Abstract
Until recently, it was assumed that insects lack immune memory since they do not have vertebrate-like specialized memory cells. Therefore, their most well studied evolutionary response against pathogens was increased basal immunity. However, growing evidence suggests that many insects also exhibit a form of immune memory (immune priming), where prior exposure to a low dose of infection confers protection against subsequent infection by the same pathogen that acts both within and across generations. Most strikingly, they can rapidly evolve as a highly parallel and mutually exclusive strategy from basal immunity, under different selective conditions and with divergent evolutionary trade-offs. However, the relative importance of priming as an optimal immune strategy also has contradictions, primarily because supporting mechanisms are still unclear. In this review, we adopt a comparative approach to highlight several emerging evolutionary, ecological and mechanistic features of priming vs basal immune responses that warrant immediate attention for future research.
Keywords: Cost of immunity, Coinfections, Immune priming, Resistance, Specificity, Tolerance1
1. Introduction
In recent decades, the discovery of immune memory-like responses in insects, where previous sublethal exposure to a pathogen confers subsequent protection against the same pathogen (i.e., immune priming), remains as one of the most important conceptual advancements in invertebrate immunology. The phenomenon was initially puzzling because insects apparently lack the legacy of immune cells or antibodies functionally equivalent to vertebrate lymphocytes (B and T-memory cells; components of adaptive immunity) that form the mechanistic basis of immune memory. Therefore, the most well studied evolutionary response against a virulent pathogen in insects was their increased basal immune function activated upon live pathogen exposure (Rolff and Siva-Jothy, 2003; Corby-Harris and Promislow, 2008). However, the long-standing notion is now replaced by a large number of evidence for diverse insect priming forms where their effects are detected within the same developmental stage, across life stages, or even to offspring (Contreras-Garduño et al., 2016; Khan et al., 2016; Tetreau et al., 2019; Sheehan et al., 2020). They are abundantly found in a wide range of insects species as diverse as dipterans (Pham et al., 2007; Ramirez et al., 2015), coleopterans (Khan et al., 2019), lepidopterans (Fallon et al., 2015), hymenopterans (Sadd and Schmid-Hempel, 2006) and arachnids (Galvez et al., 2020). Recent studies document the widespread presence of priming in wild-caught insect populations as well, providing the much-needed boost to justify its natural relevance (Tate and Graham, 2015; Khan et al., 2016). Mathematical models have also proposed that immune priming or memory-like responses have large impacts in reducing the infection severity and disease spread in a population (Tate and Rudolf, 2012; Best et al., 2013; Tate, 2016), clearly highlighting their adaptive roles. Priming has thus rapidly emerged as a highly efficient immune strategy in insects. Incidentally, it also implies that although vertebrate immune memory is achieved using mechanistically distinct immune responses (Netea et al., 2020), it is perhaps functionally not as unique as traditionally believed.
2. Evolving Immune Priming: Where, When And How?
Despite the surge of phenomenological evidence, the evolutionary basis of priming remained obscured until recently (Khan et al., 2017a). It was unclear whether selective parameters underlying the evolution of priming and their potential evolutionary trade-offs were different from basal immune responses (which functions without any prior exposure to pathogens). Also, we lack estimates of how much genetic variation exists for priming in natural populations (Khan et al., 2016), thereby restricting our ability to predict its evolvability. Unlike basal immunity which positively correlates with infection prevalence and strength of pathogen pressure (Linhart and Grant, 1996; Reznick and Ghalambor, 2001; Corby-Harris and Promislow, 2008; Mayer et al., 2015), it was unclear whether the same parameters play a similar role for priming as well. Wild populations inhabiting pathogen-rich environments certainly have a higher risk of reinfection by the same pathogen (see Corby-Harris and Promislow, 2008), but the link between the strength of priming and infection severity was not very clear. This was confirmed very recently in wild-caught flour beetle populations, where benefits of priming in natural populations were determined by the infection severity, with increased pathogen susceptibility imposing selection for stronger priming response (Khan et al., 2019). While these results could establish the ubiquitous role of pathogen pressure behind both priming and basal immunity, a source of major confusion is— how does then pathogen selection distinguish the evolutionary trajectory between priming vs basal immunity? From an evolutionary perspective, this certainly needs to be clarified before we can analyse the relative importance of priming vs stronger basal immunity as effective immune function in insects.
While experiments were lacking, a general mathematical model was able to offer some critical insights (Mayer et al., 2015). Although not specific to insects, this model, for the first time, brought more detailed features of the pathogenic environment such as frequency and duration of exposure at the forefront. It was also coupled with critical analyses of relative costs vs benefits of different immune functions to explain why organisms evolve divergent immune strategies. For example, when pathogen exposure is relatively rare, inducible immune responses can be adaptively favoured to minimise the costs of constitutively expressed innate immunity, suggesting complex interactions between pathogenic conditions, pathogen exposure statistics and fitness effects of counteractive immune components behind evolving optimal immune responses (Mayer et al. 2015). The first experimental evidence came from Khan and co-workers where they manipulated the frequency of pathogen exposure (e.g., once vs twice every generation) in flour beetle Tribolium castaneum populations evolving against a lethal dose of their natural pathogen Bacillus thuringiensis (Khan et al., 2017a). Within 14 generations of experimental evolution, they found populations that were exposed to a single large dose of infection every generation evolves both within- and across-generation priming (Khan et al. 2017a; Prakash et al. 2019). In contrast, populations that were exposed repeatedly to the same pathogen (Khan et al., 2017a; Prakash et al., 2019), evolved inherently higher post-infection survival, with no additional effects of priming (henceforth, basal infection response). Although the study implicitly assumes that the observed higher post-infection survival was achieved by increasing the basal immune function or resistance, but this causal link has not yet been established (also see the discussion later). Interestingly, these results on evolving immune strategies in insect models as a function of pathogen exposure statistics also closely mirrored the predictions of Mayer et al., (2015), with strong implications for the general evolution of immune responses.
Two years later, another flour beetle study by Ferro and co-workers also reported the evolution of priming against B. thuringiensis, using a different experimental set-up from Khan et al. (2017a). Instead of using one pathogenic strain, they used combinations of different bacteria and B. thuringiensis strains to prime and infect beetles for 14 generations. However, priming only evolved against a focal B. thuringiensis strain that primed and infected beetles at every three generations (e.g., generation 1, 4, 7 and so on) (Ferro et al., 2019), suggesting that the selection for priming against B. thuringiensis doesn’t have to be continuous across generations. Interestingly, the selective conditions for evolving inherently high basal infection response remained comparable between Ferro et al. (2019) and Khan et al. (2017a)— i.e., in both the studies, beetles evolving high basal infection response was exposed to the focal B. thuringiensis antigen twice (priming followed by the live infection) at every generation. Hence, despite some differences in experimental designs, comparable results from separate studies with independently evolving beetle populations unequivocally supported the role of lower pathogen exposure frequency in priming evolution, whereas basal immune function increasing survival is favoured in populations exposed to the same pathogen more frequently (Khan et al., 2017a; Ferro et al., 2019). Most importantly, these two studies established priming as a rapidly evolving independent strategy from basal infection responses, ending all the prior speculations on whether they have distinct evolutionary origins. However, it is important to note that since both studies addressed the evolution of priming in flour beetles, more studies are thus needed to verify whether similar selective conditions are also relevant for other insect species. Till then, they can serve as important benchmarks for future studies, presenting exciting possibilities of several previously unknown comparative features of priming vs basal immunity or infection responses. Below, we outline how our current understanding based on these studies might provide the impetus for further research on insect immune priming, integrating evolutionary, ecological and mechanistic insights.
3. Emerging Features Of Priming
While recent data clearly state the adaptive significance of priming independently of basal infection responses, they also attract a new level of complications vis-à-vis diverse fitness effects, unexpected evolutionary trade-offs and intertwined mechanisms. For example, it is surprising that evolution of priming does not increase the basal infection response in flour beetles at all (Khan et al., 2017a)—i.e., without a primary antigen exposure, beetles evolving priming can still be highly susceptible to infection, suggesting an intrinsic difference in how priming vs basal immunity might respond to infections. However, one of the most striking features of these results is the consistently higher survival benefits of increased basal infection response relative to priming (i.e., 85% vs 50% survival after pathogen exposure), raising further questions on the actual adaptive potential of these diverse responses. Most importantly, if evolving better basal infection response and immunity can confer more survival benefit, it is surprising that why it does not evolve in all the populations facing the pathogen pressure? One plausible explanation for this observation could be that basal infection response is also associated with other fitness costs, but contrary to much of our expectation, later experiments revealed that evolving basal infection response is consistently beneficial for multiple reasons. For example, these beetles have increased reproduction and longer lifespan (Prakash et al., 2019). They also seem to have better body condition as they have extended lifespan under starvation as well (Prakash et al., 2019). Also, these results might strongly contradict the traditional view of evolving immunity-fitness trade-offs, but lack of any measurable fitness costs has been experimentally proven in other studies as well (see Ye et al., 2009; Ma et al., 2012).
In contrast, both maintenance and activation of evolved priming can impose diverse suits of fitness costs such as reduced offspring survival, developmental time and reproduction (Ferro et al., 2019; Prakash et al., 2019). These results also corroborate outcomes from other single-generation experiments, where inducing priming responses can directly reduce the reproductive fitness in flour beetles (Contreras-Garduño et al., 2014), mosquitoes (Ramirez et al., 2015) and wax moth (Trauer and Hilker, 2013) or delayed development in mealworm beetles (Zanchi et al., 2011). Together, these pervasive costs might constrain the survival benefits of priming responses at a much lower level than consistently beneficial basal infection responses. Yet, it remains a mystery why putative alleles for basal immunity and infection response either did not arise or failed to outcompete priming alleles in all populations (Khan et al., 2019b; Ferro et al., 2019). Can the answer be hidden underneath mechanistic constraints? Possibly, selective conditions favouring the evolution of priming (e.g., lower pathogen exposure frequency, Khan et al. 2017a) could somehow also produce inhibitory signals that mechanistically preclude the alleles for more beneficial basal infection response to appear and fix in host populations. As more experiments combining both organismal and molecular insights will be needed to test these possibilities, we first need to pinpoint various distinctive features of priming that separate its effects from basal immunity and infection responses. To facilitate these initiatives, below, we have combined recent evolutionary as well as mechanistic studies to synthesise a few emerging features of insect immunity in the context of priming vs basal immunity, warranting special attention—
3.1. Multifaceted insect immune responses: where and how?
Insects are widely known to possess a robust immune system against infection that includes both cellular and humoral components (Hoffman, 2003; Stofanko et al., 2010, Buchon et al., 2014; Parsons et al., 2016; Hanson et al., 2019). While some of them are inducible and slow-acting against persistent infections such as anti-microbial peptides (henceforth AMPs), there are also components such as enzymatic cascades of phenoloxidase (henceforth PO) and melanisation responses that are constitutively expressed and can act rapidly (Haine et al., 2008). However, despite knowing how they might broadly function, assigning immune responses distinctly to priming vs basal immunity and their fitness consequences is not straightforward. It can be more complicated if a relatively limited repertoire of immune cells is stretched into playing diverse roles across species and pathogens. For example, while fast-acting PO is required to control the pathogen growth and increase survival in Drosophila melanogaster during Salmonella typhimurium and Listeria monocytogenes infections (Ayres and Schneider, 2008), it is not needed against Providencia rettgeri infection (Duneau et al., 2017). The same PO activity is also cytotoxic to Drosophila cell lines (Zhao et al., 2011) and highly immunopathological in the mealworm beetle Tenebrio molitor, causing a drastic reduction in beetle lifespan by damaging key organs such as Malpighian tubules (Khan et al., 2017b). It becomes even more intriguing when fast-acting nonspecific PO activity (Khan et al. 2017b) can also partake in evolving pathogen-specific priming (Ferro et al., 2019), a response that can be selected independently of basal immunity and infection responses. Together, these results underscore the astounding level of functional complexity of molecules involved in a so-called simple insect immune system. More recently, another interesting example emerged from fruit flies, where traditionally known cytotoxic reactive oxygen species (Lennicke and Cocheme, 2020) such as H2O2 produced by Toll-and JAK/STAT pathway activation & damage associated signalling molecules (Draper) in haemocytes could signal the priming of haemocytes against future infections (Chakrabarti and Visweswariah, 2020). The absence of ROS response in haemocytes leads to loss of priming, indicating its causal role in training innate immunity. Although this study only used a single pathogen Enterococcus faecalis, it exemplifies how immune effectors should be analysed using the diverse lens of their functionalities.
In general, exploring candidate genes and molecules for priming vs basal immunity should be integrated with their functional verifications and phenotypic effects (a graphical summary of some of the currently discussed mechanisms of immune priming across species is provided in Fig. 1). For example, the only study that tracked mechanisms underlying the evolution of priming, using flour beetle populations infected with B. thuringiensis, found a positive correlation with the overactivity of dopa decarboxylase (ddc), a gene involved in PO response (Ferro et al., 2019). AMPs such as attacins, defensins and coleoptericin were also upregulated, but priming was more consistently associated with higher expression of ddc, possibly indicating higher importance of PO response than AMPs. This is corroborated by other single-generation or short-term studies as well, including higher PO activity in primed greater wax moths Galleria mellonella (Vertyporokh and Wojda, 2020), or a transgenerational study in flour beetles where paternal priming can cause a dramatic increase of haemolymph PO activity in their offspring (Roth et al., 2010; Eggert et al., 2014). However, the fitness benefits of priming responses that are critically dependent on PO activity (Nappi and Ottaviani, 2000; Khan et al., 2017b) should also be interpreted with caution. This is because the net fitness gain can be constrained by an upper limit to reduce the immunopathological costs of highly activated PO (Khan et al., 2017b). This perhaps also indicate a mechanistic reason why evolved priming has lower survival benefits against a lethal infection relative to evolved basal infection response (see Khan et al., 2017a). It could have been interesting to analyse such mechanistic changes in beetle populations parallelly evolving stronger basal immunity and infection responses as well, but unfortunately such a comparative is missing.
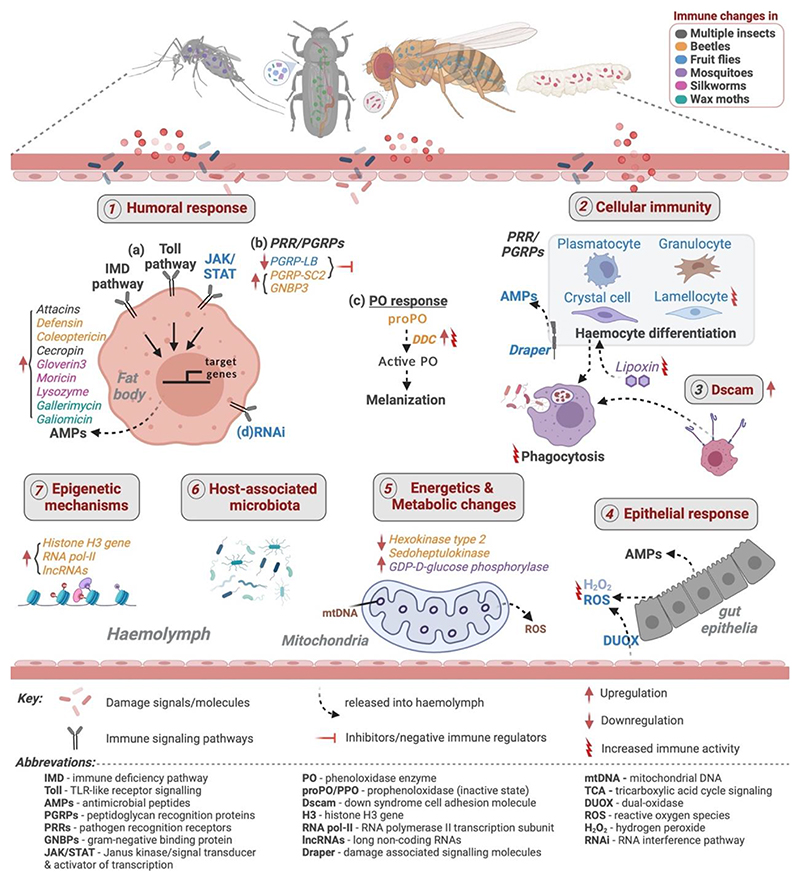
Figure 1. A brief summary of known (1-3) vs proposed (4-7) priming mechanisms from recent studies.
(1) Humoral response: (a) Activation of IMD- (immune deficiency), Toll- (TLR-like receptor), and JAK/STAT (Janus kinase signal transducer and activator of transcription) signalling pathways leading to synthesis & production of inducible AMPs (Sheehan et al., 2020) such as Attacins, Defensins & Coleoptericins in flour beetles (Greenwood et al., 2017; Ferro et al., 2019); Cecropins in fruit flies (Chakrabarti and Visweswariah, 2020); Cecropin, Attacin, Gloverin, Moricin & Lysozyme in silkworms (Yi et al., 2019); Gallerimycin & Galiomicin in wax-moths (Bergin et al., 2006); Cecropin in tobacco moths (Roesel et al., 2020) (b) Upregulated PGRP-SC (peptidoglycan recognition proteins - receptor for IMD pathway) and GNBPs (gram-negative binding proteins - receptor for Toll-pathway) in flour beetles (Ferro et al., 2019); Downregulated PGRP-LB (negative regulator of IMD-pathway) in fruit flies (Bozler et al., 2019) (c) Phenoloxidase (PO) response: Increased PO activity in flour beetles and mealworm beetles (Tetreau et al., 2019); Increased DDC (dopa-decarboxylase – a gene involved in PO cascade) in flour beetles (Ferro et al., 2019). (d) RNAi-pathway mediated specific priming against viral pathogens in fruit flies (Mondotte et al., 2018). (2) Cellular immunity: Increased phagocytosis activity (Pham et al., 2007; Weavers et al., 2016) and production of lamellocytes (Bozler et al., 2019) in fruit flies; Haemocyte differentiation induced by lipoxin (a lipid carrier) in mosquitoes (Rodrigues et al., 2010; Ramirez et al., 2015). (3) Down syndrome cell adhesion molecule (Dscam): Suspected role in specific immunity of flour beetles, fruit flies and other insects (Armitage et al., 2015); Acts as receptor during phagocytosis in crabs (Li et al., 2018); Upregulated in primed silkworms (Yi et al., 2019). (4) Epithelial response: Activation of haemocytes via intracellular accumulation of H2O2 (hydrogen peroxide) produced by DUOX (Reactive oxygen species producing dual-oxidase) through Toll & JAK/STAT pathway activation & Draper (damage associated signal molecules) in fruit flies (Chakrabarti and Visweswariah, 2020) (5) Metabolism and energetics: Downregulated metabolism-associated genes - hexokinase type 2 and sedoheptulokinase in flour beetles (Ferro et al., 2019); Upregulated trehalose transporter, GDP-D-glucose phosphorylase in mosquito Anopheles. albimanus (Maya-Maldonado et al., 2021) (6) Host-associated microbiota: Loss of priming in flour beetles (Futo et al., 2017) and Anopheles. gambiae mosquitoes (Rodrigues et al., 2010) after depleting microbiota. (7) Epigenetic mechanisms and reprogramming: Upregulated histone H3 gene, RNA polymerase II (transcription subunit 15) & exosome complex exonuclease (RRP6-like) (Ferro et al., 2019); Upregulated lncRNAs (long non-coding RNAs – necessary for regulating- metabolic, immune signalling and epigenetic processes (Ali et al., 2019) in flour beetles.
Finally, there are also exciting possibilities of intimate cross-talk between the immune system and other physiological processes such as metabolic changes which may influence the host’s evolutionary response against pathogen attacks. Indeed, in addition to traditional immune effectors, a recent experiment using flour beetles found a tight correlation between downregulated metabolism-associated genes such as hexokinase type 2 and sedoheptulokinase with increased priming (Ferro et al., 2019). These results draw close comparisons with the metabolic basis of macrophage polarisation (Kelly and O’Neill, 2015) and trained immunity in vertebrates (Cheng et al., 2014). More recently, another study analysing transcriptome from the midgut of primed mosquitoes Anopheles albimanus also reported upregulated genes related to metabolic activity, including trehalose transporter, GDP-D-glucose phosphorylase and fatty acid hydroxylase (Maya-Maldonado et al., 2021), suggesting the importance of metabolic variations in priming across species. In addition, several mutations in mitochondrial DNA are also emerging as key mediators of both humoral and cellular immunity in insects (Riley and Tait, 2020; Salminen and Vale, 2020). In Drosophila, a rapid metabolic switch explicitly triggers anabolic lipid metabolism to favour more phospholipid synthesis and endoplasmic reticulum expansion to compensate for the increasing demand for antimicrobial peptide synthesis to neutralize pathogens (Martinez et al., 2020). Based on these results, it thus appears that future studies might open up new avenues to explore whether and how energetics explain the divergent mechanisms of evolving priming vs basal infection response (Fig. 1).
3.2. Role of competing immune strategies: Tolerance vs resistance
Although most studies on pathogen defence primarily focus on the mechanisms that hosts employ to resist infection, recent experiments also provide ample evidence for tolerance to pathogenic infections— i.e., hosts can coexist with pathogens and withstand their negative fitness effects by reducing pathogen- or immune-mediated damage (McCarville and Ayres, 2018; Råberg et al., 2007; Råberg et al., 2009; Schneider and Ayres, 2008; Medzhitov et al., 2012; Seal et al., 2021). Theoretical models suggest that tolerance is consistently beneficial and hence, can reduce the levels of genetic variation by rapidly fixing tolerance-related alleles in the population under directional selection (Miller et al., 2005; Roy and Kirchner, 2000). In contrast, pathogen resistance and clearance by immune responses which typically reduces pathogen fitness, often involve costly immune activation and life-history trade-offs (Khan et al., 2017b). Consequently, resistance trait might converge to an intermediate optimum under stabilizing selection (Råberg, 2014). Besides, it might also have features of balancing selection, maintaining highly polymorphic infection outcomes within the population (Lefèvre et al., 2011; Råberg, 2014). However, despite these distinct evolutionary trajectories of pathogen resistance vs tolerance, their relevance to diverse forms of insect immunity and infection responses has never been analysed systematically— e.g., how does the choice between immune strategies (i.e., resistance vs tolerance) affect the evolution of priming relative to basal infection responses (see Fig. 2 for the conceptual link)? Previous studies suggest that the link between priming and tolerance can be species-specific. For instance, in fruit flies, larval priming with the Drosophila C virus increases tolerance to subsequent exposure of adults with the same virus (Mondotte et al., 2018), and also to their progeny (Mondotte et al., 2020). In contrast, transgenerational priming of crustacean Daphnia magna (Little et al., 2003) increases antibacterial activity to inhibit the growth of Pasteuria ramosa in their offspring. More recently, flour beetles primed with B. thuringiensis also exhibit enhanced survival by suppressing the pathogen burden (Khan et al., 2019), suggesting a form of pathogen resistance. Such priming-induced pathogen clearance in flour beetles may be mediated via reactive and cytotoxic PO response (Zhao et al., 2011; Khan et al., 2017b; Ferro et al., 2019), increasing the overall physiological costs associated with priming (Ferro et al., 2019; Prakash et al., 2019). Perhaps, it also offers more detailed and composite (evolutionary & mechanistic) explanation for why priming, as described in Khan et al. (2017a), has limited fitness gain. If priming indeed relies on immunopathological PO responses (Zhao et al., 2011) to resist pathogen growth, priming allele (increasing pathogen resistance; Khan et al., 2019) can only evolve an intermediate trait value under stabilising selection (also see above, Miller et al., 2005; Roy and Kirchner, 2000) and is unlikely to be fixed in the population due to fitness constraints.
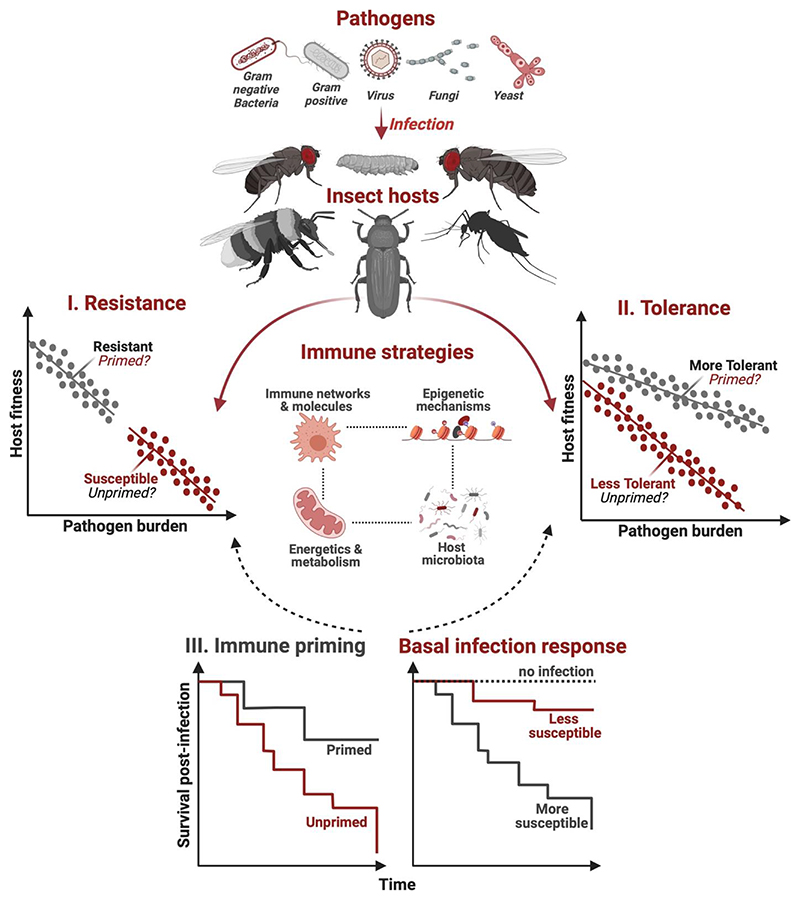
Figure 2.
A diagrammatic representation linking (I) hosts ability to either resist pathogen growth, by immune activation, or (II) tolerate pathogen burden, by reducing the fitness costs of infection or immune activation without directly reducing the pathogen numbers to (III) the outcome of immune priming (i.e., post-infection survival benefits after priming relative to unprimed controls) or basal infection response (i.e., survival response to infection without prior priming relative to uninfected control). At the mechanistic level, a complex interplay between immune pathways & molecules, host metabolism, epigenetic reprogramming and host-associated microbiota might be collectively responsible for evolving priming vs basal immunity and infection responses. Figures on tolerance and resistance are adapted from Råberg et al., 2007.
On the contrary, evolution under frequent exposure to pathogens, as reported in Khan et al., (2017a) and Ferro et al., (2019), might hint at a tolerance-like response to infections. Notably, both the studies interpreted the improved basal infection response primarily as resistance, but ~85% post-infection survival (Khan et al., 2017a), coupled with increased reproduction, better body condition and no other fitness costs (Khan et al., 2019) perhaps warrants an alternative explanation. For example, the rapid increase from 50% to ~85% post-infection survival within <5 generations (see Khan et al., 2017a) can also be achieved by the rapid spread of tolerance alleles under directional selection (Miller et al., 2005), increasing the survival by reducing the cost of infection or immune responses, rather than by employing efficient pathogen killing mechanisms (Ayres and Schneider, 2012). Future experiments directly estimating changes in fitness with increasing immune function or pathogen clearance (slope of fitness-by-pathogen load curve, see Fig. 2II) might resolve the ambiguity here.
Also, it remains to be seen what happens when immune strategies (i.e., pathogen clearance vs infection tolerance) are swapped across priming vs basal infection responses— e.g., if priming evolution is mediated by increased tolerance (see Mondotte et al., 2018; for fruit flies against viral infections), does it increase fitness more than basal immunity (compare with Khan et al., 2017a)? Certainly, more studies are needed to understand whether or to what extent fitness trajectories of priming vs basal immunity and infection responses across diverse insect host-pathogen systems depend on the choice of immune strategies. They will also serve as critical resources for mechanistic studies where the role of transcriptionally and translationally active molecules and pathways can be analysed and compared across diverse host-pathogen systems to impart a deeper understanding of dynamic immune strategies and molecular mechanisms therein.
We also speculate that the regulators of major insect immune responses (e.g., IMD, Toll or JAK/STAT pathway; Kuraishi et al., 2013; Kojour et al., 2020; Frank and Schmid-Hempel, 2019) might hold the key for understanding the relative impacts of resistance vs tolerance, thereby influencing the outcome of basal infection response vs priming. For instance, in fruit flies, G9a (an epigenetic negative regulator of the damage sensing JAK/STAT pathway) has been previously identified as being an important molecule in reducing immunopathology by tolerating Drosophila C Virus infections (Merkling et al., 2015; Gupta and vale, 2017). Recently, a negative regulator PGRP-LB (peptidoglycan recognition protein LB) from the IMD pathway has also been identified as a key candidate during transgenerational immune priming in Drosophila against parasitoid wasps (Leptopilina heterotoma and L. victoriae) (Bozler et al., 2020)— global downregulation of PGRP-LB is necessary to increase lamellocytes for pathogen clearance in the offspring. In another instance, the absence of PGRP-LB renders flies more resistant to Eschericia coli, whereas its normal activation leads to greater tolerance (Vincent and Dionne, 2021). Overall, we have just begun to uncover the potential role of tolerance via various insect immune regulation in infection outcomes. We certainly need more experiments to explore how targeted modulation of various receptors, signal transduction and immune effectors can be combined with functional characterization and phenotypic response across insect species. They might offer important clues about how negative feedback mechanisms can evolve to support diverse priming types vs basal infection response and immunity (Khan et al., 2017a; Ferro et al., 2019). Lastly, another indirect possibility, though not mutually exclusive, is that selection acts on the composition and function of the host-associated microbiota which functions as a signalling hub to regulate pathways underlying inflammatory responses (Thaiss et al., 2016) (also see Fig. 1). This may significantly contribute to maintaining tolerance, reducing the damage caused by inflammatory responses. Such a balanced immunity-microbiota alliance is also known to reduce oxidative damage to DNA and its repair machinery, whereas an imbalance leads to the development of health hazards in humans (Ray and Kidane, 2016; Belkaid and Hand, 2014). Based on these facts, future studies should also explore whether and how the microbiome might evolve in insects under pathogenic conditions to act as an additional regulatory switch between immune strategies, subsequently influencing priming vs basal infection responses (also see Rodrigues et al., 2010; Futo et al., 2017).
3.3. Remarkable specificity of priming: Why and how?
One of the most striking features of insect priming is their ability to differentiate between strains of the same pathogen (Roth et al., 2009; Wu et al., 2015; Futo et al., 2017), revealing an unexpectedly high degree of specificity, a feature that is previously thought to be an exclusive feature of vertebrate adaptive immunity. Recent flour beetle studies have not only reported phenomenological evidence of such exquisite specificity (Roth et al., 2009; Khan et al., 2016), rather they could also experimentally evolve priming response specific to a single B. thuringiensis strain that was used to infect beetles across generations (Khan et al., 2017a). In contrast, priming with another strain of the same pathogen could not confer any survival advantage in the evolved beetles (Khan et al., 2017a). More recently, the evolution of specific priming is corroborated by another study where priming could evolve only against the most virulent B. thuringiensis strain, whereas other less virulent strains or non-natural pathogens failed to evoke sufficient selection pressure (Ferro et al., 2019). While these results indicate that priming evolution is perhaps effective only against natural pathogens, they also suggest that even within natural pathogens, priming response might be strongly contingent upon their strain information which actually imposed the selection pressure.
How do insects mechanistically exercise such specific priming responses? Until recently, the answer to this question has been conceptually challenging because of our incomplete understanding of insect immunity. A longstanding notion was that insect immunity has limited ability to distinguish between specific pathogens (Cooper and Eleftherianos, 2017). Antimicrobial peptides (AMPs), the closest candidate for imparting specific immunity in insects, was known only to differentiate between broadly classified microbial families such as Gram-positive vs. Gram-negative bacteria or fungi, but functional distinctions between individual AMPs and their specific effects on pathogens were largely overlooked (Rolff and Schmid-Hempel, 2016; Unckless and Lazzaro, 2016). Newer evidence, however, contradicts this conservative view (Unckless et al., 2016; Hanson et al., 2019), by revealing an unexpectedly high degree of non-redundancy and specificity: Even a single AMP can be wholly responsible to prevent infections caused by a specific pathogen. For example, in flies, AMPs Diptericins or Drosocin alone can confer complete protection against Providencia rettgeri or Enterobacter cloacae infection respectively (Hanson et al., 2019). Although experiments are lacking, such targeted AMP actions might have a role in specific priming too.
In recent years, several other candidates/ immune genes have also been implicated in pathogen-specific priming. For example, increased phagocytosis (Pham et al., 2007; Weavers et al., 2016) or blood cell differentiation into granulocytes (Rodrigues et al., 2010) plays a role during pathogen-specific immune priming in fruit flies and mosquito Anopheles gambiae respectively. Flour beetles that experimentally evolved specific priming have increased dopa-decarboxylase gene expression which in addition to activating the PO response, can also promote nodulation and phagocytosis (Ferro et al., 2019; Sideri et al., 2008), although the causal link is not yet tested. Fruit flies can also use an RNA interference pathway to produce virus-specific priming responses, using information from the remnants of viral DNA (Mondotte et al., 2018, 2020; also see Maori et al., 2007 for honeybee Apis melifera). Taken together, these examples can collectively explain pathogen-specific responses in diverse insect species, but the mechanisms underlying the evolution of astonishing strain-specific priming still remains unknown. For instance, it is unclear how nonspecific insect immune responses such as increased PO activity (Cerenius and Söderhäll, 2004), which has been previously implicated in evolving specific priming (Ferro et al., 2019), can mechanistically differentiate between multiple B. thuringiensis strains in flour beetles (Khan et al., 2017a). Thus, future studies might also expand their horizon by exploring (i) the production of receptor diversity via alternative splicing of Down syndrome cell adhesion molecule (Dscam) to discriminate between different pathogen strains (Kurtz and Armitage, 2006; Armitage et al., 2015), or mechanisms equivalent to (ii) functional reprogramming of vertebrate myeloid cells or (iii) cytolytic innate immune effectors such as natural killer cells which can produce long-lasting and antigen-specific immune memory independent of B- and T cells (see O’Leary et al., 2006; Vivier et al., 2011; Netea et al., 2020). Moreover, recent studies suggest the role of genes involved in the epigenetic reprogramming of immune cells such as histone H3 gene that are upregulated during the evolution of specific priming responses (Ferro et al., 2019; also see Netea et al., 2020). It is quite possible that plasticity in immune responses evolve due to quantitative (rather than qualitative) changes in gene expression patterns and downstream pathways, regulated via epigenetic processes (Morandini et al., 2016), to produce nuanced differences in both pathogen- and strain-specific responses. In fact, epigenetic mechanisms such as DNA methylation, histone acetylation and microRNA expression can be particularly relevant for the transgenerational effects of paternal priming (Vilcinskas, 2021).
Finally, another striking feature is that the same insect populations that evolve strain-specific priming can also favour the evolution of generalized protection against multiple Bt strains under more frequent antigen exposure (in flour beetles, Khan et al., 2017a), suggesting an added level of functional complexity of insect immunity vis-à-vis specificity vs non-specificity. While further work is needed to understand how they can be mechanistically supported in such situations, distinctions perhaps reside in what immune strategies they choose to evolve. For example, if priming is achieved via pathogen resistance (Khan et al., 2019), it might favour specific modulation of immune responses that are just sufficient to clear infections, minimising the cost of general immune activation as much as possible. In contrast, increased basal infection response by plausible evolution of tolerance under frequent antigen exposure might also improve the overall body condition (Khan et al., 2017a), with reduced inflammation or more investment in antioxidant mechanisms to compensate immune-mediated cytotoxicity (Ha et al., 2005). The overall physiological effects of such tolerance response are thus more likely to be generalised and non-specific across pathogens and their strains.
3.4. Priming against multiple infections
Under natural conditions, hosts are frequently exposed to infections caused by multiple pathogens, ranging from close-related strains and pathogens to very diverse phyla, imposing complex selection pressure on their immune systems (Tate, 2019). In such cases, spatial and temporal constraints on immune system development, maturation or expression might play critical roles in disease outcomes (Tate and Graham, 2015). Previous infections might leave an immunological imprint and create a historical contingency upon the second pathogen, as found in lab mice where infection with nematodes increases resistance against later infections caused by Plasmodium parasites (Griffiths et al., 2015). By contrast, co-infection with gut protozoa (Gregarine sp.) and B. thuringiensis in flour beetles can severely curtail their progeny survival against B. thuringiensis infection (Tate and Graham, 2015). Depending on pathogen species, coinfection might also induce myriad effects on host immunity. One possibility is that it can activate shared immune pathways against pathogens, exemplified by Plasmodium parasites coexisting with several bacterial species inside the mosquito midgut (Pumpuni et al., 1996; Contreras-Garduño et al., 2015). Alternatively, coinfection can also lead to the expansion of immune repertoires catering to divergent immunological pursuits against invading pathogens, increasing the overall costs of immune activation (Viney and Graham, 2013). Tentative situations in insects might arise when they might need activation of both (i) IMD and Toll pathways against co-infecting Gram-negative and -positive bacteria (Lemaitre and Hoffmann, 2007); or (ii) rapid-acting PO vs inducible AMPs against fast- and slow-growing pathogens (Haine et al., 2008).
How do such mixed pathogenic environments affect the evolution of priming? Although direct experiments are lacking, answer to this question also has strong implications for evaluating the natural relevance of previously reported strain-specific priming evolution in flour beetles (Khan et al., 2017a). It is unclear whether and how such strain-specificity is affordable in the wild particularly when there is a high chance of experiencing multiple co-infecting B. thuringiensis strains (Abdel-Razek et al., 1999). Unless there is a large difference in virulence between strains, it is perhaps more favourable to evolve responses against the pathogen itself, disregarding its strain identity. The closest evidence for the evolution of priming under coinfection comes from Ferro et al., (2019), where selection treatments consisted of either exposing beetle to the same type of bacterium for both priming and challenge (specific treatment) or different ones (unspecific selection treatment) every generation. Although the study design does not include simultaneous infections by different pathogens, thereby limiting their direct interactions, it tests (a) the effects of selection for vs. against the ability to mount specific priming responses (b) mimics the effects of mixed pathogen selection in natural conditions where specific adaptation to one particular pathogen might not be an optimal strategy for protection against the next set of pathogens. Despite experiencing mixed selection by multiple pathogen species (Pseudomonas fluorescens, Lactococcus lactis, and 4 strains of B. thuringiensis) for 14 generations, priming could evolve only against the most virulent strain of B. thuringiensis (i.e., DSM 2046; also used in Khan et al., 2017a), reemphasizing how the strength of priming selection might be most strongly determined by the virulence level of specific strains of a natural pathogen (see Khan et al., 2019). However, more studies are needed to test situations where multiple natural pathogenic strains with comparable virulence (e.g., B. thuringiensis strain DSM 2046 vs MTCC 6905 described in Khan et al., 2017a; also see Bose and Schulte, 2014) are coinfecting and how the level of priming specificity might be modulated. Perhaps, such conflicting situations between strains might prevent the evolution of strain-specific priming.
4. Summary And Future Perspectives
In closing, we want to highlight that recent studies established different immune priming types as distinct strategies in insects, separate from basal immunity and infection response, indicating an unprecedented level of functional diversity of their immune functions (Khan et al., 2019, Ferro et al., 2019). However, in-depth analyses of their selective conditions, fitness effects and various life-history trade-offs are posing several open questions that are paradoxical. For example, a stronger basal infection response is consistently beneficial than priming. Both, early survival vs reproductive costs of priming can constrain its evolution, much more so than resistance and yet, it evolves in many populations as a mutually exclusive parallel strategy. It is puzzling what prevents alleles responsible for evolving consistently beneficial basal infection response from sweeping across all the populations? Can certain selective conditions demarcate the non-overlapping evolutionary as well as mechanistic space for these divergent responses? Evolutionarily, it is perhaps expected, because, in both the studies that successfully evolved priming (Khan et al., 2017a, Ferro et al., 2019), there is a clear connection between pathogen exposure statistics and evolved infection responses (Mayer et al., 2015). However, the underlying mechanistic constraints that might prevent the evolution of more beneficial basal immunity or infection responses during the low frequency of pathogen exposure are unknown. Certainly, the first hurdle here is to ascertain how evolved priming and basal infection response are linked to the host immune strategies— e.g., do they increase pathogen resistance by activating immunity or show tolerance to reduce the fitness costs of infection and immunity or both? This might not only reflect in their relative fitness impacts but also determine the evolution of the underlying immune mechanisms (see Miller et al., 2005). Finally, do they involve different or overlapping sets of immune pathways? If so, how are the cross-talks mediated? At present, although there are multiple immune mechanisms known to influence immune priming in several insect models (reviewed in Milutinović et al., 2016; Contreras-Garduño et al., 2016), there is a lack of consensus on how these diverse mechanisms might explain the adaptive potential of priming relative to basal immunity and infection response across species. In this perspective, while we have highlighted the importance of considering the role of remarkable diversity and flexibility of insect innate immune adaptation against infections, we suggest that future studies should also carefully identify the source of diverse complexities in characterizing modalities of these immune responses, such as various regulatory networks underlying immune strategies; the metabolic basis; the role of epigenetic reprogramming and finally, plausible immune modulations by host-associated microbiota. Such a multifaceted approach is needed to identify and validate various potential trade-offs between immune components and constraints underlying immune strategies that are responsible for mutually exclusive evolutionary space of basal infection response vs priming. It is also required to demystify the emergent properties of insect immunity such as strain-specificity which appears counterintuitive without a functional adaptive immune system.
Finally, we note that our understanding of directly evolving priming in insect populations is limited to only a few studies on flour beetles (Khan et al., 2017a; Ferro et al., 2019; Prakash et al., 2019). While they certainly represent the first steps uncovering the evolutionary basis of diverse immune strategies in response to selection imposed by the same pathogen, more studies are required to test repeatability in other insects too and perhaps, also adjust with their hypotheses on how underlying mechanisms might change. For example, are the fitness benefits of priming always lower than basal immunity and infection response? It may not be true in a species like Drosophila where, unlike in beetles (Khan et al., 2019), priming increases tolerance against viral infections (Mondotte et al., 2018) and hence, can be fixed in a population more rapidly than basal response (Miller et al., 2005). We also want to end by emphasising that an integrated evolutionary understanding of divergent immunity and infection outcomes as well as decoding the underlying mechanistic basis are not any more insect-specific problems. By all means, this is a pressing issue in higher animals as well where the evolution of diverse immune types and respective fitness landscapes lack thorough experimental validation (Mayer et al., 2015; Netea et al., 2020). We suggest that insects, owing to their rapidly emerging complex immune forms and functions analogous to vertebrate immunity, can be a powerful system to model the evolution and mechanistic basis of divergent animal immune responses.
Acknowledgements
We are grateful to Basabi Bagchi, Biswajit Shit, Devashish Kumar, Joy Bose, Malavika Menon, Saubhik Sarkar and Srijan Seal for helpful discussions and feedback on the manuscript. Figures were designed in the Biorender platform.
Funding
AP and IK acknowledge financial support from the Darwin trust, the University of Edinburgh and DBT-Wellcome Trust India Alliance Intermediate Fellowship under award number IA/I/20/I/504930 respectively.
Footnotes
Author Contributions
IK and AP developed the idea; IK and AP wrote the manuscript.
Competing Interests
We have no competing interests
References
- Abdel-Razek AS, Salama HS, White NDG, Morris ON. Effect of Bacillus thuringiensis on feeding and energy use by Plodia interpunctella (Lepidoptera: Pyralidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) The Can Entomol. 1999;131:433–440. [Google Scholar]
- Ali Mohammadie, Kojour M, Han YS, Jo YH. An overview of insect innate immunity. Entomol Res. 2020;50:282–291. [Google Scholar]
- Armitage SA, Peuss R, Kurtz J. Dscam and pancrustacean immune memory-a review of the evidence. Dev Comp Immunol. 2015;48:315–323. doi: 10.1016/j.dci.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:305. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006;8:2105–2112. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Best A, Tidbury H, White A, Boots M. The evolutionary dynamics of within-generation immune priming in invertebrate hosts. J R Soc Interface. 2013;10:20120887. doi: 10.1098/rsif.2012.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Schulte RD. Testing GxG interactions between coinfecting microbial parasite genotypes within hosts. Front Genet. 2014;5:124. doi: 10.3389/fgene.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler J, Kacsoh BZ, Bosco G. Maternal priming of offspring immune system in Drosophila. G3-Genes Genom Genet. 2020;10:165–175. doi: 10.1534/g3.119.400852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Visweswariah SS. Intramacrophage ROS Primes the Innate Immune System via JAK/STAT and Toll Activation. Cell reports. 2020;33:108368. doi: 10.1016/j.celrep.2020.108368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR. mTOR-and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:6204. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Garduño J, Rodríguez MC, Hernández-Martínez S, Martínez-Barnetche J, Alvarado-Delgado A, Izquierdo J, Herrera-Ortiz A, Moreno-García M, Velazquez-Meza ME, Valverde V, Argotte-Ramos R. Plasmodium berghei induced priming in Anopheles albimanus independently of bacterial co-infection. Dev Comp Immunol. 2015;52:172–181. doi: 10.1016/j.dci.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Contreras-Garduño J, Rodríguez MC, Rodríguez MH, Alvarado-Delgado A, Lanz-Mendoza H. Cost of immune priming within generations: trade-off between infection and reproduction. Microbes Infect. 2014;16:261–267. doi: 10.1016/j.micinf.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Contreras-Garduño Jorge, Lanz-mendoza Humberto, Franco B, Nava A, Pedraza-reyes mario, Canales-lazcano Jorge. Insect immune priming: ecology and experimental evidences. Ecol Entomol. 2016;41:351–366. [Google Scholar]
- Cooper D, Eleftherianos I. Memory and specificity in the insect immune system: current perspectives and future challenges. Front Immunol. 2017;8:539. doi: 10.3389/fimmu.2017.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby-Harris V, Promislow DE. Host ecology shapes geographical variation for resistance to bacterial infection in Drosophila melanogaster. J Anim Ecol. 2008;77:768. doi: 10.1111/j.1365-2656.2008.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhinaut J, Chogne M, Moret Y. Trans-generational immune priming in the mealworm beetle protects eggs through pathogen-dependent mechanisms imposing no immediate fitness cost for the offspring. Dev Comp Immunol. 2018;79:105–112. doi: 10.1016/j.dci.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Duneau DF, Kondolf HC, Im JH, Ortiz GA, Chow C, Fox MA, et al. The Toll pathway underlies host sexual dimorphism in resistance to both Gram-negative and Gram-positive bacteria in mated Drosophila. BMC Biology. 2017;15:124. doi: 10.1186/s12915-017-0466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert H, Kurtz J, Diddens-de Buhr MF. Different effects of paternal trans-generational immune priming on survival and immunity in step and genetic offspring. Proc R Soc B Biol Sci. 2014;281:20142089. doi: 10.1098/rspb.2014.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JP, Troy N, Kavanagh K. Pre-exposure of galleria mellonella larvae to different doses of aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence. 2015;2:413–421. doi: 10.4161/viru.2.5.17811. [DOI] [PubMed] [Google Scholar]
- Ferro K, Peuß R, Yang W, Rosenstiel P, Schulenburg H, Kurtz J. Experimental evolution of immunological specificity. Proc Natl Acad Sci. 2019;116:20598–20604. doi: 10.1073/pnas.1904828116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, Schmid-Hempel P. Evolution of negative immune regulators. PLoS Pathog. 2019;15:1007913. doi: 10.1371/journal.ppat.1007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futo M, Sell MP, Kutzer MA, Kurtz J. Specificity of oral immune priming in the red flour beetle Tribolium castaneum. Biol Let. 2017;13:20170632. doi: 10.1098/rsbl.2017.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez D, Añino Y, Vega C, Bonilla E. Immune priming against bacteria in spiders and scorpions? PeerJ. 2020;8:9285. doi: 10.7717/peerj.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JM, Milutinović B, Peuß R, Behrens S, Esser D, Rosenstiel P, Schulenburg H, Kurtz J. Oral immune priming with Bacillus thuringiensis induces a shift in the gene expression of Tribolium castaneum larvae. BMC genomics. 2017;18:1–14. doi: 10.1186/s12864-017-3705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EC, Fairlie-Clarke K, Allen JE, Metcalf CJE, Graham AL. Bottom-up regulation of malaria population dynamics in mice co-infected with lung-migratory nematodes. Ecol Let. 2015;18:1387–1396. doi: 10.1111/ele.12534. [DOI] [PubMed] [Google Scholar]
- Gupta V, Vale PF. Nonlinear disease tolerance curves reveal distinct components of host responses to viral infection. R Soc Open Sci. 2017;4:170342. doi: 10.1098/rsos.170342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008;322:1257–1259. doi: 10.1126/science.1165265. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B. Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. Elife. 2019;8:e44341. doi: 10.7554/eLife.44341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Kelly B, O’neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Agashe D, Rolff J. Early-life inflammation, immune response and ageing. Proc R Soc B Biol Sci. 2017b;284:20170125. doi: 10.1098/rspb.2017.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Prakash A, Agashe D. Divergent immune priming responses across flour beetle life stages and populations. Ecol Evol. 2016;6:7847–7855. doi: 10.1002/ece3.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Prakash A, Agashe D. Experimental evolution of insect immune memory versus pathogen resistance. Proc R Soc B Biol Sci. 2017a;284:20171583. doi: 10.1098/rspb.2017.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Prakash A, Agashe D. Pathogen susceptibility and fitness costs explain variation in immune priming across natural populations of flour beetles. J Anim Ecol. 2019;88:1332–1342. doi: 10.1111/1365-2656.13030. [DOI] [PubMed] [Google Scholar]
- Kuraishi T, Hori A, Kurata S. Host-microbe interactions in the gut of Drosophila melanogaster. Front Physiol. 2013;4:375. doi: 10.3389/fphys.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J, Armitage SA. Alternative adaptive immunity in invertebrates. Trends Immunol. 2006;27:493–496. doi: 10.1016/j.it.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Williams AJ, de Roode JC. Genetic variation in resistance, but not tolerance, to a protozoan parasite in the monarch butterfly. Proc R Soc B Biol Sci. 2011;278:751–759. doi: 10.1098/rspb.2010.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lennicke C, Cochemé HM. Redox signalling and ageing: insights from Drosophila. Biochem Soc Trans. 2020;48:367–377. doi: 10.1042/BST20190052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart YB, Grant MC. Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst. 1996;27:237–277. [Google Scholar]
- Little TJ, O’Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. [DOI] [PubMed] [Google Scholar]
- Ma J, Benson AK, Kachman SD, Hu Z, Harshman LG. Drosophila melanogaster selection for survival of Bacillus cereus infection: Life history trait indirect responses. Int J Evol Biol. 2012;2012:935–970. doi: 10.1155/2012/935970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maori E, Tanne E, Sela I. Reciprocal sequence exchange between non-retro viruses and hosts leading to the appearance of new host phenotypes. Virology. 2007;362:342–349. doi: 10.1016/j.virol.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Martínez BA, Hoyle RG, Yeudall S, Granade ME, Harris TE, Castle JD, Leitinger N, Bland ML. Innate immune signaling in Drosophila shifts anabolic lipid metabolism from triglyceride storage to phospholipid synthesis to support immune function. PLoS Genetics. 2020;16:e1009192. doi: 10.1371/journal.pgen.1009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Maldonado K, Cime-Castillo J, Maya-Lucas O, Argotte-Ramos R, Rodríguez MC, Lanz-Mendoza H. Transcriptome analysis uncover differential regulation in cell cycle, immunity, and metabolism in Anopheles albimanus during immune priming with Plasmodium berghei. Dev Comp Immunol. 2021;120:104046. doi: 10.1016/j.dci.2021.104046. [DOI] [PubMed] [Google Scholar]
- Mayer A, Mora T, Rivoire O, Walczak AM. Diversity of immune strategies explained by adaptation to pathogen statistics. Proc Natl Acad Sci. 2015;113:8630–8635. doi: 10.1073/pnas.1600663113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville J, Ayres J. Disease tolerance: concept and mechanisms. Curr Opin Immunol. 2018;50:88–93. doi: 10.1016/j.coi.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkling SH, Overheul GJ, Van Mierlo JT, Arends D, Gilissen C, Van Rij RP. The heat shock response restricts virus infection in Drosophila. Sci Rep. 2015;5:1–15. doi: 10.1038/srep12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. The evolution of host resistance: Tolerance and control as distinct strategies. J Theor Biol. 2005;236:198–207. doi: 10.1016/j.jtbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Milutinović B, Peuß R, Ferro K, Kurtz J. Immune priming in arthropods: an update focusing on the red flour beetle. Zoology. 2016;119:254–261. doi: 10.1016/j.zool.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Mondotte JA, Gausson V, Frangeul L, Blanc H, Lambrechts L, Saleh MC. Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat Microbiol. 2018;3:1394–1403. doi: 10.1038/s41564-018-0265-9. [DOI] [PubMed] [Google Scholar]
- Mondotte JA, Gausson V, Frangeul L, Suzuki Y, Vazeille M, Mongelli V, Blanc H, Failloux AB, Saleh MC. Evidence For Long-Lasting Transgenerational Antiviral Immunity in Insects. Cell Reports. 2020;33(11):108506. doi: 10.1016/j.celrep.2020.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandini AC, Santos CF, Yilmaz Ö. Role of epigenetics in modulation of immune response at the junction of host-pathogen interaction and danger molecule signaling. Pathog Dis. 2016;74 doi: 10.1093/femspd/ftw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi AJ, Ottaviani E. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays. 2000;22:469–480. doi: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LA, van der Meer JW, Mhlanga MM, Mulder WJ, Riksen NP. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell-and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Parsons B, Foley E. Cellular immune defenses of Drosophila melanogaster. Dev Comp Immunol. 2016;58:95–101. doi: 10.1016/j.dci.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Agashe D, Khan I. Basal resistance against a pathogen is more beneficial than immune priming responses in flour beetles. bioRxiv. 2019 [Google Scholar]
- Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- Råberg L. How to Live with the Enemy: U nderstanding Tolerance to Parasites. PLoS Biol. 2014;12:e1001989. doi: 10.1371/journal.pbio.1001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Phil Trans Proc R Soc B Biol Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Sim D, Read AF. Disentangling Genetic Variation for Resistance and Tolerance to Infectious Diseases in Animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- Ramirez JL, de Almeida Oliveira G, Calvo E, Dalli J, Colas RA, Serhan CN, Ribeiro JM, Barillas-Mury C. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat Commun. 2015;6:1–7. doi: 10.1038/ncomms8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Kidane D. Gut microbiota imbalance and base excision repair dynamics in colon cancer. JCancer. 2016;7:1421. doi: 10.7150/jca.15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Microevolution rate, pattern, process. 2001:183–198. [PubMed] [Google Scholar]
- Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020;21:49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy MT. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- Rolff J, Schmid-Hempel P. Perspectives on the evolutionary ecology of arthropod antimicrobial peptides. Philos Trans R Soc B: Biol Sci. 2016;371:20150297. doi: 10.1098/rstb.2015.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesel CL, Rosengaus RB, Smith W, Vollmer SV. Transcriptomics reveals specific molecular mechanisms underlying transgenerational immunity in Manduca sexta. Ecol Evol. 2020;10:11251–11261. doi: 10.1002/ece3.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, Kurtz J. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. J Anim Ecol. 2010;79:403–413. doi: 10.1111/j.1365-2656.2009.01617.x. [DOI] [PubMed] [Google Scholar]
- Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc R Soc B Biol Sci. 2009;276:145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy BA, Kirchner JW. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54:51–63. doi: 10.1111/j.0014-3820.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Salminen TS, Vale PF. Drosophila as a model system to investigate the effects of mitochondrial variation on innate immunity. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal S, Dharmarajan G, Khan I. Evolution of pathogen tolerance and emerging infections: A missing experimental paradigm. 2021 doi: 10.7554/eLife.68874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan G, Farrell G, Kavanagh K. Immune priming: the secret weapon of the insect world. Virulence. 2020;11:238–246. doi: 10.1080/21505594.2020.1731137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideri M, Tsakas S, Markoutsa E, Lampropoulou M, Marmaras VJ. Innate immunity in insects: surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly haemocytes. Immunology. 2008;123:528–537. doi: 10.1111/j.1365-2567.2007.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofanko M, Kwon SY, Badenhorst P. Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PloS one. 2010;5:14051. doi: 10.1371/journal.pone.0014051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate AT. The interaction of immune priming with different modes of disease transmission. Front Microbiol. 2016;7:1102. doi: 10.3389/fmicb.2016.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate AT. Role of multiple infections on immunological variation in wild populations. Msystems. 2019;4:e00099-19. doi: 10.1128/mSystems.00099-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate AT, Graham AL. Trans-generational priming of resistance in wild flour beetles reflects the primed phenotypes of laboratory populations and is inhibited by co-infection with a common parasite. Funct Ecol. 2015;29:1059–1069. [Google Scholar]
- Tate AT, Rudolf VH. Impact of life stage specific immune priming on invertebrate disease dynamics. Oikos. 2012;121:1083–1092. [Google Scholar]
- Tetreau G, Dhinaut J, Gourbal B, Moret Y. Trans-generational immune priming in invertebrates: current knowledge and future prospects. Front Immunol. 2019;10:1938. doi: 10.3389/fimmu.2019.01938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Trauer U, Hilker M. Parental legacy in insects : Variation of transgenerational immune priming during offspring development. PLoS One. 2013;8:e63392. doi: 10.1371/journal.pone.0063392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Howick VM, Lazzaro BP. Convergent balancing selection on an antimicrobial peptide in Drosophila. Curr Biol. 2016;26:257–262. doi: 10.1016/j.cub.2015.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Lazzaro BP. The potential for adaptive maintenance of diversity in insect antimicrobial peptides. Phil Trans Proc R Soc B Biol Sci. 2016;371:20150291. doi: 10.1098/rstb.2015.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertyporokh L, Wojda I. Immune response of Galleria mellonella after injection with non-lethal and lethal dosages of Candida albicans. J Invertebr Pathol. 2020;170:107327. doi: 10.1016/j.jip.2020.107327. [DOI] [PubMed] [Google Scholar]
- Vilcinskas A. Mechanisms of transgenerational immune priming in insects. Dev Comp Immunol. 2021 doi: 10.1016/j.dci.2021.104205. [DOI] [PubMed] [Google Scholar]
- Viney ME, Graham AL. Patterns and processes in parasite co-infection. Adv Parasitol. 2013;82:321–369. doi: 10.1016/B978-0-12-407706-5.00005-8. [DOI] [PubMed] [Google Scholar]
- Vincent CM, Dionne MS. Disparate regulation of IMD signaling drives sex differences in infection pathology in Drosophila melanogaster. Proc Natl Acad Sci. 2021;118(32) doi: 10.1073/pnas.2026554118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H, Evans IR, Martin P, Wood W. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li M, Liu Y, Ding Y, Yi Y. The specificity of immune priming in silkworm, Bombyx mori, is mediated by the phagocytic ability of granular cells. J Insect Physiol. 2015;81:60–68. doi: 10.1016/j.jinsphys.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Ye YH, Chenoweth SF, McGraw EA. Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PLoS Pathog. 2009;5:e1000385. doi: 10.1371/journal.ppat.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Xu H, Li M, Wu G. RNA-seq profiles of putative genes involved in specific immune priming in Bombyx mori haemocytes. Infect Genet Evol. 2019;74:103921. doi: 10.1016/j.meegid.2019.103921. [DOI] [PubMed] [Google Scholar]
- Zanchi C, Troussard J, Martinaud G. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J Anim Ecol. 2011;80:1174–1183. doi: 10.1111/j.1365-2656.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- Zhao P, Lu Z, Strand MR, Jiang H. Antiviral, anti-parasitic, and cytotoxic effects of 5, 6-dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response. Insect Biochem Mol Biol. 2011;41:645–652. doi: 10.1016/j.ibmb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]