Abstract
Purpose
Survival in stage I seminoma is almost 100%. Computed tomography (CT) surveillance is an international standard of care, avoiding adjuvant therapy. In this young population, minimizing irradiation is vital. The Trial of Imaging and Surveillance in Seminoma Testis (TRISST) assessed whether magnetic resonance images (MRIs) or a reduced scan schedule could be used without an unacceptable increase in advanced relapses.
Methods
A phase III, noninferiority, factorial trial. Eligible participants had undergone orchiectomy for stage I seminoma with no adjuvant therapy planned. Random assignment was to seven CTs (6, 12, 18, 24, 36, 48, and 60 months); seven MRIs (same schedule); three CTs (6, 18, and 36 months); or three MRIs. The primary outcome was 6-year incidence of Royal Marsden Hospital stage ≥ IIC relapse (> 5 cm), aiming to exclude increases ≥ 5.7% (from 5.7% to 11.4%) with MRI (v CT) or three scans (v 7); target N = 660, all contributing to both comparisons. Secondary outcomes include relapse ≥ 3 cm, disease-free survival, and overall survival. Intention-to-treat and per-protocol analyses were performed.
Results
Six hundred sixty-nine patients enrolled (35 UK centers, 2008-2014); mean tumor size was 2.9 cm, and 358 (54%) were low risk (< 4 cm, no rete testis invasion). With a median follow-up of 72 months, 82 (12%) relapsed. Stage ≥ IIC relapse was rare (10 events). Although statistically noninferior, more events occurred with three scans (nine, 2.8%) versus seven scans (one, 0.3%): 2.5% absolute increase, 90% CI (1.0 to 4.1). Only 4/9 could have potentially been detected earlier with seven scans. Noninferiority of MRI versus CT was also shown; fewer events occurred with MRI (two [0.6%] v eight [2.6%]), 1.9% decrease (–3.5 to –0.3). Per-protocol analyses confirmed noninferiority. Five-year survival was 99%, with no tumor-related deaths.
Conclusion
Surveillance is a safe management approach—advanced relapse is rare, salvage treatment successful, and outcomes excellent, regardless of imaging frequency or modality. MRI can be recommended to reduce irradiation; and no adverse impact on long-term outcomes was seen with a reduced schedule.
Introduction
Half of testicular tumors are seminoma.1 For early-stage disease, survival following orchiectomy is approximately 100%, regardless of management.2 Although adjuvant radiotherapy effectively reduces relapses, use has declined dramatically in recent decades because of concerns over long-term toxicity and emergence of alternative approaches.3–6 Use of adjuvant carboplatin, shown to be as effective for reducing relapses, has increased.7,8 However, given 80%-85% will not relapse, and relapses are generally treated successfully, risk of overtreatment is clear.9 Therefore, surveillance, on the basis of periodic cross-sectional imaging, tumor markers, and clinical examination, is now recommended in international guidelines, often as the preferred approach, particularly for lower-risk patients.10–13
Despite increasing adoption of surveillance, there is no evidence base to inform optimal modality and frequency of imaging. Schedules vary widely, and guidelines are not specific. Lower expression of serum tumor markers and greater variability in timing of relapse (compared with nonseminoma) raise concerns about reducing intensity and/or duration of radiologic surveillance. However, risk of second malignancy from a single chest, abdominal, and pelvic computed tomography (CT) is approximately 1/2000.14,15 In a 2009 survey of UK management practices, the most common surveillance schedule used seven CT scans over 5 years,3 a risk of 1/300 of second malignancy related to imaging. In these young patients, unlikely to die from seminoma, avoiding unnecessary radiation exposure is vital.
TRISST (ISRCTN65987321) sought to evaluate whether scan frequency could be reduced, or CT replaced with magnetic resonance imaging (MRI), without an unacceptable increase in advanced relapses.
Methods
Study Design
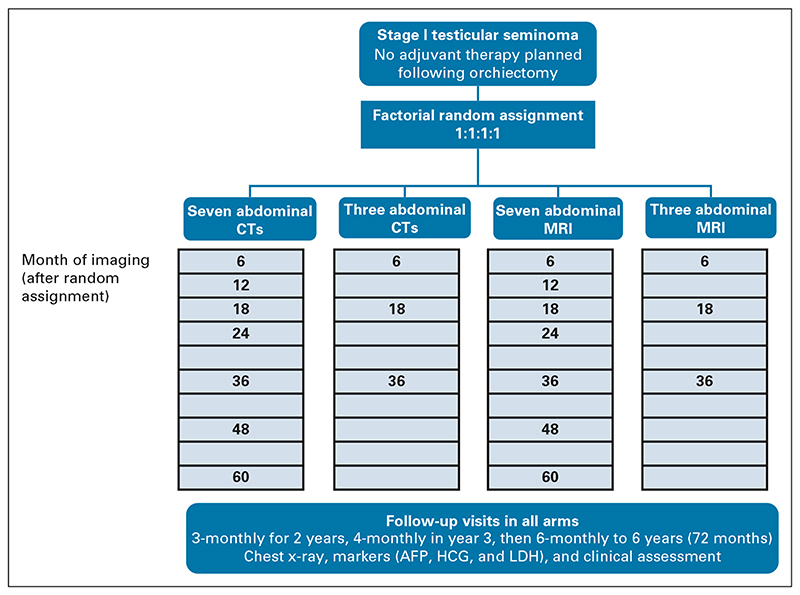
TRISST is a phase III, multicenter, open-label, randomized, noninferiority trial with 2 × 2 factorial design. Allocation, using minimization with a random element (1:1:1:1), was to seven CTs, three CTs, seven MRIs, or three MRIs of the retroperitoneum (Fig 1). Seven-scan schedules involved imaging 6, 12, 18, 24, 36, 48, and 60 months after random assignment, a common schedule in the United Kingdom when the trial was developed.6 Three-scan schedules involved imaging 6, 18, and 36 months after random assignment. Minimization factors were center, maximum tumor diameter (≤ 4 cm, > 4 cm), and presence of rete testis invasion. Central allocation by the trials unit ensured allocation concealment.
Fig 1. TRISST trial schema.
AFP, alpha-fetoprotein; CT, computed tomography; HCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging.
Patients
Eligible patients, recruited at UK hospitals/cancer centers, were age ≥ 16 years; had histologically confirmed stage I testicular seminoma; orchiectomy < 10 weeks before random assignment; normal postorchiectomy serum alpha-fetoprotein (AFP) and Β-human chorionic gonadotropin (AFP not known to be raised preorchiectomy); and no adjuvant therapy planned. Patients with bilateral seminoma were eligible. Exclusions were coexistent of previously treated malignancy; inability to comply with assessments; contraindication to MRI; and spermatocytic tumors. Regulatory, national, and local ethical approvals were obtained. Participants provided written informed consent.
Imaging and Follow-Up
Imaging of the retroperitoneum was performed according to allocation. Where there was a history of ipsilateral inguinoscrotal surgery, the pelvis was also imaged. Minimum imaging requirements were: CT using spiral or multi-detector scanner with a maximum reconstructed slice thickness of 5 mm and images acquired after oral and intravenous contrast media injection in the portal venous phase; MRI on at least a 1 Tesla system with phased array coils and contiguous axial 5-mm section T1 and T2 weighted images. Additional images/sequences were acquired at local radiologists’ discretion. Node measurement was taken on axial section only.
In all arms, follow-up visits took place 3-monthly in years 1-2, 4-monthly in year 3, and 6-monthly thereafter, to 6 years. Visits included clinical examination, chest x-ray (CXR), tumor markers, and imaging according to allocation.
Relapse Detection and Treatment
Relapsing patients underwent chest CT. Those with relapse detected by markers, CXR, clinical examination, or symptoms underwent retroperitoneal CT or MRI (according to allocation). Those with relapse detected on MRI underwent confirmatory CT (within 2 weeks), providing comparative tumor measurements. IGCCCG (International Germ Cell Consensus Classification Group) intermediate-risk patients16 underwent brain CT and/or bone scan where indicated. Relapses were staged according to Royal Marsden Hospital (RMH) criteria.17 Scans between baseline and relapse underwent independent central review (to be reported separately).
Relapse treatment was at the discretion of the investigator but, for limited-stage disease (< 5 cm), recommended approach was carboplatin area under the curve 7 followed by para-aortic radiotherapy.18 For more advanced disease, three or four bleomycin, etoposide, and cisplatin (BEP) cycles (or four cycles of etoposide and cisplatin) were recommended, according to IGCCCG group. Follow-up continued for a minimum of 6 years after random assignment.
Outcomes
The primary outcome was relapse with RMH stage ≥ IIC disease (para-aortic nodes > 5 cm or more extensive metastatic disease equivalent to TNM Tany N3 M0 or Tany Nany M1 disease). For clarity, ≥ IIC disease is used in the text, chosen to reflect a common threshold for giving BEP when the trial was designed. A 2020 survey of UK practice confirmed the relevance of this threshold, but also indicated an alternative: tumor size ≥ 3 cm.19 This was, therefore, prespecified as a key secondary outcome before analysis. For both, 6-year incidence was evaluated, with censoring for patients who did not experience the event, or died from another cause during follow-up. The 6-year time point ensured inclusion of any relapses missed because of the omission of the 60-month scan (in 3-scan arms), which might arise clinically thereafter.
Other secondary outcomes were mean abdominal mass size at relapse (on CT); method of detecting relapse; IGCCCG prognostic group at relapse; new primary malignancy; disease-free survival (DFS); and overall survival (OS).
Statistical Analysis
There were two comparisons (CT v MRI; three v seven scans), with all patients contributing to both. Assuming that the population would largely comprise patients with one or no risk factors,20 it was expected that 15% would relapse and 38% of relapses would be RMH stage ≥ IIC.8,21 This equates to 5.7% of the randomized cohort. The study was designed to exclude an increase of ≥ 5.7% (noninferiority margin), to ≥ 11.4%, through a move to MRI or less frequent scanning. Treating the primary outcome as a binary measure, an estimated 660 patients were needed to achieve 80% power on the basis of 90% CIs (ie, one-sided 5% significance level, reflecting the noninferiority design) and allowing for dropout. During the follow-up period of the trial, it was decided (and prespecified with approval from oversight committees) that time-to-event analysis would facilitate incorporation of partial data from patients who did not complete follow-up. Additionally, a revision to the sample size software used suggested the original sample size was overestimated. Both of these factors meant the trial was likely to have more than 80% power.
The primary and key secondary outcomes are assessed using both intent-to-treat (ITT) and per-protocol (PP) analyses (Appendix Table A1, online only); noninferiority was to be demonstrated in both to conclude a positive result. Comparisons are based on absolute differences in 6-year incidence from the Kaplan-Meier estimator (with 90% CI), using inverse probability of treatment weighting to account for minimization factors (tumor size and rete testis invasion) as well as the other comparison (modality or frequency of scans).22 Standard errors are calculated using bootstrapping.
The primary and key secondary outcomes are also considered in an analysis restricted to relapsing patients. Proportions of relapsing patients with ≥ stage IIC disease (or ≥ 3 cm) are compared between factorial groups using χ2 tests. The trial was sufficiently powered to exclude an increase of 38% (to ≥ 76%). Method of relapse detection is also compared using χ2 tests; IGCCCG classification is presented but no test was performed because of small numbers. Abdominal mass size at relapse (on the basis of CT) is compared using a Mann-Whitney test.
For DFS and OS, hazard ratios (HRs; with 90% CIs) and 5-year estimates are presented from Cox regression models, adjusting for factors as above. Second primary malignancies are presented by factorial group (no formal test because of low numbers).
In addition to outcome data, surveillance details, timing of relapse, and relapse treatment are described. Median follow-up is reported on the basis of reverse Kaplan-Meier.
Results
Patients
A total of 669 participants were enrolled from 35 UK centers (2008-2014), mean age 39 years. Arms were well balanced in terms of key characteristics (Table 1). Mean tumor size was 2.9 cm, 581 (87%) were pT1, and 358 (54%) were low risk.
Table 1. Patient Characteristics at Random Assignment.
| Age, years | |||||
| Mean (SD) | 39 (10.1) | 38 (9.2) | 39 (10.9) | 39 (10.0) | 39 (10.0) |
| Range | 23-76 | 19-64 | 18-64 | 19-72 | 18-76 |
| Max. tumor diameter, cm | |||||
| Mean (SD) | 2.9 (1.8) | 2.9 (1.7) | 2.9 (1.6) | 2.9 (1.8) | 2.9 (1.7) |
| ≤ 2, No. (%) | 71 (42) | 62 (37) | 53 (32) | 69 (41) | 255 (38) |
| 2-3, No. (%) | 39 (23) | 44 (27) | 52 (31) | 36 (22) | 171 (26) |
| 3-4, No. (%) | 28 (17) | 27 (16) | 29 (17) | 27 (16) | 111 (17) |
| > 4, No. (%) | 31 (18) | 33 (20) | 33 (20) | 35 (21) | 132 (20) |
| Rete testis invasion, No. (%) | |||||
| No | 110 (65) | 111 (67) | 109 (65) | 109 (65) | 439 (66) |
| Unknown | 4 (2) | 1 (1) | 1 (1) | 3 2) | 9 (1) |
| Yes | 55 (33) | 54 (33) | 57 (34) | 55 (33) | 221 33) |
| Pagetoid | 17 (31) | 16 (30) | 15 (26) | 14 (25) | 62 (28) |
| Interstitial | 10 (18) | 10 (19) | 15 (26) | 10 (18) | 45 (20) |
| Not defined | 25 (45) | 20 (37) | 22 (39) | 28 (51) | 95 (43) |
| Not known | 3 (5) | 8 (15) | 5 (9) | 3 (5) | 19 (9) |
| Warde risk factors, No. (%) | |||||
| Neither | 95 (56) | 90 (54) | 87 (52) | 86 (51) | 358 (54) |
| ≤ 4 cm and rete testis invasion | 41 (24) | 43 (26) | 46 (28) | 45 (27) | 175 (26) |
| > 4 cm and no rete testis invasion | 15 (9) | 21 (13) | 22 (13) | 23 (14) | 81 (12) |
| > 4 cm and rete testis invasion | 14 (8) | 11 (7) | 11 (7) | 10 (6) | 46 (7) |
| Unknown (rete testis invasion) | 4 (2) | 1 (1) | 1 (1) | 3 (2) | 9 (1) |
| Side of tumor, No. (%) | |||||
| Left | 76 (45) | 83 (50) | 83 (50) | 82 (49) | 324 (48) |
| R ight | 92 (54) | 82 (49) | 83 (50) | 85 (51) | 342 (51) |
| Both | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 3 (< 1) |
| T stage, No. (%) | |||||
| T1 | 153 (91) | 137 (83) | 144 (86) | 147 (88) | 581 (87) |
| T2 | 13 (8) | 20 (12) | 20 (12) | 19 (11) | 72 (11) |
| T3 | 3 (2) | 9 (5) | 3 (2) | 1 (1) | 16 (2) |
| Postoperative LDH,a No. (%) | |||||
| In normal range | 146 (86) | 145 (87) | 148 (89) | 144 (86) | 583 (87) |
| Raised | 15 (9) | 16 (10) | 10 (6) | 17 (10) | 58 (9) |
| Not assessed | 8 (5) | 5 (3) | 9 (5) | 6 (4) | 28 (4) |
Abbreviations: AFP, alpha-fetoprotein; CT, computed tomography; HCG, human chorionic gonadotropin; MRI, magnetic resonance imaging.
Normal postoperative AFP and Β-HCG were required for eligibility.
Surveillance and Follow-Up
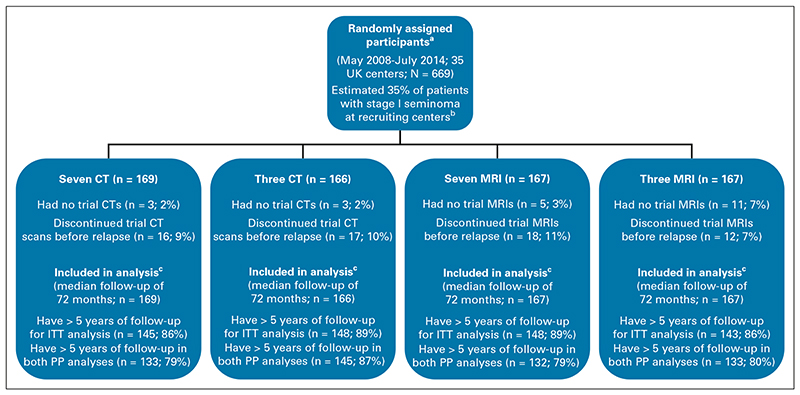
Compliance with allocated schedule was generally good. Eighty-five (13%) participants discontinued early, similar across arms (Fig 2); most commonly, patients were lost to follow-up or moved away (48). Only small numbers were unable to tolerate MRI (seven) or were too large for the scanner (two). Where patients discontinued trial surveillance, data collection continued wherever possible. Median follow-up (including postrelapse) was 72 months with 589 (88%) monitored to 5 years or relapse; the remainder withdrew, were lost to follow-up, or died from another cause before 5 years.
Fig 2. TRISST CONSORT diagram.
aOne patient prospectively identified as ineligible because of preop and postop AFP being slightly above center ULN (9 and 10 IU/L, respectively; ULN = 7 IU/L), but was allowed to enroll on the basis that these marker values were considered normal for the individual. bOn the basis of screening logs completed for a discrete period during trial recruitment. cAnalysis of primary and key secondary outcomes is based on time-to-event methods; hence, all patients are included with censoring at the time of being lost to follow-up (or noncompliance in the case of per-protocol analysis, see Appendix Table A1 for per-protocol definitions). AFP, alpha-fetoprotein; CT, computed tomography; ITT, intention-to-treat; MRI, magnetic resonance imaging; PP, per-protocol; ULN, upper limit of normal.
On the basis of PP definitions, the numbers of patients who were compliant throughout the 5-year surveillance period in terms of scan modality were 287 (86%) and 265 (80%) for CT and MRI, respectively; in terms of compliance with scan frequency, the numbers were 278 (83%) and 288 (86%) for seven- and three-scan schedules, respectively.
One hundred nineteen patients had some form of unscheduled imaging likely related to their cancer, which was not prompted by another trial investigation (ie, clinical examination, symptoms, rising markers, or equivocal finding on scheduled imaging; 187 scans). The majority of these were booked in error (118, 63%; most commonly where chest was included on scheduled CT). Others were in patients who had withdrawn from trial surveillance (38, 20%) or were performed to make up for previous missed scans (16, 9%). A further 67 unscheduled scans were reported with no reason given. No trends emerged suggesting systematic differences between arms.
Primary and Secondary Outcomes: CT Versus MRI
Eighty-two patients (12%) relapsed, 41 in each of the CT and MRI groups. Most were detected on scheduled abdominal imaging (31, 76% CT; 30, 73% MRI) and tended to be identified earlier with MRI (Table 2, Appendix Fig A1, online only). Median abdominal mass size was 2.2 cm in both groups; only one patient (three CT) was IGCCCG intermediate prognosis.16 Of those without confirmed relapse, five in each of the MRI and CT groups had one or more equivocal scan.
Table 2. Method of Detection and Tumor Characteristics at Relapse.
| First sign of relapse,a No. (%) | |||||
| Symptoms | 3 (7) | 2 (5) | 2 (6) | 3 (7) | |
| Tumor markers | 2 (5) | 6 (15) | 3 (8) | 5 (11) | |
| Scheduled abdominal scan | 31 (76) | 30 (73) | 30 (83) | 31 (67) | |
| Unscheduled abdominal CT scan | 5 (12) | 2 (5) | 1 (3) | 6 (13) | |
| Scheduled abdominal scan and scheduled chest x-rayb | 0 (0) | 1 (2) | 0 (0) | 1 (2) | |
| Abdominal mass size on CT, cmc | |||||
| No. | 40 | 37 | 34 | 43 | |
| Median | 2.2 | 2.2 | 2.2 | 2.3 | |
| Range | 1.0-9.0 | 1.0-6.2 | 1.0-5.3 | 1.0-9.0 | |
| IGCCCG intermediate, No. (%) | 1 (0) | 0 (0) | 0 (0) | 1 (2) | |
| RMH stage on the basis of CT, No. (%) | |||||
| IIA | 16 (39) | 16 (39) | 14 (39) | 18 (39) | |
| IIB | 17 (41) | 23 (56) | 21 (58) | 19 (41) | |
| IIC | 3 (7) | 2 (5) | 1 (3) | 4 (9) | |
| IIIA | 1 (2) | 0 (0) | 0 (0) | 1 (2) | |
| IIIB | 1 (2) | 0 (0) | 0 (0) | 1 (2) | |
| IIIC | 1 (2) | 0 (0) | 0 (0) | 1 (2) | |
| IVB | 2 (5) | 0 (0) | 0 (0) | 2 (4) | |
| Total, No. (%) | 41 (100) | 41 (100) | 36 (100) | 46 (100) | |
Abbreviations: CT, computed tomography; CXR, chest x-ray; MRI, magnetic resonance imaging; PP, per-protocol; RMH, Royal Marsden Hospital.
P value for CT versus MRI = .342; P value for seven versus three scans = .389.
Both scheduled abdominal MRI and CXR, performed on the same day, were equivocal.
P value CT versus MRI = .853; P-value for seven versus three scans = .580.
In total, 10 patients (1.5%) had stage ≥ IIC relapse. There were fewer events in the MRI group (two, 0.6%) compared with the CT group (eight, 2.6%); noninferiority was demonstrated (decrease of 1.9%, 90% CI, –3.5 to –0.3, Table 3A) and confirmed in both PP analysis and analysis on the basis of central review results. Taking the relapsed group as the denominator, this was a decrease of 14.6% (–26.2 to –2.6), from 19.5% with CT to 4.9% with MRI, in the proportion with ≥ IIC disease.
Table 3. Six-Year Incidence of Advanced Relapse According to Factorial Comparison Group.
| Six-Year Incidence, % (No. of events) | |||
|---|---|---|---|
| CT | MRI | ||
| Primary outcome: stage ≥ IIC | |||
| ITT analysis | 2.6 (8) | 0.6 (2) | -1.9 (-3.5 to -0.3) |
| PP analysis | 2.6 (8) | 0.6 (2) | -1.9 (-3.6 to -0.3) |
| Key secondary outcome: size ≥ 3 cm | |||
| ITT analysis | 4.1 (13) | 3.4 (11) | –0.8 (–3.3 to 1.7) |
| PP analysis | 4.2 (13) | 3.4 (11) | –0.7 (–3.3 to 1.8) |
| Six-Year Incidence, % (No. of events) | |||
|---|---|---|---|
| Seven Scans | Three Scans | ||
| Primary outcome: stage ≥ IIC | |||
| ITT analysis | 0.3 (1) | 2.8 (9) | 2.5 (1.0 to 4.1) |
| PP analysis | 0.3 (1) | 2.9 (9) | 2.6 (1.0 to 4.1) |
| Key secondary outcome: size ≥ 3 cm | |||
| ITT analysis | 2.7 (9) | 4.7 (15) | 2.0 (–0.4 to 4.4) |
| PP analysis | 2.7 (9) | 4.7 (15) | 2.0 (–0.4 to 4.4) |
Abbreviations: CT, computed tomography; ITT, intention-to-treat; MRI, magnetic resonance imaging; PP, per-protocol.
Incidence with three scans – seven scans (ie, positive values reflect an increase with three scans).
Incidence with MRI – incidence with CT (ie, negative values reflect a decrease with MRI).
Incidence of tumor size ≥ 3 cm on relapse was 3.6% (24 events). Again, slightly fewer events occurred in the MRI group (11, 3.4%; 13, 4.1% CT; Table 3A). Noninferiority was demonstrated (0.8% decrease, CI –3.3 to 1.7); PP results were similar.
Primary and Secondary Outcomes: Seven Versus Three Scans
More relapses occurred in the three-scan group (46) compared with seven scans (36), which is unexpected, given that scanning frequency only has the potential to affect timing/stage of relapse. Although the majority were detected by scheduled abdominal imaging in both groups (30, 83% seven scans; 31, 67% three scans), detection by markers or unscheduled imaging was more common with three scans (Table 2). Timing also tended to be slightly later, reflecting schedule, although relapse beyond 3 years was rare (Appendix Fig A1).
There were more ≥ IIC relapses with three scans (9, 2.8%) compared with seven scans (1, 0.3%), a 2.5% increase (1.0%, 4.1%; Table 3B), but within the noninferiority margin (5.7%). Noninferiority was confirmed in PP analysis and analysis on the basis of central review. Taking the relapsed group as the denominator, there was an increase of 16.8% (0.6%, 27.4%), from 2.8% with seven scans to 20.0% with three scans, in the proportion with ≥ IIC disease. This was, again, within the noninferiority margin (38%).
Incidence of tumor size ≥ 3 cm on relapse was 2.0% higher (CI –0.4 to 4.4) with three scans (15, 4.7%) compared with seven (nine, 2.7%), although within the non-inferiority margin (Table 3B). PP results were similar.
Considering outcome events in the four individual trial arms, the difference between three CT and seven CT arms as more marked than for three MRI versus seven MRI (Table 4).
Table 4. Six-Year Incidence of Advanced Relapse According to Individual Trial Arm.
| Seven CT | Three CT | Seven MRI | Three MRI | |
|---|---|---|---|---|
| Primary outcome: stage ≥ IIC | ||||
| ITT analysis | 0 (0) | 5.1 (8) | 0.6 (1) | 0.6 (1) |
| PP analysis | 0 (0) | 5.1 (8) | 0.6 (1) | 0.6 (1) |
| Key secondary outcome: size ≥ 3 cm | ||||
| ITT analysis | 1.8 (3) | 6.4 (10) | 3.6 (6) | 3.0 (5) |
| PP analysis | 1.8 (3) | 6.4 (10) | 3.7 (6) | 3.1 (5) |
| Total patients | 169 | 166 | 167 | 167 |
Abbreviations: CT, computed tomography; ITT, intention-to-treat; MRI, magnetic resonance imaging; PP, per-protocol.
Relapse Treatment and Long-Term Outcomes
Relapses were treated with low-dose carboplatin and paraaortic radiotherapy (33), combination chemotherapy (normally 4× BEP, 28), or high-dose carboplatin (17; Appendix Fig A2, online only).23 Combination chemotherapy was slightly more common in the three-scan group, given the greater number of advanced relapses. Sixty-seven/80 (84%) of patients had a complete response (in one case following surgery); the remainder (13, 16%) had residual mass and normal markers. Two patients were treated for further progression, but none had active disease at the end of follow-up. There were no tumor-related deaths.
Five-year DFS was similar in CT and MRI groups (88% v 86%; HR = 1.12; CI, 0.79 to 1.59) and in seven- and three-scan groups (89% v 85%; HR = 1.38; CI, 0.97 to 1.97; Appendix Fig A3, online only). Events included seven deaths from other causes. Five-year OS was ≥ 98% for all groups. Nine patients developed secondary malignancies (three prostate, three skin, two lung, and one colon), similar numbers in each arm.
Discussion
TRISST provides the first multicenter, randomized evidence comparing different imaging modalities and schedules for surveillance of stage I seminoma. Outcomes were excellent in all arms, and survival approached 100% after median 6 years. This confirms observational data showing that surveillance is a safe approach.24,25
TRISST was designed to exclude an increase in advanced relapse (RMH stage ≥ IIC) of 5.7% or more associated with the use of MRI or fewer scans. Noninferiority against this criterion was demonstrated in ITT and PP analyses for both comparisons. However, given the lower-than-expected incidence of events, the prespecified noninferiority margin may be less relevant and it is important to consider other aspects of the data to confirm this conclusion.
There were fewer ≥ IIC relapses with MRI compared with CT; 90% CIs exclude the possibility of any increase associated with MRI. Noninferiority was also confirmed with the alternative definition of advanced relapse (≥ 3 cm), where incidence was closer to that expected in the design. Data on relapse timing show a trend toward earlier detection with MRI. Findings align with observational studies indicating that MRI, with experienced radiologists, is a safe alternative in this setting.26–28 Numbers of advanced relapses on MRI were too small to assess center variation. Independent central scan review (to be reported separately) will provide further insights into impact of radiologist experience. Although some national guidelines already recommend MRI,29 to date, supportive evidence has been insufficient, and is crucial, given the costs associated with MRI. Health economics data (to be reported separately) will facilitate a holistic evaluation of MRI in this setting; it may not be deemed cost-effective unless a reduction in number of scans can also be implemented.
Incidence of ≥ IIC relapse in the seven-scan group was particularly low (one event), making it challenging to perform a statistically valid and relevant noninferiority assessment of three-scan schedules. Although more events occurred in three-scan arms, the absolute number was still low (nine, 2.8%). Furthermore, there were more relapses verall in three-scan arms. Since scan frequency will not affect the number of relapses, this suggests the group was slightly higher risk despite random assignment and apparent balance in terms of known risk factors. Only 4/9 ≥ IIC relapses in the three-scan group could potentially have been identified at an earlier scan with the seven-scan schedule: two at the 12-month scan, one at the 24-month scan, and one at either 48 or 60 months. Given the small number and variation in timing, these do not suggest an obvious modification to improve the three-scan schedule. Treatment/response data are not available for one of these patients; outcomes for the other three were good (one complete response after carboplatin and surgery; one complete response after BEP; one residual mass, normal markers after BEP; none had further progression). It is possible that earlier detection may have avoided use of BEP.
In keeping with other studies,2,24,25,30 relapse beyond 3 years was uncommon (5/558 at risk, < 1%). These were not necessarily later-stage relapses (two IIA, two IIB, and one IIC), and only 2/5 were treated with BEP. The TRISST Protocol (online only) included regular marker assessment and clinical examination up to 6 years in all arms, which may be important for detecting the small number of later relapses if scans are stopped earlier.
Considering the alternative advanced relapse definition (≥ 3 cm), the increase associated with the three-scan schedule was small (six events, 2.0% increase, ITT) and noninferiority was demonstrated. Thus, the impact of a less frequent imaging schedule on the selection for the use of local treatment at relapse (either radiotherapy or, as recently reported, minimally invasive retroperitoneal surgery31,32) is likely to be small, especially as our data suggest some centers use the same approach (either high-dose carboplatin23 or BEP) regardless of relapse stage/size. Hence, numbers of advanced relapses would not affect care. Perhaps, most importantly, outcomes in three-scan arms were excellent, despite more advanced relapses (5-year survival 99%), suggesting no longer-term detriment associated with a reduced schedule. The use of more sensitive biomarkers, such as miRNA-371, in future practice may further reduce the need for frequent scanning.33
Although the trial is not powered to assess interactions between scan frequency and modality, it is notable that 8/9 stage ≥ IIC relapses occurred in the three-CT arm, suggesting less impact of reducing the number of scans with MRI. A three-MRI schedule is attractive, avoiding irradiation but limiting increased costs.
As an alternative approach to reduce radiation exposure, a single-arm, prospective study (209 patients with seminoma) has suggested that quality of low-dose CT for this purpose is also acceptable.34–36 However, more robust randomized evidence is not yet available.
A limitation of the trial is that the cohort was relatively low risk; only 7% had both Warde risk factors.20 Thus, generalizability of findings for this group are less clear. A risk-adapted approach may be appropriate.37 However, evidence to validate these risk factors remains limited38; further analysis of TRISST data will inform this area.
A limitation of any study assessing technology is their continuing advancements. Both CT and MRI have undergone significant recent developments (specifically, CT dose optimization and MRI image acquisition techniques). A key development over the past 10 years has been diffusion-weighted MRI (DW-MRI). DW-MRI is the most sensitive imaging technique to detect lymph nodes but lacks specificity.39,40 However, the superior sensitivity of DW-MRI over other cross-sectional imaging means that detection of retroperitoneal lymph node relapse is best suited to this technique.
Further potential limitations relate to compliance and loss to follow-up. Effectiveness of surveillance relies on good adherence to schedules. Here, 13% did not start their allocated schedule or discontinued early. However, PP analyses indicate that the impact of noncompliance on conclusions was negligible. Follow-up of patients who stopped surveillance continued wherever possible and, with a median follow-up of 72 months, likelihood of missed relapses is small.
In conclusion, surveillance is a safe management approach for stage I seminoma—advanced relapse was rare, salvage treatment successful, and long-term outcomes excellent, regardless of imaging frequency or modality. MRI can be recommended to avoid irradiation. Furthermore, no adverse impact on long-term outcomes was seen with a reduced imaging schedule.
Supplementary Material
Context.
Key Objective
In the context of computed tomography (CT) surveillance for stage I testicular seminoma, the TRISST trial sought to evaluate whether scan frequency could be reduced, or CT replaced with magnetic resonance imaging (MRI), without an unacceptable increase in advanced relapses requiring intensive treatment.
Knowledge Generated
This phase III, factorial, noninferiority trial (N = 669) compared (1) seven scans over 60 months versus three scans over 36 months; and (2) CT versus MRI. The number of advanced relapses was very small in all groups (1.5% overall); noninferiority was demonstrated. Relapse treatment was successful, there were no tumor-related deaths, and survival approached 100% in all groups.
Relevance
In this young patient group who are very unlikely to die from their seminoma cancer, minimizing exposure to potentially harmful radiation is important. TRISST demonstrates that the intensity and duration of CT surveillance can be reduced, or MRI can be used, to reduce irradiation, and that long-term outcomes remain excellent.
Acknowledgment
The authors would like to thank the trial participants and their families. They are grateful for the efforts of investigators and research teams at participating centers; in addition to those included as coauthors, they would like to acknowledge the contribution of Dr Thiagarajan Sreenivasan and his team at the United Lincolnshire Hospitals NHS Trust.
Participating centers received support from the National Institute for Health Research through the UK National Cancer Research Network. Finally, they thank members of the independent Data Monitoring Committee (chaired by Judith Bliss) and Trial Steering Committee (chaired by Jeremy Whelan) for their support and guidance throughout the trial.
Footnotes
Author contributions
Conception and design: Johnathan K. Joffe, Gordon J.S. Rustin, Syed A. Sohaib, Rhian Gabe, Sally P. Stenning, Robert A. Huddart
Administrative support: Dipa Noor, Simona Wade, Francesca Schiavone
Provision of study materials or patients: Johnathan K. Joffe, Gordon J.S. Rustin, Sarah Swift, Elaine Dunwoodie, Marcia Hall, Anand Sharma, Jonathan Shamash, John Logue, Sarah Rudman, Jane Worlding, David Bloomfield, Guy Faust, Hilary Glen, Rachel Jones, Michael Seckl, Graham MacDonald, Satish Kumar, Andrew Protheroe, Victoria Coyle, Tom Geldart, Robert A. Huddart
Collection and assembly of data: Johnathan K. Joffe, Fay H. Cafferty, Laura Murphy, Gordon J.S. Rustin, Syed A. Sohaib, Rhian Gabe, Dipa Noor, Simona Wade, Francesca Schiavone, Sarah Swift, Marcia Hall, Anand Sharma, Jeremy Braybrooke, Jonathan Shamash, John Logue, Henry H. Taylor, Sarah Rudman, Jane Worlding, David Bloomfield, Guy Faust, Hilary Glen, Rachel Jones, Michael Seckl, Graham MacDonald, Satish Kumar, Andrew Protheroe, Ramachandran Venkitaraman, Danish Mazhar, Victoria Coyle, Martin Highley, Tom Geldart, Robert Laing, Richard S. Kaplan, Robert A. Huddart
Data analysis and interpretation: Johnathan K. Joffe, Fay H. Cafferty, Laura Murphy, Gordon J.S. Rustin, Syed A. Sohaib, Rhian Gabe, Elizabeth James, Sarah Swift, Elaine Dunwoodie, Anand Sharma, Jeff White, Richard S. Kaplan, Robert A. Huddart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
Prior Presentation
Presented at the ASCO Genitourinary Cancer Symposium 2021 (Oral abstract session: Urothelial Carcinoma; and Adrenal, Penile, Testicular, and Urethral Cancers, February 12, 2021, abstract 374).
Authors’ Disclosures of Potential Conflicts of Interest
Imaging Modality and Frequency in Surveillance of Stage I Seminoma Testicular Cancer: Results From a Randomized, Phase III, Noninferiority Trial (TRISST)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
| Gordon J.S. Rustin | Michael Seckl |
| Consulting or Advisory Role: AbbVie | Consulting or Advisory Role: Bristol Myers Squibb |
| Expert Testimony: Teva | |
| Syed A. Sohaib | Travel, Accommodations, Expenses: Bristol Myers Squibb |
|
Honoraria: Pfizer |
|
| Marcia Hall | Graham MacDonald |
| Honoraria: GlaxoSmithKline, Clovis Oncology | Honoraria: Janssen |
| Consulting or Advisory Role: GlaxoSmithKline, Clovis Oncology | Andrew Protheroe |
| Research Funding: BMS (Inst), Clovis Oncology (Inst), Merck (Inst) | Employment: Genesis Cancer Care |
| Honoraria: Astellas Pharma, MSD Oncology, Ipsen, Astellas Pharma, Merck | |
| Jeremy Braybrooke | Serono, Eisai, Merck |
| Consulting or Advisory Role: GenesisCare, Sciensus | Travel, Accommodations, Expenses: Bayer, EUSA Pharma |
| Research Funding: AstraZeneca (Inst), Roche/Genentech (Inst) | |
| Victoria Coyle | |
| Jonathan Shamash | Honoraria: Gilead Sciences |
| Speakers’ Bureau: Pfizerzer/EMD Serono | Consulting or Advisory Role: Servie |
| Henry H. Taylor | Research Funding: Bayer (Inst), CellCentric (Inst), Astex Pharmaceuticals (Inst) |
| Stock and Other Ownership Interests: GlaxoSmithKline | Travel, Accommodations, Expenses: Servier |
| Ivo Hennig | Tom Geldart |
| Honoraria: Immedica | Research Funding: Eisai (Inst), GlaxoSmithKline (Inst), Oncohost (Inst) |
| Travel, Accommodations, Expenses: Immedica | |
| Robert Laing | |
| Sarah Rudman | Employment: BXTAccelyon |
| Honoraria: Bristol Myers Squibb Foundation, Bristol Myers Squibb Foundation, | Stock and Other Ownership Interests: BXTAccelyon |
| EUSA Pharma, Ipsen, Pfizer | |
| Consulting or Advisory Role: Ipsen, Ipsen, Pfizer, EUSA Pharma | |
|
Richard S. Kaplan Research Funding: AstraZeneca (Inst) | |
| Guy Faust | Robert A. Huddart |
| Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Novartis, Pfizer | Employment: Aspen Parkside Hopsital |
| Speakers’ Bureau: Roche, Bristol Myers Squibb, Novartis, Eisai, Pierre Fabre, | Leadership: Cancer Clinic London Limited liability partnership |
| Merck Serono, Merck/Pfizer, Roche, Janssen Oncology, MSD Oncology, Sanofi | Consulting or Advisory Role: Roche, Merck Sharp & Dohme, Janssen Oncology, |
| Travel, Accommodations, Expenses: Novartis, Janssen, Bayer | |
| Nektar, Bayer | |
| Hilary Glen | Speakers’ Bureau: MSD, Roche |
| Honoraria: Ipsen, Astellas Pharma, Bristol Myers Squibb/Pfizer, Eisai, Janssen | Research Funding: Merck Sharp & Dohme (Inst), Roche (Inst), Bristol Myers |
| Oncology, Ferring, Merck Serono | Squibb (Inst), Janssen (Inst), Nektar (Inst), Astellas Pharma (Inst), Basilea (Inst) |
| Consulting or Advisory Role: Other | Patents, Royalties, Other Intellectual Property: Royalties for dug discovery from Janssen (Inst) |
| Rachel Jones | Travel, Accommodations, Expenses: Roche/Genentech, Nektar |
| Honoraria: Clovis Oncology | |
| Consulting or Advisory Role: Tesaro/GSK, Clovis Oncology, Amgen | No other potential conflicts of interest were reported. |
| Travel, Accommodations, Expenses: Tesaro/GSK, AstraZeneca |
Support
Supported funded by Cancer Research UK (C17084/A8690) and the Medical Research Council Clinical Trials Unit at UCL (MC_UU_12023/28).
Data Sharing Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.01199.
References
- 1.Bray F, Ferlay J, Devesa SS, et al. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol. 2006;3:532–543. doi: 10.1038/ncpuro0606. [DOI] [PubMed] [Google Scholar]
- 2.Tandstad T, Smaaland R, Solberg A, et al. Management of seminomatous testicular cancer: A binational prospective population-based study from the Swedish Norwegian testicular cancer study group. J Clin Oncol. 2011;29:719–725. doi: 10.1200/JCO.2010.30.1044. [DOI] [PubMed] [Google Scholar]
- 3.Cafferty FH, Gabe R, Huddart RA, et al. UK management practices in stage I seminoma and the Medical Research Council Trial of Imaging and Schedule in Seminoma Testis managed with surveillance. Clin Oncol (R Coll Radiol) 2012;24:25–29. doi: 10.1016/j.clon.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Thong AE, Lichtensztajn DY, Almario L, et al. Stage I testicular seminoma: A SEER analysis of contemporary adjuvant radiotherapy trends. J Urol. 2013;190:1240–1244. doi: 10.1016/j.juro.2013.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieckmann KP, Dralle-Filiz I, Heinzelbecker J, et al. Seminoma clinical stage 1-patterns of care in Germany. Urol Int. 2016;96:390–398. doi: 10.1159/000443214. [DOI] [PubMed] [Google Scholar]
- 6.Huddart RA, Joffe JK. Preferred treatment for stage I seminoma: A survey of Canadian radiation oncologists. Clin Oncol (R Coll Radiol) 2006;18:693–695. doi: 10.1016/j.clon.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: Mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214) J Clin Oncol. 2011;29:957–962. doi: 10.1200/JCO.2009.26.4655. [DOI] [PubMed] [Google Scholar]
- 8.Horwich A. Testicular Cancer: Investigation and Management. Chapman & Hall Medical; London, United Kingdom: 1996. [Google Scholar]
- 9.Chung P, Warde P. Stage I seminoma: Adjuvant treatment is effective but is it necessary? J Natl Cancer Inst. 2011;103:194–196. doi: 10.1093/jnci/djq535. [DOI] [PubMed] [Google Scholar]
- 10.Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: A report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): Part I. Eur Urol. 2008;53:478–496. doi: 10.1016/j.eururo.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg J, Fossa SD, Nuver J, et al. Testicular seminoma and non-seminoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi125-32. doi: 10.1093/annonc/mdt304. [DOI] [PubMed] [Google Scholar]
- 12.Gilligan T, Lin DW, Aggarwal R, et al. Testicular cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:1529–1554. doi: 10.6004/jnccn.2019.0058. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich A, Paffenholz P, Nestler T, et al. European Association of Urology guidelines on testis cancer: Important take home messages. Eur Urol Focus. 2019;5:742–744. doi: 10.1016/j.euf.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic x-rays: Estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 15.Wall BF, Haylock R, Jansen JTM, et al. Radiation Risks From Medical X-Ray Examinations as a Function of the Age and Sex of the Patient. Oxfordshire, United Kingdom: HPA Centre for Radiation, Chemical and Environmental Hazards; 2011. Document No.: HPA-CRCE-028. [Google Scholar]
- 16.International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 17.Peckham MJ. In: The Management of Testicular Tumours. Peckham MJ, editor. Hodder Arnold; London, United Kingdom: 1981. Investigation and staging: General aspects and staging classification; pp. 89–101. [Google Scholar]
- 18.Horwich A, Dearnaley DP, Sohaib A, et al. Neoadjuvant carboplatin before radiotherapy in stage IIA and IIB seminoma. Ann Oncol. 2013;24:2104–2107. doi: 10.1093/annonc/mdt148. [DOI] [PubMed] [Google Scholar]
- 19.Joffe JK, Cafferty F, Butcher E, et al. 794P UK practices for treatment of relapse in seminoma testicular cancer. Ann Oncol. 2020;31:S603 [Google Scholar]
- 20.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: A randomised trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 22.Raad H, Cornelius V, Chan S, et al. An evaluation of inverse probability weighting using the propensity score for baseline covariate adjustment in smaller population randomised controlled trials with a continuous outcome. BMC Med Res Methodol. 2020;20:70. doi: 10.1186/s12874-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamash J, Syed R, Sarker SJ, et al. A phase II study of carboplatin AUC-10 guided by positron emission tomography-defined metabolic response in metastatic seminoma. Eur J Cancer. 2019;115:128–135. doi: 10.1016/j.ejca.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33:51–57. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen MS, Lauritsen J, Gundgaard MG, et al. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur Urol. 2014;66:1172–1178. doi: 10.1016/j.eururo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Sohaib SA, Koh DM, Barbachano Y, et al. Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin Radiol. 2009;64:362–367. doi: 10.1016/j.crad.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Narine R, Osman H, Wongwaisayawan S, et al. Unenhanced MRI of the abdomen and pelvis for surveillance of patients with stage 1 testicular cancer post-radical orchiectomy. Abdom Radiol (NY) 2020;46:1157–1162. doi: 10.1007/s00261-020-02715-z. [DOI] [PubMed] [Google Scholar]
- 28.Laukka M, Mannisto S, Beule A, et al. Comparison between CT and MRI in detection of metastasis of the retroperitoneum in testicular germ cell tumors: A prospective trial. Acta Oncol. 2020;59:660–665. doi: 10.1080/0284186X.2020.1725243. [DOI] [PubMed] [Google Scholar]
- 29.SWENOTECA Swedish & Norwegian Testicular Cancer group. SWENOTECA X: A Cancer Care Program for Germ Cell Tumours 2020. 2021. https://www.swenoteca.org/
- 30.Hosni A, Warde P, Jewett M, et al. Clinical characteristics and outcomes of late relapse in stage I testicular seminoma. Clin Oncol (R Coll Radiol) 2016;28:648–654. doi: 10.1016/j.clon.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Albers P, Hiester A, Grosse Siemer R, et al. The PRIMETEST trial: Interim analysis of a phase II trial for primary retroperitoneal lymph node dissection (RPLND) in stage II A/B seminoma patients without adjuvant treatment. J Clin Oncol. 2019;37(suppl 7):507. [Google Scholar]
- 32.Daneshmand S, Cary C, Masterson TA, et al. SEMS trial: Result of a prospective, multi-institutional phase II clinical trial of surgery in early metastatic seminoma. J Clin Oncol. 2021;39(suppl 6):375. [Google Scholar]
- 33.Dieckmann KP, Radtke A, Geczi L, et al. Serum levels of MicroRNA-371a-3p (M371 test) as a new biomarker of testicular germ cell tumors: Results of a prospective multicentric study. J Clin Oncol. 2019;37:1412–1423. doi: 10.1200/JCO.18.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung P, O’Malley ME, Jewett MAS, et al. Detection of relapse by low-dose computed tomography during surveillance in stage I testicular germ cell tumours. Eur Urol Oncol. 2019;2:437–442. doi: 10.1016/j.euo.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Lieng H, Warde P, Bedard P, et al. Recommendations for followup of stage I and II seminoma: The Princess Margaret Cancer Centre approach. Can Urol Assoc J. 2018;12:59–66. doi: 10.5489/cuaj.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Malley ME, Chung P, Haider M, et al. Comparison of low dose with standard dose abdominal/pelvic multidetector CT in patients with stage 1 testicular cancer under surveillance. Eur Radiol. 2010;20:1624–1630. doi: 10.1007/s00330-009-1710-1. [DOI] [PubMed] [Google Scholar]
- 37.Cohn-Cedermark G, Stahl O, Tandstad T. Surveillance vs. adjuvant therapy of clinical stage I testicular tumors—A review and the SWENOTECA experience. Andrology. 2015;3:102–110. doi: 10.1111/andr.280. [DOI] [PubMed] [Google Scholar]
- 38.Boormans JL, Mayor de Castro J, Marconi L, et al. Testicular tumour size and rete testis invasion as prognostic factors for the risk of relapse of clinical stage I seminoma testis patients under surveillance: A systematic review by the testicular cancer guidelines panel. Eur Urol. 2018;73:394–405. doi: 10.1016/j.eururo.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Gao S, Li S. A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: A meta-analysis based on 67 studies. Gynecol Obstet Invest. 2017;82:209–222. doi: 10.1159/000456006. [DOI] [PubMed] [Google Scholar]
- 40.Mir N, Sohaib SA, Collins D, et al. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol. 2010;54:358–364. doi: 10.1111/j.1754-9485.2010.02182.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.01199.