Abstract
The term superficial siderosis is derived from the Greek word sideros, meaning iron. It includes two subtypes, distinguished by their anatomical distribution, causes and clinical features: “classical” infratentorial superficial siderosis (iSS, which sometimes also affects supratentorial regions); and cortical superficial siderosis (cSS, which affects only supratentorial regions). This paper considers iSS, a potentially disabling disorder usually associated with very slow persistent or intermittent subarachnoid bleeding from a dural defect and characterised by progressive hearing and vestibular impairment, ataxia, myelopathy, and cognitive dysfunction. The causal dural defect - most often spinal but sometimes in the posterior fossa - typically follows trauma or neurosurgery occurring decades before diagnosis. Increasing recognition of iSS with paramagnetic-sensitive -weighted MRI is leading to an unmet clinical need. Given the diagnostic challenges and complex neurological impairments in iSS, we have developed a multidisciplinary approach involving key teams. We discuss pathophysiology, diagnosis and management, including a proposed clinical care pathway.
Keywords: Superficial siderosis, infratentorial, central nervous system, haemosiderin, clinical care pathway
What Is Superficial Siderosis?
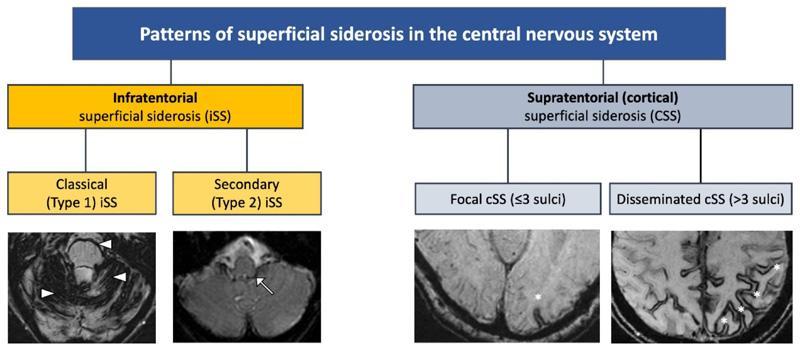
The term superficial siderosis – derived from the Greek word sideros, meaning iron – refers to the deposition of iron-containing compounds generated by blood breakdown (mainly haemosiderin) in the most superficial layers of the tissue of the central nervous system (CNS). The increased detection of siderosis (in large part due to the advent of paramagnetic-sensitive MRI sequences has led to the recognition of two siderosis subtypes, distinguished by their anatomical distribution, causes and clinical features. Classical or infratentorial superficial siderosis (iSS) always affects infratentorial but sometimes also supratentorial regions, while cortical superficial siderosis (cSS) affects only the supratentorial convexities (Figure 1, Table 1) 1 2. We term the first type of siderosis, the main topic of this review, “classical” or Type 1 infratentorial siderosis (iSS), although a range of different terms have previously been used 2 3. We chose the word “classical” as this is the most established form of siderosis, first diagnosed in 1908 and well recognised pathologically and clinically (with a syndrome of deafness, imbalance and ataxia, and sometimes myelopathy) in the pre-MRI era 4 5. The term infratentorial is used because deposition of haemosiderin in this form invariably involves the infratentorial structures but, perhaps confusingly, supratentorial (cortical) involvement is usually also present by the time of diagnosis 1 6. The mechanism underlying classical iSS is a slow, persistent or intermittent low-volume haemorrhage into the subarachnoid space - most often spinal but sometimes in the posterior fossa - typically following cranio-spinal trauma (including brachial plexus avulsion) or neurosurgery decades before diagnosis. Based on this understanding of the pathophysiology we suggested radiological diagnostic criteria of symmetrical haemosiderin deposition in at least two of the infratentorial regions on MRI (cerebellum, brainstem, cranio-cervical junction or spine) 1. We also distinguished a second form of iSS (Type 2 or “secondary” iSS) in which there is more restricted haemosiderin deposition in infratentorial structures, but not as symmetrical or widespread as in Type 1, and with a history of a definite single incident intracranial bleeding event (subarachnoid haemorrhage (SAH) or intracerebral haemorrhage (ICH)); this category is based on a presumption that it is due to “overspill” of blood and haemosiderin deposition from a single intracranial haemorrhage rather than a persistent low-volume leak into the cerebrospinal fluid (CSF).
Figure 1.
Classification and types of superficial siderosis of the central nervous system (CNS). Shown in the left hand panels, type 1 (classical) infratentorial superficial siderosis (iSS) refers to a symmetrical pattern of infratentorial siderosis affecting 2/3 areas of the cerebellum, brainstem or craniocervical junction, often associated with the clinical syndrome of hearing loss, ataxia and imbalance, and myelopathy while type 2 iSS is distinguished by limited and often asymmetrical infratentorial haemosiderin (white arrow). In cortical superficial siderosis (cSS; shown in the right hand panels), haemosiderin is limited to supratentorial structures and can be focal (asterisk) or disseminated (multiple asterisks).
Table 1. Classification and nomenclature of superficial siderosis involving the central nervous system (CNS).
| Superficial siderosis types | Term used | Haemosiderin distribution | Description | |
|---|---|---|---|---|
| Infratentorial superficial siderosis (iSS) | Type 1 | Classical | Infratentorial, involving superior cerebellar vermis and at least one other infratentorial region, as described in proposed radiological diagnostic criteria* In our experience “restricted” iSS at an early stage of the disease (often with minimal clinical symptoms) can involve the superior cerebellar vermis only Note: the proximal VIII (vestibulo-cochlear) cranial nerves are also frequently involved (and probably early in the disease) but siderosis affecting these structures is more difficult to visualise reliably radiologically |
Likely due to chronic low-volume low-pressure extravasation of blood into CSF, usually over decades before diagnosis |
| Type 2 | Secondary | Infratentorial, extensive involvement of the site of intracranial haemorrhage and a thin rim of haemosiderin in adjacent regions often surrounding the outlet of 4th ventricle | Associated with “overspill” of blood from a large single acute intracranial haemorrhagic event: intracranial haemorrhage, subarachnoid haemorrhage, CNS surgery or trauma | |
| Supratentorial cortical superficial siderosis (cSS) | Focal | Supratentorial only, involving cerebral convexities and ≤3 sulci | Associated with previous convexity subarachnoid cortical haemorrhage; in older people (>60 years) the most common cause is cerebral amyloid angiopathy | |
| Disseminated | Supratentorial only, affecting cerebral convexities and >3 sulci | |||
Infratentorial distribution of superficial siderosis that is bilateral, symmetrical and involves at least two of the infratentorial regions: 1) cerebellum (superior cerebellar vermis, folia, peduncles); 2) brainstem (midbrain, pons, medulla), 3) craniocervical junction or spinal cord (if imaging available) 1. Legend: CSF cerebrospinal fluid
More recently, a clinically and radiologically distinct type of superficial siderosis, cortical superficial siderosis (cSS) has been described2 in which haemosiderin is limited to a supratentorial (cortical) distribution (Figure 1, Table 1), in a “tramline” configuration either side of cerebral sulci. In older people, it is most often associated with cerebral amyloid angiopathy (CAA), where rupture of amyloid-laden leptomeningeal arterioles leads to subarachnoid bleeding over the cerebral convexities which evolves to form subpial cortical haemosiderin 2. Similar appearances to cSS can, less commonly, follow other forms of bleeding including traumatic brain injury, aneurysmal SAH, reversible cerebral vasoconstriction syndrome, haemorrhagic transformation of an infarct, dural fistulae or cortical vein thrombosis, but these can usually be differentiated by their clinical or radiological features. cSS is an entirely different disease entity to classical iSS and is not associated with the clinical syndrome of progressive deafness, ataxia and myelopathy 2.
Pathophysiology
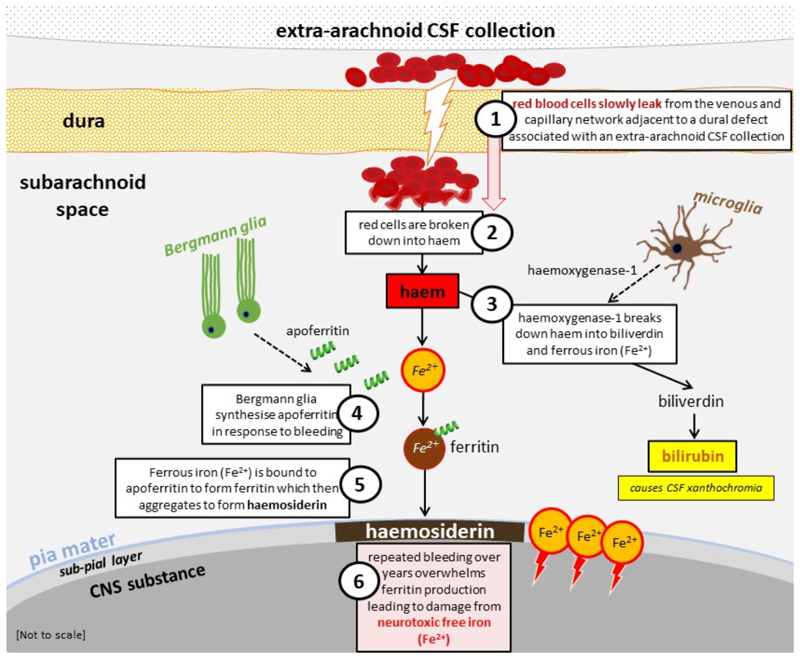
Animal studies and clinical observations indicate that classical iSS is due to a chronic low volume leakage of blood into the subarachnoid space, usually associated with a dural defect occurring many years before clinical diagnosis 1 4 6. Once red cells are extravasated into the CSF their resultant breakdown to haem stimulates microglia to release haemoxygenase-1 and Bergmann glia to synthesise apoferritin 7 8 (Figure 2). Haemoxygenase-1 breaks down haem into biliverdin and neurotoxic free (ferrous) iron. The neurotoxic free (ferrous) iron is bound by apoferritin to form ferritin which then aggregates to form haemosiderin deposited in the subpial layers and leptomeninges 7 8. Histopathologically, haemosiderin is found as large granules in macrophages. Because it neutralises toxic free iron, haemosiderin is hypothesised to be neuroprotective rather than neurotoxic8. However, once ferritin synthesis and the capacity to form haemosiderin is saturated, the unbound (ferrous) iron is the toxic agent that causes tissue injury from lipid peroxidation resulting in gliosis, demyelination and, ultimately, axonal damage 7. Thus, monitoring the extent of haemosiderin deposition as a neuroimaging biomarker may not be a clinically meaningful way of monitoring disease progression as it may not be relevant to clinical disability.
Figure 2.
Diagram of pathophysiological processes implicated in infratentorial superficial siderosis (classical, Type 1); adapted, with permission from Chan et al, 2021 27. Legend: CNS central nervous system; CSF cerebrospinal fluid.
The invariable involvement of cerebellum (particularly, superior vermis, but also flocculus and cerebellar convexities), is likely due to their abundant ferritin-reactive Bergmann microglia (necessary to make haemosiderin), and the pattern of CSF flow which causes early and extensive irrigation of these structures with red cells and iron degradation products 4 7. Both segments of the vestibulo-cochlear nerve are susceptible because of the long glial segment exiting the brainstem at the cerebellopontine angle to reach the porus acousticus of the internal auditory canal 4 8. Selective involvement of other cranial nerves including the olfactory and optic nerves also probably relates to the relative length of their glial portions 4 6. Visual impairment is rare in iSS, possibly due to the macular fibres being most central within the optic nerve protected from the superficial pathophysiological changes 9. Other commonly involved regions in iSS are the brainstem, craniocervical junction, spinal cord and spinal roots, basal frontal lobes and medial temporal lobes.
Underlying Causes
Previous studies suggest long lists of possible causes of iSS but these did not use standardised clinical or radiological criteria for iSS and may have included radiologically detected siderosis not related to the classical syndrome or a mixture of classical (Type 1) and secondary (Type 2) iSS. In our experience, by far the most common cause of iSS, identified in over 80% of patients, is a dural abnormality leak, either in the spine or the posterior fossa 1. Chronic bleeding into the subarachnoid space probably arises from small vessels at the free edges of an acquired dural defect, typically associated with either an extradural collection or pseudomeningocoele. The most common causes of a dural abnormality are previous cranio-spinal trauma (including brachial plexus avulsions), or neurosurgery (Table 2) 1 4.However, why only a small portion of people develop iSS is unknown.
Table 2.
List of likely causes of infratentorial superficial siderosis (iSS), including cranial and spinal tumours reported in the setting of iSS 1 4 10. Legend: AVM arteriovenous malformation, CSF cerebrospinal fluid, SAH subarachnoid haemorrhage.
| Likely causes of iSS (in order of most-to-least frequently reported) | |
|---|---|
| Infratentorial superficial siderosis (classical, Type 1) | |
| Dural abnormalities | Dural defect with extra-arachnoid CSF collection or pseudomeningocoele from previous craniospinal trauma (including brachial plexus avulsion) or neurosurgery |
| Dural ectasia presumed associated with a dural defect | |
| CNS tumours with capacity for slow sustained bleeding into the subarachnoid space | Ependymoma |
| Pituitary adenoma | |
| Melanocytoma | |
| Astrocytoma | |
| Paraganglioma | |
| Metastases | |
| Described in single cases: craniopharyngioma, germ-cell tumour, haemangioblastoma, oligodendroglioma, meningioma, neurinoma, papillary glioneuronal tumour, pinealoma, teratoma | |
| Atypical aneurysms or cavernomas close to the cortical or ependymal surfaces with slow sustained low volume SAH (likely rare) | |
| Infratentorial superficial siderosis (secondary, Type 2) resulting from “overspill” due to a single intracranial bleeding event | |
| Intracerebral haemorrhage (may be “primary” due to cerebral small vessel disease or “secondary” due to a macrovascular cause (e.g., AVM, aneurysm) | Lobar |
| Deep | |
| Aneurysmal SAH | |
| Subdural haemorrhage | |
| Intraoperative haemorrhage | |
In our experience, brachial plexus injury is a frequent cause of iSS in our experience, probably due to the damage to small fragile venous structures related to the nerve roots. Dural abnormalities (e.g., ectasia) in patients with ankylosing spondylitis, neurofibromatosis and Marfan’;s syndrome can also be associated with iSS, presumably also due to slow bleeding from the abnormal distorted dura 1 4.
Vascular tumours - particularly those related to the ependymal surface - can also generate chronic SAH and thus classical siderosis (Table 2) 10. Classical iSS typically does not result from “macrovascular” lesions that cause symptomatic arterial bleeding, for example aneurysms, arteriovenous malformations, cavernomas, or dural fistulae. Thus, cerebral or spinal angiography – previously traditionally widely used to investigate iSS – is not likely to be helpful and is not indicated in the routine investigation of iSS. However, a small proportion of atypical macrovascular lesions can be associated with a slow subarachnoid leak (Table 2).
In our experience, a few patients have no definite history of trauma or neurosurgery, but, rather, a neurological “event” (usually a sudden-onset persistent orthostatic postural headache) attributable to a spontaneous CSF leak, often many years previously which was either ignored or felt to be a non-aneurysmal SAH. The critical history of postural headache following thunderclap headache (often with failed lumbar puncture due to low CSF pressure or identified blood products but no opening pressure recorded and negative tests for aneurysmal SAH) was identified in retrospect. There is limited evidence describing iSS in the setting of spinal dural defects with concomitant spontaneous intracranial hypotension 11–13. In a recent study of 51 patients with persistent spontaneous intracranial hypotension and ventral spinal CSF leaks, superficial siderosis developed in 12% patients and bibrachial amyotrophy in 4% during 280 patient-years of follow-up 14. Whether symptoms of intracranial hypotension, iSS, or both develop may depend on the type of dural defect and rate of subarachnoid bleeding.
Diagnosis of Classical Superficial Siderosis: Radiological Features
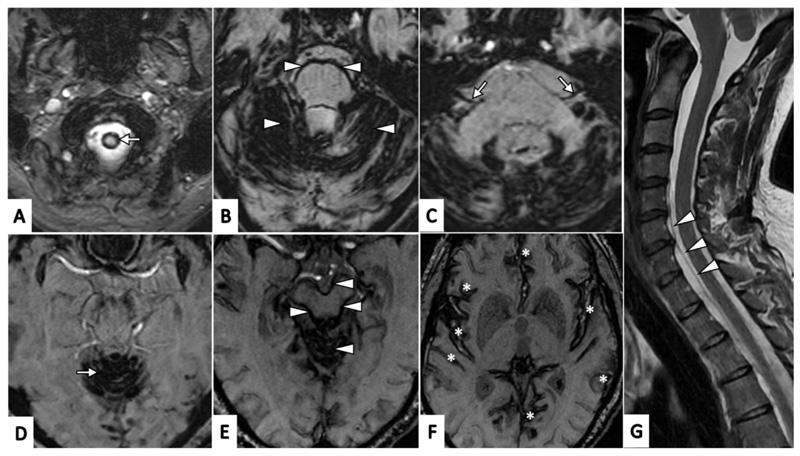
The diagnosis of iSS – previously often achieved at autopsy, intra-operatively or clinically - has become much easier with the widespread use of MRI, which is much more sensitive and specific than computerised tomography (CT) 1 4 9 15. The first radiological report of iSS in 1985 described hypointense rims of haemosiderin along the surfaces of the brain, brainstem, cerebellar folia, optic, trigeminal and vestibulocochlear nerves, and spinal cord on T2-weighted MR images 16. Paramagnetic-sensitive sequences – Gradient-Recalled Echo (GRE) T2* and susceptibility-weighted imaging (SWI) – are more sensitive and specific than T2-weighted MRI for identifying haemosiderin deposits 1 9 17 18. These sequences are therefore essential in confirming or excluding the diagnosis. As mentioned previously, radiological diagnostic criteria for iSS are based on symmetrical haemosiderin distribution involving the superior cerebellar vermis (often extending to cerebellar folia, peduncles or both) and at least one other infratentorial structure (including the brainstem, cranio-cervical junction or spinal cord (Figure 3)) 1. In our experience people with few or no symptoms can have siderosis confined to the superior cerebellar vermis, which may be a very early site of involvement in the disease course. Hypointense appearances on paramagnetic-sensitive MR sequences consistent with haemosiderin deposits can occur with other clinical entities (“mimics”) with distinct patterns and distribution: these include CAA, in which cortical haemosiderin has a “tramline” appearance either side of a cortical sulcus (Figure 1), dural fistulae or cortical vein thrombosis; haemorrhagic transformation of an infarct; or head trauma. These alternative entities usually have distinct clinical and radiological features so are usually not likely to be confused with iSS.
Figure 3. Typical (classical) radiological appearances of haemosiderin deposits in infratentorial superficial siderosis (axial susceptibility weighted MR images, SW MRI, A-F).
A. Craniocervical junction (arrow); B. Haemosiderin involving cerebellar folia with a rim of haemosiderin surrounding the pons (all marked with arrow heads); C. Haemosiderin involving the vestibulo-cochlear nerves (CN VIII) bilaterally (arrows); D. Haemosiderin involving superior vermis (arrow); E. Haemosiderin involving superior vermis with a rim of haemosiderin surrounding the midbrain(all marked with arrowheads); F. Haemosiderin involving supratentorial regions (including the Sylvian fissures, temporal and frontal lobes and cingulate gyri, all marked with asterisks); G. Saggital T2-weighted MRI demonstrating ventral cerebrospinal fluid spinal collection (arrowheads).
How Common is Superficial Siderosis?
iSS is considered rare (defined as less than 1 per 2,000 people in a general European population or fewer than 200,000 in the USA) and is listed in the OrphaNet Rare Diseases Registry (ORPHA:247245) 3 19. Although there have been very few dedicated prevalence studies, iSS was identified in 0.094% (1/1062) individuals in the population-based Rotterdam imaging study, and in 0.14% (2/1412) in a population-based Mayo Clinic Study of Aging (Minnesota) 20 21. In two hospital-based MR studies the prevalence was 0.10% (9/8843) and 0.031% (30/97733)22 23.
What are the Clinical Features of Superficial Siderosis?
iSS is usually characterised by sensorineural hearing loss; imbalance, ataxia or both; and sometimes myelopathic symptoms and signs 4 6. Hearing loss is usually the first symptom. The disease onset and progression are often insidious and slow over years or decades, so that patients commonly present in their second half of life with significant functional decline and often advanced disease by the time of diagnosis. However, iSS may cause a much broader array of symptoms (Box 1).
Box 1. Clinical features associated with infratentorial superficial siderosis (iSS) 1 4 6 10 27 28.
Box 1.
| Clinical features in iSS (in order of most-to-least frequently reported) |
|---|
| Hearing loss |
| Imbalance |
| Gait/truncal ataxia |
| Pyramidal signs |
| Myelopathy-related symptoms (radiculopathy, pain, sensory disturbances) |
| Cognitive dysfunction |
| Bladder disturbance (incontinence, voiding difficulty, urgency, sensory abnormalities) |
| Bowel disturbances (constipation) |
| Symptoms of intracranial hypotension |
| Symptoms associated with arachnoiditis (lower back/sacral pain, sciatica pain) |
| Dysphagia |
| Hydrocephalus |
| Olfactory dysfunction |
| Ageusia |
| Myoclonus |
| Seizures |
| Visual disturbances |
| Cranial nerve palsies |
| Paraparesis/quadriparesis |
Hearing, vestibular and balance dysfunction
Patients nearly always have hearing disturbance and often describe an early symptom of difficulty with hearing in noisy environments. Absence of hearing difficulty should lead to questioning the diagnosis. iSS-related hearing loss is characterised as bilateral sensorineural of both cochlear and retro-cochlear origin 4 24 25. It can be asymmetric, of variable degree, ranging from mild-to-moderate to severe-to-profound 24–26.There is often sparing of low-frequencies and mid-frequencies in the early stages of disease, with down-sloping configuration on pure-tone audiometry (PTA), which may be misdiagnosed as age-related hearing loss 25. Its onset in iSS, however, may be earlier than in age-matched populations 24 25. Gradual involvement of mid- and low-frequencies and overall deterioration of hearing thresholds may occur with disease progression 4 25. There are few dedicated studies on vestibular assessment, describing mixed central (cerebellar) and peripheral vestibular impairment, likely due to involvement of the cerebellar structures, vestibular nerves and end-organs 24 26. Impaired balance is a major symptom, usually with a broad-based ataxic gait, usually worse in the dark; in several cases this may progress to truncal ataxia and inability to sit or walk unassisted.
Cognitive dysfunction
We recently reported cognitive impairment (affecting executive function and performance IQ) in 50% of people with iSS suggesting that this might be an under-recognised core feature of the syndrome 27, but studies in larger unselected cohorts are needed.
Myelopathy and sphincter disturbances
There may be symptoms suggesting myelopathy (e.g., gait disturbance, reduced dexterity, leg stiffness) at the time of diagnosis or they may develop with disease progression 4. Radicular pain may also occur. Bladder and bowel dysfunction can develop in severe disease, manifesting as neurogenic bladder, constipation, urinary or faecal incontinence and reduced sensation during micturition and defaecation 4 6 28.
Olfactory dysfunction
In our experience olfactory symptoms (reduction or loss of smell) are common in iSS, but not often volunteered by patients so should be specifically enquired about; there are no systematic studies available4 6.
Other symptoms
Given the widespread distribution of iSS in both infratentorial and supratentorial regions (see below) it is unsurprising that other neurological symptoms such as epilepsy (focal onset seizures), dysphagia may occur in iSS 1 4 6 10. Severe persistent back pain may be a feature of arachnoiditis associated with iSS 4.
Increasingly, iSS is detected on MR scans of the brain or spine performed for other reasons (including in neurologically healthy individuals). The pattern of siderosis in asymptomatic people in our experience is typically limited to the cerebellum, often the superior vermis but there are no natural history studies available. An important question is whether asymptomatic or minimally symptomatic people with iSS will remain stable or eventually progress to develop the typical syndrome, and whether early investigation and treatment is appropriate.
How should I Investigate a Patient with Superficial Siderosis?
Imaging
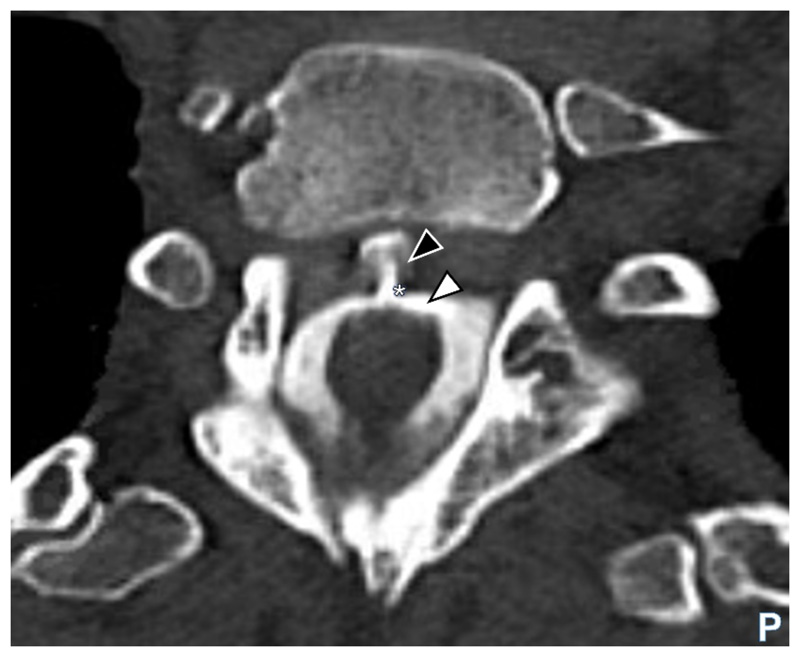
MR of both the brain and whole spine imaging (ideally with paramagnetic-sensitive sequences) is essential to define the pattern of siderosis and seek an extradural collection as a potential source of bleeding (frequently a ventral collection in the thoracic region1). A key goal is to identify a source of bleeding that could be treatable 1 28. When MRI or CT myelography identifies an epidural or extra arachnoid CSF collection (Figure 4), its exact location is often unclear, but must be determined to consider surgical repair. Current reports suggest that dynamic CT myelography and digital subtraction myelography are the most successful modalities for this purpose but the imaging remains a highly specialist technical challenge 29 30. Specialist sequences (e. g., balanced turbo-field echo (BTFE) combined with dynamic improved motion-sensitized driven-equilibrium steady-state free precession (iMSDE SSFP) might be a useful adjunct method to locate the defect but are likely limited to highly-specialist units 31. The decision on appropriate imaging should be made following a discussion with the interventional neuroradiology and neurosurgical teams and after careful consideration of the likely benefit from surgical repair if a leak is identified (Supplemental figure 1) 1. CT, MR and digital subtraction angiography are generally not helpful in investigating classical iSS because macrovascular lesions are unlikely to cause chronic low-volume subarachnoid bleeding 1 28.
Figure 4.
Axial CT myelogram image showing contrast (white arrowhead) in the cerebrospinal fluid (CSF) and clearly visible extravasation of contrast (black arrowhead) at the site of dural defect (asterisk) in the thoracic region. Legend: CT computerised tomography; P posterior.
CSF analysis
CSF analysis is usually performed to check for active or recent bleeding, confirmed by the presence of elevated red cell count (RCC) and ferritin 1 4 28. The RCC is often in the high hundreds or thousands (out of proportion to the white cell count, ratio >500:1), with highest titres in samples nearest to the bleeding site 4. The red count is presumed to reflect very recent bleeding, while CSF ferritin, upregulated in response to subarachnoid bleeding, is likely to be raised for some months in response to haemorrhage32 but the duration of ferritin detection is uncertain and it is not known whether it relates to disease severity or future progression 4 9 28 33. Two patients evaluated in our service showed marked reduction or resolution of CSF ferritin levels following dural defect repair, making it a potential biomarker of treatment success (Figure 5).
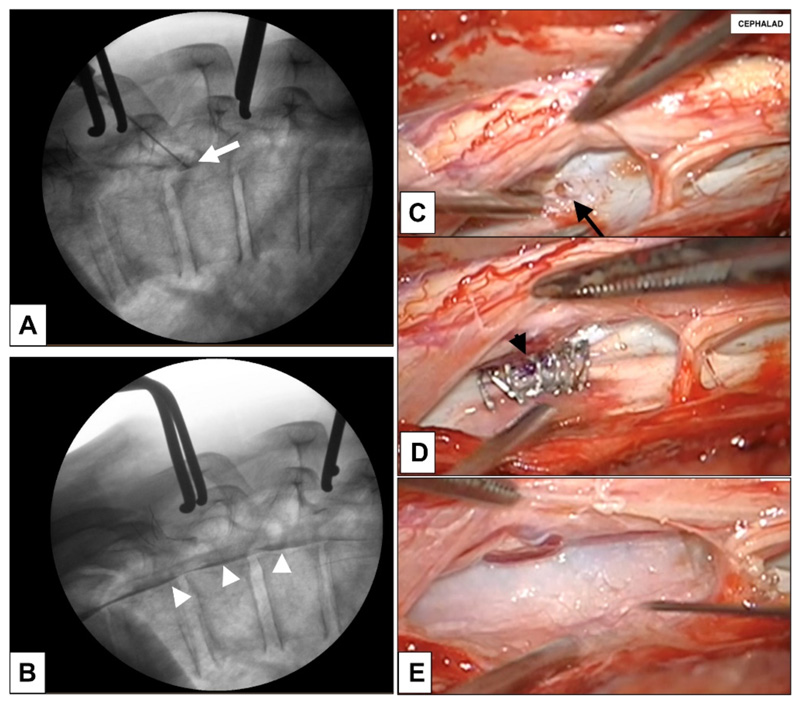
Figure 5.
(A-E). A, B. Intra-operative myelogram showing contrast injected through the ventral dural defect (white arrow) which had shown accumulation of dye in the ventral epidural space (white arrowheads) (B) prior to dural derfet repair; C. intra-operative images showing the 2mm ventral dural defect (black arrow); the arachnoid had prolapsed into the defect thus creating a CSF fistula; in the image, spinal cord has been retracted, and dorsal dura reflected to expose the ventral dural defect; D. following defect repair with 6-0 suture (black arrowhead) and Anastoclips® and (E) reinforced with Durepair™ dural patch and Evicel® tissue glue. Legend: CSF cerebrospinal fluid.
Neuro-otological evaluation
In view of commonly reported audiovestibular impairments, some of which are amenable to treatment, we refer all patients with iSS for neuro-otological evaluation to inform diagnosis, monitoring, management and rehabilitation. A full audiological evaluation in iSS should include pure-tone audiometry (PTA) and speech tests in quiet. Additional tests of otoacoustic emissions, auditory brainstem-evoked responses (ABR), speech-in-noise and “central” tests of hearing can be helpful dictated by the hearing levels, the patient’s symptoms and other characteristics (comorbidities, previous ear, nose and throat (ENT) history, CNS surgery or trauma). We previously identified progressive Interval changes on PTA and ABR in iSS, probably reflecting disease progression 26. Vestibular tests may help to determine the deficit pattern and vestibular rehabilitation plan; potentially useful tests include video-nystagmography and video head-impulse test (contraindicated if there has been recent CNS surgery, ventriculoperitoneal shunting, abnormal intracranial pressure, history of cervical immobility) 24 26 34 35. Caloric testing may be performed to assess (horizontal) semicircular canal/superior vestibular nerve involvement while vestibular evoked myogenic potentials assess otolith end-organ and vestibular nerve function 26.
Neuropsychology
Because in our experience many people with classical iSS report cognitive difficulties our neuropsychology team routinely assesses cognitive function at referral – and sometimes over time – as a means of monitoring for the pattern and progression of cognitive impairment 27. Cognitive difficulties can often be overshadowed by more obvious neurological deficits and are often difficult to assess accurately in people with hearing or movement impairment. In our opinion, specialist neuropsychological assessment is often required over and above brief cognitive and mood screening measures.
How Should I Manage iSS?
There are two broad approaches to neurological management of the bleeding associated with iSS: (1) to identify and, if possible, treat a source of ongoing subarachnoid bleeding; and (2) to reduce the amount and adverse clinical impact of neurotoxic iron using iron-chelating agents such as deferiprone (Supplemental figure 1). Although the goal of treatment is to prevent disease progression and functional decline, there are currently no randomised trial data showing definitive clinical benefit from any treatment for iSS. Nevertheless, based on current understanding of iSS pathophysiology, the most logical treatment goal is to repair the dural defect that is judged to be causing ongoing subarachnoid bleeding 28. We consider surgical dural repair for patients who: (1) are good surgical candidates (i.e. with good preoperative functional status and few severe comorbidities); (2) have confirmed active current or recent bleeding into the subarachnoid space; (3) have evidence of recet and significant clinical progression of iSS-related neurological impairment; (4) have a clear dural defect reliably identified, localised and judged amenable to repair; and (5) are likely to have benefits outweighing the risks of the surgery 28. Surgical repair of the identified dural defect – that is, closure of posterior fossa pseudomeningocoele 36, spinal ventral dural defect 37 or cervical pseudomeningocoele secondary to brachial plexus avulsion 38, can lead to biochemical resolution of red cells, xanthochromia or ferritin in CSF. However, data regarding clinical outcomes are limited by small cohorts, short follow-up, lack of control groups, and advanced symptoms at the time of surgical repair. However, most series seem to suggest that surgical repair might at least stop further neurological deterioration (Supplemental table 1).
Autologous targeted or blind blood patching may also be considered, particularly if there is a high suspicion of low CSF pressure or the dural defect cannot be identified, 12 but in our experience may not lead to CSF biochemical improvement or limit neurological progression.
The iron chelating agent, deferiprone, has been used for many years in iSS, with growing evidence suggesting possible efficacy 39 40. However, the reported radiological measures of haemosiderin deposition are not validated as a surrogate biomarker of treatment benefit, and there are no validated clinical scores that capture the full range of iSS-related impairments. We therefore consider the evidence for deferiprone efficacy to be weak, but do consider this in patients fulfilling the following criteria: (1) symptomatic with progressive functional deterioration attributed to iSS; (2) confirmed ongoing subarachnoid bleeding, with no identified surgically treatable source; (3) unwilling or unsuitable for surgical or radiological intervention (or with no evidence of biochemical or clinical benefit despite such intervention); and (4) with no contraindications to deferiprone (e.g., neutropenia). From observational reports (with all of the methodological limitations already mentioned) of deferiprone in iSS, radiological improvement seems more likely than clinical benefit, (Table 3, Supplemental table 1). There are few data on combined treatment with surgery followed by iron-chelation therapy.
Table 3.
Summary of clinical and radiological outcomes following surgical repair or iron-chelation therapy or both, reported in the time-period of year 2000 and November 2021 and limited to the English language (Supplemental table 1);
| Studies | Number of participants (n=168) | Outcomes (n, %) | |
|---|---|---|---|
| Clinical | Radiological | ||
| Deferiprone only | 63* | Improved 17 (27%) Stable 29 (46%) Worse 17 (27%) N/R 0 |
Improved 19 (42%) Stable 16 (36%) Worse 10 (22%) N/R 18 |
| Surgery only | 82 | Improved 24 (33%)** Stable 34 (47%)** Worse 14 (19%)** N/R 10 |
Improved 5 (25%) Stable 8 (40%) Worse 7 (35%) N/R 62 |
| Deferiprone and surgery combined | 19* | Improved 8 (73%) Stable 0 Worse 3 (27%) N/R 8 |
Improved 8 (100%) Stable 0 Worse 0 N/R 11 |
| Trientine only | 4 | Improved 0 Stable 2 (50%) Worse 2 (50%) N/R 0 |
Improved 0 Stable 1 (100%) Worse 0 N/R 3 |
| Side-effects of deferiprone (alone or combined with surgery; total n=82)† | (n, %)‡ | ||
| Fatigue | 10 (12%) | ||
| Neutropaenia; neutropaenic sepsis | 6 (7%) | ||
| Iron deficiency anaemia | 5 (6%) | ||
| Zinc deficiency | 5 (6%) | ||
| Joint pain | 4 (5%) | ||
| Abnormal liver function tests (transient) | 3 (4%) | ||
| Mouth ulcers | 2 (2%) | ||
| Transient nausea at start of treatment | 1 (1%) | ||
Outcomes for 2 patients who had surgery included into “deferiprone only” group 42;
side-effects not reported for trientine (4 individuals); N/R not reported;
Including participants from ’deferiprone only’ and ’deferiprone and surgery combined’ cohorts (n=82);
Total at 99% due to rounding up; n, number; N/R not reported.
In our experience, the benefit of deferiprone remains uncertain, and it may be associated with a substantial and potentially serious risks of agranulocytosis and sepsis (Table 3, Supplemental table 1) 39 41 42. In our service, the specialist haematology team (including an expert specialist nurse) regularly monitor blood counts and iron stores in all patients taking deferiprone. Deferiprone use is further limited by reduced availability in hospital formularies and costs as an off-label prescription. Randomised controlled trials of deferiprone in iSS using validated outcome measures (ideally reported by patients as well as objective clinical scales and surrogate imaging or CSF biomarkers) are needed, though the heterogeneity of the disease remains a major challenge. In our service, treatment approaches are determined on a case-by-case basis and with the input from a multidisciplinary team (MDT) including neurosurgical, interventional neuroradiology, haematology, audiovestibular and neuro-otology clinicians. The patient and their family should be involved with discussion of treatment options and their possible benefits and associated risks, with emphasis on the lack of robust evidence for their efficacy 28 41–43. Following surgery, we suggest repeat MR scan of the brain and spine and lumbar puncture at 6 months to assess resolution of the pseudomeningocoele/ventral epidural CSF collections and for resolution of CSF red cells, xanthochromia and ferritin (Supplemental figure 1).
Management of hearing impairment and vestibular deficits
Hearing is a major factor influencing quality of life in iSS. Measures to improve hearing include conventional hearing aids and other types of hearing rehabilitation. Cochlear implantation may be considered for patients with profound hearing loss, although with variable outcomes likely due to progressive cochlear nerve involvement 24. Input from neurophysiotherapy or vestibular physiotherapy teams may be indicated for balance or gait disturbance, and a personalised rehabilitative exercise programme can address the patient’s deficits and optimise and potentially maintain function.
Management of other deficits
Other deficits (cognitive, daily function, mobility, dysphagia, sphincter disturbances) should be managed by relevant neurotherapy teams (e.g., including speech and language therapy or occupational therapy) as for any complex neurological condition. Uro-neurological input should be considered depending on symptoms; for example, urinary urgency can impact markedly on quality of life and can often be effectively treated. Unsurprisingly, patients with iSS often develop clinically significant anxiety, depression (or both) secondary to the debilitating impact of the disease 27. A referral to specialist clinical neuropsychology services may be helpful in providing cognitive rehabilitation, counselling, and support.
How Should A Person with iSS be Monitored and What is the Prognosis?
There are few high-quality data describing the natural history of iSS. In our experience some individuals can be stable and minimally symptomatic for long periods, but in others the condition may lead to slow and irreversible functional decline 4 6. The factors associated with disease progression are not known, but the heterogeneity of underlying causes of iSS is likely to impact on prognosis. Due to the frequent delay in its diagnosis, patients may have established significant morbidity by the time of diagnosis limiting the potential benefit from treatments 1 4 6.
Patients at our unit are offered regular neurology follow-up to assess for clinical deterioration. However, there are no validated clinical rating scales for iSS so judging clinical progression can be very challenging. It can also be difficult to know which neurological deficits relate to iSS rather than its underlying cause (e.g., previous neurosurgery or trauma). Those taking deferiprone ideally require haematology specialist nurse follow-up to ensure safe monitoring. We arrange appointments with neuro-surgical and haematology teams are arranged if required. We offer neuro-otological or audiovestibular team reviews as-needed to monitor and manage hearing and balance objectively.
While interval imaging may an option to monitor disease progression, in our experience the extent of visible haemosiderin deposition is unlikely to change in the short-term (6-12 months) but may do so over longer time intervals (several years). Clinical evidence of deterioration or new symptoms may warrant repeat imaging. There are currently no validated imaging biomarkers of disease progression in iSS; haemosiderin is challenging to measure and currently proposed methods of haemosiderin quantification may not be clinically helpful since haemosiderin might not be directly neurotoxic 8. There are no validated measures of the anatomical extent of iSS on MRI.
The Importance of Multidisciplinary Teamworking
The complexity of iSS means that patients require input from several clinical disciplines. As a result of our unit’s experience spanning over almost two decades, we established a specialist iSS multidisciplinary team at Queen Square (London, UK). We hold bi-monthly meetings involving senior clinicians from neurology, neuroradiology, neurosurgery, haematology, neuro-otology or audiovestibular medicine, and neuropsychology. The team discusses each case individually, evaluates patients’ clinical progress, reviews recent imaging and CSF results, proposes further investigations where necessary and sets individualised management plans. Based on our experience, we recently proposed a clinical care pathway for patients with iSS, outlining suggested steps in their rational diagnosis and management (Supplemental figure 1).
Gaps in Knowledge and Future Directions
iSS is becoming more frequently recognised due to the widespread use of MRI including paramagnetic-sensitive sequences, and clinical and research evidence has grown considerably in the past two decades. However, the condition remains relatively understudied in several domains, with work currently underway to close some of the knowledge gaps. Box 2 outlines some potential directions for future research.
Box 2. Summary of the current knowledge gaps and suggested future directions for research. Legend: CNS central nervous system; iSS infratentorial superficial siderosis.
Current gaps in the knowledge about iSS
Prevalence in the general population
Natural history, including early recognition, sequence of CNS region involvement and duration of symptomatic phase, though longitudinal studies on natural history may be challenging due to long prodrome and likely long asymptomatic period
Genetic susceptibility to development or progression of acquired iSS, with a particular focus on impairment of haemoglobin clearance
- Validated and robust biochemical and clinical or surrogate biomarkers (including patientreport measures):
-
-for early detection and diagnosis of iSS
-
-to determine disease burden
-
-to monitor clinical progression and response to medical therapy or following surgical repair
-
-to assess quality of life
-
-
New approaches for quantification of radiological findings, for example measuring the extent of haemosiderin deposition or volume of key structures such as the cerebellum as a marker of disease progression
Efficacy of currently available treatment options by means of large multi-centre randomised control trials
Development of novel therapeutic approaches to reduce neurological injury
Supplementary Material
Key Learning Points.
Classical infratentorial superficial siderosis is characterised by hearing loss (almost always present), vestibular loss, ataxia, and sometimes myelopathy; recent studies suggest that cognitive impairment is also common.
Diagnosis is based on the radiological appearances and clinical syndrome to allow differentiation from other types of superficial siderosis.
The cause is very often a dural defect in the spinal cord or posterior fossa, related to either craniospinal trauma or neurosurgery, usually decades before the diagnosis; a history of thunderclap headache with features suggesting low CSF pressure or volume may be a causative event for subsequent radiological iSS, especially if the patient cannot mobilise at the onset of the headache.
Repair of the dural defect probably gives the best prospect of preventing disease progression; chelating agents, including deferiprone, have been used but without clear evidence of efficacy to date; multidisciplinary tea input is essential to guide diagnosis and management
Funding
This work was funded by the NIHR UCLH BRC Deafness and Hearing Problems Theme. NK’s work (doctoral studentship grant BRC-1215-20016-546624) and DEB’s time for this manuscript were funded by the NIHR UCLH BRC Deafness and Hearing Problems Theme. SFF receives funding support from the NIHR UCLH BRC. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Additional support was provided by the Bernice Bibby Research Charity grant (UK Registered Charity Number 1058703).
Footnotes
Author contributions
DJW had the idea for the article. NK wrote the first draft with extensive editing by DJW. NK and DJW prepared the figures. All authors checked the article t for important intellectual content.
Competing interests
The authors declare otherwise no conflict of interest regarding the authorship, content or publication.
Ethics statement
Ethics committee approval was not required. Formal consent was obtained from the patient for use of intraoperative images (Figure 5).
Patient consent for publication
Not required
Data availability
No datasets were generated for this work.
References
- 1.Wilson D, Chatterjee F, Farmer SF, et al. Infratentorial superficial siderosis: Classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017;81(3):333–43. doi: 10.1002/ana.24850. [published Online First: 2016/12/27] [DOI] [PubMed] [Google Scholar]
- 2.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138(Pt 8):2126–39. doi: 10.1093/brain/awv162. [published Online First: 2015/06/28] [DOI] [PubMed] [Google Scholar]
- 3.ORPHANET. Disease: superficial siderosis. 2022. [accessed 2022/05/23]. [Available from https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=247245]
- 4.Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. 1995;118(Pt 4):1051–66. doi: 10.1093/brain/118.4.1051. [published Online First: 1995/08/01] [DOI] [PubMed] [Google Scholar]
- 5.Hamill RC. Report of a case of melanosis of the brain, cord, and meninges. J Nerv Ment Dis. 1908;35:594. [Google Scholar]
- 6.Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nature clinical practice Neurology. 2007;3(1):54–8. doi: 10.1038/ncpneuro0356. quiz 59. [DOI] [PubMed] [Google Scholar]
- 7.Koeppen AH, Dickson AC, Chu RC, et al. The pathogenesis of superficial siderosis of the central nervous system. Ann Neurol. 1993;34(5):646–53. doi: 10.1002/ana.410340505. [published Online First: 1993/11/01] [DOI] [PubMed] [Google Scholar]
- 8.Koeppen AH, Borke RC. Experimental superficial siderosis of the central nervous system. I. Morphological observations. J Neuropathol Exp Neurol. 1991;50(5):579–94. doi: 10.1097/00005072-199109000-00005. [published Online First: 1991/09/01] [DOI] [PubMed] [Google Scholar]
- 9.Bracchi M, Savoiardo M, Triulzi F, et al. Superficial siderosis of the CNS: MR diagnosis and clinical findings. AJNR Am J Neuroradiol. 1993;14(1):227–36. [published Online First: 1993/01/01] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa Rodriguez EE, Moro RC, Martinez San Millan JS, et al. Rare association of secondary superficial siderosis caused by a fourth ventricle hemorrhagic ependymoma mimicking a cavernoma: Case report and literature review. Surg Neurol Int. 2017;8:14. doi: 10.4103/2152-7806.199554. [published Online First: 2017/02/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb AJ, Flossmann E, Armstrong RJ. Superficial siderosis following spontaneous intracranial hypotension. Pract Neurol. 2015;15(5):382–4. doi: 10.1136/practneurol-2015-001169. [published Online First: 2015/07/04] [DOI] [PubMed] [Google Scholar]
- 12.Schievink WI, Wasserstein P, Maya MM. Intraspinal hemorrhage in spontaneous intracranial hypotension: link to superficial siderosis? Report of 2 cases. J Neurosurg Spine. 2016;24(3):454–6. doi: 10.3171/2015.6.SPINE15428. [published Online First: 2015/11/21] [DOI] [PubMed] [Google Scholar]
- 13.Schievink WI, Maya MM, Nuno M. Chronic cerebellar hemorrhage in spontaneous intracranial hypotension: association with ventral spinal cerebrospinal fluid leaks: clinical article. J Neurosurg Spine. 2011;15(4):433–40. doi: 10.3171/2011.5.SPINE10890. [published Online First: 2011/07/12] [DOI] [PubMed] [Google Scholar]
- 14.Schievink WI, Maya M, Moser F, et al. Long-term Risks of Persistent Ventral Spinal CSF Leaks in SIH: Superficial Siderosis and Bibrachial Amyotrophy. Neurology. 2021;97(19):e1964–e70. doi: 10.1212/WNL.0000000000012786. [published Online First: 2021/09/11] [DOI] [PubMed] [Google Scholar]
- 15.Kumar N. Superficial siderosis: associations and therapeutic implications. Archives of neurology. 2007;64(4):491–6. doi: 10.1001/archneur.64.4.491. [DOI] [PubMed] [Google Scholar]
- 16.Gomori JM, Grossman RI, Bilaniuk LT, et al. Case report. High-field MR imaging of superficial siderosis of the central nervous system. J Comput Assist Tomogr. 1985;9(5):972–5. doi: 10.1097/00004728-198509000-00029. [published Online First: 1985/09/01] [DOI] [PubMed] [Google Scholar]
- 17.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74. doi: 10.1016/S1474-4422(09)70013-4. [published Online First: 2009/01/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIver CL, Ebden S, Tallantyre EC. MRI: how to understand it. Pract Neurol. 2021;21(3):216–24. doi: 10.1136/practneurol-2020-002905. [published Online First: 2009/01/24] [DOI] [PubMed] [Google Scholar]
- 19.Richter T, Nestler-Parr S, Babela R, et al. Rare Disease Terminology and Definitions-A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015;18(6):906–14. doi: 10.1016/j.jval.2015.05.008. [published Online First: 2015/09/28] [DOI] [PubMed] [Google Scholar]
- 20.Vernooij MW, Ikram MA, Hofman A, et al. Superficial siderosis in the general population. Neurology. 2009;73(3):202–5. doi: 10.1212/WNL.0b013e3181ae7c5e. [published Online First: 2009/07/22] [DOI] [PubMed] [Google Scholar]
- 21.Pichler M, Vemuri P, Rabinstein AA, et al. Prevalence and Natural History of Superficial Siderosis: A Population-Based Study. Stroke. 2017;48(12):3210–14. doi: 10.1161/STROKEAHA.117.018974. [published Online First: 2017/10/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offenbacher H, Fazekas F, Schmidt R, et al. Superficial siderosis of the central nervous system: MRI findings and clinical significance. Neuroradiology. 1996;38 Suppl 1:S51–6. doi: 10.1007/BF02278119. [published Online First: 1996/05/01] [DOI] [PubMed] [Google Scholar]
- 23.Friedauer L, R-K B, Steinmetz H, du Mesnil de Rochemont R, Foerch C. Spinal dural leaks in patients with infratentorial superficial siderosis of the central nervous system-Refinement of a diagnostic algorithm. Eur J Neurol. 2020 doi: 10.1111/ene.14611. [published Online First: 2020/10/25] [DOI] [PubMed] [Google Scholar]
- 24.Yoo A, Jou J, Klopfenstein JD, et al. Focused Neuro-Otological Review of Superficial Siderosis of the Central Nervous System. Front Neurol. 2018;9:358. doi: 10.3389/fneur.2018.00358. [published Online First: 2018/06/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sydlowski SA, Levy M, Hanks WD, et al. Auditory profile in superficial siderosis of the central nervous system: a prospective study. Otol Neurotol. 2013;34(4):611–9. doi: 10.1097/MAO.0b013e3182908c5a. [published Online First: 2013/05/15] [DOI] [PubMed] [Google Scholar]
- 26.Kharytaniuk N, Cowley P, Werring DJ, et al. Case Report: Auditory Neuropathy and Central Auditory Processing Deficits in a Neuro-Otological Case-Study of Infratentorial Superficial Siderosis. Front Neurol. 2020;11:610819. doi: 10.3389/fneur.2020.610819. [published Online First: 2021/02/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan E, Sammaraiee Y, Banerjee G, et al. Neuropsychological and neuroimaging characteristics of classical superficial siderosis. J Neurol. 2021;268(11):4238–47. doi: 10.1007/s00415-021-10548-z. [published Online First: 2021/04/19] [DOI] [PubMed] [Google Scholar]
- 28.Kumar N. Superficial Siderosis: A Clinical Review. Ann Neurol. 2021;89(6):1068–79. doi: 10.1002/ana.26083. [published Online First: 2021/04/17] [DOI] [PubMed] [Google Scholar]
- 29.Thielen KR, Sillery JC, Morris JM, et al. Ultrafast dynamic computed tomography myelography for the precise identification of high-flow cerebrospinal fluid leaks caused by spiculated spinal osteophytes. J Neurosurg Spine. 2015;22(3):324–31. doi: 10.3171/2014.10.SPINE14209. [published Online First: 2015/01/03] [DOI] [PubMed] [Google Scholar]
- 30.Kumar N, Lindell EP, Wilden JA, et al. Role of dynamic CT myelography in identifying the etiology of superficial siderosis. Neurology. 2005;65(3):486–8. doi: 10.1212/01.wnl.0000172345.05810.14. [published Online First: 2005/08/10] [DOI] [PubMed] [Google Scholar]
- 31.Katoh H, Shibukawa S, Yamaguchi K, et al. A Combination of Magnetic Resonance Imaging Techniques to Localize the Dural Defect in a Case of Superficial Siderosis-A Case Report. Medicines (Basel) 2020;7(6):36. doi: 10.3390/medicines7060036. [published Online First: 2020/07/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petzold A, Worthington V, Appleby I, et al. Cerebrospinal fluid ferritin level, a sensitive diagnostic test in late-presenting subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2011;20(6):489–93. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.021. [published Online First: 2010/08/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirinzi T, Sancesario G, Anemona L, et al. CSF biomarkers in superficial siderosis: a new tool for diagnosis and evaluation of therapeutic efficacy of deferiprone--a case report. Neurol Sci. 2014;35(7):1151–2. doi: 10.1007/s10072-014-1709-5. [published Online First: 2014/03/15] [DOI] [PubMed] [Google Scholar]
- 34.Takeda T, Kawashima Y, Hirai C, et al. Vestibular Dysfunction in Patients With Superficial Siderosis of the Central Nervous System. Otol Neurotol. 2018;39(6):e468–e74. doi: 10.1097/MAO.0000000000001844. [published Online First: 2018/06/12] [DOI] [PubMed] [Google Scholar]
- 35.Kang KW, Lee C, Kim SH, et al. Bilateral Vestibulopathy Documented by Video Head Impulse Tests in Superficial Siderosis. Otol Neurotol. 2015;36(10):1683–6. doi: 10.1097/MAO.0000000000000865. [published Online First: 2015/10/07] [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Jacob JT, Welker KM, et al. Superficial siderosis of the central nervous system associated with incomplete dural closure following posterior fossa surgery: report of 3 cases. J Neurosurg. 2015;123(5):1326–30. doi: 10.3171/2014.12.JNS141920. [published Online First: 2015/06/13] [DOI] [PubMed] [Google Scholar]
- 37.Takai K, Taniguchi M. Superficial siderosis of the central nervous system associated with ventral dural defects: bleeding from the epidural venous plexus. J Neurol. 2021;268(4):1491–94. doi: 10.1007/s00415-020-10319-2. [published Online First: 2021/01/04] [DOI] [PubMed] [Google Scholar]
- 38.Aquilina K, Kumar R, Lu J, et al. Superficial siderosis of the central nervous system following cervical nerve root avulsion: the importance of early diagnosis and surgery. Acta Neurochir (Wien) 2005;147(3):291–7. doi: 10.1007/s00701-004-0460-8. discussion 97. published Online First: 2005/01/22. [DOI] [PubMed] [Google Scholar]
- 39.Kessler RA, Li X, Schwartz K, et al. Two-year observational study of deferiprone in superficial siderosis. CNS Neurosci Ther. 2018;24(3):187–92. doi: 10.1111/cns.12792. [published Online First: 2017/12/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nose Y, Uwano I, Tateishi U, et al. Quantitative clinical and radiological recovery in post operative patients with superficial siderosis by an iron chelator. J Neurol. 2021 doi: 10.1007/s00415-021-10844-8. [published Online First: 2021/10/20] [DOI] [PubMed] [Google Scholar]
- 41.Sammaraiee Y, Banerjee G, Farmer S, et al. Risks associated with oral deferiprone in the treatment of infratentorial superficial siderosis. J Neurol. 2020;267(1):239–43. doi: 10.1007/s00415-019-09577-6. [published Online First: 2019/10/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flores Martin A, Shanmugarajah P, Hoggard N, et al. Treatment Response of Deferiprone in Infratentorial Superficial Siderosis: a Systematic Review. Cerebellum. 2021;20(3):454–61. doi: 10.1007/s12311-020-01222-7. [published Online First: 2021/01/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dani KA, Murray LJ, Razvi S. Rare neurological diseases: a practical approach to management. Pract Neurol. 2013;13(4):219–27. doi: 10.1136/practneurol-2012-000379. [published Online First: 2013/03/15] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated for this work.