Abstract
Background
Children with human immunodeficiency virus type 1 (HIV-1) infection have limited options for effective antiretroviral treatment (ART).
Methods
We conducted an open-label, randomized, noninferiority trial comparing three-drug ART based on the HIV integrase inhibitor dolutegravir with standard care (non–dolutegravir-based ART) in children and adolescents starting first- or second-line ART. The primary end point was the proportion of participants with virologic or clinical treatment failure by 96 weeks, as estimated by the Kaplan–Meier method. Safety was assessed.
Results
From September 2016 through June 2018, a total of 707 children and adolescents who weighed at least 14 kg were randomly assigned to receive dolutegravir-based ART (350 participants) or standard care (357). The median age was 12.2 years (range, 2.9 to 18.0), the median weight was 30.7 kg (range, 14.0 to 85.0), and 49% of the participants were girls. By design, 311 participants (44%) started first-line ART (with 92% of those in the standard-care group receiving efavirenz-based ART), and 396 (56%) started second-line ART (with 98% of those in the standard-care group receiving boosted protease inhibitor–based ART). The median follow-up was 142 weeks. By 96 weeks, 47 participants in the dolutegravir group and 75 in the standard-care group had treatment failure (estimated probability, 0.14 vs. 0.22; difference, –0.08; 95% confidence interval, -0.14 to -0.03; P=0.004). Treatment effects were similar with first- and second-line therapies (P=0.16 for heterogeneity). A total of 35 participants in the dolutegravir group and 40 in the standard-care group had at least one serious adverse event (P=0.53), and 73 and 86, respectively, had at least one adverse event of grade 3 or higher (P=0.24). At least one ART-modifying adverse event occurred in 5 participants in the dolutegravir group and in 17 in the standard-care group (P = 0.01).
Conclusions
In this trial involving children and adolescents with HIV-1 infection who were starting first- or second-line treatment, dolutegravir-based ART was superior to standard care. (Funded by ViiV Healthcare; ODYSSEY ClinicalTrials.gov number, NCT02259127; EUDRACT number, 2014-002632-14; and ISRCTN number, ISRCTN91737921.)
WORLDWIDE, AN ESTIMATED 1.8 MILLION children and adolescents younger than 15 years of age are living with human immunodeficiency virus (HIV) infection.1 Treatment options for children and adolescents lag behind those for adults, and outcomes are consistently worse.1 Dolutegravir is a second-generation HIV integrase strand-transfer inhibitor (INSTI) that has been shown to be effective in trials involving adults2,3 and that is being rapidly rolled out in national treatment programs.
In the ODYSSEY trial, we aimed to compare the efficacy and safety of dolutegravir-based antiretroviral therapy (ART) with those of standard care in children and adolescents who were starting first- or second-line ART in resource-limited and well-resourced settings.4 We report here the results of the main trial involving children and adolescents weighing at least 14 kg.
Methods
Trial Oversight
We conducted this open-label, noninferiority, 96-week, randomized trial to compare dolutegravir-based ART with non–dolutegravir-based standard care in children and adolescents with HIV type 1 (HIV-1) infection who were starting first-line ART (the ODYSSEY A cohort) or switching to second-line ART after having treatment failure (the ODYSSEY B cohort). The Penta Foundation, the trial steering committee, and the independent data and safety monitoring committee provided trial oversight. ViiV Healthcare provided funding, and ViiV Healthcare and Mylan–Viatris provided ART drugs for the trial. National or local ethics committees and the University College London ethics committee approved the trial protocol, which is available with the full text of this article at NEJM.org. The trial committees, clinical site investigators, ViiV Healthcare, and the Penta Foundation reviewed and provided comments on the manuscript. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Participants and Randomization
Children and adolescents (age, ≥4 weeks to <18 years) with HIV-1 infection who weighed at least 14 kg were recruited to the main trial. Children who weighed at least 3 kg but less than 14 kg were enrolled in a separate randomized cohort in the trial; their follow-up is ongoing and is not reported here. Participants were randomly assigned, in a 1:1 ratio, to receive either dolutegravir plus two nucleoside (or nucleotide) reverse-transcriptase inhibitors (NRTIs) (dolutegravir group) or standard care with a nonnucleoside reverse-transcriptase inhibitor (NNRTI), boosted protease inhibitor, or non-dolutegravir integrase inhibitor plus two NRTIs. Second-line ART included a new third agent and at least one NRTI with preserved activity, as assessed on the basis of resistance tests or assumed from treatment history (in locations where resistance tests were not routinely available). The choice of NRTIs among abacavir, tenofovir, or zidovudine was made according to World Health Organization (WHO)5 or national guidelines.
Participants who were enrolled in the ODYSSEY B cohort had an HIV-1 RNA viral load of at least 500 copies per milliliter within the 4 weeks before screening or at screening. The main exclusion criteria for both the ODYSSEY A and B cohorts were clinically significant liver disease, pregnancy or breast-feeding, and previous exposure to an integrase inhibitor for more than 2 weeks.4 All the caregivers provided written informed consent, and participants who were deemed to be old enough to understand their participation in the trial provided written assent.
Randomization was stratified according to trial cohort (ODYSSEY A or B), routine availability of resistance tests (available or unavailable), intended standard care (boosted protease inhibitor ART or other third agent), and intended NRTI backbone therapy (abacavir and lamivudine, tenofovir [tenofovir disoproxil fumarate {DF} or tenofovir alafenamide] and either lamivudine or emtricitabine, or other). The computer-generated randomization list was prepared by the trial statistician and incorporated within the database, which enabled access only to the next assignment. Fast enrollment of children and adolescents who weighed at least 35 kg and had not received ART previously led to the decision to cap the recruitment in this subgroup in July 2017.4
The trial included pharmacokinetic substudies evaluating simplified administration of dolutegravir and new dispersible 5-mg dolutegravir tablets for use in children (Table S4.2 of the Supplementary Appendix, available at NEJM.org).4 Once these results were available,6,7 participants who were receiving dolutegravir moved from receiving the initial doses approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) to receiving higher doses (50-mg film-coated tablets for participants weighing ≥20 kg and 25-mg dispersible tablets [administered as five 5-mg tablets] for those weighing 14 to <20 kg) (Table S4.1). Licenses were subsequently updated by the FDA and EMA.8,9
Trial Procedures
Participants were seen at screening, at enrollment, at weeks 4 and 12, and then every 12 weeks at visits in which height, weight, HIV disease stage, adverse events, and adherence (from week 4 onward in participants in the ODYSSEY A cohort) were assessed. CD4, CD8, biochemical, and hematologic tests were performed at baseline, at weeks 4 and 24, and then every 24 weeks. Lipid and glucose levels were measured at baseline and every 48 weeks thereafter. Plasma samples for retrospective viral-load testing were obtained and stored at baseline, at weeks 4 and 12, and then every 12 weeks; real-time viral load testing was done according to local practice (every 6 to 12 months in African countries).
End Points
The primary end point was treatment failure by 96 weeks. Treatment failure was defined as the first occurrence of any of the following: a decrease of less than 1 log10 in the viral load at week 24 (or a viral load of ≥50 copies per milliliter at week 24 if the viral load had been <500 copies per milliliter at baseline) and a switch to second- or third-line ART for treatment failure; virologic failure (defined as two consecutive viral-load results of ≥400 copies per milliliter, the first occurring at or after week 36); a new or recurrent acquired immunodeficiency syndrome (AIDS)–defining event (WHO stage 4) or severe WHO stage 3 event; or death from any cause. Participants who had a virologic end-point event were retrospectively tested for post–treatment failure resistance up to week 96, with the use of the latest sample showing a viral load of at least 1000 copies per milliliter after treatment failure and before any treatment change. The corresponding baseline sample was sequenced if at least one major mutation as defined by the International AIDS Society was identified.10
The change in the total cholesterol level from baseline to week 96 was the prespecified main secondary end point for assessing the safety superiority of dolutegravir-based ART over standard care. Other secondary end points included virologic, immunologic, and safety end points (Table S2.1). Here we report the secondary end points of treatment failure by 48 weeks; the proportion of participants with a cross-sectional viral load of less than 50 copies per milliliter or less than 400 copes per milliliter at 96 weeks; the change in the CD4 count and CD4 lymphocyte percentage from baseline to 96 weeks; the proportion of participants in whom new resistance mutations developed; and the incidence of serious adverse events, new clinical and laboratory adverse events of grade 3 or 4, and adverse events of any grade leading to treatment modification. Secondary end points that were reported by participants or their caregivers included quality of life, adherence to the treatment regimen, and acceptability of the treatment as assessed on questionnaires.
All the clinical events were reviewed against prespecified criteria by an independent end-point review committee whose members were unaware of the randomized group assignments. The independent data and safety monitoring committee reviewed interim data, including all viral-load results and safety data, at four meetings. The Haybittle–Peto criterion (P<0.001) was the statistical guide for considering a recommendation of stopping or modifying the trial. Retrospective viral-load results were not returned to the treating clinicians.
Statistical Analysis
Assuming a probability of treatment failure of 0.18 in each group by 96 weeks, a 10% loss to follow-up, and a noninferiority margin of 0.10, we calculated that the planned sample of 700 participants in the overall trial (across both the ODYSSEY A and B cohorts) would provide the trial with 90% power to show that dolutegravir-based ART was noninferior to standard care. ODYSSEY A and B each had 80% power to exclude a between-group difference of more than 0.12, assuming an estimated probability of treatment failure of 0.15 in ODYSSEY A and 0.20 in ODYSSEY B.
Follow-up was censored when the last participant reached 96 weeks of follow-up; otherwise, data were censored at loss to follow-up or at the date when the last viral load was assessed for virologic end points. Comparisons between randomized groups were assessed in the intention-to-treat population (which included all the participants who underwent randomization, except those who were in major violation of the eligibility criteria), with adjustment for the stratification factors (ODYSSEY A vs. B; abacavir and lamivudine NRTI backbone vs. other NRTI backbone; routine resistance testing available vs. unavailable). Adjustment for the use of a boosted protease inhibitor or other third agent in the standard-care group was not made because the third agent was strongly associated with the ODYSSEY A and B strata.
The cumulative treatment-failure function for each randomized group was estimated as a weighted mean of the corresponding stratum-specific cumulative treatment-failure functions (estimated from a Cox model with adjustment for stratification factors and randomized group), with weights proportional to the number of participants in each stratum at baseline. The estimated probability of treatment failure by week 96 was compared between the treatment groups. The 95% bias-corrected confidence intervals were estimated with the use of the bootstrap method (Section S3.2).
The time to the first event was compared between groups with the use of Cox regression. Regimen change was defined as a change of the third agent due to treatment failure, toxic effects, pregnancy, or a major protocol deviation. The per-protocol population excluded participants who did not meet all the eligibility criteria, and follow-up was censored at regimen change or discontinuation of ART for more than 31 days.
Changes in continuous outcomes were analyzed with the use of analysis of covariance, with adjustment for baseline value and stratification factors; mean treatment differences through follow-up were estimated with the use of mixed linear models with a random effect for intercept and fixed effects for treatment group, visit week, and adjustment covariates. Participants who had a viral load of less than 50 copies per milliliter or less than 400 copies per milliliter at 48 weeks and 96 weeks were compared in the two groups on the basis of crude proportions and the FDA snapshot algorithm (Tables S7.7 through S7.14). All the P values are two-sided. No imputation was performed for missing outcomes. No adjustment was made for testing multiple secondary outcomes; secondary efficacy outcomes were mostly components of the composite primary or closely related to it, and safety outcomes were tested independently to identify any risks associated with dolutegravir.
Results
Trial Participants
A total of 710 children and adolescents underwent randomization between September 20, 2016, and June 22, 2018. Three participants were excluded because of major eligibility violations (Fig. S5.1), so 707 participants were included in the final analysis. A total of 331 participants were enrolled in Uganda, 146 in Zimbabwe, 144 in South Africa, 61 in Thailand, and 25 in Europe.
A total of 350 participants were randomly assigned to receive dolutegravir-based ART and 357 to receive standard care. In the ODYSSEY A cohort, 311 participants (44% of the trial population) started first-line ART (154 participants in the dolutegravir group and 157 in the standard-care group), and in the ODYSSEY B cohort, 396 participants (56% of the trial population) started second-line ART (196 participants in the dolutegravir group and 200 in the standard-care group).
The characteristics of the participants at baseline were similar in the treatment groups. A total of 49% of the participants were girls. The median age of the participants was 12.2 years (range, 2.9 to 18.0), and the median weight was 30.7 kg (range, 14.0 to 85.0). On average, the CD4 count was marginally higher and the viral load marginally lower in the standard-care group than in the dolutegravir group both in the total trial population and in the ODYSSEY B cohort (Table 1).
Table 1. Characteristics of the Trial Participants at Baseline.*.
| Characteristic | Dolutegravir (N = 350) |
Standard Care (N = 357) |
Total (N = 707) |
|---|---|---|---|
| Country or region — no. (%) | |||
| Uganda | 170 (49) | 161 (45) | 331 (47) |
| Zimbabwe | 79 (23) | 67 (19) | 146 (21) |
| South Africa | 61 (17) | 83 (23) | 144 (20) |
| Thailand | 28 (8) | 33 (9) | 61 (9) |
| Europe | 12 (3) | 13 (4) | 25 (4) |
| Female sex — no. (%) | 174 (50) | 171 (48) | 345 (49) |
| Age at last birthday — yr | |||
| Median | 12.2 | 12.1 | 12.2 |
| Interquartile range | 9.2-15.1 | 8.8-14.7 | 9.1-14.9 |
| Range† | 3.4-18.0 | 2.9-18.0 | 2.9-18.0 |
| Weight — kg | |||
| Median | 30.4 | 31.0 | 30.7 |
| Interquartile range | 23.7-43.7 | 23.3-42.7 | 23.4-43.0 |
| Range | 14.0-85.0 | 14.2-72.7 | 14.0-85.0 |
| Race — no. (%)‡ | |||
| Black African | 310 (89) | 313 (88) | 623 (88) |
| Asian | 28 (8) | 32 (9) | 60 (8) |
| White | 5 (1) | 1 (<1) | 6 (1) |
| Other | 7 (2) | 11 (3) | 18 (3) |
| CD4 lymphocyte percentage§ | |||
| Median | 20 | 22 | 21 |
| Interquartile range | 11-29 | 13-31 | 12-30 |
| CD4 lymphocyte count§ | |||
| Median — cells/mm3 | 444 | 486 | 459 |
| Interquartile range — cells/mm3 | 196-652 | 254-751 | 228-707 |
| Distribution — no. (%) | |||
| <200 cells/mm3 | 88 (25) | 70 (20) | 158 (22) |
| 200 to <500 cells/mm3 | 118 (34) | 114 (32) | 232 (33) |
| ≥500 cells/mm3 | 144 (41) | 173 (48) | 317 (45) |
| Viral load — no./total no. (%)§ | |||
| <10,000 copies/ml | 93/350 (27) | 123/356 (35) | 216/706 (31) |
| 10,000 to <100,000 copies/ml | 159/350 (45) | 158/356 (44) | 317/706 (45) |
| <I00,000 copies/ml | 98/350 (28) | 75/356 (21) | 173/706 (25) |
| Viral load — log10 copies/ml§¶ | |||
| Median | 4.5 | 4.4 | 4.4 |
| Interquartile range | 3.9-5.1 | 3.7-4.9 | 3.9-5.0 |
| WHO stage — no. (%) | |||
| 1 or 2 | 253 (72) | 265 (74) | 518 (73) |
| 3 | 69 (20) | 60 (17) | 129 (18) |
| 4 | 28 (8) | 32 (9) | 60 (8) |
WHO denotes World Health Organization.
All the participants were younger than 18 years of age at enrollment. The upper limit of the range is reported as 18.0 owing to rounding.
Race was reported by participants or caregivers or were determined on the basis of the participants’ records.
At a participant level, the mean of the measured values was used if measured values were available at screening and randomization.
Data on the viral load at baseline were missing for one participant.
In the standard-care group, 92% of the participants started efavirenz in the ODYSSEY A cohort, and 98% started a boosted protease inhibitor (boosted lopinavir in 72%, boosted atazanavir in 24%, and boosted darunavir in 1%) in the ODYSSEY B cohort. NRTI backbone therapies were balanced across the groups; 65% of the participants received abacavir and lamivudine, 23% received tenofovir DF and lamivudine or tenofovir DF and emtricitabine, 11% received zidovudine and lamivudine, and 1% received other combinations (Table S12.1). The last participant reached 96 weeks of follow-up on April 24, 2020. The median follow-up was 142 weeks (interquartile range, 124 to 159). A total of 687 participants (97%) were seen at or after 96 weeks or had a primary end-point event.
Efficacy
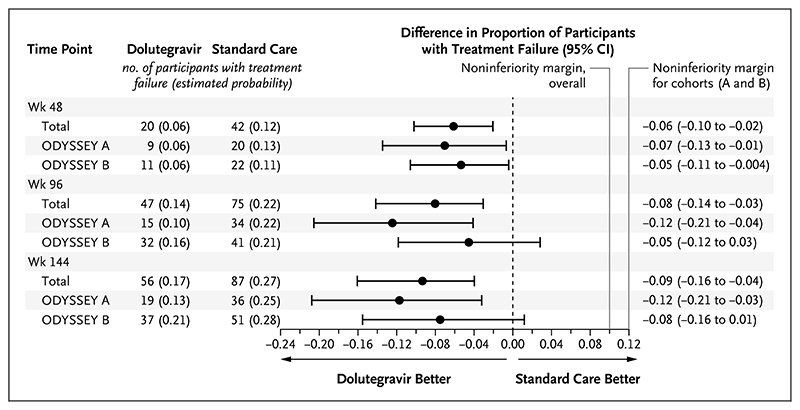
A total of 47 participants had protocol-defined treatment failure by 96 weeks (estimated probability of treatment failure, 0.14) in the dolutegravir group, as compared with 75 participants (estimated probability, 0.22) in the standard-care group (difference, –0.08; 95% confidence interval [CI], -0.14 to -0.03; P=0.004) (Fig. 1). Results were similar in the per-protocol analysis (difference in estimated probability, -0.07; 95% CI, -0.12 to -0.01) and in sensitivity analyses (Figs. S7.2 through S7.7). Among participants who met the primary end point, 40 in the dolutegravir group and 67 in the standard-care group had virologic treatment failure. Seven participants in the dolutegravir group and 8 in the standard-care group were categorized as having had treatment failure on the basis of a new or recurrent WHO stage 4 or severe WHO stage 3 event or death (Table 2). Treatment effects were similar in the ODYSSEY A and B cohorts (P=0.16 for heterogeneity) (Fig. 1).
Figure 1. Difference in the Proportion of Participants with Virologic or Clinical Treatment Failure by 48, 96, and 144 Weeks.
The primary end point was treatment failure by 96 weeks. Results are presented both overall and according to trial cohort (ODYSSEY A or B). Participants who were starting first-line antiretroviral therapy (ART) were assigned to the ODYSSEY A cohort, and those who were starting second-line ART after having treatment failure were assigned to the ODYSSEY B cohort. The noninferiority margins for the overall trial and for the trial cohorts are shown.
Table 2. Efficacy End Points for the Comparison of Dolutegravir-based ART with Standard Care.*.
| End Point | Total Population | ODYSSEY A | ODYSSEY B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dolutegravir (N = 350) |
Standard Care (N=357) |
Treatment Effect (95% Cl) |
Dolutegravir (N = I54) |
Standard Care (N = I57) |
Treatment Effect (95% Cl) |
Dolutegravir (N = I96) |
Standard Care (N = 200) |
Treatment Effect (95% Cl) |
|
| Primary end point | |||||||||
| Treatment failure by % wk — no. (%) |
47 (13) | 75 (21) | 15 (10) | 34 (22) | 32 (16) | 41 (20) | |||
| Primary end-point components — no. (%) | |||||||||
| Insufficient virologic response at 24 wk |
0 | 3(1) | 0 | 2(1) | 0 | 1(1) | |||
| Confirmed viral load ≥400 copies/ml at >36 wk |
40 (11) | 64 (18) | 10 (6) | 26 (17) | 30 (15) | 38 (19) | |||
| Severe WHO stage 3 event | 0 | l (<l) | 0 | 0 | 0 | 1 (1) | |||
| WHO stage 4 event | 7 (2) | 5 (1) | 5 (3) | 5 (3) | 2 (1) | 0 | |||
| Death | 0 | 2 (1) | 0 | 1 (1) | 0 | 1 (1) | |||
| Estimated probability of treat- ment failure (95% Cl) |
0.14 (0.10 to 0.17) |
0.22 (0.18 to 0.26) |
-0.08 (-0.14 to-0.03) † |
0.10 (0.05 to 0.15) |
0.22 (0.16 to 0.30) |
-0.12 (-0.21 to -0.04) |
0.16 (0.12 to 0.22) |
0.21 (0.15 to 0.27) |
-0.05 (-0.12 to 0.03) |
| P value | 0.004 | 0.003 | 0.22 | ||||||
| Secondary end points | |||||||||
| Viral load at 96 wk | |||||||||
| <50 copies/ml | |||||||||
| No. of participants | 270 | 252 | 117 | 113 | 153 | 139 | |||
| Percent of participants (95% Cl) |
81 (76 to 84) |
76 (71 to 80) |
5 (-1 to 11) |
80 (73 to 86) |
81 (73 to 86) |
-1 (-10 to 8) |
81 (75 to 86) |
72 (66 to 78) |
9 (0.4 to 17) |
| <400 copies/ml | |||||||||
| No. of participants | 299 | 285 | 129 | 124 | 170 | 161 | |||
| Percent of participants (95% Cl) |
89 (85 to 92) |
86 (81 to 89) |
3 (-2 to 8) |
88 (82 to 93) |
89 (82 to 93) |
0 (-8 to 7) |
89 (84 to 93) |
83 (77 to 88) |
6 (-1 to 12) |
| Mean change in CD4 count from baseline to 96 wk — cells/mm3 |
265±17 | 230±17 | 35 (-12 to 82) |
311±23 | 267±24 | 44 (-21 to 109) |
228±24 | 202±24 | 27 (-39 to 93) |
Plus-minus values are means +SE within the treatment groups. The probability of having virologic or clinical treatment failure by 96 weeks (primary end point) was adjusted for all stratification factors (Section S3.2). Proportions of participants who had other end-point events at or by 96 weeks were unadjusted. The mean change in the CD4 count from baseline to 96 weeks was calculated with the use of normal regression with adjustment for baseline measure; estimates are presented for the mean change from a baseline CD4 count of 521.8 cells per cubic millimeter in the total population, 487.4 cells per cubic millimeter in the ODYSSEY A cohort, and 548.8 cells per cubic millimeter in the ODYSSEY B cohort. Comparisons of treatment groups are adjusted for all stratification factors and are presented for the dolutegravir group as compared with the standard-care group. The between-group differences in the percentages of participants with a viral load of less than 50 copies per milliliter and of less than 400 copies per milliliter at 96 weeks are the marginal risk differences from the logistic regression model and are presented in percentage points. The between-group difference in the mean change from baseline was calculated with the use of normal regression with adjustment for baseline measure and stratification factors. ART denotes antiretroviral therapy.
P = 0.16 for the interaction between trial group (dolutegravir or standard care) and trial cohort (ODYSSEY A or B) for the primary end point.
In the overall trial population, the adjusted hazard ratio for treatment failure at 96 weeks was 0.60 (95% CI, 0.42 to 0.86). In prespecified exploratory analyses, there was no evidence that the treatment effect differed according to sex; baseline age, weight, CD4 lymphocyte percentage (CD4 percentage), and viral load; randomization stratification factors (choice of NRTI backbone therapy and availability of resistance testing); or calendar time (indicator of changes in the recommended dolutegravir doses and formulations). Adjustment for baseline viral load (for which there was by chance a slight baseline imbalance between the treatment groups) did not affect results (Fig. S7.8). The difference between treatment groups was present by week 48 and sustained to week 144 (Fig. 1).
At weeks 48 and 96, the percentages of participants with a viral load of less than 400 copies per milliliter and of less than 50 copies per milliliter were similar in the two treatment groups, both overall and in the ODYSSEY A and B cohorts. Assessment of these cross-sectional viral loads did not include consideration of previous viral rebound (in which a child or adolescent had resuppression with the same regimen or after treatment change) (Table 2). There were 10 new or recurrent WHO stage 4 or severe WHO stage 3 events or deaths (in 8 participants) in the dolutegravir group and 8 such events (in 8 participants) in the standard-care group, with no evidence of differences between groups across the total follow-up or by 96 weeks (Tables S7.18 and S7.19). At 96 weeks, the CD4 count did not differ significantly between the groups (Table 2), but we observed some evidence of a difference in the increase in the CD4 count from baseline in favor of the dolutegravir group over the standard-care group (adjusted mean difference through 96 weeks, 27 cells per cubic millimeter; 95% CI, 0 to 53); the results were similar for the CD4 percentage (Table S8.4).
Among participants receiving first-line therapy (ODYSSEY A cohort), none of those in the dolutegravir group had a major drug-resistance mutation (as defined by the International AIDS Society) after treatment failure. Of 29 participants in the standard-care group who had virologic treatment failure by week 96 and had a post–treatment failure resistance test available for the drug class, 18 participants (62%) had NRTI-related mutations, 27 (93%) had NNRTI-related mutations, and none had protease inhibitor–related mutations; most of the mutations were new (Table 3).
Table 3. Genotypic Resistance with Dolutegravir-based ART and Standard Care.*.
| End Point | ODYSSEY A | ODYSSEY B | ||
|---|---|---|---|---|
| Dolutegravir | Standard Care | Dolutegravir | Standard Care | |
| Virologic failure by 96 wk — no./total no. (%)† |
11/154 (7) | 30/157 (19) | 31/196 (16) | 40/200 (20) |
| Resistance after virologic failure — no./total no. (%)‡ | ||||
| Any drug class | 0/11 | 28/29 (97) | 23/29 (79) | 36/40 (90) |
| NRTI | 0/11 | 18/29 (62) | 21/29 (72) | 31/40 (78) |
| NNRTI | 0/11 | 27/29 (93) | 22/29 (76) | 36/40 (90) |
| Protease inhibitor | 0/11 | 0/29 | 2/29 (7) | 3/40 (8) |
| INSTI | 0/11 | — | 4/22 (18) | — |
| Emerging resistance after virologic failure — no. (%)§ | ||||
| Any drug class | 0 | 21 (97) | 6 (22) | 6 (19) |
| NRTI | 0 | 13 (62) | 2 (8) | 3 (10) |
| NNRTI | — | 19 (88) | — | 2 (100) |
| Protease inhibitor | — | — | — | 2 (5) |
| INSTI¶ | 0 | — | 4 (18) | — |
INSTI denotes integrase strand-transfer inhibitor, NNRTI nonnucleoside reverse-transcriptase inhibitor, and NRTI nucleoside (or nucleotide) reverse-transcriptase inhibitor.
A total of 112 participants had a virologic end-point event by week 96 (defined as a confirmed viral load of ≥400 copies per milliliter after week 36 or a lack of virologic response by week 24 followed by a switch in ART); 5 participants had a virologic end-point event after meeting a clinical component of the composite primary end point.
Major International AIDS Society (IAS) drug-resistance mutations were defined according to the 2019 update of the IAS drug-resistance mutations. Shown are the percentages of participants with resistance after virologic failure, among those with virologic failure by week 96 who had a post-treatment failure resistance test available for the drug class. (The integrase gene was not sequenced for the standard-care group.)
Among participants with virologic failure and exposure to the drug class, emerging resistance was estimated under an assumption of the same proportion of new resistance in participants with an available baseline resistance test and those without.
Four participants had resistance to dolutegravir (Q148R in one participant, Q148K in one, G118RS in one, and G118RS and R263K in one).
Among participants receiving second-line therapy (ODYSSEY B cohort), 23 of 29 (79%) in the dolutegravir group and 36 of 40 (90%) in the standard-care group had at least one major mutation after treatment failure. Among participants who had exposure to the drug class, an INSTI-related mutation developed in 4 participants in the dolutegravir group (of whom 3 were receiving twice-daily zidovudine and lamivudine) and 2 had new NRTI mutations; in the standard-care group, 3 had new NRTI-related mutations, 2 participants had new NNRTI-related mutations, and 2 had new protease inhibitor–related mutations (Tables 3 and S7.21).
Safety
Similar percentages of participants in each group had at least one serious adverse event (35 participants [10%] in the dolutegravir group and 40 [11%] in the standard-care group; adjusted hazard ratio, 0.87; 95% CI, 0.55 to 1.36; P=0.53) (Table 4). A total of 83% of the serious adverse events were considered to be serious owing to hospitalization; 50% of the serious adverse events were due to infection (Table S10.2). Five participants died (2 in the dolutegravir group and 3 in the standard-care group). More participants had serious adverse events in the ODYSSEY A cohort (23 participants [15%] in the dolutegravir group and 27 [17%] in the standard-care group) than in the ODYSSEY B cohort (12 [6%] and 13 [7%], respectively).
Table 4. Safety End Points for the Comparison of Dolutegravir-based ART with Standard Care.*.
| End Point | Total Population | ODYSSEY A | ODYSSEY B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dolutegravir (N = 350) |
Standard Care (N = 357) |
Treatment Effect (95% Cl) |
Dolutegravir (N = I54) |
Standard Care (N = I57) |
Treatment Effect (95% Cl) |
Dolutegravir (N = I96) |
Standard Care (N = 200) |
Treatment Effect (95% Cl) |
|
| Serious adverse event — no. of events (no. of participants)† |
65 (35) | 44 (40) | 0.87 (0.55 to 1.36) |
52 (23) | 31 (27) | 0.83 (0.48 to 1.46) |
13 (12) | 13 (13) | 0.93 (0.42 to 2.04) |
| P value | 0.53 | 0.52 | 0.86 | ||||||
| Grade ≥3 adverse event— no. of events (no. of participants)† |
113 (73) | 132 (86) | 0.83 (0.61 to 1.13) |
80 (48) | 62 (43) | 1.13 (0.75 to 1.70) |
33 (25) | 70 (43) | 0.54 (0.33 to 0.88) |
| P value | 0.24 | 0.57 | 0.01 | ||||||
| ART-modifying event— no. of events (no. of participants)† |
6 (5) | 17 (17) | 0.29 (0.11 to 0.77) |
4 (3) | 8 (8) | 0.35 (0.09 to 1.33) |
2 (2) | 9 (9) | 0.22 (0.05 to 1.03) |
| P value | 0.01 | 0.13 | 0.055 | ||||||
| Mean change in total cholesterol from baseline to 96 wk — mg/dl† |
-5.0±1.5 | 9.9±1.5 | -15.1 (-19.0 to-11.1) |
2.1±2.3 | 19.6±2.3 | -17.5 (-23.9 to-11.1) |
-10.5±1.8 | 2.8±l.8 | -13.4 (-18.5 to -8.4) |
| P value | <0.001 | <0.001 | <0.001 | ||||||
| Mean change in weight from base line to 96 wk — kg‡ |
7.1±0.3 | 6.1±0.3 | 1.0 (0.3 to 1.7) |
7.8±0.4 | 6.5±0.4 | 1.4 (0.2 to 2.5) |
6.7±0.3 | 5.9±0.3 | 0.8 (-0.1 to 1.6) |
| Mean change in BMI-for-age z score from baseline to 96 wk‡ |
0.24±0.04 | 0.11±0.04 | 0.13 (0.01 to 0.25) |
0.36±0.07 | 0.20±0.07 | 0.17 (-0.03 to 0.36) |
0.14±0.05 | 0.04±0.05 | 0.10 (-0.05 to 0.25) |
Plus-minus values are means ±SD within the treatment groups. The mean changes in the continuous measures to 96 weeks were calculated with the use of normal regression with adjustment for baseline measure. Estimates are presented for the mean change in the total cholesterol level from a baseline level of 138.1 mg per deciliter (3.55 mmol per liter) in the total population, 134.3 mg per deciliter (3.45 mmol per liter) in the ODYSSEY A cohort, and 141.1 mg per deciliter (3.65 mmol per liter) in the ODYSSEY B cohort; for the mean change in weight from baseline weights of 34.0 kg, 34.3 kg, and 33.6 kg, respectively; and for the mean change in the z score for the body-mass index (BMI) for age from baseline z scores of-0.7, -0.6, and -0.8, respectively. Comparisons of treatment groups are adjusted for all stratification factors and are presented for the dolutegravir group as compared with the standard-care group. Hazard ratios are shown for time-to-event analyses. Between-group differences in mean changes were calculated with the use of normal regression with adjustment for baseline measure and stratification factors.
This secondary safety end point was specified in the protocol. Additional secondary end points are presented in the Supplementary Appendix.
This end point was not a secondary end point that was specified in the protocol, but it was included in a planned analysis (see the statistical analysis plan) and is presented owing to data in adults that suggest an association of dolutegravir-based ART with excessive weight gain.
In the overall trial population, similar percentages of participants had one or more adverse events of grade 3 or higher (73 participants [21%] in the dolutegravir group and 86 [24%] in the standard-care group; adjusted hazard ratio, 0.83; 95% CI, 0.61 to 1.13; P=0.24); a total of 104 of 245 events (42%) were asymptomatic laboratory events of grade 3 or 4. Excess adverse events of grade 3 or higher that were observed in the standard-care group in the ODYSSEY B cohort were explained by elevated bilirubin levels in participants receiving atazanavir and ritonavir (Tables 4 and S10.3).
ART-modifying adverse events were less frequent in the dolutegravir group than in the standard-care group (5 participants [1%] vs. 17 [5%]; adjusted hazard ratio, 0.29; 95% CI, 0.11 to 0.77; P=0.01) (Tables 4 and S10.4). Ten participants in the dolutegravir group and 4 in the standard-care group had immune reconstitution inflammatory syndrome (IRIS) events (adjusted hazard ratio, 2.54; 95% CI, 0.80 to 8.09; P=0.12). A total of 12 participants had tuberculosis-associated IRIS, of whom 11 were successfully treated; 1 participant in the dolutegravir group who had severe acute malnutrition and suspected tuberculosis died.
Psychiatric events occurred in 10 participants in the dolutegravir group and in 4 in the standard-care group (adjusted hazard ratio, 2.48; 95% CI, 0.78 to 7.92; P = 0.12). A total of 12 participants (8 in the dolutegravir group and 4 in the standard-care group) had suicidality events (suicidal ideation and attempts), including 5 suicide attempts (in 2 participants in the dolutegravir group and in 3 in the standard-care group).
Participants in the dolutegravir group took currently approved doses for 81% of the follow-up; these doses were higher than the initial trial doses in participants weighing 14 to less than 40 kg. There was no evidence that increased doses of dolutegravir led to higher risks of adverse events (Table S13.1).
At the trial data-censoring date or loss to follow-up, 335 participants (96%) in the dolutegravir group and 321 (90%) in the standard-care group were receiving their initial trial regimen. Two participants (1%) in the dolutegravir group and 22 (6%) in the standard-care group had their regimen switched owing to treatment failure (Table S12.2).
The total cholesterol level was lower in the dolutegravir group than in the standard-care group. At 96 weeks, the estimated between-group difference in the mean change from baseline was -15 mg per deciliter (-0.40 mmol per liter; 95% CI, -19 to -11 mg per deciliter [-0.50 to -0.30 mmol per liter]) (P<0.001). The results were similar in the ODYSSEY A cohort (-18 mg per deciliter [-0.50 mmol per liter]; 95% CI, -24 to -11 mg per deciliter [-0.60 to -0.30 mmol per liter]) and the ODYSSEY B cohort (-13 mg per deciliter [-0.35 mmol per liter]; 95% CI, -18 to -8 mg per deciliter [-0.50 to -0.20 mmol per liter]). However, these findings involved an increase from baseline in the total cholesterol level in the standard-care group in the ODYSSEY A cohort and a decrease from baseline in the dolutegravir group in the ODYSSEY B cohort (Table 4); differences were mainly in the low-density lipoprotein (LDL) cholesterol levels (Tables S9.1 through S9.3 and S9.7 through S9.9).
Participants’ weight, height, and body-mass index (BMI)–for–age z score increased more in the dolutegravir group than in the standard-care group (the BMI is the weight in kilograms divided by the square of the height in meters). The estimated between-group differences in means at 96 weeks were as follows: for weight, 1 kg (95% CI, 0.3 to 1.7); for height, 0.8 cm (95% CI, 0.2 to 1.4); and for the BMI-for-age z score, 0.13 (95% CI, 0.01 to 0.25). Details are provided in Tables S14.1, S14.4, and S14.7.
Adherence and quality-of-life assessments, as reported by participants or caregivers, were high and similar in the two treatment groups. Acceptability of the trial treatment was high overall, with few reported problems with taste or swallowing; there were marginally more such reports in the standard-care group than in the dolutegravir group. Details are provided in Tables S11.1 through S11.3.
Discussion
The randomized ODYSSEY trial showed evidence of the superior efficacy of dolutegravir-based ART, as compared with standard care, in children and adolescents starting first-line and second-line ART. The risk of treatment failure was approximately 40% lower with dolutegravir-based ART than with standard care. Superior efficacy was evident by 48 weeks, was sustained to 144 weeks, and was consistent across age, weight bands, and NRTI backbone therapies. No anti-viral resistance was observed over a period of approximately 2 years in children and adolescents who started dolutegravir-based first-line ART, which suggests a higher barrier to INSTI resistance and protection against NRTI resistance than with mainly NNRTI-based first-line standard care. The occurrence of new INSTI resistance in 4 participants who received dolutegravir-based second-line ART highlights the need for ongoing adherence support among children and adolescents starting second-line treatment. Retention in the trial was excellent, and the incidence of treatment failure was low despite infrequent realtime viral-load monitoring in most participants and no requirement for resistance testing to guide the choice of NRTI backbone therapy for second-line treatment. Results are therefore generalizable across various settings, particularly in Africa, where most children with HIV-1 infection live.
Dolutegravir use was associated with a similar frequency of adverse events as standard care but with fewer treatment changes, although because the trial was open-label, clinicians may have been less willing to switch participants from dolutegravir therapy than from another ART. Participants who had been randomly assigned to dolutegravir had better lipid profiles at 48 weeks and 96 weeks after enrollment, particularly in regard to the LDL cholesterol level, than those who had been assigned to standard care. In contrast to adults,11–14 the children and adolescents in our trial had minimal additional weight gain with dolutegravir therapy; this gain occurred early and alongside a small increase in height, which suggests improvement in normal growth. No participants in the dolutegravir group received dolutegravir and tenofovir alafenamide, the ART combination that has been reported to be associated with the greatest weight gain in adults.14,15 There were few IRIS and psychiatric events, with no significant differences between the treatment groups.
The results of our trial are in line with those from trials of dolutegravir-based ART for first-line or second-line treatment in adults. Two meta-analyses have shown that, as compared with efavirenz and ritonavir-boosted protease inhibitors, dolutegravir-based first-line ART provided superior virologic suppression, protection against emerging drug resistance, and fewer drug discontinuations.2,3 Only the ADVANCE trial included adolescents 12 years of age or older (only 14 participants were <19 years of age). The DAWNING trial of second-line treatment also showed superior efficacy of dolutegravir therapy over lopinavir plus ritonavir therapy at 48 weeks,16 although at least one fully active NRTI was required, on the basis of genotypic resistance testing. Recently, the NADIA trial in Africa showed that dolutegravir therapy was noninferior to darunavir plus ritonavir in combination with tenofovir DF– or zidovudine-containing NRTIs at 48 weeks in adults who switched to second-line treatment empirically without resistance testing; results were consistent in the subgroup of participants with no NRTIs that were predicted to have activity.17 Similar to findings in the ODYSSEY B cohort, in the DAWNING and NADIA trials, resistance to dolutegravir developed in a few adults receiving second-line ART who had virologic rebound, results that were numerically higher than with boosted protease inhibitors.16,17 As in the NADIA trial, 3 of the 4 participants with emerging dolutegravir resistance in the ODYSSEY trial were receiving zidovudine and lamivudine.17
In 2018, while the ODYSSEY trial was ongoing, the WHO recommended dolutegravir-based ART as a preferred first- and second-line treatment for adults and children with HIV-1 infection18; recommendations for treatment in children were conditional, on the basis of low-certainty evidence. Nested ODYSSEY pharmacokinetic substudies provided evidence for updated simplified WHO guidance regarding doses, as well as FDA and EMA pediatric licensing recommendations in 2020.6,7 Once results from the pharmacokinetic substudies were available, participants in the dolutegravir group in our trial who weighed 20 to 40 kg moved from receiving 25 mg or 35 mg of dolutegravir (administered as 10-mg and 25-mg film-coated tablets, according to the initial doses approved by the FDA and EMA) to a single 50-mg film-coated tablet (intended for adults). These results made it possible for the majority of children living with HIV-1 infection worldwide to have expedited access to dolutegravir. In the trial follow-up, we saw no concerns about toxic effects in participants weighing 20 to 40 kg who were receiving the adult dose.
Children weighing less than 20 kg received 5-mg dispersible tablets of dolutegravir manufactured by ViiV Healthcare. Pharmacokinetic data from these children in our trial also contributed to timely regulatory approvals of the 5-mg dispersible formulation of dolutegravir,8,9 which enabled rapid approvals of generic 10-mg, scored, dispersible dolutegravir tablets through public–private partnerships among Unitaid, the Clinton Health Access Initiative, ViiV Healthcare, and generic-drug companies in India (Mylan–Viatris and Macleods Pharmaceuticals).19,20 Results in the 85 infants and children weighing 3 to 14 kg at enrollment (who were recruited later as a separate ODYSSEY cohort and were randomly assigned to receive dolutegravir-based ART or standard care)21 are not reported here.
Many currently used antiretroviral drugs in children have challenges associated with use. For example, nevirapine has been phased out owing to increasing primary NNRTI resistance,22,23 efavirenz is not recommended for children younger than 3 years of age owing to wide variation in drug exposure, raltegravir requires twice-daily treatment and has a low resistance threshold, and pediatric lopinavir–ritonavir is unpalatable, requires twice daily treatment, and is complex to administer with antituberculosis drugs.22 Dolutegravir formulations are easy to take; the 50-mg tablet is small, and the 5-mg tablets disperse rapidly and are palatable. Once-daily administration and easier dose adjustment with antituberculosis treatment24 mean that a transition to dolutegravir as first- and second-line therapy simplifies treatment in children and adolescents. The need for only two types of dolutegravir formulation across all weight bands in children and the availability of both formulations from generic-drug companies will allow national programs to align treatment for children with that for adults, simplifying drug procurement.25,26
This randomized trial showed the superior efficacy of dolutegravir-based ART over standard care in children and adolescents weighing at least 14 kg.
Supplementary Material
Acknowledgments
Supported by ViiV Healthcare. The Medical Research Council Clinical Trials Unit at University College London received core support from the U.K. Medical Research Council (grant number, MC_UU_00004/03). INSERM-ANRS supported the trial in France. The Penta Foundation provided support to sites in Africa, Europe, and Thailand.
We thank all the participants and their caregivers; the medical, nursing, pharmacy, laboratory, and data-management staff; the Youth Trial Board members and all those who supported community engagement at the ODYSSEY trial sites; the members of the trial steering committee, end-point review committee, and independent data monitoring committee for contributions, including oversight of the safety of the trial; and Dr. James Hakim, the principal investigator at the University of Zimbabwe Clinical Research Centre site, who died from coronavirus disease 2019 in January 2021. We express our deep appreciation for his immense contribution to the trial.
Footnotes
Contributor Information
H.A. Mujuru, University of Zimbabwe, Harare, Kampala, Uganda
A.R. Kekitiinwa, Baylor College of Medicine, Fort Portal, Kampala, Uganda
C.M. Kityo, Joint Clinical Research Center, Kampala, Uganda
A. Violari, Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg, Durban, South Africa
A. Lugemwa, Joint Clinical Research Center, Mbarara, Kampala, Uganda
T.R. Cressey, Program for HIV Prevention and Treatment–Institut de Recherche pour le Développement Research Unit, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
P. Musoke, Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda
E. Variava, Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg, Durban, South Africa.
M.F. Cotton, Family Centre for Research with Ubuntu, the Department of Paediatrics and Child Health, Stellenbosch University, Tygerberg, Durban, South Africa
M. Archary, Department of Paediatrics and Children Health, King Edward VIII Hospital, University of KwaZulu–Natal, Durban, South Africa
T. Puthanakit, Faculty of Medicine, Chulalongkorn University; HIV-NAT (HIV Netherlands Australia Thailand Research Collaboration), Thai Red Cross AIDS Research Center, Bangkok, Thailand
O. Behuhuma, Africa Health Research Institute, Durban, South Africa
R. Kobbe, First Department of Medicine, Division of Infectious Diseases, University Medical Center Hamburg–Eppendorf, Hamburg, Germany
S.B. Welch, London, and Heartlands Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
M. Bwakura-Dangarembizi, University of Zimbabwe, Harare, Kampala, Uganda
P. Amuge, Baylor College of Medicine, Fort Portal, Kampala, Uganda
E. Kaudha, Joint Clinical Research Center, Kampala, Uganda
L. Barlow-Mosha, Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda
S. Makumbi, Joint Clinical Research Center, Mbarara, Kampala, Uganda
N. Ramsagar, Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg, Durban, South Africa
C. Ngampiyaskul, Prapokklao Hospital, Chanthaburi, Thailand
G. Musoro, University of Zimbabwe, Harare, Kampala, Uganda
L. Atwine, Joint Clinical Research Center, Mbarara, Kampala, Uganda
A. Liberty, Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg, Durban, South Africa
V. Musiime, Joint Clinical Research Center, Kampala, Uganda; Makerere University, Kampala, Uganda
D. Bbuye, Baylor College of Medicine, Fort Portal, Kampala, Uganda
G.M. Ahimbisibwe, Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda
S. Chalermpantmetagul, Program for HIV Prevention and Treatment–Institut de Recherche pour le Développement Research Unit, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
A. Colbers, Department of Pharmacy, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, the Netherlands
N. Klein, University College London Great Ormond Street Institute of Child Health, United Kingdom; Africa Health Research Institute, Durban, South Africa
S. Bernays, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, United Kingdom; School of Public Health, University of Sydney, Sydney
T. Grossele, Penta Foundation, Padua, Italy
A. Compagnucci, INSERM-ANRS SC10-US019, Essais Thérapeutiques et Maladies Infectieuses, Villejuif, France
C. Giaquinto, Penta Foundation, Padua, Italy; Department of Women and Child Health, University of Padua, Padua, Italy
Dr. P. Rojo, Pediatric Infectious Diseases Unit, Hospital Universitario 12 de Octubre, Madrid
Dr. D. Ford, Medical Research Council Clinical Trials Unit at University College London, Institute of Clinical Trials and Methodology, United Kingdom
Dr. D.M. Gibb, Medical Research Council Clinical Trials Unit at University College London, Institute of Clinical Trials and Methodology, United Kingdom
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Progress towards the Start Free, Stay Free, AIDS Free targets: 2020 report. 2020 https://www.unaids.org/sites/default/files/media_asset/start-free-stay-free-aids-free-2020-progress-report_en.pdf .
- 2.Kanters S, Vitoria M, Zoratti M, et al. Comparative efficacy, tolerability and safety of dolutegravir and efavirenz 400mg among antiretroviral therapies for first-line HIV treatment: a systematic literature review and network meta-analysis. EClin-icalMedicine. 2020;28:100573. doi: 10.1016/j.eclinm.2020.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis. 2021;21:222. doi: 10.1186/s12879-021-05850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CL, Turkova A, Mujuru H, et al. ODYSSEY clinical trial design: a randomised global study to evaluate the efficacy and safety of dolutegravir-based antiretroviral therapy in HIV-positive children, with nested pharmacokinetic substudies to evaluate pragmatic WHO-weight-band based dolutegravir dosing. BMC Infect Dis. 2021;21:5. doi: 10.1186/s12879-020-05672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. 2016. Jun, https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdfjsessionid=23A66E85BCB9BC4AFADF6F52F1909422?sequence=1 . [PubMed] [Google Scholar]
- 6.Bollen PDJ, Moore CL, Mujuru HA, et al. Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial. Lancet HIV. 2020;7(8):e533–e544. doi: 10.1016/S2352-3018(20)30189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waalewijn H, Bollen PDJ, Moore C, et al. Pharmacokinetics of dolutegravir 5mg dispersible tablets in children weighing 6 to <20kg dosed using WHO weight bands; Presented at the 10th IAS Conference on HIV Science; Mexico City. July 21-24, 2019; abstract WEAB0401LB http://programme.ias2019.org/Abstract/Abstract/4782. [Google Scholar]
- 8.Committee for Medicinal Products for Human Use (CHMP) Tivicay: assessment report. Amsterdam: European Medicines Agency; 2020. Nov 12, https://www.ema.europa.eu/en/documents/variation-report/tivicay-h-c-2753-x-0058-g-epar-assessment-report-variation_en.pdf . [Google Scholar]
- 9.TIVICAY. GlaxoSmithKline; Research Triangle Park, NC: 2020. Jun, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213983s000lbl.pdf . [Google Scholar]
- 10.Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2019;27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484. doi: 10.1002/jia2.25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calmy A, Tovar Sanchez T, Kouanfack C, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020;7(10):e677–e687. doi: 10.1016/S2352-3018(20)30238-1. [DOI] [PubMed] [Google Scholar]
- 13.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of anti-retroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–89. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtri-citabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferi-ority trial. Lancet HIV. 2020;7(10):e666–e676. doi: 10.1016/S2352-3018(20)30241-1. [DOI] [PubMed] [Google Scholar]
- 15.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–15. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 16.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopi-navir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an openlabel, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19:253–64. doi: 10.1016/S1473-3099(19)30036-2. [DOI] [PubMed] [Google Scholar]
- 17.Paton N, Musaazi J, Kityo CM, et al. Nucleosides and darunavir/dolutegravir in Africa (NADIA) trial: 48 wks primary outcome; Presented at the virtual Conference on Retroviruses and Opportunistic Infections (CROI 2021), March 6-10, 2021; abstract 94 https://www.croiconference.org/wp-content/uploads/sites/2/resources/2021/vCROI-2021-Abstract-eBook.pdf. [Google Scholar]
- 18.World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. World Health Organization; Geneva: 2018. Dec, https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51 . [Google Scholar]
- 19.TIVICAY. Mylan Laboratories Limited; Canonsburg, PA: 2020. Oct, https://www.accessdata.fda.gov/drugsatfda_docs/pepfar/214521PI.pdf . [Google Scholar]
- 20.Dolutegravir. Macleods Pharmaceuticals Limited; Daman (U.T.), India: 2021. Mar, https://www.accessdata.fda.gov/drugsatfda_docs/pepfar/214566PI.pdf . [Google Scholar]
- 21.Turner B, Ford D, Moore C, et al. Analysing small groups within clinical trials, while borrowing information from larger groups; Presented at the 40th Annual Conference of the International Society for Clinical Biostatistics, Leuven; Belgium. July 16, 2019. [Google Scholar]
- 22.Penazzato M, Townsend CL, Rakhmanina N, et al. Prioritising the most needed paediatric antiretroviral formulations: the PADO4 list. Lancet HIV. 2019;6(9):e623–e631. doi: 10.1016/S2352-3018(19)30193-6. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. The 2021 optimal formulary and limited-use list for antiretroviral drugs for children: policy brief. 2021. Apr, https://www.who.int/publications/i/item/9789240023529 .
- 24.Waalewijn H, Mujuru HA, Amuge P, et al. Adequate dolutegravir exposure dosed BID with rifampicin in children 6 to <18 years; Presented at the Conference on Retroviruses and Opportunistic Infections (CROI); Boston. March 8-11, 2020; abstract 847 https://2jg4quetidw2blbbq2ixwziw-wpengine.netdna-ssl.com/wp-content/uploads/sites/2/posters/2020/1430_9_Waalewijn_00847.pdf. [Google Scholar]
- 25.Burki T. Cheaper HIV treatment for children. Lancet. 2020;396:1873. doi: 10.1016/S0140-6736(20)32665-9. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Joint statement calling for urgent country scale-up of access to optimal HI V treatment for infants and children living with HIV, December 22, 2020. 2020. https://www.who.int/news/item/22-12-2020-joint-statement-calling-for-urgent-country-scale-up-of-access-to-optimal-hiv-treatment-for-infants-and-children-living-with-hiv .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.