Abstract
Objectives
We aimed to determine the associations of non-alcoholic fatty liver disease (NAFLD) with cardio-metabolic risk factors for diabetes in adult Kenyans.
Methods
A cross-sectional study was undertaken among rural and urban Kenyans of different ethnic origin. Ultrasonography scanning (USS) methods were used for the assessment of hepatic fat accumulation for NAFLD assessment and abdominal fat distribution, and simple anthropometry measurements were performed. All participants underwent a 2-h oral glucose tolerance test, and biochemical, haemodynamic and lifestyle data were obtained. Multivariate logistic regression analyses were used to assess sex, age, residency and ethnic differences in the association between NAFLD and various metabolic parameters.
Results
In total, 743 individuals (59.1% women) with a mean age of 38.0 (range 18-68) years participated in the study. Overall, 118 individuals (15.9%) had NAFLD, of whom 94.1% had mild steatosis. Age >40 years was significantly associated with having NAFLD compared to <30 years of no difference found in NAFLD between ethnic groups (Luo, Kamba, Maasai). All body composition and clinical measurements were associated with NAFLD (p<0.045 for OR).
Conclusions
Finding lower odds for NAFLD in men was unexpected, as was the lack of differences in NAFLD among the ethnic groups, while higher odds for NAFLD with increasing age and in urban vs. rural populations was expected. Especially the sex-specific results warrant further studies in black African populations on biology of body composition for having NAFLD, and whether this translates into insulin resistance and higher risk of diabetes and consequently cardiovascular disease in black African women.
Keywords: Non-alcoholic fatty liver disease, sub-Saharan Africa, cardio-metabolic risk, fatty liver index
Sustainable Development Goals: Good Health and Wellbeing, Reduced inequalities
Introduction
Non-communicable diseases (NCDs) are becoming a significant burden in sub-Saharan Africa (SSA) (1). Two decades ago, the “Global Burden of Disease Study” showed that 20% of deaths in SSA were caused by NCDs (2). More recently, in rural and urban populations in Kenya, we showed that obesity (3), diabetes (4), as well as hypertension and dyslipidemia (5) are moderately prevalent in adults with a strong positive rural-urban and age gradient of obesity and diabetes prevalence independently of sex and ethnicity. However, central obesity as measured by visceral adipose tissue (VAT) was higher in men than women, and in Maasai compared to Luo and Kamba (3). The diets of these ethnic groups are characterised by mainly milk and cereals, fish and cereals, and cereals, respectively, as staples in traditional, rural societies (6). Furthermore, we have also shown that proprotein convertase substilisin/kexin type 9 – a key modulator of the degradation of the low-density lipoprotein cholesterol receptor – is associated with hepatic steatosis in adult Kenyans, and to some degree mediated by hepatic insulin resistance (7). Thus, it is likely that hepatic steatosis will become more prevalent in SSA and contribute to the development of cardio-metabolic disease, especially type 2 diabetes in Kenyan as well as SSA populations in the light of increasing urbanization.
Non-alcoholic fatty liver disease (NAFLD) covers a broad range of histologic manifestations ranging from simple deposition of adipose tissue to more progressive steatosis with associated hepatitis, fibrosis, cirrhosis and in some cases hepatocellular carcinoma (8). The term NAFLD is comprised of non-alcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) (9). NAFL is characterized by steatosis of the liver that involves more than 5% of the parenchyma with no evidence of hepatocyte injury (10). NASH is defined by a necro-inflammatory process where the liver cells become injured due to steatosis (10). Even though simple steatosis follows a more benign course, NASH has the potential to progress into cirrhosis and thereby increase the risk of developing hepatocellular carcinoma (11;12). To capture the hepatic steatosis a liver biopsy is the gold standard (8). A liver biopsy is an invasive technique, which may be associated with clinical complications. Ultrasonography is a cheaper, non-invasive and more practical solution and is validated and approved with regards to assessment of NAFLD (13).
In a recent meta-analysis of studies either using imaging or liver biopsy, the regional prevalence of NAFLD was estimated to be 31.8% in the Middle-East, 30.5% in South America, 24.1% in North America, 23.7% in Europe, 23.4% in Asia and 13.5% in Africa (14). It is noteworthy that the low prevalence in Africa is based on two studies with small sample sizes (15;16) hardly representative of SSA populations. Globally, the estimated prevalence of NAFLD is 25% and 23% of the affected individuals also have type 2 diabetes, hyperlipidemia (69%), hypertension (39%), or metabolic syndrome (43%) (14).
A prospective study of 1,051 middle-aged participants from the Framingham Heart Study found a bidirectional relationship between NAFLD and cardiovascular disease risk factors even if the underlying mechanisms of NAFLD and cardiovascular risk factors are not fully understood (17).
We aimed to identify anthropometric and cardio-metabolic correlates of NAFLD by sex, age, ethnicity and urban vs. rural residency in adult Kenyans. Based on current evidence, we hypothesised that NAFLD is more prevalent in urban populations, with increasing age, in men vs. women and in Maasai vs. Luo and Kamba ethnic populations.
Methods
Study area and population
A community-based cross-sectional study was conducted in 2005-2006 in rural and urban Kenya. Inclusion criteria for the study were ≥18 years of age and Luo, Kamba, or Maasai ethnicity or culturally related ethnic groups. Exclusion criteria were pregnancy, serious illnesses such as malaria, inability to walk unassisted and severe mental disease (3). Liver scans were performed in 799 individuals. All participants gave written or thumb print informed consent following information given at local community meetings. Ethical permission was obtained from the National Ethical Review Committee in Kenya (SSC Protocol No. 936), and consultative approval was given by the Danish National Committee on Biomedical Research Ethics in Denmark. For more details concerning the selection procedure and the study participants, see Christensen et al. (3).
Liver scan, scoring methods and fatty liver index
Ultrasonography scan (USS) was used for the assessment of hepatic tissue with regards to steatosis using a portable scanner (Aquila Basic Unit, Pie Medical Equipment; Esaote, Masstricht, the Netherlands) with a 3C.RS 3.5/5.0 MHz curved transducer (Probe Article no. 410638 Curved Array HiD probe R40; Pie Medical Equipment, Maastricht, the Netherlands). The abdominal ultrasound investigation was performed with the participant holding his/her breath. Multiple images were acquired on both sides of the main liver lobes by two trained operators. A sub-costal approach was used, and four sweeps were performed using standard imaging protocol (13).
A semi-quantitative grading system was used to define normal, mild, moderate and severe steatosis. The liver scoring criteria were (13):
-
1:
Increased echogenicity of liver parenchyma (bright liver in comparison with the kidney)
-
2:
Decreased visualization of intra-hepatic vasculature
-
3:
Attenuation of the ultrasound beam. The different scoring criteria were summed to derive a liver steatosis score. A score of ≤4 was classified as normal, score 5-7 for mild steatosis, score 8-10 for moderate steatosis and score ≥11 for severe steatosis. The usage of ultrasonography versus magnetic resonance spectroscopy was validated and approved with regards to assessment of NAFLD (13).
Fatty liver index (FLI) was calculated as previously described by Bedogni et al., and categorized as grade I (FLI<30), grade II (FLI≥30-59), and grade III (FLI≥60) according to the formula: (e ^ 0.953*loge (triglycerides) + 0.139*bmi + 0.718*loge (ggt) + 0.053*waist circumference - 15.745) / (1 + e ^ 0.953*ln(triglycerides) + 0.139*bmi + 0.718*loge (ggt) + 0.053*waist circumference - 15.745)*100 (18). We used the ultrasound-based scores to define NAFLD, while FLI was used as supplementary information.
Anthropometry and ultrasonography for abdominal fat distribution
Anthropometric measurements were taken with the participants standing barefoot and wearing light clothing. Body mass index (BMI, kg/m2) was calculated and waist circumference (WC) was measured with a body tape (WM02 Body Tape; Chasmors, Haechstmass, Germany) midway between the iliac crest and the costal margin following a quiet expiration. For further details, see Christensen et al. (3).
Abdominal fat distribution, i.e. VAT and subcutaneous adipose tissue (SAT), was measured in cm using USS (Aquila Basic Unit, Esaote, Pie Medical Equipment, Maastricht, the Netherlands) with a 3.5/5.0MHz transducer (Probe Article no. 410638 Curved Array HiD probe R40 Pie Medical Equipment). We followed the protocol by Stolk et al. which has been validated in relation to computed tomography and magnetic resonance imaging (19).
Laboratory tests
Following an overnight fast (≥8-h), all participants had a blood test taken and subsequently underwent a standard 75-g oral glucose tolerance test (OGTT). Venous blood glucose was determined by the glucose dehydrogenase method using haemolysation and deproteinisation on a HemoCue B-Glucose 201+ device (HemoCue AB, Ängelholm, Sweden) with 5 mL of blood. Standard procedures were followed to centrifuge blood, which was stored as plasma and serum in cryotubes at -20°C at the nearest health facility while in the field, and later at -80°C at KEMRI, Nairobi, Kenya, before being shipped to Steno Diabetes Center Copenhagen in Denmark for insulin and standard lipid profile analyses; for further details, see (5). Later, plasma samples were shipped to Canada for analysis of a standard liver enzyme profile at Montreal Clinical Research Institute; for further details, see (7). Insulin resistance was calculated by the homoeostasis model assessment of insulin resistance by computer model (HOMA-IR) (20). Based on Matsuda et al., HOMA-IR was calculated according to the formula: (fasting insulin (pmol/l) x fasting glucose (mmol/l)/135 (21). Insulin resistance was defined as follows: 1) BMI >28.9 kg/m2, or 2) HOMA-IR >4.65, or 3) HOMA-IR >3.60 if BMI >27.5 kg/m2 based on Stern et al. (22). Dyslipidemia was regarded as high triglyceride (>1.7 mmol/L) and/or low high-density lipoprotein cholesterol (HDL-C) levels (<1.03 for men, and <1.29 for women) (23).
Other clinical variables
Blood pressure was measured with each participant sitting upright and determined as the average of two measurements using a full-automatic device (Omron M6, HEM-7001-E, Kyoto, Japan). Hypertension was defined as ≥140 and/or 90 mmHg of systolic and diastolic blood pressure, respectively (24). Blood haemoglobin was determined on site using a standard Coulter counter technique (model KX-21N, Sysmex Corporation, Kobe, Japan). Information concerning alcohol consumption and tobacco use was self-reported by the participants via an interactive Medical Health Assessment Form. Excessive alcohol consumption threshold was 20 g/d and 30 g/d for women and men, respectively (25).
Statistical analysis
Continuous variables were tested for normality using histogram and normal quantile plot. Skewed data were log-transformed prior to analysis. Differences between non-NAFLD and NAFLD groups were determined by using Student’s t-test, while the chi2 test was used to test for differences in proportions. Regression diagnostics showed that the associations between the outcome and exposure variables were linear, the residuals were normally distributed, and the requirement for homoscedasticity was fulfilled. Logistic regression analyses adjusted for age and sex were used to assess the association between the binary outcome for not having or having hepatic steatosis in relation to age categories, sex, residence, ethnicity, waist circumference (per cm increase), VAT (per cm increase), SAT (per cm increase), HOMA-IR (per unit increase), glucose metabolism categories, HDL-C (per mmol/L increase), dyslipidemia, and hypertension expressed as odds ratio (OR) (95% CI). Parameters including sex, NAFLD, HOMA-IR, DM, IGT and IFG, dyslipidemia, and hypertension were categorized by FLI categories. In order to establish to what extent the differences in proportions of IFG, IGT and DM were driven by NAFLD, analyses of associations between IFG, IGT and DM without having and with NAFLD were carried out. Data that showed normal distribution are presented as means (SD) for continuous variables, and n (%) for categorical variables. Data with skewed distribution are presented as geometric means (SD). Statistical analyses were performed using Stata/MP 14.0 (Stata Corp, College Station, USA).
Results
The study population included 799 Kenyans. Of these, potential participants were excluded due to not reaching the 18-years-old age-limit (n=2), missing values for age, height, weight, sex, BMI and steatosis score (n=28), or if they exceeded the criteria for AFLD (25), i.e. excessive alcohol consumption (n=26). Thus, 743 participants with a mean age (SD) of 38.0 (11.0) (range 18-68) years participated in the study whereof 439 (59.1%) were women. In total, 587 (79%) were rural residents, and ethnic distribution was 68 (9.2%) Luo, 353 (47.5%) Kamba, and 307 (41.3%) Maasai, respectively while 15 (2%) belonged to other ethnic groups. Eight (1.1 %) participants were diagnosed with DM during the study, while 16 (2.2 %) had known DM, of whom 11 took medication. Background characteristics of participants without and with NAFLD are presented in Table 1. Based on ultrasound scanning, 118 (15.9%) had NAFLD, with 111 (94.1%) and 7 (5.9%) having mild and moderate steatosis, respectively. None had severe steatosis. The proportion of NAFLD was 13.3% (n=78) and 25.6% (n=40) of the rural and urban participants, respectively (p<0.001), respectively. Among those with NAFLD, 21 (17.8 %) and 66 (55.9 %) were women <50 and ≥50 years of age, and 31 (26.3%) were men, respectively. Men were less likely to have NAFLD than women (OR=0.48 (95% CI: 0.31, 0.75), p=0.001). When further adjusted for WC or VAT, the results for sex difference remained statistically significant and more pronounced (0.39, 95% CI: 0.23, 0.65 and 0.29, 95% CI: 0.16;0.53, respectively, both p<0.001). After further adjustment for BMI or SAT, there was no longer a difference in OR between women and men for having NAFLD (OR=0.78 95% CI: 0.47, 1.30, p=0.34)) and (OR=1.35 95% CI: 0.79, 2.30, p=0.28), respectively. For individuals having NAFLD, mean BMI in women <50 and ≥50 years was 29.7 (5.8) and 26.9 (5.5) kg/m2, respectively, and for men it was 27.0 (6.4) kg/m2. Mean SAT was 3.56 (1.00), 2.84 (1.50), and 2.40 (1.19) cm, for women <50 years, women ≥50 years, and men, respectively. Among those with NAFLD, WC and VAT was higher in men, while BMI and SAT was higher in women (results not shown). All body composition, biochemical and haemodynamics data were positively associated with NAFLD (p<0.045) (see Table 2).
Table 1. General characteristics of participants without and with non-alcoholic fatty liver disease (n=743) presented as mean (SD) and proportion (%).
| Non-NAFLD | NAFLD | ||
|---|---|---|---|
| Variable | n=625 | n=118 | p-value |
| Age (years) | 36.7 (10.8) | 41.6 (11.4) | 0.001 |
| Sex (F: M %) | 352:273 (56:44) | 87:31 (74:26) | 0.001 |
| Urban residence, n(%) | 116 (19) | 40 (34) | 0.001 |
| BMI (kg/m2) | 21.3 (3.8) | 27.4 (5.8) | 0.001 |
| Waist circumference (cm) | 76.8 (9.3) | 91.5 (14.2) | 0.001 |
| VAT (cm) | 6.3 (1.31) | 7.7 (2.0) | 0.001 |
| SAT* (cm) | 1.4 (1.0) | 2.9 (1.4) | 0.001 |
| Fasting plasma glucose | |||
| (mmol/L)* | 4.5 (1.4) | 4.9 (1.8) | 0.012 |
| Fasting serum insulin | |||
| (pmol/L) | 27.6 (20.5) | 48.6 (41.5) | 0.001 |
| HOMA-IR | 0.82 (0.76) | 1.65 (2.7) | 0.001 |
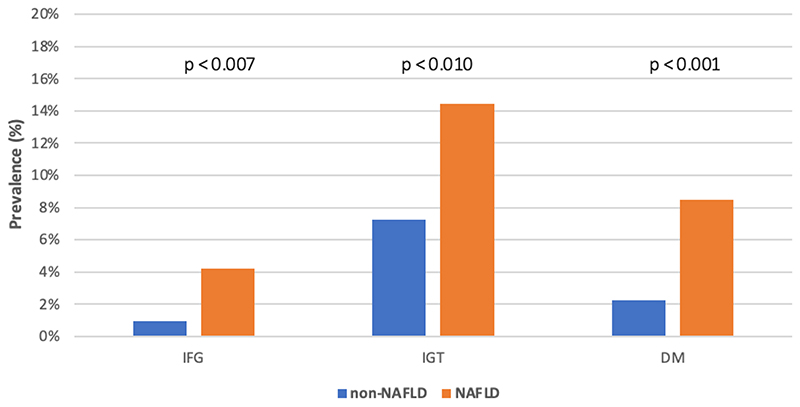
| DM, n (%) | 14 (2.2) | 10 (8.5) | 0.001 |
| IGT, n (%) | 45 (7.2) | 17 (14.4) | 0.010 |
| IFG, n (%) | 6 (1.0) | 5 (4.2) | 0.007 |
| Total cholesterol (mmol/L) | 3.9 (0.9) | 4.4 (1.1) | 0.001 |
| LDL-C (mmol/L) | 2.2 (0.9) | 2.7 (1.0) | 0.001 |
| HDL-C (mmol/L) | 1.15 (0.29) | 1.08 (0.28) | 0.015 |
| Triglycerides (mmol/L) | 0.9 (0.5) | 1.2 (0.65) | 0.001 |
| Dyslipidemia, n (%) | 167 (26.7) | 45 (38.1) | 0.017 |
| ALP (U/L) | 80.4 (31.6) | 84.1 (26.5) | 0.264 |
| ALT (U/L) | 8.7 (5.1) | 8.6 (4.4) | 0.750 |
| AST (U/L) | 21.4 (10.2) | 19.6 (7.79) | 0.107 |
| Total bilirubin (μmol/L) | 4.7 (3.8) | 6.5 (5.93) | 0.003 |
| GGT (U/L) | 22.5 (19.6) | 27.4 (31.8) | 0.040 |
| Systolic BP (mmHg) | 119 (16) | 127 (17) | 0.001 |
| Diastolic BP (mmHg) | 74 (10) | 78 (11) | 0.001 |
| Hypertension, n (%) | 60 (9.6) | 30 (25.4) | 0.001 |
| Haemoglobin (g/dL) | 13.7 (2.1) | 13.4 (2.2) | 0.145 |
| Tobacco use, n (%) ** | 76 (12.2) | 2 (1.7) | 0.001 |
| Alcohol, n (%) *** | 46 (7.4) | 7(5.9) | 0.543 |
Presented as geometric mean (SD)
For most Maasai smoking is sniff and chewing tobacco
Participants with an alcohol-consumption that does not exceed the criteria for NAFLD
Data are presented as means (SD) for continuous variables and n (%) for categorical variables.
BMI: body mass index; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; HOMA-IR: homeostasis model assessment was calculated as (fasting plasma insulin X fasting plasma glucose)/22.5; LDLC: low-density lipoprotein; HDL-C: high-density lipoprotein; Systolic BP: systolic blood pressure; Diastolic BP: diastolic blood pressure; DM: diabetes mellitus; IGT: impaired glucose tolerance; IFG: impaired fasting glycaemia.
Waist circumference: n=741; VAT: n=741; SAT: n=741; Fasting serum insulin: n=722; HOMA-IR: n=722; Total cholesterol: n=711; LDL-C: n=734; HDL-C: n=711; Triglycerides: n=711; Systolic BP: n=731; Haemoglobin: n=699; Smoking: n=706; Alcohol: n=699.
Table 2. Odds ratio for univariate and multivariate logistic regression for having nonalcoholic fatty liver disease.
| Variable | Univariate analysis Odds ratio (95%CI) |
p-value | Multivariate analysis Odds ratio (95%CI) |
p-value |
|---|---|---|---|---|
| Age (years)1 | ||||
| Age ≤ 30 | 1.00 | 1.00 | ||
| 30 < Age ≤ 40 | 1.31 (0.72, 2.40) | 0.383 | 1.22 (0.66, 2.22) | 0.527 |
| Age > 40 | 2.67 (1.58, 4.50) | 0.001 | 2.48 (1.46, 4.21) | 0.001 |
| Men2 | 0.47 (0.29, 0.71) | 0.001 | 0.48 (0.31, 0.75) | 0.001 |
| Urban residence | 2.25 (1.12, 3.46) | 0.001 | 2.85 (1.81, 4.50) | <0.001 |
| Ethnicity | ||||
| Luo | 1 | 1 | ||
| Kamba | 0.68 (0.45, 1.03) | 0.069 | 0.82 (0.53, 1.27) | 0.317 |
| Maasai | 0.70 (0.15, 3.16) | 0.641 | 1.13 (0.24, 5.38) | 0.872 |
| Body mass index (per kg/m2increase) | 1.28 (1.22, 1.34) | <0.001 | 1.27 (1.21, 1.33) | <0.001 |
| Waist circumference (pr cm increase) | 1.11 (1.09, 1.13) | <0.001 | 1.11 (1.09, 1.14) | <0.001 |
| VAT (pr cm increase) | 1.70 (1.46, 1.98) | <0.001 | 1.81 (1.53, 2.14) | <0.001 |
| SAT (pr cm increase) | 2.57 (2.15, 3.10) | <0.001 | 2.65 (2.16, 3.27) | <0.001 |
| HOMA-IR (per unit increase) | 1.83 (1.50, 2.26) | <0.001 | 1.77 (1.43, 2.19) | <0.001 |
| Glucose metabolism | ||||
| NGT | 1 | 1 | ||
| Prediabetes (IFG/IGT) | 2.64 (1.53, 4.55) | <0.001 | 2.43 (1.39, 4.24) | 0.002 |
| Diabetes mellitus | 4.63 (1.99, 10.7) | <0.001 | 3.82 (1.59, 9.17) | 0.003 |
| Total cholesterol (per mmol/L increase) | 1.64 (1.34, 2.00) | <0.001 | 1.57 (1.26, 1.95) | <0.001 |
| HDL-C (per mmol/L increase) | 0.42 (0.21, 0.85) | 0.016 | 0.41 (0.20, 0.83) | 0.014 |
| LDL-C (per mmol/L increase) | 1.18 (144, 2.29) | <0.001 | 1.73 (1.36, 2.03) | <0.001 |
| Triglycerides (per mmol/L increase) | 2.26 (1.61, 3.14) | <0.001 | 2.17 (1.51, 3.13) | <0.001 |
| Dyslipidemia | 1.65 (1.01, 2.34) | 0.018 | 1.51 (0.99, 2.31) | 0.057 |
| Hypertension | 3.22 (1.97, 5.28) | <0.001 | 3.34 (1.96, 5.70) | <0.001 |
All multivariable analyses adjusted for age and sex except for
only adjusted for sex and
only adjusted for age.
VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; HOMA-IR: homeostasis model assessment was calculated as (fasting plasma insulin x fasting plasma glucose)/135; HDL-C: high-density lipoprotein; LDLC: low-density lipoprotein; NGT: Normoglycaemia; IGT: impaired glucose tolerance; IFG: impaired fasting glycaemia.
Excludes 15 participants of other ethnicities than Luo, Kamba, Maasai
Except for sex distribution and the distribution of IFG between the three FLI grade groups, all cardio-metabolic diseases showed significant associations across FLI grade groups going from grade I to grade III (p<0.001) (see Supplemental Table).
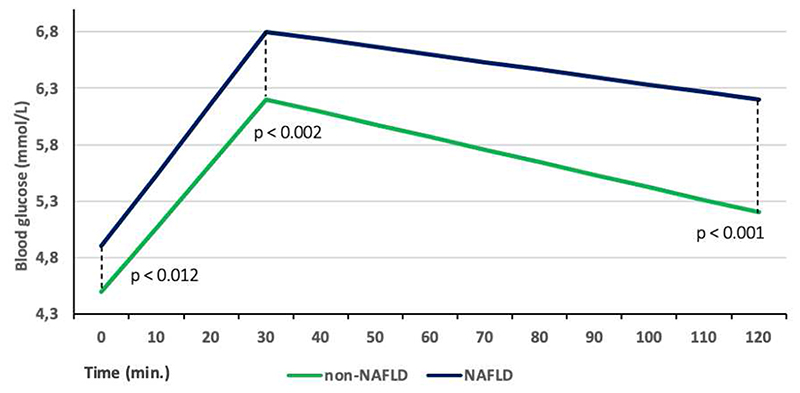
The proportion of IFG, IGT and DM in the participants with NAFLD was 4.2, 2.0 and 3.8 times higher, respectively compared to those without NAFLD (Figure 1). Blood glucose levels at 0, 30 and 120 min. were significantly higher in the participants with NAFLD (p<0.002) (Figure 2).
Figure 1.
Prevalence of impaired fasting glucose (IFG*, 6.1 to 6.9 mmol/L** and <7.8 mmol/L***), impaired glucose tolerance (IGT*, <7.0 mmol/L** and ≥7.8 and <11.1 mmol/L***) and diabetes mellitus (DM*, ≥7.0 mmol/L** or ≥11.1 mmol/L***) among participants with non-NAFLD and NAFLD. *Plasma values; **fasting glucose test; ***2-h glucose tolerance test.
Figure 2.
Whole blood glucose profile in participants with NAFLD and non-NAFLD during 2-h oral glucose tolerance test. Presented as geometric means.
Discussion
In Kenyan adults, we found lower OR (0.48) for having NAFLD in men than women with a female-to-male ratio of 2.8 to 1. Furthermore, being age >40 years showed an OR of ~2.5 for NAFLD versus being <30 years of age, and the urban population had a ~3-fold higher risk of NAFLD compared to the rural population. Cardio-metabolic risk parameters were all significantly associated with NAFLD (OR >1.5), with hypertension and DM having the highest OR of 3.4 and 3.8, respectively.
The lower OR for having NAFLD in men than women that we found in the current study is in contrast to previous findings (26;27). Furthermore, this result is despite men in the current cohort having higher abdominal fat accumulation measured as WC and VAT, i.e. metabolically active fat tissue contributing to venous drainage of fat tissue going directly to the liver via the portal vein resulting in ectopic fat accumulation and insulin resistance (28). When adjusting for WC and VAT accumulation, the OR estimates became even more pronounced. This may be explained by women being more prone to NAFLD for a given amount of intra-abdominal fat accumulation, even if at this stage this suggestion remains pure speculation. Interestingly, when adjusting for BMI and SAT accumulation, both of which were higher in women, the sex-specific difference for having NAFLD disappeared. In a systematic review, Pang et al. showed that WC and VAT had a stronger association with NAFLD than BMI, even though all three obesity measures were independently associated with increased risk of NAFLD (29). Furthermore, Fan et al. (30) found a dose-response relationship between BMI and liver steatosis risk in an approximate J-shaped fashion. In the current cohort, but including a higher number of participants (n=1459), we previously showed that in women, BMI had the highest OR for glucose intolerance risk with increasing obesity as compared to WC, VAT, and SAT, while for men VAT showed the highest OR for glucose intolerance (4). Thus, general obesity appears to be more detrimental to metabolic disorders in Kenyan women compared to men.
Nevertheless, SAT also explained the difference in sex-specific OR for having NAFLD. In the NAFLD individuals, women regardless of age group had higher SAT than men; furthermore, women <50 years (presumably premenopausal), had higher SAT accumulation than women ≥50 years (presumably postmenopausal). This difference could be explained by the high-energy demands of lactation in young women as SAT acts as an energy reservoir for women within reproductive age (31).
SAT in general is a depot for surplus triglycerides (32), and is regarded as protective in relation to cardio-metabolic disorders (33). However, the USS technique does not distinguish between superficial and deep SAT divided by the Scarpa’s fascia (34). Deep SAT adipocytes have been shown to have higher lipolytic activity compared to superficial SAT adipocytes (35), and contain more saturated fat than superficial SAT (36). Thus, deep SAT to some extent resembles VAT, and may therefore be responsible for ectopic fat accumulation, including hepatic steatosis. It is important to note that more than half of the individuals with NAFLD in the current study were postmenopausal women when using age ≥50 years as a proxy measure. Prevalence and incidence of NAFLD are higher in men and postmenopausal women compared to premenopausal women (37;38). For the postmenopausal women, this is due to the loss of oestrogens, which among others regulate liver lipogenesis (39). Nevertheless, Naran et al. have shown that SAT is a significant negative determinant of hepatic steatosis as measured by computed tomography in a small sample (n=29) of black, South African women (40).
Cardio-metabolic disorders were significantly higher in the current study population with NAFLD whether measured as absolute numbers, by OR for each unit increase, or by OR for standard cut-offs for disease. Increased HOMA-IR is regarded as the metabolic manifestation of hepatic steatosis, and as this feature progresses may result in DM (41). We found significantly higher proportions of DM as well as pre-diabetes (IFG and IGT) in those with NAFLD. The results are based on a fasting blood test followed by an OGTT, and this indicates not only hepatic IR, but also peripheral IR as demonstrated by higher 30 min and 2-h whole blood glucose levels in NAFLD individuals. Furthermore, DM showed the highest OR for having NAFLD at 3.80 compared to other cardio-metabolic and anthropometric correlates of hepatic steatosis. This is in line with previous studies showing that excess hepatic fat deposition is uniformly present before the onset of “classical” type 2 diabetes (42;43), assuming that the DM subtype in the present study was predominantly if not entirely type 2 diabetes.
We found no difference in OR for having hepatic steatosis between the three ethnic groups studied, i.e. the Luo, Kamba and Maasai. Based on results from the same but larger groups of participants, the Maasai had higher VAT accumulation (n=1430), and higher hepatic insulin resistance based on HOMA-IR (n=1087), respectively, compared to the other ethnic groups (3;44). Therefore, a higher proportion of hepatic steatosis in the Maasai was expected. Thus, this adds to the evidence of a lack of linear relationship between VAT accumulation, insulin resistance and hepatic steatosis, but points to a more complex interaction between these interrelated parameters.
FLI is an algorithm used as a prediction for liver steatosis (16), which is often employed in clinical practice as the parameters (triglycerides, BMI, GGT, and WC) included in the algorithm are routine measurements, albeit not in public clinics or hospitals in Kenya or SSA in general. According to Bedogni et al., FLI ≥60 is an indicator of hepatic steatosis when using USS as reference (18). We found a significant association for having NAFLD across the three grades of FLI. Nevertheless, FLI may underestimate hepatic steatosis in black Africans due to low triglyceride levels in black African populations in general, which we also found in the current study population (5). Importantly, HOMA-IR had an overlap of 83.8% when comparing FLI ≥60 with USS-derived diagnosis. Therefore, FLI seems to be relevant for cardio-metabolic disease risk in black African populations, and this algorithm may be easier to implement in clinical practice compared to analysing USS-captured images which is relatively time consuming.
The prevalence of NAFLD in the current study was 15.9%, which is slightly higher than the prevalence reported by Younossi et al. based on a meta-analysis (14) based on two very small studies in SSA. It is of note, that we only found mild and moderate steatosis, and that USS technique is limited by lower sensitivity for detecting milder degrees (<30%) of steatosis as compared to magnetic resonance imaging and magnetic resonance spectroscopy (45). Thus, we may have underestimated the prevalence of NAFLD.
Our results show that NAFLD is not only an important factor for adverse cardio-metabolic traits, but also that hepatic steatosis becomes more prevalent with increasing age and urbanisation. The latter two factors are important to consider in Kenya and other SSA nations where life expectancy and urbanisation have been increasing over the past few decades – a trend which is expected to continue (46;47). Thus, NAFLD prevalence is likely to increase, and along with this development cardio-metabolic disease risk is expected to increase as well. Special attention may be needed for women in this context, especially if NAFLD translates into higher incidence of diabetes and cardiovascular diseases in the female part of the population.
Strengths of the current study were the large sample size in an African context, inclusion of rural and urban participants, and a comprehensive panel of USS, simple anthropometric, biochemical and haemodynamic measurements across a population with a wide age range (18 to 68 years).
We acknowledge several limitations to this study. Neither intra- nor inter-observer validity USS were carried out, and USS is not a gold standard technique, all of which could have compromised the quality of the data. However, NAFLD screening using USS at the population level in resource-poor settings may be the most cost-effective approach in a public health context. Furthermore, this is a cross sectional study, and therefore the interpretation of the results from a cause-relationship perspective, must be done with caution. We also want to stress that using logistic regression analysis for association with frequent disease (>10% prevalence) such as hypertension or dyslipidaemia may inflate the estimates. It is of note that 10 subjects in the cohort were treated with hypotensive drugs, and none of them were on lipid-lowering, oral hypoglycemic agents or insulin therapy. Finally, we did not collect data on HIV including anti-retroviral drugs, hepatitis B or C or other biomarkers for infections, which could have affected the results.
In conclusion, NAFLD is more common in Kenyan women than men, which may be explained by general obesity (BMI) and SAT accumulation. Furthermore, increasing age and urbanisation are both associated with NAFLD, while ethnicity comparing Luo, Kamba and Maasai people showed no difference in NAFLD. Especially the sex-specific results warrant further studies in black African populations on biology of body composition for having NAFLD, and whether this translates into insulin resistance and higher risk of diabetes and consequently cardiovascular disease in black African women.
Supplementary Material
Acknowledgements
We are grateful to all study participants, the local chiefs and sub-chiefs, the local elder councils and district politicians. We kindly thank all local assistants for their excellent work mobilizing participants and measurements of anthropometry and body composition. A special thanks to Andreas W. Hansen who performed parts of the abdominal ultrasound scans. We are grateful to funding to EDLR, who is supported by the NIHR Cambridge Biomedical Research Centre (IS-BRC-1215-20014. Likewise, we are grateful to the general contributions to the Kenya Diabetes Study by Professor Knut Borch-Johnsen, Professor Inge Tetens, and Dr. David L. Mwaniki. Funding was received from DANIDA (J. no.104.DAN.8–871, RUF project no. 91202), University of Copenhagen (Cluster of International Health), Steno Diabetes Center Copenhagen, Beckett Foundation, Dagmar Marshall’s Foundation, Dr Thorvald Madsen’s Grant for the Advancement of Medical Sciences, Kong Christian den Tiende’s Foundation, and Brdr. Hartmann Foundation.
References
- (1).Baingana FK, Bos ER. Changing Patterns of Disease and Mortality in Sub-Saharan Africa: An Overview. 2006 [PubMed] [Google Scholar]
- (2).The International Bank for Reconstruction and Development / The World Bank. Global Burden of Disease and Risk Factors. Oxford University Press; New York: 2006. [PubMed] [Google Scholar]
- (3).Christensen DL, Eis J, Hansen AW, Larsson MW, Mwaniki DL, Kilonzo B, et al. Obesity and regional fat distribution in Kenyan populations: impact of ethnicity and urbanization. Ann Hum Biol. 2008 Mar;35(2):232–49. doi: 10.1080/03014460801949870. [DOI] [PubMed] [Google Scholar]
- (4).Christensen DL, Friis H, Mwaniki DL, Kilonzo B, Tetens I, Boit MK, et al. Prevalence of glucose intolerance and associated risk factors in rural and urban populations of different ethnic groups in Kenya. Diabetes Res Clin Pract. 2009 Jun;84(3):303–10. doi: 10.1016/j.diabres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- (5).Christensen DL, Faurholt-Jepsen D, Birkegaard L, Mwaniki DL, Boit MK, Kilonzo B, et al. Cardiovascular risk factors in rural Kenyans are associated with differential age gradients, but not modified by sex or ethnicity. Ann Hum Biol. 2016;43(1):42–9. doi: 10.3109/03014460.2015.1013987. [DOI] [PubMed] [Google Scholar]
- (6).Hansen AW, Christensen DL, Larsson MW, Eis J, Christensen T, Friis H, Mwaniki DL, Kilonzo B, Boit MK, Borch-Johnsen K, Tetens I. Dietary patterns, food and macronutrient intakes among adults in three ethnic groups in rural Kenya. Public Health Nutr. 2011 Sep;14(9):1671–9. doi: 10.1017/S1368980010003782. [DOI] [PubMed] [Google Scholar]
- (7).Paquette M, Gauthier D, Chamberland A, Prat A, De Lucia RE, Rasmussen JJ, et al. Circulating PCSK9 is associated with liver biomarkers and hepatic steatosis. Clin Biochem. 2020 Mar;77:20–5. doi: 10.1016/j.clinbiochem.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017 Jun 8;9(16):715–32. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016 May;20(2):205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- (10).Kanwar P, Kowdley KV. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin Liver Dis. 2016 May;20(2):225–43. doi: 10.1016/j.cld.2015.10.002. [DOI] [PubMed] [Google Scholar]
- (11).Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002 Dec;36(6):1349–54. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- (12).Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995 Dec;22(6):1714–9. [PubMed] [Google Scholar]
- (13).De Lucia RE, Brage S, Sleigh A, Finucane F, Griffin SJ, Wareham NJ, et al. Validity of ultrasonography to assess hepatic steatosis compared to magnetic resonance spectroscopy as a criterion method in older adults. PLoS One. 2018;13(11):e0207923. doi: 10.1371/journal.pone.0207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- (15).Almobarak AO, Barakat S, Khalifa MH, Elhoweris MH, Elhassan TM, Ahmed MH. Non alcoholic fatty liver disease (NAFLD) in a Sudanese population: What is the prevalence and risk factors? Arab J Gastroenterol. 2014 Mar;15(1):12–5. doi: 10.1016/j.ajg.2014.01.008. [DOI] [PubMed] [Google Scholar]
- (16).Onyekwere CA, Ogbera AO, Balogun BO. Non-alcoholic fatty liver disease and the metabolic syndrome in an urban hospital serving an African community. Ann Hepatol. 2011 Apr;10(2):119–24. [PubMed] [Google Scholar]
- (17).Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017 Feb;66(2):390–397. doi: 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006 Nov 2;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Stolk RP, Wink O, Zelissen PM, Meijer R, van Gils AP, Grobbee DE. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord. 2001 Sep;25(9):1346–51. doi: 10.1038/sj.ijo.0801734. [DOI] [PubMed] [Google Scholar]
- (20).Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- (21).Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- (22).Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005 Feb;54(2):333–9. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- (23).Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- (24).Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003 Nov;21(11):1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- (25).EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- (26).Lonardo A, Suzuki A. Nonalcoholic fatty liver disease: does sex matter? Hepatobiliary Surg Nutr. 2019 Apr;8(2):164–6. doi: 10.21037/hbsn.2018.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019 Oct;70(4):1457–69. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Pang Q, Zhang JY, Song SD, Qu K, Xu XS, Liu SS, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015 Feb 7;21(5):1650–62. doi: 10.3748/wjg.v21.i5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci Rep. 2018 Oct 15;8(1):15273. doi: 10.1038/s41598-018-33419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wells JC. The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol Rev Camb Philos Soc. 2006 May;81(2):183–205. doi: 10.1017/S1464793105006974. [DOI] [PubMed] [Google Scholar]
- (32).Al-Sulaiti H, Diboun I, Banu S, Al-Emadi M, Amani P, Harvey TM, et al. Triglyceride profiling in adipose tissues from obese insulin sensitive, insulin resistant and type 2 diabetes mellitus individuals. J Transl Med. 2018 Jun 26;16(1):175. doi: 10.1186/s12967-018-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005 Feb;48(2):301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- (34).Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De CR. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011 Dec;33(10):835–42. doi: 10.1007/s00276-010-0772-8. [DOI] [PubMed] [Google Scholar]
- (35).Monzon JR, Basile R, Heneghan S, Udupi V, Green A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes Res. 2002 Apr;10(4):266–9. doi: 10.1038/oby.2002.36. [DOI] [PubMed] [Google Scholar]
- (36).Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37(3):821–9. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- (37).Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014 May 27;6(5):274–83. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014 Apr;59(4):1406–14. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Palmisano BT, Zhu L, Stafford JM. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol. 2017;1043:227–56. doi: 10.1007/978-3-319-70178-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Naran NH, Haagensen M, Crowther NJ. Steatosis in South African women: How much and why? PLoS One. 2018;13(1):e0191388. doi: 10.1371/journal.pone.0191388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008 Oct;51(10):1781–9. doi: 10.1007/s00125-008-1116-7. [DOI] [PubMed] [Google Scholar]
- (42).Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005 Apr;48(4):634–42. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- (43).Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007 May;30(5):1212–8. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- (44).Christensen DL, Faurholt-Jepsen D, Faerch K, Mwaniki DL, Boit MK, Kilonzo B, et al. Insulin resistance and betacell function in different ethnic groups in Kenya: the role of abdominal fat distribution. Acta Diabetol. 2014 Feb;51(1):53–60. doi: 10.1007/s00592-013-0474-x. [DOI] [PubMed] [Google Scholar]
- (45).Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011 Jan;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Saghir J, Santoro J. Urbanization in sub-Saharan Africa: Meeting Challenges by Bridging Stakeholders. Center for Strategic & International Studies; Washington D.C: 2018. [Google Scholar]
- (47).World Bank. Life Expectancy at Birth, Total (Years) – sub-Saharan Africa. World Bank Group; Washington D.C: 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.