |
| Range degradation | ±2.5% | |
| Total (excluding biology) | 4.6% + 1.2 mm | |
| Total (using Monte Carlo dose calculation) | 2.4% + 1.2 mm |
Clinically, a substantial margin is added to the distal and proximal target edge in order to ensure tumor coverage, e.g. in proton therapy this range margin is on the order of 3.5% of the prescribed range, based on initial estimation of Michael Goitein at MGH [106]. The additional margin corresponds to a significant normal tissue volume uselessly irradiated with particles that reduces the conformality that would be achieved based on simple physics [41]. If this volume is reduced bringing the PTV close to the CTV, normal tissue complication probability modeling predicts a reduced toxicity for proton [107] and C-ion therapy [108] for both serial and parallel organs at risk.
Using dual-energy X-ray CT for treatment simulation instead of the typically used single-energy X-ray CT can substantially reduce necessary range margins [109], since the two applied photon energy spectra provide partially complementary information on the target tissues. This improves the accuracy of the beam range estimate in the patient [110–112], close to direct particle CT [113].
3.2. Moving targets
For IMPT, not delivering homogeneous doses per field, range uncertainties are often not explicitly considered but incorporated into robust optimization strategies designed to minimize the impact of range or setup errors [114]. With robust optimization, the definition of PTV is not necessary any longer and the treatment optimization is done directly on the CTV [115].
Robust optimization is now part of the commercial treatment planning systems in particle therapy. IMPT treatment planning is performed either by single- or multi-field optimization. Single-field is more robust against motion because it only requires the consistency of the anatomy during the treatment delivery of one field, while multi-field optimization requires the integrity of anatomy during the entire treatment session from all treatment fields [116]. Intra-fractional organ motion unavoidably modifies patient anatomy and interferes with the movement of the pencil beam in the scanning pattern. This ‘‘interplay effect’’ degrades the target coverage, generating cold- and hot-spots [74] (Supplementary Video 2). Treatment of moving targets in particle therapy therefore requires motion management techniques aiming to either reduce the anatomical motion (e.g. breath holding or abdominal compression) or to adapt the treatment during planning or delivery (e.g. 4D treatment planning [117], tracking). Despite ongoing attempts to achieve a standardized approach to motion management by PTCOG [118], currently every center is using its own approach to treat moving targets with IMPT.
Even if breath-holding [119] or compression belts [120] reduce the motion of thoracic and abdominal tumors, these passive methods, routinely used in conventional radiotherapy, have several pitfalls in particle therapy, such as large variability in patient’s breath hold capability and compression tolerance. They also do not account for inter-fractional motion [121].
More robust approaches are based on planning on 4DCT images, rather than conventional static CT images [122]. The boundaries of all CTV positions registered in the 4DCT define an internal target volume (ITV). Using the ITV as a static PTV is not adequate in IMPT, considering the interplay effect, the different density of the lung and tumor tissue, range uncertainty and irregular motions and deformations. A valid strategy to mitigate motion-induced range changes is a density override of the ITV [123], which is still in clinical use. ITV can nonetheless be used in association with other motion mitigation strategies, such as rescanning [124], gating [125] or tracking [126]. In beam tracking the motion of the pencil beams accurately follows the motion of the tumor, resulting in the optimal dose distribution comparable to static plan (Supplementary Video 2) [127]. Tracking is, however, technically far more challenging than rescanning or gating. Due to the additional degree of freedom provided by the range of the particles inside the patient, tracking can be performed either only laterally or also in depth, but tracking alone does not regain dose conformity in irregular motion scenarios [128,129]. In addition, as noted above, conformal radiotherapy needs robust optimization and, for proper handling of organ motion, this means 4D robust optimization [130]. These approaches have now been tested both for protons [131,132] and carbon ions [133–135], the latter being further complicated by the problem of non-linearity of the RBE-weighted dose [136] used in heavy ion therapy. The best possible option is the multiphase 4D dose delivery with residual tracking (MP4DRT) [129], where a dedicated quasi-static treatment plan is delivered to each motion phase of a periodic 4DCT and lateral beam tracking compensates for the displacement of the tumor center-of-mass relative to the current phase in the planning 4DCT. MP4DRT can deliver highly conformal particle therapy to irregularly moving targets (Supplementary Video 3), but it depends on clinically available motion monitoring like all tracking-based motion mitigation methods [137].
3.3. Online image guidance
Image guidance is generally considered essential for modern, conformal radiotherapy [138]. Modern radiotherapy uses cone beam CT (CBCT) for 3D patient positioning. CBCT reduces re-positioning errors and shows inter-fractional organ movements, but the image quality is lower than for conventional CT, and the image has several artifacts that make a full re-planning difficult. During therapy, optical surface monitoring [139] is offered by different vendors. Orthogonal X-ray fluoroscopy with or without markers and combined with different motion models can be used to track the tumor position. In conventional X-ray therapy, portal imaging of the MV treatment beam is offered on many machines, and is a valuable tool to monitor therapy and to support online dose calculation [140,141]. The combined MR-Linac provides excellent image quality during therapy [142,143], which is also under research for particle therapy [144,145].
All of the above imaging methods identify the target, but do not provide a direct measurement of beam range, which is critical for particle therapy. Current strategies for range estimation rely on different nuclear reactions of the high-energy projectile with the target [146]. The production of positron-emitting isotopes such as 11C (produced by nuclear fragmentation of the 12C projectile in carbon ion therapy [147]) or 15O (generated by target fragmentation of 16O in proton therapy [148]) with half-lives in the order of minutes enables PET imaging, resulting in 3D images available after delivery [149]. Prompt emission of γ -rays [150] or charged particles [151] provides faster feedback and is currently under clinical investigation [152]. The INSIDE detector at CNAO is currently in use in patients treated with protons or 12C-ions and includes both an in-beam PET [153] and a dose profiler [154] for the detection of secondary charged particles. However, all these methods are affected by caveats such as low signal-to-noise ratio and indirect correlation to the Bragg Peak that limit their applicability in clinical settings. This justifies the intensive research effort in the field of online range monitoring that is currently ongoing worldwide.
PET imaging is the oldest technique for online beam visualization and range verification, and many new ideas are under study to improve it [156]. These improvements include detection of very short-lived isotopes (such as 12N) for online imaging [157], Open-PET with a full-ring geometry developed at NIRS in Japan [158], and J-PET with inexpensive plastic scintillators developed at the Jagellonian University in Poland [159]. A caveat of PET detectors in range verification is the low signal-to-noise ratio because only secondary products are measured, and the shift between the Bragg peak and the activity peak. These problems can be solved using radioactive ion beams for both treatment and beam visualization (Fig. 9). This idea was already proposed many years ago during the pilot trial of heavy ion therapy in Berkeley [160], but was hampered by the low intensity of the radioactive ion beams [161]. With the construction of new, intense accelerators dedicated to nuclear physics with exotic beams [162], a pre-clinical test of this idea becomes possible and is under investigation in the BARB project at GSI in Germany [155,163]. Within BARB, it has been shown that sub-mm resolutions are achievable thanks to the high count rate provided 10C or 11C projectiles [164], and a pe-clinical test using an animal model is ongoing.
Fig 9. Potential advantages radioactive ion beams for image-guided radiotherapy.
The images show Monte Carlo simulation of 12C and 11C beams stopping in a spherical water volume and visualized by PET in 20 min. The graphs show the dose (red curve) and the activity (blue curve) distribution along the beam direction (z-axis) showing the shift between dose and activity when stable ions are used. Simulation by Monte Carlo code FLUKA.
Source: Image from Ref. [155], reproduced with permission.
A novel concept is range probing, which uses selected high energy beams fully penetrating the patient prior to treatment to provide a range verification of setup or control imaging [165,166]. The idea is based on proton radiography [167,168], which is under study since many years to eliminate the problem of the conversion between Hounsfield units (from the CT) to water equivalent path lengths (used in beam transport calculation), but goes toward an online exploitation using simultaneously one beam in the Bragg peak for therapy and second, of lower mass, going through the patient for online imaging. Mixed ion beam guidance has been tested in experiments with subsequent, separate 12C and 4He beams with promising results [169,170].
3.4. AI in particle therapy
There is hardly any field of science nowadays where artificial intelligence (AI) methods is not producing rapid advances. Radiotherapy is obviously no exception. Because the radiotherapy workflow is based on imaging, most AI methods in radiotherapy use deep learning and in particular convolution neural networks. AI can actually be applied in all steps of the radiotherapy chain [171]:
-
–
treatment strategy (machine learning is used in normal tissue complication models [172], radiogenomics [173], and image analysis [174], to support the decisions on individualized treatment strategy);
-
–
segmentation (in the imaging phase, segmentation algorithms reduce the human variability [175]; a notable AI system nnU-Net, a deep learning-based segmentation method that automatically configures itself and adapts to new datasets [176]);
-
–
treatment planning (automatic dose prediction algorithms are already used in conventional radiotherapy [177]);
-
–
QA (machine learning can accelerate treatment plan verification and detect errors for re-planning [178]);
-
–
beam delivery (AI-based algorithms have the potential to monitor organ motion, reduce treatment uncertainty and improve dose delivery accuracy [179]);
-
–
follow-up care (as well as before the treatment, machine learning can support decisions in adjuvant settings [180]).
All these applications in conventional radiotherapy are also venturing into particle therapy. The applications in treatment planning, QA and beam delivery are quite distinct from the problems faced in X-ray therapy, simply because the physics is profoundly different.
Can AI improve conformality of particle therapy? First, all methods described for online imaging in Section 3.3 benefit from AI algorithms. For instance, the difference between dose and activity distribution in range verification by PET can be corrected with machine learning algorithms [181,182]. Convolutional neural network can calculate proton dose [183] and improve the resolution of the beam range measurement [184] from prompt-γ images; and deep learning can contribute to the development of new methods for range verification such as ionoacoustic [185] or luminescence [186] imaging. Machine learning can also be used to improve CBCT images generating a synthetic CT where it is possible to re-plan the patient [187–189]. Second, machine learning can make dose calculation in treatment planning much faster [190,191] ,with an accuracy comparable to the time-consuming Monte Carlo calculations. Considering that AI can play a decisive role in online imaging and fast dose calculation, it is clear that it is an ideal tool for online adaptive particle therapy (Fig. 10). Adaptive therapy is a strategy to take into account re-positioning errors, inter-fractional anatomical variations including tumor shrinkage, intra-fractional motions, to make eventually radiotherapy more conformal, as it allows reduction of the margins [192,193]. Adaptive therapy is more necessary in particle therapy than in conventional radiotherapy, because the high precision of the Bragg peak and the steep gradient makes it more sensitive to geometrical uncertainties [194]. It expands to different time scales, because tumor shrinkage can occur over weeks, inter-fractional organ movements and repositioning daily, and intra-fractional movements in the time-scale of seconds. The clinical workflow, including imaging, segmentation, and treatment planning, makes online adaptation extremely difficult with conventional computational power. Here AI can play a decisive role, both for imaging, generation of synthetic CT and fast dose re-calculation to make particle therapy more conformal. The current results are very promising [195–197] and the rate of progress in this field extremely fast. The need for large training databases to develop new AI methods calls for large, international collaborations between particle therapy facilities, to overcome the limitation that each center treats only a comparatively small number of patients for each cancer site.
Fig 10. Potential contribution of AI (here represented as a neural network in the center of the figure) to online adaptive particle therapy.
The green dashed line corresponds to the possibility to use AI for online beam delivery adaptation to intra-fractional target motion.
4. Ultra-high intensities
Soon after the first attempts to use ionizing radiation to sterilize tumors, it became clear that daily dose fractionation was mandatory to reduce the treatment toxicity. The standard fractionation regime requires 2–3 Gy/fraction up to total doses of 60–80 Gy to the target at intensities of 0.5–1 Gy/min. Recently, a paradigm-shift experiment by a French-Swiss collaboration [198] showed that ultra-high dose rates (>40 Gy/s) could spare normal tissue without compromising tumor control. The FLASH effect holds the promise to widen the therapeutic window, and it is therefore currently one the most prominent topics in radiotherapy. The original experiment was performed with electrons accelerated at a high beam current 4.5 MeV linac. Later the FLASH effect has been observed in different cell and animal models using synchrotron radiation photons [199], hard X-rays [200], protons [201], helium- [202] and carbon- [203] ions. The FLASH effect has a lot of unanswered questions including molecular mechanism, dependence on total dose, instantaneous and average doserate, irradiation time, fractionation, quantitative assessment of the tissue-dependent dose-modifying factor, let alone the technological challenges [42]. Notwithstanding these uncertainties, clinical trials are ongoing with electrons for cutaneous tumors [204] and protons for bone metastasis [205], and have already shown safety and feasibility of the FLASH approach in the clinical setting.
4.1. FLASH impact on medical physics
Whether the FLASH effect will maintain the promise of reducing toxicity and will find wide applications in clinical settings remains to be demonstrated. However, the technological efforts to build machines able to deliver safely high dose at ultra-high dose rate can lead to other benefits from the medical physics side. First, the treatment time is reduced to less than a second, thus potentially improving the clinical workflow, even if the beam-on period is making up only a minor fraction of the time needed to treat the patient (that includes access to the cave, positioning, etc.). Second, FLASH is characterized by high total doses to be delivered in one or 2–4 fractions, and can potentially expand the number of tumor cases for which extreme hypofractionation is feasible today [206]. Again, this results in a benefit for the patient and for the clinics. Finally, FLASH requires a complete re-assessment of the motion management problem [116]. The concept of tumor tracking becomes impossible at the required high delivery speeds, and it also becomes irrelevant. The whole high dose is delivered in a fraction of a second, so the issue is to shoot exactly at the right time when the tumor and the normal organs are in the right position. Fractionation itself is a countermeasure for the interplay effect, because it washes-out the inhomogeneities over the different fractions, but it is lost in oligofractionated or single-fraction FLASH. Adaptive online therapy and image guidance becomes even more necessary, considering that only intra-fractional but also inter-fractional movements and positioning errors can lead to fatal overdosage in organs at risk. For online imaging, the use of high doses provides an opportunity for pulse-by-pulse imaging using Cherenkov or ionoacoustic imaging [207].
4.2. Particle FLASH
Considering the small penetration of electrons from linacs and the poor efficiency of bremsstrahlung for high-intensity X-ray generation, it can be stated that particle therapy is the most mature technology for the clinical translation of FLASH. Clinical cyclotrons can reach FLASH intensities at a fixed energy easily, considering that they have a potential to provide beam currents over an order of magnitude higher than those used in clinical mode (1–10 nA). This is especially true for isochronous cyclotrons with their higher duty cycle, typically achieving beam currents ~1 μA. Synchrotrons used for heavy ion therapy have a low repetition rate (∼1 Hz) and therefore the whole dose has to be delivered in a single synchrotron spill (~100 ms) [208]. A potential alternative is to use fast extraction, using bunch compression and a kicker magnet to deliver all the particles in the ring in a few μs, but with this method beam scanning becomes impossible.
For tumor irradiation, the 3D volumetric scanning used in clinics is too slow (each energy change takes ~1 s) to reach FLASH conditions in the whole tumor, and the speed should increase at least two orders of magnitude [209]. The most mature approach for clinical applications of particle is hybrid active–passive systems using patient-specific, 3D-range modulators [210,211]. The pencil beam is scanned in 2D over a ridge filter specifically designed to produce passively the desired SOBP. Irradiation time is therefore only limited by the raster scanning, which is extremely fast. Experimental tests for a lung cancer case demonstrated the delivery of 0.5 Gy to a ~70 cm3 target volume in just ~6 s at a contemporary heavy ion facility [212]. 3D range modulators are already employed in experimental facilities using protons [213] and heavy ions [214] (Fig. 11) and are currently under test for clinical facilities implementing proton-FLASH [215].
Fig 11. Beamline for FLASH experiments using carbon ions in the GSI cave A (Darmstadt, Germany).
Blu arrow: He/CO2 filled ionization chamber (beam monitor); red arrow: binary range shifter; white arrow: 3D-printed SOBP-modulator; green arrow: target station for animal irradiation.
Can particle FLASH be as conformal as conventional treatments? Range modulators can replace 3D volumetric scanning with virtually no loss of conformality, but the question is whether a gantry can be used to achieve high conformality with multiple fields. If irradiation is done from multiple angles, the normal tissue surrounding the tissue will experience different dose-rates, ranging from zero (while the gantry is rotated) to the maximum corresponding to the Bragg peak in the distal edge, whereas other sub-volumes will experience intermediate dose-rates due to the overlap of different beamlets [216]. Whether these conditions maintain the FLASH effect is still unclear. One alternative to gantry rotation is to use the toroidal concept described previously in Section 2.3 (Fig. 7), which could be ideal for conformal FLASH. In FLASH treatment planning [217] it will be necessary to include a dose-rate optimization, and perhaps a combination of Bragg peak and shoot-through beams to maintain high dose-rate and conformality [218].
Of course laser-driven particle accelerators, discussed in Section 2.2, would be a prime candidate for FLASH applications. While pre-clinical in vitro cell studies are ongoing [219–221], the hurdles described in Section 2.2 for clinical translation remain, but it is possible that the investment in FLASH radiotherapy will accelerate the development of appropriate solutions for ultra-high intensity lasers in clinics.
5. Conclusions
Particle therapy is growing so quickly (Fig. 1) that we should ask the question of whether it will replace conventional X-ray therapy completely. While the physical and biological (especially for heavy ions) rationale is known since long time, clinical evidence of superiority is now accumulating. Nevertheless, charged particles are not going to replace photons, at least in the near future. The cost of a particle therapy center is in fact still substantially higher than an X-ray linac, and this forces radiation oncologists to select carefully those patient that can benefit the most from particles. Moreover, particle therapy is more sensitive than conventional therapy to positioning uncertainties and organ movements. Finally, with the current outburst of FLASH radiotherapy, particle accelerators should be able reach higher intensity to remain superior to photons and high-energy electron linacs. The future of proton and heavy ion therapy therefore depends on technological improvements to make particle therapy cheaper, more conformal and faster. Promising solutions include superconductive magnets for more compact accelerators with reduced footprint; irradiation in upright position to avoid large and expensive gantries; AI-assisted online imaging and adaptive radiotherapy to reduce treatment margins; and high-intensity accelerators with 3D-printed range modulators for FLASH radiotherapy. Even if research is ongoing in several centers on these topics, for clinical translation larger collaborative efforts are needed. For example, research in proton radiography is ongoing independently in dozens of proton therapy centers, but as yet they have not produced a single device used in clinical settings. A platform for collaboration in particle therapy is traditionally PTCOG [9], but dedicated funding should come from large-scale initiatives such as the Cancer Moonshot in US [2] or Cancer Mission in EU [3].
Supplementary Material
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.ppnp.2023.104046.
Acknowledgments
The research in medical physics at GSI is partly funded by EU grant agreements No. 101008548 (HITRIplus), 863969 (RAPTOR) and 883425 (ERC AdG BARB). This paper is dedicated to the memory of Prof. Dr. Gerhard Kraft (1941–2023), founder and former director of the Biophysics Department at GSI and pioneer of heavy ion therapy.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Marco Durante reports financial support was provided by European Union.
References
- [1].Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2019;5:1749. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singer DS. Nature Med. 2022;28:1345–1347. doi: 10.1038/s41591-022-01881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawler M, Davies L, Oberst S, Oliver K, Eggermont A, Schmutz A, La Vecchia C, Allemani C, Lievens Y, Naredi P, Cufer T, et al. Lancet Oncol. 2023;24:e11–e56. doi: 10.1016/S1470-2045(22)00540-X. [DOI] [PubMed] [Google Scholar]
- [4].Thariat J, Hannoun-Levi J-M, Sun Myint A, Vuong T, Gérard J-P. Nat Rev Clin Oncol. 2012;10:52–60. doi: 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- [5].Chandra RA, Keane FK, Voncken FEM, Thomas CR. Lancet. 2021;398:171–184. doi: 10.1016/S0140-6736(21)00233-6. [DOI] [PubMed] [Google Scholar]
- [6].Waddle MR, Sio TT, Van Houten HK, Foote RL, Keole SR, Schild SE, Laack N, Daniels TB, Crown W, Shah ND, Miller RC. Int J Radiat Oncol. 2017;99:1078–1082. doi: 10.1016/j.ijrobp.2017.07.042. [DOI] [PubMed] [Google Scholar]
- [7].Nogueira LM, Jemal A, Yabroff KR, Efstathiou JA. JAMA Netw Open. 2022;5:e229025. doi: 10.1001/jamanetworkopen.2022.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].PTCOG. 2022. [accessed December 12, 2022]. http://ptcog.site .
- [9].PTCOG. 2022. [accessed December 12, 2022]. https://www.ptcog.site .
- [10].Li Y, Li X, Yang J, Wang S, Tang M, Xia J, Gao Y. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.819905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paganetti H, editor. Proton Therapy Physics. second. Taylor Francis; 2019. [Google Scholar]
- [12].Schardt D, Elsässer T, Schulz-Ertner D. Rev Modern Phys. 2010;82:383–425. doi: 10.1103/RevModPhys.82.383. [DOI] [Google Scholar]
- [13].Lomax AJ. Cancer J. 2009;15:285–291. doi: 10.1097/PPO.0b013e3181af5cc7. [DOI] [PubMed] [Google Scholar]
- [14].Smith AR. Med Phys. 2009;36:556–568. doi: 10.1118/1.3058485. [DOI] [PubMed] [Google Scholar]
- [15].Durante M, Paganetti H. Rep Progr Phys. 2016;79:096702. doi: 10.1088/0034-4885/79/9/096702. [DOI] [PubMed] [Google Scholar]
- [16].Newhauser WD, Zhang R. Phys Med Biol. 2015;60:R155. doi: 10.1088/0031-9155/60/8/R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kraft G. Prog Part Nucl Phys. 2000;45:473–544. doi: 10.1016/S0146-6410(00)00112-5. [DOI] [Google Scholar]
- [18].Bichsel H. Adv Quantum Chem. 2013;65:1–38. doi: 10.1016/B978-0-12-396455-7.00001-7. [DOI] [Google Scholar]
- [19].Lievens Y, Pijls-Johannesma M. Semin Radiat Oncol. 2013;23:134–141. doi: 10.1016/j.semradonc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [20].Verma V, Mishra MV, Mehta MP. Cancer. 2016;122:1483–1501. doi: 10.1002/cncr.29882. [DOI] [PubMed] [Google Scholar]
- [21].Durante M, Orecchia R, Loeffler JS. Nat Rev Clin Oncol. 2017;14:483–495. doi: 10.1038/nrclinonc.2017.30. [DOI] [PubMed] [Google Scholar]
- [22].Kerstiens J, Johnstone GP, Johnstone PAS. J Am Coll Radiol. 2018;15:1704–1708. doi: 10.1016/j.jacr.2018.07.020. [DOI] [PubMed] [Google Scholar]
- [23].Contreras J, Zhao T, Perkins S, Sun B, Goddu S, Mutic S, Bottani B, Endicott S, Michalski J, Robinson C, Tsien C, et al. Pract Radiat Oncol. 2017;7:e71–e76. doi: 10.1016/j.prro.2016.07.003. [DOI] [PubMed] [Google Scholar]
- [24].Li X, Kitpanit S, Lee A, Mah D, Sine K, Sherman EJ, Dunn LA, Michel LS, Fetten J, Zakeri K, Yu Y, et al. JAMA Netw Open. 2021;4:e2113205. doi: 10.1001/jamanetworkopen.2021.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Youssef I, Yoon J, Mohamed N, Zakeri K, Press RH, Chen L, Gelblum DY, McBride SM, Tsai CJ, Riaz N, Yu Y, et al. JAMA Netw Open. 2022;5:e2241538. doi: 10.1001/jamanetworkopen.2022.41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baumann BC, Mitra N, Harton JG, Xiao Y, Wojcieszynski AP, Gabriel PE, Zhong H, Geng H, Doucette A, Wei J, O’Dwyer PJ, et al. JAMA Oncol. 2020;6:237. doi: 10.1001/jamaoncol.2019.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baumann BC, Hallahan DE, Michalski JM, Perez CA, Metz JM. Br Cancer J. 2020;123:869–870. doi: 10.1038/s41416-0200919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin SH, Hobbs BP, Verma V, Tidwell RS, Smith GL, Lei X, Corsini EM, Mok I, Wei X, Yao L, Wang X, et al. J Clin Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang JT, Wijetunga NA, Pentsova E, Wolden S, Young RJ, Correa D, Zhang Z, Zheng J, Steckler A, Bucwinska W, Bernstein A, et al. J Clin Oncol. 2022;40:3858–3867. doi: 10.1200/JCO.22.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pan Z, Yang G, He H, Yuan T, Wang Y, Li Y, Shi W, Gao P, Dong L, Zhao G. Sci Rep. 2018;8:10445. doi: 10.1038/s41598-018-28662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, Jones RM, MacDonald SM, Pulsifer MB, Lavally B, Abrams AN, et al. Lancet Oncol. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- [32].Barden MM, Omuro AM. Cancer. 2023 doi: 10.1002/cncr.34711. [DOI] [Google Scholar]
- [33].Wijetunga NA, Goglia AG, Weinhold N, Berger MF, Cislo M, Higginson DS, Chabot K, Osman AM, Schaff L, Pentsova E, Miller AM, et al. Clin Cancer Res. 2022:OF1–OF9. doi: 10.1158/1078-0432.CCR-22-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Faddegon B, Ramos-Méndez J, Schuemann J, McNamara A, Shin J, Perl J, Paganetti H. Phys Medica. 2020;72:114–121. doi: 10.1016/j.ejmp.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Krämer M, Jäkel O, Haberer T, Kraft G, Schardt D, Weber U. Phys Med Biol. 2000;45:3299–3317. doi: 10.1088/0031-9155/45/11/313. [DOI] [PubMed] [Google Scholar]
- [36].Wieser H-P, Cisternas E, Wahl N, Ulrich S, Stadler A, Mescher H, Müller L-R, Klinge T, Gabrys H, Burigo L, Mairani A, et al. Med Phys. 2017;44:2556–2568. doi: 10.1002/mp.12251. [DOI] [PubMed] [Google Scholar]
- [37].Bortfeld TR, Loeffler JS. Nature. 2017;549:451–453. doi: 10.1038/549451a. [DOI] [PubMed] [Google Scholar]
- [38].Shah A, Ricci KI, Efstathiou JA. Lancet Oncol. 2016;17:559–561. doi: 10.1016/S1470-2045(16)00171-6. [DOI] [PubMed] [Google Scholar]
- [39].Mitin T, Zietman AL. J Clin Oncol. 2014;32:2855–2863. doi: 10.1200/JCO.2014.55.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Paganetti H. Phys Med Biol. 2012;57:R99–R117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Durante M, Flanz J. Semin Oncol. 2019;46:219–225. doi: 10.1053/j.seminoncol.2019.07.007. [DOI] [PubMed] [Google Scholar]
- [42].Vozenin M-C, Bourhis J, Durante M. Nat Rev Clin Oncol. 2022;19:791–803. doi: 10.1038/s41571-022-00697-z. [DOI] [PubMed] [Google Scholar]
- [43].Durante M, Debus J, Loeffler JS. Nat Rev Phys. 2021;3:777–790. doi: 10.1038/s42254-021-00368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Coutrakon GB. Technol Cancer Res Treat. 2007;6:49–54. doi: 10.1177/15330346070060S408. [DOI] [PubMed] [Google Scholar]
- [45].Collings EW, Lu L, Gupta N, Sumption MD. Front Oncol. 2022;11 doi: 10.3389/fonc.2021.737837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alonso JR, Antaya TA. Rev Accel Sci Technol, WORLD SCIENTIFIC. 2013:227–263. doi: 10.1142/9789814449953_0009. [DOI] [Google Scholar]
- [47].Owen H, Holder D, Alonso J, Mackay R. Internat J Modern Phys A. 2013;29:1–54. doi: 10.1142/S0217751X14410024. [DOI] [Google Scholar]
- [48].Iwata Y, Kadowaki T, Uchiyama H, Fujimoto T, Takada E, Shirai T, Furukawa T, Mizushima K, Takeshita E, Katagiri K, Sato S, et al. Nucl Instrum Methods Phys Res A. 2010;624:33–38. doi: 10.1016/j.nima.2010.09.016. [DOI] [Google Scholar]
- [49].Mizushima K, Furukawa T, Iwata Y, Hara Y, Saotome N, Saraya Y, Tansho R, Sato S, Fujimoto T, Shirai T, Noda K. Nucl Instrum Methods Phys Res B. 2017;406:347–351. doi: 10.1016/j.nimb.2017.03.051. [DOI] [Google Scholar]
- [50].Younkin JE, Bues M, Sio TT, Liu W, Ding X, Keole SR, Stoker JB, Shen J. Adv Radiat Oncol. 2018;3:412–420. doi: 10.1016/j.adro.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jongen Y, Abs M, Blondin A, Kleeven W, Zaremba S, Vandeplassche D, Aleksandrov V, Gursky S, Karamyshev O, Karamysheva G, Kazarinov N, et al. Nucl Instrum Methods Phys Res A. 2010;624:47–53. doi: 10.1016/j.nima.2010.09.028. [DOI] [Google Scholar]
- [52].Chevalier F, Lesueur P, Gaubert G. Nucl Phys News. 2022;32:27–31. doi: 10.1080/10619127.2022.2063002. [DOI] [Google Scholar]
- [53].Rossi L, Ballarino A, Barna D, Benedetto E, Calzolaio C, Ceruti G, De Matteis E, Echeandia A, Ekelof T, Farinon S, Felcini E, et al. IEEE Trans Appl Supercond. 2022;32:1–7. doi: 10.1109/TASC.2022.3147433. [DOI] [Google Scholar]
- [54].Ageev A, Bogdanov I, Kozub S, Tkachenko L. Nucl Instrum Methods Phys Res A. 2021;1000:165223. doi: 10.1016/j.nima.2021.165223. [DOI] [Google Scholar]
- [55].Baird YTE, Li Q. IEEE Trans Appl Supercond. 2020;30:1–8. doi: 10.1109/TASC.2019.2954681. [DOI] [Google Scholar]
- [56].Larbalestier DC, Jiang J, Trociewitz UP, Kametani F, Scheuerlein C, Dalban-Canassy M, Matras M, Chen P, Craig NC, Lee PJ, Hellstrom EE. Nature Mater. 2014;13:375–381. doi: 10.1038/nmat3887. [DOI] [PubMed] [Google Scholar]
- [57].Wang X, Gourlay SA, Prestemon SO. Instruments. 2019;3:62. doi: 10.3390/instruments3040062. [DOI] [Google Scholar]
- [58].Benedetti S, Grudiev A, Latina A. Phys Rev Accel Beams. 2017;20:040101. doi: 10.1103/PhysRevAccelBeams.20.040101. [DOI] [Google Scholar]
- [59].Amaldi U, Braccini S, Puggioni P. Rev Accel Sci Technol. 2009;2:111–131. doi: 10.1142/S179362680900020X. [DOI] [Google Scholar]
- [60].Amaldi U, Bonomi R, Braccini S, Crescenti M, Degiovanni A, Garlasch M, Garonna A, Magrin G, Mellace C, Pearce P, Pittá G, et al. Nucl Instrum Methods Phys Res A. 2010;620:563–577. doi: 10.1016/j.nima.2010.03.130. [DOI] [Google Scholar]
- [61].Degiovanni A. 9th Int. Part. Accel. Conf; Vancouver, Canada. 2018. pp. 425–428. [DOI] [Google Scholar]
- [62].Mustapha B, Aydogan B, Nolen J, Nassiri A, Noonan J, Pankuch M, Welsh J, Schulte R, Robb J. AIP Conf Proc. 2019:050009. doi: 10.1063/1.5127701. [DOI] [Google Scholar]
- [63].Trbojevic D, Alessi J, Blaskiewicz M, Cullen C, Hahn H, Lowenstein D, Marneris I, Meng W, Mi J, Pai C, Raparia D, et al. Proc IPAC. San Sebastian; Spain: 2011. [Google Scholar]
- [64].Antoine S, Autin B, Beeckman W, Collot J, Conjat M, Forest F, Fourrier J, Froidefond E, Lancelot JL, Mandrillon J, Mandrillon P, et al. Nucl Instrum Methods Phys Res A. 2009;602:293–305. doi: 10.1016/j.nima.2009.01.025. [DOI] [Google Scholar]
- [65].Caporaso GJ, Chen Y-J, Sampayan SE. Rev Accel Sci Technol. 2009;02:253–263. doi: 10.1142/S1793626809000235. [DOI] [Google Scholar]
- [66].Badziak J. J Phys Conf Ser. 2018;959:012001. doi: 10.1088/1742-6596/959/1/012001. [DOI] [Google Scholar]
- [67].Wang KD, Zhu K, Easton MJ, Li YJ, Lin C, Yan XQ. Phys Rev Accel Beams. 2020;23:111302. doi: 10.1103/PhysRevAccelBeams.23.111302. [DOI] [Google Scholar]
- [68].Karsch L, Beyreuther E, Enghardt W, Gotz M, Masood U, Schramm U, Zeil K, Pawelke J. Acta Oncol (Madr) 2017;56:1359–1366. doi: 10.1080/0284186X.2017.1355111. [DOI] [PubMed] [Google Scholar]
- [69].Linz U, Alonso J. Phys Rev Accel Beams. 2016;19:124802. doi: 10.1103/PhysRevAccelBeams.19.124802. [DOI] [Google Scholar]
- [70].Higginson A, Gray RJ, King M, Dance RJ, Williamson SDR, Butler NMH, Wilson R, Capdessus R, Armstrong C, Green JS, Hawkes SJ, et al. Nature Commun. 2018;9:724. doi: 10.1038/s41467-018-03063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kroll F, Brack F-E, Bernert C, Bock S, Bodenstein E, Brüchner K, Cowan TE, Gaus L, Gebhardt R, Helbig U, Karsch L, et al. Nat Phys. 2022;18:316–322. doi: 10.1038/s41567-022-01520-3. [DOI] [Google Scholar]
- [72].Noda K. J Phys Conf Ser. 2019;1154:012019. doi: 10.1088/1742-6596/1154/1/012019. [DOI] [Google Scholar]
- [73].Lomax AJ, Boehringer T, Coray A, Egger E, Goitein G, Grossmann M, Juelke P, Lin S, Pedroni E, Rohrer B, Roser W, et al. Med Phys. 2001;28:317–324. doi: 10.1118/1.1350587. [DOI] [PubMed] [Google Scholar]
- [74].Bert C, Durante M. Phys Med Biol. 2011;56:R113–R144. doi: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- [75].Bottura L, Felcini E, Ferrero V, Fiorina E, Monaco V, Pennazio F, de Rijk G, Cerello P. Front Phys. 2020;8 doi: 10.3389/fphy.2020.566679. [DOI] [Google Scholar]
- [76].Kamada T, Tsujii H, Mizoe J-E, Matsuoka Y, Tsuji H, Osaka Y, Minohara S, Miyahara N, Endo M, Kanai T. Radiother Oncol. 1999;50:235–237. doi: 10.1016/S0167-8140(99)00005-5. [DOI] [PubMed] [Google Scholar]
- [77].Heeg P, Schardt D, Störmer J. GSI Rep 2002-1, GSI, Darmstadt. 2002:166.
- [78].Schreuder N. Technological Developments Allowing for the Widespread Clinical Adoption of Proton Radiotherapy. University College London; 2020. https://discovery.ucl.ac.uk/id/eprint/10118672/1/Schreuder_NiekschreuderPHDThesis-GrandFinalJan52021.pdf . [Google Scholar]
- [79].Balakin VE, Belikhin MA, Pryanichnikov AA, Shemyakov AE, Strelnikova NS. KnE Energy. 2018;3:45. doi: 10.18502/ken.v3i2.1790. [DOI] [Google Scholar]
- [80].Sheng Y, Sun J, Wang W, Stuart B, Kong L, Gao J, You D, Wu X. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Buchner T, Yan S, Li S, Flanz J, Hueso-Gonzalez F, Kielty E, Bortfeld T, Rus D. Biomechatronics; 2020 8th IEEE RAS/EMBS Int. Conf. Biomed. Robot; 2020. pp. 981–988. [DOI] [Google Scholar]
- [82].Boisbouvier S, Boucaud A, Tanguy R, Grégoire V. Tech Innov Patient Support Radiat Oncol. 2022;24:124–130. doi: 10.1016/j.tipsro.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Noda K, Furukawa T, Fujimoto T, Hara Y, Inaniwa T, Iwata Y, Katagiri K, Kanematsu N, Mizushima K, Mori S, Saotome N, et al. Nucl Instrum Methods Phys Res B. 2017;406:374–378. doi: 10.1016/j.nimb.2017.04.021. [DOI] [Google Scholar]
- [84].Zhou Y, Li Y, Kubota Y, Sakai M, Ohno T. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.715025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wan W, Brouwer L, Caspi S, Prestemon S, Gerbershagen A, Schippers JM, Robin D. Phys Rev Spec Top-Accel Beams. 2015;18:103501. doi: 10.1103/PhysRevSTAB.18.103501. [DOI] [Google Scholar]
- [86].Kim J, Yoon M. Phys Rev Accel Beams. 2019;22:101601. doi: 10.1103/PhysRevAccelBeams.22.101601. [DOI] [Google Scholar]
- [87].Nesteruk KP, Bolsi A, Lomax AJ, Meer D, van de Water S, Schippers JM. Phys Med Biol. 2021;66:055018. doi: 10.1088/1361-6560/abe02b. [DOI] [PubMed] [Google Scholar]
- [88].Felcini E, Haziot A, Louzguiti A, Lehtinen T, Vernassa G, Dutoit B, Bottura L. IEEE Trans Appl Supercond. 2022;32:1–5. doi: 10.1109/TASC.2022.3160380. [DOI] [Google Scholar]
- [89].Volz L, Sheng Y, Durante M, Graeff C. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.930850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rahim S, Korte J, Hardcastle N, Hegarty S, Kron T, Everitt S. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Shah AP, Strauss JB, Kirk MC, Chen SS, Kroc TK, Zusag TW. Med Dosim. 2009;34:82–86. doi: 10.1016/j.meddos.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [92].Yang J, Chu D, Dong L, Court LE. Pract Radiat Oncol. 2014;4:e53–e58. doi: 10.1016/j.prro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- [93].Yamada Y, Yamada M, Chubachi S, Yokoyama Y, Matsuoka S, Tanabe A, Niijima Y, Murata M, Fukunaga K, Jinzaki M. Sci Rep. 2020;10:16203. doi: 10.1038/s41598-020-73240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].McCarroll RE, Beadle BM, Fullen D, Balter PA, Followill DS, Stingo FC, Yang J, Court LE. J Appl Clin Med Phys. 2017;18:223–229. doi: 10.1002/acm2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].ICRU. J ICRU. 2010;10 doi: 10.1093/jicru/10.1.Report83. NP.1–NP. [DOI] [Google Scholar]
- [96].Segedin B, Petric P. Radiol Oncol. 2016;50:254–262. doi: 10.1515/raon-2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].van Herk M, Remeijer P, Rasch C, Lebesque JV. Int J Radiat Oncol. 2000;47:1121–1135. doi: 10.1016/S0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- [98].Bernstein D, Taylor A, Nill S, Oelfke U. Phys Med Biol. 2021;66:055024. doi: 10.1088/1361-6560/abe029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lomax AJ. Br J Radiol. 2020;93:20190582. doi: 10.1259/bjr.20190582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Urie M, Goitein M, Holley WR, Chen GTY. Phys Med Biol. 1986;31:1–15. doi: 10.1088/0031-9155/31/1/001. [DOI] [PubMed] [Google Scholar]
- [101].Sawakuchi GO, Titt U, Mirkovic D, Mohan R. Phys Med Biol. 2008;53:4605–4619. doi: 10.1088/0031-9155/53/17/010. [DOI] [PubMed] [Google Scholar]
- [102].Besemer A, Paganetti H, Bednarz B. Phys Med Biol. 2013;58:887–902. doi: 10.1088/0031-9155/58/4/887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kamakura S, Sakamoto N, Ogawa H, Tsuchida H, Inokuti M. J Appl Phys. 2006;100:064905. doi: 10.1063/1.2345478. [DOI] [Google Scholar]
- [104].Deasy J. Med Phys. 1994;21:709–710. doi: 10.1118/1.597176. [DOI] [Google Scholar]
- [105].Paul H. Adv Quantum Chem. 2013;65:39–61. doi: 10.1016/B978-0-12-396455-7.00002-9. [DOI] [Google Scholar]
- [106].Goitein M. Med Phys. 1985;12:608–612. doi: 10.1118/1.595762. [DOI] [PubMed] [Google Scholar]
- [107].Tattenberg S, Madden TM, Gorissen BL, Bortfeld T, Parodi K, Verburg J. Med Phys. 2021:mp.15097. doi: 10.1002/mp.15097. [DOI] [PubMed] [Google Scholar]
- [108].Sokol O, Cella L, Boscolo D, Horst F, Oliviero C, Pacelli R, Palma G, De Simoni M, Conson M, Caroprese M, Weber U, et al. Sci Rep. 2022;12:21792. doi: 10.1038/s41598-022-26290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Peters N, Wohlfahrt P, Hofmann C, Möhler C, Menkel S, Tschiche M, Krause M, Troost EGC, Enghardt W, Richter C. Radiother Oncol. 2022;166:71–78. doi: 10.1016/j.radonc.2021.11.002. [DOI] [PubMed] [Google Scholar]
- [110].Bär E, Lalonde A, Royle G, Lu H-M, Bouchard H. Med Phys. 2017;44:2332–2344. doi: 10.1002/mp.12215. [DOI] [PubMed] [Google Scholar]
- [111].Niepel KB, Stanislawski M, Wuerl M, Doerringer F, Pinto M, Dietrich O, Ertl-Wagner B, Lalonde A, Bouchard H, Pappas E, Yohannes I, et al. Phys Med Biol. 2021;66:075009. doi: 10.1088/1361-6560/abbd14. [DOI] [PubMed] [Google Scholar]
- [112].Berthold J, Khamfongkhruea C, Petzoldt J, Thiele J, Hölscher T, Wohlfahrt P, Peters N, Jost A, Hofmann C, Janssens G, Smeets J, et al. Int J Radiat Oncol. 2021;111:1033–1043. doi: 10.1016/j.ijrobp.2021.06.036. [DOI] [PubMed] [Google Scholar]
- [113].Volz L, Collins-Fekete C-A, Bär E, Brons S, Graeff C, Johnson RP, Runz A, Sarosiek C, Schulte RW, Seco J. Phys Med Biol. 2021;66:235010. doi: 10.1088/1361-6560/ac33ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Unkelbach J, Paganetti H. Semin Radiat Oncol. 2018;28:88–96. doi: 10.1016/j.semradonc.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Unkelbach J, Alber M, Bangert M, Bokrantz R, Chan TCY, Deasy JO, Fredriksson A, Gorissen BL, van Herk M, Liu W, Mahmoudzadeh H, Nohadani O, et al. Phys Med Biol. 2018;63:22TR02. doi: 10.1088/1361-6560/aae659. [DOI] [PubMed] [Google Scholar]
- [116].Pakela JM, Knopf A, Dong L, Rucinski A, Zou W. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.806153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Richter D, Schwarzkopf A, Trautmann J, Krämer M, Durante M, Jäkel O, Bert C. Med Phys. 2013;40:051722. doi: 10.1118/1.4800802. [DOI] [PubMed] [Google Scholar]
- [118].Chang JY, Zhang X, Knopf A, Li H, Mori S, Dong L, Lu H-M, Liu W, Badiyan SN, Both S, Meijers A, et al. Int J Radiat Oncol. 2017;99:41–50. doi: 10.1016/j.ijrobp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- [119].Dueck J, Knopf A-C, Lomax A, Albertini F, Persson GF, Josipovic M, Aznar M, Weber DC, Munckaf Rosenschöld P. Int J Radiat Oncol. 2016;95:534–541. doi: 10.1016/j.ijrobp.2015.11.015. [DOI] [PubMed] [Google Scholar]
- [120].Lin L, Souris K, Kang M, Glick A, Lin H, Huang S, Stützer K, Janssens G, Sterpin E, Lee JA, Solberg TD, et al. Med Phys. 2017;44:703–712. doi: 10.1002/mp.12040. [DOI] [PubMed] [Google Scholar]
- [121].Mori S, Lu H-M, Wolfgang JA, Choi NC, Chen GTY. J Radiat Res. 2009;50:513–519. doi: 10.1269/jrr.09032. [DOI] [PubMed] [Google Scholar]
- [122].Czerska K, Emert F, Kopec R, Langen K, McClelland JR, Meijers A, Miyamoto N, Riboldi M, Shimizu S, Terunuma T, Zou W, et al. Phys Medica. 2021;82:54–63. doi: 10.1016/j.ejmp.2020.12.013. [DOI] [PubMed] [Google Scholar]
- [123].Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, Komaki R, Cox JD, Balter PA, Liu H, Zhu XR, et al. Int J Radiat Oncol. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- [124].Grassberger C, Dowdell S, Sharp G, Paganetti H. Med Phys. 2015;42:2462–2469. doi: 10.1118/1.4916662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Steidl P, Haberer T, Durante M, Bert C. Phys Med Biol. 2013;58:N295–N302. doi: 10.1088/0031-9155/58/21/N295. [DOI] [PubMed] [Google Scholar]
- [126].Prunaretty J, Boisselier P, Aillères N, Riou O, Simeon S, Bedos L, Azria D, Fenoglietto P. Rep Pract Oncol Radiother. 2019;24:97–104. doi: 10.1016/j.rpor.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Luchtenborg R, Saito N, Durante M, Bert C. Med Phys. 2011;38:5448–5458. doi: 10.1118/1.3633891. [DOI] [PubMed] [Google Scholar]
- [128].Krieger M, Giger A, Jud C, Duetschler A, Salomir R, Bieri O, Bauman G, Nguyen D, Cattin PC, Weber DC, Lomax AJ, et al. Phys Med Biol. 2021;66:035011. doi: 10.1088/1361-6560/abcde6. [DOI] [PubMed] [Google Scholar]
- [129].Steinsberger T, Donetti M, Lis M, Volz L, Wolf M, Durante M, Graeff C. Int J Radiat Oncol. 2022 doi: 10.1016/j.ijrobp.2022.11.034. [DOI] [PubMed] [Google Scholar]
- [130].Graeff C. Phys Medica. 2014;30:570–577. doi: 10.1016/j.ejmp.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [131].Ge S, Wang X, Liao Z, Zhang L, Sahoo N, Yang J, Guan F, Mohan R. Cancers (Basel) 2019;11:35. doi: 10.3390/cancers11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu W, Schild SE, Chang JY, Liao Z, Chang Y-H, Wen Z, Shen J, Stoker JB, Ding X, Hu Y, Sahoo N, et al. Int J Radiat Oncol. 2016;95:523–533. doi: 10.1016/j.ijrobp.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Graeff C, Lüchtenborg R, Eley JG, Durante M, Bert C. Radiother Oncol. 2013;109:419–424. doi: 10.1016/j.radonc.2013.09.018. [DOI] [PubMed] [Google Scholar]
- [134].Lis M, Donetti M, Newhauser W, Durante M, Dey J, Weber U, Wolf M, Steinsberger T, Graeff C. Phys Medica. 2020;76:307–316. doi: 10.1016/j.ejmp.2020.07.029. [DOI] [PubMed] [Google Scholar]
- [135].Knopf A-C, Czerska K, Fracchiolla F, Graeff C, Molinelli S, Rinaldi I, Rucincki A, Sterpin E, Stützer K, Trnkova P, Zhang Y, Chang JY, et al. Radiother Oncol. 2022;169:77–85. doi: 10.1016/j.radonc.2022.02.018. [DOI] [PubMed] [Google Scholar]
- [136].Molinelli S, Magro G, Mairani A, Matsufuji N, Kanematsu N, Inaniwa T, Mirandola A, Russo S, Mastella E, Hasegawa A, Tsuji H, et al. Radiother Oncol. 2016;120:307–312. doi: 10.1016/j.radonc.2016.05.031. [DOI] [PubMed] [Google Scholar]
- [137].Riboldi M, Orecchia PR, Baroni PG. Lancet Oncol. 2012;13:e383–e391. doi: 10.1016/S1470-2045(12)70243-7. [DOI] [PubMed] [Google Scholar]
- [138].Grégoire V, Guckenberger M, Haustermans K, Lagendijk JJW, Ménard C, Pötter R, Slotman BJ, Tanderup K, Thorwarth D, Herk M, Zips D. Mol Oncol. 2020;14:1470–1491. doi: 10.1002/1878-0261.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fattori G, Hrbacek J, Regele H, Bula C, Mayor A, Danuser S, Oxley DC, Rechsteiner U, Grossmann M, Via R, Böhlen TT, et al. Z Med Phys. 2022;32:52–62. doi: 10.1016/j.zemedi.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mijnheer B, Beddar S, Izewska J, Reft C. Med Phys. 2013;40:070903. doi: 10.1118/1.4811216. [DOI] [PubMed] [Google Scholar]
- [141].Olaciregui-Ruiz I, Beddar S, Greer P, Jornet N, McCurdy B, Paiva-Fonseca G, Mijnheer B, Verhaegen F. Phys Imaging Radiat Oncol. 2020;15:108–116. doi: 10.1016/j.phro.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, Glitzner MA, Kotte NTJ, van Asselen B, de Boer JCJ, Bluemink JJ, Hackett SL, Moerland MA, Woodings SJ, et al. Phys Med Biol. 2017;62:L41-L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- [143].Bayouth JE, Low DA, Zaidi H. Med Phys. 2019;46:3753–3756. doi: 10.1002/mp.13657. [DOI] [PubMed] [Google Scholar]
- [144].Pham TT, Whelan B, Oborn BM, Delaney GP, Vinod S, Brighi C, Barton M, Keall P. Radiother Oncol. 2022;170:37–47. doi: 10.1016/j.radonc.2022.02.031. [DOI] [PubMed] [Google Scholar]
- [145].Moteabbed M, Smeets J, Hong TS, Janssens G, Labarbe R, Wolfgang JA, Bortfeld TR. Phys Med Biol. 2021;66:195004. doi: 10.1088/1361-6560/ac1ef2. [DOI] [PubMed] [Google Scholar]
- [146].Kraan AC. Front Oncol. 2015;5:150. doi: 10.3389/fonc.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Pönisch F, Parodi K, Hasch BG, Enghardt W. [accessed June 13, 2017];Phys Med Biol. 2004 49:5217–5232. doi: 10.1088/0031-9155/49/23/002. [DOI] [PubMed] [Google Scholar]
- [148].Zhu X, El Fakhri G. Theranostics. 2013;3:731–740. doi: 10.7150/thno.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Parodi K. Med Phys. 2015;42:7153–7168. doi: 10.1118/1.4935869. [DOI] [PubMed] [Google Scholar]
- [150].Krimmer J, Dauvergne D, Létang JM, Testa É. Nucl Instrum Methods Phys Res A. 2018;878:58–73. doi: 10.1016/j.nima.2017.07.063. [DOI] [Google Scholar]
- [151].Piersanti L, Bellini F, Bini F, Collamati F, De Lucia E, Durante M, Faccini R, Ferroni F, Fiore S, Iarocci E, La Tessa C, et al. Phys Med Biol. 2014;59:1857–1872. doi: 10.1088/0031-9155/59/7/1857. [DOI] [PubMed] [Google Scholar]
- [152].Hueso-González F, Enghardt W, Fiedler F, Golnik C, Janssens G, Petzoldt J, Prieels D, Priegnitz M, Römer KE, Smeets J, Vander Stappen F, et al. Phys Med Biol. 2015;60:6247–6272. doi: 10.1088/0031-9155/60/16/6247. [DOI] [PubMed] [Google Scholar]
- [153].Moglioni M, Kraan AC, Baroni G, Battistoni G, Belcari N, Berti A, Carra P, Cerello P, Ciocca M, De Gregorio A, De Simoni M, et al. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.929949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Toppi M, Baroni G, Battistoni G, Bisogni MG, Cerello P, Ciocca M, De Maria P, De Simoni M, Donetti M, Dong Y, Embriaco A, et al. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.601784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Boscolo D, Kostyleva D, Safari MJ, Anagnostatou V, Äystö J, Bagchi S, Binder T, Dedes G, Dendooven P, Dickel T, Drozd V, et al. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.737050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Parodi K, Yamaya T, Moskal P. Z Med Phys. 2022 doi: 10.1016/j.zemedi.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Buitenhuis HJT, Diblen F, Brzezinski KW, Brandenburg S, Dendooven P. Phys Med Biol. 2017;62:4654–4672. doi: 10.1088/1361-6560/aa6b8c. [DOI] [PubMed] [Google Scholar]
- [158].Tashima H, Yoshida E, Inadama N, Nishikido F, Nakajima Y, Wakizaka H, Shinaji T, Nitta M, Kinouchi S, Suga M, Haneishi H, et al. Phys Med Biol. 2016;61:1795–1809. doi: 10.1088/0031-9155/61/4/1795. [DOI] [PubMed] [Google Scholar]
- [159].Borys D, Baran J, Brzeziński K, Gajewski J, Chug N, Coussat A, Czerwiński E, Dadgar M, Dulski K, Eliyan KV, Gajos A, et al. Phys Med Biol. 2022;67:224002. doi: 10.1088/1361-6560/ac944c. [DOI] [PubMed] [Google Scholar]
- [160].Llacer J, Chatterjee A, Alpen EL, Saunders W, Andreae S, Jackson HC. IEEE Trans Med Imaging. 1984;3:80–90. doi: 10.1109/TMI.1984.4307660. [DOI] [PubMed] [Google Scholar]
- [161].Durante M, Parodi K. Front Phys. 2020;8:00326. doi: 10.3389/fphy.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Durante M, Golubev A, Park W-Y, Trautmann C. Phys Rep. 2019;800:1–37. doi: 10.1016/j.physrep.2019.01.004. [DOI] [Google Scholar]
- [163].Boscolo D, Kostyleva D, Schuy C, Weber U, Haettner E, Purushothaman S, Dendooven P, Dickel T, Drozd V, Franczack B, Geissel H, et al. Nucl Instrum Methods Phys Res A. 2022;1043:167464. doi: 10.1016/j.nima.2022.167464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kostyleva D, Purushothaman S, Dendooven P, Haettner E, Geissel H, Ozoemelam I, Schuy C, Weber U, Boscolo D, Dickel T, Drozd V, et al. Phys Med Biol. 2023;68:015003. doi: 10.1088/1361-6560/aca5e8. [DOI] [PubMed] [Google Scholar]
- [165].Farace P, Righetto R, Meijers A. Phys Med Biol. 2016;61:4078–4087. doi: 10.1088/0031-9155/61/11/4078. [DOI] [PubMed] [Google Scholar]
- [166].Seller Oria C, Thummerer A, Free J, Langendijk JA, Both S, Knopf AC, Meijers A. Med Phys. 2021;48:4498–4505. doi: 10.1002/mp.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Poludniowski G, Allinson NM, Evans PM. Br J Radiol. 2015;88:20150134. doi: 10.1259/bjr.20150134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Johnson RP. Rep Progr Phys. 2018;81:016701. doi: 10.1088/1361-6633/aa8b1d. [DOI] [PubMed] [Google Scholar]
- [169].Mazzucconi D, Agosteo S, Ferrarini M, Fontana L, Lante V, Pullia M, Savazzi S. Med Phys. 2018;45:5234–5243. doi: 10.1002/mp.13219. [DOI] [PubMed] [Google Scholar]
- [170].Volz L, Kelleter L, Brons S, Burigo L, Graeff C, Niebuhr NI, Radogna R, Scheloske S, Schömers C, Jolly S, Seco J. Phys Med Biol. 2020;65:055002. doi: 10.1088/1361-6560/ab6e52. [DOI] [PubMed] [Google Scholar]
- [171].Huynh E, Hosny A, Guthier C, Bitterman DS, Petit SF, Haas-Kogan DA, Kann BH, Aerts JWL, Mak RH. Nat Rev Clin Oncol. 2020;17:771–781. doi: 10.1038/s41571-020-0417-8. [DOI] [PubMed] [Google Scholar]
- [172].Draguet C, Barragán-Montero AM, Vera MC, Thomas M, Populaire P, Defraene G, Haustermans K, Lee JA, Sterpin E. Radiother Oncol. 2022;176:101–107. doi: 10.1016/j.radonc.2022.08.031. [DOI] [PubMed] [Google Scholar]
- [173].Saxena S, Jena B, Gupta N, Das S, Sarmah D, Bhattacharya P, Nath T, Paul S, Fouda MM, Kalra M, Saba L, et al. Cancers (Basel) 2022;14:2860. doi: 10.3390/cancers14122860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Guiot J, Vaidyanathan A, Deprez L, Zerka F, Danthine D, Frix A, Lambin P, Bottari F, Tsoutzidis N, Miraglio B, Walsh S, et al. Med Res Rev. 2022;42:426–440. doi: 10.1002/med.21846. [DOI] [PubMed] [Google Scholar]
- [175].Primakov SP, Ibrahim A, van Timmeren JE, Wu G, Keek SA, Beuque M, Granzier RWY, Lavrova E, Scrivener M, Sanduleanu S, Kayan E, et al. Nature Commun. 2022;13:3423. doi: 10.1038/s41467-022-30841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH. Nat Methods. 2021;18:203–211. doi: 10.1038/s41592-020-01008-z. [DOI] [PubMed] [Google Scholar]
- [177].McIntosh C, Conroy L, Tjong MC, Craig T, Bayley A, Catton C, Gospodarowicz M, Helou J, Isfahanian N, Kong V, Lam T, et al. Nature Med. 2021;27:999–1005. doi: 10.1038/s41591-021-01359-w. [DOI] [PubMed] [Google Scholar]
- [178].van den Berg CAT, Meliadò EF. Semin Radiat Oncol. 2022;32:304–318. doi: 10.1016/j.semradonc.2022.06.001. [DOI] [PubMed] [Google Scholar]
- [179].Zhao W, Shen L, Islam MT, Qin W, Zhang Z, Liang X, Zhang G, Xu S, Li X. Quant Imaging Med Surg. 2021;11:4881–4894. doi: 10.21037/qims-21-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Howard FM, Kochanny S, Koshy M, Spiotto M, Pearson AT. JAMA Netw Open. 2020;3:e2025881. doi: 10.1001/jamanetworkopen.2020.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Hu Z, Li G, Zhang X, Ye K, Lu J, Peng H. Phys Med Biol. 2020;65:185003. doi: 10.1088/1361-6560/ab9707. [DOI] [PubMed] [Google Scholar]
- [182].Rahman AU, Nemallapudi MV, Chou C-Y, Lin C-H, Lee S-C. Phys Med Biol. 2022;67:185010. doi: 10.1088/1361-6560/ac8af5. [DOI] [PubMed] [Google Scholar]
- [183].Liu C-C, Huang H-M. Phys Medica. 2020;69:110–119. doi: 10.1016/j.ejmp.2019.12.006. [DOI] [PubMed] [Google Scholar]
- [184].Polf JC, Barajas CA, Peterson SW, Mackin DS, Beddar S, Ren L, Gobbert MK. Front Phys. 2022;10 doi: 10.3389/fphy.2022.838273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jiang Z, Sun L, Yao W, Wu QJ, Xiang L, Ren L. Phys Med Biol. 2022;67:215012. doi: 10.1088/1361-6560/ac9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yabe T, Yamamoto S, Oda M, Mori K, Toshito T, Akagi T. Med Phys. 2020;47:3882–3891. doi: 10.1002/mp.14372. [DOI] [PubMed] [Google Scholar]
- [187].Kurz C, Maspero M, Savenije MHF, Landry G, Kamp F, Pinto M, Li M, Parodi K, Belka C, van den Berg CAT. Phys Med Biol. 2019;64:225004. doi: 10.1088/1361-6560/ab4d8c. [DOI] [PubMed] [Google Scholar]
- [188].Thummerer A, Seller Oria C, Zaffino P, Meijers A, Guterres Marmitt G, Wijsman R, Seco J, Langendijk JA, Knopf A, Spadea MF, Both S. Med Phys. 2021;48:7673–7684. doi: 10.1002/mp.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Thummerer A, Seller Oria C, Zaffino P, Visser S, Meijers A, Guterres Marmitt G, Wijsman R, Seco J, Langendijk JA, Knopf AC, Spadea MF, et al. Med Phys. 2022;49:6824–6839. doi: 10.1002/mp.15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Pastor-Serrano O, Perkó Z. Phys Med Biol. 2022;67:105006. doi: 10.1088/1361-6560/ac692e. [DOI] [PubMed] [Google Scholar]
- [191].Zhang X, Hu Z, Zhang G, Zhuang Y, Wang Y, Peng H. Med Phys. 2021;48:2646–2660. doi: 10.1002/mp.14781. [DOI] [PubMed] [Google Scholar]
- [192].Brock KK. Semin Radiat Oncol. 2019;29:181–184. doi: 10.1016/j.semradonc.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Glide-Hurst CK, Lee P, Yock AD, Olsen JR, Cao M, Siddiqui F, Parker W, Doemer A, Rong Y, Kishan AU, Benedict SH, et al. Int J Radiat Oncol. 2021;109:1054–1075. doi: 10.1016/j.ijrobp.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Paganetti H, Botas P, Sharp GC, Winey B. Phys Med Biol. 2021;66:22TR01. doi: 10.1088/1361-6560/ac344f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Chang C-W, Zhou S, Gao Y, Lin L, Liu T, Bradley JD, Zhang T, Zhou J, Yang X. Phys Med Biol. 2022;67:215004. doi: 10.1088/1361-6560/ac9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Zhang G, Chen X, Dai J, Men K. Phys Medica. 2022;103:18–25. doi: 10.1016/j.ejmp.2022.09.018. [DOI] [PubMed] [Google Scholar]
- [197].Zhang G, Zhou L, Han Z, Zhao W, Peng H. Phys Med Biol. 2022;67:245010. doi: 10.1088/1361-6560/aca517. [DOI] [PubMed] [Google Scholar]
- [198].Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, Poupon M, Brito I, Hupé P, Bourhis J, Hall J, et al. Sci Transl Med. 2014;6:245ra93. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- [199].Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, Bräuer-Krisch E, et al. Radiother Oncol. 2018;129:582–588. doi: 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- [200].Gao F, Yang Y, Zhu H, Wang J, Xiao D, Zhou Z, Dai T, Zhang Y, Feng G, Li J, Lin B, et al. Radiother Oncol. 2022;166:44–50. doi: 10.1016/j.radonc.2021.11.004. [DOI] [PubMed] [Google Scholar]
- [201].Diffenderfer ES, Sørensen BS, Mazal A, Carlson DJ. Med Phys. 2022;49:2039–2054. doi: 10.1002/mp.15276. [DOI] [PubMed] [Google Scholar]
- [202].Tessonnier T, Mein S, Walsh DWM, Schuhmacher N, Liew H, Cee R, Galonska M, Scheloske S, Schömers C, Weber U, Brons S, et al. Int J Radiat Oncol. 2021;111:1011–1022. doi: 10.1016/j.ijrobp.2021.07.1703. [DOI] [PubMed] [Google Scholar]
- [203].Tinganelli W, Weber U, Puspitasari A, Simoniello P, Abdollahi A, Oppermann J, Schuy C, Horst F, Helm A, Fournier C, Durante M. Radiother Oncol. 2022;175:185–190. doi: 10.1016/j.radonc.2022.05.003. [DOI] [PubMed] [Google Scholar]
- [204].Gaide O, Herrera F, Jeanneret Sozzi W, Gonçalves Jorge P, Kinj R, Bailat C, Duclos F, Bochud F, Germond J-F, Gondré M, Boelhen T, et al. Radiother Oncol. 2022;174:87–91. doi: 10.1016/j.radonc.2021.12.045. [DOI] [PubMed] [Google Scholar]
- [205].Mascia AE, Daugherty EC, Zhang Y, Lee E, Xiao Z, Sertorio M, Woo J, Backus LR, McDonald JM, McCann C, Russell K, et al. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2022.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Zelefsky MJ, Yamada Y, Greco C, Lis E, Schöder H, Lobaugh S, Zhang Z, Braunstein S, Bilsky MH, Powell SN, Kolesnick R, et al. Int J Radiat Oncol. 2021;110:672–679. doi: 10.1016/j.ijrobp.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].El Naqa I, Pogue BW, Zhang R, Oraiqat I, Parodi K. Med Phys. 2022;49:4109–4122. doi: 10.1002/mp.15662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Weber UA, Scifoni E, Durante M. Med Phys. 2022;49:1974–1992. doi: 10.1002/mp.15135. [DOI] [PubMed] [Google Scholar]
- [209].Jolly S, Owen H, Schippers M, Welsch C. Phys Medica. 2020;78:71–82. doi: 10.1016/j.ejmp.2020.08.005. [DOI] [PubMed] [Google Scholar]
- [210].Simeonov Y, Weber U, Schuy C, Engenhart-Cabillic R, Penchev P, Durante M, Zink K. Z Med Phys. 2021;31:203–214. doi: 10.1016/j.zemedi.2020.06.008. [DOI] [PubMed] [Google Scholar]
- [211].Simeonov Y, Weber U, Penchev P, Ringbæk TP, Schuy C, Brons S, Engenhart-Cabillic R, Bliedtner J, Zink K. Phys Med Biol. 2017;62:7075–7096. doi: 10.1088/1361-6560/aa81f4. [DOI] [PubMed] [Google Scholar]
- [212].Simeonov Y, Weber U, Schuy C, Engenhart-Cabillic R, Penchev P, Flatten V, Zink K. Biomed Phys Eng Express. 2022;8:035006. doi: 10.1088/2057-1976/ac5937. [DOI] [PubMed] [Google Scholar]
- [213].Tommasino F, Rovituso M, Bortoli E, La Tessa C, Petringa G, Lorentini S, Verroi E, Simeonov Y, Weber U, Cirrone P, Schwarz M, et al. Phys Medica. 2019;58:99–106. doi: 10.1016/j.ejmp.2019.02.001. [DOI] [PubMed] [Google Scholar]
- [214].Luoni F, Weber U, Boscolo D, Durante M, Reidel C-A, Schuy C, Zink K, Horst F. Front Phys. 2020;8 doi: 10.3389/fphy.2020.568145. [DOI] [Google Scholar]
- [215].Kang M, Wei S, Choi JI, Lin H, Simone CB. Int J Radiat Oncol. 2022;113:203–213. doi: 10.1016/j.ijrobp.2022.01.009. [DOI] [PubMed] [Google Scholar]
- [216].MacKay R, Burnet N, Lowe M, Rothwell B, Kirkby N, Kirkby K, Hendry J. Radiother Oncol. 2021;164:122–127. doi: 10.1016/j.radonc.2021.09.011. [DOI] [PubMed] [Google Scholar]
- [217].Schwarz M, Traneus E, Safai S, Kolano A, Water S. Med Phys. 2022;49:2861–2874. doi: 10.1002/mp.15579. [DOI] [PubMed] [Google Scholar]
- [218].Ramesh P, Gu W, Ruan D, Sheng K. Med Phys. 2022;49:7826–7837. doi: 10.1002/mp.16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Chaudhary P, Milluzzo G, Ahmed H, Odlozilik B, McMurray A, Prise KM, Borghesi M. Front Phys. 2021;9 doi: 10.3389/fphy.2021.624963. [DOI] [Google Scholar]
- [220].Chaudhary P, Gwynne DC, Odlozilik B, McMurray A, Milluzzo G, Maiorino C, Doria D, Ahmed H, Romagnani L, Alejo A, Padda H, et al. Radiat Oncol. 2022;17:77. doi: 10.1186/s13014-022-02024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Chaudhary P, Milluzzo G, McIlvenny A, Ahmed H, McMurray A, Maiorino C, Polin K, Romagnani L, Doria D, McMahon SJ, Botchway SW, et al. Phys Med Biol. 2023;68:025015. doi: 10.1088/1361-6560/aca387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.