Summary
Background
Vancomycin is the most widely used antibiotic for neonatal Gram-positive sepsis, but clinical outcome data of dosing strategies are scarce. The NeoVanc programme comprised extensive preclinical studies to inform a randomised controlled trial to assess optimised vancomycin dosing. We compared the efficacy of an optimised regimen to a standard regimen in infants with late onset sepsis that was known or suspected to be caused by Gram-positive microorganisms.
Methods
NeoVanc was an open-label, multicentre, phase 2b, parallel-group, randomised, non-inferiority trial comparing the efficacy and toxicity of an optimised regimen of vancomycin to a standard regimen in infants aged 90 days or younger. Infants with at least three clinical or laboratory sepsis criteria or confirmed Gram-positive sepsis with at least one clinical or laboratory criterion were enrolled from 22 neonatal intensive care units in Greece, Italy, Estonia, Spain, and the UK. Infants were randomly assigned (1:1) to either the optimised regimen (25 mg/kg loading dose, followed by 15 mg/kg every 12 h or 8 h dependent on postmenstrual age, for 5±1 days) or the standard regimen (no loading dose; 15 mg/kg every 24 h, 12 h, or 8 h dependent on postmenstrual age for 10±2 days). Vancomycin was administered intravenously via 60 min infusion. Group allocation was not masked to local investigators or parents. The primary endpoint was success at the test of cure visit (10±1 days after the end of actual vancomycin therapy) in the per-protocol population, where success was defined as the participant being alive at the test of cure visit, having a successful outcome at the end of actual vancomycin therapy, and not having a clinically or microbiologically significant relapse or new infection requiring antistaphylococcal antibiotics for more than 24 h within 10 days of the end of actual vancomycin therapy. The non-inferiority margin was –10%. Safety was assessed in the intention-to-treat population. This trial is registered at ClinicalTrials.gov (NCT02790996).
Findings
Between March 3, 2017, and July 29, 2019, 242 infants were randomly assigned to the standard regimen group (n=122) or the optimised regimen group (n=120). Primary outcome data in the per-protocol population were available for 90 infants in the optimised group and 92 in the standard group. 64 (71%) of 90 infants in the optimised group and 73 (79%) of 92 in the standard group had success at test of cure visit; non-inferiority was not confirmed (adjusted risk difference –7% [95% CI –15 to 2]). Incomplete resolution of clinical or laboratory signs after 5 ± 1 days of vancomycin therapy was the main factor contributing to clinical failure in the optimised group. Abnormal hearing test results were recorded in 25 (30%) of 84 infants in the optimised group and 12 (15%) of 79 in the standard group (adjusted risk ratio 1·96 [95% CI 1·07 to 3·59], p=0·030). There were six vancomycin-related adverse events in the optimised group (one serious adverse event) and four in the standard group (two serious adverse events). 11 infants in the intention-to-treat population died (six [6%] of 102 infants in the optimised group and five [5%] of 98 in the standard group).
Interpretation
In the largest neonatal vancomycin efficacy trial yet conducted, no clear clinical impact of a shorter duration of treatment with a loading dose was demonstrated. The use of the optimised regimen cannot be recommended because a potential hearing safety signal was identified; long-term follow-up is being done. These results emphasise the importance of robust clinical safety assessments of novel antibiotic dosing regimens in infants.
Funding
EU Seventh Framework Programme for research, technological development and demonstration.
Introduction
Neonatal sepsis is a major public health concern, with about 3 million cases per year worldwide.1 Coagulase-negative staphylococci are skin and gut commensal bacteria and the most frequently isolated organisms in late onset sepsis in high-income countries,2 particularly occurring in hospitals as a central line-associated bloodstream infection. Although overall mortality rates are low,3 coagulase-negative staphylococci-associated late onset sepsis is associated with neurodevelopmental sequelae.4 Coagulase-negative staphylococci are often multidrug resistant,5 and the global emergence of vancomycin heteroresistant organisms is concerning, especially the increasing reports in infants.6,7
Vancomycin is the most widely used antibiotic for Grampositive late onset sepsis.8 Neonatal vancomycin dosing and durations of treatment vary markedly,9 leading to different drug exposures.10 Robust infant pharmacokinetic, safety, and clinical efficacy data for different dosing strategies are scarce,11 and the NeoVanc project attempted to address this gap.
Preclinical components of the NeoVanc programme included a hollow-fibre infection model, a rabbit model, and a population pharmacokinetic meta-analysis (appendix p 3). This work and a clinical bridging study12 determined that frequent dosing facilitated bacterial killing and led to quicker reduction in C-reactive protein, whereas continuous infusions appeared to select for vancomycin heteroresistance. The neonatal pharmacokinetic model suggested that standard dosing regimens had low vancomycin target attainment and supported the use of a loading dose to shorten the time to achieve therapeutic concentrations when combined with more frequent dosing in infants younger than 29 weeks postmenstrual age.13 The clinical bridging study and the pharmacokinetic model indicated the need for more frequent dosing in infants younger than 29 weeks postmenstrual age, in whom it can take days to achieve therapeutic concentrations. A vancomycin loading dose is routinely given in adults and has been used in infants preceding continuous infusion;14 however, the loading dose is novel within the context of intermittent dosing. The subsequent optimised dosing regimen for the NeoVanc randomised clinical trial (RCT) was a short course (5±1 days) of vancomycin with a loading dose and more frequent dosing in infants younger than 29 weeks postmenstrual age than in the standard of care with no loading dose (vancomycin for 10 ± 2 days). Shorter vancomycin durations are supported by retrospective analyses.15 A non-inferiority design was selected because shorter treatment was not expected to lead to higher efficacy than longer treatment but might possibly result in secondary benefits, including reduced rates of antimicrobial resistance and reduced toxic effects because of lower overall vancomycin exposure.
For the NeoVanc programme see https://www.neovanc.org/en/
Potential toxicity of vancomycin includes nephrotoxicity and ototoxicity. Neonatal vancomycin safety studies have historically been underpowered and relied on retrospective analyses of routinely collected data,16 and robust, preclinical, neonatal vancomycin ototoxicity models are scarce.
This NeoVanc study aimed to use a loading dose of vancomycin to provide faster target attainment with a new, shorter optimised regimen compared with the standard dosing regimen in infants with late onset sepsis that was known or suspected to be caused by Gram-positive microorganisms. The overall aim was to test whether the efficacy of the optimised regimen, which included a loading dose, was non-inferior to the standard regimen.
Methods
Study design
In this open-label, multicentre, phase 2b, parallel-group, randomised non-inferiority trial, we recruited participants across 22 neonatal intensive care units (NICUs) in Greece, Italy, Estonia, Spain, and the UK. All NICUs were tertiary care centres and prescribed vancomycin routinely. NICUs were selected to ensure representation of varying practice across Europe. NeoVanc was approved by the London–West London and Gene Therapy Advisory Committee (REC reference [16]/LO/1026) on July 18, 2016. Ethics committee and regulatory body approvals were obtained for each participating hospital. Written informed consent was obtained from all participants’ parents or guardians. Preconsent was allowed provided consent was reconfirmed if an infant became unwell. The study was performed in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines, local regulations, and standard operating procedures. The study protocol has previously been published.17 Protocol amendments are outlined in the appendix (p 2).
Participants
Eligible participants were infants at postnatal age 72 h and older but 90 days or younger at randomisation who had clinical or laboratory-defined sepsis or positive blood culture sepsis. Modified European Medicines Agency (EMA) criteria were applied to identify clinical sepsis;18 enrolment required three or more clinical or laboratory criteria or a positive culture with Gram-positive bacteria from a sterile site and one or more clinical or laboratory criteria in the 24 h before randomisation (appendix p 4). Exclusion criteria included the administration of any systemic antibiotic regimen for more than 24 h before randomisation unless a change was driven by an apparent absence of efficacy of the antibiotic regimen, treatment with vancomycin for 24 h or more within 7 days of randomisation, and known renal impairment. Post-randomisation exclusions included any participant found to have Gram-negative or fungal sepsis, osteomyelitis, septic arthritis, urinary tract infection, meningitis, or Staphylococcus aureus bacteraemia because these conditions would have required treatment with a different antibiotic or a vancomycin course longer than the optimised regimen. Full trial inclusion and exclusion criteria and post-randomisation exclusions from efficacy analysis are in the appendix (p 4). Any infant who received one or more doses of study vancomycin were followed up for safety.
Randomisation and masking
Infants were randomly assigned (1:1) to a standard regimen or an optimised regimen of vancomycin. A secure, web-based system was used for randomisation with an authorised username and password. Infants were recruited and randomised by trained investigators at each site. A minimisation algorithm was used to ensure balance between groups in relation to baseline data (NICU, postmenstrual age, and presence of an umbilical catheter or central line). Local investigators and parents or guardians were not masked to regimen allocation. The trial management group and data analysts were masked to aggregate outcomes apart from statisticians who were unmasked for interim analyses and independent data monitoring committee meetings.
Procedures
Infants in the standard regimen group received the following regimen: vancomycin at 15 mg/kg every 24 h for those at postmenstrual age younger than 29 weeks, every 12 h for those at postmenstrual age 29–35 weeks, or every 8 h for those at postmenstrual age older than 35 weeks for 10 ± 2 days. Those in the optimised regimen group received the following regimen: an initial loading dose of vancomycin 25 mg/kg on day 1 followed by a maintenance dose of 15 mg/kg every 12 h for those at postmenstrual age 35 weeks or younger or 8 h for those at postmenstrual age older than 35 weeks for a total of 5 ± 1 days. In the optimised group, the first maintenance dose was administered 8 h or 12 h after the loading dose, dependent on postmenstrual age. Vancomycin (Laboratorio Reig Jofre, Barcelona, Spain) was administered intravenously via 60 min infusion. Vancomycin durations outside the specified limits were permitted on the basis of clinician assessment. The standard treatment regimen was based on European dosing recommendations,19 with the 10 ± 2 days duration being chosen to best reflect current practice across European NICUs from pretrial surveys, because no reference information from RCTs was available. Dose adjustments were permitted through routine therapeutic drug monitoring or in renal impairment, where modifications were made on the basis of vancomycin concentrations and local policy.
Study visits are specified in the appendix (pp 5–6). Clinical and laboratory parameters were monitored in accordance with the modified EMA neonatal sepsis criteria, at day 3, day 5 (± 1), and day 10 (± 2 if still receiving study vancomycin only).18 At the end of actual vancomycin therapy (ie, when vancomycin for the episode of sepsis at enrolment is stopped), improvement in overall clinical status was assessed. Infants judged as having a successful outcome at the end of actual vancomycin therapy proceeded to the test of cure visit, 10 ± 1 days after end of actual vancomycin therapy. At this point, clinically or microbiologically significant relapse or new infections were recorded (appendix pp 7–8). Relapse and new infections were also assessed at a short-term follow-up visit 30 ± 5 days from initiation of study vancomycin.
Hearing screening was performed between the end of actual vancomycin therapy and 90 days after randomisation. Otoacoustic emissions or auditory brainstem responses were permitted according to local clinical practice, and abnormal hearing was defined as no clear response in one ear on otoacoustic emissions or auditory brainstem responses.
Data were collected in an electronic case report form managed by Consorzio per Valutazioni Biologiche e Farmacologiche (Pavia, Italy). All collected data remain strictly confidential and anonymous.
For more on neonIN see https://neonin.org.uk/
Outcomes
Given the low mortality in coagulase-negative staphylococci-associated sepsis, the primary outcome was based on clinical recovery, defined using modified EMA guidance18 and expert consensus at the test of cure visit in the per-protocol population. The primary outcome was considered successful if the participant was alive at the test of cure visit, had a successful outcome at the end of actual vancomycin therapy, and did not have a clinically or microbiologically significant relapse or new infection requiring treatment with vancomycin or other specific antistaphylococcal antibiotic (flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin, or teicoplanin) for more than 24 h between end of actual vancomycin therapy and test of cure visit. A successful outcome at the end of actual vancomycin therapy was defined as the participant being alive with a significant improvement in overall clinical status, microbiological resolution or presumed eradication of bacteria, and no new vancomycin-susceptible pathogens identified (appendix pp 7–8). Success was assessed using a clinical algorithm (appendix pp 7–8) that did not rely on physician assessment of outcome.
Secondary efficacy outcomes were success at 5 ± 1 days from the initiation of study vancomycin, success at the end of actual vancomycin therapy (ie, when therapy actually ended), success at the end of allocated therapy (ie, when therapy was intended to end according to group assignment; prespecified in the statistical analysis plan), clinically or microbiologically significant relapse or new infection requiring treatment with non-antistaphylococcal (other) antibiotics for more than 24 h at the test of cure visit, and the primary outcome extended to the short-term follow-up visit. Other secondary pharmacokinetic and microbiological outcomes will be reported separately when laboratory results are available.
Secondary safety outcomes were abnormal renal function at short-term follow-up (urinary output <0·7 mL/kg per h for 24 h or creatinine value ≥100 μmol/L [≥1·13 mg/dL]), abnormal hearing screening tests after end of actual vancomycin therapy, adverse events up to the short-term follow-up visit, vancomycin-related adverse events, all serious adverse events, and vancomycin-related serious adverse events. All adverse events and serious adverse events occurring between the administration of the first dose of study vancomycin and the final follow-up visit were recorded in the electronic case report form.
Statistical analysis
Including 150 infants per group would provide at least 90% power to demonstrate non-inferiority using a two-sided 95% CI (ie, type I error rate of 2·5%), assuming a success rate in both groups of 95% and a non-inferiority margin on the risk-difference scale of –10% (Wilson-score method; nQuery). A 5% relapse or new infection rate was based on data from neonIN, an international neonatal infection surveillance network, and the magnitude of a clinically relevant effect was obtained through consensus in the NeoVanc Consortium. There is no regulatory guidance from either the US Food and Drug Administration (FDA) or the EMA on neonatal sepsis trials, although a non-inferiority margin of 10% has been recommended by the FDA for acute pneumonia RCTs, in which treatment is believed to be highly efficacious.20 The 10% non-inferiority margin was based on relapse or new infection and is in-keeping with adult antibiotic RCTs.21,22 A power sensitivity analysis, without reference to the data, was performed when it became apparent that this sample size would not be met within the project timelines. This analysis indicated there would not be a substantial increase in power gained from the expected sample size of 100 per group (expected power of 83% using the same parameters as the original sample size calculation) to the maximum possible sample sizes, given resource and time limitations (power of 87% for 110 per group). An independent data monitoring committee reviewed the data periodically and the trial was consequently stopped before the planned recruitment target was met.
The primary analysis used binomial regression with an identity link to report risk difference and associated 95% CI, with a non-inferiority margin of –10%. Inference was based on adjusted estimates, in which postmenstrual age (<29 weeks, 29–35 weeks, or >35 weeks), and presence or absence of umbilical catheters or central venous lines were fixed effects, and study centre was a random effect. Three separate subgroup analyses were prespecified as follows: postmenstrual age at randomisation (<29 weeks, 29–35 weeks, or >35 weeks), birthweight (<1000 g, 1000–1500 g, >1500 g), and presence or absence of an umbilical catheter or central venous line at the onset of sepsis. Bayesian analysis, prespecified in the statistical analysis plan, was used to estimate the probability of the optimised regimen truly being superior to the standard regimen under different previous assumptions (appendix p 9).
Analyses of secondary outcomes used risk ratios and their 95% CIs from log binomial regression models, with the same adjustment factors as the primary outcome, except for adverse events and serious adverse events, which were reported as the incidence rate per 1000 child days (number of infant-days recorded as alive and in the study between randomisation and short-term follow-up) with comparison using incidence rate ratios and 95% CIs to allow for the possibility of multiple events occurring in the same infant and negative binomial regression to account for overdispersion. Post-hoc imputation was done for rates of abnormal hearing due to missing data, and imputation was done separately for each group. Factors included in the model were baseline variables of post-menstrual age stratum, birthweight stratum, presence or absence of umbilical catheters or central venous lines, sex, hypoxic ischaemic encephalopathy, intraventricular haemorrhage, and presence or absence of separate known risk factor antibiotics (amikacin, ciprofloxacin, gentamicin, linezolid, netilmicin, and teicoplanin). For all analyses, 95% CIs were used with no adjustment for multiple testing. Statistical analyses used Stata, version 16.
Safety analyses were done in the intention-to-treat (ITT) population of all randomly assigned infants except for post-randomisation exclusions and where consent to use data had subsequently been withdrawn. Efficacy analyses were done in the per-protocol population, which excluded infants randomly assigned in error, with a loading dose not administered as assigned, or who received vancomycin for less than 48 h from initiation of study vancomycin.
An independent data monitoring committee, composed of a neonatologist, microbiologist, and statistician met three times throughout the trial period to monitor progress, efficacy, safety, and pharmacokinetic data according to a specific charter and without formal stopping guidelines. NeoVanc is registered at ClinicalTrials.gov (NCT02790996) and EudraCT (2015-000203-89).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
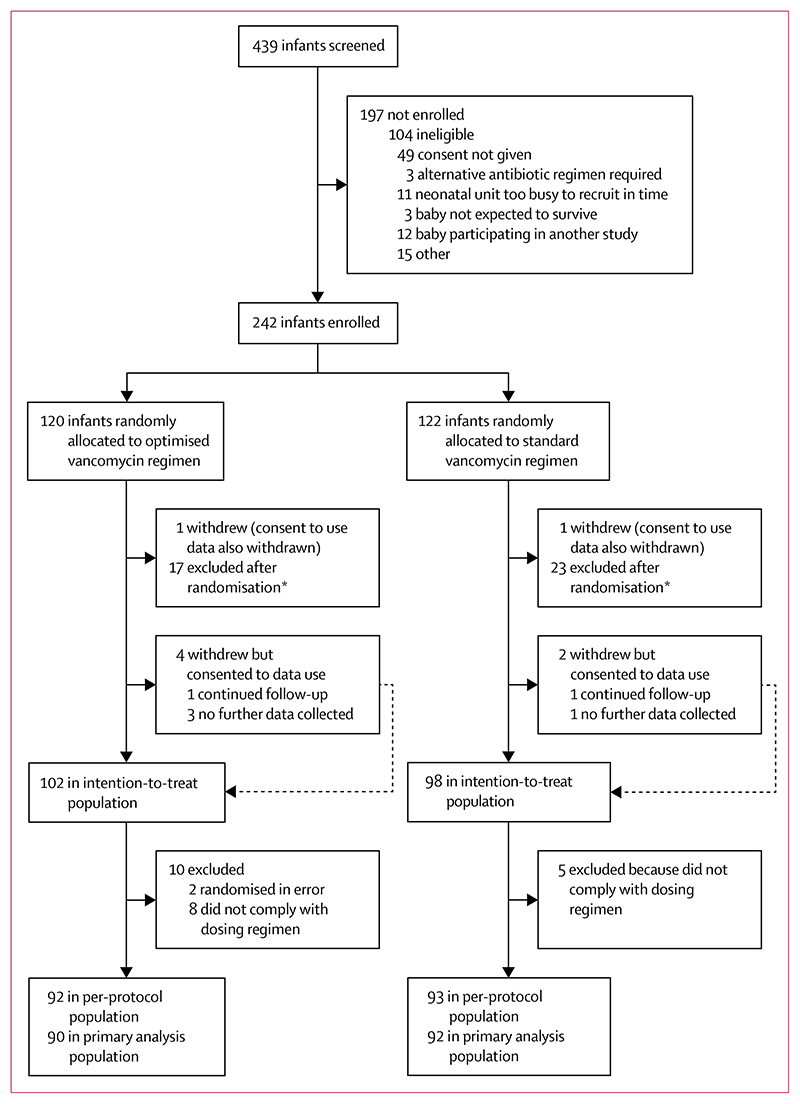
Between March 3, 2017, and July 29, 2019, 242 infants were randomly assigned to the standard regimen group (n=122) or the optimised regimen group (n=120; figure 1). Primary outcome data in the per-protocol population were available for 90 infants in the optimised group and 92 in the standard group.
Figure 1. Trial profile.
*Any participant with Gram-negative or fungal sepsis, osteomyelitis, septic arthritis, urinary tract infection, meningitis, or meticillin susceptible or resistant Staphylococcus aureus, and bacteraemia.
Baseline characteristics were broadly similar across groups (table 1; appendix pp 10–11). All but two of the 242 infants had at least three clinical or laboratory signs of neonatal sepsis at baseline. 80 Gram-positive bacteria were detected at baseline in 76 infants (69% S epidermidis, 10% S hominis, 9% S haemolyticus, with the remaining 12% comprising six different species; appendix p 11). S epidermidis was relatively more common in the standard group (34 [79%] of 43 bacteria detected) than in the optimised group (21 [57%] of 37), with S hominis being more common in the optimised group (five [14%] of 37) than in the standard group (three [7%] of 43); other organisms were detected in similar proportions between groups. No invasive organism exhibited vancomycin resistance by European Committee on Antimicrobial Susceptibility Testing breakpoints. 101 (87%) of 116 available coagulase-negative staphylococci blood culture isolates had vancomycin heteroresistance by the brain heart infusion agar method (51 in the standard group and 50 in the optimised group).23
Table 1. Participant baseline characteristics (per-protocol population).
| Optimised vancomycin regimen (n=92) | Standard vancomycin regimen (n=93) | |
|---|---|---|
| Number of participating centres | 14 | 15 |
| Number of infants per country | ||
| Greece | 44 (48%) | 44 (47%) |
| Italy | 28 (30%) | 24 (26%) |
| Estonia | 5 (5%) | 15 (16%) |
| Spain | 10 (11%) | 9 (10%) |
| UK | 5 (5%) | 1 (1%) |
| Infant sex | ||
| Girls | 45 (49%) | 40 (43%) |
| Boys | 47 (51%) | 53 (57%) |
| Postnatal age at trial entry, days | 14 (9–28) | 14 (9–23) |
| Postmenstrual age at trial entry, weeks | 33 (29–37) | 32 (29–37) |
| <29 | 20 (22%) | 23 (25%) |
| 29–35 | 44 (48%) | 43 (46%) |
| >35 | 28 (30%) | 27 (29%) |
| Infant birthweight, g | 1155 (855–1720) | 1120 (800–1930) |
| <1000 | 39 (42%) | 33 (35%) |
| 1000–1500 | 21 (23%) | 27 (29%) |
| >1500 | 32 (35%) | 33 (35%) |
| Infant weight at trial entry, g | 1465 (945–2145) | 1300 (940–2213) |
| Multiple birth | 19 (21%) | 25 (27%) |
| Infant ethnicity | ||
| White | 84 (91%) | 89 (96%) |
| Asian | 2 (2%) | 0 |
| Black | 3 (3%) | 2 (2%) |
| Other | 2 (2%) | 0 |
| Mixed | 1 (1%) | 2 (2%) |
| Umbilical catheter or central venous line present | 58 (63%) | 58 (62%) |
| Number of Gram-positive bacteria detected in baseline blood culture | ||
| sample | ||
| 0 | 54 (59%) | 44(47%) |
| 1 | 31 (34%) | 41 (44%) |
| 2 | 3 (3%) | 1 (1%) |
| No sample | 4 (4%) | 7 (8%) |
| Postnatal age at onset of late-onset sepsis, days | 14 (8·5–27·5) | 14 (8–23) |
| Congenital malformations or underlying neonatal conditions | 53 (58%) | 52 (56%) |
| Infants with surgery performed in the past month | 10 (11%) | 17 (18%) |
| Infants with risk factors for hearing impairment | 20 (22%) | 19 (20%) |
| Infants with antibiotics given in 7 days before trial entry | 62 (67%) | 73 (78%) |
| Total number of sepsis criteria at trial entry | ||
| 2 | 1 (1%) | 1 (1%) |
| 3 | 20 (22%) | 15 (16%) |
| 4 | 30 (33%) | 27 (29%) |
| 5 | 19 (21%) | 27 (29%) |
| 6 | 8 (9%) | 13 (14%) |
| 7 | 9 (10%) | 6 (6%) |
| 8 | 3 (3%) | 2 (2%) |
| 9 | 2 (2%) | 2 (2%) |
Data are n, n (%), or median (IQR).
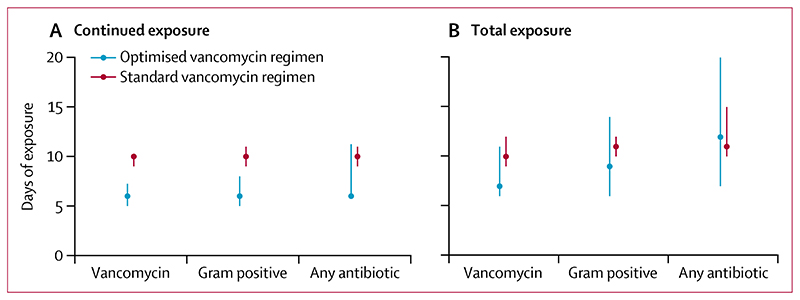
59 (64%) of 92 infants in the optimised group and 82 (88%) of 93 in the standard group received vancomycin within their randomised duration window. Continued treatment with vancomycin or another antistaphylococcal antibiotic, probably reflecting treatment for the original infection, lasted a median of 6 days (IQR 5–8) from commencement of study vancomycin in the optimised group and 10 days (9–10) in the standard group (figure 2A; appendix p 12). However, the difference between treatment groups became notably less when considering the total days of exposure to short-term follow-up, both of vancomycin (median 7 days [6–11] in the optimised group and 10 days [9–12] in the standard group) and all antibiotics (median 12 days [7–20] in the optimised group and 11 days [10–15] in the standard group; figure 2B; appendix p 12). Therapeutic drug monitoring was assessed for 46 (25%) infants in 12 centres, with 23 (50%) of assessed participants having at least one dosing adjustment; assessment rates were slightly higher in the standard group than the optimised group (appendix p 12).
Figure 2. Continued and overall antibiotic therapy duration by study group.
Data are median with IQR (bars). Per-protocol population of 185 infants. Continued exposure is defined as continuous antibiotic therapy from initiation of vancomycin. Total exposure is defined as total antibiotic exposure from initiation of vancomycin to the short-term follow-up visit (30 ± 5 days from initiation of vancomycin). Gram positive indicates antistaphylococcal antibiotics including vancomycin, flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin, or teicoplanin. Any antibiotic indicates any antibiotic including those stated above.
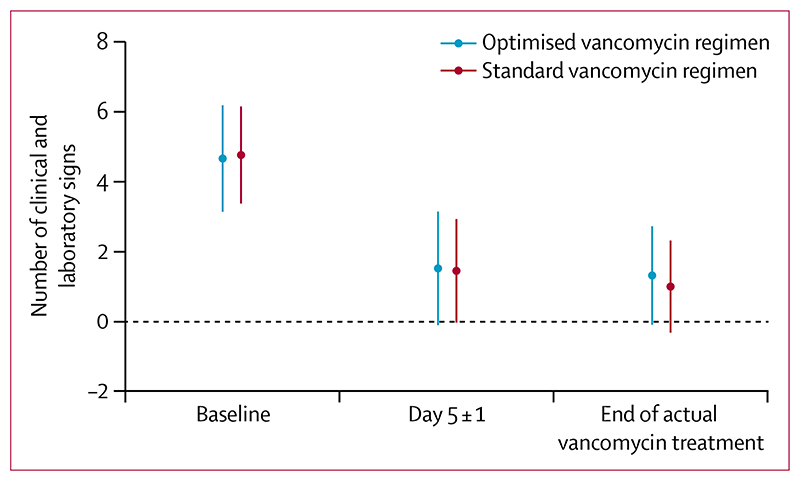
Regarding the primary outcome, 64 (71%) of 90 infants in the optimised group and 73 (79%) of 92 in the standard group had a successful outcome at the test of cure visit (table 2). The adjusted risk difference between groups was –7% (95% CI –15 to 2) and consequently non-inferiority was not concluded on the basis of a non-inferiority margin of –10% (appendix p 13 for analysis of ITT population). The slightly lower success rate in the optimised group seemed to be driven by lower rates of overall clinical improvement (ie, total number of clinical and laboratory signs of sepsis) at the end of actual vancomycin therapy (19 [21%] of 90 in the optimised group, at about day 5, and nine [10%] of 92 in the standard group, at about day 10; appendix p 13), as opposed to death or clinically or microbiologically significant relapse or new infection, despite a similar number of signs at 5 ± 1 days (figure 3). Of those 28 infants who did not have a successful outcome at the end of actual vancomycin therapy because of absence of clinical improvement, 16 (57%) had at least three clinical signs and 22 (79%) had at least one laboratory sign of sepsis. Bayesian analysis showed 79–99% probability that the optimised group was truly worse than the standard group, depending on the prior used, and 4–43% probability that the optimised group was truly worse than the standard group by at least the 10% non-inferiority margin (appendix pp 14–15). There was no evidence of heterogeneity in subgroup analyses (postmenstrual age, birthweight, presence of a central line) for the primary outcome (appendix p 15).
Table 2. Efficacy outcomes (per-protocol population).
| Optimised vancomycin regimen | Standard vancomycin regimen | Adjusted risk ratio (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Success at test of cure visit | 64/90 (71%) | 73/92 (79%) | –7% (–15 to 2)* | .. |
| Secondary outcomes | ||||
| Successful outcome at 5 ± 1 days after start of allocated vancomycin therapy | 65/91 (71%) | 76/93 (82%) | 0·90 (0·78 to 1·04) | 0·16 |
| Successful outcome at end of actual vancomycin therapy | 68/90 (76%) | 82/92 (89%) | 0·87 (0·80 to 0·95) | 0·0011 |
| Successful outcome at test of cure visit: composite including treatment with other antibiotics† | 64/90 (71%) | 68/92 (74%) | 0·98 (0·87 to 1·11) | 0·76 |
| Successful outcome at short-term follow-up‡ | 56/90 (62%) | 71/92 (77%) | 0·81 (0·71 to 0·93) | 0·0025 |
| Successful outcome at end of allocated therapy𝕊 | 65/91 (71%) | 83/93 (89%) | 0·79 (0·69 to 0·91) | 0·0017 |
| Relapses or new infections between end of actual vancomycin therapy and test of cure caused by treatment with other antibiotics† | 3/90 (3%) | 16/92 (17%) | 0·19 (0·08 to 0·39) | 0·0010 |
| Relapses or new infections between test of cure and short-term follow-up‡ | 11/90 (12%) | 4/92 (4%) | 2·81 (0·84 to 9·38) | 0·094 |
Data are n/N (%) unless otherwise stated.
Risk difference (95% CI).
Any antibiotic that is not vancomycin or another specific antistaphylococcal antibiotic, as specified in the protocol (flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin, or teicoplanin).
30 ± 5 days from initiation of study vancomycin.
Prespecified only in the statistical analysis plan.
Figure 3. Number of clinical and laboratory signs of sepsis over time.
Data are mean with SD (bars). Per-protocol population of 185 infants.
Secondary efficacy outcomes are outlined for the perprotocol population in table 2 and for the ITT population in the appendix (p 13). Success rates at day 5 ± 1 after the start of allocated vancomycin therapy were similar in the optimised group (65 [71%] of 91 infants) and the standard group (76 [82%] of 93), with an adjusted risk ratio of 0·90 (95% CI 0·78 to 1·04). Post-hoc analysis methods were the same as the primary outcome analysis, and did not conclude non-inferiority (adjusted risk difference −8% [95% CI –19 to 3]). There were fewer relapses or new infections treated with non-antistaphylococcal (other) antibiotics between the end of actual vancomycin therapy and test of cure in the optimised group (three [3%] of 90) than in the standard group (16 [17%] of 92). When the primary outcome was extended to include relapse or new infections treated with any antibiotics between the end of actual vancomycin therapy and test of cure, there was no evidence that success rates differed between the optimised group (64 [71%] of 90) and standard group (68 [74%] of 92; adjusted risk ratio 0·98 [95% CI 0·87 to 1·11]), but post-hoc analyses again could not conclude non-inferiority (adjusted risk difference 3% [95% CI –10 to 6]).
Abnormal hearing screening test results were twice as common in the optimised group (25 [30%] of 84 infants) than in the standard group (12 [15%] of 79; adjusted risk ratio 1·96 [95% CI 1·07 to 3·59], p=0·030; table 3), although only 163 (82%) of 200 infants in the ITT population had hearing assessed. 11 (30%) of the 37 infants died before a hearing assessment, and 18 (69%) of the 26 remaining individuals were from two sites. Post-hoc multiple imputation (details shown in appendix p 16) indicated slightly higher rates of abnormal hearing in both groups (34 [33%] of 102 in the optimised group and 19 [19%] of 98 in the standard group; adjusted risk ratio 1·72 [95% CI 1·0 to 2·9]; table 3). Additional post-hoc analyses, on infants with available hearing screening results, showed higher rates of abnormal hearing in the optimised group across all postmenstrual age groups but with weak evidence for a greater excess risk in those with the youngest postmenstrual age (appendix p 17) and across both hearing tests (appendix p 17). Age at hearing test did not differ between groups (post-hoc analyses mean 61 days [SD 30] in the optimised group and 62 days [SD 27] in the standard group; difference 1·6 days [95% CI –12 to 9]; p=0·77). Results were unchanged when repeated on the as-treated population because all infants with outcome data received the loading dose as randomly assigned (appendix p 18). Adding cumulative dose to the unadjusted model resulted in a very small decrease in the effect size, although the cumulative dose itself was not significantly associated with abnormal hearing (appendix p 19). Rates of abnormal renal function tests at short-term follow-up were low, including two (2%) of 84 infants in the optimised group and none of 81 in the standard group (table 3). There were six vancomycin-related adverse events in the optimised group (one serious adverse event) and four in the standard group (two serious adverse events). The frequency of adverse events and serious adverse events, both all-cause and vancomycin related, did not differ across the groups (table 3).
Table 3. Safety outcomes (intention-to-treat population).
| Optimised vancomycin regimen | Standard vancomycin regimen | Adjusted risk ratio (95% CI) | p value | |
|---|---|---|---|---|
| Abnormal renal function tests at the short-term follow-up*† | 2/84 (2%) | 0/81 (0%) | 0·85 (–1·7 to + inf) | 0·50 |
| Abnormal hearing screening test after end of actual vancomycin therapy | 25/84 (30%) | 12/79 (15%) | 1·96 (1·07 to 3·59) | 0·030 |
| Abnormal hearing screening test after imputation | 33·7/102 (33%) | 18·5/98 (19%) | 1·72 (1·0to 2·9) | 0·044 |
| Adverse events at short-term follow-up† | ||||
| All | 45·8 (138/3012) | 41·3 (122/2956) | 1·11 (0·67 to 1·87) | 0·41 |
| Vancomycin related | 2·0 (6/3012) | 1·4 (4/2956) | 1·45 (0·78 to 2·68) | 0·24 |
| Serious adverse events at short-term follow-up† | ||||
| All | 6·9 (21/3012) | 9·5 (28/2956) | 0·73 (0·29 to 1·83) | 0·50 |
| Vancomycin related | 0·3 (1/3012) | 0·7 (2/2956) | 0·49 (0·11 to 2·15) | 0·34 |
Data are n/N (%) or incidence rate per 1000 child days (events/child days). Denominator differs because all patients with available data are shown. + inf indicates infinity when the model cannot estimate the upper CI boundary.
Unadjusted output from Poisson regression.
30 ± 5 days from initiation of study vancomycin.
11 infants in the ITT population died (six [6%] of 102 infants in the optimised group and five [5%] of 98 in the standard group). Four died with necrotising enterocolitis, two with Gram-negative infection, three with respiratory pathology, one with pericarditis and S epidermidis bloodstream infection, and one with severe brain injury secondary to vein of Galen aneurysm and septic shock.
Discussion
NeoVanc, an open-label RCT, aimed to validate preclinical data to establish whether the duration of vancomycin treatment for Gram-positive late-onset sepsis could be safely reduced to 5 ± 1 days with more frequent dosing in infants younger than 29 weeks postmenstrual age, and to assess the inclusion of a loading dose. We could not conclude non-inferiority on the primary outcome. Additionally, a potential safety signal was detected in relation to higher abnormal hearing screening rates in the optimised group.
The inability to conclude non-inferiority of the optimised group in the primary outcome was multifactorial. The intended sample size was not reached, which might have led to insufficient power. In hindsight, a non-inferiority limit of 10% with an anticipated relapse rate of 5% could have been considered large. However, it did not affect inference in the study. Bayesian analysis showed 79–99% probability of the optimised group being truly worse than the standard group, implying that low power might not be the only factor. The unsuccessful primary outcome in the optimised group was predominantly related to low rates of clinical recovery at the end of actual vancomycin therapy, and not because of relapse or new infection. 21% of all infants (83% of all unsuccessful outcomes) did not have an overall clinical improvement (ie, still had at least three clinical or laboratory signs at the end of vancomycin therapy) in the optimised group compared with 10% of infants (47% of all unsuccessful outcomes) in the standard group. Gram-postive blood culture positivity rate at the end of vancomycin therapy was low, despite a rate of more than 40% at baseline. The day of end of actual vancomycin therapy differed between groups, and secondary efficacy analyses showed lower success rates in the optimised group than the standard group at the end of vancomycin therapy, both when therapy was intended to end according to group assignment (end of allocated therapy) and when therapy actually ended (end of actual vancomycin therapy). These differences might reflect the time taken for clinical or laboratory signs to normalise in infants with serious sepsis regardless of dosing regimen; assessment of both groups at day 10 ± 2 (end of allocated therapy in the standard group) might have elucidated the differences further. The new National Institute for Health and Care Excellence neonatal sepsis guidelines recommend 7 days of antibiotic treatment in babies with culture-positive late-onset sepsis.24
Only two infants showed abnormal renal function at short-term follow-up. There was no evidence that the frequency of adverse events and serious adverse events differed between study groups. Rates of abnormal hearing were almost twice as high in the optimised group, although the associated 95% CIs were wide and hearing screening results were only available for 82% of the ITT population. This result could reflect a genuine safety signal but might be due to small sample sizes and chance attributable to multiple testing. There was no evidence that age at the time of hearing screening differed between groups. Multiple imputation, factoring in other risk factors for hearing loss, including aminoglycoside and furosemide exposure and low birthweight, showed a slightly reduced effect size (1·7 times) and smaller CIs with the lower limit of the 95% CI being 1·0, and consequently the pattern of missing data might be driving some of the differences observed. The protocol definition of abnormal hearing was stricter than that used in clinical practice,25 so failure rates might be higher, although one would expect the rates to be distributed evenly between groups. If genuine, the higher abnormal hearing screening rates in the optimised group could be caused by either the loading dose or more frequent administration of vancomycin in infants less than 29 weeks postmenstrual age. There was weak evidence of an interaction between postmenstrual age group and treatment group on abnormal hearing screening rates, although sample sizes were small. Cumulative exposure of vancomycin has been described as a risk factor for abnormal hearing screening at NICU discharge in babies with very low birthweight,26 although we did not find robust evidence of this association. If the ototoxicity safety signal is being driven by the loading dose, then these NeoVanc results suggest that cumulative dose is unlikely to be the only risk factor, particularly as the number of days of vancomycin exposure up to short-term follow-up was similar in both groups. Risk factors are likely to be cumulative, and data on hearing outcomes in septic babies are sparse. Notably, an RCT assessing neonatal meropenem versus standard of care reported abnormal hearing screening rates of up to 29% in their population of infants with sepsis.27 Robust, prospective, long-term hearing data are required to ascertain whether poor results at hearing screening translate to long-term hearing loss on diagnostic auditory assessment.16 A NeoVanc long-term follow-up study is planned with the aim of obtaining missing data and collecting follow-up hearing data in infants who did not pass hearing screening.
Only two RCTs investigating vancomycin in neonates have been registered on ClinicalTrials.gov and the International Standard Randomised Controlled Trial Number trial registry in the past 20 years, emphasising the paucity of efficacy trials.28,29 Both trials were stopped before recruitment of their target sample size, demonstrating the difficulty of recruiting to neonatal antibiotics trials.
Coagulase-negative staphylococci-associated sepsis has historically been considered to have a less severe clinical course than sepsis caused by other Gram positive or Gram negative organisms. However, infants recruited into NeoVanc had serious clinical sepsis; 99% had at least three clinical or laboratory signs with blood culture positivity rate being high. The inclusion criteria clearly identified infants with sepsis.
Test of cure assessment in neonatal antibiotic trials is not standardised, and no guidance is available on neonatal sepsis trial design from the FDA or EMA.30 Test of cure in NeoVanc was based on number of days from the actual end of vancomycin therapy and not days from randomisation and was therefore at different timepoints in the optimised group than in the standard group. Very low rates of new infection and relapse were seen in both groups. The NeoVanc trial was a pragmatic open-label study, and this might have influenced clinician decisions, particularly if they were accustomed to giving longer antibiotic course durations. The short-term follow-up visit was at 30±5 days from randomisation to ensure comparability of outcome assessment with respect to the initial presenting episode and to ensure that overall antibiotic exposure was similar between groups to this timepoint, which supports the absence of a difference between the groups at this later follow-up assessment.
The NeoVanc programme incorporated extensive pre-clinical studies,12 including the largest ever meta-analysis assessing vancomycin pharmacokinetic data in infants.13 This RCT also provides valuable pharmacokinetic, safety, and efficacy information on infants less than 29 weeks postmenstrual age, who comprised nearly a quarter of the study population.
Interim NeoVanc pharmacokinetic analysis (the full analysis has been delayed by the COVID-19 pandemic and will be published later) indicate that the newly developed pharmacokinetic model from the preclinical studies, which has been externally validated, is robust, supporting the use of preclinical studies to optimise antimicrobial dosing regimens. However, modelling toxicity is more problematic and can only be detected within the context of a reasonably sized RCT. The ototoxicity safety signal, potentially associated with the loading dose in this RCT, was not predicted, particularly given the previous inconclusive data relating to ototoxicity in infants and considering that a loading dose is recommended in critically ill children and adults.31 Neonates might demonstrate unique toxicity profiles, and dosing recommendations should be adopted with caution if the data are generated from adult or childhood RCTs alone. Rates of ototoxicity have not been compared between continuous and intermittent vancomycin infusion within the setting of an RCT in infants.
Recruitment to neonatal antibiotic trials is challenging and the sample size required to detect safety signals is considerably more than that of most of the neonatal antibiotics trials that are currently recruiting.32 An approach that balances risk and unmet need seems appropriate. For antibiotics with a low risk of toxicity (eg, beta lactams) and limited clinical unmet need, it is reasonable to use pharmacokinetic studies alone to establish optimal dosing regimens. For drugs with a higher toxicity potential and high unmet clinical need, NeoVanc demonstrates that robust RCTs adequately powered to identify potential novel toxicity signals might be required. Additionally, efficacy assessment should be done at the same timepoint from randomisation in each group to allow equal time for resolution of symptoms in both groups. In view of our primary analysis results and the identified hearing safety signal, we would not currently recommend a 25 mg/kg loading dose of vancomycin in infants or more frequent dosing in infants less than 29 weeks postmenstrual age.
Supplementary Material
Research in context.
Evidence before this study
Neonatal sepsis is a global health priority. Coagulase-negative staphylococci are the most frequently identified organisms in neonatal late onset sepsis in high-income countries, and babies with low birthweight have the greatest associated morbidity and mortality. Vancomycin is the main treatment of late onset sepsis caused by coagulase-negative staphylococci. Before the NeoVanc trial, commencing in March 2017, we searched PubMed, ClinicalTrials.gov, and ISRCTN. We identified two randomised controlled trials (RCTs) of neonatal vancomycin registered in the past 20 years. These two RCTs recruited 220 babies; one was an active control trial comparing cefazolin and vancomycin, and the other compared continuous and intermittent infusion with pharmacokinetic endpoints.
The NeoVanc programme completed preclinical studies that informed the optimal dosing regimen evaluated in this RCT. The NeoVanc hollow fibre infection model and animal model determined that more frequent dosing might be beneficial in facilitating bacterial killing and discouraging the development of vancomycin resistance. A clinical bridging study established that more frequent dosing led to a quicker and more efficient reduction in C-reactive protein, particularly in infants younger than 29 weeks postmenstrual age, and the study supported a shorter vancomycin course. A retrospective study by Linder and colleagues found that infants with sepsis with an uncomplicated clinical course, treated for coagulase-negative staphylococci and who were given vancomycin for 5 days after a positive blood culture had similar outcomes to those treated for longer durations. A novel neonatal vancomycin pharmacokinetic model was developed as part of the NeoVanc programme on the basis of a population pharmacokinetic meta-analysis, which included 4894 vancomycin concentrations from 1631 infants. This model supported the need for more frequent dosing in infants younger than 29 weeks postmenstrual age and predicted an optimised regimen with a 25 mg/kg loading dose of vancomycin. The use of a loading dose of 25 mg/kg has been supported by the Infectious Diseases Society of America for the treatment of meticillin-resistant Staphylococcus aureus in seriously unwell adults and children.
Added value of this study
To our knowledge, NeoVanc is the first RCT to evaluate a loading dose of vancomycin in conjunction with intermittent dosing for neonatal sepsis, as well as the largest neonatal vancomycin clinical efficacy trial. We identified an ototoxicity safety signal, potentially associated with the use of a loading dose or more frequent dosing, or both, in infants younger than 29 weeks postmenstrual age, which has not previously been recognised in this population. Additionally, no clear advantage was seen for adopting a shorter 5 day (± 1) course compared with a standard 10 day (± 2) course of vancomycin for infants with serious clinical or confirmed sepsis. The adapted European Medicines Agency neonatal sepsis criteria were used as inclusion criteria in our study and successfully identified infants with serious clinical sepsis, with more than 40% positive baseline blood cultures.
Implications of all the available evidence
There is no evidence for reducing the vancomycin course duration to 5 ± 1 days in infants with sepsis because no benefit was identified. A vancomycin loading dose and more frequent dosing in infants younger than 29 weeks postmenstrual age should not be recommended because of a possible ototoxicity safety signal. Collection of long-term neonatal vancomycin hearing data is in progress.
Acknowledgments
The study was funded by the European Union Seventh Framework Programme for research, technological development, and demonstration (grant 602041). We thank the parents and guardians of participants and investigators from the centres who participated in this NeoVanc study (recruiting centres and NeoVanc consortium members are listed in the appendix p 1). We thank the sponsor, Fondazione Penta, and the independent data monitoring committee (John van den Anker, Jim Gray, and Corine Chazallon).
Footnotes
Contributors
MAT, IL, EJ-A, PTH, ER, and MS were responsible for the conceptualisation and funding acquisition. LFH, MAT, PTH, IL, EJ-A, ER, and MS contributed to the study design. LFH, MAT, PTH, IL, EJ-A, ER, MS, MNC, and LR contributed to the methods. MNC did the data curation. MNC and LFH completed the formal data analysis. LFH, DD, and LR were responsible for project administration. CA-D, EB, AD, M-LI, AM, TM, GM, VP, and KS did the investigation. LFH, MS, MAT, DD, IL, PTH, ER, LR, CA-D, EB, AD, M-LI, AM, TM, GM, VP, and KS performed the supervision. MNC was responsible for the statistical coding and validation. LFH, MNC, and MS wrote the first draft. LFH, MNC, MS, MAT, DD, IL, ER, PTH, EJ-A, LR, CA-D, EB, AD, M-LI, AM, TM, GM, VP, KS, and ASW wrote, reviewed, and edited the manuscript. LFH was the trial coordinator. MNC was the trial statistician. MS was the chief investigator. MAT, IL, ER, CA-D, and AD were the country coordinators. LFH and MNC have accessed and verified all the data in the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
PTH is a member of the National Institute for Health and Care Excellence neonatal infection guideline development group. LR is an employee of Therakind. DD obtained a PhD that was funded by Fondazione Penta; the capacity and remit of this PhD was independent and unrelated to involvement with NeoVanc. All other authors declare no competing interests.
Contributor Information
Louise F Hill, Institute for Infection and Immunity, St George’s, University of London, London, UK.
Michelle N Clements, Medical Research Council Clinical Trials Unit, University College London, London, UK.
Prof Mark A Turner, Institute of Translational Medicine, University of Liverpool, Liverpool, UK.
Daniele Donà, Division of Pediatric Infectious Diseases, Department of Women’s and Children’s Health, University of Padova, Padova, Italy; Fondazione Penta, Padua, Italy.
Prof Irja Lutsar, University of Tartu, Tartu, Estonia.
Prof Evelyne Jacqz-Aigrain, Department of Pediatric Pharmacology and Pharmacogenetics, Hôpital Robert Debré, Paris, France.
Prof Paul T Heath, Institute for Infection and Immunity, St George’s, University of London, London, UK.
Prof Emmanuel Roilides, 3rd Department of Pediatrics, Aristotle University, Thessaloniki, Greece.
Louise Rawcliffe, Therakind, London, UK.
Clara Alonso-Diaz, Hospital Universitario 12 de Octubre, Madrid, Spain.
Prof Eugenio Baraldi, Azienda Ospedale-Universita’ di Padova, Fondazione Istituto di Ricerca Pediatrica, Padova, Italy.
Andrea Dotta, Ospedale Pediatrico Bambino Gesù, Rome, Italy.
Mari-Liis Ilmoja, Tallinn Children’s Hospital, Tallinn, Estonia.
Ajit Mahaveer, St Mary’sHospital, Manchester, UK.
Prof Tuuli Metsvaht, Tartu University Hospital, Tartu, Estonia.
Prof George Mitsiakos, Papageorgiou Hospital, Thessaloniki, Greece.
Prof Vassiliki Papaevangelou, General University Hospital, Chaïdári, Greece.
Prof Kosmas Sarafidis, Hippokration Hospital, Thessaloniki, Greece.
Prof A Sarah Walker, Medical Research Council Clinical Trials Unit, University College London, London, UK.
Prof Michael Sharland, Institute for Infection and Immunity, St George’s, University of London, London, UK.
Data sharing
Sharing of data will be considered on the basis of a detailed proposal which should include aims, methods, and a statistical analysis plan. Requests will be checked for compatibility with regulatory and ethics committee requirements and with participant informed consent. Proposals should be addressed to the corresponding author at lhill@sgul.ac.uk and will be evaluated by the sponsor.
References
- 1.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6:223–30. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 2.Cailes B, Kortsalioudaki C, Buttery J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. 2018;103:F547–53. doi: 10.1136/archdischild-2017-313203. [DOI] [PubMed] [Google Scholar]
- 3.Piening BC, Geffers C, Gastmeier P, Schwab F. Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PLoS One. 2017;12:e0180134. doi: 10.1371/journal.pone.0180134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alshaikh B, Yee W, Lodha A, Henderson E, Yusuf K, Sauve R. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J Perinatol. 2014;34:125–29. doi: 10.1038/jp.2013.155. [DOI] [PubMed] [Google Scholar]
- 5.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent F, Butin M. Staphylococcus capitis and NRCS-A clone: the story of an unrecognized pathogen in neonatal intensive care units. Clin Microbiol Infect. 2019;25:1081–85. doi: 10.1016/j.cmi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Public Health England. Increased incidence of detections of Staphylococcus capitis in neonatal clinical samples. 2021. [accessed July 19, 2021]. https://hubble-live-assets.s3.amazonaws.com/bapm/attachment/file/411/Increased_incidence_of_detections_of_Staphylococcus_capitis_in_neonates.pdf .
- 8.Prusakov P, Goff DA, Wozniak PS, et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: the no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine. 2021;32:100727. doi: 10.1016/j.eclinm.2021.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metsvaht T, Nellis G, Varendi H, et al. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr. 2015;15:41. doi: 10.1186/s12887-015-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dao K, Guidi M, André P, et al. Optimisation of vancomycin exposure in neonates based on the best level of evidence. Pharmacol Res. 2020;154:104278. doi: 10.1016/j.phrs.2019.104278. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency. Revised priority list for studies on off-patent paediatric medicinal products. 2013. [accessed Nov 11, 2020]. https://www.ema.europa.eu/en/documents/other/revised-priority-list-studies-patent-paediatric-medicinal-products_en.pdf .
- 12.Ramos-Martín V, Johnson A, Livermore J, et al. Pharmacodynamics of vancomycin for CoNS infection: experimental basis for optimal use of vancomycin in neonates. J Antimicrob Chemother. 2016;71:992–1002. doi: 10.1093/jac/dkv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacqz-Aigrain E, Leroux S, Thomson AH, et al. Population pharmacokinetic meta-analysis of individual data to design the first randomized efficacy trial of vancomycin in neonates and young infants. J Antimicrob Chemother. 2019;74:2128–38. doi: 10.1093/jac/dkz158. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child. 2013;98:449–53. doi: 10.1136/archdischild-2012-302765. [DOI] [PubMed] [Google Scholar]
- 15.Linder N, Lubin D, Hernandez A, Amit L, Ashkenazi S. Duration of vancomycin treatment for coagulase-negative Staphylococcus sepsis in very low birth weight infants. Br J Clin Pharmacol. 2013;76:58–64. doi: 10.1111/bcp.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lestner JM, Hill LF, Heath PT, Sharland M. Vancomycin toxicity in neonates: a review of the evidence. Curr Opin Infect Dis. 2016;29:237–47. doi: 10.1097/QCO.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 17.Hill LF, Turner MA, Lutsar I, et al. An optimised dosing regimen versus a standard dosing regimen of vancomycin for the treatment of late onset sepsis due to Gram-positive microorganisms in neonates and infants aged less than 90 days (NeoVanc): study protocol for a randomised controlled trial. Trials. 2020;21:329. doi: 10.1186/s13063-020-4184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Report on the expert meeting on neonatal and paediatric sepsis. 2010. [accessed Dec 18, 2020]. https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf .
- 19.Sharland M, Butler K, Cant A, et al. Antimicrobial dosing guide Manual of childhood infections: the blue book. 4th edn. Oxford: Oxford University Press; 2016. [Google Scholar]
- 20.Food and Drug Administration. Non-inferiority clinical trials to establish effectiveness guidance for industry. 2016. [accessed Aug 17, 2021]. https://www.fda.gov/media/78504/download .
- 21.Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63:754–62. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 23.Satola SW, Farley MM, Anderson KF, Patel JB. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. J Clin Microbiol. 2011;49:177–83. doi: 10.1128/JCM.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. Neonatal infection: antibiotics for prevention and treatment NICE guideline [NG195] 2021. [accessed April 22, 2021]. https://www.nice.org.uk/guidance/NG195 . [PubMed]
- 25.Public Health England. NHS newborn hearing screening programme. 2019. [accessed Nov 13, 2021]. https://www.england.nhs.uk/wp-content/uploads/2017/04/Service-Specification-No.20-NHS-Newborn-Hearing.pdf .
- 26.Marissen J, Fortmann I, Humberg A, et al. Vancomycin-induced ototoxicity in very-low-birthweight infants. J Antimicrob Chemother. 2020;75:2291–98. doi: 10.1093/jac/dkaa156. [DOI] [PubMed] [Google Scholar]
- 27.Lutsar I, Chazallon C, Trafojer U, et al. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1):a randomised controlled trial. PLoS One. 2020;15:e0229380. doi: 10.1371/journal.pone.0229380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cernadas JMC, Jonusas SF, Márquez M, Garsd A, Mariani G. Evolución clínica en recién nacidos con presunción de sepsis nosocomial tratados con cefazolina o vancomicina. Estudio de no inferioridad, aleatorizado, controlado. Arch Argent Pediatr. 2014;112:308–14. [Google Scholar]
- 29.Gwee A, Cranswick N, McMullan B, et al. Continuous versus intermittent vancomycin infusions in infants: a randomized controlled trial. Pediatrics. 2019;143:e20182179. doi: 10.1542/peds.2018-2179. [DOI] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Evaluation of medicinal products indicated for treatment of bacterial infections. 2019. [accessed March 23, 2021]. https://www.ema.europa.eu/en/evaluation-medicinal-products-indicated-treatment-bacterial-infections .
- 31.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 32.Folgori L, Lutsar I, Standing JF, et al. Standardising neonatal and paediatric antibiotic clinical trial design and conduct: the PENTA-ID network view. BMJ Open. 2019;9:e032592. doi: 10.1136/bmjopen-2019-032592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sharing of data will be considered on the basis of a detailed proposal which should include aims, methods, and a statistical analysis plan. Requests will be checked for compatibility with regulatory and ethics committee requirements and with participant informed consent. Proposals should be addressed to the corresponding author at lhill@sgul.ac.uk and will be evaluated by the sponsor.