Abstract
The spindle assembly checkpoint (SAC) is a cellular surveillance mechanism that functions to ensure accurate chromosome segregation during mitosis. Macromolecular complexes known as kinetochores, act as the interface of sister chromatid attachment to spindle microtubules. In response to unattached kinetochores, the SAC activates its effector, the mitotic checkpoint complex (MCC), which delays mitotic exit until all sister chromatid pairs have achieved successful attachment to the bipolar mitotic spindle. Formation of the MCC (composed of Mad2, BubR1, Bub3 and Cdc20) is regulated by an Mps1 kinase-dependent phosphorylation signaling cascade which assembles and repositions components of the MCC onto a catalytic scaffold. This scaffold functions to catalyze the conversion of the HORMA-domain protein Mad2 from an “inactive” open-state (O-Mad2) into an “active” closed-Mad2 (C-Mad2), and simultaneous Cdc20 binding. Here, our current understanding of the molecular mechanisms underlying the kinetic barrier to C-Mad2:Cdc20 formation will be reviewed. Recent progress in elucidating the precise molecular choreography orchestrated by the catalytic scaffold to rapidly assemble the MCC will be examined, and unresolved questions will be highlighted. Ultimately, understanding how the SAC rapidly activates the checkpoint not only provides insights into how cells maintain genomic integrity during mitosis, but also provides a paradigm for how cells can utilize molecular switches, including other HORMA domain-containing proteins, to make rapid changes to a cell's physiological state.
Keywords: Bub1, Cdc20, Mad1, mitotic checkpoint complex, Mps1, spindle assembly checkpoint
Abbreviations
- APC/C
anaphase-promoting complex/cyclosome
- C-Mad2
closed-state Mad2
- CTD
C-terminal domain
- FRET
fluorescence resonance energytransfer
- KEN
Lys-Glu-Asp
- MCC
mitotic checkpoint complex
- MIM
Mad2-interacting motif
- NTD
N-terminal domain
- O-Mad2
open-state Mad2
- PDB
protein data bank
- RLK
Arg-Leu-Lys
- SAC
spindle assembly checkpoint
- TPR
tetratricopeptide repeat
1. Introduction
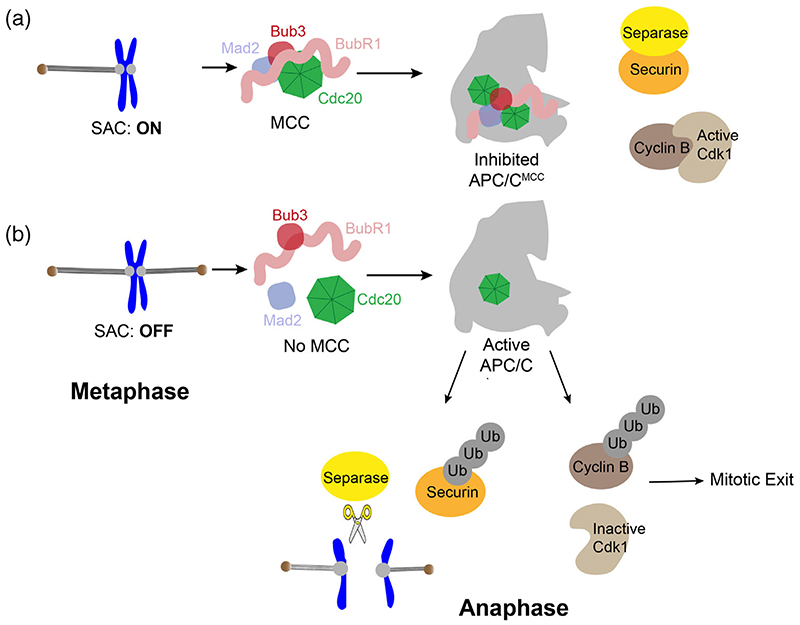
The accurate segregation of sister chromatids to daughter cells during the metaphase-to-anaphase transition of mitosis is critical to maintaining the faithful inheritance of genetic material and genomic stability.1,2 Segregation errors result in genomic abnormalities, including a loss or gain of a chromosome, known as aneuploidy, which ultimately leads to cell death and/or disease.1–3 Chromosome segregation is safeguarded by the spindle assembly checkpoint (SAC).4–9 This process is mediated by large proteinaceous assemblies known as kinetochores which are assembled onto the core of centromeric chromatin and act as the interface for microtubule attachment to sister chromatids.3,8,10,11 Kinetochores are also the signaling hub required to create communication between the state of microtubule attachment and SAC activity.12 Unattached kinetochores trigger activation of the SAC, which creates a “wait for anaphase signal,” halting anaphase onset until all kinetochore-microtubule attachments are made (Figure 1a).13,14
Figure 1. Overview of the Spindle Assembly Checkpoint (SAC).
(a) During metaphase, at unattached kinetochores, the SAC activates its diffusible inhibitor, the mitotic checkpoint complex (MCC), which binds and inhibits the anaphase-promoting complex/cyclosome (APC/C) to prevent premature securin and cycle B degradation. (b) Upon biorientation of kinetochore-microtubule attachments, SAC signaling is turned off, the MCC is disassembled, and the APC/C is free to ubiquitinate securin and cyclin B to trigger anaphase onset and subsequent mitotic exit.
The SAC delays anaphase onset by generating a diffusible inhibitor known as the mitotic checkpoint complex (MCC), comprised of two subcomplexes, BubR1: Bub3 and Cdc20:Mad2 (Figure 1a).13–15 The BubR1:Bub3 complex remains stable throughout mitosis.16–19 In contrast, Cdc20:Mad2 association involves a kinetically disfavored major structural rearrangement of Mad2 from an “inactive” open-state (O-Mad2) into an ‘active’ closedstate (C-Mad2), catalyzed by unattached kinetochores.20–23 Once formed, the MCC binds and inhibits an E3 ubiquitin ligase, the anaphase-promoting complex/cyclosome (APC/C), whose ubiquitination activity is required to target cyclin B and securin for degradation (Figure 1a).24–31 Once all sister chromatid pairs have achieved successful attachment to the bipolar mitotic spindle, the SAC must be silenced to relieve APC/C inhibition and allow timely mitotic exit (Figure 1b).4,5,32,33 This includes kinetochore-based SAC-silencing mechanisms such as the phosphatase activity of protein phosphatase 1 (PP1) and protein phosphatase 2 B56 (PP2-B56), dynein-mediated stripping of SAC components, as well as MCC-specific silencing mechanisms including TRIP13-mediated disassembly of the MCC, and MCC ubiquitination by the APC/C.4,5,32,34–43
The structure and function of the MCC and how it binds and inhibits the APC/C are well established.24–28,44 More elusive has been uncovering how the SAC overcomes the kinetic barrier to Cdc20:C-Mad2 association to rapidly produce the MCC. Recently, several new studies, building on decades of experimental work, have unveiled a compelling model for how this is achieved.5,23,45–49 Unattached kinetochores activate an Mps1-dependent phosphorylation signaling cascade which sequentially recruits checkpoint proteins to the outer kinetochore to assemble a catalytic scaffold for MCC formation.5,45,47 The scaffold functions to not only locally concentrate but also promote the accessibility and spatial repositioning of Cdc20 and Mad2 to catalyze their interaction.5,46,48,49 This review will first overview the structure and function of the MCC (Section 2) and the molecular mechanisms underlying the kinetic barrier to its formation (Section 3). After which, how Mps1 orchestrates the assembly of the catalytic scaffold will be outlined (Section 4). Our current understanding of the structure of the catalytic scaffold (Section 5) and how it acts to catalyze the Cdc20:C-Mad2 association (Section 6) will then be presented and discussed.
2. Mcc
2.1. Architecture of the MCC
A schematic of the key functional domains within BubR1, Bub3, Mad2, and Cdc20 is shown in Figure 2. Mad2 is a HORMA domain-containing protein that adopts two topologically distinct states: an “inactive” open state (O-Mad2) and an ‘active’ closed state (C-Mad2).49–53 Conversion of O-Mad2 into C-Mad2 involves a metamorphic rearrangement of its β-sheet (detailed in Section 3.1), which allows its C-terminal β-hairpin and its adjacent loop, termed the “safety-belt,” to entrap the Mad2-interacting motif (MIM) of Cdc20 to form the C-Mad2:Cdc20 complex (Figure 3a).51,54 The majority of Cdc20 is comprised of a WD40 domain which folds into a canonical seven-bladed β-propeller and binds to the tetra-tricopeptide repeat (TPR) domain, as well as the KEN1, ABBA2 (A2) and D-box2 (D2) motifs of BubRI at distinct positions on its β-propeller (Figure 3a).14,55 The N-terminal helix–loop (HLH) motif of BubR1 which contains the KEN1 box is sandwiched between the β-propeller of Cdc20 and the C-terminal α-helix of C-Mad2 (αC) (Figure 3a).14 Following the BubR1 HLH motif, the TPR domain makes further reinforcing contacts with Cdc20 and C-Mad2 (Figure 3a).14 Bub3 is made up of seven WD40 repeats that form a β-propeller arrangement and bind to the GLEBS motif of BubR1.56,57 Bub3 has never been seen in structures of the MCC suggesting it is only flexibly tethered to BubR1. Bub3 is not required for MCC association but has several important downstream functions discussed in Section 4.17,58 Bub3 is not part of the MCC in fission yeast.59
Figure 2.
A schematic diagram of mitotic checkpoint complex (MCC) subunits and key proteins required for MCC assembly onto the outer kinetochore. Functional domains are outlined in colored ovals and their residues numbers are outlined to the right of each schematic. NTE: N-terminal extension. MR: Middle region. TPR: Tetratricopeptide repeat. SILK/RVSF: Ser-Ile-Leu-Lys/Arg-Val-Ser-Phe. MELT: Met-Glu-Leu-Thr. KI: Lys-Ile. RWD: RING, WD40, DEAD. WD40: Trp-Asp 40. A: ABBA (named ABBA as the motif was originally identified in A-type cyclins, Bub1, BubR1 and Acm1, though it has also been referred to as the Phe motif). GLEBS: Gle2-binding-sequence. BDD: Bub dimerization domain. KEN: Lys-Glu-Asn-box. CD1: Conserved domain 1. MIM: Mad2-interacting motif. RLK: Arg-Leu-Lys. HORMA: Hop1p, Rev7p, Mad2 proteins. C: C-box. D: Destruction box. IR: IR-tail.
Figure 3. Structure of the mitotic checkpoint complex (MCC) and its inhibition of the anaphase-promoting complex/cyclosome (APC/C).
(a) The crystal structure of the MCC from S. pombe (Protein Data Bank (PDB) 4AEZ).14 (b) A cryo-EM reconstruction of APC/CMCC (PDB 6TLJ).44 The APC/C subunits are displayed using grey surface representation in ChimeraX,198 Cdc20APC/C is colored in cyan, BubR1 in pink, Cdc20MCC in green, and Mad2 in blue. (c) Two views of the MCC bound to and inhibiting Cdc20APC/C (PDB 6TLJ).44 (d) Schematic representation of BubR1. BubR1 domains involved in interacting with either Cdc20MCC or Cdc20APC/C are shaded dark pink and are highlighted in the structure in (c). All structures are displayed using ChimeraX.198
2.2. The molecular basis of MCC-mediated APC/C inhibition
The critical function of the MCC is to prevent the ubiqui-tination of cyclin B and securin by the APC/C when the APC/C is coactivated by Cdc20 (APC/CCdc20).13,24 The APC/C is a 1.2 MDa cullin-RING E3 ubiquitin ligase, composed of 14 distinct proteins and 19 subunits (Figure 3b).24,28,60–62 The APC/C complex is stable throughout the entire cell cycle, but its activity is highly regulated. Temporal regulation occurs through two structurally related co-activators; Cdc20 and Cdh1, APC/C inhibitors, and reversible protein phosphorylation.26,63,64 APC/C coactivators, Cd20 and Cdh1, assist APC/C substrate recognition using their WD40 domains to identify KEN-boxes and D-boxes on substrates.65–67 The ABBA motif is additionally recognized by Cdc20 in humans and by Cdh1 in S. cerevisiae.67,68
The MCC is a potent APC/CCdc20 inhibitor. It binds directly to APC/CCdc20 and represses APC/C ubiquitination by blocking substrate recognition and by impairing the E3 ligase activity of the APC/C24,25,27,28,31,69 (reviewed in Ref.24). The MCC achieves this largely by two mechanisms: steric hindrance and inhibiting conformational change of the APC/C active site. The Cdc20 molecule which binds and co-activates APC/C (Cdc20APC/C), is a separate molecule from that of the Cdc20 incorporated into the MCC (Cdc20MCC) (Figure 3b,c).70–73 This gives Cdc20 the unique role of being both a critical activator and inhibitor of the APC/C. The MCC binds to the central cavity of APC/CCdc20 by docking onto its substrate recognition module (Cdc20APC/C), forming an extensive interface to both APC/C and Cdc20APC/C (Figure 3b).24–28,31 Cdc20MCC and Cdc20APC/C engage with each other through BubR1 which weaves between them (Figure 3c). BubR1 then uses a separate set of degrons (KEN2, A1, D1) from those used to interact with Cdc20MCC (KEN1, A2, D2) to obstruct substrate recognition by Cdc20APC/C (Figure 3a,c,d).26–29 Key to this is Mad2 which helps to position KEN1 of BubR1 to bind Cdc20MCC (Figure 3c,d). The ability of the BubR1 degrons to act as pseudosubstrate inhibitors explains why these degrons are critical to MCC-mediated APC/C inhibition and robust checkpoint response.74–78 Additionally, the APC/C subunit Apc10 which contributes to Cdc20APC/C substrate recognition, is separated from the substrate recognition site upon MCC binding to APC/CCdc20. This is due to a conformational change within APC/CCdc20 induced upon MCC binding which pushes Cdc20APC/C away from Apc10 (Figure 3b). The TPR domain of BubR1 also binds to the winged-helix B (WHB) domain of APC2. This blocks UbcH10 (the E2 of APC/C) from binding the APC/C catalytic module and represses APC/C E3 ligase activity (Figure 3b).
3. The Kinetic Barrier To Cdc20:C-Mad2 Association
Rapid assembly of the MCC requires overcoming a kinetic barrier to C-Mad2:Cdc20 association.20–23,79 Our current understanding is that there are largely two impediments to their interaction: a naturally very slow conversion of O-Mad2 into C-Mad2 which is required to entrap the MIM of Cdc20,20,53,79,80 and repositioning of the Cdc20 MIM relative to O-Mad2 which is required to promote MIM accessibility and induce rapid Mad2 conversion.5,46,48,49 The former has been well studied while the latter only recently became recognized and is less well understood. The underlying mechanisms for both barriers will be presented and examined below.
3.1. The molecular details of Mad2 conversion
A variety of solution and crystal structures of Mad2 in the unbound and bound states have been determined to delineate the dramatic remodeling that Mad2 undergoes during the open-to-closed (O-to-C) Mad2 transition.52,80–86 Mad2 contains a central core (colored grey in Figure 4a), which includes a three-stranded anti-parallel β-sheet (β4-6) and three α-helices (αA-C) with a β-hairpin (β2-3) between the αA and αB helices (Figure 4a). The core of Mad2 is preserved during O-to-C Mad2 conversion while both the N- and C-terminus of Mad2 (colored yellow and red, respectively) undergo a metamorphic structural remodeling (Figure 4a). The disordered segment at the very C-terminus of O-Mad2, as well as the two adjacent β-strands (β7/8 colored red) which form a C-terminal β-hairpin, swing across the central face of Mad2, displacing the N-terminal β-strand in O-Mad2 (β1 colored yellow in O-Mad2) (Figure 4a). This ejected N-terminal β-strand then refolds into an α-helix (αN) and an extended αA-helix in C-Mad2 (colored yellow) (Figure 4a). The rearrangement of the C-terminal β-hairpin and its adjacent loop (the safety-belt), ultimately enables Mad2 to enclose the β-stranded Cdc20 MIM (colored green) (Figure 4a). This entrapment of the Cdc20 MIM during Mad2 O-to-C conversion has thus been coined the ‘safety-belt’ mechanism and results in a dramatic alteration of the secondary structure and tertiary structure of Mad2.85 The existence of transient intermediate(s) of Mad2 (often referred to as I-Mad2) which occur during Mad2 conversion have been suggested, however, the existence and/or relative importance of these intermediate states has yet to be fully examined.81,87
Figure 4. Mad2 conversion and the kinetic barrier to C-Mad2:Cdc20 association.
(a) The conformational rearrangement of Mad2 during the O-Mad2 to C-Mad2 transition. On the left O-Mad2 is depicted, and on the right C-Mad2 with the Mad2-interacting motif (MIM) entrapped by the safety belt of Mad2 (PDB 2V64).84 The crystal structure was determined in the presence of a MIM peptide selected for its enhanced C-Mad2 affinity using phage display.83 The N- and C-terminal segments which undergo remodeling during Mad2 conversion are colored in yellow and red, respectively. (b-d) Schematics outlining relative rates at cellular concentrations of Mad2 conversion and C-Mad2: Cdc20 formation with or without ligand (Cdc20) and catalysts. (b) Conversion of O-Mad2 by itself into C-Mad2 is kinetically disfavored and thus conversion occurs very slowly. (c) Conversion of O-Mad2 and C-Mad2:Cdc20 association in the presence of full-length Cdc20 without accompanying catalysts is limited by the disfavoured and rate-limiting spontaneous conversion of Mad2 which is required to form C-Mad2: Cdc20. (d) Conversion of O-Mad2 and concomitant C-Mad2:Cdc20 formation is rapid in the presence of identified catalysts (Bub1, Mad1:C-Mad2, and Mps1 phosphorylation).
3.2. Mad2 conversion is kinetically disfavored
In cells, the SAC response is established within minutes of kinetochore-microtubule detachment.65,88 In vitro, however, formation of the MCC from O-Mad2, BubR1: Bub3, and Cdc20 at physiological concentrations is extremely slow, with the half-life being about 220 mins and requiring overnight incubation to reach equilibrium (Figure 4b,c).23 This is because the topological rearrangement which occurs during the O-to-C conversion of Mad2 requires large activation energy (Figure 4a).20 This means that spontaneous O-to-C conversion of Mad2, which is required to entrap the MIM of Cdc20 to form C-Mad2:Cdc20, is kinetically disfavored (naturally very slow) and rate-limiting for MCC formation.20–22 As detailed below, subsequent studies focused on discovering the molecular mechanisms responsible for overcoming this rate-limiting barrier.
3.3. C-Mad2:Cdc20 formation occurs on the Mad1:C-Mad2 platform
One of the first proteins identified as essential to the checkpoint response is the highly evolutionarily conserved protein Mad1.89–91 Mad1 is an elongated protein that self-dimerizes through a series of coiled-coil α-helices (Figures 2 and 5a,b).90–92 Currently, there is no experimentally determined structure of full-length Mad1 or its N-terminal 485 residues. AlphaFold293,94 generates a model of the N-terminus of Mad1 which largely consists of long coiled coils that possibly fold onto themselves (Figure 5b).93,94 In contrast the C-terminus of Mad1 (Mad1485–718) is well studied. The coiled-coil formed from residues 485–584 is interrupted by a MIM, spanning residues 530–550, which entraps one molecule of Mad2 per subunit to make the Mad1:C-Mad2 tetramer (Figure 5a-c).85 Each Mad1-bound C-Mad2 can then target and self-dimerize to cytosolic O-Mad2, to make a Mad1:C-Mad2:O-Mad2 pentamer or hexamer (Figure 5c).54,85,86,95,96 It is the O-Mad2 molecule that is asymmetrically dimerized to Mad1-bound C-Mad2 which then undergoes conversion to C-Mad2 to generate the C-Mad2:Cdc20 complex (Figure 5d).85,92,95,97,98
Figure 5. Mad1:C-Mad2 is the platform for Mad2 conversion.
(a) A schematic diagram of Mad1 where non-coiled-coil segments are depicted as ovals as predicted by COILS.199 (b) An AlphaFold2 prediction of full-length dimeric Mad1, where residues 1−485 and 486−718 are colored brown and orange respectively.93,94 (c) Side and bottom views of Mad1485−718:C-Mad2:O-Mad2 hexamer. The Mad1 dimer encompassing residues 485−584 is depicted in orange, bound to two C-Mad2 molecules in light blue (PDB 1GO4).85 Two O-Mad2 molecules (dark blue) dimerized to C-Mad2 are fitted using the structure of the O:C Mad2 dimer (PDB 2V64).84 The coiled-coil is then interrupted by a flexible 16-residue segment, termed the C-terminal hinge, which is modelled by AlphaFold2 to contain a novel short α-helix.93,94 The Mad1 C-terminal hinge facilitates fold-over of the Mad1 C-terminus (Mad1CTD; residues 597−718), which comprises a coiled-coil stem ending in a globular head domain featuring an RING, WD40, DEAD (RWD) fold (PDB 4DZO).45,153 The RLK motif within Mad1CTD is highlighted in red. (d) The Mad1:C-Mad2 platform targets O-Mad2 to unattached kinetochores. O-Mad2 then undergoes conversion to C-Mad2, releasing it from Mad1:C-Mad2 and simultaneously binding the Mad2-interacting motif (MIM) of Cdc20 positioned nearby.
Several experiments have been key to unveiling this mechanism (first coined the “Mad2 template model”95), by which C-Mad2:Cdc20 formation requires Mad1:C-Mad2 as the platform for O-to-C Mad2 conversion. The SAC cannot be triggered in cells where Mad2 mutants locked in either the open or closed state are targeted to kinetochores, or by targeting a Mad2 R133A mutant which is unable to self-dimerize.95,99,100 Mad1 overexpression will titrate away free O-Mad2 and abrogate the checkpoint.97 P31comet, an adaptor protein important for MCC disassembly, which is homologous to O-Mad2 and can bind the Mad1:C-Mad2 complex, blocks O-Mad2 binding and prevents MCC formation.40,86,101–104 Checkpoint dysfunction occurs if a Mad1 MIM mutant (K541A/L543A), which cannot bind Mad2 is used.105
Originally it was proposed that on the Mad1:C-Mad2 platform O-Mad2 first converts into a ligand-free “empty” C-Mad2 which can then rapidly associate with Cdc20.52,54 However, at cellular concentrations Cdc20 does not bind to C-Mad2 at an appreciable rate.46 Binding of C-Mad2 to Cdc20 at cellular concentrations only occurs when the MIM of Cdc20 is presented as a peptide (Cdc20MIM) or when the N-terminus preceding the MIM of Cdc20 is truncated (Cdc20111-C).46 This is likely because in either scenario the MIM can thread under the already remodeled safety-belt, whereas this is prevented in full-length Cdc20. This is also supported by experiments that show that the checkpoint is abrogated in cells where C-Mad2 is the only available Mad2 species.99,106,107 Additionally, because C-Mad2 cannot dimerize with C-Mad2 already bound to Mad1 (although an unliganded C-Mad2:C-Mad2 dimer has been shown to exist82), the Mad1:C-Mad2 platform cannot be utilized when only C-Mad2 is present.95 Altogether this indicates that C-Mad2: Cdc20 formation occurs on the Mad1:C-Mad2 platform, where O-to-C Mad2 conversion is both necessary and simultaneous with Mad2 safety-belt entrapment of Cdc20 (Figure 5d). How the kinetic barrier to Mad2 conversion affects Mad1:C-Mad2 formation in cells is an interesting question. Our current understanding suggests that there is no intricate mechanism required to assemble Mad1:C-Mad2. This may be because Mad1 and Mad2 have ample time to associate under non-catalytic conditions, or it may be that the MIM of Mad1 can spontaneously thread under the safety-belt of C-Mad2, as compared to Cdc20 where this pathway is not available.
3.4. A Bub1:Mad1:C-Mad2 scaffold catalyzes C-Mad2:Cdc20 formation
Dimerization of O-Mad2 to the Mad1:C-Mad2 platform has been shown to directly accelerate the O-to-C Mad2 transition.20,23,46,79 Therefore, Mad1-bound C-Mad2, not only acts as a platform for recruitment and conversion of O-Mad2 but also acts directly to encourage Mad2 conversion.84,85,96,108 How Mad2 dimerization directly promotes O-to-C Mad2 remodeling remains unclear. One hypothesis is that O-Mad2 dimerized to Mad1-bound C-Mad2 is in a partially “activated” state, whereby the C-terminal β7/8-hairpin of O-Mad2 which must become detached during the structural metamorphosis into C-Mad2, is somehow more susceptible to spontaneous detachment (Figure 4a).81,108 However, the interaction of O-Mad2 with Mad1:C-Mad2, albeit essential for checkpoint function, promotes only a minor acceleration of MCC assembly, indicating that other components are required for full catalytic acceleration.23,46,88
An elegant FRET system that directly measures O-Mad2 conversion and concomitant Cdc20:C-Mad2 formation by assessing CFP-Cdc20 binding to Mad2 conjugated to a TAMRA fluorophore has been established.23,46,109 This assay has identified and quantified other factors which directly contribute to catalyzing C-Mad2:Cdc20 formation in vitro. An MCC-mediated APC/C inhibition substrate ubiquitination assay also contributed to these findings.47 These assays identified that in addition to Mad2 dimerization, Mad1:C-Mad2, Cdc20, phosphorylated Bub1, and phosphorylation of the Mad1 C-terminus are all key contributors to catalytic C-Mad2:Cdc20 association.23,46,47 The O-to-C transition of Mad2 and simultaneous binding to Cdc20 in the presence of identified catalysts (Mad1:C-Mad2, Bub1, and Mps1 phosphorylation—“the catalytic scaffold”) in vitro, accelerates O-Mad2 binding to Cdc20 to within minutes.23,46 This is a 35-fold increase which is near physiologically relevant rates (Figure 4d).23,46 It is important to highlight that as these assays are conducted in vitro, the catalytic contribution of Bub1 and Mad1 identified is complementary to their roles in recruiting checkpoint proteins to kinetochores (discussed in Section 4). This is confirmed by the importance of Bub1 in SAC signaling even when Mad1 is artificially tethered to kinetochores.110,111 Interestingly, the N-terminus of Mad1 is not required for catalysis in vitro, although a Mad1 truncation encompassing residues 420–718 was found to be a slightly stronger catalyst than the traditionally used Mad1485–718 (Figure 5a).23
3.5. The catalytic scaffold functions to present the Cdc20 MIM to O-Mad2
Understanding how the scaffold functions to accelerate Mad2 conversion and simultaneous Cdc20 binding is of key interest. Spontaneous O-to-C conversion of Mad2 can be induced by presenting O-Mad2 with a peptide encompassing the MIM of either of its ligands (Mad1MIM and Cdc20MIM).49,80,83 The molecular mechanisms for how the MIM directly induces Mad2 conversion remain a fundamental question. One hypothesis is that the MIM transiently binds O-Mad2, and either directly or allosterically triggers detachment of the β7/8-hairpin of Mad2, making it available to entrap the MIM (Figure 4a). It may also be that this β7/8-hairpin of O-Mad2 is in a more “breathable” intermediate state than the experimentally determined O-Mad2 structures suggest. This could allow the MIM to instantaneously trigger its own entrapment upon spontaneous β7/8-hairpin detachment and stabilize the C-Mad2 state. Further clarifying these hypotheses is key to completing our understanding of how the catalytic scaffold directly catalyzes C-Mad2:Cdc20 formation but remains a challenge due to the fleeting nature of these states.
The ability of the MIM to induce O-to-C Mad2 conversion does not however explain how rapid Mad2 conversion is achieved at unattached kinetochores. This is because even at cellular concentrations Cdc20MIM does not have a fast association rate with O-Mad2.46 At physiological concentrations Cdc20MIM and full-length Cdc20 associate with O-Mad2 at comparably slow rates.46 The ability of Cdc20MIM to induce Mad2 conversion in a concentration-dependent manner does however imply that the critical function of the catalytic scaffold in cells is to position the MIM of Cdc20 close to O-Mad2 to trigger their rapid association.49 These in vitro MCC assembly assays also identify that the catalytic scaffold accelerates full-length Cdc20 binding to O-Mad2 significantly, while binding to Cdc20MIM is accelerated only mildly.46 This suggests that the catalytic scaffold acts directly on Cdc20 to locally concentrate and spatially constrain the MIM close to O-Mad2 to trigger Mad2 conversion. In support of this, even when tested in vitro, preventing O-Mad2 dimerization to Mad1:C-Mad2 or preventing Cdc20 association to the scaffold by eliminating its interaction with Mad1 and Bub1 (discussed in Section 6) abolishes the ability of the catalytic scaffold to facilitate catalytic C-Mad2:Cdc20 formation.46
4. Assembly Of The Catalytic Scaffold By Unattached Kinetochores
4.1. The master regulator: Mps1
SAC activation and error correction is a highly regulated signal transduction cascade under the surveillance of several kinases, including Aurora B, Cdk1, and Mps1, as well as the phosphatases, PP1 and PP2A.112–114 At the apex of SAC signaling is the evolutionarily conserved kinase Mps1 (monopolar spindle 1/TTK). Mps1 is responsible for sequentially recruiting SAC components onto the outer kinetochore, facilitated by the KMN (Knl1, Mis12 and Ndc80) network.115–119 Ultimately this is how the SAC assembles the catalytic scaffold to rapidly generate the MCC.5 Mps1 also releases the Mad1:C-Mad2 complex from nuclear pores during mitotic entry,120,121 and phosphorylates Mad1 to promote catalytic C-Mad2:Cdc20 formation.46,47,49 Overexpression of Mps1 can activate the checkpoint even in the presence of intact kinetochore-microtubule attachments, a mechanism which occurs through hyperphosphorylation of Bub1 and Mad1.118 Mps1 persistence at kinetochores, such as through KMN tethering, delays anaphase onset and can induce cell death.122 Furthermore, inhibiting Mps1 by adding reversine, a selective inhibitor of Mps1 kinase activity, impedes the localization of nearly all downstream checkpoint proteins and creates a defective checkpoint.122–124
Despite extensive studies on Mps1, the mechanism of its kinetochore localization remains poorly understood. The prevailing model suggests that the Ndc80 complex of the KMN network is the direct receptor of Mps1 at kinetochores.125–128 The Ndc80 complex is composed of Ndc80 (Hec1 in humans), Spc24, Spc25, and Nuf28.129–131 The N-terminal calponin-homology (CH) domain of Ndc80 binds microtubules directly.8,11,132 Mps1 contains an N-terminal TPR domain and an N-terminal extension (NTE) (Figure 2). The TPR domain and NTE self-interact prior to mitosis, but during mitosis this autoinhibition is relieved and both bind to the CH domain of Ndc80 to recruit Mps1, with the NTE being the critical interaction site for Ndc80.126,127,133–135 Consistent with these results, deletion of the Mps1 N-terminus abolishes Mps1 kinetochore targeting and SAC signaling.125
This binding site of Mps1 to the CH domain of Ndc80 is at least partially mutually exclusive with the microtubule-binding site on Ndc80.134,135 This has led to the suggestion that Mps1 localization is a direct sensor for microtubule attachment and plays a key regulatory role in initiating the Mps1 phosphorylation-dependent signaling cascade to assemble the MCC.5,136 However, Mps1 was found to localize to microtubule-attached kinetochores and facilitate microtubule release during Aurora B-dependent error correction.137 This suggests Mps1 kinetochore localization is not only limited to recruitment by microtubule-free Ndc80, and further work will be required to clarify these pathways. Aurora B also plays a role in Mps1 localization.112,138,139 Inhibition of Aurora B prevents Mps1 recruitment, while tethering Mps1 bypasses the SAC assembly requirement for Aurora B.139 Aurora B might contribute to Mps1 kinetochore targeting by phosphorylating the Ndc80 CH domain which then enhances Mps1 binding.140 Aurora B activity also helps alleviate the inhibitory effect of the Mps1 TPR:NTE interaction.125 Another possibility is that Mps1 self-phosphorylates its NTE, to relieve autoinhibition.112,125,133
4.2. Mps1 phosphorylation sequentially assembles the catalytic scaffold for MCC formation
Stepwise assembly of the catalytic scaffold by Mps1 is outlined in Figure 6. Mps1 phosphorylates several MELT (Met-Glu-Leu-Thr) motifs on the outer kinetochore subunit Knl1.141–147 These phosphorylated MELTs recruit Bub3, which is already bound to Bub1 of the Bub1:BubR1 dimer.145,146,148–150 Mps1 then phosphorylates the conserved domain 1 (CD1) of Bub1 at Thr461, after being primed by Cdk1 phosphorylation at Ser459.47,142,151,152 This enables phosphorylated Bub1 CD1 to bind and recruit the Mad1:C-Mad2 complex through an interaction with an RLK (Arg-Leu-Lys) motif within the C-terminal domain of Mad1 (Mad1CTD).45,47,151–153 Mps1 also phosphorylates Mad1CTD to promote an interaction with the N-terminus of Cdc20, and this together with Bub1-mediated Cdc20 kinetochore recruitment, assembles the catalytic scaffold for C-Mad2:Cdc20 formation.46,47,49
Figure 6. Mps1 phosphorylation-dependent stepwise assembly of the catalytic scaffold for MCC formation.
(a) The sequential steps of catalytic MCC assembly onto unattached kinetochores are outlined. (b) A schematic of the catalytic scaffold assembled onto the outer kinetochore. Phosphorylated Knl1 Met-Glu-Leu-Thr (MELT) motifs recruit the Bub3:Bub1 complex, which is sequentially phosphorylated by Cdk1 and Mps1 on the Bub1 CD1 domain. Phosphorylated Bub1 CD1 recruits the Mad1:C-Mad2 complex and O-Mad2 is recruited through self-dimerization with Mad1-bound C-Mad2. Mad1 is further phosphorylated by Mps1 which promotes interaction with the N-terminus of Cdc20. The catalytic scaffold then functions to bring the Mad2-interacting motif (MIM) of Cdc20 near O-Mad2 to promote near instantaneous conversion of O-Mad2 into C-Mad2, releasing it from Mad1:C-Mad2 and simultaneous entrapment of the Cdc20 MIM by the C-Mad2 safety-belt. C-Mad2:Cdc20 then binds BubR1:Bub3 to generate the MCC.
4.3. Bub1 and BubR1 kinetochore targeting
4.3.1. Knl1 MELT motifs
Knl1 is phosphorylated by Mps1 at its conserved MELT motifs ([M/I/L/V]-[E/D]-[M/I/L/V]-pT) which then act as a recruitment platform for Bub3:Bub1 and possibly BubR1:Bub3 (Figures 2 and 7a).143,149,150,154 Binding occurs through the methionine and phosphorylated threonine of the MELpT sequence which dock onto the hydrophobic and basic patches of Bub3, respectively (Figure 7b).150 Interestingly the number of MELT copy numbers within Knl1 varies substantially across organisms, from 2 to 27 MELT repeats, suggesting that Knl1 is a rapidly evolving protein.145,147,155 Human Knl1 has 19 MELT-like motifs (Figure 2) as compared to S. pombe (Spc7) which has 8, and S. cerevisiae (Spc105) which only has 5. Not all MELT motifs have the same activity towards Bub3 recruitment (Figure 7a).146
Figure 7. Knl1 MELTs and the KI1 and KI2 motifs in SAC signaling.
(a) A schematic depiction of Knl1 with its 19 Met-Glu-Leu-Thr (MELT) repeats. The activity of each MELT, as defined by the ability of the MELT to recruit the Bub3:Bub1 complex for SAC signaling, corresponds to a color gradient (the darker the more active).145,146 The strength of the Bub3:MELT interaction is also depicted by the arrow length (the shorter the stronger the binding). (b) A close-up of the N-terminus of Knl1 where MELT1 is immediately proceeded by the KI1 and KI2 motifs which bind to the tetratricopeptide repeat (TPR) lobes of Bub1 and BubR1, respectively. This mechanism enhances Bub1/BubR1 recruitment and is unique to some metazoans including humans. (c) Strong MELT motif activity requires sequential multisite phosphorylation of the Knl1:Bub3 interface by Mps1. Mps1 first phosphorylates the threonine MELT residue (MELpT) which enhances Mps1 phosphorylation at the SHT motif. Strong recruiters of Bub3:Bub1 require the SHT motif to enhance the Bub3:MELT interaction.
MELT motifs also have other species-specific motifs adjacent to them which likely alter the affinity of Knl1 for Bub3:Bub1. In humans, 10 MELTs have an N-terminal TxxΩ motif (x, any amino acid; Ω, aromatic) which is critical for Bub1 recruitment by an unknown mechanism (Figure 7c).145 The most active MELTs also have an indispensable Ser-His-Thr (SHT) motif C-terminal to the MELT (Figure 7c).146 MELT motifs first recruit Mps1 after which MELT phosphorylation primes SHT motif phosphorylation and Bub3 recruitment, a mechanism thought to be unique to vertebrate Knl1.146 Furthermore, in vertebrate orthologs, the N-terminal MELT1 has two 12-residue upstream KI motifs which can recruit Bub1 and BubR1 TPR lobes (Figure 7b).149,156–159 However, mutations in the KI motifs do not abolish Bub1/BubR1 kinetochore localization and are not required for the checkpoint.143,159 Detailed analyses have shown that KI motifs are MELT enhancers, making MELT1 the strongest recruiter of Bub1:Bub3 and the only MELT which can sustain the SAC by itself.159 Overall, the abundance of MELTs and the difference in their affinities suggest a mechanism by which eukaryotic cells can control the strength of SAC signaling in various scenarios of segregation errors.146,160
4.3.2. Bub1:BubR1 dimerization
Bub1 and BubR1 evolved from several duplication events with a common ancestral gene.147 They share similar domains, including TPR, GLEBS, KEN, and ABBA motifs (Figure 2), and yet have very different roles in SAC signaling.149 Both Bub1 and BubR1 form stable mutually exclusive complexes with Bub3, both using the GLEBS domain.57,161 Initially, it was thought that both Bub1 and BubR1 were recruited to Knl1 through Bub3. However, Bub3 is not essential for BubR1 recruitment and BubR1 does not stabilize the Bub3:Knl1 interaction as Bub1 does.149,150 This is because Bub1 contains a loop upstream of the GLEBS Bub3 binding domain which enhances Bub3 binding to phosphorylated MELTs on Knl1.149 Interestingly the same loop in BubR1 is required for enhancing the ability of the MCC to inhibit APC/CCdc20.58 Subsequently, it was discovered that kinetochore localization of BubR1 relies on heterodimerization of Bub1 and BubR1 through a novel domain within their respective GLEBS domains, termed the Bub1-BubR1 dimerization (BBD) domain (Figures 2 and 7b).149 Heterodimerization of Bub1:BubR1 is conserved in fission yeast where dimerization occurs through their tetratricopeptide repeat (TPR) domains.162 However, it has also been suggested that the pool of BubR1 dimerized to Bub1 is required for BubR1-mediated chromosome alignment.16 While the pool of BubR1 required for SAC signaling interacts directly with phosphorylated MELT motifs through Bub3.16 Therefore, the exact pathways of BubR1 kinetochore targeting remain debated.
4.4. Mad1 targeting
Mad1 is targeted to kinetochores as a constitutive heterotetrameric Mad1:C-Mad2 complex.85,92,95,97,163 Kinetochore targeting of Mad1:C-Mad2 is crucial to SAC signaling. Mad1:C-Mad2 is the kinetochore receptor for the O-Mad2 molecule which undergoes conversion into C-Mad2 and is incorporated into the MCC.85,95–98 Additionally, the C-terminal domain of Mad1 plays a critical role in presenting Cdc20 MIM to O-Mad2 during catalytic C-Mad2:Cdc20 formation (discussed in Section 6).5,46,47,49,91 Artificially tethering Mad1 to kineto-chores can maintain an active checkpoint even after all kinetochores are attached.164 Additionally, reactivation of the checkpoint after SAC silencing can be achieved by using an FRB/FKBP rapamycin system to conditionally target Mad1 to kinetochores.165 Several studies have also shown that the strength of the SAC response is dictated by Mad1:C-Mad2 kinetochore levels.88,160
4.4.1. Bub1 is the principal Mad1 receptor for MCC formation
In both humans and yeast Bub1 is the primary kinetochore receptor of Mad1.5,136 Mps1 phosphorylation of a central conserved domain 1 (CD1) of Bub1, recruits the C-terminus of Mad1 through direct interaction with the RLK motif (Figures 2 and 6b).45,47,151,152,166 In budding yeast, two phosphorylation sites within Bub1 CD1 are important for Mad1 recruitment, Thr453 and Thr455, with Thr455 being the primary contributor.47,152 In humans, the equivalent two sites (Ser459 and Thr461) are required for Mad1 kinetochore targeting.47,152 Just as for budding yeast only the second site (Thr461) is critical to the human Bub1-Mad1 interaction in vitro. It was subsequently discovered that in humans these two sites are sequentially phosphorylated. Cdk1 first phosphorylates Ser459, which then primes Mps1 phosphorylation of Thr461.45,47,152 This mechanism of priming is confirmed in the crystal structure of human Bub1CD1:Mad1CTD where the phosphate of pThr461 of Bub1CD1 directly contacts Arg617 of the Mad1 RLK motif, whereas Bub1 pSer459 is part of the flexible N-terminus of Bub1CD1 and does not contact Mad1.45
4.4.2. Is Rod:Zw10:Zwilch a direct receptor for Mad1:C-Mad2?
Contrary to yeast where Bub1 is the only kinetochore receptor of Mad1, it has been proposed that in humans other pathways exist for Mad1 kinetochore localization. One of the main contenders is the metazoan-specific Rod:Zw10:Zwilch (RZZ) complex.110,167–174 The RZZ complex comprises a fibrous biopolymer that forms the corona of the kinetochore, an expansion process that promotes microtubule capture and SAC signaling.175,176 RZZ and its dynein-adaptor Spindly also recruit the major retrograde motor dynein-dynactin to coordinate chromosome congression, corona disassembly (also referred to as corona “stripping” or “shedding”), and SAC silencing.5,177–180 Key to this process is the removal of Mad1:C-Mad2 which effectively halts MCC formation.5,35,171,175
Although the role RZZ plays in the removal of Mad1: C-Mad2 from kinetochores seems quite clear, the specific contribution RZZ has on Mad1:C-Mad2 kinetochore recruitment remains highly debated.5,110,136,171–174,181 A direct interaction between Mad1 and RZZ has yet to be detected, although Mad1 has been found in RZZ immunoprecipitants.170,171,182,183 Recently, it has been shown that corona-localized cyclin B1 directly binds the N-terminus of Mad1.184 This may suggest that the Cdk1-cyclin B1 complex tethers Mad1 to the corona, and thus RZZ may only indirectly contribute to Mad1 kinetochore localization. Although only the C-terminal 485–718 residues of Mad1 are required for in vitro MCC assembly,23 the rest of Mad1 contains a long N-terminal coiled-coil that likely spans over 80 nm in length (Figure 5a,b). The interaction of Mad1 to cyclin B1 localized at the outer corona might therefore directly scaffold the SAC.184,185
Ultimately, the specific role other pathways play in Mad1 kinetochore recruitment for MCC assembly remains elusive and further investigation is required. It will be particularly difficult to determine if these pathways are independent or synergistic with Bub1-mediated Mad1 kinetochore recruitment. Clarifying pathways to Mad1 kinetochore recruitment has been complicated by recent reports that Bub1 is not essential to SAC signaling and that Mad1:C-Mad2 recruitment does not require Bub1.173,186,187 However, further efforts clarified that residual Bub1 from incomplete depletions using RNAi or CRISPR-Cas9 was sufficient to catalyze MCC formation and support SAC signaling.110,188,189 Because Bub1 (and the Mad1:C-Mad2 it recruits) work catalytically in MCC assembly, incomplete depletion may remain compatible with checkpoint function under many conditions.
4.5. Cdc20 targeting
In C. elegans, Cdc20 recruitment is solely dependent upon the ABBA motif of Bub1.190 In humans, the exact recruitment pathway of Cdc20 remains unclear. Bub1 and BubR1 contain multiple different Cdc20 binding motifs, several of which are required for SAC signaling and contribute to Cdc20 kinetochore localization.67,148,152,191,192 It seems likely that the Bub1 ABBA motif, which immediately precedes its KEN1 motif (Figure 2), is the critical kinetochore receptor of the specific pool of Cdc20 for MCC formation, after which it facilitates transfer to BubR1 during catalytic MCC formation.5,67,152,181 Removal of the Bub1 ABBA motif has a dramatic effect on SAC signaling, whereas removing KEN1/2 and the Bub1 kinase domain does not.67,152,181 The KEN1 motif of Bub1 is the predominant recognition site for Bub1 ubiquitin-mediated degradation by the APC/C.193 KEN2, which is directly N-terminal to the start of the kinase domain of Bub1, mainly functions in the recruitment of Cdc20 for Bub1-mediated phosphorylation.194–196 Ultimately, clarifying the kinetochore recruitment pathways of Cdc20, as well as BubR1 and Mad1 remains an outstanding challenge.
5. Molecular Architecture Of The Catalytic Scaffold
The catalytic scaffold for Cdc20:C-Mad2 formation is formed from Bub1 and Mad1:C-Mad2. Recent advances have provided the first structural model of the entire assembly, which allows visualizing how the catalytic scaffold would poise Cdc20 and Mad2 for their association (Figure 8).49
Figure 8. The molecular basis for catalytic C-Mad2:Cdc20 formation by the Bub1:Mad1 scaffold.
(a) Model of pBub1:Cdc20:pMad1:C-Mad2:O-Mad2 complex (the mitotic checkpoint complex [MCC]-assembly scaffold) posed for catalytic C-Mad2:Cdc20 formation.49 The model was generated using PDBs 1GO4, 2V64, 6TLJ, 7B1F,44,45,84,85 and the AlphaFold293,94 models of Bub1448–534:Mad1CTD:Cdc20 and the folded model of Mad1485–718 and are displayed using ChimeraX. Residues for the Mad1 pThr716 and Cdc20 Box1, Cdc20 α1 Asp9 and Mad1 Lys619, Mad1 Arg617 and Bub1 pThr461 interactions are depicted as sticks, as well as the Bub1 ABBA interaction with the Cdc20 WD40 domain. Key side-chain interactions are highlighted in the three boxes to the right of the model. (b) A schematic of catalytic MCC formation by the MCC-assembly scaffold. The doubly phosphorylated Bub1 CD1 domain targets the Mad1:C-Mad2 complex to kinetochores.
Phosphorylation of the C-terminus of Mad1 at Thr716 promotes its interaction with the N-terminus of Cdc20. Interaction between the WD40 domain of Cdc20 and the ABBA/KEN1 motif of Bub1 also occurs and this likely promotes Cdc20 kinetochore targeting and positions Cdc20 close to Mad1:C-Mad2. The tripartite Bub1:Mad1:Cdc20 interaction together with Mad1CTD fold-over then promotes Cdc20 Mad2-interacting motif (MIM) accessibility and repositioning close to O-Mad2 to trigger spontaneous C-Mad2:Cdc20 association. The Cdc20:C-Mad2 complex then rapidly binds BubR1:Bub3 to generate the MCC.
5.1. The Mad1:C-Mad2 platform
Tetrameric Mad1:C-Mad2 forms the base of the catalytic scaffold (Figure 8a). The coiled-coil of Mad1485–718 is first interrupted by the MIM (residues 530–550) which entraps one molecule of C-Mad2 per subunit (Figures 5c and 8a).85 O-Mad2 can then self-dimerize with Mad1-bound C-Mad2.84,108 C-terminal to this region the coiled-coil of Mad1 is again interrupted by a flexible region (residues 585–596; termed the C-terminal hinge), which Alpha-Fold2 predicts to contain a novel short α-helix (Figures 5a-c and 8a).93 After this the coiled-coil resumes and ends in a globular head domain (Mad1CTD, residues 597–718) featuring a conserved RWD-fold (Ring, WD40, DEAD).45,153 This RWD domain consists of an antiparallel β-sheet of four β-strands, a short α-helix followed by two C-terminal α-helices (Figure 5c). The C-terminal hinge of Mad1 has recently been shown both in vitro and in vivo to facilitate a flexible fold-over of the Mad1CTD back towards Mad1-bound Mad2.49,109 A combination of cryo-EM, cross-linking mass spectrometry and Alpha-Fold2 analyses provides a model of the catalytic scaffold where Mad1:C-Mad2 is in a folded state (Figure 8).49
5.2. The Bub1:Mad1 interaction
The crystal structure of Mad1CTD bound to a phosphorylated Bub1CD1 peptide has recently been solved (Figure 8a).45 Bub1CD1 forms a single α-helix which lies diagonally across the largely hydrophobic interface of the coiled-coil stem of Mad1CTD. At the N-terminus of Bub1CD1 the pThr461 site at the start of the CD1 helix contacts the coiled-coil of one monomer and directly binds to Mad1 Arg617 of the conserved RLK motif (Figure 8a, panel 2). Specificity of this interaction is augmented by the ability of the pThr461 phosphate to stabilize the N-terminal α-helix dipole of Bub1CD1. The C-terminus of Bub1CD1 α-helix then contacts the opposite Mad1 monomer at the top of the coiled-coil and its adjoining head domain β-sheet and poses Bub1 C-terminal to the CD1 domain to extend across the top of the Mad1CTD head domain.
5.3. The Mad1:Cdc20 interaction
The N-terminus of Cdc20 (Cdc20N) interacts with Mad1CTD in a phosphorylation-dependent manner.46,47,49 Recently, it has been shown that phosphorylation of Mad1 Thr716 by Mps1, just two residues before the Mad1 C-terminus, directly modulates their interaction.49 Although no experimentally determined structure of Cdc20 bound to phosphorylated Mad1 exists, a combination of NMR, biophysics and AlphaFold2 analyses has generated a structural model of their interaction (Figure 8a).49 The N-terminal 35 residues of Cdc20 bind across Mad1CTD using two regions with residual α-helicity (α1 and Box1). Cdc20 α1 lies diagonally across the coiled-coil, mimicking the Bub1CD1:Mad1CTD interaction. With its N-terminus, α1 contacts one Mad1CTD monomer at the conserved RLK site, and with its C-terminus, it contacts the top of the coiled-coil and adjoining head domain β-sheet of the adjacent monomer (Figure 8a, panel 3). The Box1 helix then binds to the β2 strand, β2/β3 loop adjacent to pThr716 (residues 652–661), and C-terminal α-helix, and positions its positively charged Arg/Lys residues to interact with the phosphothreonine site (Figure 8a, panel 2). The loop which connects Cdc20 α1 and Box1 α-helices also interacts with the conserved QYRL (residues 648–651) motif within the first β-strand of the head domain. This loop also contains four prolines which likely provide rigidity to orientate both helices.
5.4. Tripartite assembly of Bub1 and Cdc20 on Mad1CTD
Biophysical characterization has identified that only a single copy of Bub1CD1 and a single copy of Cdc20N can bind to the Mad1CTD homodimer.45,49 This unusual stoichiometry is most likely controlled by inherent asymmetry within Mad1CTD in which the coiled-coil is bent with respect to the head domain and which makes only one side of the homodimer favorable for binding.45,49 Despite the binding sites of Bub1CD1 and Cdc20N almost entirely overlapping, fluorescent anisotropy measurements have identified that Bub1CD1 and Cdc20N bind concurrently to Mad1CTD.49 An experimentally validated AlphaFold2 model outlines how Mad1CTD can accommodate both Bub1CD1 and Cdc20N on opposite sides of the Mad1CTD homodimer (Figure 8a).49 This study also reported pre-liminary evidence that Bub1 and Cdc20 binding to Mad1CTD is not cooperative.49 This suggests that Cdc20N and Bub1CD1 likely have preferential affinity for opposite sides of the asymmetric homodimer, a process which might be conferred by the additional interaction of the Cdc20 Box1 helix to the phosphorylated head domain of Mad1CTD. Further work is required to understand how the tripartite assembly of Cdc20 and Bub1 on Mad1CTD is controlled.
5.5. The Bub1ABBA:Cdc20WD40 interaction
The ABBA motif of Bub1, binds to the C-terminal WD40 β-propeller of Cdc20, specifically between blades two and three.197 This interaction likely determines how Cdc20 is targeted and repositioned at kinetochores during MCC assembly (discussed in Section 4.5).5 The parallel orientation of Cdc20N and Bub1CD1 on Mad1CTD is thus optimally positioned to be compatible with the Bub1ABBA: Cdc20WD40 interaction (Figure 8a). Additionally, the dual anchorage of both Bub1 and Cdc20 to each other and to Mad1CTD may be important for enhancing the stable assembly of the scaffold as the interaction of Bub1CD1 and Cdc20N with Mad1CTD is relatively weak (low μM range).45,47,49,152
6. Insights Into The Molecular Basis Of Catalytic C-Mad2:Cdc20 Formation
As discussed in Section 3, a wealth of biochemical and cellular data have carefully dissected out contributors to catalytic Cdc20:C-Mad2 association. Placing these experiments in the context of the structural model for the catalytic scaffold provides what seems to be a near-complete understanding of how unattached kinetochores overcome the kinetic barrier to the C-Mad2:Cdc20 association to rapidly generate the MCC.
6.1. Tripartite assembly together with Mad1 fold-over delivers the Cdc20 MIM to O-Mad2
The structural model of the catalytic scaffold outlines the molecular mechanisms for how Bub1 and Mad1 would spatially constrain and reposition the MIM of Cdc20 near its binding site on Mad2. Key to this seems to be the ability of Mad1CTD to flexibly fold-over which in the context of tripartite Bub1:Mad1:Cdc20 assembly poises Cdc20 MIM to bind to the β6 strand of Mad2 with which it pairs as an antiparallel β-sheet in the C-Mad2:Cdc20 complex (Figures 4a and 8a).
This model is supported by several key experiments and observations. The Cdc20 MIM would be positioned too far away from O-Mad2 in the context of Cdc20 bipartite anchorage to Bub1 and Mad1.46,49,109 Decreasing the distance between Box1 and the MIM of Cdc20 impairs catalysis of C-Mad2:Cdc20 formation in vitro.109 Maintaining flexibility within the C-terminal hinge which enables fold-over is essential for catalytic MCC assembly in vitro and SAC signaling in vivo.109 The importance of coupling fold-over and the spatial constraints imposed by tripartite Bub1: Cdc20:Mad1 assembly is also highlighted in experiments that identify that catalysis induced by the C-terminal Mad1 hinge is dependent upon an intact Mad1:C-Mad2 interaction with Bub1 CD1 and Cdc20 Box1.109 Additionally, the CD1 and ABBA motifs of Bub1 individually contribute to catalyzing in vitro C-Mad2:Cdc20 formation.46
6.2. Bipartite anchorage of Cdc20 may also unveil the Cdc20 MIM
It has been reported that the N-terminus of Cdc20 interacts with its C-terminal WD40 domain,22,48 as well as self-interaction within the N-terminus of Cdc20.46 In vitro MCC assembly assays also show that truncating the N-terminus or C-terminus on either side of the Cdc20 MIM promotes the formation of C-Mad2:Cdc20.46 This has led to the suggestion that Cdc20 may exist in an autoinhibited state whereby self-interaction masks the MIM of Cdc20 and not only impairs the ability of the Cdc20 MIM from inducing Mad2 conversion but impedes the ability of the Mad2 safety-belt from being able to latch on to it.5,22,48 The structure of the catalytic scaffold suggests bipartite anchorage of Cdc20N to pMad1CTD and Cdc20WD40 to Bub1ABBA as a plausible mechanism for relieving self-interaction of Cdc20 to unveil its central MIM (Figure 8). However, whether Cdc20 self-interaction impairs MIM accessibility, and whether bipartite anchorage of Cdc20 in conjunction with Mad1CTD fold-over acts to directly relieve Cdc20 autoinhibition remains unclear and requires further investigation.
It should be noted that when a minimal kinetochore-targeting region of Bub1 (residues 1–280), which does not contain the ABBA motif, is fused to Mad1 in vivo, the checkpoint is still robust, although impaired.152 This may suggest that the interaction of Cdc20N to Mad1CTD is a critical contributor to promoting MIM accessibility. However, it is also possible that the KEN or ABBA motifs in BubR1 could bind to Cdc20 to relieve Cdc20 autoinhibition instead. Bub1 and BubR1 self-dimerize, which is likely how BubR1 is targeted to kinetochores and repositioned for MCC formation.149 It would therefore be interesting to test if Bub11–280, where the BubR1 dimerization domain is removed, could also sustain a robust checkpoint in vivo and if this leads to a further decrease in catalytic MCC assembly in vitro.
7. Concluding Remarks
We now have a structural model for the catalytic Bub1: Mad1:C-Mad2 scaffold which provides key insights into how Bub1 and Mad1:C-Mad2 target and locally concentrate O-Mad2 and Cdc20 (Figure 8). This model also demonstrates how the tripartite complex of Bub1:Mad1: Cdc20 likely relieves Cdc20 autoinhibition and orchestrates the arrangement of the MIM of Cdc20 relative to its binding site on Mad2. The catalytic scaffold centered on the Mad1 platform likely functions through a “floating-and-catch” mechanism,46,109 where dual-anchorage of Cdc20 in conjunction with flexible Mad1CTD fold-over “stretches” the N-terminus of Cdc20 to unveil and situate the Cdc20 MIM for rapid entrapment by the Mad2 safety-belt.
How much Mad1CTD undergoes fold-over from a “fully extended” state to facilitate the C-Mad2:Cdc20 interaction as compared to Mad1 being constitutively folded which then has some mobility within the C-terminal hinge has yet to be addressed. Future studies should investigate whether there are any other factors, such as phosphorylation, that promote or regulate the Mad1CTD fold-over. It is also an outstanding challenge to understand how the MIM of Cdc20 directly induces the O-Mad2 to C-Mad2 transition. Our current model suggests that the Mad1:C-Mad2 platform can perform only one catalytic cycle at a time because only one Bub1 and Cdc20 can bind at the same time (Figure 8a). This raises the question of whether both O-Mad2 reaction sites on Mad1:C-Mad2 are utilized. Because the fold-over of Mad1CTD is highly flexible it is likely that either site could be used and the presence of two O-Mad2 molecules could facilitate Cdc20 MIM entrapment. Another possibility is that remodeling of the Mad1:C-Mad2 platform might occur which could then alter the favorability of either O-Mad2 reaction site. This could be related to how Mad2 conversion is accelerated by O-Mad2 dimerized to the Mad1:C-Mad2 platform, as it has been suggested that dimerized O-Mad2 presents an intermediate state which is more susceptible to spontaneous conversion.196
In summary, our new mechanistic knowledge of how cells overcome the kinetic barrier to the Mad2:Cdc20 association to rapidly assemble the MCC now sets the stage for understanding how similar cellular control mechanisms operate. This notably includes other HORMA-domain-containing molecular switches. Further insights can also be obtained by investigating how the catalytic scaffold functions in organisms where the molecular interactions of checkpoint proteins differ. This would especially be interesting in C. elegans where Mad1 and Bub1 do not interact through the CD1 of Bub1.5,48,166
Acknowledgements
The author gratefully acknowledges the Gates Cambridge Scholarship for funding her Ph.D. at the University of Cambridge and the MRC Laboratory of Molecular Biology. The author also thanks David Barford, Conny Yu, and Stanislau Yatskevich for their critical readings of the manuscript and valuable suggestions. Alexandra Newton and the IUBMB are also thanked for honoring the author with the inaugural William Whelan Young Investigator Award and giving her the opportunity to contribute to this special issue on protein phosphorylation. And lastly, to Eddy Fischer for his timeless inspiration and support of the author's career in science.
Funding information
Gates Cambridge Scholarship; University of Cambridge
Footnotes
Conflict of Interest
The author declares that she has no conflict of interest.
References
- 1.Holland AJ, Cleveland DW. Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 2012;13:501–514. doi: 10.1038/embor.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santaguida S, Amon A. Short-and long-term effects of chromosome mis-segregation and aneuploidy. Nat Rev Mol Cell Biol. 2015;16:473–485. doi: 10.1038/nrm4025. [DOI] [PubMed] [Google Scholar]
- 3.Santaguida S, Musacchio A. The life and miracles of kineto-chores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett K, Molecular D. Mechanisms of spindle assembly checkpoint activation and silencing. Prog Mol Subcell Biol. 2017;56:429–455. doi: 10.1007/978-3-319-58592-5_18. [DOI] [PubMed] [Google Scholar]
- 5.Lara-Gonzalez P, Pines J, Desai A. Spindle assembly checkpoint activation and silencing at kinetochores. Semin Cell Dev Biol. 2021;117:86–98. doi: 10.1016/j.semcdb.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–R980. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Musacchio A. The Molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol. 2015;25:R1002–R1018. doi: 10.1016/j.cub.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 8.Vader G, Musacchio A. The greatest kinetochore show on earth. EMBO Rep. 2017;18:1473–1475. doi: 10.15252/embr.201744541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia L, Kim S, Yu H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci. 2013;38:302–311. doi: 10.1016/j.tibs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Pesenti ME, Weir JR, Musacchio A. Progress in the structural and functional characterization of kinetochores. Curr Opin Struct Biol. 2016;37:152–163. doi: 10.1016/j.sbi.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Musacchio A, Desai A. A molecular view of kinetochore assembly and function. Biology. 2017;6:5. doi: 10.3390/biology6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudakin V, Chan GKT, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao WCH, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex. Nature. 2012;484:208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- 15.Liu S-T, Zhang H. The mitotic checkpoint complex (MCC): looking back and forth after 15 years. AIMS Mol Sci. 2016;3:597–634. doi: 10.3934/molsci.2016.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, Mendez BL, Sedgwick GG, Nilsson J. Two functionally distinct kinetochore pools of BubR1 ensure accurate chromosome segregation. Nat Commun. 2016;7:1–12. doi: 10.1038/ncomms12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han JS, Vitre B, Fachinetti D, Cleveland DW. Bimodal activation of BubR1 by Bub3 sustains mitotic checkpoint signaling. Proc Natl Acad Sci USA. 2014;111:E4185–E4193. doi: 10.1073/pnas.1416277111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen RH. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J Cell Biol. 2002;158:487–496. doi: 10.1083/jcb.200204048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardwick KG, Johnston RC, Smith DL, Murray AW. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J Cell Biol. 2000;148:871–882. doi: 10.1083/jcb.148.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonetta M, et al. The influence of catalysis on Mad2 activation dynamics. PLoS Biol. 2009;7:e1000010. doi: 10.1371/journal.pbio.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16:105–117. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JS, et al. Catalytic assembly of the mitotic checkpoint inhibitor BubR1-Cdc20 by a Mad2-induced functional switch in Cdc20. Mol Cell. 2013;51:92–104. doi: 10.1016/j.molcel.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faesen AC, et al. Basis of catalytic assembly of the mitotic checkpoint complex. Nature. 2017;542:498–502. doi: 10.1038/nature21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfieri C, Zhang S, Barford D. Visualizing the complex functions and mechanisms of the anaphase promoting complex/cyclosome (APC/C) Open Biol. 2017;7:170204. doi: 10.1098/rsob.170204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfieri C, et al. Molecular basis of APC/C regulation by the spindle assembly checkpoint. Nature. 2016;536:431–436. doi: 10.1038/nature19083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, et al. Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature. 2016;533:260–264. doi: 10.1038/nature17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi M, et al. Cryo-EM of mitotic checkpoint complexbound APC/C reveals reciprocal and conformational regulation of ubiquitin ligation. Mol Cell. 2016;63:593–607. doi: 10.1016/j.molcel.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson ER, Brown NG, Peters JM, Stark H, Schulman BA. Posing the APC/C E3 ubiquitin ligase to orchestrate cell division. Trends Cell Biol. 2019;29:117–134. doi: 10.1016/j.tcb.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16:82–94. doi: 10.1038/nrm3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao R, et al. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc Natl Acad Sci USA. 2016;113:E2570–E2578. doi: 10.1073/pnas.1604929113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown NG, et al. Dual RING E3 architectures regulate multiu-biquitination and ubiquitin chain elongation by APC/C. Cell. 2016;165:1440–1453. doi: 10.1016/j.cell.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokros M, Wang Y. Spindle assembly checkpoint silencing and beyond. Cell Cycle. 2016;15:1661–1662. doi: 10.1080/15384101.2016.1176396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benzi G, Piatti S. Silencing the spindle assembly checkpoint: let's play polo. J Cell Biol. 2020;219:e202010053. doi: 10.1083/jcb.202010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesage B, Qian J, Bollen M. Spindle checkpoint silencing: PP1 tips the balance. Curr Biol. 2011;21:R898–903. doi: 10.1016/j.cub.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 35.Etemad B, et al. Spindle checkpoint silencing at kinetochores with submaximal microtubule occupancy. J Cell Sci. 2019;132 doi: 10.1242/jcs.231589. jcs231589. [DOI] [PubMed] [Google Scholar]
- 36.Vanoosthuyse V, Hardwick KG. Overcoming inhibition in the spindle checkpoint. Genes Dev. 2009;23:2799–2805. doi: 10.1101/gad.1882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kops GJPL, Shah J. Connecting up and clearing out: how kinetochore attachment silences the spindle assembly check point. Chromosoma. 2012;121:509–525. doi: 10.1007/s00412-012-0378-5. [DOI] [PubMed] [Google Scholar]
- 39.Brulotte ML, et al. Mechanistic insight into TRIP13-catalyzed Mad2 structural transition and spindle checkpoint silencing. Nat Commun. 2017;8:1956. doi: 10.1038/s41467-017-02012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagan RS, et al. P31 comet acts to ensure timely spindle checkpoint silencing subsequent to kinetochore attachment. Mol Biol Cell. 2011;22:4236–4246. doi: 10.1091/mbc.E11-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espeut J, Cheerambathur DK, Krenning L, Oegema K, Desai A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J Cell Biol. 2012;196:469–482. doi: 10.1083/jcb.201111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177:1005–1015. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquiti-nation by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 44.Alfieri C, Tischer T, Barford D. A unique binding mode of Nek2A to the APC/C allows its ubiquitination during prometaphase. EMBO Rep. 2020;21:e49831. doi: 10.15252/embr.201949831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer ES, et al. Molecular mechanism of Mad1 kinetochore targeting by phosphorylated Bub1. EMBO Rep. 2021;22:e52242. doi: 10.15252/embr.202052242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piano V, et al. CDC20 assists its catalytic incorporation in the mitotic checkpoint complex. Science. 2021;371:67–71. doi: 10.1126/science.abc1152. [DOI] [PubMed] [Google Scholar]
- 47.Ji Z, Gao H, Jia L, Li B, Yu H. A sequential multi-target Mps1 phosphorylation cascade promotes spindle checkpoint signaling. Elife. 2017;6:e22513. doi: 10.7554/eLife.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara-Gonzalez P, Kim T, Oegema K, Corbett K, Desai A. A tripartite mechanism catalyzes Mad2-Cdc20 assembly at unattached kinetochores. Science. 2021;371:64–67. doi: 10.1126/science.abc1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer ES, et al. Juxtaposition of Bub1 and Cdc20 on phosphorylated Mad1 during catalytic mitotic checkpoint complex assembly. Nat Commun. 2022;13:1–18. doi: 10.1038/s41467-022-34058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardwick KG. Checkpoint signalling: Mad2 conformers and signal propagation. Curr Biol. 2005;15:R122–R124. doi: 10.1016/j.cub.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Skinner JJ, Wood S, Shorter J, Englander SW, Black BE. The Mad2 partial unfolding model: regulating mitosis through Mad2 conformational switching. J Cell Biol. 2008;183:761–768. doi: 10.1083/jcb.200808122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo X, Yu H. Protein metamorphosis: the two-state behavior of Mad2. Structure. 2008;16:1616–1625. doi: 10.1016/j.str.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo X, et al. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat Struct Mol Biol. 2004;11:338–345. doi: 10.1038/nsmb748. [DOI] [PubMed] [Google Scholar]
- 54.Yu H. Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J Cell Biol. 2006;173:153–157. doi: 10.1083/jcb.200601172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian W, et al. Structural analysis of human Cdc20 supports multisite degron recognition by APC/C. Proc Natl Acad Sci USA. 2012;109:18419–18424. doi: 10.1073/pnas.1213438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen NA, Harrison SC. Crystal structure of the spindle assembly checkpoint protein Bub3. J Mol Biol. 2004;344:885–892. doi: 10.1016/j.jmb.2004.09.094. [DOI] [PubMed] [Google Scholar]
- 57.Larsen NA, Al-Bassam J, Wei RR, Harrison SC. Structural analysis of Bub3 interactions in the mitotic spindle check-point. Proc Natl Acad Sci USA. 2007;104:1201–1206. doi: 10.1073/pnas.0610358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overlack K, et al. BubR1 promotes Bub3-dependent APC/C inhibition during spindle assembly checkpoint signaling. Curr Biol. 2017;27:2915–2927.:e7. doi: 10.1016/j.cub.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sczaniecka M, et al. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C) J Biol Chem. 2008;283:23039–23047. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King RW, et al. A 20 s complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 61.Chang L, Barford D. Insights into the anaphase-promoting complex: a molecular machine that regulates mitosis. Curr Opin Struct Biol. 2014;29:1–9. doi: 10.1016/j.sbi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature. 2014;513:388–393. doi: 10.1038/nature13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z, He M, Shah AA, Wan Y. Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div. 2016;11:9. doi: 10.1186/s13008-016-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 66.Pfleger CM, Lee E, Kirschner MW. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 2001;15:2396–2407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiFiore B, et al. The ABBA motif binds APC/C activators and is shared by APC/C substrates and regulators. Dev Cell. 2015;32:358–372. doi: 10.1016/j.devcel.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He J, et al. Insights into degron recognition by APC/C coactivators from the structure of an Acm1-Cdh1 complex. Mol Cell. 2013;50:649. doi: 10.1016/j.molcel.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herzog F, et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science (1979) 2009;323:1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izawa D, Pines J. Mad2 and the APC/C compete for the same site on Cdc20 to ensure proper chromosome segregation. J Cell Biol. 2012;199:27–37. doi: 10.1083/jcb.201205170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izawa D, Pines J. The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C. Nature. 2015;517:631–634. doi: 10.1038/nature13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- 73.Hwang LH, et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 74.Lara-Gonzalez P, Scott MIF, Diez M, Sen O, Taylor SS. Bubr1 blocks substrate recruitment to the APC/C in a KEN-box-dependent manner. J Cell Sci. 2011;124:4332–4345. doi: 10.1242/jcs.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sewart K, Hauf S. Different functionality of Cdc20 binding sites within the mitotic checkpoint complex. Curr Biol. 2017;27:1213–1220. doi: 10.1016/j.cub.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Tromer E, Bade D, Snel B, Kops GJPL. Phylogenomics-guided discovery of a novel conserved cassette of short linear motifs in BubR1 essential for the spindle checkpoint. Open Biol. 2016;6:160315. doi: 10.1098/rsob.160315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davey NE, Morgan DO. Building a regulatory network with short linear sequence motifs: lessons from the degrons of the anaphase-promoting complex. Mol Cell. 2016;64:12–23. doi: 10.1016/j.molcel.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.di Fiore B, Wurzenberger C, Davey NE, Pines J. The mitotic checkpoint complex requires an evolutionary conserved cassette to bind and inhibit active APC/C. Mol Cell. 2016;64:1144–1153. doi: 10.1016/j.molcel.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lad L, Lichtsteiner S, Hartman JJ, Wood KW, Sakowicz R. Kinetic analysis of Mad2-Cdc20 formation: conformational changes in Mad2 are catalyzed by a C-Mad2-ligand complex. Biochemistry. 2009;48:9503–9515. doi: 10.1021/bi900718e. [DOI] [PubMed] [Google Scholar]
- 80.Luo X, et al. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat Struct Biol. 2000;7:224–229. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- 81.Hara M, Özkan E, Sun H, Yu H, Luo X. Structure of an intermediate conformer of the spindle checkpoint protein Mad2. Proc Natl Acad Sci USA. 2015;112:11252–11257. doi: 10.1073/pnas.1512197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang M, et al. Insights into Mad2 regulation in the spindle checkpoint revealed by the crystal structure of the symmetric Mad2 dimer. PLoS Biol. 2008;6:643–655. doi: 10.1371/journal.pbio.0060050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo X, Tang Z, Rizo J, Yu H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol Cell. 2002;9:59–71. doi: 10.1016/s1097-2765(01)00435-x. [DOI] [PubMed] [Google Scholar]
- 84.Mapelli M, Massimiliano L, Santaguida S, Musacchio A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell. 2007;131:730–743. doi: 10.1016/j.cell.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 85.Sironi L, et al. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J. 2002;21:2496–2506. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mapelli M, et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 2006;25:1273–1284. doi: 10.1038/sj.emboj.7601033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y, et al. Stable folding intermediates prevent fast interconversion between the closed and open states of Mad2 through its denatured state. Protein Engineering, Design and Selection. 2015;29:23–29. doi: 10.1093/protein/gzv056. [DOI] [PubMed] [Google Scholar]
- 88.Dick AE, Gerlich DW. Kinetic framework of spindle assembly checkpoint signalling. Nat Cell Biol. 2013;15:1370–1377. doi: 10.1038/ncb2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 90.Hardwick KG, Murray AW. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo Y, Ahmad E, Liu ST. MAD1: kinetochore receptors and catalytic mechanisms. Front Cell Dev Biol. 2018;6:51. doi: 10.3389/fcell.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen RH, Brady DM, Smith D, Murray AW, Hardwick KG. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol Biol Cell. 1999;10:2607–2618. doi: 10.1091/mbc.10.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mirdita M, et al. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19:679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Antoni A, et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol. 2005;15:214–225. doi: 10.1016/j.cub.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 96.Nezi L, et al. Accumulation of Mad2-Cdc20 complex during spindle checkpoint activation requires binding of open and closed conformers of Mad2 in Saccharomyces cerevisiae. J Cell Biol. 2006;174:39–51. doi: 10.1083/jcb.200602109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung E, Chen RH. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol Biol Cell. 2002;13:1501–1511. doi: 10.1091/mbc.02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fava LL, Kaulich M, Nigg EA, Santamaria A. Probing the in vivo function of Mad1:C-Mad2 in the spindle assembly checkpoint. EMBO J. 2011;30:3322–3336. doi: 10.1038/emboj.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kruse T, et al. A direct role of Mad1 in the spindle assembly checkpoint beyond Mad2 kinetochore recruitment. EMBO Rep. 2014;15:282–290. doi: 10.1002/embr.201338101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sironi L, et al. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 2001;20:6371–6382. doi: 10.1093/emboj/20.22.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin HJ, et al. P31comet-induced cell death is mediated by binding and inactivation of Mad2. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0141523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Date DA, Burrows AC, Summers MK. Phosphorylation regulates the p31comet-mitotic arrest-deficient 2 (Mad2) interaction to promote spindle assembly checkpoint (SAC) activity. J Biol Chem. 2014;289:11367–11373. doi: 10.1074/jbc.M113.520841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang M, et al. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131:744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westhorpe FG, Tighe A, Lara-Gonzalez P, Taylor SS. p31 comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci. 2011;124:3905–3916. doi: 10.1242/jcs.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji W, Luo Y, Ahmad E, Liu ST. Direct interactions of mitotic arrest deficient 1 (MAD1) domains with each other and MAD2 conformers are required for mitotic checkpoint signaling. J BiolChem. 2018;293:484–496. doi: 10.1074/jbc.RA117.000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim DH, et al. TRIP13 and APC15 drive mitotic exit by turnover of interphase-and unattached kinetochore-produced MCC. NatCommun. 2018;9:1–11. doi: 10.1038/s41467-018-06774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma HT, Poon RYC. TRIP13 functions in the establishment of the spindle assembly checkpoint by replenishing O-MAD2. Cell Rep. 2018;22:1439–1450. doi: 10.1016/j.celrep.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 108.Mapelli M, Musacchio A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr Opin Struct Biol. 2007;17:716–725. doi: 10.1016/j.sbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 109.Chen C, et al. The structural flexibility of MAD1 facilitates the assembly of the mitotic checkpoint complex. bioRxiv. 2022 doi: 10.1101/2022.498198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Rodriguez JA, et al. Distinct roles of RZZ and Bub1-KNL1 in mitotic checkpoint signaling and kinetochore expansion. Curr Biol. 2018;28:3422–3429.:e5. doi: 10.1016/j.cub.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heinrich S, et al. Mad1 contribution to spindle assembly checkpoint signalling goes beyond presenting Mad2 at kineto-chores. EMBO Rep. 2014;15:291–298. doi: 10.1002/embr.201338114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saurin AT. Kinase and Gphosphatase cross-talk at the kineto chore. Front Cell Dev Biol. 2018;6:62. doi: 10.3389/fcell.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gelens L, Qian J, Bollen M, Saurin AT. The importance of kinase-phosphatase integration: lessons from mitosis. Trends Cell Biol. 2018;28:6–21. doi: 10.1016/j.tcb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Cordeiro MH, Smith RJ, Saurin AT. A fine balancing act: a delicate kinase-phosphatase equilibrium that protects against chromosomal instability and cancer. Int J Biochem Cell Biol. 2018;96:148–156. doi: 10.1016/j.biocel.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abrieu A, et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 117.Tighe A, Staples O, Taylor S. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J Cell Biol. 2008;181:893–901. doi: 10.1083/jcb.200712028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- 119.Pachis ST, Kops GJPL. Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol. 2018;8:180109. doi: 10.1098/rsob.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cunha-Silva S, et al. Mps1-mediated release of Mad1 from nuclear pores ensures the fidelity of chromosome segregation. J Cell Biol. 2020;219:e201906039. doi: 10.1083/jcb.201906039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jackman M, et al. Cyclin B1-Cdk1 facilitates MAD1 release from the nuclear pore to ensure a robust spindle checkpoint. J Cell Biol. 2020;219:e201907082. doi: 10.1083/jcb.201907082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jelluma N, Dansen TB, Sliedrecht T, Kwiatkowski NP, Kops GJPL. Release of Mps1 from kinetochores is crucial for timely anaphase onset. J Cell Biol. 2010;191:281–290. doi: 10.1083/jcb.201003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maciejowski J, et al. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190:89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sliedrecht T, Zhang C, Shokat KM, Kops GJPL. Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One. 2010;5:e10251. doi: 10.1371/journal.pone.0010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nijenhuis W, et al. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol. 2013;201:217–231. doi: 10.1083/jcb.201210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stucke VM, Baumann C, Nigg EA. Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma. 2004;113:1–15. doi: 10.1007/s00412-004-0288-2. [DOI] [PubMed] [Google Scholar]
- 127.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 128.Kemmler S, et al. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 2009;28:1099. doi: 10.1038/emboj.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wigge PA, Kilmartin Jv. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol. 2001;152:349. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Janke C, et al. The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 2001;20:777. doi: 10.1093/emboj/20.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ciferri C, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pesenti ME, et al. Reconstitution of a 26-subunit human kinetochore reveals cooperative microtubule binding by CENP-OPQUR and NDC80. Mol Cell. 2018;71:923–939.:e10. doi: 10.1016/j.molcel.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Combes G, et al. Mps1 phosphorylates its N-terminal extension to relieve autoinhibition and activate the spindle assembly checkpoint. Curr Biol. 2018;28:872–883.:e5. doi: 10.1016/j.cub.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hiruma Y, et al. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science. 2015;348:1264–1267. doi: 10.1126/science.aaa4055. [DOI] [PubMed] [Google Scholar]
- 135.Ji Z, Gao H, Yu H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science. 2015;348:1260–1264. doi: 10.1126/science.aaa4029. [DOI] [PubMed] [Google Scholar]
- 136.Dou Z, et al. Recent Progress on the localization of the spindle assembly checkpoint machinery to kinetochores. Cell. 2019;8:278. doi: 10.3390/cells8030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hayward D, Roberts E, Gruneberg U, William S. MPS1 localizes to microtubule-attached kinetochores and actively promotes microtubule release. bioRxiv. doi: 10.1101/2022.493048. [DOI] [PubMed] [Google Scholar]
- 138.Santaguida S, Tighe A, D'Alise AM, Taylor SS, Musacchio A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol. 2010;190:73–87. doi: 10.1083/jcb.201001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saurin AT, van der Waal MS, Medema RH, Lens SMA, Kops GJPL. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun. 2011;2:1–9. doi: 10.1038/ncomms1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu T, et al. Phosphorylation of microtubule-binding protein hec1 by mitotic kinase aurora b specifies spindle checkpoint kinase mps1 signaling at the kinetochore. J Biol Chem. 2013;288:36149–36159. doi: 10.1074/jbc.M113.507970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.London N, Biggins S. Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol. 2014;15:735–747. doi: 10.1038/nrm3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 144.Zhang G, Lischetti T, Nilsson J. A minimal number of MELT repeats supports all the functions of KNL1 in chromosome segregation. J Cell Sci. 2014;127:871–884. doi: 10.1242/jcs.139725. [DOI] [PubMed] [Google Scholar]
- 145.Vleugel M, et al. Arrayed BUB recruitment modules in the kinetochore scaffold KNL1 promote accurate chromosome segregation. J Cell Biol. 2013;203:943–955. doi: 10.1083/jcb.201307016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vleugel M, et al. Sequential multisite Phospho-regulation of KNL1-BUB3 interfaces at mitotic kinetochores. Mol Cell. 2015;57:824–835. doi: 10.1016/j.molcel.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 147.Vleugel M, Hoogendoorn E, Snel B, Kops GJPL. Evolution and function of the mitotic checkpoint. Dev Cell. 2012;23:239–250. doi: 10.1016/j.devcel.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 148.Vleugel M, et al. Dissecting the roles of human BUB1 in the spindle assembly checkpoint. J Cell Sci. 2015;128:2975–2982. doi: 10.1242/jcs.169821. [DOI] [PubMed] [Google Scholar]
- 149.Overlack K, et al. A molecular basis for the differential roles of Bubl and BubR1 in the spindle assembly checkpoint. Elife. 2015;2015:e05269. doi: 10.7554/eLife.05269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Primorac I, et al. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife. 2013;2013:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.London N, Biggins S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 2014;28:140–152. doi: 10.1101/gad.233700.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang G, et al. Bub1 positions Mad1 close to KNL1 MELT repeats to promote checkpoint signalling. Nat Commun. 2017;8:15822. doi: 10.1038/ncomms15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim S, Sun H, Tomchick DR, Yu H, Luo X. Structure of human Mad1 C-terminal domain reveals its involvement in kinetochore targeting. Proc Natl Acad Sci USA. 2012;109:6549–6554. doi: 10.1073/pnas.1118210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shepperd LA, et al. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tromer E, Snel B, Kops GJPL. Widespread recurrent patterns of rapid repeat evolution in the kinetochore scaffold KNL1. Genome Biol Evol. 2015;7:2383–2393. doi: 10.1093/gbe/evv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bollen M. Kinetochore signalling: the KIss that MELTs Knl1. Curr Biol. 2014;24:R68–R70. doi: 10.1016/j.cub.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 157.Kiyomitsu T, Obuse C, Yanagida M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell. 2007;13:663–676. doi: 10.1016/j.devcel.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 158.Bolanos-Garcia VM, Blundell TL. BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem Sci. 2011;36:141–150. doi: 10.1016/j.tibs.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krenn V, Overlack K, Primorac I, van Gerwen S, Musacchio A. KI motifs of human Knl1 enhance assembly of comprehensive spindle checkpoint complexes around MELT repeats. Curr Biol. 2014;24:29–39. doi: 10.1016/j.cub.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 160.Collin P, Nashchekina O, Walker R, Pines J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat Cell Biol. 2013;15:1378–1385. doi: 10.1038/ncb2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang X, et al. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J Biol Chem. 2001;276:26559–26567. doi: 10.1074/jbc.M101083200. [DOI] [PubMed] [Google Scholar]
- 162.Leontiou I, et al. The Bub1-TPR domain interacts directly with Mad3 to generate robust spindle checkpoint arrest. Curr Biol. 2019;29:2407–2414.:e7. doi: 10.1016/j.cub.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen RH, Shevchenko A, Mann M, Murray AW. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol. 1998;143:283–295. doi: 10.1083/jcb.143.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maldonado M, Kapoor TM. Constitutive Mad1 targeting to kinetochores uncouples checkpoint signalling from chromosome biorientation. Nat Cell Biol. 2011;13:475–483. doi: 10.1038/ncb2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuijt TEF, Omerzu M, Saurin AT, Kops GJPL. Conditional targeting of MAD1 to kinetochores is sufficient to reactivate the spindle assembly checkpoint in metaphase. Chromosoma. 2014;123:471–480. doi: 10.1007/s00412-014-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moyle MW, et al. A Bub1-Mad1 interaction targets the Mad1-Mad2 complex to unattached kinetochores to initiatethe spindle checkpoint. J Cell Biol. 2014;204:647–657. doi: 10.1083/jcb.201311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buffin E, Lefebvre C, Huang J, Gagou ME, Karess RE. Recruitment of Mad2 to the kinetochore requires the rod/-Zw10 complex. Curr Biol. 2005;15:856–861. doi: 10.1016/j.cub.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 168.Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Menant A, Karess RE. Mutations in the Drosophila rough deal gene affecting RZZ kinetochore function. Biol Cell. 2020;112:300–315. doi: 10.1111/boc.201900105. [DOI] [PubMed] [Google Scholar]
- 170.Défachelles L, Hainline SG, Menant A, Lee LA, Karess RE. A maternal effect rough deal mutation suggests that multiple pathways regulate drosophila RZZ kinetochore recruitment. J Cell Sci. 2015;128:1204–1216. doi: 10.1242/jcs.165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Défachelles L, et al. RZZ and Mad1 dynamics in Drosophila mitosis. Chromosome Res. 2015;23:333–342. doi: 10.1007/s10577-015-9472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calda Gv, et al. The RZZ complex requires the N-terminus of KNL1 to mediate optimal Mad1 kinetochore localization in human cells. Open Biol. 2015;5:150160. doi: 10.1098/rsob.150160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silio V, McAinsh AD, Millar JB. KNL1-bubs and RZZ provide two separable pathways for checkpoint activation at human kinetochores. Dev Cell. 2015;35:600–613. doi: 10.1016/j.devcel.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 174.Zhang G, et al. Efficient mitotic checkpoint signaling depends on integrated activities of Bub1 and the RZZ complex. EMBO J. 2019;38:e100977. doi: 10.15252/embj.2018100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suzuki A, Varma D. Cell division: the unattached kinetochore wears an expansive RZZ coat. Curr Biol. 2018;28:R1250–R1252. doi: 10.1016/j.cub.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 176.Kops GJPL, Gassmann R. Crowning the kinetochore: the fibrous Corona in chromosome segregation. Trends Cell Biol. 2020;30:653–667. doi: 10.1016/j.tcb.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 177.Sivaram MVS, Wadzinski TL, Redick SD, Manna T, Doxsey SJ. Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. EMBO J. 2009;28:902–914. doi: 10.1038/emboj.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barisic M, Geley S. Cell cycle spindly switch controls anaphase: spindly and RZZ functions in chromosome attachment and mitotic checkpoint control. 2011 doi: 10.4161/cc.10.3.14759. [DOI] [PubMed] [Google Scholar]
- 179.Raisch T, et al. Structure of the RZZ complex and molecular basis of spindly-driven corona assembly at human kineto-chores. EMBO J. 2022;41:e110411. doi: 10.15252/embj.2021110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maiato H, Gomes AM, Sousa F, Barisic M. Mechanisms of chromosome congression during mitosis. Biology. 2017;6:13. doi: 10.3390/biology6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang G, Lischetti T, Hayward DG, Nilsson J. Distinct domains in Bub1 localize RZZ and BubR1 to kinetochores to regulate the checkpoint. Nat Commun. 2015;6:7162. doi: 10.1038/ncomms8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holland AJ, et al. Preventing farnesylation of the dynein adaptor Spindly contributes to the mitotic defects caused by farne-syltransferase inhibitors. Mol Biol Cell. 2015;26:1845–1856. doi: 10.1091/mbc.E14-11-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yamamoto TG, Watanabe S, Essex A, Kitagawa R. Spdl-1 functions as a kinetochore receptor for MDF-1 in Caenorhab-ditis elegans. J Cell Biol. 2008;183:187–194. doi: 10.1083/jcb.200805185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allan LA, et al. Cyclin B1 scaffolds MAD1 at the kinetochore corona to activate the mitotic checkpoint. EMBO J. 2020;39:e103180. doi: 10.15252/embj.2019103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Conde C, Gassmann R. Spindle checkpoint: trapped by the corona, cyclin B1 goes MAD. EMBO J. 2020;39:1–3. doi: 10.15252/embj.2020105279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Currie CE, Mora-Santos M, Smith CA, McAinsh AD, Millar JBA. Bub1 is not essential for the checkpoint response to unattached kinetochores in diploid human cells. Curr Biol. 2018;28:R929–R930. doi: 10.1016/j.cub.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 187.Raaijmakers JA, Medema RH. Killing a zombie: a full deletion of the BUB1 gene in HAP1 cells. EMBO J. 2019;38:e102423. doi: 10.15252/embj.2019102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang G, et al. Response to Raaijmakers & Medema. EMBO J. 2019;38:e103547. doi: 10.15252/embj.2019103547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Meraldi P. Bub1—The zombie protein that CRISPR cannot kill. EMBO J. 2019;38:e101912. doi: 10.15252/embj.2019101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim T, et al. Kinetochores accelerate or delay APC/C activation by directing Cdc20 to opposing fates. Genes Dev. 2017;31:1089–1094. doi: 10.1101/gad.302067.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lischetti T, Zhang G, Sedgwick GG, Bolanos-Garcia VM, Nilsson J. The internal Cdc20 binding site in BubR1 facilitates both spindle assembly checkpoint signalling and silencing. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms6563. [DOI] [PubMed] [Google Scholar]
- 192.Diaz-Martinez LA, et al. The Cdc20-binding Phe box of the spindle checkpoint protein BubR1 maintains the mitotic checkpoint complex during mitosis. J Biol Chem. 2015;290:2431–2443. doi: 10.1074/jbc.M114.616490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qi W, Yu H. KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J Biol Chem. 2007;282:3672–3679. doi: 10.1074/jbc.M609376200. [DOI] [PubMed] [Google Scholar]
- 194.Jia L, Li B, Yu H. The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat Commun. 2016;7:10818. doi: 10.1038/ncomms10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 196.Kang J, Yang M, Li B, et al. Structure and substrate recruitment of the human spindle checkpoint kinase Bub1. Mol Cell. 2008;32:394–405. doi: 10.1016/j.molcel.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 198.Pettersen EF, Goddard TD, Huang CC, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lupas A, van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]