Abstract
A key issue in neurobiological studies of episodic-like memory is the geometric frame of reference in which memory traces of experience are stored. Assumptions are sometimes made that specific protocols favour either allocentric (map-like) or egocentric (body-centered) representations. There are, however, grounds for suspecting substantial ambiguity about coding strategy, including the necessity to use both frames of reference occasionally, but tests of memory representation are not routinely conducted. Using rats trained to find and dig up food in sandwells at a particular place in an event-arena (episodic-like 'action-where' encoding), we show that a protocol previously thought to foster allocentric encoding is ambiguous but more predisposed towards egocentric encoding. Two changes in training protocol were examined with a view to promoting preferential allocentric encoding - one in which multiple start locations were used within a session as well as between sessions; and another that deployed a stable home-base to which the animals had to carry food reward. Only the stable home-base protocol led to excellent choice performance which rigorous analyses revealed to be blocked by occluding extra-arena cues when this was done after encoding but before recall. The implications of these findings for studies of episodic-like memory are that the representational framework of memory at the start of a recall trial will likely include a path-direction in the egocentric case but path-destination in the allocentric protocol. This difference should be observable in single-unit recording or calcium-imaging studies of spatially-tuned cells.
Keywords: event arena, rats, hippocampus, frames of reference, path-integration
Introduction
Humans automatically form memories of attended experience, a process thought to involve the hippocampus (Marr, 1971). If later asked about our day, we readily recall some events and where they happened, whereas we forget others with many parameters of remembering and forgetting now well understood (Wixted, 2004; Richards & Frankland, 2017). In the case of spatial memory, for which recall as well as recognition can be tested in animals, it is important to establish in which geometric frame of reference such event-memory traces are encoded. Is it allocentric or egocentric? That is, is the stored representation within a frame of reference that is independent of the actor or observer (allocentric), or in a body-centered frame of reference (egocentric)? In a classic study, Packard & McGaugh (1992) showed that, faced with ambiguity about potential representations in a T-maze, rats favour allocentric representations initially but this shifts over time to an egocentric strategy. However, this shift does not always occur for, in the delayed matching-to-place protocol in the watermaze in which a new escape location has to be learned each day, rats consistently use an allocentric representations for as long as they are trained (Steele and Morris, 1999).
Bast et al., (2005) and Wang et al., (2010) have outlined a potentially powerful episodic-like ‘everyday memory' task for animals, conceptually similar to the ‘delayed-matching-to-place’ protocol in the watermaze (Steele & Morris, 1999). Procedurally an appetitive task, rats (or mice) are trained to find and dig up food reward in an event-arena over several weeks, entering the arena from one of four start locations whose location varies across days (North, S, E or W). The food is hidden in an odour-masked sandwell whose location also varies over days. They are later tested for the accuracy of their recall of where the action of finding food occurred most recently. The animals learn to do this well in about 10 days and then, successfully remember each day the location where food was dug up after only a single reward. Use of multiple reward pellets, each spontaneously carried back to the start location one by one, enhances memory retention. This recency recall is good for several hours, but then typically decays to chance levels over 24 h (Whishaw, 1998; Bast et al., 2005; Wang et al., 2010; Takeuchi et al., 2016; Nonaka et al., 2017). This task is analogous to aspects of human everyday memory in that testing takes place in a familiar environment (like a room in one's house), but the events that happen within it vary with respect to their location on a day-to-day basis (such as the action of putting down one's glasses somewhere). In a recent Technical Note published in this journal, various determinants of memory retention for this protocol were identified, including trial-spacing and peri-event novelty, along with certain molecular markers of enhanced retention derived from RNAseq (Nonaka et al., 2017). The suitability of this translationally relevant protocol to test novel cognitive enhancers related to neuronal plasticity was confirmed with a Phosphodiesterase Type 4 (PDE4) inhibitor.
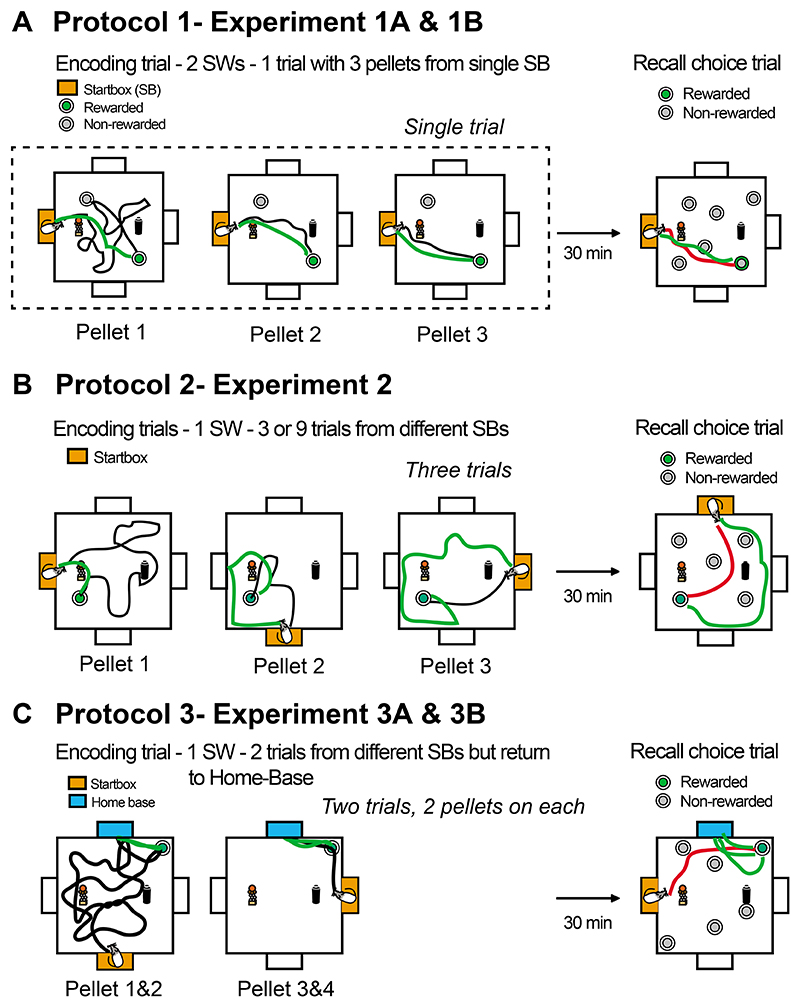
Subsequent to the Nonaka et al., (2017) publication, we discovered an unsuspected ambiguity with respect to the frame of reference of memory encoding. This emerged during tests in which the location from which the animals entered the arena was changed, within each session, between the memory encoding trial and the subsequent recall trial. Performance was very good when, as shown in Figure 1A (Protocol 1), the starting locations were the same for encoding and recall (even though these changed across sessions). However, it declined to chance levels when shifted by 90° or 180° between these two daily trial types. Experiment 1A in San Diego, replicated as Expt. 1B in Edinburgh, document this phenomenon. We were surprised by this finding as we had believed that the use of changing start locations across daily sessions in a stable environment would, as in the watermaze, promote allocentric representations. To the contrary, it seemed that being able to run back to the start-box promoted an egocentric path-integration solution that was severely disrupted by changing the start box location between the encoding and recall trials of a given session. Accordingly, we set about examining two different ways in which to promote allocentric encoding. In Protocol 2 (Figure 1B), multiple start locations were used for the several trials of daily memory encoding, while in Protocol 3 (Figure 1C), there was also a stable 'home-base' to which the animals were trained to carry the food reward. The latter protocol successfully precluded the use of path-integration. Thus, principled changes were made, step-by-step, between the successive protocols 1-3.
Figure 1. Three distinct protocols.
(A) Protocol 1: In our standard protocol (as used in Nonaka et al, 2017), there was a single encoding trial consisting of three runs (black path) out to either of two sandwells (one of which was rewarded) followed by returns to the same startbox (green paths). After a memory delay, a choice trial included a run out (red path) to choose amongst 6 sandwells (only one correct) and then a return once again to same startbox. (B) Protocol 2: The primary modification was the use of four different startboxes within a session, thereby changing from 3 rewards at encoding within a single trial (Protocol 1) to three encoding trials each with a single reward pellet. The green return paths are representative in displaying the confusion of the animals about the location to which to return. The choice trial was from the fourth location of the day. (C) Protocol 3: The primary further modification was the use of a fixed 'home-base' (blue) to which the animals had to carry the food that they had dug up (green paths). Encoding was now divided into only 2 trials, but included the opportunity to run out of the home-base back to the sandwell on each trial (i.e. 4 rewards during encoding). The recall choice trial (and any probe trial) was started from a novel location on that session, as in Protocol 2.
There is a further important procedural detail relevant to the allocentric vs. egocentric representation issue. In Protocol 1 (Nonaka et al, 2017), the animals were typically given the opportunity to retrieve 3 food pellets (0.5 gm each), one by one, within each trial. In Figure 1A, a dotted line represents the one trial with three pellets collected during memory encoding. Specifically, upon digging for a few seconds successfully in a sandwell, the animals carried each pellet back to the (dark) original startbox (orange) where it was eaten. The animals then returned to the encoding sandwell to collect pellets 2 and 3 in turn. The resulting pattern of a path-out (black) followed by a return-home (green) is a component of hoarding behaviour in laboratory-based tasks studied intensively by Whishaw in a series of papers that collectively pointed to the importance of dead-reckoning/path-integration in rodent homing (Whishaw et al., 1995; Whishaw, 1998; Whishaw et al., 2001). His group observed that, provided the food-pellet takes longer to eat than the likely travel time back to a safe place to eat, the animals will generally run accurately back to the startbox to do so (as we also observed). A consequence of this 3-pellet schedule was that, even though the finding of the goal location for the first pellet typically involved a circuitous path, finding the second and third pellet of the memory encoding trial generally involved relatively direct paths from and then back to the start location. These are conditions that could inadvertently encourage a cumulative egocentric representation of path distance and direction, using self-monitored path integration (Redish, 1999; McNaughton et al., 2006). Note that this running back and forth does not occur in the watermaze (Morris et al., 1982; Sutherland et al., 1983) for which the encoding of goal-location happens on the escape platform with no return to the starting location. Memory representation in the standard and many other protocols of the watermaze is allocentric, although some experiments have been devised that require the use of local landmark heading vectors (Pearce et al., 1998). Keen to use a dry-land task for physiological and optogenetic studies later, the challenge before us was to establish a protocol affording true allocentric memory representations for the land-based event arena on the grounds that, to be a valid model, it should mimic our ability to remember where a daily event happened rather than merely memory of how to get there.
Methods
Subjects
At Dart Neuroscience (DN), 11 adult male Long-Evans (Envigo Laboratories Huntingdon, UK; 300-400g at start of study) were used in Expt. 1A. Rats were housed 2/cage, food-restricted to 85-90% of the free-feeding weight (adjusted for growth), had free access to water and maintained on a 12:12 h light/dark cycle with training conducted during the light phase. All experimental methods were approved by the DN Institutional Animal Care and Use Committee, and followed the guidance of the National Research Council Guide for the Care and Use of Laboratory Animals Studies (2011).
At Edinburgh (EDI), a total of 32 adult male Lister-hooded rats were used in Expt. 1B (n=7; Protocol 1), Expt. 2 (n=8; Protocol 2) and Expts. 3A and 3B (n=17; Protocol 3). As described in detail in Nonaka et al. (2017), they were group housed (3-4/cage; and similarly food-deprived to 85-90% of the free-feeding weight against a growth curve, with free-access to water, 12:12 h light-dark cycle with training in the light phase). Care of the animals complied with the UK Animals (Scientific Procedures) Act conducted under a Project Licence (PPL 60/4566).
Blinding and replicability
Growing interest in the replicability of biomedical studies has led us (and others) to be explicit about blinding and other procedures CAMARADES (Camarades). As the animals were not, except in Expt. 3B, in separate groups, caution regarding artefacts and replicability took the form of adherence to specific procedures. These included careful design of the sandwells to prevent olfactory artefacts, counterbalancing of the sequencing of trials to prevent an animal from merely following the path of a previously tested animal, the experimenter(s) scoring probe tests without knowledge of the correct sandwell of the 5 or 6 being used, independent scoring from video data by two independent experimenters with cross-correlation of their data, and so on. We did not rotate the arena between trials or sessions, but instead cleaned the perspex floor surface with alcohol-impregnated wipes between all trials. In Expt. 3B, the experimenters were also blind with respect to whether an individual animal had received drug or vehicle.
Apparatus
All experiments were conducted using each of two identical ‘event-arenas’ in both Edinburgh and San Diego. Designed and made in Edinburgh, their construction and appearance is fully described in Nonaka et al (2017). Of note is that numerous precautions were taken to avoid olfactory artefacts, with behavioural checkpoints used to ensure that these worked. Specifically, Plexiglas sandwells (6 cm diameter, 4 cm depth) that contained the hidden reward pellets were placed in one or a subset of the floor panels with holes. To mask the smell of the food, the sandwells were filled with bird sand mixed with Garam Masala (P&B Foods, Bradford, UK), 150 g/5 kg sand initially (and replenished daily). Each sandwell had a spherical plastic bowl within it in which one or more reward pellets (0.5 g) were placed and thereby accessible. This plastic bowl also made it possible for an equal number of reward pellets to be placed underneath, and thereby inaccessible. The plastic bowls had holes and so were porous to odours, ensuring that the rewarded and non-rewarded sandwells contained the same number of reward pellets at approximately the same depth in the sand and thus should exude the same smell. Extensive randomising and counterbalancing was also arranged to minimize olfactory artefacts: (a) the same sandwells used in the encoding trial were not used for the recall trial of the same session; (b) all sandwells were used a rewarded or non-rewarded sandwell across days; (c) the arena floor was regularly wiped with a 70% alcohol impregnated towel between trial, and before recall and probe trials. The sandwell arrangement is shown in Figure 1B of Nonaka et al (2017).
Procedure
The arena consists of four different startboxes (on the video screen but not magnetically at North, S, E and W) that were located just outside the perimeter of a large 1.6 x 1.6 m arena. Within it, there is a 7 x 7 grid of possible sandwell locations, with one or more sandwells containing either accessible food (rewarded) or non-accessible food (non-rewarded). The arenas were each set within a laboratory room with stable extra-arena cues. The animals entered the arena from a startbox at either N, S, E or W in random sequence (depends on protocol) across successive sessions of training, continuing across many weeks. New memories were formed in each session, and usually forgotten within 24 hr. The apparently automatic one-trial encoding of where the food-digging event happened leading to one-shot memory in both recall choice-trials and recall-probe tests after daily encoding pointed to an episodic-like memory representation within an allocentric map-like framework (Tolman, 1948; O'Keefe and Nadel, 1978).
Habituation
In all protocols, the rats were first taught to dig for food in sandwells inside their home cages. In a first habituation session in the arena(s), containing no sandwells, the rats were permitted to explore the arena with two intra-arena cues and surrounding extra-arena cues for 10 min. They were then given five sessions of daily habituation, starting by being put in a startbox and given a 0.5 g ‘cue’ food pellet to eat. When the pellet was eaten (typically around 30 s), the rats were allowed 10 min access to the arena. Rats started exploration from a different startbox in each session and were trained to search and dig for control food pellets in sandwells in the various locations in the event-arena. On habituation session 2, one 0.5 g pellet was placed on top of the sandwell; rats collected the pellet and took it back to the startbox. On habituation sessions 3 & 4, one 0.5 g pellet was placed on top of the sandwell and another was buried in the middle of the sandwell. On habituation session 5, three 0.5 g pellets were buried at the bottom of the sandwell. By the end of habituation, all rats were running quickly into the arena, collecting pellets and returning to the startbox to eat each pellet. Habituation normally lasted for 6 sessions.
Protocol 1 - training - encoding and recall choice trials
The Protocol 1 experiments constituted a collaboration between two independent laboratories with only minor differences of procedure between them. The key feature of Protocol 1 is that the animals run back and forth between the correct sandwell and the startbox three times. In Expts 1A and 1B, there were 2 sandwells available during memory encoding trials, one rewarded and one non-rewarded, and 6 sandwells during memory recall trials (one sandwell with accessible food reward, and 5 sandwells with only inaccessible food). Later studies used either 1 (S+) or 2 sandwells (S+ and S-) at encoding. It makes little difference whether there is a choice on the encoding trial or not, and thus data from both procedures within each protocol are described.
A training session consisted of an encoding trial followed ~ 60 min later by a recall trial. On encoding trials, each rat was placed in the startbox designated for that session (N, S, E, or W, counterbalanced across sessions) and given a 0.5 gm flavoured pellet. Once the experimenter had exited the testing room, the startbox door was opened remotely and the rat allowed to explore the arena and sandwell(s), one of which contained accessible food (S+). The encoding trial ended once the rat had retrieved the pellets from the rewarded sandwell and returned to the startbox. The door to the arena was closed. On recall trials, the rat was returned to the original startbox and then presented in the arena with six sandwells which included the one rewarded and now five unrewarded sandwells. The focus in the recall trial was whether a rat would preferential choose the rewarded sandwell from the encoding trial(s), and ended once the rat retrieved 3 pellets from it and returned to the startbox. Each training session used a different six sandwell 'configuration', requiring the rats to learn a new rewarded sandwell location each session. The configuration map was used for all rats in each session (i.e. locations A, B, C, D, E and F); however, half of the rats were trained on a different pair of rewarded and nonrewarded encoding locations (i.e. A and B) from the rest (i.e. C and D) to control for any potential location or response biases. We saw no indication that any rat was following the path of a previously trained which would, anyway, have been an unsuccessful strategy. We also always cleaned the arena with 70% alcohol wipes between trials.
Memory for location during encoding was calculated in two separate ways. The first measure, used during training, was choice performance during recall trials - called Performance Index (PI). For clarity and comparison to 2-alternative forced choice data, this index was computed to ensure a 100% score implied perfect memory (minimal errors) whereas a 50% score implied chance [Performance Index (PI) = (maximum number of errors that can be made - number of errors made on this trial)/Maximum number of errors that can be made) x 100]. The second measure of memory, which is very sensitive, was the proportion of time spent digging at the correct or other sandwells during recall trials when no accessible food was available. The first trial type is called a "recall choice trial"; the second a "recall probe trial". Other measures during training trials included latency before digging at the correct sandwell, and qualitative aspects that we noted such as paths taken, returns to an inappropriate startbox, etc.
Protocol 1: Memory recall probe trials
Multiple recall probe trials were used to test the impact of different conditions relevant to the allocentric vs. egocentric coding issue (e.g. time delay, arena rotation, drug infusion). These probe tests consisted of a recall phase with only non-rewarded sandwells present (typically 5 or 6). The rats were allowed to search for the correct sandwell for 60 s from the first dig at any sandwell, with the time spent digging monitored carefully. After 60 s, the experimenter placed pellets in the correct sandwell (to prevent extinction) and the animal allowed to find them. Dig time during the 60 s recording period was measured, and the relative proportion of time at the correct and incorrect sandwells was calculated as percentage dig times.
Protocol 1: Experiment 1A (San Diego)
Once the rats (n=11) were consistently performing with a PI above 80% on the daily sessions, test sessions were interleaved periodically. The consistent performance across days in this longitudinal paradigm is critical for allowing different tests at different times with data that can be compared. One series of tests investigated the effects of varying the retention interval on memory. These test sessions consisted of a standard encoding trial, with the animals only retrieving 1 pellet from the rewarded sandwell on the encoding trial. Following a variable delay (0.5 – 72 h), memory recall for the event location was assessed using a probe test.
The impact of rotating the starting location between encoding and recall trials by 180° during four sessions (with intra-arena cues removed) was then examined. For example, rats released from the N startbox on the encoding trial would, on the recall trial, be released from the N startbox (control procedure) or from the S startbox (rotation condition). The impact of occluding spatial cues was also assessed. For these tests, there was a standard encoding trial, a memory delay of 60-90 min, and then half the rats received a recall trial with intra-arena cues removed and extra-arena cues occluded (by curtains around the arena). The other half of the rats received a standard recall trial with all cues visible. These two conditions were examined within-subjects in counterbalanced order, interleaved with additional training sessions.
Protocol 1: Experiment 1B (Edinburgh)
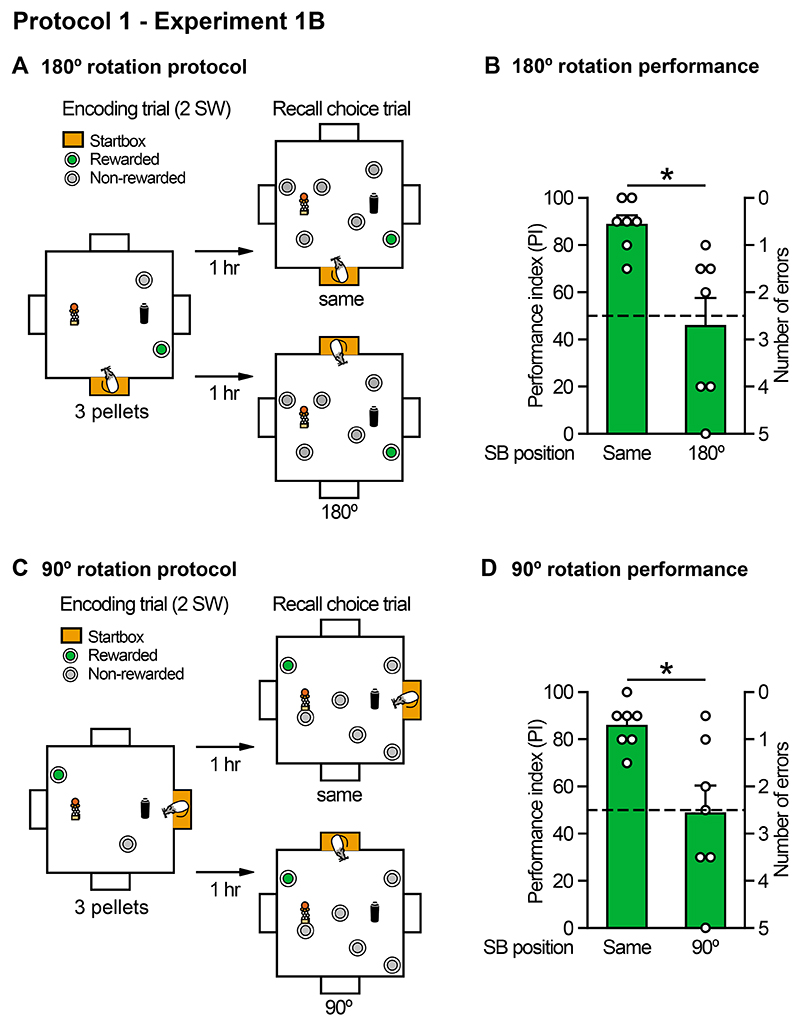
This study of the impact of startbox rotation was conducted at the end of the Nonaka et al. (2017) study using the same animals (n=7; Figure 2). By that time, they had completed 100+ training sessions over 5 months. Using essentially the same procedure in Edinburgh as in San Diego, standard control sessions were interleaved with 'rotation' sessions in which the startbox location was rotated for either a 180° rotation (as in San Diego) or a 90° rotation. These rotations could potentially have a different impact as the 180° rotation varies the relation between the goal-sandwell and the startbox in both distance and direction (for example, from 'near and to the left of during encoding to a location that was 'far and to the right of' during testing); whereas in the 90° rotation (Figure 2B) achieves a symmetrical flip between 'near vs. far' (with respect to proximity between the goal location and startbox), but with the direction (right or left) unaltered.
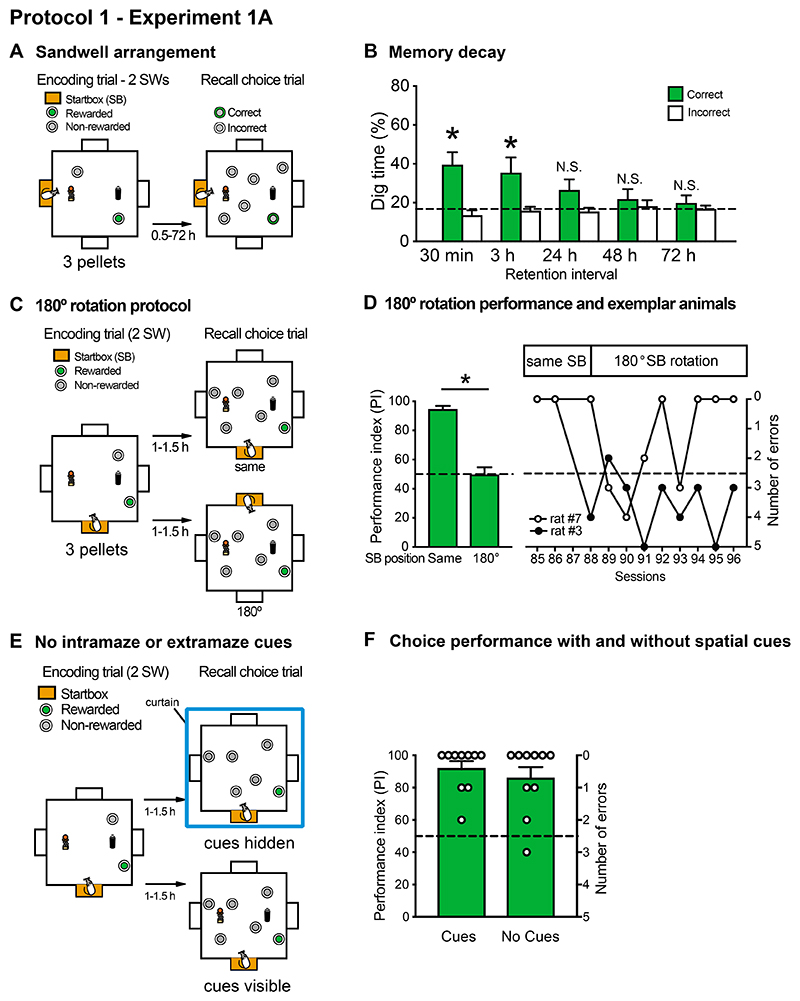
Figure 2. Protocol 1 - Experiment 1A (San Diego) - Time-dependent decay of every-day memory and the effect of 180 Startbox rotations on recall trial performance.
(A,B) Sandwell arrangement for the arena used in San Diego for the study of memory retention across delays (30 min to 72 h). Memory for the correct location was significant at short retention intervals of 30 min and 3 h, consistent with Nonaka et al (2017), but memory returned to chance within 24 h. (C, D) Impact of a 180° startbox rotation in well-trained rats. The startbox location varied across successive sessions (not shown). In the recall trial, rats were either started from the same daily location, or the startbox was rotated by 180°. Rotation resulted in a significant decline of the performance index (PI). Note that individual animals vary, with one representative animal (no. 7) displaying only temporary disruption of performance, while another (no. 3) was consistently affected over 10+ sessions. (E, F) Occlusion of extramaze cues and removal of intramaze cues had no effect on performance. Means +/- 1 SEM.
Protocol 2: Experiment 2 (Edinburgh)
In Protocol 2 (Expt. 2; Figure 1B), using new experimentally naive subjects (n=8), we made one key change. This involved having multiple start locations during the memory encoding trials rather than the single start location of Protocol 1 that had inadvertently encouraged egocentric encoding. In Protocol 2, there were also 3 encoding trials but now from 3 separate start locations within each session (and in a counterbalanced sequence across sessions). However, as in Protocol 1, the location of the rewarded sandwell location continued to vary between sessions to ensure this was an episodic-like task in which the animals had to remember where digging up food occurred most recently.
We began with 10 sessions of 'pre-training' using a 5-alternative-choice (5-AFC) sandwell discrimination protocol in which all 5 sandwells were available on the 3 encoding trials. This procedure was not of primary interest, but was included to encourage learning that one sandwell was rewarded but the others not. For main Protocol 2 model, intended to simplify the memory demands at encoding, only a single rewarded sandwell was used from session 11. The expectation was that performance would improve and reflect allocentric coding, based on the successful DMP procedure in the watermaze in which multiple start locations are used within a single session of training to a single hidden escape platform in an otherwise featureless pool (Steele & Morris, 1999).
The fatal complication that rapidly emerged from using this protocol, which does not arise in the watermaze, is that the animals had to remember from which startbox they had started and thus to which they should return with their single reward pellet. This proved very problematic, with striking interference building up within each session. This problem is captured graphically in Figure 1B with the return paths of the animals from the encoding sandwell (green) reflecting the confusion about where to go. From session 17, training was increased to as many as 9 daily encoding trials from the 3 different starting locations, ending with the recall trial on the 10th trial from a novel start location. In this final attempt to get Protocol 2 to work, all startbox doors were open with the carrying of the food reward to any startbox being permitted.
Protocol 3: Experiments 3A and 3B (Edinburgh)
In Protocol 3, a further conceptual change in protocol was added. The standard procedure in food hoarding tasks is that the return after foraging is to the starting location (as in Whishaw's studies). If this anchoring promotes egocentric encoding, perhaps the demand to carry the reward pellets to a fixed 'home-base' might change things to favour allocentric encoding. The logic behind this likely change in preferred strategy is that cumulative idiothetic path-integration could get the animal back to the start location, but could not direct an animal to the home-base from which the animals did not start on that trial. But it was the home-base to which the animals ran with the reward. In effect, the animals had no alternative but to do the task in a different way. The home-base (North) was never used as a startbox, only as the place to go with the 0.5 g food rewards. This shift ensured that carrying was never along a direct path from the rewarded sandwell back to the startbox, thereby likely precluding path-integration (Figure 6A).
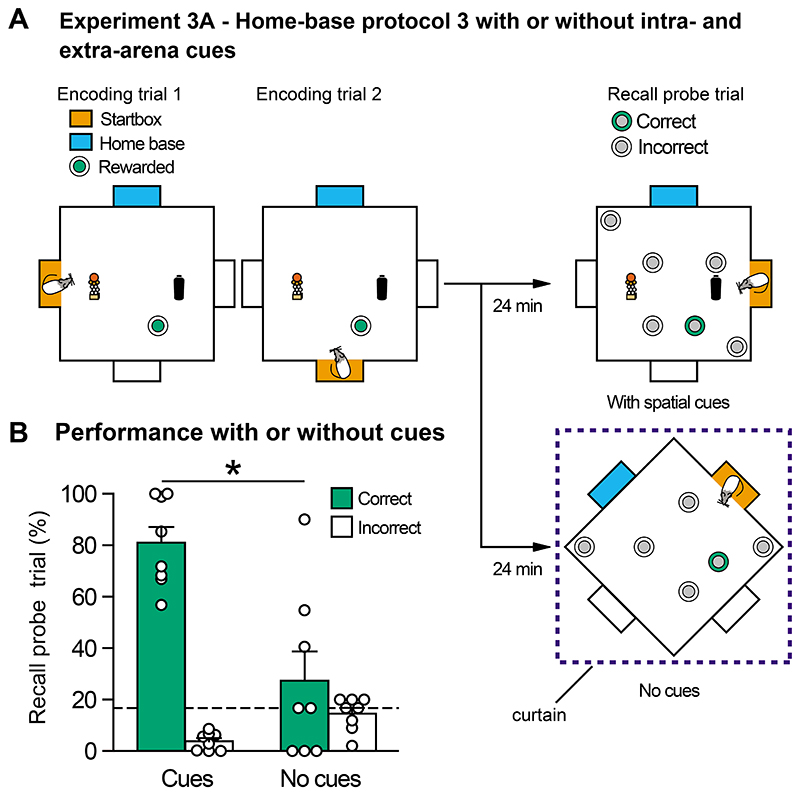
Figure 6. Protocol 3 - Experiment 3A - impact of a stable home-base on performance and its decline upon removal of spatial cues.
(A) Curtains occluding spatial cues were either drawn around the arena, and intra-maze cues removed, or these cues were fully available. (B) In a recall probe test at 24 min, performance was very good with cues available (>70%) or fell to chance (without cues). Means +/- 1 SEM and individual animal data plots.
Instead of 3 daily encoding trials (as Protocol 1 and Protocol 2), we used only 2 encoding trials that could begin at either E, W or S (in random sequence across days), with the door to the home-base (at N) opened once the animal had dug up food at the rewarded sandwell. Access to W, S and E was simultaneously disallowed by closing the door of whichever startbox door had been used on that trial. Once the animal had successfully carried food to the home-base (green paths in Figure 1C), a second run was allowed from that home-base to the rewarded sandwell (black path), enabling pellet 2 to be secured, followed by a return to the home-base (red path) whereupon the door was then closed. The second encoding trial was given approximately 1-2 min later, but from a different start-box location. After a short delay (24 min), a recall trial was scheduled using 6 sandwell protocol (of which only the encoding location contained accessible food). This trial began at the one remaining unused starting location for that trial (red path). Again, the animal had to carry the food to the home-base.
Protocol 3: Rigorous procedures for and analyses of allocentric encoding
Having established that the rats (n=8) could successfully learn this allocentric protocol, we then examined spatial memory over two retention delays (24 min vs. 24 h), predicting the same decay of memory to near-chance levels as observed by Nonaka et al. (2017). We also examined the impact of removal or alteration of the intra- and extra-arena cues between the encoding and recall trials, predicting that doing this would now have a deleterious effect on performance to chance levels.
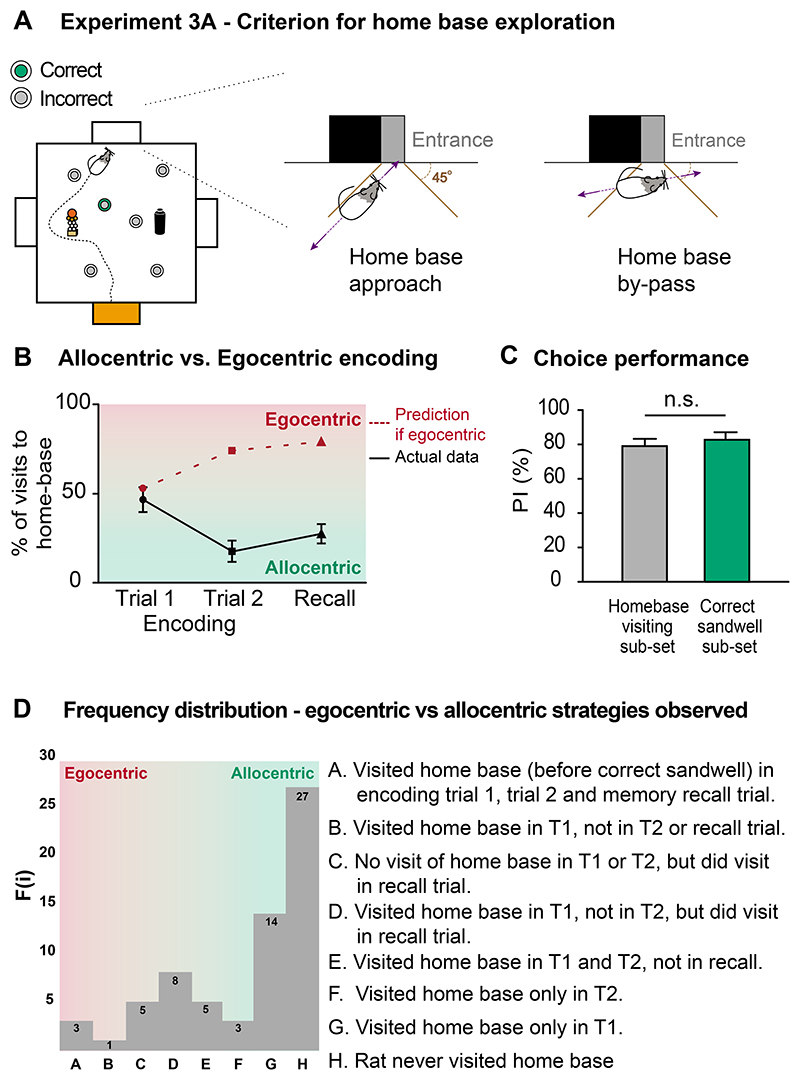
The analysis of allocentric encoding began with a post-hoc video-analysis conducted to examine the qualitative paths taken on encoding and recall trials. The reasons for doing this is because we wondered if a cryptic egocentric strategy might yet possible. Specifically, an independent observer (J-MC) blind to the experimental conditions on any trial viewed all videos ad hoc and recorded the number of times animals went directly to two different locations: one location was to the correct sandwell from the startbox (necessarily using an allocentric strategy); the other was to the home-base before going to the correct sandwell (a strategy which could potentially be permissive for egocentric encoding with a switch to path-navigation from the home-base). A direct approach beyond a 45° angle to the side-walls (Figure 7A) was used as the criterion for identifying approach to the home-base, thereby excluding occasions when the animal merely ran around the perimeter (such trials were in practice very rare). The frequency of the distribution of these distinct paths across 4 sessions was monitored.
Figure 7. Protocol 3: Experiment 3A - detailed analysis of paths taken in the arena.
(A) The criterion for identifying whether the rats approached the home-base or not before they reached the correct reward sandwell on successive daily trials. An approach was at an angle > 45°, whereas a by-pass was at < 45°. (B) A possible cryptic 'egocentric' strategy with the 'home-base' might be to run to it and then use it as an anchor-point for the start of a path-integration-associated accumulation of information. This view predicts that approaches to the home-base location would increase within each session, and be high on recall trials (red symbols and shading). In fact, the actual data (black-symbols) shows the opposite trend. Some animals visited the home-base location on encoding trial 1, but this declined as the animals learned the allocentric location of that session 's rewarded sandwell. (C) There was no difference in PI score between a subset of animals that approached the home-base first (grey) and those first visiting the correct sandwell (green). (D) The frequency of different combinations of preferential approach to the home-base before visiting the correct rewarded sandwell. The left is more egocentric, while right is more allocentric. The most egocentric category implies the rats would always visit the home-based before digging at the correct sandwell, while the most allocentric category implies they should visit the correct sandwell directly.
Experiment 3B - Electrophysiology
In Experiment 3B, male Lister-Hooded rats, weighing 250+ g, n=5 per drug (CNQX) 6-cyano-7-nitroquinoxaline-2,3-dione, 3 mM; Tocris, Abingdon, UK and artificial cerebrospinal fluid (aCSF, Sigma, Irvine, UK) were used in the non-recovery electrophysiology studies. These animals were prepared for acute surgery in a stereotaxic apparatus (David Kopf Instruments) under non-recovery urethane anaesthesia (1.3 g/kg body weight; Sigma-Aldrich, USA), with the first intraperitoneal injection given during brief isofluorane anaesthesia (4% isoflurane in 0.8 l/min O2). These studies typically lasted 6–8 hr, with the initial 2 hr being spent securing accurate placement of the stimulating and recording electrodes and cannula, and the subsequent 4 hr monitoring field-potential baseline and the impact of intrahippocampal drug infusions.
Stimulating and recording electrode positions are shown in Figure 8A. The stimulating electrode was a twisted bipolar Teflon-coated platinum-iridium electrode (20 μm diameter, 400 μm coated diameter for each of the two single strands) aimed at the angular bundle of the perforant path (anterior-posterior (AP) 0.0 mm from lambda; mediolateral (ML) 4.2 mm; dorsoventral (DV) 2.15 mm from dura). The recording electrode was a single Teflon coated platinum-iridium wire targeting the hilus of the dentate gyrus (AP 4.08 mm from bregma; ML 2.5 mm; DV 3.5 mm). The drug cannula was a 28 gauge stainless steel tube whose tip was stereotaxically located at least 0.5 to 1.0 mm (± 0.3) mm away from the recording electrode (AP 3.6 mm from bregma; ML 2.6 mm; DV 3.5 mm).
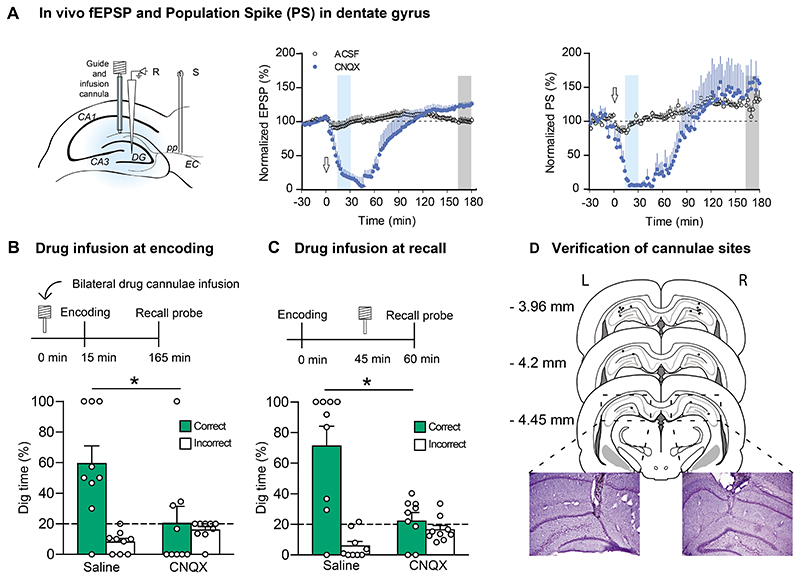
Figure 8. Experiment 3B - Electrophysiology, Histology and. Behaviour - Impact of bilateral inactivation of the dorsal hippocampus.
(A) Experimental design for electrophysiology. Dentate field potentials (fEPSP and PS) were measured over a period from -30 min to +180 min after 2 ul drug infusion of aCSF or CNQX. In the later behavioural study, the blue shading marks time-point after infusion when encoding trials were given (Data in B) and the grey shading marks the time point when recall trials were given (C). Note that both the fEPSP and the PS decline to near zero from a point about 15 min after infusion until 90 min, whereupon both measures return to baseline. (B) The hippocampus was inactivated by bilateral microinfusion of CNQX 15 min before the first of two encoding trials. The recall probe test was conducted 2.5 h later after the effects of CNQX would have dissipated (and thus hippocampal activity should be back to normal). Memory was poor after CNQX but normal after vehicle infusions. (C) The hippocampus was inactivated by bilateral microinfusion of CNQX 15 min before the recall probe test, encoding having been conducted in the absence of the drug. Memory was again poor after CNQX. Means +/- 1 SEM and individual animal data plots. (D) Histological verification of locations of the tips of the bilateral guide cannulae used for drug infusions.
Conventional field-potential recordings were made, with stimulation every 20 s, and these monitored and calculated on-line using EPS software (in house). In response to biphasic 200 μs stimulus pulses of circa 600–800 μA, we measured both the early-rising slope of the evoked potential by linear regression over several points, and the amplitude of the evoked dentate population spike. The stimulus intensity was adjusted to secure initial population spike amplitudes of circa 3–6 mV.
Once acquired using suitable electrode placements, potentials typically remained relatively stable over periods of up to 3–4 hr, with a small upward drift of the population spike (but not fEPSP) that rarely exceeded 15% over this long period. Animals for which the potentials were unstable were discarded. The same long time-period stability was observed when aCSF, was infused into the dorsal hippocampal formation at a depth targeting a region encompassing the stratum moleculare of area CA1. A volume of 2 μl aCSF (as vehicle) (in mM: 150 Na+, 3 K+, 1.4 Ca2+, 0.8 Mg2+, 155 Cl-, 0.2 H2PO4-, 0.8 HPO42-, pH 7.2) or CNQX was infused (0.5 μl/min) that, on the basis of previous autoradiographic data (Riedel et al., 1999), would be expected to diffuse throughout the entire CA1, CA3 and dentate gyrus regions of the septal (dorsal) hippocampus. After the infusion, electrophysiological recordings, measuring the same parameters and under the same conditions per animal, last for 180 min.
Protocol 3 - Experiment 3B - Impact of hippocampal inactivation
Finally, we explored the impact of temporary inactivation of the dorsal hippocampus through microinfusion of the AMPA receptor antagonist CNQX via intracerebral cannulae. Rats (n=9) were anaesthetised with 2-5% isoflurane (Abbott, UK) and positioned in a stereotaxic frame (Kopf instruments, CA, USA). Guide cannulae (26-gauge; Plastics One, UK) were implanted bilaterally into the dorsal hippocampus (coordinates relative to skull at Bregma: AP = -4.5 mm; ML = 3.0 mm; DV from dura = -2.5 mm). Dental cement (Kemdent, Purton, UK) was then sculpted around the guide cannulae. Solid stainless steel (“dummy”) cannulae were inserted into the implanted guide cannulae to prevent infection or blockages. The dummy cannulae were 33 gauge with a 0.5 mm protrusion from the end of guide cannulae when inserted. All rats were allowed a recovery period of at least 10 days to allow them to regain their pre-surgery weights before food restriction and behavioural testing commenced.
Phosphate-buffered aCSF were used as infusion vehicles and for dissolving drugs. Drug concentration for infusions was 0.89 mg/ml (3 mM) of the competitive AMPA/kainate receptor antagonist CNQX. The pH of the CNQX solution was adjusted to 7.2 by the addition of concentrated phosphoric acid. These volumes and concentrations were calibrated in a study with electrophysiological monitoring of the extent and duration for which excitatory post-synaptic potentials and population spikes were blocked by this concentration and volume of CNQX (Rossato et al., 2018).
One day before drug infusions, a mock infusion was used to habituate rats to the drug infusion conditions. Rats were restrained manually and their dummy cannulae removed. Injection cannulae were placed into the guide cannulae (for 5 min) but no solutions were infused into the rats’ brains. Thereafter, the rats were restrained manually and infusions into both hemispheres were performed simultaneously in a control testing room. Prior to infusion, the injection cannulae tips were dipped into 70% alcohol and then rinsed in saline. The tips of these infusion cannulae protruded 0.5 mm from the ends of the guide cannulae within the brain, and were connected to microsyringes (SGE brand, World Precision Instruments, FL, USA) on a microinfusion pump (World Precision Instruments, USA) via flexible polyvinyl chloride tubing (Plastics One, UK). The flexible tubing was rinsed through with bottled water for injections (Hameln, Gloucester, UK). CNQX (1 μl/hemisphere) was infused at a rate of 0.2 μl/min over 5 min, after which the infusion cannulae were left in place for a further 2 min to ensure all droplets of solution entered the brain. Dummy cannulae were then rinsed with alcohol and saline and placed back into the guide cannulae.
Finally, all rats were terminally anaesthetised with Euthanal (Merial, Roydon, UK) and then perfused intracardinally with 0.9% saline, followed by 4% formalin in saline. The brains were removed and stored in 4% formalin for several days. Coronal sections (30 μm) were cut using a cryostat for histological analysis and were mounted on slides, stained with cresylviolet, and coverslipped using DPX. The sections were examined under a light microscope with 20-fold magnification to verify cannulae placements. For each brain, the infusion site was plotted by determining the deepest point (Figure 8D) at which tissue damage was evident and marking this location on the appropriate coronal sections from a standard rat brain atlas (Paxinos & Watson, 1998).
Results
Protocol 1: Experiment 1A
In Protocol 1, rats run back and forth between the start box and the correct sandwell during the sample trial, and hopefully chose the correct sandwell in the choice trial. It was observed that the rats rapidly acquired the standard task of running into the arena from a different daily startbox, finding and digging up a single pellet of food 3 times during a single memory encoding trial (3 pellet encoding; Figure 2A). During the recall trial, they exhibited similar levels of memory recall in San Diego to that of the Nonaka et al. (2017) study conducted in Edinburgh. In a series of recall probe tests, the animals showed time-dependent forgetting of the location of the rewarded sandwell characteristic of everyday memory (Figure 2B). When the delay interval was short (0.5-3 h), rats spent significantly more percent time digging at the correct sandwell compared to chance (0.5 h = 39.6 ± 6.4%, 3 h = 35.4 ± 7.8%, one sample t-test vs. chance (16.7%), ts(10) > 2.40, ps < 0.05). Retention intervals of 24 h or longer resulted in chance performance (24 h = 26.5 ± 5.4%, 48 h = 21.9 ± 5.1%, and 72 h = 19.9 ± 3.8; ts(10) = 0.85-1.82, n.s.).
Figure 2C,D shows the effects of the 180° rotation in the starting location between encoding and recall choice trials. This resulted in a dramatic decline in the average PI score from circa 85% to chance levels (chance = 50%, before rotation: ts(10) = 7.67-18.81, p < 0.0001; after rotation: ts(10) = 0.46-2.32, n.s.). Performance did not improve across further sessions of training, although two representative animals (Nos 3 and 7) illustrate within group variability in reaction to the rotation test. The poor mean performance across the startbox-rotation sessions contrasts with that observed for the initial acquisition of the standard no-rotation version of the task.
As shown in Figure 2E,F, the rats surprisingly exhibited unimpaired performance when the intra- and extra-arena cues were obscured during a choice trial (t(8) = 0.82, n.s.; 2 animals excluded for failing to dig in the sandwells effectively). This outcome suggests that the 10 animals included were largely using a egocentric or path navigation strategy to find the correct sandwell. Although they could find the correct well with high levels of accuracy on trials where the spatial cues were obscured, they were significantly slower to make the first dig in any sandwell on these trials (latency to first dig on trials with extra-arena cues = 11.5 ± 4.7 s, latency to first dig on trials without extra-arena cues = 79.1 ± 12.3 s; t(8) = 3.9, p <0.01), indicating that the rats were at least aware of the change in the extra-arena environment.
Protocol 1: Experiment 1B
This surprising "Houston, we have a problem" finding prompted a replication, conducted in Edinburgh. This used animals that were already running well in the arena according to the identical training protocol and showing a comparable level of efficiency with a PI of >80% (as in Nonaka et al., 2017). To test the impact of startbox rotation between encoding and recall, 4 encoding/recall trials were given across 12 sessions with either no rotation between encoding and recall (2 tests) or either a 180° or a 90° rotation (2 tests; Figure 3A,C). These tests were interspersed with 8 sessions of regular training.
Figure 3. Protocol 1 - Experiment 1B (Edinburgh) - Startbox rotations impair recall trial performance.
Using the same animals as in Nonaka et al (2017), we examined the impact of 180° (A, B) or 90° (C, D) rotations of the startbox used between the encoding and memory recall choice trial. The design allowed counterbalancing for “near” vs “far” (A), and “left” vs “right”(C) in the separate tests. Repeated measures data allowed comparison of an individual animals' scores on rotated and non-rotated trials. Both rotations resulted in a significant decline of the performance index (PI) on recall to chance level. Means +/- 1 SEM and individual animal data plots.
The change in startbox location caused an immediate reduction in recall to chance levels (Figure 3B,D: 180°: t (6) = 3.1, p < 0.05; 90°: t (6) = 3.2, p < 0.05). The data is plotted with means and SEMs, together with individual data points that graphically display the increased variability of the PI scores on rotation trials, some animals showing little change in the PI score from circa 85% whereas most show a decline to as many as 5 errors per trial (i.e. to a PI = 0%). This variability may, respectively, reflect a subset using an egocentric encoding strategy (massive disruption in performance) and a smaller subset that were predominantly using an allocentric strategy (little change). The implication appears to be that the 'standard' training protocol fosters an ambiguous outcome with respect to coding strategy.
Protocol 2: Experiment 2
Our next step towards trying to find an effective allocentric strategy was to supplement daily between-session changes in startbox location with within-session changes also (as in Bast et al, 2005). Specifically, in Protocol 2, there were three sample trials but each begun in different startbox locations. Specifically, instead of allowing 3 pellets to be collected and taken one-by-one back to the same startbox to eat, Protocol 2 permitted (a) only 1 pellet for each startbox location, and (b) scheduled 3 separate memory encoding trials from different starting locations (Figure 4A,B). A fourth startbox location was used for the memory recall trial.
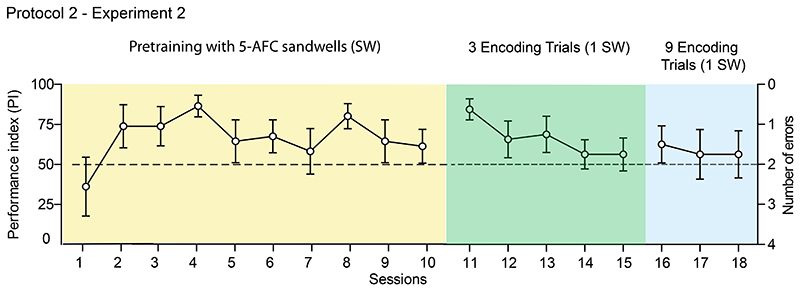
Figure 4. Protocol 2 - Experiment 2 (Edinburgh) - Different startbox locations across successive encoding trials.
Pretraining consisted of a 5-alternative discriminative choice procedure in which all 4 trials of a session started from different startboxes (yellow shading). From session 11 onwards, a single rewarded sandwell was used on each of 3 encoding trials from three different startbox locations (green shading). On the recall trial, there were 5 sandwells, with the obligation to choose the correct sandwell and then return with the food reward to the startbox location of that trial. From sessions 16-18, there were 9 encoding trials (blue shading, 1 pellet each). Performance was initially good upon transfer to the main encoding-choice protocol (session 11), but declined across further training sessions. Means +/- 1 SEM.
This protocol was begun with new animals (n = 8) in which pretraining consisted of a 10-session sandwell 5-AFC discrimination protocol (Figure 1B). Performance improved from chance levels to show a trend for above chance memory recall (Figure 4; yellow shading), but was characterised by considerable within-animal variability with only 2/8 animals showing less than 3 errors on every recall trial at some point from session S4 to S10. Average performance over these sessions was 70.1 +/- 3.1%, above chance, but it was unstable on a day-to-day basis and at only 62.50 +/- 10.6% on session 10 (N.S. compared to chance).
The main single sandwell encoding procedure of Protocol 2 training was begun at session 11 with, initially and encouragingly, performance well above chance (84.4 +/- 6.6% correct; t(7) = 5.23, p = 0.001; Figure 4; green shading). However, instead of being sustained, the within-animal variability across sessions continued to be problematic and average performance steadily declined across the next 5 sessions. We wondered if this poor performance might be overcome by additional daily encoding trials. Accordingly, over sessions 16-18, these were increased from 3 to 9 encoding trials by repeating three times the sequence of a single pellet from each of the 3 initial startbox locations. However, no change in performance was observed (Figure 4; blue shading). The PI on session 18 was at chance (t(7) = 0.42, n.s.).
Observation of the behaviour of the animals in the arena revealed the problem. Specifically, there was 'confusion' about where the animals should go with their reward pellet, triggering such behaviour as patrolling around the perimeter of the arena (see Figure 1B). This would likely have caused interference in working memory, limiting effective memory of that day's target sandwell location. In short, increasing the number of start locations into the arena from 1 to 3 did not help.
As this failure could have been due to a poor batch of rats, we sought to check that these same animals could nonetheless learn an egocentric strategy. We therefore continued training these animals beyond S18 using a single sandwell during memory encoding and allowing the animals to return repeatedly to a single start location (i.e. a return to Protocol 1). Performance improved dramatically and stabilised at levels of around 75% or better throughout (reliably above chance at 79.8 +/- 2.4 % with t(7) values ranging from 2.50 to 5.70, ps < 0.05; data not shown). There was therefore nothing odd about this batch of animals.
Protocol 3: Experiment 3A&B
The turning point of this work occurred in two studies, conducted with new cohorts of animals (n=17 total; ns = 8 and 9 respectively). In Protocol 3, the key change was to assign a stable "home-base" to which the animals should carry any reward pellets they had dug up (Figure 1C; home-base in blue). Specifically, having dug up food during either a sample or choice trial, the food was not to be carried back to the start, but always to the home-base (which may be to the left, right or straight ahead of the animal's then location, but always in the same allocentric location. Following this change, Expt. 3A established the successful use of allocentric coding, while Expt. 3B revealed hippocampal-dependence.
Even though experimentally naïve, the animals of both experiments were reluctant to approach the home-base at the outset, making frequent attempts to re-enter the startbox from which any trial had commenced (entry door was now closed). After a few sessions of training, with 2 encoding trials each from 2 different startboxes per session (varying in location across sessions), they began to more readily enter the stable home-base willingly and settled into a routine of doing this routinely by sessions 6-8. The PI measure rose to a high level quickly, was stably elevated across successive sessions, and significantly above chance. Representative paths taken by one exemplar animal on 2 encoding trials and a later recall choice trial is shown in the supplementary movie file (See Movie S1). Whereas trial 1 was characterised by exploration all over the arena until the location of the sandwell was found, encoding trial 2 from a different startbox position, shows a typically direct approach to the correct location of the sandwell (see movie). The recall trial shows good performance with one proximal digging error before the direct approach to the target (i.e. a PI of 80%).
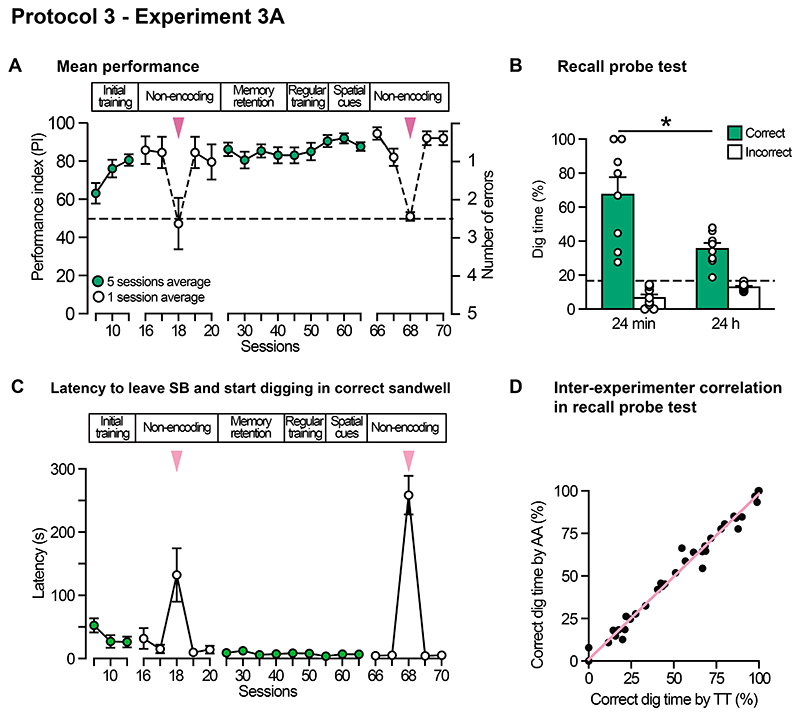
Averaged across all animals, the mean PI on the recall trial stabilised across sessions, with ever more direct paths from the rewarded sandwell to the home location. Interestingly, as they did so, signs of hesitation about leaving the startbox on the initial encoding trial of the day tended to increase, with the animals apparently inspecting the arena and extra-arena cues before venturing out. Unfortunately, this pausing behaviour was not timed (but will be in future studies). Non-encoding control trials (early and late in training), to check for any olfactory artefacts, showed choice trial performance fall exactly to chance levels when the initial encoding trials were not given (S18 and S68; Figure 5A,C) Thus, the animals were not following any differential smell cues emanating from a rewarded sandwell. They did, however, notice the change in non-encoding procedure (i.e. a recall trial without prior encoding trials) with much longer latencies to dig at the correct sandwell on ‘non-encoding’ sessions (Figure 5C).
Figure 5. Protocol 3 - Experiment 3A - impact of a stable home-base to which food-reward should be carried.
(A) Rapid acquisition of effective performance, with stable above chance performance from session 16, this maintained through to session 70. Two non-encoding control sessions were conducted at the start and end of training (s18, s68) both show performance dropping to chance. Extended regular training provided a stable >80% PI baseline permitting various memory probes tests including a test of retention over 24 h and the impact of withdrawing spatial cues (Figure 6). (B) Memory retention with 3 pellet reward declines from well above chance at 24 min to a lower but still above chance level at 24 h. (C) latency data for the time taken to dig at correct sandwell. Note massive increase in this. time on non-encoding choice-trial sessions as predicted. (D) Inter-experimenter correlation of blind probe test scoring of two experimenters (AA and TT). Means +/- 1 SEM and individual animal data plots (B).
In a critical test of 'everyday' memory, statistically significant forgetting characteristic of ‘everyday’ memory was observed over 24 h (t(7) = 2.85, p < 0.05; Figure 5B). Additionally, there was significantly lower total digging in all sandwells in the 24 h condition (t(7) = 2.97, p<0.05). Overnight forgetting is an important characteristic of episodic-like everyday memory. Two independent observers showed a close correlation in their scoring of the dig times at all sandwells during probe trials (Figure 5D), pointing to the objectivity of our 'blind' data scoring.
We then conducted two tests of allocentric encoding - one procedural, the other analytic. First, we examined whether memory recall was affected by limiting access to the extra-arena cues during the choice trials (Figure 6A). There was a clear sensitivity to the occlusion of intra- and extra-arena cues. Performance declined to chance in a statistically significant manner (t(7) = 3.37,p < 0.05; Figure 6B). The total time spent digging at all sandwells was also significantly lower when cues were occluded (t(7)=3.70, p<0.05) indicating, as in Expt. 1, that the animals noticed the change in contextual cues (Data not shown).
Second, we analytically addressed the unlikely possibility that, instead of the stable home-base (at North) aiding allocentric encoding, it was used as an 'anchor point' for a dead-reckoning-like accumulation of distances and rotations that could potentially mediate a cryptic egocentric path-navigation route to the correct sandwell. On this alternative view, the animals would have to first use allocentric memory to go from any startbox to the north location (using reference memory), and then switch to an egocentric strategy while exploring from this home-base anchor to the rewarded sandwell. We therefore monitored approaches to the home-base (Figure 7A; see Methods). The importance of this analysis derives from the fact that, if the animals did this, they would also fail on the arena cue-occlusion test (above) because they would be unable to locate the home-base (Figure 6B); they would fall to chance on the non-encoding trial (Figure 5A); and would most likely also show the overnight forgetting characteristic of everyday memory (Figure 5B). This critical additional analysis hinges upon whether (a) the animals approach the home-base preferentially on encoding or recall trials or both, and (b) display an increasing proportion of approaches to the home-base across the three daily trials.
Video files were monitored across 4 sessions for each of two sub-groups of trained animals (total n=17) to identify the frequency of approaches from any startbox to the home-base prior to approaching the correct sandwell at which to dig. These showed a declining percentage of approaches to a level of 27% on the recall trial (Figure 7B, black triangles, true data), precisely the opposite of the prediction one would make for a cryptic egocentric strategy for which it should increase (Figure 7B, red symbols, theoretical data). When the sub-set of animals (n=5) that did sometimes preferentially approach the home-base were compared with those going directly to the correct sandwell before carrying the reward to the home-base (n=12), there was no difference in PI score between the two sub-groups (Figure 7C; t (15) = 0.051, n.s.). That approaches are made to the home-base on encoding trial 1 (47%) is not itself surprising, as it likely reflects a combination of (a) exploration on trial 1 of the day (with searching all over the arena including to all of the startboxes), and (b) the north box being the place where a total of 6 food pellets are eaten each session. The home-base would thereby have acquired secondary reinforcing properties through Pavlovian context conditioning. The frequency of the different combinations of preferential approach to the home-base across encoding and recall trials before the animal went to the rewarded sandwell are shown in Figure 7D.
Protocol 3: Experiment 3B
Finally, using new animals (n=9), hippocampal-dependence of both memory encoding and memory recall were examined. In Expt. 3B, the quantitative characteristics of PI performance were very similar to those of Expt. 3A (data not shown). The critical measure was the impact of bilateral micro-infusions (2 ul) of CNQX (6-cyano-7-nitroquinoxaline-2,3-dione; 3 mM), an antagonist for AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate)/kainate-type glutamate receptor, into the dorsal and intermediate hippocampus. The first step was to examine the impact of aCSF or CNQX on perforant path evoked dentate field-potentials (fEPSPs and PS). These showed a clear drug and defined times (blue and grey shading) when relevant tests of the impact on encoding and recall could be tested (Figure 8A). In the behavioural studies, drug infusions were given 15 min prior to memory encoding or 15 min before memory recall in separate tests, and the on memory recall in a probe test at 3 h. The dose and volume used has been shown in the Edinburgh lab (Rossato et al., 2018) to cause a massive disruption of the both EPSPs and population spikes in evoked field-potentials e.g. (Rossato et al., 2018). As shown in Figures 8B,C bilateral CNQX infusion caused a complete blockade of both memory encoding and memory recall (ts(8) > 2.31, p<0.05). There were also no differences in total dig time across drug conditions (ts(8) < 0.82, n.s; Data not shown). Histological analysis of the tips of the bilateral cannulae were located in the dorsal hippocampus (Figure 8D).
Discussion
The important new finding of this study is that the provision of a stable home-base to which food should be carried on each trial in an appetitively motivated open-field foraging task favours allocentric encoding. This observation is new because previous studies of food-carrying have pointed to egocentric encoding as the predominant coding strategy when animals carry food back to the start position. Allocentric encoding emerges with the provision of a stable home-base that is distinct from a varying starting location. Detailed investigation of the first and the third training protocol showed that animals preferentially adopted an egocentric or an allocentric form of memory encoding respectively. Frequent returns to an initial startbox favoured but did not absolutely enforce egocentric encoding (Protocol 1). The use of multiple startbox locations (Protocol 2) caused confusion about where food was to be carried within a session in which both egocentric and allocentric encoding strategies could be adopted and interfere. Critically, carrying the reward to a fixed, stable home location favours allocentric coding, even when coupled to varying startbox locations within a single session of training (Protocol 3). These findings have implications for both behavioural and cognitive tests of episodic-like memory and, likely, also for physiological single-unit recording studies of the spatial localization and navigation system.
Our starting point was that unexpectedly poor spatial memory was observed, in independent across-laboratory studies, when there was a shift of startbox location between encoding and retrieval (Expts. 1A,1B). First observed in San Diego (Expt. 1A), and replicated in Edinburgh (Expt. 1B), Protocol 1 led to a relatively dominant use of egocentric encoding or a path-integration strategy to get to the correct sandwell. The across laboratory replication, which adds rigor to our observations, also revealed a small sub-set of animals in both laboratories that were relatively unaffected by within-session changes in startbox location.
The change made for Protocol 2 to multiple within-session startbox locations, intended to promote allocentric memory encoding, was not successful (Expt. 2). It became apparent that the demands of remembering the different locations of where food reward was to be carried once it had been secured caused considerable interference such that effective memory processing of the goal-location was poor (Figure 1B). Interestingly, the use of multiple start positions within a daily session is standard in watermaze experiments, but there is no ‘return to the start position' in such experiments - the animal waits on the escape platform until removed. Thus, there may have been a modest beneficial effect of multiple start locations for allocentric encoding in the event arena, but this benefit was obscured by our requirement that the animals carry the food to varying safe places to eat within a session.
The novel finding of the study emerged from the use of Protocol 3 requiring that the food reward be carried always to a stable allocentrically-defined home-base at the perimeter. This was successful in realising effective memory recall and switching animals to a truly allocentric memory representation (Expts. 3A and 3B). Performance on recall trials is (A) stably above chance across successive sessions; (B) shows gradual forgetting of everyday memory over 24 h on non-rewarded probe trials; and (C) a memory representation that is sensitive to the occlusion of intra- and extra-arena cues. However, as an extra precaution, we note that even this latter and ostensibly definitive test of allocentric encoding is ambiguous because a potential cryptic path-integration strategy might nonetheless have been deployed. This strategy would have been to run to the home-base and then use it as the anchor-point for subsequent dead-reckoning. Blind analysis of videos of the paths taken by the animals in Protocol 3 showed, however, that such a strategy was not used by the animals. Additionally, (D) hippocampal-dependence was established pharmacologically by showing that blockade of fast synaptic transmission with a selective AMPA receptor antagonist at the time of memory encoding or separately at memory recall itself impaired memory at 24 h. Thus, the key new concept to emerge from these studies is that, while the Whishaw procedure of food-carrying by the animals back to a start location usually encourages an egocentric/ path-integration strategy (Whishaw et al., 1995; Whishaw, 1998; Redish, 1999; Whishaw et al., 2001), allocentric encoding is dominant when a safe, fixed, allocentrically defined home-base location is used that is separate from the start locations.
Automatic encoding of everyday memory
The ‘episodic-like’ feature of this everyday memory task in its various forms is based on the concept shared by other tasks that much memory encoding for single events happens automatically in the course of everyday life. Tasks such as novel object recognition, object place memory and object-place-context memory also reveal 'automatic' memory encoding associated with experiences that are not rewarded (Ennaceur & Delacour, 1988; Aggleton & Pearce, 2001; Eacott & Easton, 2007). However, these are recognition tasks in which novelty-induced and context-specific novelty-induced exploration (Morris, 1983) can be triggered in various ways - by objects that are absolutely novel, by familiar objects in novel locations, or by objects in contexts different to those used during initial memory encoding (Langston & Wood, 2010). There is always something visually different in such object- or location change protocols, changes that can be recognised, but there is usually no demonstration of memory recall in such tasks. Recollection of 'what-where-which' has been successfully modelled in an object exploration task (Eacott et al., 2005), but the level of performance attained, while significantly above chance, was modest at even the shortest memory delay.
In contrast, the event-arena shares with the watermaze that it is a 'memory recall' task. Our supposition is that the animal recollects where it performed the action of digging up food most recently, and indexes that recollection by navigating effectively to it from any starting position (Morris et al., 1982; Steele & Morris, 1999; Morris, 2006). We have no direct evidence that the animal remembers the act of digging, but it is a very natural behaviour and it seems likely that they would. Nor in these studies is there direct evidence that the animals recall what food is to be found in the target location, although food-specific memory has been observed in studies of paired-associate learning involving schemas (Tse et al, 2007). The event arena has two potentially powerful advantages over the watermaze.
First, although not used in these studies, it is permissive for electrophysiological and optical recording of brain activity during behaviour. Second, as the animals only spend time at sandwells, irrespective of where they may have been located on a previous session, memory encoding can be studied in the absence of extinction by simply moving a sandwell from one location to another. As noted, a limitation of the event-arena to date is that variation in the 'what' or 'which' components of recall have not yet been investigated in the episodic-like protocols, but this could be examined by varying the target flavour of food to be secured (as in Tse et. al, 2007), or the context cues in which an arena is placed. The latter test would be analogous to manipulations examined in the context of novel object recognition memory ('object-context' and 'object-place-context'). To date, the focus in the everyday memory task has been primarily on the recency of where the discrete action of digging up food occurred. This 'action-where' conjunction ensures that, at the time of recall, there are no perceptual affordances that could mediate recognition of the correct location over any other. On choice and probe trials, the arena always looks the same.
Recency memory is conceptually related to automatic encoding (Marr, 1971; Morris, 2006). One consequence of automatic encoding is the risk of too much information being encoded in the course of a day creating potential saturation and interference. Thus, forgetting in the form of retention selectivity is, we believe, an essential feature of this form of memory. A human example might be remembering where one has parked one's bicycle at the station on the daily commute, or recalling where one's glasses have recently been mislaid around the house. Such memory is useful for a few hours, but generally not necessary for longer periods. Such memory must, almost by definition, fade over time. It is precisely this kind of everyday recollective memory that is at risk in older individuals and those in the early stages of neurodegenerative diseases that target memory formation, and for which palliative cognitive enhancement could be so useful.
Egocentric or allocentric encoding
A key issue in this study concerns the frame of reference in which the 'where' component of recency memory is encoded. In Protocol 1, there was a clear dominance of egocentric encoding from the starting position. Terrestrial rodents (e.g. the Norway Rat) do create foraging trails from their burrows that are helpful in mediating a route home for themselves and, potentially, other rats (Galef & Buckley, 1996). In laboratory settings, and in the absence of odour trials, rats may also keep track of the path they have taken using path-integration to encode distance moved, radial turning etc., as described in both quantitative experimental work (Whishaw, 1998; Whishaw & Brooks, 1999) and formal path-integration models of navigation (McNaughton et al., 1991; McNaughton et al., 1996; Redish, 1999). Egocentric encoding may have been encouraged in our first protocol by allowing the animals to repeatedly carry the reward back to the same startbox from which they emerged at the start of the first encoding trial - for which they would have become more accurate across the 3 reward pellets used in encoding. The use of such a strategy would not preclude the animals also forming an allocentric representation, but our original protocol likely allowed an egocentric representation to display trace dominance (Dudai, 2012) when the two types of memory representation were put in competition. The variability of the data secured in both the initial San Diego and the Edinburgh experiments reflected precisely that ambiguity.
However, our interest is not primarily in the navigational path the animal takes than in how its accuracy tells us something about episodic-like everyday memory encoding. The procedural change of creating a home-base in Expts. 3A and 3B disposed the animals towards a "where is it?" memory representation. In Protocol 3, our animals did continue to carry food, as in the experiments of Whishaw, but to a dark, safe, well-learned home-base rather than to the varying starting location. Eilam & Golani, (1989) have noted that, even in open-arenas, rats create a stable home-base for themselves. Critically, our 'north' home-base was encoded allocentrically and stored in long term memory, thereby obviating regular updating via working memory and so limiting interference with newly encoded information. Frustration on the part of the animals about where to go in the early sessions was reflected in frequent attempts to get back to the original startbox of the day, but they gradually settled into running directly to the home-base with their large 0.5 g reward pellet. It is also noteworthy that, upon opening the door of the startbox at the beginning of a memory encoding trial, the animals in the home-base protocol would generally pause and inspect the arena and surrounding cues, as if to identify their location within it and work out where to go. A representation of self-location, likely mediated by hippocampal encoding using place-cells, complemented by prefrontal activity contributing to vectorial representations of goal-location and then trajectory (Ito et al., 2015; Sarel et al., 2017), should also be sensitive changes in the intra- and extra-arena cues between encoding and retrieval. We observed a decline of performance to chance levels in Expt. 3A when these cues were occluded by curtains, in contrast to what was observed in Expt. 1A.
Conclusion and implications for single-unit recording studies
Does any of this matter for single-unit recording studies? We suspect it does because, for example, goal-location recall is generally not necessary in any task in which an animal deploys a praxic egocentric strategy. To the contrary, the navigational system need only keep track of the animal's movements and compute - using path-integration - a 'return vector' that would later enable the action system to carry it out. Interestingly, there is now considerable interest in the single-unit recording community about the possibility that self-location is encoded egocentrically in the medial and lateral entorhinal cortex using the metric of grid- and landmark-vector cells (Moser et al., 2008; Knierim et al., 2014). Head-direction cell firing likewise implies representation of head orientation within an environment that is perceived as polarized (Dudchenko, 2015). In such coding frameworks, goal-location encoding does not matter - only the representation of how to get there. Like tourists lost in Manhattan who are told by a local resident to walk 5 blocks north and then take a left and walk 3 blocks west, they arrive at their destination without ever knowing where it is. Our analysis suggests that in situations in which there is a single start location to which the animal returns frequently, egocentric coding will gradually come to prevail as training continues (Packard & McGaugh, 1992). The gradual shift over learning of the receptive fields of CA1 place cells to reflect reward locations observed by Boccara et al., (2019), using a single start location, may be a similar phenomenon to the dominance of routes over goals observed by Grieves et al., (2016).
In contrast, place cells recorded when there is no explicit task requiring directed navigation display allocentric encoding of self-location that is sensitive to cue-card rotation (Muller et al., 1987). Reward related distortions of the metric of space may still occur in a manner that reflects aspects of the navigational task underway (Butler etal., 2019), but the coding of goals could nonetheless be allocentric. It remains a much harder task to identify the neurobiological mechanisms by which the nervous system identifies the location of goal place G from a remote start place S (a task that Sachin Deshmukh (pers. comm.) has amusingly identified as "someone else's problem"). However, the directed performance of animals in the watermaze from any point on the circumference of the pool to the hidden platform (Morris, 1984), of rats on successive hexagons of the honeycomb maze (Wood et al., 2018), and of the animals trained in the home-location protocol in the present study, collectively indicate that spatial memory recall can be realised from a remote location. The location recalled can surely much further away than the several theta cycles of distance observed in the important studies of vicarious trial and error behaviour by (Johnson & Redish, 2007) but dissociations of remote allocentric goal-identification independent of egocentric path-directionality have not to our knowledge yet been conducted. The home-base event arena protocol may yet lend itself to such a study.
In conclusion, our findings qualify but do not invalidate our recent observations of the determinants of selective everyday memory and forgetting (Nonaka et al., 2017). Rather, they have led us to a modification of the protocol that can hopefully serve as an effective test-bed for further examining the impact of parameters such as trial-spacing, unexpected novelty and neuromodulation on memory retention using both behavioural and physiological techniques.
Acknowledgements
We thank Patrick Spooner for the event arena construction and software support, and thank the two anonymous reviewers for their constructive suggestions.
Funding
This work was supported by intra- and extramural funding from Dart Neurosciences, and by grants to RGMM from the European Research Council (NEUROSCHEMA - 268800) and the Wellcome Trust (207481/Z/17/Z).
Footnotes
Author Contributions
NB supervised Expt. 1A by BM in San Diego, with the study coordinated by MP. MC conducted the replication (Expt. 1B) in Edinburgh. LL and DT organised follow-up studies in Edinburgh (Expts. 2-3), assisted by ZI. RM conceived and, with TT, conducted the first stable home-location study; a full replication was conducted by AA and TT (Expts. 3A). DT organised various follow-up and confirmatory replication studies with the assistance of students and interns in the laboratory - EB, LS, TM and AP -collectively establishing that allocentric encoding depended on the integrity of hippocampal function (Expt. 3B). RM and DT did the surgeries to implant cannulae in dorsal hippocampus (Exp. 3B). JC did the detailed video-analysis of the animal paths in Expts. 3. MM assisted with the figures and provided a critical appraisal of the experiment and manuscript. The manuscript was written by RM, MP, NB, LL, MM and DT.
Competing interest: None.
Ethics
The studies were conducted to comply with animal experimentation regulations of DART Neuroscience, NIH and the United Animals (Scientific Procedures) Act.
Data accessibility
The database containing the primary data used in this study title Broadbent_2019_database.sav will be made available on Figshare by Wiley.
References
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2001;356:1467–1482. doi: 10.1098/rstb.2001.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, da Silva BM, Morris RGM. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25:5845–5856. doi: 10.1523/JNEUROSCI.0698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara CN, Nardin M, Stella F, O’Neill J, Csicsvari J. The entorhinal cognitive map is attracted to goals. Science. 2019;363:1443–1447. doi: 10.1126/science.aav4837. [DOI] [PubMed] [Google Scholar]
- Butler WN, Hardcastle K, Giocomo LM. Remembered reward locations restructure entorhinal spatial maps. Science. 2019;363:1447–1452. doi: 10.1126/science.aav5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarades. Review of animal data from experimental studies. 2014. http://www.dcn.ed.ac.uk/camarades/
- Dudai Y. The restless engram: consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. Spatial cognition: a tabula rasa for the sense of direction. Curr Biol. 2015;25:R143–144. doi: 10.1016/j.cub.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Eacott M, Easton A. On familiarity and recall of events by rats. Hippocampus. 2007;17:890–897. doi: 10.1002/hipo.20325. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learn Mem. 2005;12:221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Galef BG, Buckley LL. Use of foraging trails by Norway rats. Animal Behaviour. 1996;51:765–771. [Google Scholar]
- Grieves RM, Wood ER, Dudchenko PA. Place cells on a maze encode routes rather than destinations. Elife. 2016;5 doi: 10.7554/eLife.15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the Hippocampal Polyglot: The Hippocampus as a Path Integration System. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Chen LL, Markus EJ. “Dead Reckoning”, Landmark Learning, and the Sense of Direction: A Neurophysiological and Computational Hypothesis. Journal of Cognitive Neuroscience. 1991;3:190–202. doi: 10.1162/jocn.1991.3.2.190. [DOI] [PubMed] [Google Scholar]
- Morris RGM. In: Exploration in animals and humans. Archer J, Birke L, editors. Van Nostrand Reinhold; London: 1983. Neural subsystems of exploration in rats; pp. 117–146. [Google Scholar]
- Morris RGM. Developments of a watermaze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JBJ. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. Journal of Neuroscience. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Fitzpatrick R, Lapira J, Wheeler D, Spooner PA, Corcoles-Parada M, Munoz-Lopez M, Tully T, Peters M, Morris RGM. Everyday memory: towards a translationally effective method of modelling the encoding, forgetting and enhancement of memory. Eur J Neurosci. 2017;46:1937–1953. doi: 10.1111/ejn.13637. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double Dissociation of Fornix and Caudate Nucleus Lesions on Acquisition of Two Water Maze Tasks: Further Evidence for Multiple Memory Systems. Behavioral Neuroscience. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Cambridge, MA: 2013. [Google Scholar]
- Pearce JM, Roberts ADL, Good M. Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature. 1998;396:75–77. doi: 10.1038/23941. [DOI] [PubMed] [Google Scholar]
- Redish AD. Beyond the Cognitive Map: From place cells to episodic memory. MIT Press; Cambridge, MA, USA: 1999. [Google Scholar]
- Richards BA, Frankland PW. The Persistence and Transience of Memory. Neuron. 2017;94:1071–1084. doi: 10.1016/j.neuron.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff E, Martin SJ, Bridge H, Hoz L, Poeschel B, McCulloch J, Morris RGM. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature Neuroscience. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Moreno A, Genzel L, Yamasaki M, Takeuchi T, Canals S, Morris RGM. Silent Learning. Curr Biol. 2018;28:3508–3515.:e3505. doi: 10.1016/j.cub.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Sarel A, Finkelstein A, Las L, Ulanovsky N. Vectorial representation of spatial goals in the hippocampus of bats. Science. 2017;355:176–180. doi: 10.1126/science.aak9589. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate-or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RGM. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–362. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Wang SH, Redondo RL, Morris RGM. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci U S A. 2010;107:19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Rev. 1998;22:209–220. doi: 10.1016/s0149-7634(97)00002-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Brooks BL. Calibrating Space: Exploration Is Important for Allothetic and Idiothetic Navigation. Hippocampus. 1999;9:659–667. doi: 10.1002/(SICI)1098-1063(1999)9:6<659::AID-HIPO7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Coles BL, Bellerive CH. Food carrying: a new method for naturalistic studies of spontaneous and forced alternation. J Neurosci Methods. 1995;61:139–143. doi: 10.1016/0165-0270(95)00035-s. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Wood RA, Bauza M, Krupic J, Burton S, Delekate A, Chan D, O’Keefe J. The honeycomb maze provides a novel test to study hippocampal-dependent spatial navigation. Nature. 2018;554:102–105. doi: 10.1038/nature25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database containing the primary data used in this study title Broadbent_2019_database.sav will be made available on Figshare by Wiley.