Abstract
Background
Treatment of Candida albicans bloodstream infection with fluconazole is associated with significant mortality despite in vitro susceptibility to the drug.
Objectives
We sought to determine whether tolerance to fluconazole is predictive of treatment failure.
Methods
We reviewed patients with monomicrobial C albicans bloodstream infection who received primary monotherapy with fluconazole. Tolerance to fluconazole, defined as the fraction of growth above the MIC, was quantified using the disc diffusion assay and digital image analyses. Survival analyses were performed with host and treatment factors as predictive variables.
Results
Among 44 patients included in the study, all-cause mortality was 29.5% at 30 days and 43.1% at 12 weeks. Forty-one isolates (93%) were susceptible to fluconazole (MIC50, 0.5 mg/L). Fluconazole tolerance was strongly associated with death for patients treated with fluconazole within 24 h of candidemia onset (33.3% vs 0%; p = .007), but not among patients whose treatment was started later. MIC did not correlate with survival, regardless of treatment delay. A Cox regression model including time to treatment, tolerance to fluconazole, fluconazole exposure and Pitt bacteraemia score provided good prediction of treatment outcome (area under the receiveroperator curve, 0.82).
Conclusions
In patients with C albicans bloodstream infection, tolerance testing was predictive of fluconazole efficacy if the drug was started early. Further study is required to validate the utility of this metric to guide treatment choices.
Keywords: Candida albicans, candidemia, fluconazole, heteroresistance, resistance, survival, tolerance
1. Introduction
Candida species rank high among nosocomial causes of bloodstream infection and are associated with higher rates of acute mortality compared to leading bacterial pathogens.1,2 Clinical trials and meta-analyses have demonstrated the superiority of echinocandins over fluconazole for the treatment of invasive candidiasis,3,4 driving the adoption of echinocandins as front-line agents.5,6 There is concern, however, that this shift may promote the emergence of echinocandin-resistant Candida strains, a trend already apparent in some hospitals.7 Current Infectious Diseases Society of America directives consider fluconazole an acceptable alternative to echinocandins for patients who are not critically ill and are considered unlikely to harbour fluconazole-resistant Candida strains.6 Of note, resistance to fluconazole is largely restricted to tertiary medical centres caring for immunocompromised patients, where azoles are often used prophylactically.8 De-escalation from an echinocandin to fluconazole, once bloodstream infection has been cleared and the patient is clinically stable, is another strategy used to limit echinocandin use.6
Importantly, although Candida strains with intrinsic or acquired fluconazole resistance are a concern, the majority of patients who die of candidemia are infected with isolates defined as drug-susceptible by accepted MIC breakpoints.4,8,9 Mortality of patients with candidemia is influenced by host characteristics, including immune status and comorbidity, severity of sepsis and associated acute organ damage, and treatment-related factors, such as source-control, drug pharmacokinetics, pharmacodynamics, in vitro antifungal drug activity against the causative organism and timing of treatment initiation. An important unanswered question is whether treatment outcomes are affected by subtle variations in the drug response of Candida isolates that have fluconazole MIC in the susceptible range.
Tolerance refers to intrastrain cell-to-cell variability in drug response10 that is not measured in routine clinical microbiological assays. We recently described tolerance to fluconazole in clinical C albicans and C glabrata isolates11,12 which is readily detected and quantified using a simple disc diffusion assay coupled with automated image analysis.11,13 Fluconazole tolerance was associated with failure to eradicate haematogenous Candida infections in experimentally infected Galleria mellonella and in patients.11 A related phenomenon, fluconazole heteroresistance, was also associated with failure to eradicate haematogenous C glabrata infections in a mouse model.12
Here, we asked if the initial fluconazole tolerance levels of Candida bloodstream isolates affected patient mortality. We assessed the effect of fluconazole tolerance and other treatment-related factors on the clinical outcome of patients with Candida bloodstream infections that were initially treated with fluconazole. We focused on patients with bloodstream infection caused by C albicans, a species that only rarely acquires resistance to fluconazole, and is the most frequent cause of invasive candidiasis.8
2. Materials And Methods
2.1. Patients
We identified patients with C albicans bloodstream infection by querying the computerised database of the Tel Aviv Sourasky Medical Center (TASMC) clinical microbiology laboratory between January 2005 and December 2015. Patients were included in this study if they met the following criteria: (a) at least one positive blood culture for C albicans; (b) no concomitant isolation of bacteria or fungi from blood culture within 48 h of the first date of candidemia; (c) age at time of candidemia 18 years or older; (d) initial treatment with fluconazole, which continued for at least 4 days. Demographics, clinical and laboratory data were collected from patient medical records. Sequential organ failure assessment (SOFA) score, Pitt bacteraemia score and Charlson score were calculated for each patient.14–16 The primary study outcome was 30-day survival.
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The study protocol was reviewed and approved by the TASMC institutional ethics committee. Requirement for informed consent was waived given the retrospective and observational nature of the study.
2.2. Microbiological methods
Blood cultures were drawn from hospitalised patients and yeast growth was detected by the BacT/Alert system (bioMerieux, Marcy L'Etoile, France). Initial identification of C albicans was done using growth on CHROMagar (bioMerieux) and the Vitek2 system (bioMerieux) with the YST ID card. Species identification was confirmed using PCR amplification and sequencing of the internal transcribed spacer (ITS) region of ribosomal DNA. The minimum inhibitory concentrations of fluconazole were determined using broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 methodology.17 Trailing growth was defined as residual growth at drug concentrations above the MIC in at least four consecutive wells.18
2.3. Disc diffusion assay for fluconazole tolerance
Candida isolates were stored in 50% glycerol at −80°C until testing and analyses. Tolerance to fluconazole was determined using the disc diffusion assay outlined in the CLSI M44-A2 methods, with previously described modifications.11 In brief, strains were streaked from glycerol stocks onto YPD agar and incubated for 24 h at 30°C. Colonies were suspended in 1 ml PBS and diluted to 1 × 106 cells/ml. Cells (2 × 105) were spread onto 15 ml casitone plates (9 g/L Bacto casitone, 5 g/L yeast extract, 15 g/L Bacto agar, 11.5 g/L sodium citrate dehydrate and 2% glucose, 0.04 g/L adenine, 0.08 g/L uridine). A single 25 μg fluconazole disc (6 mm, Oxoid, UK or Liofilchem, Italy) was placed in the centre of each plate. Plates were then incubated at 30°C for 48 h, and each plate was photographed individually. Analysis of the disc diffusion assay was done using the diskImageR pipeline 31 and the R script available at https://github.com/acgerstein/diskImageR/blob/master/inst/walkthrough.R. The algorithm measures pixel intensity, corresponding to cell density, along 72 radii every 5° (Figure S1).13 Tolerance to fluconazole was expressed as the fraction of growth (FoG) inside the zone of inhibition, defined as the area under the curve at a given radius, normalised to growth outside the zone of inhibition. Calculations were performed for a zone of inhibition defined by an average radius corresponding to 20% growth reduction (FoG20).
2.4. Fluconazole pharmacokinetics
We assessed three fluconazole exposure parameters for their interaction with overall survival: fluconazole dose, dose/MIC and area under the fluconazole 24 h concentration-time curve (AUC24)/MIC. Renal fluconazole clearance was calculated using a previously published equation that models fluconazole clearance as a function of creatinine clearance.19 AUC24 was calculated as the fluconazole daily dose divided by fluconazole clearance. Fluconazole exposure parameter cut-offs associated with patient survival were determined using classification and regression tree (CART) analyses.
2.5. Statistical analyses
To establish a FoG20 breakpoint associated with treatment failure, we first compared the FoG20 distribution of survivors and non-survivors within 30 days from the onset of candidemia, using the non-parametric Wilcoxon rank sum test. Because delayed effective antifungal treatment was repeatedly found to be associated with treatment failure,20,21 we assessed the interaction of each treatment-related variable with overall survival separately in patients whose treatment was started within or after 24 h of candidemia onset (early fluconazole and late fluconazole groups).
Survival analyses were performed to determine the effects of patient variables (age, sex, comorbidity), infection severity variables (Pitt and SOFA scores), and treatment variables (time to initiation of fluconazole, vascular catheter removal, fluconazole exposure, resistance and tolerance) on the primary outcome. Kaplan-Meier survival curves were compared using the Cox test of equality. Multivariate Cox regression was performed using treatment and disease variables that affected outcome in the Kaplan-Meier analyses. The proportional hazards assumption was tested and confirmed for each variable after model fitting. Concordance of the model prediction with actual outcomes was calculated using the area under the receiver-operator curve (ROC).
For all statistical tests, a type 1 error of <.05 was considered to be statistically significant. Statistical analyses were performed with Stata software (College Station, Texas).
3. Results
The study cohort included 44 patients with C albicans bloodstream infection who received primary fluconazole treatment (Table 1). The median patient age was 70 years (range, 30-94 years); 27 (61.3%) were males. Median Charlson comorbidity score was 5 (interquartile range, 3 to 6), indicating a high burden of comorbidity in this cohort. Six patients (13.6%) were neutropenic. Sources of candidemia were a central venous catheter in 19 patients (43.1%), the gastrointestinal tract in 6 (13.6%), and the urinary tract in 5 (11.3%). No definitive source was found in 14 patients. Severity of sepsis was generally high, as evident from SOFA and Pitt scores, admission to the intensive care unit and requirement for inotropic support (Table 1). Overall mortality rate was 29.5% (13/44) at 30 days and 43.1% (19/44) at 12 weeks from the date of candidemia onset.
Table 1. Characteristics of study cohort.
| Characteristic | Value |
|---|---|
| Age, years median (range) | 70 (30-94) |
| Sex | |
| Male | 27 (61.3) |
| Female | 17 (38.6) |
| Solid malignancy | 17 (38.6) |
| Haematologic malignancy | 9 (20.4) |
| Neutropenia | 6 (13.6) |
| Dialysis | 8 (18.1) |
| Central venous catheter | 24 (54.5) |
| Total parenteral nutrition | 3 (6.9) |
| Dopamine/norepinephrine | 14 (32.5) |
| Intensive care unit | 12 (26) |
| Charlson score, median (IQR) | 5 (3-6) |
| SOFA score, median (IQR) | 4 (2-6) |
| Pitt score, median (IQR) | 1.5 (0-4) |
Note: All values represent number (per cent) unless stated otherwise. Abbreviations: IQR, interquartile range; SOFA, sequential organ failure assessment.
3.1. Fluconazole susceptibility and tolerance
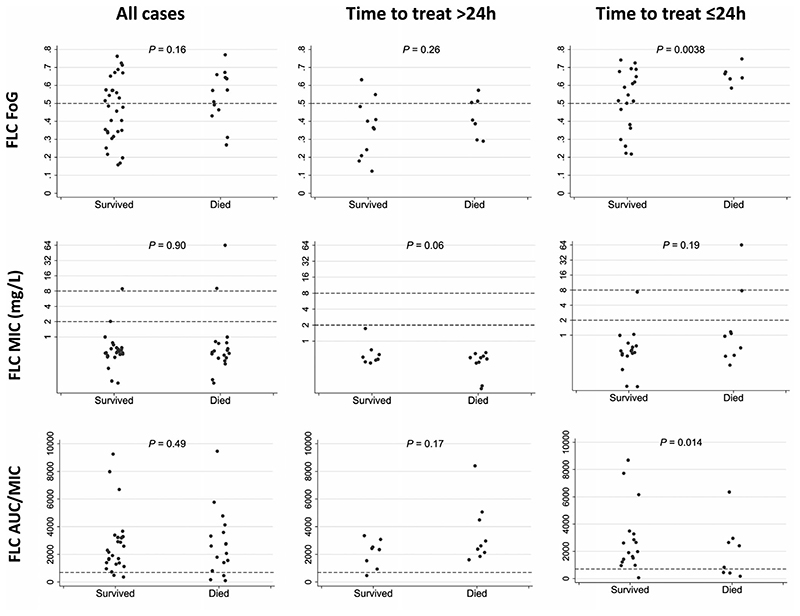
The fluconazole MIC50 and MIC90, determined by broth microdilution, were 0.5 mg/L and 1 mg/L, respectively. None of the isolates displayed significant trailing growth. Three C albicans isolates (6.8%) were non-susceptible to fluconazole (MIC > 4 mg/L). Fluconazole MIC values were similar between patients who died and those who survived to 30 days (median [interquartile range, IQR], 0.5 mg/L [0.38-1 mg/L] and 0.5 mg/L [0.5-0.5 mg/L], respectively, p = .9; Figure 1). There was no difference in MIC values between survivors and non-survivors in the subgroup of patients who received the first dose of fluconazole within 24 hours of candidemia onset (n = 26; p = .19).
Figure 1.
Distribution of fluconazole exposure and susceptibility parameters among survivors and non-survivors. FLC, Fluconazole; FoG, fraction of growth within a fluconazole disc diffusion assay zone of inhibition defined by 20% growth reduction (see 2.3 for details); MIC: minimal inhibitory concentration; AUC/MIC: (area under the 24-h fluconazole concentration curve)/(fluconazole minimal inhibitory concentration [mg/L]). Horizontal dashed lines indicate: Fluconazole FoG 0.5 breakpoint (see text); Fluconazole MIC susceptible, susceptible dose-dependent and resistant fluconazole concentration thresholds; and Fluconazole AUC24/MIC 700 breakpoint (see text)
Fluconazole tolerance was determined for all C albicans isolates using the disc diffusion assay and expressed as the fraction of growth (FoG) within a zone of inhibition defined by 20% growth reduction (FoG20; see 2.3 for details). The mean FoG20 value was 0.48 ± 0.16 (range, 0.14–0.76). FoG20 values were not significantly different between patients who died and those who survived to 30 days: 0.53 ± 0.14 and 0.46 ± 0.17, respectively, P = .16. However, for the subgroup of patients who were started on fluconazole treatment within 24 h of candidemia onset, FoG20 values differed significantly between non-survivors and survivors: 0.65 ± 0.05 versus 0.51 ± 0.16, respectively (p = .0038). In the early treatment group, 6 of 18 patients (33.3%) with FoG20 values > 0.5 died within 30 days of candidemia, versus 0 of 8 patients (0%) with FoG20 < 0.5, suggesting that a FoG20 cut-off of 0.5 may be used to predict fluconazole treatment success (Figure 1).
3.2. Fluconazole exposure parameters
The median fluconazole dose was 400 mg/day (range, 200–800 mg per day). Fluconazole median (IQR) dose/MIC and AUC24/MIC values were 800 (400-1052) and 2355 (1475-3103), respectively. Fluconazole daily dose, dose/MIC and AUC24/MIC were not significantly different between survivors and non-survivors, for both early and late fluconazole treatment groups (Figure 1). However, classification and regression tree (CART) analysis identified an association between death and fluconazole AUC24/MIC < 700 in the early treatment group.
3.3. Survival analyses
We assessed the association of chronic comorbidity, acute sepsis scores, fluconazole exposure and tolerance (FoG20) with patient survival using Kaplan-Meier analyses and Cox regression.
3.3.1. Patient characteristics and disease severity
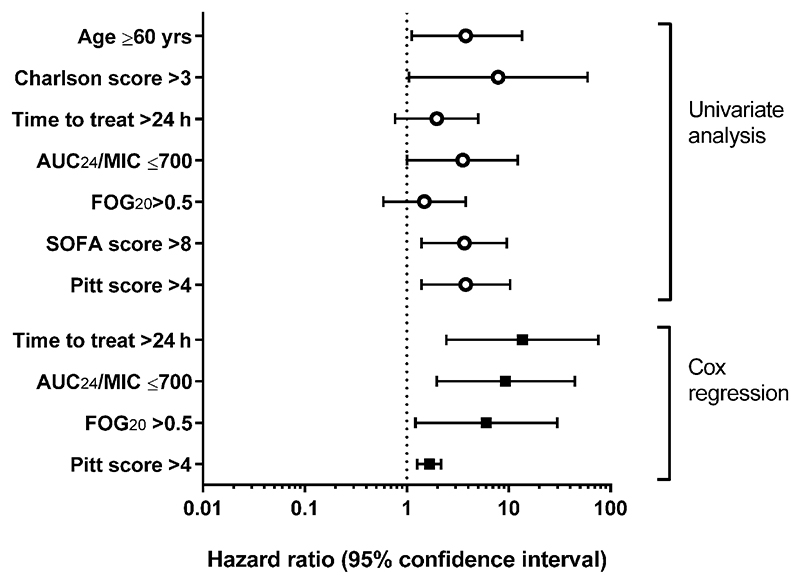
Variables found to be significantly associated with death on univariate analyses were age > 60 years (hazard ratio, 3.8; p = .03), Charlson comorbidity score > 3 (HR, 7.9; p = .04), SOFA score > 6 (HR 3.6; p = .008) and Pitt bacteraemia score > 4 (HR 3.8; p = .009; Figure 2).
Figure 2.
Association of patient and treatment variables with survival rate of patients with candidemia treated with fluconazole. SOFA, sequential organ failure assessment; AUC24/MIC: (area under the 24-hour fluconazole concentration curve)/(fluconazole minimal inhibitory concentration [mg/L]); FoG20: fraction of growth within a fluconazole disc diffusion assay zone of inhibition defined by 20% growth reduction (see Section 2.3 for details)
3.3.2. Treatment variables
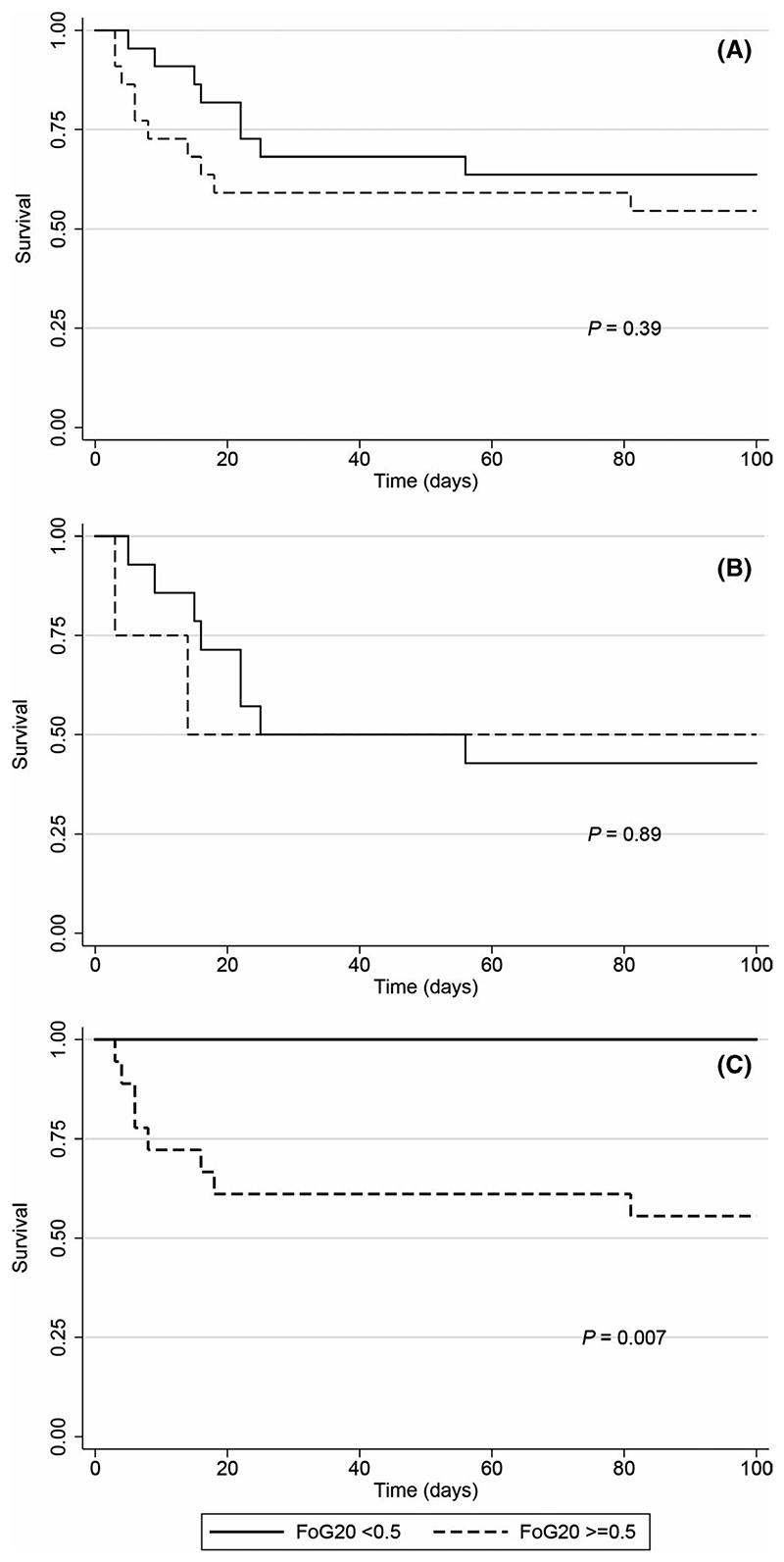
Fluconazole tolerance, as determined by FoG20 values greater than 0.5, was significantly associated with death in the early fluconazole group (HR 7.8; p = .007), but not in the late fluconazole group (Figures 2 and 3). The fluconazole exposure parameter AUC24/MIC < 700 was also significantly associated with death in the early fluconazole group (HR, 5.7; p = .018; Figure 2).
Figure 3.
Fluconazole tolerance and survival of patients with candidemia. Kaplan-Meier survival curves are shown for A. All patients; B. patients who received the first dose of fluconazole >24 h after onset of candidemia; and C. patients who received the first dose of fluconazole <24 h after the onset of candidemia. FoG20: fraction of growth within a fluconazole disc diffusion assay zone of inhibition defined by 20% growth reduction (see Section 2.3 for details)
3.3.3. Cox proportional hazards model
Incorporating treatment and disease parameters into a multivariate Cox regression model, FoG20 > 0.5 (HR, 6.0; p = .021), AUC24/MIC < 700 (HR, 9.3; p = .005), time to treatment initiation > 24 h (HR 13.6, p = .003), and Pitt bacteraemia score > 4 (HR 1.6, p < .001), were all significantly associated with mortality (Figure 2). The area under the ROC for this model was 0.82, indicating good overall prediction of patient outcomes. Patient variables (age and Charlson score) were not retained in the Cox model. Removing either tolerance or AUC24/MIC from the model reduced the ROC to below 0.8, suggesting that these parameters provide complementary information on treatment effectiveness.
4. Discussion
Candida albicans infrequently acquires genetic resistance to fluconazole.22 Genetic resistance is mediated in C albicans by gain-of-function mutations in the transcription factor Upc2, resulting in constitutive upregulation of Erg11,23 and transcription factors Mrr1 and Tac1, causing constitutive upregulation of multidrug efflux pumps. This type of resistance is readily detected in the clinical microbiology laboratory through MIC testing by standardised methods. However, most cases of treatment failure with azoles are not related to genetic resistance. Rather, infections with Candida species with low rates of resistance, such as C albicans, are associated with mortality rates at least as high as those of more resistant species, such as C glabrata.24,25 Tolerance and other modes of phenotypic heterogeneity, although potentially more frequent than genetic resistance, are poorly characterised and remain largely undetected and unreported. Only a few studies have examined the clinical importance of non-inherited resistance in Candida species, using different microbiological methods and definitions for antifungal tolerance.11,18,26 Here, we studied patients with bloodstream infection with predominantly (43/46, 93%) fluconazole-susceptible C albicans, who received primary treatment with this drug. Intrastrain variation in fluconazole response (tolerance) was a significant predictor of treatment failure among patients who received the first dose of fluconazole within 24 h of candidemia onset. Remarkably, none of the patients whose isolates exhibited low tolerance died, versus 50% of patients infected with fluconazole-tolerant isolates.
Tolerance to antimicrobial drugs lacks a clear definition.10 Furthermore, terms used to define different types of non-inherited resistance, such as tolerance, persistence, indifference and heteroresistance, are often used interchangeably. In previous work, we attempted to address this problem by using the disc diffusion assay, which is widely applied in clinical microbiology laboratories, as a highly reproducible assay for fluconazole tolerance.11
The phenomenon of trailing growth, previously reported for Candida species with azoles,18,27–32 may be related to tolerance as quantified by the disc diffusion method in this study. While there is no standardised definition, trailing growth refers to residual fungal growth at drug concentrations above the MIC, resulting in an increase in MIC determined at 48 h relative to 24 h.18,27–32 Trailing growth was shown to be associated with upregulation of efflux transporter expression,30,33 but not with azole resistance as determined using sterol quantitation.28 No association was found between trailing and response to fluconazole treatment in mice.34,35 The CLSI has therefore recommended that fluconazole MIC should be read at 24 h, ignoring any trailing growth.36
There are only a few studies on the clinical relevance of trailing growth. In a recent study by Rueda et al, trailing growth was marginally associated with lower 30-day mortality among patients treated with fluconazole.18 In a study by Hauzer et al, the presence of Candida microcolonies was determined within the E-test inhibition zone after 3 serial passages on E-test/RPMI.26 The presence of microcolonies was not associated with treatment failure.
Unlike trailing, which is a dichotomous parameter determined visually, the FoG20 parameter used here to define tolerance is a quantitative measure determined under strictly standardised conditions. Interestingly, Astvad et al recently assessed heavy C tropicalis trailing and found it to be associated with lower survival in a Galleria mellonella model and impaired Candida clearance from mouse tissue.33 These results suggest that quantitative assessment of tolerance may be important to determine its effect on in vivo treatment response. Moreover, time to initiation of treatment was not considered in any of the studies examining the clinical importance of trailing growth.18,26 This may be a critical point for understanding treatment-related effects on clinical outcomes. Since the efficacy of antifungal treatment is reduced significantly if started >24 h after the onset of candidemia,21,37,38 drug-response parameters probably have little effect on the mortality observed among patients whose treatment was delayed.
This study is limited primarily by its small size, which reflects difficulties in identifying patients with C albicans bloodstream infection treated empirically with fluconazole and by its analysis of samples from a single medical centre. Whether similar rates of tolerance to fluconazole will be found in clinical C albicans isolates from different centres remain to be seen.
5. Conclusions
Our results underscore the critical interdependence of drug exposure and response parameters, time delay from disease onset to treatment initiation, and clinical outcome. Tolerance, as defined and measured here, appears to be a promising in vitro parameter to predict response to fluconazole. Further validation using larger patient cohorts is required to determine its usefulness as a guide for treatment choices.
Supplementary Material
Additional supporting information may be found online in the Supporting Information section.
Acknowledgements
We thank Alex Rosenberg and other members of the Berman lab for helpful guidance and discussions. This work was presented at the 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID).
Funding information
This work was supported by the Israeli Science Foundation Moked programme [grant number 442/18] to RB and the European Research Council Advanced Award [grant number 340087/RAPLODAPT] to JB
Footnotes
Author Contribution
Tal Levinson: Data curation (equal); Investigation (equal); Writingoriginal draft (equal); Writing-review & editing (equal). Alon Dahan: Investigation (equal). Anna Novikov: Data curation (equal); Investigation (equal); Resources (equal). Yael Paran: Validation (equal). Judith Berman: Conceptualization (equal); Data curation (equal); Software (equal). Ronen Ben-Ami: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Project administration (equal); Validation (equal); Visualization (equal); Writing-original draft (equal).
Conflict Of Interest
The authors have no conflict of interest to declare.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 4.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–2482. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 5.Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutro-penic adult patients. ClinMicrobiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 6.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasingechinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory con-centrations. Clin Infect Dis. 2013;56(12):1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Ami R, Rahav G, Elinav H, et al. Distribution of fluconazole-resistant Candida bloodstream isolates among hospitals and inpatient services in Israel. ClinMicrobiol Infect. 2013;19:752–756. doi: 10.1111/1469-0691.12004. [DOI] [PubMed] [Google Scholar]
- 9.Lee I, Morales KH, Zaoutis TE, Fishman NO, Nachamkin I, Lautenbach E. Clinical and economic outcomes of decreased fluconazole susceptibility in patients with Candida glabrata bloodstream infections. Am J Infect Control. 2010;38(9):740–745. doi: 10.1016/j.ajic.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Halfawy OM, Valvano MA. Antimicrobial heteroresistance: an emerging field in need of clarity. ClinMicrobiol Rev. 2015;28(1):191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg A, Ene IV, Bibi M, et al. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun. 2018;9(1):2470–2484. doi: 10.1038/s41467-018-04926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Ami R, Zimmerman O, Finn T, et al. Heteroresistance to fluconazole is a continuously distributed phenotype among Candida glabrata clinical strains associated with in vivo persistence. MBio. 2016;7(4):e00655-00616. doi: 10.1128/mBio.00655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstein AC, Rosenberg A, Hecht I, Berman J. diskImageR: quantification of resistance and tolerance to antimicrobial drugs using disk diffusion assays. Microbiology. 2016;162(7):1059–1068. doi: 10.1099/mic.0.000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 15.Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31(2):146–150. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 16.Vaquero-Herrero MP, Ragozzino S, Castano-Romero F, et al. The Pitt Bacteremia Score, Charlson Comorbidity Index and Chronic Disease Score are useful tools for the prediction of mortality in patients with Candida bloodstream infection. Mycoses. 2017;60(10):676–685. doi: 10.1111/myc.12644. [DOI] [PubMed] [Google Scholar]
- 17.CaLS Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard: M27-A3. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. [Google Scholar]
- 18.Rueda C, Puig-Asensio M, Guinea J, et al. Evaluation of the Possible influence of trailing and paradoxical effects on the clinical outcome of patients with Candidemia. ClinMicrobiol Infect. 2017;23:49.e41–49.e48. doi: 10.1016/j.cmi.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Sobue S, Tan K, Layton G, Leclerc V, Weil A. The effects of renal impairment on the pharmacokinetics and safety of fosfluconazole and fluconazole following a single intravenous bolus injection of fosfluconazole. Br J Clin Pharmacol. 2004;57(6):773–784. doi: 10.1111/j.1365-2125.2004.02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008;36(11):2967–2972. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 21.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: application of CLSI Epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother. 2017;61(10):e00906–e00926. doi: 10.1128/AAC.00906-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flowers SA, Barker KS, Berkow EL, et al. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot Cell. 2012;11(10):1289–1299. doi: 10.1128/EC.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 25.Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37(5):634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 26.Hauzer M, Cohen MJ, Polacheck I, Moses A, Korem M. The prevalence and clinical significance of microcolonies when tested according to contemporary interpretive breakpoints for fluconazole against Candida species using E-test. Med Mycol. 2018;57:718–723. doi: 10.1093/mmy/myy130. [DOI] [PubMed] [Google Scholar]
- 27.Alp S, Sancak B, Hascelik G, Arikan S. Influence of different susceptibility testing methods and media on determination of the relevant fluconazole minimum inhibitory concentrations for heavy trailing Candida isolates with low-high phenotype. Mycoses. 2010;53(6):475–480. doi: 10.1111/j.1439-0507.2009.01739.x. [DOI] [PubMed] [Google Scholar]
- 28.Arthington-Skaggs BA, Lee-Yang W, Ciblak MA, et al. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother. 2002;46(8):2477–2481. doi: 10.1128/AAC.46.8.2477-2481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MK, Kim HR, Kang JO, et al. Susceptibility and trailing growth of Candida albicans to fluconazole: results of a Korean multicentre study. Mycoses. 2007;50(2):148–149. doi: 10.1111/j.1439-0507.2006.01329.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee MK, Williams LE, Warnock DW, Arthington-Skaggs BA. Drug resistance genes and trailing growth in Candida albicans isolates. J AntimicrobChemother. 2004;53(2):217–224. doi: 10.1093/jac/dkh040. [DOI] [PubMed] [Google Scholar]
- 31.Marcos-Zambrano LJ, Escribano P, Sanchez-Carrillo C, Bouza E, Guinea J. Scope and frequency of fluconazole trailing assessed using EUCAST in invasive Candida spp. isolates. Med Mycol. 2016;54(7):733–739. doi: 10.1093/mmy/myw033. [DOI] [PubMed] [Google Scholar]
- 32.Marr KA, Rustad TR, Rex JH, White TC. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43(6):1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astvad KMT, Sanglard D, Delarze E, Hare RK, Arendrup MC. Implications of the EUCAST trailing phenomenon in Candida tropicalis for the in vivo susceptibility in invertebrate and murine models. Antimicrob Agents Chemother. 2018;62(12):e01624–e01634. doi: 10.1128/AAC.01624-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arthington-Skaggs BA, Warnock DW, Morrison CJ. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 2000;44(8):2081–2085. doi: 10.1128/aac.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rex JH, Nelson PW, Paetznick VL, Lozano-Chiu M, Espinel-Ingroff A, Anaissie EJ. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42(1):129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revankar SG, Kirkpatrick WR, McAtee RK, et al. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J ClinMicrobiol. 1998;36(1):153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 38.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.