Lipoprotein(a) [Lp(a)] is a circulating lipoprotein with proatherogenic, proinflammatory, and possibly prothrombotic properties. Circulating Lp(a) levels are largely genetically determined, particularly by the LPA gene. As such, genetic variants at the LPA locus can serve as instrumental variables for investigating the clinical effects of circulating Lp(a) levels. Mendelian randomization (MR) studies have shown that elevated Lp(a) levels are associated with higher risk of coronary artery disease1–3 and aortic valve stenosis.2–4 Evidence on the causal role of elevated Lp(a) levels for other atherosclerotic and specific valvular diseases is limited, although there is MR data supporting a positive association between genetically-predicted Lp(a) levels and peripheral artery disease.2,3 Whether Lp(a) is causally related to thrombotic disease and cerebrovascular disease remains unclear.2,3,5
In this study, we used the UK Biobank cohort to perform an MR investigation into the causal effects of circulating Lp(a) levels on atherosclerotic, cerebrovascular, thrombotic and valvular diseases. As a recent MR study provided evidence of an inverse association of Lp(a) levels with Alzheimer’s disease,5 we additionally explored whether genetically-predicted Lp(a) levels are associated with Alzheimer’s disease and dementia.
This study included outcomes from 367 586 unrelated European-descent UK Biobank participants, which were defined based on International Diagnostic Classification of Diseases and Health Related Problems codes, and self-reported data validated by interview with a nurse. Incident cases were recorded until March 31, 2017 and deaths until February 14, 2018. The UK Biobank was approved by the North West Multicenter Research Ethics Committee, and all participants provided written informed consent.
We used a genetic instrument comprising 43 single-nucleotide polymorphisms (SNPs) (at the LPA locus) conditionally associated with Lp(a) levels at genome-wide significance in 27 540 European-descent participants from the CHD Exome+ Consortium (no overlap with UK Biobank).1 Genetic associations with Lp(a) were taken from the CHD Exome+ Consortium, and were additionally validated in UK Biobank. The instrument explained 61.0% of the variance in Lp(a) levels in UK Biobank. Effect sizes of the SNP–outcome associations were estimated in UK Biobank using logistic regression under an additive model with adjustment for age, sex, and 10 principal components of ancestry. MR estimates were obtained using the inverse-variance weighted method with adjustment for correlations among the SNPs.
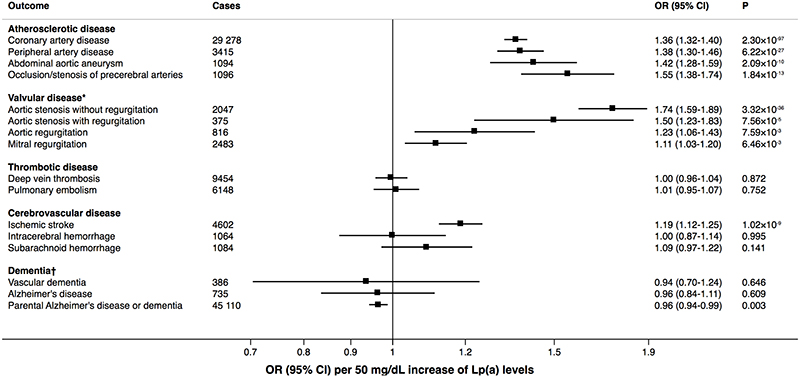
The associations with the outcomes per genetically predicted 50 mg/dL increase in Lp(a) levels are shown in the Figure. Our results supported a strong causal relationship between Lp(a) levels and coronary artery disease. The observed odds ratio was 1.36 (95% confidence interval 1.32-1.40), which is similar to the previously observed association in the CHD Exome+ Consortium (odds ratio rescaled per 50 mg/dL increment of Lp(a) levels, 1.35; 95% confidence interval 1.29-1.41).1 We observed even stronger associations between genetically-predicted Lp(a) levels and peripheral artery disease, abdominal aortic aneurysm, occlusion or stenosis of precerebral arteries, and aortic stenosis with or without regurgitation. Novel findings were positive associations of genetically predicted Lp(a) levels with both aortic and mitral regurgitation. Our findings supported a previously reported modest association between genetically-predicted Lp(a) levels and ischemic stroke2,5 as well as a null association with venous thromboembolism.2,3 We found null associations with hemorrhagic stroke subtypes. Although we found null associations with Alzheimer’s disease and vascular dementia, possibly due to lack of power, our study revealed a weak inverse association of genetically-predicted Lp(a) levels with self-reported parental history of Alzheimer’s disease or dementia. All results were consistent when using a genetic instrument comprising the two SNPs (rs10455872 and rs3798220) used in several previous MR studies.3–5
Figure. Associations of genetically-predicted Lp(a) levels with atherosclerotic, valvular, thrombotic, and cerebrovascular diseases and dementia.
*Excluding rheumatic valvular disorders. †Vascular dementia and Alzheimer’s disease are defined based on an individual’s routinely-collected medical outcomes, whereas parental Alzheimer’s disease or dementia is defined as self-report of a family history of Alzheimer’s disease or dementia. Genetic associations with parental Alzheimer’s disease or dementia are less likely to suffer from selection bias or survivor bias, as participation in UK Biobank is less likely to be influenced by the medical history of an individual’s parents.
The mechanism behind the associations of Lp(a) levels with aortic regurgitation without concomitant aortic stenosis and mitral regurgitation is unclear but could possibly be related to degenerative change from calcific aortic valve disease known to be associated with Lp(a) levels. Aortic valve sclerosis represents a significant proportion of the underlying etiology of also isolated aortic regurgitation. Likewise, mitral annular calcification may interfere with mitral valve closure and increase mitral regurgitation, which represented over 90% of all mitral valve disease cases. Finally, aortic stenosis may also create or worsen mitral regurgitation.
An advantage of our study is that we assess and compare the associations of genetically-predicted Lp(a) levels with atherosclerotic, cerebrovascular, thrombotic and valvular diseases in a single population of European-descent individuals. This, however, limited the generalizability of our results to other populations. Another limitation is that outcomes were partially defined by validated self-reported disease, which could lead to some misclassification of outcome.
In conclusion, this MR study supported Lp(a) as a causal risk factor for atherosclerotic and valvular diseases but not thrombotic disease and hemorrhagic stroke subtypes. These findings may be used to inform the design of further research towards the treatment and prevention of atherosclerotic and valvular diseases. Whether lowered Lp(a) levels increase the risk of dementia needs further investigation.
TOP statement.
The data that support the findings of this study are available from the corresponding author upon reasonable request. The UK Biobank data is available upon application (http://www.ukbiobank.ac.uk/register-apply).
Acknowledgments
This research has been conducted using the UK Biobank resource (Application 29202). The UK Biobank data is available on application (http://www.ukbiobank.ac.uk/register-apply).
Sources of Funding
S.C.L. acknowledges support from the Swedish Heart-Lung Foundation (Hjärt-Lungfonden, grant number 20190247), the Swedish Research Council (Vetenskapsrådet, grant number 2019-00977), and the Swedish Research Council for Health, Working Life and Welfare (Forte, grant number 2018-00123). D.G. is funded by the Wellcome 4i Clinical PhD Program at Imperial College London. S.B. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (award number 204623/Z/16/Z). S.B. and A.S.B. report funding from Novartis relating to the investigation of lipoprotein(a). The funder had no influence on the content of the investigation or the decision to publish.
Footnotes
Disclosures
A.S.B. has received grants from AstraZeneca, Biogen, Bioverativ, Merck, Sanofi outside of this work.
References
- 1.Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018;3:619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, Bjornsson E, Norddahl GL, Jonasdottir A, Jonasdottir A, et al. Lipoprotein(a) Concentration and Risks of Cardiovascular Disease and Diabetes. J Am Coll Cardiol. 2019;74:2982–2994. doi: 10.1016/j.jacc.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Emdin CA, Khera AV, Natarajan P, Klarin D, Won HH, Peloso GM, Stitziel NO, Nomura A, Zekavat SM, Bick AG, et al. Phenotypic Characterization of Genetically Lowered Human Lipoprotein(a) Levels. J Am Coll Cardiol. 2016;68:2761–2772. doi: 10.1016/j.jacc.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan Y, Li H, Wang Y, Meng X, Wang Y. Causal Effect of Lp(a) [Lipoprotein(a)] Level on Ischemic Stroke and Alzheimer Disease: A Mendelian Randomization Study. Stroke. 2019;50:3532–3539. doi: 10.1161/STROKEAHA.119.026872. [DOI] [PubMed] [Google Scholar]