Abstract
Background:
Patients with dementia usually have multiple comorbidities. The presence of comorbidities may exacerbate the progression of dementia and decreases the patient’s ability to participate in health maintenance activities. However, there is hardly any meta-analysis estimating the magnitude of comorbidities among patients with dementia in the Indian context.
Methods:
We searched PubMed, Scopus, and Google Scholar, and relevant studies conducted in India were included. The risk of bias was assessed and a random-effects meta-analysis model was used in which I 2 statistics were calculated to measure heterogeneity among studies.
Results:
Fourteen studies were included in the meta-analysis based on the inclusion and exclusion criteria. Altogether, we found the coexistence of comorbid conditions such as hypertension (51.10%), diabetes (27.58%), stroke (15.99%), and factors like tobacco use (26.81 %) and alcohol use (9.19%) among patients with dementia in this setting. The level of heterogeneity was high due to differences in the methodologies in the included studies
Conclusions:
Our study found hypertension as the most common comorbid condition among patients with dementia in India. The observed lacuna of methodological limitations in the studies included in the current meta-analysis provides the urgent need for good quality research to successfully meet the challenges ahead while devising appropriate strategies to treat the comorbidities among patients with dementia.
Keywords: Dementia, comorbidities, risk factors, India
Dementia, a neurodegenerative disorder associated with population aging, was estimated to have increased by 117% (95% uncertainty interval [UI] 114–121) between 1990 and 2016 globally. 1 There is emerging evidence of decreasing prevalence of dementia in high-income countries such as North America and Europe, due to the increase in educational attainment and improvements in the management of cardiovascular disease and its risk factors.2–3 On contrary, an increase of 197% change (95% UI: 160–238) and an age-standardized rate of 6.4% (95% UI: –3.6–20.4) for dementia is forecasted in India by 2050. This forecasted transition is attributed to changes in population growth, population aging, changes due to education, and risk factors. 4 Globally, 12 modifiable risk factors such as low education, hypertension, hearing impairment, smoking, midlife obesity, depression, physical inactivity, diabetes, social isolation, excessive alcohol consumption, head injury, and air pollution were associated with dementia.5–7 In India, the risk factors for dementia were observed as vascular and metabolic (diabetes, hypertension, dyslipidemia, obesity, stroke, high BMI), genetic (Apolipoprotein 8 Presenilin-1), 9 and other risk factors (gender differentials, 10 nutritional deficiencies, 11 low literacy, change in social system urban living). 12
Worldwide literature on the comorbidities in dementia suggest prevalence rates of 6%–39% for diabetes, 13 3%–34% for stroke, 13 15.9% for major depressive disorder, 14 20% for depression, 15 14% for anxiety, 15 and 4.4% for post-traumatic stress disorder. 15 The corresponding rates are likely to be different in India due to the differences in socioeconomic status, lifestyle, and literacy, as well as access to health care and the internet. Further, the increasing prevalence of multi-morbidities and the underdiagnosed and undertreated medical illnesses, particularly in rural Indian settings, complicates studying the comorbidities of dementia. 16 Understanding the burden of comorbidities among dementia patients is critical to addressing future changes in modifiable risk factors that might influence the trajectory of trends in age-specific prevalence. The changes in the brain leading to dementia start at least two decades before the presentation of overt clinical symptoms. 17 In this context, it is important to quantify the burden of comorbidities and/ or multi-morbidities among dementia patients in India, to advance the strategies for prevention.
Methods
The meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered in the PROSPERO database (CRD42022321019)
Search Strategy and Selection Criteria
We searched the following electronic bibliographic databases: PubMed, Scopus, and Google Scholar. To prevent omissions, a snowball search was conducted for references to include articles and relevant reviews to supplement the relevant literature. We used the combination of Medical Subject Headings and keywords of the following search concepts: “dementia”; “comorbidity”; “India.” The details of the search strategy in PubMed are given as Supplementary Material 1. The data search was carried out by two investigators (HH and VLN). The archives of relevant Indian journals were reviewed for maximum inclusion of the available studies. The screening of the potentially eligible studies was performed by two investigators (BV and MD) who further appraised the full texts of appropriated records to reach a common consensus regarding the inclusion and exclusion of individual studies.
Inclusion and Exclusion Criteria
Observational studies (hospital and community-based studies) conducted in the Indian setting reporting the comorbidities in patients with dementia and published in the English language were included. Studies were included if participants had dementia based on standard diagnostic or screening criteria. Global or Indian prevalence or incidence studies that did not estimate the comorbidity among patients with dementia were excluded. Besides, studies with inadequate data and studies that exclusively evaluated cognitive deficits without diagnosis/screening for dementia were excluded.
Data Extraction
The data extraction was done based on the following study characteristics: namely author (year of publication), period of study, study type, criteria for dementia, sample size, gender, age, education, comorbid conditions such as hypertension, diabetes, dyslipidemia, tobacco use, alcohol use, family history of dementia, history of stroke, etc. Two investigators (BV and SG) were involved in the preparation of the data extraction table based on the eligibility criteria. The extracted data were cross-verified by the author EM. A mutual consensus resolved disagreements between the authors (BV, SG, and EM).
Quality Assessment
The JBI Critical Appraisal Checklist was used for the risk of bias assessment (Available from https://synthesismanual.jbi.global). Each study was graded as 1—yes, 0—no, and UC—unclear after rigorous review. The total scores of the included studies were not considered for the study selection criterion in the meta-analysis. Two review authors independently assessed the risk of bias in the included studies (JJ & SS). This process was performed iteratively. First, each author reviewed the studies and made the risk of bias assessment based on the criteria. A third independent reviewer (SA) addressed discrepancies in the quality scoring of two reviewers. Disagreements were resolved by group consensus.
Statistical Analysis
The R software was used to perform this meta-analysis, and the pooled estimate of outcome measures was estimated using inverse-variance weighting methods (Available from: https://www.R-project.org/). Assuming the significant inconsistency among the studies, a random-effects meta-analysis model was used, and I 2 statistics were calculated to measure heterogeneity among studies. The heterogeneity was considered mild, moderate, or high when the I 2 values were 25%–50%, 51%–75%, and >75%, respectively. The funnel plot and Egger’s test were used to assess the potential publication bias when the number of studies is more than 10 or wherever possible.
Results
Identification of Studies
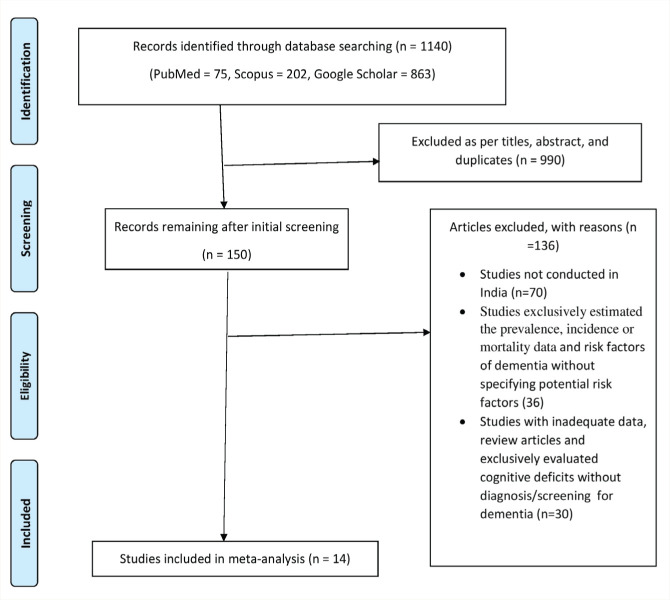
The database search identified 1140 reports: 990 were excluded based on title and abstract screening and after eliminating duplicates, 150 articles were retrieved for detailed evaluation, and 136 of these were excluded for the reasons summarized in Figure 1. Finally, 14 eligible articles were included in the meta-analysis.
Figure 1. Flow chart of search stretegy and selection process.
Study Characteristics
The sample size ranged from 27 to 1777 in which the age of the study subjects was mainly in the early sixties (Table 1).18–31 The studies recruited study participants from the hospital-based setting19, 21–26, 29 and community-based population.18, 20, 27, 28, 30, 31 Half of the studies were conducted in the southern part of India19, 20, 23, 26, 29–31 and the period of conducting the studies ranged from 2003 to 2017. The assessment of dementia was most commonly done by using a Mini-Mental status examination.19–21, 23–31 Other scales used to measure dementia include the Clinical Dementia Rating Scale, 22 Early Dementia Questionnaire, 18 Community Screening Instrument—Dementia, 29 Geriatric Mental State. 30 Around 55% of the dementia patients with comorbidities were males (55.4%; 95% CI: 46.64–63.84) while the proportion of females was 44.6% (95% CI: 36.16–53.36). The total score of risk of bias assessment according to the JBI Critical Appraisal Checklist for analytical cross-sectional studies was 8 and the score ranged from 1 to 5. The mean risk of bias score was 3 and the median score was 1 indicating a high risk of bias in the included studies. A major proportion of the studies did not provide detailed descriptions of the study settings. Out of the 14 studies, the information on the procedures for the measurement of the various comorbidities of dementia was reported only in six studies. None of the studies listed the possible specific confounding factors and did not state the strategies to deal with the same. Appropriate statistical analyses were not performed in the majority of the studies. The details of the quality assessment of the studies using the JBI checklist are described in Table 2.
Table 1.
Characteristics of the Studies Included in the Meta-analysis (N = 14).
| Author (Year of Publication)/ Study Region |
Study Population | Study Period (Year) |
Criteria for Dementia | Sample Size# |
Male/ Female |
Mean Age± SD/Range (Years) |
Illiterate/ Educated |
HTN |
DM |
Tobacco Use | Alcohol Use |
Others |
| Saikia AM et al (2018)/ Assam[14] |
CB | 2013 | EDQ | 45 | 12/33 | >65 Year | 14/31 | NM | NM | 22 | 08 | Nonvegetarian—34 Social isolation—15 |
| Lalu JS et al (2018)/ Kerala[15] | HB | 2016-2017 | MMSE | 50 | 20/30 | >65 Year | 00/50 | 16 | 16 | 10 | 07 | Sedentary life style—09 Depression—18 Dyslipidaemia—11 Nonvegetarian—36 F H/o D—11 |
| Mummadi MK (2018) Telengana[16] |
CB | 2013 | MMSE | 112 | 70/42 | >65 Year | NM | 40 | 30 | 32 | 16 | Stroke—8 |
| Kushwaha S et al (2017)/ New Delhi[17] |
HB | 2007-2014 | MMSE | 1777 | 1115/662 | NM | 1349/428 | 984 | 338 | 84 | 27 | Dyslipidaemia—797 Hypothyroidism—5 Vit B12 Deficiency—55 |
| Chandra M et al(2015)/ New Delhi[18] | HB | 2013-2014 | CDR | 159 | 87/72 | 69.35±7.51 | NM | 117 | 65 | NM | NM | Dyslipidemia—126 CAD—93 |
| Chandra SR et al(2014)/ Karnataka[19] | HB | 2004-2010 | MMSE (mHIS) |
83 | 60/23 | 65.4 ± 9.2 |
NM | 59 | 24 | 39 | 22 | Dyslipidemia—40 Stroke—16 |
| Tripathi M et al (2012)/ New Delhi[20] |
HB | 2003-2005 | MMSE |
150 | 118/32 | 65.73±9.70 |
NM | 83 | 50 | 50 | 29 | F H/o D—22 h/o of head injury—22 CAD—8 Stroke—38 Depression—60 Dyslipidaemia—150 |
| Ghosh T et al (2012)/New Delhi[21] | HB | NM | MMSE CDR |
50 | 28/22 | 66.7±3.2 | NM | NM | NM | 11 | NM | NM |
| Kota LN et al(2012)/ Karnataka[22] |
HB | NM | HMSE | 331 | 180/151 | 61.14 ± 11.40 | NM | NM | 104 | NM | NM | |
| Poddar K et al(2011/ Uttar Pradesh[23] |
CB | NM | MMSE HMSE |
146 | 69/77 | >50 Year | 88/58 | 52 | 23 | 61 | 2 | NM |
| Saldanha D et al(2010)/ Maharashtra[24] |
CB | 2005-2007 | MMSE CSI-D CERAD |
78 | NM | >65 Year | 55/23 | NM | NM | NM | NM | Social isolation—34 |
| Alladi S et al.(2006)/ Telengana[25] |
HB | 2006-2010 | MMSE CDR |
347 | 229/118 | 66.3 |
298/49 | NM | NM | NM | NM | F H/o D—46 |
| Rajkumar S et al (1997)/ Tamil Nadu[26] | CB | NM | MMSE GMS |
27 | 11/16 | >60 Year | 24/3 | NM | NM | NM | NM | NM |
| Shaji et al.(1996)/ Kerala[27] |
CB | NM | MMSE | 65 | 27/38 | >65 Year | NM | 30 | NM | 16 | NM | F H/o D—8 |
NM: not mentioned, CBS: community based, HBS: hospital based, EDQ: early dementia questionnaire, MMSE: mini-mental status examination, HTN: hypertension, DM: diabetes mellitus, AD: Alzheimer’s dementia, VD: vascular dementia, CDR: clinical dementia rating scale, MINI: mini international neuropsychiatric interview, mHIS: modified Hachinski ischaemic scale, CSI-D: community screening instrument—dementia, GMS: geriatric mental state, F H/o D: family history of dementia. #The subjects with dementia based on a criterion.
Table 2.
Risk of Bias Assessment of Included Studies.
| Author (Year of Publication) | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total |
| Saikia et al. (2018) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Lalu et al. (2018) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 5 |
| Mummadi (2018) | 1 | 0 | UC | UC | 0 | 0 | UC | 0 | 1 |
| Kushwaha et al. (2017) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 4 |
| Chandra et al. (2015) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Chandra et al. (2014) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 4 |
| Tripathi et al. (2012) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Ghosh et al. (2012) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Kota et al. (2012) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Poddar et al. (2011) | 1 | 0 | UC | UC | 0 | 0 | UC | 0 | 1 |
| Saldanha et al. (2010) | 1 | 0 | UC | 1 | 0 | 0 | UC | 0 | 2 |
| Alladi et al. (2006) | 1 | 1 | UC | 1 | 0 | 0 | UC | 0 | 3 |
| Rajkumar et al. (1997) | 1 | 1 | UC | 1 | 0 | 0 | UC | 0 | 3 |
| Shaji et al. (1996) | 1 | 1 | UC | 1 | 0 | 0 | UC | 0 | 3 |
| Mean: 3.1, Median: 3 |
Q1: Were the criteria for inclusion in the sample clearly defined? Q2: Were the study subjects and the setting described in detail? Q3: Was the exposure measured in a valid and reliable way? Q4: Were objective, standard criteria used for measurement of the condition? Q5: Were confounding factors identified? Q6: Were strategies to deal with confounding factors stated? Q7: Were the outcomes measured in a valid and reliable way? Q8: Was appropriate statistical analysis used? (1—Yes, 0—No, NA—Not applicable, UC—Unclear).
Comorbidities
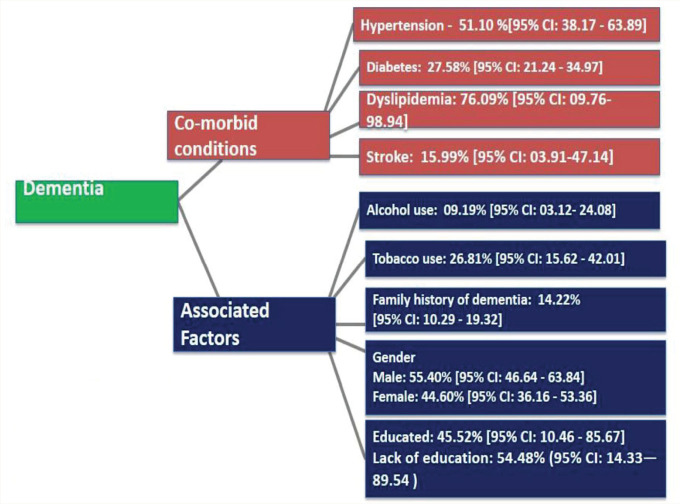
Dementia being a chronic illness, we identified the comorbidities and the associated factors based on the cross-sectional studies conducted in the Indian setting (Figure 2). Of the 2542 patients included in the analysis from 8 studies, the pooled proportion of dementia patients with hypertension as comorbid condition was 51.1% (95% CI: 38.17–63.89).19–24, 27, 31 Overall, 2808 patients were included in the analysis from 8 studies for detecting the pooled proportion of dementia patients with diabetes mellitus which was found to be 27.58% (95% CI: 21.24–34.97).19–24, 26–27 The other comorbidities associated with dementia patients were dyslipidemia (76.09%; 95% CI: 10–99; n = 5 studies)19, 21–24 and stroke (15.99%; 95% CI: 03.91–47.14; n = 3 studies).20, 23–24 Two studies reported depression (36% and 40%)19, 24 and coronary artery disease (5.3% and 58.8%)22, 24 as comorbid conditions associated with dementia in this setting. One study reported the presence of hypothyroidism (0.2%) and vitamin deficiency (3.09%) in treatment-seeking patients with dementia. 21
Figure 2. The distribution co-morbid conditions and associated factors among patients with dementia in India.
Associated Factors
The history of tobacco use, both smoking and smokeless forms was 26.81% (95% CI: –15.62–42.01; n = 9 studies)18–21, 23–25, 27, 31 and alcohol use was 9.19% (95% CI: –03.12–24.08; n = 7 studies).18–21, 23–24, 27 Approximately 55% of the patients were illiterate (54.48%; 95% CI: 14.33–89.54) and the remaining subjects had undergone formal education (45.52%; 95% CI: 10.46–85.67).18–19, 21, 27–30 The family history of dementia was reported in 14% of the subjects (14.22%; 95% CI: 10.2–19.32; n = 4 studies).19, 24, 29, 31 The presence of social isolation (33.3% and 43.6%)18, 28 and nonvegetarian diet (75% and 72%) were reported among patients with dementia in two studies.18, 19 One study reported sedentary lifestyle patterns (18%) in treatment-seeking patients with dementia. 19
We used the random-effects models to calculate the pooled estimates as there was a significant heterogeneity on the outcome measures (hypertension—I 2 = 91%, Tau Squared = 0.35, P < 0.01; diabetes mellitus—I 2 = 90%, Tau Squared = 0.13 P < 0.01; dyslipidemia—I 2 = 94%, Tau Squared = 6.81, P < 0.01; tobacco—I 2 = 98%, Tau Squared = 0.72, P < 0.01; alcohol—I 2 = 96%, Tau Squared = 1.39, P < 0.01; stroke—I 2 = 85%, Tau Squared = 0.3, P < 0.01; previous history of stroke—I 2 = 96%, Tau Squared = 0.61, Q = 447.43, P < 0.01; family history of dementia—I 2 = 92.6%, Tau Squared = 0.70, Q = 162.83, P < 0.001). The pooled analysis of the proportion of individual predisposing factors and heterogeneity are depicted in supplementary materials (S2.1–2.7). The funnel plot and Egger’s test regarding the publication bias of individual predisposing factors were not evaluated as the number of studies was less than 10.
Discussion
Early recognition of comorbidities and associated factors of dementia has been identified as a potential strategy for decreasing the severity of the disease process. Patients with dementia usually have multi-morbidities. However, there is hardly any meta-analysis estimating the magnitude of comorbidities and/or multi-morbidity among patients with dementia in the Indian context. Around the world, hypertension is a common comorbidity in adults with major chronic illnesses, with a prevalence rate of more than 50% in the Asian population. 32 Our study found that 51% of patients with dementia had hypertension as a comorbid condition. These findings are in line with a previous study conducted in Europe that identified hypertension as a major comorbid condition among patients with dementia. 33 Diabetes is also considered an established risk factor for dementia, 34 and our analyses suggested that the proportion of diabetes mellitus among patients with dementia is 27.58%. This proportion is, however, considerably lower than the results obtained in a study from Germany. 35
According to the current meta-analysis, the proportion of dyslipidemia in dementia patients was 76% (95% CI: 10%–99%); however, the wide confidence interval of the pooled proportion questions the validity of these findings within this setting. Our aggregates of evidence revealed that the proportion of stroke among patients with dementia was 15%. The evidence across the globe strongly suggests stroke is an independent and potentially modifiable risk factor for dementia. 36 As dementia is a disorder developing slowly over many years, it is often identified in the late course of the disease. Besides, it could be possible that patients with an earlier stroke were not examined for dementia, which might have affected the pooled analysis of the proportion of stroke in patients with dementia in this setting. Our study showed that around 9% and 26% of the patients with dementia reported a history of alcohol use and tobacco use. Similar findings were observed in other studies which identified a significant association between smoking and alcohol use in patients with dementia.37–38 The existing evidence indicates various genetic factors for dementia 39 and the current study identified a family history of dementia in 14% of the subjects. Our study identified that other conditions such as depression, coronary artery diseases, and social isolation were reported in dementia patients but the number of studies was relatively low for estimating a pooled proportion.
It is evident that the comorbid medical conditions may exacerbate the progression of dementia and increases the vulnerability of the patient’s ability to health maintenance activities. 40 Altogether, we found the coexistence of comorbid conditions such as hypertension, diabetes mellitus, dyslipidemia, tobacco use, and alcohol use among patients with dementia in this setting. However, the risk of bias assessment of the included studies has implications for the generalization of our findings. First, there was a high risk of bias in the studies included in the current meta-analysis. Reporting was incomplete in most studies. To cite, the existing evidence suggests that the associated factors for dementia may vary according to different age groups. 41 However, the proportion of comorbidities according to gender and age groups was not reported in the majority of the included studies. Furthermore, with increasing age, the propensity of occurrence of multi-morbidities is higher, which could worsen dementia. We could not estimate the prevalence and pattern of multi-morbidities due to the inherent data limitations. Second, as the case-control studies were very limited we could not substantiate the risk factors with dementia, but the associated factors. Further, overlapping comorbidities were not reported in the majority of the studies. Third, the meta-analysis showed significant heterogeneity which is commonly observed in epidemiological studies and could be attributed to several factors such as differences in defining, measuring, and analyzing outcomes; criteria for patient selection; study objectives; and statistical analysis. Though the studies we analyzed were cross-sectional and do not prove causation, based on evidence from other parts of the world, the factors we identified are known to increase the risk of dementia. Hence, older adults, their family members, clinicians, other health workers, and policymakers should be sensitized about these factors and the importance of their early detection and management to reduce the burden of dementia in a country where the proportion of older adults is gradually increasing.
There are certain limitations to generalizing our findings. The results are purely based on cross-sectional studies with methodological limitations. The level of heterogeneity of the included studies was high due to differences in the study contexts. No attempts were made to acquire grey/unpublished literature considering the inherent conflict of interest which might increase the risk of bias. The age and gender-specific proportion of comorbidities of dementia were not reported in the majority of the included studies. A major strength of this study was that we were able to generate the pooled estimates of the comorbidities and associated factors among patients with dementia based on the epidemiological studies conducted in the Indian setting.
Conclusion
Our study found hypertension to be the most common comorbid condition among patients with dementia in this setting. The observed lacuna of methodological limitations in the included studies in the current meta-analysis suggests that there is an urgent need for good quality research to successfully meet the challenges ahead while devising appropriate strategies to curtail the comorbidities among patients with dementia. Future research exploring the role of multi-morbidity in the onset and progression of dementia is warranted.
Supplemental Material
Supplemental material for this article is available online.
Acknowledgments
Elezebeth Mathews would like to thank DBT, India for the Clinical and Public Health Early Career Fellowship (grant number IA/CPHE/17/1/503345).
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18: 88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolters FJ, Chibnik LB, Waziry R, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020; 95: e519–e531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews FE, Stephan BCM, Robinson L, et al. A two-decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016; 7: 711398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022Feb1; 7(2): e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020; 396: 413–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017; 177: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoog I, Börjesson-Hanson A, Kern S, et al. Decreasing prevalence of dementia in 85-year olds examined 22 years apart: the influence of education and stroke. Sci Rep. 2017Jul21; 7(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Basak JM, and Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron, 2009Aug13; 63(3): 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey P, Pradhan S, and Mittal B.. Presenilin gene predisposes to late-onset degenerative but not vascular dementia: a comparative study of PS1 and ApoE genes in a North Indian Cohort. Dement Geriatr Cogn Disord, 2007; 24(3): 151–161. [DOI] [PubMed] [Google Scholar]
- 10.Kenchappa RS, Diwakar L, Annepu J, et al. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J, 2004Jul; 18(10): 1102–1104. [DOI] [PubMed] [Google Scholar]
- 11.Mahalle N, Kulkarni MV, Garg MK, et al. Vitamin B12 deficiency and hyperhomocysteinemia as correlates of cardiovascular risk factors in Indian subjects with coronary artery disease. J Cardiol, 2013Apr1; 61(4): 289–294. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon P, Ladusingh L, and Agrawal G.. Ageing and changing patterns in familial structure for older persons in India: a decomposition analysis. Qual Ageing Older Adults, 2016Jun13; 17: 83–96. [Google Scholar]
- 13.Bunn F, Burn AM, Goodman C, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med, 2014Oct31; 12: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asmer MS, Kirkham J, Newton H, et al. Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J Clin Psychiatry, 2018; 79(5): 17r11772. [DOI] [PubMed] [Google Scholar]
- 15.Kuring JK, Mathias JL, and Ward L.. Prevalence of depression, anxiety and PTSD in people with dementia: a systematic review and meta-analysis. Neuropsychol Rev, 2018Dec; 28(4): 393–416. [DOI] [PubMed] [Google Scholar]
- 16.Ravindranath V and Sundarakumar JS. Changing demography and the challenge of dementia in India. Nat Rev Neurol, 2021Dec; 17(12): 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan NS, Fox NC. Imaging presymptomatic Alzheimer’s disease. Adv Clin Neurosci Rehabil, 2014; 14: 6–9. [Google Scholar]
- 18.Saikia AM, Neelakshi M, Ajaya M, et al. Prevalence and correlates of dementia among the community-dwelling elderly of Guwahati City, Assam. Int J Health Res Medico Leg Prae, 2018July; 4(2): 31–34. [Google Scholar]
- 19.Lalu JS, Vijayakumar P, George S, et al. Risk factors of dementia: a comparative study among the geriatric age group in Ernakulam, Southern India. Int J Community Med Public Health, 2018; 5: 544–549. [Google Scholar]
- 20.Mummadi MK. A cross-sectional study on dementia in elderly persons living in old-age homes of Hyderabad, Telangana. Int J Med Sci Public Heal, 2018Sep1; 7(11): 709. [Google Scholar]
- 21.Kushwaha S, Talwar P, Anthony A, et al. Clinical spectrum, risk factors, and behavioral abnormalities among dementia subtypes in a North Indian population: a hospital-based study. Dement Geriatr Cogn Disord, 2017; 7(2): 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra M and Anand KS. Vascular disease burden in Indian subjects with vascular dementia. Aust J Med Sci, 2015; 8(7): 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra SR, Yadav R, Puneeth CS, et al. “The spectrum of vascular dementia”—a retrospective study from South India. J Assoc Physic Ind, 2014Jun1; 62(6): 498–503. [PubMed] [Google Scholar]
- 24.Tripathi M, Vibha D, Gupta P, et al. Risk factors of dementia in North India: a case–control study. Aging Ment Heal, 2012Mar1; 16(2): 228–235. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh T, Mustafa MD, Kumar V, et al. A preliminary study on the influence of glutathione S transferase T1 (GSTT1) as a risk factor for late onset Alzheimer’s disease in North Indian population. Asian J Psych, 2012Jun1; 5(2): 160–163. [DOI] [PubMed] [Google Scholar]
- 26.Kota LN, Shankarappa BM, Shivakumar P, et al. Dementia and diabetes mellitus: association with apolipoprotein e4 polymorphism from a hospital in southern India. Int J Alzheimers Dis, 2012Jun3; 2012: 702972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poddar K, Kant S, Singh A, et al. An epidemiological study of dementia among the habitants of eastern Uttar Pradesh, India. Ann Ind Acad Neurol. 2011Jul; 14(3): 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saldanha D, Mani MR, Srivastava K, Goyal S, and Bhattacharya D.. An epidemiological study of dementia under the aegis of mental health program, Maharashtra, Pune chapter. Indian J Psychiatry 2010Apr; 52(2): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alladi S, Mekala S, Chadalawada SK, et al. Subtypes of dementia: a study from a memory clinic in India. Dement Geriatr Cogn Disord, 2011; 32(1): 32–38. [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar S, Kumar S, and Thara R.. Prevalence of dementia in a rural setting: a report from India. Int J Ger Psych, 1997Jul; 12(7): 702–707. [DOI] [PubMed] [Google Scholar]
- 31.Shaji S, Promodu K, Abraham T, et al. An epidemiological study of dementia in a rural community in Kerala, India. British J Psych, 1996Jun; 168(6): 745–749. [DOI] [PubMed] [Google Scholar]
- 32.Colosia AD, Palencia R, and Khan S.. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes, 2013Sep17; 6: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelis SM, Wu YT, Matthews FE, et al. The impact of co-morbidity on the quality of life of people with dementia: findings from the IDEAL study. Age Ageing, 2019May1; 48(3): 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng G, Huang C, Deng H, and Wang H.. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int Med J, 2012May; 42(5): 484–491. [DOI] [PubMed] [Google Scholar]
- 35.Kaczynski A, Michalowsky B, Eichler T, et al. Comorbidity in dementia diseases and associated health care resources utilization and cost. J Alzheimers Dis, 2019Jan1; 68(2): 635–646. [DOI] [PubMed] [Google Scholar]
- 36.Kuźma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement, 2018Nov1; 14(11): 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anstey KJ, Mack HA, and Cherbuin N.. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Ger Psych, 2009Jul1; 17(7): 542–555. [DOI] [PubMed] [Google Scholar]
- 38.Anstey KJ, von Sanden C, Salim A, et al. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol, 2007Aug15; 166(4): 367–378. [DOI] [PubMed] [Google Scholar]
- 39.Loy CT, Schofield PR, Turner AM, et al. Genetics of dementia. Lancet, 2014Mar1; 383(9919): 828–840. [DOI] [PubMed] [Google Scholar]
- 40.Sinclair AJ, Girling AJ, and Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. Diabetes Res Clin Pract, 2000; 50: 203–212. [DOI] [PubMed] [Google Scholar]
- 41.Ong PA, Annisafitrie FR, Purnamasari N, et al. Dementia prevalence, comorbidities, and lifestyle among Jatinangor elders. Front Neurol, 2021; 12: 643480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for this article is available online.