Abstract
Objective
Depression and anxiety have both been reported to predict the worse subsequent survival of people with cancer. However, depression and anxiety are mutually associated and we lack understanding of their independent associations with survival. We therefore aimed to investigate these in a large sample of patients with common cancers.
Methods
We analysed data on 19,966 patients with common cancers (breast, colorectal, gynaecological, lung and prostate) who had attended specialist NHS outpatient clinics in Scotland, UK. Hospital Anxiety and Depression Scale (HADS) data were linked with demographic, cancer and mortality data. We estimated the independent associations of depression (HADS depression score) and anxiety (HADS anxiety score) with survival by fitting (separately for each cancer) Cox proportional hazards models which incorporated cubic splines to allow for non-linear associations. We also adjusted for potential confounders.
Results
The median time from HADS completion to death or censoring was 1.9 years. Greater depression was found to be strongly associated with worse survival from all cancers. When adjusted for anxiety, this association remained in males and increased in females. Greater anxiety was also associated with worse survival in nearly all cancers. However, when adjusted for depression, the association of anxiety with worse survival was lost. In females the association reversed direction so that greater anxiety was associated with better survival.
Conclusion
Although often considered together as aspects of ‘emotional distress’, depression and anxiety have different independent associations with survival in patients with cancer and should therefore be considered separately.
Keywords: depression, anxiety, cancer, survival, mortality, neoplasms
Introduction
There is considerable interest in the relationship between psychological factors and the survival of people with cancer. A growing body of literature suggests that depression and anxiety, in particular, are associated with worse subsequent survival [1–3].
A hitherto neglected aspect of this literature is whether depression and anxiety, which commonly co-occur, have similar associations with survival when they are considered separately. This question arises from an increasing understanding that depression and anxiety are not just aspects of ‘emotional distress,’ but have distinct psychological and biological mechanisms [4]. We are unaware of any studies published to date that have examined this question. We therefore sought to answer it by conducting an analysis of prospectively collected data from a large cohort of patients with common cancers (breast, colorectal, gynaecological, lung and prostate cancers) who had completed depression and anxiety questionnaires as part of their routine cancer care and for whom we had survival data.
The aims of our analysis were to examine the independent associations of depression and anxiety with subsequent survival in patients with common cancers by determining: (a) the association of depression with subsequent survival in patients with each cancer, with and without adjustment for anxiety and (b) the association of anxiety with subsequent survival in patients with each cancer, with and without adjustment for depression.
Methods
Study design and sample
We analysed data from patients who had attended outpatient clinics of the Edinburgh, Glasgow and Dundee National Health Service (NHS) cancer centres in Scotland, UK. Each of these cancer centres provides a full range of diagnostic and treatment services in a large urban teaching hospital with outreach clinics in the smaller hospitals of surrounding towns. Together the three centres serve a geographically defined area of approximately four million people and provide specialist care for the vast majority of patients who have been diagnosed with cancer in this region. Patients attending these clinics were asked to complete a depression and anxiety questionnaire as part of their routine cancer care. Most patients (80%) completed this questionnaire (the main reason that patients did not complete the questionnaire was that their oncology appointment had begun before they could do so).
We included a patient’s data in this analysis if: (a) they had attended an outpatient oncology consultation in a central or outreach cancer clinic between May 12, 2008 and Aug 24, 2011; (b) they had completed the depression and anxiety questionnaire (the Hospital Anxiety and Depression Scale, HADS) that was used routinely in the cancer clinics [5]; (c) the patient had no missing items on the HADS; (d) we could obtain their matched demographic and clinical data from the Scottish National Cancer Registry; (e) they had given consent for their relevant clinical data to be used for research; and (f) they had a primary breast, colorectal, gynaecological, lung or prostate cancer. We chose these cancers because they are the most common, they often form the basis for multidisciplinary cancer care (therefore the associations between depression and anxiety and survival in each group is clinically useful) and the number of patients within each grouping was sufficient to estimate these associations with acceptable accuracy.
Measures
Depression and anxiety
The HADS was routinely given to everyone who attended the cancer clinics in order to assess how much depression and anxiety they had experienced over the preceding week [5]. The HADS has a total of 14 items; seven items make up the HADS depression subscale and seven make up the HADS anxiety subscale. The individual items are each scored from zero to three, resulting in maximum depression and anxiety subscale scores of 21, with higher scores indicating greater severity.
Demographic and cancer data
We obtained data on patients’ demographic and cancer characteristics from the NHS Scotland Cancer Registry. The Registry systematically collects information from hospitals throughout Scotland for all recorded cases of cancer. The data included sex, date of cancer diagnosis, age at cancer diagnosis, social deprivation score (calculated using the Scottish Index of Multiple Deprivation, based on area of residence at the time of cancer diagnosis; see Appendix A for details), primary cancer (see Appendix B for details) and initial cancer treatment objective (curative or palliative) which we used as a proxy for cancer severity that could be applied across all the cancers studied.
Mortality data
We obtained data on deaths up to April 30, 2012 (that is, 47 months from the first HADS completion on May 12, 2008 and eight months from the last HADS completion on Aug 24, 2011). These data were obtained from the National Records of Scotland (NRS) database and included the date and recorded cause of death of each patient.
Data linkage
To ensure data security and confidentiality the dataset of the patients’ HADS (depression and anxiety) scores was sent to the Information Services Division of NHS Scotland for linkage using unique patient identification numbers (Community Health Index numbers) and dates of birth. All identifying data were then removed in a one-way linkage to produce the anonymised dataset that was used for analysis. The study was approved by the South East Scotland Research Ethics Committee, the NHS Scotland Caldicott Guardian Forum, and the NHS Scotland Privacy Advisory Committee.
Statistical analyses
For each patient, we calculated the time to their death from the date they completed the HADS. We included deaths from any cause in our analysis because most of the deaths were recorded as being due to cancer (see results). If a patient had attended the cancer clinic and completed the HADS more than once during the study period, we used the data relating to the earliest of these clinic attendances. We censored patients who had left Scotland (at their date of emigration) and patients who were not known to have died or to have emigrated at the latest date on which data were available (April 30, 2012). Patients whose mortality status was unknown were followed to their last known appointment date (within the study period) or were excluded from the analysis if this was unavailable.
We separately analysed the data from patients with each primary cancer (see Appendix B for details). Some of the cancers studied are sex-specific (prostate, breast and gynaecological). For the other, non-sex specific, cancers (colorectal cancer and lung cancer) we conducted separate analyses for males and females because inspection of the data suggested sex differences in the associations between anxiety and survival. Our main analysis consequently comprised seven sets of models with patients grouped as follows: prostate cancer, colorectal cancer – males, lung cancer – males, breast cancer, gynaecological cancer, colorectal cancer – females, lung cancer – females. For patients who had multiple primary cancers, we used the cancer diagnosis that most closely preceded their completion of the HADS to assign them to a group, except where two or more diagnoses were made on the same day (nine patients who were given two different cancer diagnoses on the same day were included in the analyses of both cancers).
We used Cox proportional hazards models to estimate the associations of depression (HADS depression score) and anxiety (HADS anxiety score) with subsequent survival. As expected, depression and anxiety scores were associated (Pearson correlation = 0.60, see Appendix C). In order to determine their independent associations with survival, we therefore fitted models that included both as predictor variables (i.e. we calculated the association of depression with survival when adjusted for anxiety and vice versa). Because the associations appeared non-linear, we used restricted cubic splines with four knots (positioned at 5th, 35th, 65th and 95th percentiles) to model the associations of depression and anxiety with survival (we also performed an analysis using cubic splines with five knots, but choose to present results for four knots as five knots sometimes produced implausibly steep increases and decreases in the fitted relationships).
We also extended these mutually adjusted models to incorporate interactions between depression and anxiety scores. In these models we first included all products of the linear term for depression and cubic spline terms for anxiety and vice-versa, as is recommended [6]. If these interaction terms were jointly statistically significant we additionally compared the fit of this model with a simpler one that included only the product of the linear terms for depression and anxiety.
Having conducted separate analyses for males and females with each of the non-sex specific cancers (colorectal cancer and lung cancer), we performed secondary analyses in which we fitted models to all patients with lung cancer and (separately) all patients with colorectal cancer that included interactions between sex and the cubic spline terms for depression scores and anxiety scores.
In all the models, we adjusted for the following covariates: age at cancer diagnosis, time between cancer diagnosis and completion of the HADS, social deprivation score, and initial treatment objective recorded at the time of cancer diagnosis. Depression, anxiety and all adjustment variables were either inherently or treated as fixed over the follow-up time. We expected the associations between continuous adjustment variables (time between cancer diagnosis and HADS completion, age at cancer diagnosis and deprivation score) and survival to be non-linear and therefore used restricted cubic splines with four knots to allow flexible parameterisation of these relationships.
The models also included two-way interactions between the time interval between cancer diagnosis and completion of the HADS and the adjustment variables described above. This was because age at cancer diagnosis, social deprivation score and initial cancer treatment objective were all measured at the time of patients’ cancer diagnoses and it is plausible that the magnitude of their confounding associations with survival may change according to the time interval between cancer diagnosis and HADS completion. For each two-way interaction between this time interval and either age at cancer diagnosis or social deprivation score, all products of linear terms were included in the models.
We used multiple imputation to deal with missing data on initial cancer treatment objective (2,533 patients) and social deprivation score (two patients). We used the substantive model compatible fully conditional specification (SMCFCS) method for each imputation in order to properly account for interactions and non-linear associations [7]. Imputation models could include extra variables that were found to be predictive of survival and missingness (see Appendix D for further details on the handling of missing data). We performed 20 imputations (separately for each cancer) using the final model. We fitted Cox regression models to each imputed dataset and combined the results using Rubin’s rules [8]. We then calculated predicted hazard ratios (HR) at all levels of depression and anxiety for each cancer. Imputations were carried out in R version 3.4.1 and all analysis models were fitted in Stata version 15 [9, 10].
Results
We included data from 19,966 patients in the analysis (see Table 1 for their characteristics). The median time from HADS completion to death or censoring was 1.9 years (IQR: 1.1, 2.8). 5,884 patients died (from all causes) during the period of follow-up. Most (91.5%) of the deaths were recorded as being due to cancer (see Appendix E).
Table 1. Demographics, depression and anxiety, and survival of patients included in the analysis.
| Prostate cancer |
Colorectal cancer-males |
Lung cancer-males |
Breast cancer |
Gynaecological cancer |
Colorectal cancer- females |
Lung cancer- females |
|
|---|---|---|---|---|---|---|---|
| Total | 1531a | 1573 | 2299 | 8467 a | 2910 a | 1154 | 2041 |
| Sex | |||||||
| Female | 0 (0%) | 0 (0%) | 0 (0%) | 8467 (100%) | 2910 (100%) | 1154 (100%) | 2041 (100%) |
| Male | 1531 (100%) | 1573 (100%) | 2299 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
|
Age at cancer diagnosis [median
years, IQR] |
66 [62, 72] | 65 [59, 72] | 68 [61, 74] | 57 [49, 66] | 60 [50, 69] | 65 [57, 72] | 67 [60, 74] |
| SIMD score quintile b | |||||||
| 1 | 262 (17%) | 281 (18%) | 749 (33%) | 1442 (17%) | 617 (21%) | 217 (19%) | 700 (34%) |
| 2 | 251 (16%) | 304 (19%) | 543 (24%) | 1547 (18%) | 617(21%) | 231 (20%) | 511 (25%) |
| 3 | 254 (17%) | 297 (19%) | 365 (16%) | 1539 (18%) | 555 (19%) | 195 (17%) | 328 (16%) |
| 4 | 334 (22%) | 279 (18%) | 309 (13%) | 1630 (19%) | 546 (19%) | 204(18%) | 249 (12%) |
| 5 | 430 (28%) | 411 (26%) | 333 (14%) | 2308 (27%) | 575 (20%) | 307 (27%) | 253 (12%) |
| Missing | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Initial cancer treatment objective | |||||||
| Curative | 635 (41%) | 1116 (71%) | 566 (25%) | 6533 (77%) | 2010 (69%) | 830 (72%) | 546 (27%) |
| Palliative | 634 (41%) | 319 (20%) | 1644 (72%) | 466 (6%) | 521 (18%) | 231 (20%) | 1391 (68%) |
| Missing | 262 (17%) | 138 (9%) | 89 (4%) | 1468 (17%) | 379 (13%) | 93 (8%) | 104 (5%) |
|
Time interval between cancer
diagnosis & HADS c completion [median years, IQR] |
2.0 [0.8, 4.4] | 1.0 [0.3, 2.5] | 0.3 [0.1, 0.8] | 2.0 [0.4, 5.2] | 1.0 [0.4, 2.9] | 1.0 [0.3, 2.6] | 0.3 [0.1, 0.9] |
| HADS | |||||||
| HADS-D (median, IQR) | 3 [1, 6] | 3 [1, 6] | 6 [3, 9] | 3 [1, 6] | 4 [1, 7] | 3 [1, 7] | 6 [3, 9] |
| HADS-A (median, IQR) | 4 [1, 7] | 4 [1, 7] | 5 [3, 9] | 5 [3, 9] | 5 [2, 8] | 5 [2, 8] | 7 [4, 10] |
|
Time from HADS completion to death
or censoring [median years, IQR] |
2.2 [1.7, 3.1] | 1.8 [1.2, 2.8] | 0.8 [0.3, 1.4] | 2.3 [1.6, 3.0] | 1.9 [1.2, 2.8] | 1.8 [1.2, 2.7] | 0.9 [0.4, 1.6] |
| Died during study period | 288 (19%) | 518 (33%) | 1603 (70%) | 1000 (12%) | 824 (28%) | 328(28%) | 1328 (65%) |
Data are n (%) unless stated otherwise.
9 patients are included in this table twice because they were diagnosed with more than one primary cancer on the same day: 1 had breast & gynaecological cancers, 3 had colorectal & gynaecological cancers, 2 had breast & lung cancers, 1 had breast & colorectal cancers, 1 had colorectal & lung cancers (male), 1 had colorectal & prostate cancers.
Scottish Index of Multiple Deprivation quintile score: 1=most deprived, 5=least deprived.
Hospital Anxiety and Depression Scale: HADS-D=depression severity, HADS-A=anxiety severity
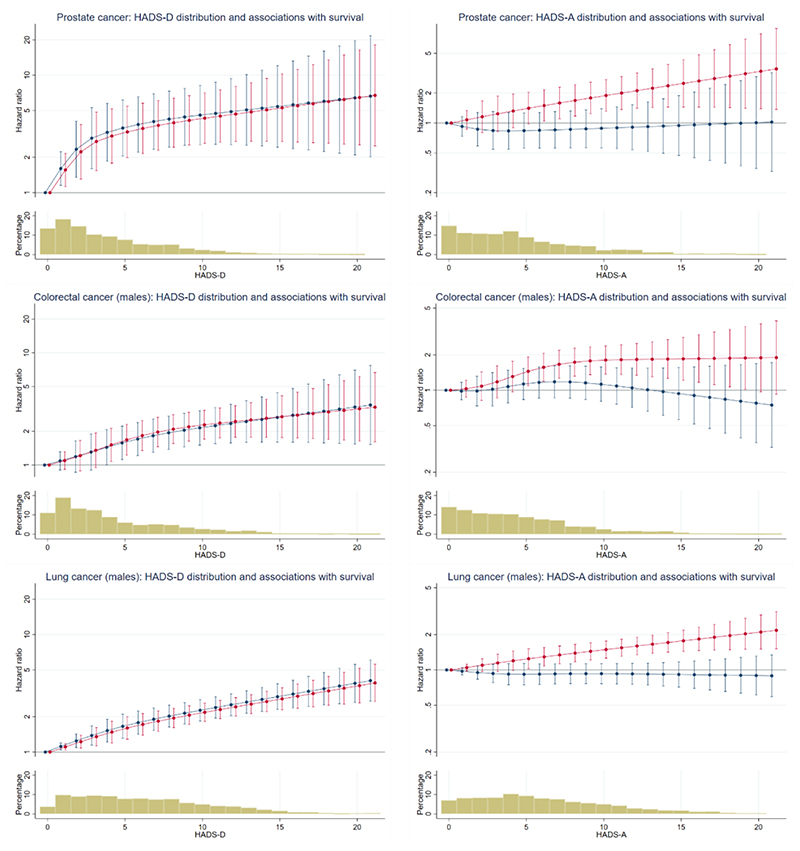
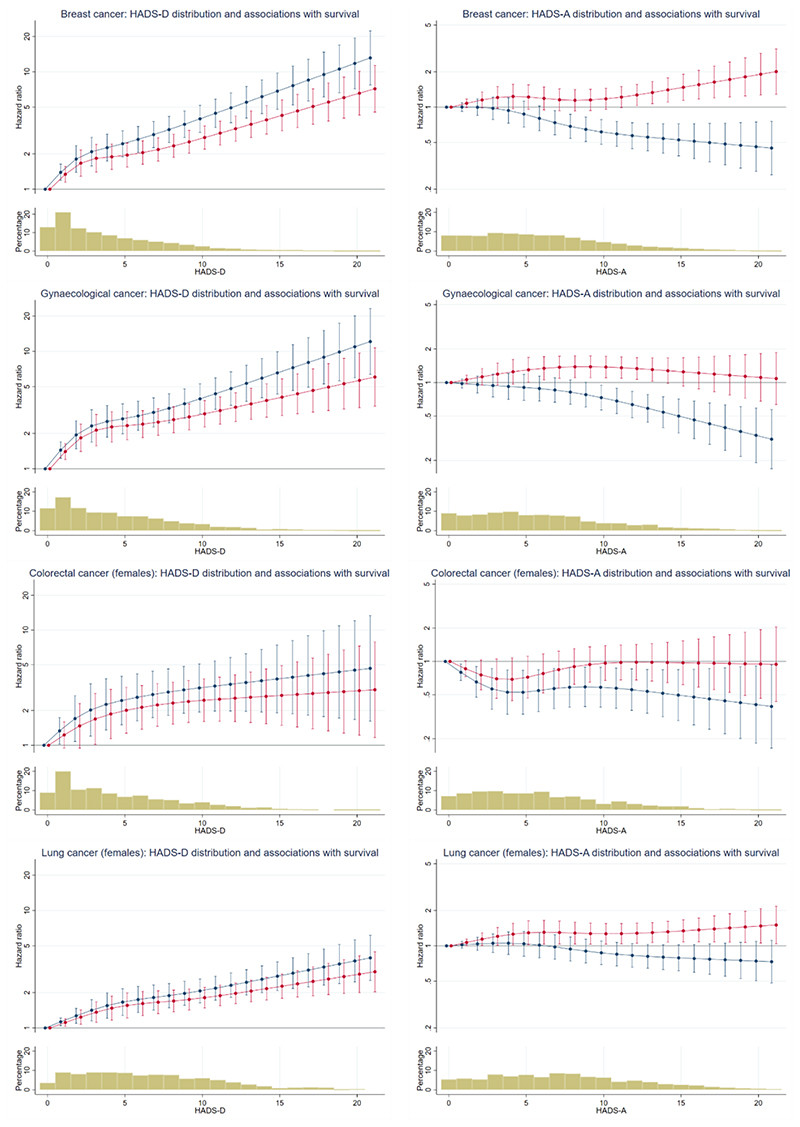
The fitted associations of depression and anxiety with survival in males and females are shown in Figures 1 and 2 respectively (see also Appendix F).
Figure 1. Associations of survival with depression and anxiety for male patients with cancer.
Plots show predicted hazard ratios (hazard of mortality for patients with each HADS-D or HADS-A score relative to patients with a score of zero). Bar Charts show the percentage of patients with each HADS-D and HADS-A score. Red lines show unadjusted hazard ratios (prostate cancer HADS-D p<0.0001, HADS-A p=0.0013; colorectal cancer – males HADS-D p<0.0001, HADS-A p=0.0001; lung cancer – males HADS-D p<0.0001, HADS-A p<0.0001). Blue lines show the hazard ratios adjusted for the other symptom (depression or anxiety) of interest (prostate cancer HADS-D p<0.0001, HADS-A p=0.8401; colorectal cancer – males HADS-D p<0.0001, HADS-A p=0.4586; lung cancer – males HADS-D p<0.0001, HADS-A p=0.8798). Note that y-axis scales are different for HADS-D and HADS-A, but consistent across cancers.
Figure 2. Associations of survival with depression and anxiety for female patients with cancer.
Plots show predicted hazard ratios (hazard of mortality for patients with each HADS-D or HADS-A score relative to patients with a score of zero). Bar Charts show the percentage of patients with each HADS-D and HADS-A score. Red lines show unadjusted hazard ratios (breast cancer HADS-D p<0.0001, HADS-A p=0.0220; gynaecological cancer HADS-D p<0.0001, HADS-A p=0.0398; colorectal cancer - females HADS-D p=0.0001, HADS-A p=0.1521; lung cancer - females HADS-D p<0.0001, HADS-A p=0.0656). Blue lines show the hazard ratios adjusted for the other symptom (depression or anxiety) of interest (breast cancer HADS-D p<0.0001, HADS-A p<0.0001; gynaecological cancer HADS-D p<0.0001, HADS-A p=0.0002; colorectal cancer – females HADS-D p<0.0001, HADS-A p=0.0371; lung cancer - females HADS-D p<0.0001, HADS-A p=0.0186). Note that y-axis scales are different for HADS-D and HADS-A, but consistent across cancers.
The figures (which are interpreted in detail in the next section) show plots for each of our seven groups (prostate cancer, colorectal cancer – males, lung cancer – males, breast cancer, gynaecological cancer, colorectal cancer – females, lung cancer – females). The plots on the left of each figure show predicted HRs for the association between depression and survival, without adjustment for anxiety (red lines) and then with adjustment for anxiety (blue lines). The plots on the right of each figure show predicted HRs for the association between anxiety and survival, without adjustment for depression (red lines) and then with adjustment for depression (blue lines). HRs refer to the hazard of mortality for patients with each HADS depression or HADS anxiety score relative to those with a score of zero.
The bar chart below each plot shows the percentage of patients with each HADS depression or HADS anxiety score; around 90% of patients had depression scores of 10 or less and around 90% had anxiety scores of 12 or less.
Association of depression with survival
Greater depression was strongly associated with worse subsequent survival in all the seven groups (p<0.0001 in all groups). The HRs were sizeable, for example the HRs comparing patients with HADS depression scores of 10 and 0 varied from 4.30 (95% CI 2.63, 7.06) for prostate cancer to 1.81 (95% CI 1.48, 2.22) for lung cancer – females. The fitted relationships were not linear, typically being steeper at the lower end of the range than at the higher end.
Association of depression with survival when adjusted for anxiety
When we adjusted for anxiety, the association between depression and survival remained statistically significant for all seven groups (p<0.0001). The HRs comparing patients with HADS depression scores of 10 and 0 varied from 4.57 (95% CI 2.56, 8.16) for prostate cancer to 2.07 (95% CI 1.64, 2.61) for lung cancer – females. For the female groups depression was more strongly associated with survival after adjustment for anxiety (see figure 2 blue lines compared with red lines).
Association of anxiety with survival
Greater anxiety was also associated with worse subsequent survival in five of the seven groups (prostate cancer p=0.001, colorectal cancer – males p=0.0001, lung cancer – males p<0.0001, breast cancer p=0.022, gynaecological cancer p=0.040, colorectal cancer – females p=0.152, lung cancer – females p=0.066). The HRs observed were however smaller than those for depression, for example the HRs comparing patients with HADS anxiety scores of 10 and 0 varied from 1.89 (95% CI 1.31, 2.72) for prostate cancer to 0.96 (95% CI 0.67, 1.39) for colorectal cancer – females.
Association of anxiety with survival when adjusted for depression
When we adjusted for depression, the association of anxiety with survival changed markedly. For males, little or no association between anxiety and survival remained. For females, the association of anxiety with survival was typically in the opposite direction to that observed before we adjusted for depression. That is to say, greater anxiety was now associated with better survival (breast cancer p<0.0001, gynaecological cancer p=0.0002, colorectal cancer – females p=0.037, lung cancer – females p=0.019). The HRs comparing patients with HADS anxiety scores of 10 and 0 varied from 0.87 (95% CI 0.69, 1.10) for lung cancer - females to 0.58 (95% CI 0.39, 0.88) for colorectal cancer - females.
The observed difference between the sexes in the association between anxiety and survival was clearest when comparing the plots for the sex-specific cancers. The same directional differences were also seen in the sex-specific analyses of the lung and colorectal cancer groups, however, formal interaction tests from models including both males and females were not statistically significant (colorectal p=0.111, lung p=0.095).
Interaction between depression and anxiety in their associations with survival
When fitting models with both depression and anxiety and an interaction between the two, there was some evidence of an interaction for those with breast cancer (p=0.025) but not for the other cancers. Results from this analysis are presented in Appendix G. We suggest caution in interpretation as this is only one statistically significant result of many tests done.
Discussion
Main findings
We found that, as expected, greater depression was strongly associated with worse subsequent survival for both male and female patients for all the cancers we studied. After adjusting for anxiety, this association remained in males and became stronger in females. We also found that greater anxiety was associated with worse survival in most of the groups analysed. However, after adjusting for depression the relationship between anxiety and survival changed, disappearing in males and changing direction in females such that greater anxiety became associated with better subsequent survival. This negative association of greater anxiety and worse survival, coupled with the fact that depression and anxiety are highly associated, explains why the association between depression and survival became stronger in females after adjusting for anxiety.
Other literature
The finding that greater depression is associated with worse subsequent survival in people with cancer has been frequently reported [1–3], but is disputed on methodological grounds [11]. Our findings, from this large methodologically robust study, support this association. Although less studied, the finding that greater anxiety is associated with worse survival in people with cancer has also been reported [3, 12]. Our finding that, after adjustment for depression, this association effectively disappears in males (so that anxiety is no longer associated with survival) and actually reverses direction in females (so that greater anxiety is associated with better survival) is novel. We are not aware of any previous study of the associations of depression and anxiety with survival in patients with cancer that has examined these independent associations. There have however been a small number of relevant studies in other populations. In people with cardiac disease, a systematic review reported an association between greater anxiety and worse survival, but also that this association was weakened when severity of depression was adjusted for, suggesting that depression was the more important factor [13]. A large study of patients with suspected cardiac disease undergoing exercise testing found, as we did in patients with cancer, that after adjustment for depression, anxiety was associated with better, rather than worse survival [14]. Studies of the general population have also found that anxiety predicts better rather than worse life expectancy [15], and that when anxiety complicates depression the association between depression and worse survival is reduced [16]. These similar findings in non-cancer populations increase our confidence that our novel findings in patients with cancer are meaningful.
Interpretation
Our results suggest that, whatever the mechanism of the association between depression and worse survival in people with cancer, it is specific to depression [17]. Potential mechanisms for this association have been proposed, but none proven [18]. It is of interest that the relationship between depression and survival was not linear, typically being steeper at the lower end of the range than at the higher end. The explanation for this observation is unclear. However, we note that mild depression has been associated with worse survival in patients with heart disease [19], and small changes in that mild depression over time have been associated with improved survival [20]. Our findings in patients with cancer and these in patients with heart disease suggest that we should not focus solely on the association between severe depression and survival in patients with medical illnesses, but also consider mild and moderate depression.
Anxiety appears to have a different relationship with survival than depression, with no association in males and an association with better, not worse, survival in females. This is most clearly seen in the female-specific cancers (breast and gynaecological). There are a number of potential mechanisms for the association of anxiety with better survival, but perhaps the most plausible is that anxiety leads to healthier behaviours, more medical care seeking and better adherence to medical treatments [21]. It is of interest that this is only clearly observed in female specific cancers and may reflect the importance of patient adherence to treatment recommendations in these cancers.
Strengths and limitations
The strengths of our study were: (a) the use of data from a large representative sample of patients with common cancers attending UK NHS cancer centres serving a geographically defined area; (b) the availability of continuous measures of depression and anxiety using a well-validated scale; (c) a cancer diagnosis and severity assessment done by oncologists; (d) an almost complete follow-up of the cohort using individually linked national registry data, including data on cause of death and (e) robust analysis of these unique data accounting for missing data and controlling for possible confounders, including not only age and sex, but also social deprivation (determined by the patient’s address) and initial cancer severity (determined by recorded treatment objective).
Despite these strengths, our study also had limitations including: (a) findings that may not necessarily generalise to other populations (such as patients in different healthcare settings or those who were diagnosed with cancer many years ago and who no longer attend clinics); (b) the assessment of depression and anxiety using self-rating scales which unlike a diagnostic interview do not provide diagnoses, but rather a continuous measure of symptom severity; (c) some missing data on the HADS score and initial cancer treatment objective (which we addressed using multiple imputation in the analysis but we cannot rule out the possibility that these data were not missing at random); (d) the completion of the HADS at varying intervals after initial cancer diagnosis (although we did take account of this in our analysis); (e) a lack of information on the time-course of depression and anxiety either prior to or subsequent to the HADS completion; (f) follow-up data on patients for a mean of approximately two years from the time of HADS completion but not on all patients to the time of their death; (g) an inability to fully adjust for all potential confounders - in particular we had to rely on initial treatment objective as a measure of cancer severity as it was not possible to combine the different staging systems used for different cancer types in our analysis; (h) an inability to control for medical comorbidities, although it is unlikely that these were important in determining survival, as almost all the patient deaths were attributed to cancer.
Conclusions
Depression and anxiety have both been associated with the worse subsequent survival of people with common cancers. The findings presented here confirm that depression is associated with survival but also indicate that, when depression is adjusted for, anxiety is not. In fact anxiety may even predict better survival in females. The implication of these findings is that whatever the mechanism of the association of depression with worse survival, it is specific to depression. Depression and anxiety should not therefore be lumped together as ‘emotional distress’ but should be considered separately in future studies of the predictors of survival in people with cancer and indeed other illnesses.
Supplementary Material
Acknowledgements
This analysis was funded by the UK National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford NHS Foundation Trust. MS is an NIHR Senior Investigator. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The original data collection was funded by the charity Cancer Research UK (grant no. C5547/A7375). The funders had no involvement in study design; collection, analysis and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Footnotes
Declaration of interests:
None.
References
- [1].Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- [2].Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, Shi J, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0595-x. [DOI] [PubMed] [Google Scholar]
- [4].Kircanski K, LeMoult J, Ordaz S, Gotlib IH. Investigating the nature of co-occurring depression and anxiety: Comparing diagnostic and dimensional research approaches. Journal of Affective Disorders. 2017;216:123–135. doi: 10.1016/j.jad.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- [6].Harrell JFE. Regression Modeling Strategies: With Applications to Linear Models. Logistic and Ordinal Regression, and Survival Analysis. 2015 [Google Scholar]
- [7].Bartlett J. smcfcs: Multiple Imputation of Covariates by Substantive Model Compatible Fully Conditional Specification. doi: 10.1177/0962280214521348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rubin D. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- [9].R Core Team. Foundation for Statistical Computing Vienna. Austria: 2017. R: A language and environment for statistical computing. [Google Scholar]
- [10].StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; College Station, TX: 2017. [Google Scholar]
- [11].Miloyan B, Fried E. A reassessment of the relationship between depression and allcause mortality in 3,604,005 participants from 293 studies. World Psychiatry. 2017;16(2):219–220. doi: 10.1002/wps.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shim EJ, Lee JW, Cho J, Jung HK, Kim NH, Lee JE, Min J, Noh WC, Park SH, Kim YS. Association of depression and anxiety disorder with the risk of mortality in breast cancer: A National Health Insurance Service study in Korea. Breast Cancer Res Treat. 2019 doi: 10.1007/s10549-019-05479-3. [DOI] [PubMed] [Google Scholar]
- [13].Celano CM, Millstein RA, Bedoya CA, Healy BC, Roest AM, Huffman JC. Association between anxiety and mortality in patients with coronary artery disease: A meta-analysis. American Heart Journal. 2015;170(6):1105–1115. doi: 10.1016/j.ahj.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Herrmann C, Brand-Driehorst S, Buss U, Rüger U. Effects of anxiety and depression on 5-year mortality in 5057 patients referred for exercise testing. Journal of Psychosomatic Research. 2000;48(4):455–462. doi: 10.1016/s0022-3999(99)00086-0. [DOI] [PubMed] [Google Scholar]
- [15].Colman I, Kingsbury M, Sucha E, Horton NJ, Murphy JM, Gilman SE. Depressive and anxious symptoms and 20-year mortality: Evidence from the Stirling County study. Depression and anxiety. 2018;35(7):638–647. doi: 10.1002/da.22750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mykletun A, Bjerkeset O, øverland S, Prince M, Dewey M, Stewart R. Levels of anxiety and depression as predictors of mortality: the HUNT study. British Journal of Psychiatry. 2009;195(2):118–125. doi: 10.1192/bjp.bp.108.054866. [DOI] [PubMed] [Google Scholar]
- [17].Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54(3):269–82. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- [18].Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Köhler CA, Fernandes BS, Stubbs B, Pavlidis N, et al. Depression in cancer: The manybiobehavioral pathways driving tumor progression. Cancer Treatment Reviews. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- [19].Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, Fauerbach JA. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. The American Journal of Cardiology. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- [20].Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-Year Risk of Cardiac Mortality in Relation to Initial Severity and One-Year Changes in Depression Symptoms After Myocardial Infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- [21].Ossola P, Gerra ML, DePanfilis C, Tonna M, Marchesi C. Anxiety, depression, and cardiac outcomes after a first diagnosis of acute coronary syndrome. Health Psychology. 2018;37(12):1115–1122. doi: 10.1037/hea0000658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.