Abstract
Background
Pulmonary arterial hypertension (PAH) is a rare disease characterised by remodelling of the pulmonary arteries, increased vascular resistance and right heart failure. Genome-wide association studies (GWAS) of idiopathic/heritable PAH established novel genetic risk variants including conserved enhancers upstream of transcription factor (TF) SOX17 containing two independent signals. SOX17 is an important transcription factor in embryonic development and in the homeostasis of pulmonary artery endothelial cells (hPAEC) in the adult. Rare pathogenic mutations in SOX17 cause heritable PAH. We hypothesised that PAH risk alleles in an enhancer region impair TF-binding upstream of SOX17, which in turn reduces SOX17 expression and contributes to disturbed endothelial cell function and PAH development.
Methods
CRISPR manipulation and small interfering RNA were used to modulate SOX17 expression. Electromobility shift assays (EMSA) were used to confirm in-silico-predicted TF differential binding to the SOX17 variants. Functional assays in hPAEC were used to establish the biological consequences of SOX17 loss. In-silico analysis using the connectivity map (CMap) were used to predict compounds that rescue disturbed SOX17 signalling. Mice with deletion of the SOX17 signal 1 enhancer region (SOX17-4593/enhKO) were phenotyped in response to chronic hypoxia and SU5416/hypoxia.
Results
CRISPR-Inhibition of SOX17-signal 2 and deletion of SOX17-signal 1 specifically decreased SOX17 expression. EMSA demonstrated differential binding of hPAEC nuclear proteins to the risk and non-risk alleles from both SOX17 signals. Candidate TFs HOXA5 and ROR-α were identified through in silico analysis and antibody EMSA. Analysis of the hPAEC transcriptomes revealed alteration of PAH-relevant pathways upon SOX17 silencing, including extracellular matrix regulation. SOX17 silencing in hPAEC resulted in increased apoptosis, proliferation, and disturbance of barrier function. Using CMap, compounds were identified that reversed the SOX17-dysfunction transcriptomic signatures in hPAECs. SOX17 enhancer knockout in mice reduced lung SOX17 expression, resulting in more severe pulmonary vascular leak and hypoxia or SU5416/hypoxia-induced pulmonary hypertension.
Conclusions
Common PAH risk variants upstream of the SOX17 promoter reduce endothelial SOX17 expression, at least in part, through differential binding of HOXA5 and ROR-α. Reduced SOX17 expression results in disturbed hPAEC function and PAH. Existing drug compounds can reverse the disturbed SOX17 pulmonary endothelial transcriptomic signature.
Keywords: Pulmonary Hypertension, PH, SOX17
Introduction
Pulmonary arterial hypertension (PAH) is a rare but lethal disease. With no intervention, the mean survival is 2.8 years (1) and with modern therapeutic intervention, the rate of mortality in the first year is around 15% (2). Increased pulmonary vascular resistance in PAH is driven by vasoconstriction, inflammation, and proliferative remodelling of the intima and media of precapillary arteries (3,4). The endothelium of healthy pulmonary arteries forms a semi-permeable barrier, which dynamically adapts to external stimuli such as shear stress or hypoxia. Injury or dysfunction of the endothelium is thought to be an early, yet poorly understood, trigger in PAH development. While genetic factors enhance susceptibility (e.g. bone morphogenetic protein receptor 2, BMPR2 variants), environmental factors like hypoxia, change in shear stress, inflammation, drugs or toxins can directly injure the endothelial barrier, leading to apoptosis, loss of barrier integrity and vascular remodelling of the pulmonary artery wall (5).
Rare pathogenic variants in several genes, most commonly BMPR2, are associated with PAH (6), but ˜75% of idiopathic cases cannot be explained by these variants. A recent large genome-wide association study (GWAS), using data from 11,744 European individuals (2,085 patients) identified two independent PAH risk variant-containing signals (SOX17-signal 1 and SOX17-signal 2) in a region located 106-200kb upstream of the SOX17 gene promoter. The risk alleles are common in the populations tested and enriched in PAH, with 59% of patients homozygous for the risk allele of both signals compared to 46% of controls (7). In addition, whole genome sequencing studies identified rare deleterious variants in the SOX17 gene associated with the development of severe PAH. Therefore, SOX17 has the potential to provide a powerful insight into PAH risk via rare and common variants.
The SOX17 gene encodes the transcription factor, SOX17, which is a member of the SoxF protein subfamily. SoxF proteins are important regulators of cell fate and differentiation (8) and have key roles in cardiovascular development (9). SOX17 is essential for developmental angiogenesis and arterial differentiation in the embryo. In the adult, SOX17 plays a role in maintaining arterial identity and tumor angiogenesis (10,11). EC deletion of SOX17 in mouse models leads to embryonic lethality due to underdeveloped arteries and a complete lack of large arteries (10). Conditional deletion of SOX17 in splanchnic mesenchyme-derivatives leads to severe vascular abnormalities, including reduced branching of pulmonary arties and dilated cardiomyopathy (12). Thus far, the role of SOX17 in the human pulmonary arterial endothelium remains unclear. In addition, it remains unclear how upstream common variants increase the risk of PAH. Variation in SOX17-signal 1 has been shown to affect SOX17 expression, but the cellular and in vivo function of this element is still poorly understood (7); clear function has not been defined for SOX17-signal 2 region to date. We hypothesised the PAH variants upstream of SOX17 drive allele-specific transcription factor (TF) binding at the two signals which affects SOX17 expression, hPAEC function and PAH development.
Methods
For full methods and materials please see the supplemental section. We have deposited the RNAseq data to GEO with accession number GSE214742. All other data are available upon reasonable request.
Patient endothelial cells
Individuals with a diagnosis of idiopathic PAH (n=11) diagnosed according to international guidelines (13) and healthy controls who did not self-report cardiovascular or respiratory conditions (n=5) were recruited between 23/Aug/2017 and 18/Sep/2019 from the National Pulmonary Hypertension service and staff at Hammersmith Hospital for derivation of endothelial colony forming cells (ECFC) from blood. Samples were obtained with written, informed consent and local research ethics committee approval. ECFC were isolated and cultured as previously described: (14) and extracted for RNA following growth in 2% FBS-supplemented EGM-2 media plus indicated treatments or vehicle for 24 hours.
CRISPR-Manipulation
To inhibit SOX17-signal 2 and -signal 1, CRISPR−Inhibition (CRISPR-I) was used as previously described (7). To delete SOX17-signal 1, CRISPR-Deletion (CRISPR-D) was used. Two guides (Table S1) against SOX17-signal 1 were cloned into a pSpCas9(BB)-T2A-HygR vector (#118153) using a one-step dual CRISPR/Cas9 guide RNA cloning protocol as previously described (15).
EMSA & Supershift
To investigate transcription factors (TF) whose binding may be affected due to the SOX17 variants, using TF databases CIS−BP, PROMO and ConSite were used. To investigate the differential binding of TFs to the risk and non-risk allele present at the SOX17 variants, an EMSA was performed with the LightShift (chemiluminescent) EMSA kit according to manufacturer’s instructions. To investigate which transcription factor binds to the SOX17 variants, supershift assays with anti-HOXa5/RORa antibodies were performed. Five hPAEC donors were also used for chromatin immunoprecipitation (ChIP) qPCR assays with the same antibodies.
siRNA
To assess the role of SOX17 in hPAECs, siRNA experiments were performed using silencer select siRNA (ThermoFisher) targeting SOX17 (#s34626). Comparable control siRNA was also used and included two scrambled-siRNA (#ASO2FOQH, #4390847) and a control targeting GAPDH (#ASO2FLIC).
RNAseq Analysis
To assess whole transcriptomic effects of knockdown of SOX17 by siRNA and CRISPR-I SOX17-signal 2 and -signal 1, RNA sequencing (RNAseq) was carried out by the Imperial College BRC Genomics Facility and analysis of the dataset was performed in RStudio. To assess gene ontology changes resulting from either siRNA-SOX17, CRISPR-I SOX17-signal 2 or signal 1, over-representation analysis was performed using the WEB-based Gene SeTAnaLysis Toolkit (Webgestalt).
qPCR
To investigate the change in expression of target genes, reverse transcription-PCR (RTPCR) and qPCR was performed with actin beta (ACTβ) used as a reference gene (2-deltaCt).
Western Blotting
To assess the levels of SOX17 protein following siRNA transduction, total protein was extracted from cells using RIPA buffer (10X, Sigma) supplemented with protease and phosphatase inhibitor cocktail (ThermoFisher) and immunoblotted with anti-SOX17 (ab224637, Abcam, 1:500).
Proteomic analysis
SomaLogic SomaScan measurements were available from a recent proteomics study by Rhodes et al (16) Patient characteristics are shown in Table S2. Peripheral venous blood was collected during patients’ routine clinical appointments.
The SNP genotypes for SOX17 signal 1 (rs13266183) were obtained from a whole genome sequencing study from the UK National Institute for Health Research BioResource (7). Linear regression models were conducted with SOX17 signal 1 genotypes being the independent variables, protein concentrations as the dependent variables and age and sex included as covariates. The p-values from the linear regression were corrected for multiple comparisons using the false discovery rate (FDR) method. All analyses were completed in R using RStudio v1.4.1106 and the volcano plots were designed using the package “EnhancedVolcano”.
Cellular function assays
To assess the effect of siRNA-SOX17 on hPAEC function, assays investigating proliferation, apoptosis, cell viability, adhesion and barrier function were used and are discussed in full in the supplemental section.
In-silico analysis using the Connectivity Map
To analyse the differential expression patterns which occur when SOX17 expression is manipulated, RNA-sequencing of SOX17-signal 1 CRISPR-I hPAECs was performed as previously stated. To discover compounds that could be repurposed for the treatment of SOX17 dysfunction, the CMap was used (17). Differential gene expression lists (Table S3) were used to create queries for the three conditions, SOX17-promoter activation, SOX17-promoter repression, and SOX17-signal 1 repression. Candidate compounds with a tau score of over 90 or under -90 were selected. The compounds selected were Sirolimus, Aminopurvalanol-a and YK-4279. The signature of each compound was compared with the condition signature to select gene for further analysis by qPCR.
Animal models of PH in SOX17 enhancer knockout
SOX17-enhancer knockout mice generated by CRISPR-cas9 technology at the MRC Imperial College London were phenotyped blinded both in London and at Brown University, in Providence, RI, USA to either normoxia, hypoxia or combined VEGFR2 blockade SUGEN/SU5416 and hypoxia using standard measures of PH (see Supplement) and in accordance with institutional guidelines.
Results
PAH common variant signals rs10958403 and rs765727 identify SOX17 enhancers
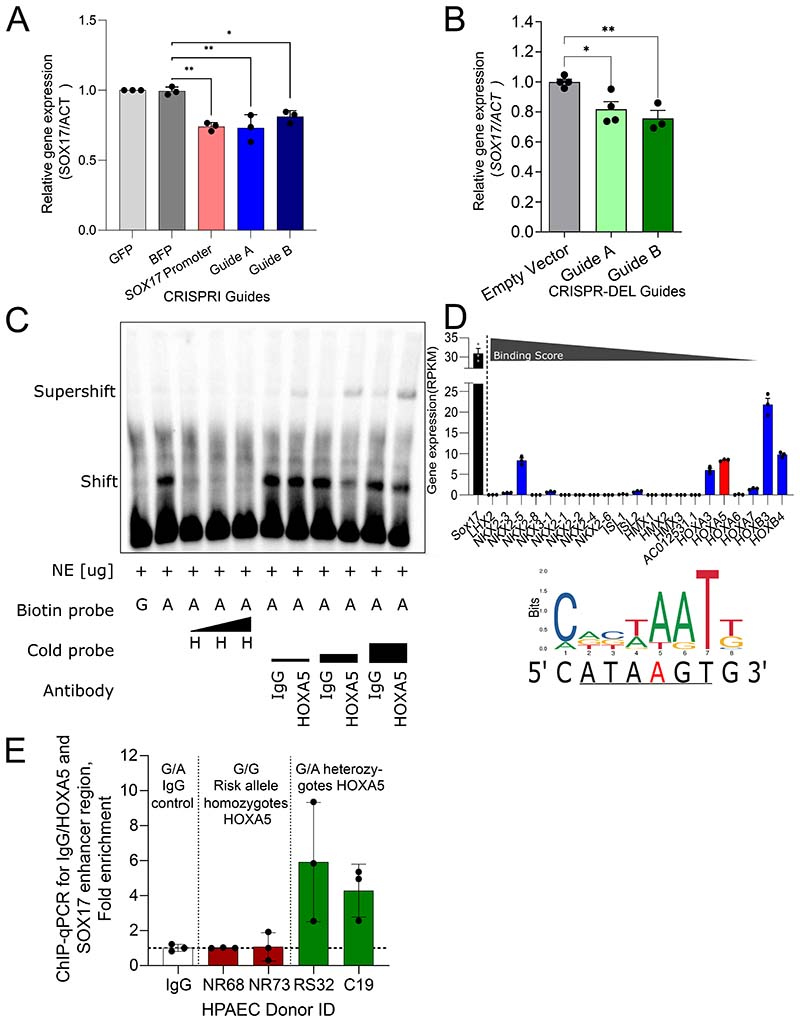
The function of the noncoding sequence containing rs765727 (SOX17-signal 2) is unknown. To test whether it is an enhancer that targets SOX17, we first targeted the region using CRISPR-inhibition in hPAECs. Guide RNAs targeting either rs765727 or the SOX17 promoter led to a significant decrease in SOX17 expression (enhancer guide A 0.73±0.055, guide B 0.81±0.025 of negative controls, both p<0.05, Figure 1A) but did not affect nearby gene TMEM68 (Supplementary Figure 1A). One guide also decreased the expression of another nearby gene, MRPL15 (guide B:0.83±0.013, Supplementary Figure 1A). Deletion of SOX17-signal 1 (rs10958403) using CRISPR-deletion guides resulted in a significant decrease in SOX17 but had no effect on nearby genes TMEM68 or MRLP15 (Figure 1B, Figure S1B).
Figure 1). Defining Upstream Regulators of SOX17 & Effects of Common Variation at the SOX17 Locus in PAH.
A) Knockdown of SOX17 through CRISPR-Inhibition of SOX17-signal 2. Relative gene expression of SOX17 compared to ACTβ in hPAEC. Ordinary 1-way ANOVA of conditions compared to BFP condition with Dunnett’s multiple comparisons test. n=3 experiments performed in triplicate. B) Knockdown of SOX17 through CRISPR-deletion of SOX17-signal 1 region. Relative gene expression of SOX17 compared to ACTβ in hPAEC. CRISPR-deletion guides A/B target SOX17-signal 1. Ordinary 1-way ANOVA of conditions compared to BFP condition with Dunnett’s multiple comparisons test. n=3. C) EMSA assay showing binding of hPAEC nuclear proteins to 21bp DNA probes containing the sequence at the rs1098403 region. Shift and supershift are highlighted. H indicates cold probes containing a putative HOXa5 binding site. Biotin-probe, biotin-labelled probe (G/A, alleles of SOX17 enhancer variant included in probe sequence). Cold-Probe, unlabelled probe. H, HOXa5 competitive probe. IgG, Mouse IgG. The black triangles show increasing molecular excess from left to right. D) RNAseq expression in hPAECs (RPKM) of potential TFs of interest for rs1098403. HOXa5 is shown in red. SOX17 is shown in black for reference. All other transcription factors are shown in blue and were found through CIS-BP. The binding score (taken from CIS-BP) refers to the predicted likelihood of the transcription factor binding to the given sequence and decreases from left to right. The underlined sequence refers to the potential binding location of HOXa5 and the A in red font is the site of rs1098403. The consensus binding sequence of HOXA5 was taken from JASPAR (jaspar.genereg.net) and is shown with the sequence of the region surrounding rs1098403 and rs765727 were taken from https://genome.ucsc.edu/. *-p<0.05, **-p<0.01. n=3. E) ChIP-qPCR for HOXA5 at rs10958493. Results of a quantitative PCR (triplet measurements per donor, n=3) performed on precipitated fraction of chromatin immunoprecipitation (ChIP) with IgG or HOXa5 antibody in 4 HPAEC donors. The ChIP with IgG control was performed in HPAEC donor C19.
In EMSA, nuclear protein from hPAEC bound to probes representing the non-risk alleles of both SOX17 signals, inducing a shift, but exhibited loss of binding to the risk alleles (Figure 1C, Supplementary Figure 1E). Competition EMSA of both loci showed removal of this shift with the addition of unlabelled competitive probes for the non-risk allele but not the risk allele, confirming the specificity of the protein binding to the non-risk sequences (Figure 1C, Supplementary Figure 1E).
In silico analyses using the TF databases CIS−BP, PROMO and ConSite, predicted multiple transcription factors more likely to bind the non-risk versus risk sequence at both SOX17 signals (Figure 1D, Supplementary Figure 1D). These transcription factors were subsequently prioritized by the level of gene expression in hPAECs, using RNAseq, and the predicted binding score (if available). TFs with no detectable or low expression in hPAECs were not investigated further. E47 was not selected despite having the highest expression in hPAECs as a splice variant (E12) was predicted to also bind to the risk allele (C). NKX2-5 was found to be undetectable in HPAEC in alternative public databases. Of the hPAEC-expressed candidates tested (HOXa5, ROR-α, Lin54, ZFX, RAR, Figure S1) only HOXa5 and ROR-a unlabeled probes were found to compete for nuclear protein binding to the non-risk sequences. For rs10958403, a TF competition EMSA with a probe containing the HOXa5 consensus binding sequence prevented the shift seen with the non-risk allele probe. Incubation with an antibody for HOXa5 produced a supershift pattern consistent with a probe-protein-antibody complex (Figure 1C). For rs765727, a TF competition EMSA with a probe containing the ROR-α consensus sequence prevented the shift seen with the non-risk allele probe. Incubation with an antibody for ROR-α also showed removal of the shift pattern (Supplementary Figure 1E). To validate the EMSA findings a ChIP was performed using an antibody against HOXa5 in HPAEC nuclear lysates and qPCR for the area containing rs10958403. The area containing SNP rs10958403 was only amplified in donors containing a non-risk A-allele, confirming that HOXa5 only binds this allele at rs10958403 (Figure 1E). For rs765727/ROR-α, none of the donors tested expressed a non-risk allele, preventing a similar comparison.
Taken together, these experiments indicated that the PAH signals identify enhancers active in hPAEC which target SOX17 and contain variants likely to drive differential binding of TFs including HOXa5 and ROR-α.
PAH-associated stimuli regulate endothelial SOX17 expression in PAH patient cells
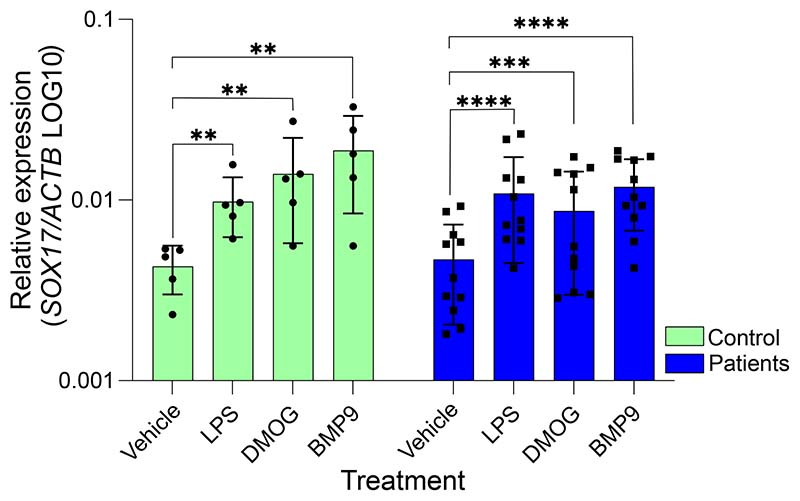
To determine whether SOX17 is regulated by factors implicated in the development of PAH, we tested SOX17 expression in endothelial colony-forming cells (ECFC) derived from healthy controls or PAH patients after stimulation with the hypoxia mimic DMOG (dimethyloxalylglycine), the inflammatory stimulus LPS (lipopolysaccharide) and the BMPR2 ligand BMP9 (bone morphogenetic protein-9). ECFC from IPAH patients have reduced barrier function versus those from control subjects and are more susceptible to LPS-induced permeability (18). LPS treatment is not only a well-established means of stimulating permeability in pulmonary EC cultures; it also linked with selective HIF-1a stabilization (DMOG treatment in vitro) and the induction of SOX17 expression in HPMVECs (19). Expression of SOX17 has a protective effect and is required for the restoration of barrier function. ECFC derived from PAH patients with pathogenic BMPR2-variants are more susceptible to LPS-induced permeability versus control ECFC and the effect is blocked by co-treatment with BMP9 (20). Stimulation with LPS, DMOG or BMP9 significantly increased SOX17 expression in both control (n=5) and PAH patient (n=11) ECFCs (Figure 2).
Figure 2). Defining the effect of upstream regulators and PAH-relevant stimuli on SOX17.
Relative gene expression of SOX17 compared to ACTβ in control and patient ECFC following exposure to known PAH stimuli. Individual data points represent individuals, n=5 controls, n=11 patients. Vehicle/treatments in 2% FBS EC media. LPS, lipopolysaccharide (2µg/ml). DMOG, Dimethyloxalylglycine (100µM), BMP9, bone morphogenetic protein-9 (10ng/ml). Ordinary 1-way ANOVA within groups compared to baseline condition with Dunnett’s multiple comparisons test. **-p<0.01, ***-p<0.005, ****-p<0.001.
SOX17 regulates pathological downstream molecular pathways and functions in hPAEC
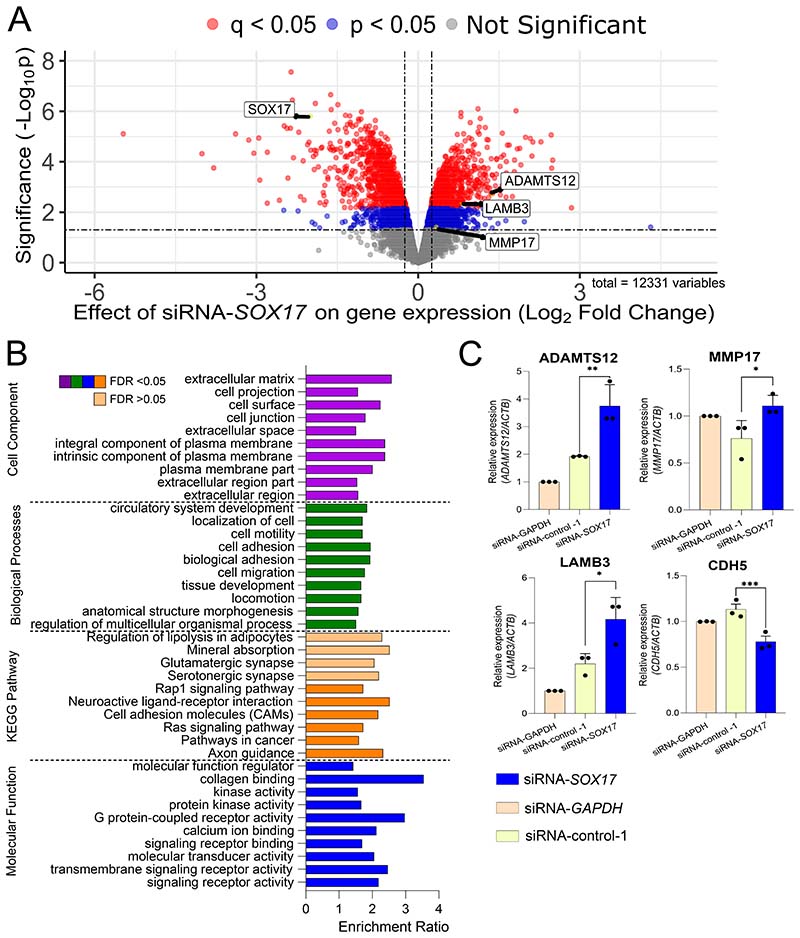
The risk alleles are associated with reduced enhancer activity and therefore reduced SOX17 expression. To determine the downstream effects of SOX17 depletion we performed RNAseq analysis of hPAEC following modulation of SOX17 by siRNA-mediated silencing or by CRISPR inhibition of SOX17-signal 1 and -signal 2 (Figure 3A, Figure S2 and S3). We identified 1717 genes that are differentially expressed following siRNA-SOX17 knockdown (absolute log2-fold change >0.25 or <-0.25, FDR q<0.05, Figure 3A). Gene ontology (GO) shows significant enrichment of the pathways involving cell adhesion and extracellular matrix organization (ECM, Figure 3B). 451 genes were significantly down- or upregulated following CRISPR-I of SOX17-signal 1 and 786 genes following CRISPR-I of SOX17-signal 2 (absolute log2-fold change >0.25 or <-0.25, p<0.05, Figure S3). There was a significant overlap of eighty-one genes differentially expressed in both siSOX17 and SOX17-signal 1 CRISPRI (p=0.0356, Supplementary Data File). Consistent with the siRNA analysis, gene ontology analysis of these differentially expressed genes shows enrichment for pathways linked to the extracellular matrix organization and cell adhesion (Figure S3). qPCR was used to validate the effect of SOX17-siRNA on affected genes enriched in ECM and adhesion pathways. ECM and adhesion genes ADAMTS12, MMP17 and LAMB3 were significantly increased when compared to a negative control siRNA, whereas CDH5 was decreased by SOX17 knockdown (Figure 3C). These data demonstrate that SOX17 loss in hPAEC drives gene expression changes in pathways relevant to PAH pathology.
Figure 3). Analysis of Sox17 Manipulation in Pulmonary Vascular Cells.
A) Differentially Expressed Genes following SOX17-siRNA in hPAECs. Volcano plot of the Log2 fold change (FC) between siRNA-SOX17 and siRNA-negative control and the negative log10 p-value. Differentially expressed genes shown in blue met the cut-off points: p<0.05 and Log2 fold change <-0.25 or >0.25. Genes shown in red also met the cut-off of q<0.05. Genes of interest are highlighted in black boxes. n=4, 12196 variables. B) Over-representation Analysis for enriched pathways and functions following SOX17-siRNA. Gene ontology analysis of Cell component (purple), biological process (green), KEGG pathway (orange) and molecular function (blue) enrichment following SOX17-siRNA in hPAECs. Darker colours indicate f<0.05. Lighter colours indicate f>0.05. Enrichment ratios were obtained from WebGestalt. C) Relative gene expression of gene ontology target genes by qPCR. The change in target gene expression is normalised to ACTβ and all siRNA conditions are relative to the GAPDH-targeting siRNA control. Target genes are ADAMTS12, MMP17, LAMB3, CDH5. All statistical tests shown are paired, one-way, student’s t-test. *-p<0.05. n=3. All in hPAECs following SOX17-siRNA treatment for 48 hours.
Plasma proteomic differences in patients with differing SOX17 variant genotypes
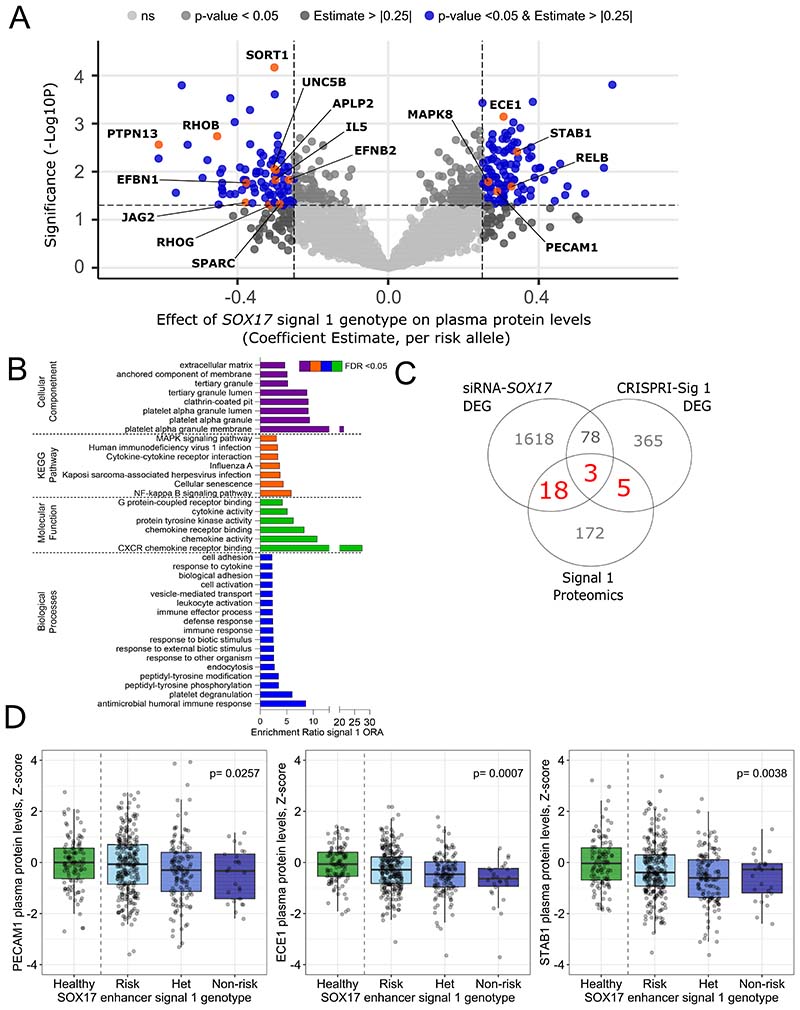
To further examine the potential effect of common variation in the enhancer area on downstream targets of SOX17, we analysed the plasma proteome of 431 PAH patients with a known genotype of the SOX17-signal 1 and SOX17-signal 2 using linear regression analysis. We identified 198 and 161 proteins where plasma levels were significantly affected by the genotype in SOX17-signal 1 and SOX17-signal 2 SNPs, respectively (beta-estimate > |0.25| and p<0.05, Figure 4A, Figure S4). In line with our data obtained from genetic interference of SOX17 in hPAEC, GO analysis of the plasma proteomics identified enrichment in proteins involved in regulation of adhesion and extracellular matrix for both signals (Figure 4B, Figure S4B). In addition, enrichment in proteins involved in regulation of proliferation, migration and apoptosis were found for SOX17-signal 2 (Figure S4). As similar processes and functions emerged from the transcriptome GO analyses, the significantly affected proteins and genes from all conditions (CRISPRI, siRNA and proteomics) were compared in detail (Figure 4C, Figure S4C). Six proteins were affected by patient genotypes at both SOX17 signals (IL5, PTPN13, STAB1, SUGT1, GAPDH and ADGRG5). There were 26 genes in common between SOX17-signal 1 proteomics and CRISPRI or siRNA analyses (Figure 4A, Supplementary Data File) and 23 genes between SOX17-signal 2 proteomics and CRISPRI or siRNA analysis (Figure S4). These included Secreted Protein Acidic And Cysteine-Rich (SPARC), Platelet And Endothelial Cell Adhesion Molecule-1 (PECAM1), Endothelin Converting Enzyme-1 (ECE1), Collagen Type XVIII Alpha-1 (COL18A1), Interleukin-5 (IL5) and Stabilin-1 (STAB1) which have been previously associated with PAH (21)(22)(23)(24) (Figure 4D, Figure S4). These analyses suggest that differences in SOX17 enhancer activity associated with PAH risk alleles lead to changes in the plasma proteome with pathologically relevant functions.
Figure 4). Effect of SOX17 enhancer variant genotype on patient proteomics.
A) Linear regression for the effect of SOX17-signal 1 on the levels of serum proteins in patient sample. Volcano plot of the coefficient estimate and the negative log10 p-value. Corrected for age and sex. Protein shown in blue met threshold b-estimate > |0.25| and p < 0.05. Proteins of interest are labelled and shown in orange n=431. B) GO analysis for the significantly affected by signal 1 genotype proteins. Gene ontology analysis of Cell component (purple), biological process (green), KEGG pathway (orange) and molecular function (blue) enrichment. Proteins met the threshold b-estimate > |0.25| and p < 0.05. Enrichment ratios were obtained from WebGestalt. C) Comparisons of transcriptomic and proteomic analysis. Venn diagram showing overlapping differentially expressed genes and proteins from transcriptomic and proteomic analysis from CRISPRi of SOX17-signal 1, siRNA-SOX17 and SOX17-signal 1 enhancer variant genotype on patient proteomics. DEG, differentially expressed genes. Numbers in red show genes and proteins which are in common between all analyses. D) Z-scored proteins in healthy controls versus patients with different genotypes in proteins of interest. Proteins included are PECAM1, ECE1 and STAB1. Risk, homozygous for risk allele, n=271. Non-risk, homozygous for non-risk allele, n=26. Het, heterozygotes, n=134. Control, n=108.
Functional impact of loss of SOX17 in cultured hPAEC
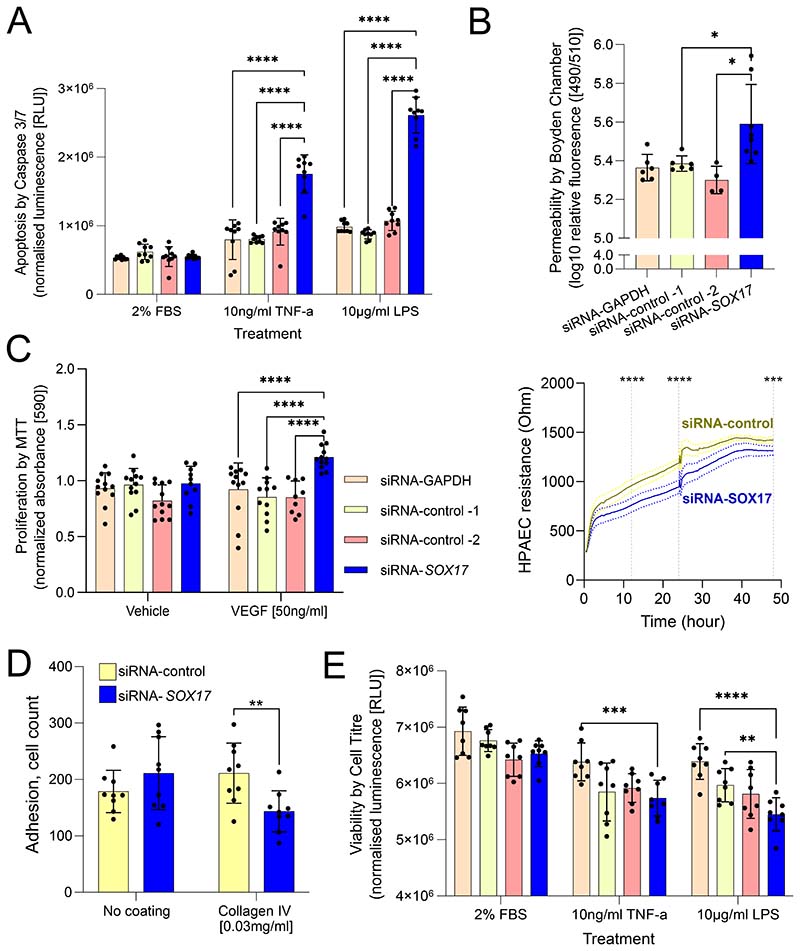
To determine the functional impact of loss of SOX17, we exposed hPAEC to relevant stimuli following siRNA-mediated SOX17 knockdown. SOX17 knockdown increased hPAEC apoptosis (caspase-3/7 activity) in response to either TNF-α or LPS as compared to siRNA controls (p<0.001, Figure 5A), with hPAEC viability being either unchanged or decreased with siRNA-SOX17 when compared to siRNA controls (Figure 5E). Knockdown of SOX17 led to an increase in hPAEC monolayer permeability, as measured by a transwell assay (both p<0.05, Figure 5B) and by electrical impedance assays (p<0.001, Figure 5B lower panel). Adhesion to collagen IV was significantly decreased in SOX17-depleted hPAECs compared to controls (Figure 5D), while SOX17 knockdown increased VEGF-induced hPAEC proliferation as determined by MTT assays (p<0.001, Figure 5C). These results suggest that SOX17 loss in hPAEC fundamentally changes their function mirroring changes observed in patient PAEC.
Figure 5). Functional Analysis of Sox17 loss in hPAEC.
All following siRNA treatment for 48hrs with scrambled controls, targeting SOX17 or unrelated gene GAPDH, with relevant stimuli as indicated. A) Caspase 3/7 apoptosis assay. 2% FBS was used as a proliferation control. TNFα and LPS were used as pro-apoptotic inflammatory stimuli. B) Permeability barrier function assays. Upper, Boyden chamber FITC dextran. Lower, Electrical cell Substrate Impedance Sensing (ECIS) measurements at 0-48h. C) MTT proliferation assay. Vehicle, 0.1% BSA in PBS. Vascular endothelial growth factor (VEGF) was used as a pro-proliferative stimulus. D) Adhesion cell counting assay. Adhesion was compared between cells in wells with no coating or pre-coated with collagen IV. E) Cell Titre viability assay. Performed under same conditions as caspase assay. TNFα, tumour necrosis factor-α; LPS, lipopolysaccharide. Statistical tests used in A, C and D are ordinary two-way ANOVA with Dunnett’s multiple comparisons test. The statistical test used in B is an ordinary one-way ANOVA with Dunnett’s multiple comparison test. *-p<0.05. **-p<0.01. ***-p<0.005. ****-p<0.001. Minimum n=3 experiments for all, culture replicates plotted.
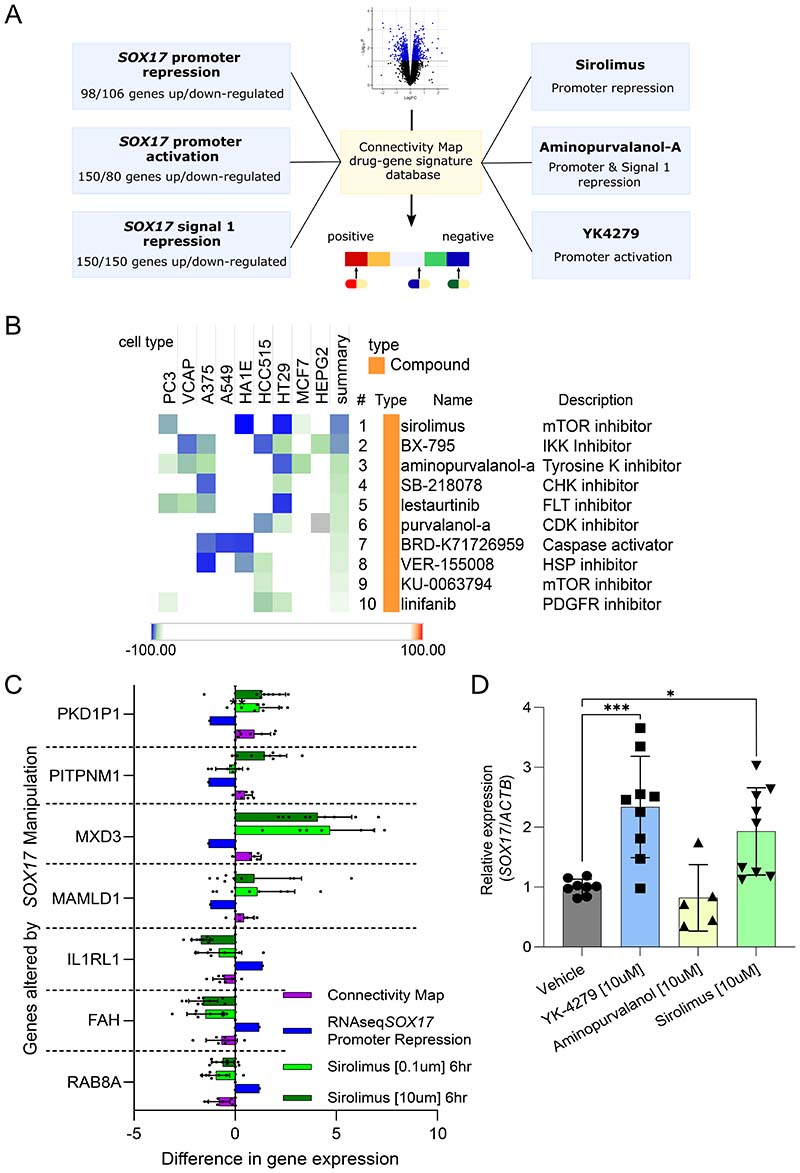
Connectivity Map prediction of rescue compounds for drug repurposing
To predict if available drug compounds could be repurposed to reverse the gene changes associated with SOX17 dysfunction, we interrogated the CMap database. The CMap contains transcriptomic signatures of thousands of compounds' effects on multiple cell lines, allowing comparison of user-generated signatures (Figure 6A). To test effects most relevant to the common and rare SOX17 variants associated with PAH, we generated SOX17 hPAEC signatures comprised of differentially expressed genes following CRISPR-inhibition of SOX17-signal 1 or the SOX17 promoter, or CRISPR-activation of the SOX17 promoter. In-silico analysis of these signatures show the compounds sirolimus, aminopurvalanol-a and YK4279 to match our SOX17 signature in hPAEC (Figure 6B). Sirolimus and aminopurvalanol-a are predicted to reverse (negatively connected to) SOX17 promoter repression (Tau score:-96.94 and -95.17 respectively). Aminopurvalanol-a is also negatively connected to SOX17-signal 1 repression (-99.65). YK4279 is predicted to mimic SOX17 promoter activation (positive connection, +93.88). Comparisons of each compound’s signature from the Cmap and our RNAseq signature resulted in the gene lists shown in Table S3. For each compound, we selected a panel of genes that showed consistent directional changes across multiple cell lines in the Cmap and tested their expression by qPCR in hPAEC following compound exposure. For sirolimus, all tested genes showed the predicted expression change (Figure 6C). For aminopurvalanol-a, only half of the genes tested changed in the direction predicted by the Cmap signature (Supplementary Figure 5). For YK4279, all the gene expression changes tested were as predicted (Supplementary Figure 5). To determine whether the changes seen could be directly through SOX17 effects we measured SOX17 levels in the treated hPAEC and found that both YK-4279 and sirolimus significantly increased SOX17 relative expression compared to vehicle (Figure 6D). These data confirmed that Cmap-predicted compounds can successfully reverse some genetic changes associated with SOX17 dysfunction in hPAEC.
Figure 6). Repurposing of Compounds to Rescue loss of SOX17 Function in PAH.
A) Summary diagram showing the prediction of compounds from omics signatures using connectivity map (CMap) database signatures of drug or gene manipulation in reference cell lines. Omics signatures used to query the Cmap database are shown on the left. Predicted compounds are shown on the right. B) Cmap analysis results for SOX17 promoter repression using CRISPR-inhibition in human pulmonary artery endothelial cells (hPAEC). Cell types are shown. Tau scores within heatmap indicate percentage of all possible compounds in CMAP and cell lines tested the specific result is more connected than. Sirolimus overall is negatively connected more strongly than other compounds, with a summary Tau of -96.94. C) Relative gene expression of target genes in the sirolimus perturbagen signature. Target genes were PKD1P1, PITPNM1, MXD3, MAMLD1, IL1RL1, FAH and RAB8A. Connectivity map, perturbagen z-score taken from the Cmap. RNAseq SOX17 promoter repression via CRISPRI (n=3), fold change from RNAseq analysis of DEG following CRISPRI of the SOX17 promoter (n=3). Sirolimus [0.1μm/10μm] (n=3), the change in target gene expression is normalised to ACTβ and all siRNA conditions are relative to the GAPDH-targeting siRNA control in hPAECs following sirolimus exposure at the stated concentrations. D) Relative expression of SOX17 following CMap compound exposure by qPCR in hPAEC. The change in SOX17 expression is normalised to ACTβ and all compounds are relative to the vehicle control. Ordinary 1-way ANOVA of conditions compared to vehicle with Dunnett’s multiple comparisons test. *-p<0.05, ***-p<0.005. n=3.
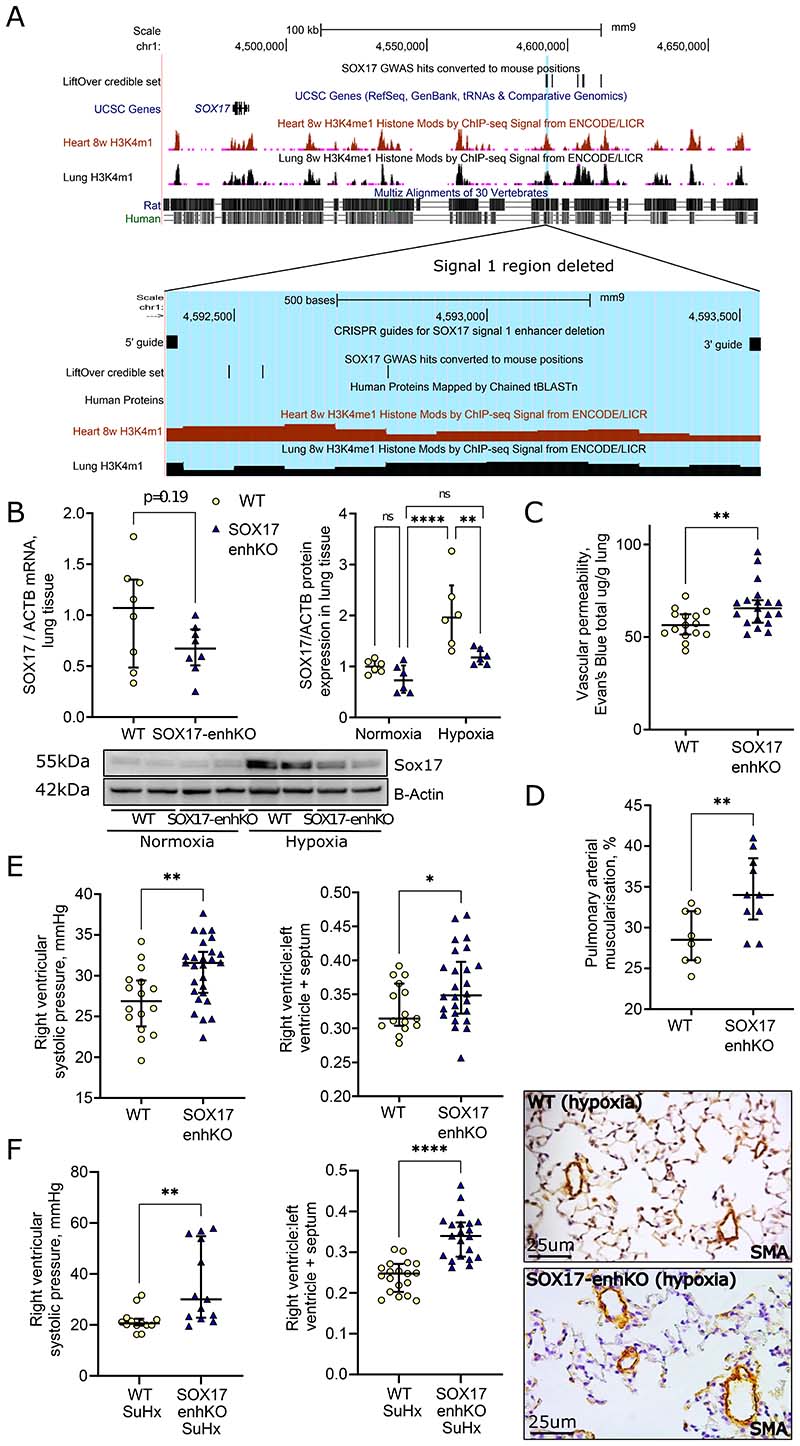
Animal knockout of SOX17 enhancer worsens PH
We have successfully generated mice lacking the 747bp enhancer region containing SOX17 GWAS signal 1 using CRISPR/Cas9-mediated deletion on C57BL/6 background (Figure 7A, Figure S6). The SOX17-enhKO and wild type (WT) mice kept in normoxia did not show significant differences in right ventricular systolic pressure (RVSP) and right ventricular hypertrophy index (RVH, RV/LV+septum, Supplementary Figure 6B). We then exposed the mice to hypoxia (normobaric, 10% oxygen) for 1 and 3 weeks. SOX17 protein levels in the lungs of the SOX17-enhKO mouse were reduced in comparison to WT when exposed to hypoxia (Figure 7B). After 1-week hypoxia exposure SOX17-enhKO mice demonstrated significantly increased lung vascular permeability in comparison to WT (Figure 7C). At 3 weeks, chronic hypoxia-induced PH severity was intensified in the SOX17-enhKO animals as demonstrated by increased peripheral pulmonary vessel muscularization (Figure 7D), RVSP (Mean+/-Std.Deviation: 26.93+/-3.916 vs. 30.66+/-3.856 mmHg, p=0.004), RVH and RV/body weight (Figure 7E, Supplementary Figure 6C).
Figure 7). SOX17-signal 1 enhancer knockout mice develop more severe PH in hypoxia.
A) Map of PAH SOX17 enhancers in mouse genome. Black bars labelled ‘Enhancer and GWAS hits LiftOver’ and ‘User track’ indicate conserved genomic regions from the human SOX17 enhancer peaks. Lines indicate positions of variants associated with PAH in the human GWAS. Mouse epigenomic H3K4m1 data show that this area is also likely to be an active regulatory region in mice. Blue region highlights enhancer targeted for deletion. B) SOX17 mRNA and protein expression. Taken from lung tissue following 3 weeks hypoxia and compared to beta-actin (ACTB) housekeeping gene/protein. *-p<0.05, **-p<0.01 versus WT (unpaired t-tests). n=6 and n=8. C) Lung vascular permeability. Determined by Evan’s blue dye in wildtype (WT) and SOX17 enhancer knockout lung tissue following 1 week of hypoxia. *-p<0.05, **-p<0.01 versus WT (unpaired t-tests). n=15 and n=18. D) Pulmonary vascular muscularisation. Determined from smooth muscle actin (SMA) and Elastic Van Gieson (EVG) staining. *-p<0.05, **-p<0.01 versus WT (unpaired t-tests). n=7 and n=10. E) Right ventricular systolic pressure (RVSP) and RV hypertrophy (RVH) indices of PH severity in WT and SOX17 enhancer knockout mice following chronic hypoxia (10% O2 3 weeks). *-p<0.05, **-p<0.01 versus WT (unpaired t-tests). F) Right ventricular systolic pressure (RVSP) and RV hypertrophy (RVH) indices of PH severity in WT and SOX17 enhancer knockout mice following SUGEN 5 mg/kg and 12% O2 for 3 weeks (SuHx). **-p<0.01, ****-p<0.001 versus WT SuHx (unpaired t-tests).
To confirm these findings using a different model of PH, the same SOX17-enhKO were studied under different levels of hypoxia and SUGEN (SU-5416). Using the standard protocol of 20 mg of SUGEN and 3 weeks of hypoxia, there were no significant differences in the severity of PH that developed between, SOX17-enhKO and WT mice. However, SOX17-enKO were more susceptible to developing SUGEN/hypoxia-PH as evidenced by the development of severe PH at lower levels of SUGEN-hypoxia (5 mg/kg and 12% O2) which did not produce PH in WT littermates (RVSP: 21.66+/-4.43 vs 35.94+/-15.36 mmHg p=0.006, Figure 7F and Supplementary Figure 7). Thus, in two independent laboratories using different PH models it was shown that SOX17 enhancer knockout increases susceptibility to and severity of PH.
Discussion
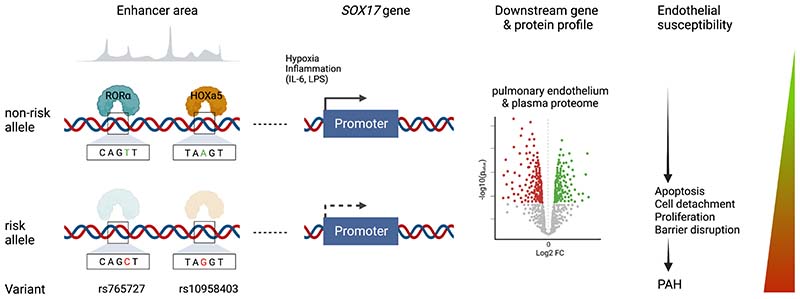
Here we provide novel insight into how two independent common genetic variants upstream of the key endothelial transcription factor, SOX17, can increase susceptibility to PH (Figure 8). In brief, variation at rs765727 and rs10958403 in putative SOX17 enhancer signals 1 and 2 determine the binding of two transcription factors, RORα and HOXA5, respectively. Allele-specific reduced binding of either factor leads to reduced SOX17 expression. SOX17 is crucial for maintaining endothelial cell homeostasis and its loss drives abnormal proliferation, apoptosis and adhesion, and impairs endothelial barrier integrity. Our prediction that this would increase susceptibility to PH was confirmed in mice lacking SOX17-signal 1 enhancer signaling exposed to hypoxia with and without Sugen.
Figure 8). Summary figure.
Schematic depicting overall study findings from identification of RORα and HOXa5 as transcription factors binding PAH-associated variants in enhancers upstream of SOX17, through regulation of SOX17 by PAH stimuli, downstream effects of SOX17 on gene and protein expression profiles and endothelial cell behaviour, culminating in worsened PAH in SOX17-enhancer knockout mice.
Defining the importance and biological function of GWAS signals in complex diseases is a challenge. Many are located in non-coding regions of the genome which complicates interpretation. Confirmed examples of variation in enhancer regions causing disease are few, including BCL11 in sickle cell disease (25) and FTO in obesity (26). SOX17-signal 1 and -signal 2 are located inside a topologically associated domain (TAD) in which SOX17 is the only gene, making it the most likely target for these two enhancers (7). A significant and specific decrease in SOX17 expression was observed following CRISPR-inhibition of SOX17-signal 2 and CRISPR-deletion of SOX17-signal 1, establishing that these genomic signals are associated with the regulation of SOX17 expression. EMSA (and ChIP-qPCR for HOXa5) demonstrated that HOXa5 or ROR-alpha bind to the non-risk alleles present at SOX17-signal 1 and signal 2 respectively. Thus, an individual homozygous for one or both risk alleles would be more resistant to HOXa5 and/or ROR-alpha induced SOX17 expression than an individual hosting non-risk alleles. While it is not a requirement that these transcription factors are themselves associated with PAH, HOX transcription factors expression have been shown to differ in PAH lung tissue (27) which may further exacerbate the effect of the differential binding affected by the disease-driving variant.
SOX17 has established roles in systemic artery endothelial cells, but little is known about its role in the pulmonary vasculature. Establishing the downstream targets of SOX17 in hPAEC is vital to understanding how it mediates the risk of developing PAH. Our transcriptomic analysis identified several specific candidates and enrichment of gene pathways implicating dysregulation of endothelial functions and extracellular matrix (ECM) associated with loss of SOX17. This was supported by our plasma proteomic analysis between patients with risk or non-risk SOX17 enhancer genotypes, where we found an enrichment of adhesion- and ECM-associated proteins. The basement membrane is thicker in the lungs of iPAH patients, and regulation of the ECM is established as an important factor in PAH (28). We have demonstrated here that the ECM-linked genes LAMB3, ADAMTS12 and MMP17 were affected at the mRNA level by SOX17 knockdown in independent experiments. ADAMTS12 and MMP17 expression increased following a loss of SOX17, suggesting they may be important contributors to the functional effects of SOX17 loss in hPAEC. ADAMTS12 is a disintegrin and matrix metalloproteinase gene with an important role in ECM composition (29). Coupled with MMP17, it is part of a large family of matrix metalloproteinase genes which may have important roles in pathophysiological functions in hPAEC in PAH (30). Although MMP17 has not been directly linked to PAH, loss of function variants, found in familial studies, confer a greater risk of aortic aneurysm in mice through dysfunctional ECM filament deposition and an enlarged aortic lumen (31).
Analysis of the effect of patient SOX17 enhancer genotypes at SOX17-signal 1 and signal 2 on plasma protein levels identified a large number of significantly affected proteins enriched in the regulation of adhesion and extracellular matrix, proliferation, migration and apoptosis, all processes crucial for the development of PAH. There were proteins and genes that were significantly affected both by the SOX17-signal 1 and signal 2 patient genotypes and by the loss of SOX17 expression via siRNA or CRISPRi in hPAEC, suggesting circulating proteins might reflect SOX17 dysfunction in PAH. Interesting candidates among these are SPARC, which was found at increased levels in lung of IPAH patients and is involved in the regulation of PASMC proliferation (21), ECE1, which is involved in the cleavage/transformation of Endothelin 1 to its active form (22), PECAM-1, which plays a role in the adaptation of endothelium to shear stress (23) and COL18A1, which when cleaved produces Endostatin, a protein whose serum levels are correlated with disease severity and survival in PAH (24).
That rare pathogenic variants in SOX17 also drive PAH development and are associated with more severe PAH and younger age (32), emphasises the importance of this gene and related pathways as a therapeutic target. To pursue this, we explored CMap for novel candidates that might rescue SOX17 activity. Three candidate compounds emerged from our screen using hPAECs, all suitable for exploratory studies in humans. Sirolimus is a specific inhibitor of mTOR and an allosteric inhibitor of mTORC1. Another immunosuppressor, tacrolimus, has entered clinical trials in PAH. Tacrolimus is a calcineurin inhibitor but was selected for study at low doses expected to modulate BMPR2 signalling based on a screening strategy and hence has a distinct mechanism to sirolimus. Tacrolimus was found to be well tolerated and to improve 6-min walk distance and echocardiographic parameters of heart failure in PAH patients in a phase IIa safety and tolerability study. Although the small number of patients studied did not result in statistical significance, the findings did support the study of tracrolimus in a phase IIb efficacy trial (33). Several prior studies have shown the efficacy of sirolimus in reversing animal models of PH (34) and an albumin-bound nanoparticle form of sirolimus is currently undergoing a phase I/II clinical trial and has so far shown no safety concerns and an early efficacy signal (35). It would be of interest to gauge the importance of regulation of SOX17 and its signalling in the efficacy of sirolimus and addition of biomarker measurements (e.g. SPARC) in future trials would be valuable.
Aminopurvalanol-a (also known as purvalanol-a) is a CDK1/cyclin B inhibitor that arrests proliferating cells in the G2/M stage of the cell cycle and prevents proliferation. It has been investigated in human microvascular endothelial cells as an anti-angiogenic drug and was shown to inhibit proliferation, increase apoptosis and prevent tube formation (36). Some CDKs are upregulated following SOX17 silencing in arterial endothelium (37). In both monocrotaline and Sugen/hypoxia PAH rat models, the CDK inhibitor palbociclib reversed PAH pathology including right heart hypertrophy and pulmonary remodelling (38). Both CDK inhibitors dinaciclib (inhibits CDK1, 2, 5 and 9) and palbociclib (inhibits CDK4 and 6) reduced proliferation in SMCs (38).
A third compound from the Cmap screen, YK-4279, is an ETS family inhibitor that has undergone pre-clinical efficiency trials as an anti-lymphoma drug (39). ETS family members are known oncogenes whose aberrant expression is found in many solid tumours (40). They also have roles in vascular development and maintenance (for full review: (41)). YK-4279 specifically inhibits ERG transcription and ERG-mediated cell migration and proliferation in prostate cancer (42). The ERG TF is essential for EC homeostasis and has recently been shown to bind to a super-enhancer upstream of the SOX17 gene in HUVEC, suggesting a possible role for ERG in the regulation of SOX17 in this cell type (43).
SOX17 knockdown in mice results in embryonic lethality with heart defects and enlarged veins (44). Endothelial-specific knockdown in either embryonic or adult mice causes defects in artery specification and in-utero lethality (10,45). SOX17 endothelial-(Cdh-CreER)-knockout exacerbated hypoxic PH which was sustained despite return to normoxia for 3 weeks. Consistent with our findings in si-SOX17-treated human PAEC, hyperproliferation of ECs was prominent in Sox17knockout/hypoxic mice by Ki67 staining (46). These studies support a role for SOX17 in arterial endothelial cells in-vivo. We can report that mice lacking SOX17-signal 1 are viable but show increased susceptibility to hypoxia or combined SUGEN-hypoxia associated PH. Consistent with our in vitro data and prior reports in other vascular beds (47,48), the SOX17 enhancer knockout mice exhibited elevated vascular permeability compared to WT animals. This illustrates the importance of fine tuning SOX17 levels to modulate endothelial barrier function under pathological conditions. Whilst there was no phenotype apparent in the mice under normoxic conditions or more severe SUGEN-hypoxia, it is remarkable that deletion of an enhancer alone, rather than a complete or partial gene deletion (as required for BMPR2) was sufficient to lead to a worse PH phenotype in two independent laboratories, under hypoxia or lower levels of SUGEN-hypoxia than drove PH in wildtype animals. The concept that a ‘second hit’ may be required to exhibit the PH phenotype is well understood and in patients harbouring a SOX17 enhancer risk allele, this may comprise inflammation or drug toxicity as well as hypoxia.
It remains possible that other transcription factors also bind differentially to PAH-associated SOX17 enhancer variants. In our EMSA experiments, use of a ROR-α antibody lead to loss of signal rather than a clear supershift, which is most likely due to the antibody preventing formation of the ROR-α-probe complex. SOX17 is not included on the proteomics platform used in this study. The numbers of homozygotes for rarer variant alleles are small so variability in these measurements is high. Validation of key findings in hPAECs with (naturally occurring or knocked-in) variants in SOX17-signal 1 or 2 in addition to the complete deletion of the enhancer would have even more robustly supported functional conclusions. The effects would likely be more subtle than deletion or inhibition of the enhancer requiring larger n numbers. Aminopurvalanol did not affect SOX17 expression but was identified as a drug which can alter the downstream transcriptomic signature produced by SOX17 loss.
In summary, we provide comprehensive insight into how common variation influences the binding of HOXa5 and RORα to enhancers upstream of SOX17 and can reduce susceptibility to PH. Loss of SOX17 leads to downstream alterations in extracellular matrix regulation and hPAEC function. SOX17 is a priority for therapeutic rescue and predicted compounds which restore endothelial gene expression offer candidates for future investigation.
Supplementary Material
Clinical Perspective.
1). What is new?
SOX17 enhancer common variants associated with PAH development alter binding of transcription factors and thereby levels of SOX17.
Loss of SOX17 enhancer region drives worsening of PH in animal models.
Gene and protein signatures driven by SOX17 dysfunction are identified and potential rescue therapeutic candidates proposed.
2). What are the clinical implications?
Higher confidence in the causal variants of common genetic risk of PAH is provided.
This research describes the biological pathways that are likely affected in PAH patients carrying risk genotypes of SOX17, thereby improving our understanding of the pathogenesis of this deadly condition.
Compounds targeting the pathways to restore physiological SOX17 signaling in PAH patients are a priority for further investigation.
Acknowledgements
We thank National Institute for Health Research (NIHR) BioResource volunteers for their participation, and gratefully acknowledge NIHR BioResource centres, NHS Trusts and staff for their contribution. We thank the NIHR Imperial Clinical Research Facility, NIHR Sheffield Biomedical Research Centre, Cambridge NIHR Cardiorespiratory BRC and NHS Blood and Transplant. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. We thank the members of the UK PAH Cohort consortium listed in full in the supplement. Figure 8 was produced using Biorender.
Funding Sources
This work was supported by the National Institute for Health and Care Research BioResource which supports the UK National Cohort of Idiopathic and Heritable PAH; the British Heart Foundation (BHF PG/19/17/34275, SP/12/12/29836) and the UK Medical Research Council (MR/K020919/1). This work was supported in part by a British Heart Foundation Centre for Research Excellence award to MRW (RE/18/4/34215). CJR is supported by a BHF Intermediate Basic Science Research fellowship (FS/15/59/31839) and Academy of Medical Sciences Springboard fellowship (SBF004\1095). NWM is a BHF Professor and NIHR Senior Investigator. JA is supported by an European Respiratory Society/European Molecular Biology Organisation Longterm Research Fellowship and by an Nederlandse Organisatie voor Wetenschappelijk Onderzoek VENI grant (#09150161910155). IC is recipient of a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (224662/Z/21/Z). JF is supported by Medical Research Council (MR/L02036X/1), Wellcome Trust Senior Investigator Award (WT101033) and European Research Council Advanced Grant (789055) awards. This work was also supported in part by grants from the National Institutes of Health P20 GM119943 (ODL and JRK), by the American Heart Association, Transformational Project Award 18TPA34110329 (ODL), and by the Brown Physicians, Inc., Academic Assessment Research Award (JRK and ODL).
Footnotes
Conflict of Interest Disclosures
The authors declare no direct conflicts specific to the work in this manuscript.
References
- 1.Nakhleh MK, Haick H, Humbert M, Cohen-Kaminsky S. Volatolomics of breath as an emerging frontier in pulmonary arterial hypertension. The European respiratory journal. 2017;49(2):1601897. doi: 10.1183/13993003.01897-2016. [DOI] [PubMed] [Google Scholar]
- 2.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. The European respiratory journal. 2007;30(6):1103–1110. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nature Reviews Cardiology. 2011;8(8):443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan N, Massam B, Kulkarni S, Lang C. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases. 2018;6(2):38. doi: 10.3390/diseases6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaillancourt M, Ruffenach G, Meloche J, Bonnet S. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Canadian Journal of Cardiology. 2015:407–415. doi: 10.1016/j.cjca.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Southgate L, Machado RD, Gräf S, Morrell NW. Molecular genetic framework underlying pulmonary arterial hypertension. Nature Reviews Cardiology. 2020:85–95. doi: 10.1038/s41569-019-0242-x. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, et al. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. The Lancet Respiratory medicine. 2019;7(3):227–238. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development (Cambridge, England) 2013;140(20):4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 9.Francois M, Koopman P, Beltrame M. SoxF genes: Key players in the development of the cardio-vascular system. The International Journal of Biochemistry & Cell Biology. 2010;42(3):445–448. doi: 10.1016/J.BIOCEL.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, Nyqvist D, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nature Communications. 2013;4(1):2609. doi: 10.1038/ncomms3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Williams J, Smallwood PM, Nathans J. Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina. Chen J, editor. PloS one. 2015;10(12):e0143650. doi: 10.1371/journal.pone.0143650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange AW, Haitchi HM, LeCras TD, Sridharan A, Xu Y, Wert SE, et al. Sox17 is required for normal pulmonary vascular morphogenesis. Developmental Biology. 2014;387(1):109–120. doi: 10.1016/j.ydbio.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Respiratory Journal. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 14.Wojciak-Stothard B, Abdul-Salam VB, Lao KH, Tsang H, Irwin DC, Lisk C, et al. Aberrant Chloride Intracellular Channel 4 Expression Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Circulation. 2014;129(17):1770. doi: 10.1161/CIRCULATIONAHA.113.006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebola I. Deletion of Regulatory Elements with All-in-One CRISPR-Cas9 Vectors. Methods in Molecular Biology. 2021;2351:321–334. doi: 10.1007/978-1-0716-1597-3_18/COVER. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes CJ, Wharton J, Swietlik EM, Harbaum L, Girerd B, Coghlan JG, et al. Using the Plasma Proteome for Risk Stratifying Patients with Pulmonary Arterial Hypertension. American Journal of Respiratory and Critical Care Medicine. 2022;205(9):1102–1111. doi: 10.1164/RCCM.202105-1118OC/SUPPL_FILE/DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171(6):1437–1452.:e17. doi: 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang H, Leiper J, Lao KH, Dowsett L, Delahaye MW, Barnes G, et al. Role of asymmetric methylarginine and connexin 43 in the regulation of pulmonary endothelial function. Pulmonary Circulation. 2013;3(3):675. doi: 10.1086/674440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Zhang L, Marsboom G, Jambusaria A, Xiong S, Toth PT, et al. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nature Communications. 2019;10(1):2126. doi: 10.1038/s41467-019-10134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nature Medicine. 2015;21(7):777–785. doi: 10.1038/nm.3877. 2015 21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veith C, Vartürk-Özcan I, Wujak M, Hadzic S, Wu CY, Knoepp F, et al. SPARC, a Novel Regulator of Vascular Cell Function in Pulmonary Hypertension. Circulation. 2022;145(12):916–933. doi: 10.1161/CIRCULATIONAHA.121.057001. [DOI] [PubMed] [Google Scholar]
- 22.Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovascular Research. 2004;61(2):227–237. doi: 10.1016/J.CARDIORES.2003.11.026/2/61-2-227-FIG3.GIF. [DOI] [PubMed] [Google Scholar]
- 23.Szulcek R, Happe CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension: Role of platelet endothelial cell adhesion molecule-1 cleavage. American Journal of Respiratory and Critical Care Medicine. 2016;193(12):1410–1420. doi: 10.1164/RCCM.201506-1231OC/SUPPL_FILE/DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damico R, Kolb TM, Valera L, Wang L, Housten T, Tedford RJ, et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2015;191(2):208–218. doi: 10.1164/RCCM.201409-1742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, et al. Correction of Sickle Cell Disease in Adult Mice by Interference with Fetal Hemoglobin Silencing. Science (New York NY) 2011;334(6058):993. doi: 10.1126/SCIENCE.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371. doi: 10.1038/NATURE13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golpon HA, Geraci MW, Moore MD, Miller HL, Miller GJ, Tuder RM, et al. HOX Genes in Human Lung: Altered Expression in Primary Pulmonary Hypertension and Emphysema. The American Journal of Pathology. 2001;158(3):955. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jandl K, Marsh LM, Hoffmann J, Mutgan AC, Baum O, Bloch W, et al. Basement membrane remodeling controls endothelial function in idiopathic pulmonary arterial hypertension. American Journal of Respiratory Cell and Molecular Biology. 2020;63(1):104–117. doi: 10.1165/RCMB.2019-0303OC/SUPPL_FILE/DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 29.Mohamedi Y, Fontanil T, Cal S, Cobo T, Obaya ÁJ. ADAMTS-12: Functions and Challenges for a Complex Metalloprotease. Frontiers in Molecular Biosciences. 2021;8:378. doi: 10.3389/FMOLB.2021.686763/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. European Respiratory Journal. 2012;40(3):766–782. doi: 10.1183/09031936.00209911. [DOI] [PubMed] [Google Scholar]
- 31.Alonso M, García-Redondo AB, Guo D, Camafeita E, Martínez F, Alfranca A, et al. Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circulation Research. 2015;117(2):e13–e26. doi: 10.1161/CIRCRESAHA.117.305108. [DOI] [PubMed] [Google Scholar]
- 32.Zhu N, Welch CL, Wang J, Allen PM, Gonzaga-Jauregui C, Ma L, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Medicine. 2018;10(1):56. doi: 10.1186/s13073-018-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, del Rosario P, Bill M, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. European Respiratory Journal. 2017;50(3):1602449. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 34.Paddenberg R, Stieger P, von Lilien AL, Faulhammer P, Goldenberg A, Tillmanns HH, et al. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Respiratory Research. 2007;8(1):1–12. doi: 10.1186/1465-9921-8-15/FIGURES/6_553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon M, Gomberg-Maitland M, Oudiz RJ, Machado RF, Rischard FP, Elinoff JM, et al. ABI-009, nab-Sirolimus, an mTOR Inhibitor with High Lung Accumulation in Preclinical Models Is Active in Patients with Severe Pulmonary Arterial Hypertension; American Thoracic Society International Conference Meetings Abstracts American Thoracic Society International Conference Meetings Abstracts; 2019. A4409. [DOI] [Google Scholar]
- 36.Zahler S, Liebl J, Fürst R, Vollmar AM. Anti-angiogenic potential of small molecular inhibitors of cyclin dependent kinases in vitro. Angiogenesis. 2010;13(3):239–249. doi: 10.1007/S10456-010-9181-1/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Kim IKI, Ahn JS, Woo DC, Kim ST, Song S, et al. Deficiency of endothelium-specific transcription factor Sox17 induces intracranial aneurysm. Circulation. 2015;131(11):995–1005. doi: 10.1161/CIRCULATIONAHA.114.012568. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L, et al. Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nature Communications. 2019;10(1):1–17. doi: 10.1038/s41467-019-10135-x. 2019 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamhamedi SE, Menegaz BA, Ramamoorthy V, Aiyer RA, Maywald RL, Buford AS, et al. An Oral Formulation of YK-4-279: Preclinical Efficacy and Acquired Resistance Patterns in Ewing Sarcoma. Molecular Cancer Therapeutics. 2015;14(7):1591–1604. doi: 10.1158/1535-7163.MCT-14-0334. [DOI] [PubMed] [Google Scholar]
- 40.Sizemore GM, Pitarresi JR, Balakrishnan S, Ostrowski MC. The ETS family of oncogenic transcription factors in solid tumours. Nature Reviews Cancer. 2017;17(6):337–351. doi: 10.1038/nrc.2017.20. 2017 17:6. [DOI] [PubMed] [Google Scholar]
- 41.Craig MP, Sumanas S. ETS Transcription Factors in Embryonic Vascular Development. Angiogenesis. 2016;19(3):275. doi: 10.1007/S10456-016-9511-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, et al. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114(5):1123–1130. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalna V, Yang Y, Peghaire CR, Frudd K, Hannah R, Shah Av, et al. The Transcription Factor ERG Regulates Super-Enhancers Associated With an Endothelial-Specific Gene Expression Program. Circulation Research. 2019;124(9):1337. doi: 10.1161/CIRCRESAHA.118.313788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development (Cambridge, England) 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 45.Clarke RL, Yzaguirre AD, Yashiro-Ohtani Y, Bondue A, Blanpain C, Pear WS, et al. The expression of Sox17 identifies and regulates haemogenic endothelium. Nature Cell Biology. 2013;15(5):502–510. doi: 10.1038/ncb2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soon Park C, Hyun Kim S, Young Yang H, Kim JH, Theo Schermuly R, Seul Cho Y, et al. Sox17 Deficiency Promotes Pulmonary Arterial Hypertension via HGF (Hepatocyte Grow Factor)/c-Met Signaling. Circulation research. 2022:101161CIRCRESAHA122320845. doi: 10.1161/CIRCRESAHA.122.320845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu M, Zhang L, Marsboom G, Jambusaria A, Xiong S, Toth PT, et al. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nature Communications. 2019;10(1):2126. doi: 10.1038/s41467-019-10134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corada M, Orsenigo F, Bhat GP, Conze LL, Breviario F, Cunha SI, et al. Fine-Tuning of Sox17 and Canonical Wnt Coordinates the Permeability Properties of the Blood-Brain Barrier. Circulation Research. 2019;124(4):511. doi: 10.1161/CIRCRESAHA.118.313316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beucher A, Cebola I. One-step dual CRISPR/Cas9 guide RNA cloning protocol. 2019 doi: 10.21203/RS.2.1831/V1. [DOI] [Google Scholar]
- 50.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nature Methods. 2017;14(4):417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences [version 2; referees: 2 approved] F1000Research. 2016;4 doi: 10.12688/F1000RESEARCH.7563.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blighe K, Sharmila R, Myles L. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. 2018 [Google Scholar]
- 54.Yates B, Braschi B, Gray KA, Seal RL, Tweedie S, Bruford EA. Genenames.org: the HGNC and VGNC resources in 2017. Nucleic acids research. 2017;45(D1):D619–D625. doi: 10.1093/nar/gkw1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.