Abstract
Purpose
Accurate interpretation of variants detected in dilated cardiomyopathy (DCM) is crucial for patient care but has proven challenging. We applied a set of proposed refined ACMG/AMP criteria for DCM, re-classified all detected variants in robust genes, and associated these results to patients´ phenotype.
Methods
The study included 902 DCM probands from the Maastricht Cardiomyopathy Registry, who underwent genetic testing. Two gene-panel sizes (extended n=48; and robust panel n=14) and two standards of variant classification (standard versus the proposed refined ACMG/AMP criteria) were applied to compare genetic yield.
Results
A pathogenic or likely pathogenic (P/LP) variant was found in 17.8% of patients, and a variant of uncertain significance (VUS) was found in 32.8% of patients when using method 1 (extended panel (n=48)+standard ACMG/AMP), compared to respectively 16.9% and 12.9% when using method 2 (robust panel (n=14)+standard ACMG/AMP), and respectively 14% and 14.5% using method 3 (robust panel (n=14)+refined ACMG/AMP). Patients with P/LP variants had significantly lower event-free survival compared to genotype-negative DCM patients.
Conclusion
Stringent gene selection for DCM genetic testing reduced the number of VUSs whilst retaining ability to detect similar P/LP variants. The number of genes on diagnostic panels should be limited to genes which have the highest signal to noise ratio.
Introduction
Dilated cardiomyopathy (DCM) has become a globally common cardiac disease with an approximate prevalence of up to 1:250 1. It is one of the leading causes of heart failure (HF), predominantly affects younger people compared to ischemic HF, and is the most frequent indication for cardiac transplantation 2. DCM has a large and complex genetic component characterized by variable disease penetrance and expression 3. Genetic testing has become an integral part of patient care in DCM, and current diagnostic gene panels constituting of ˜50 genes identify a pathogenic or likely pathogenic (P/LP) gene variant in 20% to 40% of DCM patients 3,4. However, most diagnostic panels also include many genes that lack robust evidence supporting a causal role in DCM leading to the identification of many variants with uncertain molecular and clinical relevance 5. As such, newly reported genes mainly increase the number of reported variants of unknown significance (VUS) as opposed to the intended increase in clinically relevant variants. This leads to a reduction in the clinical utility and cost-effectiveness of genetic testing, but also increases the risk for the patient in the form of misdiagnosis or false reassurance of their relatives, i.e. performing predictive testing for the wrong variant. One of the main reasons to perform genetic testing in DCM patients is cascade screening and family management, wherein accurate classification and interpretation of detected variants is of utmost importance. This is an increasingly bigger challenge with the large-scale molecular data that becomes available with more extensive genetic testing. The American College of Medical Genetics (ACMG) together with the Association for Molecular Pathology (AMP) have made a tremendous effort in creating guidelines and standards to interpret variants in a systematic and structural manner 6. As the ACMG/AMP standards are very broad, and need further disease specification, the domain working groups of the ClinGen consortium tailored the guidelines in this manner. The first specification for dilated and hypertrophic cardiomyopathies focused on the interpretation of MYH7 variants 7. The DCM Precision Medicine Study used these guidelines as a foundation to propose an adaptation of the ACMG/AMP criteria specifically for DCM 8. In parallel, an analysis including ˜2,500 DCM patients demonstrated a robust disease association for only 12 genes, implying that some variants in these 12 genes cause disease 9.
In this study, we have applied the newly proposed DCM framework of the ACMG/AMP criteria to our own DCM registry. We re-classified all variants limiting to the robust DCM-associated genes, in order to evaluate the broad sequencing panels which are currently used in clinical practice. Subsequently, we tested the influence of this re-classification on the clinical phenotype and prognosis of the genetic DCM subgroup.
Materials and Methods
Dilated cardiomyopathy patients
The study population consisted of 902 consecutive, unrelated, DCM probands from the Maastricht Cardiomyopathy Registry which prospectively included patients from the out-patient clinic between 2004 and 2020. Inclusion criteria for the inclusion of patients referred to our center with unexplained systolic dysfunction were: (i) DCM defined as LVEF<50% with an indexed left ventricular end-diastolic diameter (LVEDDi) >33 mm/m2 (men) or >32 mm/m2 (women) measured by echocardiography; or a hypokinetic non-dilated cardiomyopathy (HNDC) defined as LVEF <50% with an LVEDDi) <33 mm/m2 (men) or <32 mm/m2 (women) measured by echocardiography. This mixed population is further referred to as DCM in this paper; (ii) age ≥18 years; (iii) written informed consent. Exclusion criteria for the Maastricht Cardiomyopathy Registry included: (i) myocardial infarction and/or significant coronary artery disease; (ii) primary valvular disease; (iii) hypertensive or congenital heart disease; (iv) acute myocarditis; (v) arrhythmogenic right ventricular cardiomyopathy; (vi) hypertrophic, restrictive or peripartum cardiomyopathy, in accordance with the latest European Society of Cardiology (ESC) proposal 10.
All patients underwent a physical examination, blood sampling,12-lead ECG, 24-hour Holter monitoring, a complete echocardiographic and Doppler evaluation, and coronary angiography at baseline. As part of the protocol, patients were referred to the clinical genetics department of the Maastricht University Medical Center (MUMC, Maastricht, the Netherlands) for genetic counseling and DNA testing between 2012 and 2020. The study was performed according to the declaration of Helsinki and was approved by the institutional Medical Ethics Committee.
Genetic analysis
The 902 patients at the genetics outpatient clinic received genetic counseling and testing using our 47 cardiomyopathy-associated gene panel either with exome sequencing or single molecule Molecular Inversion Probes (smMIP) (Table S1). FLNC was added to the gene panel in June 201811. Consequently, in 385 patients (42.7%) a total of 48 genes was sequenced. All detected variants were confirmed by Sanger sequencing. The 48 cardiomyopathy-associated gene panel is further referred to as the ‘extended panel’.
Twelve genes were previously stated as robust DCM-associated genes based on a demonstrable excess of rare variation in these genes in DCM patients: TTN, DSP, MYH7, LMNA, BAG3, TNNT2, TNNC1, PLN, ACTC1, NEXN, TPM1 and VCL 9. The other genes from the extended panel did show only a small yield, providing a low signal to noise ratio, meaning that the yield of pathogenic variants (signal) is lower compared to VUSs (noise). This does not rule out these genes as disease-causing, but rather as low-yield genes which remain under investigation. We added two additional genes to the robust genes: FLNC and RBM20, based on available literature and personal experience, creating a total set of 14 robust DCM genes collectively referred to as the ‘robust panel’.
A family history of cardiac-related disease and sudden cardiac death was obtained by a 3-generation pedigree analysis at the initial visit of the patient. Familial inheritance was defined as recommended by the ESC 10: (i) two or more individuals (first or second-degree relatives) have DCM fulfilling diagnostic criteria for ‘definite’ disease OR (ii) in the presence of an index patient fulfilling diagnostic criteria for DCM and a first-degree relative with autopsy-proven DCM and sudden death at <50 years of age.
Gene selection and variant classification
We used two different gene sets and two different standards of variant classification in order to compare three methods (Figure S1):
-
-
Method 1: including variants present in the extended panel of 48 genes, which are classified according to the 2015 clinical guidelines of the ACMG/AMP6.
-
-
Method 2: only including variants in the robust panel of 14 genes, which are classified according to the 2015 clinical guidelines of the ACMG/AMP6.
-
-
Method 3: only including variants in the robust panel of 14 genes, which are classified according to the 2020 DCM adaptation of the ACMG/AMP guidelines as proposed by the DCM precision study and the ClinGen MYH7-cardiomyopathy variant interpretation framework7,8.
The main difference between the 2015 ACMG/AMP guidelines and the 2020 DCM proposed adaptation, is the fact that the adaptation contains gene-specific recommendations, e.g. a distinction in criteria strength for loss of function variants in specific genes (very strong in LMNA, strong for TTN, moderate for PLN). The full list of the proposed ACMG/AMP adaptation for DCM can be found in Table S2, highlighting the differences compared to the 2015 ACMG/AMP guidelines. Variants were classified as a variant of unknown significance (VUS), likely pathogenic or pathogenic variant according to the used clinical guideline. For the analyses, pathogenic and likely pathogenic variants were combined into one patient group (P/LP), as they both represent actionable variants warranting clinical consequences.
Follow-up
The median follow-up time was 4.2 years (interquartile range 2-7.8 years). Information about the occurrence of adverse events at follow-up was retrieved from the hospital medical records, the Dutch Personal Records Database and/or telephone contact with the patient or their general practitioners. We collected information regarding three different adverse events: 1) death due to cardiovascular disease, 2) heart transplantation or LVAD implantation, 3) heart failure that required a non-elective hospitalization despite optimal heart failure therapy according to the ESC/ACC/AHA guidelines) life-threatening arrhythmias (LTA) defined as non-fatal ventricular fibrillation (with or without ICD-shock), and/or sustained ventricular tachycardia with appropriate ICD shock. The combined end-point was defined as the occurrence of at least one of the above-mentioned adverse events.
Statistical analysis
Categorical data were compared using Pearson’s chi-square test or Fisher’s exact test. For continuous variables, unpaired Student’s t-tests or Mann-Whitney U-test were used. Kaplan-Meier survival curves were estimated and differences between groups were assessed by the log-rank test, using time at diagnosis as time zero. Multivariable binary logistic regression analysis was performed to find associations between clinical variables and P/LP variants. All univariable associated factors were added in a backward selection fashion with p<0.1 and p<0.05 as the cut-off for entry and retention, respectively. Calculations were done using SPSS version 23.0 (SPSS Inc., Chicago, Illinois).
Results
Clinical, demographic, and family history
902 unrelated DCM probands were included in this study. The mean age of disease diagnosis was 54 years (SD 12.71, range 18-90). 25 percent (225/902) of probands self-reported a family history of DCM; all 225 had at least one relative who had a diagnosis of DCM confirmed through retrieved medical files. Patients with a family history of DCM had a slightly earlier onset of disease compared to the probands without a reported family history (52±12 years versus 55±13 years, p=0.001). 62 percent (558/902) of the DCM probands were male. The median ejection fraction was 32 percent (interquartile range 24-41, range 8-49), with a mean indexed left ventricular end-diastolic diameter of 31 mm/m2 (SD 5.0, range 18-53). 24 percent of probands (214/902) had atrial fibrillation, 28 percent (249/902) had non-sustained ventricular tachycardias, and 29 percent (258/902) had a left bundle branch block at initial presentation.
Genetic analysis
We used a pan-cardiomyopathy panel consisting of 48 genes (Table S1), including the 14 robust DCM-associated genes TTN, DSP, MYH7, LMNA, BAG3, TNNT2, TNNC1, PLN, ACTC1, NEXN, TPM1, VCL, FLNC and RBM20. Our panel is representative for the size and constitution of the gene panels used in the last decade to diagnose DCM patients (Table S3). In comparison, the average number of genes on commercial pan-cardiomyopathy panels is 67 (Figure S2 and Table S4). Two genes (CALR3 and CTNNA3) were unique to our panel and three genes (FHL1, FLNC and MIB1) were included in less than half of the available commercial panels. 22 genes are on all commercial DCM gene panels, including 12 of the 14 robust DCM genes except for FLNC and NEXN.
The extended panel has the highest genetic yield, accompanied by a high rate of VUS
Method 1 (extended panel (n=48) + 2015 ACMG/AMP standards)
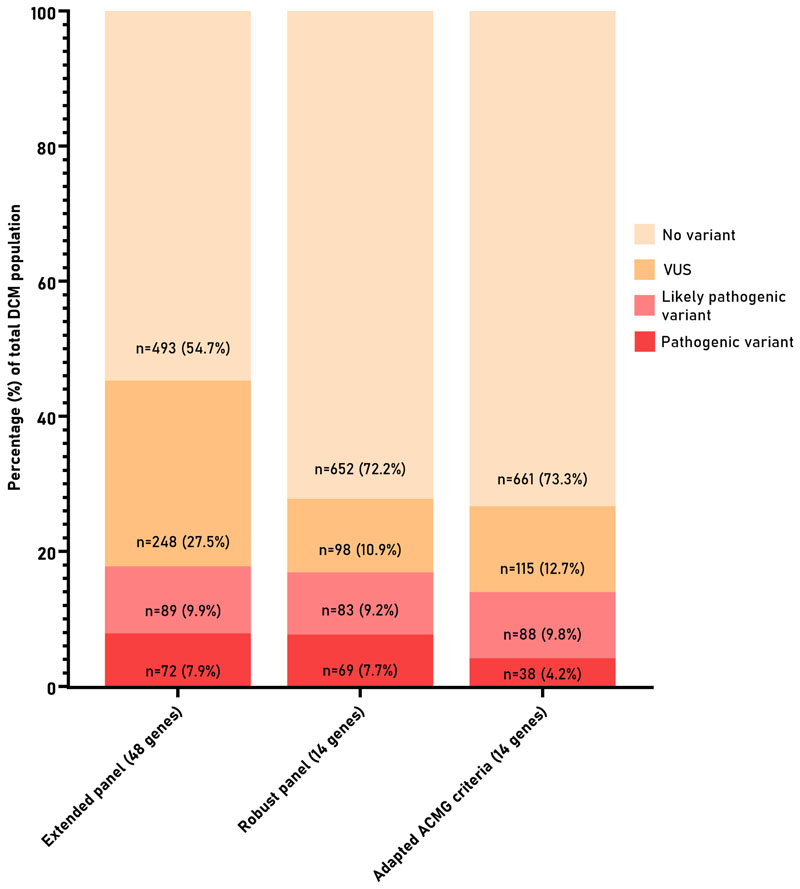
A total of 164 P/LP variants were detected in 18 of the 48 genes in 161 patients (17.8%; 7.9% pathogenic, 9.9% likely pathogenic) (Fig.1 and Figure S3). A VUS was reported in 41 genes, with a total of 364 VUSs in 296 patients (32.8%). 104 patients (11.5%) had more than 1 reported variant, of which 61 patients (6.8%) had multiple VUSs (Figure S4).
Fig. 1. Proportion of DCM patients with either no variant, a variant of unknown significance, a likely pathogenic, or pathogenic variant as result after genetic testing.
The use of a restricted panel including robust genes with a high signal to noise ratio identified nearly all actionable variants, but greatly reduces the number of VUSs.
Method 1 resembles the extended panel of 48 genes in which variants are classified according to the 2015 ACMG/AMP standards. Method 2 uses the robust panel of 14 DCM-associated genes and classified variants according to the 2015 ACMG/AMP standards. Method 3 uses the robust panel of 14 DCM-associated genes and classified them according to the 2020 DCM adaptation of the ACMG/AMP standards.
Method 2 (robust panel(n=14) + 2015 ACMG/AMP standards)
After reducing the analysis to the robust panel, there was only a minor non-significant decrease in the genetic yield of P/LP variants to 16.9% (7.7% pathogenic, 9.2% likely pathogenic; 155 variants in in 152 patients; Fig. 1; Table S5 and S6), missing P/LP variants in EMD, MYBPC3, MYL2, SCN5A and TTR (Table S5). Remarkably, the number of reported VUSs significantly decreased by 65% to 12.9% (129 variants in 116 patients; Figure S3 and Table S6). One hundred eighty families did not have a VUS as a test result anymore, a decrease of 20%. Only 29 patients (3.2%) received genetic results with more than 1 reported variant (Figure S4).
Method 3 (robust genes (n=14) + ACMG/AMP adaptation)
Nineteen P/LP variants in 27 patients were reclassified after applying the adapted ACMG/AMP criteria to variants in the robust DCM genes, resulting in 126 patients (14%) with a P/LP variant (4.2% pathogenic, 9.8% likely pathogenic; Table S6 and S7). Twelve variants were reclassified from a P/LP variant to a VUS (3 pathogenic and 9 likely pathogenic; Table 1 and Table S7 and S8), and 13 variants were reclassified from a P variant to a LP variant (Table S7 and S8). Additionally, seven variants in nine patients (MYH7 and TPM1) were initially considered as a P/LP secondary finding, as these variants are strongly associated with a HCM phenotype in literature (Table S9). The re-appraisal of genetic variants within a DCM framework does not allow these HCM founder variants to be classified as causal for a DCM patient, as the variant-disease association is not robust. In total, 10 P/LP HCM-associated variants were detected in 12 DCM patients (1.3%), also including variants in MYBPC3.
Table 1. Variants which are classified as a variant of unknown significance using the adapted ACMG/AMP criteria for DCM. The variants were initially classified as likely pathogenic or pathogenic.
| Gene | Number of index patients | Allele | Nucleotide consequencea | Amino acid consequencea | Classification (criteria)b | Previous classification (criteria)c |
|---|---|---|---|---|---|---|
| DSP | 1 | Heterozygous | c.3383_3384del | Val1128Glyfs*5 | VUS (PM2; PP1; PVS1_mod) | Pathogenic (PM2; PP1; PP3; PVS1) |
| DSP | 1 | Heterozygous | c.6393del | p.Gly2133Valfs*2 | VUS (PM2; PVS1_mod) | Pathogenic (PM2; PP3; PVS1; PP5) |
| DSP | 2 | Heterozygous | c.7773_7776del | p.Ser2591Argfs*11 | VUS (PM2: PS4_sup; PVS1_mod) | Pathogenic (PM2; PP3; PVS1) |
| MYH7 | 1 | Heterozygous | c.5722G>A | p.Glu1908Lys | VUS (PM2; PP3) | Likely pathogenic (PM1; PM2; PP1; PP3) |
| MYH7 | 1 | Heterozygous | c.732+1G>A | Disruptscanonicalsplice site | VUS (PM2; PS4_sup: PVS1_mod) | Likely pathogenic (PM2; PP1; PP3; PP4; PP5) |
| NEXN | 2 | Heterozygous | c.1909_1912del | p.Tyr637Alafs*48 | VUS (PM2; PP1; PS4_sup) | Likely pathogenic (PM2; PP1; PP3; PP4; PP5) |
| RBM20 | 1 | Heterozygous | c.1528-1G>C | p.? | VUS (PM2) | Likely pathogenic (PM2; PP1; PP3; PP4; PVS1) |
| RBM20 | 1 | Heterozygous | c.1764T>G | p.Ile588Met | VUS (PM2) | Likely pathogenic (PM1; PM2; PP3; PP4) |
| RBM20 | 1 | Heterozygous | c.419del | p.Pro140Argfs*3 | VUS (PM2) | Pathogenic (PM2; PP1; PP3; PP4; PVS1) |
| TNNC1 | 1 | Heterozygous | c.317+1G>A | Disruptscanonicalsplice site | VUS (PM2; PP1_mod; PS4_sup) | Likely pathogenic (PS3; PM2; PP1; PP3;) |
| TNNT2 | 5 | Heterozygous | c.742T>G | p.Phe248Val | VUS (PM2; PP1; PP3; PS4_sup) | Likely pathogenic (PM1; PM2; PP1; PP3; PP4) |
| TNNT2 | 1 | Heterozygous | c.442C>T | p.Arg148Trp | VUS (PM2; PM5; PP3) | Likely pathogenic (PM5; PM1; PM2; PP3; PP2) |
Re-evaluating phenocopies: HCM-causing variants in a DCM cohort
All patients included in this study were referred for genetic testing after being diagnosed with DCM. We re-evaluated the available cardiac imaging of the twelve patients who were heterozygous for P/LP HCM (founder) variant for any signs of HCM. None of the patients fulfilled the echocardiographic criteria for HCM, but the majority had left ventricular hypertrophy (LVH; Table 2). Interestingly, 10 of 12 patients had AF and signs of end-stage HCM, including a decreased LVEF, myocardial fibrosis and cavity dilation. Therefore we cannot exclude that these patients presented with a dilated and/or hypokinetic LV in the late phase of HCM.
Table 2. Clinical characteristics of twelve patients diagnosed with DCM who were referred for genetic testing in which a pathogenic HCM variant was detected.
| Gene | Nucleotide substitutiona | Amino acid substitutiona | Late gadolinium enhancement | Wall thickness (PW/IVS) | Left ventricle volume | Left atrium volume | Ejection fraction | Atrial fibrillation | Endpoint |
|---|---|---|---|---|---|---|---|---|---|
| MYH7 | c.1207C>T | p.Arg403Trp | + | 11/12mm | 190ml | 140ml | 31% | Yes | - |
| MYH7 | c.2167C>T | p.Arg723Cys | ++ | 14/14mm | 230ml | 138ml | 26% | Yes | Death |
| MYH7 | c.2594A>G | p.Lys865Arg | + | 6/7mm | 292ml | 60ml | 14% | Yes | - |
| MYH7 | c.5774G>A | p.Arg1925His | + | 10/10mm | 230ml | 129ml | 45% | Yes | - |
| TPM1 | c.184G>C | p.Glu62Gln | + | 14/9mm | 169ml | 165ml | 45% | Yes | - |
| TPM1 | c.184G>C | p.Glu62Gln | + | 13/13mm | 202ml | 152ml | 40% | Yes | - |
| TPM1 | c.284T>C | p.Val95Ala | NA | 10/10mm | 316ml | 150ml | 35% | Yes | Htx |
| TPM1 | c.829G>A | p.Ala277Thr | - | 7/8mm | 321ml | 72ml | 26% | No | - |
| TPM1 | c.829G>A | p.Ala277Thr | NA | 10/9mm | NA | NA | 35% | No | - |
| MYBPC3 | c.1696T>C | p.Cys566Arg | ++ | 10/10mm | 133ml | 97ml | 18% | Yes | Death |
| MYBPC3 | c.2905C>T | p.Gln969* | + | 12/12mm | 159ml | 139ml | 22% | Yes | Death |
| MYBPC3 | c.2373dup | p.Trp792Valfs*41 | + | 11/11mm | 210ml | 122ml | 41% | Yes | - |
See Table S1 for the reference refseq transcripts
+ = Midwall fibrosis; ++ = Extensive fibrosis in whole left ventricle; - = no fibrosis; NA = not available
Abbreviations: PW = posterior wall thickness; IVS = interventricular septum; Htx = heart transplantation
Clinical relevance of variant classification
A model of seven clinical parameters (i.e. family history of DCM, age, NYHA class ≥3, AF, NSVT, AVB and LBBB) could be considered characteristics of a genetic DCM patient, since there is a strong association between these parameters and the detection of a P/LP variant (Table 3). The method of genetic testing and classification is important in determining the gold standard: a patient that is heterozygous for a P/LP gene variant. The clinical model became more calibrated when the size of the gene panel was decreased (method 2), and even moreso when the adapted ACMG/AMP criteria were applied (method 3; Figure S5). This underscores that strict criteria aid the discovery of P/LP gene variants with the highest signal to noise ratio, and leads to a more homogeneous DCM subgroup characterized by; a positive familial history of DCM, younger onset and cardiac arrhythmias such as NSVT, AF and AVB.
Table 3. Association between clinical variables and a likely pathogenic or pathogenic (P/LP) gene variant at univariate and multivariate logistic regression analysis (p<0.05).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Odds ratio | 95% confidence interval | p-value | Odds ratio | 95% confidence interval | p-value | Wald-score |
| Familial history | 4.64 | 3.24-6.65 | <0.001 | 4.36 | 2.96-6.42 | <0.001 | 55.3 |
| Non-sustained VT | 3.34 | 2.35-4.76 | <0.001 | 2.91 | 1.98-4.29 | <0.001 | 29.2 |
| Atrial fibrillation | 2.51 | 1.75-3.61 | <0.001 | 2.62 | 1.71-4.01 | <0.001 | 19.6 |
| AV-block | 2.66 | 1.7-4.17 | <0.001 | 2.52 | 1.49-4.62 | <0.001 | 11.9 |
| Age | 0.98 | 0.97-0.99 | 0.021 | 0.98 | 0.97-0.99 | 0.012 | 6.3 |
| NYHA ≥III | 1.73 | 1.21-2.64 | 0.003 | 1.59 | 1.06-2.36 | 0.024 | 5.1 |
| Left bundle branch block | 0.54 | 0.35-0.82 | 0.004 | 0.6 | 0.37-0.97 | 0.038 | 4.3 |
| Sex | 0.62 | 0.43-0.89 | 0.01 | … | … | … | … |
| Left ventricular ejection fraction | 0.98 | 0.97-1.0 | 0.09 | … | … | … | … |
| Body mass index | 0.99 | 0.96-1.02 | 0.54 | … | … | … | … |
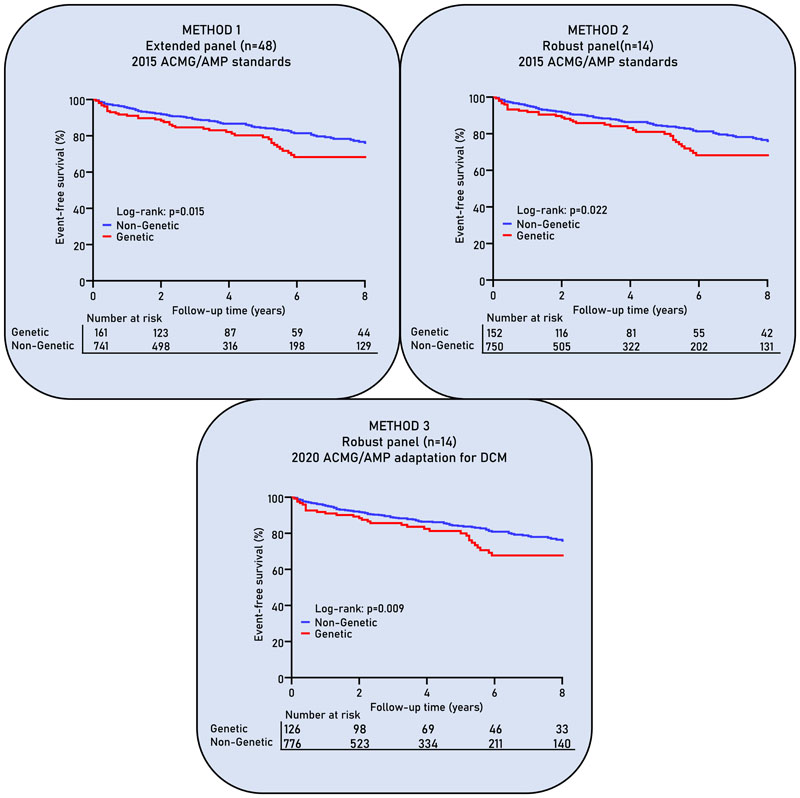
To determine the influence of variant classification on prognostic outcome of DCM patients with a P/LP gene variant, each classification method was followed by a survival analysis. DCM patients with a P/LP gene variant have a lower event-free survival compared to non-genetic DCM patients, i.e. DCM patients with no detected P/LP variant (method 1; p=0.015; Fig. 2). Limiting the number of genes (method 2), and applying the adapted ACMG/AMP criteria afterwards (method 3) did not change the prognosis of the genetic DCM subgroup (p=0.022 and 0.009 respectively). This implies that limiting the number of genes used for genetic testing still identifies the high-risk genetic DCM patients; constituting mainly of DCM patients with a pathogenic LMNA variant (Figure S6).
Fig. 2. Survival curves show freedom from combined endpoint (cardiac death or transplantation, heart failure hospitalization or life-threatening arrhythmia) stratified on genetic status.
Method 1 resembles the extended panel of 48 genes in which variants are classified according to the 2015 ACMG/AMP standards. Method 2 uses the robust panel of 14 DCM-associated genes and classified variants according to the 2015 ACMG/AMP standards. Method 3 uses the robust panel of 14 DCM-associated genes and classified them according to the 2020 DCM adaptation of the ACMG/AMP standards.
Discussion
The introduction of more stringent gene selection for DCM clinical genetic testing reduced the number of VUSs, whilst retaining the ability to identify P/LP variants. The DCM patients with a P/LP variant represented a homogeneous clinical subgroup with a lower event-free survival, compared to non-genetic DCM patients.
Rare variants in non-robust DCM genes
Variants in non-robust DCM genes are not a prevalent cause of DCM. This does not exclude a disease-causing role for variants in these genes, but implies that the majority of variants in these genes will not be interpretable in a clinical context; which defines genes with a low signal to noise ratio (i.e. genes with few pathogenic variants (signal) and many VUSs (noise)). The additional value of every gene on a test panel should be carefully considered. As most variants in non-robust genes will not be pathogenic, strong additional evidence for pathogenicity will be necessary. There are insufficient resources to perform segregation and functional studies for all detected variants. Family sizes are relatively smaller now compared to previous decades, which does not allow extensive segregation - preventing the acquisition of additional evidence to classify rare variants as P/LP. High throughput screening of VUSs in functional studies is absent for almost all genes, as a reliable readout for loss-of-function in in-vitro models is challenging. Prioritizing variants in genes from the extended panel will be of utmost importance, in order to ensure that benefit outweighs cost.
Including non-robust genes in clinical test panels may have an adverse impact on patient management. A VUS can cause uncertainty and fear in patients and their family, and requires laborious counselling, in order to handle the controversies and ambiguities surrounding VUSs. Moreover, the potential of erroneous interpretation of these variants by other healthcare professionals can warrant harm by unnecessary check-ups or even procedures12,13. The noise introduced by including non-robust genes in routine genetic testing will eventually lead to unnecessary patient anxiety and use of clinical resources.
Following a period of increasing gene panel size, there is now enhanced awareness of the issues surrounding non-robust genes on test panels. ClinGen is making great effort to curate genes associated with (cardiac) diseases such as HCM14, Brugada15 and long QT syndrome16. At the moment of writing this manuscript, 48 genes associated with DCM have been curated, of which 31 are on our extended panel 17. 17 of the 48 curated genes are classified as moderate, strong or definitive; and 4 of these genes are not included on our robust panel: SCN5A (definitive), DES (definitive), JPH2 (moderate) and TNNI3 (moderate) (Table S1). The genes on our robust panel were mainly chosen based on significant enrichment of rare variants in DCM patients 9, the ClinGen curation takes more facets of gene-disease validity into consideration 17. Including these four genes on the robust panel would have increased the P/LP variants by 2 (0.2%; SCN5A), and the number of VUSs by 39 (4.3%).
Clinical context of variant interpretation
Tailoring the ACMG/AMP standards for DCM was an essential step, as the standards serve a broad spectrum of single-gene conditions, not considering the unique genetic features of DCM 8. Using the adapted ACMG/AMP criteria does, by definition, not allow pathogenic HCM variants to be classified as pathogenic for the DCM phenotype, as the variant-disease association is not robust. However, this does not decrease the pathogenicity of such HCM variants. These are initially ‘secondary’ findings and require us to re-evaluate the clinical presentation and natural history of the patient, but is important information that can be used for better clinical management. Eighty three percent of the patients in our study with a pathogenic HCM variant had AF -known to aggravate the clinical course of HCM 18. With the LVH, pronounced cardiac fibrosis, and decreased systolic function, it is very likely that the AF complicated the course of HCM, associated with increased mortality 19.
An estimated 0.5% of HCM patients per year progress to ‘burnt-out’ HCM, characterized by wall thinning, cavity dilation, and systolic dysfunction 20. In clinical practice it is difficult to distinguish the dilated HCM phenotype from primary DCM. The clinical context is important in the interpretation of the initial ‘secondary’ findings and depends on prior documentation of hypertrophy and/or family history. Phenotyping remains a crucial pillar in understanding genetic variants and determine their pathogenicity. The results of genetic testing in patients with a DCM phenotype irrespective of a proven etiology can help us to understand the disease in a specific patient, and may provide clues for additional phenotyping (i.e. reverse phenotyping). The current study reports on the prevalence of a masqueraded ‘burnt-out’ HCM in a DCM cohort, diagnosed by genetic sequencing. When a ‘burnt-out’ HCM is probable, there should be consideration to take a broader genetic approach to include all genes associated with cardiomyopathy when clinical genetic testing is indicated. Otherwise the pathogenic MYBPC3 variants would not have been detected, since variants in this gene are known to cause HCM but not DCM. Clinicians should be aware of these ‘phenocopies’ in the gene selection and subsequent interpretation of genetic results, and as such, some of the genes absent on the robust panel should be considered in some circumstances. In general, actionable variants in cardiomyopathy genes are among the most frequent secondary findings in clinical exome and genome sequencing 21, and their management is further depicted in corresponding practice resource papers 22.
Genotype influences outcome
The prognostic influence of pathogenic gene variants remains under debate in the literature, which is due to two important issues: [1] the large genetic heterogeneity of different cohorts inherent to the population (i.e. the division of genes with pathogenic variants), and [2] the variation in gene selection and variant interpretation used for genetic testing (i.e. the (number of) genes included on panels). We addressed these points by critically re-evaluating the gene constitution of our panel and applying the adapted ACMG/AMP criteria for DCM, thereby limiting the genetic heterogeneity of our cohort to only 11 genes with 64.8% of pathogenic variants in TTN. A systematic approach to these criteria revealed a clinically more homogeneous patient population, in which pathogenic gene variants are associated with cardiac arrhythmias and a lower event-free survival. Applying this approach post-hoc on previous studies investigating genetic variants in DCM in association with event-free survival, would address the two issues and provide statistical power to analyze the role of individual genes on the outcome of DCM patients. Such efforts are necessary to determine the clinical impact of gene-specific pathogenic variants, and subsequently improve patient- and family-directed care.
Future directions of routine genetic testing in DCM
Based on our results and recent literature, we strongly suggest to limit the number of genes on routine diagnostic panels 9, with the goal to increase the signal to noise ratio 23. Genes outside the robust panel can still be pathogenic, although have a low yield of actionable variants (i.e. signal), and a high yield of VUSs (i.e. noise). This was the case for genes curated by the ClinGen consortium as definitive (SCN5A and DES) or moderate (JPH2 and TNNI3). The principle of a diagnostic panel should be to balance the number of genes on a panel to a high signal to noise ratio. The precise panel composition is likely to evolve with our understanding in the upcoming years. As our diagnostic panel was representative for the genetic sequencing in DCM in the past decade (Table S3), the results of this study can be generalized to other centers conducting genetic sequencing in DCM patients.
When limiting the gene panel size to a targeted DCM-specific panel, there is a slim chance that a P/LP variant is missed, which are mainly syndromic, pediatric and rare genetic causes of (isolated) DCM. These patients often have clear clinical symptoms and signs that are indicative for a pathogenic variant in specific genes (e.g. EMD and TTR). However, the associated symptoms can also be more subtle, as pathogenic variants in DMD are associated with adulthood DCM with relatively mild skeletal muscle findings. The specific signs and symptoms of the genetic disease should raise awareness to take a broader approach and include the associated genes in the genetic sequencing, it is advised to discuss challenging cases in a multidisciplinary cardiogenetic team.
Two genes had already been removed from our diagnostic panel in the past months (CALR3 and MYH6), representing the beginning of re-appraising routine genetic testing in DCM. SCN5A is one of the genes curated by the ClinGen assortium as being definitively associated with DCM, and was not included in the list of robust genes. In addition, specific missense variants in the gene are associated with arrhythmogenic DCM, including segregation and functional data. Loss of function (LOF) variants were reported to have an increased odds ratio of 16.5 in DCM cases versus controls. We suggest to add SCN5A to the robust genes, reporting only the LOF and the missense variants that are strongly evidenced (e.g. p.Arg216His, p.Arg222Gln) in clinical practice. Fundamental knowledge on important hotspots of the protein will help improve variant classification. In the ACMG/AMP adaptation for DCM, there is only such knowledge for RBM20 and MYH7 (PM1 criterium), leaving evidence missing for all other genes. In line with this, genes with a low yield still remain under investigation and our evolving knowledge can increase the signal to noise ratio for these genes. Overall, the adapted ACMG/AMP criteria appear more stringent, which is reflected in the decrease of pathogenic variants from 7.7% to 4.2%. For example, novel truncating variants in DSP would be classified as VUS in the absence of additional supporting evidence, mainly due to the strength specification of the PVS1 criterium which is applied at moderate, rather than very strong, for predicted loss of function variants in DSP. Additional studies are needed to fully assess the impact and accuracy of these adaptations. The composition of a robust panel should be considered as dynamic and editable, although the principle should be to limit the number of genes to improve the signal to noise ratio.
In conclusion, limiting the number of genes on diagnostic DCM sequencing panels will decrease the number of uncertain results, but still identifies patients with a high-risk P/LP gene variant. The number of genes on a diagnostic panel should be limited to the genes which have the highest signal (i.e. number of P/LP variants) to noise (i.e. number of variants of uncertain significance) ratio.
Supplementary Material
Acknowledgments
The authors are grateful to Pablo Garcia-Pavia and Juan Pablo Ochoa (Department of Cardiology, Hospital Universitario Puerta de Hierro, Madrid, Spain) for their input and revising of the manuscript.
J.W.: This work was supported by Wellcome Trust [107469/Z/15/Z], Medical Research Council (UK), British Heart Foundation [RE/18/4/34215], NIHR Royal Brompton Cardiovascular Biomedical Research Unit, and the NIHR Imperial College Biomedical Research Centre.
S.H.: The research leading to these results has received funding from the European Union Commission’s Seventh Framework programme under grant agreement N° 305507 (HOMAGE). This manuscript has been possible thanks to the support of the the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2016-Early HFPEF, 2015-10, CVON She-PREDICTS, grant 2017-21.
The views expressed in this work are those of the authors and not necessarily those of the funders.
Footnotes
Author Information
Conceptualization: J.V., H.B., J.W.; Data curation: J.V., D.H., G.C.; Formal Analysis: J.V.; Funding acquisition: S.H., H.B., A.vd.W.; Investigation: S.S., D.H., G.C., I.K., E.V., J.V.; Methodology: J.V., Resources: H.B., A.vd.W.; Supervision: H.B.; Visualization: S.S., J.V.; Writing – original draft: S.S., J.V.; Writing – review & editing: D.H., G.C., U.T., I.K., E.V., M.H., J.W.
Ethics Declaration
The study was performed according to the declaration of Helsinki and was approved by the institutional Medical Ethics Committee of the Maastricht University Medical Center. All patients gave written informed consent.
Conflict of Interest
The authors declare no conflict of interest.
Data Availability
The data that support the findings of this study are availablefrom the corresponding author on request.
References
- 1.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature reviews Cardiology. 2013;10(9):531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 2.Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The Diagnosis and Evaluation of Dilated Cardiomyopathy. Journal of the American College of Cardiology. 2016;67(25):2996–3010. doi: 10.1016/j.jacc.2016.03.590. [DOI] [PubMed] [Google Scholar]
- 3.Verdonschot JAJ, Hazebroek MR, Krapels IPC, et al. Implications of Genetic Testing in Dilated Cardiomyopathy. Circ Genom Precis Med. 2020 doi: 10.1161/CIRCGEN.120.003031. [DOI] [PubMed] [Google Scholar]
- 4.Gigli M, Merlo M, Graw SL, et al. Genetic Risk of Arrhythmic Phenotypes in Patients With Dilated Cardiomyopathy. Journal of the American College of Cardiology. 2019;74(11):1480–1490. doi: 10.1016/j.jacc.2019.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh R, Thomson KL, Ware JS, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genetics in medicine : official journal of the American College of Medical Genetics. 2017;19(2):192–203. doi: 10.1038/gim.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly MA, Caleshu C, Morales A, et al. Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen's Inherited Cardiomyopathy Expert Panel. Genetics in medicine : official journal of the American College of Medical Genetics. 2018;20(3):351–359. doi: 10.1038/gim.2017.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales A, Kinnamon DD, Jordan E, et al. Variant Interpretation for Dilated Cardiomyopathy: Refinement of the American College of Medical Genetics and Genomics/ClinGen Guidelines for the DCM Precision Medicine Study. Circ Genom Precis Med. 2020;13(2):e002480. doi: 10.1161/CIRCGEN.119.002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzarotto F, Tayal U, Buchan RJ, et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation. 2020;141(5):387–398. doi: 10.1161/CIRCULATIONAHA.119.037661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016;37(23):1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 11.Verdonschot JAJ, Vanhoutte EK, Claes GRF, et al. A mutation update for the FLNC gene in myopathies and cardiomyopathies. Hum Mutat. 2020 doi: 10.1002/humu.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson KL, Ormondroyd E, Harper AR, et al. Analysis of 51 proposed hypertrophic cardiomyopathy genes from genome sequencing data in sarcomere negative cases has negligible diagnostic yield. Genetics in medicine : official journal of the American College of Medical Genetics. 2019;21(7):1576–1584. doi: 10.1038/s41436-018-0375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manrai AK, Funke BH, Rehm HL, et al. Genetic Misdiagnoses and the Potential for Health Disparities. The New England journal of medicine. 2016;375(7):655–665. doi: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingles J, Goldstein J, Thaxton C, et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ Genom Precis Med. 2019;12(2):e002460. doi: 10.1161/CIRCGEN.119.002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini SM, Kim R, Udupa S, et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation. 2018;138(12):1195–1205. doi: 10.1161/CIRCULATIONAHA.118.035070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler A, Novelli V, Amin AS, et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation. 2020;141(6):418–428. doi: 10.1161/CIRCULATIONAHA.119.043132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan E, Peterson L, Ai T, et al. An Evidence-based Assessment of Genes in Dilated Cardiomyopathy. Circulation. 2021 doi: 10.1161/CIRCULATIONAHA.120.053033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517–2524. doi: 10.1161/hc4601.097997. [DOI] [PubMed] [Google Scholar]
- 19.Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114(3):216–225. doi: 10.1161/CIRCULATIONAHA.105.583500. [DOI] [PubMed] [Google Scholar]
- 20.Sen-Chowdhry S, Jacoby D, Moon JC, McKenna WJ. Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nature reviews Cardiology. 2016;13(11):651–675. doi: 10.1038/nrcardio.2016.140. [DOI] [PubMed] [Google Scholar]
- 21.Kalia SS, Adelman K, Bale SJ, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genetics in medicine : official journal of the American College of Medical Genetics. 2017;19(2):249–255. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- 22.Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG) Genetics in medicine : official journal of the American College of Medical Genetics. 2018;20(9):899–909. doi: 10.1038/s41436-018-0039-z. [DOI] [PubMed] [Google Scholar]
- 23.Bean LJH, Funke B, Carlston CM, et al. Diagnostic gene sequencing panels: from design to report-a technical standard of the American College of Medical Genetics and Genomics (ACMG) Genetics in medicine : official journal of the American College of Medical Genetics. 2020;22(3):453–461. doi: 10.1038/s41436-019-0666-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are availablefrom the corresponding author on request.